Introduction
Thyroid cancer is the most common malignant tumor in
the endocrine system, among which papillary carcinoma is the most
common, accounting for ~88% of thyroid tumors (1). In patients with papillary thyroid
carcinoma, the early symptoms are not obvious (2). Thyroid nodules are identified in the
majority of patients using B-mode ultrasound during physical
examination, and diagnosis is confirmed following histopathological
examination (3). Generally, the
survival rate of patients without metastasis of papillary thyroid
carcinoma is >80% at 10 years following surgical treatment
(4). However, in patients with
lymph node metastasis, the tumor cannot be completely removed by
surgery, thus the prognosis following palliative treatment remains
poor (2). Therefore, early
detection, diagnosis and surgical treatment are important means to
improve the prognosis of patients with papillary thyroid
carcinoma.
Traditional chemotherapy drugs generally have no
significant effect on thyroid tumors, and surgical treatment is
still the preferred treatment for patients with thyroid tumors
(5). With the development of
molecular biology and immunohistochemical technology in medical
diagnosis and auxiliary diagnosis, the application value of
molecular markers with high sensitivity and high specificity in
auxiliary diagnosis of tumor is gradually increasing. The
application of specific molecular markers in tumor screening can
indicate early tumor lesions and the presence of lymph node
metastasis (6). In addition,
targeting and regulating the expression of molecules in cancer can
influence the occurrence and development of diseases, thus
providing a theoretical basis for targeted therapy in the treatment
of cancer.
Potassium channels exist in a variety of cells and
are an important determinant of membrane potential (7). By controlling the opening and closing
of potassium channels, membrane potential and signal transduction
pathways, such as calcium ions, can be changed (8). Previous studies have demonstrated that
there are various potassium channels on tumor cells, and these
potassium channels play an important role in the regulation of
tumor cell proliferation and apoptosis, and their molecular
mechanisms are involved in multiple pathways (9–11). In
addition, some potassium channels have specific high expression in
some tumor cell membranes, which can be used as a basis for novel
antitumor targets (12,13).
Potassium inwardly rectifying channel subfamily J
member 2 (KCNJ2) gene is located on chromosome 17, 17 q23, its
coding protein is inward rectifier potassium channel Kir2.1
(14). The association between
KCNJ2 and tumor progression has been demonstrated; however,
mechanistic studies have not yet been performed (15). A study has reported that in small
cell lung cancer, inhibition of KCNJ2 expression can promote cell
apoptosis, inhibit the cell cycle and promote the sensitivity of
cancer cells to chemotherapy drugs (16). In addition, silencing KCNJ2
expression can significantly decrease the invasive and metastatic
abilities, and epithelial-to-mesenchymal transition (EMT) of
gastric cancer cells (17). It has
been reported that KCNJ2 expression is upregulated in thyroid
cancer tissues (18,19). However, the specific role of KCNJ2
in papillary thyroid carcinoma remains unknown.
Heterotrimeric G protein has been reported to be
involved in several biological activities, including cell
proliferation, differentiation, invasion and angiogenesis (20,21). G
protein γ2 subunit (Gng2/GNG2) is one of the subunits of the
Gβγ-dimer, composed of a heterotrimeric G protein with a Gα-subunit
(22). Overexpression of GNG2
inhibits metastasis of human malignant melanoma cells (23). Thus, GNG2 may be a molecular target
for the prevention and treatment of malignant melanoma metastasis
(23).
The present study aimed to investigate the specific
role of KCNJ2 in papillary thyroid carcinoma, and its regulatory
molecular mechanism to provide a theoretical basis for molecular
biological screening and targeted therapy for papillary thyroid
carcinoma.
Materials and methods
Cell culture
The papillary thyroid carcinoma cell lines, BCPAP
and TPC-1, and the human thyroid normal cell line, Nthy-ori 3-1,
were purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences and maintained in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% (v/v) penicillin/streptomycin
(Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2.
Bioinformatics analysis
StarBase website (version 2.0, http://starbase.sysu.edu.cn/) was used to predict the
expression of KCNJ2 and GNG2 in all types of thyroid cancer
(24). The STRING website (version
11.0, http://string-db.org/) was used to
predict the relationship between KCNJ2 and GNG2. The expression of
KCNJ2 in thyroid cancer tissues was predicted using the GEPIA
database (gepia.cancer-pku.cn/) (25).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA at ~65°C for 10
min using the SuperScript™ III Reverse Transcriptase kit (cat. no.
18080093; Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. qPCR was subsequently performed
using the SYBR Green qPCR Master mix (Takara Biotechnology Co.,
Ltd.) and StepOnePlus Real-Time PCR system (Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
95°C for 10 min, 40 cycles of 95°C for 10 sec, 55°C for 10 sec, and
72°C for 30 sec. KCNJ2 and GNG2 were normalized to the internal
reference gene GAPDH. Relative expression levels were calculated
using the 2−ΔΔCq method (26). The following primer sequences were
used for qPCR: GNG2 forward, 5′-ATCGATATGGCCACCAACAACACAGCTA-3′ and
reverse, 5′-TTACAGGATGGCAGAAGAAC-3′; KCNJ2 forward,
5′-TGGATGCTGGTTATCTTCTGC-3′ and reverse,
5′-AGCCTATGGTTGTCTGGGTCT-3′; and GAPDH forward,
5′-TCAAGGCTGAGAACGGGAAG-3′ and reverse,
5′-TGGACTCCACGACGTACTCA-3′.
Western blotting
Total protein was extracted from each group of cells
using RIPA lysis buffer (Sigma-Aldrich; Merck KGaA) and the protein
concentrations were determined using a BCA protein assay kit
(Bio-Rad Laboratories, Inc.). A total of 40 µg protein/well was
separated via SDS-PAGE on 10% gel. The separated proteins were
subsequently transferred onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc.) and pre-blocked with 5% non-fat milk
for 1 h at room temperature. The membranes were then incubated with
rabbit anti-human polyclonal antibody and mouse anti-human GAPDH
monoclonal antibody (1:1,000; Abcam) overnight at 4°C. Membranes
were washed with PBS and incubated with secondary HRP-conjugated
anti-rabbit antibody (1:5,000; cat. no. ab205718; Abcam) for 1 h at
room temperature. Protein bands were visualized using an enhanced
chemiluminescence detection system (EMD Millipore), and protein
band intensity was determined using ImageJ software (version 146;
National Institutes of Health). The ratio of gray value of target
protein band to that of GAPDH was regarded as the relative protein
expression. The following primary antibodies were used: Anti-KCNJ2
(1:1,000; cat. no. ab109750; Abcam), anti-matrix metalloproteinase
(MMP)2 (1:1,000; cat. no. ab92536; Abcam), anti-MMP9 (1:1,000; cat.
no. ab76003; Abcam), anti-N-cadherin (1:1,000; cat. no. ab76011;
Abcam), anti-Snail (1:1,000; cat. no. ab216347; Abcam), anti-zinc
finger E-box binding homeobox 1 (ZEB1; 1: 1,000; cat. no. ab203829;
Abcam), anti-GNG2 (1:1,000; cat. no. ab198225; Abcam) and
anti-GAPDH (1:1,000; cat. no. ab8245; Abcam).
Plasmid construction and
transfection
The KCNJ2 gene was knocked down using two different
KCNJ2 short hairpin (sh)RNAs (shKCNJ2-1 and shKCNJ2-2) with
lentiviral expression vector GV 493. A negative control shRNA
(sh-NC) was used to control for off-target and non-specific effects
of shRNA treatment at a concentration of 20 nM. All purchased from
Shanghai GenePharma Co., Ltd. Transfection was performed using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). TPC-1 cells were seeded in 6-well plates at a
density of 1×106 cells/well. Small interfering RNA
(si)-GNG2-1, si-GNG2-2 and NC at a concentration of 20 nM, all
purchased from Shanghai GenePharma Co., Ltd. The vectors (Shanghai
GenePharma Co., Ltd.) were transfected into TPC-1 cells using
Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. After
transfection for 48 h at 37°C with 5% CO2, the
transfection efficiency was measured via RT-qPCR analysis, and then
subsequent assays were performed after 48 h. The following
sequences were used: si-GNG2, 5′-ATAGATCTCCGGACTCTAAGATGAAGTT-3′
and 5′-ATAGATCTCCGGACTCTAAGATGAAGTT-3′; and si-NC,
5′-GGAUUGAAUCAAGUCAUUC-3′ and 5′-GAAUGACUUGAUUCAAUCC-3′. Cells were
divided into the following groups: Control, sh-NC, sh-KCNJ2-2,
sh-KCNJ2-2 + si-NC and sh-KCNJ2-2 + si-GNG2-2.
MTT assay
The viability of cancer cells was assessed via an
MTT assay. Cells (1×103 cells/well) were seeded into
96-well plates with 0.1 ml for each well and incubated at 37°C with
5% CO2. Cells were transfected for 24 h at 37°C, and 10
µl MTT was subsequently added to each well. Cells were cultivated
for an additional 4 h and 150 µl DMSO was added to each well and
shaken at low speed for 10 min to make the crystals fully
dissolved. Viability was subsequently analyzed at a wavelength of
570 nm, using the Fisherbrand™ accuSkan™ GO UV/Vis microplate
spectrophotometer (Thermo Fisher Scientific, Inc.).
Colony formation assay
Following cell transfection, cells were seeded into
6-well plates at a density of 500 cells/well and cultured in DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.), under normal
conditions for 14 days at 37°C until visible colonies appeared.
Cells were fixed with methanol for 15 min at room temperature and
subsequently stained with 0.5% crystal violet (Sigma-Aldrich; Merck
KGaA) for 30 min at room temperature, prior to observing the cell
colonies with a light contrast microscope (Olympus Corporation;
magnification, ×1).
Wound healing assay
Cells (1×105 cells/well) were seeded into
6-well plates and cultured (serum-starved) until they reached 80%
confluence. Cell monolayers were subsequently wounded (width, 2 mm)
using sterile plastic pipette tips and washed with PBS to remove
cell debris. The cells were then cultured with fresh DMEM. Cells
were observed in five randomly selected fields under a light
microscope (magnification, ×100) at 0 and 24 h. The distance of
cell migration into the wound area was calculated using ImageJ
software v1.48u (National Institutes of Health).
Transwell Matrigel assay
Cells in 200 µl serum-free DMEM were plated in the
upper chambers of 24-well Transwell plates (Corning, Inc.) with
8-µm pore inserts coated with Matrigel (BD Biosciences), at a
concentration of ~1×106 cells/ml. DMEM (0.5 ml)
supplemented with 10% FBS was plated in the lower chambers. For the
invasion assay, Transwell membranes were coated with Matrigel (BD
Biosciences) at 37°C for 30 min, according to the manufacturer's
instructions. Following incubation for 24 h at 37°C with 5%
CO2, cells were fixed with paraformaldehyde for 15 min
at 25°C and stained with 0.1% crystal violet for 30 min at room
temperature. Cells in the upper chambers were removed using cotton
swabs, while stained cells were observed under a light contrast
microscope (Olympus Corporation; magnification, ×100).
Statistical analysis
Data are presented as the mean ± standard deviation
(n≥3). Statistical analyses were performed using SPSS statistical
software (version 22.0; IBM Corp.). Comparisons among multiple
groups were analyzed using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
KCNJ2 expression in papillary thyroid
carcinoma cells
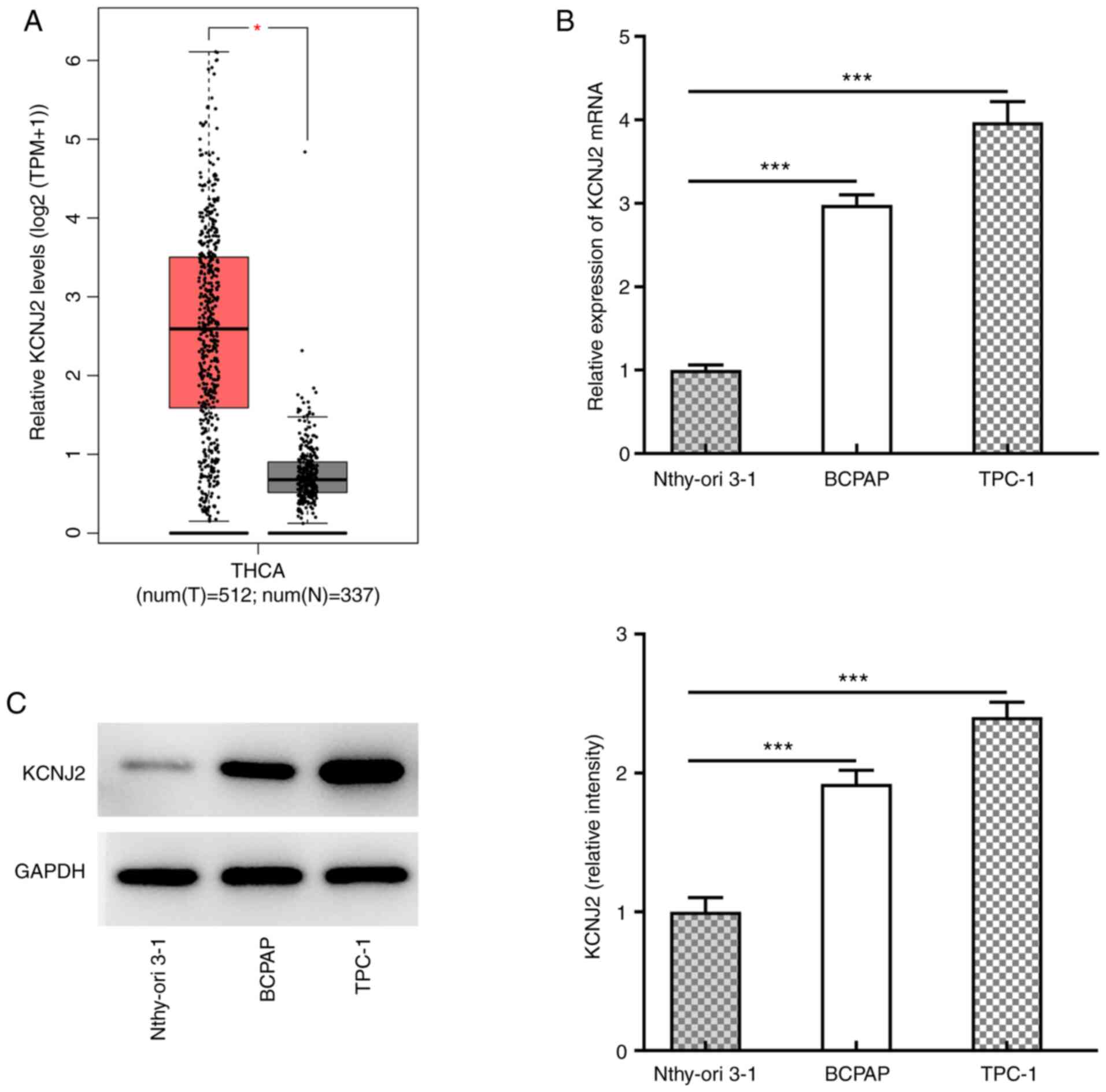
The prediction results from the GEPIA database
demonstrated that KCNJ2 expression was upregulated in thyroid
cancer tissues (Fig. 1A). KCNJ2
expression in papillary thyroid carcinoma cell lines was
subsequently analyzed. RT-qPCR (Fig.
1B) and western blot analyses (Fig.
1C) demonstrated that KCNJ2 expression was significantly
increased in papillary thyroid carcinoma cell lines, with the most
significant increase in TPC-1 cells. Thus, TPC-1 cells were
selected for subsequent experimentation.
Interfering with KCNJ2 inhibits the
proliferation of papillary thyroid carcinoma cells
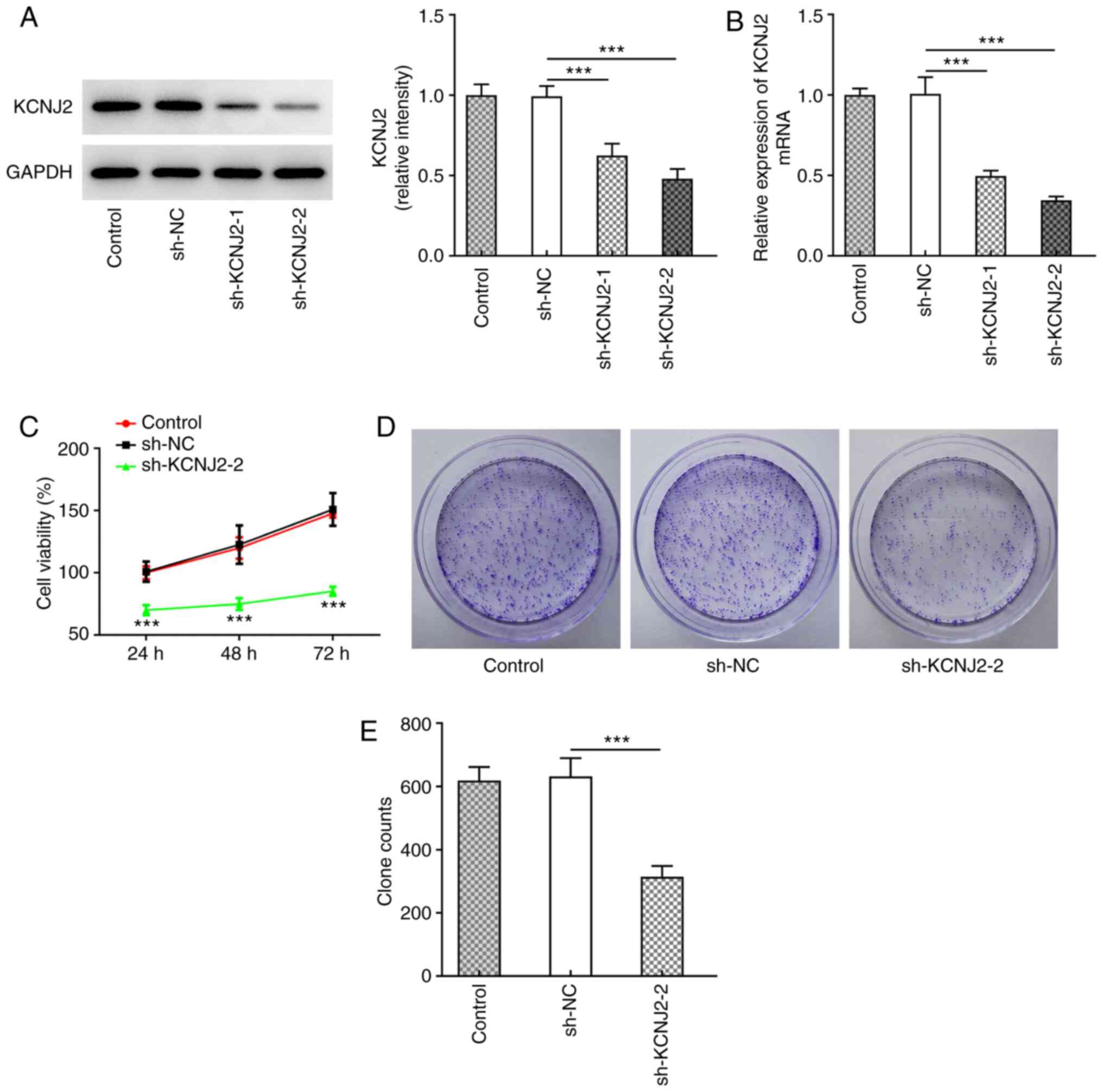
KCNJ2 expression in papillary thyroid carcinoma
cells was inhibited following cell transfection, as assessed via
western blotting (Fig. 2A) and
RT-qPCR (Fig. 2B). The results
demonstrated that KCNJ2 expression was significantly decreased in
the sh-KCNJ2-1 and SH-KCNJ2-2 groups compared with the sh-NC group,
and the decrease was more notable in the sh-KCNJ2-2 group. Thus,
the sh-KCNJ2-2 group was selected for subsequent experimentation.
Cell viability was assessed via the MTT (Fig. 2C) and colony formation (Fig. 2D and E) assays. The results
demonstrated that the cell survival rate and proliferative ability
of cells in the sh-KCNJ2-2 group significantly decreased compared
with the sh-NC group. Taken together, these results suggested that
interfering with KCNJ2 inhibited the proliferation of papillary
thyroid carcinoma cells.
Interfering with KCNJ2 inhibits
invasion, migration and the EMT process of papillary thyroid
carcinoma cells
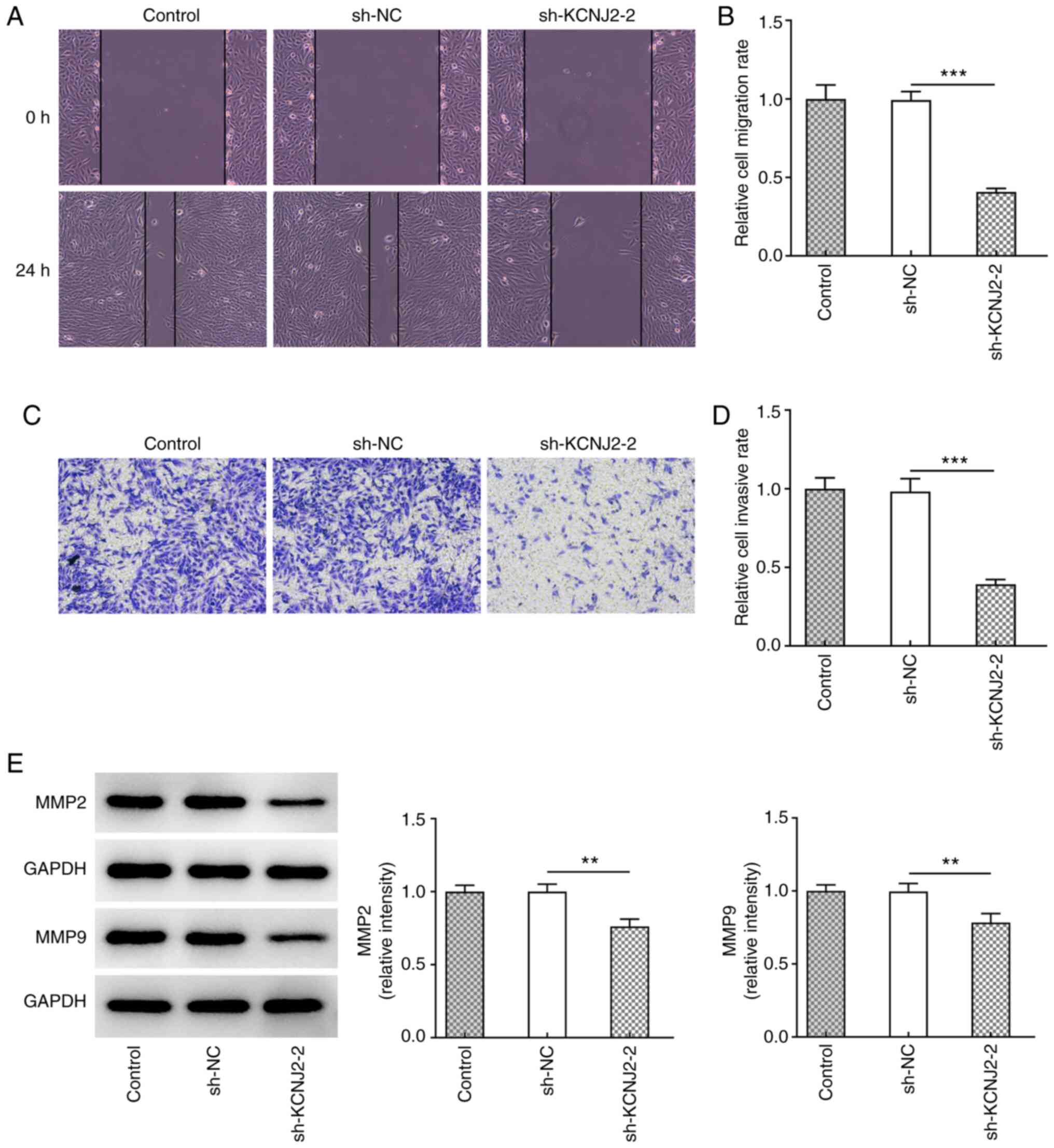
The invasive and migratory abilities of papillary
thyroid carcinoma cells were assessed. The results of the wound
healing assay demonstrated that KCNJ2 knockdown significantly
inhibited the migratory ability of papillary thyroid carcinoma
cells (Fig. 3A and B). In addition,
the results of the Transwell assay demonstrated that KCNJ2
knockdown significantly inhibited the invasive ability of papillary
thyroid carcinoma cells (Fig. 3C and
D). Western blot analysis was performed to detect the
expression levels of the transport-related proteins, MMP2 and MMP9.
The results demonstrated that MMP2 and MMP9 expression
significantly decreased in the sh-KCNJ2-2 group compared with the
sh-NC group (Fig. 3E). The
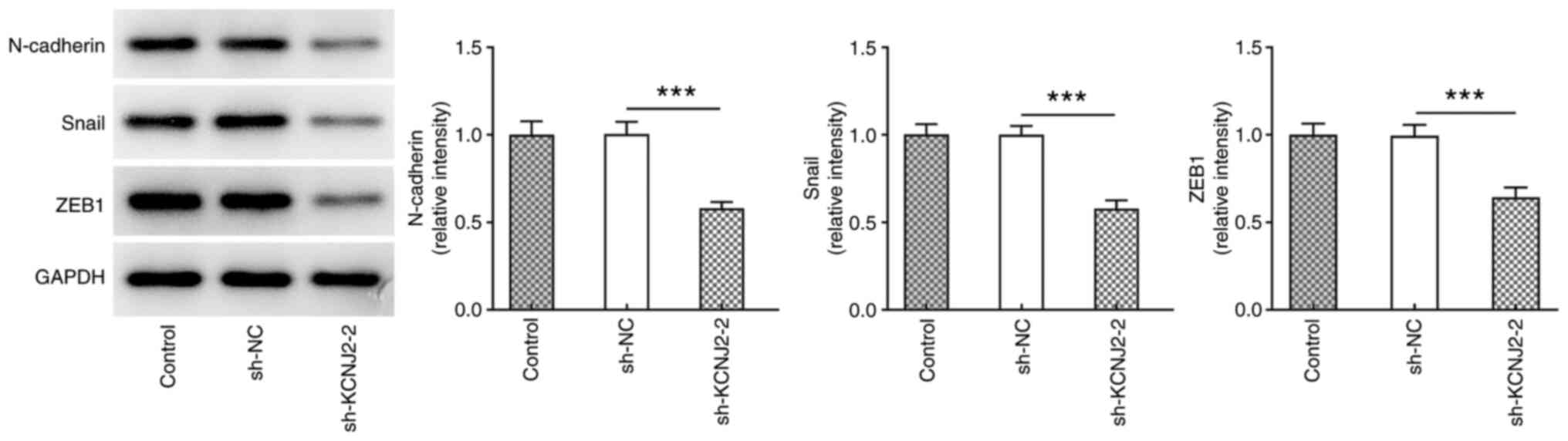
expression levels of the EMT-related proteins, N-cadherin, Snail
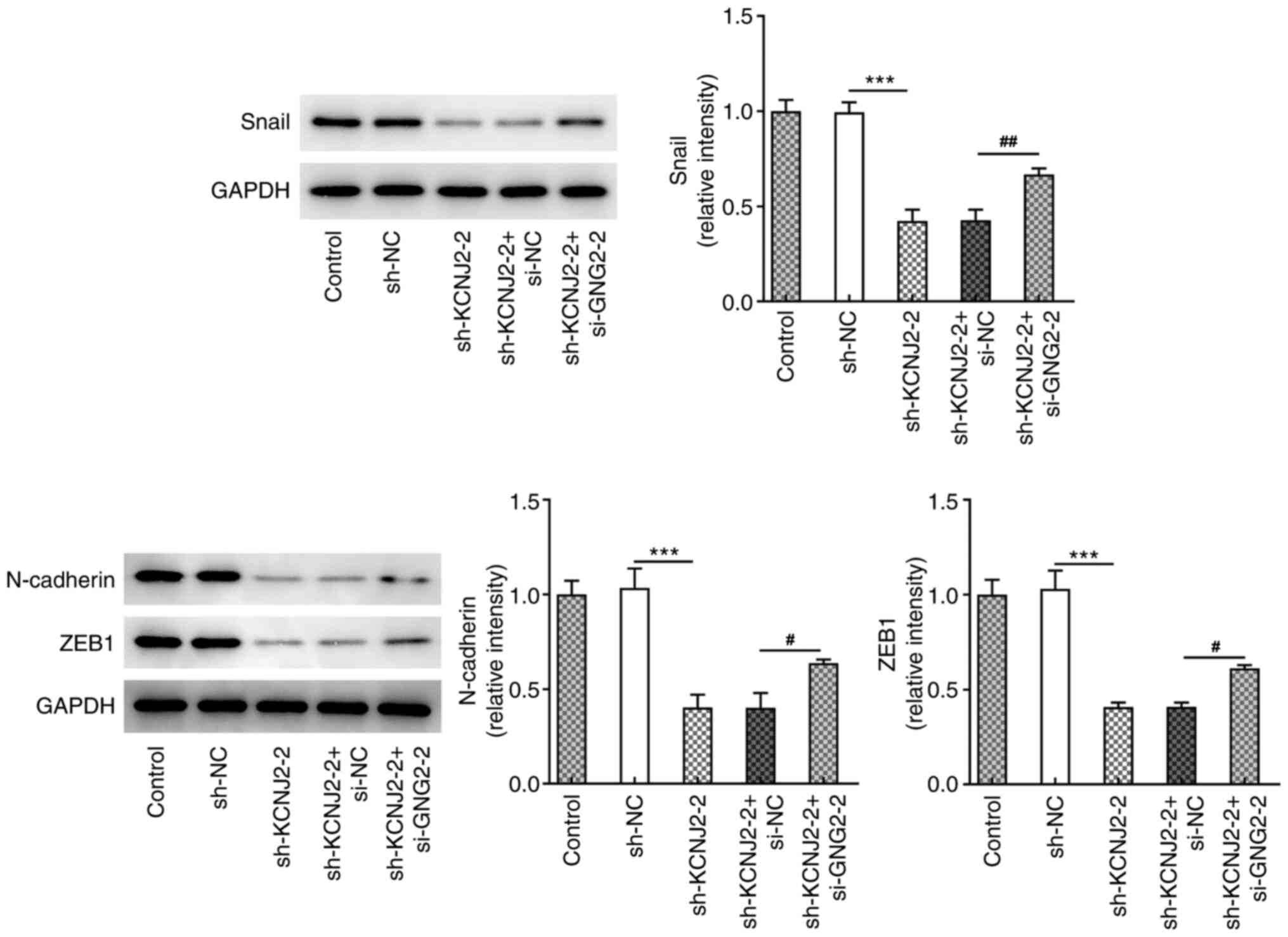
and ZEB1 were also detected. The results demonstrated that
N-cadherin, Snail and ZEB1 expression significantly decreased in
sh-KCNJ2-2 group compared with the sh-NC group (Fig. 4).
Interfering with KCNJ2 upregulates
GNG2 expression in papillary thyroid carcinoma cells
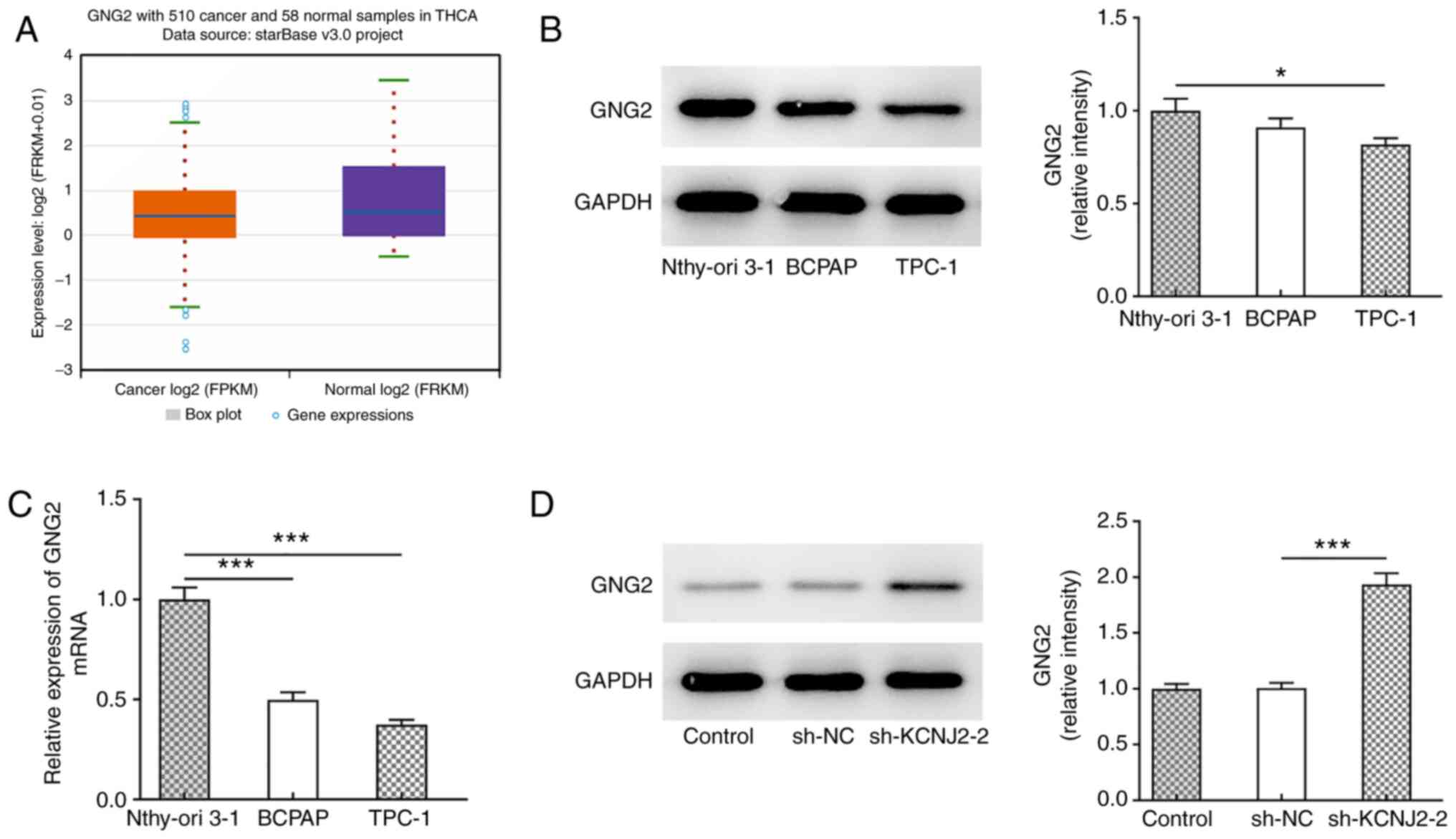
The StarBase database predicted that GNG2 expression
was downregulated in thyroid cancer (Fig. 5A), and western blotting (Fig. 5B) and RT-qPCR (Fig. 5C) were performed to detect GNG2
expression in papillary thyroid carcinoma cells. The results
demonstrated that GNG2 expression was significantly decreased in
papillary thyroid carcinoma cells compared with the normal thyroid
cells, Nthy-ori 3-1. Following transfection to suppress KCNJ2
expression, GNG2 expression significantly increased in the
sh-KCNJ2-2 group compared with the sh-NC (Fig. 5D). TPC-1 cells were selected for
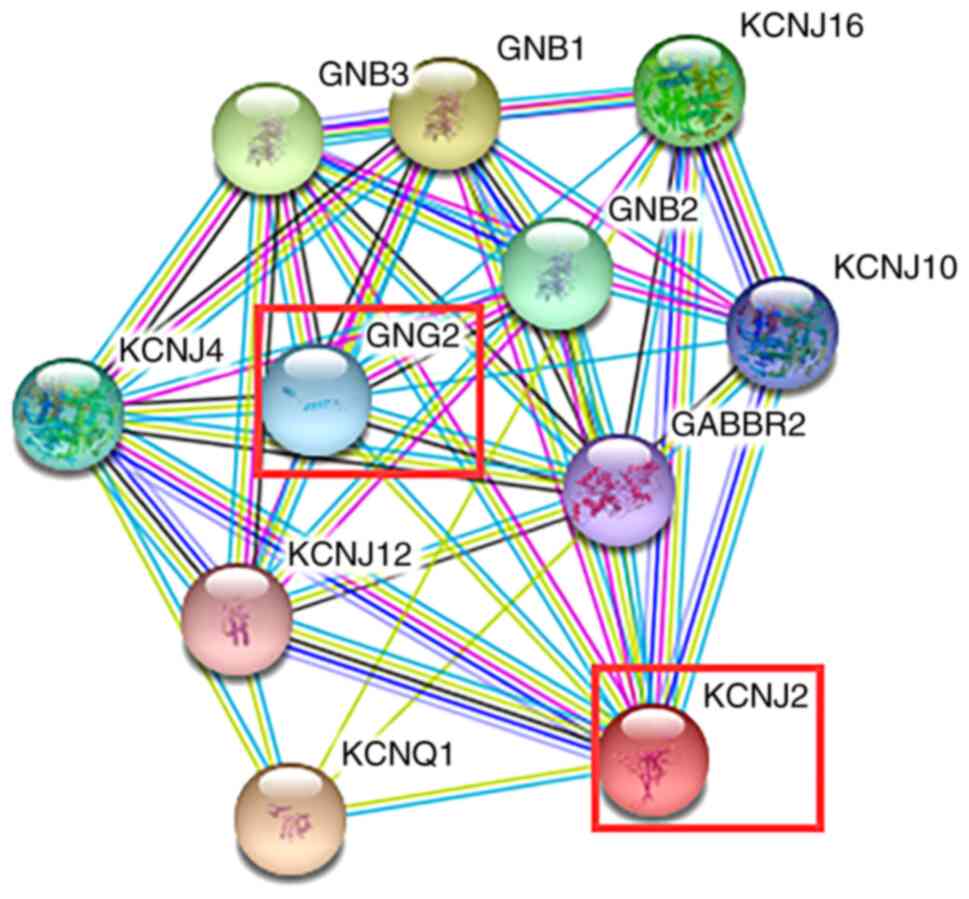
subsequent experimentation. The results from the STRING database
demonstrated that KCNJ2 could regulate GNG2 expression (Fig. 6). Collectively, these results
suggested that interference with KCNJ2 upregulated GNG2
expression.
Knockdown of GNG2 expression partially
reverses the effects of KCNJ2 interference on the proliferation,
migration and EMT process of papillary thyroid carcinoma cells
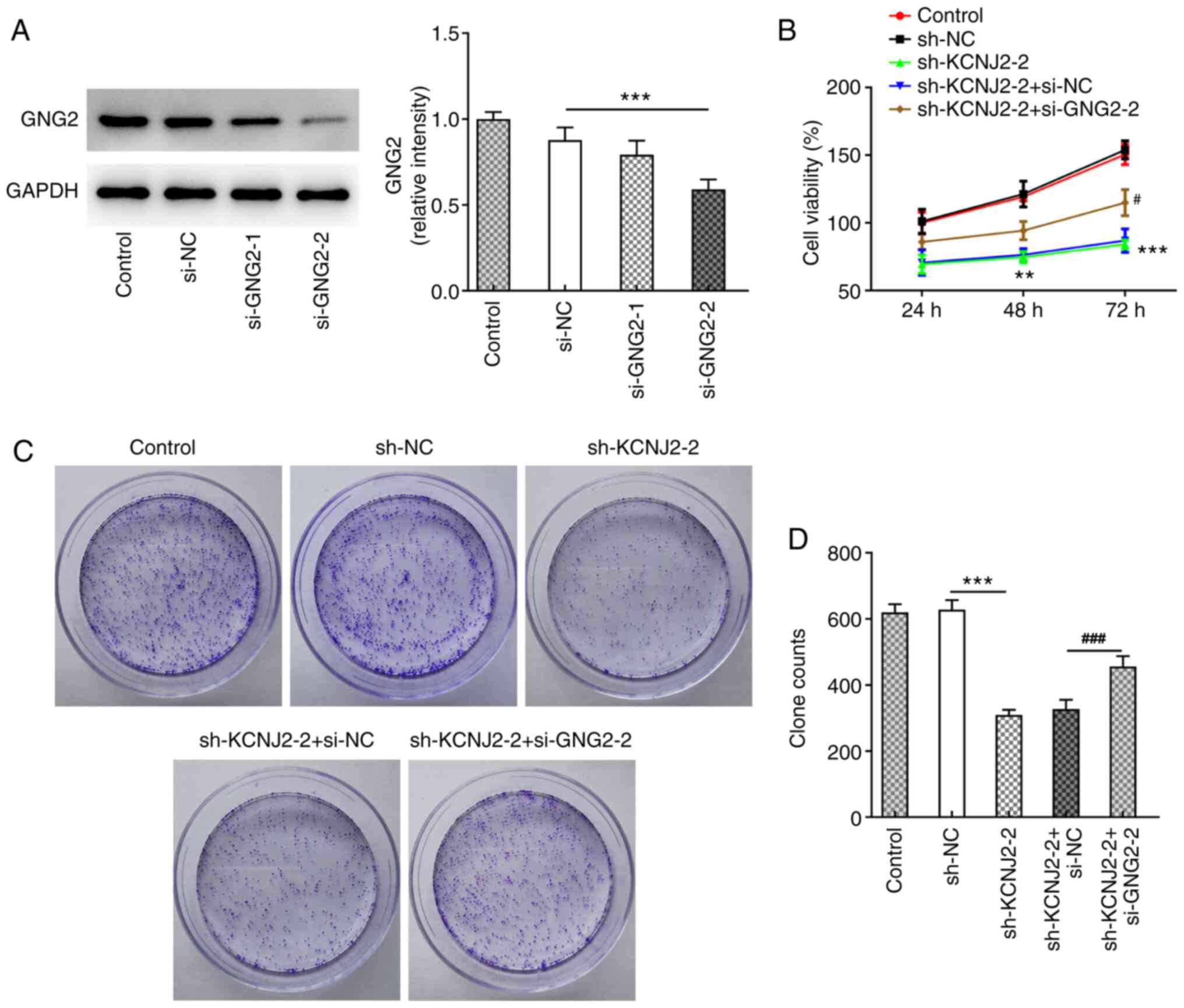
Cell transfection was performed to interfere with
GNG2 expression in papillary thyroid carcinoma cells, and western
blot analysis was performed to detect the interference effect
(Fig. 7A). The results demonstrated
that si-GNG2-1 and si-GNG2-2 expression levels significantly
decreased compared with the si-NC group, and the decrease was more
notable in the si-GNG2-2 group, thus si-GNG2-2 was selected for
subsequent experimentation. The MTT (Fig. 7B) and colony formation (Fig. 7C and D) assays were performed to
detect the proliferative ability of cells. The results demonstrated
that cell viability increased in the sh-KCNJ2-2 + si-GNG2-2 group
compared with the sh-KCNJ2-2 + si-NC group, suggesting that GNG2
interference could partially reverse the effect of KCNJ2
interference on papillary thyroid carcinoma cell proliferation. In
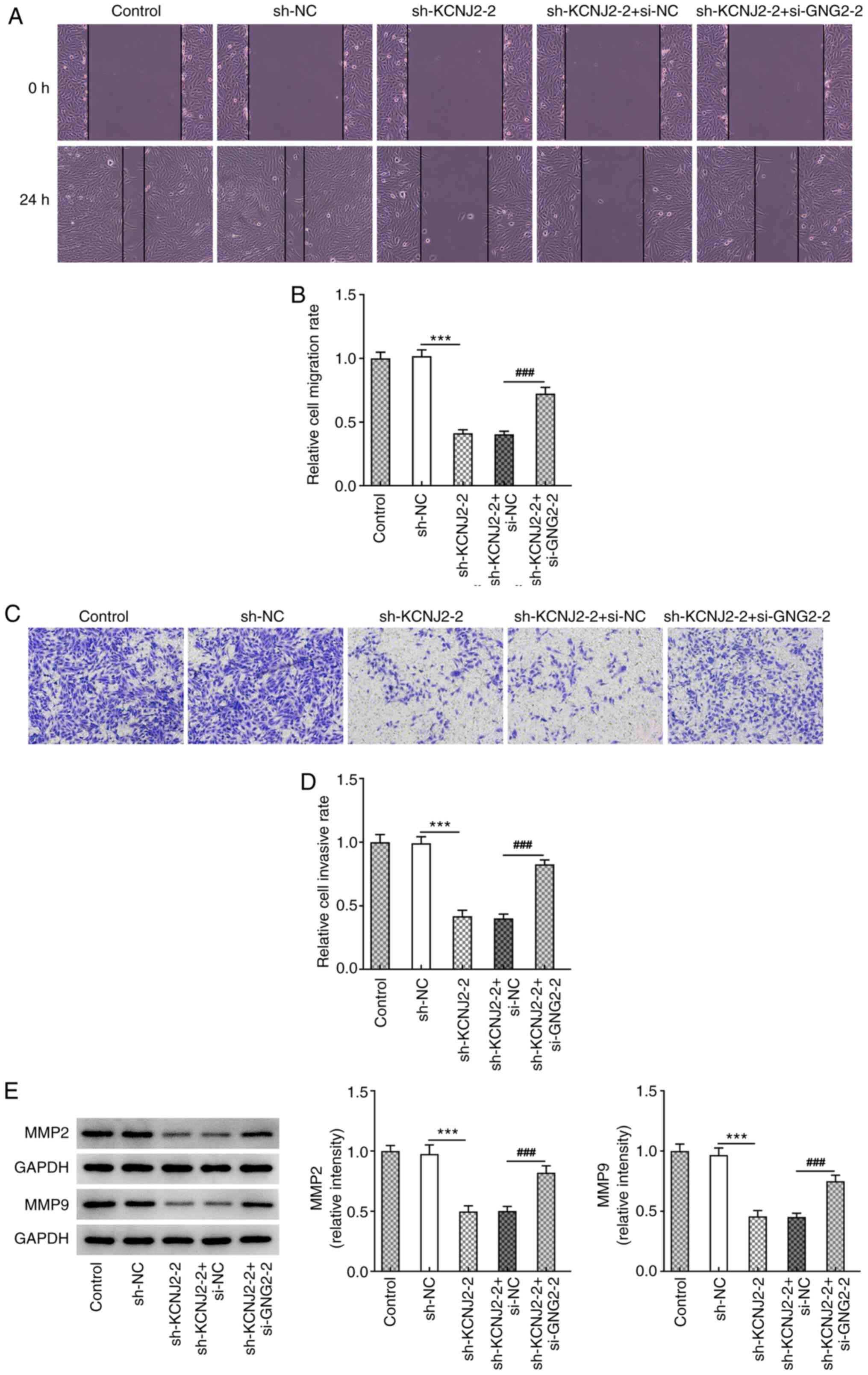
addition, the results of the wound healing assay demonstrated that
cell migration significantly increased in the sh-KCNJ2-2 +
si-GNG2-2 group compared with the sh-KCNJ2-2 + si-NC (Fig. 8A and B). The results of the
Transwell assay demonstrated that cell invasion significantly
increased in the sh-KCNJ2-2 + si-GNG2-2 group compared with the
sh-KCNJ2-2 + si-NC group (Fig. 8C and
D), accompanied by increased MMP2 and MMP9 expression (Fig. 8E). Furthermore, the expression trend
of the EMT-related proteins (N-cadherin, Snail and ZEB1) was
consistent with that of the migration-related proteins (Fig. 9). Taken together, these results
suggested that interference with GNG2 could partially reverse the
effects of KCNJ2 interference on papillary thyroid carcinoma cell
invasion, migration and the EMT process.
Discussion
According to the GEPIA database, KCNJ2 expression
was found to be upregulated in patients with thyroid cancer in the
present study. Furthermore, this study also confirmed that KCNJ2
expression was significantly increased in papillary thyroid
carcinoma cells, and KCNJ2 knockdown inhibited cell proliferation,
invasion, migration and the EMT process. According to the STRING
database, there is a regulatory relationship between KCNJ2 and
GNG2. In addition, the StarBase database demonstrated that GNG2
expression was downregulated in thyroid cancer. The results of the
present study demonstrated that GNG2 expression was significantly
downregulated in papillary thyroid carcinoma cells, and GNG2
interference partially reversed the effects of KCNJ2 interference
on the proliferation, migration and the EMT process of papillary
thyroid carcinoma cells.
The present study demonstrated that KCNJ2 expression
was significantly upregulated in TCP-1 papillary thyroid carcinoma
cells. This finding was consistent with a previous study by Kim
et al (18), which reported
that KCNJ2 expression is significantly upregulated in papillary
thyroid carcinoma tissues compared with normal tissues. KCNJ2 is a
potassium ion channel, which plays an important role in the
occurrence and development of tumors (27). A previous study demonstrated that
KCNJ2 can be used as a biomarker for prognosis of gastric cancer
(28). In addition, KCNJ2 can
promote the invasion, metastasis and the EMT process of gastric
cancer cells by interacting with serine/threonine-protein kinase 38
(17). However, only a few studies
have investigated the role of KCNJ2 in other types of cancer and
determined its molecular mechanisms (15,29).
The results of the present study demonstrated that interference
with KCNJ2 expression in papillary thyroid carcinoma cells
significantly decreased the proliferative, invasive and migratory
abilities of the cells, and inhibited the EMT process. Of note, the
BCPAP thyroid cancer cells used in the present study have been
proven to be a problematic cell line as they are considered to be
poorly differentiated thyroid cancer cells (30). However, these effects did not have
any impact on the experimental results of the present study, as it
was confirmed that KCNJ2 was expressed at low levels in TPC-1
papillary thyroid carcinoma cells, as predicted by the GEPIA
database. Thus, all experiments were performed using TPC-1
cells.
Previous studies on GNG2 focus on its role in
melanoma, for example a previous study demonstrated that GNG2
expression is downregulated in mouse malignant melanoma and human
melanoma cell lines (31).
Overexpression of GNG2 enhances the proliferation of melanoma
cells, suggesting that GNG2 may be a novel molecular target for the
treatment of malignant melanoma (32). The results of the present study
demonstrated that GNG2 expression was downregulated in papillary
thyroid carcinoma cells; however, its specific molecular mechanism
has not yet been investigated. Prediction analysis revealed that
KCNJ2 can combine to regulate GNG2. Following interference with
KCNJ2 expression, GNG2 expression significantly increased in
papillary thyroid carcinoma cells, suggesting that KCNJ2 may
regulate GNG2 expression in papillary thyroid carcinoma. In
addition, the results confirmed that GNG2 interference could
partially reverse the effects of KCNJ2 interference on the
proliferation, migration and the EMT process of papillary thyroid
carcinoma cells.
In conclusion, the results of the present study
demonstrated the role of KCNJ2 in papillary thyroid carcinoma and
discussed its molecular mechanism, providing a theoretical basis
for molecular targeted therapy of papillary thyroid carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed generated during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
XH wrote the manuscript and analyzed the data. SC
and MH performed the experiments and literature search, supervised
the present study and revised the manuscript. XH and SC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howitt BE, Chang S, Eszlinger M, Paschke
R, Drage MG, Krane JF and Barletta JA: Fine-needle aspiration
diagnoses of noninvasive follicular variant of papillary thyroid
carcinoma. Am J Clin Pathol. 144:850–857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewinski A and Adamczewski Z: Papillary
thyroid carcinoma: A cancer with an extremely diverse genetic
background and prognosis. Pol Arch Intern Med. 127:388–389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambrosi F, Righi A, Ricci C, Erickson LA,
Lloyd RV and Asioli S: Hobnail variant of papillary thyroid
carcinoma: A literature review. Endocr Pathol. 28:293–301. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arianpoor A, Asadi M, Amini E, Ziaeemehr
A, Ahmadi Simab S and Zakavi SR: Investigating the prevalence of
risk factors of papillary thyroid carcinoma recurrence and
disease-free survival after thyroidectomy and central neck
dissection in Iranian patients. Acta Chir Belg. 120:173–178. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuchida N, Ikeda MA, Iotashino U, Grieco
M and Vecchio G: FUCA1 is induced by wild-type p53 and expressed at
different levels in thyroid cancers depending on p53 status. Int J
Oncol. 50:2043–2048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bogolyubova AV, Abrosimov AY, Selivanova
LS and Belousov PV: Histopatological and molecular genetic
characteristics of clinically aggressive variants of papillary
thyroid carcinoma. Arkh Patol. 81:46–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson WF: Potassium channels in
regulation of vascular smooth muscle contraction and growth. Adv
Pharmacol. 78:89–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martelli A: Potassium channels: A big
family, many different targets, great pharmacological
opportunities. Curr Med Chem. 25:26262018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel SH, Edwards MJ and Ahmad SA:
Intracellular ion channels in pancreas cancer. Cell Physiol
Biochem. 53:44–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Zou W, Zhou SS and Chen DD:
Potassium channels and proliferation and migration of breast cancer
cells. Sheng Li Xue Bao. 61:15–20. 2009.PubMed/NCBI
|
|
11
|
Zhang P, Yang X, Yin Q, Yi J, Shen W, Zhao
L, Zhu Z and Liu J: Inhibition of SK4 potassium channels suppresses
cell proliferation, migration and the epithelial-mesenchymal
transition in triple-negative breast cancer cells. PLoS One.
11:e01544712016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teisseyre A, Gasiorowska J and Michalak K:
Voltage-gated potassium channels Kv1.3-potentially new molecular
target in cancer diagnostics and therapy. Adv Clin Exp Med.
24:517–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang S, Zhu L, Yang J, Hu L, Gu J, Xing
X, Sun Y and Zhang Z: Integrated expression profiling of potassium
channels identifys KCNN4 as a prognostic biomarker of pancreatic
cancer. Biochem Biophys Res Commun. 494:113–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang C, Sindic A, Hill CE, Hujer KM, Chan
KW, Sassen M, Wu Z, Kurachi Y, Nielsen S, Romero MF and Miller RT:
Interaction of the Ca2+-sensing receptor with the
inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in
inhibition of channel function. Am J Physiol Renal Physiol.
292:F1073–F1081. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franke M, Ibrahim DM, Andrey G, Schwarzer
W, Heinrich V, Schopflin R, Kraft K, Kempfer R, Jerković I, Chan
WL, et al: Formation of new chromatin domains determines
pathogenicity of genomic duplications. Nature. 538:265–269. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, Huang J, Peng J, Wu X, Zhang Y, Zhu
W and Guo L: Upregulation of the inwardly rectifying potassium
channel Kir2.1 (KCNJ2) modulates multidrug resistance of small-cell
lung cancer under the regulation of miR-7 and the Ras/MAPK pathway.
Mol Cancer. 14:592015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji CD, Wang YX, Xiang DF, Liu Q, Zhou ZH,
Qian F, Yang L, Ren Y, Cui W, Xu SL, et al: Kir2.1 interaction with
Stk38 promotes invasion and metastasis of human gastric cancer by
enhancing MEKK2-MEK1/2-ERK1/2 signaling. Cancer Res. 78:3041–3053.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HS, Kim DH, Kim JY, Jeoung NH, Lee IK,
Bong JG and Jung ED: Microarray analysis of papillary thyroid
cancers in Korean. Korean J Intern Med. 25:399–407. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oczko-Wojciechowska M, Pfeifer A, Jarzab
M, Swierniak M, Rusinek D, Tyszkiewicz T, Kowalska M, Chmielik E,
Zembala-Nozynska E, Czarniecka A, et al: Impact of the tumor
microenvironment on the gene expression profile in papillary
thyroid cancer. Pathobiology. 87:143–154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung T, Chen H, Stauffer AM, Giger KE,
Sinha S, Horstick EJ, Humbert JE, Hansen CA and Robishaw JD:
Zebrafish G protein gamma2 is required for VEGF signaling during
angiogenesis. Blood. 108:160–166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwindinger WF and Robishaw JD:
Heterotrimeric G-protein betagamma-dimers in growth and
differentiation. Oncogene. 20:1653–1660. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pirone A, Cozzi B, Edelstein L, Peruffo A,
Lenzi C, Quilici F, Antonini R and Castagna M: Topography of Gng2-
and NetrinG2-expression suggests an insular origin of the human
claustrum. PLoS One. 7:e447452012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yajima I, Kumasaka MY, Yamanoshita O, Zou
C, Li X, Ohgami N and Kato M: GNG2 inhibits invasion of human
malignant melanoma cells with decreased FAK activity. Am J Cancer
Res. 4:182–188. 2014.PubMed/NCBI
|
|
24
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42((Database Issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Handklo-Jamal R, Meisel E, Yakubovich D,
Vysochek L, Beinart R, Glikson M, McMullen JR, Dascal N, Nof E and
Oz S: Andersen-tawil syndrome is associated with impaired
PIP2 regulation of the potassium channel Kir2.1. Front
Pharmacol. 11:6722020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng C, Wang Q, Zhu M, Liu K and Zhang Z:
Integrated analysis reveals potential long non-coding RNA
biomarkers and their potential biological functions for disease
free survival in gastric cancer patients. Cancer Cell Int.
19:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang H, Lin HC, Liu H, Gan D, Jin W, Cui
C, Yan Y, Qian Y, Han C and Wang Z: A 6 lncRNA-based risk score
system for predicting the recurrence of colon adenocarcinoma
patients. Front Oncol. 10:812020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Staveren WC, Solís DW, Delys L, Duprez
L, Andry G, Franc B, Thomas G, Libert F, Dumont JE, Detours V and
Maenhaut C: Human thyroid tumor cell lines derived from different
tumor types present a common dedifferentiated phenotype. Cancer
Res. 67:8113–8120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yajima I, Kumasaka MY, Naito Y, Yoshikawa
T, Takahashi H, Funasaka Y, Suzuki T and Kato M: Reduced GNG2
expression levels in mouse malignant melanomas and human melanoma
cell lines. Am J Cancer Res. 2:322–329. 2012.PubMed/NCBI
|
|
32
|
Yajima I, Kumasaka MY, Tamura H, Ohgami N
and Kato M: Functional analysis of GNG2 in human malignant melanoma
cells. J Dermatol Sci. 68:172–178. 2012. View Article : Google Scholar : PubMed/NCBI
|