Introduction
Glioma is the most common primary malignant tumor
arising from the neuroectodermal central nervous system with poor
prognosis, accounting for 50–60% of intracranial tumors (1). Glioma is usually treated by surgery,
followed by chemotherapy combined with radiotherapy (2). However, the average survival time of
the patients with low grade glioma is only 3–5 years and 1–2 years
in the case of patients with high grade glioma (3). The conventional treatment of glioma
involves the removal of most of the tumor by craniotomy, followed
by several methods that are used to inhibit the proliferation of
residual tumor cells or to kill the remaining tumor cells (4). However, even for patients with the
same WHO level and pathological type of glioma, such as WHO I, II
and III the prognosis is quite different (5). This is due to the difference in
treatment strategy. The biological characteristics of tumors,
especially the differences in heredity, gene, and protein, also
play an important role (2). With
the development of molecular biology, immunology, biochemistry, and
other related disciplines, researchers have taken the treatment of
glioma from the traditional approach, as outlined above, to the
targeting of glioma cells, oncogene genes and related proteins
(6).
Epidermal growth factor receptor (EGFR), a membrane
receptor with tyrosine kinase activity, is widely expressed in
various human cancers (7). The
EGFR-mediated signal transduction pathway is complex and has
various effects, which can induce cell proliferation, migration and
differentiation, and is closely related to cell regeneration and
the development of malignant tumors (8). EGFR is the first receptor in solid
tumors to be used as a therapeutic target (9). Previous findings have shown that there
is a high expression of EGFR in human glioma (10–12).
Thus, it can be used as a target for human glioma and for combining
with the ligands of chemotherapeutic drugs and be involved in
targeted therapy (11).
Previous studies have shown that the application of
targeted therapy is relatively safe and effective; however, there
are issues that remain to be solved, including the therapeutic
effect of targeted therapy and its predictability, how to combine
the targeted therapy with the conventional therapy and improve the
curative effect, the drug resistance of the tumor cells, and the
single factor and the single target drug research and development
strategy, which is inadequate to prevent and control multiple
factors and multiple targets (12–14).
Gefitinib, an epidermal growth factor receptor
antagonist, is currently widely used in the treatment of non-small
cell lung cancer (15–17). Although glioma also expresses high
levels of epidermal growth factor receptor, it has been shown that
gefitinib is not effective in the treatment of glioma (18). The present study investigated the
underlying molecular mechanism, modification and synthesis of
gefitinib. A series of gefitinib derivatives were produced by the
chemical modification of gefitinib, introducing hydrophilic groups
and anti-tumor groups.
To develop more active small molecules targeting
anticancer drugs, the present study used quinazoline as the
research subject because it exhibits relatively suitable biological
activities, including anticancer, bactericidal, insecticidal and
antiviral properties (19–21). Quinoline compounds play an important
role in tumor therapy and inhibit EGFR tyrosine kinase, vascular
endothelial growth factor receptor, platelet derived growth factor
receptor, FMS like tyrosine kinase 3 and other targets (22–25).
In previous studies, a few anticancer compounds were
derived by introducing small molecular heterocycles on the four
sites of quinazoline (including pyridine, pyrimidine and pyrazole)
(26,27). Non-quinazoline heterocycles, such as
Tozasertib, also have relatively effective anticancer activities.
Therefore, the present study used gefitinib as the precursor
compound and introduced small molecular heterocyclic rings to its
four sites, in order to achieve the ‘superposition’ of the activity
and obtain more anticancer compounds.
Materials and methods
Chemicals, antibodies, and
reagents
LPY-9 and gefitinib were provided by Guizhou
University School of Pharmacy. U251-MG cells were obtained from
Procell. Fetal bovine serum (FBS) was purchased from Gibco (Thermo
Fisher Scientific, Inc.; 10091148). DMEM was purchased from Hyclone
(GE Healthcare Life Sciences; SH30022.01). DMSO was purchased from
Sigma-Aldrich (Merck KGaA). CCK-8 proliferation toxicity test kit
was obtained from Dojindo Molecular Technologies, Inc. (CK04),
Giemsa staining kit was obtained from Beijing Solarbio Science
& Technology Co., Ltd. (G4641), PI-Annexin V double dye flow
reagent kit was obtained from Nanjing KeyGen Biotech. Co., Ltd.
(40303ES20). Caspase-3 Enzyme Activity kit was purchased from
Nanjing KeyGen Biotech. Co., Ltd. (41345ES50), Transwell assay kit
was obtained from Corning Inc. (3428), BCA protein quantitative kit
was obtained from GenStar Biosolutions (E162-05).
Cell culture
The U251-MG cells were obtained from Procell and
cultured using DMEM supplemented with 10% FBS, 1% sodium pyruvate
(100 mM), 1% non-essential amino acids (10 mM), and 1%
penicillin/streptomycin solution (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were maintained at 37°C under a 5%
CO2 atmosphere. The cells were passaged after every 2
days.
Cell Counting Kit-8
U251-MG cells (2×104 cells/well) were
seeded in 96-well plates and incubated for 24 h at 37°C followed by
treatment with different concentrations of gefitinib and LPY-9.
Then, 10 µl of CCK-8 solution (Dojindo Molecular Technologies,
Inc.) was added to each well and incubated for 4 h. The samples
were then detected at 450 nm by a microplate reader (Bio-Rad
Laboratories, Inc.), and analyzed using the formula: [(OD value of
test-OD value of blank)/(OD value of control-OD value of blank)],
to quantify the cell viability.
Giemsa staining
According to the results of CCK-8 assay, U251-MG
cells were treated with 15, 30 or 60 µmol/l LPY-9, or the same
concentrations of gefitinib. After 24 h, U251-MG cells were fixed
with methanol and washed carefully twice, and were then incubated
with 10% Giemsa stain at room temperature for 10 min and washed
carefully twice. Finally, the cell morphology was observed, and
images captured, under an inverted microscope in three different
fields at random.
Caspase-3 activity
To investigate caspase-3 activity, 4×106
cells were collected according to the manufacturer's protocol of an
ELISA kit (Biovision, K4221-100). After washing with PBS twice,
lysis buffer was added into cells, mixed, incubated for 30 min on
ice, and oscillated four times (each time 10 sec at 3,000 rpm).
Then, the cells were centrifuged at 8,000 × g at 4°C for 30 min,
and protein was quantified by a BCA kit.
Wound healing assay
Prior to seeding cells, three straight lines were
drawn parallel to the bottom of a 6-well using marker pen. Cells
were seeded in 6-well plates and cultured in a DMEM containing 1%
FBS, 1% penicillin/streptomycin solution in order to protect cell
proliferation, although the usage of FBS is a limitation of the
present study. After the cells adhered to the wall and formed a
monolayer, a 200 µl pipette tip was used to make three parallel
scratches, followed by washing with PBS to remove the delimiting
cells. Then the cell migration was observed with a light microscope
(magnification ×10) in three different fields.
Effects of LPY-9 and gefitinib on VEGF
secretion
The effects of LPY-9 and gefitinib on VEGF secretion
were detected using an ELISA kit. U251-MG cells were treated with
15, 30 or 60 µmol/l LPY-9, or 15, 30 or 60 µmol/l gefitinib. ELISA
for VEGF (ab65345, Abcam) was performed following the
manufacturer's protocol.
Transwell assay
U251-MG cells were digested at log phase and washed
with PBS. The cells were suspended in 3% FBS containing DMEM, and
the cell density was adjusted to 5×105 cells/ml. Then,
~200 µl of cell suspension treated with 60 µmol/l of LPY-9 and
gefitinib were added to the Transwell (cat. no. 3460, Corning Life
Sciences, 8 µm) upper chamber. Then, ~500 µl DMEM medium containing
10% FBS was added to the lower chamber and cultured for 12 h in
37°C under 5% CO2 incubator. The cells were fixed with
10% formaldehyde at room temperature for 10 min and stained using
10% Giemsa stain at room temperature for 10 min and then observed,
and images captured, under a light microscope (magnification ×10)
in three different fields at random.
Cell apoptosis detection
The proportion of cells actively undergoing
apoptosis was quantified by annexin and propidium iodide staining
using the Annexin V-FITC Apoptosis Detection kit (Nanjing KeyGen
Biotech, Co., Ltd.) and a Beckman flow cytometer (Beckman Coulter,
Inc.) according to the manufacturer's instructions. Briefly,
according to results of CCK-8 assay, the cells were treated with
either 15 µmol/l gefitinib or 15 µmol/l LPY-9 and then harvested
and washed with PBS twice. Then, 5 µl Annexin V-FITC and 5 µl PI
were added and after mixing, the mixture was incubated at room
temperature for 5 min in the dark and analyzed using FlowJo
Software v10.5.3 (FlowJo LLC).
Western blotting
U251-MG cells were treated with 15, 30 or 60 µmol/l
LPY-9 or gefitinib for 24 h and lysed with RIPA buffer (Yeasen
Biotechnology (Shanghai) Co., Ltd.) on ice for 30 min. Then 50–150
mg of thermally denatured protein as detected by a BCA kit (GenStar
Biosolutions) extract was loaded on a 10% polyacrylamide gel,
electroblotted onto a nitrocellulose membrane and blocked at room
temperature for 1 h with 5% BSA. The membrane was then incubated
with antibodies against caspase-3 (Cell Signaling Technology, Inc.,
9662; 1:1,000), cleaved caspase-3 (Cell Signaling Technology, Inc.,
9664; 1:1,000), VEGF (Bioworld Technology, Inc., BS91432; 1:1,000),
EGFR (Sigma-Aldrich; Merck KGaA, SAB1306008; 1:1,000), AKT (Cell
Signaling Technology, Inc., 4691; 1:1,000), p-AKT (Cell Signaling
Technology, Inc., 9611; 1:600), PI3K (Cell Signaling Technology,
Inc., 4249; 1:1,000) and GADPH antibody (Hangzhou Goodhere
Biotechnology Co., Ltd., AB-P-R001; 1:2,500) at 4°C overnight. The
membranes were washed with PBS three times and incubated with
HRP-labeled goat anti rabbit antibody from Boster Biological
Technology Co., Wuhan, China (BA1054; 1:5,000) at room temperature
for 2 h. ECL buffer (Yeasen Biotechnology (Shanghai) Co., Ltd.) was
used to develop the blots. The X-ray film was dried and scanned
with a scanner. Glyko Bandscan 5.0 software (Glyko Inc.) was used
to analyze the gray value of the film after scanning the obtained
image. The relative expression level of the protein was expressed
by normalizing against internal reference protein.
Statistical analysis
Statistical analysis was performed using SPSS17.0
software. The differences were evaluated using one way or two-way
ANOVA followed by Tukey's test. Error bars represent standard
deviation (SD), and three independent experiments were performed
for each assay All statistical analyses and comparisons were made
against a control group. Histograms were drawn using GraphPad Prism
5.01 (GraphPad Software, Inc.) and every figure was combined using
Photoshop CS6 (Adobe Systems, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Construction of gefitinib derivative,
GLPY-9
In the present study, gefitinib was used as a parent
compound to introduce some novel heterocycles into its four sites
in order to achieve the superposition of activity and obtain
compounds with higher anti-cancer activity than gerfitinib. In
addition, the functional group at 6 and 7 position of quinazoline
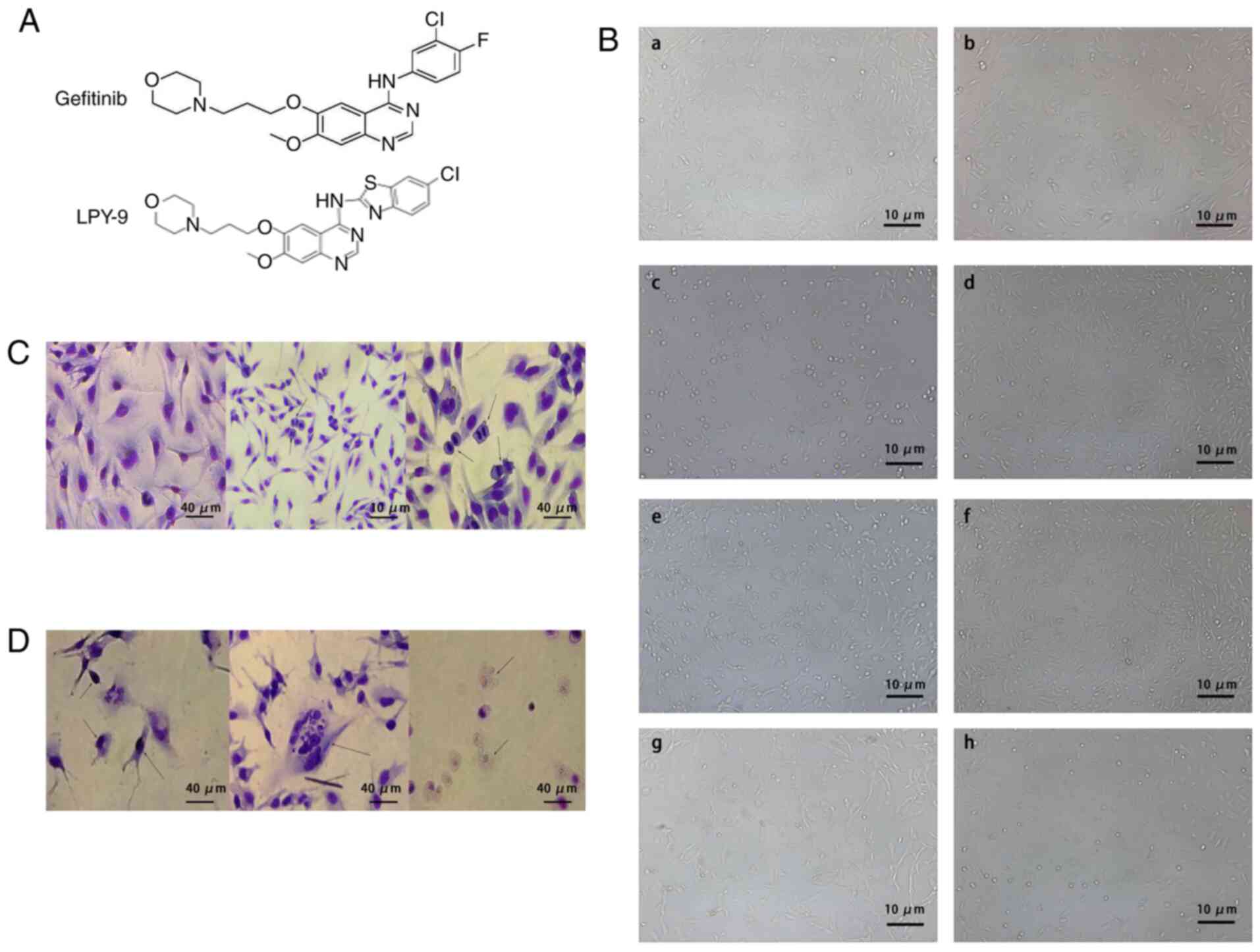
was also exchanged (Fig. 1A).
Observation of cell morphology
The human glioma U251-MG cells grew rapidly and
adhered. The shape of U251-MG cells included spindle, star and
irregular shapes. The cells had strong luminance, strong refraction
in the cell body, and the nuclei were round and oval (Fig. 1Ba and b). The cells that were
treated with different concentrations of LPY-9 grew slowly, their
cell bodies became smaller, cell synapse decreased and their
transparency decreased. Their cell morphology was significantly
different compared with normal glioma cells; the intercellular
space was enlarged and the cell debris were visible. The number of
parietal cells decreased significantly with the increase of drug
concentration (Fig. 1Bc and e).
Compared with the same concentration of gefitinib, LPY-9 exhibited
more significant inhibitory effect on proliferation of glioma cells
(Fig. 1B). Following Giemsa
staining, the nuclei of normal U251-MG cells were stained uniformly
purple, a large number of mitotic cells were observed and the cells
were healthy (Fig. 1C). Following
treatment with LPY-9, the cells showed nuclear condensation,
nuclear fragmentation and nuclear dissolution (Fig. 1D). With an increase in drug
concentration, the abovementioned effects became more prominent.
Compared with cells treated with the same concentration of
gefitinib, the changes in LPY-9-treated cells were significantly
more evident compared with gefitinib-treated cells.
Effect of LYP-9 on the proliferation
and apoptosis of U251-MG cells
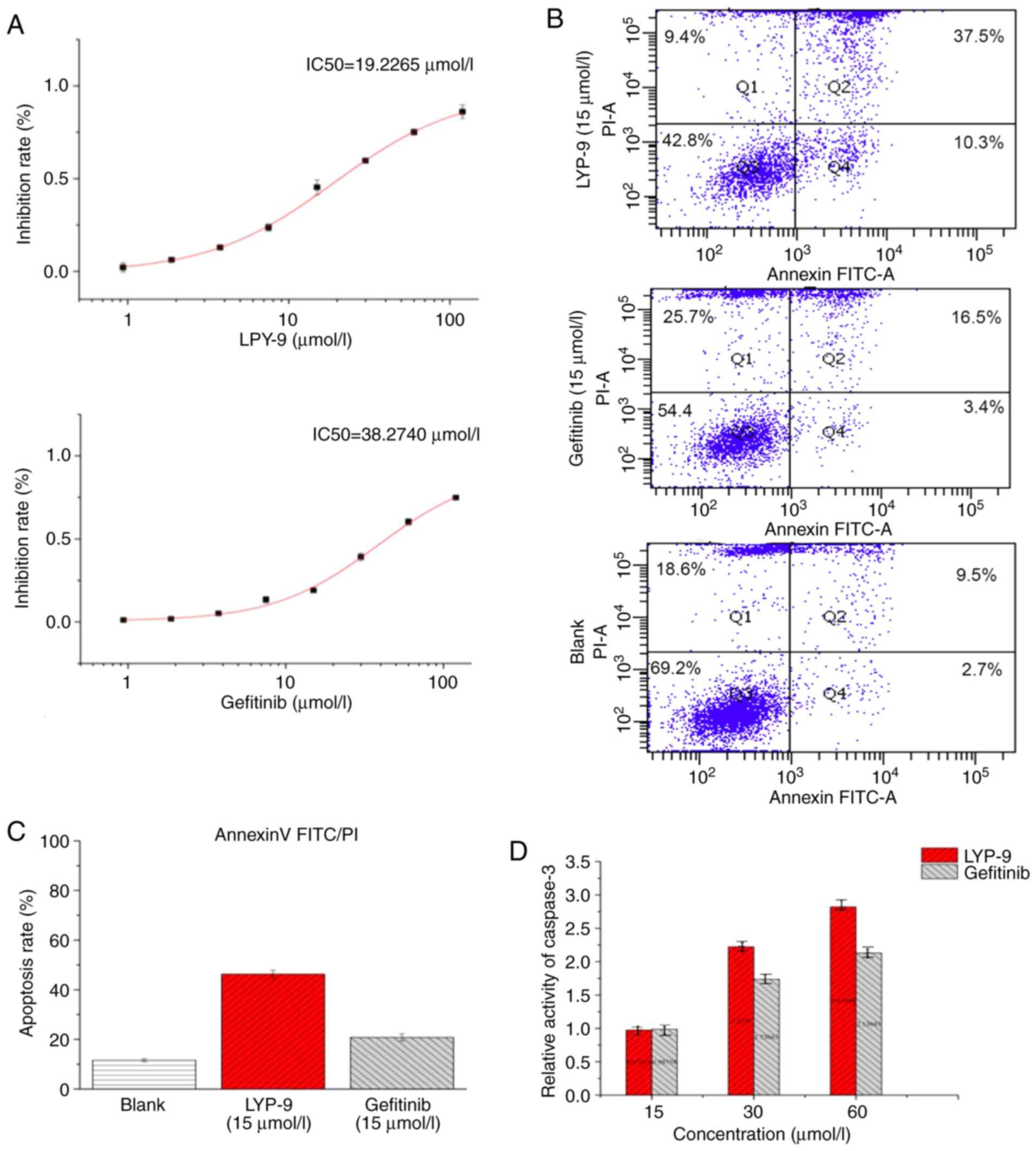
After treatment of human glioma U251-MG cells with
LPY-9 and gefitinib for 24 h, a dose-dependent inhibitory effect
(P<0.01) was identified, except at the dose of 0.9375 µmol/l
(P=0.208). The higher the LPY-9 and gefitinib concentration, the
stronger the inhibition of U251-MG cells. The effect of LPY-9 was
more prominent compared with that of gefitinib (P<0.01). The
median inhibitory concentration (IC50) of LPY-9 was
19.2265 µmol/l, and IC50 of gefitinib was 38.2740 µmol/l
(Fig. 2A).
The effects of LPY-9 and gefitinib on apoptosis of
U251-MG cells were detected by the flow Annexin V/PI FITC
double-labeling method. The induction of a significant level of
apoptosis was found in the LPY-9 (46.3±1.5875%) and gefitinib
(20.8±1.4731%) groups compared with the control group
(11.6±0.5568%; P<0.01). Thus, the LPY-9-treated cells exhibited
a higher apoptosis rate compared with gefitinib-treated cells
(P<0.01; Fig. 2B and C).
Caspase-3 activity
In order to detect caspase-3 activity, ELISA were
used to detect caspase-3 enzyme activity. As shown in Fig. 2D, both LPY-9 and gefitinib increased
the activity of caspase-3 in a dose-dependent manner and the
difference was statistically significant (P<0.01). Specifically,
at 15 µmol/l, no significant difference in casepase-3 enzyme
activity was observed between LPY-9 and gefitinib (P>0.05). At
30 and 60 µmol/l, the enzyme activity of the two drugs was
statistically different (P<0.01). At low concentrations, the
effect of LPY-9 was similar to that of gefitinib. However, when the
concentration increased, LPY-9 significantly increased the activity
of casepase-3 enzyme, and the effect was more evident compared with
gefitinib (P<0.01) at the same concentration.
Effect of LPY-9 on cell migration and
invasion
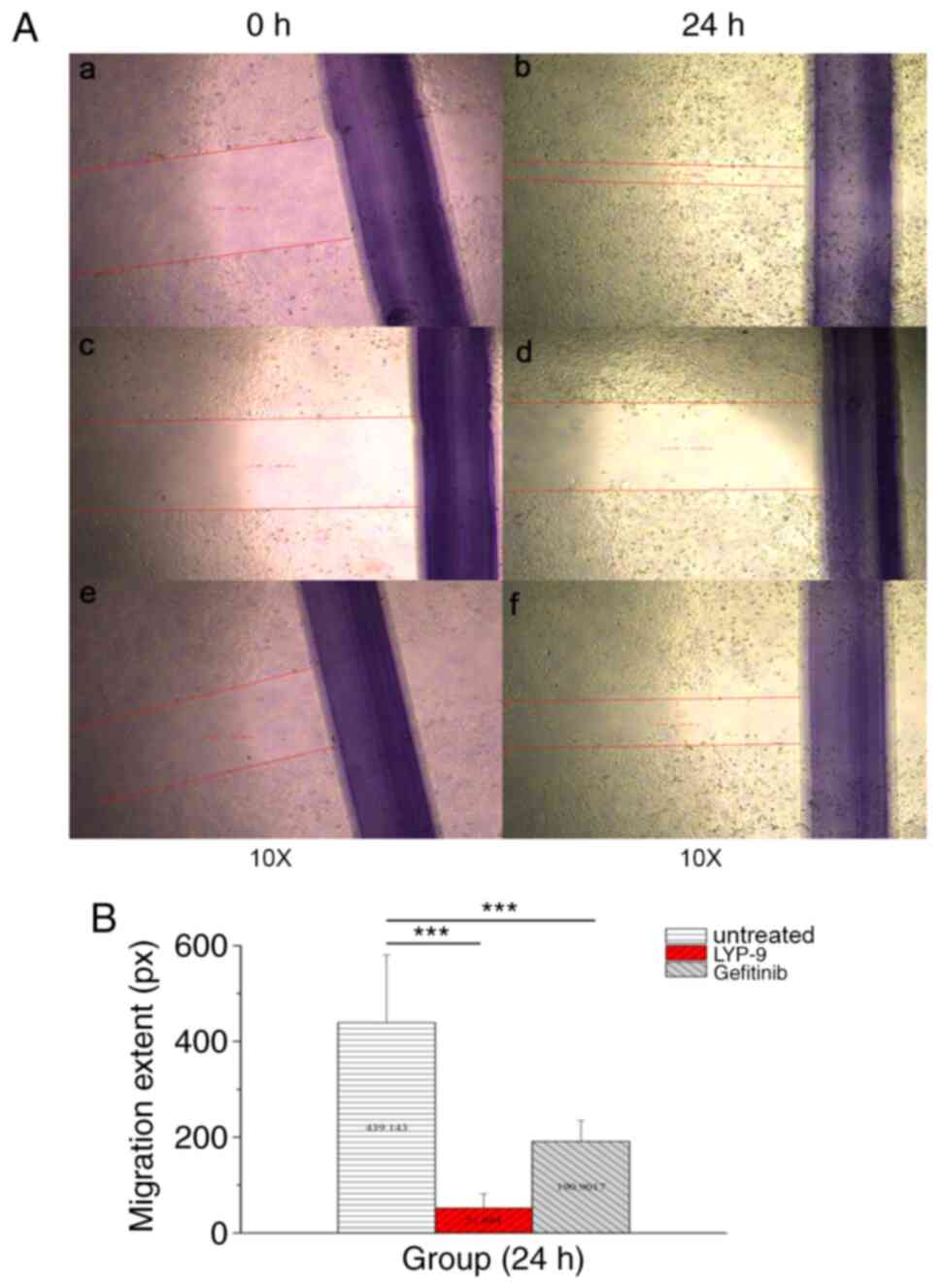
In order to explore the effect of LPY-9 and
gefitinib on migration of U251-MG cells, scratch assay was used.
After treatment with drugs for 24 h, the cells in the control group
exhibited normal migration (Fig. 3Aa
and b); however, the migration of cells of the LPY-9 group was
not significant (Fig. 3Ac and d),
and was even less than that of cells of the gefitinib group
(Fig. 3Ae and f). The difference of
migration distance was not statistically significant (P>0.05;
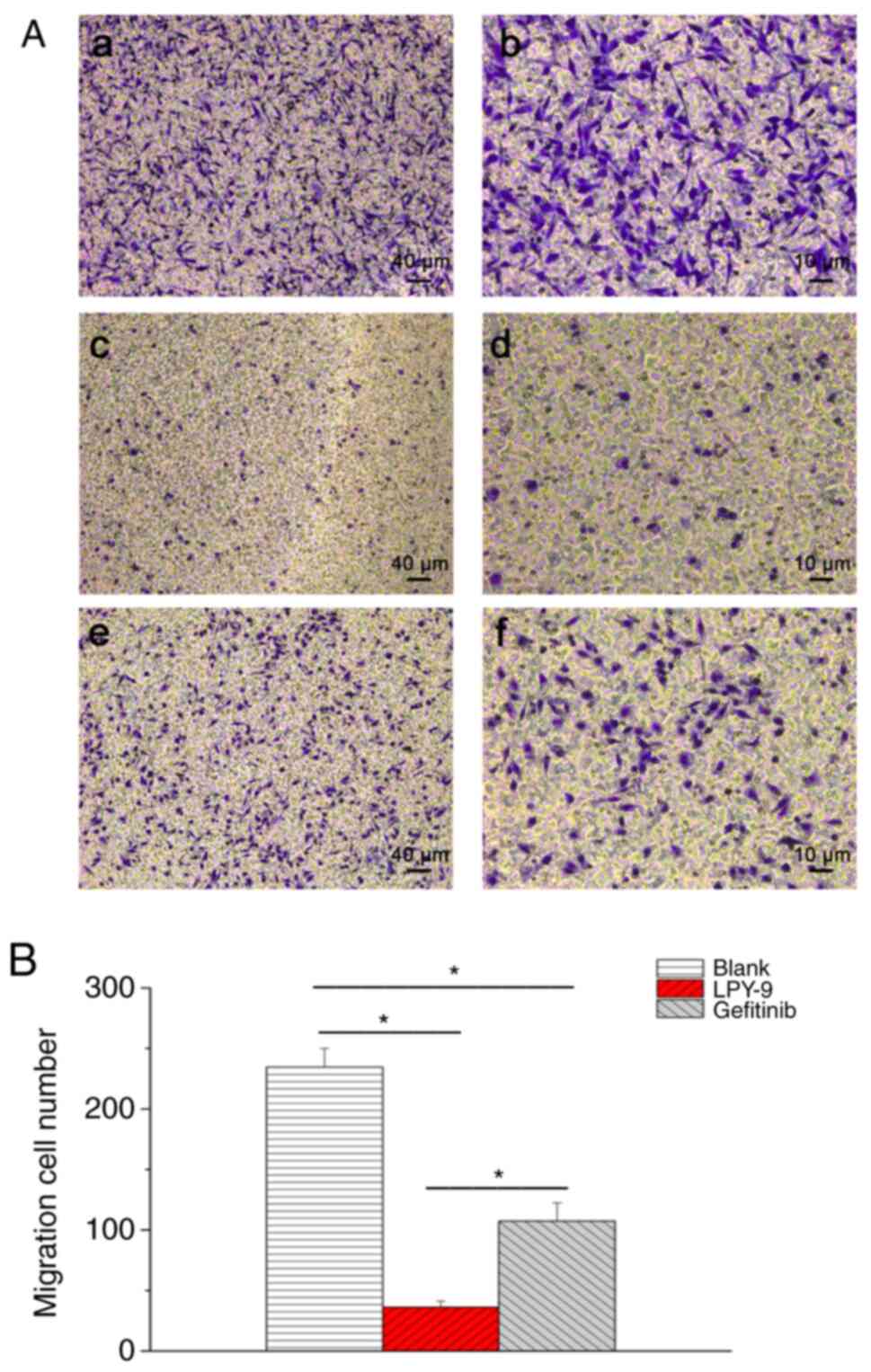
Fig. 3B). The Transwell assay was
also used to detect cell migration. There were more cells in the
blank control group (Fig. 4Aa and
b), while LPY-9 (Fig. 4Ac and
d) inhibited the migration of U251-MG cells, and the inhibitory
effect of LPY-9 was significantly more compared with gefitinib
(P<0.01; Fig. 4Ae and Af and
B).
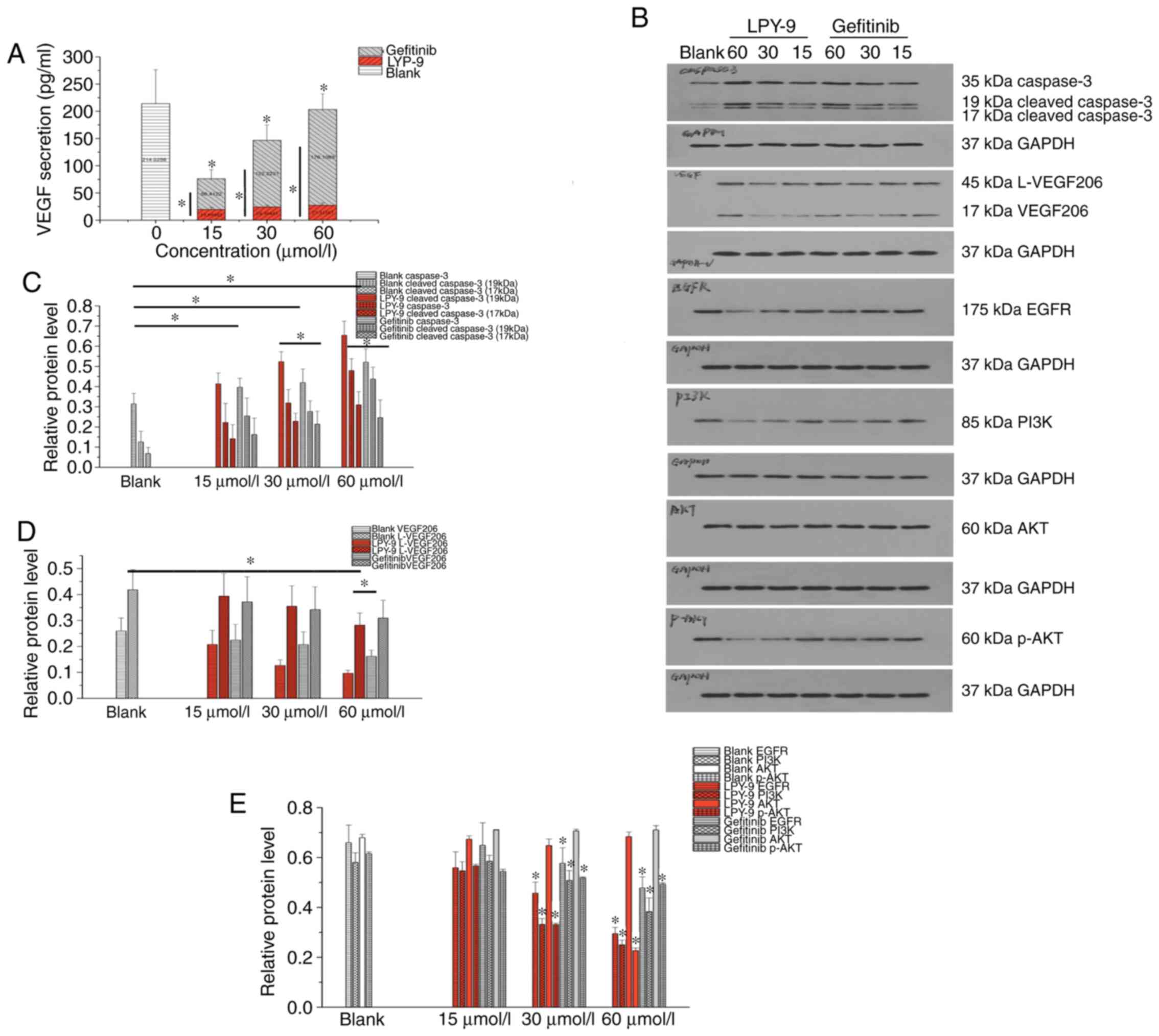
Effects of LPY-9 and gefitinib on VEGF
secretion
VEGF secretion was detected in the U251-MG cell
media of different groups using ELISA. It was found that low and
high concentration of LPY-9 significantly inhibited the secretion
of VEGF protein, and the difference was statistically significant
(P<0.01). The effect of LPY-9 did not change significantly with
concentration (P>0.05). Compared with the gefitinib treatment
group, the effect of LPY-9 was more evident (Fig. 5A) and the result was statistically
significant (P<0.01).
Effects of LPY-9 and gefitinib on
expression of caspase-3 and cleaved Caspase-3 protein
The expression of caspase-3 and cleaved caspase-3
protein in the normal cells was low. Following treatment with LPY-9
and gefitinib, the expression of caspase-3 and cleaved caspase-3 in
U251-MG cells increased in a drug-dependent manner, and the
difference was statistically significant (P<0.05). The
expression level of caspase-3 protein in LPY-9-treated group was
higher compared with the gefitinib-treated group (P<0.05). At a
drug concentration of 15 µmol/l, the expression of cleaved
caspase-3 protein in the gefitinib treatment group was higher
compared with that of the LPY-9 treatment group. At drug
concentrations of 30 and 60 µmol/l, the expression of cleaved
caspase-3 protein in the LPY-9 treatment group was higher compared
with the gefitinib treatment group, and the difference was
statistically significant (P<0.05) (Fig. 5B and C).
Effects of LPY-9 and gefitinib on VEGF
and its subunit protein
The expression of VEGF and its subunit protein in
the normal cells was high. Following treatment of LPY-9 and
gefitinib, the expression of VEGF and its subunit decreased with
the increase of drug concentration. At drug concentrations of 15
and 30 µmol/l, the difference was not statistically significant
(P>0.05). At 60 µmol/l concentration, the effect of LPY-9 and
gefitinib increased significantly, and the difference was
statistically significant (P<0.05). LPY-9 exhibited a greater
inhibitory effect on VEGF, and the difference was statistically
significant (P<0.05; Fig. 5B and
D).
Effects of LPY-9 and gefitinib on EGFR
protein
The expression of EGFR protein in the normal cells
was high. Following treatment with LPY-9 and gefitinib, the
expression of EGFR decreased, and the difference was statistically
significant (P<0.01 or P<0.05). By contrast, LPY-9 exhibited
a greater inhibitory effect on EGFR expression. There was no
significant difference in EGFR expression between LPY-9 and
gefitinib groups when the concentrations of LPY-9 and gefitinib
were 30 and 60 µmol/l (P>0.05), but the effect was more
prominent than that observed after treatment with 15 µmol/l drug
concentration (P<0.05; Fig. 5B and
E).
Effects of LPY-9 and gefitinib on PI3K
protein
The expression of PI3K protein in the normal cells
was high. Following LPY-9 and gefitinib treatment, the expression
of PI3K decreased in a dose-dependent manner (P<0.05). The
effect at 15 µmol/l concentration was not significant (P>0.05
and P>0.05). At relatively high concentrations, both gefitinib
and LPY-9 showed a significant inhibitory effect on PI3K protein
(P<0.05), while the effect of LPY-9 was greater compared with
gefitinib (P<0.01; Fig. 5B and
E).
Effects of LPY-9 and gefitinib on AKT
and p-AKT protein
The expressions of AKT and p-AKT proteins in the
normal cells were high. Following treatment with LPY-9 and
gefitinib, no significant change was observed in the expression
level of AKT (P>0.05). However, the expression of p-AKT
decreased significantly as the concentration of LPY-9 and gefitinib
increased (P<0.01). By contrast, the inhibitory effect of LPY-9
was greater than gefitinib at the same concentration (P<0.01;
Fig. 5B and E).
Discussion
Human glioma is one of the most common malignant
tumors of the nervous system (1).
Routine treatment includes surgical excision, followed by
chemotherapy combined with radiotherapy (2). Patients with low malignancy usually
have a survival period of 3–5 years; however, patients with higher
malignancy have only 1–2 years of survival (3). As the incidence of glioma is on the
increase annually, and the treatment not effective enough, it is
necessary to develop new and improved treatment strategies.
Gefitinib is a type of epidermal growth factor
receptor antagonist, which has been widely used in the treatment of
non-small cell lung cancer, and glioma cells also express high
levels of epidermal growth factor receptor (7). However, previous findings have shown
that gefitinib is not effective in the treatment of glioma
(28,29).
The present study studied the molecular mechanism of
glioma and the transformation and synthesis of chemotherapeutic
drugs. These drugs were derived after chemical modification of
gefitinib, such as introducing hydrophilic groups and antitumor
active groups. The present study used the gefitinib derivative,
LPY-9 and its effects on the glioma U251-MG cell line were
investigated, to verify whether it was effective, and to explore
the possible underlying mechanism of its action.
Cell migration is the movement of cells induced by
stimulation of migration signals or gradients of concentration of
certain substances and plays an important role in the metastasis of
tumor cells. Using wound healing and Transwell assays, it was found
that the cell migration in the normal U251-MG cell group was high
and the cells could migrate in a short time period. The migration
of U251-MG human glioma cells reduced after LPY-9 and gefitinib
treatment. LPY-9 inhibited the migration of cells more
significantly compared to gefitinib, which provided a basis for
further research on the inhibition of cell invasiveness by
LPY-9.
Apoptosis is a common feature in tumor cells and
tissues. However, in tumor cells, due to the anti-apoptotic effect,
the apoptosis rate of tumor cells decreases, and the abnormal
proliferation of cells increases; the specific mechanism of tumor
cells remains unclear (30,31). The caspase family is the core link
in the process of apoptosis. The degree of apoptosis depends on the
activation and abnormal expression of caspase proteins (32,33).
The activation of caspase-3 can lead to abnormal cell cycle and
cell structure, and protein kinase inactivation, leading to cell
apoptosis (34,35). The present study found that the
expression of caspase-3 in glioma cells without any drug
intervention was relatively low compared with drug intervention,
which is consistent with the results of Chhanabhai et al
(16). LPY-9 and gefitinib
treatment significantly increased the expression of caspase-3 and
the expression of cleaved caspase-3 to induce apoptosis
(P<0.05), and the effect of LPY-9 was more prominent than
gefitinib (P<0.05). The findings suggested that both drugs can
promote cell apoptosis by increasing the expression level of
caspase-3 and increasing its activation.
VEGF is a vasogenic factor commonly found in cells
and tissues and promotes endothelial cell division, proliferation
and angiogenesis by interacting specifically with VEGF receptor,
which is closely related to the development, metastasis, and
infiltration of tumors (36–38).
In 1971, Folkman (36) first
proposed that the tumor development depended on the
neovascularization of its internal formation, and Nesbit (37) proposed that tumor tissue will not
increase and may even deteriorate when the tumor volume is larger
than 1 mm3 and no new blood vessels grow. As a result,
researchers have turned their attention to inhibiting tumor growth
by inhibiting tumor angiogenesis. Kil et al (38) showed that increased expression of
VEGF promoted glioma U251-MG cell proliferation.
The present study analyzed the effects of LPY-9 and
gefitinib on VEGF by ELISA and western blotting. It was found that
the expression of both intracellular and extracellular VEGF was
high. Following LPY-9 and gefitinib treatment, the levels of VEGF
in the intracellular and secreted medium decreased significantly,
in a dose-dependent manner. The effect of LPY-9 was greater than
that of gefitinib.
EGFR is a transmembrane protein receptor, exhibiting
the activity of tyrosine kinase. It is closely associated with the
proliferation, invasion and anti-apoptotic ability of tumor cells.
It has been found that the activity of EGFR in malignant tumors is
significantly higher than that in normal cells (39,40).
The high expression of EGFR protein in glioma is considered a sign
of abnormal differentiation, and with the increase of tumor
malignancy the expression of the protein is significantly increased
(41).
At present, a number of EGFR downstream signal
transduction pathways are known, among which the main signal
transduction pathways are the Ras/Raf/mitogen-activated protein
kinase kinase/mitogen-activated protein kinase kinase 1 pathway,
the PI3K/3-phosphoinositide dependent protein kinase 1/AKT/mTOR
pathway and the Janus kinase/STAT pathway (42). PI3K plays an anti-apoptotic role in
many kinds of tumors (43). It is
also one of the causes of induction of drug resistance in malignant
tumor cells (44). AKT, also named
PKB, is a serine/threonine protein kinase, which plays an important
role in cell survival and apoptosis (45). Abnormal activation of PI3K/AKT
pathway can increase the level of phosphorylated AKT in cells,
leading to tumor resistance, reduced tumor necrosis and the
promotion of the progression of malignant tumors (46,47).
The present study found that LPY-9 decreased the expression of
EGFR, reduced the expression of PI3K and significantly inhibited
the phosphorylation of AKT, thus promoting the apoptosis of tumor
cells. Compared with gefitinib, LPY-9 exhibited a greater
inhibitory effect on AKT phosphorylation.
Although the present study found that LPY-9 is more
useful than gefitinib in treatment of U251-MG cells, whether LPY-9
was effective in other types of cancer cells will be investigated
in the future. An effective anti-cancer drug does not only kill
cancer cells, but also minimizes damage to normal cells. Future
studies will further explore the effectiveness for other cancer
cells and toxicity for normal cells.
In conclusion, LPY-9 and gefitinib effectively
inhibited the proliferation and growth of human glioma U251-MG
cells. By comparing two drugs, it was found that the effectiveness
of LPY-9 was superior to gefitinib and that LPY-9 could exhibit
significant effects at relatively low concentrations. By comparing
the effects of LPY-9 and gefitinib on the migration of U251-MG
cells in human glioma, it was found that both the drugs
significantly inhibited the migration of tumor cells, and the
effect of LPY-9 was more pronounced compared with gefitinib. In
addition, LPY-9 also increased the levels of caspase-3 and cleaved
caspase-3 in tumor cells, and increased the activity of caspase-3
enzyme to promote the apoptosis of tumor cells; again, the effect
was more pronounced compared with gefitinib. As a multi-target
drug, LPY-9 exerted significant effects on caspase-3,
cleaved-caspase-3, VEGF, EGFR, p-AKT, PI3K and other proteins.
Several target sites synergistically promoted apoptosis of tumor
cells, and the effect of LPY-9 was more pronounced compared with
gefitinib.
The experimental results of the present study
demonstrated that the gefitinib derivative LPY-9 can be used as a
multi-target targeted drug for the treatment of glioma, and it
shows a more pronounced effect than Gefitinib. The authors are
currently applying for the patent for LPY-9. After the patent
document is approved, the authors will further disclose the
preparation process of LPY-9, as well as the further data of water
property, and toxicity, in order to conduct further research.
Acknowledgements
The authors thank Professor Guiping Ouyang of
pharmacy school of Guizhou University for designing, producing and
providing LPY-9 compound.
Funding
This work was funded by The Foundation of National
Natural Science Foundation of China (grant no. 81560409) and
Science Foundation of Guizhou Province of China [grant no. (2014)
6008].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conceived and designed the study. YS performed
most of the experiments. JL wrote the manuscript and approved its
publication, LC, HW and HP performed some of the experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have the patent rights for LPY-9.
References
|
1
|
Giesexs A and Westphal M: Glioma invasion
in the central nervous system. Neurosurgery. 39:235–250. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bush NA, Chang SM and Berger MS: Current
and future strategies for treatment of glioma. Neurosurg Rev.
40:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omuro A and Deangelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hopkins K, Chandler C, Eatough J, Moss T
and Kemshead JT: Direct injection of 90Y MoAbs into glioma tumor
resection cavities 1eads to limited diffusion of the
radioimmunoconjugates into nomal brain parenchyma: A model to
estimate absorbed radiation dose. Int J Radiat Oncol Biol Phys.
40:835–844. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rudà R, Reifenberger G, Frappaz D, Pfister
SM, Laprie A, Santarius T, Roth P, Tonn JC, Soffietti R, Weller M
and Moyal EC: EANO guidelines for the diagnosis and treatment of
ependymal tumors. Neuro Oncol. 27:445–456. 2017.
|
|
6
|
Freije WA, Castro-Vargas FE, Fang Z,
Horvath S, Cloughesy T, Liau LM, Mischel PS and Nelson SF: Gene
expresion profiling of glioma strongly predicts survival. Cancer
Res. 64:6503–6510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 (Suppl 4):S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gadji M, Crous AM, Fortin D, Krcek J,
Torchia M, Mai S, Drouin R and Klonisch T: EGF receptor inhibitors
in the treatment of glioblastma multiform: Old clinicalallies and
newly emerging therapeutic concepts. EurJ Pharmacol. 25:23–30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muracciole X, Romain S, Dufour H, Palmari
J, Chinot O, Ouafik L, Grisoli F, Branger DF and Martin PM: PAI-1
and EGFR expression in adult glioma tumors: Toward a molecular
prognostic classification. Int J Radiat Oncol Biol Phys.
52:592–598. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Zhang W, Chen D, Lv Y, Zheng J,
Lilljebjörn H, Ran L, Bao Z, Soneson C, Sjögren HO, et al: A glioma
classification scheme based on coexpression modules of EGFR and
PDGFRA. Proc Natl Acad Sci USA. 111:3538–3543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin T, Wang M, Liang HS and Liu EZ: The
expression of p53, mgmt and egfr in brain glioma and clinical
significance. J Biol Regul Homeost Agents. 29:143–149.
2015.PubMed/NCBI
|
|
13
|
Ladanyi M and Pao W: Lung adenocarcinoma:
Guiding EGFR-targeted therapy and beyond. Mod Pathol. 21 (Suppl
2):S16–S22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lo HW: EGFR-Targeted therapy in malignant
glioma: Novel aspects and mechanisms of drug resistance. Curr Mol
Pharmacol. 3:37–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giaccone G: The role of gefitinib in lung
cancer treatment. Clin Cancer Res. 10:4233s–4237s. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hotta K, Ueoka H, Kiura K, Tabata M, Ogino
A, Umemura S, Harita S, Gemba K, Yonei T, Bessho A, et al: Safety
and efficacy of gefitinib treatment in elderly patients with
non-small-cell lung cancer: Okayama lung cancer study group
experience. Acta Oncol. 44:717–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ostoros G, Harisi R, Kovacs G, Horti J,
Geczi L, Szondy K, Orosz M, Ferenczi E, Ruby E and Dome B:
Inhibition of EGFR tyrosine-kinase in NSCLC treatment: The
hungarian experience with gefitinib in the context of an expanded
access programme. Anticancer Res. 25:4759–4762. 2005.PubMed/NCBI
|
|
18
|
Antipenko L, Karpenko A, Kovalenko S,
Katsev A, Komarovska-Porokhnyavets E, Novikov V and Chekotilo A:
Synthesis of New 2-Thio-(1,2,4)triazolo(1, 5-c)quinazoline
derivatives and its antimicrobial activity. Chem Pharm Bull
(Tokyo). 57:580–585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Latli B, Wood E and Casida EJ:
Insecticidal quinazoline derivatives with (trifluor-omethyl)
diazirinyl and azido substituents as NADH: Ubiquinone
oxidoreductase inhibitors and candidate photoaffinity probes. Chem
Res Toxicol. 9:445–450. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schleiss M, Eickhoff J, Auerochs S, Leis
M, Abele S, Rechter S, Choi Y, Anderson J, Scott G and Rawlinson W:
Protein kinase inhibitors of the quinazoline class exert
anti-cytomegaloviral activity in vitro and in vivo. Antiviral Res.
79:49–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barker AJ: Quinazoline derivatives useful
for treatment of neoplastic disease. US Patent 5457105A. Filed
August 2, 1994; issued October 10, 1995.
|
|
22
|
Lokker NA, Sullivan CM, Hollenbach SJ,
Israel MA and Giese NA: Platelet-Derived growth factor (PDGF)
autocrine signaling regulates survival and mitogenic pathways in
glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D
ligands may play a role in the development of brain tumors. Cancer
Res. 62:3729–3735. 2002.PubMed/NCBI
|
|
23
|
Cheng Y and Paz K: Tandutinib, an oral,
small-molecule inhibitor of FLT3 for the treatment of AML and other
cancer indications. IDrugs. 11:46–56. 2008.PubMed/NCBI
|
|
24
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li GH, Zhang H, Liu Y, Kong L, Guo Q and
Jin F: Effect of temozolomide on livin and caspase-3 in U251 glioma
stem cells. Exp Ther Med. 9:744–750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Hou L, Sun W, Yu Z, Wang J, Gao H
and Yang G: Synthesis of p-O-Alkyl salicylanilide derivatives as
novel EGFR inhibitors. Drug Dev Res. 77:37–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin KH, Hsieh YH, Sulake RS, Wang SP, Chao
JI and Chen C: Optimization of gefitinib analogues with potent
anticancer activity. Bioorg Med Chem Lett. 24:5247–5250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou J, Kwang KJ, Wu Z, Yang D, Li J,
Chang M, Song Y, Zeng H, Lee LJ, Hu J and Bai C: PLAUR confers
resistance to gefitinib through EGFR/P-AKT/Survivin signaling
pathway. Cell Physiol Biochem. 47:1909–1924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blazar IN: Differential effects of
epidermal growth factor receptor inhibitors on glioblastoma
multiforme (unpublished PhD thesis). Boston University Theses &
Dissertations. 2015.
|
|
30
|
Li Y, Raffo AT, Drew L, Mao Y, Tran A,
Petrylak DP and Fine RL: Fas-Mediated apoptosis is dependent on
wild-type p53 status in human cancer cells expressing a temperature
sensitive p53 mutant alanine-143. Cancer Res. 63:1527–1533.
2003.PubMed/NCBI
|
|
31
|
Chhanabhai M, Krajewski S, Krajewska M,
Wang HG, Reed JC and Gascoyne RD: Immunohistochemical analysis of
interleukin-1beta-convertin enzyme/Ced-3 family protease,
CPP32/Yama/Caspase-3, in Hodgkin's disease. Blood. 15:2451–2455.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Creagh EM, Conroy H and Martin SJ:
Caspase-Activation pathways in apoptosis and immunity. Immunol Rev.
193:10–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leung SY, Chan AS, Wong MP, Yuen ST,
Cheung N and Chung LP: Expression of vascular endothelial growth
factor and its receptors in pilocytic astrocytoma. Am J Surg
Pathol. 21:941–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lassoued W, Murphy D, Tsai J, Oueslati R,
Thurston G and Lee WM: Effect of VEGF and VEGF trap on vascular
endothelial cell signaling in tumor. Cancer Biol Ther.
10:1326–1333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nesbit M: Abrogation of tumor vasculature
using gene therapy. Cancer Metastasis Rev. 19:45–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kil WJ, Tofilon PJ and Camphausen K:
Post-Radiation in crease in VEGF enhances glioma cell motility in
vitro. Radiat Oncol. 7:252012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herbst RS and Shin DM: Monoclonal antibody
to target epidermal growth factor receptor positive tumors: A new
paradigm for cancer therapy. Cancer. 94:1593–1611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pore N, Jiang Z, Gupta A, Cerniglia G, Kao
GD and Maity A: EGFR tyrosine kinase inhibitors decrease VGFR
expression by both hypoxia-inducible factor(HIF)-1-Dependent
mechanism. Cancer Res. 66:3197–3204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bronte G, Terrasi M, Rizzo S, Sivestris N,
Ficorella C, Cajozzo M, Di Gaudio F, Gulotta G, Siragusa S, Gebbia
N and Russo A: EGFR genomic alterations in cancer: Prognostic and
predictive value. Front Biosci (Elite ED). 1:879–887.
2011.PubMed/NCBI
|
|
42
|
Tao JJ, Castel P, Radosevic-Robin N,
Elkabets M, Auricchio N, Aceto N, Weitsman G, Barber P, Vojnovic B,
Ellis H, et al: Antagonism of EGFR and HER3 enhances the response
to inhibitors of the PI3K-Akt pathway in triple-negative breast
cancer. Sci Signal. 7:ra29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McCubrey JA, Steelman LS, Abrams SL, Lee
JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA,
D'Assoro AB, et al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT
pathways in malignant transformation and drug resistance. Adv
Enzyme Regul. 46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Freudlsperger C, Burnett JR, Friedman JA,
Kannabiran VR, Chen Z and Van Waes C: EGFR-PI3K-AKT-mTOR signaling
in head and neck squamous cell carcinomas-attractive targets for
molecular-oriented therapy. Expert Opin Ther Targets. 15:63–74.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luwor RB, Taylor LE, Wang B and Zhu HJ:
Tumor-associated EGFR over-expression specifically activates Stat3
and Smad7 resulting in desensitization of TGF-β signaling. Nat
Prec. 2008. View Article : Google Scholar
|
|
47
|
Kim SH, Juhnn YS and Song YS: Akt
involvement in paclitaxel chemoresistance of human ovarian cancer
cells. Ann N Y Acad Sci. 10:82–89. 2007. View Article : Google Scholar
|