Introduction
Transient forebrain ischemia (tFI) causes the
pathophysiological alterations and a selective neuronal death in
the brain. Especially, the pyramidal neurons in the hippocampal
cornu ammonis 1 (CA1) region are well known to be the most
vulnerable to tFI (1). tFI-induced
neuronal death in the pyramidal neurons of hippocampal CA1 region
is called as the delayed neuronal death (DND), because it occurs a
few days following tFI (1). To
explain the tFI-induced DND, many underlying mechanisms has been
suggested, including neuroinflammatory processes. Following
cerebral ischemia, neuroinflammatory responses and upregulation of
inflammatory cytokines occurs generally in the brain, and the
imbalance between pro-inflammatory cytokines, such as tumor
necrosis factor (TNF)-α, and anti-inflammatory cytokines could lead
to the neuronal damage after ischemic injury (2–5). In
addition, the cerebral ischemia-induced neuronal damage has been
also known to be affected by various factors, including ischemic
duration, the age and sex of experimental animals (6–9).
TNF-α plays a diverse biological role in regulating
inflammatory responses, and its biological actions is mediated
through two plasma membrane receptors, TNF receptor 1 (TNF-R1) and
TNF-R2, which are present in all cell types, including neurons and
glial cells, in the CNS (10–12).
It has been well known that toxic effects of TNF-α begin with and
require binding to TNF-R1, and that TNF-R1 is responsible for
mediating the detrimental effects of TNF-α, such as apoptotic cell
death and cytokine production (13,14).
However, another study also reported that the protective role of
TNF-α against excitotoxic hippocampal neurodegeneration was closely
associated with TNF-R1 signaling (15). In addition, TNF-α has been thought
to be one of important mediators in modulating neuronal death in
in vitro and in vivo models of ischemic damage
(13,16,17).
In our previous studies, we reported the tFI-induced
changes of TNF-α and/or TNF-R1 protein expression in the
hippocampus of adult gerbils (4,18,19).
However, the relationship between TNF-α and neuronal damage in the
hippocampus of young animals has not been fully elucidated yet.
Therefore, in the present study, we compared the time-dependent
changes of TNF-α and TNF-R1 protein expression in the hippocampal
CA1 region of adult and young gerbils after tFI.
Materials and methods
Experimental animals
Male gerbils of one month (body weight: 25–30 g) and
six months (body weight: 65–75 g) of age were used as young and
adult groups. The animals were provided by the Experimental Animal
Center of Kangwon National University (Chuncheon, Kangwon, Republic
of Korea). They were housed in a conventional state (room
temperature, 23±0.5°C; humidity, 55±5%; 12:12 light/dark cycle)
with freely accessible pellet feed and water. The protocol of this
experiment was approved (approval no. KW-200113-1) by the
Institutional Animal Care and Use Committee.
Experimental groups and induction of
tFI
The gerbils used in this research were divided into
four groups: i) adult sham group (n=28); ii) tFI-operated
adult gerbils (adult ischemia group) (n=56); iii) young sham
group (n=28); and iv) young ischemia group
(n=56).
As previously described in our published papers
(4,19,20),
the surgical procedure of tFI in the gerbils was performed as
follows. Briefly, the gerbils were adequately anesthetized using
mixture of 2.5% isoflurane (Baxtor) in 67% nitrous oxide and 33%
oxygen (21). Under the anesthesia,
both common carotid arteries, which locate at the neck and supply
arterial blood to the brain, were isolated and ligated for 5 min
with aneurysm clips. Body temperature was checked using a rectal
temperature probe and controlled at normothermic condition
(37±0.5°C) using a thermometric blanket before and during tFI.
After tFI, body temperature was controlled at normothermia until
they awaked. The sham gerbils received the same tFI operation
without the occlusion of the arteries.
Western blotting for TNF-α and
TNF-R1
The gerbils in the sham groups were sacrificed at 1
day (n=7) and 4 days (n=7, data not shown) after tFI,
the gerbils in the ischemia groups were sacrificed at 1 day
(n=7), 4 days (n=7), 7 days (n=7) and 15 days
(n=7) after tFI. The gerbils in all groups were deeply
anesthetized with 70 mg/kg pentobarbital sodium, and the dose was
increased to 200 mg/kg for euthanasia. The confirmation of death
was evaluated with vital signs including heart beats, pupillary
response, and respiratory pattern (lack of cardiac activity for 5
min through cardiac palpation, unresponsiveness to light with
dilated pupils using light into the eyes of the animal, and lack of
spontaneously breathing pattern with shallow and irregular
breathing pattern). As previously described (22), hippocampal proteins (50 µg for each
sample) were denatured, electrophoretically separated, and
transferred to a nitrocellulose membranes. The membrane was blocked
with TBS-T buffer containing non-fat dry milk (5%) and incubated
for 8 h at 4°C with primary antibodies-rabbit anti-TNF-α or rabbit
anti-TNF-R1 (diluted 1:1,000; Abcam). The next day, the membrane
was incubated with secondary antibody [peroxidase conjugated goat
anti-rabbit IgG (Pierce; Thermo Fisher Scientific)] for 1 h at room
temperature. Thereafter, the membranes was analyzed using
chemiluminescence system according to the manufacturer (Amersham;
GE Healthcare.).
The western blot bands of TNF-α and TNF-R1 were
scanned by ChemiDoc Imaging System (Bio-Rad Laboratories, Inc. The
protein signal was quantified by scanning densitometry using Scion
Image software of Scion Corp. Data were normalized to appropriate
references (β-actin for total protein).
Preparation of histological
sections
The gerbils in the sham groups were sacrificed at 1
day (n=7) and 4 days (n=7, data not shown) after tFI,
and the gerbils in the ischemia groups were sacrificed at 1 day
(n=7), 4 days (n=7), 7 days (n=7) and 15 days
(n=7) after tFI. In our current study, to reduce the numbers
of the gerbils, the brain sections of the young and adult sham
groups were obtained at 1 and 4 days after sham operation. The
brain sections containing the hippocampi were prepared as
previously described in our studies (4,19,20).
In brief, the gerbils in all groups were deeply anesthetized with
70~90 mg/kg pentobarbital sodium (21) at 1, 4, 7 and 15 days after tFI.
Under anesthesia, the gerbils were perfused transcardially with
saline, and then with 4% paraformaldehyde solution. The brains were
obtained and more fixed in the same fixative for 6 h at room
temperature. For cutting the brains, they were infiltrated with 30%
sucrose solution to avoid tissue damage from freeze. Finally, the
brain tissues were coronally sectioned into 30-µm thickness in a
cryostat.
Fluoro-Jade B (FJB)
histofluorescence
To examine neuronal damage/death (loss) in CA1 after
tFI, FJB histofluorescence was done as described previously
(23). In short, the tissues
(sections) were incubated in 0.06% potassium permanganate and
stained with 0.0004% FJB (Histochem) for 10 min using a rotator.
Thereafter, the sections were rinsed and incubated in 0.0004% FJB
(Histochem) for 20 min After washing, the sections were placed on a
slide warmer for the reaction of F-J B. Thereafter, the sections
were fully dried, cleared by immersion in xylene and coverslipped
with dibutylphthalate polystyrene xylene (DPX; Sigma-Aldrich; Merck
KGaA).
To count the FJB positive cells (neurons) in CA1,
five sections/gerbil were chosen and analyzed using previously
described method (4,19,20).
The image of FJB positive cells was taken using fluorescence
microscope from Carl Zeiss with blue excitation fluorescence filter
between 450–490 nm. The FJB positive cells were captured using
image capture software (cellSens Standard; Olympus). The captured
FJB positive cells were totally counted in 250 µm2 at
the middle in CA1. The mean number was calculated using NIH Image
1.59 software (NIH; National Institutes of Health).
Immunohistochemistry for neuronal
nuclear antigen (NeuN), TNF-α and TNF-R1
According to published methods (4,19,20)
with modification, immunohistochemical staining using a single
antibody (NeuN, TNF-α and TNF-R1) was performed by
streptavidin-biotin-peroxidase method. In brief, the sections were
immersed with 0.3% hydrogen peroxide to block endogenous peroxidase
activity (20 min at room temperature), and 5% normal horse serum
was treated to block unspecific proteins in the tissues (30 min at
room temperature). And then, the tissues were incubated with each
antibody-mouse anti-NeuN (1:1,100, Chemicon), rabbit anti-TNF-α
(1:1,100, Abcam) or rabbit anti-TNF-R1 (1:1,000, Abcam)-for 10 h at
4°C. The tissues were rinsed and incubated with biotinylated horse
anti-mouse or anti-rabbit IgG (Vector Laboratories, Inc.) for 2 h
at room temperature, and continuously reacted with Elite
avidin-biotin enzyme complex (ABC; Vector Laboratories, Inc.) for 1
h at room temperature. The visualization of each immunoreaction was
achieved with solution of 3,3′-diaminobenzidine (Vector
Laboratories, Inc.).
To count NeuN-immunoreactive cells, and to analyze
TNF-α- and TNF-R1-immunoreactive structure, we used published
methods (4,19,20) In
short, five sections/gerbil with 120-µm interval were chosen. The
digital images of NeuN-immunoreactive cells were captured in 250
µm2 at the middle of CA1 using light microscope (BX53)
from Olympus. For TNF-α- and TNF-R1-immunoreactive structure, their
digital images were also captured in all layers using BX53
microscope. TNF-α- and TNF-R1-immunoreactivity was evaluated by
relative optical density (ROD) according to published methods
(19,20) with some modification. Namely, the
color image was converted to 8-bit greyscale images with a rage of
0–255 (from black to white). The background level and the variance
of the staining intensity in CA1 was calculated on the 0–255
greyscale. The percentile in the corresponding area was analyzed
using image analysis software from Image J (NIH).
Double immunofluorescence
To confirm the type of TNF-α- and
TNF-R1-immunoreactive cells, double immunofluorescence was
performed using rabbit anti-TNF-α (diluted 1:400; Abcam)/mouse
anti-GFAP (diluted 1:1,000, Chemicon) or rabbit anti-TNF-R1
(diluted 1:400, Abcam)/mouse anti-GFAP (diluted 1:1,000, Chemicon)
for astrocytes. The sections obtained at 7 days after tFI were
incubated in the mixture of antisera for 8 h at 4°C. They were
briefly washed and reacted with the mixture of both Cy3- or
FITC-conjugated donkey anti-rabbit or anti-mouse IgG (diluted
1:200; Jackson ImmunoResearch) for 2 h at room temperature. The
double immunoreaction was examined with confocal MS from LSM510
META NLO (Carl Zeiss).
Statistical analysis
The data presented in this study represent the means
± SEM. The data were statistically analyzed using SPSS 18.0 (SPSS,
Inc.). The two-way analysis of variance (ANOVA) with a post
hoc Bonferroni's multiple comparison test was done to determine
differences among groups. P<0.05 was used for statistical
significance.
Results
Difference in tFI-induced DND between
the adult and young
We examined the difference of tFI-induced DND in CA1
of gerbil hippocampus between adult and young gerbils using
neuronal nuclear antigen (NeuN, a marker for neuron)
immunohistochemistry and Fluoro-Jade B (FJB, a marker for
degenerating or dead neuron) histofluorescence (Fig. 1).
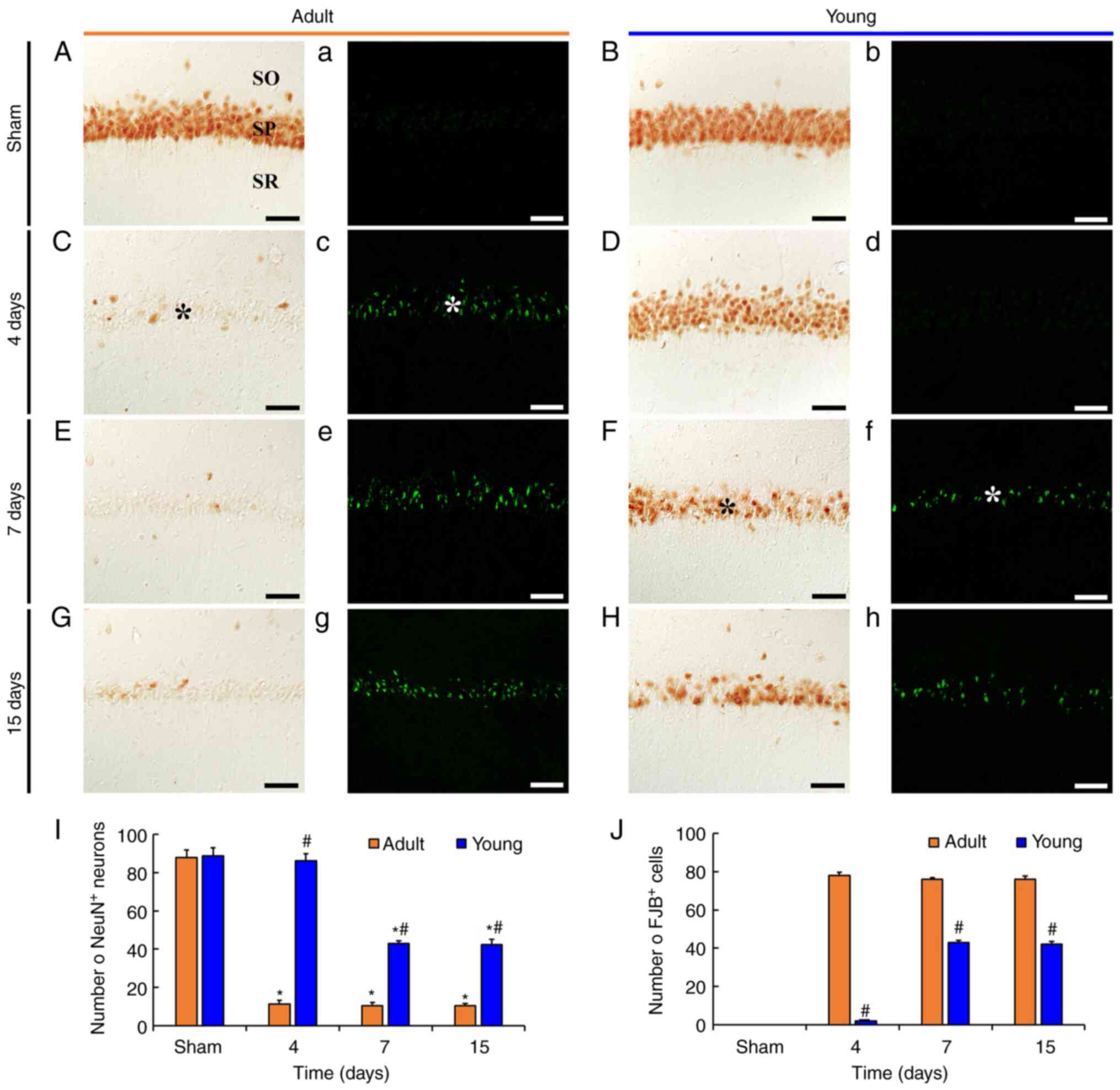 | Figure 1.tFI-induced delayed neuronal death.
(A-H) NeuN immunohistochemistry and (a-h) FJB histofluorescence
staining in the CA1 in the (A, a, B and b) sham and (C-H and c-h)
ischemia groups of adult (left two columns) and young (right two
columns) gerbils. In the adult ischemia group, a few
NeuN-immunoreactive pyramidal neurons and several FJB-positive
pyramidal neurons were observed in the SP (asterisks) 4 days after
tFI. In the young ischemia group, the number of NeuN-immunoreactive
neurons significantly decreased and FJB-positive neurons increased
7 days after tFI. Scale bar, 50 µm. (I and J) Mean numbers of
NeuN-immunoreactive and FJB-positive neurons in 250 µm2
at the center of the CA1, respectively. Data are presented as the
mean ± SEM. *P<0.05 vs. sham group; #P<0.05 vs.
corresponding adult group. NueN, neuronal nuclear antigen; FJB,
Fluoro-Jade B; CA1, cornu ammonis 1; tFI, transient forebrain
ischemia; SP, stratum pyramidale; SO, stratum oriens; SR, stratum
radiatum. |
In adult and young sham groups, NeuN
immunoreactivity was easily identified in pyramidal neurons
(88.0±3.8/250 µm2) located in the stratum pyramidale
(SP) (Fig. 1A and 1B). In addition, FJB-positive cells were
not detected in SP (Fig. 1A and B).
This finding means that no damaged cells were present in the sham
groups.
In adult ischemia group, a significant loss of
NeuN-immunoreactive CA1 pyramidal neurons (11.3±1.9/250
µm2) and many FJB-positive neurons (78.1±1.7/250
µm2) were observed in SP at 4 days after tFI. In this
group, the number of NeuN-immunoreactive neurons was approximately
13% of that of the adult sham group (Fig. 1C and Cc). Thereafter, the numbers of
NeuN-immunoreactive neurons and FJB-positive cells at 7 and 15 days
after tFI were similar to those at 4 days after tFI (Fig. 1E, Ee, G, Gg, I and J).
In the young ischemia group, the number of
NeuN-immunoreactive pyramidal neurons at 4 days after tFI was
similar to that in young sham group, although the NeuN
immunoreactivity was decreased (Fig.
1D, Dd and I). However, at 7 and 15 days after tFI, the number
of NeuN-immunoreactive pyramidal neurons was distinctly decreased
(approximately 48.5 and 47.9%, respectively), compared with that in
the young sham group (Fig. 1F, H and
I). In addition, we found that many FJB-positive pyramidal
neurons were observed at 7 and 15 days after tFI, showing that the
mean percentage of the neurons was approximately 57 and 55%,
respectively, of that in the adult ischemia group, respectively
(Fig. 1f, h and J).
Difference in tFI-induced changes of
TNF-α and TNF-R1 levels between the adult and young
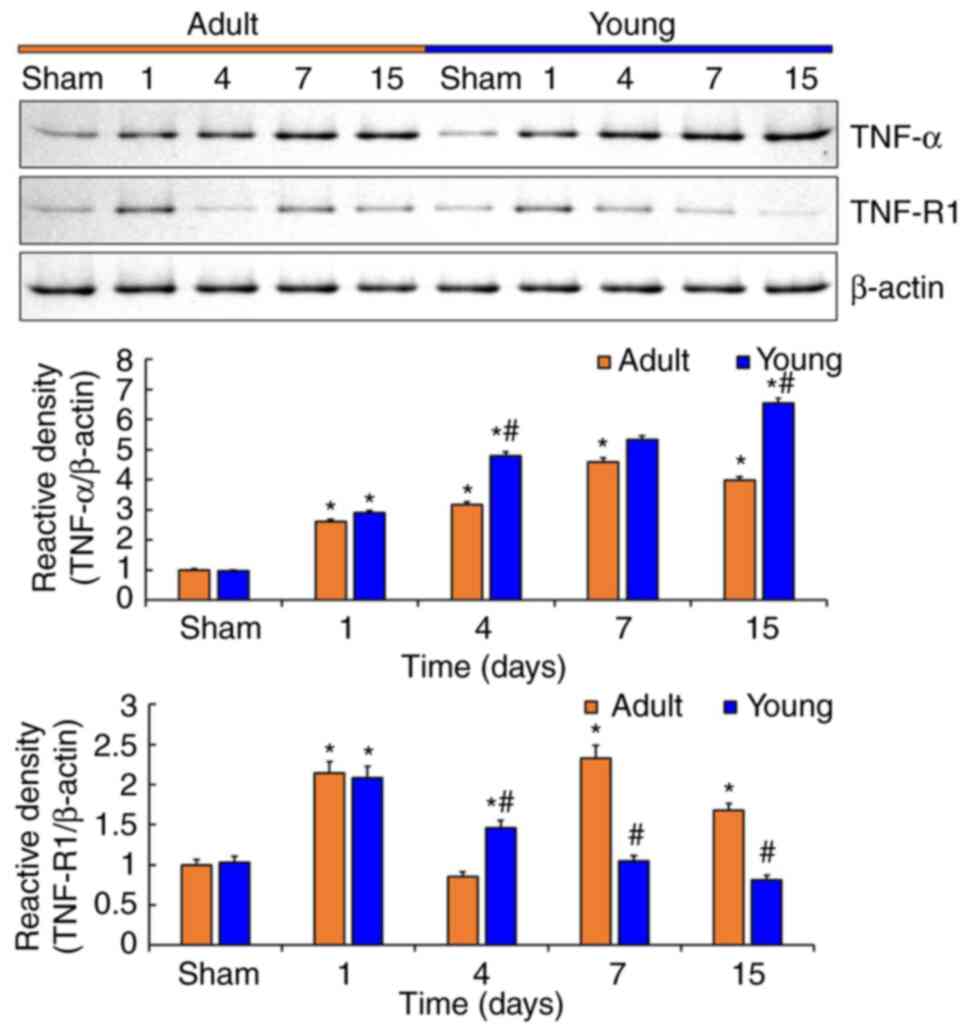
To compare the changes of TNF-α and TNF-R1 protein
levels in ischemic CA1 between adult and young gerbils, western
blot was conducted in CA1 after tFI (Fig. 2).
Protein levels of TNF-α in the adult and young CA1
were significantly changed with time after tFI (Fig. 2). In both groups, TNF-α protein
levels were gradually increased from 1 day after tFI, however,
TNF-α protein levels were significantly higher in the young group
than that in the adult group, showing that relative density in the
young was 1.1-fold at 1 day, 1.5-fold at 4 days, 1.2-fold at 7 days
and 1.6-fold at 15 days after tFI compared with that in the
corresponding adult groups (Fig.
2).
The change pattern of TNF-R1 protein levels in the
adult and young was not similar to the changes of TNF-α protein
levels, but the changes were significant (Fig. 2). At 1 day after tFI, TNF-R1 protein
levels were dramatically increased in both groups (2.1-fold in the
adult and 2.1-fold in the young) compared with those in each sham
group (Fig. 2). Thereafter, TNF-R1
protein levels in both groups were irregularly altered, showing
that relative density in the young groups was 1.7-fold at 4 days,
0.4-fold at 7 days, and 0.5-fold at 15 days, respectively, after
tFI compared with that in the corresponding adult groups (Fig. 2).
Difference in tFI-induced change of
TNF-α immunoreactivity between the adult and young
To investigate the change of TNF-α immunoreactivity
in CA1, immunohistochemistry for TNF-α was conducted in adult and
young gerbils after tFI (Fig.
3).
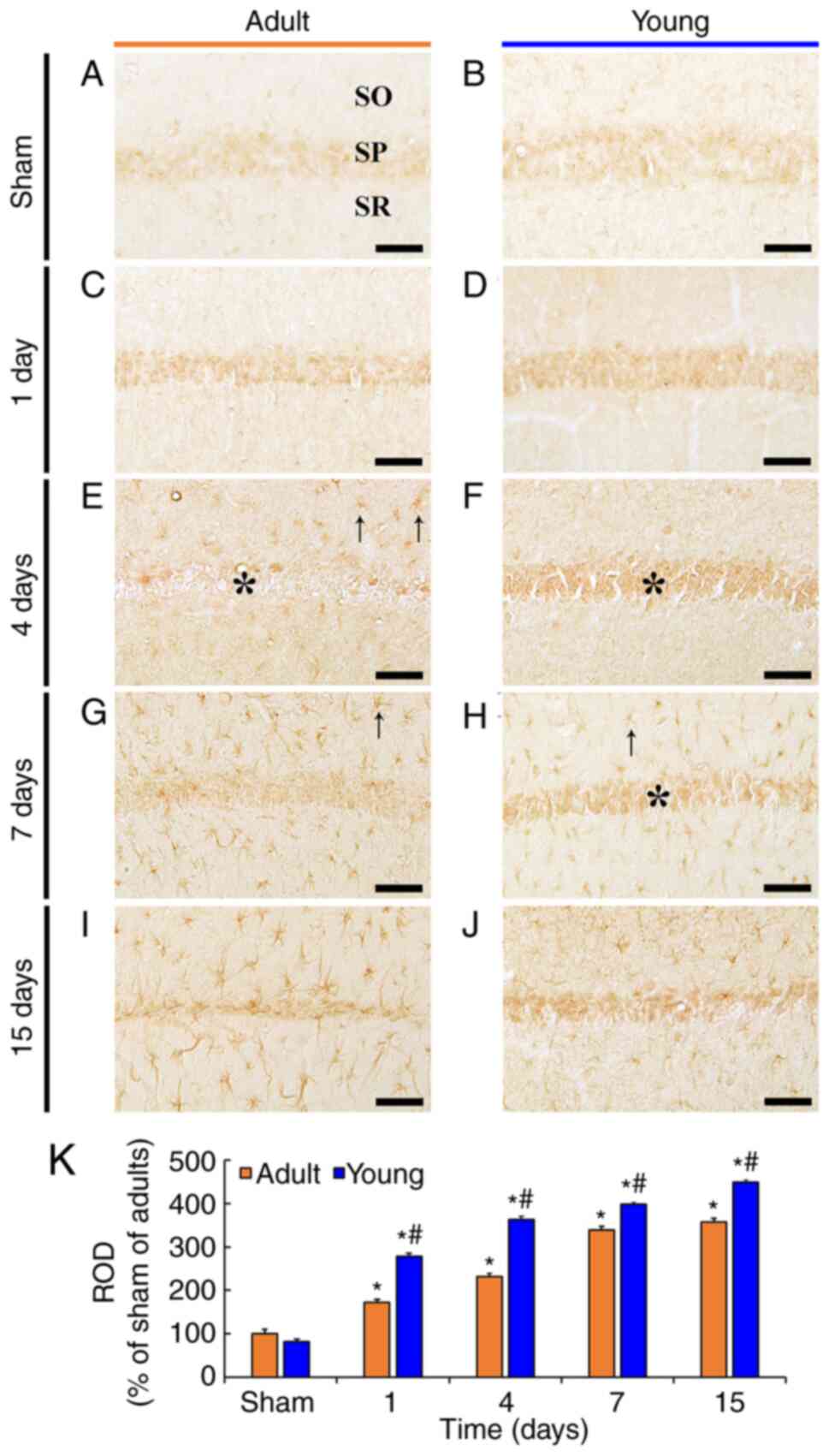 | Figure 3.TNF-α immunohistochemistry. (A-J)
TNF-α immunohistochemistry in the CA1 in the (A and B) sham and
(C-J) ischemia groups of adult (left column) and young (right
column) gerbils. In the adult ischemia group, TNF-α
immunoreactivity was rarely exhibited in the SP (asterisks) and
observed in non-pyramidal cells (arrows) located in the SO and SR 4
days after tFI. In the young ischemia group, TNF-α immunoreactivity
in the SP was observed 15 days after tFI, whereas TNF-α
immunoreactivity was observed in non-pyramidal cells 7 days after
tFI. Scale bar, 50 µm. (K) ROD as % of TNF-α immunoreactivity in
CA1 (n=7). *P<0.05 vs. sham group; #P<0.05
vs. corresponding adult group. Data are presented as the mean ±
SEM. TNF, tumor necrosis factor; CA1, cornu ammonis 1; SP, stratum
pyramidale; SO, stratum oriens; SR, stratum radiatum; tFI,
transient forebrain ischemia; ROD, relative optical density. |
Weak TNF-α immunoreactivity was mainly found in CA1
pyramidal neurons in the adult sham group (Fig. 3A and K). In the adult ischemia
group, a significant increase of TNF-α immunoreactivity (172.5% of
the adult sham group) was found in the pyramidal neurons at 1 day
after tFI, compared with that in the adult sham group (Fig. 3C and K). At 4 days after tFI, TNF-α
immunoreactivity was hardly observed in the pyramidal neurons;
however, at this time, TNF-α-immunoreactivity began to be expressed
in non-pyramidal cells located in the stratum oriens (SO) and
stratum radiatum (SR) (Fig. 3E and
K). Thereafter, TNF-α-immunoreactivity was mainly observed in
the non-pyramidal cells, and the relative optical density (ROD) of
the TNF-α-immunoreactivity in CA1 was markedly increased (339.1% in
the adult and 357.4% in the young) at 7 and 15 days after tFI
compared to that in the adult sham group (Fig. 3G, I and K).
In the young sham group, no significant difference
in TNF-α immunoreactivity in the pyramidal neurons was found when
compared with that in the adult sham group (Fig. 3B and K). In the young ischemia
group, TNF-α immunoreactivity in the pyramidal was significantly
increased at 1 and 4 days after tFI, and the ROD in CA1 was
increased (161.2% in the adult and 156.7% in the young) compared to
that in the corresponding adult ischemia group (Fig. 3D, F and K). At 7 and 15 days after
tFI, TNF-α immunoreactivity in the pyramidal neurons was still
observed, and non-pyramidal cells newly expressed TNF-α
immunoreactivity, showing that the ROD in CA1 was increased (117.4%
in the adult and 125.8% in the young) compared to that in the
corresponding adult ischemia group (Fig. 3H, J and K).
As described above, TNF-α immunoreactivity was shown
in non-pyramidal cells at late time after tFI. To identify the cell
types of the non-pyramidal cells containing TNF-α immunoreactivity,
we performed double immunofluorescence staining for TNF-α/GFAP (a
marker for astrocyte), and we found that most of
TNF-α-immunoreactive non-pyramidal cells were colocalized with
GFAP-immunoreactive astrocytes in the adult and young ischemia
groups (Fig. 4).
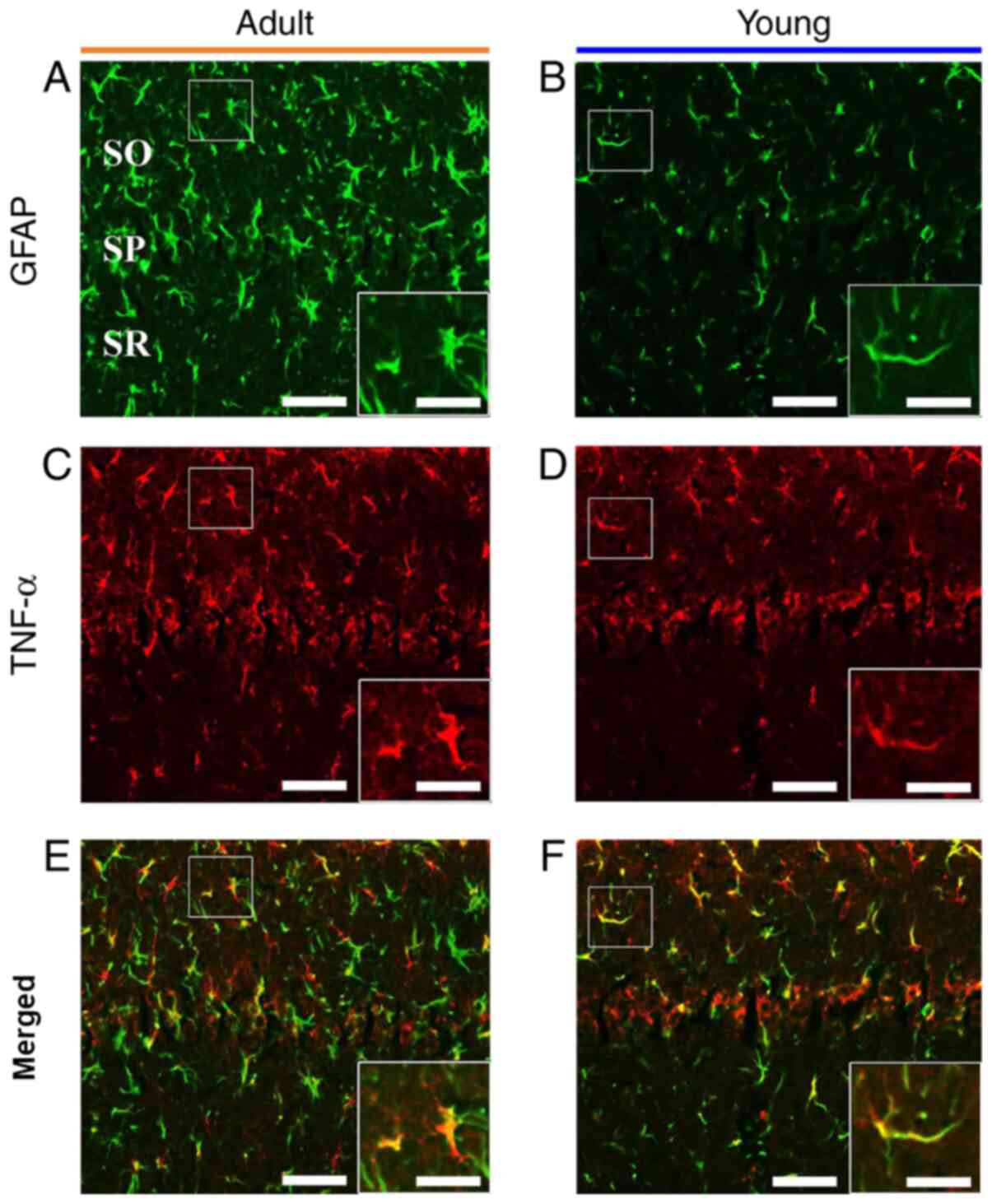 | Figure 4.GFAP and TNF-α double
immunofluorescence. Double immunofluorescence for (A and B) GFAP,
(C and D) TNF-α and (E and F) merged images in the CA1 in the (A, C
and E) adult and (B, D and F) young ischemia groups 7 days after
transient forebrain ischemia. GFAP immunoreactive astrocytes
expressed TNF-α in both groups. Scale bar, 50 µm (enlarged images,
100 µm). GFAP, glial fibrillary acidic protein; TNF, tumor necrosis
factor; CA1, cornu ammonis 1; SO, stratum oriens; SP, stratum
pyramidale; SR, stratum radiatum. |
Difference in tFI-induced change of
TNF-R1 immunoreactivity between the adult and young
To examine the change of TNF-R1 immunoreactivity in
CA1, immunohistochemistry was done in the adult and young groups
(Fig. 5).
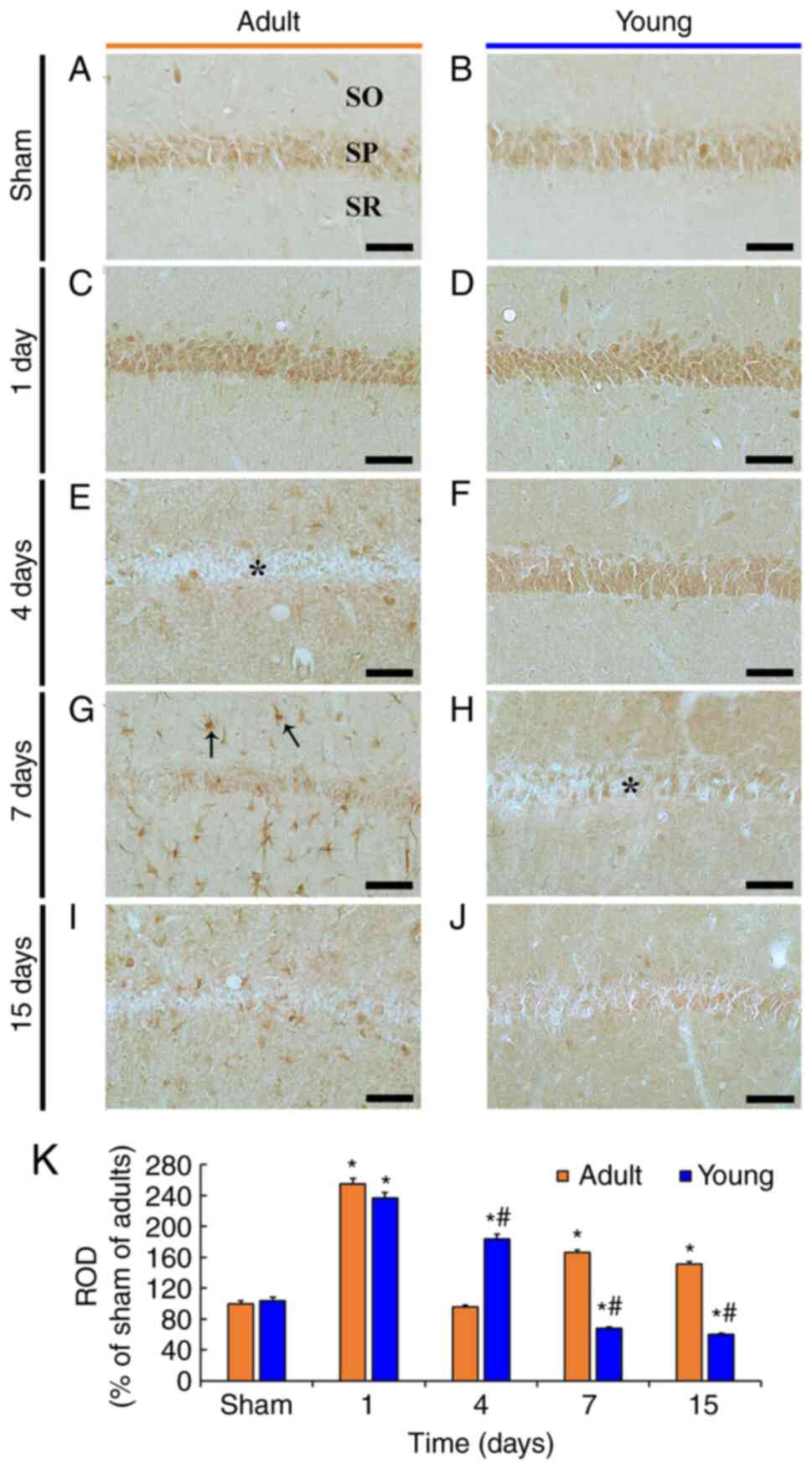 | Figure 5.TNF-R1 immunohistochemistry. (A-J)
TNF-R1 immunohistochemistry in the CA1 in the (A and B) sham and
(C-J) ischemia groups of adult (left column) and young (right
column) gerbils. In the adult ischemia group, TNF-R1
immunoreactivity increased in the SP (asterisk) 1 day after tFI and
was rarely exhibited in the SP 4 days after tFI. TNF-R1
immunoreactivity was present in non-pyramidal cells (arrows). In
the young ischemia group, TNF-R1 immunoreactivity increased in the
SP 4 days after tFI. Thereafter, TNF-R1 immunoreactivity weakened
in the CA1. The asterisk in (H) indicates that TNF-R1
immunoreactivity in the SP was exhibited in some pyramidal neurons.
Scale bar, 50 µm. (K) ROD as % of TNF-R1 immunoreactivity in CA1
(n=7) after tFI. *P<0.05 vs. sham group;
#P<0.05 vs. corresponding adult group. Data are
presented as the mean ± SEM. TNF-R1, tumor necrosis factor receptor
1; CA1, cornu ammonis 1; SP, stratum pyramidale; tFI, transient
forebrain ischemia; ROD, relative optical density; SO, stratum
oriens; SR, stratum radiatum. |
In the adult sham group, TNF-R1 immunoreactivity was
easily shown in pyramidal neurons (Fig.
5A). In the adult ischemia group, TNF-R1 immunoreactivity was
significantly increased (254.9% of the adult sham group) in the
pyramidal neurons at 1 day after tFI (Fig. 5C and K). Thereafter, TNF-R1
immunoreactivity in the pyramidal neurons was hardly shown at 4
days after tFI, but TNF-R1 immunoreactivity was newly shown in
non-pyramidal cells, and TNF-R1 immunoreactivity in CA1 was 96.4%
of the adult sham group (Fig. 5E and
K). At 7 and 15 days after tFI, TNF-R1 immunoreactivity was
more increased in the non-pyramidal cells, and TNF-R1
immunoreactivity in CA1 was 166.6 and 151.6% of the adult sham
group, respectively (Fig. 5G, I and
K). These non-pyramidal cells containing TNF-R1
immunoreactivity were identified as GFAP-immunoreactive astrocytes
by double immunofluorescence (Fig. 6A,
C and E).
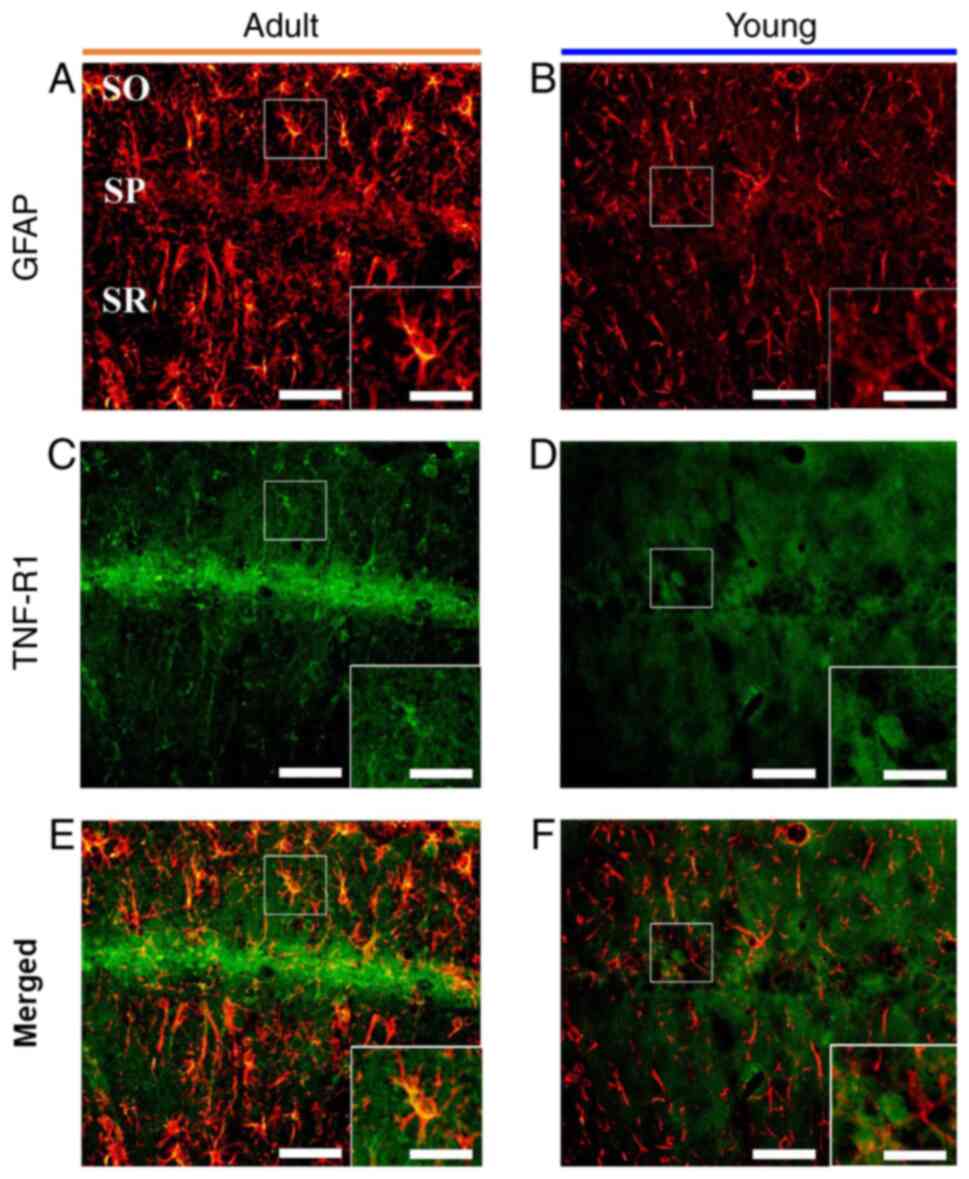 | Figure 6.GFAP and TNF-R1 double
immunofluorescence. Double immunofluorescence for (A and B) GFAP,
(C and D) TNF-R1 and (E and F) merged images in the CA1 in the (A,
C and E) adult and (B, D and F) young ischemia groups 7 days after
transient forebrain ischemia. GFAP immunoreactive astrocytes only
expressed TNF-R1 in adults. Scale bar, 50 µm (enlarged images, 100
µm). GFAP, glial fibrillary acidic protein; TNF-R1, tumor necrosis
factor receptor 1; CA1, cornu ammonis 1; SO, stratum oriens; SP,
stratum pyramidale; SR, stratum radiatum. |
In the young sham group, TNF-R1 immunoreactivity was
also found in pyramidal neurons, and the immunoreactivity was
similar to that in the adult sham group (Fig. 5B and K). In the young ischemia
group, TNF-R1 immunoreactivity in the pyramidal neurons was
increased only in the pyramidal neurons, showing that the TNF-R1
immunoreactivity was 93.0% at 1 day and 190.9% at 4 days after tFI
compared with that in the corresponding adult ischemia group
(Fig. 5D, F and K). At 7 and 15
days after tFI, TNF-R1 immunoreactivity in CA1 was markedly
decreased (68.1% in the adult and 59.9% in the young) compared to
that in the young sham group (Fig. 5H,
J and K). At these times, TNF-R1 immunoreactivity was only
shown in a few pyramidal neurons; TNF-R1 immunoreactivity was
hardly detected in astrocytes by double immunofluorescence
(Fig. 6B, D and F).
Discussion
In this study, we found that tFI-induced DND
occurred in pyramidal neurons located in CA1 of the adult at 4 days
after tFI, whereas the DND of pyramidal neurons in the young
ischemia group was observed at 7 days after tFI. In addition, the
number of dead pyramidal neurons (DND) at 7 and 15 days after tFI
was significantly lower in the young ischemia group than that in
the adult ischemia group. This result is consistent with our and
other previous studies reporting that tFI-induced DND in the
hippocampal CA1 in young gerbils was shown in fewer pyramidal
neurons and more delayed in time than that shown in adult gerbils
(9,24,25).
Taken together, we suggest that the difference of DND between young
and adult groups might be associated with the differences of
various factors to affect DND, including glial activation,
inflammatory cytokine expression and NMDA receptor expression
(26–28).
It has been widely accepted that both TNF-α and
TNF-R1 expressions are highly increased in ischemic brains and
upregulated TNF-α plays diverse roles in modulating cerebral
ischemia-induced neuronal damage (4,18,19,29–32).
However, the exact role of TNF-α in ischemic brains is still
controversial: Detrimental or protective role. A previous study
showed that an intracerebroventricular injection of exogenous TNF-α
led to neurological deficit and brain injury following focal brain
ischemia induced by middle cerebral artery occlusion in rats,
showing that the blockage of endogenous TNF-α by
intracerebroventricular administration with a neutralized
anti-TNF-α monoclonal antibody attenuated the focal
ischemia-induced brain injury and infarct size compared with
control rats (33). It was also
reported that TNF-α was involved in oxygen-glucose
deprivation-mediated cortical cell death in a dose-dependent manner
(13). In contrast, Nawashiro et
al (34) reported that
intracisternal pretreatment with TNF-α reduced infarct size in a
time- and dose-dependent manner in a mouse model of focal brain
ischemia. In addition, it was reported that TNFα-expressing neurons
were protected from glutamate-induced excitotoxicity in
TNFα-transgenic mice compared with wild-type mice, and a
pre-exposure of TNF-α to neurons resulted in protective effect
against excitotoxicity in wild-type mice (35). Furthermore, it was reported that
neuronal damage following focal cerebral ischemia or epileptic
seizure was greater in TNFR-knockout mice than that in wild-type
mice (36). Additionally, Lu et
al (15) reported that kainic
acid-induced seizure activity, and neuronal damage and glial
activation in the hippocampus were significantly enhanced in
TNF-R1-knockout mice compared with wild-type mice, suggesting that
the protective role of TNF-α was mediated through TNF-R1 signaling.
Therefore, in the present study, to disclose the reason why the
tFI-induced DND of the CA1 pyramidal was different between adult
and young gerbils, we investigated the changes of TNF-α and TNF-R1
protein expressions in the hippocampal CA1 region of adult and
young gerbils after tFI. In our current study, TNF-α
immunoreactivity was shown in the pyramidal neurons in both sham
gerbils. After tFI, TNF-α immunoreactivity was gradually increased
in the pyramidal neurons or astrocytes both groups, but the change
pattern was different from each other and the immunoreactivity was
higher in the young. TNF-R1 immunoreactivity in both sham groups
was also shown only in the pyramidal neurons. After tFI, in both
groups, TNF-R1 immunoreactivity was transiently enhanced in the
pyramidal neurons at 1 day after tFI, thereafter, TNF-R1
immunoreactivity gradually decreased, showing that TNF-R1
immunoreactivity was newly expressed in astrocytes only in the
adult group. Based on our and the above-mentioned previous studies,
it can be postulated that a marked increase of TNF-α and TNF-R1
expression in the pyramidal neurons at early phase after tFI might
be related to a protective potential against ischemic damage, and
the different reduction of TNF-α and TNF-R1 expression might be
associated with the difference in the tFI-induced DND of the
pyramidal neurons between the adult and young.
It is well known that TNF-α is involved in
inflammatory response and stimulates astrocyte and microglia
activation at the site of ischemic injury following brain ischemic
insults (37,38). Reactive astrocytes have been known
to be protective against ischemic injury by controlling immune
response following cerebral ischemia (39) and to play an important role in the
structural and funtional remodeling of ramainined brain tissue
(40). In our current study, in the
adult ischemia group, non-pyramidal cells located in SO and SR
began to express TNF-α and TNF-R1 immunoreactivity from 4 days
after tFI, and most of the TNF-α and TNF-R1 immunoreactive
non-pyramidal cells were identified as astrocytes. Similar to this
finding, TNF-α immunoreactivity was observed in astrocytes in human
brain after ischemic stroke and traumatic brain injury (41,42).
In addition, it was reported that astrocytes expressing TNF-α and
TNF-R1 were increased in the spinal cord after sciatic nerve injury
in mice, suggesting that reactive astrocytes expressed more TNF-α,
which regulated the synthesis and expression of TNF-α through
TNF-R1 on astrocytes in a positive feedback mechanism (43). Therefore, it is likely that an
increased expression of TNF-α and TNF-R1 in activated astrocytes in
the adult ischemia group might be related to the neurorestorative
role of the reactive astrocytes after neuronal death induced by
tFI. On the other hand, in the young ischemia group,
TNF-α-immunoreactive astrocytes began to be shown from 7 days after
tFI, and TNF-R1 immunoreactivity was found in the pyramidal
neurons, not astrocytes, until 15 days after tFI. We cannot clearly
explain why TNF-R1 immunoreactivity was not found in astrocytes at
the late phase after tFI in the young ischemia group, which was
apparently different from the adult ischemia group. For this
finding, there is a paper showing that tFI-induced activation of
astrocytes was much lower in young gerbils than that in adult
gerbils between 4 and 15 days after tFI (28). Based on this finding, the decreased
TNF-R1 expression in the pyramidal neurons in the young ischemia
group at 7 days after tFI might be associated with more delayed
DND; this DND was similar to that in the adult ischemia group at 4
days after tFI. Therefore, it can be mentioned that TNF-R1
expression in astrocytes following tFI might be dependent on
difference in the degree of tFI-induced DND.
There are some limitations in the current study. We
did not investigate how TNF-α and TNF-R1 expressions were affected
by ischemia at various ages, such as 12 and 24 months. In addition,
the levels of TNF-α and TNF-R1 expressions in serum and
cerebrospinal fluid should be measured in future studies to support
the conclusion of the present study. Furthermore, rodent researches
should be extended to primate or human researches, which provide
more convincing evidences of significant treatment strategy for
patients with ischemic injuries.
In summary, both TNF-α and TNF-R1 protein levels and
immunoreactivities were differently changed in ischemic hippocampal
CA1 between young and adult gerbils following tFI. This result
indicates that the different expressions of TNF-α and TNF-R1 may be
one of underlying mechanisms of tFI-induced DND between the young
and adult. Therefore, therapeutic strategy of ischemic insults must
be considered in the age of patients with ischemic injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant nos.
NRF-2019R1A6A1A11036849 and NRF-2020R1F1A1052380).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CHL and JHA drafted the initial manuscript. CHL,
JHA, BHC, MHW and SYC were responsible for experimental
conceptualization, data curation and analysis. BHC, DWK, HS, TKL
and JHP performed the experiments, and collected and analyzed the
data. MHW and SYC reviewed the manuscript for important
intellectual content and acquired funding. MHW and SYC confirmed
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee (approval no.
KW-200113-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farhadi Moghadam B and Fereidoni M:
Neuroprotective effect of menaquinone-4 (MK-4) on transient global
cerebral ischemia/reperfusion injury in rat. PLoS One.
15:e02297692020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim DW, Lee JC, Cho JH, Park JH, Ahn JH,
Chen BH, Shin BN, Tae HJ, Seo JY, Cho JH, et al: Neuroprotection of
ischemic preconditioning is mediated by anti-inflammatory, not
pro-inflammatory, cytokines in the gerbil hippocampus induced by a
subsequent lethal transient cerebral ischemia. Neurochem Res.
40:1984–1995. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee TK, Kang IJ, Kim B, Sim HJ, Kim DW,
Ahn JH, Lee JC, Ryoo S, Shin MC, Cho JH, et al: Experimental
pretreatment with chlorogenic acid prevents transient
ischemia-induced cognitive decline and neuronal damage in the
hippocampus through anti-oxidative and anti-inflammatory effects.
Molecules. 25:35782020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Victoria ECG, Toscano ECB, Oliveira FMS,
de Carvalho BA, Caliari MV, Teixeira AL, de Miranda AS and Rachid
MA: Up-regulation of brain cytokines and metalloproteinases 1 and 2
contributes to neurological deficit and brain damage in transient
ischemic stroke. Microvasc Res. 129:1039732020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH, Yoo KY, Choi JH, Park OK, Hwang
IK, Kim SK, Kang IJ, Kim YM and Won MH: Neuronal damage is much
delayed and microgliosis is more severe in the aged hippocampus
induced by transient cerebral ischemia compared to the adult
hippocampus. J Neurol Sci. 294:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee TK, Kim H, Song M, Lee JC, Park JH,
Ahn JH, Yang GE, Kim H, Ohk TG, Shin MC, et al: Time-course pattern
of neuronal loss and gliosis in gerbil hippocampi following mild,
severe, or lethal transient global cerebral ischemia. Neural Regen
Res. 14:1394–1403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manwani B, Liu F, Scranton V, Hammond MD,
Sansing LH and McCullough LD: Differential effects of aging and sex
on stroke induced inflammation across the lifespan. Exp Neurol.
249:120–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan BC, Wang J, Cao J and Won MH: Less
hippocampal neuronal death in young gerbils following transient
global cerebral ischemia is associated with long-term maintenance
of insulin-like growth factor 1 and its receptors in the
hippocampal CA1 region. Mol Med Rep. 17:3055–3061. 2018.PubMed/NCBI
|
|
10
|
Kinouchi K, Brown G, Pasternak G and
Donner DB: Identification and characterization of receptors for
tumor necrosis factor-alpha in the brain. Biochem Biophys Res
Commun. 181:1532–1538. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz-Fernández MA and Fresno M: The role
of tumour necrosis factor, interleukin 6, interferon-gamma and
inducible nitric oxide synthase in the development and pathology of
the nervous system. Prog Neurobiol. 56:307–340. 1998. View Article : Google Scholar
|
|
12
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differe. 10:45–65.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Badiola N, Malagelada C, Llecha N, Hidalgo
J, Comella JX, Sabriá J and Rodríguez-Alvarez J: Activation of
caspase-8 by tumour necrosis factor receptor 1 is necessary for
caspase-3 activation and apoptosis in oxygen-glucose deprived
cultured cortical cells. Neurobiol Dis. 35:438–447. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doll DN, Rellick SL, Barr TL, Ren X and
Simpkins JW: Rapid mitochondrial dysfunction mediates
TNF-alpha-induced neurotoxicity. J Neurochem. 132:443–451. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu MO, Zhang XM, Mix E, Quezada HC, Jin T,
Zhu J and Adem A: TNF-alpha receptor 1 deficiency enhances kainic
acid-induced hippocampal injury in mice. J Neurosci Res.
86:1608–1614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin-Villalba A, Hahne M, Kleber S,
Vogel J, Falk W, Schenkel J and Krammer PH: Therapeutic
neutralization of CD95-ligand and TNF attenuates brain damage in
stroke. Cell Death Differ. 8:679–686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Feuerstein GZ, Xu L, Wang H,
Schumacher WA, Ogletree ML, Taub R, Duan JJ, Decicco CP and Liu RQ:
Inhibition of tumor necrosis factor-alpha-converting enzyme by a
selective antagonist protects brain from focal ischemic injury in
rats. Mol Pharmacol. 65:890–896. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JC, Park CW, Shin MC, Cho JH, Lee HA,
Kim YM, Park JH, Ahn JH, Cho JH, Tae HJ, et al: Tumor necrosis
factor receptor 2 is required for ischemic preconditioning-mediated
neuroprotection in the hippocampus following a subsequent longer
transient cerebral ischemia. Neurochem Int. 118:292–303. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohk TG, Ahn JH, Park YE, Lee TK, Kim B,
Lee JC, Cho JH, Park JH, Won MH and Lee CH: Comparison of neuronal
death and expression of TNF-α and MCT4 in the gerbil hippocampal
CA1 region induced by ischemia/reperfusion under hyperthermia to
those under normothermia. Mol Med Rep. 22:1044–1052. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn JH, Kim DW, Park JH, Lee TK, Lee HA,
Won MH and Lee CH: Expression changes of CX3CL1 and CX3CR1 proteins
in the hippocampal CA1 field of the gerbil following transient
global cerebral ischemia. Int J Mol Med. 44:939–948.
2019.PubMed/NCBI
|
|
21
|
Kanda I: Exotic Animal Formulary. Can
Veterinary J. 56:7362015.
|
|
22
|
Park CW, Ahn JH, Lee TK, Park YE, Kim B,
Lee JC, Kim DW, Shin MC, Park Y, Cho JH, et al: Post-treatment with
oxcarbazepine confers potent neuroprotection against transient
global cerebral ischemic injury by activating Nrf2 defense pathway.
Biomed Pharmacother. 124:1098502020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmued LC and Hopkins KJ: Fluoro-Jade B:
A high affinity fluorescent marker for the localization of neuronal
degeneration. Brain Res. 874:123–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bae EJ, Chen BH, Yan BC, Shin BN, Cho JH,
Kim IH, Ahn JH, Lee JC, Tae HJ, Hong S, et al: Delayed hippocampal
neuronal death in young gerbil following transient global cerebral
ischemia is related to higher and longer-term expression of p63 in
the ischemic hippocampus. Neural Regen Res. 10:944–950. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertrand N, Ishii H and Spatz M: Cerebral
ischemia in young and adult gerbils: Effects on cholinergic
metabolism. Neurochem Int. 28:293–297. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo JY, Yan BC, Park JH, Ahn JH, Kim IH,
Lee JC, Kwon YG, Kim YM, Cho JH and Won MH: Comparison of the
immunoreactivities of NMDA receptors between the young and adult
hippocampal CA1 region induced by experimentally transient cerebral
ischemia. J Neurol Sci. 325:108–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan BC, Kim SK, Park JH, Ahn JH, Lee CH,
Yoo KY, Choi JH, Lee DS, Kim MJ, Kim YM and Won MH: Comparison of
inflammatory cytokines changes in the hippocampal CA1 region
between the young and adult gerbil after transient cerebral
ischemia. Brain Res. 1461:64–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan BC, Park JH, Ahn JH, Choi JH, Yoo KY,
Lee CH, Cho JH, Kim SK, Lee YL, Shin HC and Won MH: Comparison of
glial activation in the hippocampal CA1 region between the young
and adult gerbils after transient cerebral ischemia. Cell Mol
Neurobiol. 32:1127–1138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murakami Y, Saito K, Hara A, Zhu Y, Sudo
K, Niwa M, Fujii H, Wada H, Ishiguro H, Mori H and Seishima M:
Increases in tumor necrosis factor-alpha following transient global
cerebral ischemia do not contribute to neuron death in mouse
hippocampus. J Neurochem. 93:1616–1622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saito K, Suyama K, Nishida K, Sei Y and
Basile AS: Early increases in TNF-alpha, IL-6 and IL-1 beta levels
following transient cerebral ischemia in gerbil brain. Neurosci
Lett. 206:149–152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uno H, Matsuyama T, Akita H, Nishimura H
and Sugita M: Induction of tumor necrosis factor-alpha in the mouse
hippocampus following transient forebrain ischemia. J Cereb Blood
Flow Metab. 17:491–499. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xing J and Lu J: HIF-1α activation
attenuates IL-6 and TNF-α pathways in hippocampus of rats following
transient global ischemia. Cell Physiol Biochem. 39:511–520. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barone FC, Arvin B, White RF, Miller A,
Webb CL, Willette RN, Lysko PG and Feuerstein GZ: Tumor necrosis
factor-α: A mediator of focal ischemic brain injury. Stroke.
28:1233–1244. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nawashiro H, Tasaki K, Ruetzler CA and
Hallenbeck JM: TNF-alpha pretreatment induces protective effects
against focal cerebral ischemia in mice. J Cereb Blood Flow Metab.
17:483–490. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marchetti L, Klein M, Schlett K,
Pfizenmaier K and Eisel UL: Tumor necrosis factor (TNF)-mediated
neuroprotection against glutamate-induced excitotoxicity is
enhanced by N-methyl-D-aspartate receptor activation: Essential
role of a TNF receptor 2-mediated phosphatidylinositol
3-kinase-dependent NF-kappa B pathway. J Biol Chem.
279:32869–32881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bruce AJ, Boling W, Kindy MS, Peschon J,
Kraemer PJ, Carpenter MK, Holtsberg FW and Mattson MP: Altered
neuronal and microglial responses to excitotoxic and ischemic brain
injury in mice lacking TNF receptors. Nat Med. 2:788–794. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watters O and O'Connor JJ: A role for
tumor necrosis factor-α in ischemia and ischemic preconditioning. J
Neuroinflammation. 8:872011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai AY and Todd KG: Microglia in cerebral
ischemia: Molecular actions and interactions. Can J Physiol
Pharmacol. 84:49–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang R, Zhang X, Zhang J, Fan Y, Shen Y,
Hu W and Chen Z: Oxygen-glucose deprivation induced glial scar-like
change in astrocytes. PLoS One. 7:e375742012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Z and Chopp M: Astrocytes, therapeutic
targets for neuroprotection and neurorestoration in ischemic
stroke. Prog Neurobiol. 144:103–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dziewulska D and Mossakowski M: Cellular
expression of tumor necrosis factor a and its receptors in human
ischemic stroke. Clin Neuropathol. 22:35–40. 2003.PubMed/NCBI
|
|
42
|
Knoblach SM, Fan L and Faden AI: Early
neuronal expression of tumor necrosis factor-alpha after
experimental brain injury contributes to neurological impairment. J
Neuroimmunol. 95:115–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ohtori S, Takahashi K, Moriya H and Myers
RR: TNF-alpha and TNF-alpha receptor type 1 upregulation in glia
and neurons after peripheral nerve injury: Studies in murine DRG
and spinal cord. Spine (Phila Pa 1976). 29:1082–1088. 2004.
View Article : Google Scholar : PubMed/NCBI
|