Introduction
Globally, colorectal cancer (CRC) is the third most
common form of cancer, and is the second most common cause of
cancer-associated mortality (1,2).
Although CRC is considered to be mainly prevalent in developed
countries, the incidence is also rising rapidly in developing
countries (3,4). Further basic and clinical research
would undoubtedly accelerate the progress in the treatment of
CRC.
Oxaliplatin has been proven for its specificity
against colorectal tumors, thereby becoming a standard therapeutic
in the management of this malignancy (5). However, its clinical application is
restricted due to drug resistance and toxic side effects (6). Although research has been conducted
through laboratory-based and clinical studies to improve and
optimize therapeutic potency, high efficiency and safe treatment
options remain the focus of ongoing studies. In recent years,
chemotherapeutic drugs and gene-based combination therapy have
become one of the most promising and active research fields in
medicine (7).
Oxaliplatin functions predominantly via the
formation of drug-DNA adducts that block DNA synthesis, thereby
triggering a cellular response and eventually leading to cell
apoptosis (8). Translesion DNA
synthesis (TLS) is a strategy for tolerating DNA damage, which
serves an essential role in the maintenance of genome stability
(9). Recent studies have revealed
that TLS polymerase contributes to the development of platinum
resistance in cancer cells (10),
particularly polymerase ζ (11).
REV3, the catalytic subunit of polymerase ζ, has attracted
increased attention from researchers and a series of related
reports can be found (12–15), while another subunit, mitotic arrest
deficient 2 like 2 (MAD2L2; also known as REV7), has been rarely
studied (11).
The ubiquitin (Ub) proteasome pathway (UPP) is the
most important pathway involved in intracellular protein
degradation (16). The 26S
proteasome is an essential multi-catalytic protease complex, which
serves key roles in the function of the UPP. The 26S proteasome
consists of proteasome 26S subunit, non-ATPase 13 (PSMD13) and a
few additional components, such as proteasomal Ub receptor,
proteasome 26S subunit, ATPase 1, Ub specific peptidase 14 and Ub
C-terminal hydrolases 37 (17,18).
Specific proteins involved in DNA damage repair, cell cycle
regulation and apoptosis are the targets of the UPP (19). However, the underlying regulatory
mechanism between the expression level of MAD2L2 and the UPP has
not yet been elucidated. The current study proposed that the UPP
may be involved in DNA damage repair of tumor cells caused by TLS
and oxaliplatin, which may be mediated by regulating the expression
levels of MAD2L2, thereby affecting cell apoptosis. MG132, a
specific inhibitor of the 26S proteasome, was selected as an
appropriate experimental compound to be used in the present
study.
The present study aimed to investigate the antitumor
effect and possible mechanism of MAD2L2, in order to provide a
rationale for the clinical treatment of colon cancer.
Materials and methods
Cell culture and treatment
Colon cancer cell lines, HCT116 and SW620, were
obtained from the American Type Culture Collection. Cells were
incubated in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and Penicillin-Streptomycin Solution (10,000 U/ml; Beyotime
Institute of Biotechnology) at 37°C and 5% CO2.
Gene expression was knocked down using small
interfering (si)RNA. Cells were transfected with 10 µM MAD2L2 siRNA
and negative control siRNA using Lipofectamine® RNAi MAX
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h.
siRNA were designed and synthesized by Shanghai GenePharma Co.,
Ltd. The siRNA sequences were as follows: MAD2L2 forward,
5′-AAGAUGCAGCUUUACGUGGAATT-3′ and reverse,
5′-UUCCACGUAAAGCUGCAUCUUTT-3′; and negative control forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. Reverse transcription-quantitative PCR
(RT-qPCR) and western blotting were performed to identify
transfection efficiency after 48-h transfection.
Cells were treated with oxaliplatin(Jiangsu Hengrui
Medicine Co., Ltd.) and MG132 (MedChemExpress) at 37°C for 24 h.
The concentrations of both drugs under the current experimental
condition were recalculated according to previous studies:
Oxaliplatin, 50 µM in HCT116 cells and 90 µM in SW620 cells; and
MG132, 18 µM in HCT116 cells and 36 µM in SW620 cells. As a
control, the blank group received the same volume of 1% dimethyl
sulfoxide (DMSO) vehicle as the other groups.
RT-qPCR
RT-qPCR was performed to detect and quantify mRNA.
Total RNA was extracted from experimental cells using
TRIzol® reagent (Beyotime Institute of Biotechnology).
cDNA was produced using SYBR Premix Ex TaqII (TliRNaseH Plus;
Takara Biotechnology Co., Ltd.) from the extracted RNA. RT
reactions were conducted under the following conditions: 37°C for
15 min, 85°C 5 sec, maintained at 4°C. qPCR amplification reactions
were carried out in the Applied Biosystems™ 7500 Fast Real Time PCR
system using a PowerUp™ SYBR™ Green Master Mix (both Thermo Fisher
Scientific, Inc.). Available primers were obtained from Sangon
Biotech, Co., Ltd., and the sequences of primers are as follows:
MAD2L2 forward, 5′-CCAGGCTGTACCTTCACAGTC-3′ and reverse,
5′-TCTTCCACGTAAAGCTGCATC-3′; and GAPDH forward,
5′-ACCCACTCCTCCACCTTTGAC-3′ and reverse,
5′-CACCACCCTGTTGCTGTAGCC-3′. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 2 min; followed by 40
cycles of denaturation at 95°C for 3 sec and annealing/elongation
at 60°C for 30 sec; followed by a final extension step at 60°C for
1 min. Finally, the relative gene expression values were calculated
using the 2−∆∆Cq method (20).
Western blotting
Western blotting was performed to separate and
identify proteins. Total proteins were extracted using RIPA buffer
(Beyotime Institute of Biotechnology), followed by the
determination of the protein concentration using a BCA kit (Nanjing
KeyGen Biotech. Co., Ltd.). Proteins (20–30 µg/well) were separated
via 12% SDS-PAGE at 120 V and then transferred into 0.2-µm PVDF
membrane under wet conditions at 300 mA. The blotted membranes were
blocked with 5% non-fat milk for 2 h at room temperature.
Antibodies were diluted in TBS-Tween-20 (1% TBS and 0.1% Tween).
Primary antibodies were incubated at 4°C overnight and secondary
antibodies were incubated at room temperature for 1 h.
Chemiluminescent signals were detected using Pierce™ ECL Western
Blotting substrate (cat. no. 32109; Thermo Fisher Scientific,
Inc.). Images were captured using Bio-Rad ChemiDOC XRS+
system (Bio-Rad Laboratories, Inc.) and analyzed by Image Lab
Software (version 5.2.1; Bio-Rad Laboratories, Inc.). The following
antibodies were used: Primary antibodies, MAD2L2 (cat. no.
sc135977; 1:500; Santa Cruz Biotechnology, Inc.), PSMD13 (cat. no.
ab229812; 1:1,000; Abcam), Bax (cat. no. bs-0127R; 1:200; BIOSS),
Bak (cat. no. bs-1284R; 1:200; BIOSS), Bcl-2 (cat. no. bs-0032R;
1:200; BIOSS) and GAPDH (cat. no. AB-P-R 001; 1:1,000; Hangzhou
Goodhere Biotechnology Co., Ltd.); Secondary antibodies, goat
Anti-Rabbit IgG H&L (HRP) (cat. no. ab6721; 1:10,000; Abcam)
and goat Anti-Mouse IgG H&L (HRP) (cat. no. ab205719; 1:10,000;
Abcam).
MTT assay
Cell viability was investigated using an MTT assay
kit (Nanjing KeyGen Biotech Co., Ltd.). The cell count was adjusted
to 1×104 cells/ml. Cells were treated with oxaliplatin
and MG132 in 96-well plate at 37°C for 24 h. MTT solution was added
and incubated for 30 min at 37°C until the solution turned purple.
DMSO was used for dissolving the purple crystals. Absorbance was
measured using Multiskan™ GO microplate spectrophotometer (Thermo
Fisher Scientific, Inc.) at 570 nm. The percentage of cell
viability was calculated.
Flow cytometry
Cell apoptosis was detected via flow cytometry (BD
Accuri™ C6; BD Biosciences). EDTA-free trypsin (Hyclone
Laboratories, Inc.) was used to detach the experimental cells.
According to the instructions of the Annexin V-FITC/PI kit (BD
Biosciences), each group of cells was incubated with 5 µl Annexin
V-FITC for 15 min at 2–8°C. Subsequently, 10 µl PI was added and
incubated for 5 min at 2–8°C. Cells were analyzed within 30 min
after staining using BD Accuri C6 software (version 5.0; BD
Biosciences). The apoptotic rate was calculated as the percentage
of early and late apoptotic cells.
TUNEL assay
DNA fragmentation, which is a marker of cell
apoptosis (2), was detected using
the TUNEL BrightGreen Apoptosis Detection kit (cat. no. A112-02;
Vazyme Biotech Co., Ltd.). The cells were cultured on microscope
slides, fixed with 4% formaldehyde at room temperature for 15 min
and permeabilized with 0.25% Triton®X-100 at room
temperature for 20 min. TUNEL staining was performed according to
the manufacturer's instructions; cells were stained with 50 µl
Recombinant TdT Enzyme at 37°C for 60 min and with 50 µl
BrightGreen Labeling Mix at 37°C for 60 min. The slides were then
immersed into 2 µg/ml DAPI solution and stained in the dark for 5
min. The samples were examined via fluorescence microscopy (DFC450
C; Leica Microsystems, Inc.); 10 visual fields were randomly
selected per slide at ×400 magnification.
ELISA
Total cell protein was extracted using RIPA buffer
(Beyotime Institute of Biotechnology) from experimental cells. The
activity of PSMD13 was detected using an ELISA kit (cat. no.
ml-55255; Enzyme-linked Biotechnology Co., Ltd.). According to the
manufacturer's instructions, standard wells, testing sample wells
and blank wells were set. Standard and blank proteins were obtained
from the ELISA kit. Standard protein was diluted to a range of 0,
50, 100, 150, 200, 250 and 300 ng/ml. Each concentration of these
standard samples was added to standard wells, 50 µl/well; extracted
protein samples were added to the testing well, 50 µl/well; And 50
µl blank protein was added to the blank well. Subsequently, 80 µl
HRP-conjugated antibody was immediately added to the wells and
incubated at 37°C for 1 h. Chromogenic substrate A (50 µl) and
chromogenic substrate B (50 µl) were added and incubated at 37°C
for 10 min, in the dark. Finally, 50 µl stop solution were added to
each well to terminate the reaction. The optical density was
measured at 450 nm. A standard curve was constructed and the
corresponding concentration of PSMD13 was calculated.
Statistical analysis
SPSS 21.0 (IBM Corp.) was used for statistical
analysis and GraphPad Prism 5 (GraphPad Software, Inc.) was used to
generate the figures and mark the statistical difference. Data are
presented as the mean ± SD. An unpaired Student's t-test was used
for comparison between two independent samples. One-way ANOVA was
used for comparisons between groups, followed by Tukey's multiple
comparisons test as the post-hoc test. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were repeated ≥3 times.
Results
MAD2L2 is regulated by oxaliplatin and
MG132 in human colon cancer cells
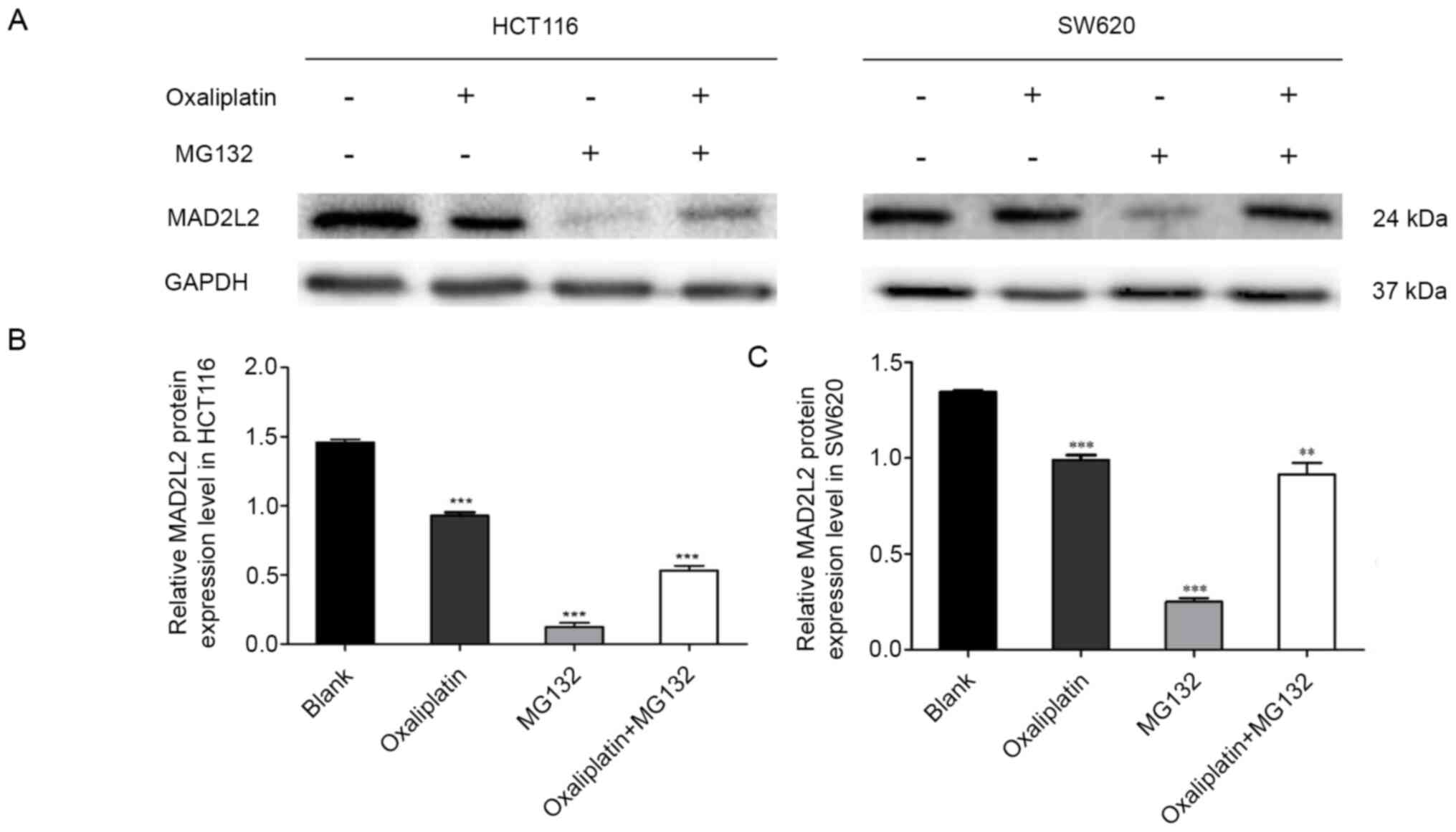
Cells were treated with oxaliplatin and MG132. The
MTT data indicated that cells treated with oxaliplatin or MG132 had
a significant decrease in their viability compared with the blank
group, but a synergistic effect was not observed during the
co-treatment of oxaliplatin and MG132 (Table I). The western blotting results
demonstrated that oxaliplatin and MG132 caused a significant
decrease in the protein expression level of MAD2L2, but a
synergistic effect was not observed in the co-treatment group
(Fig. 1). These results indicated
that the expression level of MAD2L2 was decreased by oxaliplatin
and MG132 in human colon cancer cells, but no synergistic effects
were observed.
 | Table I.Effects of oxaliplatin and/or MG132
on the viability of human colorectal cancer cells. |
Table I.
Effects of oxaliplatin and/or MG132
on the viability of human colorectal cancer cells.
| Cells | Blank | Oxaliplatin | MG132 | Oxaliplatin and
MG132 |
|---|
| HCT116 | 0.904±0.001 |
0.656±0.043a |
0.257±0.011a |
0.351±0.005a |
| SW620 | 0.909±0.0002 |
0.665±0.011a |
0.215±0.006a |
0.258±0.007a |
Knockdown of MAD2L2 promotes
Bcl-2-mediated apoptosis of colon cancer cells
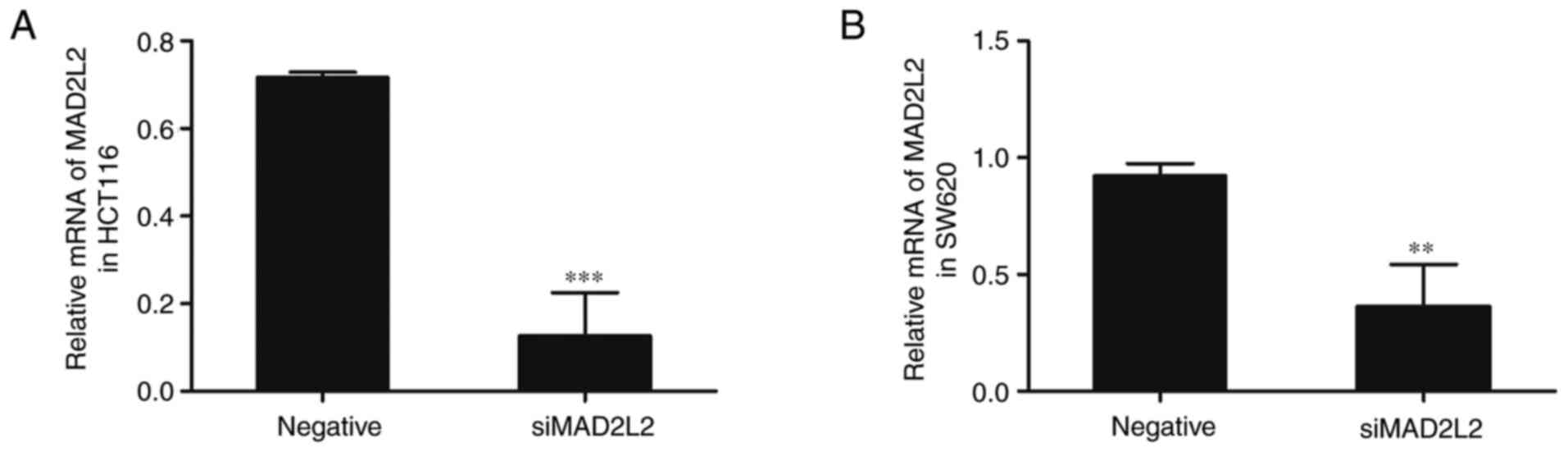
Since oxaliplatin and MG132 were identified to
affect the expression level of MAD2L2, the associations between
them were investigated. Cells were transfected with siMAD2L2;
compared with the negative control group, the mRNA expression
levels of MAD2L2 were significantly downregulated in the siMAD2L2
group (Fig. 2). Subsequently, the
experimental cells were treated with oxaliplatin and MG132. The MTT
data revealed that, compared with the negative control group,
siMAD2L2 caused a significant decrease in cell viability (Table II). Compared with the siMAD2L2
group, cell viability was suppressed by the co-treatment of
siMAD2L2 and oxaliplatin, whereas it was increased by the
co-treatment of siMAD2L2 and MG132. A synergistic effect was not
observed during triple co-treatment with siMAD2L2, oxaliplatin and
MG132 (Table II).
 | Table II.Effects of siMAD2L2 in combination
with oxaliplatin and/or MG132 on the viability of human colorectal
cancer cells. |
Table II.
Effects of siMAD2L2 in combination
with oxaliplatin and/or MG132 on the viability of human colorectal
cancer cells.
| Treatment | HCT116 | SW620 |
|---|
| Negative | 0.905±0.009 | 0.942±0.013 |
| siMAD2L2 |
0.573±0.013a |
0.539±0.002a |
| siMAD2L2 and
oxaliplatin |
0.270±0.005a,b |
0.191±0.004a,b |
| siMAD2L2 and
MG132 |
0.676±0.008a–c |
0.666±0.006a–c |
| siMAD2L2,
oxaliplatin and MG132 |
0.363±0.009a–d |
0.377±0.0004a–d |
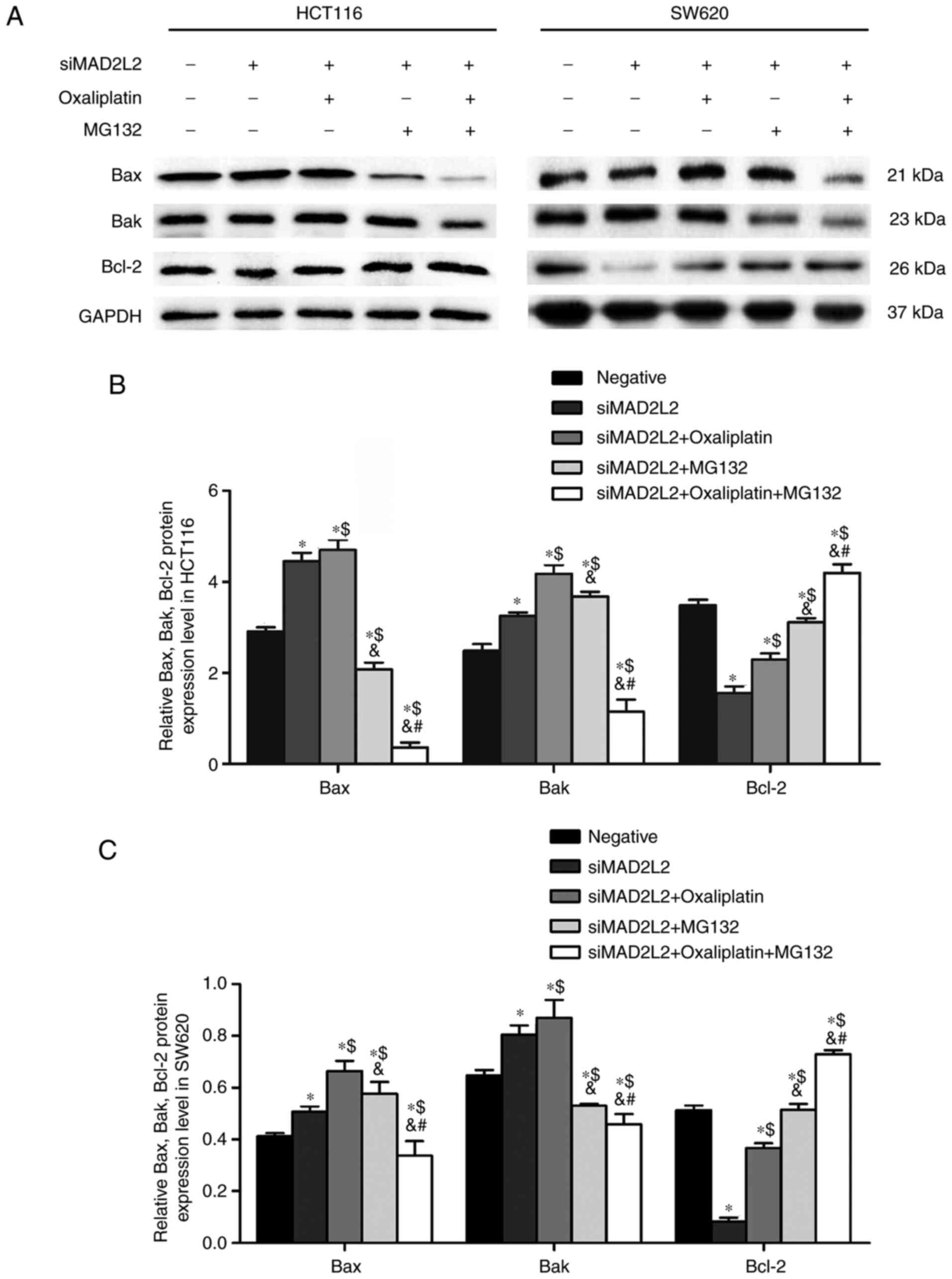
As shown by western blotting, compared with negative
control group, siMAD2L2 significantly increased the expression
levels of the pro-apoptotic proteins Bax and Bak, as well as
decreased the expression level of the anti-apoptotic protein Bcl-2
(Fig. 3). Compared with siMAD2L2
group, cells co-treated with siMAD2L2 and oxaliplatin had increased
Bax and Bak expression, and increased Bcl-2 expression. Compared
with siMAD2L2 and oxaliplatin co-treatment group, the expression
levels of Bax and Bak were significantly decreased after
co-treatment with siMAD2L2, oxaliplatin and MG132, while Bcl-2
expression was higher (Fig. 3).
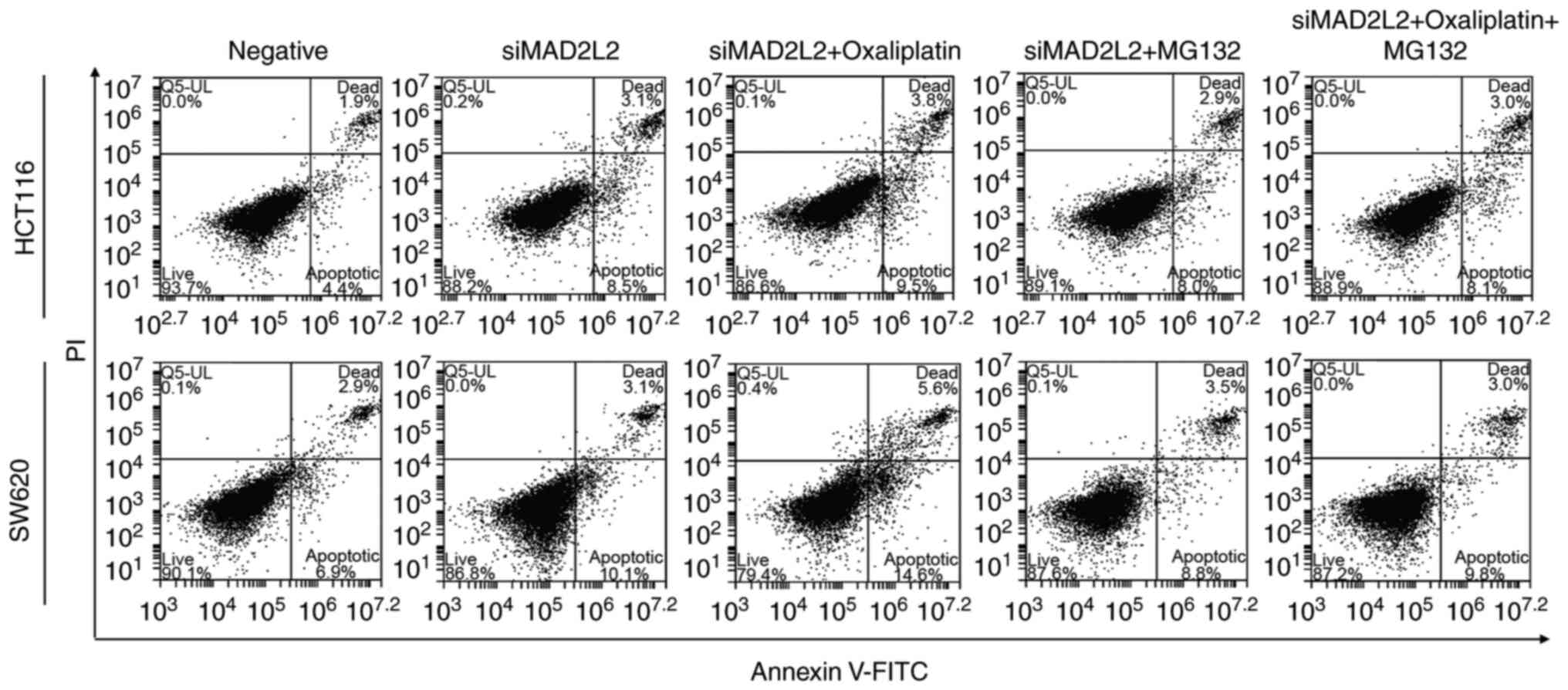
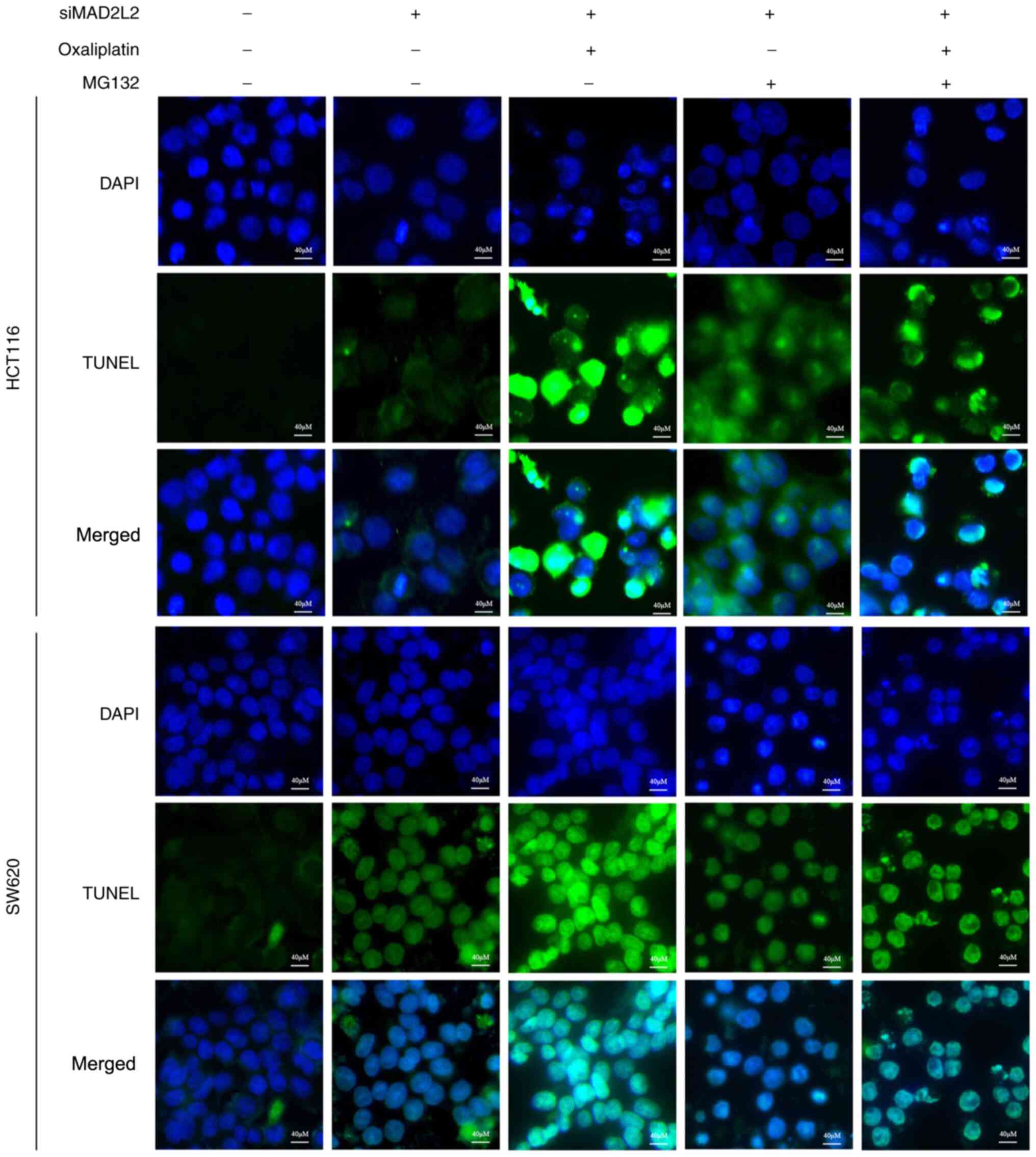
This apoptotic effect was further confirmed by the flow cytometry
and TUNEL assay results (Figs. 4
and 5). These observations
suggested that the Bcl-2 pathway may be involved in the cell
apoptosis mediated by MAD2L2.
siMAD2L2-induced suppression of PSMD13
is regulated by oxaliplatin and MG132
Previous studies reported that DNA damage-induced
cytotoxic effects were inhibited by MG132 (21–23).
Therefore, the activity of PSMD13, a key protein of the UPP, was
evaluated via ELISA. The results demonstrated that, compared with
the negative control group, the activity and expression level of
PSMD13 protein were significantly increased in the siMAD2L2 group.
Compared with the siMAD2L2 group, PSMD13 activity was mitigated by
the co-treatment of siMAD2L2 and MG132, whereas it was promoted by
the co-treatment of siMAD2L2 and oxaliplatin. A synergistic effect
was not observed during triple co-treatment with siMAD2L2,
oxaliplatin and MG132 (Table
III), and this effect was further confirmed via western
blotting (Fig. 6). These
observations indicated that the UPP was involved in the regulation
of TLS.
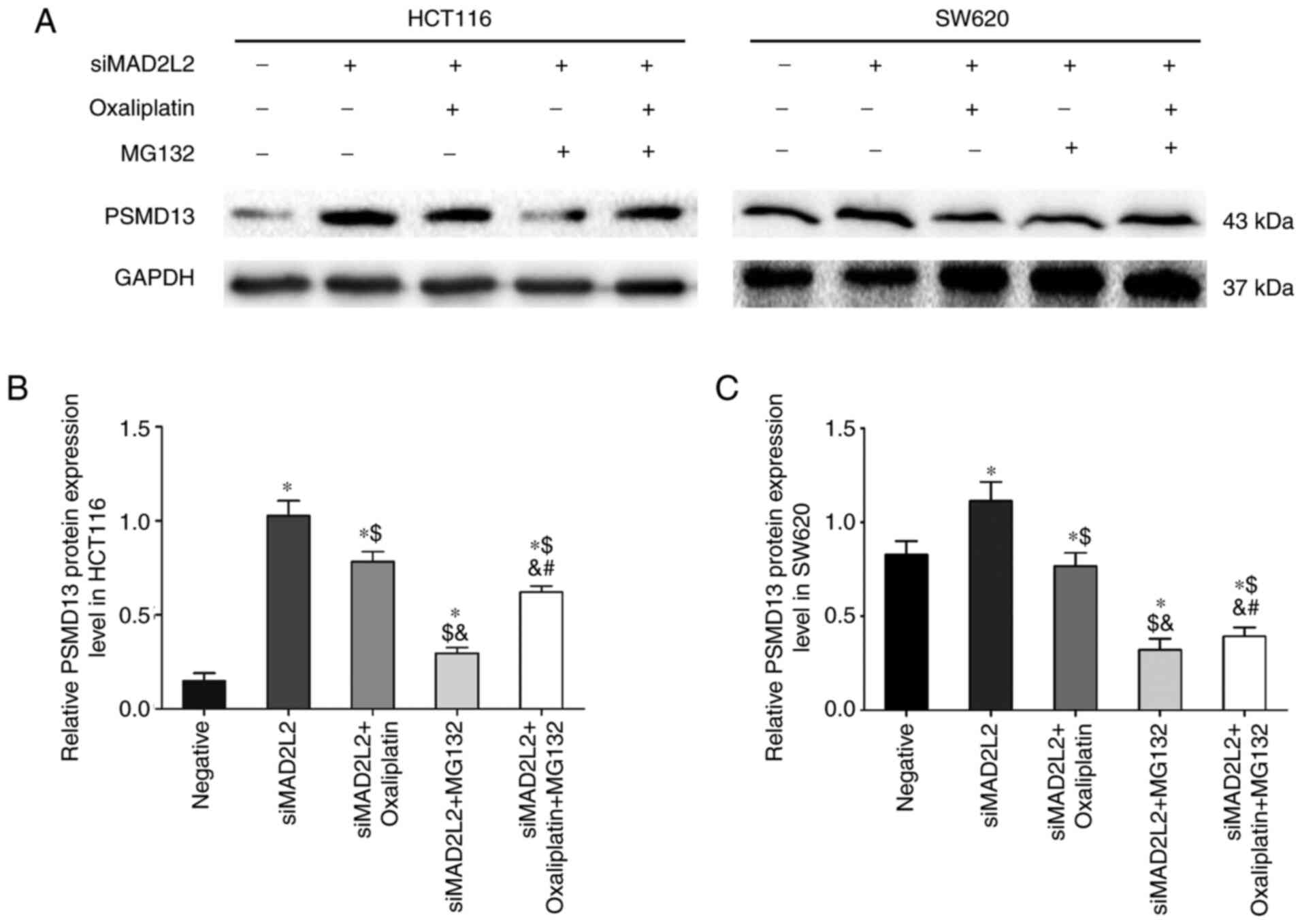 | Figure 6.siMAD2L2-induced suppression of
PSMD13 is regulated by oxaliplatin and MG132. The cells were
treated with negative siRNA, siMAD2L2, siMAD2L2 + oxaliplatin,
siMAD2L2 + MG132, siMAD2L2 + oxaliplatin + MG132 (oxaliplatin was
incubated for 24 h at 50 and 90 µM with HCT116 and SW620 cells,
respectively; MG132 was incubated for 24 h at 18 and 36 µM with
HCT116 and SW620 cells, respectively). (A) Western blotting was
performed to detect the protein levels of PSMD13 in HCT116 and
SW620 cells. One-way ANOVA followed by Tukey's multiple comparisons
test was used to determine the statistical significance in (B)
HCT116 and (C) SW620 cells. The data are presented as the mean ±
SD. n=3. *P<0.05 vs. negative group; $P<0.05 vs.
siMAD2L2 group; &P<0.05 vs. siMAD2L2 +
oxaliplatin group; #P<0.05 vs. siMAD2L2 + MG132
group. MAD2L2, mitotic arrest deficient 2 like 2; si, small
interfering RNA; PSMD13, proteasome 26S subunit, non-ATPase 13. |
 | Table III.Activity of PSMD13 in human
colorectal cancer cells. |
Table III.
Activity of PSMD13 in human
colorectal cancer cells.
| Treatment | HCT116 | SW620 |
|---|
| Negative | 293.21±31.25 | 280.31±6.93 |
| siMAD2L2 |
385.68±37.13a |
370.62±22.43a |
| siMAD2L2 and
oxaliplatin |
485.07±44.17a,b |
459.15±28.11a,b |
| siMAD2L2 and
MG132 |
359.72±36.52a–c |
334.73±3.71a–c |
| siMAD2L2,
oxaliplatin and MG132 |
418.46±33a–d |
411.28±2.67a–d |
Discussion
MAD2L2, also called REV7 or MAD2B, encodes a core
subunit of DNA polymerase ζ (24).
Previous studies have reported that, in addition to maintaining
genomic stability, MAD2L2 was also involved in multiple cellular
functions, such as drug resistance reversal, epithelial stromal
transformation transcription and signal transduction events
(25–27). In the present study, the aim was to
investigate the effects of MAD2L2-induced cell apoptosis.
Previous studies have shown that DNA polymerase ζ
plays an important role in the regulation of platinum resistance
and REV3 has been considered the main focus (28); however, the role of MAD2L2 has been
underestimated. In the present study, MAD2L2 was selected as the
target gene; the results revealed that MAD2L2 promoted
oxaliplatin-induced apoptosis. In the present study, human colon
cancer cells, HCT116 and SW620, were selected. Cells were
characterized for DNA damage by oxaliplatin. The protein expression
level of MAD2L2 was found to be significantly downregulated. Flow
cytometry and TUNEL results demonstrated that treatment with
siMAD2L2 or oxaliplatin alone increased the apoptosis of both
HCT116 and SW620 cells, whereas cells co-treated with siMAD2L2 and
oxaliplatin significantly promoted apoptosis. The results of
western blotting showed that knockdown of MAD2L2 expression caused
by RNA interference or oxaliplatin increased the expression levels
of pro-apoptotic proteins Bax and Bak and decreased the expression
levels of anti-apoptotic protein Bcl-2, compared with the negative
control group. These results indicated that oxaliplatin promoted
siMAD2L2-induced apoptosis of colon cancer cells, and this process
was associated with the Bcl-2 family mediated cell apoptosis
pathway.
In order to investigate the causes of MAD2L2-induced
cell apoptosis, the present study focused on the UPP, which is
responsible for the majority of intracellular protein degradation
(29). This system exerts its
biological effect via the cooperation of E1/E2/E3. Ub is activated
by E1 and is then transferred to E2, which permits it to be
sequentially conjugated to E3. E3 recognizes target substrates and
catalyzes the covalent attachment of Ub to it (30). Finally, the substrates modified with
polyubiquitin chains are delivered to the 26S proteasome for
proteolytic destruction. Here, PSMD13 serves an important role
(31).
In the present study, the activity of PSMD13 was
evaluated via ELISA and further confirmed by western blotting. The
results showed that the activity and protein expression level of
PSMD13 were significantly increased by siMAD2L2. In addition,
MG132, the inhibitor of proteasome, decreased the expression of
MAD2L2, while reducing the siMAD2L2-induced cell apoptosis. These
results suggest that the UPP was implicated in the regulation of
TLS.
TLS depends on the orderly assembly of DNA
polymerases (32). Cells are
constantly exposed to DNA damage agents, such as UV, methyl
methanesulfonate and other cytotoxic factors (33). Once DNA damage occurs, proliferating
cell nuclear antigen (PCNA) can be recruited and mono-ubiquitinated
by Rad18-Rad6 at Lys164 (K164), and Ub-PCNA mediates cellular
response by TLS polymerases (34,35).
MAD2L2 helps to coordinate the nucleotide insertion and extension
steps of lesion bypass (36).
Interestingly, Rad18 and Rad6 are particularly important E2 and E3
enzymes, which function as Ub conjugating and Ub ligase enzymes,
respectively, and have been shown to be involved in the UPP, which
mediates the degradation of proteins (37).
In the present study, the protein expression level
of MAD2L2 was inhibited when the cells were treated with MG132. In
addition, cell apoptosis was reduced by MG132 compared with the
co-treatment of siMAD2L2 and oxaliplatin. Based on these results,
it was suggested that the UPP may be one of the responders of
TLS-related DNA damage in colon cancer cells. DNA lesions that are
induced by oxaliplatin or siMAD2L2 may promote the cooperation
between Rad6 and Rad18, which then activate PSMD13, finally
resulting in the degradation of MAD2L2 protein. Such agents arrest
TLS, trigger the accumulation of DNA damage and promote cancer cell
apoptosis (38).
Although the present study indicated that both
oxaliplatin and MG132 exerted impacts on siMAD2L2-induced cell
apoptosis, these factors were not simply synergistic or
antagonistic. More direct evidence is needed for effective
treatment of colon cancer in the future. In conclusion, the present
study demonstrated oxaliplatin promoted siMAD2L2-induced colon cell
apoptosis, which was regulated by the UPP. Overall, the present
study provides a theoretical basis for improving the clinical
efficacy of colon cancer by combining chemotherapy and gene
therapy.
Acknowledgements
I would like to extend my sincere gratitude to
Professor Hao Wang (School of Pharmacy, Minzu University of China),
Professor Jianmin Sun (School of Basic Medical Sciences, Ningxia
Medical University) for their intellectual guidance, valuable
comments and enlightening suggestions for my study and manuscript
preparation.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31360251) and West
China first-class Disciplines Basic Medical Sciences at Ningxia
Medical University (grant no. NXYLXK2017B07).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
LM and FX designed the study. YL performed cell
culture, transfection experiments and drug sensitivity tests. HTS
contributed to the ELISA and western blotting assays. XPZ and XL
performed the flow cytometry and TUNEL assays. LM and HTS acquired
and interpretated the data. FFK and YS assisted with data analysis.
LM, XL and YL drafted the manuscript. HTS and LM are responsible
for confirming the authenticity of the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crowley LC, Marfell BJ and Waterhouse NJ:
Detection of DNA fragmentation in apoptotic cells by TUNEL. Cold
Spring Harb Protoc. 20162016.
|
|
3
|
Zhou J, Zheng R, Zhang S, Zeng H, Wang S,
Chen R, Sun K, Li M, Gu J, Zhuang G and Wei W: Colorectal cancer
burden and trends: Comparison between China and major burden
countries in the world. Chin J Cancer Res. 33:1–10. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Health Commission Of The People's
Republic Of China, . National guidelines for diagnosis and
treatment of colorectal cancer 2020 in China (English version).
Chin J Cancer Res. 32:415–445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma G, Anghore D, Khare R and Rawal RK:
Oxaliplatin for colorectal cancer therapy: A review. Clin Cancer
Drugs. 5:13–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown A, Kumar S and Tchounwou PB:
Cisplatin-based chemotherapy of human cancers. J Cancer Sci Ther.
11:972019.PubMed/NCBI
|
|
7
|
McQuade RM, Stojanovska V, Bornstein JC
and Nurgali K: Colorectal cancer chemotherapy: The evolution of
treatment and new approaches. Curr Med Chemistry. 24:1537–1557.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sigel A, Riddell IA, Lippard SJ, Sigel H,
Freisinger E and Sigel RKO: Cisplatin and oxaliplatin: Our current
understanding of their actions. 1–42. 2018.PubMed/NCBI
|
|
9
|
Ma X, Tang TS and Guo C: Regulation of
translesion DNA synthesis in mammalian cells. Environ Mol Mutagen.
61:680–692. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shanbhag V, Sachdev S, Flores JA, Modak MJ
and Singh K: Family A and B DNA polymerases in cancer:
Opportunities for therapeutic interventions. Biology (Basel).
7:52018.PubMed/NCBI
|
|
11
|
Sharma S, Shah NA, Joiner AM, Roberts KH
and Canman CE: DNA polymerase zeta is a major determinant of
resistance to platinum-based chemotherapeutic agents. Mol
Pharmacol. 81:778–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang HG, Chen P, Su JY, Wu M, Qian H,
Wang Y and Li J: Knockdown of REV3 synergizes with ATR inhibition
to promote apoptosis induced by cisplatin in lung cancer cells. J
Cell Physiol. 232:3433–3443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malik R, Kopylov M, Gomez-Llorente Y, Jain
R, Johnson RE, Prakash L, Prakash S, Ubarretxena-Belandia I and
Aggarwal AK: Structure and mechanism of B-family DNA polymerase
zeta specialized for translesion DNA synthesis. Nat Struct Mol
Biol. 27:913–924. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Washington MT and Gildenberg MS: Structure
of DNA polymerase ζ: Capturing the getaway driver. Nat Struct Mol
Biol. 27:1–2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki T, Sassa A, Gruz P, Gupta RC,
Johnson F, Adachi N and Nohmi T: Error-prone bypass patch by a
low-fidelity variant of DNA polymerase zeta in human cells. DNA
Repair (Amst). 100:1030522021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaczynska M and Osmulski PA: Targeting
protein-protein interactions in the ubiquitin-proteasome pathway.
Adv Protein Chem Struct Biol. 110:123–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bi W, Zhu L, Zeng Z, Jing X, Liang Y, Guo
L, Shi Q, Xu A and Tao E: Investigations into the role of 26S
proteasome non-ATPase regulatory subunit 13 in neuroinflammation.
Neuroimmunomodulation. 21:331–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bard JAM, Goodall EA, Greene ER, Jonsson
E, Dong KC and Martin A: Structure and function of the 26S
proteasome. Ann Rev Biochem. 87:697–724. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Htet ZM, Lopez-Alfonzo E, Martin A
and Walters KJ: Proteasome interaction with ubiquitinated
substrates: From mechanisms to therapies. FEBS J. Nov 19–2020.(Epub
ahead of print). View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tu Y, Chen C, Pan J, Xu J, Zhou ZG and
Wang CY: The Ubiquitin Proteasome Pathway (UPP) in the regulation
of cell cycle control and DNA damage repair and its implication in
tumorigenesis. Int J Clin Exp Pathol. 5:726–738. 2012.PubMed/NCBI
|
|
22
|
Sakai W, Yuasa-Sunagawa M, Kusakabe M,
Kishimoto A, Matsui T, Kaneko Y, Akagi JI, Huyghe N, Ikura M, Ikura
T, et al: Functional impacts of the ubiquitin-proteasome system on
DNA damage recognition in global genome nucleotide excision repair.
Sci Rep. 10:197042020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramadan K and Meerang M:
Degradation-linked ubiquitin signal and proteasome are integral
components of DNA double strand break repair: New perspectives for
anti-cancer therapy. FEBS Lett. 585:2868–2875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rizzo AA, Vassel FM, Chatterjee N, D'Souza
S, Li Y, Hao B, Hemann MT, Walker GC and Korzhnev DM: Rev7
dimerization is important for assembly and function of the
Rev1/Polζ translesion synthesis complex. Proc Natl Acad Sci USA.
115:E8191–E8200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng L, Wei W, Heng Z, Yantao H and Chunbo
W: Knockdown of REV7 inhibits breast cancer cell migration and
invasion. Oncol Res. 24:315–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosenberg SC and Corbett KD: The
multifaceted roles of the HORMA domain in cellular signaling. J
Cell Biol. 211:745–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hara K, Taharazako S, Ikeda M, Fujita H,
Mikami Y, Kikuchi S, Hishiki A, Yokoyama H, Ishikawa Y, Kanno SI,
et al: Dynamic feature of mitotic arrest deficient 2-like protein 2
(MAD2L2) and structural basis for its interaction with chromosome
alignment-maintaining phosphoprotein (CAMP). J Biol Chem.
292:17658–17667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sanders MA, Haynes B, Nangia-Makker P,
Polin LA and Shekhar MP: Pharmacological targeting of RAD6
enzyme-mediated translesion synthesis overcomes resistance to
platinum-based drugs. J Biol Chemistry. 292:10347–10363. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jantrapirom S, Piccolo LL, Pruksakorn D,
Potikanond S and Nimlamool W: Ubiquilin networking in cancers.
Cancers. 12:1206–1586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leestemaker Y and Ovaa H: Tools to
investigate the ubiquitin proteasome system. Drug discovery today.
Technologies. 26:25–31. 2017.PubMed/NCBI
|
|
31
|
Wehmer M, Rudack T, Beck F, Aufderheide A,
Pfeifer G, Plitzko JM, Förster F, Schulten K, Baumeister W and
Sakata E: Structural insights into the functional cycle of the
ATPase module of the 26S proteasome. Proc Natl Acad Sci USA.
114:1305–1310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rizzo AA and Korzhnev DM: The Rev1-Polζ
translesion synthesis mutasome: Structure, interactions and
inhibition. Enzymes. 45:139–181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fernandes E, Fonseca TG, Carriço T, Mestre
N, Tavares Á and Bebianno MJ: Cytotoxic responses of the anticancer
drug cyclophosphamide in the mussel Mytilus galloprovincialis and
comparative sensitivity with human cells lines. Chemosphere.
261:1276782020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanao R and Masutani C: Regulation of DNA
damage tolerance in mammalian cells by post-translational
modifications of PCNA. Mutat Res. 803-805:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lau WCY, Li Y, Zhang Q and Huen MSY:
Molecular architecture of the Ub-PCNA/Pol η complex bound to DNA.
Sci Rep. 5:157592015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Quinet A, Lerner LK, Martins DJ and Menck
CFM: Filling gaps in translesion DNA synthesis in human cells.
Mutat Res Genet Toxicol Environ Mutagen. 836:127–142. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
An H, Yang L, Wang C, Gan Z, Gu H, Zhang
T, Huang X, Liu Y, Li Y, Chang SJ, et al: Interactome analysis
reveals a novel role for RAD6 in the regulation of proteasome
activity and localization in response to DNA damage. Mol Cell Biol.
37:e00419–e00416. 2017. View Article : Google Scholar
|
|
38
|
Masuda Y and Masutani C: Spatiotemporal
regulation of PCNA ubiquitination in damage tolerance pathways.
Crit Rev Biochem Mol Biol. 54:418–442. 2019. View Article : Google Scholar : PubMed/NCBI
|