Introduction
Endometriosis is a common benign disease in women,
which is characterized by endometrial tissue (glands and stroma)
appearing outside the uterus. Endometriosis is associated with
chronic pelvic pain, dysmenorrhea, dyspareunia and infertility
(1). Numerous theories and
hypotheses have attempted to explain the pathogenesis of
endometriosis, and the most widely accepted theory is that
endometriosis results from retrograde menstruation (2,3).
During menstruation, glandular epithelium and endometrial
mesenchymal cells can enter the pelvic cavity through the fallopian
tube, where they implant in the ovary or adjacent pelvic
peritoneum, and continue to proliferate and spread to form an
endometriotic lesion (4).
According to Sampson's theory, retrograde
endometrial fragments suffer from heavier hypoxic stress compared
with their eutopic tissues (5,6).
Hypoxia inducible factor (HIF)-1α is a key regulator of hypoxia,
which makes it a useful marker of cellular hypoxic stress. Our
previous studies revealed that HIF-1α expression was increased in
ectopic lesions compared with that in eutopic tissues and normal
endometrium (7,8). Ectopic endometrial cells may
ultimately use the hypoxic microenvironment to promote cell
adhesion, proliferation and angiogenesis (5,6);
however, how ectopic cells convert the metabolism to overcome and
adapt to hypoxic microenvironments before new vasculature forms in
ectopic lesions is not clear. Several studies have suggested that
the Warburg-like effect, a shift in cell metabolism from
mitochondrial oxidative phosphorylation to aerobic glycolysis that
favors tumor progression, may be induced by transforming growth
factor β1 to promote the development of endometriosis (9,10).
Pyruvate dehydrogenase kinase 1 (PDK1), a critical enzyme for
regulating hypoxia-induced glucose metabolism, has been reported to
alter cellular metabolism and to inhibit apoptosis in endometriotic
stromal cells (11). Whether other
glycolysis regulators are involved in the growth of ectopic lesions
is, to the best of our knowledge, unknown.
Lactate dehydrogenase A (LDHA) converts pyruvate to
lactic acid during the last step of glycolysis. Increased
expression of LDHA is required for the maintenance of glycolysis,
and has been associated with the evolution of aggressive and
metastatic cancer in a variety of tumor types, including pancreatic
cancer (12), squamous cell
carcinoma of the head and neck (13), and non-Hodgkin's cell lymphoma
(14). Silencing of LDHA has been
shown to significantly attenuate the colony-forming ability and
invasive capacity of cervical cancer cells (15). In endometriosis, LDHA expression has
been reported to be significantly increased in endometriosis
lesions compared with that in eutopic endometrium from women with
endometriosis (9). However, the
underlying mechanism of the elevated regulation of LDHA in
endometriotic cells and cellular functions remains to be
investigated.
LDHA is one of the important rate-limiting enzymes
in the glycometabolism pathway (16). Myc and HIF can regulate the
expression of LDHA at the level of transcription and translation
(17). HIF-1α increases glycolysis
and promotes lactic acid production through the PI3k/Akt/mTOR
pathway and can be upregulated by combining with the promoter of
LDHA (17,18). HIF is a key transcription factor
that mediates the cell response to hypoxia, which plays a notable
role in the adaptation of ectopic endometrial cells to hypoxia
(19). Previous studies have shown
that HIF-1α is a determinant of tumor development, and is
associated with tumor invasion, metastasis and prognosis (20,21).
In addition, HIF-1α expression has been reported to be increased in
endometriosis, where it can induce vascular endothelial growth
factor, leptin, cysteine-rich angiogenic inducer, osteopontin and
other factors to promote the establishment of an effective vascular
network, and the occurrence and development of endometriosis
(5,22–24).
HIF-1α may also activate the expression of glycolysis
pathway-related genes, such as LDHA, phosphoglycerate kinase 1 and
pyruvate kinase M2, by binding to hypoxia response elements
(25). Given that HIF-1α and LDHA
are coincidently expressed in endometriotic lesions (9) and their functions are strongly
associated with hypoxia in other diseases, it was hypothesized that
hypoxia-induced LDHA may serve a notable role in the development of
endometriosis. The present study compared the expression of LDHA in
normal endometrium, eutopic endometrium and ectopic lesions, and
explored the function of LDHA in endometriotic cells.
Materials and methods
Study approval and sample
collection
The present study was initiated on May 1, 2018 and
was terminated on April 30, 2019 at the Department of Obstetrics
and Gynecology, Sir Run Run Shaw Hospital, Zhejiang University
School of Medicine (Hangzhou, China). All patients provided written
informed consent before sample collection. Endometriosis was
confirmed by pathologists of Sir Run Run Shaw Hospital who were not
involved in the study. Endometriosis was classified according to
the revised American Society for Reproductive Medicine scoring
system (26). Patients with no
hormone treatment 6 months prior to gynecological surgery were
included. Individuals with other endocrine diseases or diseases
associated with the uterus or ovaries, such as uterine cavity
polyps and uterine fibroids, were excluded. Both eutopic and
ectopic endometrium specimens of peritoneal endometriosis were
collected. Endometrial fragments obtained from patients with
another benign gynecological disease were recruited as normal
controls. The baseline data of the included patients are presented
in Table I. The present study was
approved by The Ethics Committee of Sir Run Run Shaw Hospital at
Zhejiang University. A total of 35 normal endometrial tissues, 39
eutopic tissues and 62 ectopic lesions were examined. Paired
eutopic and ectopic tissues were collected from 25 patients with
endometriosis; in total, tissues were obtained from 111 patients,
of which 35 were normal control individuals, in the present
study.
 | Table I.Baseline data for cases included in
the study. |
Table I.
Baseline data for cases included in
the study.
| Characteristic | Normal | Endometriosis |
P-valuea |
|---|
| Age, years | 30.88±4.10 | 30.99±4.8 | 0.818 |
| Body mass index,
kg/m2 | 21.70±2.37 | 20.97±2.71 | 0.053 |
| Age of menarche,
years | 14.20±1.12 | 14.12±1.10 | 0.824 |
| Menstrual cycle,
days | 31.68±5.57 | 29.49±5.81 | 0.078 |
| Menstrual period,
days | 5.42±1.17 | 6.12±1.17 | 0.004 |
Isolation, purification, culture and
authentication of endometrial stromal cells (ESCs)
The protocol for isolation of ESCs from patients
with or without endometriosis was performed as previously described
(27). In brief, clinical specimens
were washed with PBS, cut into small fragments in DMEM/nutrient
mixture F-12 (F12) (cat. no. MA0590; Meilunbio) and digested with
collagenase I (cat. no. C0130; Sigma-Aldrich; Merck KGaA) for >1
h at 37°C. The resulting cell suspension was filtered and
centrifuged at 800 × g for 5 min, at room temperature. Pelleted
cells were cultured in DMEM/F12 supplemented with 10% FBS (cat. no.
10099; Gibco; Thermo Fisher Scientific, Inc.), penicillin (66.67
mg/l) and streptomycin (100 mg/l) in a humidified atmosphere of 5%
CO2 at 37°C. For hypoxic treatment, cells were incubated
in a hypoxia chamber with 1% O2, 94% N2 and
5% CO2 at 37°C (28,29).
Cells were incubated in a hypoxia chamber for 0, 2, 4, 8, 12, 24
and 48 h. Immunofluorescent authentication of ESCs was then
conducted. Briefly, primary cultured cells were cultured on
coverslips (cell confluence, 30–50%), washed gently with PBS twice
and fixed with 4% paraformaldehyde (cat. no. P1110; Beijing
Solarbio Science & Technology Co., Ltd.) for 20 min at room
temperature. After permeabilization with PBS- 0.1% Triton X-100 for
10 min at 4°C, cells were blocked with 5% BSA (cat. no. AR0004;
Wuhan Boster Biological Technology Co., Ltd) for 30 min at room
temperature and then incubated with vimentin (cat. no. 5741; 1:100;
Cell Signaling Technology, Inc.) and pan-cytokeratin (cat. no.
ab215838; 1:500; Abcam) at 4°C overnight. Fluorescent-conjugated
secondary antibody solution [1:100; green fluorescence, cat. no.
70-GAR4882; red fluorescence, cat. no. 70-GAR5492; Hangzhou Multi
Sciences (Lianke) Biotech Co., Ltd.] at room temperature in the
dark for 30 min was used to visualize the signal. DAPI solution
(cat. no. H-1200; Vector Laboratories) was used to stain cell
nuclei. Images were captured under a fluorescence microscope
(Olympus BX51; Olympus Corporation). Cell identification is shown
in Fig. S1.
Immunohistochemistry assay
Tissue samples were fixed with 4% paraformaldehyde
for 24 h at room temperature, embedded in paraffin, sliced into
5-µm sections and stained according to standard immunohistochemical
staining procedures with an antibody against LDHA (cat. no. 3582S;
Cell Signaling Technology, Inc.). Briefly, the tissue sections were
immersed in blocking reagent (500 ml methanol + 0.5 ml 3% hydrogen
peroxide) and incubated at room temperature for 5 min, then boiled
in citrate buffer (cat. no. C1010-2L; Beijing Solarbio Science
& Technology Co., Ltd.) for 2.5 min and sealed with 5% BSA at
room temperature in the dark for 30 min. Subsequently, sections
were incubated with the LDHA antibody (1:400) at room temperature
for 40 min and with the secondary antibody (cat. no. GK500710;
Dako) at room temperature for 30 min. Non-immune normal IgG serum
(cat. no. BMR371; Beijing Bersee Technology Co., Ltd.) was used as
the control, and the control staining was performed at the same
dilution. Staining was developed by diaminobenzidine (cat. no.
GK500710; Dako) at room temperature in the dark for 5 min and cell
nuclei were stained with hematoxylin (cat. no. G1140; Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature
for 60 sec. H-scores of staining results were calculated using the
equation: H-Score=∑Pi (i + 1), where i refers to the intensity of
staining (1, weak; 2, moderate; or 3, strong) and Pi represents the
positive rate of stained cells, with intensities ranging from
0–100%. LDHA expression was classified into two groups: Low
expression group when H-Score was <1 or the high expression
group when H-Score was ≥1. H-score data were analyzed using
Kruskal-Wallis test with Bonferroni post hoc test.
Western blotting
Western blotting was carried out according to
standard experimental procedures. ImageJ 1.52a (National Institutes
of Health) analysis was applied to determine the signal intensity
of immunoblotted target proteins. Antibodies used in the study are
listed in Table SI.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from treated cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (1 µg) was reverse transcribed into cDNA using
reverse transcriptase (M-MLV; Promega Corporation), dNTP (Promega
Corporation) and Oligo(dT)15 (Promega Corporation). RT was
conducted at 42°C for 1 h, according to the reverse transcriptase
manufacturer's instructions. RT-qPCR was performed with FastStart
Universal SYBR® Green Master (Roche Diagnostics) and a
CFX96 real-time quantitative system (Bio-Rad Laboratories, Inc.).
qPCR was performed according to the SYBR® Green Master
manufacturer's instructions: 95°C for 3 min, followed by 40 cycles
at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec, and a
final cycle at 95°C for 15 sec, 60°C for 60 sec and 95°C for 15
sec. 18S was used as the internal control, and expression values
were normalized to the mean value of 18S rRNA. Quantification of
mRNA abundance was based on the threshold cycle (Cq) as
2−Δ(ΔCq), where ΔCq = Cq(LDHA) - Cq(18S) and Δ(ΔCq) =
Cq(experimental group) - Cq(control group) (30). Primer sequences are listed in
Table SII.
LDHA interference
The infection efficiency and knockdown rate of
primary cultured cells were both too low; therefore, the following
experiments were conducted in immortalized THESC cells (American
Type Culture Collection; cat. no. 4003TM) and Ishikawa cells (cat.
no. BNCC 338693; BeNa Culture Collection; Beijing Beina Chunglian
Institute of Biotechnology). Both of these cell lines are model
cell lines of the endometrium (31,32).
Lentivirus LDHA interference sequence [short hairpin (sh-)LDHA in a
GV248 lentiviral plasmid; Shanghai GeneChem Co., Ltd] with the
target sequence 5′-GGCAAAGACTATAATGTAA-3′ (vehicle components:
hU6-MCS-Ubiquitin-EGFP-IRES-puromycin) and the non-targeting
negative control (sh-Con) were infected into Ishikawa cells and
THESC cells with a MOI of 10. After infection for 12 h at 37°C, the
culture medium was replaced with complete medium. After 72 h,
proteins were harvested, and western blotting was applied to assess
the effect of infection.
Apoptosis analysis
LDHA-silenced Ishikawa cells, THESC cells and their
counterpart controls were inoculated into 6-cm dishes at a density
of 3×105 cells/dish. Subsequently, 12 h after adhesion,
cells were cultured under normoxia (21% O2) or hypoxia
(1% O2) for 24 h. An Annexin V-FITC/propidium iodide
(PI) apoptosis assay kit (cat. no. AD10; Dojindo Molecular
Technologies, Inc.) was used to detect apoptotic cells. Trypsinized
cells were washed twice with PBS and suspended in 100 µl 1X Annexin
V binding solution. Subsequently, 5 µl each of Annexin V-FITC and
PI staining solutions were added to the cell suspension, which was
incubated at room temperature in the dark for 15 min. After adding
400 µl 1X Annexin V binding solution, apoptosis was detected using
flow cytometry (CytoFLEX; Beckman Coulter, Inc.) and analyzed with
CytExpert for DxFLEX (2.0.0.274; Beckman Coulter, Inc.). The
apoptotic cell rate (%) was calculated as Annexin V-positive
cells/the total number of cells.
Cell cycle analysis
LDHA-silenced Ishikawa cells, THESC cells and their
counterpart controls were exposed to normoxia or hypoxia for 24 h.
Subsequently, cells were collected and fixed overnight with 70%
ethanol at 4°C. After removing the fixative solution by
centrifugation at 200 × g for 5 min at room temperature, cell
pellets were washed twice with ice-cold PBS, and treated with RNase
A (10 µg/ml; cat. no. ST579; Beyotime Institute of Biotechnology)
and PI (final concentration, 50 µg/ml) at room temperature in the
dark for 30 min. PI-stained cells were evaluated by flow cytometry
(CytoFLEX; Beckman Coulter, Inc.) for cell cycle phase distribution
within 1 h. Cell phase distribution was analyzed with FlowJo v10
(Flowjo, LLC).
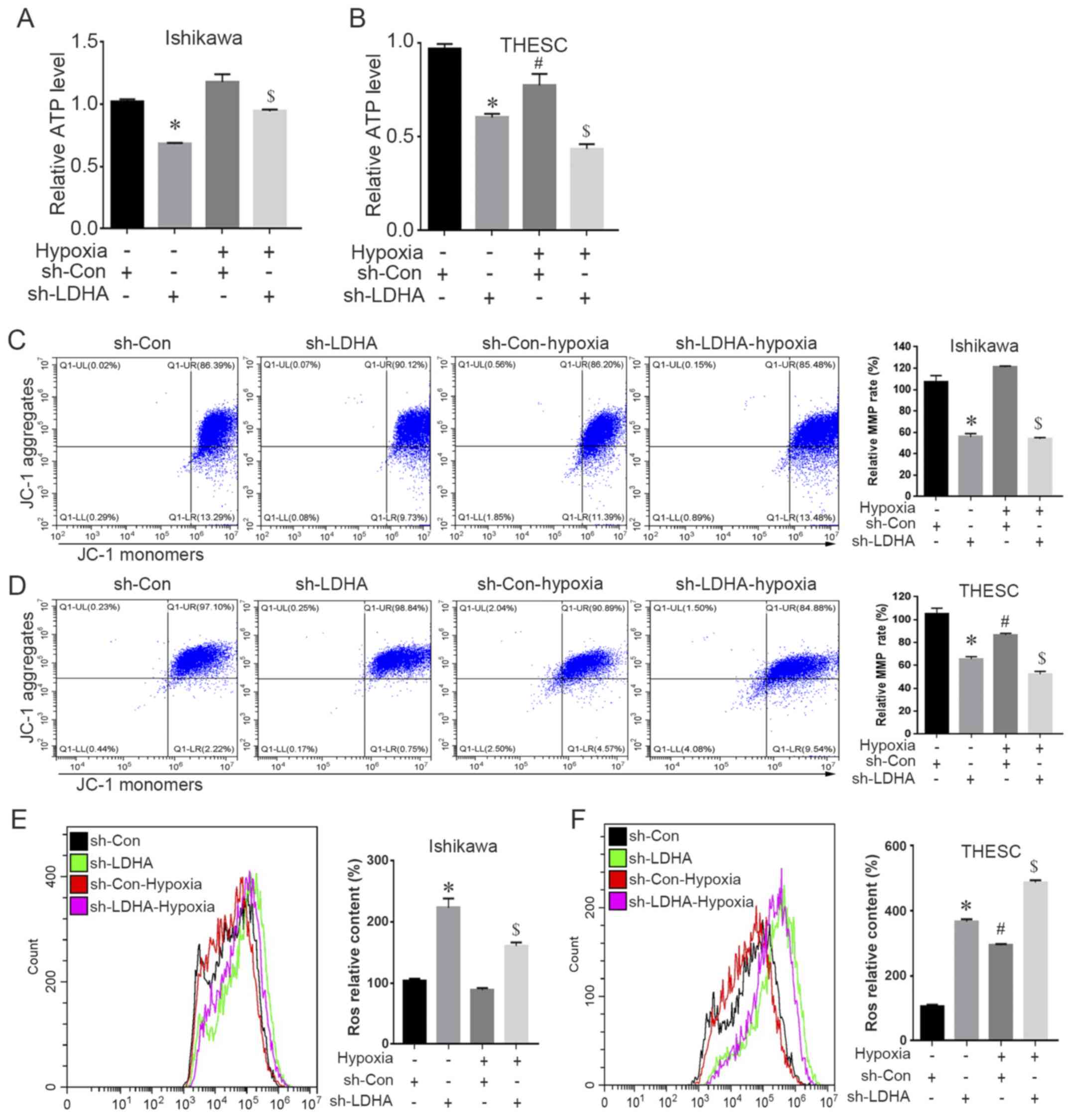
Detection of cellular ATP levels
LDHA-silenced Ishikawa cells, THESC cells and their
counterpart controls were exposed to normoxia or hypoxia for 24 h.
ATP levels in GC lysates were measured using a luminometer (Synergy
H4; BioTek Instruments, Inc.) according to the manufacturer's
instructions of the ATP kit (cat. no. S0027; Beyotime Institute of
Biotechnology).
Analysis of mitochondrial membrane
potential (MMP)
MMP was determined by flow cytometry using the JC-1
assay kit (cat. no. C2006; Beyotime Institute of Biotechnology).
LDHA-silenced Ishikawa cells, THESC cells and their counterpart
controls were exposed to normoxia or hypoxia for 24 h. The
collected cells were treated according to the manufacturer's
instructions. Each sample was assessed by flow cytometry (CytoFLEX;
Beckman Coulter, Inc.) for red (JC-1 aggregates) and green (JC-1
monomers) fluorescence and analyzed with CytExpert for DxFLEX
(2.0.0.274; Beckman Coulter, Inc.). Relative MMP ratio was
calculated as red fluorescence intensity/green fluorescence
intensity. The relative signal intensities of JC-1 aggregates and
monomers were normalized to the control group.
Analysis of reactive oxygen species
(ROS)
ROS content was determined by flow cytometry using a
ROS assay kit (cat. no. S0033; Beyotime Institute of
Biotechnology). LDHA-silenced Ishikawa cells, THESC cells and their
counterpart controls, were exposed to normoxia or hypoxia for 24 h.
The collected LDHA-silenced and sh-Con cells were incubated with 10
µM 2′-7′dichlorofluorescin diacetate (DCFH-DA) dye (cat. no. S0033;
Beyotime Institute of Biotechnology) for 20 min at 37°C. Each
sample was assessed by flow cytometry (CytoFLEX; Beckman Coulter,
Inc.) for fluorescence intensity. Data were analyzed with CytExpert
for DxFLEX (2.0.0.274; Beckman Coulter, Inc.).
Cell Counting Kit-8 (CCK-8) assay
LDHA-silenced Ishikawa cells and THESC cells and
their counterpart controls were grown to logarithmic phase,
trypsinized, counted and inoculated into 96-well plates at a
density of 2×103 cells/well. Cell proliferation rates
were measured for 7 days using the CCK-8 assay (cat. no. CK04;
Dojindo Molecular Technologies, Inc.). CCK-8 reagent (10 µl) was
added to 100 µl DMEM/F12 with 10% FBS, and was added to cells in a
humidified atmosphere containing 5% CO2 at 37°C for 1 h
and absorbance was measured at 450 nm at the same time each day,
according to the manufacturer instructions.
Transwell migration assay
LDHA-silenced Ishikawa cells, THESC cells and their
counterpart controls were trypsinized and resuspended in serum-free
medium. Cell suspensions containing 2×104 cells in a
200-µl volume were added to the upper chamber of each Transwell
(8.0-µm aperture; Corning, Inc.), whereas 700 µl complete culture
medium supplemented with 10% FBS was added to the lower chamber.
Ishikawa cells were cultured for 48 h, whereas THESC cells were
cultured for 24 h. Cells in the upper chamber were fixed in 75%
alcohol for 20 min at room temperature and stained with crystal
violet for 5 min at room temperature. Subsequently, cells remaining
in the upper chamber were wiped off, and cells that migrated
through the membrane were counted under a light microscope.
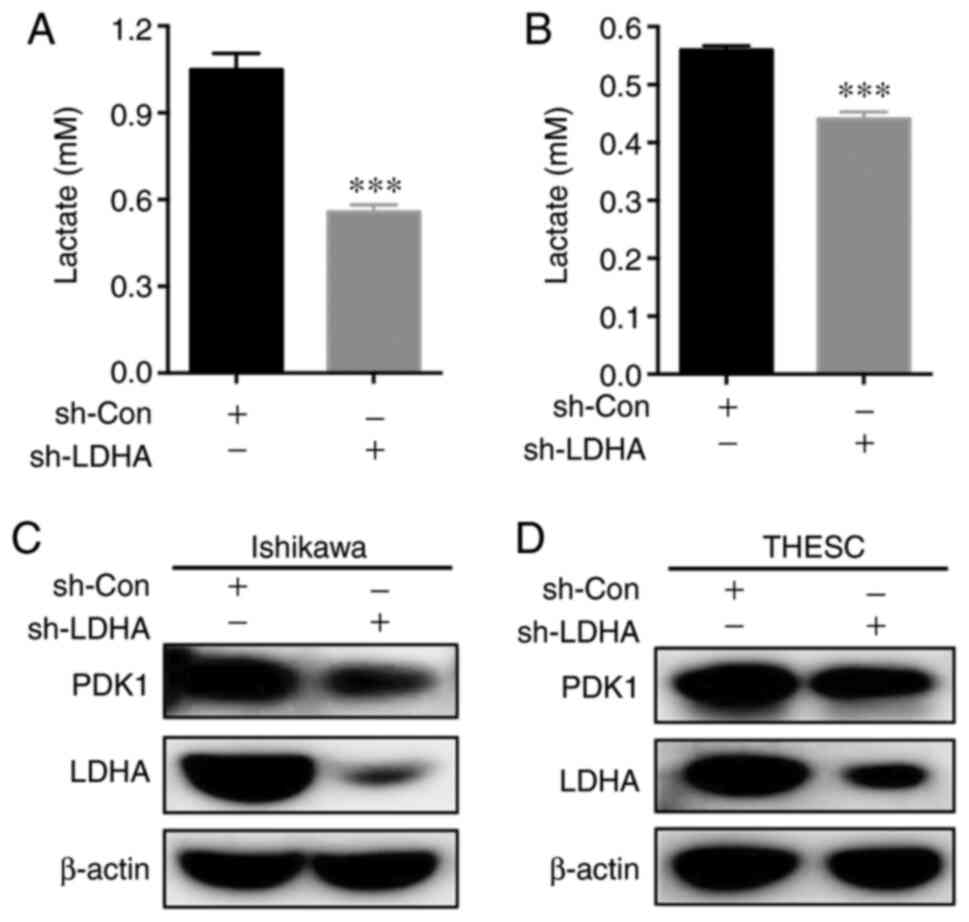
Lactic acid detection
LDHA-silenced Ishikawa cells, THESC cells and their
counterpart controls were cultured for 24 h, trypsinized and
counted. The lactic acid concentration in the collected medium was
determined with a blood gas analyzer (Cobas B 221; Roche
Diagnostics). Lactate production is presented as the amount of
lactate normalized to cell number.
Statistical analysis
The experiments were performed in triplicate and
repeated three times. Data are expressed as the mean ± standard
deviation. SPSS version 25.0 (IBM Corp.) and GraphPad Prism version
7 (GraphPad Software, Inc.) were used for statistical analysis.
Student's t-test was used for comparison of two groups of
data and one-way or two-way ANOVA was used for comparison of
multiple groups of data, followed by the Bonferroni correction.
Fisher's exact test was used to analyze normal endometrium and
eutopic endometrium groups, and χ2 test was used to
analyze ectopic endometrium groups. H-score data were analyzed
using Kruskal-Wallis test with Bonferroni post hoc test.
Differences in the expression levels of LDHA (based on H-Score) in
paired eutopic and ectopic tissues obtained from 25 patients with
endometriosis were analyzed using Wilcoxon signed-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
LDHA expression is increased in
endometriotic lesions
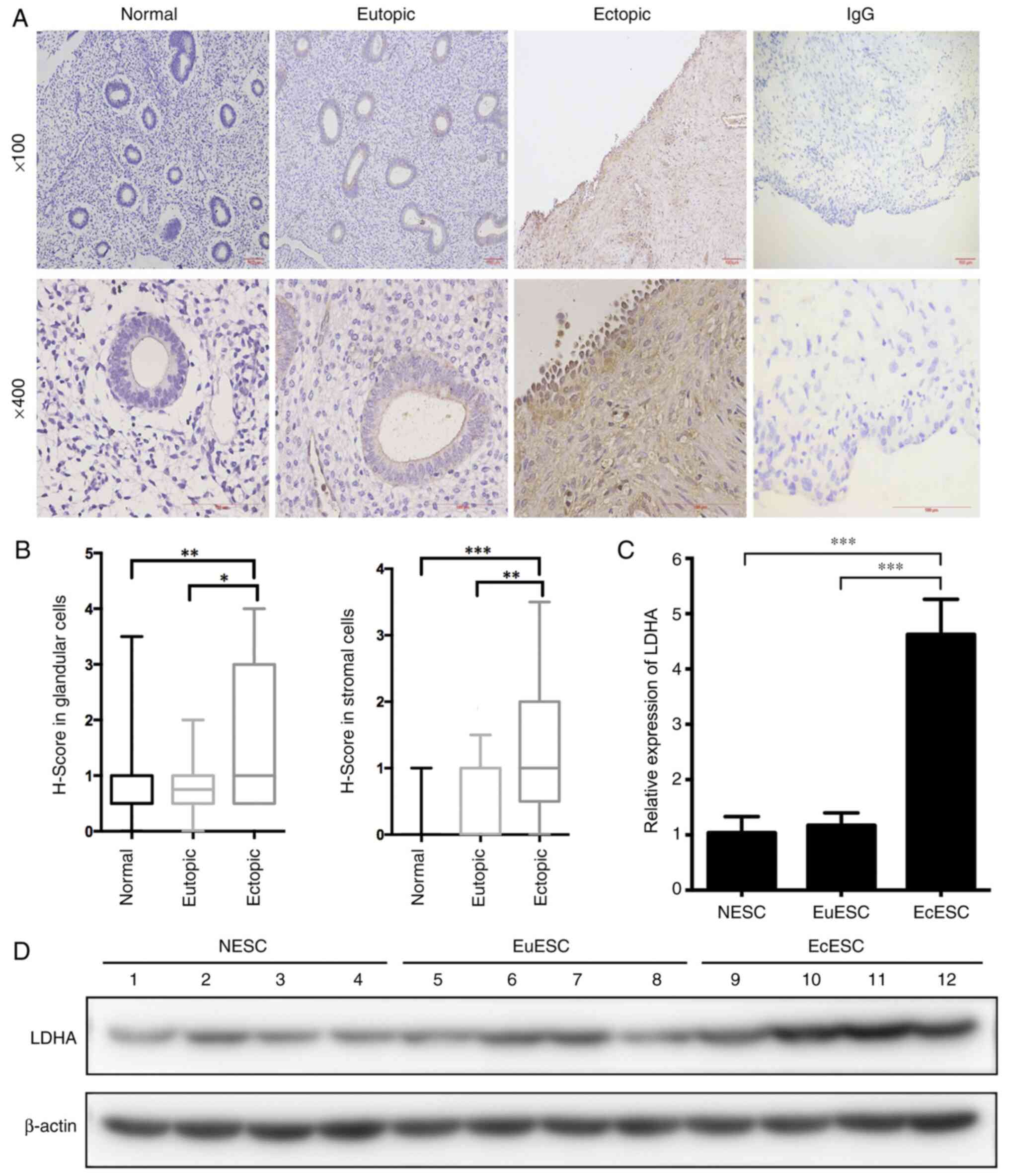
The results from immunohistochemical analysis of
normal endometrium showed that LDHA protein was expressed in both
endometrial glandular cells and stromal cells, and was mainly
located in the cytoplasm (Fig. 1A).
In the independent samples, the LDHA protein expression levels in
ectopic endometrium were significantly increased compared with
those in normal and eutopic endometria, with no significant
difference in LDHA expression observed between the latter two
groups (Fig. 1A and B). The protein
expression levels of LDHA in ectopic endometrial glandular cells
and stromal cells were significantly higher than in paired eutopic
endometrial in 25 patients with endometriosis (Table SIII). Additionally, the LDHA
protein expression levels in ectopic endometrial glandular cells
were significantly higher during the proliferative phase compared
with during the secretive phase (Table
II). However, no difference in LDHA protein expression levels
was observed in ectopic endometrial stromal cells (EcESCs) at
different endometrial growth cycles (Table II).
 | Table II.Lactate dehydrogenase A expression in
glandular and stromal cells during menstrual phases. |
Table II.
Lactate dehydrogenase A expression in
glandular and stromal cells during menstrual phases.
|
| Glandular
cells |
| Stromal cells |
|
|---|
|
|
|
|
|
|
|---|
| Sample type | Low | High | P-value | Low | High | P-value |
|---|
| Normal
endometriuma |
|
| 0.366 |
|
| 0.715 |
|
Proliferative phase | 13 | 10 |
| 21 | 2 |
|
|
Secretive phase | 9 | 3 |
| 12 | 0 |
|
| Eutopic
endometriuma |
|
| 0.602 |
|
| 0.099 |
|
Proliferative phase | 12 | 17 |
| 21 | 8 |
|
|
Secretive phase | 7 | 3 |
| 10 | 0 |
|
| Ectopic
endometriumb |
|
| 0.03 |
|
| 0.355 |
|
Proliferative phase | 7 | 32 |
| 14 | 25 |
|
|
Secretive phase | 10 | 13 |
| 11 | 12 |
|
To evaluate LDHA expression in primary cultured
cells, samples of normal, eutopic and ectopic endometria were
collected to obtain normal endometrial stromal cells (NESCs),
eutopic endometrial stromal cells (EuESCs) and EcESCs,
respectively. RT-qPCR and western blotting showed that LDHA
expression in EcESCs was markedly increased compared with that in
NESCs and EuESCs (Fig. 1C and D),
whereas LDHA expression in NESCs and EuESCs was not significantly
different, which is consistent with the immunohistochemical
results. Increased expression of LDHA in endometriotic cells
suggested that it may serve a role in the development of
endometriosis.
Hypoxia induces LDHA expression in
endometrial cells
Hypoxia, a feature of the endometriotic
microenvironment (21), has
previously been reported to regulate LDHA expression in tumor cells
(33). The current study
investigated whether hypoxia influenced LDHA expression in ESCs and
in immortalized endometrial glandular cells using the Ishikawa cell
line. RT-qPCR showed that both HIF-1α and LDHA mRNA expression
levels were increased in ESCs (including NESCs, EuESCs and EcESCs)
and in Ishikawa cells after exposure to hypoxia, with the mRNA
expression levels of these proteins significantly increasing with
the prolongation of hypoxia (Fig.
S2).
Western blotting results also showed that HIF-1α
increased significantly within a short time (4 h) in ESCs and
Ishikawa cells exposed to hypoxia, and remained high for 24 or even
48 h. However, the changes in LDHA expression lagged behind the
increase in HIF-1α expression. A significant difference in LDHA
expression was shown after 4 h of hypoxia and continued for 48 h
(Fig. 2A-D). Additionally, the
HIF-1α and LDHA expression levels in EcESCs without hypoxia were
significantly increased compared with NESCs and EuESCs. Notably,
LDHA protein expression levels in EcESCs remained higher compared
with those in NESCs and EuESCs after hypoxia treatment for 12 or 48
h (Fig. 2E).
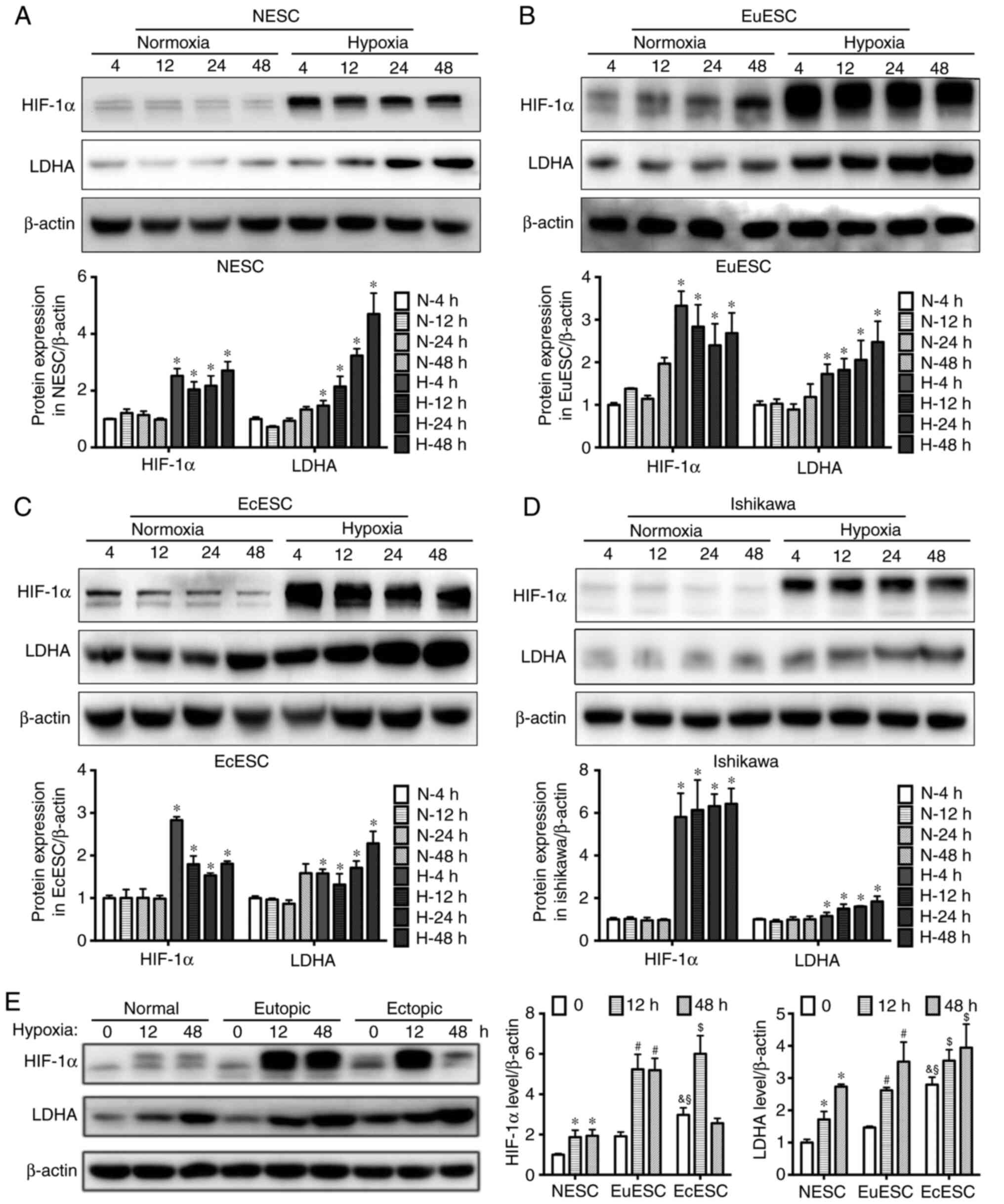 | Figure 2.Hypoxia induces LDHA expression in
endometrial cells. Western blot analysis of HIF-1α and LDHA
expression in (A) NESCs, (B) EuESCs, (C) EcESCs and (D) Ishikawa
cells at 4, 12, 24 and 48 h after culture in normoxic or hypoxic
conditions. Upper panel shows protein bands, whereas the lower
panel shows a histogram semi-quantifying the results of
corresponding treatments. One-way ANOVA and Bonferroni post hoc
test were used to analyze multiple comparisons. *P<0.05, hypoxia
vs. normoxia group at the same time point. (E) Western blot
analysis of HIF-1α and LDHA expression in NESCs, EuESCs and EcESCs
after 12 or 48 h of normoxia or hypoxia. The left panel shows
protein bands, the middle panel shows a histogram of HIF-1α
expression in the three cell types, and the right panel shows a
histogram of LDHA expression. β-actin was used as a loading
control. Values are expressed as mean ± SD of three independent
experiments. Two-way ANOVA and Bonferroni post hoc test were used
to analyze multiple comparisons. *P<0.05, hypoxia vs. normoxia
group in NESCs; #P<0.05, hypoxia vs. normoxia group
in EuESCs; $P<0.05, hypoxia vs. normoxia group in
EcESCs; &P<0.05, EcESCs vs. NESCs in normoxia;
§P<0.05, EcESCs vs. EuESCs in normoxia. LDHA, lactate
dehydrogenase A; HIF-1α, hypoxia inducible factor 1α; NESC, normal
endometrial stromal cells; EuESC, eutopic endometrial stromal
cells; EuESC, eutopic endometrial stromal cells. |
LDHA-knockdown increases apoptosis of
Ishikawa cells and THESC cells
To examine the function of LDHA in endometrial
glandular epithelial and mesenchymal cells, Ishikawa cells and
THESC cells were infected with a sh-LDHA lentivirus. Compared with
control virus-infected cells (sh-Con), LDHA protein expression
levels in sh-LDHA virus-infected Ishikawa cells and THESC cells
were reduced (Figs. 3C and 4C). Flow cytometry was conducted to
examine the effect of LDHA-knockdown on apoptosis. The results
showed that LDHA-knockdown significantly increased the rate of
apoptotic Ishikawa cells (Fig. 3A and
B). Western blotting showed that the expression levels of
proapoptotic proteins, including BID, BAX, BIK, BIM, PUMA,
phosphorylated (P)-BAD/BAD and cleaved (C)-caspase 3, were
significantly increased after LDHA-knockdown (Fig. 3C and D). Consistent with these
results, LDHA-knockdown significantly increased apoptosis of THESC
cells compared with that in the control cells (Fig. 4A and B). Moreover, BID, BAX, BIK,
P-BAD/BAD and C-caspase 3 protein expression levels in the
LDHA-knockdown groups were significantly upregulated compared with
those in the control group (Fig. 4C and
D).
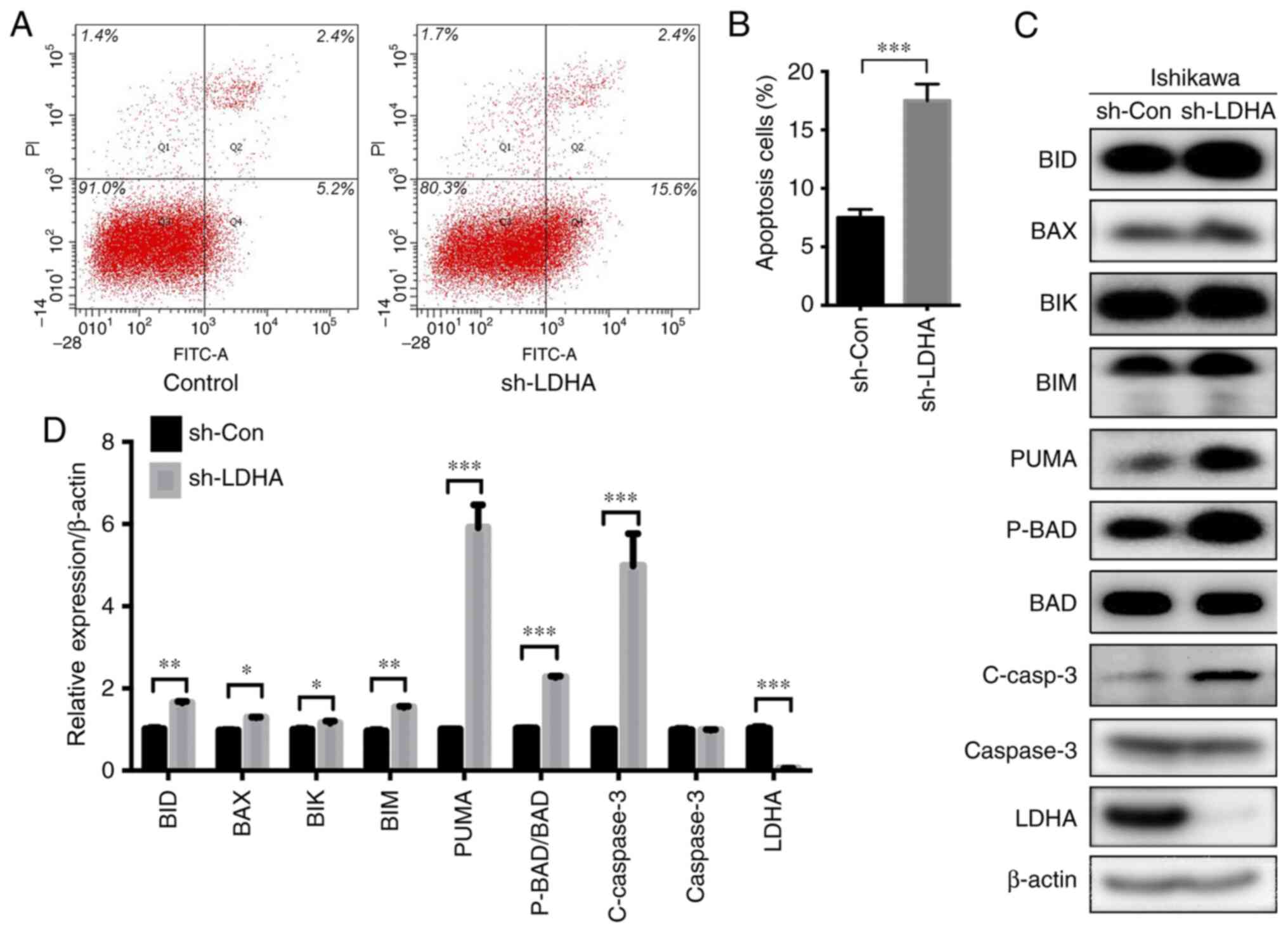 | Figure 3.Effect of LDHA on apoptosis of
Ishikawa cells. (A) Representative graphs of apoptosis detected by
flow cytometry. (B) A higher number of Ishikawa cells underwent
apoptosis when LDHA was knocked down (sh-LDHA) compared with the
control (sh-Con). (C) Western blot analysis of apoptosis-related
proteins. (D) Histogram of BID, BAX, BIK, BIM, PUMA, P-BAD/BAD,
C-caspase 3, total caspase 3 and LDHA protein expression in
Ishikawa cells. Values are expressed as mean ± SD of three
independent experiments. Student's t-test was used to
compare two groups. *P<0.05, **P<0.01 and ***P<0.001.
LDHA, lactate dehydrogenase A; C-, cleaved; sh-, short hairpin;
Con-, control; P-, phosphorylated. |
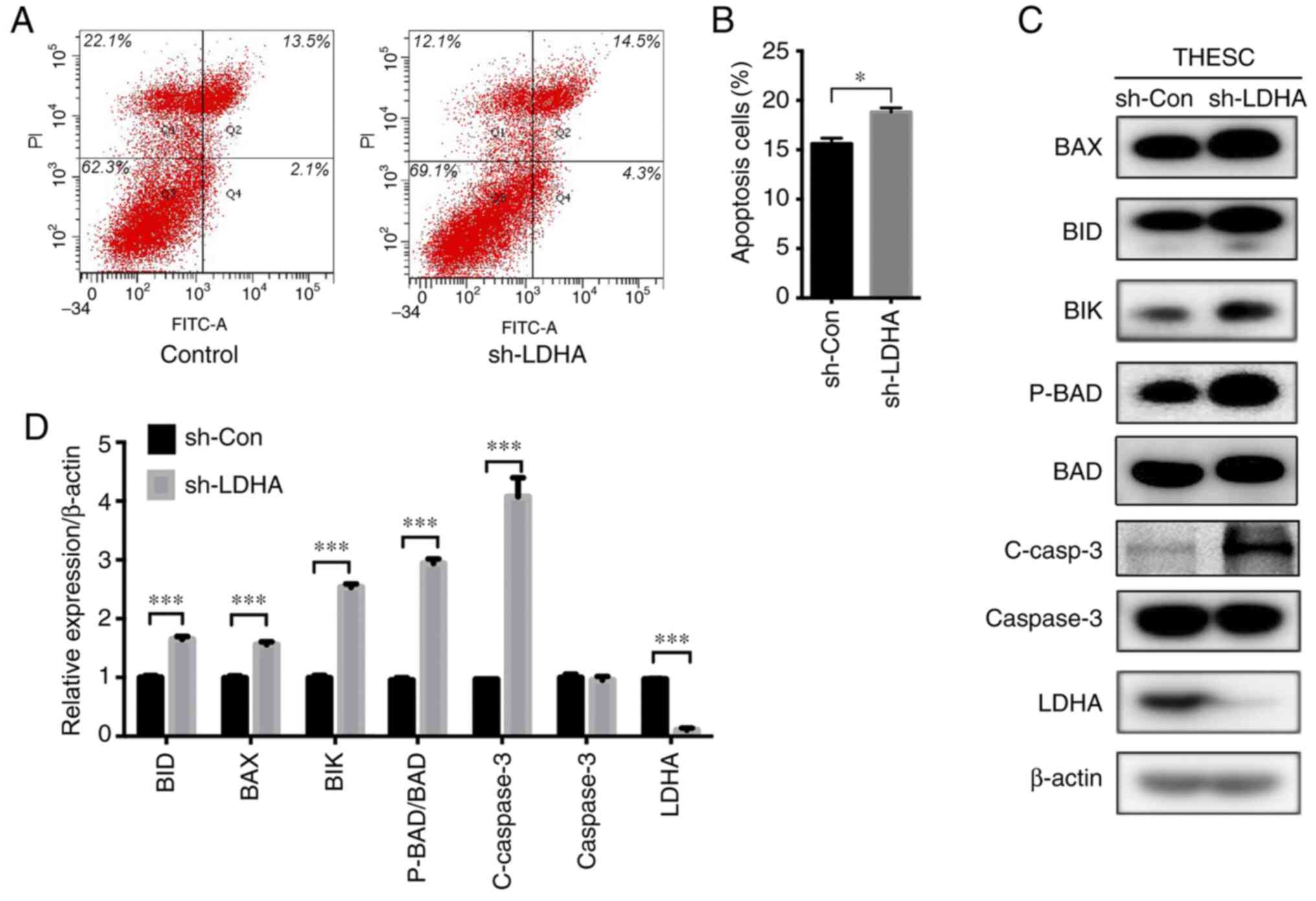 | Figure 4.Effect of LDHA on apoptosis of
immortalized human endometrial stromal cells (THESC cells). (A)
Representative graphs of apoptosis detected by flow cytometry. (B)
An increased number of THESC cells underwent apoptosis when LDHA
was knocked down (sh-LDHA) compared with the control (sh-Con). (C)
Western blot analysis of apoptosis-related proteins. (D) Histogram
of BID, BAX, BIK, P-BAD/BAD, C-caspase 3, total caspase 3 and LDHA
protein expression in THESC cells. Values are expressed as mean ±
SD of three independent experiments. Student's t-test was
used to compare two groups. *P<0.05 and ***P<0.001. LDHA,
lactate dehydrogenase A; sh-, short hairpin; Con-, control; C-,
cleaved; P-, phosphorylated. |
Effect of LDHA-knockdown on
mitochondrial function in Ishikawa cells and THESC cells
Mitochondria are the key energy-producing organelles
and cellular source of ROS, which are responsible for managing cell
life and death by controlling cell respiration and ATP synthesis
(34). Changes in MMP caused by
hypoxia can lead to mitochondrial permeability transition and the
release of mitochondrial pro-apoptotic factors, resulting in
apoptosis (35).
The present study investigated mitochondrial
functions in LDHA-silenced cells under hypoxic or normoxic
conditions. Hypoxia led to suppressed ATP production, decreased MMP
levels and increased ROS levels in THESC cells (Fig. 5B, D and F), whereas no significant
difference was found in Ishikawa cells. Under both normoxic and
hypoxic conditions, knockdown of LDHA in Ishikawa cells and THESC
cells significantly reduced ATP and MMP levels, and induced
increased aggregation of ROS (Fig.
5A-F).
Effects of LDHA-knockdown on cell
cycle distribution of Ishikawa cells and THESC cells
To examine the effect of LDHA-knockdown on the cell
cycle, LDHA-silenced Ishikawa cells and THESC cells were analyzed
by flow cytometry after PI staining. The results showed that
compared with the control group, LDHA interference significantly
increased the proportion of G1-phase Ishikawa cells and
decreased that of S-phase cells (Fig.
S3A and C). However, LDHA-knockdown did not affect the cell
cycle distribution of THESC cells (Fig. S3B and D). Consistently, western
blotting results showed that p21 expression levels were increased
in the Ishikawa knockdown group compared with the control group
(Fig. S3E), whereas no change in
p21 expression was observed in THESC cells (Fig. S3F).
Effects of LDHA-knockdown on migration
and proliferation of Ishikawa cells and THESC cells
The results of the Transwell assay showed that the
numbers of migrated Ishikawa cells and THESC cells in the
LDHA-knockdown group were significantly decreased compared with
those in the control groups (Fig.
S4A-C). Furthermore, western blotting showed that the
expression levels of the epithelial marker E-cadherin in Ishikawa
cells were increased when LDHA was knocked down, whereas the
mesenchymal marker, β-catenin, was slightly decreased (Fig. S4D). By contrast, the mesenchymal
marker, vimentin, was unchanged in Ishikawa cells (Fig. S4D). Similarly, in LDHA-knockdown
THESC cells, β-catenin expression was decreased, whereas the
expression levels of vimentin did not change (Fig. S4D). The CCK-8 assay results showed
no significant changes in the proliferative capacity of Ishikawa
cells or THESC cells after LDHA-knockdown (Fig. S4E and F).
LDHA-knockdown impairs the metabolism
of Ishikawa cells and THESC cells
LDHA, a key catalytic enzyme for the transformation
of pyruvate into lactic acid, is involved in glycolysis regulation
(16). To investigate the role of
LDHA in endometrial cells, the lactic acid levels in LDHA-knockdown
and control cells were measured. The results showed that lactate
production in Ishikawa cells and THESC cells was significantly
decreased when LDHA was knocked down (Fig. 6A and B). Moreover, western blot
analysis of key glycolysis enzymes showed that PDK1 expression was
decreased in Ishikawa cells and THESC cells (Fig. 6C and D).
Discussion
Hypoxia plays critical roles in promoting
pathological processes that facilitate the development of
endometriosis (36). Moreover,
glucose metabolism and energy production in ectopic endometriotic
cells under hypoxia have been reported to influence the occurrence
and invasion of endometriosis (36,37).
LDHA is a key enzyme that catalyzes the conversion of pyruvate into
lactic acid for energy metabolism (16). Exploring the expression pattern of
LDHA and its potential function in endometriosis may provide
critical clues for understanding the regulatory mechanisms of
energy production in ectopic endometriotic cells during hypoxia,
and may help identify new targets for the treatment of
endometriosis.
Previous research has confirmed that HIF-1α and LDHA
expression in ectopic tissues of endometriosis is higher compared
with that in normal and eutopic endometrial tissues (9). In the present study, analysis of
clinical samples revealed significantly higher LDHA expression
levels in ectopic tissues compared with those in normal and eutopic
tissues. Additionally, exposure of ESCs and glandular epithelial
Ishikawa cells to hypoxia increased LDHA mRNA and protein
expression levels. Furthermore, the LDHA expression levels were
significantly increased in EcESCs compared with EuESCs and NESCs.
These results indicated that hypoxia may play a notable role in the
progression of endometriotic cysts in association with upregulated
LDHA expression.
Apoptosis is a key cellular process for the
development of endometriosis (38,39).
Proapoptotic molecules, such as BAD, BID, BIM, BIK, BAX and BAK,
induce apoptosis, whereas antiapoptotic molecules, such as BCL-2,
BCL-X and BCL-W, inhibit this process (40). Several previous studies reported
that patients with endometriosis had a high number of BAX-negative
and BCL-2-positive macrophages in their ectopic lesions (41–44).
Additionally, the proportion of BCL-2-positive macrophages in the
abdominal fluid was significantly increased compared with that in
healthy women, which may be associated with the anti-apoptotic
effect of immune cells in patients with endometriosis (44). BAX and BAK have been shown to be
downregulated in patients with endometriosis, further demonstrating
a notable role in the occurrence and development of endometriosis
(45). The results of the present
study showed that LDHA-knockdown promoted apoptosis of Ishikawa
cells and THESC cells, suggesting that LDHA played a role in
inhibiting apoptosis of endometrial cells.
Mitochondria are important organelles in the
majority of cells and are closely associated with apoptosis
(34). Generally, ATP levels and
MMP decrease during apoptosis, whereas levels of ROS increase,
indicating that mitochondrial function is impaired or decreased
(46,47). In mammalian cells, hypoxia induces
dephosphorylation of FUN14 domain-containing protein 1 and enhances
its interaction with LC3 to promote mitochondrial autophagy
(48). The present study showed
that after LDHA-knockdown, mitochondrial functions of THESC cells
and Ishikawa cells were impaired, which may lead to apoptosis. In
addition, hypoxia influenced THESC mitochondrial function but not
that in Ishikawa cells; the specific mechanism remains unclear.
Increased expression of E-cadherin, a
calcium-dependent adhesion molecule found on the surface of
epithelial cells, may enhance the connection between cells, thus
inhibiting their migratory and invasive abilities (49). Downregulated expression of
E-cadherin has been reported to be potentially associated with
susceptibility to endometriosis, resulting in elevated migration
and invasion potentials of endometriotic cells (50). β-catenin protein localizes in the
cell membrane and cytoplasm, where it is mainly involved in cell
proliferation, differentiation and apoptosis (51). β-catenin can also bind to
intracellular E-cadherin (52) to
form an E-cadherin/β-catenin complex that regulates cell adhesion
and motility. Suppression of β-catenin expression has been shown to
inhibit cell proliferation and migration, and promote apoptosis of
mesenchymal cells in the endometrium, thus inhibiting the
occurrence and development of endometriosis (53). The present study showed that
LDHA-knockdown inhibited migration of Ishikawa cells and THESC
cells, which was associated with increased E-cadherin expression
and decreased β-catenin expression. These results indicated that
LDHA promoted endometriotic cell migration in the progression of
endometriotic cysts. LDHA is involved in the process of
epithelial-mesenchymal transition and changes the ability of cells
to migrate (54); however, how LDHA
participates in the regulation of the relevant signaling pathways
and affects downstream signaling molecules still needs further
research.
Hypoxia may increase the expression of PDK1, a key
enzyme in glucose metabolism, in ESCs, resulting in altered cell
metabolism and lactic acid accumulation that inhibits apoptosis
(11). The present study found that
LDHA-knockdown in Ishikawa cells and THESC cells decreased lactic
acid production and inhibited PDK1 expression, suggesting that
glycolysis was inhibited. However, the current study did not
confirm whether the effect of LDHA on lactic acid production was
associated with its regulatory roles in apoptosis, cell cycle or
cell motility.
In conclusion, the present study confirmed that LDHA
was significantly increased in endometriotic tissues. Moreover,
hypoxia-induced LDHA may serve an important role in the occurrence
and development of endometriosis. Finally, LDHA-knockdown inhibited
ESCs and glandular epithelial cell migration, promoted apoptosis
and inhibited glycolysis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant nos. 81871135,
81971358 and 81671435); Natural Science Foundation of Zhejiang
Province (grant no. LY18H040008); National Key Research and
Development Program (grant no. 2018YFC1004800); Key Research and
Development Program of Zhejiang Province (grant no. 2017C03022);
and The Medical and Health Program in Zhejiang Province (grant no.
2019KY411).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ, YD and XL performed the experiments. JZ, XL, QH,
LS, NL, FZ and XJ collected clinical samples, analyzed and
interpreted the data. JZ and YD wrote the manuscript. YD made the
figures. JZ, YD and SZ critically reviewed the article. JZ and YD
confirm the authenticity of all the raw data. YD and SZ conceived
and supervised the project. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Sir Run Run Shaw Hospital at Zhejiang University. All
patients provided written informed consent before sample
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batt RE; Invited comment on the paper by
Benagiano, ; et al: Entitled ‘the history of endometriosis’.
Gynecol Obstet Invest. 78:10–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shubina AN, Egorova AA, Baranov VS and
Kiselev AV: Recent advances in gene therapy of endometriosis.
Recent Pat DNA Gene Seq. 7:169–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu MH, Chen KF, Lin SC, Lgu CW and Tsai
SJ: Aberrant expression of leptin in human endometriotic stromal
cells is induced by elevated levels of hypoxia inducible
factor-1alpha. Am J Pathol. 170:590–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsiao KY, Lin SC, Wu MH and Tsai SJ:
Pathological functions of hypoxia in endometriosis. Front Biosci
(Elite Ed). 7:309–321. 2015.PubMed/NCBI
|
|
7
|
Lin X, Dai Y, Xu W, Shi L, Jin X, Li C,
Zhou F, Pan Y, Zhang Y, Lin X and Zhang S: Hypoxia promotes ectopic
adhesion ability of endometrial stromal cells via TGF-β1/smad
signaling in endometriosis. Endocrinology. 159:1630–1641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai Y, Lin X, Xu W, Lin X, Huang Q, Shi L,
Pan Y, Zhang Y, Zhu Y, Li C, et al: MiR-210-3p protects
endometriotic cells from oxidative stress-induced cell cycle arrest
by targeting BARD1. Cell Death Dis. 10:1442019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Young VJ, Brown JK, Maybin J, Saunders PT,
Duncan WC and Horne AW: Transforming growth factor-β induced
Warburg-like metabolic reprogramming may underpin the development
of peritoneal endometriosis. J Clin Endocrinol Metab. 99:3450–3459.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molinari E, Bar H, Pyle AM and Patrizio P:
Transcriptome analysis of human cumulus cells reveals hypoxia as
the main determinant of follicular senescence. Mol Hum Reprod.
22:866–876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HC, Lin SC, Wu MH and Tsai SJ:
Induction of pyruvate dehydrogenase kinase 1 by hypoxia alters
cellular metabolism and inhibits apoptosis in endometriotic stromal
cells. Reprod Sci. 26:734–744. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D
and Lou W: Lactate dehydrogenase A is overexpressed in pancreatic
cancer and promotes the growth of pancreatic cancer cells. Tumour
Biol. 34:1523–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koukourakis MI, Giatromanolaki A, Winter
S, Leek R, Sivridis E and Harris AL: Lactate dehydrogenase 5
expression in squamous cell head and neck cancer relates to
prognosis following radical or postoperative radiotherapy.
Oncology. 77:285–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giatromanolaki A, Koukourakis MI, Pezzella
F, Sivridis E, Turley H, Harris AL and Gatter KC: Lactate
dehydrogenase 5 expression in non-Hodgkin B-cell lymphomas is
associated with hypoxia regulated proteins. Leuk Lymphoma.
49:2181–2186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase A in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: HIF-1 mediates metabolic
responses to intratumoral hypoxia and oncogenic mutations. J Clin
Invest. 123:3664–3671. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeung SJ, Pan J and Lee MH: Roles of p53,
MYC and HIF-1 in regulating glycolysis-the seventh hallmark of
cancer. Cell Mol Life Sci. 65:3981–3999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holmes RS and Goldberg E: Computational
analyses of mammalian lactate dehydrogenases: Human, mouse, opossum
and platypus LDHs. Comput Biol Chem. 33:379–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhan L, Wang W, Zhang Y, Song E, Fan Y and
Wei B: Hypoxia-inducible factor-1alpha: A promising therapeutic
target in endometriosis. Biochimie. 123:130–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Griffiths EA, Pritchard SA, Valentine HR,
Whitchelo N, Bishop PW, Ebert MP, Price PM, Welch IM and West CM:
Hypoxia-inducible factor-1alpha expression in the gastric
carcinogenesis sequence and its prognostic role in gastric and
gastro-oesophageal adenocarcinomas. Br J Cancer. 96:95–103. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun HC, Qiu ZJ, Liu J, Sun J, Jiang T,
Huang KJ, Yao M and Huang C: Expression of hypoxia-inducible
factor-1 alpha and associated proteins in pancreatic ductal
adenocarcinoma and their impact on prognosis. Int J Oncol.
30:1359–1367. 2007.PubMed/NCBI
|
|
22
|
Maybin JA, Hirani N, Brown P, Jabbour HN
and Critchley HO: The regulation of vascular endothelial growth
factor by hypoxia and prostaglandin F2α during human endometrial
repair. J Clin Endocrinol Metab. 96:2475–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maybin JA, Hirani N, Jabbour HN and
Critchley HO: Novel roles for hypoxia and prostaglandin E2 in the
regulation of IL-8 during endometrial repair. Am J Pathol.
178:1245–1256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Amico F, Skarmoutsou E, Quaderno G,
Malaponte G, La Corte C, Scibilia G, D'Agate G, Scollo P, Fraggetta
F, Spandidos DA and Mazzarino MC: Expression and localisation of
osteopontin and prominin-1 (CD133) in patients with endometriosis.
Int J Mol Med. 31:1011–1016. 2013. View Article : Google Scholar
|
|
25
|
He G, Jiang Y, Zhang B and Wu G: The
effect of HIF-1alpha on glucose metabolism, growth and apoptosis of
pancreatic cancerous cells. Asia Pac J Clin Nutr. 23:174–180.
2014.PubMed/NCBI
|
|
26
|
Revised American Society for Reproductive
Medicine classification of endometriosis, . 1996. Fertil Steril.
67:817–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryan IP, Schriock ED and Taylor RN:
Isolation, characterization, and comparison of human endometrial
and endometriosis cells in vitro. J Clin Endocrinol Metab.
78:642–649. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsiao KY, Wu MH, Chang N, Yang SH, Wu CW,
Sun HS and Tsai SJ: Coordination of AUF1 and miR-148a destabilizes
DNA methyltransferase 1 mRNA under hypoxia in endometriosis. Mol
Hum Reprod. 21:894–904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsiao KY, Chang N, Lin SC, Li YH and Wu
MH: Inhibition of dual specificity phosphatase-2 by hypoxia
promotes interleukin-8-mediated angiogenesis in endometriosis. Hum
Reprod. 29:2747–2755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiappini F, Baston JI, Vaccarezza A,
Singla JJ, Pontillo C, Miret N, Farina M, Meresman G and Randi A:
Enhanced cyclooxygenase-2 expression levels and metalloproteinase 2
and 9 activation by Hexachlorobenzene in human endometrial stromal
cells. Biochem Pharmacol. 109:91–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Luo Y, Wang G and Yang Q:
CircATRNL1 promotes epithelial-mesenchymal transition in
endometriosis by upregulating Yes-associated protein 1 in vitro.
Cell Death Dis. 11:5942020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui XG, Han ZT, He SH, Wu XD, Chen TR,
Shao CH, Chen DL, Su N, Chen YM, Wang T, et al: HIF1/2α mediates
hypoxia-induced LDHA expression in human pancreatic cancer cells.
Oncotarget. 8:24840–24852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abate M, Festa A, Falco M, Lombardi A,
Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia
M, et al: Mitochondria as playmakers of apoptosis, autophagy and
senescence. Semin Cell Dev Biol. 98:139–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Liao W, Gao W, Huang J and Gao Y:
Intermittent hypoxia protects cerebral mitochondrial function from
calcium overload. Acta Neurol Belg. 113:507–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu MH, Hsiao KY and Tsai SJ: Hypoxia: The
force of endometriosis. J Obstet Gynaecol Res. 45:532–541. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasvandik S, Samuel K, Peters M, Eimre M,
Peet N, Roost AM, Padrik L, Paju K, Peil L and Salumets A: Deep
quantitative proteomics reveals extensive metabolic reprogramming
and cancer-like changes of ectopic endometriotic stromal cells. J
Proteome Res. 15:572–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gebel HM, Braun DP, Tambur A, Frame D,
Rana N and Dmowski WP: Spontaneous apoptosis of endometrial tissue
is impaired in women with endometriosis. Fertil Steril.
69:1042–1047. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vaskivuo TE, Stenback F, Karhumaa P,
Risteli J, Dunkel L and Tapanainen JS: Apoptosis and
apoptosis-related proteins in human endometrium. Mol Cell
Endocrinol. 165:75–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oltvai ZN and Korsmeyer SJ: Checkpoints of
dueling dimers foil death wishes. Cell. 79:189–192. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Harada M, Suganuma N, Furuhashi M,
Nagasaka T, Nakashima N, Kikkawa F, Tomoda Y and Furui K: Detection
of apoptosis in human endometriotic tissues. Mol Hum Reprod.
2:307–315. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meresman GF, Vighi S, Buquet RA,
Contreras-Ortiz O, Tesone M and Rumi LS: Apoptosis and expression
of Bcl-2 and Bax in eutopic endometrium from women with
endometriosis. Fertil Steril. 74:760–766. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McLaren J, Prentice A, Charnock-Jones DS,
Sharkey AM and Smith SK: Immunolocalization of the apoptosis
regulating proteins Bcl-2 and Bax in human endometrium and isolated
peritoneal fluid macrophages in endometriosis. Hum Reprod.
12:146–152. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Depalo R, Cavallini A, Lorusso F, Bassi E,
Totaro I, Marzullo A, Bettocchi S and Selvaggi L: Apoptosis in
normal ovaries of women with and without endometriosis. Reprod
Biomed Online. 19:808–815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bulthuis EP, Adjobo-Hermans MJW, Willems P
and Koopman WJH: Mitochondrial morphofunction in mammalian cells.
Antioxid Redox Signal. 30:2066–2109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu L, Feng D, Chen G, Chen M, Zheng Q,
Song P, Ma Q, Zhu C, Wang R, Qi W, et al: Mitochondrial
outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in
mammalian cells. Nat Cell Biol. 14:177–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nagafuchi A, Shirayoshi Y, Okazaki K,
Yasuda K and Takeichi M: Transformation of cell adhesion properties
by exogenously introduced E-cadherin cDNA. Nature. 329:341–343.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saliminejad K, Edalatkhah H, Kamali K,
Memariani T, Nasiri M, Saket M and Khorram Khorshid HR: Association
of common variations of the E-cadherin with endometriosis. Gynecol
Endocrinol. 31:899–902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma T, Fu B, Yang X, Xiao Y and Pan M:
Adipose mesenchymal stem cell-derived exosomes promote cell
proliferation, migration, and inhibit cell apoptosis via
Wnt/beta-catenin signaling in cutaneous wound healing. J Cell
Biochem. 120:10847–10854. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hinck L, Nathke IS, Papkoff J and Nelson
WJ: Dynamics of cadherin/catenin complex formation: Novel protein
interactions and pathways of complex assembly. J Cell Biol.
125:1327–1340. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang X, Liu Z, Chen M, Cao Q and Huang D:
Effects of S100A6 gene silencing on the biological features of
eutopic endometrial stromal cells and betacatenin expression. Mol
Med Rep. 15:1279–1285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao J, Huang X, Xu Z, Dai J, He H, Zhu Y
and Wang H: LDHA promotes tumor metastasis by facilitating
epithelialmesenchymal transition in renal cell carcinoma. Mol Med
Rep. 16:8335–8344. 2017. View Article : Google Scholar : PubMed/NCBI
|