Introduction
Multiple myeloma (MM), a hematopoietic malignancy,
is caused by the malignant expansion of plasma cells in the bone
marrow (1,2). It is the second most diagnosed
hematological malignancy in China with an incidence rate of
~1.1/100,000, which accounted for 2.1% of all new cancer cases in
2017 (2). Clinical manifestations
of MM include bone fractures, hypercalcemia, renal impairment and
anemia (3,4). MM involves numerous mechanisms
associated with molecules and cells, such as chromosomal
abnormality, epigenetic alteration, mutations and the imbalance of
stromal cells in the microenvironment of the bone marrow (5). According to statistics, the survival
time of patients with MM ranges from several months to >10 years
(6). Despite the fact that
significant progress has been achieved with conventional
chemotherapy, nanomedicine and stem cell transplantation (7,8), the
survival rate of patients with MM remains poor (3,9).
Therefore, it is imperative to identify the underlying molecular
mechanisms of MM and develop innovative approaches for MM
therapy.
Long non-coding (lnc)RNAs, consisting of >200
base pairs, are a set of RNA transcripts that do not encode
proteins (10,11). Previous studies have revealed that
lncRNAs exert pivotal roles in the progression of MM (12,13).
Over the past few years, 176 lncRNAs have been identified as
biomarkers for MM prognosis (14,15).
For example, Liu et al (12)
reported that silencing lncRNA metastasis associated lung
adenocarcinoma transcript 1 repressed cell viability and invasion
in MM cells. Moreover, Wang et al (13) observed that the upregulation of
lncRNA OIP5-antisense RNA (AS) 1 suppressed the viability,
migration and invasion of MM cells, as well as inhibited MM
tumorigenesis in vivo. As an anti-differentiation ncRNA,
differentiation antagonizing non-protein coding RNA (DANCR) has
been implicated in the development of multiple cancer types
(16,17). For example, silencing of lncRNA
DANCR has been shown to suppress the proliferative, migratory and
invasive abilities of cells in cervical cancer (16). Furthermore, knockdown of DANCR
represses the proliferative, migratory and invasive abilities of
osteosarcoma cells (17).
Importantly, a previous study has reported the downregulation of
DANCR expression in blood samples of patients with MM compared with
blood samples from healthy controls (18). Nevertheless, the precise function
and mechanism of lncRNA DANCR it yet to be elucidated in MM.
Previous studies have shown that lncRNAs regulate
the translation of mRNAs by sponging microRNAs (miRNAs/miRs)
(19). miRNAs are a genre of
single-stranded RNA molecules with a length of 20–23 nucleotides
that affect the stability and translation of their target mRNAs
(20,21). It has been revealed that miRNAs
participate in MM development (22). For example, Liu et al
(23) reported that the inhibitory
effect of circular RNA SMARCA5 on cell activity was partially
abolished by miR-767-5p in MM. Kong et al (24) also observed that decreasing
miR-17-5p inhibited the proliferation and colony formation of MM
cells, and repressed tumor growth in mouse models of MM. Moreover,
miR-135b is upregulated and associated with MM (25,26).
Hao et al (25) revealed
that miR-135b was upregulated in the serum of patients with MM and
was essential for the prediction of MM prognosis. Furthermore, Xu
et al (26) reported that
miR-135b was notably upregulated in human mesenchymal stem cells
from patients with MM (MM-hMSCs), and repression of miR-135b
facilitated osteogenic differentiation in MM-hMSCs. However, the
regulatory mechanism between lncRNA DANCR and miR-135b-5p in MM
remains largely unknown.
Krüppel-like factor 9 (KLF9) is a basic
transcription element-binding protein (27,28).
An increasing number of studies have shown that KLF9 exerts
regulatory roles in the pathogenesis of several cancer types
(29,30). Zhong et al (29) found that KLF9 decreased the
proliferative, migratory and invasive abilities of tumor cells in
pancreatic cancer. Moreover, Kong et al (30) demonstrated that KLF9 partially
reversed the promoting effect of miR-141 on cell invasion and
proliferation in non-small cell lung cancer (NSCLC). Notably,
Mannava et al (31) revealed
that KLF9 was a crucial regulator of drug-induced apoptosis in MM
cells. However, to the best of our knowledge, the regulatory impact
of miR-135b-5p on KLF9 has not been examined in MM.
Therefore, the current study focused on
investigating the expression levels and functions of lncRNA DANCR
and miR-135b-5p in MM cells, and the relationship between lncRNA
DANCR, miR-135b-5p and KLF9 in MM cells was further examined. Thus,
the findings from this study may lay a foundation for the molecular
therapy of MM.
Materials and methods
Collection of clinical samples
Serum samples (10 ml) were obtained from patients
with MM (n=55; age range, 49–86 years old; 29 female patients and
26 male patients) and healthy donors (n=40) at the Anhui No. 2
Provincial People's Hospital (Hefei, China) between March 2017 and
January 2020. The inclusion criteria were as follows: i) The
patients were diagnosed with MM by pathological examinations, which
were in accordance with the updated 2014 International Myeloma
Working Group criteria for MM diagnosis (32); and ii) the clinical data for MM were
complete. The exclusion criteria included patients who had received
chemotherapy before the operation. The patients were selected
according to the aforementioned conditions with an eventual sample
size of 55. In addition, all MM patients were divided into a low
group (n=27) and high group (n=28), according to the median
expression level of lncRNA DANCR (0.491), as shown in Table I. This study was approved by the
Ethics Committee of Anhui No. 2 Provincial People's Hospital
(approval no. 2020-16-27), and adhered to the guidelines of the
Declaration of Helsinki. Written informed consent was obtained from
all participants.
 | Table I.Clinical parameters of the patients
with multiple myeloma who were included in this study. |
Table I.
Clinical parameters of the patients
with multiple myeloma who were included in this study.
|
|
| DANCR
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Total | Low (n=27) | High (n=28) | P-value |
|---|
| Age, years |
|
|
| 0.882 |
|
<60 | 30 | 15 | 15 |
|
|
≥60 | 25 | 12 | 13 |
|
| Sex |
|
|
| 0.504 |
|
Male | 26 | 14 | 12 |
|
|
Female | 29 | 13 | 26 |
|
| WBC,
×109/l |
|
|
| 0.139 |
|
<5.40 | 27 | 16 | 11 |
|
|
≥5.40 | 28 | 11 | 17 |
|
| ISS stage |
|
|
| 0.002a |
| I | 13 | 1 | 12 |
|
| II | 20 | 11 | 9 |
|
|
III | 22 | 15 | 7 |
|
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from serum samples and cells
using TRIzol® reagent (Beyotime Institute of
Biotechnology). Total RNA was quantified at 260 nm with a
spectrophotometer, and the purity was evaluated using the ratio of
readings at 260 and 280 nm. A PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd.) was used to synthesize cDNA at 42°C for 45
min. RT-qPCR was conducted using a SYBR ExScript qRT-PCR kit
(Takara Biotechnology Co., Ltd.). The reaction procedures were as
follows: Initial denaturation at 95°C for 10 min; followed by 38
cycles of 95°C for 30 sec, annealing at 58°C for 30 sec, elongation
at 72°C for 1 min; and a final extension at 72°C for 5 min. Primer
sequences acquired from Sangon Biotech Co., Ltd., are listed in
Table II. The relative expression
levels of DANCR, miR-135b-5p and KLF9 were quantified according to
the 2−ΔΔCq calculation (33) and normalized to GAPDH (for DANCR and
KLF9) or U6 (for miR-135b-5p).
 | Table II.Primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Forward | Reverse |
|---|
| DANCR |
5′-GCCACAGGAGCTAGAGCAGT-3′ |
5′-GCAGAGTATTCAGGGTAAGGGT-3′ |
| miR-135b-5p |
5′-GGTATGGCTTTTCATTCCT-3′ |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| KLF9 |
5′-TGGCTGTGGGAAAGTCTATGG-3′ |
5′-CTCGTCTGAGCGGGAG-3′ |
| GAPDH |
5′-GCATCCTGGGCTACACTG-3′ |
5′-TGGTCGTTGAGGGCAAT-3′ |
| U6 |
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
Cell culture
Human normal plasma cells (nPCs) were purchased from
Hunan Fenghui Biotechnology Co., Ltd., and MM cells (RPMI-8226,
H929, U266 and MM1S cells) were purchased from the American Type
Culture Collection. All cells were cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and penicillin-streptomycin
mixed solution (1%). Cultures were incubated at 37°C in a
humidified atmosphere with 5% CO2.
Cell transfection
The overexpression vectors of DANCR (pcDNA-DANCR),
pcDNA-KLF9 and pcDNA-negative control (NC) were provided by Hanbio
Biotechnology Co., Ltd. Short hairpin RNA (sh)-KLF9 and sh-NC were
purchased from Guangzhou RiboBio Co., Ltd. miR-135b-5p inhibitor
(5′-UCACAUAGGAAUGAAAAGCCAUA-3′), inhibitor NC
(5′-UUCAUCGUGUUAUUAGCGUUCCU-3′), miR-135b-5p mimics
(5′-UAUGGCUUUUCAUUCCUAUGUGA-3′) and miR-NC
(5′-UAUAUCGUGUUAUUAGCGUUCCU-3′) were purchased from Shanghai
GenePharma Co., Ltd. The transcripts (20 nM) were transfected into
MM cells using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Transfection was performed for 48 h at
37°C. Subsequently, 48 h after transfection, the transfected cells
were harvested to perform further experiments.
Cell proliferation assay
Cell viability was measured using the MTT assay.
Briefly, RPMI-8226 and H929 cells were seeded in 96-well plates
(5×103 cells/well). Following incubation for 24, 48, 72
and 96 h at 37°C, MTT (20 µl; Sigma-Aldrich; Merck KGaA) was
pipetted into each well and the reaction mixture was incubated at
37°C for 4 h. Thereafter, 100 µl dimethyl sulfoxide (200 µl/well;
Sigma-Aldrich; Merck KGaA) was added to dissolve the formazan. To
determine the cell viability, the optical density value was
monitored at 490 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
The 5-ethynyl-2′-deoxyuridine (EdU) assay kit (cat.
no. C10310-1; Guangzhou RiboBio Co., Ltd.) was used to measure the
number of aforementioned cells undergoing DNA replication, as the
thymidine analogue EdU is incorporated into DNA during DNA
replication. Briefly, cells were first cultured with 50 µM EdU for
2 h at 37°C, followed by fixing with 4% formaldehyde for 20 min at
room temperature, permeabilization with 0.5% Triton X-100 for 20
min and incubation with 1X Apollo reaction cocktail for 30 min at
room temperature. Following which, cell nuclei were stained with
DAPI (1 µg/ml; 10 min; Sigma-Aldrich; Merck KGaA) at room
temperature for 2 h. EdU-positive cells were determined using
fluorescence microscopy (magnification, ×400).
Cell migration and invasion
assays
The cell invasive ability was assessed using
Transwell chambers precoated with Matrigel (8-µm pore size;
Corning, Inc.) overnight at 37°C. Briefly, cells (1×105)
resuspended in serum-free medium were inoculated into the upper
chamber, accompanied by the addition of RPMI-1640 medium containing
10% FBS to the lower chamber. After incubation for 48 h at 37°C,
non-invaded cells were wiped off. Invaded cells were fixed with 4%
paraformaldehyde and stained with 0.5% crystal violet for 15 min at
37°C. Finally, the stained cells were analyzed using an inverted
light microscope (Olympus Corporation; magnification, ×400).
For the assessment of cell migration, the operation
was similar to the cell invasion assay, but the Transwell chambers
were not pre-coated with Matrigel.
Target prediction
The miRNA targets of DANCR were predicted using
starBase software version 2.0 (http://starbase.sysu.edu.cn/), and 53 targets were
predicted. Among these miRNA targets, miR-135b-5p was selected for
the following assays due to its important role in MM (25,26)
and its unknown regulatory relationship. In addition, the mRNA
targets of miR-135b-5p were also predicted using starBase software.
In total, 3,791 targets were predicted. KLF9 was selected for the
following assays due to its important role in MM (31) and its unknown relationship with
miR-135b-5p in MM.
Dual-luciferase reporter (DLR)
assay
The 3′-untranslated region (UTR) fragment of DANCR
or KLF9, including the assumed binding sites for miR-135b-5p, was
introduced into psiCHECK2 (Promega Corporation), and named as DANCR
wild-type (wt) or KLF9 wt. Similarly, the 3′-UTR portion of DANCR
or KLF9 harboring the mutated complementary sites for the
miR-135b-5p seed region were inserted into psiCHECK2 (Promega
Corporation), and named DANCR mutant (mut) or KLF9 mut.
Subsequently, the aforementioned vectors (80 ng), along with the
miR-135b-5p mimics or miR-NC (50 nM), were incubated with RPMI-8226
and H929 cells using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 48 h at 37°C. The luciferase
activity of cell lysates was examined using a DLR Assay system
(Promega Corporation). Relative luciferase activity was defined as
the ratio of firefly luciferase activity/Renilla luciferase
activity.
Western blotting
RIPA buffer (Beyotime Institute of Biotechnology)
was used to extract the protein from RPMI-8226 and H929 cells. The
BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.) was
used to measure the protein concentration. Protein samples (30 µg)
were separated via 10% SDS-PAGE, and then transferred onto PVDF
membranes. After blocking the membranes with 5% skimmed milk for 2
h at room temperature, the membranes were incubated at 4°C
overnight with primary antibodies, including anti-KLF9 (1:1,000;
cat. no. sc-12996; Santa Cruz Biotechnology, Inc.) and anti-GAPDH
(1:1,000; cat. no. ab8245; Abcam). Then, the membranes were washed
with TBS containing 0.1% Tween-20. The secondary antibody (1:2,000;
cat. no. ab205723; Abcam) was then added and the membranes were
incubated with the protein samples at 37°C for 1 h. The
immunoreactive signals were visualized using an ECL system (BD
Biosciences), and the relative protein expression level of KLF9 was
analyzed using Alpha Innotech imaging software version 3.1.2
(ProteinSimple).
Statistical analysis
All experiments were conducted in triplicate in at
least three independent experiments. Experimental data were
analyzed using SPSS 22.0 software (IBM Corp.) and are presented as
the mean ± SD. Differences between two groups were compared using
an unpaired Student's t-test. Comparisons between multiple groups
were assessed by one-way ANOVA, which was then followed by the
Tukey's post hoc test. A χ2 test was used for analysis
the data in Table I. Correlations
among DANCR, miR-135b-5p and KLF9 were assessed using Pearson
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
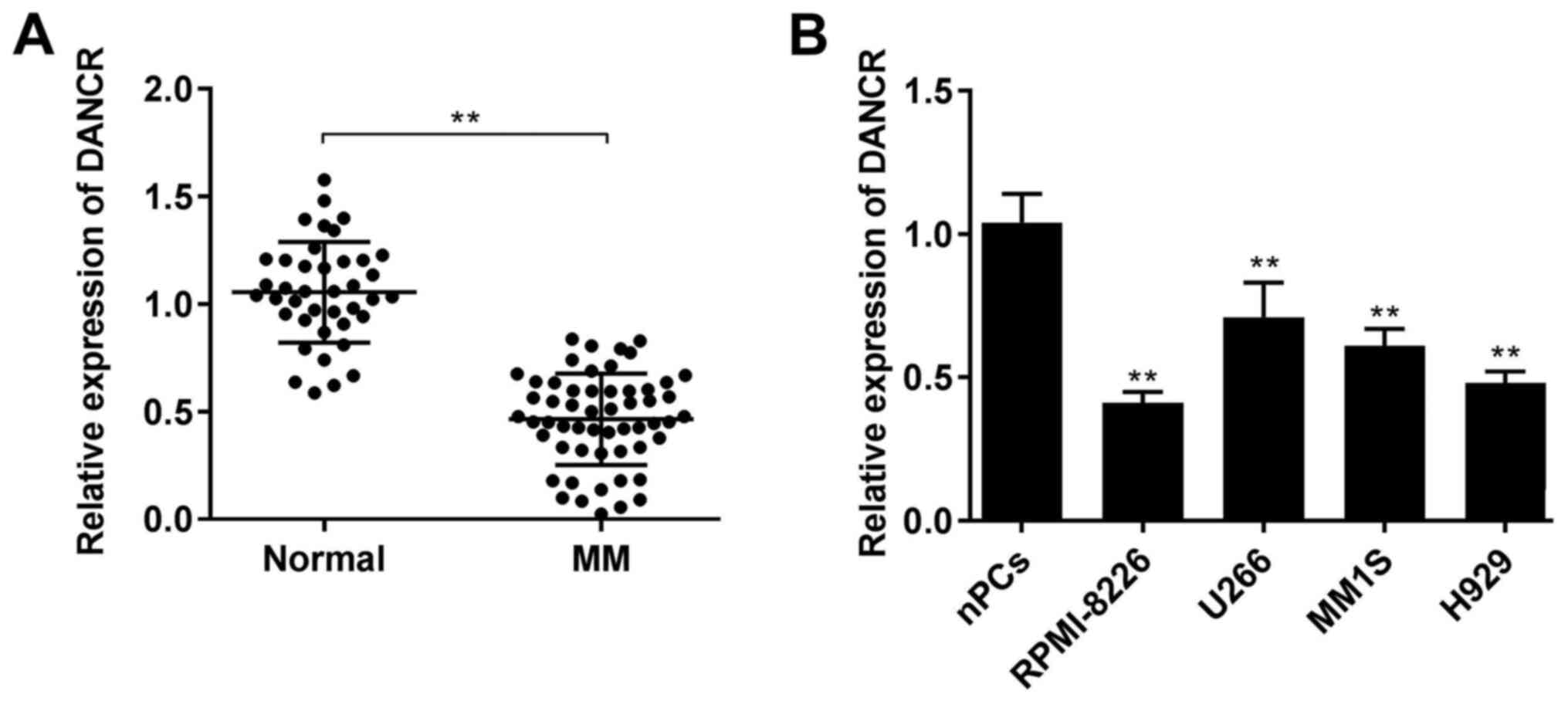
lncRNA DANCR is downregulated in the
serum of patients with MM and MM cells
The expression level of lncRNA DANCR was determined
using RT-qPCR. The relative expression level of DANCR was lower in
serum samples from patients with MM compared with that in serum
samples from healthy donors (P<0.01; Fig. 1A). Moreover, DANCR expression was
consistently decreased in the MM cell lines (RPMI-8226, U266, MM1S
and H929 cells) compared with that in the nPCs (all P<0.01;
Fig. 1B), especially in RPMI-8226
and H929 cells. Therefore, RPMI-8226 and H929 cells were chosen for
further experiments.
The association between DANCR and the
clinicopathological characteristics of MM cases was also studied,
and it was found that DANCR expression was associated with the
International Staging System (ISS) stage (P=0.002; Table I).
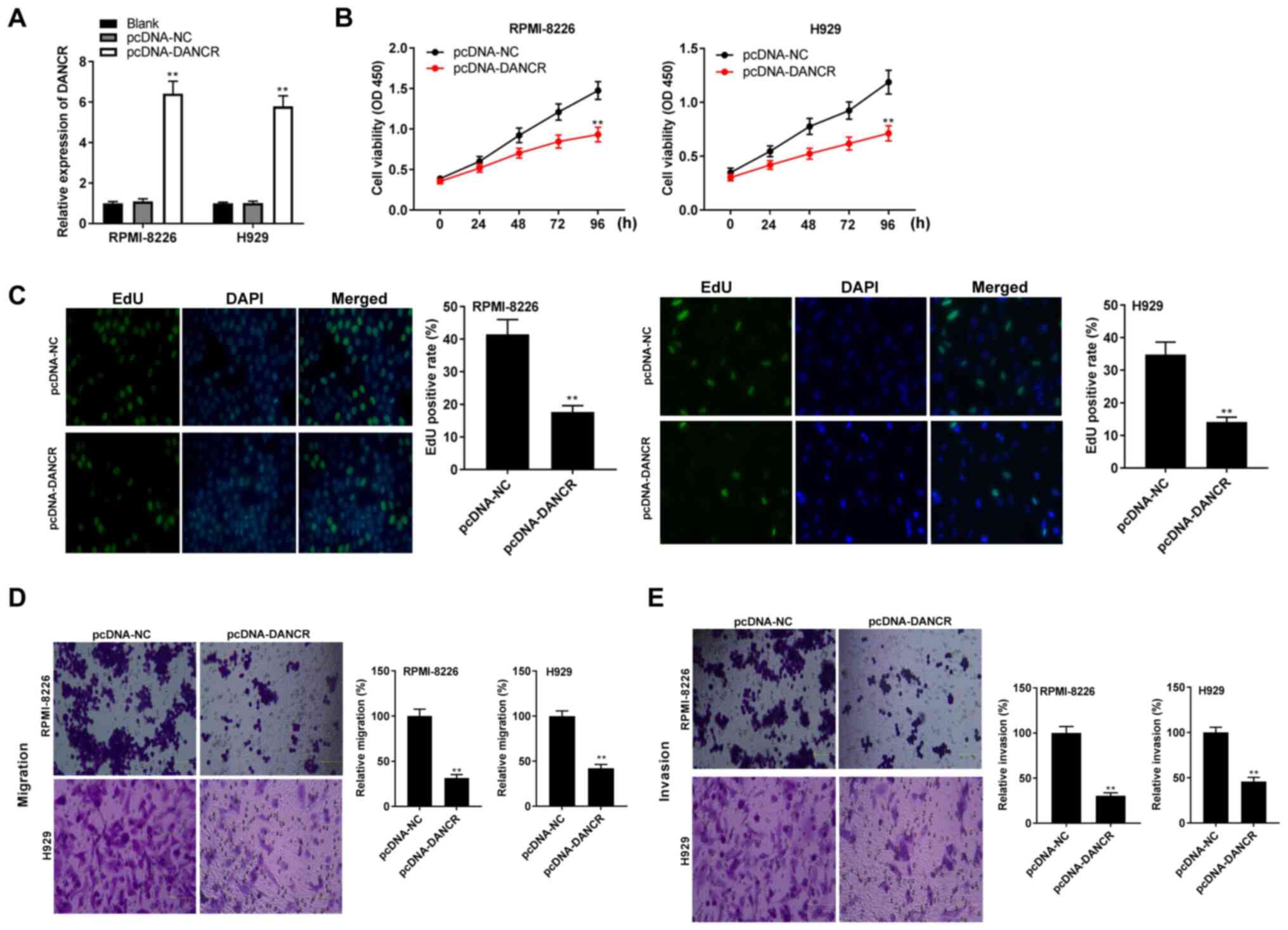
Overexpression of lncRNA DANCR
suppresses the viability, migration and invasion of RPMI-8226 and
H929 cells
To evaluate the role of lncRNA DANCR in MM cells,
DANCR was initially overexpressed by transfection with pcDNA-DANCR.
The addition of pcDNA-DANCR led to a significant increase in the
expression level of DANCR compared with the addition of pcDNA-NC in
RPMI-8226 and H929 cells (all P<0.01; Fig. 2A). Functional experiments were also
performed. The results of the MTT assay indicated that
overexpression of DANCR significantly reduced cell viability at 96
h of incubation compared with the pcDNA-NC group in RPMI-8226 and
H929 cells (all P<0.01; Fig.
2B). Moreover, the number of RPMI-8226 or H929 cells
incorporating EdU in the pcDNA-DANCR group was significantly lower
compared with that in the pcDNA-NC group (all P<0.01; Fig. 2C). The results of the Transwell
assay also demonstrated that the relative migration and invasion of
RPMI-8226 and H929 cells were decreased by overexpression of DANCR
(all P<0.01; Fig. 2D and E).
lncRNA DANCR directly binds to
miR-135b-5p
To examine the downstream mechanism of lncRNA DANCR
in RPMI-8226 and H929 cells, bioinformatics analysis was performed
using starBase and it was shown that DANCR had combinative sites
for miR-135b-5p (Fig. 3A),
indicating that miR-135b-5p was the target of DANCR. Accordingly,
the expression level of miR-135b-5p was determined and the
association between DANCR and miR-135b-5p in MM cells was
investigated. It was identified that miR-135b-5p was upregulated in
the MM cell lines compared with that in nPCs (all P<0.01;
Fig. 3B). Moreover, miR-135b-5p
expression was decreased by the overexpression of DANCR in
RPMI-8226 and H929 cells (all P<0.01; Fig. 3C). Subsequently, a DLR assay was
applied to further confirm the cooperation of DANCR and
miR-135b-5p, and it was found that the relative luciferase activity
of RPMI-8226 and H929 cells co-transfected with DANCR wt and
miR-135b-5p mimics was lower compared with that of RPMI-8226 and
H929 cells co-transfected with DANCR wt and miR-NC (all P<0.01;
Fig. 3D).
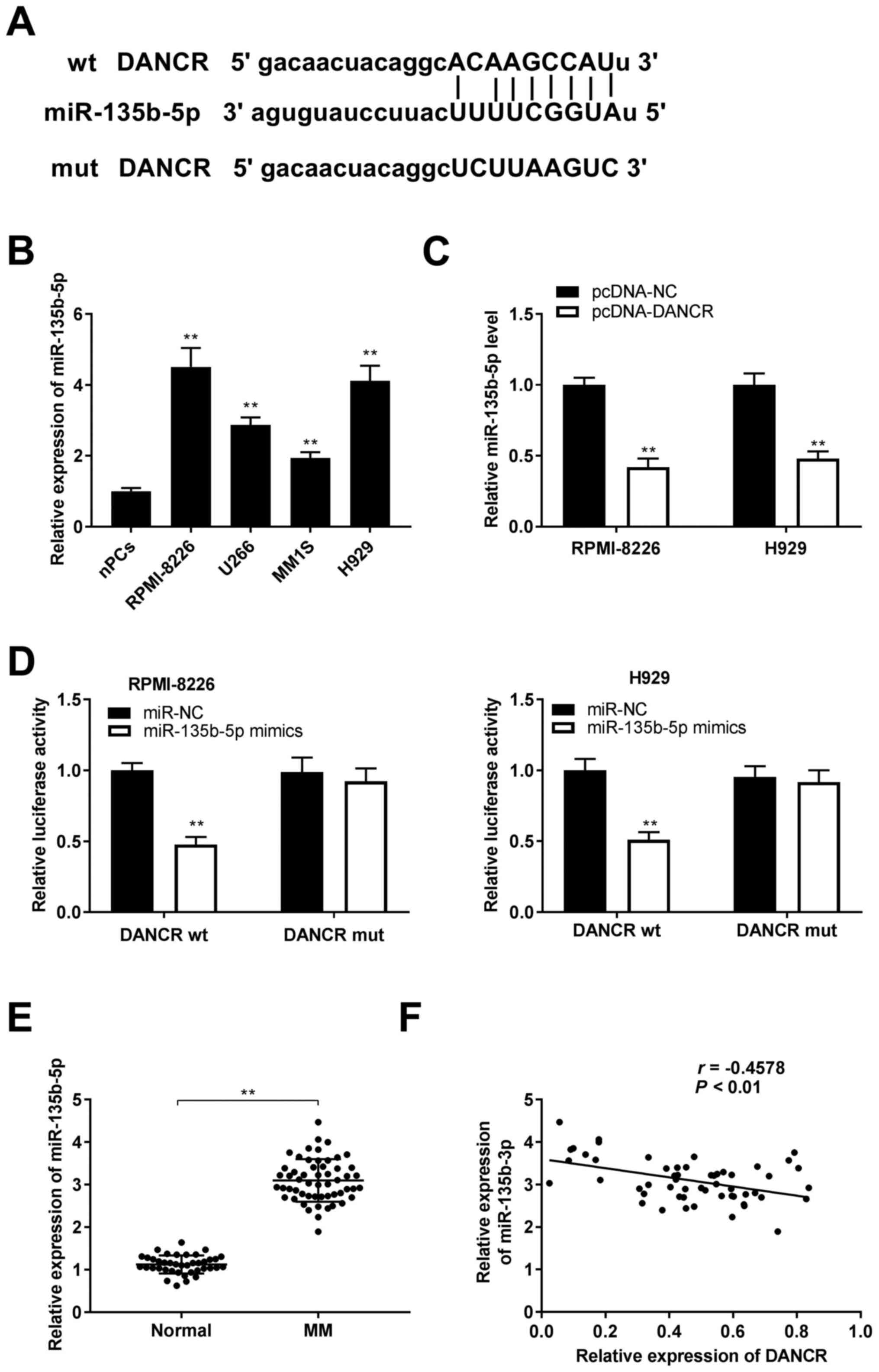 | Figure 3.DANCR directly binds to miR-135b-5p.
(A) Binding sequence between DANCR and miR-135b-5p was predicted
using starBase. (B) Relative expression level of miR-135b-5p was
detected via RT-qPCR in RPMI-8226, U266, MM1S, H929 cells and nPCs.
**P<0.01 vs. nPCs. (C) After transfection of pcDNA-DANCR and
pcDNA-NC, the relative expression level of miR-135b-5p was detected
via RT-qPCR in RPMI-8226 and H929 cells. **P<0.01 vs. pcDNA-NC.
(D) The interaction between DANCR and miR-135b-5p was confirmed
using a dual luciferase reporter assay in RPMI-8226 and H929 cells.
**P<0.01 vs. miR-NC. (E) Relative expression level of
miR-135b-5p was detected via RT-qPCR in serum from patients with MM
and healthy donors. **P<0.01 vs. Normal. (F) Correlation between
DANCR expression and miR-135b-5p expression in serum of patients
with MM was analyzed via Pearson's correlation analysis. NC,
negative control; DANCR, differentiation antagonizing non-protein
coding RNA; miR, microRNA; wt, wild-type; mut, mutant; nPCs, normal
plasma cells; MM, multiple myeloma; RT-qPCR, reverse
transcription-quantitative PCR. |
It was demonstrated that miR-135b-5p was highly
expressed in serum samples of patients with MM as opposed to serum
samples from healthy donors (P<0.01; Fig. 3E). There was also a moderate
negative correlation between miR-135b-5p expression and DANCR
expression in serum samples from patients with MM (P<0.01;
Fig. 3F).
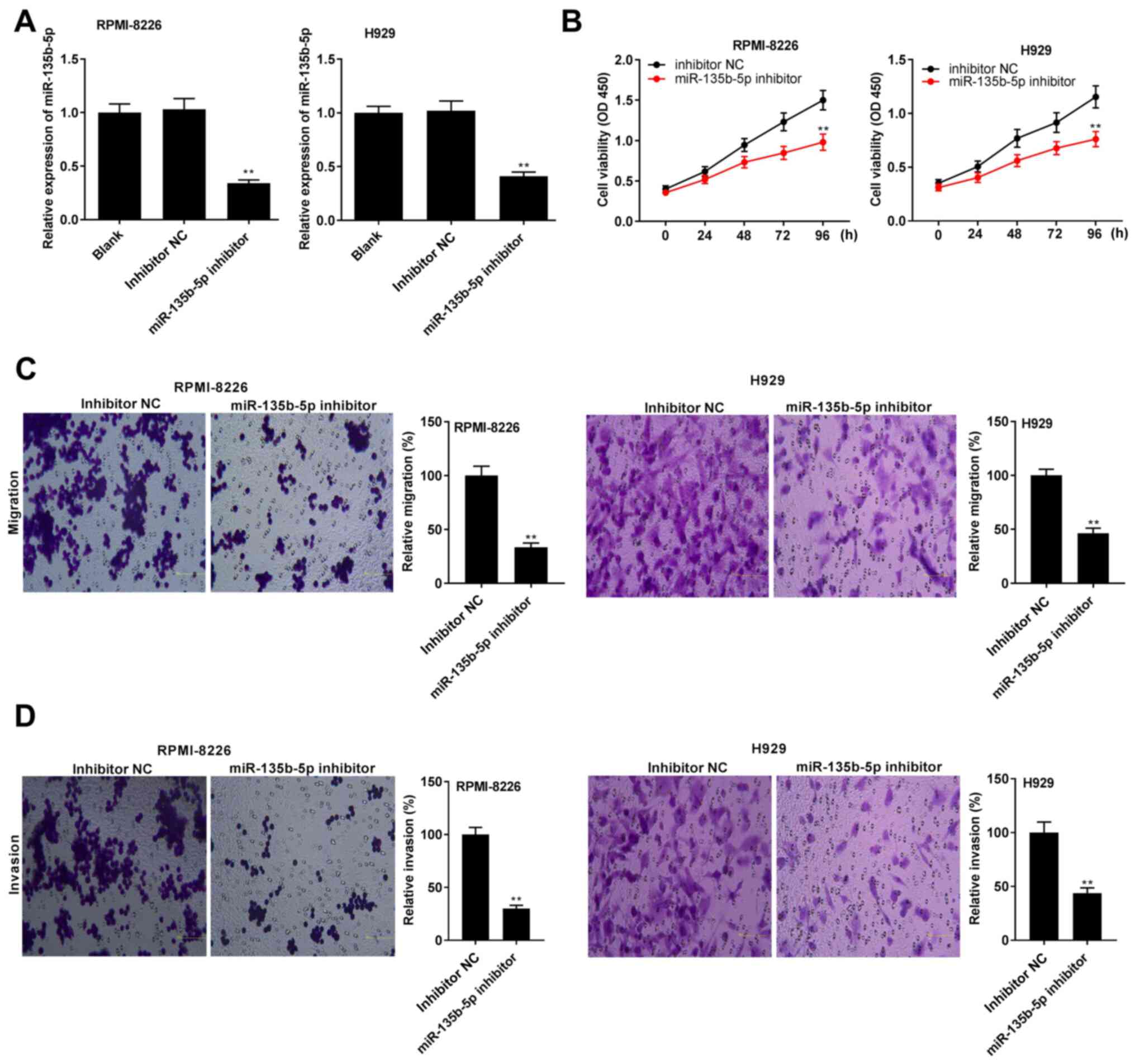
Knockdown of miR-135b-5p represses
viability, migration and invasion in RPMI-8226 and H929 cells
The expression of miR-135b-5p was knocked down by
the addition of miR-135b-5p inhibitor. The expression level of
miR-135b-5p was significantly decreased after the addition of
miR-135b-5p inhibitor in RPMI-8226 and H929 cells (all P<0.01;
Fig. 4A). The specific effects of
miR-135b-5p on RPMI-8226 and H929 cells were then studied. It was
found that the knockdown of miR-135b-5p caused a significant
decrease in the viability of RPMI-8226 and H929 cells (all
P<0.01; Fig. 4B), as well as
reduced the migratory and invasive abilities of RPMI-8226 and H929
cells (all P<0.01; Fig. 4C and
D).
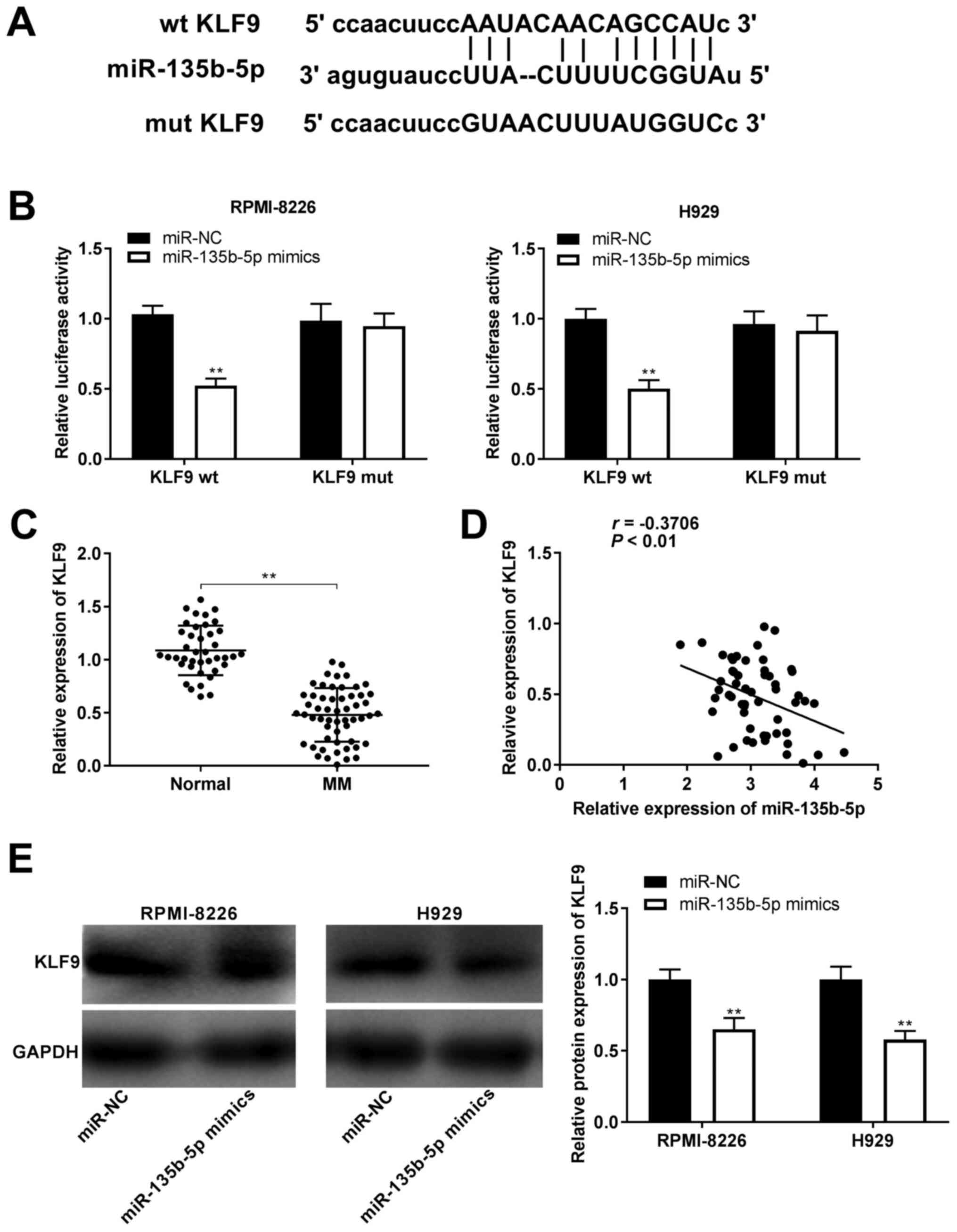
KLF9 is targeted by miR-135b-5p
To elucidate the regulatory mechanism of miR-135b-5p
in MM cells, the targets of miR-135b-5p were predicted using
starBase and it was found that there were base pairing sites
between miR-135b-5p and KLF9 (Fig.
5A). A DLR assay was also performed to corroborate the
association between miR-135b-5p and KLF9. It was demonstrated that
the addition of miR-135b-5p mimics significantly decreased the
relative luciferase activity of the KLF9 wt compared with the
addition of miR-NC (all P<0.01; Fig.
5B), but showed no significant effect on the luciferase
activity of the KLF9 mut in RPMI-8226 and H929 cells (Fig. 5B). Simultaneously, it was observed
that KLF9 expression was significantly downregulated in the MM
group compared with the normal group (P<0.01; Fig. 5C). It was found that KLF9 expression
was weakly, negatively correlated with miR-135b-5p expression in
serum samples from patients with MM (P<0.01; Fig. 5D). In addition, the regulatory
association between KLF9 and miR-135b-5p was verified via western
blotting, and it was found that the relative protein expression
level of KLF9 was downregulated by miR-135b-5p overexpression in
RPMI-8226 and H929 cells (all P<0.01; Fig. 5E).
Overexpression of KLF9 inhibits the
viability, migration and invasion in RPMI-8226 cells
The expression level of KLF9 in MM cell lines was
determined. As presented in Fig.
6A, it was found that KLF9 expression was significantly
downregulated in MM cell lines compared with that in nPCs
(P<0.01). Next, pcDNA-KLF9 or NC was transfected into RPMI-8226
cells to determine transfection efficiency. The results
demonstrated that KLF9 expression was significantly increased after
pcDNA-KLF9 transfection (P<0.01; Fig. 6B). The effects of KLF9
overexpression on cell viability, migration and invasion were also
studied. As shown in Fig. 6C-E, the
overexpression of KLF9 significantly repressed the viability,
migration and invasion of RPMI-8226 cells (P<0.01).
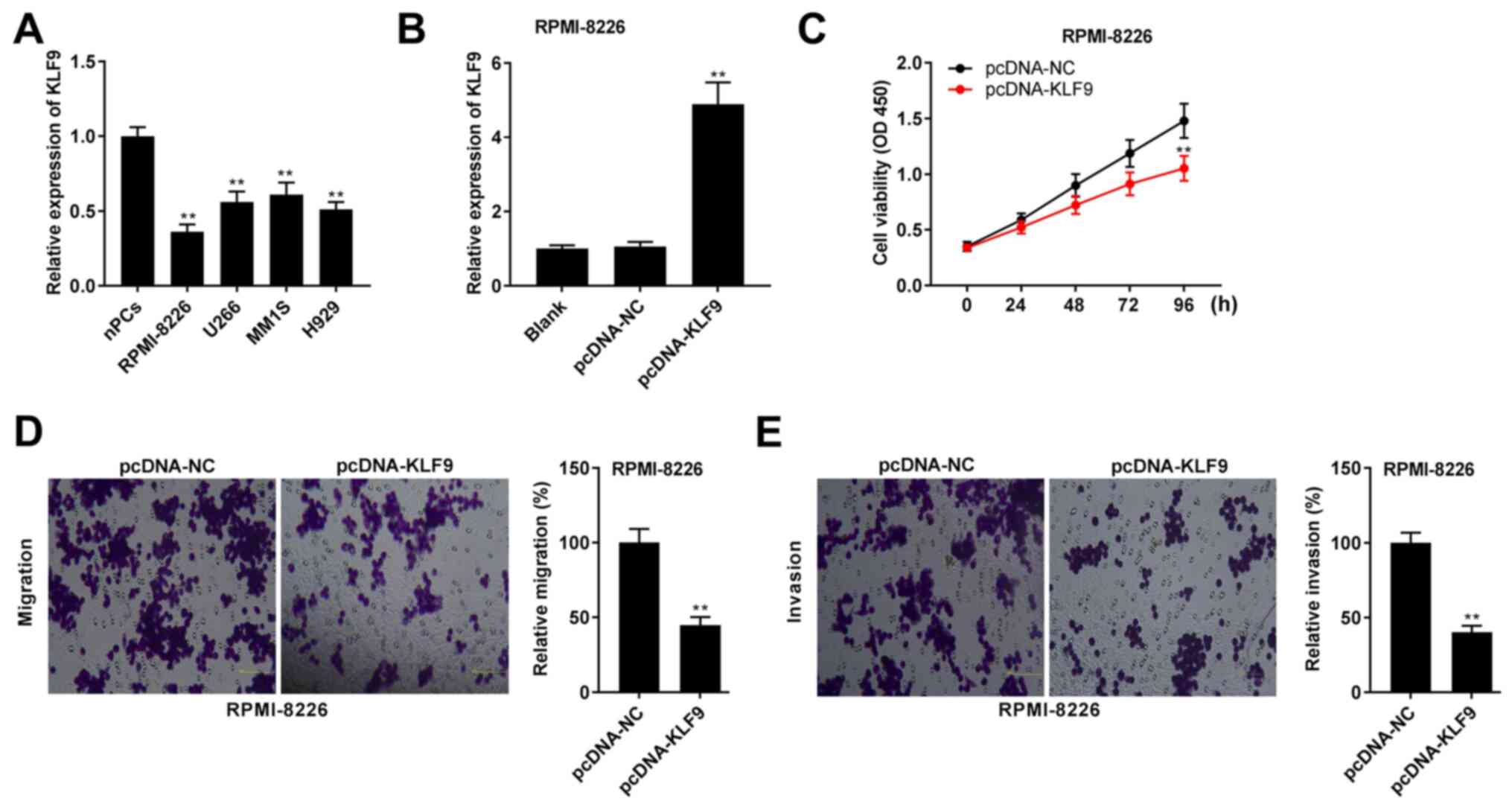 | Figure 6.Overexpression of KLF9 inhibits the
viability, migration and invasion of RPMI-8226 cells. (A) Relative
expression level of KLF9 was detected via RT-qPCR in RPMI-8226,
U266, MM1S, H929 cells and nPCs. **P<0.01 vs. nPCs. (B) After
transfection of pcDNA-KLF9 and pcDNA-NC, relative expression level
of KLF9 was detected via RT-qPCR in RPMI-8226 cells. (C) Cell
viability was determined using an MTT assay in RPMI-8226 cells. (D)
Relative migration of RPMI-8226 cells was determined using a
Transwell assay (magnification, ×400). (E) Relative invasion of
RPMI-8226 cells was determined using a Transwell assay
(magnification, ×400). **P<0.01 vs. pcDNA-NC. NC, negative
control; KLF9, Krüppel-like factor 9; nPCs, normal plasma cells;
RT-qPCR, reverse transcription-quantitative PCR; OD, optical
density. |
lncRNA DANCR represses cell viability,
migration and invasion by sponging miR-135b-5p to target KLF9 in
RPMI-8226 cells
To determine the association between lncRNA DANCR,
KLF9 and miR-135b-5p, KLF9 was silenced and miR-135b-5p was
overexpressed in the RPMI-8226 cells. As presented in Fig. 7A and B, the introduction of sh-KLF9
significantly decreased the relative expression level of KLF9, and
the introduction of miR-135b-5p mimics elevated the relative
expression level of miR-135b-5p in RPMI-8226 cells (all P<0.01).
Rescue experiments were then performed. It was demonstrated that
the viability of RPMI-8226 cells was reduced in the pcDNA-DANCR
group compared with the pcDNA-NC group, whereas this reduction in
cell viability mediated by DANCR was partially abrogated by the
overexpression of miR-135b-5p or the knockdown of KLF9 in RPMI-8226
cells (all P<0.01; Fig. 7C).
Additionally, the relative migration and invasion of RPMI-8226
cells were repressed in the pcDNA-DANCR group compared with the
pcDNA-NC group, which was partially abrogated by the overexpression
of miR-135b-5p or the knockdown of KLF9 in RPMI-8226 cells (all
P<0.01; Fig. 7D and E).
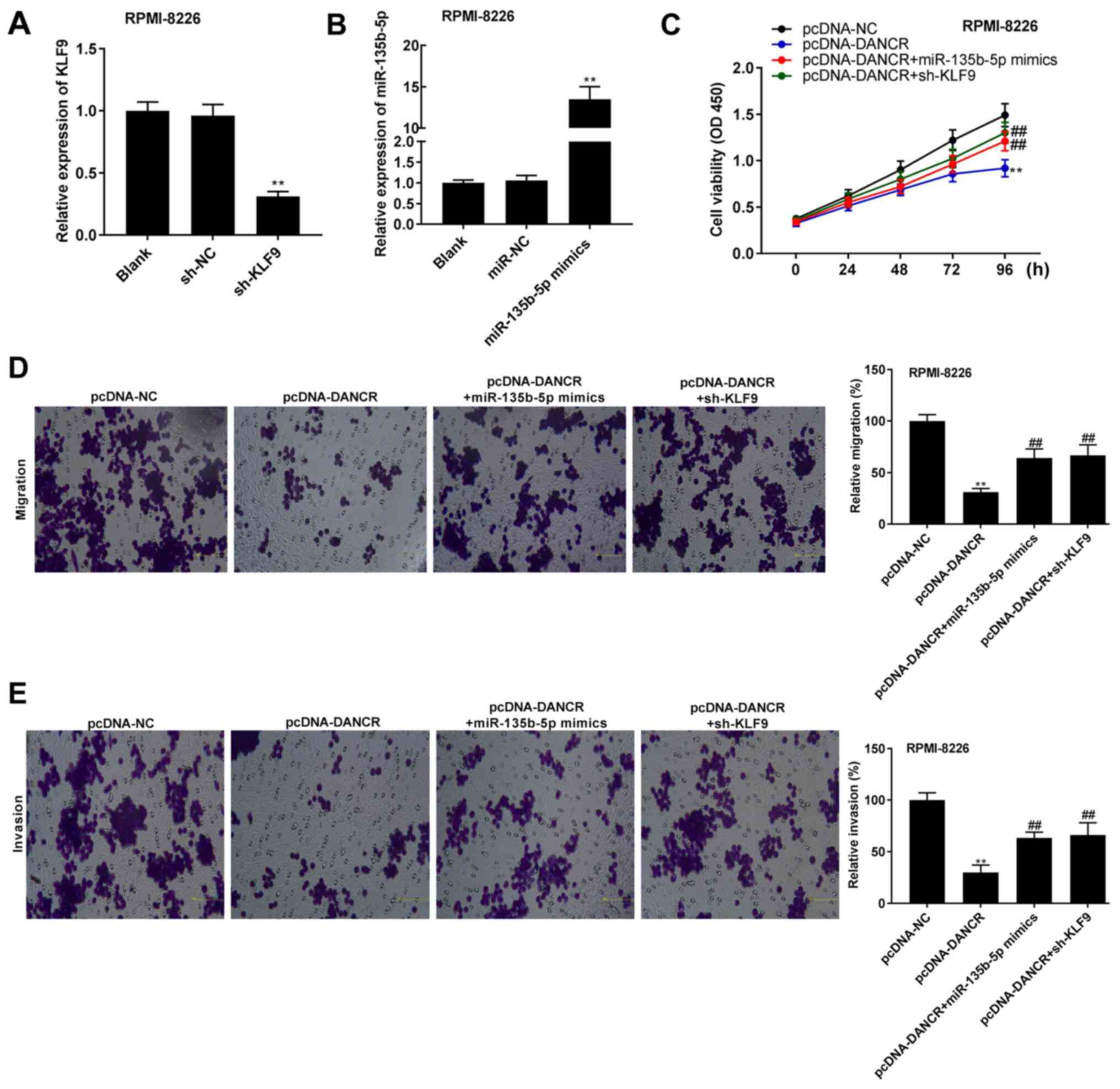 | Figure 7.lncRNA DANCR represses the viability,
migration and invasion of RPMI-8226 cells by sponging miR-135b-5p
to target KLF9. (A) After transfection of sh-KLF9 and sh-NC,
relative expression level of KLF9 was detected via RT-qPCR in
RPMI-8226 cells. **P<0.01 vs. sh-NC. (B) After transfection of
miR-135b-5p mimics and miR-NC, relative expression level of
miR-135b-5p was detected via RT-qPCR in RPMI-8226 cells.
**P<0.01 vs. miR-NC. (C) Cell viability was determined using an
MTT assay in RPMI-8226 cells. (D) Relative migration of RPMI-8226
cells was determined using a Transwell assay (magnification, ×400).
(E) Relative invasion of RPMI-8226 cells was detected using a
Transwell assay (magnification, ×400). **P<0.01 vs. pcDNA-NC;
##P<0.01 vs. pcDNA-DANCR. NC, negative control; KLF9,
Krüppel-like factor 9; sh, short hairpin RNA; DANCR,
differentiation antagonizing non-protein coding RNA; miR, microRNA;
RT-qPCR, reverse transcription-quantitative PCR. |
Discussion
MM is the second most common hematological cancer
type, with high incidence and mortality (3,34).
Given that the molecular mechanism of MM is largely unknown, the
treatment of MM remains a great challenge (35). Currently, it has been revealed that
lncRNA DANCR was abnormally expressed in various tumors and was
considered as a prognostic biomarker for cancer (36,37).
For instance, Chen et al (36) reported that DANCR expression was
distinctly higher in bladder cancer tissues compared with in normal
tissues, and its expression was associated with tumor stage, lymph
node (LN) metastasis and histological grade. Moreover, Bai et
al (37) indicated that DANCR
expression was increased in NSCLC tissues compared with normal lung
tissues, and its expression was correlated with advanced TNM stage,
larger tumor size and LN metastasis. Importantly, a previous study
by Allegra et al (18)
identified the downregulation of DANCR in serum samples of patients
with MM compared with serum samples from healthy controls. The
present study also observed that DANCR was downregulated in the
serum of patients with MM and MM cells as opposed to their
controls, which was in agreement with the results of Allegra et
al (18). Concurrently, it was
discovered that DANCR was associated with ISS, suggesting the
potential of DANCR as a molecular marker for MM diagnosis.
Several studies have suggested that lncRNA DANCR was
a critical regulator in the development of different cancer types
(38,39). For example, Jia et al
(38) revealed that lncRNA DANCR
facilitated the invasive and migratory abilities of cells in
prostate cancer. Moreover, Lu et al (39) reported that silencing DANCR resulted
in a decrease in cell proliferation, migration and invasion in lung
adenocarcinoma in vitro. Similar to previous reports, in the
present study, the overexpression of DANCR was shown to prevent
RPMI-8226 and H929 cells from migrating and invading, as well as
reduce the viability of RPMI-8226 and H929 cells. Based on the
aforementioned findings, it was suggested that lncRNA DANCR may act
as a tumor suppressor in MM.
Previous studies have shown that miR-135b was highly
expressed in patients with MM compared with healthy controls, and
miR-135b-5p has a crucial impact on the progression of several
cancer types (25,26,40).
For example, miR-135b expression is increased in the serum of
patients with MM relative to that in the serum of healthy controls
(25). miR-135b also shows distinct
upregulation in MM-hMSC (26). It
has been observed that miR-135b-5p enhances cell viability and
facilitates the invasive and migratory abilities of gastric
carcinoma cells (40). Moreover,
miR-135b-5p has been shown to facilitate the invasive and migratory
abilities of cells in pancreatic cancer (41). Similar to the aforementioned
results, the present study identified that miR-135b-5p expression
was elevated in the serum of patients with MM and MM cells as
opposed to their controls, and knockdown of miR-135b-5p reduced
cell viability and restrained RPMI-8226 and H929 cell migration and
invasion. These findings suggest that decreasing miR-135b-5p
expression repressed the progression of MM in vitro.
miR-135b-5p has been verified to be targeted by
lncRNAs in several cancer types, including lncRNA GAS8-AS1 in
papillary thyroid carcinoma (42)
and lncRNA SMAD5-AS1 in diffuse large B cell lymphoma (43). The present study demonstrated that
lncRNA DANCR could directly target miR-135b-5p, and there was a
negative correlation between DANCR and miR-135b-5p in the serum
samples of patients with MM. It was then investigated whether the
impact of DANCR on MM cells was affected by miR-135b-5p, and it was
identified that the inhibitory effects of DANCR on the viability,
invasion and migration of RPMI-8226 cells were partially reversed
by miR-135b-5p. Based on these findings, it was concluded that
DANCR served an anti-tumor role by interacting with miR-135b-5p in
MM cells.
Over the past few years, KLF9 has gained increased
attention as it is downregulated and involved in the pathological
process of multiple cancer types (29,30).
For instance, KLF9 is downregulated in pancreatic cancer tissues
and cells, reduces cell viability and suppresses the migratory and
invasive abilities of tumor cells (29). KLF9 expression was also shown to be
decreased in NSCLC tissues and cells, and partially reversed the
promoting effects of miR-141 on the proliferative and invasive
abilities of NSCLC cells (30). It
has been reported that the expression level of KLF9 was
downregulated in the serum of patients with MM and MM cells
compared with their controls, indicating that KLF9 may participate
in the pathological processes of MM. In addition, KLF9 was shown to
be a downstream target of miR-135b-5p in colorectal cancer
(44). Consistently, the present
study verified that KLF9 was a downstream target of miR-135b-5p and
was inversely correlated with miR-135b-5p in serum samples of
patients with MM, indicating that inhibition of miR-135b-5p
suppressed MM tumorigenesis by targeting KLF9 in vitro.
Moreover, the suppressive effects of lncRNA DANCR on cell
viability, migration and invasion were partially abolished by
sh-KLF9 in MM cells. Collectively, the present findings suggest
that lncRNA DANCR represses the development of MM via the
miR-135b-5p/KLF9 axis in vitro.
In summary, the present study indicated that lncRNA
DANCR was downregulated in MM tissues and cells compared with their
controls, and it repressed the viability, invasion and migration of
MM cells. Furthermore, DANCR directly targeted miR-135b-5p, which
binds to KLF9. Thus, lncRNA DANCR repressed the malignant behavior
of MM cells by sponging miR-135b-5p to target KLF9. The
DANCR/miR-135b-5p/KLF9 axis offers a neoteric perspective for MM
treatment. However, the current failed to verify the
DANCR/miR-135b-5p/KLF9 axis in MM in vivo, and further
investigation is required to confirm the current results.
Acknowledgements
Not applicable.
Funding
This study was funded by the Natural Science
Foundation of Anhui Province Universities (grant no.
KJ2018ZD020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and LXi were involved in the conception and
design, data analysis and were the major contributors in writing
the manuscript. HJ contributed to the conception of the study and
manuscript preparation. YH contributed significantly to analysis
and manuscript preparation. LL performed the data analyses and
wrote the manuscript. RX and LXu helped perform the analysis with
constructive discussions. All the authors took part in the
experiment, confirm the authenticity of all the raw data, and read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Anhui No. 2 Provincial People's Hospital (Hefei, China). Written
informed consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harding T, Baughn L, Kumar S and Van Ness
B: The future of myeloma precision medicine: Integrating the
compendium of known drug resistance mechanisms with emerging tumor
profiling technologies. Leukemia. 33:863–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar SK, Rajkumar V, Kyle RA, van Duin M,
Sonneveld P, Mateos MV, Gay F and Anderson KC: Multiple myeloma.
Nat Rev Dis Primers. 3:170462017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laubach J, Richardson P and Anderson K:
Multiple myeloma. Annu Rev Med. 62:249–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Röllig C, Knop S and Bornhäuser M:
Multiple myeloma. Lancet. 385:2197–2208. 2015. View Article : Google Scholar
|
|
5
|
Zhou W, Yang Y, Gu Z, Wang H, Xia J, Wu X,
Zhan X, Levasseur D, Zhou Y, Janz S, et al: ALDH1 activity
identifies tumor-initiating cells and links to chromosomal
instability signatures in multiple myeloma. Leukemia. 28:1155–1158.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fonseca R, Bergsagel PL, Drach J,
Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, Van Ness B, Chesi
M, Minvielle S, et al: International myeloma working group
molecular classification of multiple myeloma: Spotlight review.
Leukemia. 23:2210–2221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Detappe A, Bustoros M, Mouhieddine TH and
Ghoroghchian PP: Advancements in nanomedicine for multiple myeloma.
Trends Mol Med. 24:560–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mateos MV, Ludwig H, Bazarbachi A, Beksac
M, Bladé J, Boccadoro M, Cavo M, Delforge M, Dimopoulos MA, Facon
T, et al: Insights on multiple myeloma treatment strategies.
Hemasphere. 3:e1632018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu N, Feng S, Li H, Chen X, Bai S and Liu
Y: Long non-coding RNA MALAT1 facilitates the tumorigenesis,
invasion and glycolysis of multiple myeloma via miR-1271-5p/SOX13
axis. J Cancer Res Clin Oncol. 146:367–379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Wang H, Ruan J, Zheng W, Yang Z
and Pan W: Long non-coding RNA OIP5-AS1 suppresses multiple myeloma
progression by sponging miR-27a-3p to activate TSC1 expression.
Cancer Cell Int. 20:1552020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen Y, Feng Y, Chen H, Huang L, Wang F,
Bai J, Yang Y, Wang J, Zhao W, Jia Y, et al: Focusing on long
non-coding RNA dysregulation in newly diagnosed multiple myeloma.
Life Sci. 196:133–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu AX, Huang ZY, Zhang L and Shen J:
Potential prognostic long non-coding RNA identification and their
validation in predicting survival of patients with multiple
myeloma. Tumour Biol. 39:10104283176945632017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang H, Zhang C, Guan H, Liu J and Cui Y:
LncRNA DANCR promotes cervical cancer progression by upregulating
ROCK1 via sponging miR-335-5p. J Cell Physiol. 234:7266–7278. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan Z, Wu C, Li Y, Li H, An Y, Wang G, Dai
J and Wang Q: LncRNA DANCR silence inhibits SOX5-medicated
progression and autophagy in osteosarcoma via regulating
miR-216a-5p. Biomed Pharmacother. 122:1097072020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allegra A, Mania M, D'Ascola A, Oteri G,
Siniscalchi EN, Avenoso A, Innao V, Scuruchi M, Allegra AG,
Musolino C and Campo S: Altered long noncoding RNA expression
profile in multiple myeloma patients with bisphosphonate-induced
osteonecrosis of the Jaw. Biomed Res Int. 2020:98798762020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang G, Pian C, Chen Z, Zhang J, Xu M,
Zhang L and Chen Y: Identification of cancer-related miRNA-lncRNA
biomarkers using a basic miRNA-lncRNA network. PLoS One.
13:e01966812018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Handa H, Murakami Y, Ishihara R,
Kimura-Masuda K and Masuda Y: The role and function of microRNA in
the pathogenesis of multiple myeloma. Cancers (Basel). 11:17382019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Wu Y, Wang S, Jiang J, Zhang C,
Jiang Y, Wang X, Hong L and Huang H: Circ-SMARCA5 suppresses
progression of multiple myeloma by targeting miR-767-5p. BMC
Cancer. 19:9372019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong Y, Hu L, Lu K, Wang Y, Xie Y, Gao L,
Yang G, Xie B, He W, Chen G, et al: Ferroportin downregulation
promotes cell proliferation by modulating the Nrf2-miR-17-5p axis
in multiple myeloma. Cell Death Dis. 10:6242019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hao M, Zang M, Zhao L, Deng S, Xu Y, Qi F,
An G, Qin Y, Sui W, Li F, et al: Serum high expression of miR-214
and miR-135b as novel predictor for myeloma bone disease
development and prognosis. Oncotarget. 7:19589–19600. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu S, Cecilia Santini G, De Veirman K,
Vande Broek I, Leleu X, De Becker A, Van Camp B, Vanderkerken K and
Van Riet I: Upregulation of miR-135b is involved in the impaired
osteogenic differentiation of mesenchymal stem cells derived from
multiple myeloma patients. PLoS One. 8:e797522013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sweet DR, Fan L and Jain MK: Taking KLF9
to ‘Cort’ for crimes against metabolism. J Clin Invest.
129:2178–2180. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imataka H, Sogawa K, Yasumoto K, Kikuchi
Y, Sasano K, Kobayashi A, Hayami M and Fujii-Kuriyama Y: Two
regulatory proteins that bind to the basic transcription element
(BTE), a GC box sequence in the promoter region of the rat P-4501A1
gene. EMBO J. 11:3663–3671. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong Z, Zhou F, Wang D, Wu M, Zhou W, Zou
Y, Li J, Wu L and Yin X: Expression of KLF9 in pancreatic cancer
and its effects on the invasion, migration, apoptosis, cell cycle
distribution, and proliferation of pancreatic cancer cell lines.
Oncol Rep. 40:3852–3860. 2018.PubMed/NCBI
|
|
30
|
Kong YJ, Tan XX, Zhang Y, He QJ, Zhao L
and Meng Q: MiR-141 promotes cell proliferation and invasion in
non-small cell lung cancer by targeting KLF9. Eur Rev Med Pharmacol
Sci. 23:10370–10378. 2019.PubMed/NCBI
|
|
31
|
Mannava S, Zhuang D, Nair JR, Bansal R,
Wawrzyniak JA, Zucker SN, Fink EE, Moparthy KC, Hu Q, Liu S, et al:
KLF9 is a novel transcriptional regulator of bortezomib- and
LBH589-induced apoptosis in multiple myeloma cells. Blood.
119:1450–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
International Myeloma Working Group, :
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: A report of the
international myeloma working group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Beule N, Menu E, Bertrand MJM, Favreau
M, De Bruyne E, Maes K, De Veirman K, Radwanska M, Samali A, Magez
S, et al: Experimental African trypanosome infection suppresses the
development of multiple myeloma in mice by inducing intrinsic
apoptosis of malignant plasma cells. Oncotarget. 8:52016–52025.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Z, Chen X, Xie R, Huang M, Dong W,
Han J, Zhang J, Zhou Q, Li H, Huang J and Lin T: DANCR promotes
metastasis and proliferation in bladder cancer cells by enhancing
IL-11-STAT3 signaling and CCND1 expression. Mol Ther. 27:326–341.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai Y, Zhang G, Chu H, Li P and Li J: The
positive feedback loop of lncRNA DANCR/miR-138/Sox4 facilitates
malignancy in non-small cell lung cancer. Am J Cancer Res.
9:270–284. 2019.PubMed/NCBI
|
|
38
|
Jia J, Li F, Tang XS, Xu S, Gao Y, Shi Q,
Guo W, Wang X, He D and Guo P: Long noncoding RNA DANCR promotes
invasion of prostate cancer through epigenetically silencing
expression of TIMP2/3. Oncotarget. 7:37868–37881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu QC, Rui ZH, Guo ZL, Xie W, Shan S and
Ren T: LncRNA-DANCR contributes to lung adenocarcinoma progression
by sponging miR-496 to modulate mTOR expression. J Cell Mol Med.
22:1527–1537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Z, Gao Y, Gao S, Song D and Feng Y:
MiR-135b-5p promotes viability, proliferation, migration and
invasion of gastric cancer cells by targeting Krüppel-like factor 4
(KLF4). Arch Med Sci. 16:167–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Z, Che X, Yang N, Bai Z, Wu Y, Zhao
L and Pei H: miR-135b-5p Promotes migration, invasion and EMT of
pancreatic cancer cells by targeting NR3C2. Biomed Pharmacother.
96:1341–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen N, Yin D, Lun B and Guo X: LncRNA
GAS8-AS1 suppresses papillary thyroid carcinoma cell growth through
the miR-135b-5p/CCND2 axis. Biosci Rep. 39:BSR201814402019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao CC, Jiao Y, Zhang YY, Ning J, Zhang
YR, Xu J, Wei W and Kang-Sheng G: Lnc SMAD5-AS1 as ceRNA inhibit
proliferation of diffuse large B cell lymphoma via Wnt/β-catenin
pathway by sponging miR-135b-5p to elevate expression of APC. Cell
Death Dis. 10:2522019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Zhang Z, Yi Y, Wang Y and Fu J:
CircNOL10 acts as a sponge of miR-135a/b-5p in suppressing
colorectal cancer progression via regulating KLF9. Onco Targets
Ther. 13:5165–5176. 2020. View Article : Google Scholar : PubMed/NCBI
|