Introduction
Diabetic nephropathy (DN) is one of the leading
causes of mortality and morbidity in individuals with chronic
kidney disease, accounting for 20–30% of deaths in patients with
chronic kidney disease (1), and it
is characterized by deposition of extracellular matrix proteins,
tubulointerstitial fibrosis, glomerular sclerosis, mesangial matrix
and glomerular basement membrane expansion, as well as loss of
waste removal functions over time (2,3).
Currently, various factors are thought to be involved in the
pathogenesis of DN, including hyperglycemia, oxidative stress,
inflammatory response, activation of renin angiotensin system,
lipid metabolism disorder, accumulation of advanced glycosylation
end products, fibroblast activation, induction of transforming
growth factor β-1 (TGF-β1) expressions, pyroptosis and
depletion of adenosine triphosphate (4–7).
Although our current understanding of the mechanisms of DN has
substantially increased, the molecular mechanisms that lead to the
occurrence and development of DN remain to be elucidated.
Identification of novel therapeutic targets and strategies may
uncover renoprotective effects in DN. Since the recognition of
ferroptosis as an iron-dependent form of cell death in 2012
(8), interest in the functions of
ferroptosis are continuously increasing (9). Ferroptosis occurs through two main
types of pathway; transport dependent pathways and enzymatic
regulatory pathways (10).
Ferroptosis is dependent on intracellular iron and is primarily
caused by a redox imbalance between oxidants and antioxidants in
cells, resulting in the formation of free radicals and lipid
oxidation products (10). Studies
have demonstrated that ferroptosis is involved in the pathogenesis
of inflammation, types of cancer, neurodegeneration and tissue
injury (11,12). Emerging evidence has shown that
ferroptosis is observed in several renal diseases, including
nephrotoxic folic acid-induced acute kidney injury (13), rhabdomyolysis-induced renal damage
(14), renal ischaemia-reperfusion
injury (15), obesity-related
kidney injury (16), carbon
tetrachloride-induced renal toxicity (17) and DN (18,19).
These findings suggest that modulation of ferroptosis may function
as a future direction in the treatment of chronic or acute kidney
injury.
With the aid of computer-assisted analysis of cDNA
and genomic DNA sequence information, salusins are found to be
translated from an alternatively spliced mRNA of torsion
dystonia-related gene (TOR2A) (20). Proteolytic processing of prosalusin
(216-amino acid) yields two related peptides of 28 and 20 amino
acids, termed salusin-α and salusin-β, respectively (20). Salusins are widely expressed in
human, rat and mouse tissues, such as the central nervous system,
digestive system, vasculature and kidneys (20–22).
It is noteworthy to mention that salusin-α and salusin-β serve
critical roles in the pathogenesis of cardiovascular and metabolic
diseases (23–25). Recently, it was found that renal
salusin-α levels are lower in diabetic rats and exogenous salusin-α
markedly ameliorates the development of DN (26). Evidence is emerging that salusin-β
may serve a role in the etiologies of diabetic kidney disease. For
example, circulating levels of salusin-β tend to be higher in
diabetic patients (27–29). Patients undergoing hemodialysis who
exhibit higher serum levels of salusin-β and higher salusin-β
levels may have cardiovascular risk implications (30). Immunohistochemical analysis has
demonstrated that salusin-β is mainly expressed in epithelium cells
of the glomeruli and proximal and distal tubule cells (31). The expression of salusin-β is
increased in kidneys from rats with insulin resistance (31). These studies hint a possible role of
salusin-β in diabetes-induced renal damage. Notably, salusin-β is a
potential pro-oxidant in cardiomyocytes (32), vascular smooth muscle cells
(33), endothelial cells (34) and kidney cells (35). These studies suggest that salusin-β
may be a critical regulator in oxidative stress-related diseases.
In addition, the cellular redox system governs a pervasive
non-apoptotic form of cell death, ferroptosis (36). These results prompted the
investigation of the roles of salusin-β in diabetes-related kidney
damage and the involved downstream signaling pathways, such as
ferroptosis-related events, are also probed.
Materials and methods
Chemicals and reagents
DMEM/F-12 medium (cat. no. 12400-024), fetal bovine
serum (FBS; cat. no. 16140-071), penicillin and streptomycin
antibiotic mixture (cat. no. 15140122) and cell culture supplies
were obtained from Gibco (Thermo Fisher Scientific, Inc.).
D-glucose (cat. no. G8270), D-mannitol (cat. no. M4125),
2′,7′-dichlorofluorescin diacetate (DCFH-DA; cat. no. D6883),
4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; cat. no.
D8417), ferrostatin-1 (cat. no. SML0583), bardoxolone methyl (CDDO
Methyl Ester; cat. no. SMB00376), erastin (cat. no. E7781) and
buthionine sulfoximine (cat. no. B2515) were purchased from
Sigma-Aldrich; Merck KGaA. RSL3 (cat. no. S8155) and FIN56 (cat.
no. S8254) were obtained from Selleck Chemicals. Antibody against
salusin-β (cat. no. PAC026Hu01) and enzyme linked immunosorbent
assay (ELISA) kits for salusin-β (cat. no. CEC026Hu) measurement
were obtained Wuhan USCN Business Co., Ltd. Click-iT Plus EdU
(5-ethynyl-2′-deoxyuridine) Alexa Fluor 594 imaging kits were
procured from Thermo Fisher Scientific, Inc. (cat. no. C10639).
Goat Anti-Rabbit IgG H&L (Alexa Fluor 488; cat. no. ab150077)
was purchased from Abcam. The specific primers used in the present
study were synthesized by Santa Cruz Biotechnology, Inc. Control
short interfering (si)RNA (cat. no. sc-44239) and nuclear
factor-erythroid-2-related factor 2 (Nrf-2) siRNA cat. no.
(sc-37030) were bought from Santa Cruz Biotechnology, Inc.
Lentivirus particles carrying salusin-β short hairpin (sh)RNA or
negative control shRNA were produced by Jiman Biotechnology
(Shanghai) Co., Ltd., as previously described (35,37).
Lentivirus empty vectors or vectors expressing salusin-β were
constructed by Jiman Biotechnology (Shanghai) Co., Ltd as
previously described (33,35,38).
Antibodies against β-actin (cat. no. 66009-1-Ig or cat. no.
20536-1-AP) and HRP-conjugated secondary antibodies (cat. nos.
SA00001-1 and SA00001-2) were obtained from ProteinTech Group, Inc.
Antibodies against glutathione peroxidase 4 (GPX4; cat. no.
52455), solute carrier family 7 (cationic amino acid transporter,
y+ system) member 11 (SLC7A11; cat. no. 12691), ferritin
heavy polypeptide 1 (FTH-1; cat. no. 4393), transferrin
receptor 1 (TFR-1; cat. no. 13113) and Nrf-2 (cat.
no. 12721) were purchased from Cell Signaling Technology, Inc. Cell
Counting Kit-8 (CCK-8; cat. no. G021-1-1), lactate dehydrogenase
(LDH) release kits (cat. no. A020-2-2), malondialdehyde (MDA) assay
kit (cat. no. A003-1-2) and reduced glutathione (GSH) assay kits
(cat. no. A006-2-1) were purchased from Nanjing Jiancheng
Bioengineering Institute. An iron assay kit (cat. no. ab83366) was
from Abcam. The selected concentrations of chemicals were in accord
with previously published papers (19,39,40).
Cell culture
Human proximal tubular (HK-2) cells were procured
from the American Type Culture Collection (cat. no. CRL-2190). HK-2
cells were cultured in DMEM/F-12 medium contained 10% FBS,
streptomycin (100 mg/ml) and penicillin (100 U/ml) under a
humidified atmosphere of 5% CO2 at 37°C. When HK-2 cells
reached ~70-80% confluence, these cells were challenged by 30 mM
glucose (Sigma-Aldrich; Merck KGaA) at different time points.
D-mannitol was used as a hyperosmolar control in the current study
(5.5 mM D-glucose plus 24.5 mM D-mannitol) (41). In some experiments, HK-2 cells were
pretreated with bardoxolone methyl (100 nM), or Ferrostatin-1 (1
µM) for 30 min before high glucose (HG) stimulation.
Western blotting
After treatment, HK-2 cells were washed with
ice-cold PBS and lysed in RIPA lysis buffer (20 mM Tris at pH 7.5,
150 mM NaCl, 1 mM EDTA and 2% Triton X-100) supplemented with
protease inhibitor cocktail (Roche Diagnostics) and the protein
contents were determined by using BCA colorimetric protein kit
(P0012; Beyotime Institute of Biotechnology). The sodium dodecyl
sulfate-polyacrylamide gel electrophoresis loading buffer was added
into cell lysates and boiled for 5 min. Samples and pre-stained
markers were added as required. Equal amount of cell lysates (30
µg) was separated onto SDS-PAGE gels (8–12%) and transferred onto
polyvinylidene fluoride membranes. Membranes were sealed with 5%
skimmed milk powder for 1 h at room temperature, followed by
incubation with primary antibodies at 4°C overnight. The following
primary antibodies were used: Salusin-β (1:500; cat. no.
PAC026Hu01; Wuhan USCN Business Co., Ltd), β-actin (1:1,000;
cat. no. 66009-1-Ig; ProteinTech Group, Inc.), GPX4
(1:1,000; cat. no. 52455; Cell Signaling Technology, Inc.),
SLC7A11 (1:1,000; cat. no. 12691; Cell Signaling Technology,
Inc.), FTH-1 (1:1,000; cat. no. 4393; Cell Signaling
Technology, Inc.), TFR-1 (1:1,000; cat. no. 13113; Cell
Signaling Technology, Inc.) and Nrf-2 (1:1,000; cat. no.
12721; Cell Signaling Technology, Inc.). Membranes were then
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:5,000; cat. nos. SA00001-1 and SA00001-2; ProteinTech
Group, Inc.) for 1 h at room temperature. Immunodetection was
conducted using an ECL system (Fusion FX7 imaging system; Vilber
China). Protein expression was semi-quantified using Image Lab
software (version 5.2.1; Bio-Rad Laboratories, Inc.).
Immunofluorescence staining
HK-2 cells were fixed with 4% paraformaldehyde for
20 min at room temperature. The fixed cells were then permeabilized
with 0.1% Triton X-100 for 10 min at room temperature and 5% goat
serum (cat. no. C0265; Beyotime Institute of Biotechnology) for 1
h. The expressions of salusin-β in HK-2 cells were detected by
incubation of primary antibody against salusin-β at 4°C overnight.
Afterwards, these cells were incubated with Goat Anti-Rabbit IgG
H&L (Alexa Fluor 488) for 1 h at 37°C and the nucleus was
stained by DAPI for 10 min at room temperature. The positive
immunofluorescence images were captured by a fluorescence
microscopy (Olympus BX6 with DP72 camera; Olympus Corporation;
magnification, ×200). A total of six visual fields in each group
were randomly selected in each group.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated from cells (2×105)
by TRIzol® reagent (cat. no. 9108; Thermo Fisher
Scientific, Inc.). All steps were performed according to the
manufacturer's protocols. The purity and concentration of RNA were
detected using NanoDrop (Thermo Fisher Scientific, Inc.). The first
cDNA synthesis was conducted using GoScript Reverse Transcription
System (Promega Corporation) in the presence of 0.5 g of total RNA
in each sample. Following the manufacturer's instructions, RT-qPCR
was carried out using the qPCR SYBR-Green Master Mix (cat. no.
11119ES60; Shanghai Yeasen Biotechnology Co., Ltd.) under the
Applied Biosystems 7500 Real Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 120
sec, followed by 40 amplification cycles at 95°C for 15 sec, and
annealing and extension at 60°C for 20 sec. The relative expression
levels were calculated based on the 2−ΔΔCq method
(42). These experiments were
repeated three independent times. The primer sequences used for
RT-qPCR are listed in Table
SI.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of salusin-β in cellular lysate were
analyzed through the commercial ELISA kits according to the
instructions as previously described (43,44).
Briefly, the samples and diluted standards (50 µl) were added into
the appropriate wells, respectively. 50 µl of Detection Reagent A
was added into each well and incubated for 1 h at 37°C. After
washing three times with 1X Wash Solution, 100 µl of Detection
Reagent B working solution was added into each well and incubated
for 30 min at 37°C. Substrate Solution and Stop Solution were
subsequently added into each well and the liquid turned yellow. The
measurement of optical density was performed using a microplate
reader (SYNERGY H4; BioTek Instruments, Inc.) at 450 nm
immediately. The levels of salusin-β were expressed as pg per mg
protein by normalizing to the protein contents in each sample.
Determination of cell viability
Cell viability was evaluated using the CCK-8
according to the manufacturer's instructions. In brief, HK-2 cells
were seeded onto 96-well plates (1×104 cells/well) and
treated with normal glucose (NG) and high glucose (HG),
respectively. The culture medium was replaced with 100 µl fresh
medium supplemented with 10 µl of the CCK-8 solution in each well.
After incubating for 2 h at 37°C, measurement of the absorbance at
450 nm was carried out by using a microplate reader (SYNERGY H4;
BioTek Instruments, Inc.). In addition, Click-iT Plus EdU
(5-ethynyl-2′-deoxyuridine) Alexa Fluor™ 594 imaging kits were used
for measurement of HK-2 cell proliferation according to the
manufacturer's protocols. The red and blue fluorescence signals
were imaged by a fluorescence microscopy (Olympus BX6 with DP72
camera; Olympus Corporation; magnification, ×200). A total of 6
visual fields were randomly selected in each group and the average
number of positive cells was calculated.
Cell transfection
HK-2 cells in logarithmic growth were transduced
with lentivirus particles carrying salusin-β shRNA or negative
control shRNA (109 titer/ml; MOI=50, 50 µl lentivirus
particles per 1×106 cells) for 48 h at 37°C and then
challenged by HG stimulation for another 48 h at 37°C. For
overexpression of salusin-β in HK-2 cells, HK-2 cells were
transduced with lentivirus expressing salusin-β vectors or empty
vectors (1010 titer/ml; MOI=50, 50 µl lentivirus
particles per 1×106 cells) for 48 h at 37°C before
administration of NG or HG for 48 h. After that, cells were
harvested for biochemical testing at the desired time points. For
Nrf-2 knockdown, HK-2 cells were plated the day before siRNA
transfection. A non-targeting control siRNA (50 nM) and
Nrf-2 siRNA (50 nM) were transduced to HK-2 cells for 48 h
at 37°C by using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's instructions.
The medium was changed after 4–6 h of transfection. Subsequent
experiments were performed at 48 h post-transfection.
Iron assay
Total iron levels in cell lysates were quantified by
an iron assay kit (ab83366, Abcam). Briefly, the collected HK-2
cells were lysed in the iron assay buffer and the supernatants were
obtained by centrifugation at 16,000 × g for 10 min at 4°C. A total
of 5 ml of iron reducing agent was mixed into cell samples (50 ml)
for total iron analysis. Next, the iron probe solution (100 ml) was
added and incubated for 1 h at 25°C under dark conditions. Later,
the absorbance at the wavelength of 593 nm was detected by
spectrophotometry.
Measurement of MDA contents
The collected cells were homogenized by the MDA
lysis buffer and the supernatants were collected after
centrifugation at 13,800 × g for 10 min at 4°C. The MDA
concentrations in cell lysates were assessed using a lipid
peroxidation assay kit according to the manufacturer's
instructions. In short, 200 µl of the supernatant from each
homogenized sample was placed into a tube, followed by incubation
of the thiobarbituric acid solution (200 µl) for 40 min at 95°C.
These samples were cooled to room temperature and centrifuged
(1,500 × g for 10 min) to remove insoluble sediment. The
supernatants were collected and the absorbance was measured at 532
nm using a microplate reader (SYNERGY H4; BioTek Instruments,
Inc.).
Measurement of GSH levels
HK-2 cells were washed with ice-cold PBS and
homogenized by vibrating homogenizer with cold PBS and the
supernatants were collected after centrifugation at 13,800 × g for
10 min at 4°C. GSH assay mixture (125 µl) was added into each well
for 5 min at room temperature and the optical density was
determined at the wavelength of 405 nm. The concentrations of GSH
were then quantified by comparing the optical density of the
samples to the standard curve, respectively.
Measurement of reactive oxygen species
(ROS)
Intracellular ROS levels in HK-2 cells were
evaluated by dichloro-dihydro-fluorescein diacetate (DCFH-DA)
staining as previously described (45,46).
HK-2 cells were initially cultured in 6-well plates for 48 h and
DCFH- DA fluorescent dye (10 µM) was added and incubated for 20 min
at 37°C in the dark and then were washed with PBS. The fluorescence
intensity was measured by a fluorescence microscope (Olympus BX6
with DP72 camera; Olympus Corporation) with an excitation
wavelength at 488 nm and an emission wavelength at 525 nm. The
images were observed and captured in 10 randomly selected fields of
view from 4–6 repeated experiments in 1–2 randomly selected fields
of view (magnification, ×200).
LDH release assay
LDH contents were measured using LDH cytotoxicity
assay kits as previously reported (47). The standards and culture
supernatants were obtained and added into the appropriate wells (20
µl), matrix buffer (25 µl) and coenzyme I (5 µl) were sequentially
added. The liquid system was incubated for 15 min at 37°C. Later,
2,4,-Dinitrophenylhydrazine (25 µl) was added and incubated for 15
min at 37°C. Sodium hydroxide solution (0.4 M, 250 µl) were then
added and incubated for 5 min at room temperature. The reactions
were read at 450 nm by using a microtiter plate reader (SYNERGY H4;
BioTek Instruments, Inc.). Mean LDH release of control cells was
indexed to 100%, the value used for normalization.
Statistical analysis
Results were expressed as mean ± standard deviation.
All results were analyzed by GraphPad Prism 6.0 (GraphPad Software,
Inc.). All data were tested for normality and equal variance. If
the data passed these tests, comparisons between two groups were
made by unpaired Student's t tests. One way ANOVA was used for
multiple-group comparisons. When ANOVA was significant, post hoc
testing of differences between groups was conducted by using
Bonferroni tests. The independent experiments were repeated at
least four times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of salusin-β in
HG-incubated HK-2 cells
To test whether HG altered salusin-β expression in
HK-2 cells, HK-2 cells were treated with increasing doses of
glucose at various time points. Upon HG treatment (25 mM), the
protein expression of salusin-β was upregulated in HK-2 cells and
this was further increased with increasing glucose concentrations,
reaching its maximal effect at 30 mM (Fig. 1A and B). Thus, the dose of glucose
was selected as 30 mM for the following experiments. Time-course
experiments showed that the protein expression of salusin-β was
increased slightly after HG treatment for 24 h (Fig. 1C and D). However, the protein
expression of salusin-β was markedly augmented in HK-2 cells
exposed to HG for 48 and 72 h (Fig. 1C
and D). Accordingly, HG treatment for 48 h was chosen as a
model of cell damage during the subsequent experiments.
Furthermore, HG-induced upregulation of salusin-β in HK-2 cells was
confirmed by immunofluorescence (Fig.
1E), RT-qPCR (Fig. 1F) and
ELISA results (Fig. 1G),
respectively. To study the effects of osmolality on the expression
of salusin-β, D-mannitol was used as a hyperosmolar control (5.5 mM
D-glucose plus 24.5 mM D-mannitol) (41,48).
Notably, the protein and mRNA expressions of salusin-β were
markedly incremented in HG-incubated HK-2 cells, but not in
hyperosmolar D-mannitol-treated cells (Fig. S1A-C). These results suggested that
aberrant expressions of salusin-β in HK-2 cells were an effect of
glucotoxicity, rather than from hyperglycemia-induced
hyperosmolarity.
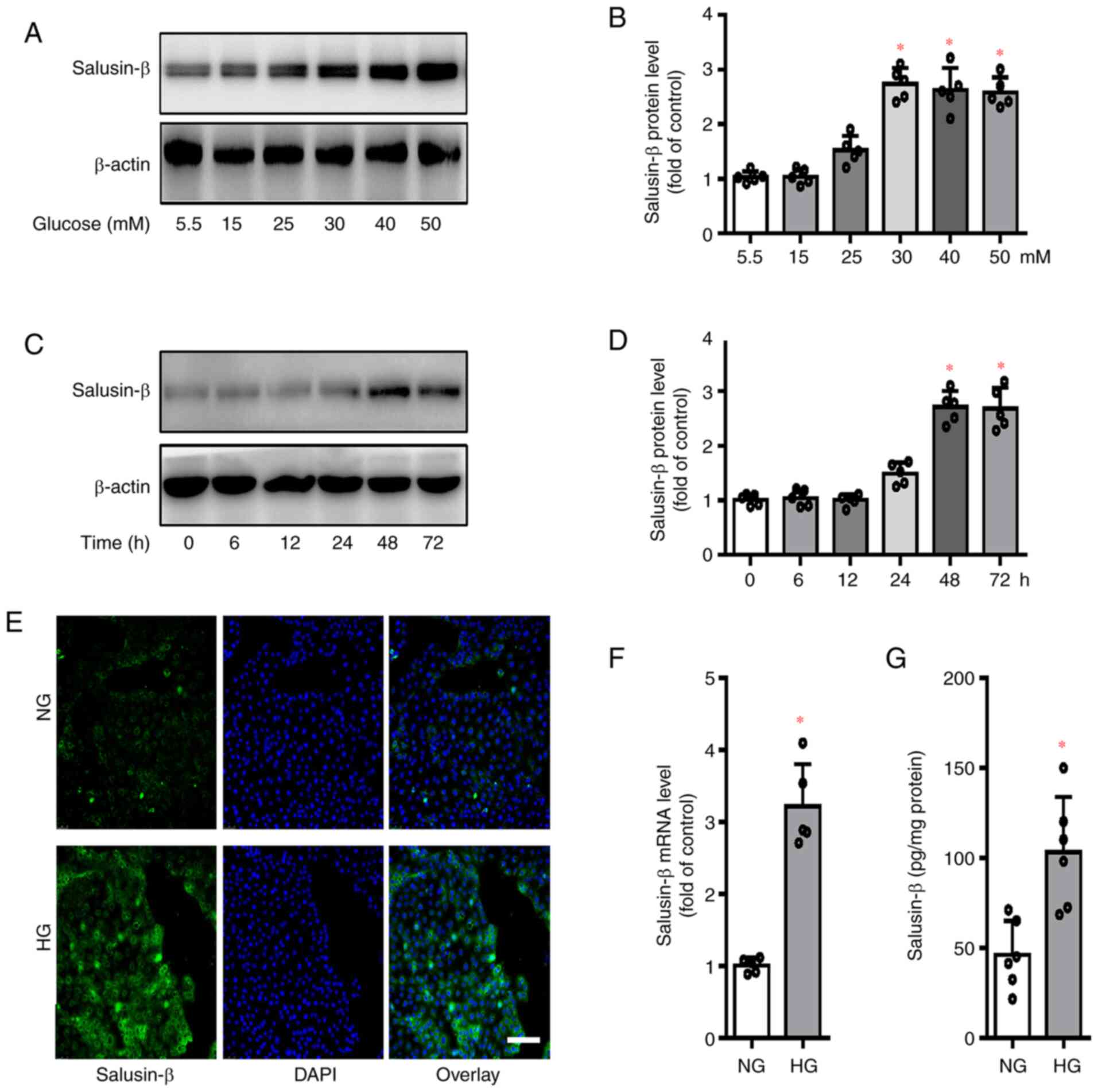 | Figure 1.Expression of salusin-β in
HG-incubated HK-2 cells. (A and B) HK-2 cells were incubated with
various doses of glucose (5.5, 15, 25, 30, 40 and 50 mM) for 48 h,
and the protein expression of salusin-β examined. (C and D) HK-2
cells were incubated with high glucose (HG, 30 mM) for various time
points and the protein expression of salusin-β was examined. (E)
Effects of HG (30 mM) incubation for 48 h on the protein expression
of salusin-β as determined by immunofluorescence staining. Scale
bar=200 µm. (F) Effects of HG (30 mM) incubation for 48 h on the
mRNA expression of salusin-β determined by reverse
transcription-quantitative PCR. (G) Effects of HG (30 mM)
incubation for 48 h on the protein expression of salusin-β as
determined by ELISA. Data are presented as the mean ± SD (n=4–5 per
group). *P<0.05 vs. 5.5 mM, 0 h, or NG. n=4–5 for each group.
HG, high glucose; NG, normal glucose. |
Effects of salusin-β knockdown on
HG-induced ferroptosis in HK-2 cells
Previous studies have highlighted the importance of
ferroptosis in the development of DN as inhibition of ferroptosis
can effectively delay the progression of DN (18,19).
It is reported that salusin-β serves as an inducer of oxidative
stress in HK-2 cells through increased ROS levels and decreased GSH
activities (35), key
characteristics of ferroptosis (15,49).
On this basis it was hypothesized that salusin-β might be involved
in HG-induced ferroptosis in HK-2 cells. To test this hypothesis,
the protein and mRNA expressions of ferroptosis-related genes, such
as GPX4, SLC7A11, FTH-1 and TFR-1, in
salusin-β-deficient HK-2 cells under HG conditions were examined.
Similar to a previous report (19),
the protein expressions of GPX4, SLC7A11 and FTH-1
were downregulated, while TFR-1 protein expression was
upregulated in HK-2 cells exposed to HG (Fig. 2A and B). However, deletion of
salusin-β (Fig. S2A) reversed
abnormal expressions of these proteins induced by HG (Fig. 2A and B). In line with this, RT-qPCR
results showed a parallel change in mRNA expression levels of
GPX4, SLC7A11, FTH-1 and TFR-1 (Fig. 2C). Meanwhile, incubation of HK-2
cells with HG promoted the concentration of iron and induced the
end products of lipid peroxidation, MDA, accompanied by a decrease
in GSH contents (Fig. 2D-F).
Notably, the ferroptosis-related changes in HG-induced HK-2 cells
were rescued when salusin-β was absent (Fig. 2D-F). In addition, knockdown of
salusin-β attenuated HG-elicited massive generation of ROS, as
evidenced by DCFH-DA staining (Fig. 2G
and H). Importantly, knockdown of salusin-β significantly
inhibited HG-triggered cell viability decline and LDH release,
suggesting the protective effects of salusin-β deficiency against
HG-induced cytotoxicity in HK-2 cells (Fig. 2I-K). Furthermore, induction of
ferroptosis and decreased cell viability were not observed in
D-mannitol-incubated HK-2 cells (Fig.
S1D-G). These preliminary results suggested that the
antagonistic effects of salusin-β knockdown on ferroptosis may be
dependent on the expressions of antioxidant genes (GPX4 and
SLC7A11) and iron metabolism-related genes (FTH-1 and
TFR-1).
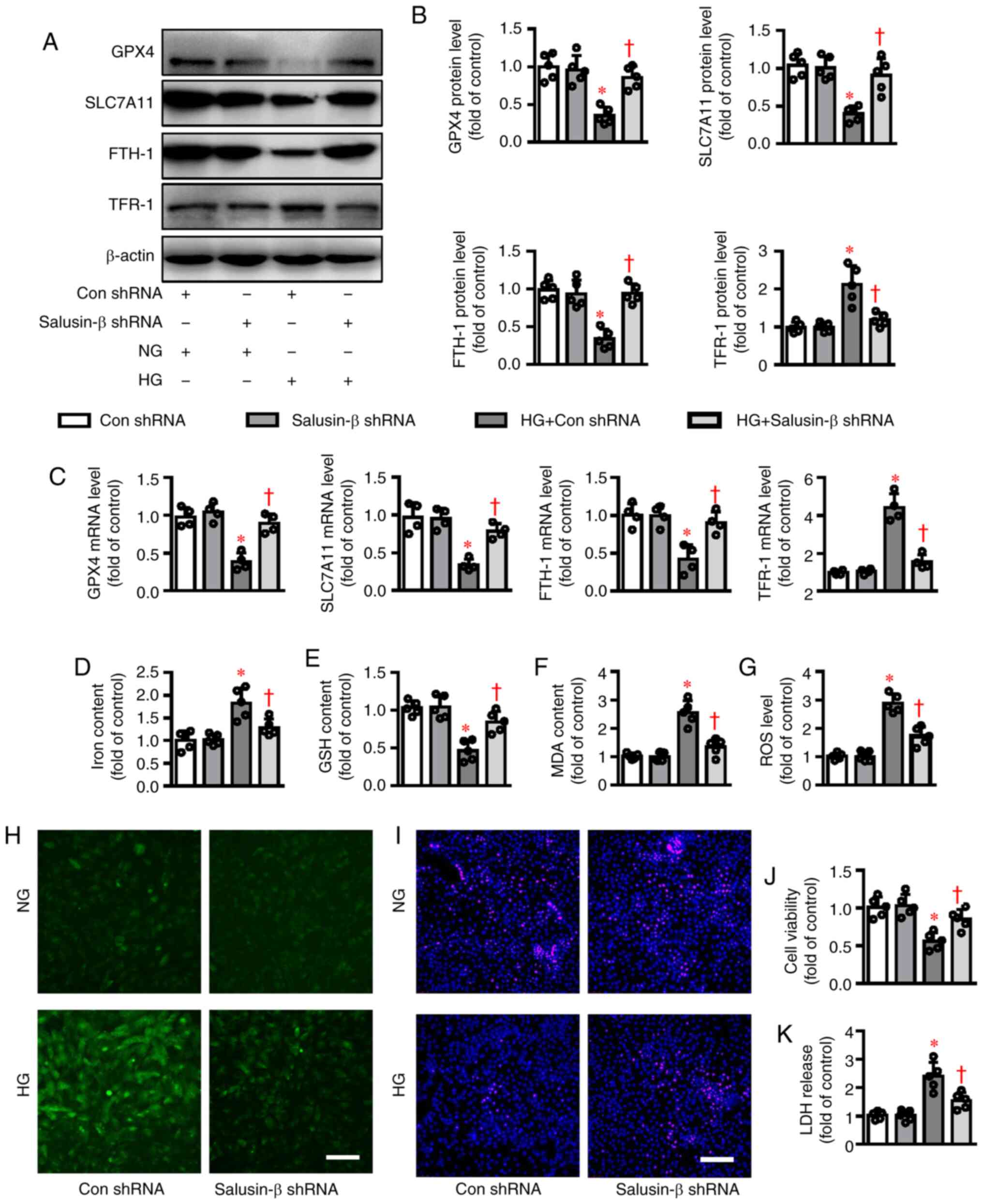 | Figure 2.Effects of salusin-β knockdown on
HG-induced ferroptosis in HK-2 cells. HK-2 cells in logarithmic
growth were transduced with lentivirus particles carrying salusin-β
shRNA or negative control shRNA at MOI=50 for 48 h and then
challenged by HG stimulation for another 48 h. (A) Representative
blot images and (B) quantitative analysis of GPX4, SLC7A11,
FTH-1 and TFR-1. (C) Relative mRNA levels of GPX4,
SLC7A11, FTH-1 and TFR-1. (D) Iron contents. (E) GSH
contents. (F) MDA levels. (G and H) ROS generation was assessed by
dichloro-dihydro-fluorescein diacetate fluorescence. (I) The
proliferation of HK-2 cells was evaluated by EdU staining. (J) The
cell proliferation was assessed by CCK-8 assay. (K) The
cytotoxicity was determined by LDH release assay. Scale bar=200 µm.
Data are presented as the mean ± SD (n=4–5 per group). *P<0.05
vs. Con shRNA. †P<0.05 vs. HG + Con shRNA. HG, high
glucose; NG, normal glucose; sh, short hairpin RNA; MOI,
multiplicity of infection; GPX4, glutathione peroxidase 4;
SLC7A11, solute carrier family 7 (cationic amino acid
transporter, y+ system) member 11; FTH-1, ferritin heavy
polypeptide 1; TFR-1, transferrin receptor 1; GSH,
glutathione; MDA, malondialdehyde; ROS, reactive oxygen species;
LDH, lactate dehydrogenase; Con, control. |
Effects of salusin-β overexpression on
HG-induced ferroptosis in HK-2 cells
In contrast to the above results, overexpression of
salusin-β (Fig. S2B) aggravated
the effects of HG on the protein (Fig.
3A-B) and mRNA (Fig. 3C)
expressions of GPX4, SLC7A11, FTH-1 and TFR-1 in HK-2
cells. Notably, salusin-β overexpression itself also changed the
expressions of GPX4, SLC7A11, FTH-1 and TFR-1 in HK-2
cells at both protein and mRNA levels (Fig. 3A-C), indicating the critical
implication of salusin-β in the process of ferroptosis. In
addition, ectopic overexpression of salusin-β further deteriorated
HG-induced ferroptosis in HK-2 cells, as manifested by more iron
accumulation, MDA contents and lower GSH contents (Fig. 3D-F). DCFH-DA staining results showed
that overexpression of salusin-β worsened the effects of HG on ROS
generation in HK-2 cells (Fig. 3G and
H). HG-induced decrease in cell viability and increase in LDH
release were further exacerbated by ectopic overexpression of
salusin-β (Fig. 3I and K).
Additionally, salusin-β overexpression led to spontaneous
cytotoxicity on HK-2 cells, as indicated by measurement of cell
viability and LDH release (Fig. 3I and
K).
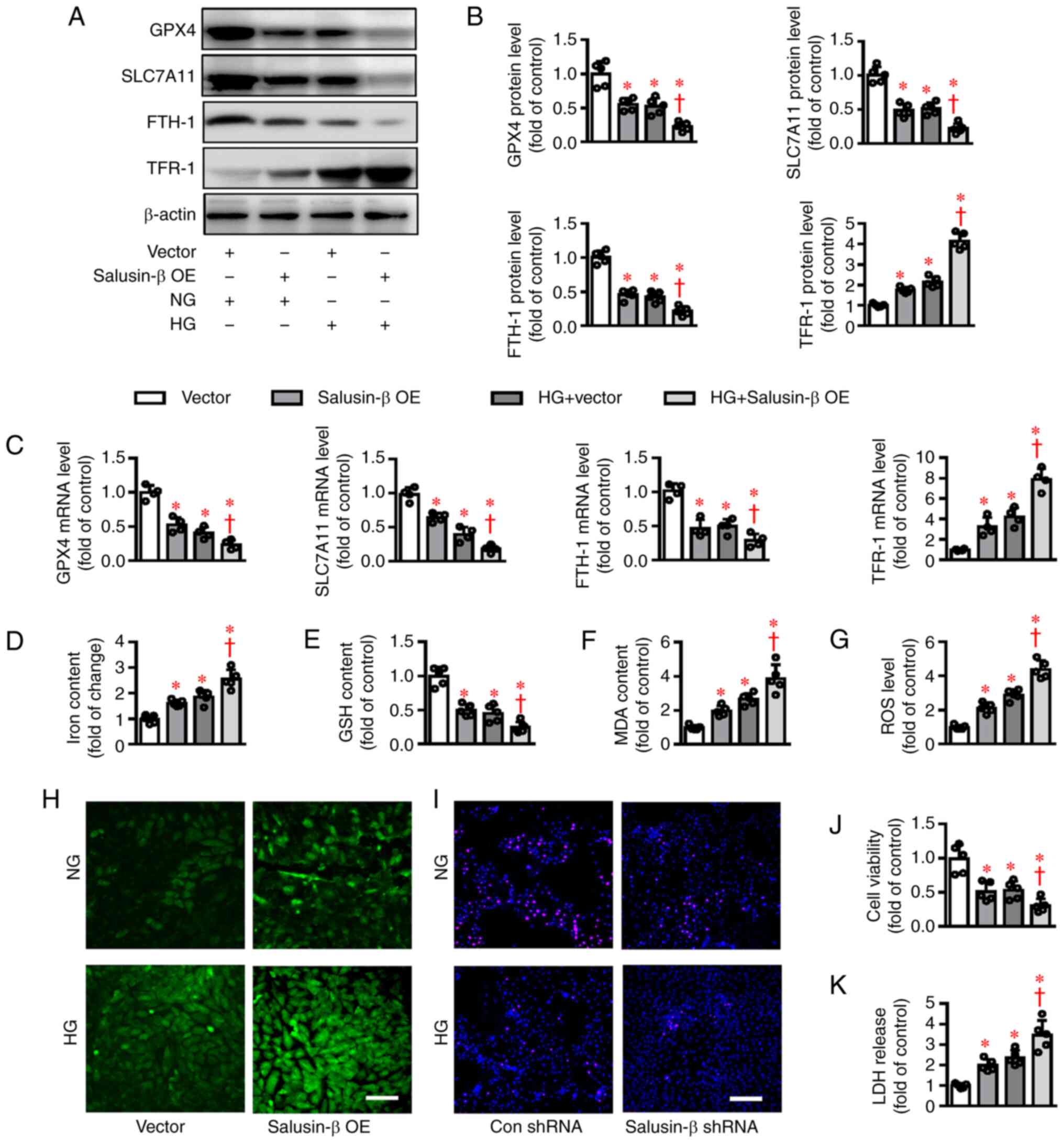 | Figure 3.Effects of salusin-β overexpression
on HG-induced ferroptosis in HK-2 cells. HK-2 cells were transduced
with lentivirus expressing salusin-β vectors or empty vectors
(MOI=50) for 48 h before administration of NG or HG for another 48
h. (A) Representative blot images and (B) quantitative analysis of
GPX4, SLC7A11, FTH-1 and TFR-1. (C) Relative mRNA
levels of GPX4, SLC7A11, FTH-1 and TFR-1. (D) Iron
contents. (E) GSH contents. (F) MDA levels. (G and H) ROS
generation was assessed by dichloro-dihydro-fluorescein diacetate
fluorescence. (I) The proliferation of HK-2 cells was evaluated by
EdU staining. (J) The cell proliferation was assessed by CCK-8
assay. (K) The cytotoxicity was determined by LDH release assay.
Scale bar=200 µm. Data are presented as the mean ± SD (n=4–5 per
group). *P<0.05 vs Vector. †P<0.05 vs. HG +
Vector. HG, high glucose; MOI, multiplicity of infection; NG,
normal glucose; sh, short hairpin RNA; GPX4, glutathione
peroxidase 4; SLC7A11, solute carrier family 7 (cationic
amino acid transporter, y+ system) member 11; FTH-1,
ferritin heavy polypeptide 1; TFR-1, transferrin receptor 1;
GSH, glutathione; MDA, malondialdehyde; ROS, reactive oxygen
species; LDH, lactate dehydrogenase; Con, control; OE,
overexpression. |
Effects of salusin-β on Nrf-2
signaling in HG-induced HK-2 cells
As a master antioxidant regulatory transcription
factor, Nrf-2 is found to prevent ferroptosis-related cell
death via upregulating antioxidant enzymes, including heme
oxygenase-1 (HO-1), GPX4 and SLC7A11 (50). In addition, Nrf-2 serves a
vital role in the regulation of ferroptosis-related genes, such as
genes for thiol-dependent antioxidant system, ion metabolism,
enzymatic detoxification of reactive carbonyl species and
carbonyls, nicotinamide adenine dinucleotide phosphate system and
ROS generation from mitochondria or extra-mitochondria (51). Activation of Nrf-2 signaling
is anticipated to open up a novel way to treat
ferroptosis-associated diseases, including DN (19). As expected, Bardoxolone methyl, an
inducer of Nrf-2 (Fig. 4A and
B), upregulated the mRNA expression levels of GPX4 and
SLC7A11 and reversed HG-induced inhibition of GPX4
and SLC7A11 mRNA levels (Fig. 4C
and D). The contents of iron and MDA were enhanced (Fig. 4E and G), whereas GSH levels and cell
viability were diminished in HK-2 cells challenged by HG (Fig. 4F and 4H) and these effects were noticeably
dampened by application of Bardoxolone methyl. The present study
next investigated whether Nrf-2 signaling was involved in
salusin-β-mediated ferroptosis in HG-stimulated HK-2 cells. As
shown in Fig. 4I, the protein
expression of Nrf-2 was significantly increased in
HG-incubated cells being treated with salusin-β shRNA compared with
the control shRNA group. By contrast, the decreased expression of
Nrf-2 was further exacerbated by overexpression of salusin-β
in HG-stimulated HK-2 cells (Fig.
4J). The data clearly demonstrated that salusin-β might be
responsible for HG-induced ferroptosis via inhibiting the
Nrf-2 signaling pathway.
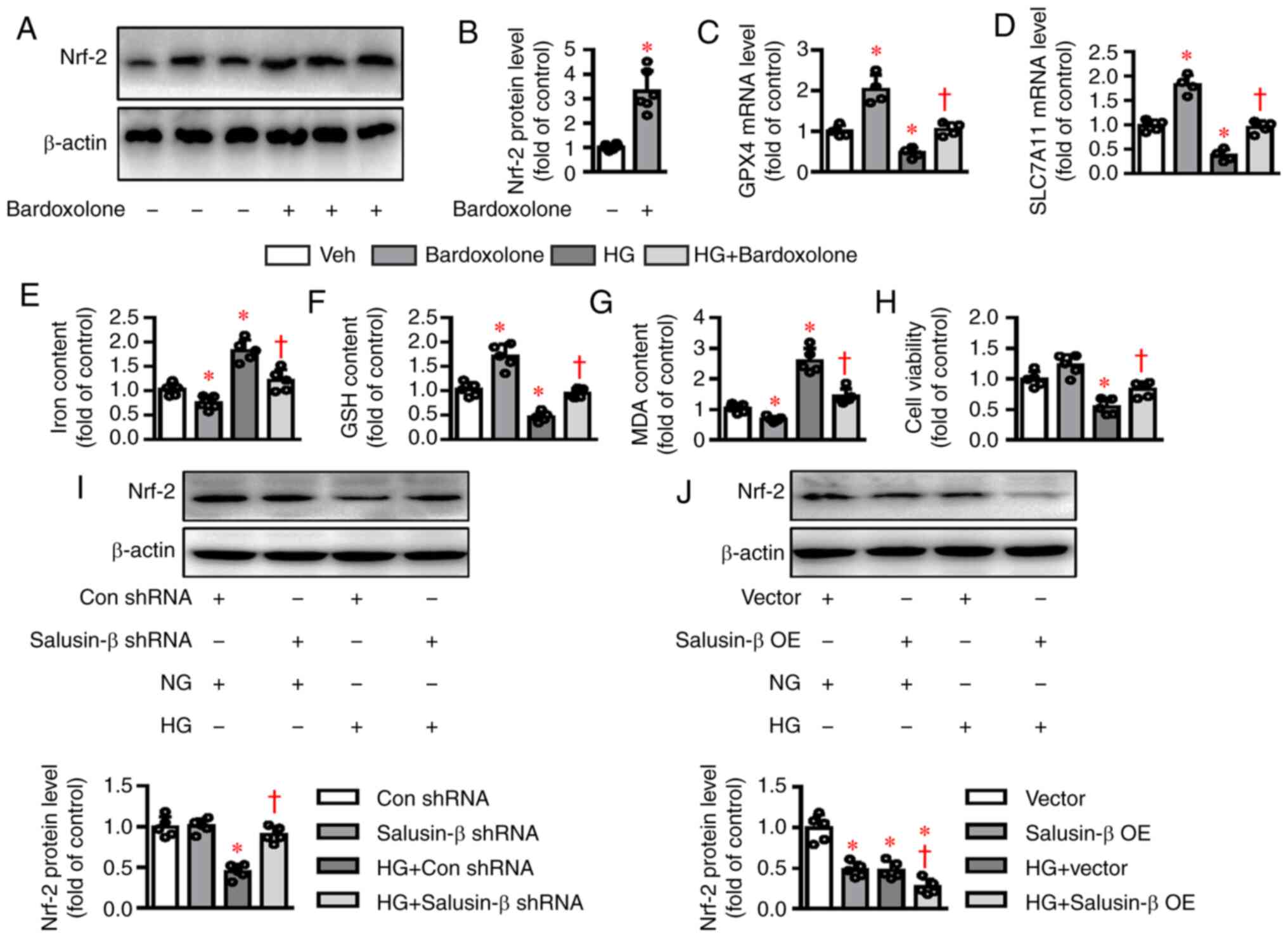 | Figure 4.Effects of salusin-β on Nrf-2
signaling in HG-induced HK-2 cells. (A and B) Effects of
Bardoxolone methyl (100 nM) incubation for 24 h on the protein
expression of Nrf-2. (C and D) HK-2 cells were pretreated
with Nrf-2 inducer bardoxolone methyl (100 nM) for 30 min
and then challenged by HG (30 mM) for 48 h. mRNA levels of
GPX4 and SLC7A11 were examined by reverse
transcription-quantitative PCR. (E) Effects of bardoxolone methyl
(100 nM) on (E) iron contents (F) GSH contents and (G) MDA contents
induced by HG. (H) Effects of bardoxolone methyl (100 nM) on cell
viability decline induced by HG. (I) Silencing of salusin-β
restored the protein expression of Nrf-2 in HG-incubated
HK-2 cells. (J) Overexpression of salusin-β further inhibited the
protein expression of Nrf-2 in HG-incubated HK-2 cells. Data
are presented as the mean ± SD (n=4–5 per group). *P<0.05 vs.
Veh, Con shRNA, or Vector. †P<0.05 vs. HG, HG + Con
shRNA, or HG + Vector. Nrf-2, nuclear factor
erythroid-derived 2-like 2; GPX4, glutathione peroxidase 4;
SLC7A11, solute carrier family 7 (cationic amino acid
transporter, y+ system) member 11; Veh, Vehicle; Con, Control; sh,
short hairpin RNA; NG, normal glucose; HG, high glucose; OE,
overexpression. |
To further verify the implication of the
Nrf-2 signaling cascade in salusin-β-mediated ferroptosis in
HK-2 cells, Nrf-2 siRNA and Nrf-2 inducer Bardoxolone
methyl were used in the present study. In the absence of Nrf-2
(Fig. S3), the suppressive effects
of salusin-β shRNA on HG-induced cell damage, including iron
deposition, GSH deletion, MDA accumulation and cell viability
decline, were abolished (Fig. 5A and
D). In parallel to this, the stimulatory effects of salusin-β
on HG-induced ferroptosis occurrence were clearly compromised by
Bardoxolone methyl pretreatment (Fig.
5E-H). Collectively, the molecular mechanism by which salusin-β
contributed to ferroptosis appeared to involve
Nrf-2-mediated regulation of antioxidant system and ion
metabolism.
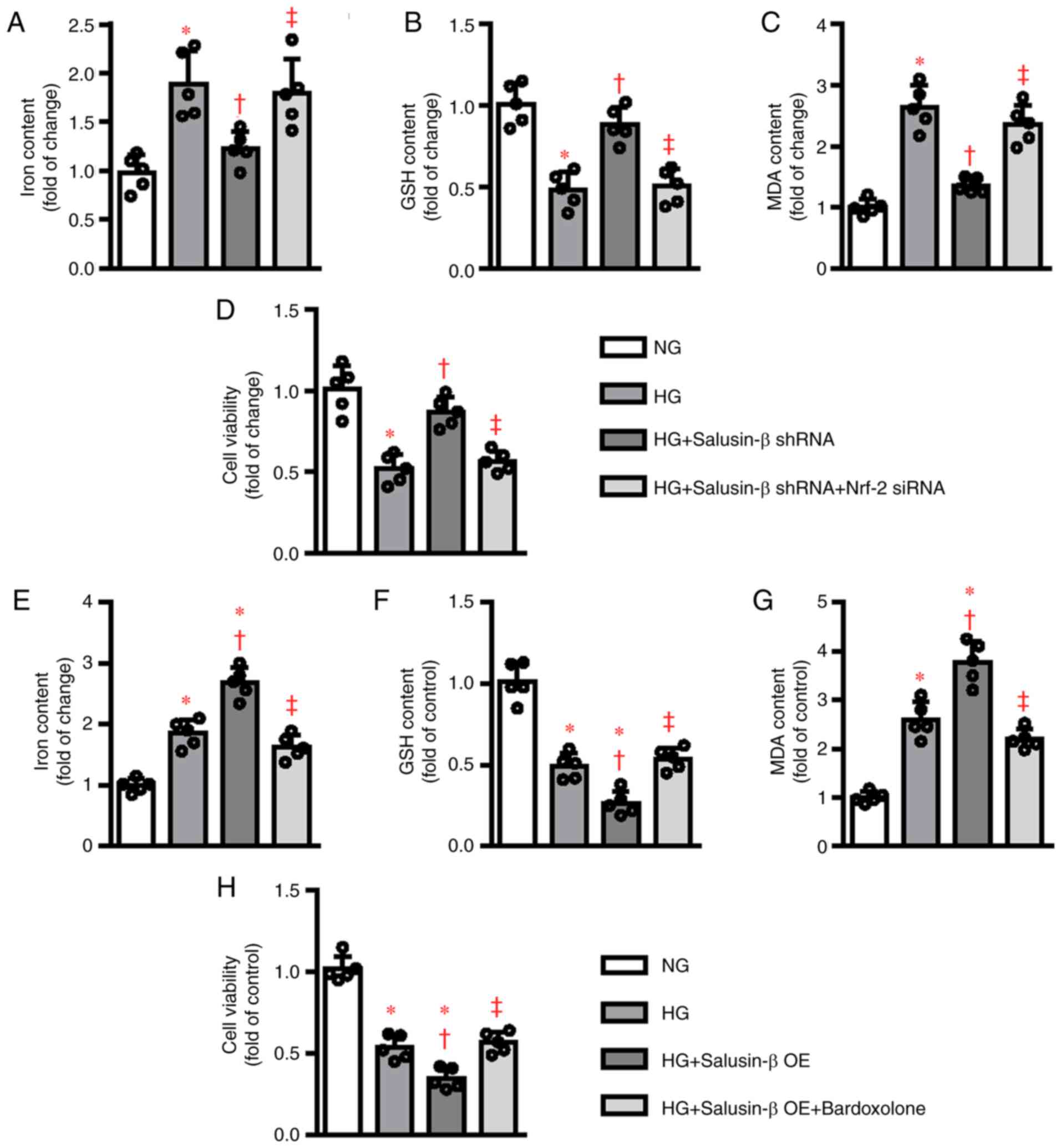 | Figure 5.Modulation of Nrf-2 signaling
affected the roles of salusin-β in HG-induced ferroptosis. HK-2
cells were transduced with control siRNA and Nrf-2 siRNA (50
nM) for 6 h and then transduced with lentivirus particles carrying
salusin-β shRNA or negative control shRNA at MOI = 50 for 24 h and
then challenged by HG stimulation for another 48 h. The (A) iron
contents, (B) GSH contents, (C) MDA levels and (D) cell viability
were then measured, respectively. HK-2 cells were pretreated with
Nrf-2 inducer bardoxolone methyl (100 nM) for 30 min and
transduced with lentivirus expressing salusin-β vectors or empty
vectors (MOI=50) for 48 h before administration of NG or HG for
another 48 h. Afterwards, the (E) iron contents, (F) GSH contents,
(G) MDA levels and (H) cell viability were then measured,
respectively. Data are presented as the mean ± SD (n=5 per group).
*P<0.05 vs. NG. †P<0.05 vs. HG.
‡P<0.05 vs. HG + Salusin-β shRNA or HG + Salusin-β
OE. Nrf-2, nuclear factor erythroid-derived 2-like 2; HG,
high glucose; si, short interfering RNA; MOI, multiplicity of
infection; NG, normal glucose; GSH, glutathione; MDA,
malondialdehyde; sh, short hairpin RNA; OE, overexpression. |
Expression of salusin-β during the
process of ferroptosis
The above results demonstrated that salusin-β
participated in HG-induced HK-2 cell ferroptosis in a
Nrf-2-dependent manner, it may be interesting to know
whether salusin-β was changed in the process of ferroptosis.
Western blotting results showed that the protein expression of
salusin-β was unexpectedly upregulated by several ferroptosis
activators, including erastin (Xc−
inhibitors), GPX4 inhibitors (RSL3 and FIN56) and GSH
synthase inhibitor (buthionine sulfoximine) (Fig. 6A). Conversely, pretreatment of
ferrostatin-1 (a ferroptosis inhibitor) downregulated the increased
expression of salusin-β in HG-treated HK-2 cells (Fig. 6B). These results suggested that
interaction of salusin-β with ferroptosis formed a positive
feedback, thereby contributing to HG-induced HK-2 cell injury and
DN.
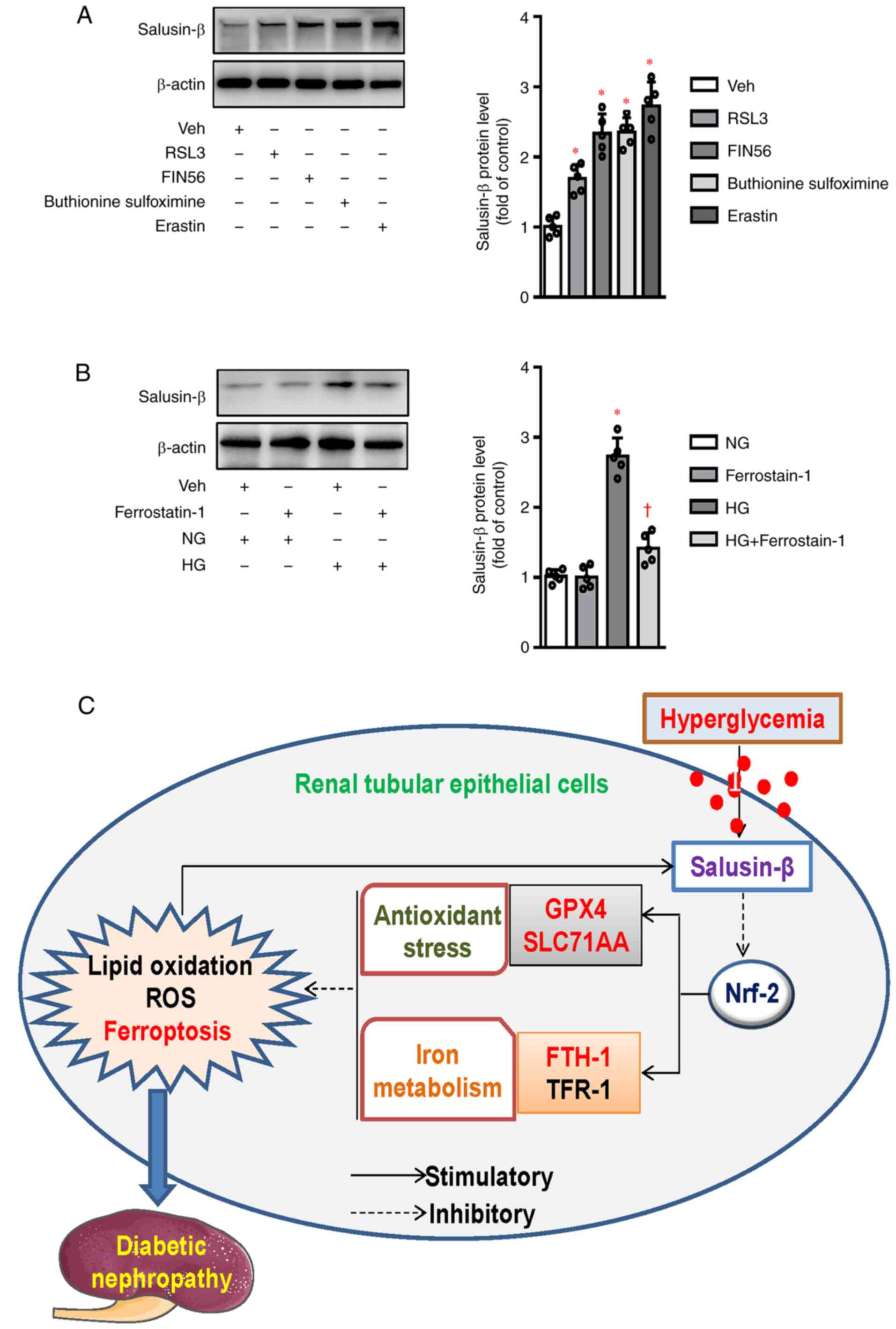 | Figure 6.Expression of salusin-β during the
process of ferroptosis. (A) HK-2 cells were treated with
ferroptosis activators for 24 h, including erastin (5 µM), GPX4
inhibitors (1 µM for RSL3 and 5 µM for FIN56) and GSH synthase
inhibitor (100 µM for buthionine sulfoximine), the protein
expression of salusin-β was analyzed by western blotting. (B) HK-2
cells were pretreated with ferrostatin-1 (1 µM) for 30 min and
incubated with HG for 48 h, the protein expression of salusin-β was
then detected by western blotting. (C) The action mechanism of
salusin-β involved in ferroptosis in diabetic kidneys via
inactivation of Nrf-2 signaling. Data are presented as the
mean ± SD (n=5 per group). *P<0.05 vs. Veh or NG.
†P<0.05 vs. HG. GPX4, glutathione peroxidase 4; GSH,
glutathione; Nrf-2, nuclear factor erythroid-derived 2-like
2; NG, normal glucose; HG, high glucose; Veh, Vehicle SLC7A11,
solute carrier family 7 (cationic amino acid transporter, y+
system) member 11; FTH-1, ferritin heavy polypeptide 1; TFR-1,
transferrin receptor 1; ROS, reactive oxygen species. |
Discussion
The main findings in the current study were that
salusin-β expression was notably increased at protein and mRNA
levels in HK-2 cells upon exposure to HG. In vitro results
showed that deficiency of salusin-β curbed, while overexpression of
salusin-β aggravated, lipoxygenase activation, iron accumulation,
GSH depletion, ROS accumulation in renal tubular epithelial cells
induced by HG. Furthermore, it was found that salusin-β knockdown
upregulated Nrf-2 signaling to reduce HG-induced
ferroptosis-related events, whereas salusin-β overexpression
conferred the opposite effects. The importance of Nrf-2 in
salusin-β-mediated ferroptosis was further confirmed by the
findings that knockdown of Nrf-2 abolished the protective effects
of salusin-β shRNA against HG-induced ferroptosis occurrence. By
contrast, induction of Nrf-2 prevented the positive effects
of salusin-β overexpression on ferroptosis progression induced by
HG. Crucially, salusin-β protein expression was also increased by
ferroptosis inducers, whereas ferroptosis inhibitor abrogated
HG-induced salusin-β overexpression in HK-2 cells. Collectively,
the results demonstrated for the first time, to the best of the
authors' knowledge, that a salusin-β-ferroptosis signaling loop
promoted the initiation and progression of renal tubular cell
injury in vitro.
Iron, an essential mineral, is required for a
variety of vital biological functions in health (52). The processes of iron metabolism are
precisely regulated by numerous biological signals, including
oxygen and lipid metabolism, protein production, cellular
respiration and DNA synthesis (53). Iron-dependent cell death, generally
known as ferroptosis, is a disorder of iron metabolism (54). Ferroptosis, a special type of
regulated cell death that is different from necroptosis, apoptosis
and other forms of cell death, is defined as iron overload,
deposition of lipid peroxidation products, ROS overproduction and
weakened antioxidant capacity (55). Studies have demonstrated that
oxidative stress-induced ROS accumulation activates various
signaling pathways, resulting in DNA damage and cell apoptosis
(56,57). These studies indicate that oxidative
stress is closely associated with both apoptosis and ferroptosis in
cells. It has been indicated that ferroptosis is involved in the
pathological mechanisms of numerous diseases, including diabetes,
neurodegenerative diseases, atherosclerosis, obesity, non-alcoholic
fatty liver disease and types of cancer (58). Notably, ferroptosis-related
pathophysiological changes are also observed in DN (18,19).
Fenofibrate, a ferroptosis inhibitor, reduces diabetes-induced
ferroptosis in the kidneys and delays the progression of diabetic
kidney disease (19). Salusin-β is
reported to be responsible for cisplatin-induced HK-2 cell injury
via increasing MDA contents and ROS generation and decreasing GSH
activity, implying the regulatory role of salusin-β in acute kidney
injury (35). The results present
of the study, showed that HG stimulation dose- and time-dependently
increased the protein and mRNA levels of salusin-β in HK-2 cells,
indicating that HG might be taken as a potential inducer for
salusin-β expression in HK-2 cells. Notably, knockdown of salusin-β
mitigated cell apoptosis, oxidative stress and iron deposition, but
restored GSH contents in HG-incubated HK-2 cells. By contrast,
overexpression of salusin-β aggravated HG-induced cell apoptosis,
ROS accumulation, antioxidant capacity reduction and iron
deposition in HK-2 cells. These results clearly suggested that
salusin-β might serve as an important regulator in HG-triggered
apoptosis and ferroptosis in HK-2 cells.
GPX4 and SLC7A11 are considered as
important biomarkers of ferroptosis as deficiency of them might
induce massive ROS production and GSH biogenesis dysfunction; key
features for ferroptosis (59,60).
TFR-1 and FTH-1, two key genes involved in iron
metabolism homeostasis, are closely linked with the development of
ferroptosis (61,62). In line with previous results
(19), the protein and mRNA
expressions of GPX4, SLC7A11 and FTH-1 were
downregulated, while TFR-1 protein and mRNA expression
levels were inhibited in HK-2 cells under HG treatment. All the
above effects were reversed by silencing of salusin-β, but were
aggravated by overexpression of salusin-β. When salusin-β was
absent, a homeostasis in GPX4, SLC7A11, FTH-1 and
TFR-1 expressions decreased the sensitivity of HK-2 cells to
ferroptosis induced HG. By contrast, overexpression of salusin-β
limited the ability of HK-2 cells to resist HG-induced ferroptosis
through abnormal expressions of GPX4, SLC7A11, FTH-1 and
TFR-1. Overall, these observations suggested that salusin-β
contributed to HG-induced ferroptosis through regulation of genes
for antioxidant system (GPX4 and SLC7A1) and iron
metabolism regulation system (FTH-1 and TFR-1). Apart
from these ferroptosis markers, abnormal ultrastructural changes in
the mitochondria are also observed in ferroptotic cells, including
shrunken mitochondria, increased bilayer membrane density,
mitochondrial ridge reduction or even disappearance and outer
mitochondrial membrane disruption (8,10,63).
Transmission electron microscopy is a well-known approach to reveal
the presence of small mitochondria with increased mitochondrial
membrane density and vanishing of mitochondrial cristae in
ferroptotic cells (63). In the
present study, the absence of mitochondrial transmission electron
microscopy images is one of the limitations and thus transmission
electron microscopy will be used to examine whether salusin-β
affects the alterations in mitochondrial morphology and cristae
structure of HK-2 cells in the future. It is expected that
mitochondrial transmission electron microscopy images would be
required to further ascertain the role of salusin-β in HG-induced
HK-2 cell ferroptosis.
Studies reveal that Nrf-2 is a dominant
transcription factor that is responsible for cellular redox balance
and Nrf-2 degradation is observed in DN (19,64,65).
Downregulation of Nrf-2 is closely associated with
diabetes-aggravated renal oxidative damage (66). Notably, Nrf-2 signaling is
implicated in the process of ferroptosis via regulating GSH
homeostasis, lipid metabolism and mitochondrial functions (67). Nrf-2 is documented to promote
ferroptosis resistance via regulation of enzymes responsible for
GSH synthesis (SLC7A11) and lipid peroxides neutralization
(GPX4), as well as proteins fundamental for iron signaling
(ferritin and ferroportin) (68,69).
Nrf-2 might afford a protection against ferroptosis-related
diseases, including DN (19). Due
to the importance of Nrf-2 in anti-oxidative stress and iron
metabolism, the present study investigated whether activation of
Nrf-2 signaling was associated with the protective effects
of salusin-β shRNA against HG-induced ferroptosis in HK-2 cells. In
line with expectations, HG-induced downregulation of Nrf-2
was largely rescued by silencing of salusin-β, but was further
decreased by overexpression of salusin-β. Following Nrf-2
silencing, the antagonistic effects of salusin-β shRNA on
ferroptosis disappeared. By contrast, induction of Nrf-2
markedly limited the positive effects of salusin-β overexpression
on ferroptosis development in the context of diabetes. The data
established that salusin-β might be involved in the pathologies of
DN-induced ferroptosis through negative regulation of the
Nrf-2 signaling.
The results also demonstrated that ferroptosis
activators promoted the protein expression of salusin-β, while
inhibition of ferroptosis counteracted HG-induced expression of
salusin-β in HK-2 cells. However, the mechanisms that underlie
ferroptosis- or HG-induced salusin-β expressions in HK-2 cells
remain to be elucidated, which merits further study. These
preliminary results indicated that salusin-β and ferroptosis form a
positive feedback loop to contribute to HG-induced renal tubular
cell injury. The second limitation of the present study was that
the role of salusin-β in HG-induced ferroptosis was only explored
in cellular experiments. Thereafter, animal studies are required to
further test the exact roles of salusin-β in the pathologies of
diabetes-induced renal ferroptosis and injury. These findings might
provide novel insights into the molecular mechanisms underlying the
roles of salusin-β in diabetic kidney disease-related
ferroptosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and XJ conducted the experiments and data
analysis, preparation of figures, as well as manuscript preparation
of manuscript. ZC and CG participated in interpretation of results
and finalizing the manuscript for submission. ZC and CG were
responsible for the drafting, revision and submission of this
manuscript. WW and ZC confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang Y, Shi K, Patel DM, Liu F, Wu T and
Chai Z: How to inhibit transforming growth factor beta safely in
diabetic kidney disease. Curr Opin Nephrol Hypertens. 30:115–122.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feliers D, Lee HJ and Kasinath BS:
Hydrogen sulfide in renal physiology and disease. Antioxid Redox
Signal. 25:720–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alicic RZ, Cox EJ, Neumiller JJ and Tuttle
KR: Incretin drugs in diabetic kidney disease: Biological
mechanisms and clinical evidence. Nat Rev Nephrol. 17:227–244.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin J, Cheng A, Cheng K, Deng Q, Zhang S,
Lan Z, Wang W and Chen J: New insights into the mechanisms of
pyroptosis and implications for diabetic kidney disease. Int J Mol
Sci. 21:70572020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun HJ, Wu ZY, Cao L, Zhu MY, Liu TT, Guo
L, Lin Y, Nie XW and Bian JS: Hydrogen sulfide: Recent progression
and perspectives for the treatment of diabetic nephropathy.
Molecules. 24:28572019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dugbartey GJ: Diabetic nephropathy: A
potential savior with ‘rotten-egg’ smell. Pharmacol Rep.
69:331–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun HJ, Xiong SP, Cao X, Cao L, Zhu MY, Wu
ZY and Bian JS: Polysulfide-mediated sulfhydration of SIRT1
prevents diabetic nephropathy by suppressing phosphorylation and
acetylation of p65 NF-κB and STAT3. Redox Biol. 38:1018132020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D and Chen L: Ferroptosis: A novel cell
death form will be a promising therapy target for diseases. Acta
Biochim Biophys Sin (Shanghai). 47:857–859. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang D and Kroemer G: Ferroptosis. Curr
Biol. 30:R1292–R1297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Latunde-Dada GO: Ferroptosis: Role of
lipid peroxidation, iron and ferritinophagy. Biochimica et
biophysica acta. Biochim Biophysc Acta Gen Subj. 1861:1893–1900.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin-Sanchez D, Ruiz-Andres O, Poveda J,
Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, Ortega MR, Egido J,
Linkermann A, Ortiz A and Sanz AB: Ferroptosis, but not
necroptosis, is important in nephrotoxic folic acid-induced AKI. J
Am Soc Nephrol. 28:218–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guerrero-Hue M, García-Caballero C,
Palomino-Antolín A, Rubio-Navarro A, Vázquez-Carballo C, Herencia
C, Martín-Sanchez D, Farré-Alins V, Egea J, Cannata P, et al:
Curcumin reduces renal damage associated with rhabdomyolysis by
decreasing ferroptosis-mediated cell death. FASEB J. 33:8961–8975.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee H, Zandkarimi F, Zhang Y, Meena JK,
Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, et al:
Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat
Cell Biol. 22:225–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Li J, Miao X, Cui W, Miao L and Cai
L: A minireview: Role of AMP-activated protein kinase (AMPK)
signaling in obesity-related kidney injury. Life Sci.
15:1188282020.
|
|
17
|
Unsal V, Cicek M and Sabancilar I:
Toxicity of carbon tetrachloride, free radicals and role of
antioxidants. Rev Environ Health. 24:doi: 10.1515. 2020.PubMed/NCBI
|
|
18
|
Wang Y, Bi R, Quan F, Cao Q, Lin Y, Yue C,
Cui X, Yang H, Gao X and Zhang D: Ferroptosis involves in renal
tubular cell death in diabetic nephropathy. Eur J Pharmacol.
888:1735742020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li S, Zheng L, Zhang J, Liu X and Wu Z:
Inhibition of ferroptosis by up-regulating Nrf2 delayed the
progression of diabetic nephropathy. Free Radic Biol Med.
162:435–449. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shichiri M, Ishimaru S, Ota T, Nishikawa
T, Isogai T and Hirata Y: Salusins: Newly identified bioactive
peptides with hemodynamic and mitogenic activities. Nat Med.
9:1166–1172. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki N, Shichiri M, Tateno T, Sato K and
Hirata Y: Distinct systemic distribution of salusin-α and salusin-β
in the rat. Peptides. 32:805–810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki N, Shichiri M, Akashi T, Sato K,
Sakurada M, Hirono Y, Yoshimoto T, Koyama T and Hirata Y: Systemic
distribution of salusin expression in the rat. Hypertension Res.
30:1255–1262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato K, Watanabe R, Itoh F, Shichiri M and
Watanabe T: Salusins: Potential use as a biomarker for
atherosclerotic cardiovascular diseases. Int J Hypertension.
2013:9651402013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe T, Sato K, Itoh F, Iso Y,
Nagashima M, Hirano T and Shichiri M: The roles of salusins in
atherosclerosis and related cardiovascular diseases. J Am Soc
Hypertens. 5:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kolakowska U, Olanski W and Wasilewska A:
Salusins in hypertension and related cardiovascular diseases. Curr
Drug Metab. 17:827–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang WJ, Jiang X, Gao CC and Chen ZW:
Salusin-α mitigates diabetic nephropathy via inhibition of the
Akt/mTORC1/p70S6K signaling pathway in diabetic rats. Drug Chem
Toxicol. 31:1–8. 2019. View Article : Google Scholar
|
|
27
|
Fujimoto K, Hayashi A, Kamata Y, Ogawa A,
Watanabe T, Ichikawa R, Iso Y, Koba S, Kobayashi Y, Koyama T and
Shichiri M: Circulating levels of human salusin-beta, a potent
hemodynamic and atherogenesis regulator. PLoS One. 8:e767142013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kolakowska U, Kuroczycka-Saniutycz E,
Wasilewska A and Olanski W: Is the serum level of salusin-beta
associated with hypertension and atherosclerosis in the pediatric
population? Pediatr Nephrol. 30:523–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yassien M, Fawzy O, Mahmoud E and Khidr
EG: Serum salusin-β in relation to atherosclerosis and ventricular
dysfunction in patients with type 2 diabetes mellitus. Diabetes
Metab Syndr. 14:2057–2062. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sipahi S, Genc AB, Acikgoz SB, Yildirim M,
Aksoy YE, Vatan MB, Dheir H and Altındis M: Relationship of
salusin-alpha and salusin-beta levels with atherosclerosis in
patients undergoing haemodialysis. Singapore Med J. 60:210–215.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sahin I and Aydin S: Serum concentration
and kidney expression of salusin-α and salusin-β in rats with
metabolic syndrome induced by fructose. Biotech Histochem.
88:153–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao MX, Zhou B, Ling L, Xiong XQ, Zhang
F, Chen Q, Li YH, Kang YM and Zhu GQ: Salusin-β contributes to
oxidative stress and inflammation in diabetic cardiomyopathy. Cell
Death Dis. 8:e26902017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun H, Zhang F, Xu Y, Sun S, Wang H, Du Q,
Gu C, Black SM, Han Y and Tang H: Salusin-β promotes vascular
calcification via nicotinamide adenine dinucleotide
phosphate/reactive oxygen species-mediated klotho downregulation.
Antioxid Redox Signal. 31:1352–1370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun HJ, Chen D, Wang PY, Wan MY, Zhang CX,
Zhang ZX, Lin W and Zhang F: Salusin-β is involved in diabetes
mellitus-induced endothelial dysfunction via degradation of
peroxisome proliferator-activated receptor gamma. Oxid Med Cell
Longev. 2017:69052172017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu QB, Du Q, Wang HP, Tang ZH, Wang YB and
Sun HJ: Salusin-β mediates tubular cell apoptosis in acute kidney
injury: Involvement of the PKC/ROS signaling pathway. Redox Biol.
30:1014112020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu X, Zhou Y, Cai W, Sun H and Qiu L:
Salusin-β mediates high glucose-induced endothelial injury via
disruption of AMPK signaling pathway. Biochem Biophys Res Commun.
491:515–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun HJ, Liu TY, Zhang F, Xiong XQ, Wang
JJ, Chen Q, Li YH, Kang YM, Zhou YB, Han Y, et al: Salusin-β
contributes to vascular remodeling associated with hypertension via
promoting vascular smooth muscle cell proliferation and vascular
fibrosis. Biochim Biophys Acta. 1852:1709–1718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Szczesny-Malysiak E, Stojak M, Campagna R,
Grosicki M, Jamrozik M, Kaczara P and Chlopicki S: Bardoxolone
methyl displays detrimental effects on endothelial bioenergetics,
suppresses endothelial ET-1 release and increases endothelial
permeability in human microvascular endothelium. Oxid Med Cell
Long. 2020:46782522020.PubMed/NCBI
|
|
40
|
Song X, Zhu S, Chen P, Hou W, Wen Q, Liu
J, Xie Y, Liu J, Klionsky DJ, Kroemer G, et al: AMPK-mediated BECN1
phosphorylation promotes ferroptosis by directly blocking system
Xc− activity. Curr Biol. 28:2388–2399. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu X, Wu S and Guo H: Active vitamin D
and vitamin D receptor help prevent high glucose induced oxidative
stress of renal tubular cells via AKT/UCP2 signaling pathway.
Biomed Res Int. 2019:90139042019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun HJ, Zhang LL, Fan ZD, Chen D, Zhang L,
Gao XY, Kang YM and Zhu GQ: Superoxide anions involved in
sympathoexcitation and pressor effects of salusin-β in
paraventricular nucleus in hypertensive rats. Acta physiol.
210:534–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun HJ, Zhao MX, Ren XS, Liu TY, Chen Q,
Li YH, Kang YM, Wang JJ and Zhu GQ: Salusin-β promotes vascular
smooth muscle cell migration and intimal hyperplasia after vascular
injury via ROS/NFκB/MMP-9 pathway. Antioxid Redox Signal.
24:1045–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akpınar O, Özşimşek A, Güzel M and
Nazıroğlu M: Clostridium botulinum neurotoxin A induces apoptosis
and mitochondrial oxidative stress via activation of TRPM2 channel
signaling pathway in neuroblastoma and glioblastoma tumor cells. J
Recept Signal Transduct Res. 40:620–632. 2020. View Article : Google Scholar
|
|
46
|
Ma X, Zhang J, Wu Z and Wang X: Chicoric
acid attenuates hyperglycemia-induced endothelial dysfunction
through AMPK-dependent inhibition of oxidative/nitrative stresses.
J Receptor Signal Trans Res. 9:1–15. 2020.
|
|
47
|
Lu QB, Sun JF, Yang QY, Cai WW, Xia MQ, Wu
FF, Gu N and Zhang ZJ: Magnetic brain stimulation using iron oxide
nanoparticle-mediated selective treatment of the left prelimbic
cortex as a novel strategy to rapidly improve depressive-like
symptoms in mice. Zool Res. 41:381–394. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Q, Li Y, Luo J, Yang Y, Li J, Sun L,
Xiao L, Xu X, Peng Y and Liu F: Effect of norcantharidin on the
expression of FN, Col IV and TGF-β1 mRNA and protein in HK-2 cells
induced by high glucose. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
37:278–284. 2012.(In Chinese). PubMed/NCBI
|
|
49
|
Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT,
Tai WC, Tsim KW, Chen YT and Xi T: Ginkgetin derived from ginkgo
biloba leaves enhances the therapeutic effect of cisplatin via
ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR
wild-type non-small-cell lung cancer. Phytomedicine. 80:1533702021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yu H, Guo P, Xie X, Wang Y and Chen G:
Ferroptosis, a new form of cell death and its relationships with
tumourous diseases. J Cell Mol Med. 21:648–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song S, Gao Y, Sheng Y, Rui T and Luo C:
Targeting NRF2 to suppress ferroptosis in brain injury. Histol
Histopathol. 36:383–397. 2021.PubMed/NCBI
|
|
52
|
Kobayashi M, Suhara T, Baba Y, Kawasaki
NK, Higa JK and Matsui T: Pathological roles of iron in
cardiovascular disease. Curr Drug Targets. 19:1068–1076. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ravingerová T, Kindernay L, Barteková M,
Ferko M, Adameová A, Zohdi V, Bernátová I, Ferenczyová K and Lazou
A: The molecular mechanisms of iron metabolism and its role in
cardiac dysfunction and cardioprotection. Int J Mol Sci.
21:78892020. View Article : Google Scholar
|
|
54
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Y, Peng X, Zhang M, Jia Y, Yu B and
Tian J: Revisiting tumors and the cardiovascular system:
Mechanistic intersections and divergences in ferroptosis. Oxid Med
Cell Longev. 2020:97381432020.PubMed/NCBI
|
|
56
|
Yang L, Guan G, Lei L, Liu J, Cao L and
Wang X: Oxidative and endoplasmic reticulum stresses are involved
in palmitic acid-induced H9c2 cell apoptosis. Biosci Rep.
39:BSR201902252019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ma B, Guan G, Lv Q and Yang L: Curcumin
ameliorates palmitic acid-induced saos-2 cell apoptosis via
inhibiting oxidative stress and autophagy. Evid Based Complement
Alternat Med. 2021:55636602021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nechushtai R, Karmi O, Zuo K, Marjault HB,
Darash-Yahana M, Sohn YS, King SD, Zandalinas SI, Carloni P and
Mittler R: The balancing act of NEET proteins: Iron, ROS, calcium
and metabolism. Biochim Biophys Acta Mol Cell Res. 1867:1188052020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 26:1–28. 2020.
View Article : Google Scholar
|
|
60
|
Chen X, Yu C, Kang R and Tang D: Iron
metabolism in ferroptosis. Front Cell Dev Biol. 8:5902262020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li LB, Chai R, Zhang S, Xu SF, Zhang YH,
Li HL, Fan YG and Guo C: Iron exposure and the cellular mechanisms
linked to neuron degeneration in adult mice. Cells. 8:1982019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fang X, Cai Z, Wang H, Han D, Cheng Q,
Zhang P, Gao F, Yu Y, Song Z, Wu Q, et al: Loss of cardiac ferritin
H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ
Res. 127:486–501. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Battaglia AM, Chirillo R, Aversa I, Sacco
A, Costanzo F and Biamonte F: Ferroptosis and cancer: Mitochondria
meet the ‘Iron Maiden’ cell death. Cells. 9:15052020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mathur A, Pandey VK and Kakkar P:
Activation of GSK3β/β-TrCP axis via PHLPP1 exacerbates Nrf2
degradation leading to impairment in cell survival pathway during
diabetic nephropathy. Free Radic Biol Med. 120:414–424. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang S, Nie P, Lu X, Li C, Dong X, Yang F,
Luo P and Li B: Nrf2 participates in the anti-apoptotic role of
zinc in Type 2 diabetic nephropathy through wnt/β-catenin signaling
pathway. J Nutr Biochem. 84:1084512020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rubin A, Salzberg AC, Imamura Y,
Grivitishvilli A and Tombran-Tink J: Identification of novel
targets of diabetic nephropathy and PEDF peptide treatment using
RNA-seq. BMC Genomics. 17:9362016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
La Rosa P, Petrillo S, Turchi R,
Berardinelli F, Schirinzi T, Vasco G, Lettieri-Barbato D, Fiorenza
MT, Bertini ES, Aquilano K and Piemonte F: The Nrf2 induction
prevents ferroptosis in friedreich's ataxia. Redox Biol.
38:1017912020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kerins MJ and Ooi A: The roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|




















