Introduction
The liver is an important detoxification organ in
the human body that can be damaged by hepatitis virus infection,
various drugs (1,2), dietary supplements (3) and hepatotoxic poisons (4). Without effective intervention, this
damage may induce acute liver injury (ALI), which has high
morbidity and mortality rates (5,6).
Carbon tetrachloride (CCl4) is a common chemical reagent
that is used to establish animal models of acute or chronic liver
injury (7,8).
The urotensin II (UII)/urotensin receptor (UT)
system is composed of UII and its specific receptor G
protein-coupled receptor (GPR14) or UT, and is present in the liver
(9), heart (10), kidney (11) and other organs. The UII/UT system
has a variety of biological effects, such as inducing contraction,
expanding blood vessels and influencing metabolism (12). Furthermore, when liver disease
occurs, the UII/UT system is activated (13). Therefore, UII may be a specific
target for the treatment of liver injury.
The MAPK pathway is an important cytokine-associated
signalling pathway that regulates a variety of biological effects,
such as oxidative stress, the inflammatory response, apoptosis,
proliferation and differentiation (14,15).
The MAPK signalling pathway is abnormally activated in ALI
(16,17). Therefore, inhibiting its activation
serves a vital role in the prevention and treatment of ALI.
Urantide is the most effective UII receptor
antagonist as it has fewer side effects and is 50–100 times more
potent than other compounds (10,18).
Previous studies have shown that urantide could antagonize the
expression of UII/UT system components, thereby effectively
alleviating ALI and inhibiting the proliferation of Kupffer cells
(9,19). However, to the best of our
knowledge, there has been no relevant report on whether the
antagonistic UII/UT system affects the MAPK signalling pathway in
preventing liver injury.
Therefore, the purpose of the present study was to
investigate whether urantide could prevent ALI caused by
CCl4 and to identify its regulatory mechanism. We
hypothesized that urantide prevented ALI by blocking the UII/UT
system and regulating the MAPK signalling pathway.
Materials and methods
Animals
In total, 50 specific pathogen free healthy male
Sprague Dawley rats (weight, 180–200 g; age, 3 weeks) were
purchased from Beijing Huafukang Biotechnology Co., Ltd. [licence
no. SCXK (Jing) 2019–0008], and one cage for every five rats met
the national standard for the minimum area of cages occupied by
experimental animals. The temperature was set as 22±2°C, the
humidity was 40–60% and the light/dark cycle was 12 h to meet the
basic needs of laboratory animals. Animals were provided with free
access to food and water. During the experiment, the rats were
nursed and treated according to the international guidelines for
the use and care of laboratory animals (20).
ALI rat model establishment and
preventive administration of urantide
The rats were randomly divided into six groups:
Control group, ALI model group, magnesium isoglycyrrhizinate (MgIG)
group, urantide low concentration group, urantide medium
concentration group and urantide high concentration group, with 10
rats in the ALI model group and 8 rats in each other group. After 1
week of being fed with the basic diet, the mice were fasted for 24
h. The control group and ALI model group were injected with normal
saline (20 mg/kg) through the caudal vein every day for 1 week, the
MgIG group was injected with MgIG (20 mg/kg; Zhengda Tianqing
Pharmaceutical Group Co., Ltd.) through the caudal vein every day
for 1 week and the treatment group was given 5, 10 and 20 mg/kg
urantide (Suzhou Qiangyao Biological Technology Co., Ltd.) every
day for 1 week. After fasting for 2 h, except for those in the
control group, the rats were intraperitoneally injected 50%
CCl4 in olive oil (1 ml/kg) (21), and control group rats were injected
with the same amount of olive oil. Each rat was weighed daily.
Blood collection and specimen
treatment
After intraperitoneal injection of CCl4
mixed solution for 24 h, an intraperitoneal injection of 150 mg/kg
sodium pentobarbital (Tianjin Fuchen Chemical Industry) was used to
euthanise the rats and obtain liver samples. A disposable negative
pressure blood collection container (Cangzhou Yongkang Medical
Supplies Co., Ltd.) was used to collect blood directly from the
abdominal aorta. The blood was centrifuged at 1,500 × g for 10 min
at 4°C to obtain plasma. The liver was stored in 4°C in 4%
formaldehyde or at −196°C in liquid nitrogen.
Evaluation of liver injury
The body weight of each rat was analysed before
administration, before modelling and after the experiment. The
serological indexes alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) were analysed using the Roche Diagnostics
(Shanghai) Co., Ltd. Liver lesions were observed via H&E
staining (Nanchang Yulu Experimental Equipment Co., Ltd.). After
fixation using 4% formaldehyde at 4°C for 24 h, 5-µm thick sections
were stained at room temperature with hematoxylin for 5 min and
eosin for 3 min. Stained sections were visualized using a light
microscope (magnification, ×200 or 1,000). For oil red O staining,
cut frozen sections of liver tissue (8-µm thick) were fixed with
10% formaldehyde at room temperature for 15 min, then stained with
oil red O working solution (Beijing Solarbio Technology Co., Ltd.)
for 15 min. Stained sections were visualized using a light
microscope (magnification, ×200 or 1,000). According to the changes
in liver tissue structure, the changes in liver cell morphology,
the degree of necrosis and the degree of inflammatory cell
infiltration, liver injury was comprehensively judged and
semi-quantitative scored as the Liver Injury Score (22). The higher the score, the more severe
the liver tissue damage. The specific criteria were as follows: 0
points, liver tissue structure and cell morphology are normal, no
obvious degeneration or necrosis, no cell swelling and inflammatory
cell infiltration; 1 point, liver cells show limited degeneration
or necrosis, and cell swelling can be found, occasionally visible
inflammatory cell infiltration and occasionally observed necrosis;
2 points, diffuse degeneration and swelling of liver cells,
localized infiltration of inflammatory cells can be found, multiple
necrotic areas, occasional focal necrosis; 3 points, liver cells
showed watery degeneration and obvious swelling, a large number of
inflammatory cells infiltrated, multiple focal necrosis, liver
tissue structure disorder; and 4 points, multiple flaky necrosis,
liver cord arrangement disorder, liver lobules lose normal
structure.
Immunohistochemistry and
immunofluorescence analysis
After fixation with 4% formaldehyde at 4°C for 24 h,
liver paraffin embedded sections (thickness, 5 µm) were hydrated
with gradient concentrations of alcohol (100% ethanol I, 100%
ethanol II, 95% ethanol, 90% ethanol, 80% ethanol, 70% ethanol; 2
min each) for immunohistochemistry and immunofluorescence analysis.
Each step was carried out in strict accordance with the
manufacturer's instructions.
Immunohistochemistry was performed using a polymer
detection kit (cat. no. PV-6000; OriGene Technologies, Inc.).
Following blocking with peroxidase blocker at 37°C for 45 min,
primary antibodies [α-smooth muscle actin (α-SMA) and osteopontin
(OPN) rabbit monoclonal antibodies; 1:100; cat. nos. A1011 and
A1499; ABclonal] were added and incubated overnight at 4°C. Goat
anti-rabbit IgG polymer (cat. no. PV-6000; OriGene Technologies,
Inc.) was added and incubated at 37°C for 45 min. Then, DAB
solution (substrate: concentrate =1:20) was added and incubated at
37°C for 5 min, following which haematoxylin staining was performed
for 5 min. Finally, stained sections were observed using a light
microscope (magnification, ×400).
Immunofluorescence detection was performed using a
polymer detection kit (Shanghai Botai Biotechnology Institute). The
nuclei were stained with DAPI. The following primary antibodies,
phosphorylated (p)-JNK (mouse; 1:200; cat. no. 9255), p-ERK
(rabbit; 1:200; cat. no. 4370) and p-P38 (rabbit; 1:1,600; cat. no.
4511), all from Cell Signalling Technology, Inc., were added and
incubated overnight at 4°C. FITC-labelled goat anti-rabbit IgG
(1:1,000; cat. no. 02-15-06; KPL, Inc.) was added and incubated at
37°C for 45 min. A fluorescence microscope equipped with a digital
high-resolution camera was used for observation (Olympus
Corporation). Quantitative analysis was performed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract RNA from the liver
tissue, and cDNA was reverse transcribed using the FastQuant RT kit
(Beijing Tiangen Biotechnology Co., Ltd.) containing gDNase
according to the manufacturer's protocol. A SuperReal Premix Plus
kit (Tiangen Biotechnology Co., Ltd.) was used to measure the
expression of related genes. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95°C for 15 min; 40 of
cycles of denaturation at 95°C for 10 sec, annealing and elongation
at 62°C for 30 sec; and final extension at 65–95°C at increments of
0.5°C for 5 sec. Relative gene expression was calculated using the
2−∆∆Cq method (23,24).
The primer and probe sequences (Takara Biotechnology Co., Ltd.) are
shown in Table I.
 | Table I.Primer sequences of rat genes. |
Table I.
Primer sequences of rat genes.
| Gene | Primer
sequence |
|---|
| UII | F:
5′-GGAGGAGCTGGAGAGGACTG-3′ |
|
| R:
5′-GAGTCTCGGCACTGGGATCT-3′ |
| GPR14 | F:
5′-AATGGCTCTAGGGTCCTCCT-3′ |
|
| R:
5′-AACAGCCTCTGTGATGGACA-3′ |
| α-SMA | F:
5′-CAGCCAGTCGCCATCAGGAAC-3′ |
|
| R:
5′-CCAGCAAAGCCCGCCTTACAG-3′ |
| OPN | F:
5′-GACGATGATGACGACGACGATGAC-3′ |
|
| R:
5′-GTGTGCTGGCAGTGAAGGACTC-3′ |
| JNK | F:
5′-TGATCGCCAGATCTCAGAAG-3′ |
|
| R:
5′-CTGAGGGACACAGGTGACAG-3′ |
| ERK | F:
5′-TGCACTAGTGTCCCATCAGG-3′ |
|
| R:
5′-GTCTGCTCTGCACTTCTTGC-3′ |
| P38 | F:
5′-AAGCCATGAGGCAAGAAACT-3′ |
|
| R:
5′-TGCTGTGATCCTCTTATCCG-3′ |
| β-actin | F:
5′-CAGGCATTGCTGACAGGATG-3′ |
|
| R:
5′-TGCTGATCCACATCTGCTGG-3′ |
Western blotting
RIPA protein lysis buffer and PMSF (Beijing Solarbio
Technology Co., Ltd.) were used to extract total protein from liver
tissue at a ratio of 100:1. The protein content was quantified
using a BCA protein concentration assay kit (Beijing Solarbio
Biotechnology Co., Ltd.). In total, 45 µg protein was
electrophoresed via 10% SDS-PAGE, transferred to PVDF membrane and
blocked with 5% skimmed milk powder at room temperature for 2 h.
Subsequently, the primary antibody was added and incubated at 4°C
for 12–16 h. The primary antibodies used in the experiment included
UII rabbit monoclonal antibody (1:1,000, cat. no. DF7281; Affinity
Biosciences), GPR14 mouse monoclonal antibody (1:1,000, cat. no.
sc-514460; Santa Cruz Biotechnology, Inc.), JNK/p-JNK rabbit/mouse
monoclonal antibodies (1:1,000/1:2,000; cat. nos. 9252 and 9255;
Cell Signalling Technology, Inc.), ERK/p-ERK rabbit monoclonal
antibodies (1:1,000/1:2,000; cat. nos. 4695 and 4370; Cell
Signalling Technology, Inc.), P38/p-P38 rabbit monoclonal
antibodies (1:1,000; cat. nos. 8690 and 4511; Cell Signalling
Technology, Inc.), GAPDH monoclonal antibody (1:5,000; cat. no.
AP0066; BioWorld, Inc.) and β-actin rabbit monoclonal antibody
(1:800; cat. no. AP0060; BioWorld, Inc.). HRP-labelled secondary
antibodies (1:5,000; cat. nos. AS014 and 074-1806; ABclonal Biotech
Co., Ltd. and Beijing XMJ Scientific Co., Ltd., respectively) were
added and incubated for 1 h at room temperature, and the blots were
developed using a highly sensitive ECL kit (Beijing Ximeijie
Technology Co., Ltd.). Image-Pro Plus 6.0 (Media Cybernetics, Inc.)
optical density analysis software was used to determine the grey
values of the protein bands. GAPDH or β-actin were used as a
reference. The relative expression level is the ratio of the grey
value of the target protein and reference protein.
Statistical analysis
The data are presented as the mean ± SEM, and were
analysed using SPSS 25.0 (IBM Corp.). One-way ANOVA and Tukey's
test were used for comparisons among groups. The liver damage score
was analysed using the Kruskal Wallis test, and then the Dunn test
was performed for multiple comparisons. GraphPad Prism (version 7;
GraphPad Software, Inc.) software was used to generate the graphs.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes in liver function in ALI model
rats
Serum ALT and AST levels in the ALI model group were
significantly higher compared with those in the control group
(P<0.01; Table II). After 7
days of pre-treatment with different concentrations of urantide,
the serum levels of ALT and AST did not increase significantly
compared with the control group. After urantide pretreatment, ALT
in each group was significantly lower compared with that in the ALI
group (P<0.01). After pretreatment with 10 and 20 mg/kg
urantide, AST was significantly lower compared with those in the
ALI model group (P<0.01). After pretreatment with 20 mg/kg
urantide, the preventive effect was significantly higher compared
with that in the MgIG group (P<0.01).
 | Table II.Effects of different concentrations
of urantide on the serum levels of ALT and AST in rats. |
Table II.
Effects of different concentrations
of urantide on the serum levels of ALT and AST in rats.
| Group | ALT (U/l) | AST (U/l) |
|---|
| Control | 28.75±3.40 | 123.75±12.82 |
| ALI |
107.25±20.95b |
487.75±97.62b |
| MgIG |
97.75±6.08b |
361.25±16.86b,c |
| Urantide dose |
|
Low |
76.50±13.53b,c,d |
423.25±62.71b |
|
Middle |
52.00±12.73a,c,e |
333.25±61.40b,c |
|
High |
46.75±9.00c,e |
173.50±38.79c,e |
The liver weight and liver index of the ALI model
group were significantly higher compared with those of the control
group (P<0.01; Table III),
indicating that CCl4 caused changes in liver weight and
liver organ index. When urantide (10 or 20 mg/kg) was administered
to the rats, the increase in liver weight and liver index were
lower compared with those in the ALI model group (P<0.01 or
P<0.05), and the protective effect was similar to that in the
MgIG group.
 | Table III.Liver weight and liver index in ALI
model rats. |
Table III.
Liver weight and liver index in ALI
model rats.
| Group | Liver weight
(g) | Liver index
(%) |
|---|
| Control | 8.36±1.60 | 3.68±0.63 |
| ALI |
10.37±1.06a |
4.44±0.43a |
| MgIG |
8.51±0.85c |
3.93±0.37b |
| Urantide dose |
|
Low |
8.50±0.42c | 4.11±0.08 |
|
Middle |
8.44±0.40c |
3.94±0.20b |
|
High |
7.98±0.51c |
3.86±0.27b |
These results suggested that urantide could protect
rats against CCl4-induced liver injury by reducing serum
markers, the liver index and liver weight.
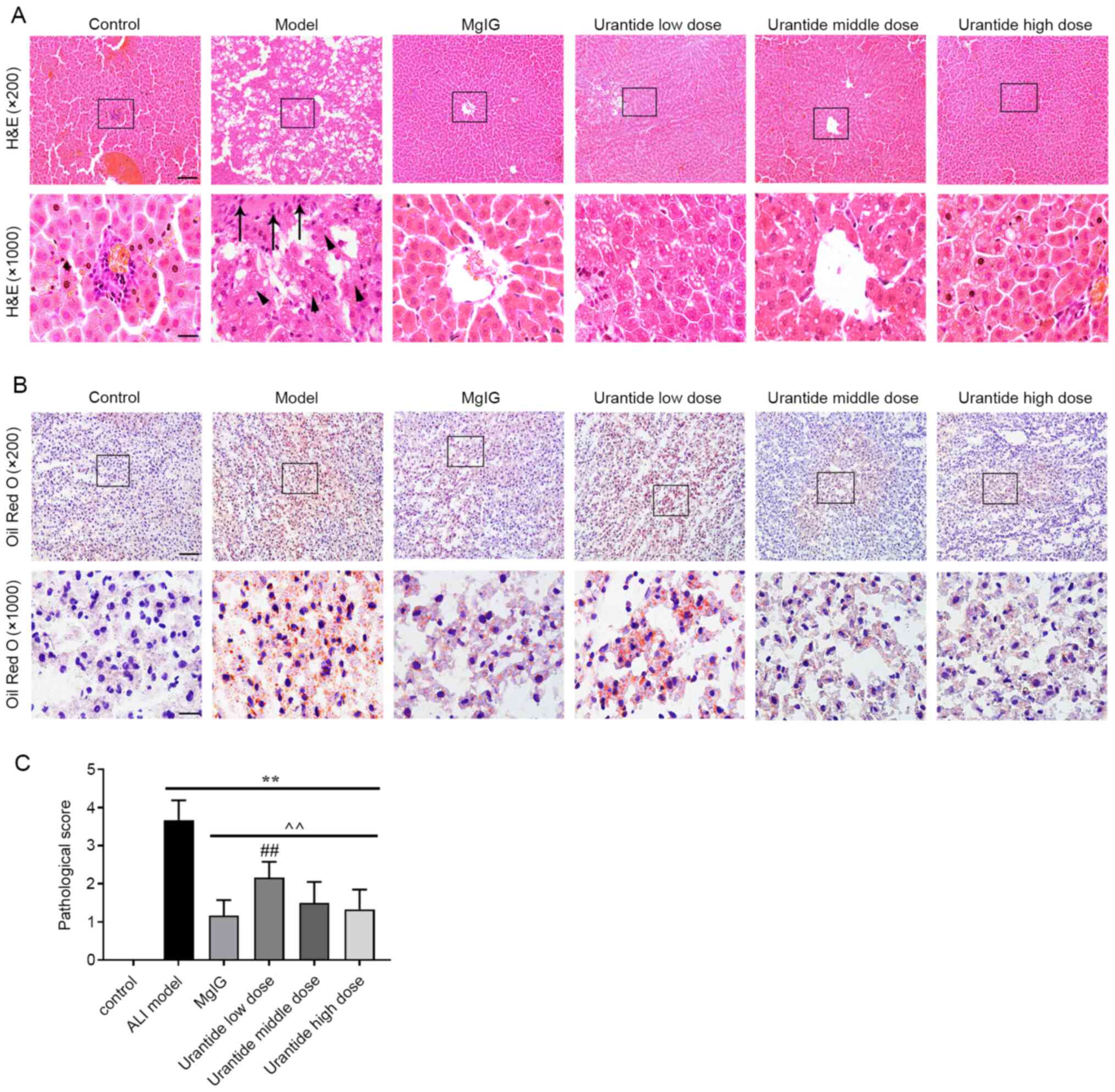
Effect of urantide on liver morphology
and structure in ALI model rats
In the control group, the liver lobules were
normally distributed; the hepatic cords were arranged regularly or
distributed in strips, with little fatty degeneration; and there
was no blood stasis in the liver sinuses. In the ALI model group,
the liver showed a large area of oedema, hepatocyte atrophy
occurred, severe steatosis was present in the cytoplasm, there was
severe structural damage and necrosis of some hepatocytes, nuclei
dissolved and disappeared, and the liver sinus was widened
(Fig. 1A and B). Compared with that
of the ALI model group, the liver tissue morphology was
correspondingly protected in the MgIG group and urantide groups.
According to liver histopathology scoring standard, the
pathological scores of liver tissues in each group were evaluated.
There was almost no pathological change in the control group, while
the average pathological score of the ALI model group was 3.67.
Compared with those of the ALI model group, the average
pathological scores of the urantide low, medium and high
concentration groups were 2.17, 1.50 and 1.33, respectively
(Fig. 1C). The histological and
pathological scores demonstrated that urantide could effectively
prevent CCl4-induced ALI and protect the liver tissue of
ALI model rats.
Expression levels of α-SMA and OPN in
the liver
α-SMA and OPN are oxidative stress-sensitive
cytokines that are associated with the degree of liver injury
(25–27). Compared with those in the control
group, the mRNA and protein expression levels of α-SMA and OPN in
the liver tissue of the ALI model group were significantly
increased (P<0.01; Fig. 2A-E).
ALI also led to a significant increase in the total number of
positive granules. α-SMA was mainly found in the common bile duct
of the liver in ALI model rats, but OPN was mainly found in the
cytoplasm of liver cells with degeneration and necrosis, while a
small amount was distributed in the stroma (Fig. 2F-H). After preventive administration
of urantide, the expression levels of α-SMA and OPN, and positive
particles were decreased (P<0.01). Moreover, the preventive
effect of urantide was greater than that of MgIG. These results
indicated that α-SMA and OPN could serve important roles in ALI.
Thus, inhibiting α-SMA and OPN could effectively prevent ALI.
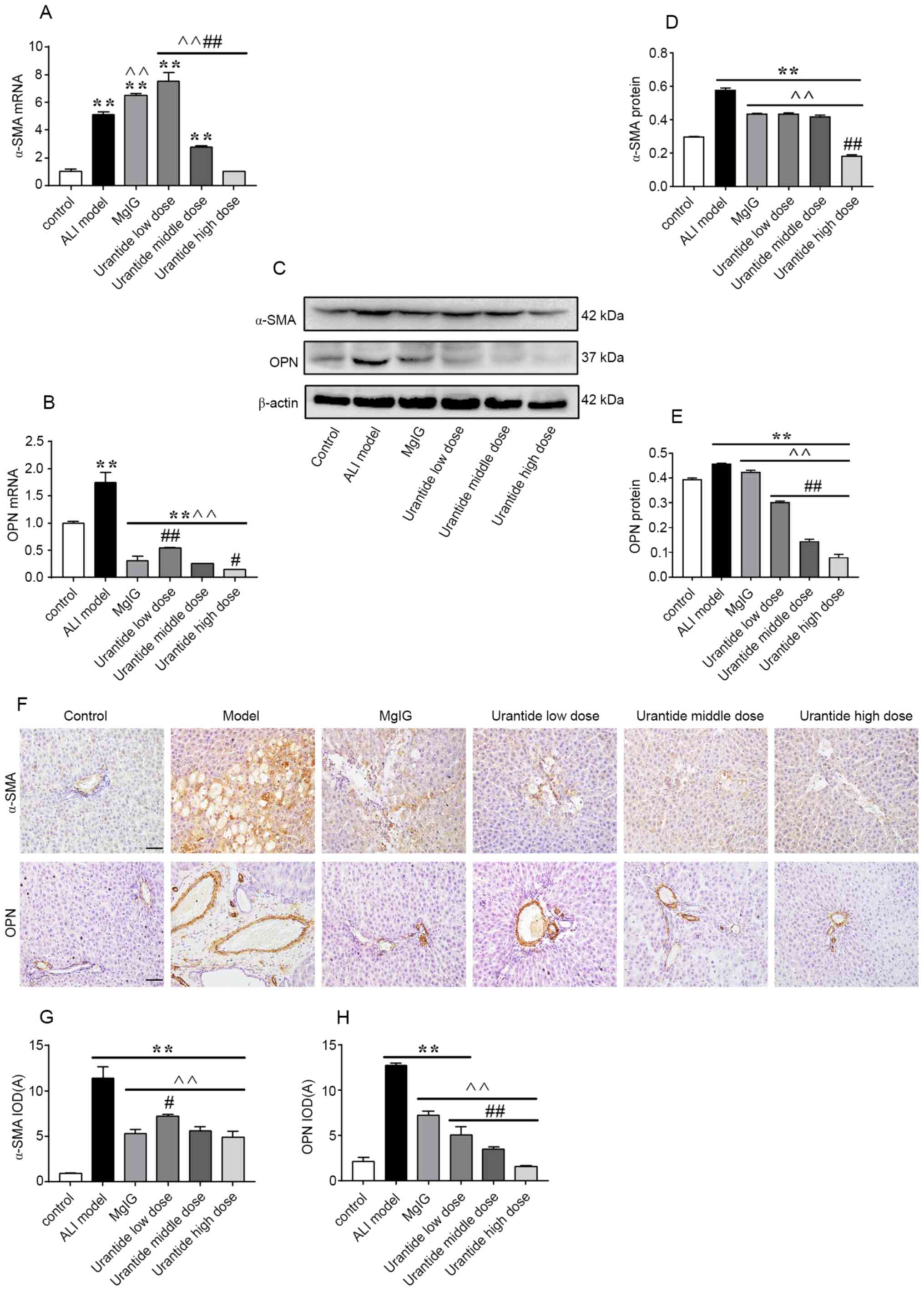 | Figure 2.Expression levels of α-SMA and OPN in
ALI livers were attenuated following urantide administration. The
relative (A) gene and (C and D) protein expression levels of α-SMA
was measured via RT-qPCR and western blotting, respectively. (F)
α-SMA protein expression was determined via immunohistochemistry.
Scale bars, 50 µm. Corresponding (G) α-SMA protein IOD. The
relative (B) gene and (C and E) protein expression levels of OPN
was measured via RT-qPCR and western blotting, respectively. (F)
OPN protein expression was determined via immunohistochemistry
(scale bar, 50 µm). Corresponding (H) OPN protein IOD. The data are
presented as the mean ± SEM. For RT-qPCR, western blotting and
morphological analysis, n=6 per group. **P<0.01 vs. control;
^^P<0.01 vs. ALI model; #P<0.05 and
##P<0.01 vs. MgIG. α-SMA, α-smooth muscle actin; OPN,
osteopontin; RT-qPCR, reverse transcription quantitative PCR; IOD,
integrated optical density; MgIG, magnesium isoglycyrrhizinate;
ALI, ALI, acute liver injury. |
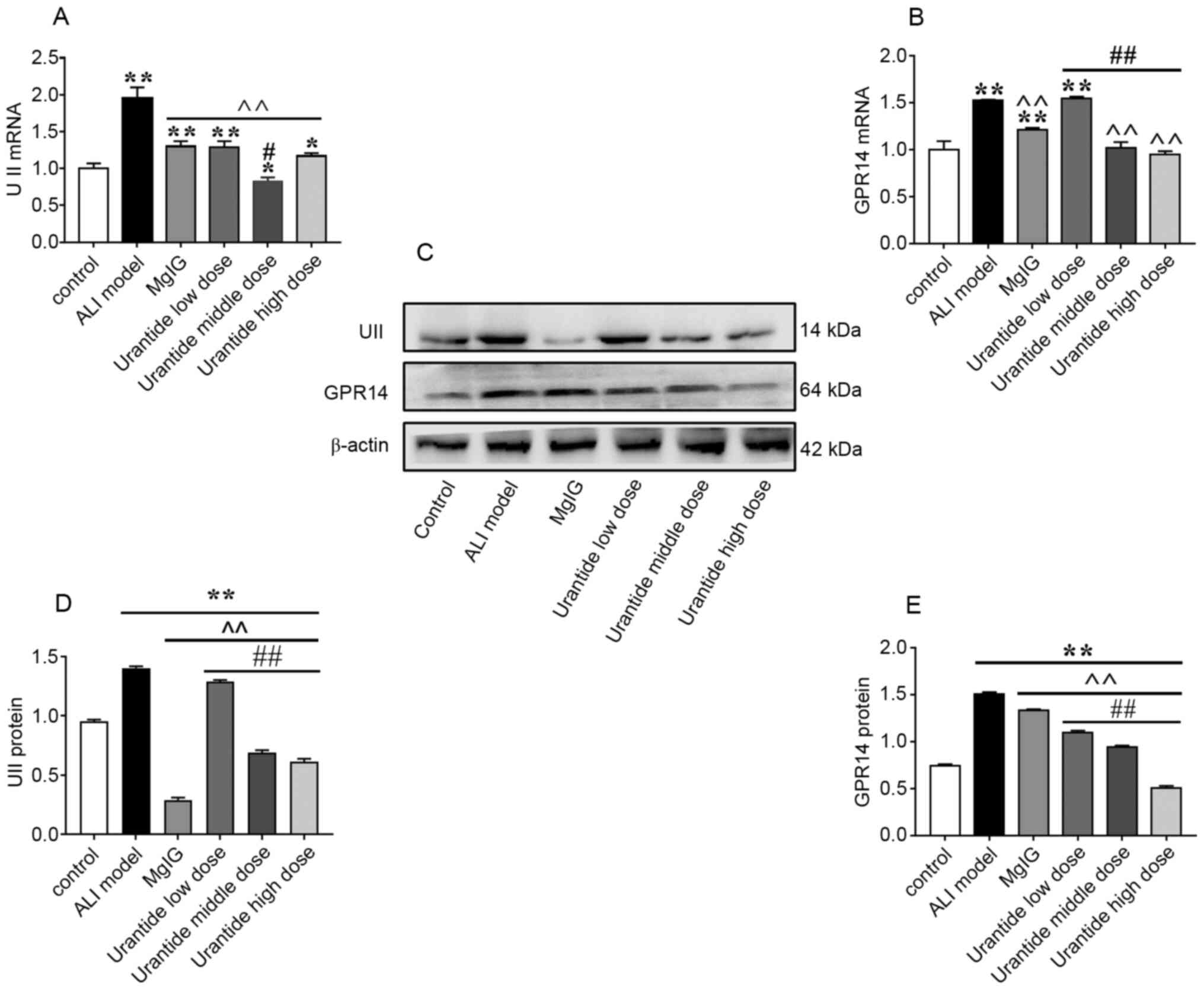
Expression of UII/UT system components
in the liver
Compared with that in the control group, the liver
expression of UII/UT in the ALI model group was significantly
increased (P<0.01; Fig. 3A-E).
After the preventive administration of the UII receptor antagonist
urantide, the expression of the GPR14 protein and gene was
downregulated in a concentration-dependent manner, and the
expression of the UII protein and gene was downregulated compared
with the ALI model group, indicating that the activation state of
the UII/UT system was effectively regulated, and the stress ability
of rats was effectively improved. Furthermore, the therapeutic
effect of urantide was greater than that of MgIG. These results
indicate that the UII/UT system may play an important role in ALI
and that inhibiting the UII/UT system could effectively protect the
liver.
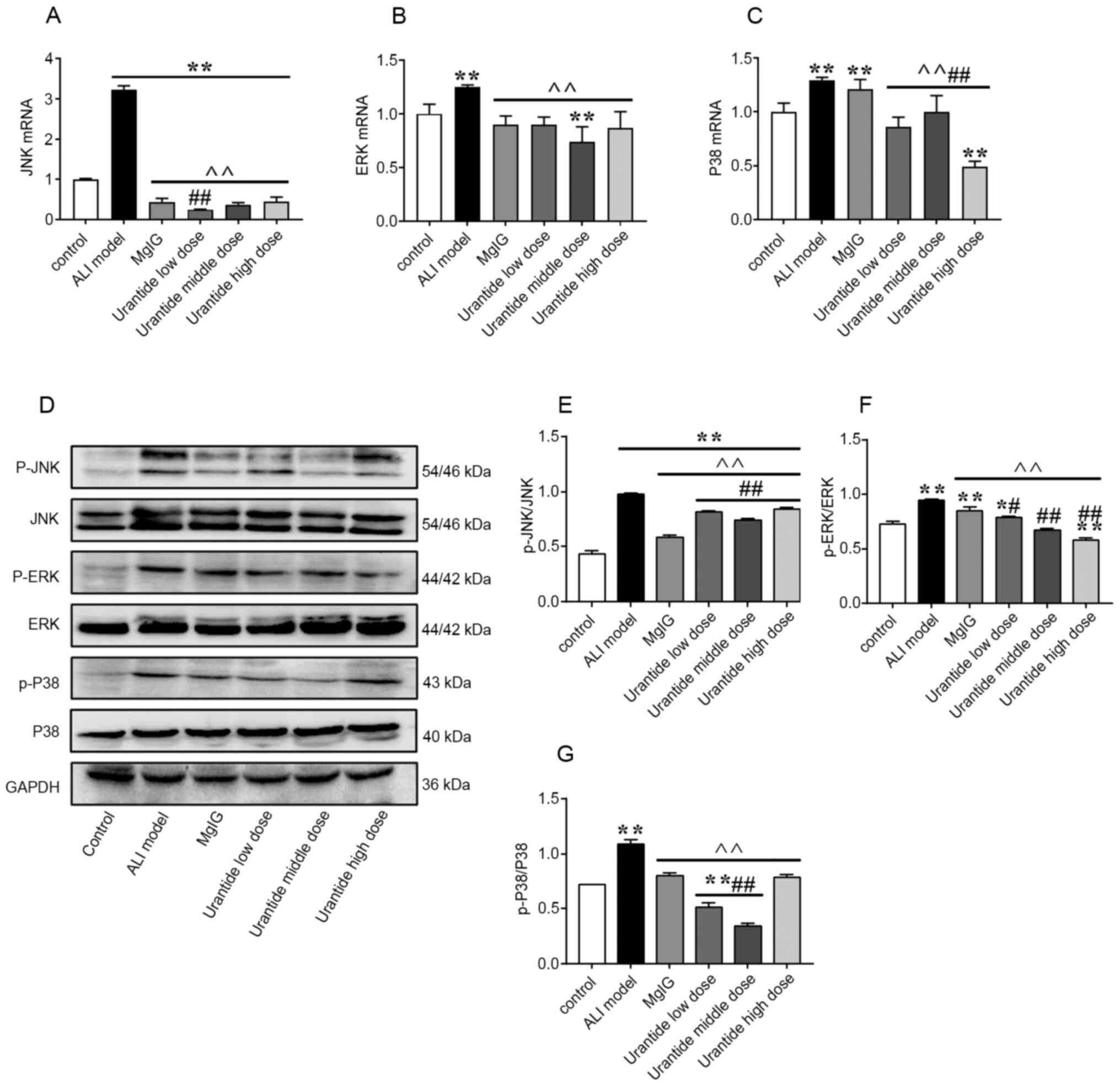
MAPK signalling pathway in the
liver
To verify how the UII/UT system works, the gene and
protein expression of MAPK signalling pathway factors that are
associated with liver injury were examined in ALI model rats. ALI
induced the gene expression of JNK, ERK and P38 in the rat liver
(Fig. 4A-C). Moreover, the protein
expression levels of p-JNK/JNK, p-ERK/ERK and p-P38/P38 were
increased after ALI induction (Fig.
4D-G). Next, it was investigated whether the binding of UII to
its receptor on the cell membrane has a series of biological
effects that regulate the MAPK signalling pathway. Changes in the
MAPK signalling pathway were further observed in ALI model rats
that were pretreated with urantide for 1 week. Compared with that
in the ALI model group, the expression of MAPK signalling
pathway-related genes and proteins were significantly reduced in
the urantide groups (P<0.01). These results suggested that
urantide could effectively protect the liver function of ALI model
rats by regulating the MAPK signalling pathway.
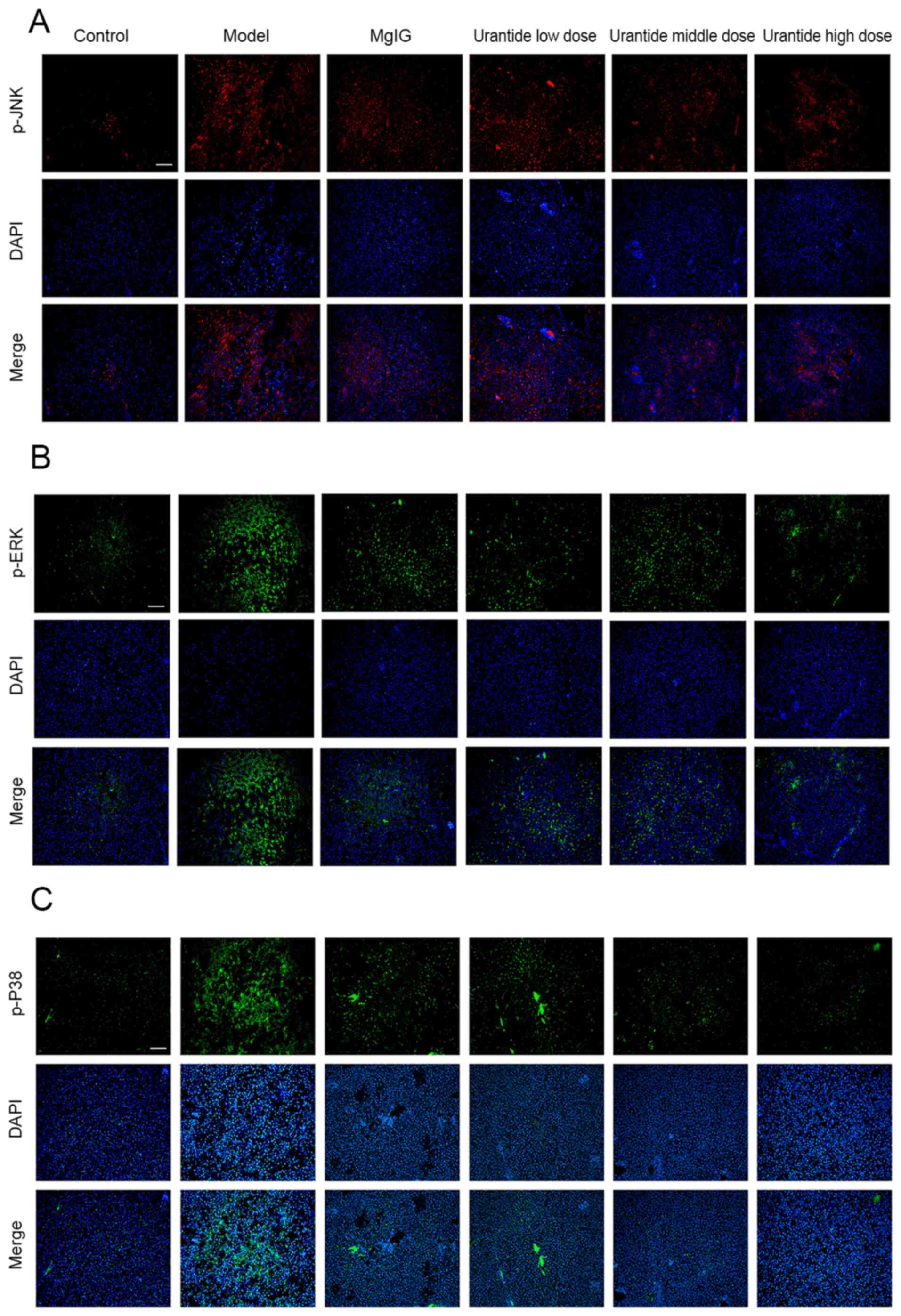
Localization of MAPK signalling
pathway proteins
When analysing the localization of α-SMA and OPN, it
was found that these proteins were mainly expressed in the
cytoplasm of hepatocytes of ALI model rats. Immunofluorescence
analysis confirmed that p-JNK, p-ERK and p-P38 exhibited similar
expression patterns (Fig. 5A-C). It
was concluded that the MAPK signalling pathway was activated during
ALI, and that the localized expression of MAPK signalling proteins
was similar to that of α-SMA and OPN. Moreover, the expression
levels of p-JNK, p-ERK and p-P38 in rats gradually became normal
after the preventive administration of urantide.
Discussion
A 2017 global burden of disease study showed that
~500,000 individuals die of liver injury every year in China
(28). Although there are numerous
hepatoprotective drugs used in clinical practice, their side
effects are significant; therefore, new targeted drugs are urgently
required for clinical use (2,29). For
this reason, the current study used more specific and safer peptide
compounds for ALI research to provide new options for the clinical
prevention and treatment of diseases.
The present study demonstrated that when
CCl4 induced ALI, the liver function indexes ALT and AST
increased significantly. Moreover, the histomorphology of the liver
showed significant pathological changes, and the two trends were
consistent with results by Munakarmi et al (30) and other studies (31,32).
In addition, the current results indicated that the UII/UT system
in the liver was activated, and the oxidative stress-sensitive
cytokines α-SMA and OPN, which can be used to assess liver injury,
also underwent significant changes. Interestingly, the expression
of proteins and genes related to the MAPK signalling pathway were
also changed with the development of the disease. We hypothesize
that antagonizing the UII/UT system and regulating the oxidative
stress-induced MAPK signalling pathway through preventive
administration may have a protective effect against ALI. However,
the role of the UII/UT system in oxidative stress in ALI model rats
has rarely been reported, and thus, the current study conducted
related research. Studies have shown that, although MgIG could
protect the liver in ALI and exert a variety of biological effects,
its preventive and therapeutic efficacy is not yet ideal.
Therefore, it remains necessary to further evaluate the occurrence
and development of ALI.
Urantide is a UII receptor antagonist peptide based
on human UII, which is currently the strongest known UII receptor
antagonist. Our previous study showed that urantide could
effectively inhibit the MAPK signalling pathway in the tissues of
atherosclerotic rats (10). Studies
have revealed that urantide has antioxidant and anti-inflammatory
effects and served a key role in CCl4-induced ALI
(33–36). The present results indicated that,
after 1 week of preventive urantide administration, the serum
levels of ALT and AST did not increase significantly. Consistent
with this finding, the pathological changes gradually approached
normal states.
Liu et al (9)
and other studies reported that preventive administration of
urantide could effectively improve the body's ability to resist
damage, and in the presence of pathogenic factors, urantide could
effectively antagonize the UII/UT system to protect against damage
(19,37). At the same time, it was found that
MAPK signalling pathway was activated after liver injury (16,38,39).
Moreover, the present study conducted related research to determine
whether antagonizing the UII/UT system has an impact on the
regulation of the oxidative stress-induced MAPK signalling
pathway.
The current results indicated that, after
prophylactic urantide administration, the activity of the MAPK
signalling pathway in liver tissue was effectively inhibited
compared with that in the ALI model group. Similarly, the
expression levels of the oxidative stress-sensitive cytokines,
α-SMA and OPN, were also decreased. This finding indicates that
effective regulation of oxidative stress may have a significant
protective effect against strong pathogenic factors that induce
liver damage.
In conclusion, urantide alleviated
CCl4-induced ALI. The objective of the present study was
to investigate the preventive effect of urantide on
CCl4-induced liver injury in rats. It was suggested that
after urantide pretreatment, the serological indexes ALT and AST
did not increase significantly, and the liver tissue morphology was
protected accordingly. At the same time, urantide could reduce the
CCl4-induced ALI toxicity by antagonizing the UII/UT
system and regulating the activation of the MAPK signalling
pathway, as well as improved the stress ability of ALI model rats,
thereby serving a role in protecting the liver. Therefore, uratide
may become a hepatoprotective drug clinically. However, whether the
UII/UT system directly or indirectly affects the MAPK signalling
pathway requires further research and verification. Although it was
found that urantide has a preventive and protective effect against
CCl4-induced ALI, the exact mechanism remains unclear.
In the future, further research will be conducted to explain the
specific mechanism and provide detailed experimental data for liver
damage caused by various aetiologies. This study only evaluated
animals in the early stage after CCl4 administration,
and late effects will be examined in future studies.
Acknowledgements
The authors would like to thank Mr. Long Chen
(Chengde Medical University, Basic Research Institute, China) for
the support with the technology for oil red O staining.
Funding
This study received funding from the Hebei
Provincial Natural Science Foundation (grant no. H2020406011),
Hebei Provincial Party Committee Organization Department Youth Top
Talent Project [grant no. JZZ (2016) No. 9], Hebei Provincial
Science and Technology Department Science and Technology Innovation
Guidance Special Project, Hebei Provincial Department of Education
Key Project (grant no. ZD2019098), Hebei Provincial Department of
Education Outstanding Youth Fund Project (grant no. YQ2013005),
Chengde Medical College National Natural Science Foundation Project
Cultivation Fund (grant no. 201916) and Key Subjects (Pathology and
Pathophysiology) at Colleges and Universities of Hebei
Province.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JZ and YL conceived and designed the study. YL, ZG,
HC, TW and YX carried out the experiments. TW and YL carried out
the animal model experiments. YL analysed the data. JZ, YL and TW
confirmed the authenticity of the raw data. YL wrote the
manuscript. All authors carefully reviewed the manuscript, and read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics Committee of
Experimental Animal Center of Chengde Medical University (approval
no. CDMULAC-201917-017, January 1, 2020). This study adhered to
ARRIVE guidelines/methodology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen M, Borlak J and Tong W: High
lipophilicity and high daily dose of oral medications are
associated with significant risk for drug-induced liver injury.
Hepatology. 58:388–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGill MR and Jaeschke H: Biomarkers of
drug-induced liver injury: Progress and utility in research,
medicine, and regulation. Expert Rev Mol Diagn. 18:797–807. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crescioli G, Lombardi N, Bettiol A,
Marconi E, Risaliti F, Bertoni M, Menniti Ippolito F, Maggini V,
Gallo E, Firenzuoli F, et al: Acute liver injury following
Garcinia cambogia weight-loss supplementation: Case series
and literature review. Intern Emerg Med. 13:857–872. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allard J, Le Guillou D, Begriche K and
Fromenty B: Drug-induced liver injury in obesity and nonalcoholic
fatty liver disease. Adv Pharmacol. 85:75–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai C, Xiao X, Li D, Tun S, Wang Y, Velkov
T and Tang S: Chloroquine ameliorates carbon tetrachloride-induced
acute liver injury in mice via the concomitant inhibition of
inflammation and induction of apoptosis. Cell Death Dis.
9:11642018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wendon J, Cordoba J, Dhawan A, Larsen FS,
Manns M, Samuel D, Simpson KJ, Yaron I, Bernardi M; European
Association for the Study of the Liver. Electronic address:
easloffice@easloffice.eu, ; et al: EASL Governing Board
representative: EASL Clinical Practical Guidelines on the
management of acute (fulminant) liver failure. J Hepatol.
66:1047–1081. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramos-Tovar E, Hernández-Aquino E,
Casas-Grajales S, Buendia-Montaño LD, Galindo-Gómez S, Camacho J,
Tsutsumi V and Muriel P: Stevia prevents acute and chronic liver
injury induced by carbon tetrachloride by blocking oxidative stress
through Nrf2 upregulation. Oxid Med Cell Longev. 2018:38234262018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schueller F, Roy S, Loosen SH, Alder J,
Koppe C, Schneider AT, Wandrer F, Bantel H, Vucur M, Mi QS, et al:
miR-223 represents a biomarker in acute and chronic liver injury.
Clin Sci (Lond). 131:1971–1987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu LM, Tu WJ, Zhu T, Wang XT, Tan ZL,
Zhong H, Gao DY and Liang DY: IRF3 is an important molecule in the
UII/UT system and mediates immune inflammatory injury in acute
liver failure. Oncotarget. 7:49027–49041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Miao G, Wang T, Li J and Xie L:
Urantide attenuates myocardial damage in atherosclerotic rats by
regulating the MAPK signalling pathway. Life Sci. 262:1185512020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Xie YQ, Miao GX, Cui HP, Liu K, Li
Y, Li Y and Zhao J: Urotensin receptor antagonist urantide improves
atherosclerosis-related kidney injury by inhibiting JAK2/STAT3
signaling pathway in rats. Life Sci. 247:1174212020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ross B, McKendy K and Giaid A: Role of
urotensin II in health and disease. Am J Physiol Regul Integr Comp
Physiol. 298:R1156–R1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leifeld L, Clemens C, Heller J, Trebicka
J, Sauerbruch T and Spengler U: Expression of urotensin II and its
receptor in human liver cirrhosis and fulminant hepatic failure.
Dig Dis Sci. 55:1458–1464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haston S, Pozzi S, Carreno G, Manshaei S,
Panousopoulos L, Gonzalez-Meljem JM, Apps JR, Virasami A, Thavaraj
S, Gutteridge A, et al: MAPK pathway control of stem cell
proliferation and differentiation in the embryonic pituitary
provides insights into the pathogenesis of papillary
craniopharyngioma. Development. 144:2141–2152. 2017.PubMed/NCBI
|
|
15
|
Peng Z, Gong X, Yang Y, Huang L, Zhang Q,
Zhang P, Wan R and Zhang B: Hepatoprotective effect of quercetin
against LPS/d-GalN induced acute liver injury in mice by inhibiting
the IKK/NF-κB and MAPK signal pathways. Int Immunopharmacol.
52:281–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ,
Han JH, Lee S, Kim HI, Shin HP and Jeon JW: Polydeoxyribonucleotide
exerts protective effect against CCl4-induced acute
liver injury through inactivation of NF-κB/MAPK signaling pathway
in mice. Int J Mol Sci. 21:78942020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Wei X, Wei X, Sun X, Huang X,
Liang Y, Xu W, Zhu X, Lin X and Lin J:
4-hydroxybenzo[d]oxazol-2(3H)-one ameliorates LPS/D-GalN-induced
acute liver injury by inhibiting TLR4/NF-κB and MAPK signaling
pathways in mice. Int Immunopharmacol. 83:1064452020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Xie LD, Song CJ, Mao XX, Yu HR, Yu
QX, Ren LQ, Shi Y, Xie YQ, Li Y, et al: Urantide improves
atherosclerosis by controlling C-reactive protein, monocyte
chemotactic protein-1 and transforming growth factor-β expression
in rats. Exp Ther Med. 7:1647–1652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu LM, Liang DY, Ye CG, Tu WJ and Zhu T:
The UII/UT system mediates upregulation of proinflammatory
cytokines through p38 MAPK and NF-κB pathways in LPS-stimulated
Kupffer cells. PLoS One. 10:e01213832015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 8th Edition. The National
Academies Press; 2011, doi: 10.17226/12910.
|
|
21
|
Sarhadynejad Z, Sharififar F, Pardakhty A,
Nematollahi MH, Sattaie-Mokhtari S and Mandegary A: Pharmacological
safety evaluation of a traditional herbal medicine
‘Zereshk-e-Saghir’ and assessment of its hepatoprotective effects
on carbon tetrachloride induced hepatic damage in rats. J
Ethnopharmacol. 190:387–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan J, Jie Z, Hou L, Wanderley JL, Soong
L, Gupta S, Qiu S, Chan T and Sun J: Parenchymal expression of CD40
exacerbates adenovirus-induced hepatitis in mice. Hepatology.
53:1455–1467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Li X, Luo R, Wang W, Wang T and Tang
H: Detection of KIT genotype in pigs by TaqMan MGB Real-time
quantitative polymerase chain reaction. DNA Cell Biol. 37:457–464.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YY, Ge QX, Cao J, Zhou YJ, Du YL, Shen
B, Wan YJ and Nie YQ: Association of Fusobacterium nucleatum
infection with colorectal cancer in Chinese patients. World J
Gastroenterol. 22:3227–3233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu Y, Lin Y, Huang X, Wu S, Wei J and Yang
C: Oxaliplatin aggravates hepatic oxidative stress, inflammation
and fibrosis in a non-alcoholic fatty liver disease mouse model.
Int J Mol Med. 43:2398–2408. 2019.PubMed/NCBI
|
|
26
|
Urtasun R, Lopategi A, George J, Leung TM,
Lu Y, Wang X, Ge X, Fiel MI and Nieto N: Osteopontin, an oxidant
stress sensitive cytokine, up-regulates collagen-I via integrin
α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology.
55:594–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Srungaram P, Rule JA, Yuan HJ, Reimold A,
Dahl B, Sanders C and Lee WM; Acute Liver Failure Study Group, :
Plasma osteopontin in acute liver failure. Cytokine. 73:270–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen
W, Li X, Wang L, Wang L, Liu Y, et al: Mortality, morbidity, and
risk factors in China and its provinces, 1990–2017: A systematic
analysis for the Global Burden of Disease Study 2017. Lancet.
394:1145–1158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Jiang L, Ren Y, Shen M and Xie J:
Characterizations and hepatoprotective effect of polysaccharides
from Mesona blumes against tetrachloride-induced acute liver injury
in mice. Int J Biol Macromol. 124:788–795. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munakarmi S, Chand L, Shin HB, Jang KY and
Jeong YJ: Indole-3-carbinol derivative DIM mitigates carbon
tetrachloride-induced acute liver injury in mice by inhibiting
inflammatory response, apoptosis and regulating oxidative stress.
Int J Mol Sci. 21:20482020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang W, Dong Z, Chang X, Zhang C, Rong G,
Gao X, Zeng Z, Wang C, Chen Y, Rong Y, et al: Protective effect of
the total flavonoids from Apocynum venetum L. on carbon
tetrachloride-induced hepatotoxicity in vitro and in vivo. J
Physiol Biochem. 74:301–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zira A, Kostidis S, Theocharis S, Sigala
F, Engelsen SB, Andreadou I and Mikros E: 1H NMR-based metabonomics
approach in a rat model of acute liver injury and regeneration
induced by CCl4 administration. Toxicology. 303:115–124.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuang Y, Han X, Xu M, Wang Y, Zhao Y and
Yang Q: Oxaloacetate ameliorates chemical liver injury via
oxidative stress reduction and enhancement of bioenergetic fluxes.
Int J Mol Sci. 19:16262018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Y, Wen PH, Zhang XX, Dai Y and He Q:
Breviscapine ameliorates CCl4-induced liver injury in
mice through inhibiting inflammatory apoptotic response and ROS
generation. Int J Mol Med. 42:755–768. 2018.PubMed/NCBI
|
|
35
|
Wang M, Niu J, Ou L, Deng B, Wang Y and Li
S: Zerumbone protects against carbon tetrachloride
(CCl4)-induced acute liver injury in mice via inhibiting
oxidative stress and the inflammatory response: Involving the
TLR4/NF-κB/COX-2 pathway. Molecules.
24:19642019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu T, Shen M, Liu S, Yu Q, Chen Y and Xie
J: Ameliorative effect of Cyclocarya paliurus
polysaccharides against carbon tetrachloride induced oxidative
stress in liver and kidney of mice. Food Chem Toxicol.
135:1110142020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang DY, Liu LM, Ye CG, Zhao L, Yu FP,
Gao DY and Wang YY, Yang ZW and Wang YY: Inhibition of UII/UTR
system relieves acute inflammation of liver through preventing
activation of NF-κB pathway in ALF mice. PLoS One. 8:e648952013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu Y, Hu D, Ma S, Zhao X, Wang S, Wei G,
Wang X, Wen A and Wang J: Protective effect of wedelolactone
against CCl4-induced acute liver injury in mice. Int
Immunopharmacol. 34:44–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mo W, Wang C, Li J, Chen K, Xia Y, Li S,
Xu L, Lu X, Wang W and Guo C: Fucosterol protects against
concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB
pathway activity. Gastroenterol Res Pract. 2018:28241392018.
View Article : Google Scholar : PubMed/NCBI
|