Introduction
Population aging has become a common issue
worldwide; it is estimated that individuals aged ≥60 years will
constitute ≥20% of the world's total population by 2050 (1). The challenges associated with an aging
population already pose great difficulties; however, it is
estimated that in the coming years, an increasing number of
countries will be faced with graver public health concerns and
heavier healthcare burdens, of which cardiovascular disease (CVD)
will account for a large percentage (2).
CVD, known for its high morbidity and mortality, is
caused by a number of factors, among which intrinsic aging plays a
prominent role; it is also the leading cause of mortality for
seniors of >65 years of age (3).
Oxidative stress is considered to be a crucial factor that triggers
heart aging. A growing body of evidence has confirmed that reactive
oxygen species (ROS) are produced in increasing amounts in
myocardial tissues as age increases (4). Therefore, the effective inhibition of
ROS is expected to attenuate heart aging, thus relieving its
detrimental effects on human health (5). D-galactose (D-gal) treatment has been
used in a number of rodent models of aging (6,7),
including brain and heart aging, and has been reported to notably
induce aging-related changes, such as reducing thymus coefficients
and increasing pathological injury and cellular senescence in the
liver, spleen and hippocampus (8).
D-gal is a reducing sugar that, when it accumulates in the body,
reacts with free amines from amino acids in proteins and peptides
to form an unstable compound Schiff base, which persists for
several months after as the compound is oxidized to very stable
advanced glycation end products (AGEs) (9). AGEs increase during aging and are
considered to be one of the signs of aging (10).
Adiponectin, an adipocyte-specific hormone derived
from fat, performs an essential anti-inflammatory and antioxidant
function in CVDs (11). A previous
study indicated that the transfection of adiponectin into
endothelial progenitor cells protected the cognitive function of
rats with D-gal-induced aging (12). However, at present, detailed
research on the effectiveness and mechanisms of adiponectin in the
aging of myocardial fibers, namely cardiomyocyte senescence, is
limited. Adiponectin has two cell surface homologous receptors,
adiponectin receptor 1 (AdipoR1) and AdipoR2, both of which are
expressed in various types of tissues (13). In addition, adaptor protein
phosphotyrosine interacting with PH domain and leucine zipper 1
(APPL1), as an adapter protein, has been identified as the signal
transductor of AdipoR1/2 (14).
The current state of the aging population and the
previous research findings prompted further investigations into the
association between adiponectin and heart aging induced by D-gal,
in addition to determining whether adiponectin exerts an inhibitory
effect on cardiomyocyte senescence through the AdipoR1/APPL1
signaling pathway. The present study aimed to provide an innovative
approach that may be used to attenuate heart aging. The findings
presented herein may provide novel insight into the treatment of
CVDs.
Materials and methods
Animals and treatments
BALB/c mice (n=16; age, 2 and 15 months old; weight,
25 g-30 g; male) were purchased from the Guangdong Medical
Laboratory Animal Center and housed in the following two groups
(eight mice per group): Young mice (2 months old) and aged mice (15
months old). The mice were housed in a controlled environment at a
temperature of 20–25°C and a humidity level of 50–70% under a 12-h
light/dark cycle and were provided with food and water ad
libitum. Following acclimation to the laboratory environment,
all 16 mice were sacrificed by cervical dislocation following
anesthesia with 350 mg/kg chloral hydrate (10%, abdominal
injection). Mice were checked for complete cardiac arrest and pupil
dilation to confirm death and then their blood samples and
myocardial tissue samples were collected for subsequent analysis.
All procedures related to animal experiments in the present study
were approved by the Shanghai Municipal Hospital of Traditional
Chinese Medicine (approval no. dw2019018; Shanghai, China).
Western blot analysis
Total proteins were extracted from the tissues using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and protein
concentration was determined using BCA kits (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. SDS-PAGE
loading buffer (Beyotime Institute of Biotechnology) was added to
the protein samples, which were then boiled in a water bath for 3–5
min to achieve protein denaturation. Subsequently, 40 µg
protein/lane was electrophoresed with 12% SDS-PAGE running buffer
(Beyotime Institute of Biotechnology) at room temperature, and then
transferred to a PVDF membrane (Thermo Fisher Scientific, Inc.),
which was blocked in 5% skimmed milk on a shaker (Beyotime
Institute of Biotechnology) for 1 h at room temperature. TBS with
Tween-20 (0.2%, TBST) buffer (Shanghai Aladdin Bio-Chem Technology
Co., Ltd.) was then used to wash the membrane three times for 1 min
each time, prior to incubation with the primary antibody dilution
buffer (Nanjing Channel Technology Group) overnight at 4°C. On the
second day, TBST buffer was again used to wash the membrane three
times for 5 min each time, followed by incubation with the
secondary antibody dilution buffer (Nanjing Channel Technology
Group) at room temperature for 2 h. Finally, the membrane was
washed three times with TBST for 5 min each time prior to
chemiluminescence detection (ECL Western substrate; Thermo Fisher
Scientific, Inc.) using Image Lab software (Version 4.0; Bio-Rad
Laboratories, Inc.). GAPDH (1:1,000; cat. no. 5174; Cell Signaling
Technology, Inc.) was selected as the internal control. All
experiments were performed in triplicate. The antibodies were as
follows: Anti-p16 (1:1,000; cat. no. 18769; Cell Signaling
Technology, Inc.), anti-p21 (1:1,000; cat. no. 2947; Cell Signaling
Technology, Inc.), anti-adiponectin (1:1,000; cat. no. 2789; Cell
Signaling Technology, Inc.), anti-AdipoR1 (1:800; cat. no.
bs-0610R; BIOSS), anti-APPL1 (1:1,000; cat. no. 3858; Cell
Signaling Technology, Inc.), anti-heme oxygenase (HO)-1 (1:1,000;
cat. no. 43966; Cell Signaling Technology, Inc.), anti-high
mobility group box 1 (HMGB1; 1:1,000; cat. no. 3935; Cell Signaling
Technology, Inc.) and mouse anti-rabbit secondary antibody
(1:1,000; cat. no. 5127; Cell Signaling Technology, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the mouse blood samples
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The RNA
purity was examined using a NanoDrop 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.). Total RNA (2.5 µg) was reverse
transcribed into cDNA using an EasyScript® First-Strand
cDNA Synthesis SuperMix (TransGen Biotech Co., Ltd.) according to
the manufacturer's instructions. qPCR was performed on a Veriti™
96-Well Thermal Cycler (Thermo Fisher Scientific, Inc.) with the
following thermocycling conditions: Initial denaturation at 85°C
for 30 sec, followed by 22 cycles at 55°C for 30 sec and 72°C for
30 sec. A mixture of 10 µl SYBR™ Green PCR Master Mix (Thermo
Fisher Scientific, Inc.), 7 µl water and 1 µl primer working
solution (Shanghai GenePharma Co., Ltd.) was added to each well
containing 2 µl cDNA for each reaction. Each sample was tested in
triplicate. The plate was then centrifuged (8,000 × g, 20 min) to
spin down the solution on a Centrifuge 5810 device (Eppendorf) at
4°C. GAPDH was used as the internal reference gene. The analysis of
the data was performed using the 2−ΔΔCq method (15). The sequence of primer pairs were as
follows: Adiponectin forward (F), 5′-GCATTCAGTGTGGGATTGGAG-3′ and
reverse (R), 5′-AGACTGTGATGTGGTAGGCAAAG-3′; AdipoR1 F,
5′-CAAGGCTGAAGAAGAACAAGC-3′ and R, 5′-AAGGAGGGCATAGGTGGTCT-3′;
APPL1 F, 5′-AGCCAGTGACCCTTTATATCTGC-3′ and R,
5′-AGGTATCCAGCCTTTCGGGTT-3′; and GAPDH F,
5′-CTCTCTGCTCCTCCCTGTTC-3′ and R, 5′-CGATACGGCCAAATCCGTTC-3′.
Cell culture and treatments
H9c2 rat cardiomyocytes were obtained from The Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences
and cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% high-quality FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin;
the medium was replaced every 2 days. Cells were maintained in a
humidified incubator of 95% air and 5% CO2 at 37°C. Upon
reaching 80% confluence, the H9c2 cells were seeded into 96-well
plates at a density of 2×103 cells/well and treated with
2.5, 5 and 10 g/l D-gal (Macklin, Inc.) at 37°C for 24 h before
being harvested for use in further experiments and analysis.
Subsequently, When the density of H9c2 cells reached ~70%,
overexpression plasmids for adiponectin (Ov-Adiponectin) were
constructed (Shanghai GenePharma Co., Ltd.) and were transfected
into H9c2 cells (1.5 µg/well) using Lipofectamine® 3000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
untransfected H9c2 cells induced by 10 g/l D-gal were used as the
negative control (NC) group for the Ov-Adiponectin group.
Subsequently, small interfering RNAs (siRNAs/si) targeting AdipoR1
(siAdipoR1, 5′-AAGGTACTACTCAACTAGAATGT-3′) and APPL1 (siAPPL1,
5′-ATGATAGGATGTGAGATAAGTCC-3′) were constructed (Shanghai
GenePharma Co., Ltd.) and were transfected into Ov-Adiponectin H9c2
cells using Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and negative control (si-NC; cat. no. 12935300)
were obtained from Thermo Fisher Scientific, Inc. The D-gal-induced
cells transfected with Ov-Adiponectin plasmid were defined as the
NC group for both the D-gal 10 g/l + Ov-Adiponectin + si-AdipoR1
and D-gal 10 g/l + Ov-Adiponectin + si-APPL1 groups. The plasmids
were transfected into cells at a concentration of 50 ng/ml. After
12 h incubation at 37°C, the medium was replaced with fresh DMEM
and cells were cultured for 72 h at 37°C before subsequent
experiments.
Senescence-associated β-galactose
(SA-β-gal) staining
SA-β-gal staining was performed using the Senescence
Assay kit (cat. no. ab65351; Abcam) to observe signs of aging in
H9c2 cells induced by D-gal, according to the manufacturer's
protocol. Briefly, H9c2 cells treated with D-gal were washed with
PBS and fixed with fixative solution for 10 min at room
temperature, after which the cells were washed with PBS again and
incubated in a staining solution mix for 1 h at 37°C. The solutions
and staining supplement mentioned were all included with the kit.
Finally, seven representative images (magnification, ×100) were
obtained using a fluorescence microscope (Leica Microsystems GmbH)
from randomly selected fields of view. The procedures were
performed in triplicate for each group.
ROS level detection
A ROS Assay kit (cat. no. S0033S, Beyotime Institute
of Biotechnology) was used to examine the production of ROS in H9c2
cells. Zinc protoporphyrin (ZnPP; 100 µg/ml; Sigma-Aldrich; Merck
KGAA, an inhibitor of HO-1, was co-incubated with cells at 37°C for
20 min. Cells at a density of 1×106 cells/ml were
collected and suspended in diluted 2′-7′Dichlorofluorescin
diacetate (DCFH-DA) at a concentration of 10 µmol/l. Following
incubation for 20 min at 37°C, the cells were washed three times
with serum-free cell culture medium to sufficiently remove the
DCFH-DA that did not enter the cells. Finally, an excitation
wavelength of 488 nm and emission wavelength of 525 nm were applied
using a microplate reader to detect the intensity of fluorescence
before and after stimulation in real-time or at different time
points.
Malondialdehyde (MDA) content
detection
The lipid peroxidation levels of H9c2 cells
subjected to the different treatments were examined using a Lipid
Peroxidation MDA Assay kit (cat. no. S0131S, Beyotime Institute of
Biotechnology). The preparation of TBA stock solution and MDA
working solution, as well as the dilution of the standard
substances were all performed in accordance with the product
manual. The MDA content in the sample solution was calculated using
a standard curve. The experiment was replicated in triplicate.
Statistical analysis
Data are presented as the mean ± SD. Data were
analyzed using GraphPad Prism 8.0.1 software (GraphPad Software,
Inc.). Statistical differences were determined using a one-way
ANOVA followed by Tukey's post hoc test for group comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Adiponectin, AdipoR1 and APPL1
expression levels are downregulated in aged mouse plasma and
myocardial tissues
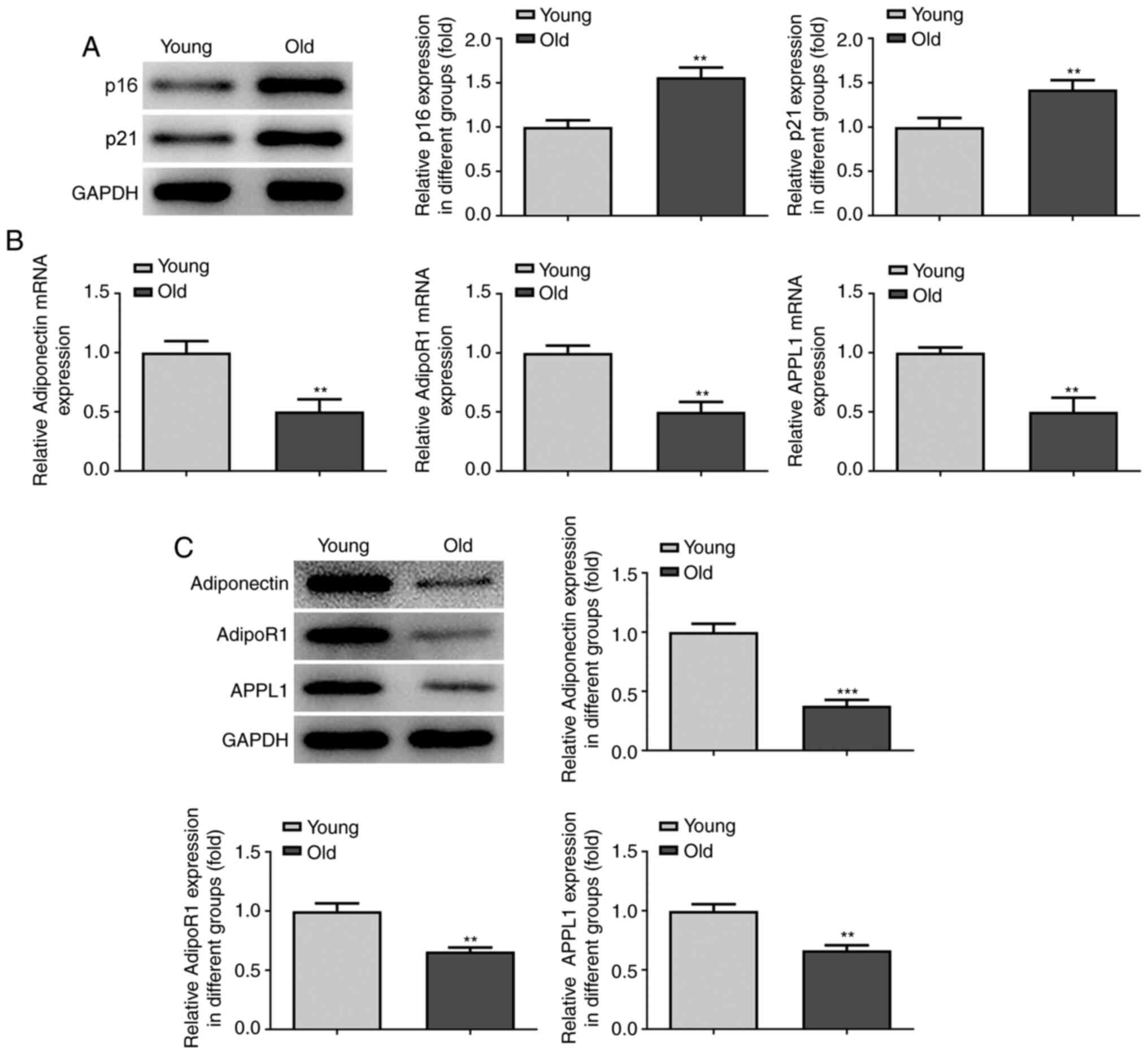
Western blot analysis was first performed to analyze
the expression levels of the senescence-related genes, p16 and p21,
in the myocardial tissues of both young mice and aged mice. A
markedly higher expression of p16 and p21 was found in the tissues
of the older group compared with the younger group (Fig. 1A). Furthermore, to explore the
association between adiponectin and heart aging, the expression
levels of adiponectin, AdipoR1 and APPL1 in the plasma of both the
young and aged mice were analyzed by RT-qPCR, followed by the
detection of these levels in the mouse myocardial tissues by
western blot analysis. The results revealed that the relative mRNA
and protein expression levels of adiponectin, AdipoR1 and APPL1
were downregulated in the older group compared with the younger
group in both the plasma and myocardial tissues (Fig. 1B and C). These findings suggested a
negative association between adiponectin expression and heart
aging.
Expression levels of adiponectin,
AdipoR1 and APPL1 are downregulated in D-gal-treated
cardiomyocytes
To establish the cardiomyocyte model of senescence,
western blot analysis was performed to detect the expression levels
of p16 and p21 in H9c2 cardiomyocytes treated with 2.5, 5 and 10
g/l D-gal. The results revealed that, compared with the control
group, the relative expression levels of p16 and p21 were elevated
in a concentration-dependent manner in the groups with D-gal
induction, and cells treated with 10 g/l D-gal exhibited the most
prominent elevation of p16 and p21 expression (Fig. 2A). Therefore, D-gal at the
concentration of 10 g/l was selected for use in subsequent
experiments.
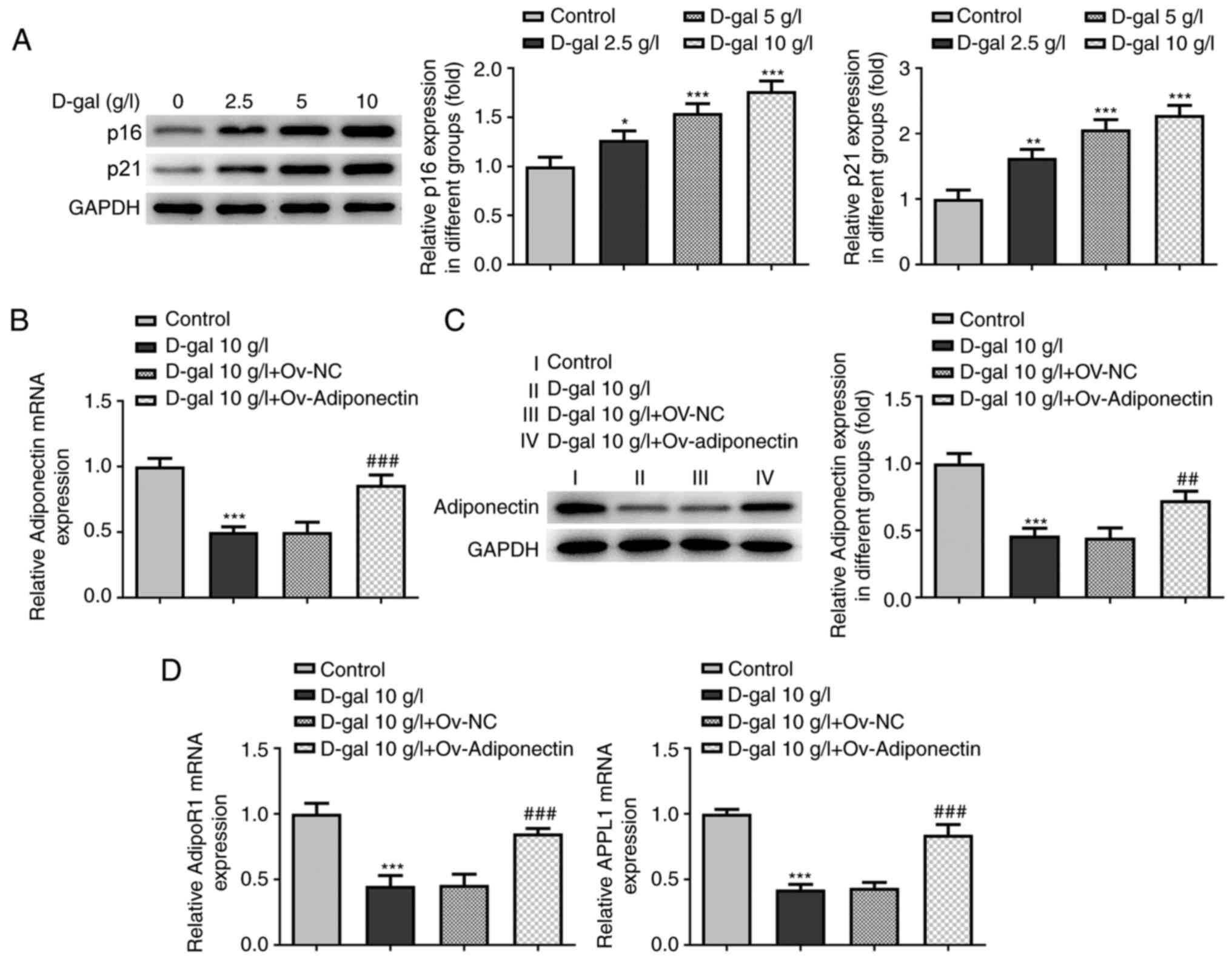 | Figure 2.Expression of adiponectin, AdipoR1
and APPL1 is downregulated in D-gal-treated cardiomyocytes. (A)
Relative expression levels of p16 and p21 in H9c2 cells treated
with 2.5, 5 and 10 g/l of D-gal were analyzed using western blot
analysis. *P<0.05, **P<0.01, ***P<0.001 vs. control.
Transfection with Ov-adiponectin promotes the expression of AdipoR1
and APPL1 in D-gal-treated cardiomyocytes. (B) Relative mRNA
expression of adiponectin in H9c2 cells before and after
transfection with Ov-adiponectin plasmid was analyzed via RT-qPCR.
***P<0.001 vs. control; ###P<0.001 vs. D-gal +
Ov-NC. (C) Relative protein expression of adiponectin in H9c2 cells
before and after transfection with Ov-adiponectin plasmid was
analyzed using RT-qPCR. ***P<0.001 vs. control;
##P<0.01 vs. D-gal + Ov-NC. (D) Relative mRNA
expression levels of AdipoR1 and APPL1 in H9c2 cells before and
after transfection with Ov-adiponectin plasmids were detected using
RT-qPCR. ***P<0.001 vs. control; ###P<0.001 vs.
D-gal + Ov-NC. AdipoR1, adiponectin receptor 1; APPL1, adaptor
protein phosphotyrosine interacting with PH domain and leucine
zipper 1; RT-qPCR, reverse transcription-quantitative PCR; NC,
negative control; D-gal, D-galactose; Ov-, overexpression. |
Adiponectin overexpression promotes
the expression of AdipoR1 and APPL1 in D-gal-treated
cardiomyocytes
To investigate the association between adiponectin
and AdipoR1/APPL1 in senescent cardiomyocytes, RT-qPCR was
performed to determine the expression of adiponectin following the
transfection of adiponectin overexpression plasmid into
D-gal-treated H9c2 cells. As shown in Fig. 2B-D, downregulated mRNA and protein
expression levels of adiponectin, AdipoR1 and APPL1 were observed
in the cells treated with 10 g/l D-gal compared with the control;
these levels were markedly increased following the overexpression
of adiponectin compared with the NC group, suggesting a promoting
effect of adiponectin overexpression on AdipoR1/APPL1 expression in
D-gal-treated cardiomyocytes.
Adiponectin inhibits D-gal-induced
cardiomyocyte senescence via AdipoR1/APPL1
To elucidate the mechanisms through which
adiponectin affects cardiomyocyte senescence and whether its
effects are through the AdipoR1/APPL1 signaling pathway, siRNAs
targeting AdiopoR1 and APPL1 were constructed and transfected into
H9c2 cells overexpressing adiponectin (Fig. S1A). The transfection efficiency was
examined by RT-qPCR and western blotting, which demonstrated that
the relative mRNA and protein expression levels of AdipoR1 and
APPL1 were notably downregulated in the OV-adiponectin cells
transfected with the siRNAs compared with the NC group (Fig. 3A-D). In addition, siRNA transfection
in OV-adiponectin cells without D-gal treatment also exhibited
downregulated mRNA and protein expression levels of AdipoR1 and
APPL1 (Fig. S1B and C). It was
further observed by SA-β-gal staining that transfection with
OV-adiponectin significantly inhibited D-gal-induced cell
senescence, which was, however, reversed to a certain degree by
transfection with si-AdiopoR1 and si-APPL1 (Fig. 3E). Additionally, western blot
analysis revealed that the expression levels of senescence-related
proteins, p16 and p21, which were downregulated by adiponectin
overexpression, were partially increased by transfection with
si-AdiopoR1 and si-APPL1 in the D-gal-treated H9c2 cells (Fig. 3F). Taken together, these results
indicated an inhibitory effect of adiponectin on D-gal-induced
cardiomyocyte senescence via regulating AdipoR1/APPL1.
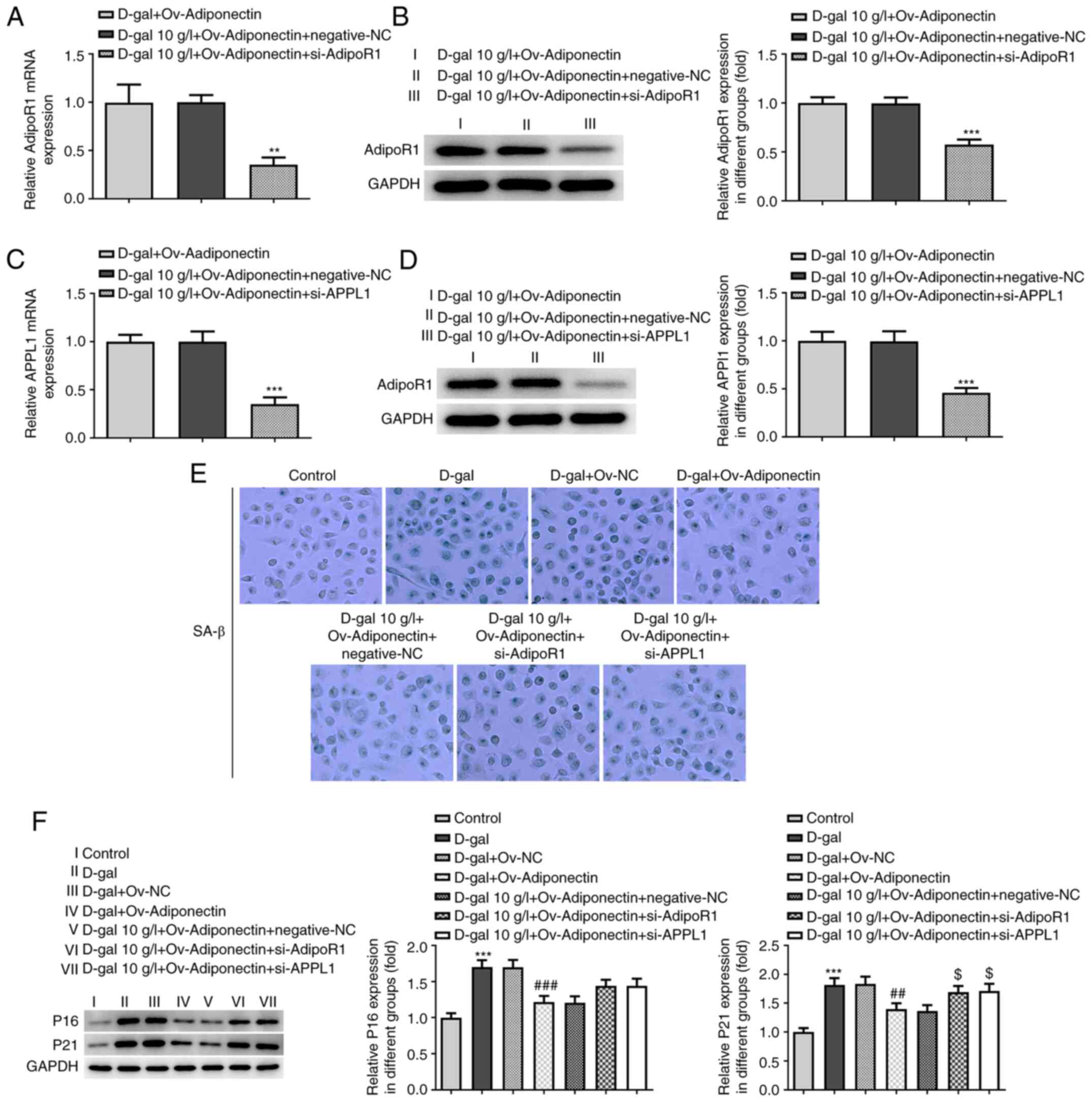 | Figure 3.Adiponectin inhibits D-gal-induced
cardiomyocyte senescence via AdipoR1/APPL1. (A and B) Relative
expression levels of AdipoR1 in D-gal-treated H9c2 cells with
Ov-adiponectin before and after transfection with si-AdipoR1 and
si-APPL were measured using RT-qPCR. **P<0.01, ***P<0.001 vs.
D-gal + Ov-adiponectin. (C and D) Relative expression levels of
APPL1 in D-gal-treated H9c2 cells with Ov-adiponectin before and
after transfection with si-AdipoR1 and si-APPL were measured using
RT-qPCR. ***P<0.001 vs. D-gal + Ov-adiponectin. (E) Cellular
senescence in the different groups was detected using SA-β-gal
staining. (F) Relative expression levels of p16 and p21 in the
different groups were detected using western blot analysis.
***P<0.001 vs. control; ##P<0.01,
###P<0.001 vs. D-gal + Ov-NC; $P<0.05
vs. D-gal + Ov-adiponectin + negative-NC. AdipoR1, adiponectin
receptor 1; APPL1, adaptor protein phosphotyrosine interacting with
PH domain and leucine zipper 1; Ov-, overexpression; RT-qPCR,
reverse transcription-quantitative PCR; NC, negative control; si-,
small interfering RNA; D-gal, D-galactose; SA-β-gal,
senescence-associated β-galactose. |
Adiponectin inhibits the oxidative
stress levels in D-gal-treated cardiomyocytes via
AdipoR1/APPL1
ROS production was detected in D-gal-treated H9c2
cells using commercial kits for a further exploration of the
mechanisms through which adiponectin inhibits cardiomyocyte
senescence. As shown in Fig. 4A,
the relative ROS levels in the D-gal-treated H9c2 cells were
significantly decreased by transfection with OV-adiponectin,
whereas they were increased by transfection with si-AdipoR1 and
si-APPL1 (Fig. 4A and B).
Furthermore, an MDA assay kit was utilized to detect the lipid
peroxidation levels in H9c2 cells; it was observed that adiponectin
overexpression markedly reduced relative MDA expression in the
D-gal treated H9c2 cells, whereas this was enhanced following
transfection with si-AdipoR1 and si-APPL1 compared with the NC
group (Fig. 4C). These results
collectively suggested that adiponectin decreased the levels of
oxidative stress in D-gal-induced cellular senescence through the
AdipoR1/APPL1 signaling pathway.
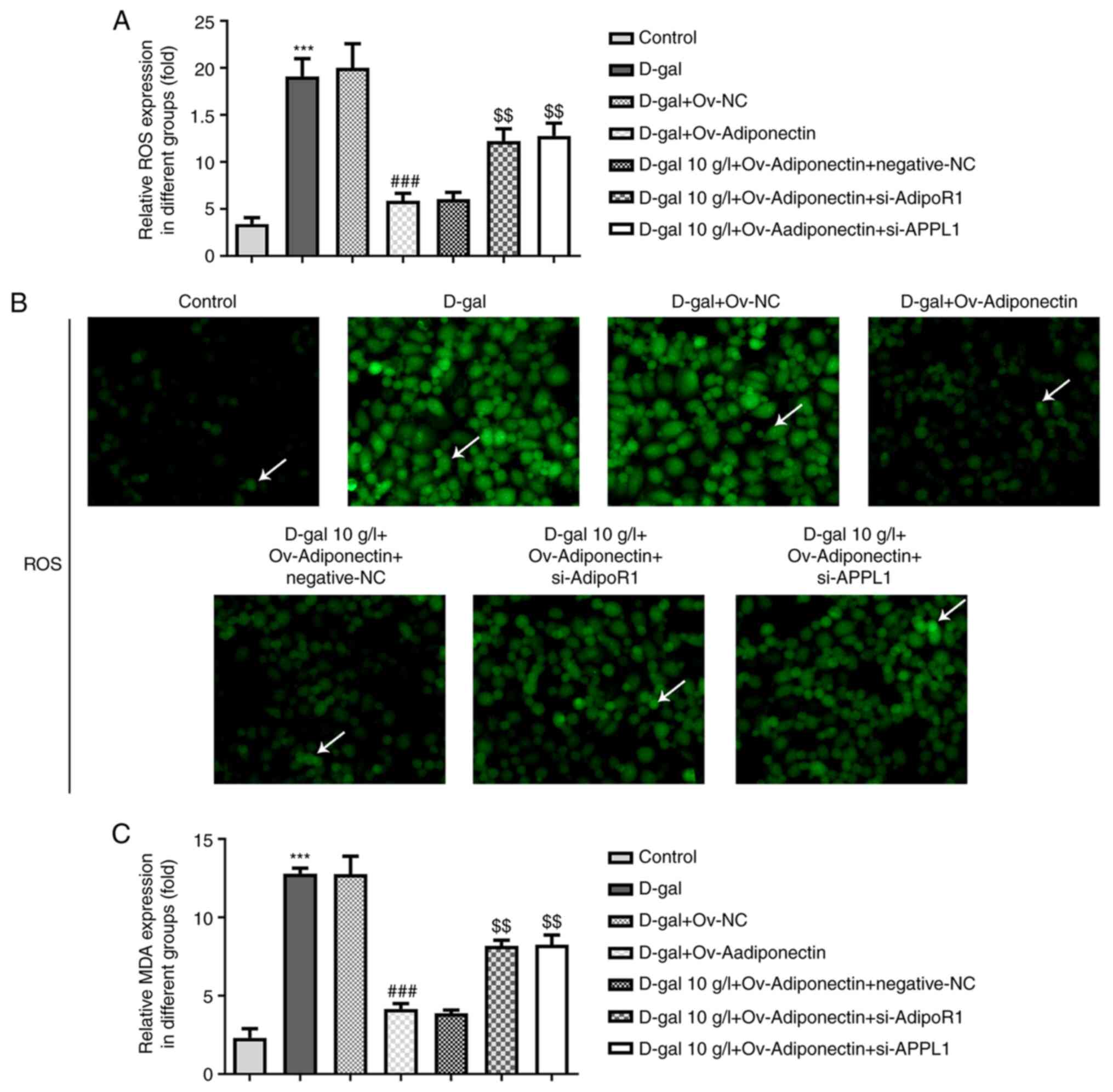 | Figure 4.Adiponectin inhibits oxidative stress
levels in D-gal-treated cardiomyocytes via AdipoR1/APPL1. (A)
si-AdipoR1 and si-APPL1 were transfected into D-gal-treated H9c2
cells overexpressing adiponectin. The relative expression of ROS in
the different groups was detected using a ROS Assay kit.
***P<0.001 vs. control; ###P<0.001 vs. D-gal +
Ov-NC; $$P<0.01 vs. D-gal + Ov-adiponectin +
negative-NC. (B) ROS production in the different groups was
detected using the ROS Assay kit (magnification, ×200). DCF
fluorescence staining of the cells are indicated by arrows in the
figure, the intensity of staining can reflect the level of ROS. (C)
Relative MDA expression in the different groups was detected using
the Lipid Peroxidation MDA Assay kit. ***P<0.001 vs. control;
###P<0.001 vs. D-gal + Ov-NC; $$P<0.01
vs. D-gal + Ov-adiponectin + negative-NC. AdipoR1, adiponectin
receptor 1; ROS, reactive oxygen species; MDA, malondialdehyde;
APPL1, adaptor protein phosphotyrosine interacting with PH domain
and leucine zipper 1; si-, small interfering RNA; Ov-,
overexpression; D-gal, D-galactose; NC, negative control. |
Adiponectin affects the release of
HO-1/HMGB1 via AdipoR1/APPL1
To determine whether HO-1/HMGB1, an oxidative stress
response-related pathway in the body (16), plays a role in the mechanisms of
action of adiponectin in cardiomyocyte senescence, the expression
levels of HO-1 and HMGB1 in the different groups were detected by
western blot analysis. It was observed that the relative expression
levels of HO-1 were significantly upregulated in the D-gal-treated
H9c2 cells following adiponectin overexpression, while following
transfection with si-AdipoR1 and si-APPL1, HO-1 expression was
decreased to a certain degree compared with the NC group (Fig. 5A). On the other hand, the relative
expression of HMGB1 in D-gal-treated H9c2 cells was decreased by
adiponectin overexpression, and was significantly increased by
transfection with si-AdipoR1 and si-APPL1 compared with the NC
group (Fig. 5A). These findings
suggested that adiponectin promotes HO-1 expression, while it
inhibits HMGB1 expression through the AdioR1/APPL1 signaling
pathway.
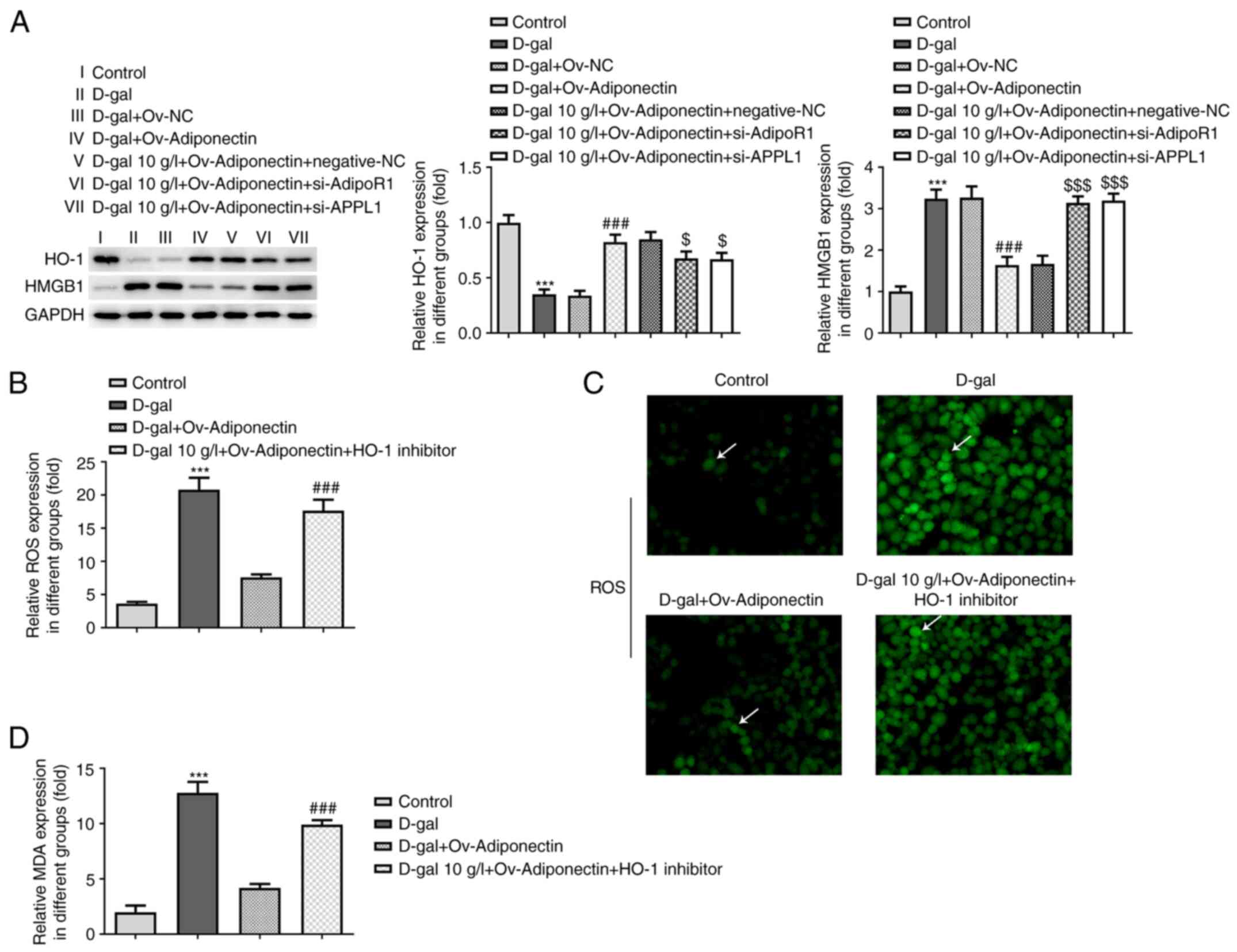 | Figure 5.Adiponectin affects the release of
HO-1/HMGB1 via AdipoR1/APPL1. (A) Relative expression of HO-1 in
the different groups was detected using western blot analysis.
Relative expression of HMGB1 in the different groups was detected
using western blot analysis. ***P<0.001 vs. control;
###P<0.001 vs. D-gal + Ov-NC; $P<0.05,
$$$P<0.001 vs. D-gal + Ov-adiponectin + negative-NC.
Adiponectin/AdipoR1/APPL1 inhibits oxidative stress via HO-1/HMGB1.
(B) H9C2 cells overexpressing adiponectin were treated with HO-1
inhibitor.; the relative expression of ROS in the different groups
was detected using a ROS Assay kit. ***P<0.001 vs. control;
###P<0.001 vs. D-gal + Ov-adiponectin group. (C) ROS
production in H9c2 cells in the different groups was detected using
a ROS Assay kit. DCF fluorescence staining of the cells is
indicated by arrows in the figure, the intensity of staining can
reflect the level of ROS (magnification, ×200). (D) Relative
expression of MDA in the different groups was detected using a
Lipid Peroxidation MDA Assay kit. ***P<0.001 vs. control;
###P<0.001 vs. D-gal + Ov-adiponectin group. AdipoR1,
adiponectin receptor 1; ROS, reactive oxygen species; HO-1, heme
oxygenase 1; HMGB1, high mobility group box 1; APPL1, adaptor
protein phosphotyrosine interacting with PH domain and leucine
zipper 1; NC, negative control; Ov-, overexpression; si-, small
interfering RNA; D-gal, D-galactose; MDA, malondialdehyde. |
Adiponectin/AdipoR1/APPL1 inhibits
oxidative stress via HO-1/HMGB1
To ascertain whether adiponectin reduces oxidative
stress in cardiomyocyte senescence through HO-1/HMGB1 signaling,
commercial kits were once again used to determine the ROS and MDA
levels in D-gal-treated H9c2 cells. In this experiment, ZnPP was
used to treat the D-gal-treated cells overexpressing adiponectin.
As shown in Fig. 5B and C, the
relative ROS levels in D-gal-treated H9c2 cells were markedly
decreased following adiponectin overexpression compared with the
cells treated with D-gal alone; these levels were noticeably
elevated following treatment with the HO-1 inhibitor. As shown in
Fig. 5D, a similar trend was
observed in MDA levels, indicating that adiponectin/AdipoR1/APPL1
suppresses the release of oxidative stress in H9c2 cells treated
with D-gal through HO-1/HMGB1 signaling.
Discussion
At 40 years of age, the remaining lifetime risk for
developing certain types of CVD increases significantly in
previously disease-free individuals; these CVDs can present in
various forms, including chronic CVD, hypertension or heart failure
(17). China, along with a number
of other countries, has entered the stage of population aging
(18,19); thus, CVDs have become a leading
cause of mortality worldwide, particularly among the elderly
(20). It is also estimated that
~20% of the world population will reach ≥65 years of age by 2030,
which signifies not only an increase in the prevalence of CVDs, but
also increased healthcare costs (2). Aging leads to structural and
functional alterations in the cardiovascular system in an
unfavorable manner (21).
Cardiomyocyte senescence, as a part of the aging process, is
directly associated with the dysfunction of myocardial tissues
(22), the deterioration of which
is often induced by oxidative stress (23). D-gal is commonly used in a number of
rodent models of aging, including models of brain (24,25),
renal (26,27) and heart (28,29)
aging. In the present study, H9c2 cells were treated with D-gal at
various concentrations, and the concentration of 10 g/l was
selected to establish a cardiomyocyte senescence model to simulate
the results of the animal experiment carried out in advance. It was
found that the expression levels of adiponectin, AdipoR1 and APPL1
were decreased in the D-gal-treated cardiomyocytes, which was
consistent with the results obtained in the animal experiments.
Jin et al (30) demonstrated that adiponectin, an
endocrine factor secreted mainly by adipose tissues, reduced
cellular senescence and led to the functional recovery of
keratinocytes. Furthermore, adiponectin binds to its two receptors,
AdipoR1 and R2, to initiate signaling transduction events, which
are regulated by adaptor proteins, such as APPL1, that directly
bind to the intracellular regions of AdipoRs (13). Visceral fat and adipocytes are risk
factors for different forms of heart disease and heart failure
(31). In the present study, an
adiponectin overexpression plasmid was constructed and this was
transfected into H9c2 cells treated with D-gal. The expression of
AdipoR1 and APPL1 was then detected in the cells. A markedly higher
expression of AdipoR1 and APPL1 was noted in the Ov-adiponectin
group compared with the NC group, which indicated that adiponectin
overexpression promoted AdipoR1 and APPL1 expression in
D-gal-treated cardiomyocytes. For the purpose of identifying the
mechanisms of adiponectin in cardiomyocyte senescence, si-AdipoR1
and si-APPL1 were transfected into D-gal-treated H9c2 cells
overexpressing adiponectin to observe cellular senescence. The
results revealed that adiponectin reduced senescent cells,
potentially by regulating AdipoR1 and APPL1.
It has been demonstrated that increased levels of
ROS are implicated in cell senescence-related pathogenesis
(32). Adiponectin has previously
been proven to exert protective effects against oxidative stress in
a number of diseases, playing an antioxidant role in oxidative
stress-associated skeletal muscle diseases (33) and reducing oxidative stress in
diabetic nephropathy (34).
Accordingly, the present study hypothesized that adiponectin may
inhibit ROS production in D-gal-treated H9c2 cells, thus
attenuating cardiomyocyte senescence. The examination of oxidative
stress often involves measuring ROS levels, as well as MDA content,
which is commonly used as a lipid peroxidation marker (35). Therefore, ROS production and the MDA
content were detected in the present study, which verified that
adiponectin inhibited oxidative stress levels in D-gal-induced
cardiomyocyte senescence via AdipoR1/APPL1 signaling. Choubey et
al (36) noted that adiponectin
treatment improved the levels of increased oxidative stress during
aging and thus improved testicular function during aging. Previous
studies have also reported that some compounds (such as onion
juice; vitamin C and hesperidin) show antioxidant properties
(29–39). In addition, pretreatment with n-3
polyunsaturated fatty acids, such as fish oil and flaxseed oil,
significantly inhibits myocardial injury (40).
A previous study found that adiponectin can activate
HO-1 signaling, which thereby attenuated the production of ROS in
HepG2 cells (41). Shan et
al (42) revealed that HO-1
improves cardiac function and attenuates ischemic injury and
aging-induced cardiomyocyte senescence. Additionally, it has been
well-established that the expression of HMGB1, a proinflammatory
adipocytokine, can be inhibited by both adiponectin and upstream
HO-1. Shimizu et al (43)
demonstrated that adiponectin can inhibit TNF-α-induced HMGB1
secretion from 3T3-L1 adipocytes. Furthermore, Luo et al
(44) observed that HO-1 can
inhibit HMGB1 activity in lipopolysaccharide-induced acute lung
injury in vitro. Based on the aforementioned evidence, the
present study performed a series of experiments to examine the
association between adiponectin, AdipoR1/APPL1 and HO-1/HMGB1 in
D-gal-induced cardiomyocyte senescence. It was found that
adiponectin overexpression elevated HO-1 expression levels and
decreased HMGB1 expression levels in D-gal-treated H9c2 cells,
which was consistent with the findings of previous studies.
Moreover, in contrast to the NC group, HO-1 expression was
decreased and HMGB1 expression was increased following transfection
of the D-gal-treated H9c2 cells overexpressing adiponectin with
si-AdipoR1 and si-APPL1. It can be concluded from the results that
adiponectin affects the release of HO-1/HMGB1 through AdipoR1/APPL1
signaling.
Furthermore, the present study further explored the
inhibitory effects of adiponectin on the levels of oxidative stress
in senescent cardiomyocytes, as well as the role of HO-1/HMGB1 in
such a mechanism. In a previous study, Chen et al (45) reported that HO-1 overexpression
reduced the production of mitochondrial oxidation to prevent
myocardial hypoxia-reoxygenation injury in H9c2 cells. Similarly,
the present study used ZnPP to inhibit HO-1 activity, and detected
the expression levels of ROS and MDA in H9c2 cells in different
groups. The results demonstrated that while adiponectin
overexpression notably decreased the oxidative stress levels, the
inhibition of HO-1 restored the levels of oxidative stress to a
large extent, suggesting that adiponectin/AdipoR1/APPL1 inhibits
oxidative stress through HO-1/HMGB1 signaling.
In conclusion, the present study provided evidence
to suggest that adiponectin protected against cardiomyocyte
senescence induced by D-gal via AdipoR1/APPL1 signaling, and that
it attenuated oxidative stress in senescent H9c2 cells by
inhibiting the HO-1/HMGB1 signaling pathway. To the best of our
knowledge, the present study was the first to elucidate the
mechanisms of action of adiponectin in cardiomyocyte senescence
induced by D-gal and shed light on the application of adiponectin
in the treatment of age-related CVDs. These results also provided
viable targets and research directions for clinical myocardial
senescence treatment. The majority of the experiments in the
present study were conducted in vitro; thus, further in
vivo experiments are required to examine the efficacy of
adiponectin on cardiomyocyte senescence-associated CVDs in order to
validate the current findings and to assist future clinical
trials.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Medical Guidance
(TCM) Science and Technology Support Project of Science and
Technology Commission of Shanghai Municipality (grant no.
17401933700), the Scientific Research Project of Shanghai Municipal
Health Commission (grant no. 201640039) and the Peak Plateau
Subject of Shanghai University of Traditional Chinese Medicine
(special project for clinical talents, grant no. 171319).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL and RL contributed to conception and design of
the study. DL, DL and JM performed the experiments and data
collection. RL and JM contributed to analysis and interpretation of
data. DL revised the manuscript critically for important
intellectual content. DL and RL confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from The Shanghai
Municipal Hospital of Traditional Chinese Medicine (approval no.
dw2019018; Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sander M, Oxlund B, Jespersen A, Krasnik
A, Mortensen EL, Westendorp RG and Rasmussen LJ: The challenges of
human population ageing. Age Ageing. 44:185–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Costantino S, Paneni F and Cosentino F:
Ageing, metabolism and cardiovascular disease. J Physiol.
594:2061–2073. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao D, Liu J, Wang M, Zhang X and Zhou M:
Epidemiology of cardiovascular disease in China: Current features
and implications. Nat Rev Cardiol. 16:203–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liguori I, Russo G, Curcio F, Bulli G,
Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce
D and Abete P: Oxidative stress, aging, and diseases. Clin Interv
Aging. 13:757–772. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin-Fernandez B and Gredilla R:
Mitochondria and oxidative stress in heart aging. Age (Dordr).
38:225–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang LF, Cao Q, Wen K, Xiao YF, Chen TT,
Guan XH, Liu Y, Zuo L, Qian YS, Deng KY and Xin HB: CD38 deficiency
alleviates D-galactose-induced myocardial cell senescence through
NAD+/Sirt1 signaling pathway. Front Physiol. 10:11252019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du H, Wang Y, Liu X, Wang S, Wu S, Yuan Z
and Zhu X: miRNA-146a-5p mitigates stress-induced premature
senescence of D-galactose-induced primary thymic stromal cells.
Cytokine. 137:1553142021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun K, Yang P, Zhao R, Bai Y and Guo Z:
Matrine attenuates D-galactose-induced aging-related behavior in
mice via inhibition of cellular senescence and oxidative stress.
Oxid Med Cell Longev. 2018:71086042018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bo-Htay C, Palee S, Apaijai N,
Chattipakorn SC and Chattipakorn N: Effects of d-galactose-induced
ageing on the heart and its potential interventions. J Cell Mol
Med. 22:1392–1410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frimat M, Daroux M, Litke R, Neviere R,
Tessier FJ and Boulanger E: Kidney, heart and brain: Three organs
targeted by ageing and glycation. Clin Sci (Lond). 131:1069–1092.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ZV and Scherer PE: Adiponectin, the
past two decades. J Mol Cell Biol. 8:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang J, Hou B, Zhang S, Wang M, Lu X,
Wang Q and Liu Y: The protective effect of adiponectin-transfected
endothelial progenitor cells on cognitive function in
D-galactose-induced aging rats. Neural Plast. 2020:12731982020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang H and Judd RL: Adiponectin regulation
and function. Compr Physiol. 8:1031–1063. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu N, Zhang Y, Doycheva DM, Ding Y, Zhang
Y, Tang J, Guo H and Zhang JH: Adiponectin attenuates neuronal
apoptosis induced by hypoxia-ischemia via the activation of
AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats.
Neuropharmacology. 133:415–428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Z, Wang F, Yang Y, Wang J, Sun S, Xia
H and Yao S: Resolvin D1 attenuates ventilator-induced lung injury
by reducing HMGB1 release in a HO-1-dependent pathway. Int
Immunopharmacol. 75:1058252019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lakatta EG: So! What's aging? Is
cardiovascular aging a disease? J Mol Cell Cardiol. 83:1–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bundy JD and He J: Hypertension and
related cardiovascular disease burden in China. Ann Glob Health.
82:227–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Jia J and Yang Z: Mini-mental state
examination in elderly Chinese: A population-based normative study.
J Alzheimers Dis. 53:487–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans MA, Sano S and Walsh K:
Cardiovascular disease, aging, and clonal hematopoiesis. Annu Rev
Pathol. 15:419–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obas V and Vasan RS: The aging heart. Clin
Sci (Lond). 132:1367–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson R, Lagnado A, Maggiorani D,
Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik
M, Jurk D, et al: Length-independent telomere damage drives
post-mitotic cardiomyocyte senescence. EMBO J. 38:e1004922019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsushima S and Sadoshima J: The role of
sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol.
309:H1375–H1389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kou X, Liu X, Chen X, Li J, Yang X, Fan J,
Yang Y and Chen N: Ampelopsin attenuates brain aging of
D-gal-induced rats through miR-34a-mediated SIRT1/mTOR signal
pathway. Oncotarget. 7:74484–74495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sadigh-Eteghad S, Majdi A, McCann SK,
Mahmoudi J, Vafaee MS and Macleod MR: D-galactose-induced brain
ageing model: A systematic review and meta-analysis on cognitive
outcomes and oxidative stress indices. PLoS One. 12:e01841222017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu B, Tu Y, He W, Liu Y, Wu W, Fang Q,
Tang H, Tang R, Wan Z, Sun W and Wan Y: Hyperoside attenuates renal
aging and injury induced by D-galactose via inhibiting AMPK-ULK1
signaling-mediated autophagy. Aging (Albany NY). 10:4197–4212.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Horany HE, Gaballah HH and Helal DS:
Berberine ameliorates renal injury in a rat model of
D-galactose-induced aging through a PTEN/Akt-dependent mechanism.
Arch Physiol Biochem. 126:157–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dehghani A, Hafizibarjin Z, Najjari R,
Kaseb F and Safari F: Resveratrol and 1,25-dihydroxyvitamin D
co-administration protects the heart against D-galactose-induced
aging in rats: Evaluation of serum and cardiac levels of klotho.
Aging Clin Exp Res. 31:1195–1205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bei Y, Wu X, Cretoiu D, Shi J, Zhou Q, Lin
S, Wang H, Cheng Y, Zhang H, Xiao J and Li X: miR-21 suppression
prevents cardiac alterations induced by d-galactose and
doxorubicin. J Mol Cell Cardiol. 115:130–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin T, Kim MJ, Heo WI, Park KY, Choi SY,
Lee MK, Hong SP, Kim SJ, Im M, Moon NJ and Seo SJ: Adiponectin
corrects premature cellular senescence and normalizes antimicrobial
peptide levels in senescent keratinocytes. Biochem Biophys Res
Commun. 477:678–684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez N, Moreno-Villegas Z,
Gonzalez-Bris A, Egido J and Lorenzo O: Regulation of visceral and
epicardial adipose tissue for preventing cardiovascular injuries
associated to obesity and diabetes. Cardiovasc Diabetol. 16:442017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davalli P, Mitic T, Caporali A, Lauriola A
and D'Arca D: ROS, cell senescence, and novel molecular mechanisms
in aging and age-related diseases. Oxid Med Cell Longev.
2016:35651272016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren Y, Li Y, Yan J, Ma M, Zhou D, Xue Z,
Zhang Z, Liu H, Yang H, Jia L, et al: Adiponectin modulates
oxidative stress-induced mitophagy and protects C2C12 myoblasts
against apoptosis. Sci Rep. 7:32092017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JY, Yang JW, Han BG, Choi SO and Kim
JS: Adiponectin for the treatment of diabetic nephropathy. Korean J
Intern Med. 34:480–491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morales M and Munne-Bosch S:
Malondialdehyde: Facts and artifacts. Plant Physiol. 180:1246–1250.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choubey M, Ranjan A, Bora PS, Baltazar F,
Martin LJ and Krishna A: Role of adiponectin as a modulator of
testicular function during aging in mice. Biochim Biophys Acta Mol
Basis Dis. 1865:413–427. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shokoohi M, Madarek EOS, Khaki A, Shoorei
H, Khaki AA, Soltani M and Ainehchi N: Investigating the effects of
onion juice on male fertility factors and pregnancy rate after
testicular torsion/detorsion by intrauterine insemination method.
Int J Womens Health and Reprod Sci. 6:499–505. 2018. View Article : Google Scholar
|
|
38
|
Moghimian M, Soltani M, Abtahi H and
Shokoohi M: Effect of vitamin C on tissue damage and oxidative
stress following tunica vaginalis flap coverage after testicular
torsion. J Pediatr Surg. 52:1651–1655. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shokoohi M, Khaki A, Shoorei H, Khaki AA,
Moghimian M and Abtahi-Eivary SH: Hesperidin attenuated
apoptotic-related genes in testicle of a male rat model of
varicocoele. Andrology. 8:249–258. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ivary SHA, Jajarmy N, Shahri MK, Shokoohi
M, Shoorei H, Ebadi A, Moghimian M and Sigaroodi F: Effect of fish
and flaxseed oil supplementation on isoprenaline-induced myocardial
infarction in rats: Inhibition of mitochondrial permeability
transition pore opening. Crescent J Med Biol Sci. 6:158–163.
2019.
|
|
41
|
Shrestha A and Park PH: Globular
adiponectin attenuates LPS-induced reactive oxygen species
production in HepG2 cells via FoxO3A and HO-1 signaling. Life Sci.
148:71–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shan H, Li T, Zhang L, Yang R, Li Y, Zhang
M, Dong Y, Zhou Y, Xu C, Yang B, et al: Heme oxygenase-1 prevents
heart against myocardial infarction by attenuating ischemic
injury-induced cardiomyocytes senescence. EBioMedicine. 39:59–68.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shimizu T, Yamakuchi M, Biswas KK, Aryal
B, Yamada S, Hashiguchi T and Maruyama I: HMGB1 is secreted by
3T3-L1 adipocytes through JNK signaling and the secretion is
partially inhibited by adiponectin. Obesity (Silver Spring).
24:1913–1921. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luo M, Hong XQ, Zhu H, Li G and Tang L:
The HO-1 signal prevents HMGB1-mediated activation of NLRP3
inflammasomes in lipopolysaccharide-induced acute lung injury in
vitro. J Surg Res. 247:335–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen D, Jin Z, Zhang J, Jiang L, Chen K,
He X, Song Y, Ke J and Wang Y: HO-1 protects against
hypoxia/reoxygenation-induced mitochondrial dysfunction in H9c2
cardiomyocytes. PLoS One. 11:e01535872016. View Article : Google Scholar : PubMed/NCBI
|