Introduction
Breast cancer (BC) is a common malignancy with the
highest incidence and the second highest mortality rate worldwide
(1). Numerous factors contribute to
the occurrence of BC, including age, obesity, alcoholism and
exposure to radiation (2). Although
substantial progress has been made in the treatment of BC in recent
decades, the overall prognosis of patients with BC remains poor.
Therefore, there is an urgent need to discover additional
mechanisms underlying the progression of BC and identify more
effective therapeutic targets.
Circular RNAs (circRNAs) are a novel type of
noncoding RNA (ncRNA) with covalently closed loop characteristics
(3). It has been verified that
circRNAs are derived from pre-mRNA transcripts (4). In addition, it has been suggested that
circRNAs serve essential roles in gene regulation and may thereby
be involved in the progression of various human diseases, including
BC (5). For example, Wu et
al (6) reported that circIRAK3
could sponge microRNA (miRNA/miR)-3607 to enhance the metastasis of
BC. Furthermore, circMYO9B has been shown to increase the
proliferation and invasiveness of BC by sponging miR-4316 and
upregulating forkhead box P4 (7).
However, the functions and underlying mechanisms of circRNAs are
still not fully understood in BC.
miRNAs are another group of ncRNAs with a length of
20–25 nucleotides. miRNAs can bind to the 3′ untranslated region
(UTR) of mRNAs and thereby inhibit the expression of genes
(8). Accumulating evidence has
indicated that dysregulation of miRNAs is involved in the
progression of various types of cancer, including BC. For example,
miR-93 has been reported to act as a tumor suppressor via
inhibition of WASP family member 3 and metastasis of BC (9). miR-6744 has also been reported to
target the N-acetyltransferase 1 enzyme and promote anoikis in
breast cancer (10). However,
little is currently known about the upstream regulatory mechanisms
of miRNAs.
Sirtuin 4 (SIRT4) is a mitochondrial matrix protein
that belongs to the SIRT family (11). SIRT4 has been shown to participate
in various biological activities, such as cellular proliferation,
migration, metabolism and inflammation (12). According to previous studies, SIRT4
is strongly associated with the tumorigenesis of various tumors,
including prostate cancer, liver cancer, thyroid cancer and
neuroblastoma (13–15). However, the role of SIRT4 in BC is
still unclear.
The aim of this study was to identify circRNAs
associated with the tumorigenesis of BC. circOMA1 was identified as
a novel circRNA that acted as an oncogene in BC. The diagnostic and
therapeutic value of circOMA1 and its molecular mechanism were also
examined.
Materials and methods
Clinical samples
This prospective study involved 64 patients who
underwent tumor resection at The Zhenghai Longsai Hospital for
primary BC between December 2018 and October 2019. All patients
were newly diagnosed with primary BC. Patients who were <18
years old or >75 years old, presented with initial distant
metastasis, complicated with other malignancies or received
neoadjuvant therapy were excluded from the study. Tumor and
matched, adjacent normal tissue were collected and stored in liquid
nitrogen. The present study was approved by the Ethics Committee of
Zhenghai Longsai Hospital (Ningbo, China) and was conducted in
accordance with the Declaration of Helsinki. Written informed
consent was obtained from all participants prior to the study.
Cell culture
Human normal breast epithelial cells (MCF-10A), BC
cells (MDA-MB-468, MDA-MB-231, BT-549 and MCF-7) and 293T cells
were obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. All cells were maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin (all Gibco; Thermo Fisher
Scientific, Inc.). The cells were cultured in a humidified
atmosphere containing 5% CO2 at 37°C.
Cell transfection
Three different short hairpin RNA (shRNA) molecules
against circOMA1 (shRNA#1, shRNA#2, shRNA#3), SIRT4 (sh-SIRT4) and
negative control (sh-NC) were subcloned into the GV248
(hU6-MCS-Ubiquitin-EGFP-IRES-puromycin) vector (Shanghai GeneChem
Co., Ltd.). The following sequences were used: i) sh-circOMA1#1,
5′-ACCUCAGGUAGUCAAGUGATT-3′; ii) sh-circOMA1#2,
5′-AUGAUUCUUAAACCAGUACAG-3′; iii) sh-circOMA1#3
5′-CUAACAAGAAGGAAGUAGU-3′ iv) sh-SIRT4, 5′-TCCTATACAGCTACGGCTC-3′;
and v) sh-NC′ 5′-TTCTCCGAACGTGTCACGT-3′. In order to overexpress
SIRT4 or circOMA1, full-length SIRT4 and circOMA1 were synthesized
by Shanghai GenePharma Co., Ltd. and were subcloned into the
pcDNA3.1 vector (Shanghai GenePharma Co., Ltd.). The empty vector
was used as a negative control. The plasmids were used to transfect
293 cells using the ViraPower kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's guidelines to generate the
lentivirus. miR-1276 mimics/inhibitors and negative control (NC)
mimics/inhibitors were purchased from Shanghai GenePharma Co.,
Ltd., and transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The miR
sequences were: i) miR-NC mimics, 5′-UUGUACUACACAAAAGUACUG-3′; ii)
miR-1276 mimics, 5′-UAAAGAGCCCUGUGGAGACA-3′; iii) miR-NC inhibitor,
5′-UCACAACCUCCUAGAAAGAGUAGA-3′; and iv) miR-1276 inhibitor,
5′-UGUAUCCACAGGGCUCUUUA-3′; For the plasmids or miRNA
mimics/inhibitors transfection, MCF-7 and MDA-MB-478 cells were
seeded into 6-well plates at a density of 1×106 cells/well and
cultured to 80% confluence. For each well, 1 µg of plasmids or 20
nM miRNA mimics/inhibitor were mixed with Lipfectamine®
2000 at a ratio of 1:4. The cells were then cultured for 24 h and
transfection efficiency was assessed. For lentivirus infection,
MCF-7 and MDA-MB-478 cells were seeded into 6-well plates at a
density of 1×106 cells/well and cultured to 80% confluence. The
lentiviral vector solution was mixed with 2 ml culture medium to
5×106 transduction units (TU), then added to each well and
incubated overnight before the medium was changed. Transduction
efficiency was assessed 24 h later.
For co-transfection of sh-circOMA1 and pcDNA3.1
SIRT4, MCF-7 and MDA-MB-478 cells were seeded into 6-well plates at
a density of 1×106 cells/well and cultured to 80%
confluence. For each well, 1 µg plasmid was mixed with
Lipfectamine® 2000 at a ratio of 1:4 and 1 ml total
volume of lentiviral vector solution (5×106 TU) was
added to MCF-7 and MDA-MB-478 cells at the same time,. The culture
medium was changed with fresh medium 4 I mh later. The cells were
then kept in the incubator and subjected to different assays at
indicated time point. The cells were screened using puromycin (2
µg/ml) for 3 days.
RNA purification and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from breast cancer and
adjacent normal tissue samples or cells (MCF-10A, MDA-MB-468,
MDA-MB-231, BT-549 and MCF-7) using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The quantity and quality of RNA were measured using a
NanoDrop ND 2000 Spectrophotometer (NanoDrop; Thermo Fisher
Scientific Inc.). Total RNA was reverse transcribed into cDNA using
PrimeScript RT Reagent (Takara Biotechnology Co., Ltd.), according
to the manufacturer's protocol.
The expression levels of circOMA1 and miRNAs were
evaluated using SYBR Premix Ex Taq (Takara Biotechnology). GAPDH
was used as internal control for circOMA1/SIRT4 and U6 was used as
internal control for and miR-1276, respectively. The following
primers were used: i) circOMA1 forward, 5′-AACCCAAGATGCCAGAATGGT-3′
and reverse, 5′-TTGATGACAGCCCCGTGAG-3′; ii) SIRT4 forward,
5′-TGTGGTGAACTGACTCCTCGTGCTGAGC-3′ and reverse,
5′-CGGAAGTTTTCTTTCACTAGCAGCGAGG-3′; iii) GAPDH forward,
5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAATG-3′;
and iv) U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′ The reaction conditions were as
follows: 95°C for 10 min for 1 cycle, followed by denaturation at
95°C for 30 sec, annealing at 56°C for 1 min and extension at 72°C
for 30 sec for a total of 40 cycles. The relative gene expression
was calculated using the 2−ΔΔCq method (16), and the samples were run in
triplicate.
Actinomycin D and RNase R
treatment
To test the stability of circOMA1 expression,
actinomycin D (2 mg/ml; Sigma-Aldrich; Merck KGaA) or DMSO was
added to inhibit transcription. Total RNA (2 µg) was treated with
or without 3U/µg of RNase R (Sigma-Aldrich; Merck KGaA). The
resulting RNA was purified with the RNeasy MinElute Cleanup kit
(Qiagen, Inc.)
circRNA microarray
Total RNA purified from three pairs of BC and
adjacent normal tissues was subjected to circRNA array analysis.
Purification of RNA and microarray hybridization were conducted
according to the standard protocols from Arraystar, Inc. Briefly,
total RNA was treated with RNase R at 37°C for 1 h (Sigma-Aldrich;
Merck KGaA) to remove linear RNA and to enrich circRNAs.
Subsequently, antisense RNA was amplified and labeled using the
Arraystar Super RNA labeling kit (cat. no. AS-LE-005; Arraystar,
Inc.) according to the manufacturer's protocol. Briefly,
first-strand cDNA synthesis was conducted using an
oligo(dT)24 primer containing a T7 promoter sequence.
After RNA template degradation and second-strand cDNA synthesis,
antisense RNA was transcribed using biotinylated ribonulceotides
and T7 RNA polymerase provided in the kit. The labeled RNAs were
then hybridized using Arraystar circRNA Array (v2.0; Arraystar,
Inc.), and hybridization was scanned using the G2505C scanner
(Agilent Technologies, Inc.).
RNA pull-down assay
RNA pull-down assays were performed using the
protocol from GeneSeed. Briefly, 1×106 MCF-7 or MDA-MB-478 cells
were fixed in 2% formaldehyde at room temperature for 20 min, then
sonicated at room temperature for 15 min. Subsequently, the
supernatant was incubated with biotinylated circOMA1 or miR-1276
probes or control probe (Guangzhou RiboBio Co., Ltd.) and magnetic
streptavidin Dynabeads (Sigma-Aldrich; Merk KGaA) at 4°C for 1 h in
an orbital shaker. The lysates were then discarded and total RNA
was extracted for RT-qPCR analysis using as aforementioned
RNA-binding protein
immunoprecipitation (RIP) assay
RIP experiments were conducted using the Magna RIP
RNA-binding protein immunoprecipitation kit (cat. no. 17-10523; EMD
Millipore) according to the manufacturer's guide. Briefly, 1×107
MCF-7 or MDA-MB-478 cells were transfected with Flag-Ago2 or
Flag-GFP plasmids, then cultured for 24 h. The cells were then
collected and re-suspended in 0.1 ml RIPA lysis buffer (Beyotime
Institute of Biotechnology). The cell lysates were incubated with 5
antibody against Flag (cat. no. F7425) or IgG-coated beads (cat.
no. I8600) (both from Sigma-Aldrich; Merck KGaA) with rotation at
4°C overnight. After treating with proteinase K, the
immunoprecipitated RNA was extracted with the RNeasy MinElute
Cleanup kit (Qiagen, Inc.) and reverse transcribed PrimeScript RT
Reagent (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. The abundance of circOMA1 was measured by
RT-PCR as aforementioned.
Cell viability assay
Cell viability was assessed using the Cell Counting
Kit (CCK)-8 assay (Beyotime Institute of Biotechnology) at 24, 48
and 72 h. MCF-7 and MDA-MB-478 cells were seeded into 96-well
plates at a density of 1×104 cells/well. Subsequently 10 µl CCK-8
solution was added to each well and incubated at 37°C for a further
2 h. The absorbance was measured at 450 nm using a microplate
reader (Biotek Corporation).
Cellular apoptosis assay
Cell apoptosis was assessed using the Annexin
V-fluorescein isothiocyanate/propidium iodide (PI) detection kit
(Invitrogen), according to the manufacturer's instructions.
Briefly, 1×105 MCF-7 and MDA-MB-478 cells were stained with Annexin
V/PI at 4°C for 15 min in the dark. Cells were then washed with PBS
and resuspended in binding buffer provided in the kit. The status
of early and late apoptotic cells (Annexin V+) was determined by
flow cytometry (FACSCalibur; BD Biosciences). The data were
analyzed using the FlowJo 10.0.7 (FlowJo LLC)
Migration and invasion assays
For migration and invasion assays, similar
procedures were performed. Briefly, 5×105 cells were seeded into
8-µm pore Transwell chambers (Corning, Inc.) For the invasion
assay, the Transwell chamber was precoated with Matrigel (Thermo
Fisher Scientific, Inc.) before cell seeding. The chambers were
then inserted into a 24-well plate; the upper chambers were filled
with 200 µl serum-free medium, whereas the bottom chambers were
filled with 500 µl complete medium. After incubation for 24 h at
room temperature, cells were fixed in 4% paraformaldehyde (Beyotime
Institute of Biotechnology) for 10 min and stained with 0.1%
crystal violet (Beyotime Institute of Biotechnology) for 10 min at
room temperature. Non-migrating cells in the upper chambers were
removed; migratory and invasive cells were counted in three
randomly selected fields and images were captured using an IX70
inverted optical microscope (magnification, ×100; Olympus
Corporation).
Subcellular fractionation assay
The location of RNAs was evaluated using the PARIS™
kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Briefly, cells were suspended in cytoplasm
lysis buffer (cat. no. 78840; Thermo Fisher Scientific, Inc.) and
centrifuged at 500 × g for 5 min at room temperature. The
cytoplasmic supernatant was then collected, and the pellet was
resuspended in nucleus lysis buffer at 4°C for 1 h, followed by
centrifugation at 500 × g for 10 min at room temperature. The RNAs
derived from cytoplasmic and nuclear extracts were extracted using
TRIzol, according to the manufacturer's instructions. The
expression levels of GAPDH (cytoplasmic control), U6 (nuclear
control) and circOMA1 in the nucleus and cytoplasm were assessed by
RT-qPCR as aforementioned.
Dual-luciferase reporter assay
Dual-luciferase reporter assays were performed by
cotransfection of cells with recombinant luciferase reporter
vectors and the indicated transfection plasmids. Partial fragments
of circOMA1 and SIRT4 3′-UTRs containing wild-type (wt) or mutant
(mut) miR-1276 interacting sites were obtained. Subsequently, these
fragments were subcloned into psiCHECK luciferase reporter vectors
(Promega Corporation). Co-transfection was performed using
Lipofectamine 2000. Briefly, MCF-7 and MDA-MB-468 were seeded into
6-well plates at a density of 1×106 cells/well., Cells were
co-transfected 24 h later with 1 µg circOMA-WT/MUT or SIRT4-WT/MUT
plasmids and 100 pmol miR-1276 mimics or miR-NC mimics using
Lipofectamine 2000. After transfection, cells were cultured at 37°C
for 24 h. Luciferase activity was monitored after using the Dual
Luciferase Reporter Assay system (Promega Corporation). The data
were normalized to Renilla luciferase activity. The mut
3′-UTRs were constructed using the QuickChange™ II Site-Directed
Mutagenesis kit (Stratagene; Agilent Technologies, Inc.) according
to the manufacturer's protocol. All experiments were conducted in
triplicate and repeated at least three times.
Western blotting
Total cellular proteins were obtained using RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with a proteinase and phosphatase inhibitor cocktail
(Sigma-Aldrich). Total proteins (20 µg) were separated by SDS-PAGE
on 12% gels and were transferred onto PVDF membranes (EMD
Millipore). The membranes were blocked with skimmed milk for 1 h at
room temperature and were then incubated with primary antibodies
overnight at 4°C. Subsequently, the membranes were washed three
times with PBS and incubated with the corresponding HRP-conjugated
secondary antibody at room temperature for 1 h. The membranes were
visualized using an ECL Prime Western Blotting kit (Beyotime
Institute of Biotechnology). The following primary and secondary
antibodies were used: i) Anti-SIRT4 (cat. no. ab10140; 1:1,000;
Abcam); ii) anti-tubulin (cat. no. 2146; 1:5,000; Cell Signaling
Technology); iii) anti-mouse, HRP-linked antibody (cat. no. 7076;
1:5,000; Cell Signaling Technology, Inc.).
Bioinformatical analysis
Prediction of the circOMA1-miRNA-target gene was
performed using the StarBase 3.0 (http://starbase.sysu.edu.cn) and CircInteractome
(https://circinteractome.nia.nih.gov/).
Statistical analysis
Statistical analyses were performed with SPSS 12.0
(IBM Corp.). Data are presented as the mean ± SD. Differences
between two groups were assessed using a paired Student's t-test.
Differences between multiple groups were analysed using one-way
ANOVA followed by Tukey's post hoc test. The diagnostic value of
circOMA1 was evaluated using receiver operating characteristic
(ROC) curves as described previously (17). Survival curves for patients with BC
were generated using the Kaplan-Meier method, and the difference
was analyzed by log-rank test. The χ2 test was used to compare the
clinicopathological characteristics of patients. Pearson's
correlation was performed to assess correlation. P<0.05
(two-tailed) was considered to indicate a statistically significant
difference. All statistical analyses were performed using the Prism
7.0 (GraphPad Software, Inc.).
Results
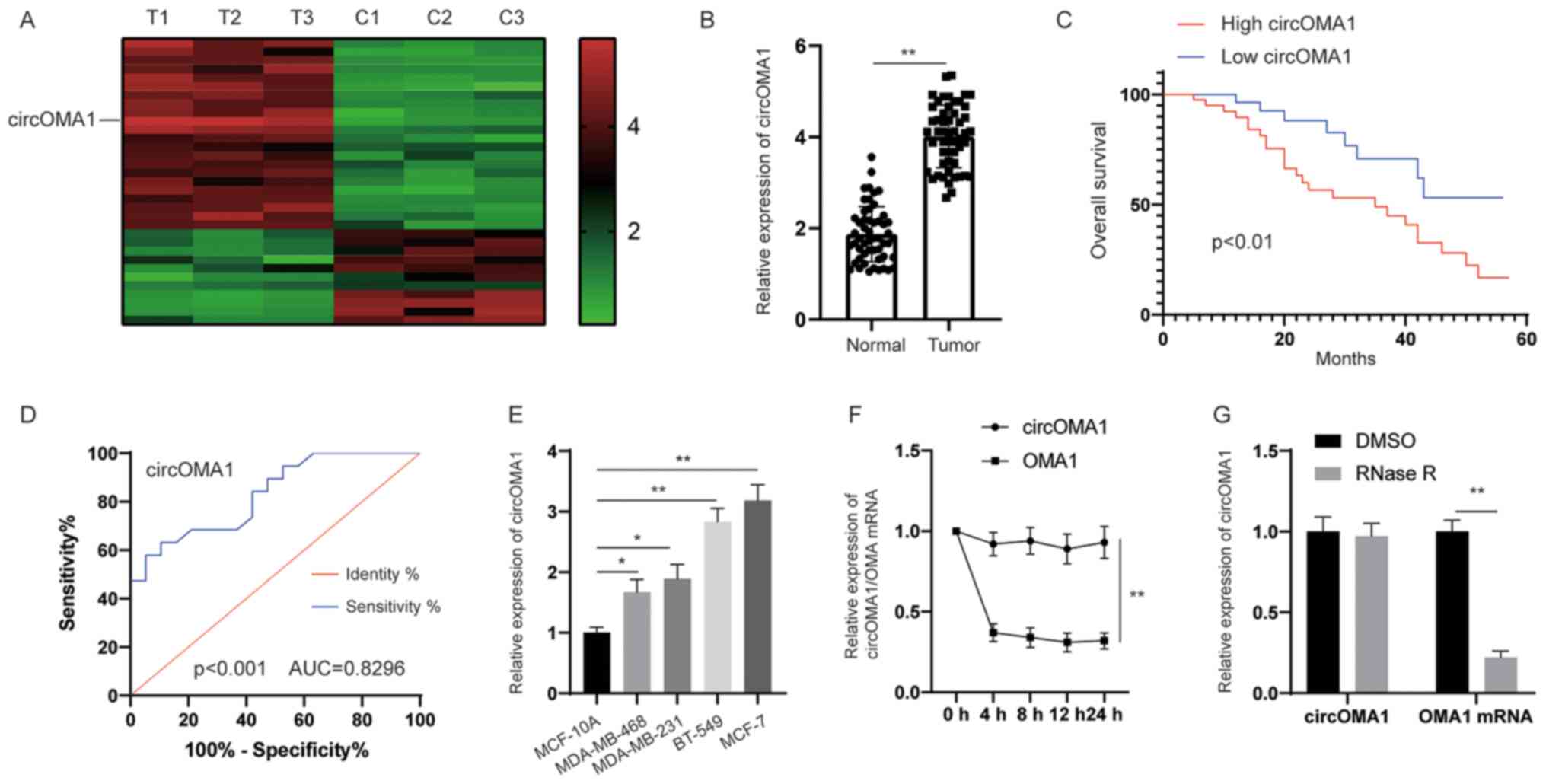
circOMA1 is upregulated in BC tissues
and is associated with poor prognosis in patients with BC
To identify the potential dysregulated circRNAs in
BC, circRNA expression profiling was performed in BC tissues and
adjacent normal tissues. The circRNA microarray assay revealed that
hsa_circRNA_0000073 was the most upregulated circRNA in BC tissues
(Fig. 1A). According to the human
reference genome (https://www.ncbi.nlm.nih.gov/grc/human),
hsa_circRNA_0000073, which was derived from the gene OMA1, was
termed circOMA1. Subsequently, the expression levels of circOMA1
were measured in 64 pairs of BC tissues and adjacent normal
tissues. The results revealed that the expression levels of
circOMA1 were significantly higher in BC tissues compared with
those in normal tissues (Fig. 1B).
According to the median ratio of circOMA1 expression, patients with
BC were divided into two groups (high and low expression of
circOMA1). Kaplan-Meier analysis and log-rank test revealed that
patients with BC and high expression of circOMA1 had poorer overall
survival compared with in patients with BC and low expression of
circOMA1 (Fig. 1C). In addition, to
assess whether the expression of circOMA1 may be used discriminate
between BC tissues and adjacent normal tissues, receiver operating
characteristic (ROC) analysis was performed. An area under the ROC
curve of 0.8296 indicated that the expression of circOMA1 could
discriminate between BC tissues and adjacent normal tissues
(Fig. 1D). Furthermore, the
expression levels of circOMA1 were higher in BC cells than those in
normal breast epithelial cells (Fig.
1E). RNA stability analysis demonstrated that the circular
transcript circOMA1 was much more stable than the linear mRNA
transcript in BC cells incubated with a transcription inhibitor
(actinomycin D) (Fig. 1F). After
exposure to RNase R or control (DMSO), RT-qPCR results demonstrated
that the expression levels of the linear form of OMA1 were
significantly decreased under RNase R treatment, whereas there was
little change in circOMA1 expression levels. Resistance to
digestion by RNase R exonuclease further confirmed that OMA1 RNA
species were present in circular form (Fig. 1G). circOMA1 expression was also
revealed to be closely associated with tumor size and lymph node
metastasis in patients (Table I).
These data suggested that circOMA1 expression was increased in BC
tissues and cell lines, and may be associated with poor prognosis
of BC. Among the BC cell lines assessed, circOMA1 expression was
the highest in MCF-7, whereas it was lowest in MDA-MB-468 cells.
Therefore, circOMA1 overexpression was induced in MDA-MB-468 cells
and circOMA1 expression was knocked down in MCF-7 cells, in order
to elucidate the function of circOMA1 in BC cells.
 | Table I.Association between the circOMA1
expression and the clinicopathological features of patients of
breast cancer. |
Table I.
Association between the circOMA1
expression and the clinicopathological features of patients of
breast cancer.
|
|
| Expression of
circOMA1 |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameters | Numbers (n=64) | High (n=32) | Low (n=32) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.068 | 0.794 |
|
≤50 | 23 | 12 | 11 |
|
|
|
>50 | 41 | 20 | 21 |
|
|
| Tumor size, cm |
|
|
| 6.478 | 0.011 |
| ≤2 | 26 | 8 | 18 |
|
|
|
>2 | 38 | 24 | 14 |
|
|
| TNM stage |
|
|
| 1.697 | 0.193 |
|
I–II | 41 | 18 | 23 |
|
|
| III-
IV | 23 | 14 | 9 |
|
|
| Lymphatic
metastasis |
|
|
| 6.667 | 0.01 |
|
Yes | 24 | 17 | 7 |
|
|
| No | 40 | 15 | 25 |
|
|
| T stage |
|
|
| 1.564 | 0.211 |
| T1 | 31 | 13 | 18 |
|
|
|
T2-T3 | 33 | 19 | 14 |
|
|
| N stage |
|
|
| 1.036 | 0.309 |
| N0 | 38 | 21 | 17 |
|
|
|
N1-N3 | 26 | 11 | 15 |
|
|
circOMA1 promotes the tumorigenesis of
BC cells
To examine the biological functions of circOMA1,
shRNA was used to knock down circOMA1 in BC cells (Fig. 2A). Among the three shRNAs tested,
shRNA#1 was the most efficient and was therefore chosen for
subsequent experiments. In addition, the expression levels of
circOMA1 were increased by transfection of BC cells with the
circOMA1 expression vector (Fig.
2B). The CCK-8 assay revealed that silencing circOMA1 inhibited
proliferation, whereas overexpression of circOMA1 promoted the
proliferation of BC cells (Fig.
2C). Subsequently, Transwell assays indicated that knockdown of
circOMA1 suppressed migration/invasion, whereas overexpression of
circOMA1 increased the migration/invasion of BC cells (Fig. 2D and E). Flow cytometric analysis of
early and late apoptotic cells demonstrated that knockdown of
circOMA1 increased apoptosis, whereas overexpression of circOMA1
reduced apoptosis of BC cells (Fig.
2F). Taken together, these data indicated that circOMA1
promoted the progression of BC.
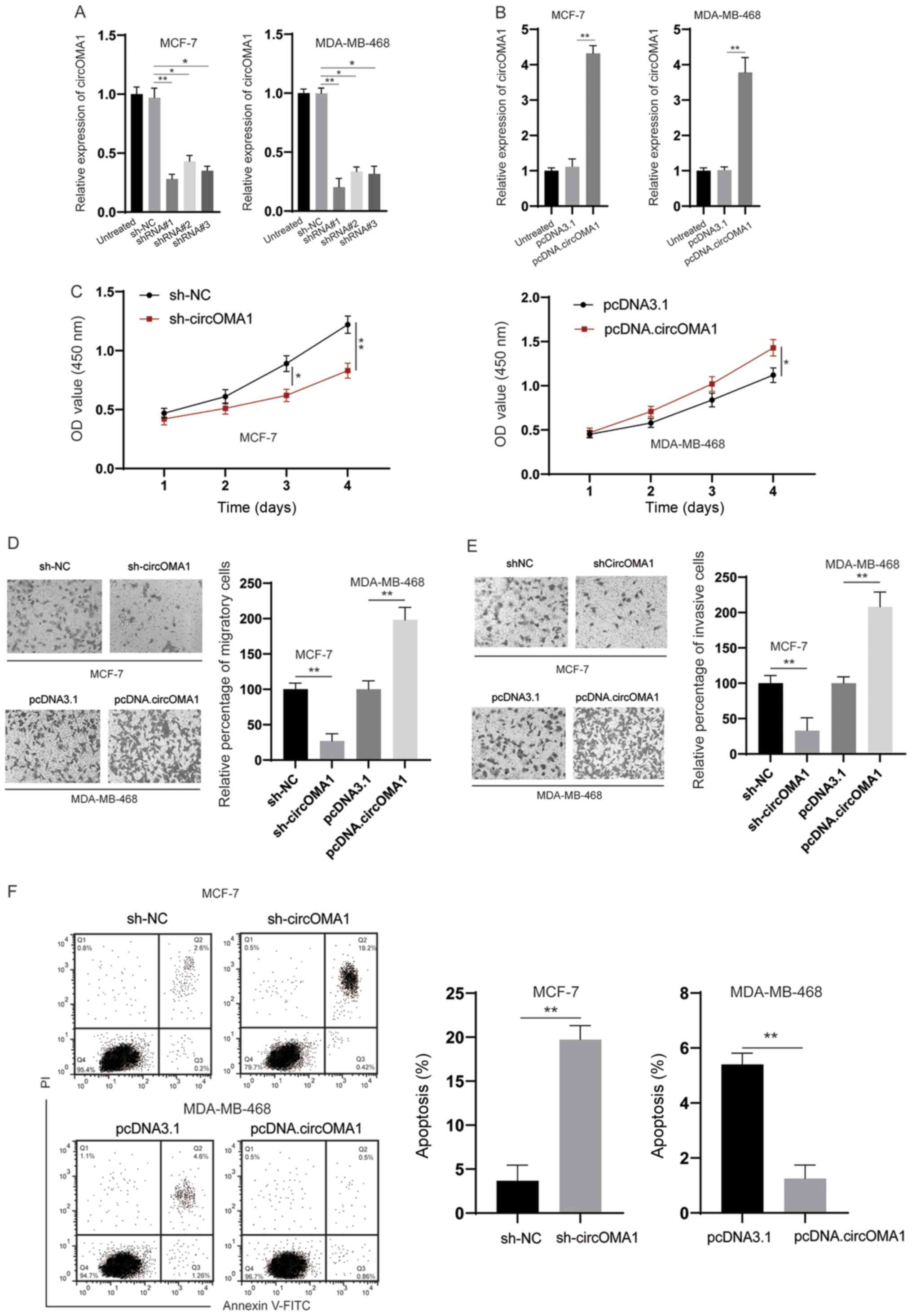 | Figure 2.circOMA1 promotes the tumorigenesis of
BC. (A) MCF-7 cells were transfected as indicated and the
expression of circOMA1 was evaluated by RT-qPCR. (B) MDA-MB-468
cells were transfected with pcDNA3.1 or pcDNA.circOMA1, and the
expression of circOMA1 was measured by RT-qPCR. (C) BC cells were
transfected as indicated and the proliferation of cells was
measured by Cell Counting Kit-8 assay at different time points. (D)
BC cells were transfected as indicated and cellular migration was
assessed (magnification, ×100). (E) BC cells were transfected as
indicated and cellular invasion was assessed (magnification, ×100).
(F) BC cells were transfected as indicated and cellular apoptosis
was assayed. Data are presented as the mean ± SD. Experiments were
performed at least three times. *P<0.05, **P<0.01. BC, breast
cancer; circ, circular RNA; RT-qPCR, reverse
transcription-quantitative PCR; sh, short hairpin RNA; NC, negative
control; OD, optical density; PI, propidium iodide. |
circOMA1 acts as a sponge of
miR-1276
To further analyze the role of circOMA1, a
subcellular fractionation assay was conducted, and it was revealed
that circOMA1 was mainly distributed in the cytoplasm rather than
the nucleus (Fig. 3A). The
cytoplasmic location of circOMA1 strongly indicated that circOMA1
may be involved in post-transcriptional regulation. A previous
study has demonstrated that circRNA molecules act as competing
endogenous RNAs (ceRNAs) by blocking the Ago2-mediated silencing
complex (18). Therefore, an
RNA-protein immunoprecipitation assay was conducted; the results
revealed that circOMA1 could bind Ago2 protein in BC cells
(Fig. 3B). Based on these findings,
it was hypothesized that circOMA1 might regulate gene expression at
the post-transcriptional level. To verify this, bioinformatics
tools (StarBase 3.0 and CircInteractome) were used, and several
candidate miRNAs were identified. To test this hypothesis, an RNA
pull-down assay was performed and revealed that a specific probe
against circOMA1 could enrich circOMA1 over 30-fold compared with
the control (Fig. 3C). Furthermore,
miR-1276 was significantly increased in the circOMA1 probe group,
whereas there was little change in the expression levels of other
miRNAs in BC cells (Fig. 3D). Thus,
miR-1276 was chosen for subsequent experiments. Dual-luciferase
reporter assays revealed that the luciferase activities of the
circOMA1 wt reporter were significantly decreased when cells were
transfected with miR-1276 mimics compared with those transfected
with the miR-NC or circOMA1 mut luciferase reporter (Fig. 3E). In addition, it was revealed that
silencing circOMA1 led to the upregulation of miR-1276 (Fig. 3F), whereas overexpression of
circOMA1 induced downregulation of miR-1276 in BC cells (Fig. 3G). The expression levels of miR-1276
were also downregulated in BC tissues compared with those in normal
tissues (Fig. 3H), and there was a
negative correlation between miR-1276 and circOMA1 expression in BC
tissues (Fig. 3I). Moreover, to
further analyze the role of miR-1276, miR-1276 mimics and miR-1276
inhibitor were used to increase or decrease miR-1276 levels,
respectively (Fig. 3J). It was
demonstrated that miR-1276 mimics inhibited the tumorigenesis of BC
cells, whereas the miR-1276 inhibitor had the opposite effects of
silencing circOMA1 on the proliferation, migration, invasion and
apoptosis of BC cells (Fig. 3K-N).
Taken together, these data suggested that circOMA1 may act as a
sponge of miR-1276.
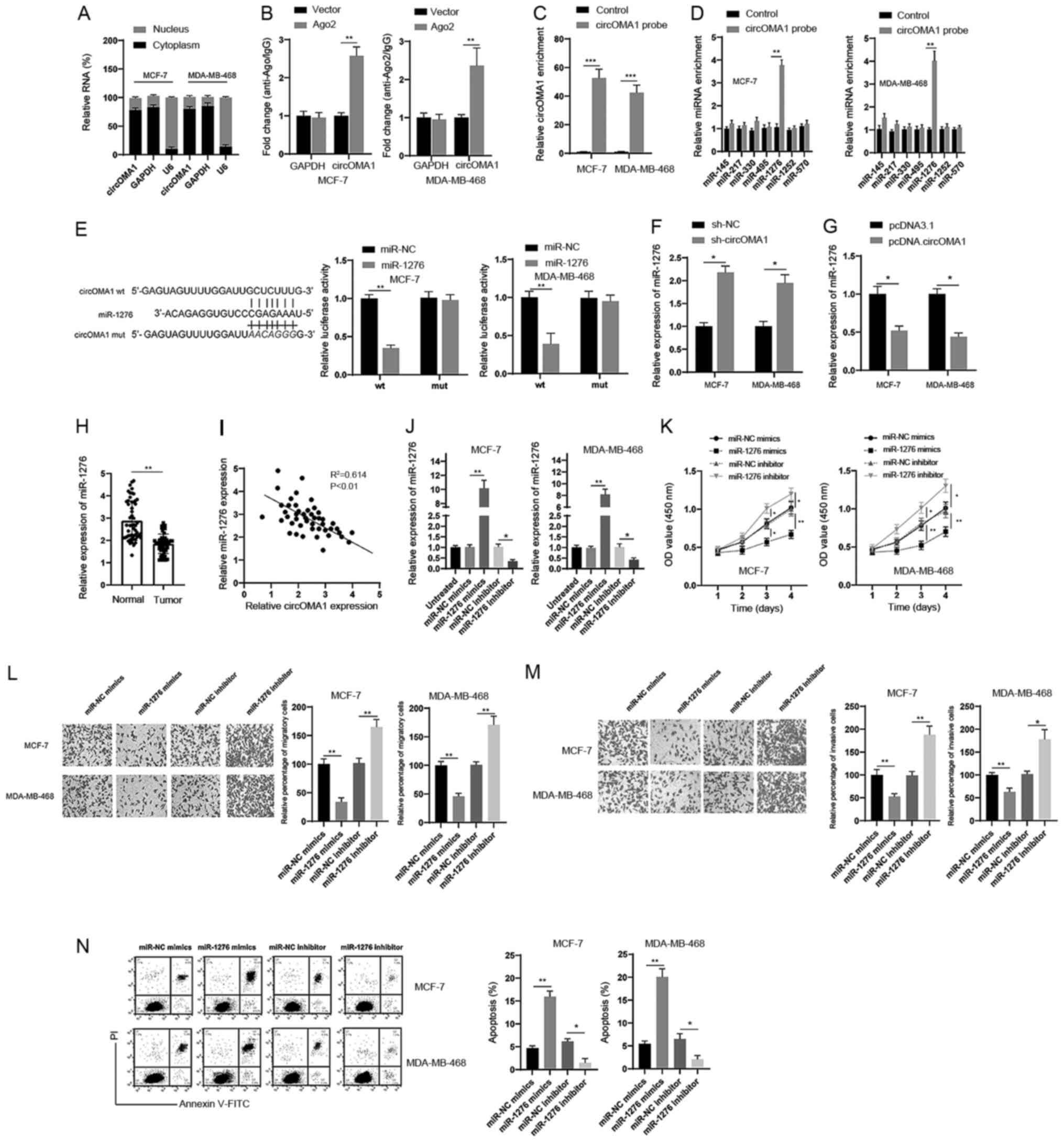 | Figure 3.circOMA1 acts as sponge of miR-1276.
(A) Subcellular fraction analysis of the location of circOMA1 in BC
cells. (B) Ago2 RNA-protein immunoprecipitation assay was used for
the detection of circOMA1 in BC cells expressing Flag-Ago2 or
Flag-tag. (C) RNA pull-down assay was used for the detection of
circOMA1 using a circOMA1-specific probe in BC cells. (D) Relative
expression of circOMA1 putative binding miRNAs was examined by
reverse transcription-quantitative PCR analysis using a
circOMA1-specific probe. (E) Predictive binding sites between
circOMA1 and miR-1276; relative luciferase activity was assessed in
BC cells co-transfected with wt or mut luciferase reporters and
miR-1276 mimics or corresponding NC. (F) BC cells were transfected
with sh-NC or sh-circOMA1, and the expression of miR-1276 was
assessed. (G) BC cells were transfected with pcDNA3.1 or
pcDNA.circOMA1, and the expression of miR-1276 was assessed. (H)
Expression of miR-1276 was assessed in BC tissues and adjacent
normal tissues. (I) Correlation between the expression of miR-1276
and circOMA1 was analyzed in BC tissues. (J) BC cells were
transfected as indicated and the expression levels of miR-1276 were
measured. (K) BC cells were transfected as indicated and cell
proliferation was measured by Cell Counting Kit-8 assay at
different time points. *P<0.05, **P<0.01 vs. NC group. (L) BC
cells were transfected as indicated and cellular migration was
assessed (magnification, ×100). (M) BC cells were transfected as
indicated and cellular invasion was assessed (magnification, ×100).
(N) BC cells were transfected as indicated and cellular apoptosis
was assessed. Data are presented as the mean ± SD. Experiments were
performed at least three times. *P<0.05, **P<0.01. BC, breast
cancer; circ/circRNA, circular RNA; sh, short hairpin RNA; NC,
negative control; miR, microRNA; mut, mutant; wt, wild-type; OD,
optical density; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
miR-1276 targets SIRT4 in BC
cells
Through bioinformatics analysis, SIRT4 was predicted
to be a direct target of miR-1276 (Fig.
4A). Subsequently, a dual-luciferase reporter assay was
conducted, and it was revealed that miR-1276 could inhibit the
luciferase activity of the wt reporter but had no effect on the mut
reporter system in BC cells (Fig.
4A). In addition, miR-1276 mimics decreased the expression of
SIRT4 at both the mRNA and protein levels in BC cells (Fig. 4B and C). Conversely, the miR-1276
inhibitor increased the expression of SIRT4 at both the mRNA and
protein levels in BC cells (Fig. 4D and
E). RNA pull-down assays also demonstrated that miR-1276 could
markedly enrich SIRT4 in BC cells (Fig.
4F). Furthermore, SIRT4 was significantly upregulated in BC
tissues compared with in normal tissues (Fig. 4G), and there was a negative
correlation between miR-1276 and SIRT4 expression in BC tissues
(Fig. 4H). Subsequently, shRNA was
used to knock down SIRT4 in BC cells (Fig. 4I). Similar to the observations made
following knockdown of circOMA1, silencing SIRT4 suppressed the
proliferation, migration and invasion, and promoted the apoptosis
of BC cells (Fig. 4J-M). These data
suggested that miR-1276/SIRT4 acted downstream of circOMA1.
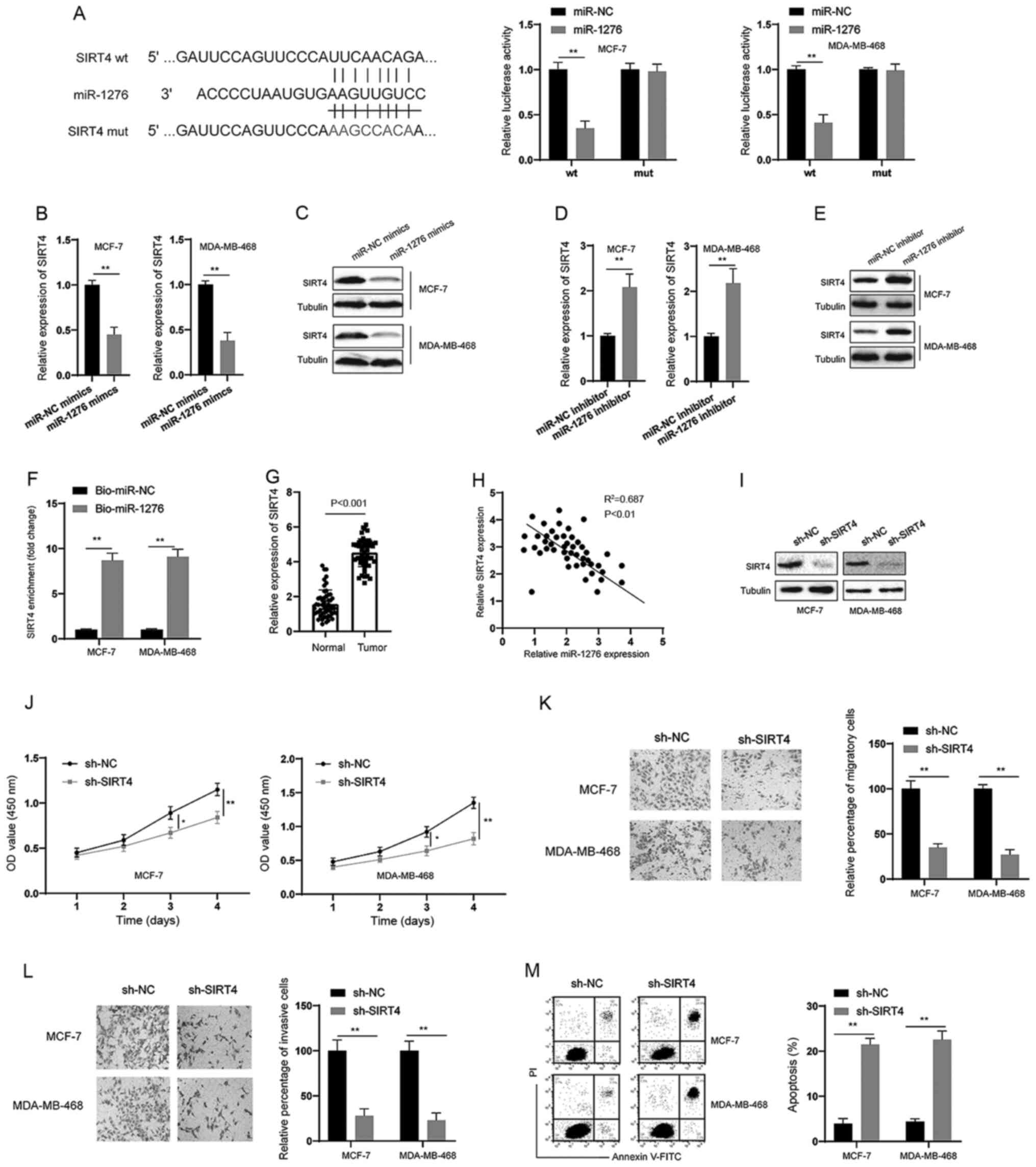 | Figure 4.SIRT4 is a target of miR-1276. (A)
Predictive binding sites between 3’ untranslated region of SIRT4
and miR-1276; relative luciferase activity was assessed in BC cells
co-transfected with wt or mut luciferase reporters and miR-1276
mimics or corresponding NC. (B and D) BC cells were transfected as
indicated and the mRNA expression levels of SIRT4 were measured. (C
and E) BC cells were transfected as indicated and the protein
expression levels of SIRT4 were measured. (F) RNA pull-down assay
was used for the detection of SIRT4 using miR-1276 and miR-NC
probes in BC cells. (G) mRNA expression levels of SIRT4 were
measured in BC tissues and adjacent normal tissues. (H) Correlation
analysis of the expression of SIRT4 and miR-1276 in BC tissues. (I)
BC cells were transfected as indicated and the protein expression
levels of SIRT4 were measured by western blotting. (J) BC cells
were transfected as indicated and proliferation of BC cells was
measured by Cell Counting Kit-8 assay at different time points. (K)
BC cells were transfected as indicated and cellular migration was
assessed (magnification, ×100). (L) BC cells were transfected as
indicated and cellular invasion was assessed (magnification, ×100).
(M) BC cells were transfected as indicated and cellular apoptosis
was assessed. Data are presented as the mean ± SD. Experiments were
performed at least three times. *P<0.05, **P<0.01. BC, breast
cancer; sh, short hairpin RNA; NC, negative control; miR, microRNA;
mut, mutant; wt, wild-type; SIRT4, sirtuin 4; OD, optical density;
PI, propidium iodide; FITC, fluorescein isothiocyanate. |
circOMA1 exerted its oncogenic effects
via the miR-1276/SIRT4 axis
The correlation between circOMA1 and SIRT4
expression was assessed and a positive correlation was detected
between them in BC tissues (Fig.
5A). In vitro experiments demonstrated that silencing
circOMA1 led to the downregulation of SIRT4 at both the mRNA and
protein levels in BC cells (Fig.
5B). Moreover, overexpression of circOMA1 induced an
upregulation of SIRT4 at both the mRNA and protein levels in BC
cells (Fig. 5C). To further analyze
the role of SIRT4 in the function of circOMA1, SIRT4 was
overexpressed in BC cells via transfection with the pcDNA3.1 vector
containing full-length SIRT4 (pcDNA.SIRT4) (Fig. 5D). The CCK-8 assay revealed that
overexpression of SIRT4 reversed the inhibitory effects of circOMA1
knockdown on the proliferation of BC cells (Fig. 5E). In addition, overexpression of
SIRT4 inhibited the effects of circOMA1 knockdown on the migration,
invasion and apoptosis of BC cells (Fig. 5F-H). Taken together, these data
suggested that circOMA1 exerted its oncogenic effects via
regulation of the miR-1276/SIRT4 axis in BC cells.
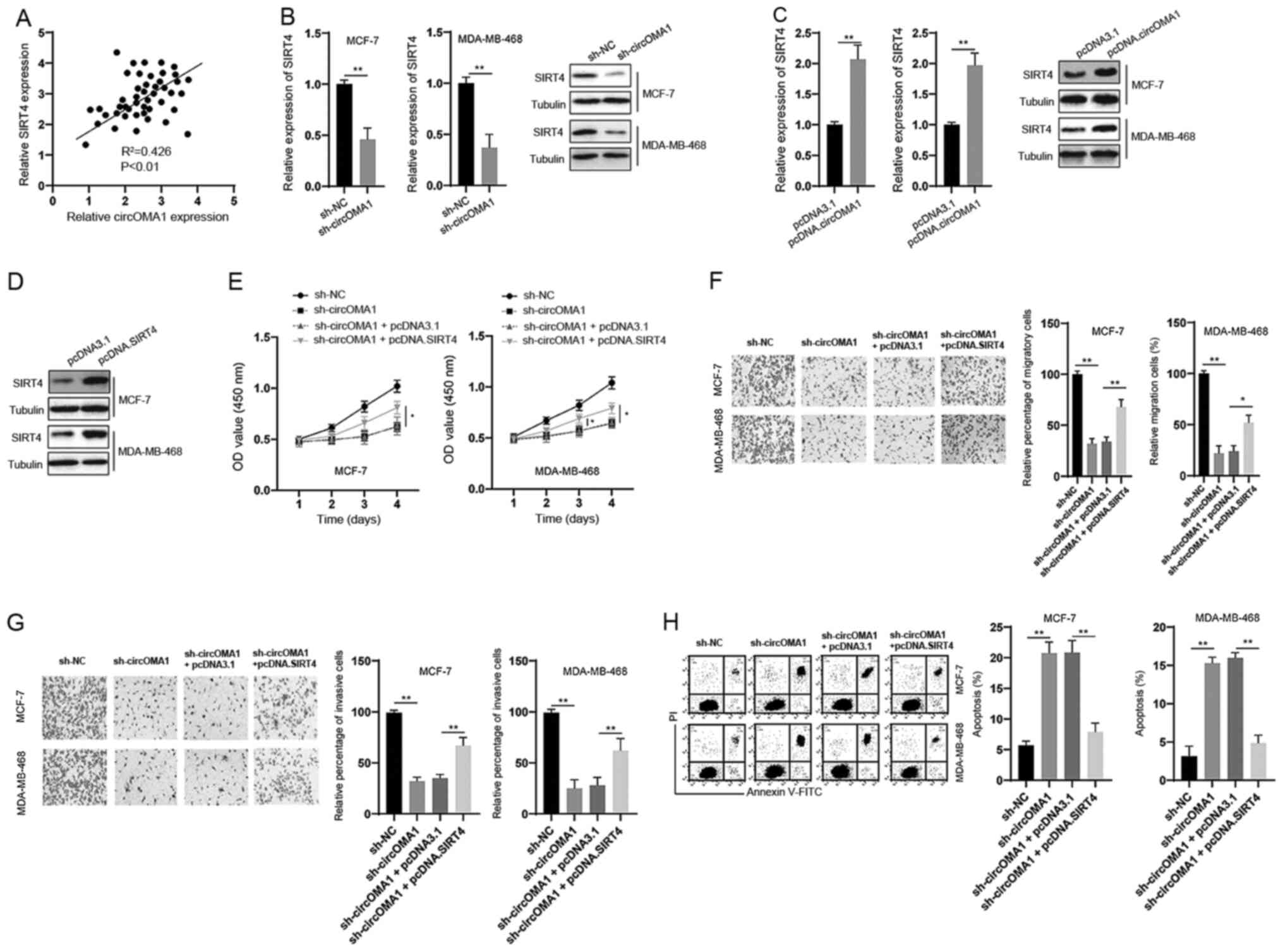 | Figure 5.Overexpression of SIRT4 abrogates the
effects of circOMA1 silencing in BC cells. (A) Correlation between
circOMA1 and SIRT4 expression was analyzed in BC tissues. (B) BC
cells were transfected as indicated and the mRNA expression levels
of SIRT4 were measured by reverse transcription-quantitative PCR.
(C) BC cells were transfected as indicated and the protein
expression levels of SIRT4 were measured by western blotting. (D)
BC cells were transfected with pcDNA3.1 or pcDNA.SIRT4, and protein
expression levels of SIRT4 were measured by western blotting. (E)
BC cells were transfected as indicated and cell viability was
measured by Cell Counting Kit-8 assay. *P<0.05 vs. sh-circOMA1 +
pcDNA3.1. (F) BC cells were transfected as indicated and cellular
migration were assessed (magnification, ×100). (G) BC cells were
transfected as indicated and cellular invasion were assessed
(magnification, ×100). (H) BC cells were transfected as indicated
and cellular apoptosis was measured by flow cytometry. Data are
presented as the mean ± SD. Experiments were performed at least
three times. *P<0.05, **P<0.01. BC, breast cancer; circ,
circular RNA; sh, short hairpin RNA; NC, negative control; SIRT4,
sirtuin 4; OD, optical density; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Discussion
BC is one of the most prevalent types of cancer with
a high incidence and poor prognosis. In recent years, accumulating
evidence has indicated that circRNAs, a new type of ncRNA, may have
essential roles in the initiation and progression of various types
of cancer, including BC. For example, circSKA3 was demonstrated to
bind integrin β1 to induce invadopodium formation and thereby
enhance invasion of BC (19).
Another recent study reported that circCDYL was able to promote
autophagy and increase the progression of BC (20).
The present study examined the expression pattern
and prognostic value of circOMA1 in BC. The results demonstrated
that the expression levels of circOMA1 were significantly higher in
BC tissues compared with those in normal tissues. Higher expression
of circOMA1 was also associated with poorer survival of patients
with BC. In vitro functional studies revealed that circOMA1
acted as an oncogene in BC cells. The present findings are in
accordance with a previous study, which reported that circOMA1
acted as an oncogene and promoted the progression of nonfunctioning
pituitary adenomas (21). It is
well documented that circRNAs can function as ceRNAs to interact
with miRNAs and thus modulate the expression of target genes. In
the present study, the interaction between circOMA1 and miR-1276
was verified. It was observed that silencing circOMA1 increased
miR-1276 expression, whereas overexpression of circOMA1 inhibited
the expression of miR-1276. Furthermore, a dual-luciferase reporter
assay confirmed the binding between circOMA1 and miR-1276. To date,
knowledge about the role of miR-1276 in tumors is limited. A
previous study reported that miR-1276 could bind to lncRNA HCG1,
and inhibit the proliferation and migration of gastric cancer cells
(22). However, another study
reported that miR-1276 exhibited high expression tendencies in
liver cancer tissues compared with in normal liver tissues
(23). The present study revealed
that miR-1276 was downregulated in BC tissues and acted as a tumor
suppressor in BC cells. These discrepancies may be due to the
different cancer types, and further investigation into the role of
miR-1276 in different types of cancer is required.
It is well documented that miRNAs exert their
functions via inhibition or deregulation of targeted mRNAs
(24). The present study aimed to
investigate the target genes of miR-1276, and SIRT4 was identified
as a potential candidate. SIRT4 belongs to the SIRT family and has
been shown to serve controversial roles in tumors. It has been
demonstrated that overexpression of SIRT4 may inhibit the
proliferation of HeLa and Burkitt lymphoma cells by inhibiting
glutamine metabolism in vitro (25,26).
In addition, SIRT4 has been shown to inhibit the tumorigenesis of
colorectal and lung cancer cells (27,28).
However, SIRT4 may act as an oncogene in cancer. For example, in a
previous study, SIRT4 protein levels were higher in esophageal
cancer tissue compared with those in adjacent nontumor esophageal
tissue (29). The present study
indicated that SIRT4 acted as an oncogene in BC, and these findings
are in line with a previous study that also demonstrated that SIRT4
was upregulated in BC tissues and promoted the migration,
proliferation and invasion of BC cells (30). Moreover, the present study revealed
that overexpression of SIRT4 abrogated the effects of circOMA1
silencing on the proliferation, migration, invasion and apoptosis
of BC cells. Collectively, it was concluded that circOMA1 was
involved in BC progression via sponging miR-176 and upregulating
SIRT4.
In conclusion, the present study revealed that the
expression levels of circOMA1 were increased in BC and that
circOMA1 was associated with poor prognosis of BC. Functional
investigations demonstrated that circOMA1 may be involved in the
proliferation, migration, invasion and apoptosis of BC cells.
Furthermore, the pivotal role of the circOMA1/miR-1276/SIRT4 axis
in BC was revealed in the current study. Therefore, circOMA1 may
have potential as a therapeutic target and novel prognostic
biomarker for BC.
Acknowledgements
The authors would like to thank Dr Renxi Zhang
(Zhejiang University, School of Medicine) for his helpful
suggestions.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LXu was responsible for the acquisition of data, KX
and LXiang analysed and interpreted the data, JY conceived and
designed this study, drafted the manuscript and revised it. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhenghai Longsai Hospital. Written informed consent
was obtained from all participants prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.Erratum in: CA
Cancer J Clin 70: 313, 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagini S: Breast Cancer: Current Molecular
Therapeutic Targets and New Players. Anticancer Agents Med Chem.
17:152–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilusz JE and Sharp PA: Molecular biology.
A circuitous route to noncoding RNA. Science. 340:440–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jahani S, Nazeri E, Majidzadeh-A K, Jahani
M and Esmaeili R: Circular RNA; a new biomarker for breast cancer:
A systematic review. J Cell Physiol. 235:5501–5510. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu J, Jiang Z, Chen C, Hu Q, Fu Z, Chen J,
Wang Z, Wang Q, Li A, Marks JR, et al: CircIRAK3 sponges miR-3607
to facilitate breast cancer metastasis. Cancer Lett. 430:179–192.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang N, Gu Y, Li L, Wang F, Lv P, Xiong Y
and Qiu X: Circular RNA circMYO9B facilitates breast cancer cell
proliferation and invasiveness via upregulating FOXP4 expression by
sponging miR-4316. Arch Biochem Biophys. 653:63–70. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibuya N, Kakeji Y and Shimono Y:
MicroRNA-93 targets WASF3 and functions as a metastasis suppressor
in breast cancer. Cancer Sci. 111:2093–2103. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malagobadan S, Ho CS and Nagoor NH:
MicroRNA-6744-5p promotes anoikis in breast cancer and directly
targets NAT1 enzyme. Cancer Biol Med. 17:101–111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahuja N, Schwer B, Carobbio S, Waltregny
D, North BJ, Castronovo V, Maechler P and Verdin E: Regulation of
insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase.
J Biol Chem. 282:33583–33592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sebastián C, Satterstrom FK, Haigis MC and
Mostoslavsky R: From sirtuin biology to human diseases: An update.
J Biol Chem. 287:42444–42452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li T, Li Y, Liu T, Hu B, Li J, Liu C, Liu
T and Li F: Mitochondrial PAK6 inhibits prostate cancer cell
apoptosis via the PAK6-SIRT4-ANT2 complex. Theranostics.
10:2571–2586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Li H, Zhao ZB, Zhu W, Feng PP, Zhu
XW and Gong JP: SIRT4 silencing in tumor-associated macrophages
promotes HCC development via PPARδ signalling-mediated alternative
activation of macrophages. J Exp Clin Cancer Res. 38:4692019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Guo Y, Gao J and Yuan X:
Tumor-suppressive function of SIRT4 in neuroblastoma through
mitochondrial damage. Cancer Manag Res. 10:5591–5603. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Obuchowski NA: ROC analysis. AJR Am J
Roentgenol. 184:364–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du WW, Yang W, Li X, Fang L, Wu N, Li F,
Chen Y, He Q, Liu E, Yang Z, et al: The Circular RNA circSKA3 binds
integrin β1 to induce invadopodium formation enhancing breast
cancer invasion. Mol Ther. 28:1287–1298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang G, Ling Y, Mehrpour M, Saw PE, Liu
Z, Tan W, Tian Z, Zhong W, Lin W, Luo Q, et al:
Autophagy-associated circRNA circCDYL augments autophagy and
promotes breast cancer progression. Mol Cancer. 19:652020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du Q, Hu B, Feng Y, Wang Z, Wang X, Zhu D,
Zhu Y, Jiang X and Wang H: circOMA1-Mediated miR-145-5p Suppresses
Tumor Growth of Nonfunctioning Pituitary Adenomas by Targeting
TPT1. J Clin Endocrinol Metab. 104:2419–2434. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Huang H, Xu X, Wang H, Wang J,
Yao Z, Xu X, Wu Q and Xu F: LncRNA HCG11 promotes proliferation and
migration in gastric cancer via targeting miR-1276/CTNNB1 and
activating Wnt signaling pathway. Cancer Cell Int. 19:3502019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong DD, Dang YW, Lin P, Wen DY, He RQ,
Luo DZ, Feng ZB and Chen G: A circRNA-miRNA-mRNA network
identification for exploring underlying pathogenesis and therapy
strategy of hepatocellular carcinoma. J Transl Med. 16:2202018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Afonso-Grunz F and Müller S: Principles of
miRNA-mRNA interactions: Beyond sequence complementarity. Cell Mol
Life Sci. 72:3127–3141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong SM, Lee A, Lee J and Haigis MC:
SIRT4 protein suppresses tumor formation in genetic models of
Myc-induced B cell lymphoma. J Biol Chem. 289:4135–4144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong SM, Xiao C, Finley LW, Lahusen T,
Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, et al:
SIRT4 has tumor-suppressive activity and regulates the cellular
metabolic response to DNA damage by inhibiting mitochondrial
glutamine metabolism. Cancer Cell. 23:450–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu L, Dong Q, He J, Wang X, Xing J, Wang
E, Qiu X and Li Q: SIRT4 inhibits malignancy progression of NSCLCs,
through mitochondrial dynamics mediated by the ERK-Drp1 pathway.
Oncogene. 36:2724–2736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyo M, Yamamoto H, Konno M, Colvin H,
Nishida N, Koseki J, Kawamoto K, Ogawa H, Hamabe A, Uemura M, et
al: Tumour-suppressive function of SIRT4 in human colorectal
cancer. Br J Cancer. 113:492–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakahara Y, Yamasaki M, Sawada G, Miyazaki
Y, Makino T, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S,
Mimori K, et al: Downregulation of SIRT4 Expression Is Associated
with Poor Prognosis in Esophageal Squamous Cell Carcinoma.
Oncology. 90:347–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang G, Lin Y and Zhu G: SIRT4 is
upregulated in breast cancer and promotes the proliferation,
migration and invasion of breast cancer cells. Int J Clin Exp
Pathol. 10:11849–11856. 2017.PubMed/NCBI
|