Introduction
Multiple myeloma (MM) is a heterogeneous disorder of
plasma cells that is characterized by uncontrolled proliferation of
monoclonal plasma cells, leading to the accumulation of
nonfunctional intact immunoglobulins or immunoglobulin chains in
the bone marrow (1). As the second
most prevalent hematological malignancy, MM accounts for ~1% of all
cancer cases and the current 5-year survival rate is estimated at
~46.6% worldwide (2). The emergence
of several advanced treatments for MM, including autologous stem
cell transplants, chemotherapy, targeted drugs and immunomodulatory
drugs, has resulted in a marked improvement in the clinical
outcomes of patients with MM. However, the majority of patients
with MM still suffer from a high risk of relapse and the
development of refractory MM, as a result of current therapies
(3–5). Therefore, it is essential to explore
novel, promising therapeutic targets, which can be applied for the
improvement of treatment efficacy in MM.
Sirtuin 2 (SIRT2) is a member of the human sirtuin
family, which has a unique NAD+-dependent protein
deacetylase activity (6). Emerging
evidence has demonstrated that SIRT2 functions as an α-tubulin
deacetylase, which is implicated in numerous biological processes,
including microtubule dynamics, mitotic arrest, cell motility and
cell differentiation (7–12). Furthermore, the involvement of SIRT2
in specific pathological processes, such as carcinogenesis,
development of leukemia, neurodegeneration and formation of drug
resistance has been previously reported (13–16).
For example, the role of SIRT2 in regulating aberrant proliferation
and survival of myeloid leukemia cells has been reported in former
studies. SIRT2 has been shown to promote proliferation and survival
of acute myeloid leukemia (AML) cells via regulating ERK1/2
signaling and RAS/ERK/JNK/MMP-9 signaling (16–18).
In addition, RAS/ERK signaling has been reported to regulate the
p85-fibroblast growth factor receptor 3 (FGFR3) interaction in MM,
whereas FGFR3 knockdown may inhibit proliferation and promote
apoptosis of MM cells (16,17,19).
Furthermore, activation of RAS/ERK has been associated with the
increased proliferation and survival of MM cells, contributing to
the pathogenesis of this disease (20). Based on the aforementioned data, it
was hypothesized that SIRT2 might have an important role in the
development and progression of MM. However, to the best of our
knowledge, the interaction of SIRT2 with MM progression has not
been previously reported. Therefore, in the present study, the
regulatory effect of SIRT2 knockdown on cell proliferation,
induction of apoptosis and regulation of the cell cycle was
investigated in MM cells. Moreover, the interaction of the RAS/ERK
signaling pathway in MM was examined with regards to the
aforementioned processes.
Materials and methods
Participants
A total of 30 MM bone marrow samples were collected
from 30 patients (age range, 28–72 years; sex, 18 males and 12
females) with de novo MM treated at Huashan Hospital
(Shanghai, China) or Shanghai Jing'an District Beizhan Hospital
(Shanghai, China) between June 2016 and December 2018. All patients
had a confirmed diagnosis of de novo symptomatic MM,
according to The International Myeloma Working Group criteria of MM
(21). The patients were all >18
years old, and had not received radiation or chemotherapy prior to
sample collection. The patients were also devoid of other
hematological malignancies or solid tumors. In addition, 15 healthy
bone marrow samples were collected from 15 bone marrow donors (age
range, 29–45 years; sex, 10 males and 5 females) during the same
period. The present study was approved by the Institutional Review
Board of Shanghai Jing'an District Beizhan Hospital (approval
number 2019–021). All participants provided written informed
consent for their participation in the study.
Cell culture
Human MM cell lines, KMS-28BM, U266, RPMI-8226 and
NCI-H929, were purchased from the American Type Culture Collection.
All cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% (KMS-28BM, RPMI-8226 and
NCI-H929) or 15% fetal bovine serum (U266) (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin and streptomycin at 37°C in a
humidified incubator containing 5% CO2. The plasma cells
were isolated from bone marrow mononuclear cells, which were
derived from the bone marrow samples of patients with MM and
healthy bone marrow donors. Briefly, after collection, the bone
marrow samples were processed with gradient density centrifugation
(865 × g at 37°C for 20 min) for separating the bone marrow
mononuclear cells; subsequently, the separated bone marrow
mononuclear cells were purified using CD138-coated magnetic beads
(Miltenyi Biotec GmbH) to obtain plasma cells (22,23).
The plasma cells were then stored in liquid nitrogen for further
analysis. After incubation at 37°C for 24 h, SIRT2 expression in MM
cell lines and normal plasma cells (from healthy bone marrow donors
that were used as the control group) was determined by RT-qPCR and
western blot analysis.
Plasmid transfection
SIRT2 short hairpin RNA (shRNA) and a nonsense shRNA
sequence were designed and synthesized by Changchun Changsheng Gene
Pharmaceutical Co., Ltd. The sequences for the shRNAs were as
follows: shSIRT2 type 1, 5′-GCTAAGCTGGATGAAAGAGAA-3′; shSIRT2 type
2, 5′-GCCAACCATCTGTCACTACTT-3′; shSIRT2 type 3,
5′-CCTGCTCATCAACAAGGAGAA-3′; and shNC, 5′-GCAACAAGATGAAGAGCACCAA-3′
(24). The sequences were
subsequently cloned into the GenePharma SuperSilencing shRNA™
vector (pGPU6/RFP/Neo) (Shanghai GenePharma Co., Ltd.) to construct
shRNA-SIRT2 (Sh-SIRT2) recombinant plasmid and shRNA-negative
control (Sh-NC) recombinant plasmid. Lipofectamine® 3000
Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
was used to transfect the RPMI-8226 and NCI-H929 cells
(1×105 cells/well) with 0.8 µg recombinant plasmids at
37°C for 24 h, which resulted in the corresponding Sh-SIRT2 and
Sh-NC cells. After 24 h, SIRT2 expression in the cells was detected
by RT-qPCR and western blot analysis.
Cell proliferation and apoptosis
assays
At 0, 24, 48 and 72 h post-transfection, the
proliferation of Sh-SIRT2 and Sh-NC cells was detected using the
Cell Counting Kit-8 assay (Dojindo Molecular Technologies, Inc.) in
accordance with the manufacturer's instructions. The optical
density value of the samples was measured at 450 nm using the iMark
microplate reader (Bio-Rad Laboratories, Inc.). The determination
of apoptosis of Sh-SIRT2 and Sh-NC cells was performed 48 h
post-transfection using the Annexin V-fluorescein isothiocyanate
Apoptosis Detection kit (Sigma-Aldrich; Merck KGaA), as described
previously (17). The apoptotic
cells (early + late apoptotic cells; PI−/Annexin
V+ staining represents early apoptotic cells and
PI+/Annexin V+ staining represents late
apoptotic cells) were analyzed using a CytoFLEX™ flow cytometer
(Beckman Coulter, Inc.) and FlowJo 7.0 software (FlowJo LLC).
Cell cycle analysis
A total of 48 h post-transfection, the transfected
Sh-SIRT2 and Sh-NC cells were harvested by trypsinization and
subjected to cell cycle analysis using a BD FACSCalibur flow
cytometer (BD Biosciences). The assay was performed as described
previously (25). At least 10,000
events were acquired for each sample in order to obtain a
measurable signal. The cell percentages at the
G0/G1, S and G2/M phases were
quantified using FlowJo 7.0 software (FlowJo LLC).
SIRT2-associated pathway
detection
The previous study indicated that SIRT2 was
associated with the activity of the ERK1/2 signaling pathway in AML
cells (16). Furthermore, it was
previously shown that SIRT2 induced the migration and invasion of
gastric cancer cells through the RAS/ERK/JNK/MMP-9 pathway
(17). As a result, in order to
investigate whether SIRT2 was associated with regulation of the
RAS/ERK pathway in MM cells, the expression levels of HRAS, ERK and
phosphorylated ERK (p-ERK) were assessed using western blot
analysis and RT-qPCR in Sh-SIRT2 and Sh-NC cells after 48 h of
incubation.
Western blot analysis
Western blot analysis was performed as described
previously (26). Briefly, the
cells were lysed in RIPA lysis buffer (Sigma-Aldrich; Merck KGaA)
and protein concentration was measured using a Pierce™ Rapid Gold
BCA Protein assay kit (Thermo Fisher Scientific, Inc.).
Subsequently, 20 µg protein/lane was separated via 4–20% SDS-PAGE.
The proteins were then transferred to nitrocellulose membranes
(Qiagen GmbH) and after blocking with 5% BSA (Beyotime Institute of
Biotechnology) at 37°C for 1.5 h, the membranes were incubated with
the following specific primary antibodies overnight at 4°C: Rabbit
monoclonal anti-SIRT2 (1:2,000; Abcam; cat. no. ab211033), rabbit
polyclonal anti-HRAS (1:2,000; ProteinTech Group, Inc.; cat. no.
15531-1-AP), rabbit monoclonal anti-ERK1/2 (1:10,000; Abcam; cat.
no. ab109282), rabbit monoclonal anti-p-ERK1/2 (1:10,000; Abcam;
cat. no. ab223500), rabbit polyclonal anti-PI3K (1:1,000; Abcam;
cat. no. ab154598), rabbit polyclonal anti-p-PI3K (1:1,000; Abcam;
cat. no. ab182651) and anti-GAPDH (1:10,000; Abcam; cat. no.
ab245355). The following day, the membranes were incubated with
goat anti-rabbit IgG H&L (HRP) (1:20,000; Abcam; cat. no.
ab97047) at 37°C for 1.5 h. The EasyBlot ECL kit (Sangon Biotech
Co., Ltd.) was used for chemiluminescence detection. GAPDH was used
as a loading control.
RT-qPCR
Total RNA was extracted from the cells using
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and was subsequently reverse transcribed into cDNA using the
ReverTra Ace® qPCR RT Kit (Toyobo Life Science) at 42°C
for 30 min. qPCR was performed using the TB Green™ Fast qPCR Mix
(Takara Bio, Inc.). The following thermocycling conditions were
used: Initial denaturation at 95°C for 30 sec; followed by 40
cycles of denaturation at 95°C for 5 sec, and annellation and
extension, both at 61°C for a total of 15 sec. The experiments
aimed to quantify SIRT2 and HRAS mRNA expression levels. The
results were calculated using the 2−∆∆Cq method with
GAPDH as the internal reference (27). The primer sequences used for RT-qPCR
were as follows: SIRT2, forward 5′-ACGCTGTCGCAGAGTCAT-3′, reverse
5′-CGCTCCAGGGTATCTATGTT-3′; HRAS, forward
5′-TGCCATCAACAACACCAAGTCTT-3′, reverse 5′-CTGAGCCTGCCGAGATTCCA-3′;
and GAPDH, forward 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse
5′-GAAGATGGTGATGGGATTTC-3′.
Statistical analysis
All data are presented as the mean ± standard
deviation and all experimental studies were conducted in
triplicate. The comparison between two groups was determined by
unpaired Student's t-test and the comparison among multiple groups
was determined by one-way ANOVA followed by the Dunnett-t test. All
figure plotting and statistical analyses were performed using
GraphPad Prism 7.01 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
SIRT2 expression in MM cell lines and
control cells
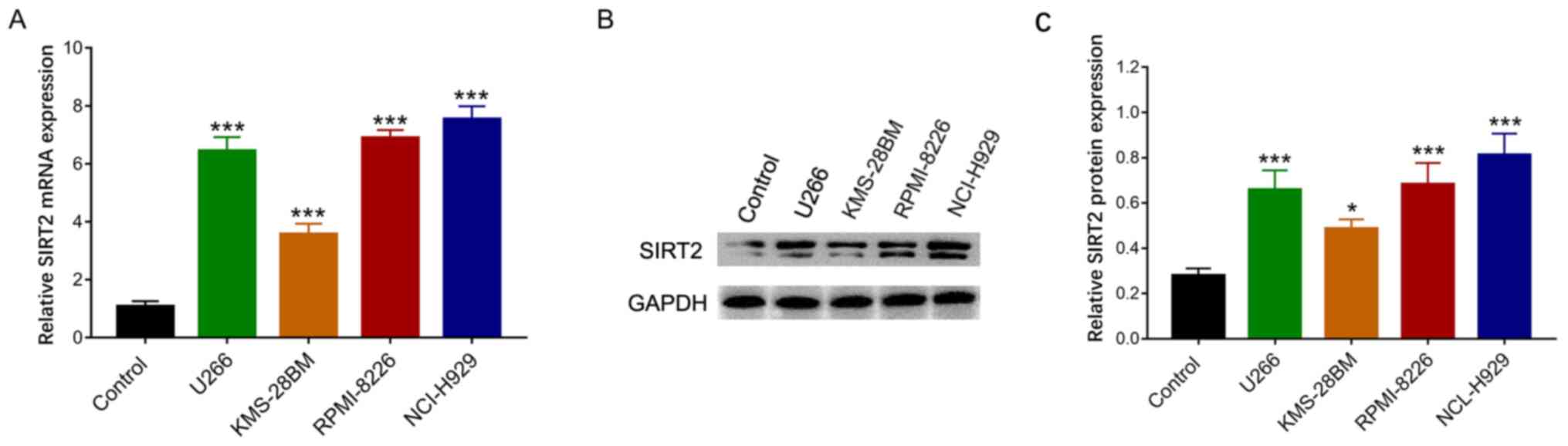
The relative mRNA expression levels of SIRT2 were
increased in U266 (P<0.001), KMS-28BM (P<0.001), RPMI-8226
(P<0.001) and NCI-H929 (P<0.001) cells compared with those in
the control cells (Fig. 1A). In
addition, western blot analysis indicated that the relative protein
expression levels of SIRT2 were increased in U266, KMS-28BM,
RPMI-8226 and NCI-H929 cell lines compared with those in the
control cells (Fig. 1B and C).
These data suggested that SIRT2 levels were increased in MM cell
lines.
SIRT2 expression in patients with MM
and healthy subjects
The relative mRNA expression levels of SIRT2 were
higher in samples from patients with MM compared with those in
samples from healthy donors (P<0.001; Fig. S1).
SIRT2 expression following
transfection of NCI-H929 and RPMI-8226 cells
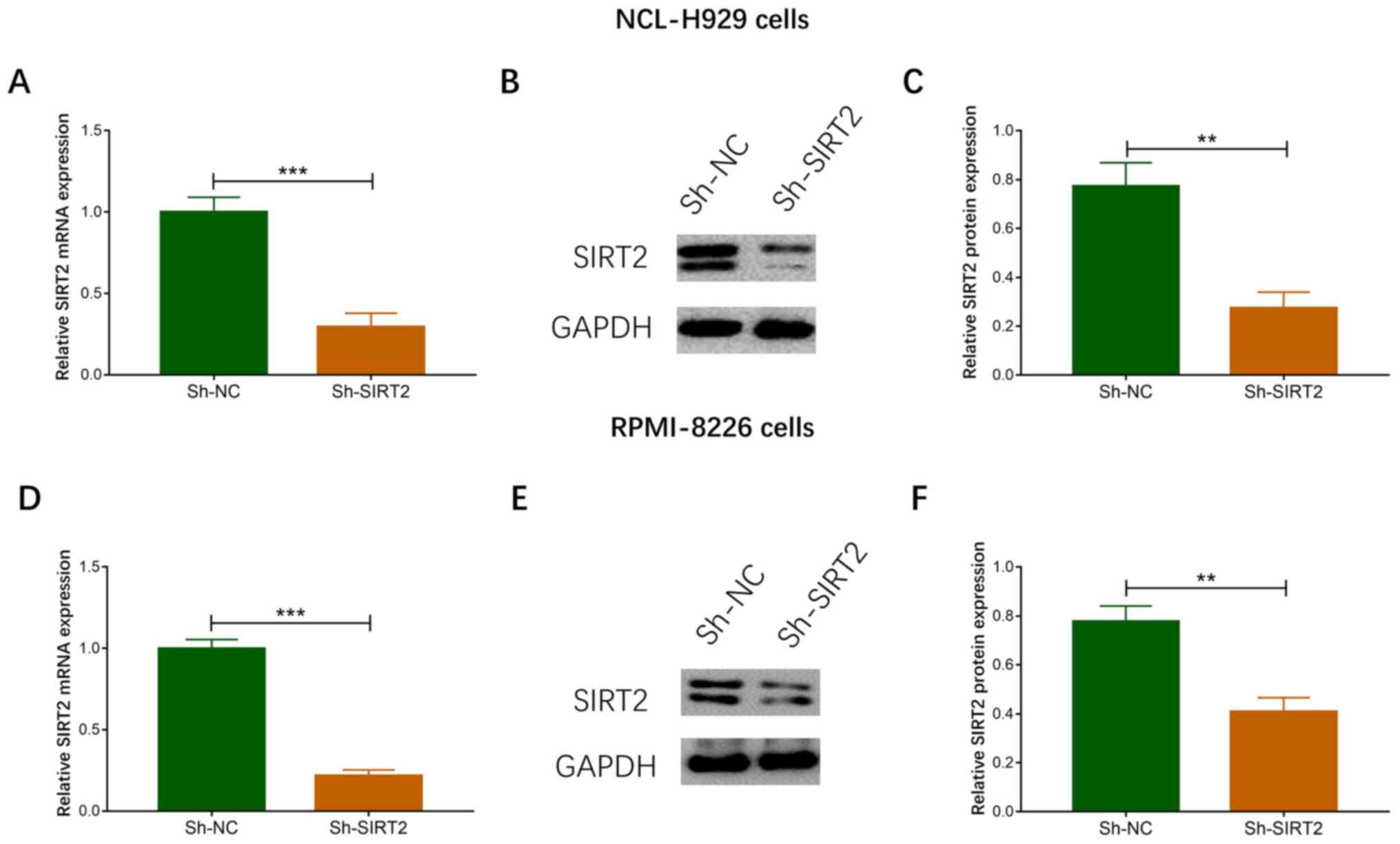
As the increased expression of SIRT2 was the most
significant in NCL-H929 and RPMI-8226 cells, these two cell lines
were chosen for subsequent experiments. Post-transfection of MM
cells with three shRNA-SIRT2 recombinant plasmids, the plasmid
(shSIRT2 type 3) with the best knockdown effect on SIRT2 expression
was selected for use in subsequent experiments (data not shown).
The expression levels of SIRT2 mRNA (P<0.001; Fig. 2A) and protein (Fig. 2B and C) were decreased in the
Sh-SIRT2 group compared with those in the Sh-NC group in NCI-H929
cells. In addition, the expression levels of SIRT2 mRNA
(P<0.001; Fig. 2C and D) and
protein (Fig. 2E and F) were
reduced in the Sh-SIRT2 group compared with those in the Sh-NC
group in RPMI-8226 cells. These data suggested that the
transfection was successful.
Effects of SIRT2 knockdown on the
proliferation of NCI-H929 and RPMI-8226 cells
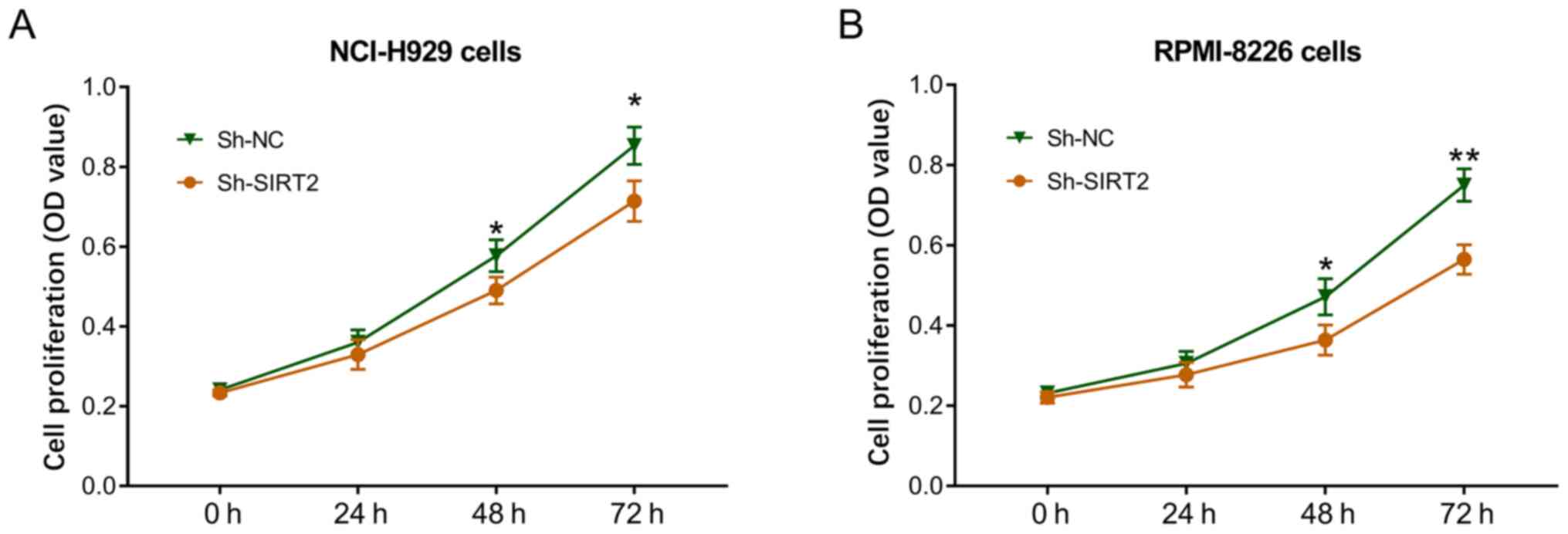
In NCI-H929 cells, cell proliferation was
significantly decreased in the Sh-SIRT2 group compared with that in
the Sh-NC group at 48 and 72 h post-transfection (P<0.05;
Fig. 3A). In RPMI-8226 cells, cell
proliferation was also reduced in the Sh-SIRT2 group compared with
that in the Sh-NC group at 48 (P<0.05) and 72 h (P<0.01)
post-transfection (Fig. 3B). These
data indicated that SIRT2 knockdown inhibited MM cell
proliferation.
Effects of SIRT2 knockdown on
apoptosis of NCI-H929 and RPMI-8226 cells
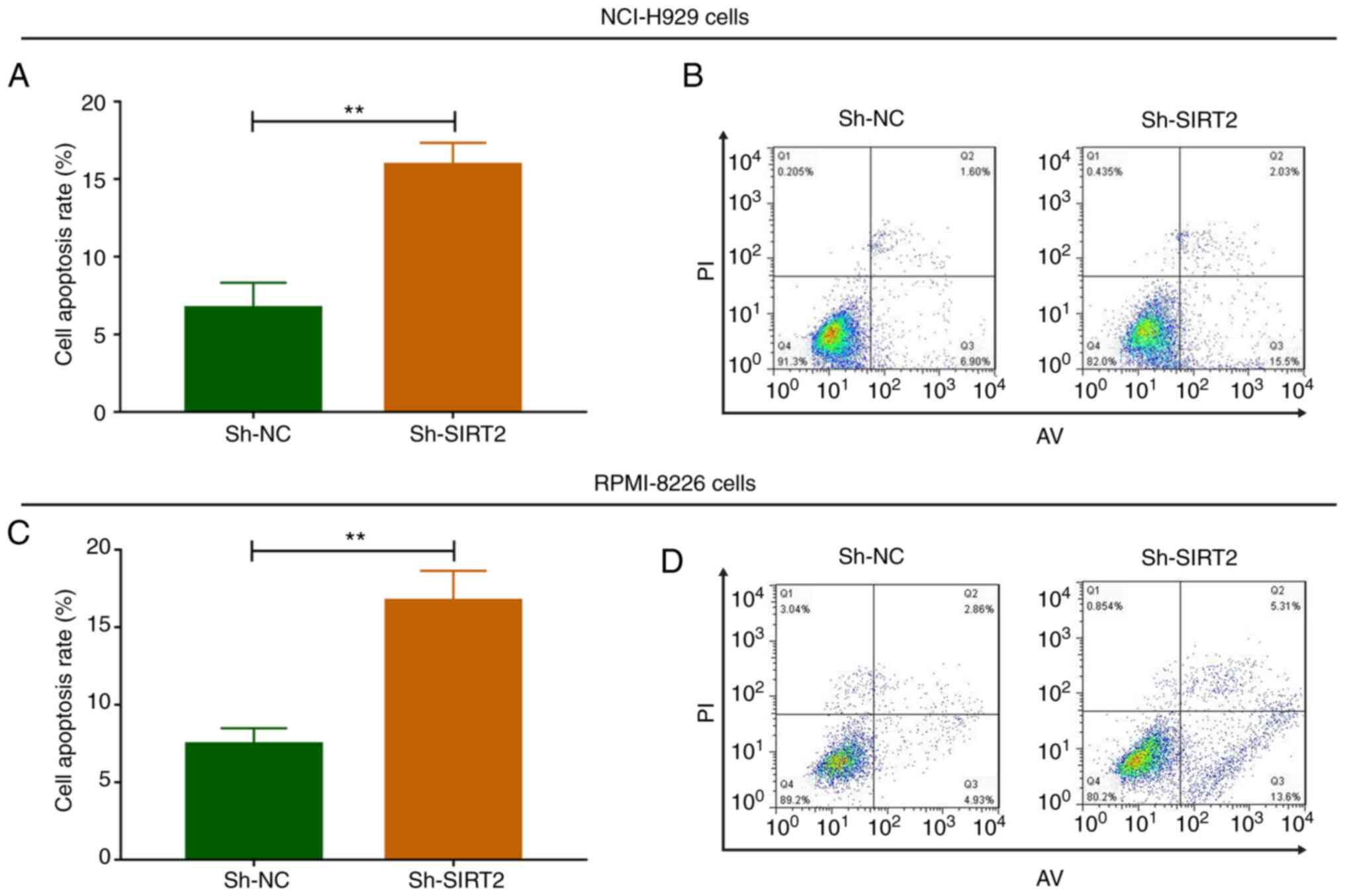
In NCI-H929 cells, the apoptotic rate was increased
in the Sh-SIRT2 group compared with that in the Sh-NC group at 48 h
post-transfection (P<0.01; Fig. 4A
and B). Similar results were noted in RPMI-8226 cells; the
apoptotic rate was significantly increased in the Sh-SIRT2 group
compared with that in the Sh-NC group at 48 h post-transfection
(P<0.01; Fig. 4C and D). These
data suggested that SIRT2 knockdown promoted MM cell apoptosis.
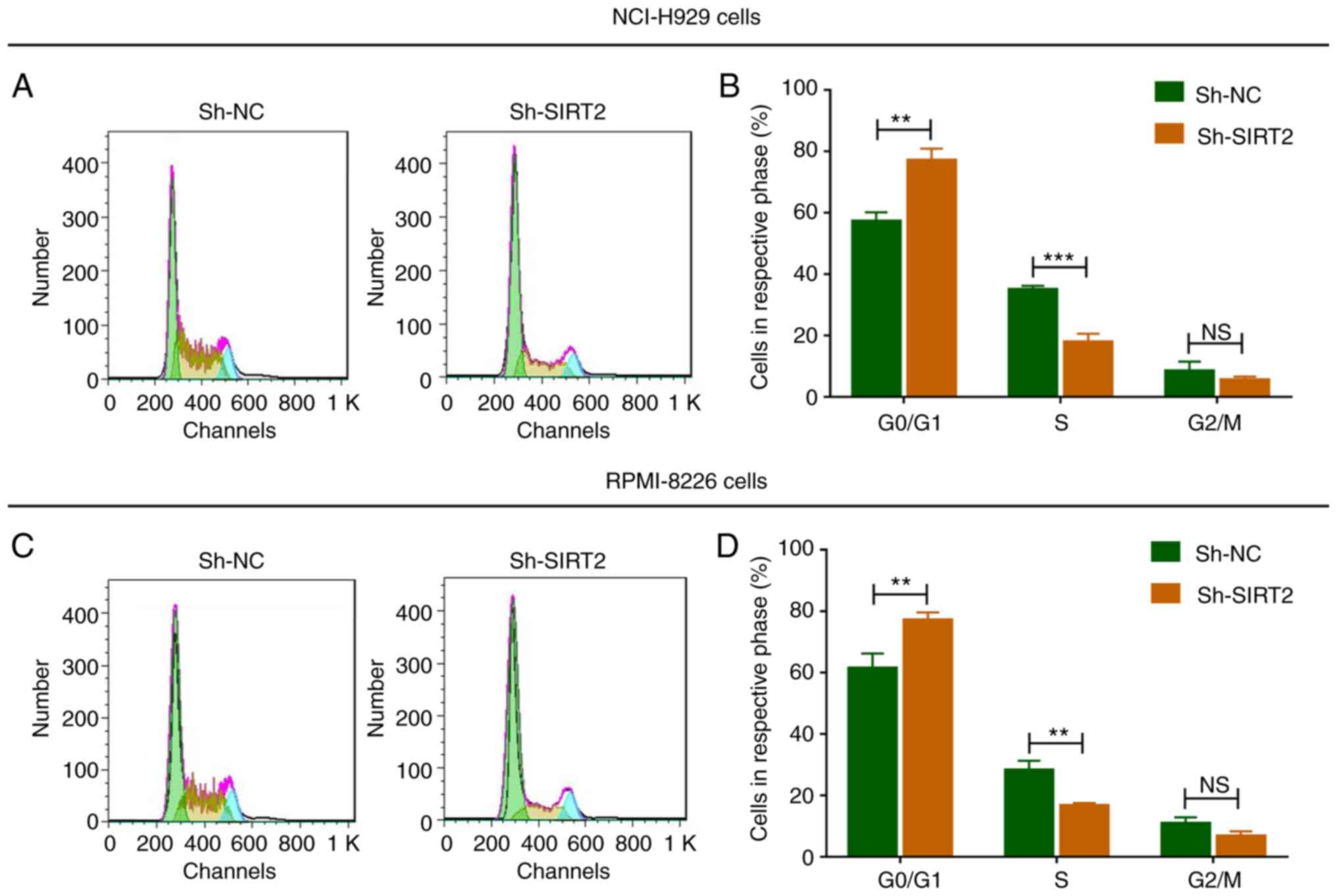
Effects of SIRT2 knockdown on cell
cycle progression in NCI-H929 and RPMI-8226 cells
In NCI-H929 cells, the percentage of the cells at
G0/G1 phase was significantly increased
(P<0.01), whereas the percentage of cells at S phase was
significantly decreased (P<0.001) in the Sh-SIRT2 group compared
with in the Sh-NC group (Fig. 5A and
B). In RPMI-8226 cells, cell cycle arrest at
G0/G1 arrest was also noted (P<0.01),
which was accompanied by a reduction in the percentage of cells at
S phase (P<0.01) in the Sh-SIRT group compared with in the Sh-NC
group (Fig. 5C and D). These
findings indicated that SIRT2 knockdown induced cell cycle arrest
in MM cells.
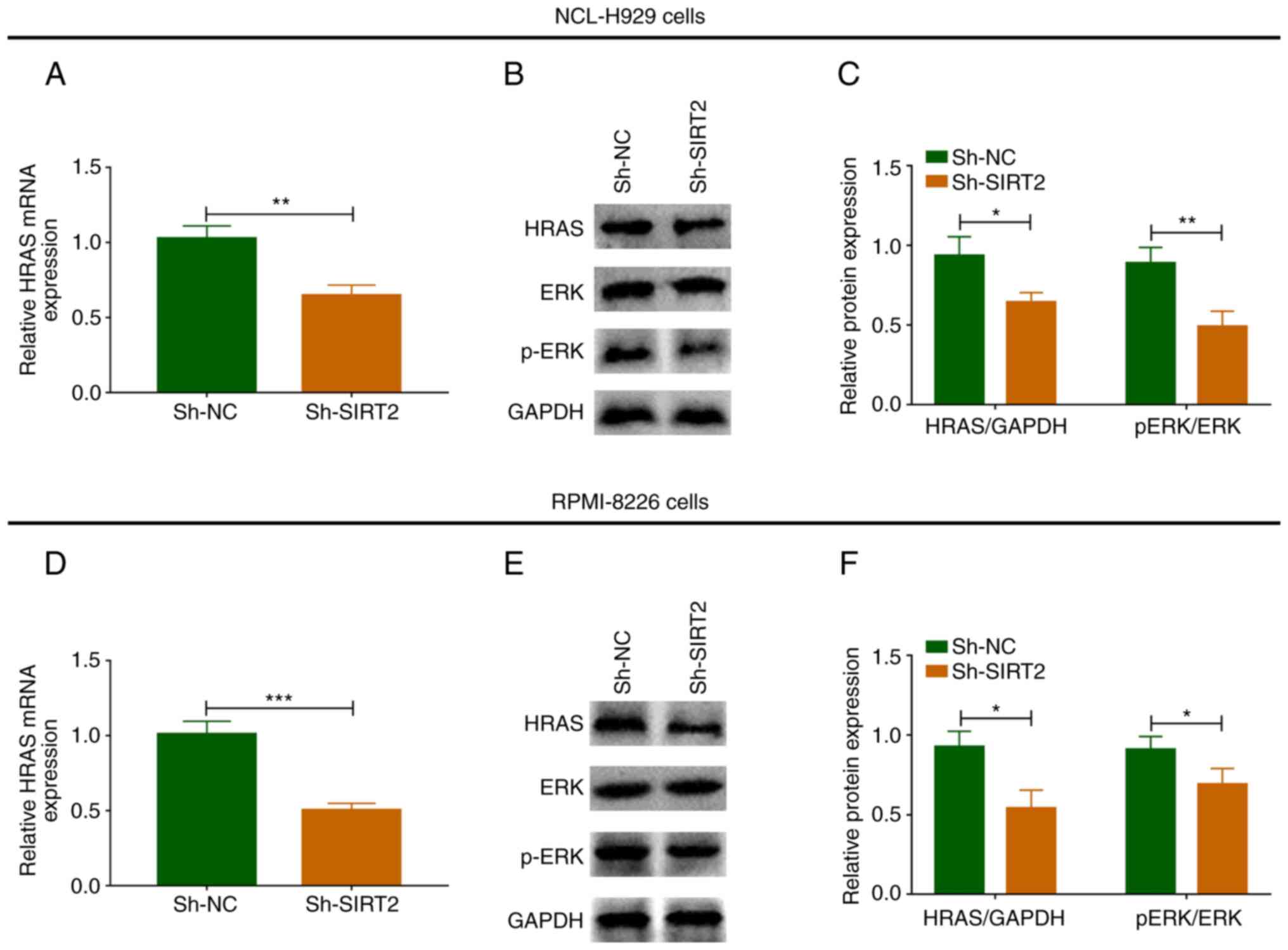
Effects of SIRT2 knockdown on the
RAS/ERK signaling pathway in NCI-H929 and RPMI-8226 cells
In NCI-H929 cells, the mRNA expression levels of
HRAS were significantly decreased in the Sh-SIRT2 group compared
with those in the Sh-NC group (P<0.01; Fig. 6A). The protein expression levels of
HRAS and p-ERK/ERK were also reduced in the Sh-SIRT2 group compared
with those in the Sh-NC group (Fig. 6B
and C). In RPMI-8226 cells, the mRNA expression levels of HRAS
were significantly decreased in the Sh-SIRT2 group compared with
those in the Sh-NC group (P<0.001; Fig. 6D). The protein expression levels of
HRAS and p-ERK were also reduced in the Sh-SIRT2 group compared
with those in the Sh-NC group (Fig. 6E
and F). In addition, in NCI-H929 (Fig. S2A and B) and RPMI-8226 (Fig. S2C and D) cells, the protein
expression levels of p-PI3K/PI3K were lower in the Sh-SIRT2 group
compared with those in the Sh-NC group. These findings suggested
that SIRT2 knockdown inactivated the RAS/ERK signaling pathway in
MM cells.
Discussion
In the present study, the data demonstrated that
SIRT2 was highly expressed in U266, KMS-28BM, RPMI-8226 and
NCI-H929 cell lines compared with those in normal plasma cells.
Although SIRT2 knockdown inhibited cell proliferation, it promoted
cell apoptosis and cell cycle arrest in MM cells. Furthermore,
SIRT2 knockdown inactivated the RAS/ERK signaling pathway in MM
cells.
SIRT2 is a NAD+-dependent deacetylase,
which serves as a regulator of α-tubulin acetylation, and is
localized in the cytoplasmic and nuclear regions of the cell
(11,12). SIRT2 has been reported to serve an
important role in regulating a variety of cellular physiological
and biological processes. Notably, the implication of SIRT2 in the
pathogenesis of malignancies, neurodegenerative diseases and
inflammation-related diseases, has attracted increasing attention
(8,13,28,29).
The involvement of SIRT2 in the pathological process of
hematological malignancies has been confirmed by a previous study
demonstrating that it was highly expressed in primary AML blasts
compared with in hematopoietic progenitor cells from healthy
donors. Moreover, its inhibition decreased cell proliferation and
promoted apoptosis in AML via acetylation of AKT and further
inactivation of β-catenin (18). In
addition, another functional experiment (16) revealed that overexpression of SIRT2
led to increased multidrug resistance protein 1 expression,
decreased drug accumulation and attenuated drug sensitivity. These
effects were caused by activation of the ERK1/2 signaling pathway
in AML cells and demonstrated the ability of SIRT1 to regulate the
RAS/ERK/JNK/MMP-9 pathway, further promoting the development and
progression of malignancies. Accumulating evidence has shown that
activation of the RAS/ERK signaling cascade may result in
FGFR3-mediated transformation, which is responsible for oncogenic
cellular transformation in MM (19). According to these data, the current
study examined whether SIRT2 was implicated in the pathological
process of MM, which, to the best of our knowledge, has not been
previously investigated. Initially, the expression levels of SIRT2
were compared between several human MM cell lines (KMS-28BM, U266,
RPMI-8226 and NCI-H929) and normal plasma cells; the data
demonstrated that SIRT2 expression levels were upregulated in MM
cells compared with those in normal plasma cells. Furthermore, the
data indicated that SIRT2 expression levels were higher in samples
from patients with MM compared with those in samples from healthy
donors.
The present study established the Sh-SIRT2 group by
transfecting RPMI-8226 and NCI-H929 cells with a Sh-SIRT2
recombinant plasmid. These transfected MM cell lines aimed to
explore the effects of SIRT2 knockdown on cell proliferation,
apoptosis and regulation of the cell cycle. The data demonstrated
that SIRT2 knockdown inhibited cell proliferation, whereas it
promoted cell apoptosis and cell cycle arrest in MM cells. The
possible reasons for these observations may include the following:
i) According to previous evidence, SIRT2 expression may be
positively associated with nicotinamide phosphoribosyltransferase
(NAMPT) expression, and NAMPT knockdown has been shown to promote
expression of the serine-threonine kinase glycogen synthase kinase
3β by inducing AKT phosphorylation. This in turn may inactivate
proto-oncogene β-catenin and suppress MM cell proliferation
(18,30). Therefore, SIRT2 knockdown may
inhibit cell proliferation and cell cycle, while promoting
apoptosis of MM cells. ii) Based on the previous studies reported,
SIRT2 knockdown may inactivate the RAS/ERK signaling cascade
(17), which could lead to
suppression of FGFR3-mediated phenotypes (16,17,19),
further contributing to decreased proliferation, cell cycle arrest
and increased apoptosis of MM cells.
SIRT2 has been reported to regulate the RAS/ERK
signaling pathway in certain types of cancer (16,17).
For example, SIRT2 expression has been revealed to be positively
associated with activation of the ERK1/2 signaling pathway in AML
(16). An additional study revealed
that the deacetylase activity of SIRT2 was inhibited by SirReal2
via the regulation of the RAS/ERK/JNK/MMP-9 signaling pathway
(16,17). Furthermore, interruption of RAS/ERK
signaling promoted the expression of the checkpoint kinase 1
inhibitor, resulting in an increase in apoptosis and cell cycle
arrest of hematopoietic malignant cells (31). Taken together, the aforementioned
studies indicated that SIRT2 knockdown may decrease the expression
levels of HRAS and p-ERK, which was also found in the current
study, suggesting that this process could inactivate RAS/ERK
signaling in MM. This may occur due to several possible reasons: i)
SIRT2 knockdown may decrease PI3K expression; notably, a previous
study indicated that PI3K interacts with RAS/ERK signaling
(32). Therefore, reduction of PI3K
expression may alleviate the stimulation of PI3K, leading to the
inhibition of RAS/ERK signaling and a further decrease in the
development of cancer (33). This
speculation was verified by cellular experiments in which SIRT2
knockdown was shown to decrease PI3K expression in MM. ii) In
addition, SIRT2 knockdown may decrease KRAS acetylation, which
could further reduce activation of RAS downstream signaling
markers, contributing to inhibition of RAS/ERK signaling (33). However, this hypothesis requires
additional studies in order to be fully verified in MM. Although
the present data demonstrated that SIRT2 knockdown inactivated
RAS/ERK signaling in MM, its ability to inhibit MM cell malignant
behavior via the RAS/ERK signaling pathway requires additional
experiments. However, due to the limited budget, the relevant
experiments were not performed, which is a limitation of the
current study.
In conclusion, SIRT2 was revealed to be highly
expressed in MM cell lines, and its knockdown inhibited cell
proliferation, inactivated RAS/ERK signaling, and promoted cell
apoptosis and cell cycle arrest in MM. Collectively, the present
study implied that targeting SIRT2 may be a novel therapeutic
option for the treatment of MM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Health Research
Project of Jing'an District of Shanghai (grant no. 2016MS10).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
JH conceived and designed the study. TD analyzed and
interpreted data, and drafted the manuscript. TD and JH performed
the experiments. Both authors participated in the writing and
revision of the manuscript. JH supervised the project. JH and TD
confirm the authenticity of all the raw data. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Institutional
Review Board of Shanghai Jing'an District Beizhan Hospital. All
participants provided written informed consent for their
participation in the study and for donation of bone marrow.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MM
|
multiple myeloma
|
|
SIRT2
|
sirtuin 2
|
|
FGFR3
|
fibroblast growth factor receptor
3
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
p-ERK
|
phosphorylated-ERK
|
|
NAMPT
|
nicotinamide
phosphoribosyltransferase
|
References
|
1
|
Brigle K and Rogers B: Pathobiology and
diagnosis of multiple myeloma. Semin Oncol Nurs. 33:225–236. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kazandjian D: Multiple myeloma
epidemiology and survival: A unique malignancy. Semin Oncol.
43:676–681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajkumar SV: Multiple myeloma: 2016 update
on diagnosis, risk-stratification, and management. Am J Hematol.
91:719–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishida H and Yamada T: Monoclonal
antibody therapies in multiple myeloma: A challenge to develop
novel targets. J Oncol. 2019:60840122019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isa R, Uoshima N, Takahashi R,
Nakano-Akamatsu S, Kawata E, Kaneko H, Shimura K, Kamitsuji Y,
Takimoto-Shimomura T, Mizutani S, et al: Sequential therapy of four
cycles of bortezomib, melphalan, and prednisolone followed by
continuous lenalidomide and dexamethasone for transplant-ineligible
newly diagnosed multiple myeloma. Ann Hematol. 99:137–145. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Swyter S, Schiedel M, Monaldi D, Szunyogh
S, Lehotzky A, Rumpf T, Ovádi J, Sippl W and Jung M: New chemical
tools for probing activity and inhibition of the NAD(+)-dependent
lysine deacylase sirtuin 2. Philos Trans R Soc Lond B Biol Sci.
373:201700832018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu G, Park SH, Imbesi M, Nathan WJ, Zou
X, Zhu Y, Jiang H, Parisiadou L and Gius D: Loss of NAD-dependent
protein deacetylase sirtuin-2 alters mitochondrial protein
acetylation and dysregulates mitophagy. Antioxid Redox Signal.
26:849–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel VP and Chu CT: Decreased SIRT2
activity leads to altered microtubule dynamics in
oxidatively-stressed neuronal cells: Implications for Parkinson's
disease. Exp Neurol. 257:170–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szego EM, Gerhardt E and Outeiro TF:
Sirtuin 2 enhances dopaminergic differentiation via the
AKT/GSK-3β/β-catenin pathway. Neurobiol Aging. 56:7–16. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sunami Y, Araki M, Hironaka Y, Morishita
S, Kobayashi M, Liew EL, Edahiro Y, Tsutsui M, Ohsaka A and Komatsu
N: Inhibition of the NAD-dependent protein deacetylase SIRT2
induces granulocytic differentiation in human leukemia cells. PLoS
One. 8:e576332013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong SG and Cho GW: The tubulin
deacetylase sirtuin-2 regulates neuronal differentiation through
the ERK/CREB signaling pathway. Biochem Biophys Res Commun.
482:182–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa T and Guarente L: Sirtuins at a
glance. J Cell Sci. 124:833–838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HS, Vassilopoulos A, Wang RH, Lahusen
T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, et al: SIRT2
maintains genome integrity and suppresses tumorigenesis through
regulating APC/C activity. Cancer Cell. 20:487–499. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luthi-Carter R, Taylor DM, Pallos J,
Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce
O, et al: SIRT2 inhibition achieves neuroprotection by decreasing
sterol biosynthesis. Proc Natl Acad Sci USA. 107:7927–7932. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Funato K, Hayashi T, Echizen K, Negishi L,
Shimizu N, Koyama-Nasu R, Nasu-Nishimura Y, Morishita Y, Tabar V,
Todo T, et al: SIRT2-mediated inactivation of p73 is required for
glioblastoma tumorigenicity. EMBO Rep. 19:e455872018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu H, Li Y, Chen L, Wang C, Wang Q, Zhang
H, Lin Y, Li Q and Pang T: SIRT2 mediates multidrug resistance in
acute myelogenous leukemia cells via ERK1/2 signaling pathway. Int
J Oncol. 48:613–623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Zhang M, Dorfman RG, Pan Y, Tang D,
Xu L, Zhao Z, Zhou Q, Zhou L, Wang Y, et al: SIRT2 promotes the
migration and invasion of gastric cancer through RAS/ERK/JNK/MMP-9
pathway by increasing PEPCK1-related metabolism. Neoplasia.
20:745–756. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dan L, Klimenkova O, Klimiankou M, Klusman
JH, van den Heuvel-Eibrink MM, Reinhardt D, Welte K and Skokowa J:
The role of sirtuin 2 activation by nicotinamide
phosphoribosyltransferase in the aberrant proliferation and
survival of myeloid leukemia cells. Haematologica. 97:551–559.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salazar L, Kashiwada T, Krejci P,
Muchowski P, Donoghue D, Wilcox WR and Thompson LM: A novel
interaction between fibroblast growth factor receptor 3 and the p85
subunit of phosphoinositide 3-kinase: Activation-dependent
regulation of ERK by p85 in multiple myeloma cells. Hum Mol Genet.
18:1951–1961. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu J and Hu WX: Targeting signaling
pathways in multiple myeloma: Pathogenesis and implication for
treatments. Cancer Lett. 414:214–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moreau P, San Miguel J, Ludwig H, Schouten
H, Mohty M, Dimopoulos M and Dreyling M; ESMO Guidelines Working
Group: Multiple myeloma, : ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 (Suppl
6):vi133–vi137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shaughnessy J Jr, Gabrea A, Qi Y, Brents
L, Zhan F, Tian E, Sawyer J, Barlogie B, Bergsagel PL and Kuehl M:
Cyclin D3 at 6p21 is dysregulated by recurrent chromosomal
translocations to immunoglobulin loci in multiple myeloma. Blood.
98:217–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Draube A, Pfister R, Vockerodt M, Schuster
S, Kube D, Diehl V and Tesch H: Immunomagnetic enrichment of CD138
positive cells from weakly infiltrated myeloma patients samples
enables the determination of the tumor clone specific IgH
rearrangement. Ann Hematol. 80:83–89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piracha ZZ, Kwon H, Saeed U, Kim J, Jung
J, Chwae YJ, Park S, Shin HJ and Kim K: Sirtuin 2 isoform 1
enhances hepatitis B virus RNA transcription and DNA synthesis
through the AKT/GSK-3β/β-catenin signaling pathway. J Virol.
92:e00955–18. 2018. View Article : Google Scholar
|
|
25
|
Dryden SC, Nahhas FA, Nowak JE, Goustin AS
and Tainsky MA: Role for human SIRT2 NAD-dependent deacetylase
activity in control of mitotic exit in the cell cycle. Mol Cell
Biol. 23:3173–3185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nie H, Li Y, Wang C, Chen X, Liu B, Wu D
and Ying W: SIRT2 plays a key role in both cell cycle regulation
and cell survival of BV2 microglia. Int J Physiol Pathophysiol
Pharmacol. 6:166–171. 2014.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Ding J, Gao ZG and Wang ZJ: A
variant in SIRT2 gene 3′-UTR is associated with susceptibility to
colorectal cancer. Oncotarget. 8:41021–41025. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma K, Lu N, Zou F and Meng FZ: Sirtuins as
novel targets in the pathogenesis of airway inflammation in
bronchial asthma. Eur J Pharmacol. 865:1726702019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y and Chi D: Overexpression of SIRT2
alleviates neuropathic pain and neuroinflammation through
deacetylation of transcription factor nuclear Factor-Kappa B.
Inflammation. 41:569–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai Y, Chen S, Pei XY, Almenara JA, Kramer
LB, Venditti CA, Dent P and Grant S: Interruption of the
Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA
damage in vitro and in vivo in human multiple myeloma cells. Blood.
112:2439–2449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song HY, Biancucci M, Kang HJ, O'Callaghan
C, Park SH, Principe DR, Jiang H, Yan Y, Satchell KF, Raparia K, et
al: SIRT2 deletion enhances KRAS-induced tumorigenesis in vivo by
regulating K147 acetylation status. Oncotarget. 7:80336–80349.
2016. View Article : Google Scholar : PubMed/NCBI
|