Introduction
Sepsis is a systemic inflammatory disease caused by
severe trauma, burn, infection and major surgery (1–3).
Sepsis is often accompanied by multiple organ failure (4). Sepsis-induced excessive inflammation,
immunosuppression or excessive tissue damage may increase
susceptibility to secondary infection (5). Therefore, the molecules and mechanisms
associated with sepsis-induced inflammatory response are important
to explore. Septic shock is associated with half of patients with
septic myocarditis (6);
inflammatory cytokines play an important role in this process.
Among them, tumor necrosis factor (TNF-α) inhibits myocardial
contractility, which results in cardiac dysfunction (7).
Long non-coding RNA (lncRNA) plays an important
regulatory role in the occurrence and development of inflammatory
response, rheumatoid arthritis, vascular aging and cancer (8,9).
LncRNA IL-1β7R is involved in the inflammatory response induced by
bacterial endotoxin lipopolysaccharide (LPS) (7). LncRNA HOTAIR promotes TNF-α production
in mice with LPS-induced sepsis (4). FGD5-AS1 has low expression level in
periodontitis, and FGD5-AS1 overexpression could inhibit the
development of periodontitis (10).
However, reports on the mechanism of action of FGD5-AS1 and its
role in sepsis are few.
MicroRNAs (miRNAs/miRs) are single-stranded
endogenous non-coding RNAs with a length of 18–25 nt (11). miRNAs are involved in gene
expression, cell development, differentiation and other processes.
miRNAs also play an important role in autoimmune diseases (12,13).
Feng et al (14) found that
miRNA plays an important role in the development of liver fibrosis.
miR-133a-3p belongs to the myocyte-specific miR-206 family and
inhibits the proliferation and differentiation of myocytes
(15). In addition, miR-133a has
been identified as a tumor suppressor gene in several tumors, such
as colorectal cancer, ovarian cancer, breast cancer and bladder
cancer (16–18). However, the role of miR-133a-3p in
sepsis has been rarely reported. Aquaporin 1 (AQP1) belongs to a
small-molecule transmembrane protein family, which is involved in
the rapid transmembrane transport of water (19). AQP1 is one of the earliest
identified members and expressed in erythrocyte membrane and
vascular endothelial cells (20).
AQP1 plays an important role in cell migration, differentiation,
proliferation and ion transport (21). AQP1 is a channel for the exchange of
intracellular and extracellular water and the transport and
exchange of oxygen in erythrocytes (22). The function of AQP1 is not only
limited to the membrane aquaporin, and its abnormal expression is
closely associated with the occurrence and development of a variety
of common diseases (23–25).
In the present study, LPS was used to establish
animal and cell models of sepsis, and the expression levels of
FGD5-AS1, miR-133a-3p and AQP1 in cells were detected; their effect
on sepsis and mechanism of action were also explored. The present
study lays a theoretical foundation for further revealing the
molecular mechanism of sepsis occurrence and development.
Materials and methods
Establishment of animal models of
sepsis
A total of 36 female BALB/C mice (weight, 25–30 g;
age, 4–6 weeks old) were purchased from Charles River Co., Ltd. Six
mice were included in each group. The mice were raised in a sterile
environment at a room temperature of 26–28°C, a humidity of 50–60%,
and illumination time of 10 h. The mice had free access to
autoclaved water and sterile food. The experiment was conducted
after 1 week of adaptive feeding. The control group was
intraperitoneally injected with 5 ml/kg sterile saline. The model
group was intraperitoneally injected with 15 mg/kg LPS (a bacterial
endotoxin, Escherichia coli LPS serotype 0111:B4;
Sigma-Aldrich; Merck KGaA). Len-NC and Len-FGD5-AS1
(1×109 PFU/ml), were injected into the tail vein after
24 h of modeling. The control group was given an equal volume of
sterilized saline. All indexes were detected 24 h after caudal vein
administration. A 0.3% pentobarbital sodium solution was given at a
dose of 50 mg/kg to anesthetize the mice. Then, 0.2 ml of blood was
collected from the orbital vein, and the upper serum was collected
after centrifugation (4,000 × g, 10 min, 4°C). The mice were
euthanized with carbon dioxide immediately after blood extraction
while still under anesthesia. The euthanasia chamber was filled
with CO2 at a rate of 20% of the volume of the
euthanasia chamber per minute after the mice were placed in the
chamber. The mice were not moving or breathing, and their pupils
were dilated when the administration of CO2 was stopped.
The mice were watched for another 2 min to confirm their death. The
animal experiments were approved by the Ethics Committee of Tianjin
Academy of Traditional Chinese Medicine Affiliated Hospital
(Tianjin, China; approval no. 20190135). The animal experiments
were conducted between May 25 and June 14, 2019.
Lentiviral vector packaging
RNAi lentiviral recombinant vector system, including
Len-FGD5-AS1 vector with green fluorescent protein (GFP), pHelper
1.0 vector and pHelper 2.0 vector and negative control lentivirus
(Len-NC). All plasmid vectors were purchased from Shanghai GeneChem
Co., Ltd. 293T cells in logarithmic growth phase were inoculated
into the culture dish at a cell number of 6×106/ml, and
placed in an incubator for 24 h (CO2, 37°C). When the
cell confluence reached 70–80%, each DNA solution (plasmid vector,
helper plasmid vector pHelper 1.0, pHelper 2.0) and
Lipofectamine® 2000 liposome were added for
co-transfection. After 48 h, the supernatant of 293T cells was
collected and centrifuged at 4,000 × g at 4°C for 10 min. The
supernatant was then filtered using a 0.45-µm diameter filter and
packed. The 96-well plate was inoculated with 4×104
cells per well. The virus was added into 8 experimental wells at a
total volume of 100 µl by multiple dilution. Then, 24 h later,
puromycin was added for screening, and the final concentration was
2.7 µg/ml. When the cell density reached 30%,
lentivirus-transfected cells were transfected at a MOI of 30. A
total of 16 h after lentivirus transfection, the solution was
changed, and the downstream experiment was carried out after 96
h.
Cardiac function detection in
mice
Cardiac function was evaluated using transthoracic
Doppler ultrasound. Mice were prematurely fasted and weighed using
a GE Vivid E9 ultrasound system (GE Healthcare). Mice were
anesthetized to ideal depth and maintained with isoflurane (3%
induction and 1–2% maintenance). The mice were fixed in supine
position on a heating mat to maintain their body temperature, and
the skin was prepared on the chest area. Measurement data: B-mode
ultrasound was selected, and the left ventricle short axis image
and the left ventricle long axis image were obtained at the left
ventricle middle level using a high-frequency probe. Ejection
fraction (EF%) and left ventricular fraction shorting rate (FS%)
were measured under M-mode ultrasound. The data were measured three
times, averaged and recorded.
Cell culture
Mice HL-1 cardiac muscle cells were purchased from
the American Type Culture Collection. The cells were cultured in
Dulbeccos modified Eagles medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (Gibco; Thermo Fisher
scientific, Inc.) with 100 U/ml penicillin and 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc.). The cells were
precultured at 37°C with 5% CO2 for 24 h. LPS
intervention was performed in some cells. These cells were plated
in a 6-well plate (2×106) and cultured for 48 h, and
then 1 g/ml LPS was added to the medium. Normal saline was added to
the control group. The cells were cultured for 12 h under the same
conditions. The harvested cells were used in subsequent
experiments.
Cell transfection
The FGD5-AS1-pcDNA3.1-overexpression plasmid
[vector-FGD5-AS1; OBiO Technology (Shanghai) Corp., Ltd.] was
constructed in strict accordance with the manufacturers
instructions. A total of 4 µg vector-FGD5-AS1 and its negative
control (vector-NC) were transfected into LPS-induced cells using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.)
under 37°C. The cells were collected after 48 h of transfection,
and transfection efficiency was detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
After 48 h of transfection, follow-up experiments were performed.
Small interfering RNAs (siRNAs) targeting FGD5-AS1 (100 nM,
5′-CAUUUGUAAUAGUGUUCAAUA-3′) and si-NC (100 nM,
5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by Shanghai
GenePharma Co., Ltd., and transfected using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.).
Vector-FGD5-AS1 (1 µg) and miR-133a-3p (100 nM) or sh-AQP1 (1 µg;
Shanghai GenePharma Co., Ltd.) were co-transfected using
Lipofectamine 3000. After 48 h of transfection, follow-up
experiments were performed. Cells were treated with 100 nM mimic
control (5′-CAGCUGGUUGAAGGGGACCAAA-3′) and 100 nM miR-133a-3p mimic
(5′-UUUGGUCCCCUUCAACCAGCUG-3′). miRNAs were purchased from Shanghai
GenePharma Co., Ltd.
Enzyme-linked immunosorbent assay
(ELISA)
Blood was drawn from the orbital venous plexus and
centrifuged at 4°C for 10 min (4,000 × g). The serum was separated
and stored in the refrigerator at 80°C for later use. The contents
of TNF-α (cat. no. BMS607-3; Thermo Fisher Scientific, Inc.),
interleukin (IL)-6 (cat. no. BMS603-2; Thermo Fisher Scientific,
Inc.), and IL-1β (cat. no. BMS6002; Thermo Fisher Scientific, Inc.)
in serum were detected by ELISA. ELISA was performed strictly in
accordance with the manufacturers instructions of the kits.
RT-qPCR
RNA from tissue or cell samples was isolated using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). RNA
was reverse transcribed into cDNA using the PrimeScript One Step
RT-PCR kit (Takara Biotechnology Co., Ltd.), according to the
manufacturers protocol. The reaction conditions for RT were as
follows: 16°C for 30 min, 42°C for 30 min, 85°C for 5 min. The
following primers were used in the present study: miR-133a-3p
forward, 5′-ACACTCCAGCTGGGTTGGTCCCCTTCAACC-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACAGCTGG-3′; AQP1 forward,
5′-ACCTCCTGGCTATTGACTAC-3′ and reverse, 5′-CCAGGATGAAGTCGTAGATG-3′;
Bcl-2 forward, 5′-ATGCCTTTGTGGAACTATATGGC-3′ and reverse,
5′-GGTATGCACCCAGAGTGATGC-3′; and Bax forward,
5′-TGAAGACAGGGGCCTTTTTG-3′ and reverse, 5′-AATTCGCCGGAGACACTCG-3′.
U6 RNA and GAPDH were used as the internal references. The primer
sequences were as follows: U6 forward, 5′-CTCGCTTCGGCAgcacA-3′ and
reverse, 5′-aACGCttcacgaatttGCGT-3′; and GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-TTgatttTGGATCTCG-3′. The
fluorescence quantitative detection conditions were as follows:
Pre-denaturation at 95°C for 30 sec, amplification at 95°C with
extension for 15 sec, and 40 cycles of annealing at 60°C for 30
sec. The 2−ΔΔCq (26)
was calculated as follows: ΔCq (experimental group)=Cq
(experimental group target genes)-Cq (experimental group internal
genes); ΔCq (control group)=Cq (control target gene)-Cq (control
group).
Western blotting
HL-1 cells were collected after different treatments
and lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology), 100 µM PMSF (X100; Sigma-Aldrich; Merck KGaA) and a
protease inhibitor cocktail (Thermo Fisher Scientific, Inc.). The
lysed solution was centrifuged (12,000 × g) at 4°C for 60 min and
then at 12,000 × g for 20 min. PBS washing and precipitation were
performed twice. Protein concentration was determined with the
Bradford method. Then, 10 µg protein was added into 10% sodium
dodecyl sulfate-polyacrylamide gel and subjected to
electrophoresis. Subsequently, the gel was transferred to a
polyvinylidene difluoride membrane, the membrane was then blocked
with 5% skimmed milk powder at room temperature for 2 h. Following
which, the membranes were incubated at 4°C overnight with primary
antibodies against the following: Bax (1:1,000; cat. no. 89477;
Cell Signaling Technology, Inc.), Bcl-2 (1:1,000; cat. no. 15071;
Cell Signaling Technology, Inc.), GAPDH (1:2,000; cat. no. 97166;
Cell Signaling Technology, Inc.) and AQP1 (1:1,000; cat. no.
ab9566; Abcam). Then, horseradish peroxidase-conjugated secondary
antibodies (1:10,000; cat. nos. 31430 and 31460; Thermo Fisher
Scientific, Inc.) were added to the membrane and incubated at room
temperature for 2 h. Chemiluminescence (ECL kit; Cytiva) was used
to detect the target bands. After the strips were scanned, the
optical density of the strips was determined using QuantityOne
version 4.3.0 software (Bio-Rad Laboratories, Inc.). The relative
expression level of each sample was calculated using GADPH as the
internal reference.
Cell counting Kit-8 (CCK-8)
experiments
Cell viability was detected by the CCK-8 method. The
cells were made into a single cell suspension and inoculated at a
density of 1×105/well to a 6-well plate. The cells were
randomly grouped when cell confluence reached 60–70%. The control
group was added with the corresponding volume of solvent. The LPS
group was treated with 10 µg/ml LPS for 12 h.
Lipofectamine® 2000 was used to transfect the target
gene plasmid in the transfection group. The cells were incubated at
37°C with 5% CO2 for 48 h. CCK-8 experiment was
performed after 48 h of cell treatment. CCK-8 reagent (Beyotime
Institute of Biotechnology) was added to each well, and culture was
continued for 4 h. The optical density at 490 nm was measured with
a microplate reader.
Dual-luciferase reporter gene
assay
StarBase version 2.0 (http://starbase.sysu.edu.cn/) online prediction
software was used to predict the lncRNA-targeted miRNAs. TargetScan
version 7.1 (http://www.targetscan.org/) online prediction software
was used to predict the miRNA target genes. The interaction of the
FGD5-AS1, miR-133a-3p and AQP1 cascade reaction was detected using
a Dual-Luciferase Reporter Assay System (Promega Corporation).
Wild-type (WT) and mutant (MT) 3UTR were designed and amplified
using Primer Premier 5.0 primer design software (Premier Biosoft
International). XhoI and NotI were introduced into
the 5 end of the WT forward primer and reverse primer,
respectively. The WT and MT recombinant plasmids were constructed
by ligating the vector psiCHECK™-2 with XhoI and
NotI. Human 293T cells (American Type Culture Collection)
were transfected with 100 nmol/µl miR-133a-3p mimics
(5′-UUUGGUCCCCUUCAACCAGCUG-3′) and its negative control (miR-NC;
5′-UUGUACUACACAAAAGUACUG-3′), 20 ng WT FGD5-AS1 (FGD5-AS1-WT) and
MT FGD5-AS1 (FGD5-AS1-MT), or 20 ng 3-untranslated region (UTR) of
WT AQP1 (AQP1-WT) and MT AQP1 (AQP1-MT) with
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Luciferase activity was detected 48 h after
transfection (Promega Corporation). According to the requirements
of the kit instructions, the ratio of Firefly/Renilla
luciferase activity was calculated. The unit of the control group
ratio was one. The relative luciferase activity of different
treatment groups was obtained.
miRNA pull-down experiment
miR-133a-3p mimic (biotinylated miR-133a-3p or
miR-NC probe) with biotinylated modification was synthesized at the
3 ends. A random sequence was used as a control. Transfection with
miR-133a-3p probe (1 µg; Sangon Biotech Co., Ltd.) was performed,
and cells were harvested after 48 h. The cells were washed with PBS
and added with lytic extract (20 mM Tris, pH 7.5; 100 mM KCl; 5 mM
MgCl2; 0.5% NP-40; and 1 U/µl recombinant RNAse
inhibitor). Cell fragments were removed by centrifugation after
lysis (4°C, 12,000 × g, 10 min). DNase l was added to the lysate to
digest the DNA. Afterward, the lysates were heated to 65°C in a
metal bath for 5 min and then quickly plunged into ice to cool. The
lysates and 25 µl avidin-coated magnetic beads (New England
BioLabs, Inc.) were mixed and incubated at 4°C for 4 h with gentle
shaking. After incubation, the beads were washed twice with the
lysis buffer. TRIzol was used to extract the RNA bound to the
magnetic beads for RT-qPCR analysis. Cell lysate was used as a
control (input group).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Comparison between two groups was performed using an
unpaired Students t-test. Differences between two groups were
calculated using one-way analysis of variance (ANOVA) followed by
Tukeys multiple comparison test. All data were statistically
analyzed using SPSS 19.0 (SPSS, Inc.). Results were obtained from
three independent experiments. P<0.05 was used to indicate a
statistically significant difference.
Results
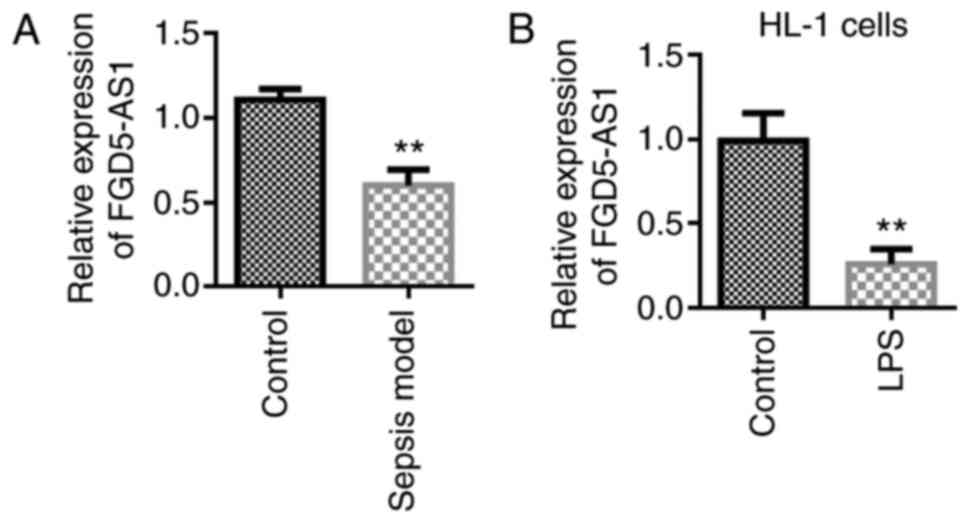
FGD5-AS1 is downregulated in
sepsis
A septic animal model was established to investigate
the role of FGD-AS1 in sepsis. Experimental results showed that
FGD5-AS1 was downregulated in the septic animal model (Fig. 1A). Furthermore, a septic
cardiomyocyte model was established using LPS. It was found that
FGD5-AS1 was downregulated in the septic cell model (mouse HL-1
cells, Fig. 1B).
Lentivirus overexpression of FGD5-AS1
can inhibit sepsis
It was investigated whether FGD5-AS1 overexpression
has a protective effect on sepsis. FGD5-AS1 was overexpressed in an
animal model of sepsis by the lentivirus technique. The
experimental results showed that the lentivirus overexpression
system could upregulate the expression of FGD5-AS1 (Fig. 2A). Inflammatory factor detection
results showed that the concentrations of TNF-α, IL-1β and IL-6 in
the sepsis model group were significantly higher compared with
those in the control group (P<0.01). The concentrations of
TNF-α, IL-1β and IL-6 in the sepsis model + Len-FGD5-AS1 group was
significantly decreased compared with the sepsis model + Len-NC
group (P<0.01; Fig. 2B-D).
Furthermore, it was found that FGD5-AS1 overexpression protected
heart functions in septic mice. The ejection fraction, fractional
shortening and the maximum [LVdP/dt (max)] and minimum rates of the
rise in left ventricular pressure [LVdP/dt (min)] were
significantly decreased in the sepsis model group compared with the
control group (P<0.01; Fig.
2E-H). Ejection fraction, fractional shortening, LVdP/dt (max),
and LVdP/dt (min) were significantly higher in the sepsis model +
Len-FGD5-AS1 group compared with the sepsis model + Len-NC group
(P<0.01; Fig. 2E-H).
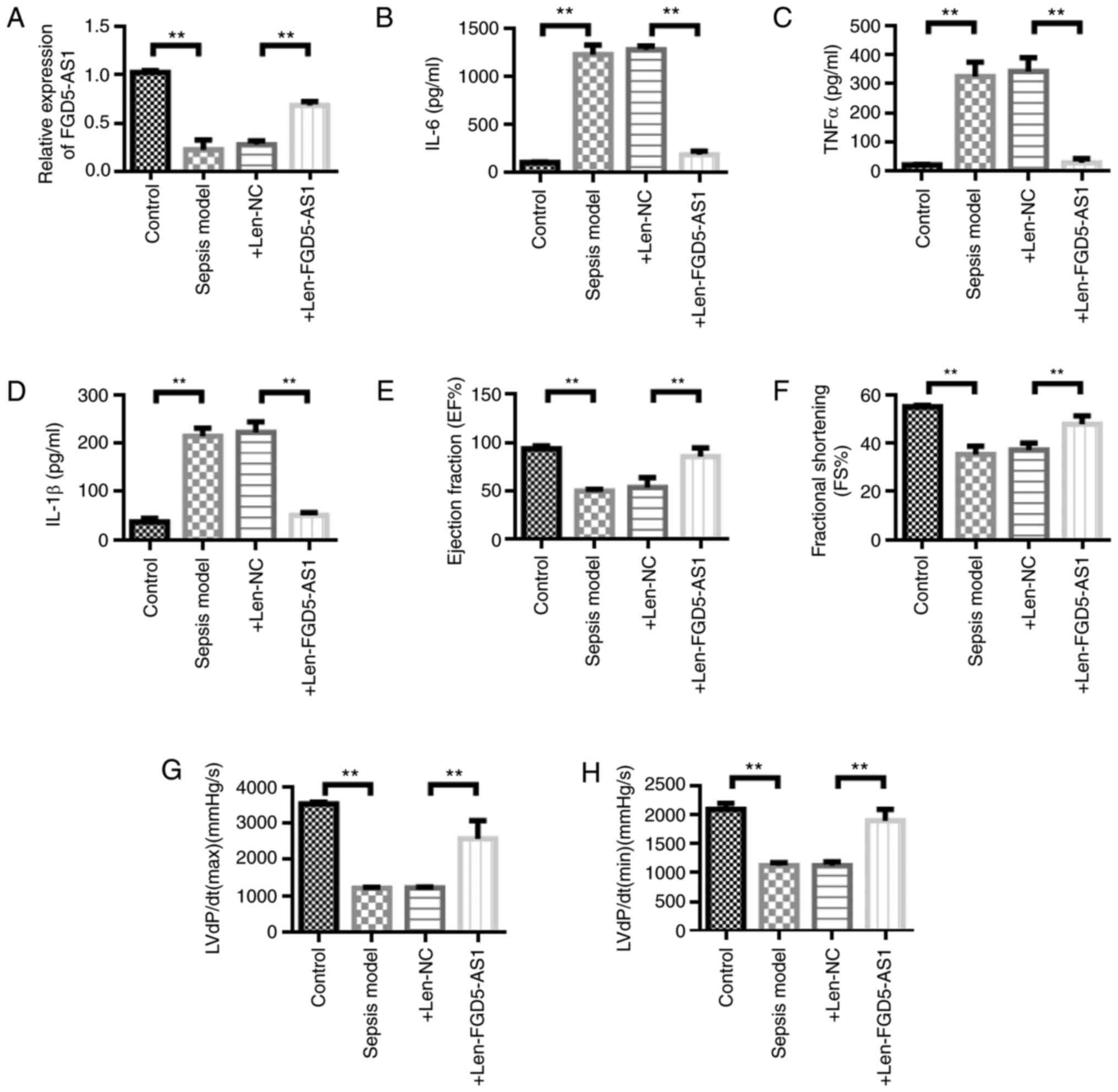 | Figure 2.Lentiviral overexpression of FGD5-AS1
can inhibit sepsis. (A) Lentiviral overexpression of FGD5-AS1 could
inhibit FGD5-AS1 expression in sepsis model mice. Detection of (B)
IL-6, (C) TNF-α and (D) IL-1β activity in serum. (E) Detection of
changes in mouse heart index (EF%). (F) Detection of changes in FS%
of mouse heart index. (G) Detection of changes in mouse heart index
LVdP/dt(max)(mmHg/sec). (H) Detection of changes in mouse heart
indicators LVdP/dt(min)(mmHg/sec). n=6. Data are represented as the
means ± SD. **P<0.01. Len-, lentivirus; IL, interleukin; TNF,
tumor necrosis factor; EF, ejection fraction; FS, fractional
shortening; NC, negative control; max, maximum; min, minimum; LVdP,
left ventricular pressure. |
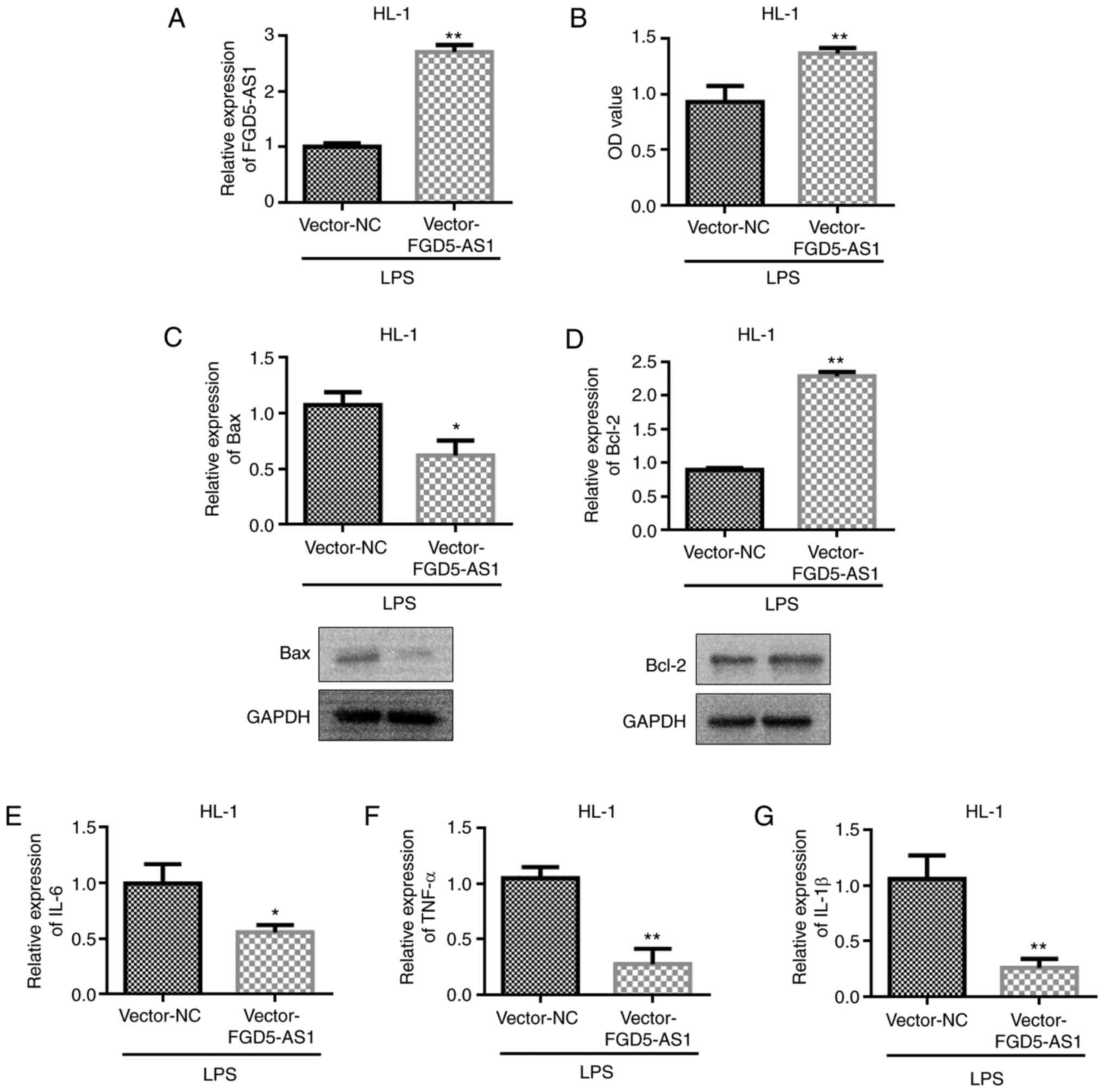
FGD5-AS1 overexpression decreases
LPS-induced HL-1 cell injury in vitro
A cell model was established by LPS, and the
transfection efficiency of vector-FGD5-AS1 was first detected.
Experimental results showed that vector-FGD5-AS1 could upregulate
the expression of FGD5-AS1 (Fig.
3A). Subsequently, the effect of vector-FGD5-AS1 on cell
viability was examined. The experimental results showed that
transfection with vector-FGD5-AS1 could upregulate the viability of
HL-1 cells compared with the vector-NC group (Fig. 3B). In addition, vector-FGD5-AS1
could inhibit Bax expression and upregulate Bcl-2 expression
(Fig. 3C and D). The influence of
FGD5-AS1 on the protein expression levels of Bax and Bcl-2 was
further analyzed. The western blot analysis results were consistent
with the RT-qPCR results; that is, vector-FGD5-AS1 transfection
could inhibit Bax expression and upregulate Bcl-2 expression
(Fig. 3C and D). Previous studies
have shown that LPS treatment increases the levels of
pro-inflammatory cytokines TNF-α, IL-6 and IL-1β (27–29).
Transfection with vector-FGD5-AS1 in the cell models reverted
LPS-induced changes in TNF-α, IL-6 and IL-1β levels (Fig. 3E-G).
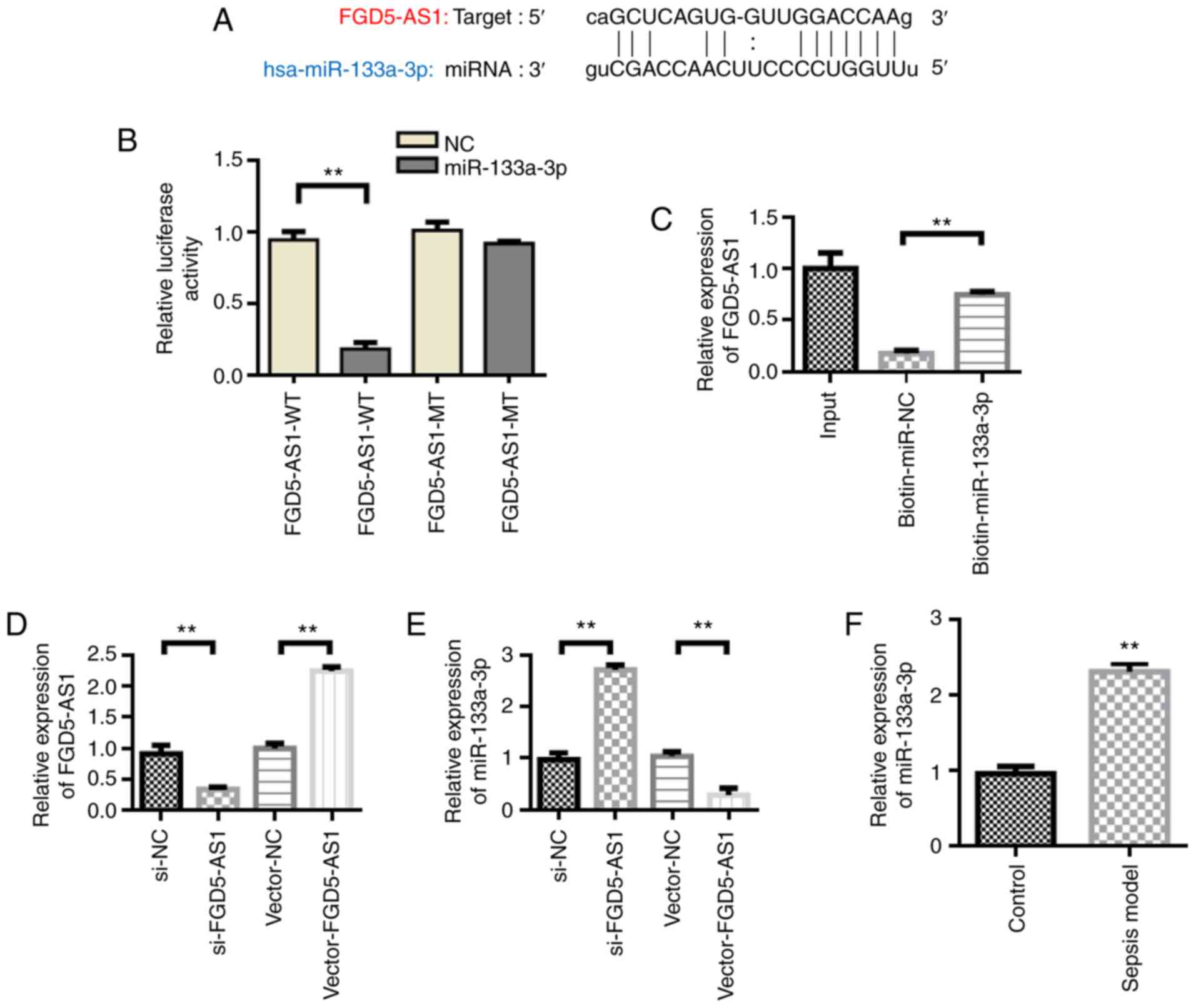
Regulation and interaction of FGD5-AS1
on miR-133a-3p
lncRNA-targeted miRNA prediction analysis using
StarBase showed that FGD5-AS1 has binding sites with miR-133a-3p
(Fig. 4A). Dual-luciferase reporter
gene validation results showed that miR-133a-3p significantly
inhibited the luciferase activity of FGD5-AS1-WT (P<0.01).
However, miR-133A-3p had no effect on the luciferase activity of
FGD5-AS1-MT (Fig. 4B). miRNA
pull-down also verified the interaction between FGD5-AS1 and
miR-133a-3p (Fig. 4C).
Subsequently, the effect of FGD5-AS1 on the expression of
miR-133a-3p was detected. Following transfection with si-FGD5-AS1,
FGD5-AS1 expression was found to be downregulated compared with the
si-NC group, whereas transfection with vector-FGD5-AS1 increased
the expression levels of FGD5-AS1 compared with the vector-NC group
(Fig. 4D). Furthermore, the
experimental results showed that transfection with vector-FGD5-AS1
inhibited miR-133a-3p expression, whereas si-FGD5-AS1 transfection
led to the upregulation of miR-133a-3p (Fig. 4E). miR-133a-3p was also upregulated
in septic animal models compared with the control group (Fig. 4F).
miR-133a-3p overexpression reverses
the protective effect of FGD5-AS1 on HL-1 cells
After confirming the regulatory effect of FGD5-AS1
on miR-133a-3p, the effect of miR-133a-3p on the function of
FGD5-AS1 was detected. The viability of HL-1 cells was measured by
CCK-8 assay. The results showed that the viability of HL-1 cells
was decreased in the LPS-stimulated group compared with the control
group. Transfection with vector-FGD5-AS1 could upregulate the
viability of HL-1 cells compared with the LPS group. However, the
viability of HL-1 cells was decreased after the co-transfection of
vector-FGD5-AS1 and miR-133a-3p mimics (Fig. 5A). The Bax expression detection
results showed that, compared with the LPS group, transfection with
vector-FGD5-AS1 could inhibit Bax expression. However, Bax
expression was upregulated after the co-transfection of
vector-FGD5-AS1 and miR-133a-3p mimics (Fig. 5B). The trend in Bcl-2 expression
changes was opposite to that of Bax. FGD5-AS1-vector transfection
upregulated the expression of Bcl-2 (Fig. 5C). LPS stimulation upregulated the
expression of inflammatory cytokines IL-6, TNF and IL-1β compared
with the control group. Transfection with vector-FGD5-AS1 decreased
the levels of inflammatory factors compared with the LPS group.
However, the inflammatory factors were upregulated after the
co-transfection of vector-FGD5-AS1 and miR-133a-3p mimics (Fig. 5D-F).
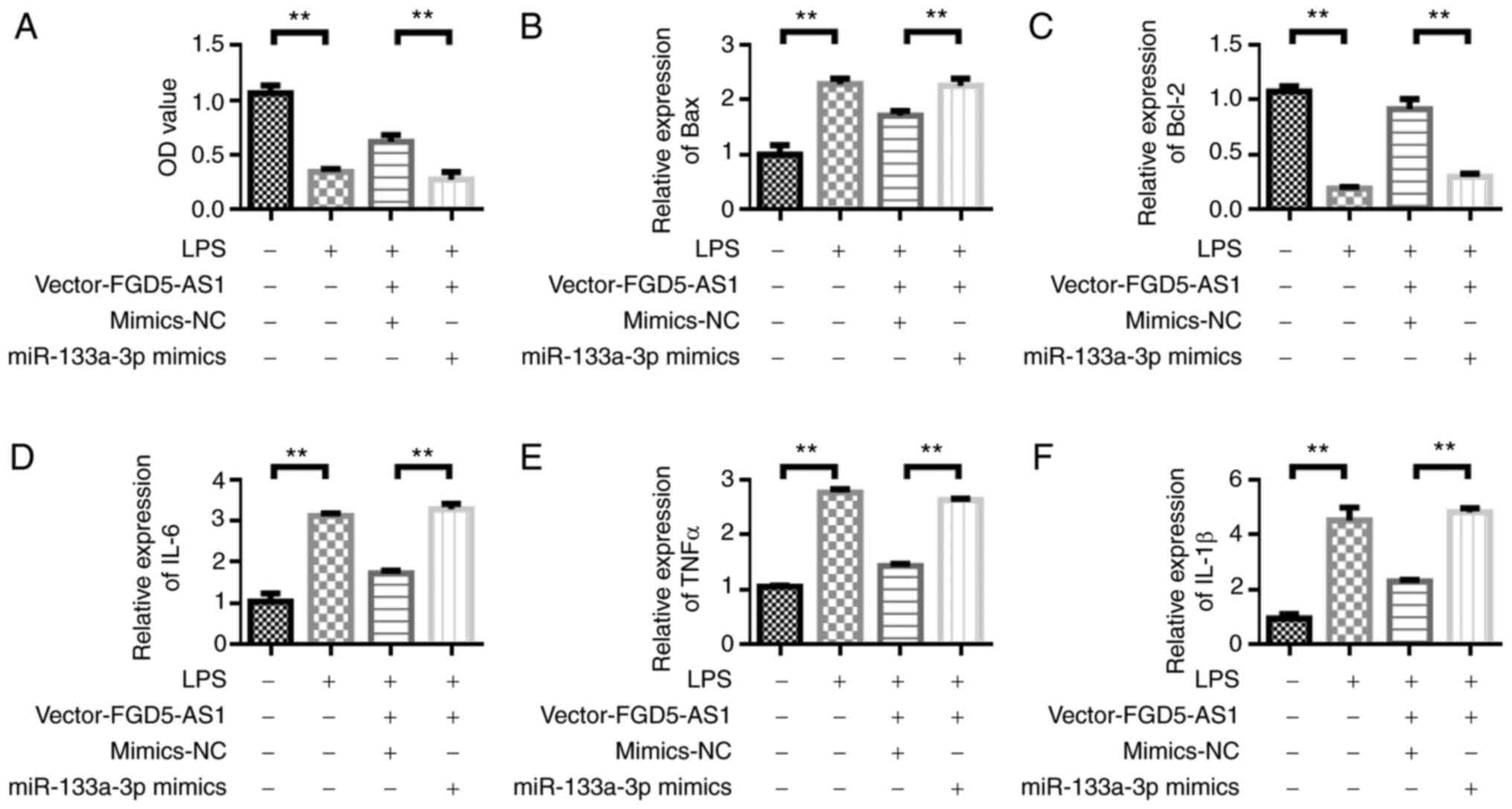 | Figure 5.Overexpression of miR-133a-3p
reverses the protective effect of FGD5-AS1 on HL-1 cells. (A) Cell
Counting Kit-8 detection of cell viability. Reverse
transcription-quantitative PCR was performed to detect the
expression of (B) Bax, (C) Bcl-2, (D) IL-6, (E) TNF-α and (F)
IL-1β. Results were obtained from three independent experiments,
each performed in triplicate, the error bars represent SD.
**P<0.01. miR, microRNA; IL, interleukin; TNF, tumor necrosis
factor; NC, negative control; LPS, lipopolysaccharide. |
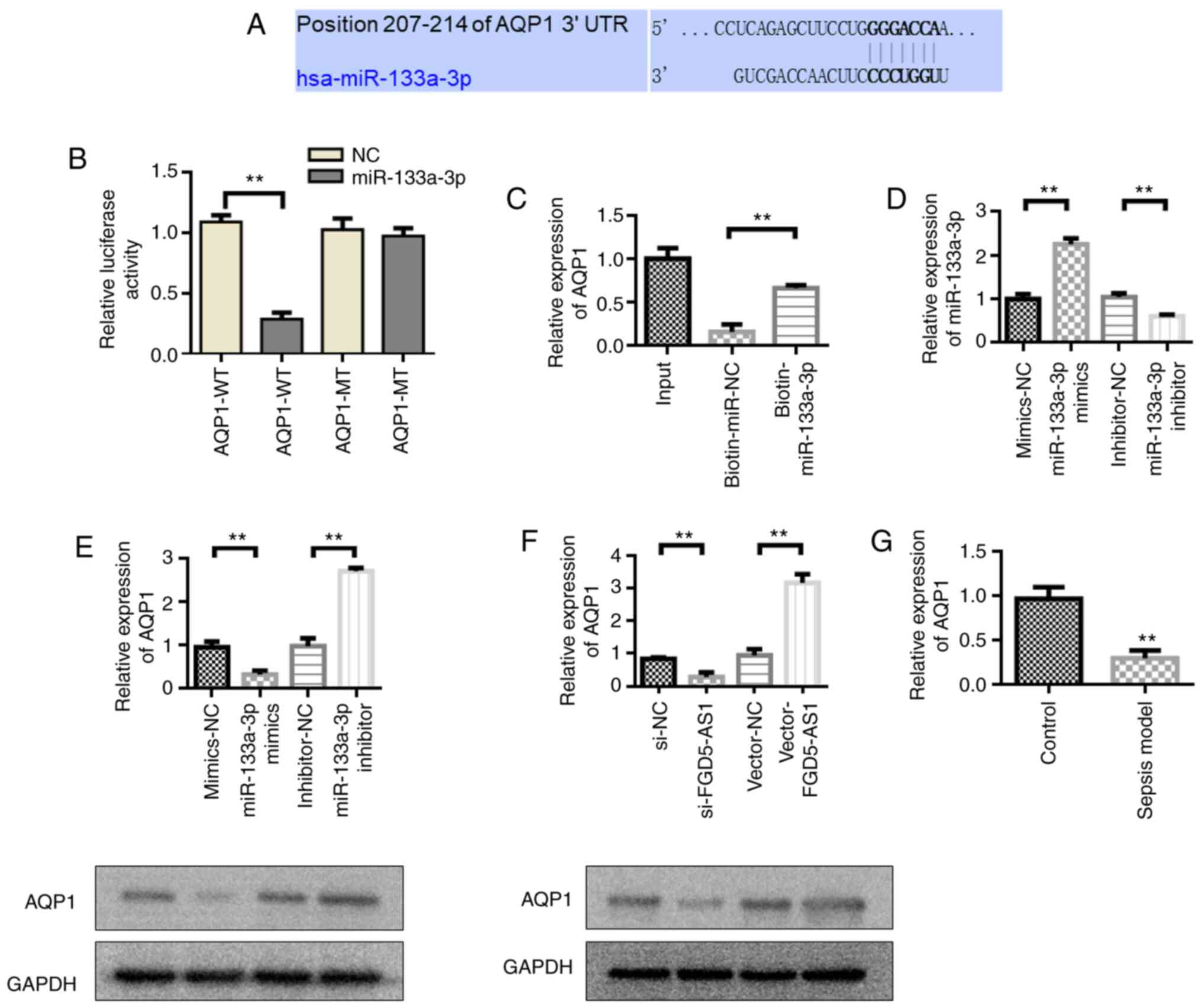
AQP1 acts as the target gene mediating
miR-133a-3p expression
The complementary binding sites of miR-133a-3p and
AQP1-3′-UTR-WT were predicted by TargetScan (Fig. 6A). Dual-luciferase reporter gene
detection results showed that the relative luciferase activity of
AQP1-WT + miR-133a-3p mimics group was significantly decreased
compared with the AQP1-WT + miR-NC group (Fig. 6B). These results indicated that
miR-133a-3p could inhibit luciferase activity by binding to
AQP13-UTR. However, the relative luciferase activity of the AQP1-MT
+ miR-133a-3p mimics group was not significantly different from
that of the AQP1-MT + miR-NC group. These results indicated that
miR-133a-3p and miR-NC could not inhibit the luciferase activity of
the mutant plasmid. miRNA pull-down also verified the binding of
miR-133a-3p to AQP1 (Fig. 6C).
Subsequently, the effect of miR-133a-3p on the expression level of
AQP1 was detected. Transfection with the miR-133a-3p mimics
upregulated the expression of miR-133a-3p compared with the
mimics-NC group, whereas transfection with the miR-133a-3p
inhibitor led to the downregulation of miR-133a-3p expression
compared with the inhibitor-NC (Fig.
6D). The experimental results showed that transfection with the
miR-133a-3p mimics inhibited AQP1 expression, whereas AQP1
expression was upregulated in the miR-133a-3p inhibitor group
(Fig. 6E). Transfection with
vector-FGD5-AS1 upregulated AQP1 expression compared with the
vector-NC group, whereas transfection with si-FGD5-AS1 led to the
downregulation of AQP1 expression compared with the si-NC group
(Fig. 6F). The western blotting
results were consistent with the RT-qPCR results (Fig. 6F). AQP1 was downregulated in septic
animal models (Fig. 6G).
AQP1 knockdown reverses the protective
effect of FGD5-AS1 on HL-1 cells
RT-qPCR was used to detect the change in AQP1
expression (Fig. S1).
FGD5-AS1-vector transfection could upregulate AQP1 expression, but
the expression of AQP1 was decreased after the addition of sh-AQP1
(Fig. 7A). The viability of HL-1
cells was measured by CCK-8 assay. The results showed that the
viability of HL-1 cells was decreased in the LPS-stimulated group
compared with the control group. Transfection with vector-FGD5-AS1
could upregulate the viability of HL-1 cells compared with the LPS
group. However, the viability of HL-1 cells decreased after the
co-transfection of vector-FGD5-AS1 + sh-AQP1 (Fig. 7B). The Bax expression detection
results showed that, compared with the LPS group, vector-FGD5-AS1
transfection could inhibit Bax expression. However, Bax expression
was upregulated after the co-transfection of vector-FGD5-AS1 +
sh-AQP1 (Fig. 7C). The change in
Bcl-2 expression was opposite to that of Bax. Vector-FGD5-AS1
transfection upregulated Bcl-2 expression (Fig. 7D). LPS stimulation upregulated the
expression of inflammatory cytokines IL-6, TNF and IL-1β compared
with the control group. Transfection with vector-FGD5-AS1 decreased
the levels of inflammatory factors compared with the LPS group.
However, the inflammatory factors were upregulated after the
co-transfection of vector-FGD5-AS1 + sh-AQP1 (Fig. 7E-G). Fig. 7H illustrates the proposed underlying
mechanism for the signaling pathway that involves
FGD-AS1-miR-133a-3p-AQP1 in sepsis.
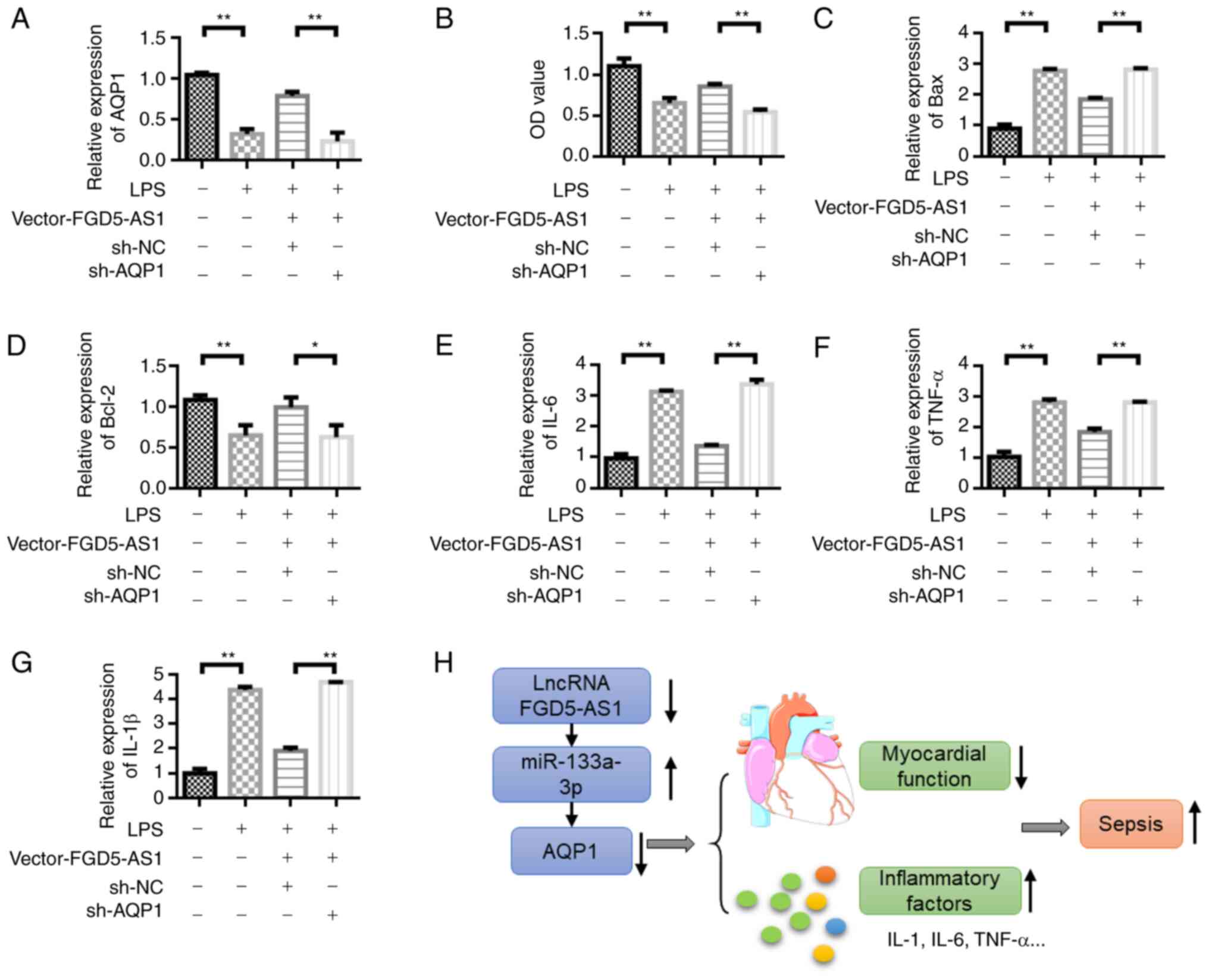 | Figure 7.Knockdown of AQP1 reverses the
protective effect of FGD5-AS1 on HL-1 cells. (A) Detection of AQP1
expression level in HL-1 cells under different treatment
conditions. (B) Cell Counting Kit-8 cell viability detection.
Reverse transcription-quantitative PCR was performed to detect the
expression of (C) Bax, (D) Bcl-2, (E) IL-6, (F) TNF-α and (G)
IL-1β. (H) Schematic diagram of the proposed mechanisms of the
signaling pathway involving FGD-AS1-miR-133a-3p-AQP1 in sepsis.
Results were obtained from three independent experiments, each
performed in triplicate, the error bars represent SD. *P<0.05,
**P<0.01. AQP1, aquaporin 1; IL, interleukin; TNF, tumor
necrosis factor; miR, microRNA; NC, negative control; sh-, short
hairpin RNA; LPS, lipopolysaccharide; lncRNA, long non-coding
RNA. |
Discussion
Sepsis is a systemic inflammatory response caused by
infection that leads to multiple organ failure, in which the heart
is one of the most vulnerable organs (30,31).
Current treatments, such as antimicrobial therapy and supportive
therapies, are still at the early stages (32,33).
The study of non-coding RNA provides a novel idea for the treatment
of myocardial inflammation caused by sepsis (4,34).
The role of non-coding RNA in physiological and
pathological processes, including cell proliferation, apoptosis,
inflammatory response and immunity, has received extensive
attention (35). Studies have shown
that lncRNAs play a crucial role in a number of inflammatory
diseases, such as sepsis (36,37).
For example, lncRNA growth arrest specific transcript 5 is involved
in regulating sepsis-induced podocyte injury by inhibiting the
expression of phosphatase and tension protein homologous gene
(38). In addition, miRNAs are
valuable markers for the diagnosis and prognosis of sepsis. Lan
et al (39) showed that the
expression of serum miR-155-5p and miR-133a-3p could be used as a
specific indicator for the diagnosis of sepsis. Further results
showed that the expression of miR-155-5p could be an independent
influencing factor for the prognosis of sepsis (39).
In the present study, in vitro cell models
were used to investigate the expression and mechanism of FGD5-AS1
in sepsis inflammatory response. Experiments confirmed that plasma
FGD5-AS1 expression decreased, miR-133a-3p expression increased,
AQP1 expression was downregulated, and the release of serum
proinflammatory cytokines was increased in the sepsis model.
Inflammatory cytokines are involved in sepsis-induced myocardial
damage. Studies have shown that TNF-α and IL-1β initiate an
inflammatory response that has a negative inotropic effect on the
myocardium (40,41). The negative regulation of IL-6 on
myocardial systolic performance is associated through the protein
kinase pathway (42,43). In the present study, FGD5-AS1
overexpression inhibited the expression of inflammatory cytokines
TNF-α, IL-1β and IL-6 in the sepsis models. Thus, these results
indicated that FGD5-AS1 is involved in the regulation of the
expression of inflammatory cytokines.
In the present study, miR-133a-3p was upregulated in
the blood samples of LPS mice. These results suggested that
miR-133a-3p plays a regulatory role in sepsis-induced inflammatory
response. Further results confirmed that miR-133a-3p and its
predicted target gene AQP1 were remarkably upregulated in plasma
and downregulated in the LPS-induced cells, respectively. AQP1
plays an important regulatory role in the inflammatory response
caused by neonatal toxic erythema, rheumatoid arthritis and
pulmonary edema (44–46). Thus, AQP1 can regulate inflammatory
response. In the present study, FGD5-AS1 overexpression decreased
LPS-induced upregulation of TNF-α, IL-6 and IL-1β by upregulating
AQP1. This result suggested that FGD5-AS1 and miR-133a-3p are
antagonistic regulators of AQP1 expression and inflammatory
response. FGD5-AS1 was indicated to have a protective effect on
sepsis-induced inflammatory response.
In summary, the present study focused on the
mechanisms and signaling pathways that regulate inflammatory
responses in sepsis and investigated whether FGD5-AS1 is a
potential therapeutic target for sepsis and complications. In the
present study, FGD5-AS1 and AQP1 expression was decreased in animal
models of sepsis, and LPS-induced cells; the expression of
miR-133a-3p was increased. Pro-inflammatory cytokines TNF-α, IL-6
and IL-1β were remarkably elevated in LPS-induced cells. FGD5-AS1
overexpression could reverse LPS-induced changes in the levels of
miR-133a-3p, AQP1 and pro-inflammatory factors. Therefore, these
results indicated that FGD5-AS1 is the competing endogenous RNA of
miR-133a-3p on AQP1.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors contributions
JK designed the experiments. YC and XW performed the
experiments and data analysis. YC, NY and YL performed data
analysis and wrote the manuscript, with contributions from all
authors. YC and JK confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Committee of Tianjin Academy of Traditional Chinese Medicine
Affiliated Hospital (Tianjin, China; grant no. 20190135).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Russell JA: Management of sepsis. N Engl J
Med. 355:1699–1713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bone RC: The pathogenesis of sepsis. Ann
Intern Med. 115:457–469. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
IDSA Sepsis Task Force, . Infectious
diseases society of America (IDSA) position statement: Why IDSA did
not endorse the surviving sepsis campaign guidelines. Clin Infect
Dis. 66:1631–1635. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu H, Liu J, Li W, Liu G and Li Z:
LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of
LPS-induced sepsis mice by activating NF-κB pathway. Biochem
Biophys Res Commun. 471:240–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cawcutt KA and Peters SG: Severe sepsis
and septic shock: Clinical overview and update on management. Mayo
Clinic Proceedings. Elsevier; pp. 1572–1578. 2014, View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo W, Liu W, Chen G, Hong S, Qian C, Xie
N, Yang X, Sun Y and Xu Q: Water-soluble andrographolide sulfonate
exerts anti-sepsis action in mice through down-regulating p38 MAPK,
STAT3 and NF-κB pathways. Int Immunopharmacol. 14:613–619. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y
and Fang G: Long noncoding RNA CCAT1, which could be activated by
c-Myc, promotes the progression of gastric carcinoma. J Cancer Res
Clin Oncol. 139:437–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearson MJ and Jones SW: Review: Long
noncoding RNAs in the regulation of inflammatory pathways in
rheumatoid arthritis and osteoarthritis. Arthritis Rheumatol.
68:2575–2583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Lan Z, Li Q and Li Y: Abnormal
expression of long noncoding RNA FGD5-AS1 affects the development
of periodontitis through regulating miR-142-3p/SOCS6/NF-κB pathway.
Artif Cells Nanomed Biotechnol. 47:2098–2106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xi X, Chu Y, Liu N, Wang Q, Yin Z, Lu Y
and Chen Y: Joint bioinformatics analysis of underlying potential
functions of hsa-let-7b-5p and core genes in human glioma. J Transl
Med. 17:1292019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JQ, Papp G, Szodoray P and Zeher M:
The role of microRNAs in the pathogenesis of autoimmune diseases.
Autoimmun Rev. 15:1171–1180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng L, Cui J, Wu H and Lu Q: The emerging
role of circulating microRNAs as biomarkers in autoimmune diseases.
Autoimmunity. 47:419–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng Y, Niu LL, Wei W, Zhang WY, Li XY,
Cao JH and Zhao SH: A feedback circuit between miR-133 and the
ERK1/2 pathway involving an exquisite mechanism for regulating
myoblast proliferation and differentiation. Cell Death Dis.
4:e9342013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Navickas R, Gal D, Laucevičius A,
Taparauskaitė A, Zdanytė M and Holvoet P: Identifying circulating
microRNAs as biomarkers of cardiovascular disease: A systematic
review. Cardiovasc Res. 111:322–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong Y, Zhao J, Wu CW, Zhang L, Liu X,
Kang W, Leung WW, Zhang N, Chan FK, Sung JJ, et al: Tumor
suppressor functions of miR-133a in colorectal cancer. Mol Cancer
Res. 11:1051–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui W, Zhang S, Shan C, Zhou L and Zhou Z:
MicroRNA-133a regulates the cell cycle and proliferation of breast
cancer cells by targeting epidermal growth factor receptor through
the EGFR/Akt signaling pathway. FEBS J. 280:3962–3974. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamasaki T, Yoshino H, Enokida H, Hidaka
H, Chiyomaru T, Nohata N, Kinoshita T, Fuse M, Seki N and Nakagawa
M: Novel molecular targets regulated by tumor suppressors
microRNA-1 and microRNA-133a in bladder cancer. Int J Oncol.
40:1821–1830. 2012.PubMed/NCBI
|
|
19
|
Tsunoda SP, Wiesner B, Lorenz D, Rosenthal
W and Pohl P: Aquaporin-1, nothing but a water channel. J Biol
Chem. 279:11364–11367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shanahan CM, Connolly DL, Tyson KL, Cary
NR, Osbourn JK, Agre P and Weissberg PL: Aquaporin-1 is expressed
by vascular smooth muscle cells and mediates rapid water transport
across vascular cell membranes. J Vasc Res. 36:353–362. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verkman AS: More than just water channels:
Unexpected cellular roles of aquaporins. J Cell Sci. 118:3225–3232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu K, Tsujimoto H, Cha SJ, Agre P and
Rasgon JL: Aquaporin water channel AgAQP1 in the malaria vector
mosquito anopheles gambiae during blood feeding and humidity
adaptation. Proc Natl Acad Sci USA. 108:6062–6066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamazato Y, Shiozaki A, Ichikawa D, Kosuga
T, Shoda K, Arita T, Konishi H, Komatsu S, Kubota T, Fujiwara H, et
al: Aquaporin 1 suppresses apoptosis and affects prognosis in
esophageal squamous cell carcinoma. Oncotarget. 9:29957–29974.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomita Y, Dorward H, Yool AJ, Smith E,
Townsend AR, Price TJ and Hardingham JE: Role of aquaporin 1
signalling in cancer development and progression. Int J Mol Sci.
18:2992017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin F, Zhang H, Shao Y, Liu X, Yang L,
Huang Y, Fu L, Gu F and Ma Y: Expression of aquaporin1, a water
channel protein, in cytoplasm is negatively correlated with
prognosis of breast cancer patients. Oncotarget. 7:8143–8154. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogino H, Fujii M, Ono M, Maezawa K, Kizu J
and Hori S: In vivo and in vitro effects of fluoroquinolones on
lipopolysaccharide-induced pro-inflammatory cytokine production. J
Infect Chemother. 15:168–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skelly DT, Hennessy E, Dansereau MA and
Cunningham C: A systematic analysis of the peripheral and CNS
effects of systemic LPS, IL-1β, [corrected] TNF-α and IL-6
challenges in C57BL/6 mice. PLoS One. 8:e691232013.Erratum in: PLoS
One 8: 10.1371/annotation/90c76048-2edd-4315-8404-4d9d8cbd411e,
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eggesbø JB, Hjermann I, Høstmark AT and
Kierulf P: LPS induced release of IL-1 beta, IL-6, IL-8 and
TNF-alpha in EDTA or heparin anticoagulated whole blood from
persons with high or low levels of serum HDL. Cytokine. 8:152–160.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hotchkiss RS, Moldawer LL, Opal SM,
Reinhart K, Turnbull IR and Vincent JL: Sepsis and septic shock.
Nat Rev Dis Primers. 2:160452016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iba T, Watanabe E, Umemura Y, Wada T,
Hayashida K, Kushimoto S; Japanese Surviving Sepsis Campaign
Guideline Working Group for disseminated intravascular coagulation,
; Wada H: Sepsis-associated disseminated intravascular coagulation
and its differential diagnoses. J Intensive Care. 7:322019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prescott HC and Angus DC: Enhancing
recovery from sepsis: A review. JAMA. 319:62–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reinhart K, Daniels R, Kissoon N, Machado
FR, Schachter RD and Finfer S: Recognizing sepsis as a global
health priority-a WHO resolution. N Engl J Med. 377:414–417. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giza DE, Fuentes-Mattei E, Bullock MD,
Tudor S, Goblirsch MJ, Fabbri M, Lupu F, Yeung SJ, Vasilescu C and
Calin GA: Cellular and viral microRNAs in sepsis: Mechanisms of
action and clinical applications. Cell Death Differ. 23:1906–1918.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hombach S and Kretz M: Non-coding RNAs:
Classification, biology and functioning. Non-coding RNAs in
colorectal cancer. Springer; pp. 3–17. 2016, View Article : Google Scholar
|
|
36
|
Zhang CC and Niu F: LncRNA NEAT1 promotes
inflammatory response in sepsis-induced liver injury via the
Let-7a/TLR4 axis. Int Immunopharmacol. 75:1057312019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Y, Fu Y, Song YF and Li N: Increased
expression of lncRNA UCA1 and HULC is required for pro-inflammatory
response during LPS induced sepsis in endothelial cells. Front
Physiol. 10:6082019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang Y, Hu JF, Wang ZH, Zhang SG, Zhang
RF, Sun LM, Cui HW and Yang F: GAS5 promotes podocyte injury in
sepsis by inhibiting PTEN expression. Eur Rev Med Pharmacol Sci.
22:8423–8430. 2018.PubMed/NCBI
|
|
39
|
Lan C, Shi X, Guo N, Pei H and Zhang H:
Value of serum miR-155-5p and miR-133a-3p expression for the
diagnosis and prognosis evaluation of sepsis. Zhonghua Wei Zhong
Bing Ji Jiu Yi Xue. 28:694–698. 2016.(In Chinese). PubMed/NCBI
|
|
40
|
Ahn J and Kim J: Mechanisms and
consequences of inflammatory signaling in the myocardium. Curr
Hypertens Rep. 14:510–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang L, Moore XL, Dart AM and Wang LM:
Systemic inflammatory response following acute myocardial
infarction. J Geriatr Cardiol. 12:305–312. 2015.PubMed/NCBI
|
|
42
|
Hochstadt A, Meroz Y and Landesberg G:
Myocardial dysfunction in severe sepsis and septic shock: More
questions than answers? J Cardiothorac Vasc Anesth. 25:526–535.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pathan N, Franklin JL, Eleftherohorinou H,
Wright VJ, Hemingway CA, Waddell SJ, Griffiths M, Dennis JL, Relman
DA, Harding SE and Levin M: Myocardial depressant effects of
interleukin 6 in meningococcal sepsis are regulated by p38
mitogen-activated protein kinase. Crit Care Med. 39:1692–1711.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marchini G, Ståbi B, Kankes K, Lonne-Rahm
S, Østergaard M and Nielsen S: AQP1 and AQP3, psoriasin, and nitric
oxide synthases 1–3 are inflammatory mediators in erythema toxicum
neonatorum. Pediatr Dermatol. 20:377–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Trujillo E, González T, Marin R,
Martin-Vasallo P, Marples D and Mobasheri A: Human articular
chondrocytes, synoviocytes and synovial microvessels express
aquaporin water channels; upregulation of AQP1 in rheumatoid
arthritis. Histol Histopathol. 19:435–444. 2004.PubMed/NCBI
|
|
46
|
Rana S, Shahzad M and Shabbir A: Pistacia
integerrima ameliorates airway inflammation by attenuation of
TNF-α, IL-4, and IL-5 expression levels, and pulmonary edema by
elevation of AQP1 and AQP5 expression levels in mouse model of
ovalbumin-induced allergic asthma. Phytomedicine. 23:838–845. 2016.
View Article : Google Scholar : PubMed/NCBI
|