Introduction
Wilms tumor, also known as nephroblastoma, is the
most common type of renal malignancy diagnosed in children
(1–3). In the past few years, the treatment of
nephroblastoma has significantly improved following the application
of various treatment regimens and techniques, including surgery,
radiotherapy, chemotherapy and autologous stem cell transplantation
(4). Thus, nephroblastoma is one of
the most successful types of pediatric malignant tumor in terms of
prognosis (5). At present, the
5-year survival rate of patients with nephroblastoma has reached
90%, but there remains 10% of children who die due to recurrence,
metastasis and insensitivity to chemotherapy drugs (6–7).
However, the current understanding of the pathogenesis and
metastatic mechanism of action of nephroblastoma is insufficient,
and corresponding effective targeted treatments are lacking.
Therefore, current clinical research is focused on investigating
more effective targeted treatments for nephroblastoma to reduce its
metastatic and recurrence rate.
The forkhead transcription factor O (FOXO) family,
also named FKHR, consists of four subtypes: FOXO1/FKHR/FOXO
subfamily 1a (FOXO1a), FOXO3/FKHRL1/FOXO3a, FOXO4/AFX and FOXO6,
which have similar structures, functions and regulatory mechanisms
(8). FOXOs are an essential protein
family that have been discovered to regulate cell apoptosis, the
cell cycle, DNA damage repair, oxidative stress, energy metabolism
and longevity, and cancer development, amongst other cell functions
(9–11). In particular, FOXO3a is a tumor
suppressor gene belonging to the FOXO family (12), which has been reported to control
various signaling pathways and biological processes of tumor cells.
FOXO3a can inhibit the invasion of breast cancer cells by
activating ER-α signaling pathway (13). The low expression of FOXO3a may
promote the invasion and migration of non-small cell lung cancer
cells through PI3K/Akt signaling pathway (14). It was found that FOXO3a can induce
EMT and promote the metastasis of renal cancer cell (15).
Downregulated expression levels of FOXO3a have been
discovered to be associated with the occurrence, progression and
drug resistance of breast, pancreatic, liver and bladder cancer,
amongst other types of tumor (16–18).
Notably, it has been reported that FOXO3a may exert an antitumor
effect in breast cancer, non-small cell lung cancer and renal
cancer (19).
The Wnt and PI3K/AKT signaling pathways are reported
to be closely related to the development of nephroblastoma
(20). AKT serves an important role
in a variety of interrelated cellular signaling mechanisms involved
in cellular metabolism, growth and division, the inhibition of
apoptosis and angiogenesis (21,22).
The activation of Wnt/β-Catenin has been identified to have a
crucial role in tumor development (23,24).
β-catenin is a multifunctional protein, which has not only been
discovered to serve as the main structural component of cell
adhesion, but also to participate in embryogenesis and tumor
formation (25). However, to the
best of our knowledge, the relationship between nephroblastoma and
the Wnt signaling pathway remains poorly understood, and the
biological role and pathogenic mechanism of the Wnt signaling
pathway in the development of nephroblastoma has not been reported.
Since nephroblastoma is an embryonic renal tumor, abnormalities
during renal development have been identified to be closely
associated with its occurrence (26). As the Wnt signaling pathway is an
important signaling transduction pathway involved in the process of
renal development (27–29), it may be of great significance to
investigate the influence of the abnormalities in the Wnt signaling
pathway in association with the occurrence of nephroblastoma.
The present study aimed to analyze the expression
levels of FOXO3a in nephroblastoma and to determine the role of
FOXO3a in the proliferation, migration and invasion of
nephroblastoma. The results suggested that FOXO3a may regulate the
Wnt/β-catenin signaling pathway to inhibit nephroblastoma
progression, thus providing a potential, novel therapeutic target
for the treatment of the disease.
Materials and methods
Cell culture and transfection
The normal human renal cell line (HK-2) and
nephroblastoma cell lines (17–94, AG01615, HFWT, WILTU-1 and WIT49)
were obtained from the American Type Culture Collection. Cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin, and maintained at 37°C in an
humidified atmosphere of 5% CO2.
For cell transfection, 1×105 17–94
cells/well were plated into six-well plates and transfected with
overexpression (OE)-FOXO3a, overexpression (OE)-NC, short hairpin
RNA (shRNA)-targeting Axin-2 (shRNA-Axin-2-1 and shRNA-Axin-2-2)
and shRNA-NC (100 nM) were using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to
manufacturer's protocol. All plasmids were supplied from Guangzhou
RiboBio Co., Ltd. Subsequent experiments were performed 48 h after
transfection.
Cell Counting Kit-8 (CCK-8) assay
A total of 5×103 17–94 cells/well were
plated into 96-well plates. Following 24, 48 or 72 h of incubation
at 37°C, a CCK-8 kit (Dojindo Molecular Technologies, Inc.) was
used to analyze the proliferative capacity/cell viability of
transfected 17–94 cells, according to manufacturer's protocol.
Briefly, 10 µl CCK-8 reagent was added/well and incubated for 1 h
at 37°C. The absorbance was subsequently measured at a wavelength
of 450 nm using a microplate reader.
Colony formation assay
Following 48 h of transfection at 37°C, 800 cells
were seeded into 6-well plates and incubated at 37°C in complete
medium for 21 days with the medium changed every 3 days. Following
the incubation at 37°C, these cells were fixed with the 70% ethanol
solution and the plates were stained with 0.5% crystal violet at
room temperature for 20 min. (Santa Cruz Biotechnology, Inc.).
Wound healing assay
A total of 5×105 17–94 cells/well were
plated into six-well plates and cultured until 100% confluence in
complete medium at 37°C. Subsequently, the monolayer of cells was
scratched by a sterilized pipette tip (20 µl) and the cells were
incubated in serum-free DMEM for 24 h at 37°C. The migratory
distance of the cells was observed under a light microscope
(magnification, ×200; Olympus Corporation) at 0 h (and the scratch
width is recorded as W0.) and 24 h (the scratch width was recorded
as W24), and analyzed using ImageJ version 1.49 software (National
Institutes of Health). The migration rate was calculated as
Migration
rate=(W24-W0)/W0x100%.
Transwell Matrigel assay
A total of 1×104 cells/well were plated
into the upper chambers of 24-well Transwell plates (8.0-µm PET
membrane; Corning, Inc.) in 400 µl serum-free DMEM. The membranes
were precoated with Matrigel at 37°C for 2 h. The lower chambers
were filled with 600 µl DMEM supplemented with 10% FBS. Following
incubation for 24 h at 37°C, the cells on the bottom of the lower
chamber were fixed with 90% ethanol solution for 30 min at 37°C and
stained with 0.1% crystal violet for 10 min at room temperature.
The invasive cells were visualized using a light microscope
(Olympus FV500; Olympus Corporation, magnification, ×200) and
analyzed using ImageJ version 1.49 software (National Institutes of
health).
Flow cytometric analysis of
apoptosis
The rate of cell apoptosis was analyzed using an
Annexin V-FITC/propidium iodide (PI) flow cytometry assay kit (BD
Biosciences), according to the manufacturer's protocol. Briefly,
cells were centrifuged at ~200 × g for 3–5 min for precipitation
and collection, then fixed in precooled 70% ethanol at 4°C
overnight. The cells were then resuspended in 300 µl cold binding
buffer and incubated with 5 µl Annexin V-FITC for 10 min in the
dark at room temperature. Following the addition of 5 µl PI and 200
µl binding buffer, the samples were incubated in the dark at room
temperature for a further 5 min. Apoptotic cells were subsequently
analyzed using the FACSCalibur flow cytometer (BD Biosciences) and
data were analyzed using BD Accuri C6 (BD Biosciences). The
percentage of early and late apoptotic cells was calculated. The
experiments were independently repeated 3 times.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 17–94 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) at −40°C. Total RNA was reverse transcribed into cDNA using
the TaqMan Reverse Transcription kit (Takara Bio, Inc.), according
to the manufacturer's protocol. qPCR was subsequently performed
using a SYBR Taq kit (Roche Diagnostics) to detect the relative
expression of FOXO3a and AXIN2 on an a Bi 7500 real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
Primers were FOXO3a, forward, 5′-CGGACAAACGGCTCACTCT-3′ and
reverse, 5′-GGACCCGCATGAATCGACTAT-3′; β-actin, forward,
5′-ATCACCATTGGCAATGAGCG-3′ and reverse, 5′-TTGAAGGTAGTTTCGTGGAT-3′;
AXIN2, forward, 5′-CACGGAAACTGTTGACAGTGGATAC-3′ and reverse,
5′-GGTGGCTGGTGCAAAGACATAG-3′; GAPDH, forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-GTGAAGACGCCAGTGGA-3′.
Gene expression levels were then normalized to that of GAPDH,
whereas fold changes were calculated using the 2−ΔΔCq
method (30).
Western blotting
Total protein was extracted from 17–94 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology) with
protease inhibitors (Roche Diagnostics). Total protein was
quantified using a bicinchoninic acid assay kit (Beyotime Institute
of Biotechnology) and 30 µg protein/lane was separated using 10%
SDS-PAGE. The proteins were subsequently transferred onto PVDF
membranes (EMD Millipore) and blocked with 5% non-fat milk for 2 h
at room temperature. The membranes were then incubated overnight at
4°C with the following primary antibodies: Anti-FOXO3A (1:5,000;
cat. no. ab109629; Abcam); anti-MMP2 (1:4,000; cat. no. ab92536;
Abcam); anti-MMP9 (1:10,000; cat. no. ab76003; Abcam); anti-MMP13
(1:1,000; cat. no. ab51072; Abcam); anti-Caspase-3 (1:500; cat. no.
ab13847; Abcam); anti-Bim (1:1,000; cat. no. ab32158; Abcam);
anti-β Catenin (1:1,000; cat. no. ab16051; Abcam); Rabbit
anti-Cyclin D1 (1:200; cat. no. ab16663; Abcam); anti-Axin 2
(1:500; cat. no. ab32197; Abcam); Anti-Bcl-2 (1:1,000; cat. no.
3498; Cell Signaling Technology, Inc.), anti-Bax (1:1,000; cat. no.
5023; Cell Signaling Technology, Inc.), anti-cleaved-caspase-3
(1:500; cat. no. 9661; Cell Signaling Technology, Inc.) and
anti-GAPDH (1:2,000; cat. no. MAB374; EMD Millipore). Following the
primary antibody incubation, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated anti-rabbit (1:10,000; cat.
no. sc-2357; Santa Cruz Biotechnology, Inc.) secondary antibodies
at room temperature for 2 h. Densitometric analysis was performed
using ImageJ software (version 1.49; National Institutes of
Health). Protein bands were detected using an ECL detection reagent
(EMD Millipore) and protein expression levels were normalized to
GAPDH. The experiments were performed in triplicate.
Statistical analysis
All data are presented as the mean ± SEM and all
experiments were repeated three times. Statistical analysis was
performed using GraphPad 6.0 software (GraphPad Software, Inc).
Statistical differences among groups were determined using a
one-way ANOVA followed by a Tukey's or Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
FOXO3a expression levels are
significantly downregulated in nephroblastoma cells
To investigate the expression levels of FOXO3a in
nephroblastoma cells, 17–94, AG01615, HFWT, WILTU-1 and WIT49, and
the normal kidney cell HK-2, RT-qPCR and western blotting were
performed. The expression levels of FOXO3a were significantly
downregulated at both the mRNA and protein level in nephroblastoma
cells compared with the normal kidney HK-2 cells (Fig. 1A-C). The expression levels of FOXO3a
in 17–94 cells were downregulated the most, thus, 17–94 cells were
used for subsequent experiments.
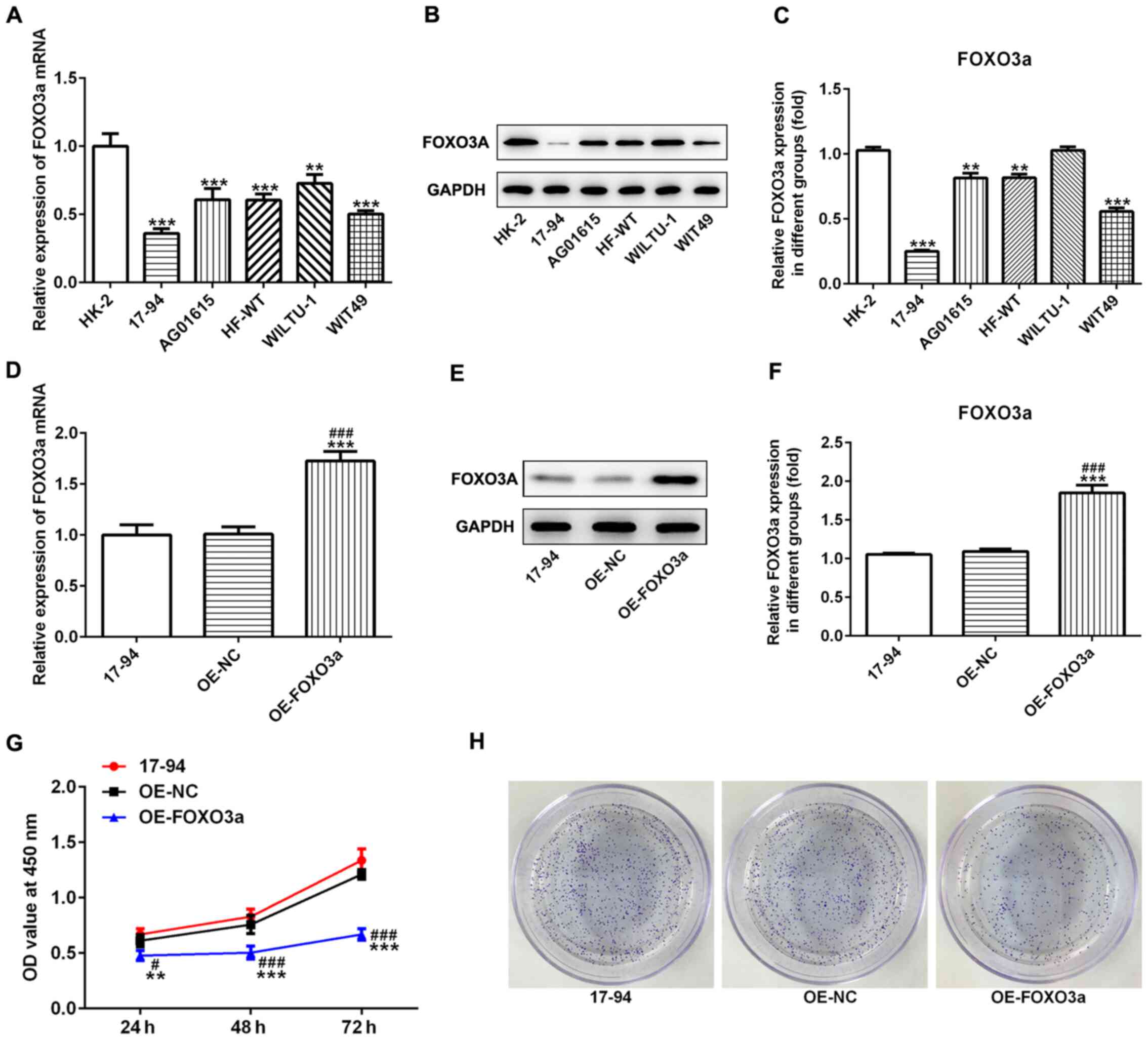 | Figure 1.Overexpression of FOXO3a inhibits the
proliferation of nephroblastoma cells. Expression levels of FOXO3a
in nephroblastoma cell lines (17–94, AG01615, HFWT, WILTU-1 and
WIT49) and the normal kidney cell line HK-2 were analyzed using (A)
RT-qPCR and (B) western blotting. (C) Semi-quantification of the
expression levels of FOXO3a presented in part (B). **P<0.01,
***P<0.001 vs. HK-2. Effect of OE-FOXO3a on the expression
levels of FOXO3a in 17–94 cells was determined using (D) RT-qPCR
and (E) western blotting. (F) Semi-quantification of the expression
levels of FOXO3a from part (E). (G) Cell Counting Kit-8 assay was
used to determine that OE-FOXO3a-transfected 17–94 cells had
significantly decreased rates of cell proliferation. (H)
OE-FOXO3a-transfected 17–94 cells demonstrated decreased rates of
colony formation. **P<0.01, ***P<0.001 vs. control;
#P<0.01, ###P<0.001 vs. OE-NC. n=3.
FOXO3a, forkhead transcription factor O subfamily 3A; OE,
overexpression; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR; OD, optical density. |
Overexpression of FOXO3a inhibits
nephroblastoma cell proliferation, cell invasion and migration
To determine the role of FOXO3a in nephroblastoma
cells, OE-FOXO3a and OE-NC plasmids were transfected into 17–94
cells. The results of the RT-qPCR and western blotting demonstrated
that the OE-FOXO3a plasmid was successfully transfected into 17–94
cells; the expression levels of FOXO3a were significantly
upregulated in the OE-FOXO3a group compared with the control and
OE-NC groups (Fig. 1D-F). The
results of the CCK-8 assay indicated that the cell proliferation
rate was significantly reduced in 17–94 cells transfected with the
OE-FOXO3a plasmid compared with the control and OE-NC groups
(Fig. 1G). Similar results were
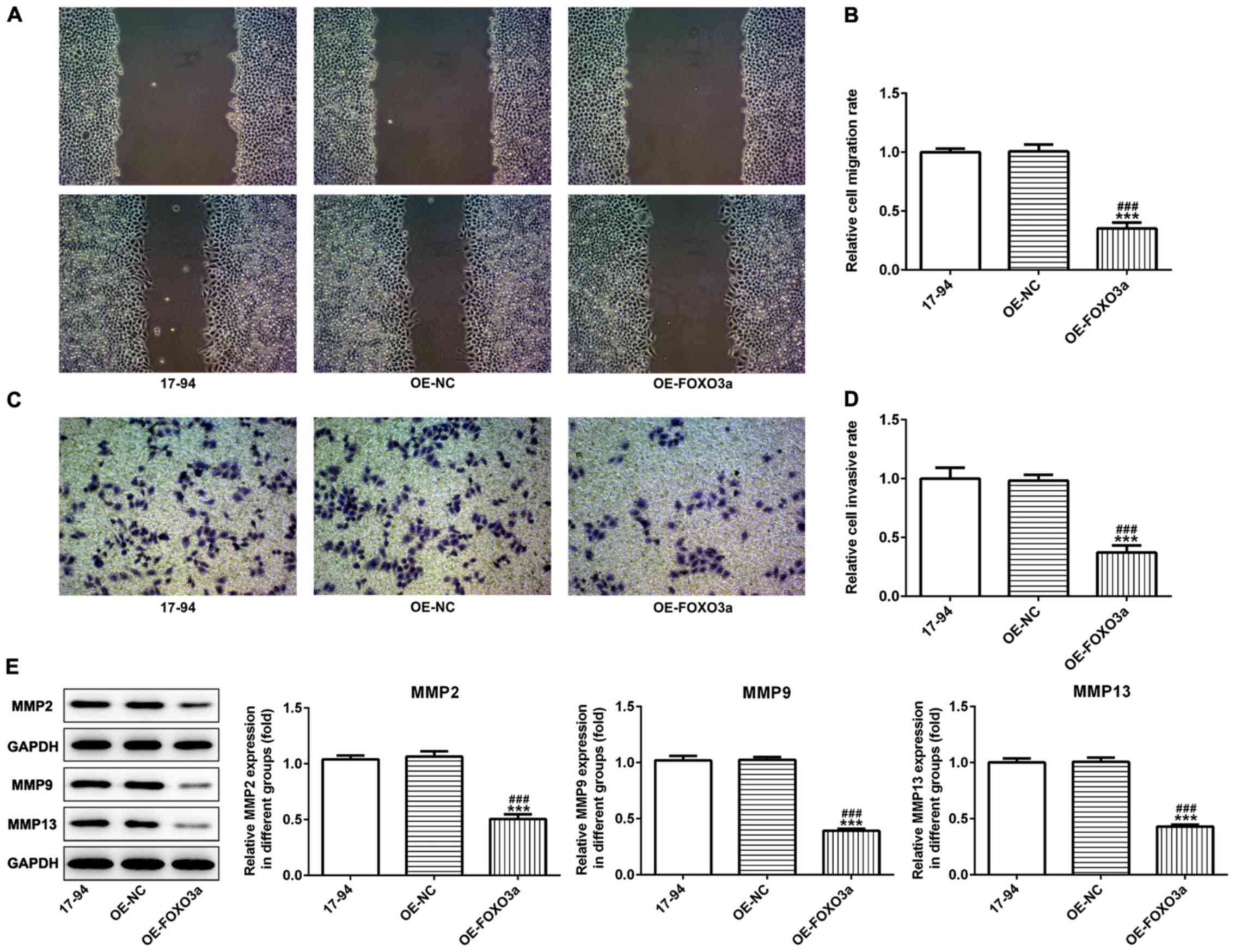
observed in the colony formation abilities of 17–94 cells (Fig. 1H). Furthermore, the overexpression
of FOXO3a significantly reduced the invasive and migratory
abilities of 17–94 cells compared with the OE-NC and control groups
(Fig. 2A-D). Western blotting
results also indicated that the overexpression of FOXO3a
significantly downregulated the expression levels of the invasion
and migration-related proteins, matrix metalloproteinase (MMP)2,
MMP9 and MMP13 in 17–94 cells compared with the control and OE-NC
groups (Fig. 2E).
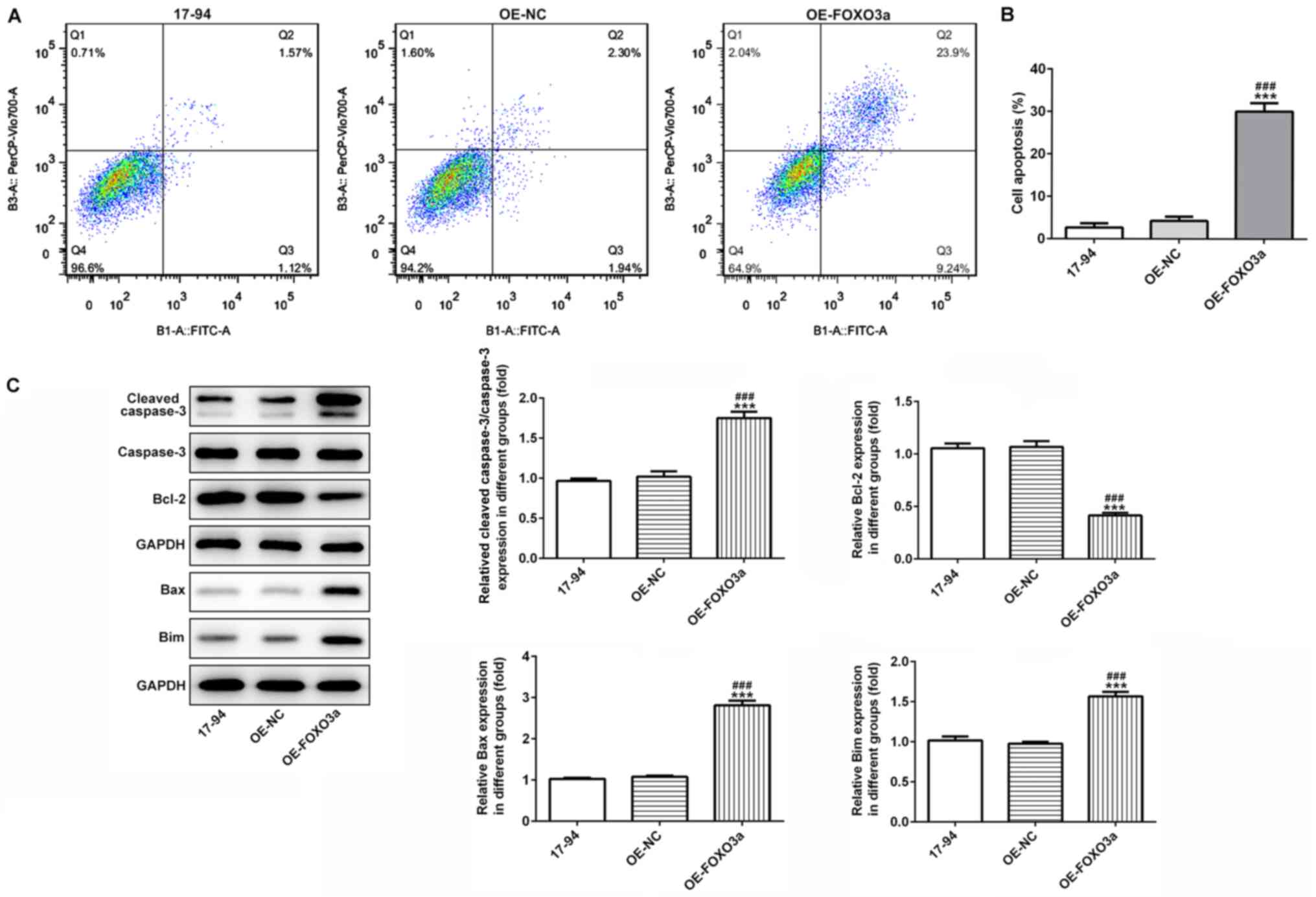
Overexpression of FOXO3a promotes
nephroblastoma cell apoptosis
Flow cytometric analysis was used to detect the rate
of cell apoptosis. The apoptotic rate was significantly increased
in the 17–94 cells overexpressing FOXO3a compared with the control
and OE-NC groups (Fig. 3A and B).
To further verify the role of FOXO3a in cell apoptosis, western
blotting was used to detect the expression levels of the
apoptosis-related proteins, Bax, Bcl-2, Bim, cleaved caspase-3 and
caspase-3. The results revealed that the protein expression levels
of Bax, Bim and cleaved caspase-3 were significantly upregulated,
while those of the Bcl-2 protein were significantly downregulated
in the OE-FOXO3a group compared with the control and OE-NC groups
(Fig. 3C).
FOXO3a-induced suppression over cell
proliferation, migration and invasion is regulated by Wnt/β-catenin
signaling
To further investigate whether FOXO3a regulated cell
viability, migration and invasion by activating Wnt/β-catenin
signaling, western blotting was used to analyze the expression
levels of the Wnt/β-catenin signaling pathway-related proteins,
Axin-2, β-catenin and cyclin D1. The results identified that the
overexpression of FOXO3a significantly upregulated the protein
expression levels of Axin-2, while the protein expression levels of
β-catenin and cyclin D1 were significantly downregulated, compared
with the control and OE-NC groups (Fig.
4A). The transfection efficiency of shRNA-Axin-2-1 and
shRNA-Axin-2-2 was analyzed using western blotting and RT-qPCR; the
results demonstrated that the inhibitory effect of shRNA-Axin-2-1
on the Axin-2 expression levels was superior compared with
shRNA-Axin-2-2 in 17–94 cells, thus shRNA-Axin-2-1 was chosen for
subsequent experiments (Fig. 4B and
C).
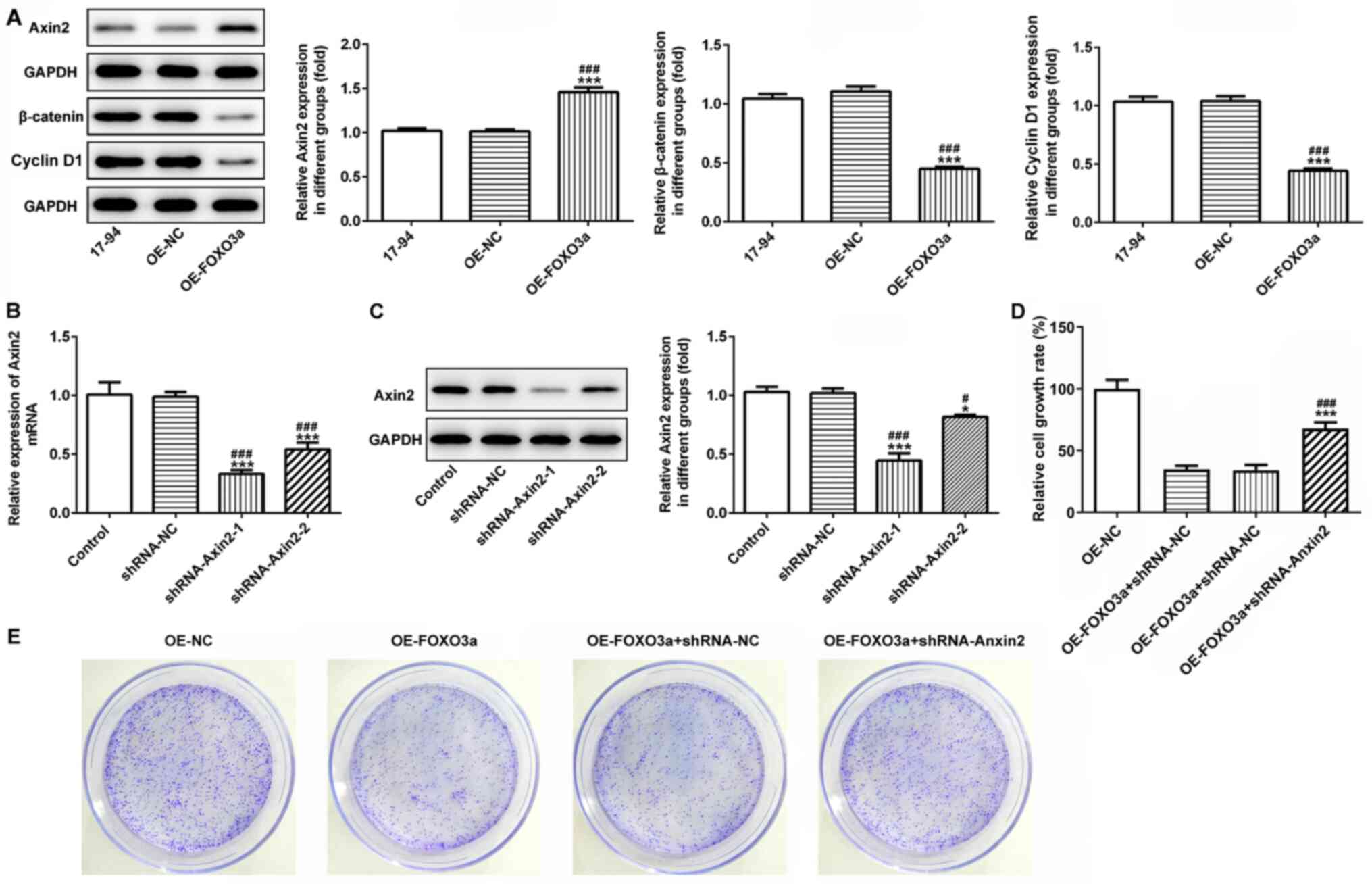 | Figure 4.Activation of Wnt/β-catenin signaling
reverses FOXO3a overexpression-induced inhibition of the
proliferation of nephroblastoma cells. (A) Western blotting was
used to analyze the expression levels of the Wnt/β-catenin
signaling-related proteins, Axin-2, β-catenin and Cyclin D1.
***P<0.001 vs. control; ###P<0.001 vs. OE-NC; n=3.
The transfection efficiency of shRNA-Axin-2-1 and shRNA-Axin-2-2
were detected using (B) reverse transcription-quantitative PCR and
(C) western blotting. OE-FOXO3a and shRNA-Axin-2 were
co-transfected into 17–94 cell lines and the cell viability and
proliferation of 17–94 cells was detected using a (D) Cell Counting
Kit-8 assay and (E) colony formation assay, respectively.
*P<0.05, ***P<0.001 vs. control; #P<0.05,
###P<0.001 vs. shRNA-NC; n=3. FOXO3a, forkhead
transcription factor O subfamily 3A; OE, overexpression; NC,
negative control; OD, optical density; shRNA, short hairpin
RNA. |
Subsequently, OE-FOXO3a and shRNA-Axin-2 were
co-transfected into 17–94 cell lines. The CCK-8 assay revealed that
the cell viability of 17–94 cells was significantly increased in
the OE-FOXO3a + shRNA-Axin-2 group compared with the OE-FOXO3a and
OE-FOXO3a + shRNA-NC groups (Fig.
4D). Furthermore, similar results were recorded in the colony
formation ability of 17–94 cells (Fig.
4E). Taken together, these findings suggested that the
activation of Wnt/β-catenin signaling may reverse the FOXO3a
overexpression-induced inhibition of cell viability and
proliferation in nephroblastoma.
The migratory and invasive abilities of 17–94 cells
in the OE-FOXO3a + shRNA-Axin-2 group were significantly increased
compared with the OE-FOXO3a and OE-FOXO3a + shRNA-NC groups
(Fig. 5A-D). In addition, western
blotting was used to analyze the expression levels of the invasion
and migration-related proteins, MMP2, MMP9 and MMP13. Compared with
the OE-FOXO3a and OE-FOXO3a + shRNA-NC groups, significantly
upregulated expression levels of MMP2, MMP9 and MMP13 were observed
in the OE-FOXO3a + shRNA-Axin-2 group (Fig. 5E).
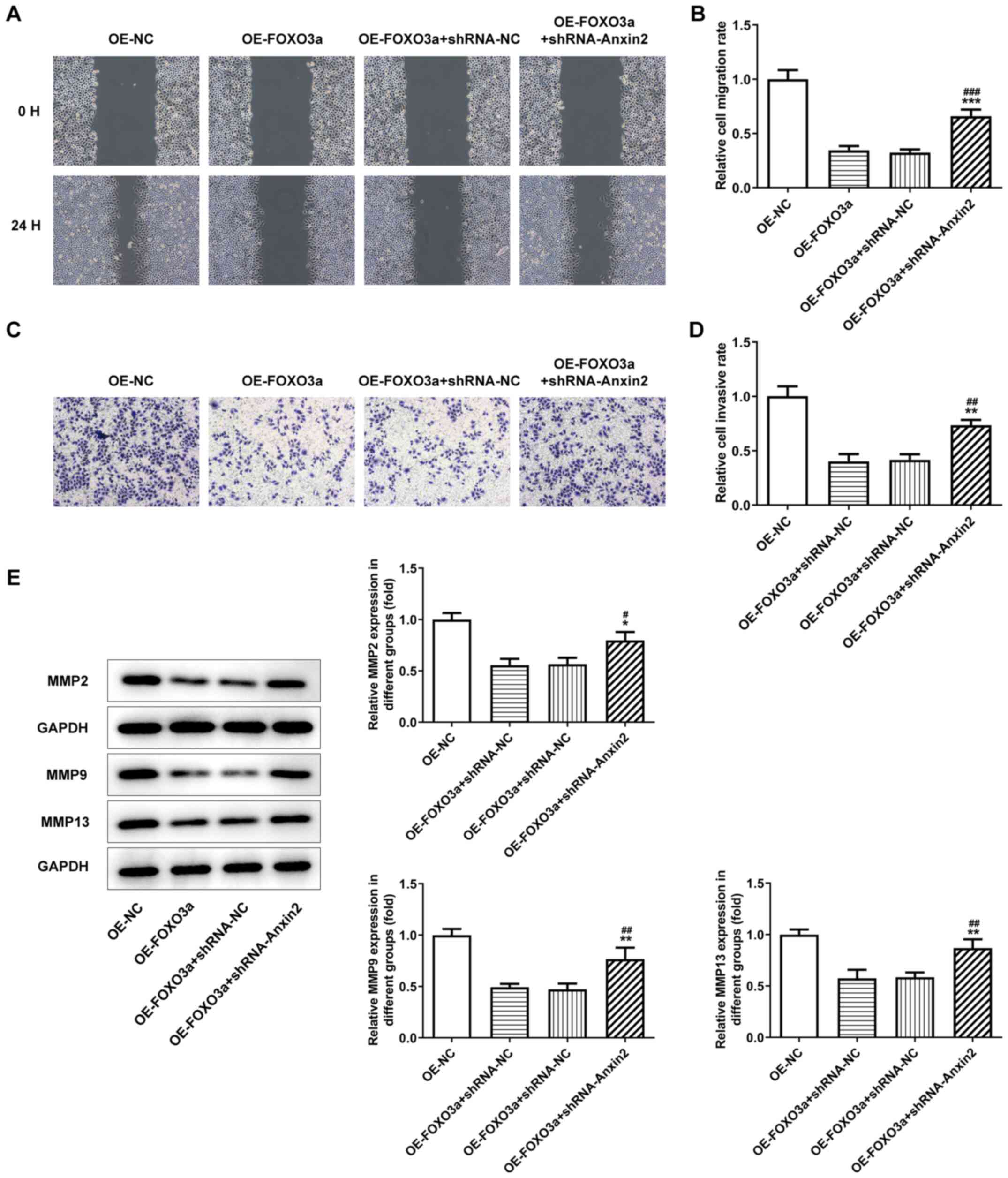 | Figure 5.Activation of Wnt/β-catenin signaling
reverses FOXO3a overexpression-induced inhibition of the invasion
and migration of nephroblastoma cells. (A) Wound healing assay was
used to analyze the migratory ability of cells transfected with
OE-FOXO3a + shRNA-Axin-2 (magnification, ×200). (B)
Semi-quantification of the cell migration rate from part (A). (C)
Transwell Matrigel assay was used to analyze the invasive ability
of cells transfected with OE-FOXO3a + shRNA-Axin-2 (magnification,
×200). (D) Semi-quantification of the cell invasive rate from part
(C). (E) Western blotting was performed to analyze the protein
expression levels of MMP2, MMP9 and MMP13 in cells transfected with
OE-FOXO3a + shRNA-Axin-2. *P<0.05, **P<0.01, ***P<0.001
vs. OE-FOXO3a; #P<0.05, ##P<0.01,
###P<0.001 vs. OE-FOXO3a + shRNA-NC; n=3. FOXO3a,
forkhead transcription factor O subfamily 3A; OE, overexpression;
NC, negative control; shRNA, short hairpin RNA; MMP, matrix
metalloproteinase. |
FOXO3a-induced cell apoptosis is
regulated by Wnt/β-catenin signaling
17–94 cells were co-transfected with OE-FOXO3a and
shRNA-Axin-2 and flow cytometry was used to determine the rate of
cell apoptosis. Compared with the OE-FOXO3a + shRNA-NC and
OE-FOXO3a groups, the cell apoptotic rate was significantly
decreased in the OE-FOXO3a + shRNA-Axin-2 group (Fig. 6A and B). Furthermore, western
blotting was performed to analyze the expression levels of the
apoptosis-related proteins, Bax, Bcl-2, Bim, cleaved caspase-3 and
caspase-3. The results revealed that the upregulated expression
levels of Bax, Bim and cleaved caspase-3, and downregulated
expression levels of Bcl-2, induced by OE-FOXO3a were significantly
reversed following the co-transfection with OE-FOXO3a and
shRNA-Axin-2 (Fig. 6C).
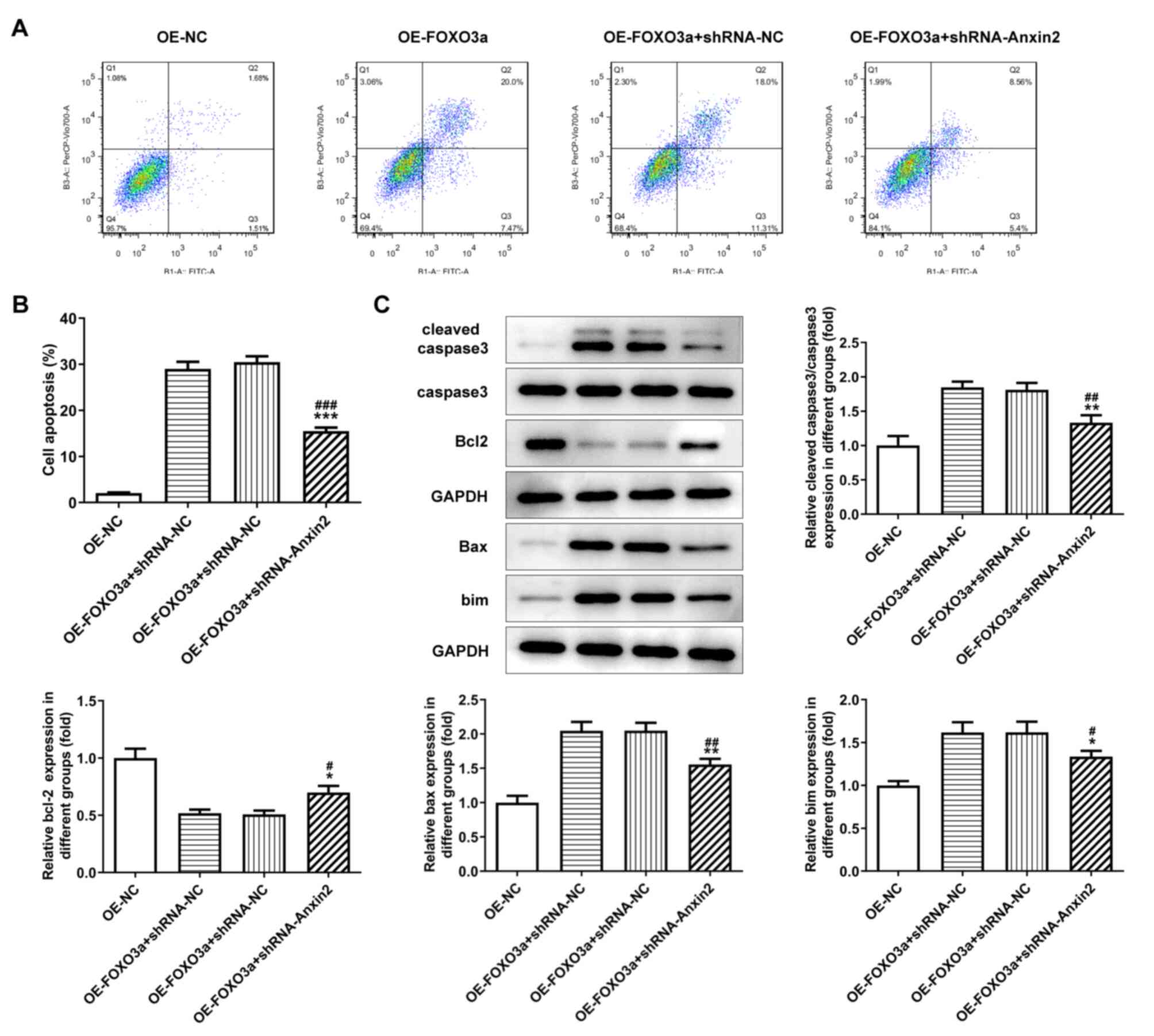 | Figure 6.Activation of Wnt/β-catenin signaling
reverses the FOXO3a overexpression-induced apoptosis of
nephroblastoma cells. (A) Flow cytometric analysis of cell
apoptosis in cells transfected with OE-FOXO3a + shRNA-Axin-2. (B)
Quantification of cell apoptosis from part (A). (C) Western
blotting was used to analyze the protein expression levels of the
apoptosis-related molecules, Bcl-2, Bax, Bim, caspase-3/cleaved
caspase-3. *P<0.05, **P<0.01, ***P<0.001 vs. OE-FOXO3a;
#P<0.05, ##P<0.01,
###P<0.001 vs. OE-FOXO3a + shRNA-NC, n=3. FOXO3a,
forkhead transcription factor O subfamily 3A; OE, overexpression;
NC, negative control; shRNA, short hairpin RNA; Bim, Bcl-2-like
protein 2. |
Discussion
Over the past few years, the incidence of childhood
tumors has increased significantly (31). Renal cancer is the most type of
common malignant tumor to occur in children, accounting for 7% of
childhood cancers (32). The most
common pathological type of renal cell carcinoma in children is
nephroblastoma (2). The rare
pathological types include rhabdomyoma sarcoma, clear cell sarcoma,
congenital mesodermal nephroma, renal Ewing's sarcoma, primary
renal myoepithelial carcinoma, cystic partially differentiated
nephroblastoma, multilocular cystic nephroma, primary synovial
sarcoma and anaplastic sarcoma (2,31–33).
Nephroblastoma has been discovered to affect the function of
unilateral or bilateral kidneys (33,34).
Although the diagnosis and treatment of nephroblastoma has
significantly improved, the mortality rate of nephroblastoma in
children remains high (10.7%) (35,36).
Thus, studying the effects of factors related to early
nephroblastoma may provide a novel target and treatment method for
nephroblastoma.
FOXO3a is a member of the FOXO family; it enters the
nucleus after binding with β-catenin, where it was reported to
accelerate the self-renewal of cancer cells, thus promoting the
formation of tumors (37,38). FOXO3a has also been discovered to
combine with the transcription regulatory machinery of different
target genes in the nucleus to serve a role in transcriptional
regulation, in addition to participating in the proliferation,
migration, invasion and apoptosis of cells (12,39).
FOXO3a was also discovered to serve a role in abnormal activity,
stress tolerance and the metabolic homeostasis of tumor cells by
regulating the expression of cell cycle-related factors and
apoptosis-related factors effect (40,41).
In the present study, FOXO3a expression levels were discovered to
be downregulated in nephroblastoma cell lines, suggesting that
FOXO3a may serve an essential role in nephroblastoma. Furthermore,
the results revealed that the overexpression of FOXO3a
significantly attenuated nephroblastoma cell proliferative,
migratory and invasive abilities. In addition, the overexpression
of FOXO3a promoted the apoptosis of nephroblastoma cells. A
previous study suggested that FOXO3a may be a potential biomarker
for the diagnosis, prognosis and treatment of a variety of types of
malignant tumor, including ovarian, prostate and pancreatic cancers
(42,43). Similarly, previous studies also
reported that FOXO3a expression levels could be used as a
prognostic biomarker for clear cell renal cell carcinoma, cervical
carcinoma and colorectal cancer (14,29,42).
Interestingly, the overexpression of FOXO3a has been associated
with the poor prognosis of patients with triple negative breast
cancer, glioblastoma and gastric cancer, while downregulated
expression levels of FOXO3a were associated with the poor prognosis
of patients with glioma and ovarian cancer (44–45).
Therefore, these studies suggested that FOXO3a may be a potential
biomarker for the diagnosis of nephroblastoma, and may potentially
serve as an oncogene or suppressor factor to be investigated in
relation to nephroblastoma progression, which requires further
investigations. Based on these previous studies (46–50)
and the present data, FOXO3a was hypothesized to be a potential
molecular target for nephroblastoma therapy and diagnosis.
The Wnt/β-catenin signaling pathway is an important
signaling pathway involved in regulating cell proliferation and
differentiation, and serves a crucial role in tumor development,
metastasis and embryonic development (23,24).
In addition, it was reported that the Wnt/β-catenin signaling
pathway was highly activated in various types of cancer, such as
ovarian epithelial cancer and prostate cancer (51,52),
thus, the inhibition of this signaling pathway has become a
research hotspot. A previous study revealed that the inhibition of
the Wnt/β-catenin signaling pathway inhibited growth and promoted
apoptosis in ovarian cancer cells (53), while microRNA-218 promoted the
apoptosis of ovarian cancer cells by inhibiting the Wnt/β-catenin
signaling pathway (53). In
addition, lysyl oxidase homolog 2, a member of the lysyl oxidase
family, was suggested to affect the growth of ovarian cancer cells
by inhibiting the Wnt/β-catenin signaling pathway (54). Thus, to further determine the
possible mechanisms by which FOXO3a participated in nephroblastoma,
the relationship between FOXO3a and the Wnt/β-catenin signaling
pathway in nephroblastoma cells was investigated. The present study
detected the expression of WNT/β-catenin signaling proteins Axin2,
β-catenin and cyclin D1. Following overexpression of FOXO3a, the
expression of Axin2 increased significantly, and the expression of
β-catenin and cyclin D1 decreased significantly. Following
co-transfection with shRNA-Axin-2, WNT signaling was activated, the
activation of the Wnt/β-catenin signaling pathway reversed the
FOXO3a overexpression-induced suppression of proliferation,
invasion and migration in nephroblastoma cells, in addition to
reversing the FOXO3a-induced apoptosis of nephroblastoma cells.
In conclusion, the findings of the present study
suggested that FOXO3a may inhibit nephroblastoma cell
proliferation, invasion and migration, while inducing apoptosis
through downregulating the Wnt/β-catenin signaling pathway. These
results may provide a novel method for the early diagnosis and
treatment of nephroblastoma. A limitation of the present study was
that FOXO3a was only studied in a nephroblastoma cell line; its
application in vivo and in clinic was not studied but will
be investigated in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL designed the study, collected the data, performed
the data analysis and wrote the manuscript. CQ conceived the study,
participated in designing the study and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leslie SW, Sajjad H and Murphy PB: Wilms
Tumor (Nephroblastoma). StatPearls; Treasure Island, FL: 2019
|
|
2
|
Anderson TE and Conran RM: Educational
case: Wilms tumor (Nephroblastoma). Acad Pathol.
6:23742895188213812019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Gao X, Zhou X, Qin Z, Wang Y, Li
R, Tang M, Wang W and Zhang W: Identification of key genes and
microRNAs involved in kidney wilms tumor by integrated
bioinformatics analysis. Exp Ther Med. 18:2554–2564.
2019.PubMed/NCBI
|
|
4
|
Chakumatha E, Weijers J, Banda K, Bailey
S, Molyneux E, Chagaluka and Israels T: Outcome at the end of
treatment of patients with common and curable childhood cancer
types in Blantyre, Malawi. Pediatr Blood Cancer. 67:e283222020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamb MG, Aldrink JH, O'Brien SH, Yin H,
Arnold MA and Ranalli MA: Renal tumors in children younger than 12
months of Age: A 65-year single institution review. J Pediatr
Hematol Oncol. 39:103–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Illhardt T, Ebinger M, Schwarze CP,
Feuchtinger T, Furtwangler R, Schlegel PG, Klingebiel T, Greil J,
Beck JF, Handgretinger R and Lang P: Children with relapsed or
refractory nephroblastoma: Favorable long-term survival after
high-dose chemotherapy and autologous stem cell transplantation.
Klin Padiatr. 226:351–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weirich A, Ludwig R, Graf N, Abel U,
Leuschner I, Vujanic GM, Mehls O, Boos J, Beck J, Royer-Pokora B
and Voûte PA: Survival in nephroblastoma treated according to the
trial and study SIOP-9/GPOH with respect to relapse and morbidity.
Ann Oncol. 15:808–820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi C, Shi R, Guo H, Shi Y and Liu X:
β-amyloid-induced gonadotropin-releasing hormone decline involving
forkhead transcription factor FOXO3a and nuclear factor-κB.
Neuroreport. 31:923–927. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Q, Tang H, Hu F and Qin C: Silencing of
FOXO6 inhibits the proliferation, invasion, and glycolysis in
colorectal cancer cells. J Cell Biochem. 120:3853–3860. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Menghini R, Casagrande V, Iuliani G, Rizza
S, Mavilio M, Cardellini M and Federici M: Metabolic aspects of
cardiovascular diseases: Is FoxO1 a player or a target? Int J
Biochem Cell Biol. 118:1056592020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Li Y and Luo B: Current perspective
on the regulation of FOXO4 and its role in disease progression.
Cell Mol Life Sci. 7:651–663. 2019.
|
|
12
|
Usami M, Kikuchi S, Takada K, Ono M,
Sugama Y, Arihara Y, Hayasaka N, Nakamura H, Ikeda Y, Hirakawa M,
et al: FOXO3a activation by HDAC class IIa inhibition induces cell
cycle arrest in pancreatic cancer cells. Pancreas. 49:135–142.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Belguise K, Guo S and Sonenshein GE:
Activation of FOXO3a by the green tea polyphenol
epigallocatechin-3-gallate induces estrogen receptor alpha
expression reversing invasive phenotype of breast cancer cells.
Cancer Res. 67:5763–5770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou
J, Li L, Chen Y, Zhang T, Wang X, et al: PI3K/Akt to
GSK3beta/beta-catenin signaling cascade coordinates cell
colonization for bladder cancer bone metastasis through regulating
ZEB1 transcription. Cell Signal. 24:2273–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y,
Ai Q, Zhang P, Song EL, Huang QB, et al: Downregulation of FOXO3a
promotes tumor metastasis and is associated with metastasis-free
survival of patients with clear cell renal cell carcinoma. Clin
Cancer Res. 20:1779–1790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramis G, Villalonga-Planells R,
Serra-Sitjar M, Brell M, Fernandez de Mattos S and Villalonga P:
The tumor suppressor FOXO3a mediates the response to EGFR
inhibition in glioblastoma cells. Cell Oncol (Dordr). 42:521–536.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Yang R, Dong Y, Chen M, Wang Y and
Wang G: Knockdown of FOXO3a induces epithelial-mesenchymal
transition and promotes metastasis of pancreatic ductal
adenocarcinoma by activation of the β-catenin/TCF4 pathway through
SPRY2. J Exp Clin Cancer Res. 38:382019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Xu L and Wang L: Expression of
miR-182 and Foxo3a in patients with bladder cancer correlate with
prognosis. Int J Clin Exp Pathol. 12:4193–4203. 2019.PubMed/NCBI
|
|
19
|
Zhao R, Li Y, Gorantla S, Poluektova LY,
Lin H, Gao F, Wang H, Zhao J, Zheng JC and Huang Y: Small molecule
ONC201 inhibits HIV-1 replication in macrophages via FOXO3a and
TRAIL. Antiviral Res. 168:134–145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao W, Li J, Li P, Guo F, Gao P, Zhang J,
Yan Z, Wang L, Zhang D and Qin P: Wilms tumor-suppressing peptide
inhibits proliferation and induces apoptosis of Wilms tumor cells
in vitro and in vivo. J Cancer Res Clin Oncol. 145:2457–2468. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (Review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
22
|
Lachmandas E, Beigier-Bompadre M, Cheng
SC, Kumar V, van Laarhoven A, Wang X, Ammerdorffer A, Boutens L, de
Jong D, Kanneganti TD, et al: Rewiring cellular metabolism via the
AKT/mTOR pathway contributes to host defence against Mycobacterium
tuberculosis in human and murine cells. Eur J Immunol.
46:2574–2586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian J, Huang X, Zhang Y, Ye X and Qian W:
γ-catenin overexpression in AML patients may promote tumor cell
survival via activation of the wnt/beta-catenin axis. Onco Targets
Ther. 13:1265–1276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao Q, Wu J, Wang WJ, Chen S, Zheng Y, Yu
X, Meeth K, Sahraei M, Bothwell AL, Chen L, et al: DKK2 imparts
tumor immunity evasion through beta-catenin-independent suppression
of cytotoxic immune-cell activation. Nat Med. 24:262–270. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anton R, Chatterjee SS, Simundza J, Cowin
P and Dasgupta R: A systematic screen for micro-RNAs regulating the
canonical wnt pathway. PLoS One. 6:e262572011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Re GG, Hazen-Martin DJ, Sens DA and Garvin
AJ: Nephroblastoma (Wilms' tumor): A model system of aberrant renal
development. Semin Diagn Pathol. 11:126–135. 1994.PubMed/NCBI
|
|
27
|
Hu Z, Li L, Cheng P, Liu Q, Zheng X, Peng
F and Zhang Q: lncRNA MSC-AS1 activates Wnt/β-catenin signaling
pathway to modulate cell proliferation and migration in kidney
renal clear cell carcinoma via miR-3924/WNT5A. J Cell Biochem.
121:4085–4093. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rim EY, Kinney LK and Nusse R:
β-catenin-mediated wnt signal transduction proceeds through an
endocytosis-independent mechanism. Mol Biol Cell. 31:1425–1436.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haque I, Banerjee S, Mehta S, De A,
Majumder M, Mayo MS, Kambhampati S, Campbell DR and Banerjee SK:
Cysteine-rich 61-connective tissue growth
factor-nephroblastoma-overexpressed 5 (CCN5)/Wnt-1-induced
signaling protein-2 (WISP-2) regulates microRNA-10b via
hypoxia-inducible factor-1α-TWIST signaling networks in human
breast cancer cells. J Biol Chem. 286:43475–43485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fair D, Potter SL and Venkatramani R:
Challenges and solutions to the study of rare childhood tumors.
Curr Opin Pediatr. 32:7–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chong WC and Cain JE: Lessons learned from
the developmental origins of childhood renal cancer. Anat Rec
(Hoboken). 303:2561–2577. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hang S, Wang X and Li H: Triptolide
inhibits viability and migration while promotes apoptosis in
nephroblastoma cells by regulation of miR-193b-3p. Exp Mol Pathol.
108:80–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu H, Ren SY, Qu Y, Liu C, Zhang Y, Li XQ
and Ma H: MiR-194-5p inhibited metastasis and EMT of nephroblastoma
cells through targeting Crk. Kaohsiung J Med Sci. 36:265–273. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakabayashi A, Kanno T, Takahashi N,
Akizawa Y, Onizuka H and Matsui H: A case of ovarian teratoma with
nephroblastoma presenting spontaneous rupture. J Obstet Gynaecol
Res. 45:1079–1083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bellalah A, Abdeljelil NB, Njima M,
Hamdani M, Hamdouni W, Hadhri R, Moussa A, Zakhama A and Njim L:
Fetal rhabdomyomatous nephroblastoma in a 31-year-old woman: A case
report. Urology. 133:e5–e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu Y and Yan J: Aberrant expression and
mechanism of miR-130b-3p/phosphatase and tensin homolog in
nephroblastoma in children. Exp Ther Med. 18:1021–1028.
2019.PubMed/NCBI
|
|
38
|
Jones BC, Youlden DR, Cundy TP,
O'Callaghan ME, Karpelowsky J, Aitken JF and Mcbride CA: Renal
tumours in Australian children: 30 years of incidence, outcome and
second primary malignancy data from the Australian childhood cancer
registry. J Paediatr Child Health. 56:908–916. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pomponio MK, Burkbauer L, Goldbach M,
Nazarian SM, Xie F, Clark AS, Matro JM, Fox KR, Shulman LN, Keele
LJ and Tchou J: Refining the indications for neoadjuvant
chemotherapy for patients with HER2+ breast cancer: A single
institution experience. J Surg Oncol. 121:447–455. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Su D, Qin Z and Chen Z:
Identification of FOXN4 as a tumor suppressor of breast
carcinogenesis via the activation of TP53 and deactivation of notch
signaling. Gene. 722:1440572020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Strassheim D, Karoor V, Nijmeh H, Weston
P, Lapel M, Schaack J, Sullivan T, Dempsey EC, Stenmark KR and
Gerasimovskaya E: c-Jun, Foxo3a, and c-Myc transcription factors
are key regulators of ATP-mediated angiogenic responses in
pulmonary artery vasa vasorum endothelial cells. Cells. 9:4162020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grossi V, Fasano C, Celestini V, Signorile
ML, Sanese P and Simone C: Chasing the FOXO3: Insights into its new
mitochondrial lair in colorectal cancer landscape. Cancers (Basel).
11:4142019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vasickova K, Horak P and Vanhara P: TUSC3:
functional duality of a cancer gene. Cell Mol Life Sci. 75:849–857.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yue X, Zhang C, Zhao Y, Liu J, Lin AW, Tan
VM, Drake JM, Liu L, Boateng MN, Li J, et al: Gain-of-function
mutant p53 activates small GTPase Rac1 through SUMOylation to
promote tumor progression. Genes Dev. 31:1641–1654. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pang X, Zhou Z, Yu Z, Han L, Lin Z, Ao X,
Liu C, He Y, Ponnusamy M, Li P and Wang J: Foxo3a-dependent miR-633
regulates chemotherapeutic sensitivity in gastric cancer by
targeting fas-associated death domain. RNA Biol. 16:233–248. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ge YF, Sun J, Jin CJ, Cao BQ, Jiang ZF and
Shao JF: AntagomiR-27a targets FOXO3a in glioblastoma and
suppresses U87 cell growth in vitro and in vivo. Asian Pac J Cancer
Prev. 14:963–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu H, Song Y, Qiu H, Liu Y, Luo K, Yi Y,
Jiang G, Lu M, Zhang Z, Yin J, et al: Downregulation of FOXO3a by
DNMT1 promotes breast cancer stem cell properties and
tumorigenesis. Cell Death Differ. 27:966–983. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin L, Zhang J, Fu HQ, Zhang X and Pan YL:
FOXO3a inhibits the EMT and metastasis of breast cancer by
regulating TWIST-1 mediated miR-10b/CADM2 axis. Transl Oncol.
14:1010962021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang X, Chen Z, Shi W, Zhang R, Li L, Liu
H and Wu L: TMF inhibits miR-29a/Wnt/β-catenin signaling through
upregulating Foxo3a activity in osteoarthritis chondrocytes. Drug
Des Devel Ther. 13:2009–2019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun T, Zhang J, Deng B, Fan X, Long T, Jin
H, Tao S, Kang P and Tan Q: FOXO1 and FOXO3a sensitize
non-small-cell lung cancer cells to cisplatin-induced apoptosis
independent of Bim. Acta Biochim Biophys Sin. 52:1348–1359. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu Z, Zhang H, Lang F, Liu G, Gao S, Li B
and Liu Y: Pin1 promotes prostate cancer cell proliferation and
migration through activation of Wnt/beta-catenin signaling. Clin
Transl Oncol. 18:792–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kotrbova A, Ovesna P, Gybel T,
Radaszkiewicz T, Bednaříková M, Hausnerová J, Jandáková E, Minář L,
Crha I, Weinberger V, et al: WNT signaling inducing activity in
ascites predicts poor outcome in ovarian cancer. Theranostics.
10:537–552. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang Y, Liang SH, Xiang LB, Han XT, Zhang
W, Tang J, Wu XH and Zhan MQ: miR-218 promoted the apoptosis of
human ovarian carcinoma cells via suppression of the
WNT/beta-catenin signaling pathway. Mol Biol (Mosk). 51:629–636.
2017.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Han XF, Zhang XX, Liu KM and Zhang Q:
Apelin-13 deficiency alters cortical bone geometry, organic bone
matrix, and inhibits Wnt/β-catenin signaling. Gen Comp Endocrinol.
267:29–35. 2018. View Article : Google Scholar : PubMed/NCBI
|