Introduction
Heat stroke is a life threatening illness
characterized by extreme elevations in core temperature reaching
>40.5°C and damage to multiple organ systems (1). Previous studies have suggested that
inflammatory injury and severe hypoxemia induced by heat stroke
lead to acute lung injury and respiratory failure (2,3).
Clinical and experimental evidence suggested that endothelial cell
injury served a vital role in the early acute phase of heat stroke
(4). Heat stress (HS) refers to
conditions involving increased core body temperature above the
normal level due to thermoregulatory failure, which leads to heat
stroke (5). However, the molecular
mechanisms underlying the endothelial cell injury in heat
stroke-induced acute lung injury remain to be elucidated. Thus,
further investigation into the molecular mechanisms underlying
tissue injury caused by direct HS are required to develop clinical
strategies for heat stroke.
HS induces endothelial cell injury through oxidative
stress, which stimulates reactive oxygen species (ROS) generation,
lipid peroxidation and DNA damage (6). ROS are by-products of oxygen
metabolism, including superoxide anions, hydrogen peroxide and
hydroxyl radicals (7). ROS target
proteins, polysaccharides, DNA and lipids and increase the rate of
cell damage (8). Furthermore,
results of our previous study demonstrated that NF-κB activation
decreased HS-induced oxidative stress in HUVECs (9). However, the potential mechanisms
underlying NF-κB in HS-induced oxidative stress remain to be
elucidated.
The results of previous studies have demonstrated
that NF-κB serves a critical role in regulating cell proliferation,
inflammatory responses, survival and apoptosis (10,11).
When bound, NF-κB and IκB remain inactive in the cytoplasm
(12). A main component of IκB is
IκBα, the first protein described for the IκB family and the most
extensively studied IκB protein to date (13,14).
NF-κB-activating stimuli activate IκB kinase, which induces the
phosphorylation at Ser32 and Ser36 on IκBα (15). NF-κB is subsequently activated and
translocated into the nucleus to control transcription (16). As a dominant regulatory kinase in
the classic NF-κB signaling pathway, IκBα serves a prominent role
in apoptosis (17). In addition,
the IκBα/NF-κB signaling pathway is one of the most popular
antitumor targets (18). The
results of the present study revealed a novel mechanism for
HS-induced NF-κB activation in rat pulmonary microvascular
endothelial cells (PMVECs) without IκBα degradation, which differs
from the classic NF-κB signaling pathway. However, the precise
molecular mechanism underlying the generation of ROS by NF-κB
without IκBα degradation and phosphorylation remains to be
elucidated.
The present study aimed to determine whether the
NF-κB/IκBα pathway inhibited HS-induced ROS generation and cell
death in PMVECs. The association between HS-induced NF-κB
activation and the phosphorylation of upstream IκBα was also
investigated.
Materials and methods
Animals
Male Sprague Dawley (SD) rats (n=50; body weight,
125–155 g; age, 7 weeks) were purchased from the Experimental
Animal Center of Southern Medical University. Rats were housed at a
constant temperature of 22–25°C and 50–60% relative humidity under
a controlled 16-h light/8-h dark cycle for at ≥5 days; they had
free access to rat chow and water. Experimental protocols involving
animals followed the guidelines approved by the Chinese Association
of Laboratory Animal Care and approved by the Institutional Animal
Care and Use Committee of Nanfang Hospital (approval no.
NFYY-2019-176; Guangzhou, China).
Cell culture and treatment
Primary PMVECs were isolated from SD rats as
previously described (19).
Briefly, after anesthesia with pentobarbital sodium (50 mg/kg,
i.p.), the distal lung tissues (diameter, 3–5 mm) were resected
from rat lungs, cut into small pieces and digested with type I
collagenase (2 mg/ml) and neutral protease (0.6 U/ml) for 1 h. The
mixture was centrifuged at 800 × g for 10 min at 37°C.
Subsequently, cells were resuspended and plated with Dynabeads
(Invitrogen; Thermo Fisher Scientific, Inc.) and rat anti-mouse
platelet endothelial adhesion molecule-1 antibody (Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h. The cell-bead mixture was
separated using a magnetic particle concentrator (Invitrogen;
Thermo Fisher Scientific, Inc.) and incubated in EGM-2 culture
media containing 10% FBS with growth factor bullet kit (Lonza
Group, Ltd.). For HS treatment, the bottom of each culture dish was
placed into a circulating water bath maintained at 43±0.5°C for 2 h
and PMVECs were further incubated at 37°C for periods as
indicated.
Cell Counting Kit-8 (CCK-8) assay
Cells were seeded in a 96-well plate at a density of
1×105/ml and incubated at 37°C overnight. Subsequently,
cells were cultured at 43°C for 2 h or treated with p65 small
interfering (si)RNA or IκBα mutant construct, mixed with 100 µl
CCK-8 solution (Dojindo Molecular Technologies, Inc.) and incubated
for 1 h. Cell viability was assessed following the manufacturer's
protocol.
Apoptosis assay
Cell apoptosis was detected using an Annexin V-FITC
apoptosis detection kit and flow cytometry according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc). A total of 1×106 cells were collected and washed
three times in PBS. Subsequently, cells were incubated in binding
buffer containing Annexin V-FITC for at 4°C 15 min, the buffer was
removed by centrifugation at 1,000 × g for 5 min at 4°C and the
cells were resuspended in buffer containing PI solution. After 5
min, apoptotic cells were detected using a FACSCalibur cytometer
(Becton, Dickinson and Company). The data were analyzed using
Flowing version 2.5.3 software (Turku Bioscience) and Origin
version 7 software (OriginLab). The results of a previous study
have suggested that Annexin V-positive cells in the right quadrant
are apoptotic, including the early and late apoptotic cells
(20).
Florescence confocal assay of
intracellular ROS
Cells were treated with 2′-7′-dichlorofluorescin
diacetate (Beyotime Institute of Biotechnology) at 37°C for 20 min
in the dark as previously described (21). For live imaging, DNA was stained
with 0.2 mg/ml Hoechst 33342 (Thermo Fisher Scientific, Inc.) at
37°C for 10 min and PMVECs were washed with DMEM Fluoro Brite
(Gibco; Thermo Fisher Scientific, Inc.). Cells were analyzed using
a confocal fluorescent microscope (magnification, ×400; LI-COR
Biosciences, Inc.).
Western blot analysis
Cells were exposed to HS treatment and a nuclear
extraction kit (Active Motif, Inc.) was used to obtain cytoplasmic
and nuclear protein extracts, according to the manufacturer's
protocol. Total protein was quantified using an Enhanced BCA
Protein Assay kit (Beyotime Institute of Biotechnology). Western
blotting was performed as previously described (22), the proteins (40 µg per lane) were
separated via 12% SDS-PAGE and further transferred onto PVDF
membranes. Then, the membranes were blocked with 5% skimmed milk
for 1 h at room temperature, and cultured with the following
primary antibodies: p65 (cat. no. ab16502; 1:1,000; Abcam), IκBα
(cat. no. 4812; 1:1,000; Cell Signaling Technology, Inc.),
phosphorylated (p)-IκBα (cat. no. 2859; 1:1,000; Cell Signaling
Technology, Inc.), p-p65 (cat. no. 3303; 1:1,000; Cell Signaling
Technology, Inc.), Lamin B (cat. no. 13435; 1:1,000; Cell Signaling
Technology, Inc.) and GAPDH (cat. no. 5174; 1:1,000; Cell Signaling
Technology, Inc.) overnight at 4°C. Following primary incubation,
membranes were incubated with HRP-conjugated anti-rabbit IgG
antibody (cat. no. ZB-2301; 1:5,000; OriGene Technologies, Inc.) at
room temperature for 1 h. Protein bands were visualized using ECL
reagents (Pierce; Thermo Fisher Scientific, Inc.) with GAPDH and
Lamin B as the internal control. ImageJ (version 6.0; National
Institutes of Health) was used for the semi-quantification of
protein expression.
NF-κB dual-luciferase assay
The luciferase activity was determined using the
Dual-Glo luciferase assay system (cat. no. E2980; Promega
Corporation) following the manufacturer's protocol. Briefly,
plasmids were purified from bacterial cultures using the QIAGENP
plasmid Midi kit (Qiagen GmbH). Rat PMVECs were transfected with
the vector constructs using Lipofectamine® 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and the pGL3-Basic plasmid without promoter as the negative
control. The pGl4.74 vector (Promega Corporation) was used as an
internal control to determine the efficiency of transfection. After
24 h of transfection, cells were washed with PBS and resuspended in
Passive Lysis Buffer (Promega Corporation). Cells were treated with
HS at 43°C for 2 h, followed by a recovery period at 37°C. Activity
was measured using a Centro LB 960 Luminometer
(Titertek-Berthold).
Transfection of plasmids
The pcDNA3.1-IκBα-mutant (M) and
pcDNA3.1-IκBα-wild-type (wt) were synthesized by Cyagen
Biosciences, Inc. Cells were transfected with the corresponding
vectors according to the Lipofectamine® 2000 reagent
procedure (Thermo Fisher Scientific, Inc.) and transfected cells
were incubated for 48–72 h at 37°C before further experiments.
Transfection of siRNA
siRNA targeting p65 was designed and synthesized by
Shanghai GenePharma Co., Ltd. The siRNA sequences for each gene and
their corresponding negative controls are demonstrated in Table I. PMVECs were seeded into 6-well
plates (Wuxi NEST Biotechnology, Co., Ltd.) at 30–50% confluence
for transfection. After 24 h, PMVECs were incubated with TurboFect
siRNA Transfection reagent (Shanghai GenePharma Co., Ltd.) and 10
nM siRNAs for 6 h at 37°C according to the manufacturer's protocol.
Transfected cells were incubated for 24 h at 37°C before further
experiments.
 | Table I.Sequences of oligonucleotide
primers. |
Table I.
Sequences of oligonucleotide
primers.
| Gene | Direction | Sequence |
|---|
| p65 | Forward |
5′-GCCCUAUCCCUUUACGUCATT-3′ |
|
| Reverse |
5′-UGACGUAAAGGGAUAGGGCTT-3′ |
| Negative
control | Forward |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
| Reverse |
5′-ACGUGACACGUUCGGAGAATT-3′ |
Co-immunoprecipitation (Co-IP)
Cells were subjected to HS treatment at 43°C for 2
h, followed by an additional incubation period at 37°C, or exposed
to 1 µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA) for
the times indicated. Co-IP assays were subsequently performed using
the Co-IP kit (Pierce; Thermo Fisher Scientific, Inc.) following
the manufacturer's protocol. Cells were lysed with immunol
precipitation lysis buffer (Beyotime Institute of Biotechnology)
for 30 min on ice, and the buffer was removed via centrifugation at
16,000 × g for 10 min at 4°C. The cell lysates were then
immunoprecipitated at 4°C overnight using anti-IκBα (cat. no. 4812;
1:1,000; Cell Signaling Technology, Inc.) and normal IgG complexes.
Then, 40 µl protein A+G agarose beads were used and spun at 4°C for
3 h. After centrifugation at 250 × g for 5 min at 4°C, the
supernatant was discarded. Finally, 1X loading buffer was added and
boiled for 5 min for western blot analysis. Protein expression
levels were semi-quantified using ImageJ (version 6.0; National
Institutes of Health).
Statistical analysis
All data were analyzed for statistical significance
using SPSS V.23.0 software (IBM Corp.). Data are presented as the
mean ± standard deviation from at least three independent
experiments, each performed in duplicate. The comparisons of
multiple groups were analyzed using the Kruskal-Wallis test
followed by a Dunn's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Influence of NF-κB p65 on HS-induced
ROS accumulation and cytotoxicity in rat PMVECs
As demonstrated in Fig.
1A, nuclear and cytosolic fractions were isolated from rat
PMVECs and PMVECs were exposed to HS for 2 h followed by further
incubation for 0, 6 and 12 h. The results of the present study
indicated a significant amount of NF-κB p65 and IκBα nuclear
translocation in a time-dependent manner. As shown in Fig. S1A, the infection efficiency of
p65siRNA was observed with p65 expression. Knockdown of p65
significantly decreased the viability of PMVECs, as demonstrated by
CCK-8 assays (Fig. 1B).
Furthermore, fluorescence confocal assays revealed that siRNA
knockdown of NF-κB p65 significantly aggravated ROS accumulation in
HS-induced rat PMVECs compared with the control (Fig. 1C). Collectively, the results of the
present study demonstrated that NF-κB p65 served a key role in
HS-induced cytotoxicity and ROS production.
 | Figure 1.Influence of NF-κB on HS-induced ROS
accumulation and cytotoxicity in rat PMVECs. (A) Cells were
incubated at 37°C (CONT) or were subjected to HS treatment at 43°C
for 2 h, followed by a recovery period at 37°C for 0 (R0h), 2
(R2h), 6 (R6h) or 12 h (R12h). Expression levels of p65 and IκBα
were detected in cytoplasmic and nuclear fractions of PMVECs using
western blotting. (B) PMVECs were subjected to HS treatment at 43°C
for 2 h and incubated at 37°C for 12 h before cell proliferation
was determined using a Cell Counting Kit-8 assay. (C)
Representative images of intracellular ROS visualized using
fluorescence microscopy. Sections were co-stained with ROS (green)
and Hoechst (blue). Scale bar, 20 µm. Each value represents the
mean ± SD of three independent experiments. *P<0.05,
**P<0.01, and &P<0.05 vs. the control group.
HS, heat stress; ROS, reactive oxygen species; PMVEC, pulmonary
microvascular endothelial cells; CONT, control; Nu, nuclear
fraction; Cyto, cytoplasmic fraction; si, small interfering. |
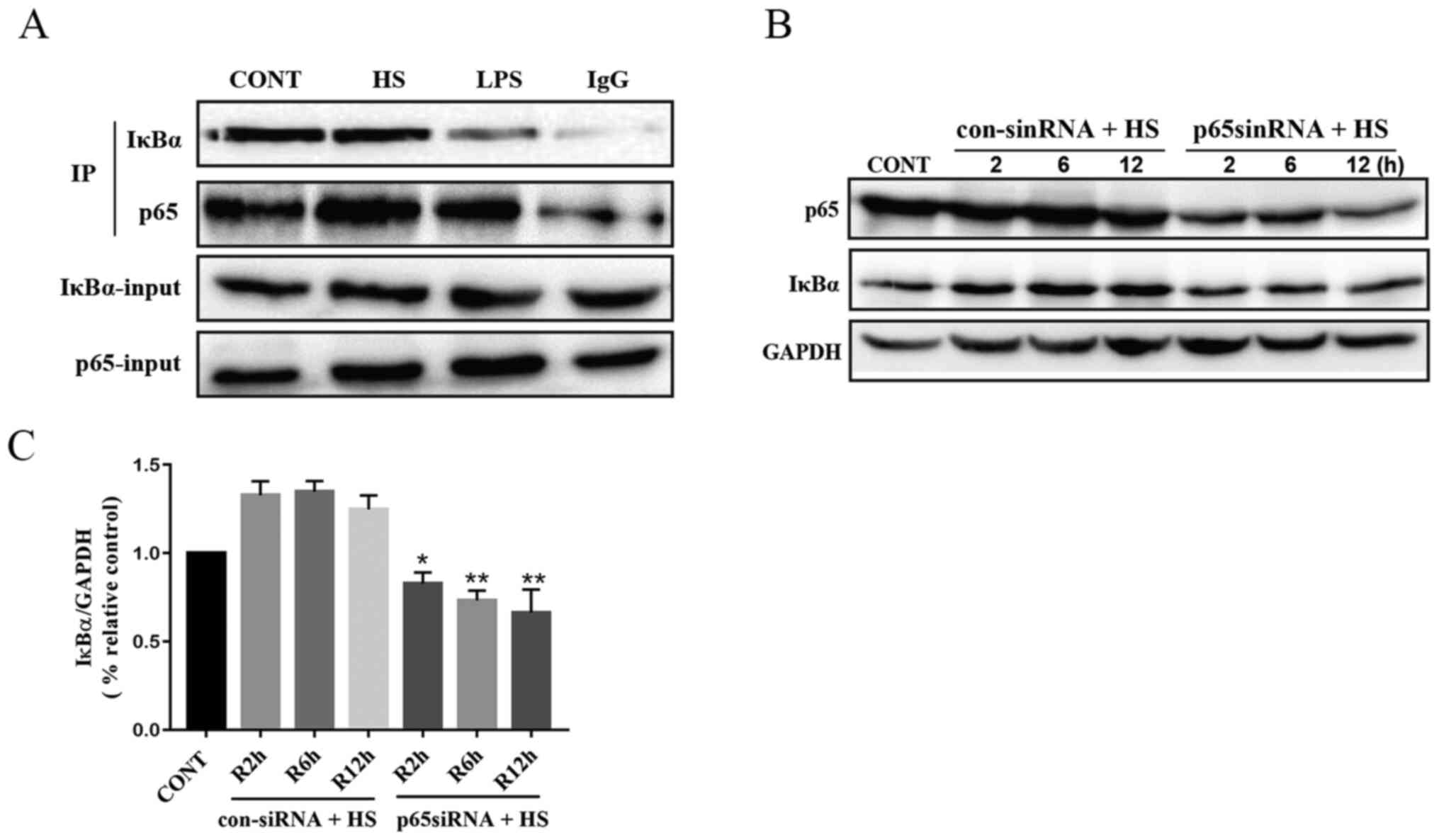
NF-κB mediates the HS-induced
expression of IκBα during the recovery period following HS
IκB is an inhibitor of NF-κB, which interacts with
the nuclear localization sequences of the Rel homology domain. IκBα
was the first protein described in the IκB protein family (23). To investigate the interaction
between NF-κB p65 and IκBα, Co-IP experiments of NF-κB p65 and IκBα
were performed. As demonstrated in Fig.
2A, Co-IP assays revealed that NF-κB p65 co-immunoprecipitated
with IκBα following HS treatment. Furthermore, treatment with LPS
lead to a decrease in the co-immunoprecipitation of IκBα and NF-κB
p65. The results of the present study demonstrated that IκBα had
the ability to bind to NF-κB and IκBα was not degraded or
dissociated from the NF-κB complex during the recovery period
following HS. To further investigate the direct interaction between
NF-κB p65 and IκBα in HS-induced PMVECs, western blot analysis was
used. Western blotting revealed that protein expression of IκBα was
significantly reduced following knockdown of NF-κB p65 during the
recovery period following HS (Fig. 2B
and C). Collectively, the findings of the present study
suggested that upstream NF-κB p65 mediated the HS-induced
expression of IκBα in HS-induced rat PMVECs.
HS mediates NF-κB activity and nucleus
translocation-independent IκBα phosphorylation
The results of our previous study confirmed that HS
induced the phosphorylation and subsequent activation of IκBα on
Ser32 and Ser36 in rat PMVECs (9).
To determine whether phosphorylation of Ser32 and Ser36 was
responsible for NF-κB activity and nucleus translocation induced by
HS, IκBα mutants with substitutions of Ser/Thr with Ala residues at
Ser32 and Ser36 were used (pcDNA3.1-IκBα-M; Fig. 3A). The infection efficiency of
pcDNA3.1-IκBα-M was observed with levels of phosphorylated IκBα
(Fig. S1B). A significant decrease
in the phosphorylation of IκBα was observed in the pcDNA3.1-IκBα-M
group compared with the pcDNA3.1-IκBα-wt and control groups
(Fig. 3B). By contrast, no
significant changes in the phosphorylation and nucleus
translocation of p65 were observed following transfection with
pcDNA3.1-IκBα-M (Fig. 3C).
Furthermore, measurement of NF-κB activity indicated that
pcDNA3.1-IκBα-M failed to attenuate activation of NF-κB in a
time-dependent manner following HS (Fig. 3D). Thus, the results of the present
study revealed that HS-mediated NF-κB activity and nucleus
translocation are independent of IκBα phosphorylation.
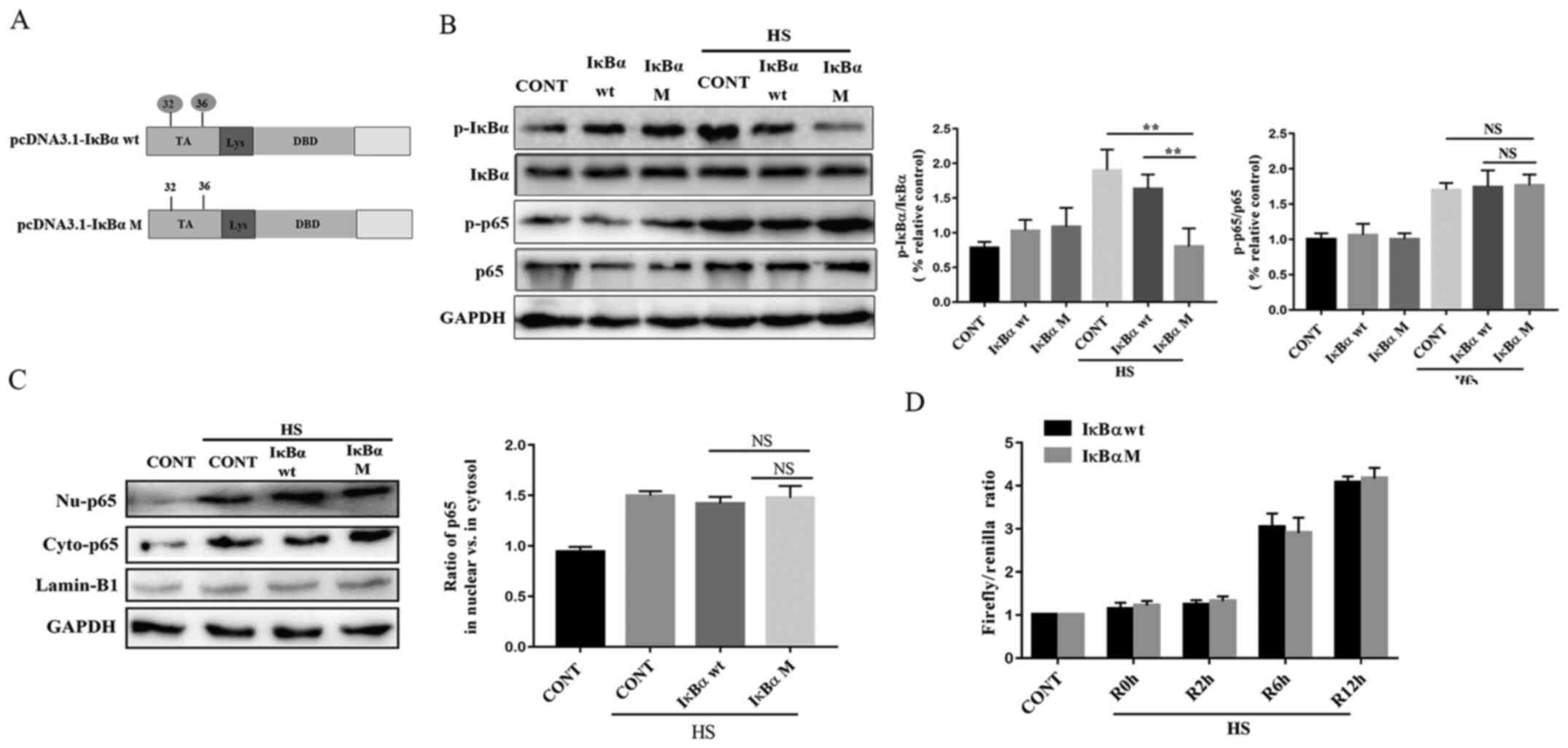 | Figure 3.HS mediated NF-κB activity and
nucleus translocation-independent IκBα phosphorylation. PMVECs were
transfected with the pcDNA3.1-IκBα wt (IκBα wt) or pcDNA3.1-IκBα-M
(IκBα M) constructs. After 48 h, cells were exposed to HS at 43°C
for 2 h and were further incubated at 37°C for 12 h. (A) Schematic
of IκBα consensus sites (p-Ser/Ub-Lys). pcDNA3.1-IκBα-M had Ala
substitutions at two phosphorylation sites, Ser32 and Ser36. (B)
Western blot analysis of the phosphorylation of IκBα and p65. (C)
pcDNA3.1-IκBα-M regulated phosphorylation levels of NF-κB p65 in
both the cytoplasm and nuclei. (D) Cells were incubated at 37°C
(CONT) or were subjected to HS treatment at 43°C for 2 h, followed
by a recovery period at 37°C for 0 (R0h), 2 (R2h), 6 (R6h) or 12 h
(R12h). Cells were analyzed for NF-κB activation using
dual-luciferase assays. Each value represents the mean ± SD from
three independent experiments. **P<0.01 vs. the control group.
HS, heat stress; PMVEC, pulmonary microvascular endothelial cells;
wt, wild-type; M, mutant; NS, not significant; TA, transactivation
domain; Lys, lysine enrichment domain; DBD, DNA binding domain; wt,
wild-type; M, mutant; Ser, serine; Ala, alanine; CONT, control; p,
phosphorylated; Nu, nuclear fraction; Cyto, cytoplasmic
fraction. |
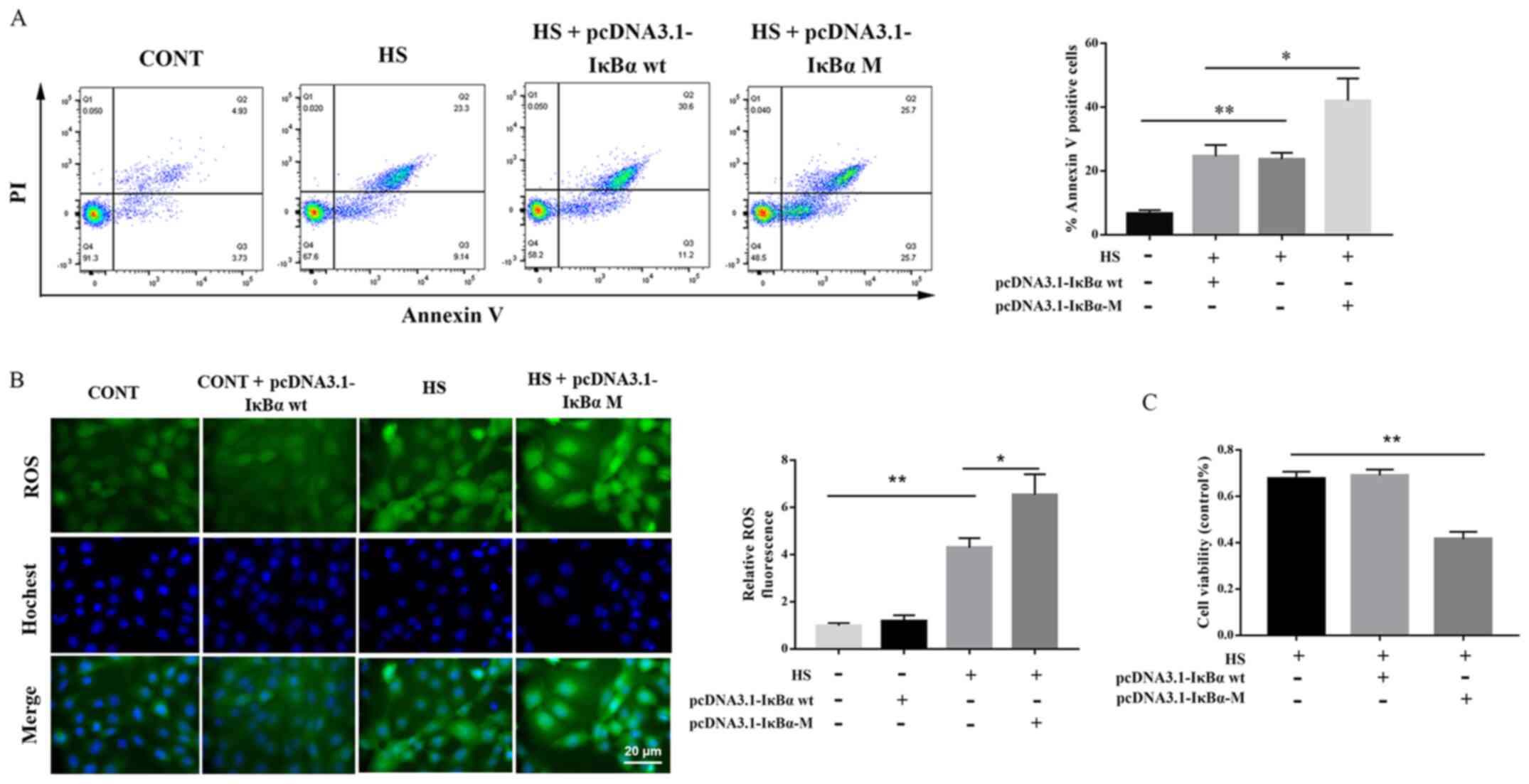
Roles of IκBα phosphorylation in
HS-induced apoptosis and ROS accumulation
The role of IκBα phosphorylation on Ser32 and Ser36
in ROS accumulation and cell apoptosis induced by HS was
investigated, following transfection of PMVECs with
pcDNA3.1-IκBα-M. Compared with the control group, pcDNA3.1-IκBα-M
significantly increased the level of apoptosis and significantly
decreased the proliferation of PMVECs, as demonstrated by flow
cytometry and CCK-8 assays, respectively (Fig. 4A and C). Additionally, the results
of the fluorescence confocal assay demonstrated that
pcDNA3.1-IκBα-M significantly aggravated ROS generation in
HS-induced rat PMVECs (Fig. 4B).
Collectively, these findings revealed that IκBα may serve an
essential role in HS-induced rat PMVEC apoptosis and ROS
generation.
Discussion
The incidence of heat stroke is increasing with the
proliferation in heatwaves caused by global warming (24). The results of a previous study
suggested that systemic and cellular responses to heat stroke
include heat cytotoxicity, coagulopathies and systemic inflammatory
response syndrome (25). Notably,
the primary injury of vascular endothelium induced by HS has been
considered as a key factor for multi-organ system dysfunction,
which acts as an early target of thermal injury (26). Thus, further investigation into the
promotion of HS-induced vascular endothelium damage is
required.
The results of a previous study suggest that
HS-induced ROS accumulation mediates oxidative damage and
disturbance of mitochondrial homeostasis and cellular function
(27). Gu et al (28) reveal that HS induces the
accumulation of intracellular ROS, which ultimately acts as an
upstream signal to promote the mitochondrial apoptotic pathways in
HUVECs. Yu et al (29) also
demonstrate that HS increased ROS and malondialdehyde generation
and significantly attenuates anti-oxidase activity resulting in
intestinal damage. The results of previous studies demonstrate that
the NF-κB/IκBα pathway exhibits a potent inhibitory potential
against inflammatory and oxidative stress (9,15). The
present study used PMVECs induced by heat stress to demonstrate the
role of NF-κB/IκBα pathway. The findings uncovered an alternative
NF-κB/IκBα pathway involving oxidative stress in HS-induced
vascular endothelial cell damage.
NF-κB is ubiquitously expressed and regulates the
expression of a number of genes involved in immune, inflammatory
and apoptotic processes both in vitro and in vivo
(30). The results of our previous
study demonstrate that the NF-κB signaling pathway is essential for
resistance to HS-induced apoptosis in HUVECs (9). NF-κB is mainly sequestered in the
cytoplasm and associated with the IκB inhibitor family (31). When NF-κB-activating stimuli
activate IκB kinase, p-IκB proteins are subsequently
poly-ubiquitinated and degraded by the 26S proteasome, which in
turn activates NF-κB to relocate to the nucleus for transcription
(32). We previously revealed that
no signs of IκBα degradation are observed in HS-induced HUVECs
compared with the control. By contrast, levels of phosphorylated
IκBα increase during the recovery period following heat stress
(9). However, the results of the
present study demonstrated that both IκBα and NF-κB translocated
from the cytoplasm into the nucleus in HS-induced PMVECs.
Furthermore, the degradation of IκBα and subsequent dissociation
from NF-κB did not occur and transfection of PMVECs with
pcDNA3.1-IκBα-M failed to inhibit the migration of NF-κB into the
nucleus. NF-κB remained transcriptionally active. Thus, the results
of the present study suggested that upstream NF-κB mediated the
expression of IκBα in HS-induced rat PMVECs.
Mechanistically, a number of potential mechanisms,
such as the role of NF-κB in the expression of target genes that
mediate cell proliferation and release antimicrobial molecules and
cytokines, may explain this phenomenon (33). All IκB proteins, including IκBα,
IκBβ, IκBε, p105/IκBγ and p100/IκBδ, are reported as target genes
of NF-κB (34). Previous studies
have demonstrated that IκBα is a transcriptional target for NF-κB,
creating a negative feedback loop (16,35).
Thus, it was hypothesized that IκBα was not degraded, as NF-κB
regulated the synthesis of new IκBα, which entered the nucleus and
inhibited the transcriptional function of NF-κB. However, the
potential mechanisms underlying NF-κB activation following HS have
yet to be elucidated. Thus, it was hypothesized that heat
cytotoxicity or alternate mechanisms may contribute to NF-κB
activation.
However, there are limitations of the present study.
The intracellular nature of NF-κB may require upstream activation
via cytokines and pathogen-associated molecular patterns. By
contrast, the results of the present study revealed that HS induced
activation of NF-κB independent of the phosphorylation of upstream
IκBα. Further investigation into the specific molecular mechanisms
underlying the activation of NF-κB without the degradation of IκBα
is required. Furthermore, prevention of oxidative stress in
patients with heat stroke is a crucial therapeutic intervention.
Further experiments are required to uncover the mechanisms
underlying oxidative stress and potential therapeutic targets. The
results of the present study were also limited by the use of PMVECs
in vitro and further mechanisms underlying endothelial cell
injury in vivo remain to be elucidated.
Collectively, the results of the present study
suggested that the NF-κB/IκBα pathway was essential for resistance
to HS-induced ROS production and cytotoxicity in rat PMVECs.
Further investigation will demonstrate the molecular mechanisms
underlying the functional role of NF-κB/IκBα proteins in HS.
Moreover, the current study suggested roles for NF-κB/IκBα as a
potential target to modulate treatment of heat stroke.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 82172181) and the Southern
Medical University Southern Hospital Dean's Fund (grant no.
2016C016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX, WH, YL and SC performed the study and composed
this manuscript. WX, WF and YL were responsible for primary data
generation and analysis. HC and ZC participated in cell culture and
transfection. WH, WX and YL performed the western blot analysis. YL
was the principal investigators and corresponding authors for these
studies. WX, WH, YL and SC are responsible for confirming the
authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Experimental protocols involving animals followed
the guidelines approved by the Chinese Association of Laboratory
Animal Care and approved by the Institutional Animal Care and Use
Committee of Nanfang Hospital (approval no. NFYY-2019-176;
Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bouchama A and Knochel JP: Heat stroke. N
Engl J Med. 346:1978–1988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin C-H, Tsai C-C, Chen T-H, Chang C-P and
Yang H-H: Oxytocin maintains lung histological and functional
integrity to confer protection in heat stroke. Sci Rep.
9:183902019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Li C, Liu N, Wang M, Zhou X, Kim
IH and Wu Z: Ursolic acid alleviates heat stress-induced lung
injury by regulating endoplasmic reticulum stress signaling in
mice. J Nutr Biochem. 89:1085572021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li L, Tan H, Gu Z, Liu Z, Geng Y, Liu Y,
Tong H, Tang Y, Qiu J and Su L: Heat stress induces apoptosis
through a Ca2+-mediated mitochondrial apoptotic pathway
in human umbilical vein endothelial cells. PLoS One. 9:e1110832014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodriguez-Fernandez M, Grosman B,
Yuraszeck TM, Helwig BG, Leon LR and Doyle FJ III: Modeling the
intra- and extracellular cytokine signaling pathway under heat
stroke in the liver. PLoS One. 8:e733932013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Li Y, Xing D and Gao C:
Characterization of mitochondrial dynamics and subcellular
localization of ROS reveal that HsfA2 alleviates oxidative damage
caused by heat stress in Arabidopsis. J Exp Bot. 60:2073–2091.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Das M, Solanki A, Ganesh A and Thakore S:
Emerging hybrid biomaterials for oxidative stress induced
photodynamic therapy. Photodiagnosis Photodyn Ther. 34:1022592021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma A, Tewari D, Nabavi SF, Nabavi SM
and Habtemariam S: Reactive oxygen species modulators in pulmonary
medicine. Curr Opin Pharmacol. 57:157–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Zhou G, Wang Z, Guo X, Xu Q, Huang
Q and Su L: NF-κB signaling is essential for resistance to heat
stress-induced early stage apoptosis in human umbilical vein
endothelial cells. Sci Rep. 5:135472015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kostyuk SV, Porokhovnik LN, Ershova ES,
Malinovskaya EM, Konkova MS, Kameneva LV, Dolgikh OA, Veiko VP,
Pisarev VM, Martynov AV, et al: Changes of KEAP1/NRF2 and IKB/NF-κB
Expression Levels Induced by Cell-Free DNA in Different Cell Types.
Oxid Med Cell Longev. 2018:10524132018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lalle G, Twardowski J and Grinberg-Bleyer
Y: NF-κB in Cancer Immunity: Friend or Foe? Cells. 10:3552021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao H, Wang Y, Liu Y, Yin K, Wang D, Li
B, Yu H and Xing M: ROS-Induced Hepatotoxicity under Cypermethrin:
Involvement of the Crosstalk between Nrf2/Keap1 and NF-κB/iκB-α
Pathways Regulated by Proteasome. Environ Sci Technol.
55:6171–6183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen H, Ji Y, Xiong Y, Kim H, Zhong X, Jin
MG, Shah YM, Omary MB, Liu Y, Qi L, et al: Medullary thymic
epithelial NF-κB-inducing kinase (NIK)/IKKα pathway shapes
autoimmunity and liver and lung homeostasis in mice. Proc Natl Acad
Sci USA. 116:19090–19097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen J, Cheng J, Zhu S, Zhao J, Ye Q, Xu
Y, Dong H and Zheng X: Regulating effect of baicalin on
IKK/IKB/NF-κB signaling pathway and apoptosis-related proteins in
rats with ulcerative colitis. Int Immunopharmacol. 73:193–200.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang W-J, Yang H-W, Liu H-N, Qian W and
Chen X-L: HMGB1 upregulates NF-κB by inhibiting IKB-α and
associates with diabetic retinopathy. Life Sci. 241:1171462020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nelson DE, Ihekwaba AE, Elliott M, Johnson
JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG,
et al: Oscillations in NF-kappaB signaling control the dynamics of
gene expression. Science. 306:704–708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amaro-Leal Â, Shvachiy L, Pinto R,
Geraldes V, Rocha I and Mota-Filipe H: Therapeutic effects of IkB
kinase inhibitor during systemic inflammation. Int Immunopharmacol.
84:1065092020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fordjour FA, Asiedu E, Larbi A and
Kwarteng A: The role of nuclear factor kappa B (NF-κB) in filarial
pathology. J Cell Commun Signal. 15:185–193. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang W, Xie W, Gong J, Wang W, Cai S,
Huang Q, Chen Z and Liu Y: Heat stress induces RIP1/RIP3-dependent
necroptosis through the MAPK, NF-κB, and c-Jun signaling pathways
in pulmonary vascular endothelial cells. Biochem Biophys Res
Commun. 528:206–212. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Ji J, Liu Q and Xu S: MUC1
downregulation promotes TNF-α-induced necroptosis in human
bronchial epithelial cells via regulation of the RIPK1/RIPK3
pathway. J Cell Physiol. 234:15080–15088. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Tan H, Zou Z, Gong J, Zhou J, Peng
N, Su L, Maegele M, Cai D and Gu Z: Preventing necroptosis by
scavenging ROS production alleviates heat stress-induced intestinal
injury. Int J Hyperthermia. 37:517–530. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Z, Wu SQ, Liang Y, Zhou X, Chen W,
Li L, Wu J, Zhuang Q, Chen C, Li J, et al: RIP1/RIP3 binding to
HSV-1 ICP6 initiates necroptosis to restrict virus propagation in
mice. Cell Host Microbe. 17:229–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng D, Li J, Deng Y, Zhu X, Zhao L, Zhang
Y, Li Z, Ou S, Li S and Jiang Y: Sodium para-aminosalicylic acid
inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis
by inhibiting NF-κB pathway activation and oxidative stress. J
Neuroinflammation. 17:3432020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Epstein Y and Yanovich R: Heatstroke. N
Engl J Med. 380:2449–2459. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dokladny K, Myers OB and Moseley PL: Heat
shock response and autophagy - cooperation and control. Autophagy.
11:200–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Su Z, Zou Z, Tan H, Cai D, Su L and
Gu Z: Ser46 phosphorylation of p53 is an essential event in
prolyl-isomerase Pin1-mediated p53-independent apoptosis in
response to heat stress. Cell Death Dis. 10:962019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu ZF, Zheng D, Fan GC, Peng T and Su L:
Heat stress prevents lipopolysaccharide-induced apoptosis in
pulmonary microvascular endothelial cells by blocking calpain/p38
MAPK signalling. Apoptosis. 21:896–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu ZT, Wang H, Li L, Liu YS, Deng XB, Huo
SF, Yuan FF, Liu ZF, Tong HS and Su L: Heat stress induces
apoptosis through transcription-independent p53-mediated
mitochondrial pathways in human umbilical vein endothelial cell.
Sci Rep. 4:44692014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu J, Liu F, Yin P, Zhao H, Luan W, Hou X,
Zhong Y, Jia D, Zan J, Ma W, et al: Involvement of oxidative stress
and mitogen-activated protein kinase signaling pathways in heat
stress-induced injury in the rat small intestine. Stress.
16:99–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hop HT, Arayan LT, Reyes AWB, Huy TXN, Min
WG, Lee HJ, Rhee MH, Chang HH and Kim S: Heat-stress-modulated
induction of NF-κB leads to brucellacidal pro-inflammatory defense
against Brucella abortus infection in murine macrophages and
in a mouse model. BMC Microbiol. 18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rasmi RR, Sakthivel KM and Guruvayoorappan
C: NF-κB inhibitors in treatment and prevention of lung cancer.
Biomed Pharmacother. 1105692020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hellweg CE: The Nuclear Factor κB pathway:
A link to the immune system in the radiation response. Cancer Lett.
368:275–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alharbi KS, Fuloria NK, Fuloria S, Rahman
SB, Al-Malki WH, Javed Shaikh MA, Thangavelu L, Singh SK, Rama Raju
Allam VS, Jha NK, et al: Nuclear factor-kappa B and its role in
inflammatory lung disease. Chem Biol Interact. 345:1095682021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai J, Guan H, Jiao X, Yang J, Chen X,
Zhang H, Zheng Y, Zhu Y, Liu Q and Zhang Z: NLRP3 inflammasome
mediated pyroptosis is involved in cadmium exposure-induced
neuroinflammation through the IL-1β/IkB-α-NF-κB-NLRP3 feedback loop
in swine. Toxicology. 453:1527202021. View Article : Google Scholar : PubMed/NCBI
|