Introduction
Vitiligo, one of the most common acquired autoimmune
skin disorders, is caused primarily through the selective
destruction of melanocytes (1).
Oxidative stress (OS), defined as an imbalance between
radical-generating and radical-scavenging activity, has been
associated with a variety of diseases, including hypertension,
diabetes, cancer and cardiovascular diseases (2,3). An
increasing number of studies have indicated that the pathogenesis
of vitiligo is associated with OS-mediated toxicity in melanocytes
(4–7). Previous studies have indicated that
the state of OS commonly results in increased intracellular
reactive oxygen species (ROS) production, which influences various
signaling pathways leading to inhibition of melanin synthesis,
destruction of cell structures and reduced survival of melanocytes
(8–11). Therefore, improving oxidative status
may be an effective therapeutic strategy for vitiligo.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a key transcription factor that regulates the protein synthesis
of phase II antioxidants, and is commonly known to play a
contributory role in protecting cells from oxidative damage
(12). A previous study confirmed
that Nrf2 protects cells from damage by scavenging free radicals in
cells (13). Under normal
physiological conditions, Nrf2 binds to kelch-like ECH-associated
protein-1 (Keap1) in the cytoplasm and remains inactivated. Upon
OS, Nrf2 dissociates from Keap1 and Nrf2 is activated (14). The activated Nrf2 translocates into
the nucleus, to induce heme oxygenase-1 (HO-1) expression. As a
member of the intracellular phase II enzyme family, HO-1 is
considered to play an important role in the regulation of redox
balance. Furthermore, HO-1 has been demonstrated to exert
anti-apoptotic and anti-inflammatory effects (15). Thus, activation of the Nrf2/HO-1
signaling pathway is critical for the protection of human
melanocytes. It has previously been revealed that PI3K/AKT is a key
pathway for promoting cell survival and metabolism. Recent results
have demonstrated that PI3K/AKT, as an upstream signaling pathway,
can control the defense system of cells against inflammation and
oxidative damage by mediating the Nrf2/HO-1 signaling pathway
(16).
Panax ginseng (PG) is one of the most ancient
traditional herbs, which has been used as an herbal remedy in Asia
for its tonic and restorative actions for years (17). Studies have revealed that PG has
various pharmacological activities, including antioxidant,
anti-inflammatory, anti-viral, neuroprotective and anticancer
effects (18–21). Ginsenosides are the major
biologically active ingredients of ginseng (the root of PG), which
are divided into protopanaxadiol [ginsenosides Rk1 (RK1), Rg5 and
Rg3] and protopanaxatriol (ginsenoside Rg1) on the basis of their
steroidal structure and sugar moieties (22). A previous study reported that RK1
may protect the liver from OS and apoptosis caused by paracetamol
in rats (23). However, to the best
of our knowledge, the protective effect of RK1 against hydrogen
peroxide (H2O2)-induced oxidative damage of
melanocytes has not yet been reported, either in vitro or
in vivo. Thus, the aim of the present study was to
investigate whether RK1 could protect against
H2O2-induced oxidative damage of human
melanocytes via activation of the PI3K/AKT/Nrf2/HO-1 signaling
pathway.
Materials and methods
Reagents
RK1 (cat. no. 42754) and LY294002 (cat. no. L9908)
were purchased from Sigma-Aldrich (Merck KGaA) and the purity was
≥95 and ≥98%, respectively. Additional reagents used in the present
study were commercially available and of analytical purity.
Cell culture
The PIG1 immortalized human melanocyte cell line was
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences, and cultured in Medium 254 (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(Beijing Solarbio Science & Technology Co., Ltd.) at 37°C in a
humidified atmosphere with 5% CO2.
Cell viability assay
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8; Dojindo, Molecular Technologies, Inc.) assay. The
PIG1 cell line was seeded into 96-well plates at a density of
7×103/well. When 60–70% confluence was reached, the
cells were pretreated with different concentrations of RK1 (0,
0.05, 0.1, 0.2, 0.4, 0.8 or 1.6 mM) for 2 h, and then treated with
1.0 mM H2O2 for 24 h at 37°C with 5%
CO2. Subsequently, 10 µl CCK-8 solution was added to
each well and cultured for 90 min. The absorbance was measured
using a microplate reader (Molecular Devices, LLC) at 450 nm. Cell
viability was calculated according to the following formula:
Viability rate (%) = (absorbance of test sample-absorbance of
blank)/(absorbance of control-absorbance of blank) ×100. In
addition, following treatment with RK1 (0.1,0.2 and 0.4 mM) for 2
h, and treatment with 1.0 mM H2O2 for 24 h at
37°C with 5% CO2, cell morphology was observed using an
inverted light microscope (Olympus Corporation).
Determination of apoptosis
Cell apoptosis was detected using an Annexin
V-fluorescein isothiocyanate (FITC)-PI apoptosis detection kit (BD
Biosciences). Briefly, PIG1 cells were seeded into 6-well plates at
a density of 3×105/well and cultured until they had
reached 60–70% confluence. The cells were exposed to 1 mM
H2O2 for 24 h after pretreatment with
different concentrations of RK1 (0, 0.1, 0.2 or 0.4 mM) or LY294002
(10 µM) for 2 h at 37°C with 5% CO2. Then, the collected
PIG1 cells were resuspended in 100 µl binding buffer
(1×105 cells), stained with 5 µl Annexin V-FITC and 5 µl
PI, and incubated at room temperature (20–25°C) for 15 min in the
dark. The cell apoptosis rate (early + late apoptosis) was detected
using a FACSCalibur flow cytometer and CellQuest software (version
3.3; BD Biosciences) within 1 h.
Detection of superoxide dismutase
(SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) activity
levels
The cells from each group were disrupted using an
ultrasonic cell disruptor (3 times; each for 3 sec with an interval
of 1 sec on ice) and the lysate was centrifuged at 4°C at 16,000 x
g for 5 min to obtain the total protein. The activity levels of
SOD, CAT and GSH-Px were determined using a SOD (cat. no.
A001-3-2), CAT (cat. no. A007-1-1), GSH-Px (cat. no. A005-1-2)
analysis kit (Nanjing Jiancheng Bioengineering Institute) according
to the manufacturer's instructions.
Western blot analysis
Total protein from PIG1 cells in each group was
extracted using RIPA lysis buffer. Nuclear and plasma proteins were
extracted using the Nuclear Protein Extraction Kit (cat. no. R0050;
Beijing Solarbio Science & Technology Co., Ltd.). Protein
concentrations were determined using a BCA kit (cat. no. AR0146;
Wuhan Boster Biological Technology, Ltd). Protein samples (20 µg)
were separated via SDS-PAGE on 10% gels, and then transferred onto
PVDF membranes. The membranes were blocked with 5% skimmed milk in
TBS-Tween-20 (0.05%) for 1 h at room temperature, followed by
incubation at 4°C overnight with primary antibodies against Bax
(cat. no. ab53154; 1:500), caspase-3 (cat. no. ab4051; 1:500),
Bcl-2 (cat. no. ab196495; 1:500), phosphorylated (p)-AKT (cat. no.
ab38449; 1:500), AKT (cat. no. ab18785; 1:500), Nrf2 (cat. no.
ab137550; 1:500), HO-1 (cat. no. ab13243; 1:2,000), lamin A/C (cat.
no. ab227176; 1:1,000), tubulin (cat. no. ab6130; 1:2,000) and
β-actin (cat. no. ab8227; 1:1,000) (all from Abcam). Subsequently,
the membranes were incubated with HRP-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (cat. no. BA1054; Wuhan Boster
Biological Technology, Ltd.) at 1:5,000 dilution for 1 h at room
temperature. The chemiluminescent signals were developed using an
enhanced chemiluminescence kit (MilliporeSigma). The images of the
proteins were collected and the intensity was analyzed using ImageJ
software (version 2.0; National Institutes of Health). β-actin was
used as a whole cell internal reference, lamin A/C was used as a
nuclear internal reference and tubulin was used as a cytosolic
internal reference.
Immunofluorescence
The PIG1 cell line was seeded into 12-well plates
containing single layer glass slides and cultured until it reached
60–70% confluence. The cells were then pretreated with 0.4 mM RK1
or 10 µM LY294002 for 2 h, and with 1 mM H2O2
for 24 h at 37°C with 5% CO2. Following treatment, the
PIG1 cell line was fixed with 4% formaldehyde solution for 20 min
at room temperature, permeabilized with 0.1% Triton X-100/PBS for 5
min at room temperature, blocked with 10% newborn calf serum (cat.
no. 22012-8612; Zhejiang Tianhang Biotechnology Co., Ltd.) for 30
min at room temperature and incubated with an anti-Nrf2 primary
antibody (cat. no. ab137550; 1:500; Abcam) overnight at 4°C. The
slides were washed twice with PBS/0.1% Tween-20 and incubated with
Alexa Fluor® 488 goat anti-rabbit IgG (green color; cat.
no. ab150077; 1:1,000; Abcam) for 1 h at room temperature. DAPI was
used to stain the cell nuclei (blue) for 15 min at room
temperature. The protein expression of Nrf2 was observed using an
Olympus confocal microscope (Olympus FV300; Olympus
Corporation).
Statistical analysis
SPSS v20.0 software (IBM Corp.) was used for
statistical analysis. All data are presented as the mean ± standard
deviation from three separate experiments. Differences among
multiple groups were compared by one-way analysis of variance with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
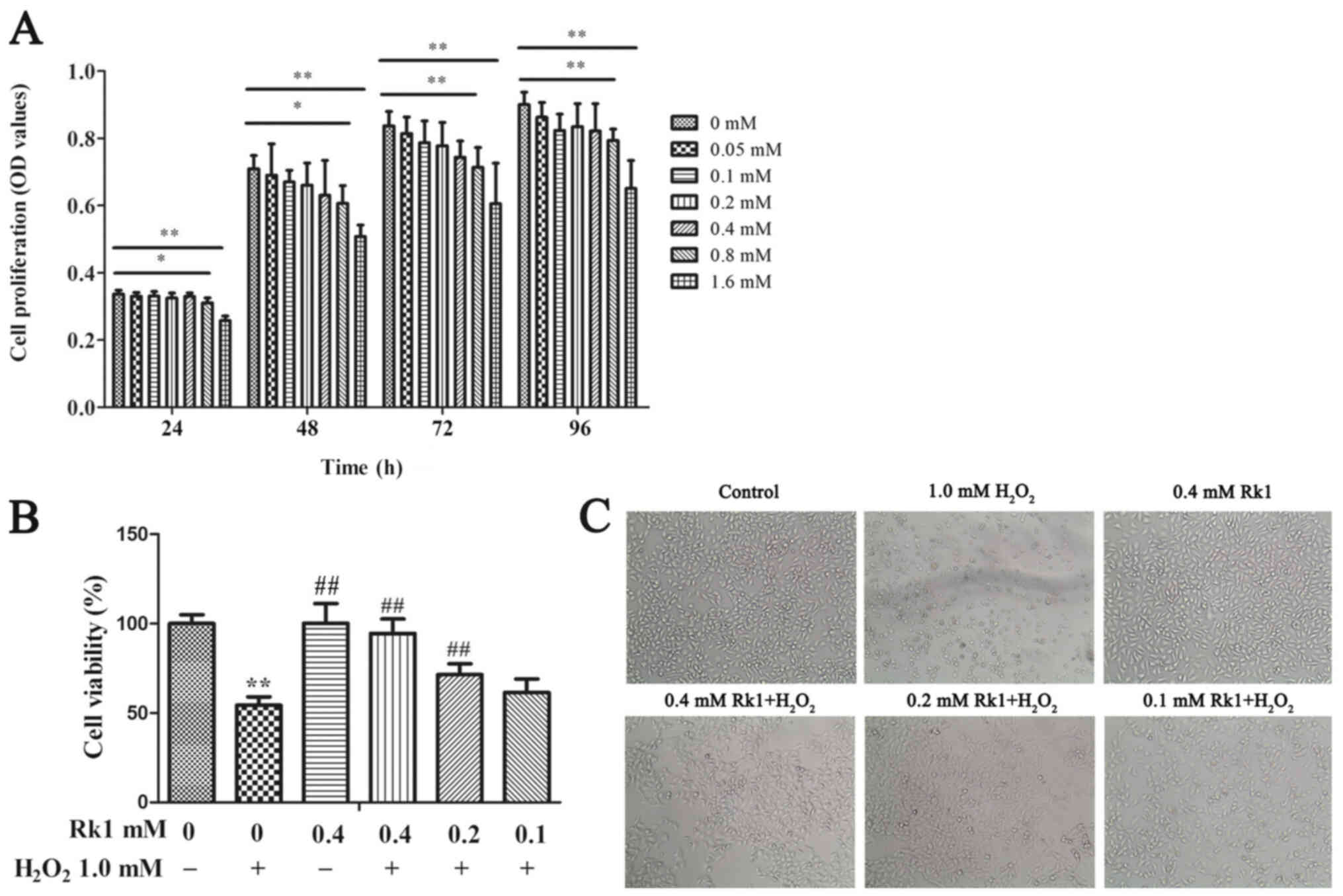
RK1 protects against
H2O2-induced human melanocyte death
To identify the cytotoxic concentration of RK1, the
effects of RK1 on PIG1 cell viability were assessed using a CCK-8
assay. As shown in Fig. 1A,
compared with that in the control group, there were no significant
changes in cell viability at doses of RK1 between 0.05 and 0.4 mM.
Nevertheless, RK1 at a concentration of 0.8 and 1.6 mM
significantly suppressed cell viability. Therefore, 0.4 mM RK1 was
used as the highest concentration in the subsequent experiments. To
investigate the protective effect of RK1 on the PIG1 cell line, the
cells were treated with RK1 (0, 0.1, 0.2 and 0.4 mM) for 2 h
followed by exposure to 1.0 mM H2O2 for 24 h.
It was previously found that 1 mM H2O2 was
the optimal dose for inducing a sufficient cytotoxic effect on
melanocytes (24,25). As shown in Fig. 1B and C, treatment with 1.0 mM
H2O2 for 24 h significantly inhibited cell
viability, and the dendrites of the melanocytes were also shortened
compared with that in the control cells. However, pretreatment with
RK1 (0.2 and 0.4 mM) for 2 h significantly increased cell viability
and decreased the amount of cell shrinkage compared with that in
cells treated with H2O2. Taken together,
these data suggested that RK1 could effectively reduce
H2O2-induced human melanocyte death.
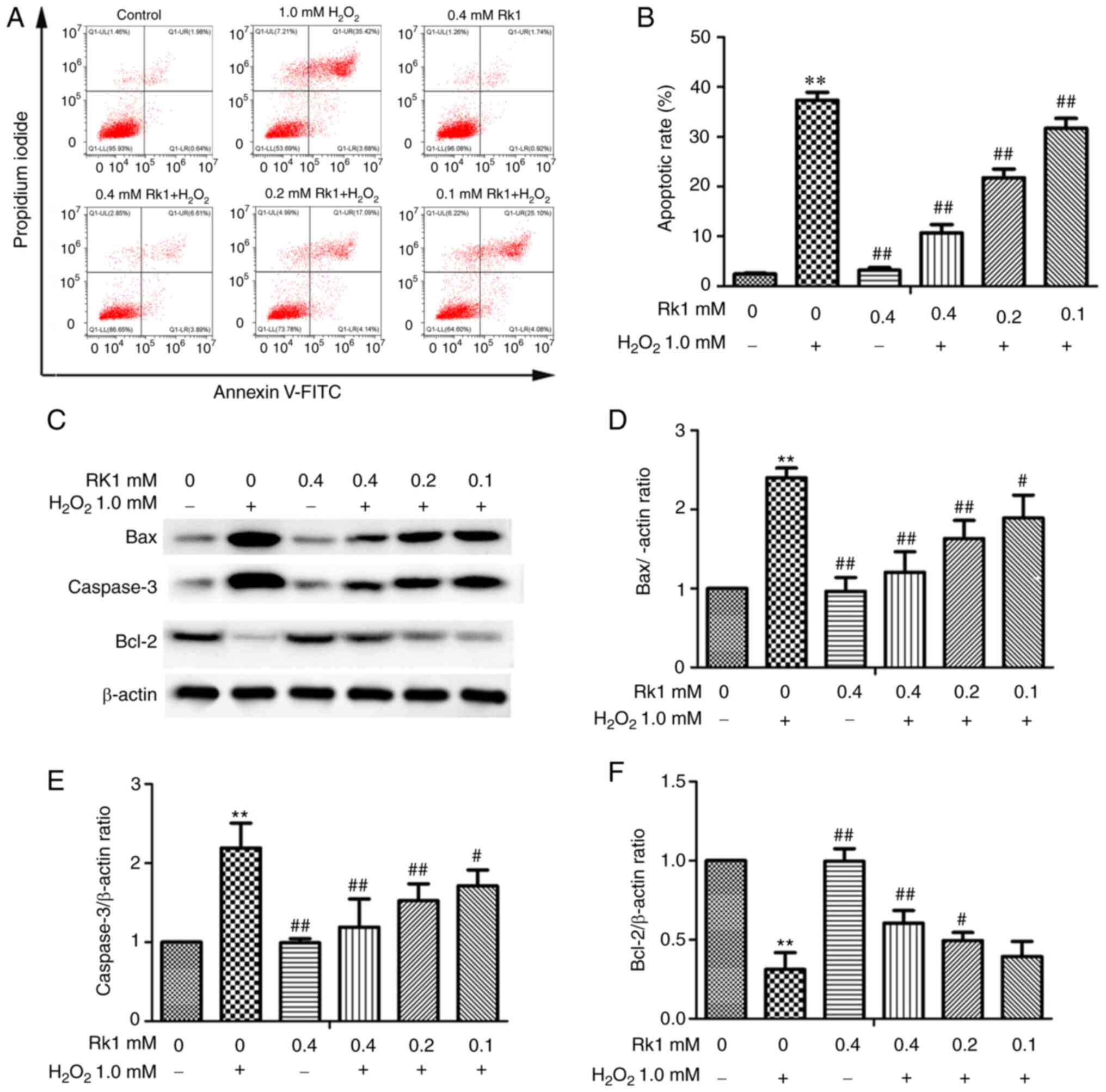
RK1 protects human melanocytes from
H2O2-induced apoptosis
To determine the protective effect of RK1 against
H2O2-induced apoptosis, the PIG1 cell line
was pretreated with various concentrations of RK1 (0.1, 0.2 and 0.4
mM) for 2 h, then treated with or without 1.0 mM
H2O2 for 24 h. As shown in Fig. 2A and B, the number of apoptotic
cells was significantly increased in the H2O2
group compared with that in the control group; however, RK1
significantly reversed these effects in a dose-dependent manner. In
addition, the western blot analysis results revealed that
H2O2 treatment resulted in a significant
increase in the anti-apoptotic protein expression levels of
caspase-3 and Bax, and a decrease in the expression of Bcl-2
protein compared with that in the control group. However, treatment
with RK1 (0.2 and 0.4 mM) significantly decreased caspase-3 and Bax
protein expression levels, and increased Bcl-2 expression levels
compared with that in H2O2-treated cells
(Fig. 2C-F). These data indicated
that RK1 could protect human melanocytes from
H2O2-induced apoptosis.
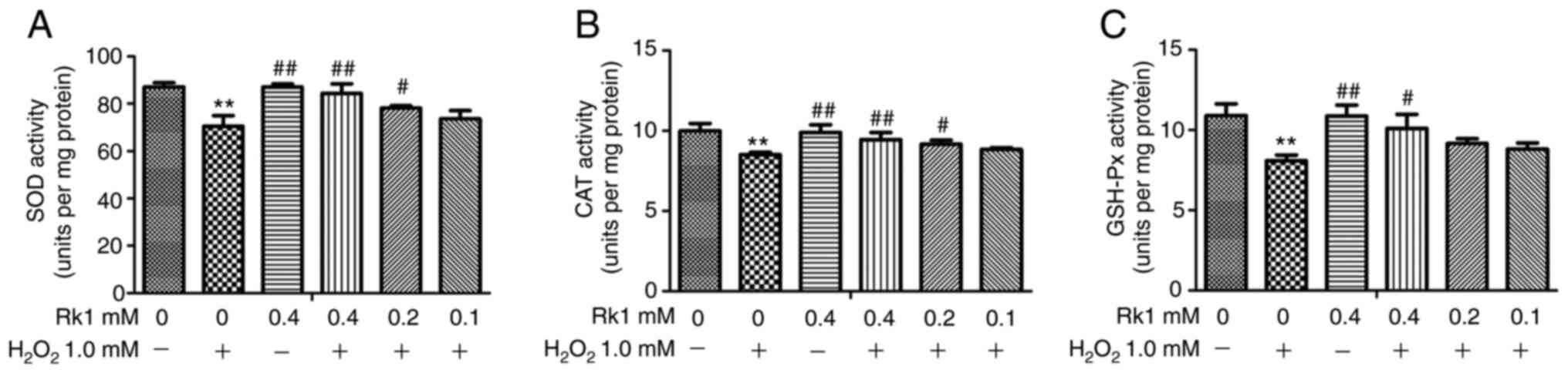
RK1 strengthens the anti-oxidant
ability in human melanocytes
To investigate whether RK1 protects melanocytes
against H2O2-induced OS, the PIG1 cell line
was pretreated with different concentrations of RK1 for 2 h,
followed by incubation with 1.0 mM H2O2 for
24 h, then the activity levels of SOD, CAT and GSH-Px were
determined. As shown in Fig. 3A-C,
the activity levels of SOD, CAT and GSH-Px were significantly
decreased in the H2O2 group compared with
that in the control group, whereas RK1 significantly reversed this
effect. These results indicated that RK1 could protect melanocytes
against H2O2-induced oxidative damage.
RK1 activates the AKT/Nrf2/HO-1
signaling pathway and promotes Nrf2 nuclear translocation in human
melanocytes
The effect of RK1 on the AKT/Nrf2/HO-1 signaling
pathway was further evaluated in the PIG1 cell line. The results
showed that H2O2 treatment significantly
decreased the protein expression levels of HO-1, the ratio of p-AKT
to AKT, and Nrf2 nuclear to cytosolic translocation in the PIG1
cell line (Fig. 4A-D). In addition,
compared with that in the H2O2 group, the
protein expression levels of HO-1, the ratio of p-AKT to AKT, and
Nrf2 nuclear to cytosolic translocation were increased in the RK1
group (Fig. 4A-D). The Nrf2
translocation in the PIG1 cell line following treatment with RK1
(0.4 mM) was subsequently investigated using immunofluorescence
staining. As expected, RK1 promoted nuclear translocation of Nrf2
(Fig. 4E). These results indicated
that H2O2 treatment inhibited the activation
of the AKT/Nrf2/HO-1 signaling pathway and promoted Nrf2
translocation from the nucleus to the cytoplasm, while RK1
pretreatment could reverse this effect.
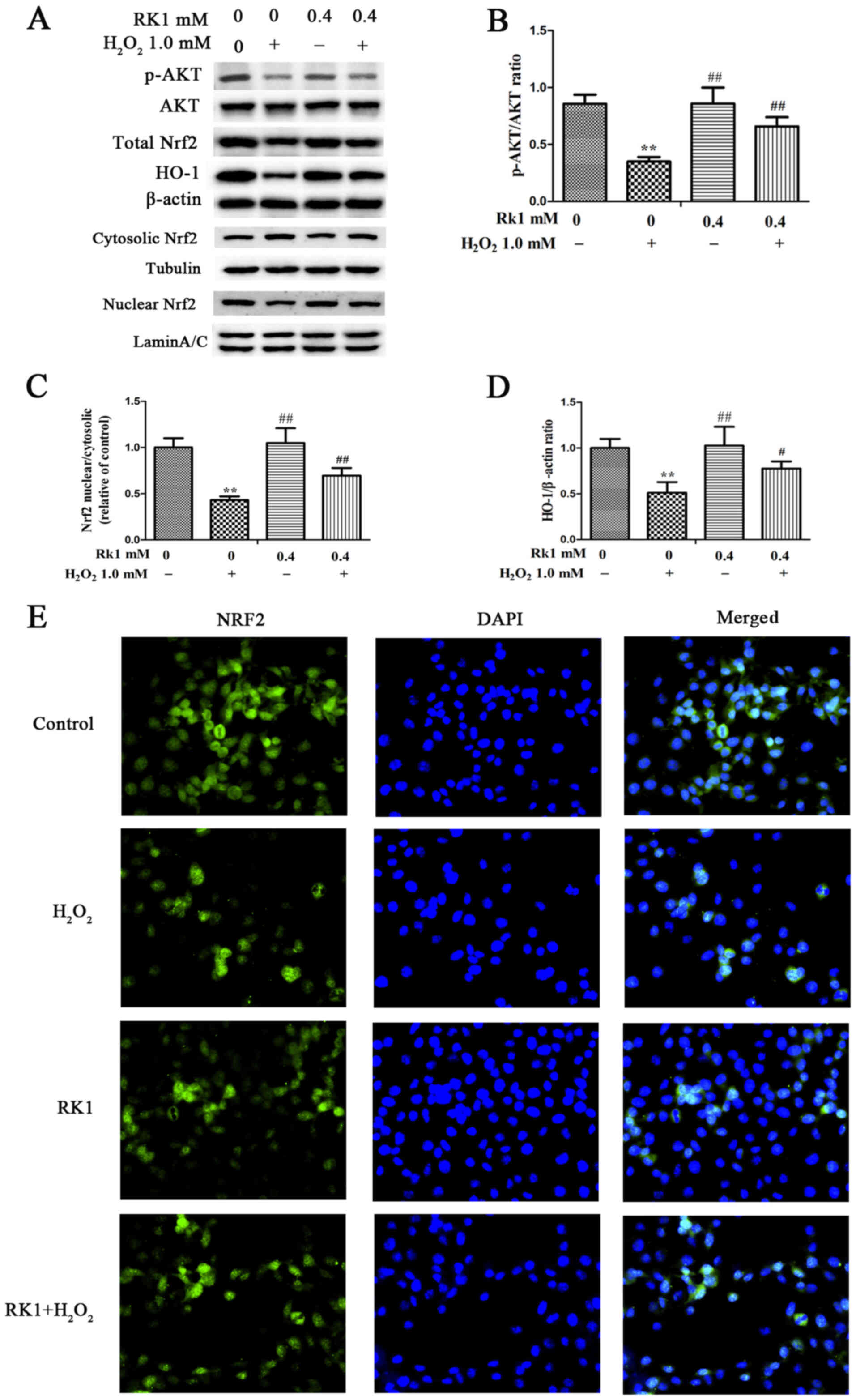 | Figure 4.Effects of RK1 on the protein
expression levels of p-AKT, AKT, HO-1 and Nrf2, and the
translocation of Nrf2 in human melanocytes. Human melanocytes were
pretreated with different concentrations of RK1 for 2 h, then
exposed to 1.0 mM H2O2 for 24 h. (A) Protein
expression levels of p-AKT, AKT, HO-1, total Nrf2, cytosolic Nrf2
and nuclear Nrf2 in the PIG1 cell line were measured using western
blot analysis. (B) Ratio of p-AKT to AKT shown as a bar graph. (C)
Ratio of Nrf2 nuclear to cytosolic translocation shown as a bar
graph. (D) Protein expression levels of HO-1 shown as a bar graph.
(E) Nrf2 distribution in nucleus/cytoplasm was observed using
immunofluorescence assay (magnification, ×400). Nrf2 was stained
with Nrf2-specific antibody (green staining), while the nucleus was
stained with DAPI (blue staining). All the data are presented as
the mean ± SD. **P<0.01 vs. control group; #P<0.05
and ##P<0.01 vs. H2O2 group.
RK1, ginsenoside Rk1; H2O2, hydrogen
peroxide; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1,
heme oxygenase-1; p, phosphorylated. |
LY294002 reverses the protective
effect of RK1 on H2O2-induced PIG1 cells
To clarify whether the PI3K/AKT signaling pathway is
involved in the protective effects of RK1 in the PIG1 cell line
against H2O2-induced damage, the PI3K
inhibitor LY294002 was used to treat the melanocytes. As shown in
Fig. 5A, pretreatment with LY294002
significantly decreased the effects of RK1 on the protein
expression levels of p-AKT, Nrf2 and HO-1. In addition, LY294002
also reversed the nuclear translocation of Nrf2 induced by RK1
(Fig. 5C). The findings suggested
that the protective effect of RK1 against
H2O2-induced downregulation of the Nrf2/HO-1
signaling pathway and Nrf2 nuclear translocation was mediated by
the PI3K/AKT signaling pathway in the PIG1 cell line.
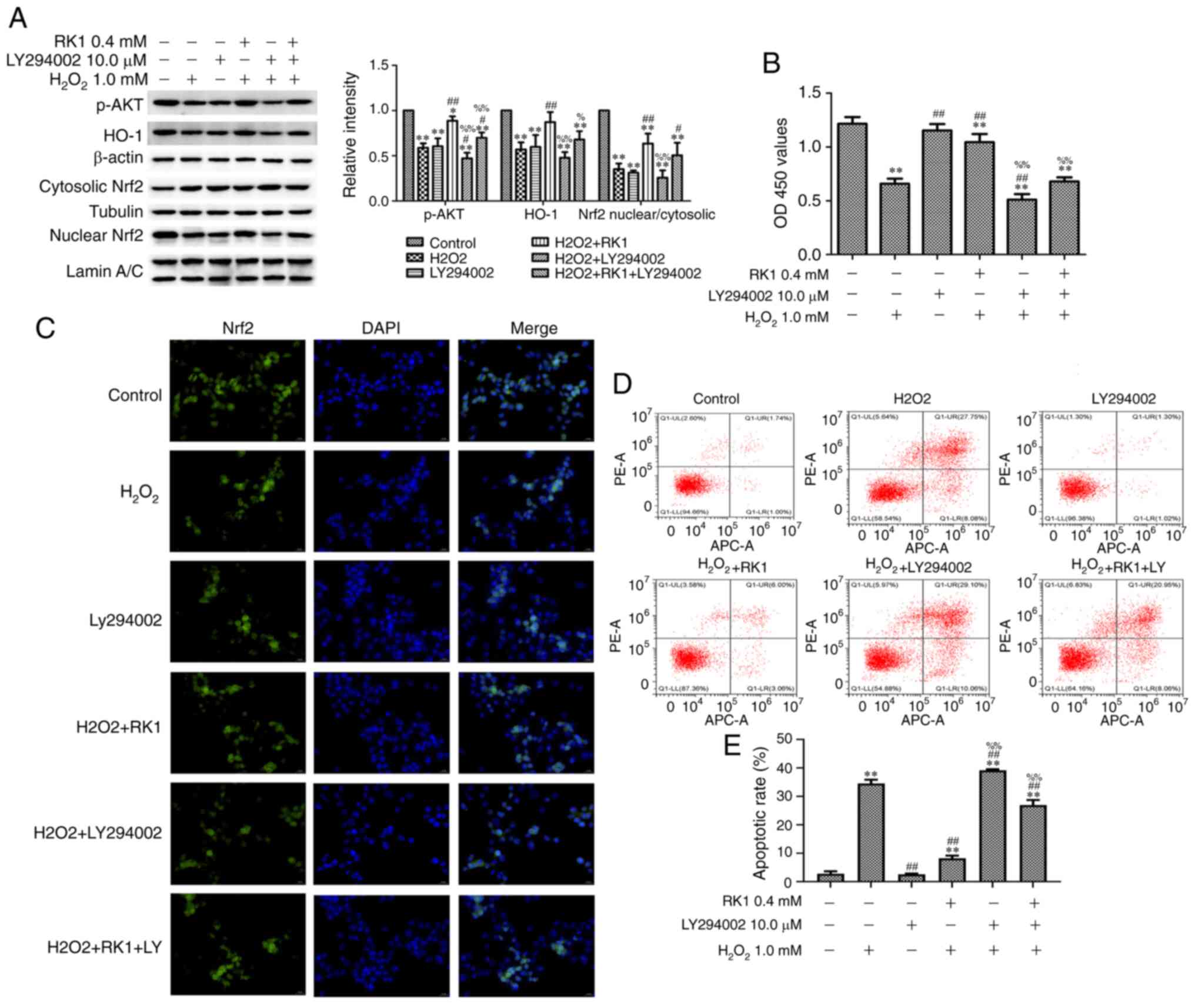 | Figure 5.LY294002 reduces the protective
effects of RK1 against H2O2-induced cell
injury in human melanocytes. Human melanocytes were pretreated with
or without RK1 (0.4 mM) or LY294002 (10.0 µM) for 2 h, then exposed
to H2O2 (1.0 mM) for 24 h. (A) Protein
expression levels of p-AKT, Nrf2 and HO-1 in the PIG1 cell line
were measured using western blot analysis. (B) Cell viability was
determined using a Cell Counting Kit-8 assay. (C) Nrf2 distribution
in the nucleus/cytoplasm was observed using an immunofluorescence
assay (magnification, ×400). Nrf2 was stained with Nrf2-specific
antibody (green staining), and the nucleus was stained with DAPI
(blue staining). (D and E) Cell apoptosis was determined using flow
cytometry. All the data are presented as the mean ± SD. *P<0.05,
**P<0.01 vs. control group; #P<0.05 and
##P<0.01 vs. H2O2 group;
%P<0.05 and %%P<0.01 vs. RK1 +
H2O2 group. RK1, ginsenoside Rk1;
H2O2, hydrogen peroxide; Nrf2, nuclear factor
erythroid 2-related factor 2; HO-1, heme oxygenase-1; p,
phosphorylated. |
The effect of LY294002 on the cell viability of the
PIG1 cell line was subsequently investigated. As shown in Fig. 5B, LY294002 significantly reduced the
protective effect of RK1 on the cell viability of the PIG1 cell
line. Then, the effect of LY294002 on apoptosis in the PIG1 cell
line exposed to H2O2 was investigated. As
shown in Fig. 5D and E, RK1
treatment significantly decreased the apoptotic rate of the PIG1
cell line exposed to H2O2, while the
pretreatment of LY294002 significantly inhibited the protective
effect of RK1 on the PIG1 cell line. These results demonstrated
that pretreatment with RK1 increased the viability of the PIG1 cell
line and resulted in a significant decrease of apoptotic cells via
the PI3K/AKT signaling pathway.
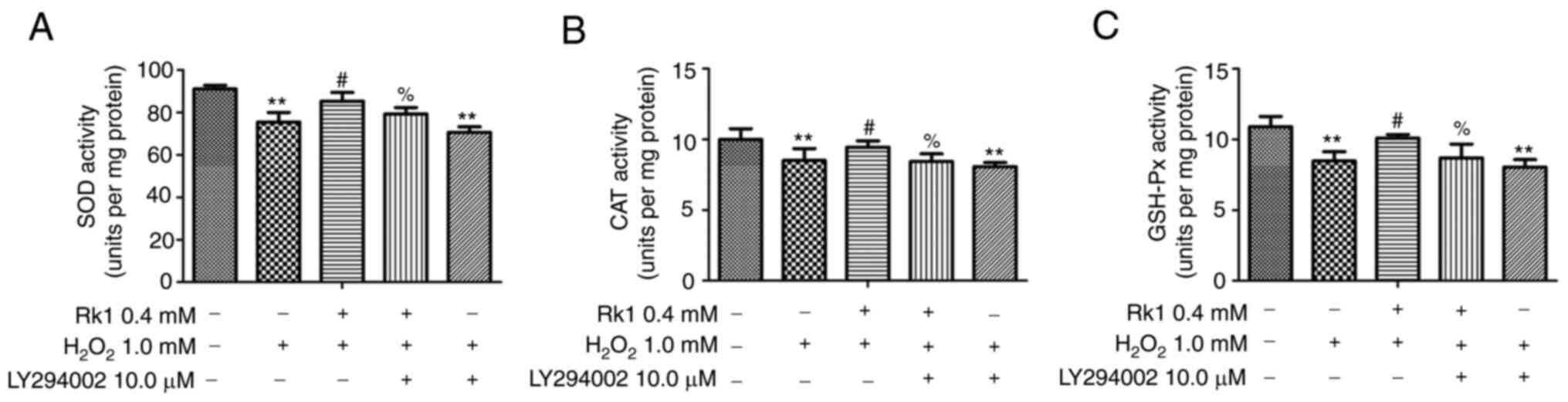
LY294002 reverses the protective
effect of RK1 against H2O2-induced OS in the
PIG1 cell line
To verify whether RK1 had a protective effect on
H2O2-induced OS via the PI3K/AKT signaling
pathway, the PIG1 cell line was treated with LY294002. As shown in
Fig. 6A-C, treatment with RK1
significantly increased the activity level of SOD, CAT and GSH-Px
in the PIG1 cell line exposed to H2O2,
whereas LY294002 could significantly reverse this effect. These
data indicated that RK1 alleviated the oxidative damage of the PIG1
cell line induced by H2O2 via the PI3K/AKT
signaling pathway.
Discussion
In the present study, the protective effect of RK1
against H2O2-induced OS in human melanocytes
was initially determined and the underlying molecular mechanism was
elucidated. It was revealed that RK1 could effectively protect the
PIG1 cell line by promoting cell viability, inhibiting cell
apoptosis, and increasing the activity levels of SOD, CAT and
GSH-Px. Notably, RK1 could protect the melanocytes against OS by
increasing Nrf2 and HO-1 protein expression levels, and increasing
Nrf2 translocation from the cytoplasm to the nucleus. In addition,
it was verified that RK1 could significantly activate the PI3K/AKT
signaling pathway. Notably, LY294002 could inhibit the protective
effect of RK1 on melanocytes.
The precise etiology remains to be investigated;
however, accumulating evidence has demonstrated that OS plays a
crucial role in the pathological changes in the onset and
progression of vitiligo, as it can directly induce the loss or
complete destruction of functioning melanocytes (26). Several studies have also confirmed
that the enzyme activity levels of CAT, SOD and GSH-Px in patients
with vitiligo were reduced (26–29).
RK1 has been reported to protect human keratinocytes by reducing
ROS levels to attenuate OS, which reflects its antioxidant ability
(30). Furthermore, the
pro-oxidative effect of RK1 was reported in triple-negative breast
cancer cells (31). Thus, the
pro-oxidative and antioxidative activities of RK1 may be associated
with different cell types. In the present study, it was revealed
that RK1 could improve cell viability, protect the change in cell
morphology, and increase the activity levels of SOD, CAT and GSH-Px
in melanocytes. Apoptosis, a mode of cell death, is crucial for the
initiation of vitiligo pathologies (32). Cellular apoptosis causes cell
shrinkage, chromatin condensation and DNA fragmentation. Previous
studies have reported that the accumulation of ROS could promote
normal human epidermal melanocyte apoptosis (33–36),
while RK1 could inhibit hepatocyte apoptosis induced by OS
(14). In the present study, it was
revealed that treatment with RK1 decreased the cell apoptotic rate,
indicating that RK1 prevented apoptosis in human melanocytes. It
has been hypothesized that there are three distinct signaling
pathways for apoptosis: The mitochondrial pathway, the death
receptor pathway and the endoplasmic reticulum stress pathway
(37). Activation of caspase-3
plays a crucial role in these pathways, which further drive the
terminal events of apoptosis (38).
In addition, it was found to regulate the transfer and activation
of the Bcl-2 family proteins (37).
The Bcl-2 family, particularly the ratio of Bcl-2 to Bax, plays a
critical role in the regulation of apoptosis. In the present study,
it was found that pretreatment with RK1 significantly increased the
protein expression levels of Bax and caspase-3, and reduced the
protein expression level of Bcl-2. These data demonstrated that the
anti-apoptotic effect of RK1 may be mediated via the
caspase-dependent pathway in human melanocytes. Lu et al
(39) reported that geniposide, an
iridoid glycoside purified from the fruit of the herb Gardenia
jasminoides, also protects melanocytes from
H2O2-induced oxidative damage and apoptosis
via the PI3K/AKT signaling pathway. Next, the present study focused
on the effect of RK1 on Nrf2 gene expression and translocation.
A previous study revealed that Nrf2, as a
transcription factor, plays an important role in regulating the
mRNA expression of antioxidant enzymes in melanocytes, and is a
major target for vitiligo treatment (40). Nrf2 translocates from the cytoplasm
to the nucleus to activate HO-1. HO-1 is a stress-response protein,
which removes oxygen free radicals, thus preventing oxidative
injury in cells (41). The PI3K/AKT
signaling pathway has been reported to be involved in regulating
cell survival, metabolism and apoptosis (42). Previous studies have demonstrated
that the PI3K/AKT signaling pathway could regulate Nrf2 expression
and activity levels (43–45). H2O2 was found
to decrease the PI3K/AKT signaling pathway in human epidermal
melanocytes (46). In the present
study, it was revealed that H2O2 inhibited
the PI3K/AKT/Nrf2/HO-1 signaling pathway; however, RK1 could not
only promote the protein expression levels of p-AKT, Nrf2 and HO-1,
but could also promote the nuclear translocation of Nrf2. These
results illustrated that the PI3K/AKT/Nrf2/HO-1 signaling pathway
may be involved in the protective effect of RK1 on
H2O2-induced oxidative injury in melanocytes.
Unfortunately, the present study did not examine the influence of
different incubation times of H2O2 on Nrf2
protein expression and Nrf2 nuclear translocation, which will be
considered in future studies. To further investigate the role of
the PI3K/AKT signaling pathway, LY294002 and RK1 were used to
determine their combined effect on melanocytes. The results showed
that the protective effect of RK1 on melanocytes was significantly
inhibited following pretreatment with LY294002, indicating that Rk1
protected melanocytes from H2O2-induced
oxidative injury by activating the PI3K/AKT signaling pathway.
However, vitiligo is an autoimmune disease, and whether RK1 could
inhibit the accumulation of melanocyte-specific CD8+ T
cells has not been clarified in the present study.
In conclusion, the present study showed that RK1
served a protective role against OS-related injury in melanocytes
by activating the PI3K/AKT/Nrf2/HO-1 signaling pathway to enhance
cell viability and attenuate apoptosis. Furthermore, RK1 may be a
potential therapeutic strategy for the treatment of vitiligo;
however, further investigation is required to clarify the specific
underlying mechanism of RK1-mediated PI3K/AKT activation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81573986 and
81873310).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author.
Authors' contributions
JX and JG conceived and designed the experiments.
JX, JY and KY performed the experiments and analyzed the data. JX
wrote the paper. JX and JG confirm the authenticity of all the raw
data. JG reviewed and revised the manuscript. All authors have read
and approved the final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dey-Rao R and Sinha AA: Interactome
analysis of gene expression profile reveals potential novel key
transcriptional regulators of skin pathology in vitiligo. Genes
Immunity. 17:30–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saleh MA, Mahran OM and Bassam Al-Salahy
M: Circulating oxidative stress status in dromedary camels infested
with sarcoptic mange. Vet Res Commun. 35:35–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fatehi-Hassanabad Z, Chan CB and Furman
BL: Reactive oxygen species and endothelial function in diabetes.
Eur J Pharmacol. 636:8–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Q, Zhang W, Guo S, Jian Z, Li S, Li K,
Ge R, Dai W, Wang G, Gao T and Li C: Oxidative stress-induced
overexpression of miR-25: The mechanism underlying the degeneration
of melanocytes in vitiligo. Cell Death Differ. 23:496–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo S and Zhang Q: Paeonol protects
melanocytes against hydrogen peroxide-induced oxidative stress
through activation of Nrf2 signaling pathway. Drug Dev Res. Jan
24–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuang T, Li S, Yi X, Guo S, Wang Y, Chen
J, Liu L, Jian Z, Gao T, Kang P and Li C: Tranilast directly
targets NLRP3 to protect melanocytes from keratinocyte-derived
IL-1β under oxidative stress. Front Cell Dev Biol. 8:5882020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Yang F, Teng L and Katayama I:
6-shogaol protects human melanocytes against oxidative stress
through activation of the Nrf2-antioxidant response element
signaling pathway. Int J Mol Sci. 21:35372020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, Li S, Zhu L, Guo S, Yi X, Cui T, He
Y, Chang Y, Liu B, Li C and Jian Z: Baicalein protects human
vitiligo melanocytes from oxidative stress through activation of
NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic Biolo
Med. 129:492–503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li XS, Tang XY, Su W and Li X: Vitexin
protects melanocytes from oxidative stress via activating
MAPK-Nrf2/ARE pathway. Immunopharmacol Immunotoxicol. 42:594–603.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiao Z, Xu Z, Xiao Q, Yang Y, Ying J,
Xiang L and Zhang C: Dysfunction of ATG7-dependent autophagy
dysregulates the antioxidant response and contributes to oxidative
stress-induced biological impairments in human epidermal
melanocytes. Cell Death Discov. 6:312020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y, Huang J, Li Y, Jiang L, Ouyang Y, Li
Y, Yang L, Zhao X, Huang L, Xiang H, et al: Cistanche deserticola
polysaccharide induces melanogenesis in melanocytes and reduces
oxidative stress via activating NRF2/HO-1 pathway. J Cell Mol Med.
24:4023–4035. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SJ and Kim HS: Deregulation of
Nrf2/ARE signaling pathway causes susceptibility of
dystrophin-deficient myotubes to menadione-induced oxidative
stress. Exp Cell Res. 364:224–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramprasath T, Vasudevan V, Sasikumar S,
Puhari SS, Saso L and Selvam GS: Regression of oxidative stress by
targeting eNOS and Nrf2/ARE signaling: A guided drug target for
cardiovascular diseases. Curr Top Med Chem. 15:857–871. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ndisang J: Synergistic interaction between
heme oxygenase (HO) and nuclear-factor E2-related factor-2 (Nrf2)
against oxidative stress in cardiovascular related diseases. Curr
Pharm Design. 23:1465–1470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CC, Hsiao LD, Cho RL and Yang CM:
CO-releasing molecule-2 induces Nrf2/ARE-dependent heme oxygenase-1
expression suppressing TNF-α-induced pulmonary inflammation. J Clin
Med. 8:4362019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Li X, Wang Y, Mu P, Chen C, Huang
P and Liu D: Ginsenoside Rb1 attenuates intestinal
ischemia/reperfusioninduced inflammation and oxidative stress via
activation of the PI3K/Akt/Nrf2 signaling pathway. Mol Med Rep.
19:3633–3641. 2019.PubMed/NCBI
|
|
17
|
Shen R, Laval S, Cao X and Yu B: Synthesis
of Δ 20-ginsenosides Rh4,
(20E)-Rh3, Rg6, and Rk1: A general
approach to access dehydrated ginsenosides. J Org Chem.
83:2601–2610. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JE, Lee W, Yang S, Cho SH, Baek MC,
Song GY and Bae JS: Suppressive effects of rare ginsenosides, Rk1
and Rg5, on HMGB1-mediated septic responses. Food Chem Toxicol.
124:45–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Wang S, Liu J, Cai E, Zhu H, He Z,
Gao Y, Li P and Zhao Y: Sesquiterpenoids from the root of Panax
Ginseng protect CCl4-induced acute liver injury by
anti-inflammatory and anti-oxidative capabilities in mice. Biomed
Pharmacother. 102:412–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi ZL, Wang Z, Li W, Hou JG, Liu Y, Li XD,
Li HP and Wang YP: Nephroprotective effects of anthocyanin from the
fruits of Panax ginseng (GFA) on cisplatin-induced acute kidney
injury in mice. Phytother Res. 31:1400–1409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang CC, Chen CY, Wu CC, Koo M, Yu ZR and
Wang BJ: Panax ginseng fraction F3 extracted by supercritical
carbon dioxide protects against oxidative stress in ARPE-19 CElls.
Int J Mol Sci. 17:17172016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen W, Wei Y, Tang D, Jia X and Chen B:
Metabolite profiles of ginsenosides Rk1 and Rg5 in zebrafish using
ultraperformance liquid chromatography/quadrupole-time-of-flight
MS. J Ginseng Res. 41:78–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu JN, Xu XY, Li W, Wang YM, Liu Y, Wang Z
and Wang YP: Ginsenoside Rk1 ameliorates paracetamol-induced
hepatotoxicity in mice through inhibition of inflammation,
oxidative stress, nitrative stress and apoptosis. J Ginseng Res.
43:10–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang Y, Li S, Guo W, Yang Y, Zhang W,
Zhang Q, He Y, Yi X, Cui T, An Y, et al: Simvastatin protects human
melanocytes from H2O2-induced oxidative
stress by activating Nrf2. J Invest Dermatol. 137:1286–1296. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang W, Li S, Chen X, Zhang W, Chang Y,
He Y, Zhang S, Su X, Gao T, Li C and Jian Z: Berberine protects
immortalized line of human melanocytes from HO-induced oxidative
stress via activation of Nrf2 and Mitf signaling pathway. J
Dermatol Sci. 94:236–243. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto A, Yang L, Kuroda Y, Guo J, Teng
L, Tsuruta D and Katayama I: Local epidermal endocrine estrogen
protects human melanocytes against oxidative stress, a novel
insight into vitiligo pathology. Int J Mol Sci. 22:2692020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shalbaf M, Gibbons NC, Wood JM, Maitland
DJ, Rokos H, Elwary SM, Marles LK and Schallreuter KU: Presence of
epidermal allantoin further supports oxidative stress in vitiligo.
Exp Dermatol. 17:761–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Becatti M: Oxidative stress and
high-mobility group box 1 (HMGB1) protein release in vitiligo. Br J
Dermatol. 176:1436–1437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui T, Zhang W, Li S, Chen X, Chang Y, Yi
X, Kang P, Yang Y, Chen J, Liu L, et al: Oxidative stress-induced
HMGB1 release from melanocytes: A paracrine mechanism underlying
the cutaneous inflammation in vitiligo. J Invest Dermatol.
139:2174–2184.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn S, Siddiqi MH, Aceituno VC, Simu SY,
Zhang J, Jimenez Perez ZE, Kim YJ and Yang DC: Ginsenoside Rg5: Rk1
attenuates TNF-α/IFN-γ-induced production of thymus- and
activation-regulated chemokine (TARC/CCL17) and LPS-induced NO
production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in
human keratinocytes and macrophages. In Vitro Cell Dev Biol Anim.
52:287–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong Y and Fan D: Ginsenoside Rk1 induces
cell cycle arrest and apoptosis in MDA-MB-231 triple negative
breast cancer cells. Toxicology. 418:22–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tulic MK, Cavazza E, Cheli Y, Jacquel A,
Luci C, Cardot-Leccia N, Hadhiri-Bzioueche H, Abbe P, Gesson M,
Sormani L, et al: Innate lymphocyte-induced CXCR3B-mediated
melanocyte apoptosis is a potential initiator of T-cell
autoreactivity in vitiligo. Nat Commun. 10:21782019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou J, An X, Dong J, Wang Y, Zhong H,
Duan L, Ling J, Ping F and Shang J: IL-17 induces cellular stress
microenvironment of melanocytes to promote autophagic cell
apoptosis in vitiligo. FASEB J. 32:4899–4916. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haasler L, Kondadi AK, Tsigaras T, von
Montfort C, Graf P, Stahl W and Brenneisen P: The BH3 mimetic (±)
gossypol induces ROS-independent apoptosis and mitochondrial
dysfunction in human A375 melanoma cells in vitro. Arch Toxicol.
95:1349–1365. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang L, Li J, Fu W, Wu W and Xu J:
Suppression of FADS1 induces ROS generation, cell cycle arrest, and
apoptosis in melanocytes: Implications for vitiligo. Aging (Albany
NY). 11:11829–11843. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang P, Xing X, Wu J, Song J and Liu Q:
PM2.5 promotes apoptosis of human epidermal melanocytes through
promoting oxidative damage and autophagy. Gen Physiol Biophys.
39:569–577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Yang J, Wang J and Kopeček J:
Drug-free macromolecular therapeutics induce apoptosis via calcium
influx and mitochondrial signaling pathway. Macromol Biosci. Jan
18–2018.(Epub ahead of print). doi: 10.1002/mabi.201700196.
|
|
38
|
Gashegu J, Ladha R, Vanmuylder N,
Philippson C, Bremer F, Rooze M and Louryan S: HSP110, caspase-3
and −9 expression in physiological apoptosis and apoptosis induced
by in vivo embryonic exposition to all-trans retinoic acid or
irradiation during early mouse eye development. J Anat.
210:532–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu W, Zhao Y, Kong Y, Zhang W, Ma W, Li W
and Wang K: Geniposide prevents H2O2-induced
oxidative damage in melanocytes by activating the PI3K-Akt
signalling pathway. Clin Exp Dermatol. 43:667–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ko H and Kim M: HO promotes the aging
process of melanogenesis through modulation of MITF and Nrf2. Mol
Biol Rep. 46:2461–2471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jian Z, Tang L, Yi X, Liu B, Zhang Q, Zhu
G, Wang G, Gao T and Li C: Aspirin induces Nrf2-mediated
transcriptional activation of haem oxygenase-1 in protection of
human melanocytes from H2 O2-induced oxidative stress. J Cell Mol
Med. 20:1307–1318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan X and Wu X: MicroRNA-122-5p promotes
the development of non-small cell lung cancer via downregulating
p53 and activating PI3K-AKT pathway. J BUON. 24:273–279.
2019.PubMed/NCBI
|
|
43
|
Kim H, Park CS and Lee AY: Reduced Nrf2
activation in PI3K phosphorylation-impaired vitiliginous
keratinocytes increases susceptibility to ROS-generating
chemical-induced apoptosis. Environ Toxicol. 32:2481–2491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sugimoto M, Ko R, Goshima H, Koike A,
Shibano M and Fujimori K: Formononetin attenuates
H2O2-induced cell death through decreasing
ROS level by PI3K/Akt-Nrf2-activated antioxidant gene expression
and suppressing MAPK-regulated apoptosis in neuronal SH-SY5Y cells.
Neurotoxicology. 85:186–200. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Y, Guo Y, Feng Z, Bergan R, Li B, Qin
Y, Zhao L, Zhang Z and Shi M: Involvement of the PI3K/Akt/Nrf2
signaling pathway in resveratrol-mediated reversal of drug
resistance in HL-60/ADR cells. Nutr Cancer. 71:1007–1018. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou J, Ling J, Song J, Wang Y, Feng B and
Ping F: Interleukin 10 protects primary melanocyte by activation of
Stat-3 and PI3K/Akt/NF-κB signaling pathways. Cytokine. 83:275–281.
2016. View Article : Google Scholar : PubMed/NCBI
|