Introduction
Patients suffering from moderate to severe angina
pectoris, which often results from chronic myocardial ischemia
(CMI) despite optimal medical therapy, and judged unsuited to both
percutaneous coronary intervention and coronary artery bypass graft
surgery, are a challenging issue for cardiologists (1). Such pain severely limits daily
activities and impairs the quality of life among these patients
(2). Owing to the current
inadequate understanding of the underlying mechanisms, the
management of chronic cardiac pain remains difficult.
It has been shown that cardiac visceral nociceptive
information is preliminarily transmitted to the spinal dorsal horn
of thoracic segments 1–5 (T1-T5) through
dorsal root ganglia (DRG), thereby ascending to higher brain
regions (3). In patients with
chronic cardiac pain, increased activity is found in the insular
cortex, believed to be a pivitol region that modulates neuropathic
pain (4,5). CMI rats show potentiated synaptic
transmission in the nucleus of the solitary tract (NTS) which
strongly indicates sensitization, an important phenomenon that
occurrs in neuropathic pain as well (6,7).
Consequently, cardiac pain in CMI shares similarities with
neuropathic pain (5). Moreover,
glial activation has been demonstrated to be involved in the spinal
cord under neuropathic pain conditions (7). However, to the best of our knowledge,
whether spinal glial cells are involved in chronic cardiac pain in
CMI has not been previously investigated.
Spinal cord stimulation (SCS) is an alternative
therapeutic approach that has been clinically utilized for decades
to treat patients with CMI, providing an improvement in intractable
angina symptoms (8,9). SCS owes its inception to the classical
‘gate-control’ theory, in which the selective stimulation of
non-nociceptive afferent fibers (Aβ) inhibits nociceptive afferent
fibers (Aδ and C) through the activation of inhibitory interneurons
in the substantia gelatinosa of the spinal dorsal horns (10). Although this mechanism may explain
the immediate and short-term action of SCS, it does not readily
account for the prolonged analgesic effect, which may reflect a
potential anti-central sensitization mechanism by SCS in
neuropathic pain. A previous study suggested that SCS could reduce
mechanical hyperalgesia by inhibiting spinal glial activation in
rats with neuropathic pain (11).
Therefore, we hypothesized that SCS may produce a cardiac analgesic
effect partially by inhibiting the activation of spinal glia in CMI
model rats. The current study tested this hypothesis directly in
model rats with CMI induced by left anterior descending artery
(LAD) ligation to produce cardiac pain (6).
Materials and methods
Animals
Experiments were performed on male adult
Sprague-Dawley rats (age, 12 weeks; weight, 250–300 g; purchased
from the Laboratory Animals Center of the General Hospital of
Western Command Theater; n=88), housed in transparent plastic cages
at 22–25°C and 55±5% relative humidity with free access to food and
water, under a 12-h light/dark cycle. Rats were acclimated to the
observation room for 30 min prior to all behavioral experiments.
Rats were randomly separated into eight groups: Naive group (n=8),
sham-operated group (n=16), CMI group (n=16), CMI+SCS group (n=16),
CMI+vehicle (Veh) group (n=8), CMI+minocycline (Mino) group (n=8),
CMI+SCS+Veh group (n=8), CMI+SCS+fractalkine (Frac) group (n=8).
Animal experiments were performed in accordance with the ethical
guidelines of the International Association for the Study of Pain
(12) and were approved by the
Animal Use and Care Committee for Research and Education at the Air
Force Military Medical University and General Hospital of Western
Command Theater (Chengdu, China; approval no. 10070). Rats were
anesthetized with an intraperitoneal (i.p.) injection of
pentobarbital (60 mg/kg) prior to operation. At the end of the
study, rats were euthanized with an overdose of pentobarbital (100
mg/kg; i.p.). All efforts were made to minimize animal suffering
and the number of animals used.
Establishment of the CMI model
LAD ligation was performed to produce a CMI rat
model based on a previously described procedure (13). Briefly, rats were anesthetized with
pentobarbital (60 mg/kg; i.p.) and, after endotracheal intubation
and assisted ventilation (tidal volume, 4 ml; ventilation rate,
60/min), the heart was exposed through a left thoracotomy at the
level of the fifth intercostal space. The LAD was then occluded 3
mm from its origin with a 6-0 prolene suture. The chest was then
closed, and each rat was allowed to recover before the subsequent
experiments. Sham-operated rats underwent an identical surgery but
without LAD ligation. Successful establishment of the CMI model was
confirmed by observing elevation of the ST-segment on
electrocardiography and pale discoloration of the myocardium
post-mortem.
SCS
After LAD ligation, a laminectomy was performed at
the level of T5 vertebra, and the SCS lead was inserted
epidurally in the rostral direction. The lead was fixed with
sutures to the muscle, the wound was sutured in layers, and the
lead was tunneled to exit the skin at the base of the neck. A
spinal cord lead designed for use in rats (Medtronic plc) that is
similar to that used in patients was inserted (11); the proximal end of the lead was
tunneled outside the animal for later connection to an external
neurostimulator (model no. 37021) and programmer (model no. 8840;
both Medtronic plc). Based on a previous study (6), 60 Hz SCS at 90% motor threshold
(amplitude; 0.35 V) was chosen in the current study. The duration
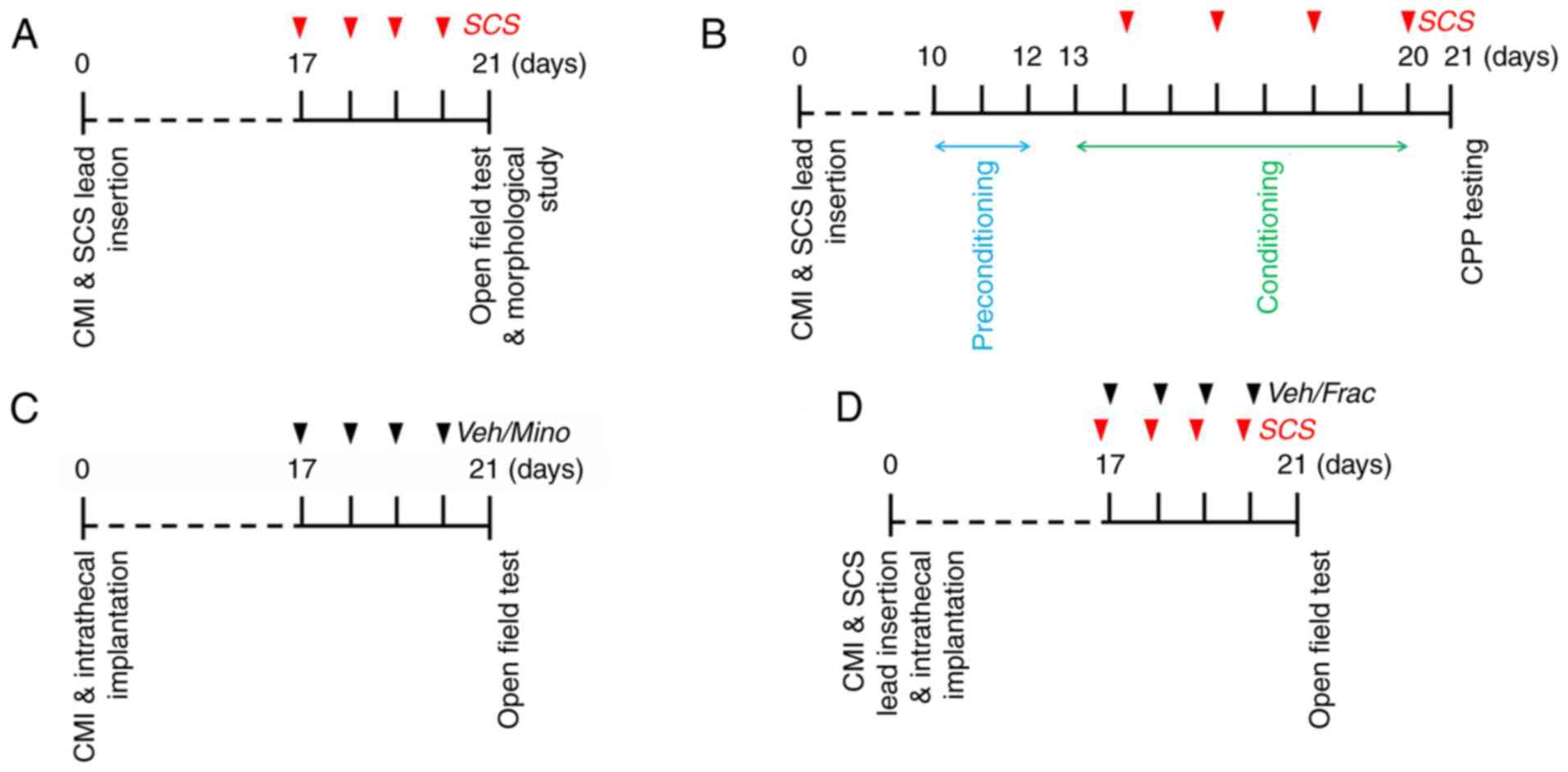
of SCS in the present study is shown in Fig. 1.
Behavioral experiments
Open field (OF) test
Rats were placed in an OF (100×100×40
cm3) inside a dimly lit isolation chamber (<50 lux in
the center of the open field) with a fan. An activity-monitoring
system (Panlab) was used to record horizontal locomotor activity.
Briefly, this system used paired sets of photobeams to detect
movement in the open field and movement was recorded as beam
breaks. Each animal was placed in the center of the OF box, and its
vertical (rearing; rats standing up on their hindlimbs) activity
and distance traveled were recorded and measured for 15 min. Fewer
rearing motions of the rats reflected more severe spontaneous pain
conditions, and vice versa (14).
SCS was applied 30 min daily for 4 consecutive days before the OF
test (Fig. 1A).
Conditioned place preference (CPP)
test
A multi-trial conditioning protocol was used for
CPP, as previously described (15).
Preconditioning to an automated three-chamber CPP box was performed
for 3 days, starting 10 days after CMI surgery (D10-D12). All
animals were acclimated with full access to all chambers for 30 min
each day. On D12, behavior was recorded for 15 min and analyzed to
confirm the absence of a preference for a particular
preconditioning chamber. Following the preconditioning phase, the
rats underwent conditioning for 8 days with alternating
SCS-treatment chamber pairings. Rats received non-SCS-chamber
pairing (rats were tethered to the neurostimulator system but did
not receive SCS) on odd days (D13, 15, 17 and 19) and SCS-chamber
pairing on even days (D14, 16, 18 and 20). Rats were placed in the
paired chamber with no access to the other chamber following
non-SCS or SCS. SCS and chamber pairings were counterbalanced. The
conditioning time was 30 min in each chamber. On the testing day
(D21), rats were placed into the neutral chamber of the CPP box
with free access to all chambers and their behavior was recorded
for 15 min to analyze their chamber preference (Fig. 1B). Increased time spent in a chamber
(for example, increased time in the SCS-paired chamber) indicated a
preference for that chamber, suggesting a pain-relieving effect
(analgesia) (15).
Immunofluorescence and Masson's
trichrome staining
Immunofluorescence staining was performed as
previously described (16). The
rats were deeply anesthetized with an overdose of pentobarbital
(100 mg/kg; i.p.) and transcardially perfused with 150 ml 0.01 M
PBS (pH 7.4), followed by 500 ml 4% paraformaldehyde in 0.1 M
phosphate buffer (pH 7.4). The heart was harvested and embedded in
paraffin. The embedded heart was sectioned at 6 µm and Masson's
trichrome staining kit (cat. no. HT15; Sigma-Aldrich; Merck KGaA)
was used according to the manufacturer's protocol. Masson's
trichrome staining was performed to verify myocardial fibrosis
induced by CMI at 3 weeks post-surgery. The spinal cord was
transversely sliced into 25-µm-thick coronal sections using a
freezing microtome (CM1950; Leica Microsystems GmbH).
Double-immunofluorescence staining for i) ionized
calcium-binding adaptor protein-1 (Iba-1; microglial marker) and
phosphorylated (p)-p38, ii) glial fibrillary acidic protein (GFAP;
astrocyte marker) and p-p38, or iii) neuronal nuclei (NeuN;
neuronal marker) and p-p38 was performed; the antibodies used are
shown in Table I. The spinal cord
sections were sequentially incubated at room temperature with
primary antisera in 0.01 M PBS containing 5% normal donkey serum
(NDS, cat. no. 566460; MilliporeSigma), 0.3% Triton X-100, 0.05%
NaN3 and 0.25% carrageenan (PBS-NDS; pH 7.4) for 24 h.
Then, the sections were incubated with the corresponding Alexa 488
or 594-conjugated secondary antibodies for 6 h at room temperature.
A negative control experiment, in which the primary antibodies were
omitted, and a peptide competition assay were performed. No
immunopositive products were detected.
 | Table I.Antisera used in each group. |
Table I.
Antisera used in each group.
| Group | Primary
antibody | Secondary
antibody |
|---|
| NeuN/p-p38 | Mouse anti-NeuN
(1:500; cat. no. ZMS377; MilliporeSigma) | Alexa 488 Donkey
anti-mouse (1:500; cat. no. A-11001; Invitrogen; Thermo Fisher
Scientific, Inc.) |
|
| Rabbit anti-p-p38
(1:200; cat. no. 4511 Cell Signaling Technology, Inc.); | Alexa 594 Donkey
anti-rabbit (1:500; cat. no. R37119; Invitrogen; Thermo Fisher
Scientific, Inc.) |
| GFAP/p-p38 | Mouse anti-GFAP
(1:500; cat. no. SAB5201104; MilliporeSigma) and rabbit anti-p-p38
(1:200; cat. no. 4511; Cell SignalingTechnology, Inc.) | Alexa 488 Donkey
anti-mouse (1:500; cat. no. A-11001; Invitrogen; Thermo Fisher
Scientific, Inc.) and Alexa 594Donkey anti-rabbit (1:500; cat. no.
R37119; Invitrogen; Thermo Fisher Scientific, Inc.) |
| Iba-1/p-p38 | Goat anti-Iba-1
(1:500, cat. no. 011-27991; Wako) | Alexa 488 Donkey
anti-goat (1:500; cat. no. A-11055; Invitrogen; Thermo Fisher
Scientific, Inc.) |
|
| Rabbit anti-p-p38
(1:200, cat. no. 4511; Cell Signaling Technology) | Alexa 594 Donkey
anti-rabbit (1:500; cat. no. R37119; Invitrogen; Thermo Fisher
Scientific, Inc.) |
After the immunofluorescence staining, the sections
were observed and images were captured using a confocal
laser-scanning microscope (FV1000; Olympus Corporation) using
FLUOVIEW software (FV10-ASW 1.7 Viewer; Olympus Corporation). In
total, five non-adjacent sections from the
T1-T5 segments were selected at random per
rat (n=8). Images were evaluated using a computer-assisted image
analysis program (MetaMorph 6.1; Molecular Devices, LLC) which set
the low and high thresholds for the immunofluorescent intensity
that was determined to be a signal. Imaging data were collected
using the same region and the same size of field within the spinal
dorsal horn. The same configuration was used to measure cell areas
in all experimental groups. The measured areas were transferred to
Excel (Microsoft Corporation) automatically for the statistical
analysis. MetaMorph 6.1 was calibrated to provide standardization
of area measurements. The immunoreactivities for GFAP and Iba-1
within the superficial dorsal horn were averaged across the five
spinal sections for the experimental group (17).
Western blotting
Rats were deeply anesthetized with an overdose of
pentobarbital (100 mg/kg; i.p.), and the
T1-T5 segments of the spinal dorsal cord were
carefully dissected and harvested for western blotting. To obtain
total protein extracts, the tissues were lysed in 300 µl lysis
buffer containing 10 mM Tris, 150 mM NaCl, 1% Triton X-100, 0.5%
NP-40 and 1 mM EDTA at pH 7.4. The samples were adequately mixed
with protease inhibitor cocktail and phosphatase inhibitor cocktail
(Roche Diagnostics) at a 100:1 (v/v) ratio. Subsequently, 30 µg
cell lysis material (quantitatively measured using the BCA protein
assay; Thermo Fisher Scientific, Inc.) was resolved by SDS-PAGE
(10% SDS-polyacrylamide gels) and transferred to PVDF membranes
(Immobilon-P; MilliporeSigma). After blocking in 3% non-fat milk
for 1 h at room temperature, the membranes were incubated overnight
at 4°C with the following primary antibodies: Rabbit anti-p38
(1:1,000; cat. no. 8690; Cell Signaling Technology, Inc.) rabbit
anti-p-p38 (1:1,000; cat. no. 4511; Cell Signaling Technology,
Inc.) and rabbit anti-GAPDH (1:1,000; cat. no. 2118; Cell Signaling
Technology, Inc.). The immunoblots were then incubated with an
HRP-conjugated goat anti-rabbit secondary antibody (1:5,000; cat.
no. ER48616; Amersham; Cytiva) at room temperature for 2 h. All of
the reactions were detected using the ECL detection method
(Amersham; Cytiva) and exposure to film. The scanned images were
semi-quantified and analyzed with ImageJ 1.52v software (National
Institutes of Health). A square of the same size was drawn around
each band to measure the density, and the background near that band
was subtracted. The density of specific p-p38 bands was measured
and normalized against total p38 expression.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed in as previously described
(18). Rats were anesthetized with
sodium pentobarbital (100 mg/kg; i.p.), the
T1-T5 spinal dorsal horn was rapidly
harvested, and total RNA was extracted with TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.). In accordance with
the manufacturer's protocols, cDNA was synthesized with oligo
(dT)12-18 using Superscript™ III reverse transcriptase
for RT-PCR (Invitrogen; Thermo Fisher Scientific, Inc.). The
primers used in the present study are shown in Table II. Equal amounts of RNA were used
to prepare cDNA using SYBR Premix Ex Taq (Takara Bio, Inc.) and
analyzed by qPCR (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The amplification protocol was as follows: Initial
denaturation for 3 min at 95°C; followed by 45 cycles of
denaturation for 10 sec at 95°C and annealing and extension for 45
sec at 60°C. Target cDNA quantities were estimated from the
quantification amplification cycle number (C1) using Sequence
Detection System software (Applied Biosystems; Thermo Fisher
Scientific, Inc.). A ΔCq value was calculated for each
sample by subtracting its Cq value from the Cq value for the
corresponding GAPDH to normalize the differences in cDNA aliquots.
Each cDNA quantity was then calculated with the following formula:
2ΔCq (19).
 | Table II.Primers sequences used for reverse
transcription-quantitative PCR. |
Table II.
Primers sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) | Accession no. |
|---|
| TNF-α | F:
TGATCGGTCCCAACAAGG A | AY427675 |
|
| R:
TGCTTGGTGGTTTGCTACGA |
|
| IL-1β | F:
TGCTGATGTACCAGTTGGGG | NM031512 |
|
| R:
CTCCATGAGCTTTGTACAAG |
|
| GAPDH | F:
CCCCCAATGTATCCGTTGTG | NM01008 |
|
| R:
TAGCCCAGGATGCCCTTTAGT |
|
Intrathecal catheter implantation and
drug administration
Intrathecal catheter implantation surgery was
performed immediately following CMI or SCS operation under
pentobarbital anesthetization. It was performed by inserting
polyethylene tubing through which the drug (minocycline,
fractalkine, or vehicle as mentioned below) was directly injected
into the subarachnoid space of the thoracic segment following CMI
or SCS operation. The catheters were primed with vehicle (sterile
saline solution, cat. no. BTYY1000T; Nanjing Bianzhen Biological
Technology) before implantation and the neck incision was closed
with sutures. The catheter was left in place until the required
experiments had been completed. At the end of each experiment, the
position of the polyethylene tubing in the intrathecal space was
visually confirmed by exposing the spinal cord post-mortem.
To investigate the involvement of microglial
activation in the CMI-induced cardiac pain, the following
behavioral pharmacological experiments were performed. Intrathecal
(i.t.) administration of minocycline (100 µg/10 µl; cat. no. M9511;
Sigma-Aldrich; Merck KGaA) was selected to inhibit microglial
activation, in accordance with a previous report (20). Fractalkine (60 ng/10 µl; i.t.; cat.
no. F135; Sigma-Aldrich; Merck KGaA), a microglia-activating
factor, was used to induce microglial activation (21). After CMI establishment, minocycline
or vehicle was applied daily from D17 to D20. OF tests were then
performed to verify the effects of the drug on cardiac pain
behaviors of CMI model rats (Fig.
1C). In CMI model rats receiving SCS treatment, either vehicle
or fractalkine was administered intrathecally 1 h after SCS to
detect the reversing influence of fractalkine on the SCS-produced
analgesia by OF testing (Fig.
1D).
Statistical analysis
Statistical analyses were performed using SPSS 22.0
software (IBM, Corp.). The results are expressed as the mean ± SEM.
Two-way ANOVA with Bonferroni multiple-comparison tests or one-way
ANOVA with Tukey's multiple-comparison post hoc tests were used for
between-group comparisons. For CPP experiments, data were analyzed
before conditioning (baseline) and after conditioning using
two-factor ANOVA (chambers vs. treatment) followed by Student's
t-test with Bonferroni correction. P<0.05 was considered to
indicate a statistically significant difference.
Results
CMI established by LAD ligation
produces significant spontaneous cardiac pain in rats
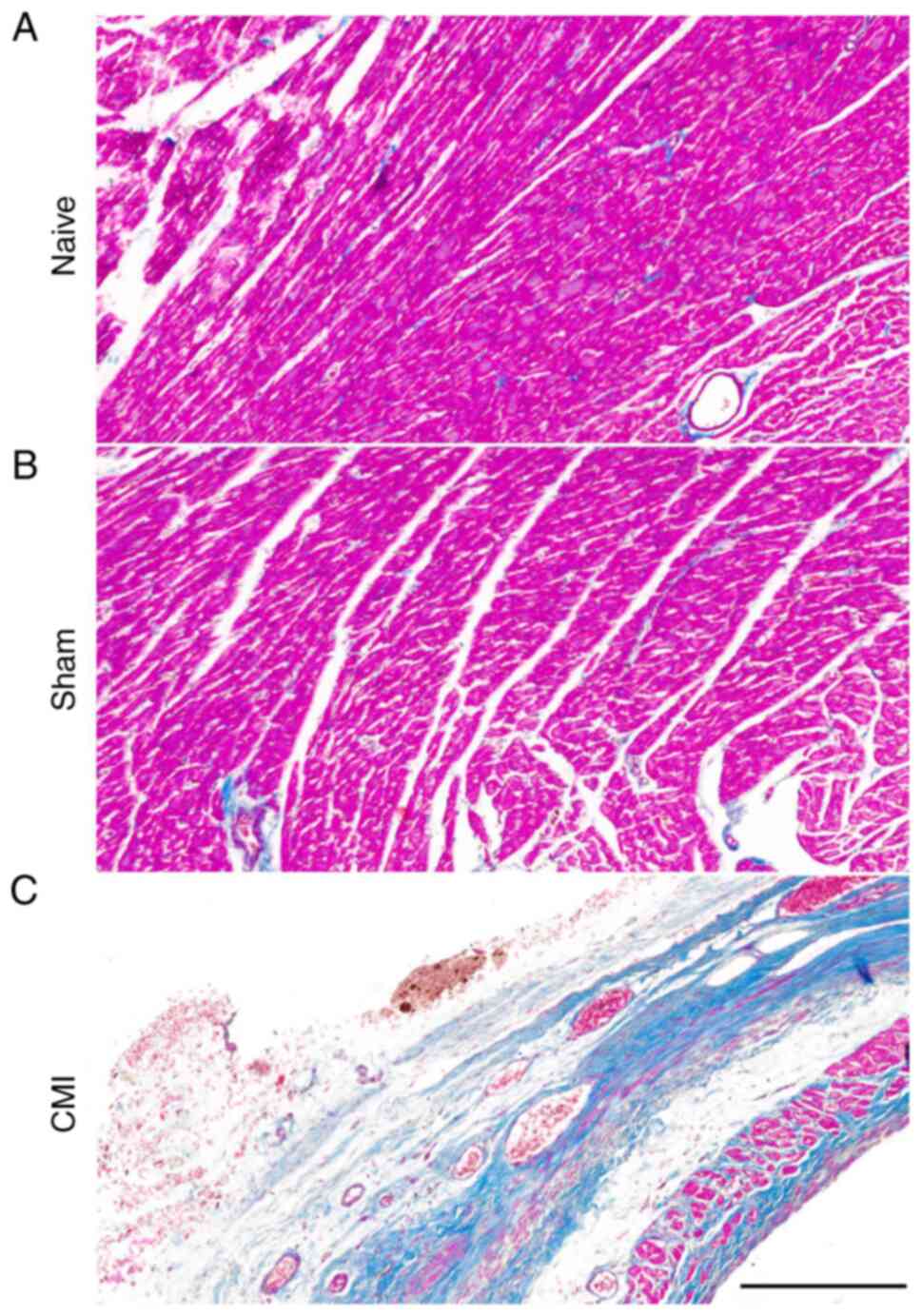
CMI model establishment was confirmed by examining a
remote area of the myocardium becoming pale after the LAD ligation.
Chronic infarction was confirmed using Masson's trichrome stain to
label collagen scar tissue (blue) and cardiac muscle (red) 3 weeks
post-operation (Fig. 2). No
difference was found between the naive and sham groups of rats;
however, clear collagen scarring rather than normal cardiac muscle
tissue was observed at cross sections of ventricular papillary
muscles in CMI model rats, in contrast to the findings in naive and
sham rats (Fig. 2), indicating
successful establishment of the CMI model.
Cardiac pain induced by CMI belongs to visceral pain
(3). Thus, OF tests were performed
to further observe the spontaneous pain behaviors based on previous
reports (14,22). The current study evaluated the
rearing count and distance traveled, which could reflect visceral
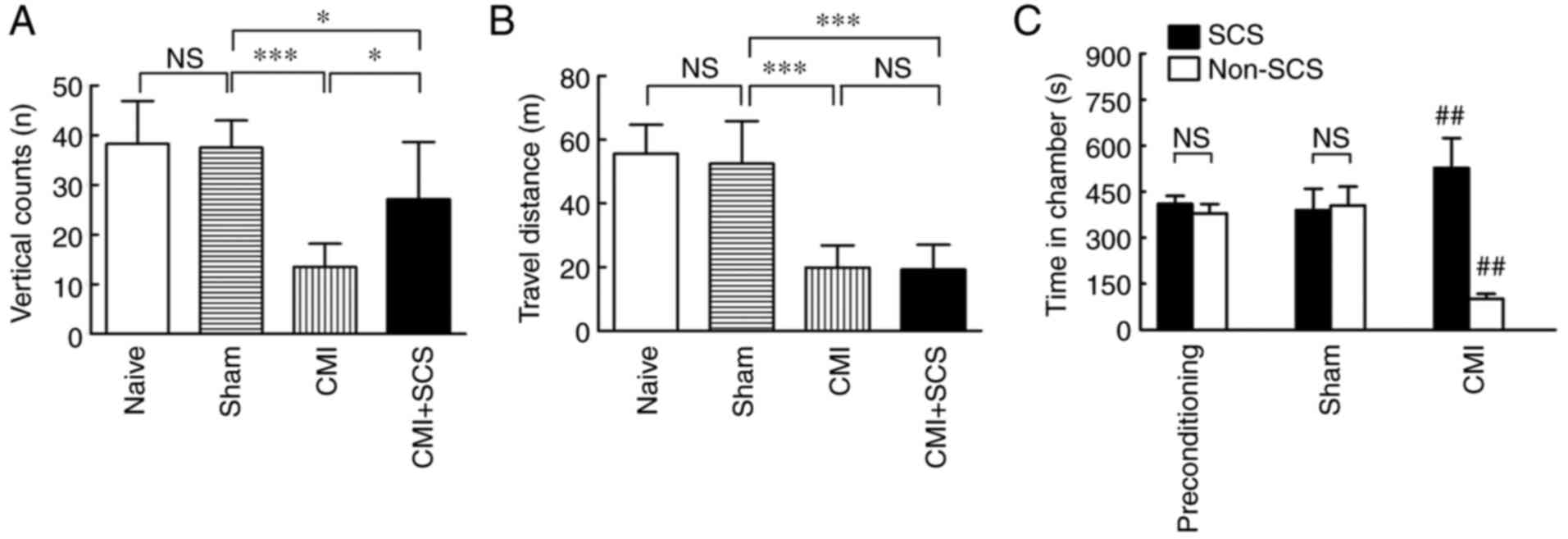
pain. No statistically significant differences in either vertical
count or distance traveled were observed between the naive and
sham-operated groups (n=8; Fig. 3A and
B, respectively). However, the vertical count was significantly
decreased in CMI model rats on day 21 post-operation compared with
that in the sham group (n=8; Fig.
3A). The results demonstrated that CMI established by LAD
ligation produced pronounced spontaneous cardiac pain. Similarly,
the distance traveled was also greatly decreased in CMI model rats
compared with that in the sham group (n=8; Fig. 3B), indicating that locomotion was
negatively affected by LAD ligation owing to cardiac insufficiency
in CMI model rats.
SCS effectively alleviates cardiac
pain in CMI model rats
Based on several studies on animals and human
patients, SCS is considered to be an effective alternative therapy
to treat many types of intractable pain, especially chronic pain
(23,24). Thus, it was next determined whether
SCS could alleviate such spontaneous cardiac pain in CMI model
rats. Before OF testing, SCS was performed on 4 consecutive days in
CMI model rats (Fig. 1A).
Interestingly, SCS improved the spontaneous cardiac pain, as
revealed by an increasing number of vertical counts in CMI model
rats compared with untreated CMI rats (n=8; Fig. 3A). However, there was no significant
difference between CMI and CMI + SCS groups in terms of locomotion,
as revealed by the distance traveled during the 15-min recording
time in the OF test (n=8; Fig. 3B).
The findings suggested that the effects of SCS on increasing
vertical counts did not depend on an improved locomotor ability in
CMI model rats. Thus, SCS could effectively alleviate the
spontaneous pain in CMI model rats without changing the declined
locomotor abilities.
It has been shown that chronic visceral pain
produces not only spontaneous pain but also ongoing pain conditions
(25). Thus, a CPP test was
conducted to investigate the effects of SCS on ongoing pain in CMI
model rats. Following a 3-day preconditioning phase, rats underwent
conditioning (8 days) using SCS or non-SCS treatment paired with
alternate chambers on alternate days (Fig. 1B). On the testing day, rats were
placed in the CPP box with free access to all of the chambers. Only
CMI model rats showed an increase in the time spent in the
SCS-paired chambers (n=8; Fig. 3C).
All groups spent equivalent time in the neutral chamber. SCS did
not produce any preference in the sham-operated rats, indicating
that it was not beneficial in the absence of CMI. The finding that
SCS produced CPP in CMI model rats demonstrated that SCS could
improve the ongoing pain under CMI conditions.
SCS inhibits microglial but not
astrocyte activation via the p-p38 pathway in spinal cord of CMI
model rats
Spinal cord glial cells have been reported to be
involved in the induction and maintenance of chronic pain (26). Therefore, glial activation in the
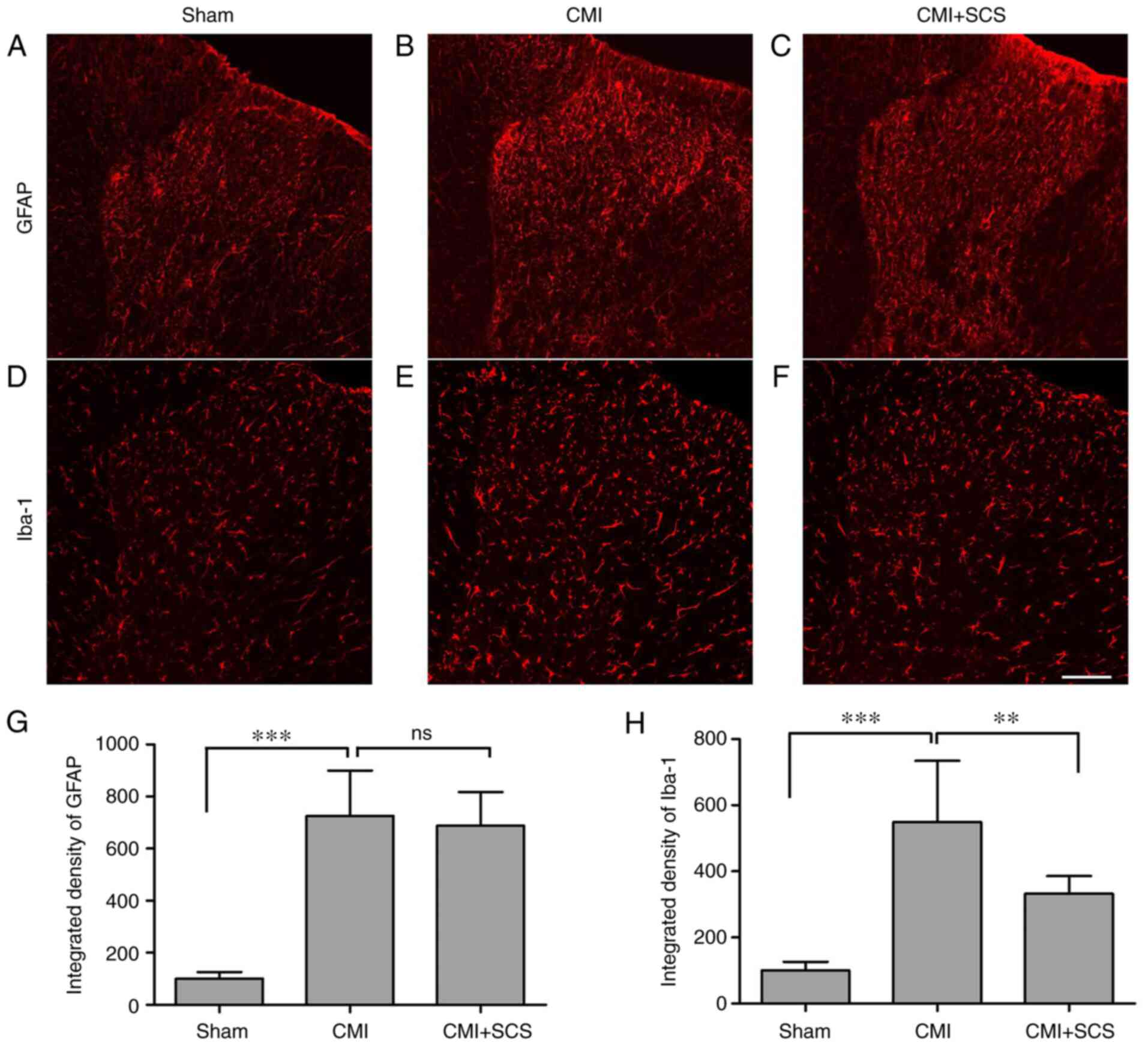
spinal cord of CMI model rats after SCS treatment was examined.
Results from immunofluorescence staining revealed that the
integrated densities of Iba-1-labeled microglia, as well as
GFAP-labeled astrocytes, were significantly increased in the
T1-T5 spinal dorsal horn 3 weeks after LAD
ligation (Fig. 4). However, SCS
could effectively suppress spinal dorsal horn Iba-1 upregulation
(Fig. 4H), but not that of GFAP in
CMI model rats (Fig. 4G), which
indicated that SCS could inhibit CMI-induced microglia instead of
astrocyte activation during the maintenance of chronic cardiac
pain.
As previous studies reported that functional
microglial activation was associated with the phosphorylation of
p38 MAPK (21,27), double-immunofluorescence staining
for spinal p-p38 was performed in rats with CMI. As shown in
Fig. 5, the immunoreactivity for
p-p38 was exclusively colocalized with Iba-1-positive cells but not
with NeuN-positive (neuronal marker) or GFAP-positive ones
(astrocyte marker). These results suggested that spinal dorsal horn
p-p38 expression may be a specific downstream signaling pathway in
spinal dorsal microglia after CMI.
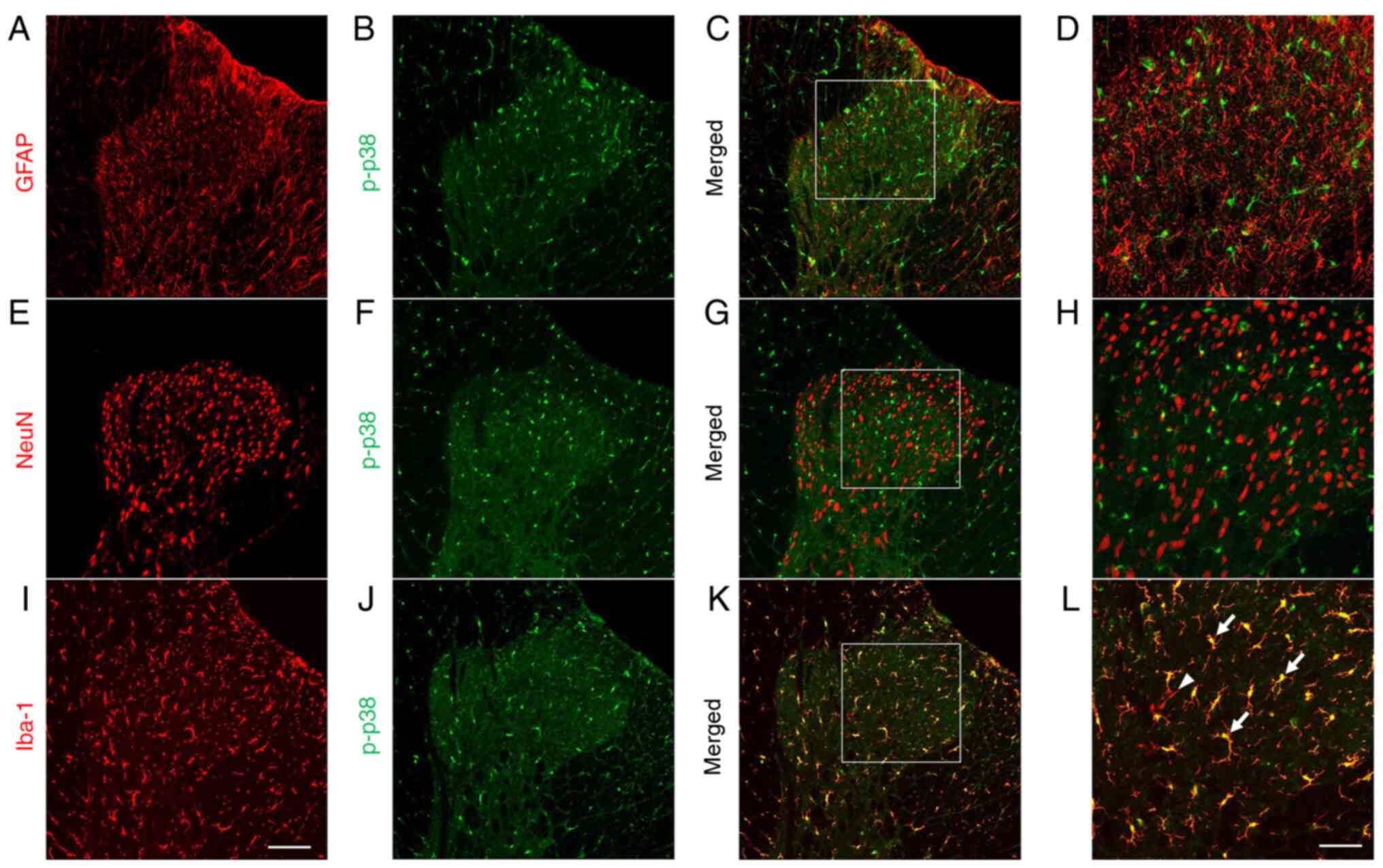 | Figure 5.p-p38 MAPK is exclusively expressed
in microglia in the spinal cord. Double-immunofluorescence staining
showed that spinal p-p38 was not co-expressed with (A-D)
GFAP-positive astrocytes or (E-H) NeuN-positive neurons. Instead,
p-p38 was colocalized with (I-L) Iba-1-positive microglia. (D, H
and L) Magnified images of the rectangles in (C, G and K,
respectively). Arrows indicate typical double-labeled (yellow)
cells and arrowhead indicates Iba-1 single-labeled microglia (red)
in panel L. Scale bar, 100 µm in panels A-C, E-G and I-K; scale
bar, 50 µm in panels D, H and L. GFAP, glial fibrillary acidic
protein; Iba-1, ionized calcium-binding adaptor protein-1; NeuN,
neuronal nuclei; p-, phosphorylated. |
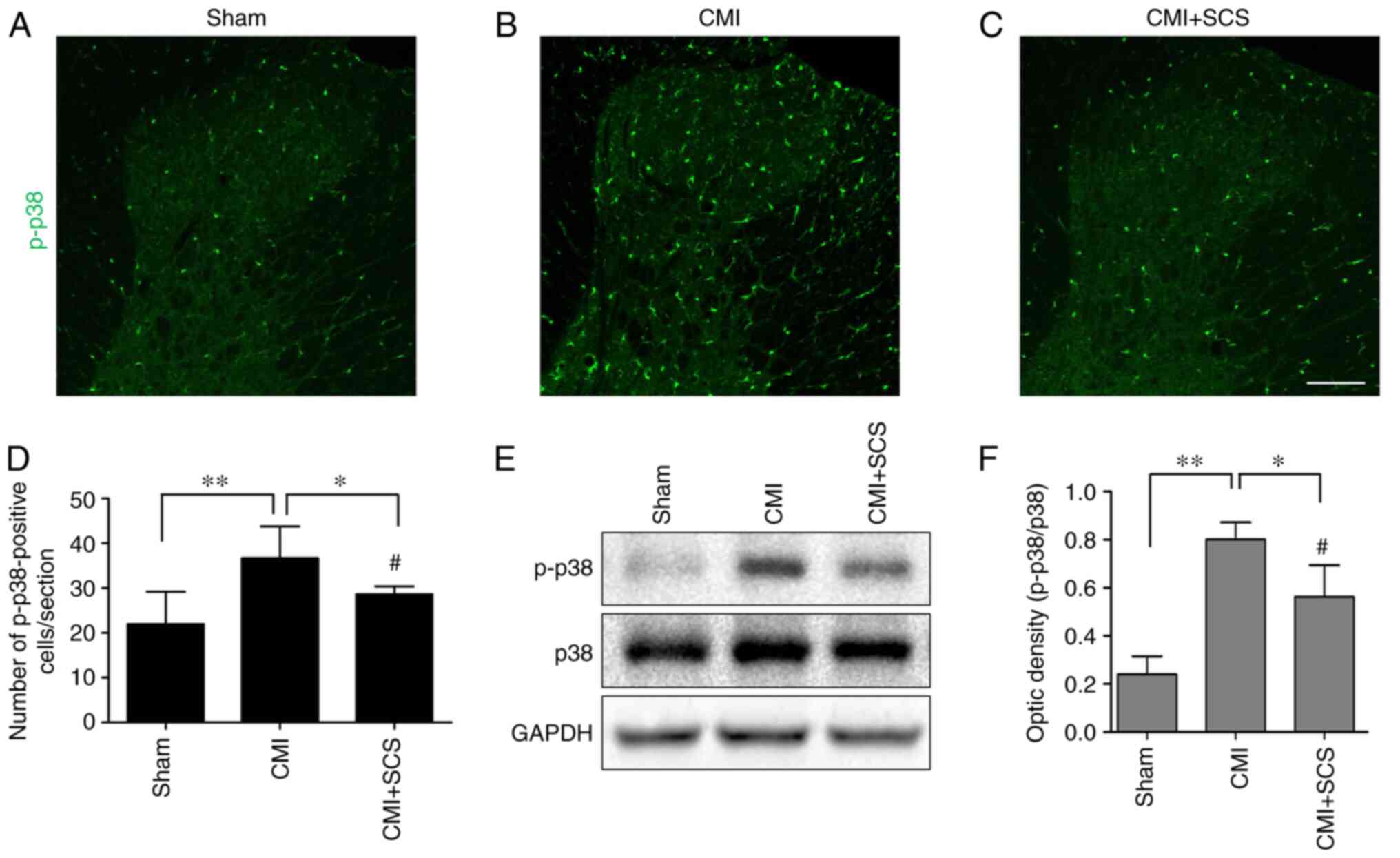
It was also observed that SCS could effectively
inhibit the upregulation of p-p38 expression in CMI model rats
(Fig. 6A-D). Subsequently, western
blotting was conducted to further analyze p-p38 expression in the
thoracic spinal dorsal horn in the different groups. Increased
ratios of p-p38/p38 were noted in rats with CMI compared with those
in the sham group (Fig. 6E and F).
However, the ratio of p-p38/p38 from the CMI + SCS group was
significantly decreased compared with that in the CMI group
(Fig. 6E and F). These results
suggested that the p-p38 signaling pathway was activated after CMI,
and SCS could effectively inhibit this pathway and microglial
activation.
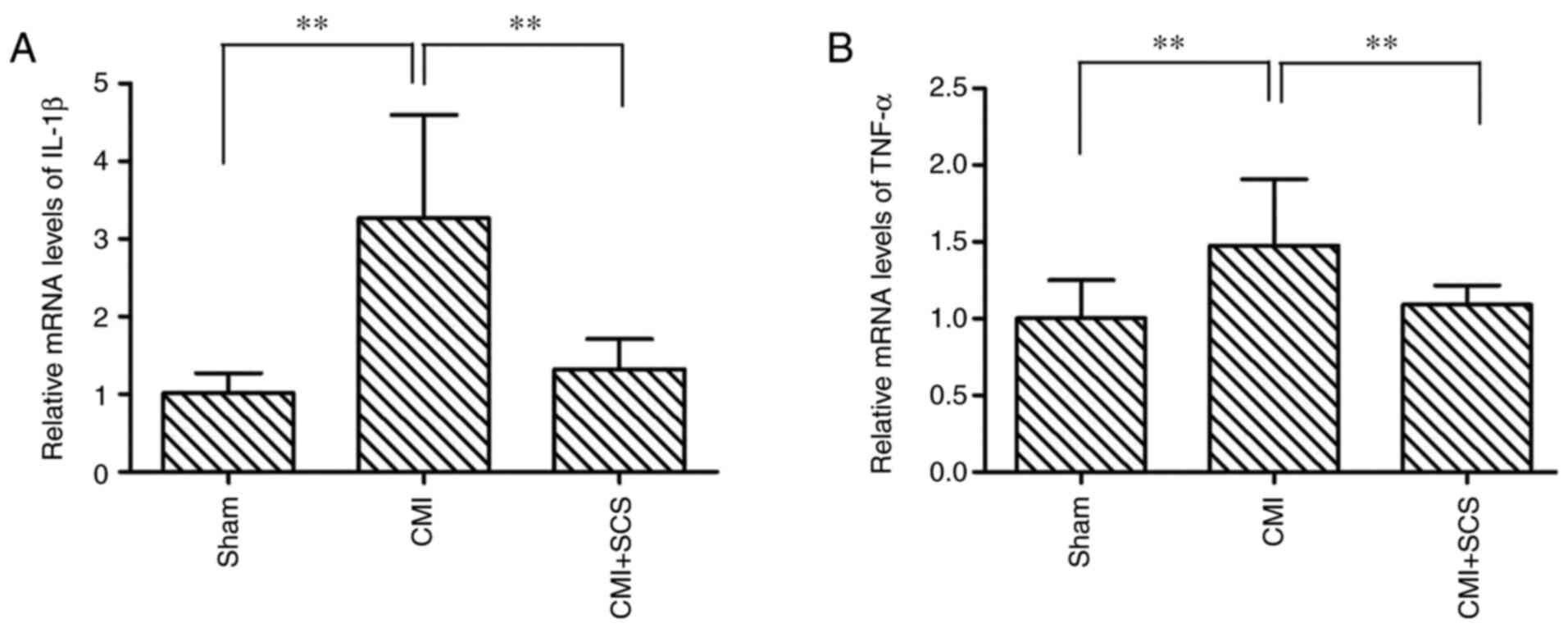
Immune responses (glial activation and cytokine
release) in the spinal dorsal horn may serve essential roles in the
induction and maintenance of chronic pain (28). Thus, the changes in expression
levels of pro-inflammatory mediators, including IL-1β and TNF-α, in
the spinal cord were determined. Using RT-qPCR, it was observed
that compared with sham group, CMI induced significant increases in
the mRNA expression levels of pro-inflammatory mediators in the
spinal dorsal horn: IL-1β (332.6±146.7%; Fig. 7A) and TNF-α (149.3±45.2%; Fig. 7B). However, SCS treatment
effectively attenuated the mRNA expression of these mediators:
IL-1β (141.7±36.1%) and TNF-α (113.7±13.1%). These findings
indicated that SCS may prevent the production of pro-inflammatory
mediators in the spinal dorsal horn in CMI model rats.
Microglial inhibition reduces the
CMI-induced cardiac pain, whereas microglial activation partially
reverses the therapeutic effects of SCS on CMI model rats
To further examine the involvement of microglia in
the SCS-produced analgesia in CMI model rats, a behavioral
pharmacological study was performed. As shown in Fig. 1C and D, CMI model rats with LAD
ligation were simultaneously implanted with an intrathecal
catheter. The vertical counts in the OF tests were recorded in the
different groups to reflect the pain behaviors. The intrathecal
administration of minocycline, a microglial inhibitor, to CMI model
rats significantly increased the vertical counts compared with
CMI+Veh group (Fig. 8), which
indicated that microglial activation may contribute to the
CMI-induced cardiac pain. Nonetheless, the vertical activity times
in the CMI + minocycline group remained lower compared with those
in the CMI + SCS group. The microglial activator fractalkine was
then administered intrathecally to activate spinal cord microglia
in CMI model rats with SCS treatment. Interestingly, compared with
the vehicle group, the improved cardiac pain behaviors after SCS
treatment were in turn aggravated in the fractalkine group, as
revealed by decreased vertical counts in the OF test. These results
demonstrated that microglial inhibition improved the cardiac pain
behaviors in CMI model rats. By contrast, rescuing the deactivated
microglia in the spinal cord could in part reverse the analgesic
effects of SCS, suggesting that the SCS-induced cardiac
pain-relieving effects were partially exerted by inhibiting
microglial activation.
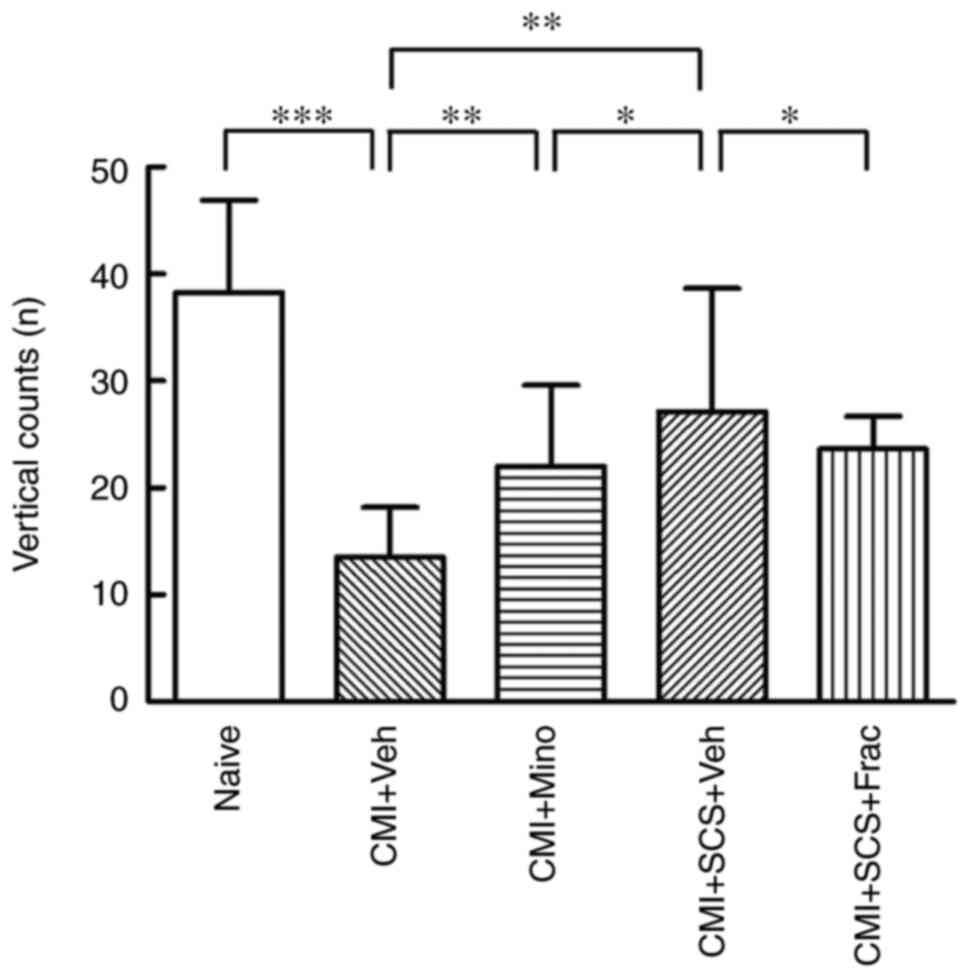 | Figure 8.Behavioral pharmacological
assessments of CMI rats in open field tests. Microglial inhibition
reduces CMI-induced cardiac pain, whereas microglial activation
partially reverses the therapeutic effects of SCS on CMI model
rats, as revealed by the open field test. The declined vertical
count in CMI model rats was increased by i.t. Mino administration.
This therapeutic effect was weaker compared with that of SCS
treatment on CMI model rats. However, following i.t. Frac
administration, the vertical count of CMI model rats after SCS was
decreased. N=8 rats/group. *P<0.05, **P<0.01, ***P<0.001.
CMI, chronic myocardial ischemia; Frac, fractalkine; i.t.,
intrathecal; Mino, minocycline; SCS, spinal cord stimulation; Veh,
vehicle. |
Discussion
Cardiac pain information is now considered to be
transmitted into the brain via two main ascending pathways: Spinal
afferents and vagal afferents (3).
Cardiac spinal afferents originating from the heart terminate at
the T1-T5 segments of the spinal cord dorsal
horn through DRG and, thus, ascend to higher brain structures. In
addition, vagal afferents also convey cardiac nociceptive
information to the NTS in the brainstem and then to higher brain
regions (5). It is well known that
chronic pain, including CMI-induced chronic cardiac pain, shares
several similar characteristics, among which central sensitization
is the pivotal mechanism that differs from acute pain (7). In total, three key mechanisms have
been implicated in central sensitization: i) Alteration in
excitatory glutamatergic neurotransmission, ii) loss of tonic
inhibitory controls (disinhibition) and iii) glial-neuronal
interactions (7). Our previous work
reported that the excitatory synaptic transmission in the NTS was
potentiated after CMI (6),
indicating that central sensitization developed under chronic
cardiac pain conditions. However, to the best of our knowledge,
whether central sensitization occurs at the spinal cord level after
CMI-induced chronic cardiac pain remains to be investigated.
The present study demonstrated that microglia and
astrocytes were activated in the thoracic spinal cord dorsal horn 3
weeks after LAD ligation. The intrathecal administration of the
specific microglial inhibitor minocycline alleviated cardiac pain
behaviors in CMI model rats. These results suggested that
microglial and astrocyte activation was involved in the chronic
cardiac pain. The present results coincide with previous findings
in nerve injury-induced chronic neuropathic pain as well as
inflammatory visceral pain (21,29).
In fact, spinal microglia have been demonstrated to contribute to
the initiation and maintenance of chronic pain, whereas astrocytes
participate in the persistence of chronic pain in later phases
(7). However, whether the roles of
microglia and astrocytes in CMI-induced cardiac pain are similar to
those in neuropathic pain warrants further investigation.
p38 MAPK is activated (phosphorylated) in spinal
microglia after nerve injury and is implicated in the production of
neuropathic pain (30). A number of
studies have also demonstrated that the inhibition of p38 MAPK
alleviates allodynia and hyperalgesia in several models of
inflammatory and neuropathic pain, suggesting a crucial role of
this signaling pathway in pain processing (31,32).
In a chronic pancreatitis pain model, spinal p-p38 expression was
found to be greatly elevated at 3 weeks after the establishment of
pancreatitis and was reversed by i.t. minocycline treatment
(21). The current study
demonstrated that spinal p-p38 expression was exclusively
colocalized with microglia and was significantly increased in CMI
model rats. MAPK activation initiates signaling cascades and
increases the synthesis of pro-inflammatory mediators. These
cytokines can drive central sensitization by increasing excitation
in spinal dorsal horn neurons (30). It has been reported that cytokines
such as IL-1β released from glial cells could bind to their
receptors on neurons and facilitate N-methyl-D-aspartic acid
receptor-related synaptic transmission (33). Consistent with this, the current
data also suggested that IL-1β and TNF-α were increased in the
spinal dorsal horn in CMI model rats, indicating that the
microglial p38 MAPK pathway may be activated, and its downstream
pro-inflammatory mediators were then elevated under chronic cardiac
pain conditions.
Given its efficacy, SCS has been clinically utilized
in patients suffering from chronic intractable angina pectoris,
especially refractory angina pectoris, as well as cardiac syndrome
X (2,9). It has been reported that SCS could
produce an anti-ischemic effect, which was caused by modulation of
the sympathetic nervous system, in refractory angina pectoris
(34). Moreover, redistribution of
coronary blood flow in the heart after SCS was observed in a
radiographic study (35).
Additionally, echocardiographic studies on both porcine and canine
ischemic heart failure models showed that SCS treatment improved
left ventricular dilation and cardiac function (36). Although numerous studies on its
effects have been performed, the underlying analgesic mechanisms of
the cardiac pain-relieving effects of SCS remain to be elucidated.
However, accumulating evidence suggests that SCS therapy focuses
primarily on neuronal effects (23). Nonetheless, glial cell mechanisms
have also been reported to be involved in the SCS-induced
analgesia. In a neuropathic pain rat model, SCS was revealed to
inhibit spinal glial activation and produce analgesia (11), although the downstream intracellular
signaling pathways underlying glial cell modulation induced by SCS
were not studied. Moreover, spinal cord glia-related genes were
shown to be altered by SCS in nerve injury rats (37). Additionally, SCS was reported to
increase M1-like rather than M2-like microglial mRNA in a chronic
constriction injury model (38).
These reports indicate that SCS modulates the activation of glial
cells in the spinal cord in neuropathic pain.
In the present work, it was observed that SCS
effectively alleviated chronic cardiac pain and inhibited
microglial activation in CMI model rats. In addition, SCS inhibited
CMI-induced spinal activation of p38 MAPK, which was specifically
expressed in microglia rather than astrocytes and neurons.
Moreover, the pro-inflammatory mediators IL-1β and TNF-α were
downregulated by SCS in CMI model rats. Finally, the activation of
microglia partly abolished the SCS-produced pain-relieving effects.
These findings suggested that SCS inhibits the microglial p38 MAPK
pathway and downstream neuroinflammation, thereby contributing to
the alleviation of chronic cardiac pain. The present results agree
with previous work that showed SCS decreased microglial expression
and p38 MAPK phosphorylation in spared nerve injury-induced
neuropathic pain rats (11).
Moreover, it has been demonstrated that SCS could reduce the
activation of spinal microglia in a spinal cord ischemic
reperfusion model in rabbits (39).
However, another study suggested that SCS enhanced spinal
microglial activation in a chronic constriction injury model
(38). It was suggested that this
discrepancy may partly be due to differences in animal models,
post-surgery time points and SCS protocols among the studies.
How SCS inhibits spinal microglia and relieves
cardiac pain remains unknown. Interestingly, other neuromodulation
therapeutic treatments, including electroacupuncture (40) and transcutaneous electrical nerve
stimulation (41), have been
reported to reduce glial activation. It has been proven that SCS
could increase the release of the inhibitory neurotransmitters
γ-aminobutyric acid (GABA), serotonin, acetylcholine and opioids in
the spinal dorsal horn (23).
Microglia express corresponding inhibitory neurotransmitter
receptors, including GABAergic, serotonergic and opioidergic
receptors, and thus are directly deactivated by such release
(42,43). Alternatively, these inhibitory
neurotransmitters could also reduce the release of neuronal
excitatory neurotransmitters, such as glutamate and ATP, which in
turn decrease glial cell activity indirectly (44). Specifically, it has been
demonstrated that P2X purinoceptor 7 (P2X7) and P2X4 are expressed
on microglia (45,46). Once these purinergic receptors are
activated by ATP, the MAPK pathway (including p38) is
phosphorylated, and the downstream pro-inflammatory mediators IL-1β
and TNF-α are synthesized and influence neurons to perpetuate the
nociceptive response. Conversely, SCS inhibits chronic cardiac pain
partially by inhibiting microglial p38 MAPK and its downstream
pro-inflammatory mediators, such as IL-1β and TNF-α. Consequently,
besides the well-known reinforcing ‘gate-control’ effect, we
proposed that SCS could also modulate glial-neuronal interactions
to reverse central sensitization in chronic cardiac pain
conditions.
The present study has some limitations. As it only
used conventional SCS, the effects of other SCS paradigms, such as
high-frequency, sub-sensory threshold and burst SCS, on chronic
cardiac pain require further investigation. Moreover, transgenic
animals should be introduced to confirm the role of SCS in such a
chronic cardiac pain model.
In summary, the present study provided evidence that
SCS reduces chronic cardiac pain partially by inhibiting the spinal
microglial p38 MAPK pathway and thus potentially downregulating the
expression of pro-inflammatory mediators, such as IL-1β and TNF-α.
Therefore, SCS therapy may be a promising way to treat intractable
chronic angina pectoris.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural
Science Foundation of China (grant no. 81701115) and The Key
Research and Development Program of Shannxi Province (grant no.
2020SF-242).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, YQL and JBZ designed the study, wrote the
manuscript and confirmed the authenticity of the raw data. JW, XCW
and MMZ completed the animal experiments and the statistical
analysis. JHR and YS participated in the morphological and
behavioral experiments. JZL participated in the western blot
analysis. XQW and SYH participated in statistical analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were performed in accordance with
the ethical guidelines of the International Association for the
Study of Pain and were approved by the Animal Use and Care
Committee for Research and Education at the General Hospital of
Western Command Theater and Air Force Military Medical University
(Chengdu, China; approval no. 10070).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Waltenberger J: Chronic refractory angina
pectoris: Recent progress and remaining challenges. Eur Heart J.
38:2556–2558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hale J, Bailey-Classen A and Cheng J:
Spinal cord stimulation for refractory angina pectoris. Pain Med.
21:198–200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foreman RD, Garrett KM and Blair RW:
Mechanisms of cardiac pain. Compr Physiol. 5:929–960. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosen SD, Paulesu E, Wise RJ and Camici
PG: Central neural contribution to the perception of chest pain in
cardiac syndrome X. Heart. 87:513–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosen SD: From heart to brain: The genesis
and processing of cardiac pain. Can J Cardiol. 28 (2 Suppl):S7–S19.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Zhang MM, Tu K, Wang J, Feng B,
Zhang ZN, Lei J, Li YQ, Du JQ and Chen T: The excitatory synaptic
transmission of the nucleus of solitary tract was potentiated by
chronic myocardial infarction in rats. PLoS One. 10:e01188272015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Basbaum AI, Bautista DM, Scherrer G and
Julius D: Cellular and molecular mechanisms of pain. Cell.
139:267–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lanza GA, Grimaldi R, Greco S, Ghio S,
Sarullo F, Zuin G, De Luca A, Allegri M, Di Pede F, Castagno D, et
al: Spinal cord stimulation for the treatment of refractory angina
pectoris: A multicenter randomized single-blind study (the SCS-ITA
trial). Pain. 152:45–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan X, Bao H, Si Y, Xu C, Chen H, Gao X,
Xie X, Xu Y, Sun F and Zeng L: Spinal cord stimulation for
refractory angina pectoris: A systematic review and meta-analysis.
Clin J Pain. 33:543–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marchand S: Spinal cord stimulation
analgesia: Substantiating the mechanisms for neuropathic pain
treatment. Pain. 156:364–365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato KL, Johanek LM, Sanada LS and Sluka
KA: Spinal cord stimulation reduces mechanical hyperalgesia and
glial cell activation in animals with neuropathic pain. Anesth
Analg. 118:464–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Zhan Y, Wang Y, Han D, Tao B, Luo
Z, Ma S, Wang Q, Li X, Fan L, et al: Chitosan/silk fibroin modified
nanofibrous patches with mesenchymal stem cells prevent heart
remodeling post-myocardial infarction in rats. Acta Biomater.
80:154–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang MM, Liu SB, Chen T, Koga K, Zhang T,
Li YQ and Zhuo M: Effects of NB001 and gabapentin on irritable
bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol
Brain. 7:472014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
King T, Vera-Portocarrero L, Gutierrez T,
Vanderah TW, Dussor G, Lai J, Fields HL and Porreca F: Unmasking
the tonic-aversive state in neuropathic pain. Nat Neurosci.
12:1364–1366. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Feng DY, Li ZH, Feng B, Zhang H,
Zhang T, Chen T and Li YQ: Activation of the mammalian target of
rapamycin in the rostral ventromedial medulla contributes to the
maintenance of nerve injury-induced neuropathic pain in rat. Neural
Plast. 2015:3948202015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuang ZY, Wen YR, Zhang DR, Borsello T,
Bonny C, Strichartz GR, Decosterd I and Ji RR: A peptide c-Jun
N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after
spinal nerve ligation: Respective roles of JNK activation in
primary sensory neurons and spinal astrocytes for neuropathic pain
development and maintenance. J Neurosci. 26:3551–3560. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao YH, Wang J, Wei YY, Zhang T, Zhang Y,
Zuo ZF, Teng XY and Li YQ: Histone deacetylase 2 is involved in
µ-opioid receptor suppression in the spinal dorsal horn in a rat
model of chronic pancreatitis pain. Mol Med Rep. 17:2803–2810.
2018.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhandare AM, Kapoor K, Powell KL, Braine
E, Casillas-Espinosa P, O'Brien TJ, Farnham MMJ and Pilowsky PM:
Inhibition of microglial activation with minocycline at the
intrathecal level attenuates sympathoexcitatory and
proarrhythmogenic changes in rats with chronic temporal lobe
epilepsy. Neuroscience. 350:23–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu PY, Lu CL, Wang CC, Lee IH, Hsieh JC,
Chen CC, Lee HF, Lin HC, Chang FY and Lee SD: Spinal microglia
initiate and maintain hyperalgesia in a rat model of chronic
pancreatitis. Gastroenterology. 142:165–173.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Li ZH, Feng B, Zhang T, Zhang H,
Li H, Chen T, Cui J, Zang WD and Li YQ: Corticotrigeminal
projections from the insular cortex to the trigeminal caudal
subnucleus regulate orofacial pain after nerve injury via
extracellular signal-regulated kinase activation in insular cortex
neurons. Front Cell Neurosci. 9:4932015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sdrulla AD, Guan Y and Raja SN: Spinal
cord stimulation: Clinical efficacy and potential mechanisms. Pain
Pract. 18:1048–1067. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dones I and Levi V: Spinal cord
stimulation for neuropathic pain: Current trends and future
applications. Brain Sci. 8:1382018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Winston JH, Fu Y, Guptarak J,
Jensen KL, Shi XZ, Green TA and Sarna SK: Genesis of anxiety,
depression, and ongoing abdominal discomfort in ulcerative
colitis-like colon inflammation. Am J Physiol Regul Integr Comp
Physiol. 308:R18–R27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji RR, Chamessian A and Zhang YQ: Pain
regulation by non-neuronal cells and inflammation. Science.
354:572–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berta T, Qadri YJ, Chen G and Ji RR:
Microglial signaling in chronic pain with a special focus on
caspase 6, p38 MAP kinase, and sex dependence. J Dent Res.
95:1124–1131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen G, Zhang YQ, Qadri YJ, Serhan CN and
Ji RR: Microglia in pain: Detrimental and protective roles in
pathogenesis and resolution of pain. Neuron. 100:1292–1311. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Mei XP, Chen L, Tang J, Li JL, Wu
SX, Xu LX and Li YQ: Triptolide prevents and attenuates neuropathic
pain via inhibiting central immune response. Pain Physician.
15:E995–E1006. 2012.PubMed/NCBI
|
|
30
|
Mai L, Zhu X, Huang F, He H and Fan W: p38
mitogen-activated protein kinase and pain. Life Sci.
256:1178852020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiyomoto M, Shinoda M, Honda K, Nakaya Y,
Dezawa K, Katagiri A, Kamakura S, Inoue T and Iwata K: p38
phosphorylation in medullary microglia mediates ectopic orofacial
inflammatory pain in rats. Mol Pain. 11:482015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taves S, Berta T, Liu DL, Gan S, Chen G,
Kim YH, Van de Ven T, Laufer S and Ji RR: Spinal inhibition of p38
MAP kinase reduces inflammatory and neuropathic pain in male but
not female mice: Sex-dependent microglial signaling in the spinal
cord. Brain Behav Immun. 55:70–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castany S, Gris G, Vela JM, Verdu E and
Boadas-Vaello P: Critical role of sigma-1 receptors in central
neuropathic pain-related behaviours after mild spinal cord injury
in mice. Sci Rep. 8:38732018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park KE and Conti CR: Non-PCI/CABG
therapies for refractory angina. Trends Cardiovasc Med. 28:223–228.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saraste A, Ukkonen H, Varis A, Vasankari
T, Tunturi S, Taittonen M, Rautakorpi P, Luotolahti M, Airaksinen
KE and Knuuti J: Effect of spinal cord stimulation on myocardial
perfusion reserve in patients with refractory angina pectoris. Eur
Heart J Cardiovasc Imaging. 16:449–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lopshire JC and Zipes DP: Spinal cord
stimulation for heart failure: Preclinical studies to determine
optimal stimulation parameters for clinical efficacy. J Cardiovasc
Transl Res. 7:321–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vallejo R, Kelley CA, Gupta A, Smith WJ,
Vallejo A and Cedeno DL: Modulation of neuroglial interactions
using differential target multiplexed spinal cord stimulation in an
animal model of neuropathic pain. Mol Pain.
16:17448069209180572020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shu B, He SQ and Guan Y: Spinal cord
stimulation enhances microglial activation in the spinal cord of
nerve-injured rats. Neurosci Bull. 36:1441–1453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong X, Li H, Lu J, Yang Y, Jing H, Cheng
Y, Jin M and Cheng W: Spinal cord stimulation postconditioning
reduces microglial activation through down-regulation of ERK1/2
phosphorylation during spinal cord ischemic reperfusion in rabbits.
Neuroreport. 29:1180–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang Y, Qiu Y, Du J, Liu J and Fang J,
Zhu J and Fang J: Inhibition of spinal microglia and astrocytes
contributes to the anti-allodynic effect of electroacupuncture in
neuropathic pain induced by spinal nerve ligation. Acupunct Med.
34:40–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matsuo H, Uchida K, Nakajima H, Guerrero
AR, Watanabe S, Takeura N, Sugita D, Shimada S, Nakatsuka T and
Baba H: Early transcutaneous electrical nerve stimulation reduces
hyperalgesia and decreases activation of spinal glial cells in mice
with neuropathic pain. Pain. 155:1888–1901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maduna T, Audouard E, Dembele D, Mouzaoui
N, Reiss D, Massotte D and Gaveriaux-Ruff C: Microglia express Mu
opioid receptor: Insights from transcriptomics and fluorescent
reporter mice. Front Psychiatry. 9:7262019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
El Oussini H, Bayer H, Scekic-Zahirovic J,
Vercruysse P, Sinniger J, Dirrig-Grosch S, Dieterlé S,
Echaniz-Laguna A, Larmet Y, Müller K, et al: Serotonin 2B receptor
slows disease progression and prevents degeneration of spinal cord
mononuclear phagocytes in amyotrophic lateral sclerosis. Acta
Neuropathol. 131:465–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen T, Willoughby KA and Ellis EF: Group
I metabotropic receptor antagonism blocks depletion of calcium
stores and reduces potentiated capacitative calcium entry in
strain-injured neurons and astrocytes. J Neurotrauma. 21:271–281.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Trang T, Beggs S, Wan X and Salter MW:
P2X4-receptor-mediated synthesis and release of brain-derived
neurotrophic factor in microglia is dependent on calcium and
p38-mitogen-activated protein kinase activation. J Neurosci.
29:3518–3528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan X, Ma W, Zhang Y and Zhang L: P2X7
receptor (P2X7R) of microglia mediates neuroinflammation by
regulating (NOD)-like receptor protein 3 (NLRP3)
inflammasome-dependent inflammation after spinal cord injury. Med
Sci Monit. 26:e9254912020. View Article : Google Scholar : PubMed/NCBI
|