Introduction
Spontaneous abortion refers to the termination of
pregnancy at <20 weeks or a fetal weight of <500 g. Recurrent
spontaneous abortion (RSA) refers to ≥3 consecutive spontaneous
abortions with the same spouse and this is one of the most common
refractory diseases in pregnancy (1,2).
The causes of RSA are complex and include genetic predispositions,
female anatomy, hormones, infection and mental health (3,4).
The causes underlying >50% of RSAs remain to be determined and
further investigation is required in the context of clinical
practice (5).
The occurrence and pathogenesis of RSA is closely
associated with the abnormal biological behavior and dysfunction of
trophoblasts (6). Dysfunction of
extravillous trophoblasts, invasion defects and uterine spiral
artery remodeling are key pathological characteristics of RSA
(7). However, the mechanisms
underlying the dysfunctional invasion of trophoblasts and abnormal
uterine spiral artery remodeling in RSA remain to be
elucidated.
Formyl peptide receptor 2 (FPR2) is involved in the
occurrence and development of inflammation, tumors and glucose and
lipid metabolism disorders, which remain key potential therapeutic
targets for a number of diseases, such as colorectal cancer, acute
lung injury, COPD and diabetic retinopathy. The results of a
previous study demonstrate that FPR2 is expressed in trophoblasts
(8), but the specific functions
remain unclear. The results of previous studies have highlighted
the role of FPR2 in regulating the biological function of
trophoblasts in complications associated with pregnancy (8–11).
FPR2 expression is associated with the activation of
PI3K/AKT, MAPK, phospholipase C/protein kinase C, NF-κB, Janus
kinase (JAK)/STAT and other signaling pathways that regulate the
proliferation, migration and apoptosis of tumor cells (12–14). The PI3K/AKT signaling pathway is a
classical signaling pathway involved in the regulation of cell
function and metabolism through either direct regulation of
nutrient transporters and metabolic enzymes, or the control of
transcription factors that mediate the expression of key components
of metabolic pathways (15).
Targeting the PI3K/AKT signaling pathway is an effective method to
treat a number of diseases, such as types of cancer, and may act as
an effective therapeutic target in the clinic (16). However, the role of FPR2 in
affecting trophoblast function via the PI3K/AKT signaling pathway
in RSA remains to be elucidated.
In the present study, clinical villi tissue samples
were used to determine the expression levels and effects of FPR2 in
women who had experienced RSA. The role of FPR2 in regulating the
biological function of trophoblasts was elucidated using FPR2
knockdown in human HTR-8/SVneo trophoblasts in vitro. The
associated signaling pathway was confirmed through the use of a
PI3K inhibitor. The present study clarified the role and mechanisms
underlying FPR2 in the regulation of trophoblast function and
provided novel targets for the treatment of RSA.
Materials and methods
Patients and sample collection
Clinical samples were obtained from the Maternal and
Child Health Care Hospital of Shandong Province between January
2020 and June 2021. The present study was approved by the Ethics
Committee of the Maternal and Child Health Care Hospital of
Shandong province. All patients signed informed consent for
scientific research. Placental villi tissue samples were obtained
from 10 women who were 6–10 weeks pregnant and had experienced
spontaneous abortion ≥2 times, 24–33 years old. Patients with
genetic factors, endocrine imbalances, immune disruption,
infection, genital malformation and other factors were excluded. In
addition, 10 age-matched pregnant women voluntarily admitted to
hospital for induced abortion due to unwanted pregnancy at 6–10
weeks of gestation were selected as the control (CT) samples. These
healthy CTs had no history of hypertension, diabetes or adverse
pregnancy. Samples were collected immediately after caesarean and
washed with PBS. Half of the sample was stored in liquid nitrogen
and the other half were fixed in 4% paraformaldehyde at room
temperature overnight, embedded in paraffin and sectioned at 4
µm.
Immunohistochemical staining
The aforementioned sections were boiled in 0.01
mol/l citrate buffer (pH 6.0) for 10 min for antigen retrieval and
subsequently cooled to room temperature. Methanol containing 3%
H2O2 was used to eliminate endogenous
peroxidases. After being blocked with 5% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min, the sections were
incubated with the anti-FPR2 primary antibody (cat. no. NLS1878;
Novus Biologicals, LLC; 1:500) in a wet box overnight at 4°C.
Following primary incubation, the sections were incubated with the
rabbit IgG (H+L) secondary antibody (cat. no. 31460; Invitrogen;
1:500) at 37°C for 1 h. Subsequently, a diaminobenzidine reagent
kit (1:1,000; Sigma-Aldrich) was used. For cell culture slides, a
secondary antibody conjugated to FITC (cat. no. AP132F;
Sigma-Aldrich; Merck KGaA; 1:1,000) was used at 37°C for 1 h.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The quality and
concentration of RNA were confirmed using a NanoDrop1000 (Thermo
Fisher Scientific, Inc.). Total RNA was reverse transcribed into
cDNA using High-Capacity cDNA Reverse Transcription kit (cat. no.
4368814; Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. qPCR was subsequently
performed using the LightCycler II 480 (Roche Diagnostics GmbH).
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95°C for 30 sec, followed by 40 cycles at
95°C for 5 sec, 60°C for 10 sec, 72°C for 15 sec, and melting from
65°C to 95°C, every 0.11°C/1 sec, and 40°C for 10 sec. The
expression level of mRNA was assessed using the 2−ΔΔCq
method (17). The following
primer pairs were used for qPCR: FPR2 forward,
5′-ATGTCCATTGTTGCCATCTGC-3′ and reverse,
5′-GACGTAAAGCATGGGGTTGAG-3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′.
Cell culture
The human trophoblast cell line HTR8/SVneo,
originating from human placental trophoblast cells, was purchased
from the American Type Culture Collection. Cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2 incubator. When ~80% confluence was reached, cells
were plated onto a six-well culture plate at 1×105
cells/well for subsequent experiments. The PI3K inhibitor-LY294002
(10 µM) was purchased from MedChemExpress. As control, cells in
solvent groups were treated with the solvent of LY294002 (10% DMSO,
40% PEG300, 5% Tween-80 and 45% saline).
Cell transfection
Transfection of FPR2 small interfering (si)RNA
purchased from Thermo Fisher Scientific, Inc. (cat. no. AM16708)
was used to knockdown the FPR2 gene in trophoblasts. siR NC #1 was
purchased from Guangzhou RiboBio Co., Ltd. (cat. no.
siN0000001-1-5) as the negative control (NC). HTR8/SVneo cells were
inoculated into a 6-well plate and divided into two groups:
siRNA-NC and siRNA-FPR2. When ~50% confluence was reached, complete
medium (RPMI-1640 medium supplemented with 10% FBS) was replaced by
reduced-serum medium. A total of 4 µl Lipofectamine®
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and 6 µl FPR2 siRNA were mixed, incubated at room temperature
for 15 min and subsequently added into the aforementioned
reduced-serum medium. Cells were incubated at 37°C in 5%
CO2 for 8 h. The medium was discarded and replaced with
complete medium and cultured for a further 48 h. Proteins were
extracted and the transfection efficiency was detected using
western blotting.
Transwell assay
Transwell chambers (8-µm; Corning, Inc.) were coated
with 60 µl Matrigel (1:8; BD Biosciences) at 37°C for 1 h. A total
of 5×105 HTR8/SVneo cells suspended in 100 µl serum-free
medium were seeded into the upper chamber and the lower chamber was
filled with 600 µl RPMI-1640 medium containing 10% FBS. Following
incubation at 37°C in 5% CO2 for 48 h, cells that
migrated to the lower surface of the chamber were fixed with 4%
paraformaldehyde at room temperature and stained with 0.1% crystal
violet for 20 min at room temperature. Images were captured using
an inverted microscope (Zeiss GmbH) and invasive cells were counted
in at least five randomly selected fields for each chamber
(magnification, ×200). Untreated Transwell chambers were used to
detect cell migration.
Gap closure assay
HTR-8/SVneo cells were seeded in a 6-well plate at a
density of 2×105/well and cultured for 24–36 h. When
~90% confluence was reached, the wound was scratched using a
culture insert (ibidi, Germany). Cell debris were removed by
washing three times in PBS and the medium was replaced with
FBS-free medium with other treatments for 24 h. Cell migration was
observed at 0 and 12 h using an inverted phase contrast microscope
(Zeiss GmbH; magnification, ×4) and analyzed using a High Content
Analysis system (HCS; Molecular Devices, LLC).
Cell Counting Kit-8 (CCK-8) assay
HTR-8/SVneo cells were seeded into a 96-well plate
at a density of 300–5,000 cells/well and cultured at 37°C in 5%
CO2 for 24 h. Subsequently, 10 µl CCK-8 solution
(Elabscience Biotechnology, Inc.) was added to each well and
incubated for 1–4 h. Absorbance was measured at 450 nm using a
microplate reader (BioTek Instruments, Inc.).
Flow cytometry
Adherent HTR-8/SVneo cells were washed with PBS for
three times and trypsinized. Cells were resuspended in 195 µl
Annexin V-FITC binding solution (BD Biosciences) at a density of
5×105/tube. Cells were subsequently mixed with 5 µl
Annexin V-FITC and 10 µl PI staining solution (BD Biosciences) and
incubated for 20 min at room temperature in the dark. Cell
apoptotic rates were calculated by detecting the percentage of
early and late apoptotic cells using flow cytometry (DxFLEX;
Beckman Coulter, Inc.) and CytExpert software (version 2.0.0.283;
Beckman Coulter, Inc.).
Western blotting
Villi tissues were cut into small pieces and
homogenized using Tissue Grinders (Potter-Elvehjem) on ice and
lysed in RIPA buffer (Sigma-Aldrich; Merck KGaA) with 1 mmol/l
PMSF. Total protein was quantified using a BCA assay and 20 µg
protein of each sample was separated by 7.5% SDS-PAGE. The
separated proteins were subsequently transferred to PVDF membranes
and blocked with 5% skimmed milk at room temperature for 1 h. The
membranes were incubated with specific primary antibodies,
including anti-FPR2 (cat. no. 63023, Abcam, 1:1,000),
anti-phosphorylated (p)-AKT (cat. no. 4060; Cell Signaling
Technology, Inc.; 1:1,000), anti-AKT (cat. no. 9272; Cell Signaling
Technology, Inc.; 1:1,000), anti-PI3K (cat. no. 4292; Cell
Signaling Technology, Inc.; 1:1,000), anti-p-PI3K (cat. no. 4228;
Cell Signaling Technology, Inc.; 1:1,000) and anti-β-actin (cat.
no. 66009; 1:2,000; ProteinTech Group, Inc.) overnight at 4°C.
Following primary incubation, membranes were incubated with
HRP-conjugated secondary antibody (cat. no. 15015; 1:5,000;
ProteinTech Group, Inc.) at room temperature for 1 h. The blots
were developed using the ECL Detection kit (Thermo Fisher
Scientific, Inc.). Protein bands were semi-quantified using the
Amersham Imager 600 (Cytiva) and analyzed using ImageJ 1.51
software (National Institutes of Health).
Statistical analysis
All experiments were repeated at least three times.
Data were collected, analyzed and visualized using GraphPad Prism
version 8.0 (GraphPad Software, Inc.). The continuous variables
with normal distribution are presented as the mean ± SD.
Differences between the si-NC group and si-FPR2 groups for
migration, proliferation, invasion, apoptosis, tube formation rate
and the phosphorylation of PI3K and AKT were analyzed using
unpaired Student's t-tests. Comparisons between three or more
groups were conducted using one way analysis of variance (ANOVA)
and the P-values were adjusted using Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of FPR2 significantly
increases in placenta tissues from patients with RSA
To confirm the association between FPR2 and RSA, the
location and expression of FPR2 in the placenta of women with RSA
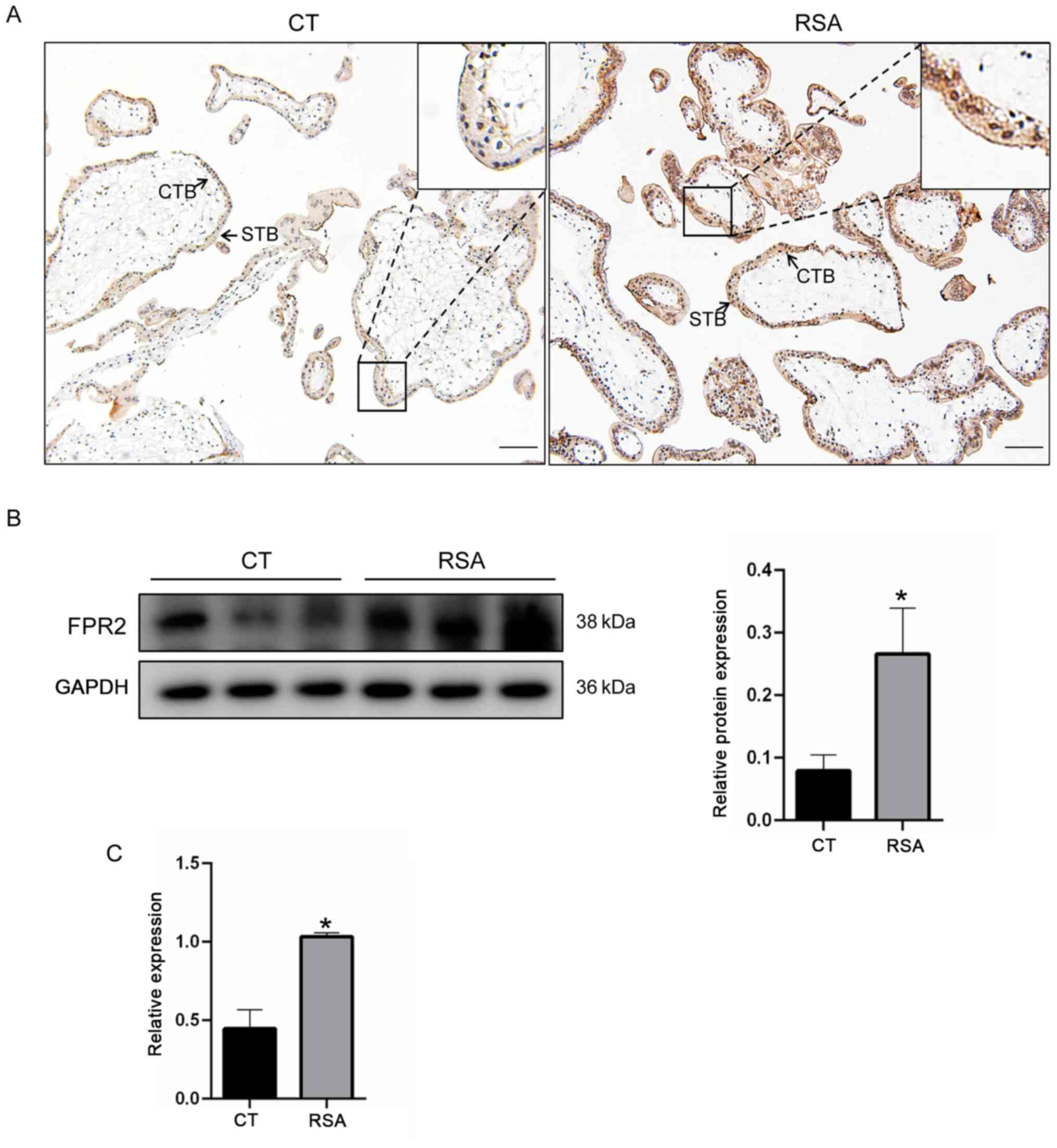
was investigated. As demonstrated in Fig. 1A, results of the
immunohistochemical analysis revealed that FPR2 was located on the
cytotrophoblasts of villi in the placenta tissue. In addition, the
expression levels of FPR2 in the villi of patients with RSA were
significantly increased, compared with the CT group. In order to
verify the changes in expression levels of FPR2 in the villi of
patients with RSA, western blotting and RT-qPCR were performed
using villi tissues derived from 10 patients in each of the RSA and
CT groups. Consistent with the results of the immunohistochemical
staining, the protein expression levels of FPR2 in the villi of
patients with RSA were increased compared with the CT group
(Fig. 1B). The mRNA expression
levels of FPR2 in women with RSA was significantly increased
compared with the CT group (Fig.
1C). These results suggested that an increase in the levels of
FPR2 is associated with RSA.
FPR2 knockdown inhibits apoptosis in
trophoblasts
As the biological dysfunction of trophoblasts is
closely associated with the pathogenesis of RSA (18), the impact of FPR2 knockdown on the
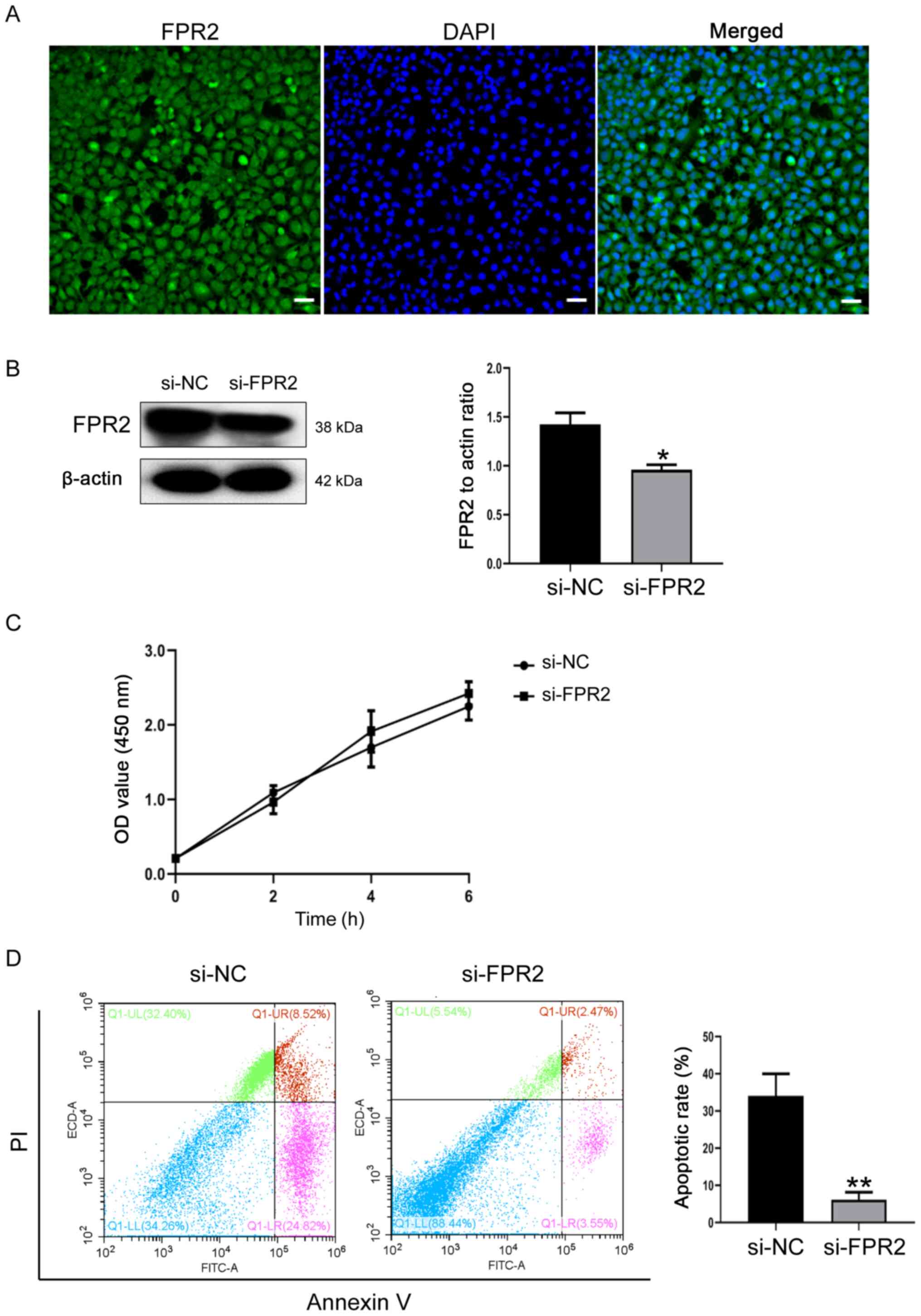
biological function of trophoblasts was investigated. As
demonstrated in Fig. 2A, FPR2 was
expressed in HTR8/SVneo cells and predominantly located in the
cytoplasm and cell membrane. Following the knockdown of FPR2 using
siRNA, the transfection efficiency was verified using western
blotting. Compared with si-NC cells, the protein expression levels
of FPR2 were significantly decreased in si-FPR2 cells (Fig. 2B). CCK-8 assays demonstrated that
FPR2 knockdown exhibited no significant effect on cell
proliferation, compared with si-NC cells (Fig. 2C). In addition, the knockdown of
FPR2 significantly attenuated the apoptosis of trophoblasts
compared with the si-NC group (Fig
2D).
FPR2 knockdown ameliorates migration,
invasion and tube formation ability of trophoblasts
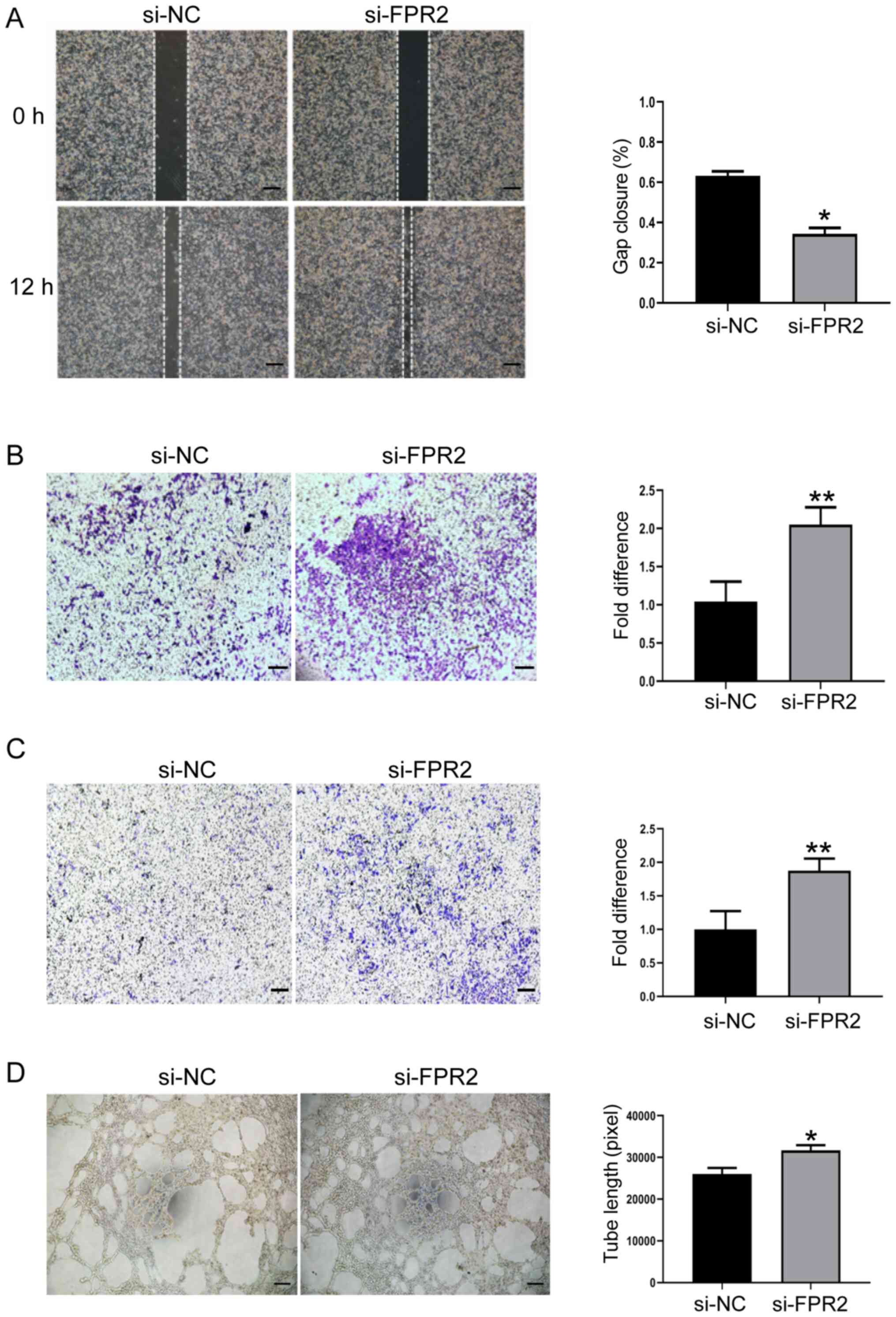
To further explore the biological significance of
FPR2 in trophoblast function, the role of FPR2 in the regulation of
trophoblast migration and invasion was investigated using gap
closure and Transwell assays. FPR2 knockdown mitigated the
migration rate of HTR8/SVneo cells compared with the si-NC group
(Fig. 3A and B). In addition,
results of the Transwell assay demonstrated that the levels of
invasion decreased in HTR8/SVneo cells following FPR2 knockdown
compared with the si-NC group (Fig.
3C). Tube formation assays demonstrated that HTR8/SVneo cells
treated with si-FPR2 exhibited an increased capability of forming
capillary-like structures, compared with the si-NC group (Fig. 3D).
FPR2 affects the migration and
invasion of trophoblasts via the PI3K/AKT pathway
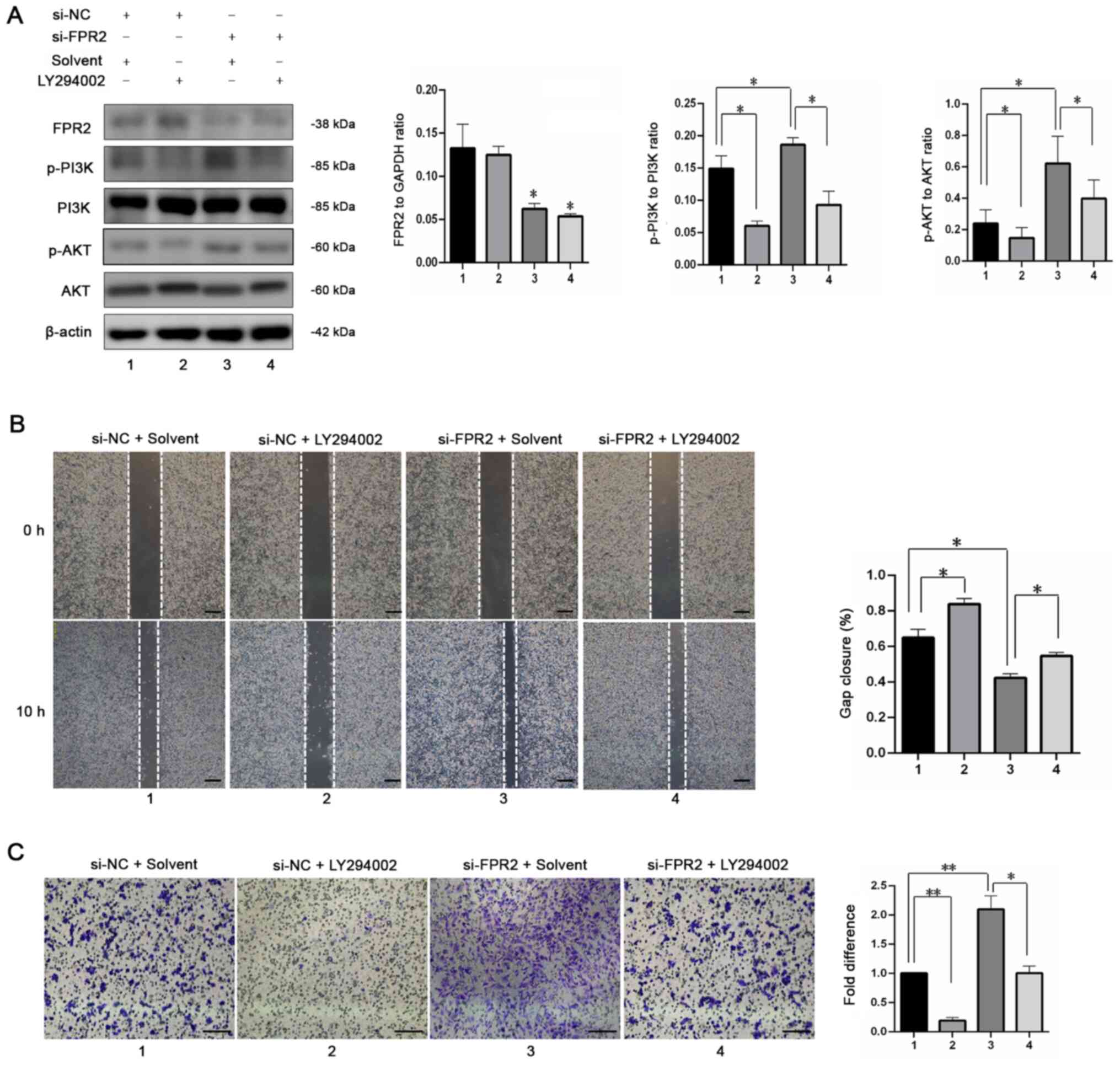
The results of a previous study revealed that
activation of FPR2 triggers the activation of PI3K in cancer cells
(19,20). Thus, to investigate the
involvement of PI3K in the regulation of trophoblast proliferation
by FPR2, western blotting was used to detect the phosphorylation
levels of PI3K and AKT. The results demonstrated that FPR2
knockdown increased the activation of the PI3K signaling pathway
compared with the si-NC+ Solvent group. In addition, in the
presence of LY294002 (10 µM; MCE), a specific PI3K inhibitor, the
si-FPR2-mediated increase in p-AKT was reversed, with no
significant changes in FPR2 expression (Fig. 4A). Gap closure assays revealed no
significant difference in the migration of HTR8/SVneo cells
following treatment with si-FPR2 and LY294002, compared with the
si-NC+ Solvent group (Fig. 4B).
Similarly, the si-FPR2-mediated increase in cell invasion was
inhibited following treatment with LY294002 (Fig. 4C). Collectively, these findings
indicated that FPR2 affects the migration and invasion of
HTR8/SVneo cells via the PI3K-AKT signaling pathway.
Discussion
The occurrence of RSA is closely associated with the
abnormal biological function of trophoblasts (18). Trophoblasts are cells that
constitute the maternal-fetal interface and serve a key role during
pregnancy. The normal biological function of trophoblasts is
important to ensure an adequate nutrient supply and the secretion
of essential hormones for fetal growth and development (21). When a fertilized egg is implanted,
the anchoring villi in the placenta differentiate into
syncytiotrophoblasts and cytotrophoblasts. Cytotrophoblasts
gradually differentiate to the infiltrating surface layer, forming
extravillous trophoblasts (EVTs) (22). EVTs are divided into mesenchymal
trophoblasts and intravascular trophoblasts, according to their
different functions. The main function of mesenchymal trophoblasts
is to infiltrate the basal layer of the uterus, a mechanism that is
strictly regulated to avoid excessive invasion and implantation
(23). The main role of EVTs is
to invade the uterine spiral artery, participate in the remodeling
of the spiral artery and ensure blood perfusion of the placenta
(24). Insufficient invasion of
trophoblast causes spiral arteriole remodeling in the decidual
layer, which is markedly shallower in uterine muscle layer compared
with normal pregnancy, leading to the poor formation of the
placental vascular network (25).
Pathological changes associated with placental ischemia, such as
hypoxia and metabolic disorders then occur, leading to pregnancy
complications. However, the mechanisms underlying trophoblast
dysfunction in patients with RSA remain to be elucidated.
The results of previous studies have demonstrated
that dysfunctional EVTs that affect the remodeling of uterine
spiral artery during the development of the placenta are a key
pathological feature of pregnancy complications, such as RSA and
pulmonary embolisms (26,27). HTR-8/SVneo cells are generated
using freshly isolated extravillous cytotrophoblasts from the first
trimester placenta transfected with a plasmid containing the simian
virus 40 large T antigen (SV40), which represents early human
villous trophoblasts (28). These
cells have previously been used as models of RSA for the research
of diseases associated with the placenta and to observe the effect
of FPR2 expression on trophoblast function (29). The results of a previous study
demonstrate that HTR-8/SVneo cells contain two populations, one of
epithelial and one of mesenchymal origin (30). Furthermore, the results of
previous studies demonstrate that these cells act as ideal models
to study a number of diseases, such as chorionic amnionitis
(8) and the reproductive toxicity
of silica nanoparticles (31–33).
FPR2, a member of the formyl-peptide receptor
family, is a G protein-coupled receptor (34). The physiological and pathological
functions of FPR2 have been extensively studied using FPR2
agonists, RNA interference technology and FPR2 gene knockout mice,
combined with in vitro and in vivo experiments. FPR2
not only regulates the chemotaxis, migration and phagocytosis of
leukocytes, but also serves a role in defense reactions and
inflammatory diseases (22). FPR2
is also involved in the occurrence of neurodegenerative diseases,
tumors and disorders associated with glucose and lipid metabolism
and is a potential therapeutic target for a number of diseases
(35–37). In addition, abnormal expression of
FPR2 has been discovered in a number of types of tumor tissues and
is involved in the proliferation and invasion of tumor cells
(38,39). FPR2 also affects tumor growth and
metastasis by acting on the tumor microenvironment to cause the
abnormal autophagy of tumor cells (40). Trophoblasts have biological
characteristics similar to tumor cells and are highly invasive.
However, unlike tumor cells, trophoblasts are controlled by a
number of regulatory factors that are space- and time-dependent and
do not have the potential for infinite proliferation and
differentiation (41). The
results of our previous study demonstrated that human placental
trophoblasts express FPR2 (8),
but the mechanism underlying FPR2 in regulating the biological
function of trophoblasts remains to be elucidated and its role in
pregnancy requires further investigation.
In the present study, FPR2 was expressed in the
trophoblasts of villi in early pregnancy and the expression levels
were significantly increased in patients with RSA, suggesting that
the abnormal expression of FPR2 in trophoblasts may be associated
with the pathogenesis of RSA. In addition, the levels of
trophoblast migration, invasion and proliferation were markedly
increased, while the levels of apoptosis were reduced following
FPR2 knockdown in HTR8/SVneo cells. This highlighted the potential
role of FPR2 in the regulation of trophoblast function.
Collectively, these results indicated that FPR2 may be involved in
the occurrence of RSA by regulating the biological function of
trophoblasts.
The signaling pathways involved in regulating the
biological function of trophoblasts include tyrosine-protein kinase
JAK1/STAT, PI3K/AKT, MAPK and TGF-β (42). The results of a previous study
demonstrate that activation of the NFκB signaling pathway is also
involved in the regulation of trophoblast cell function (43). The expression of FPR2 is
associated with the activation of PI3K/AKT, MAPK, NF-κB and other
signaling pathways, which also serve key roles in the regulation of
tumor cells, including cell proliferation, migration and apoptosis.
Kyoto Encyclopedia of Genes and Genomes functional enrichment
analysis reveals an association between FPR2 and the PI3K/AKT
signaling pathway (12). The
association between FPR2 and the PI3K/AKT signaling pathway
requires further investigation in trophoblasts.
The results of the present study demonstrated that
FPR2 knockdown caused the phosphorylation of AKT and PI3K. AKT is
the direct target protein of the PI3K signaling pathway and is
associated with cell proliferation, differentiation, apoptosis and
migration (44–46). Functional analyses in the present
study revealed that FPR2 may be involved in the regulation of
trophoblast function through the PI3K/AKT signaling pathway.
Furthermore, use of a PI3K/AKT signaling pathway agonist or an FPR2
inhibitor in the early pregnancy of patients with RSA may improve
the function of trophoblasts in the placenta (47) and may provide novel therapeutic
targets for the treatment of diseases associated with the placenta.
However, increasing the activation of the PI3K/AKT signaling
pathway may cause adverse side effects, such as excessive cell
proliferation and cancer development (48). Thus, further investigations into
the specific dosages are required. In addition, the PI3K/AKT
signaling pathway mediates cellular metabolism through a number of
key downstream substrates, such as tuberin, protein kinase gsk3 and
the FOXO transcription factors (15). Thus, the effects of downstream
substrates of the PI3K/AKT signaling pathway on the functions of
FPR2 in trophoblasts require further investigation.
The present study had a number of limitations, such
as a lack of in vivo investigations. Thus, future
investigations should use a placenta-specific FPR2 knockout mouse
model to study the FPR2-associated dysfunction of trophoblasts
in vivo. An in vivo model is essential to further
elucidate the pathogenic mechanisms underlying RSA.
In conclusion, FPR2 serves a key role in the
regulation of trophoblasts through the PI3K/AKT signaling pathway
and may be involved in RSA development. Further elucidating the
mechanisms underlying FPR2 in cell proliferation, migration,
invasion and apoptosis may uncover novel disease markers and
therapeutic targets in RSA.
Acknowledgements
Not applicable.
Funding
This present study was supported by the
Hospital-level Topic of Maternal and Child Health Care Hospital of
Shandong Province (grant no. 2020SFY001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AL drafted the manuscript and performed the
experiments. SL and CZ performed the experiments. ZF, YS and YP
analyzed the data and organized the figures. YP revised the
manuscript. XW and MZ designed the experiments and confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was in accordance with the principles set
out in the Declaration of Helsinki and its later amendments for
ethical research involving human subjects. All procedures involving
human participants were approved by the Ethics Committee of
Maternal and child health care hospital of Shandong province
(permit no: 2021–037). All patients signed informed consent for
scientific research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joob B and Wiwanitkit V: Zika and
spontaneous abortion. Bol Med Hosp Infant Mex. 77:462020.PubMed/NCBI
|
|
2
|
Van Leer P: Preventing spontaneous
abortion with progestin therapy. Am Fam Physician.
100(1)2009.PubMed/NCBI
|
|
3
|
Lee YH and Kim YC: Spontaneous resolution
of serous retinal detachment caused by choroidal mass after a first
trimester abortion. Yeungnam Univ J Med. 37:242–245. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiang H, Yan H, Sun B, Feng F and Chen P:
Decreased expression of long non-coding RNA SNHG7 cause recurrent
spontaneous abortion through suppression proliferation and invasion
of trophoblast cells via miR-34a. Am J Transl Res. 11:463–472.
2019.PubMed/NCBI
|
|
5
|
Branch DW, Gibson M and Silver RM:
Clinical practice. Recurrent miscarriage. N Engl J Med.
363:1740–1747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zong S, Li C, Luo C, Zhao X, Liu C, Wang
K, Jia W, Bai M, Yin M, Bao S, et al: Dysregulated expression of
IDO may cause unexplained recurrent spontaneous abortion through
suppression of trophoblast cell proliferation and migration. Sci
Rep. 6:199162016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun X, Tong X, Hao Y, Li C, Zhang Y, Pan
Y, Dai Y, Liu L, Zhang T and Zhang S: Abnormal Cullin1
neddylation-mediated p21 accumulation participates in the
pathogenesis of recurrent spontaneous abortion by regulating
trophoblast cell proliferation and differentiation. Mol Hum Reprod.
26:327–339. 2020.PubMed/NCBI
|
|
8
|
Li A, Zhang L, Li J, Fang Z, Li S, Peng Y,
Zhang M and Wang X: Effect of RvD1/FPR2 on inflammatory response in
chorioamnionitis. J Cell Mol Med. 24:13397–13407. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murthi P, Rajaraman G, Erwich JJHM and
Dimitriadis E: Decreased placental FPR2 in early pregnancies that
later developed small-for-gestation age: a potential role of FPR2
in the regulation of epithelial-mesenchymal transition. Cells.
9:E9212020. View Article : Google Scholar
|
|
10
|
Lappas M, McCracken S, McKelvey K, Lim R,
James J, Roberts CT, Fournier T, Alfaidy N, Powell KL, Borg AJ, et
al: Formyl peptide receptor-2 is decreased in foetal growth
restriction and contributes to placental dysfunction. Mol Hum
Reprod. 24:94–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Z, Zhao F, Lin F, Xiang H, Wang N, Ye D
and Huang Y: Preeclampsia is associated with a deficiency of
lipoxin A4, an endogenous anti-inflammatory mediator. Fertil
Steril. 102:282–290.e4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holdfeldt A, Sundqvist M, Dahlgren C and
Forsman H: Data showing effects of a PI3K-δ inhibitor on neutrophil
superoxide production during FPR2 activation and reactivation. Data
Brief. 32:1061852020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu GJ, Tao T, Wang H, Zhou Y, Gao X, Gao
YY, Hang CH and Li W: Functions of resolvin D1-ALX/FPR2 receptor
interaction in the hemoglobin-induced microglial inflammatory
response and neuronal injury. J Neuroinflammation. 17:2392020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Korimová A and Dubový P: N-Formylated
peptide induces increased expression of both formyl peptide
receptor 2 (Fpr2) and Toll-like receptor 9 (TLR9) in Schwannoma
cells-an in vitro model for early inflammatory profiling of Schwann
cells. Cells. 9:E26612020. View Article : Google Scholar
|
|
15
|
Hoxhaj G and Manning BD: The PI3K-AKT
network at the interface of oncogenic signalling and cancer
metabolism. Nat Rev Cancer. 20:74–88. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (Review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Zhou G, Jiang L, Xiang H and Cao Y:
Effect of STOX1 on recurrent spontaneous abortion by regulating
trophoblast cell proliferation and migration via the PI3K/AKT
signaling pathway. J Cell Biochem. 120:8291–8299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cattaneo F, Russo R, Castaldo M, Chambery
A, Zollo C, Esposito G, Pedone PV and Ammendola R: Phosphoproteomic
analysis sheds light on intracellular signaling cascades triggered
by Formyl-Peptide Receptor 2. Sci Rep. 9:178942019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ammendola R, Parisi M, Esposito G and
Cattaneo F: Pro-resolving FPR2 agonists regulate NADPH
oxidase-dependent phosphorylation of HSP27, OSR1, and MARCKS and
activation of the respective upstream kinases. Antioxidants
(Basel). 10:342021.PubMed/NCBI
|
|
21
|
Larqué E, Ruiz-Palacios M and Koletzko B:
Placental regulation of fetal nutrient supply. Curr Opin Clin Nutr
Metab Care. 16:292–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waechter V, Schmid M, Herova M, Weber A,
Günther V, Marti-Jaun J, Wüst S, Rösinger M, Gemperle C and
Hersberger M: Characterization of the promoter and the
transcriptional regulation of the lipoxin A4 receptor (FPR2/ALX)
gene in human monocytes and macrophages. J Immunol. 188:1856–1867.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burton GJ, Woods AW, Jauniaux E and
Kingdom JC: Rheological and physiological consequences of
conversion of the maternal spiral arteries for uteroplacental blood
flow during human pregnancy. Placenta. 30:473–482. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Georgiades P, Ferguson-Smith AC and Burton
GJ: Comparative developmental anatomy of the murine and human
definitive placentae. Placenta. 23:3–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silva JF and Serakides R: Intrauterine
trophoblast migration: A comparative view of humans and rodents.
Cell Adhes Migr. 10:88–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dagdelen M, Temur M, Yılmaz Ö, Altındag T,
Uslu T and Özbay PO: Placental bed apoptosis is increased in
pregnant women with pre-eclampsia versus normotensive pregnant
women. J Obstet Gynaecol. 36:974–979. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu HN, Tang XM, Wang XQ, Gao J, Li N,
Wang YY and Xia HF: miR-93 inhibits trophoblast cell proliferation
and promotes cell apoptosis by targeting BCL2L2 in recurrent
spontaneous abortion. Reprod Sci. 27:152–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abou-Kheir W, Barrak J, Hadadeh O and
Daoud G: HTR-8/SVneo cell line contains a mixed population of
cells. Placenta. 50:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Tian J, Yin H, Peng Y, Liu S, Yao S
and Zhang L: Chemical conjugation of FITC to track silica
nanoparticles in vivo and in vitro: An emerging method to assess
the reproductive toxicity of industrial nanomaterials. Environ Int.
152:1064972021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan S, Zhang M, Li J, Tian J, Yin H, Wang
X and Zhang L: Uterine metabolic disorder induced by silica
nanoparticles: Biodistribution and bioactivity revealed by labeling
with FITC. J Nanobiotechnology. 19:622021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin H, Li J, Tian J, Ma L, Zhang J, Zhai
Q, Yao S and Zhang L: Uterine pyruvate metabolic disorder induced
by silica nanoparticles act through the pentose phosphate pathway.
J Hazard Mater. 412:1252342021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu Y, Xue S, Chen K, Le Y, Zhu R, Wang S,
Liu S, Cheng X, Guan H, Wang JM, et al: The G-protein-coupled
chemoattractant receptor Fpr2 exacerbates neuroglial dysfunction
and angiogenesis in diabetic retinopathy. FASEB Bioadv. 2:613–623.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matte A, Recchiuti A, Federti E, Koehl B,
Mintz T, El Nemer W, Tharaux PL, Brousse V, Andolfo I, Lamolinara
A, et al: Resolution of sickle cell disease-associated inflammation
and tissue damage with 17R-resolvin D1. Blood. 133:252–265. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rüger M, Kipp E, Schubert N, Schröder N,
Pufe T, Stope MB, Kipp M, Blume C, Tauber SC and Brandenburg LO:
The formyl peptide receptor agonist Ac2-26 alleviates
neuroinflammation in a mouse model of pneumococcal meningitis. J
Neuroinflammation. 17:3252020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vital SA, Senchenkova EY, Ansari J and
Gavins FNE: Targeting AnxA1/Formyl Peptide Receptor 2 Pathway
Affords Protection against Pathological Thrombo-Inflammation.
Cells. 9:E24732020. View Article : Google Scholar
|
|
38
|
Xiang Y, Yao X, Chen K, Wang X, Zhou J,
Gong W, Yoshimura T, Huang J, Wang R, Wu Y, et al: The G-protein
coupled chemoattractant receptor FPR2 promotes malignant phenotype
of human colon cancer cells. Am J Cancer Res. 6:2599–2610.
2016.PubMed/NCBI
|
|
39
|
Lu J, Zhao J, Jia C, Zhou L, Cai Y, Ni J,
Ma J, Zheng M and Lu A: FPR2 enhances colorectal cancer progression
by promoting EMT process. Neoplasma. 66:785–791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sainz B Jr, Alcala S, Garcia E,
Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo
I, Hidalgo M, Gomez-Lopez G, et al: Microenvironmental
hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by
activating its cancer stem cell compartment. Gut. 64:1921–1935.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Red-Horse K, Zhou Y, Genbacev O,
Prakobphol A, Foulk R, McMaster M and Fisher SJ: Trophoblast
differentiation during embryo implantation and formation of the
maternal-fetal interface. J Clin Invest. 114:744–754. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao X, Jiang Y, Jiang T, Han X, Wang Y,
Chen L and Feng X: Physiological and pathological regulation of
autophagy in pregnancy. Arch Gynecol Obstet. 302:293–303. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawczak P, Bober L and Bączek T:
Evaluation of Chemotherapeutic activity of the selected bases'
analogues of nucleic acids supported by ab initio various quantum
chemical calculations. Curr Computeraided Drug Des. 16:93–103.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang W, Zuo M, Lu J and Wang Y:
Adiponectin reduces embryonic loss rate and ameliorates trophoblast
apoptosis in early pregnancy of mice with polycystic ovary syndrome
by affecting the AMPK/PI3K/Akt/FoxO3a signaling pathway. Reprod
Sci. 27:2232–2241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen J, Yue C, Xu J, Zhan Y, Zhao H, Li Y
and Ye Y: Downregulation of receptor tyrosine kinase-like orphan
receptor 1 in preeclampsia placenta inhibits human trophoblast cell
proliferation, migration, and invasion by PI3K/AKT/mTOR pathway
accommodation. Placenta. 82:17–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Correia-Branco A, Keating E and Martel F:
Involvement of mTOR, JNK and PI3K in the negative effect of ethanol
and metformin on the human first-trimester extravillous trophoblast
HTR-8/SVneo cell line. Eur J Pharmacol. 833:16–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Y, Sun XL, Ma CL, Li C, Zhan Y, Li WT,
Li C and Wang YH: STX2 promotes trophoblast growth, migration, and
invasion through activation of the PI3K-AKT pathway in
preeclampsia. Front Cell Dev Biol. 9:6159732021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|