Introduction
Triple-negative breast cancer (TNBC) accounts for ~15–20% of all patients with breast cancer and is defined by the lack of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (1). TNBC tends to more frequently affect younger patients (2). TNBC has more aggressive biological behaviours and poorer long-term outcomes compared with other breast cancer subtypes (3). The risk of distant recurrence peaks at ~3 years. Furthermore, the average survival in metastatic TNBC is 12 months, and the majority of women with metastatic TNBC will ultimately die of the disease despite treatment (4). The therapeutic strategy for breast cancer is largely based on the tumoral expression of three cell surface markers. In the absence of approved targeted therapies, radiotherapy and cytotoxic chemotherapy remain the mainstay treatments with suboptimal outcomes (5). Therefore, there is a critical need for the exploration of novel approaches for TNBC treatment.
The extracellular matrix (ECM), as a crucial component of the tumour microenvironment, provides mechanical support for cell growth, survival and migration, and plays a key role in cancer cell malignancy (6). Leucine-rich repeat-containing protein 15 (LRRC15) is a member of the leucine-rich repeat superfamily, which is involved in cell-cell and cell-ECM interactions, including adhesion and receptor-ligand binding (7). It is expressed in cancer-associated fibroblasts (CAFs) and stromal cells in various types of solid tumours, such as breast cancer, as well as directly in cancer cells in tumours of mesenchymal origin, such as sarcomas (8). High LRRC15 expression is significantly associated with worse metastasis-free survival in patients with soft-tissue sarcomas (9). LRRC15 is one of the genes known to be associated with breast cancer invasion (10). However, the effect of CAF-derived LRRCC5 on the migratory and invasive abilities of TNBC cells remains unclear.
A variety of studies have evaluated the associations between several matrix metalloproteinases (MMPs), including MMP-2, MMP-7 and MMP-9, and breast cancer cell metastasis (11–13). β-catenin, an important mediator of the Wnt signalling pathway, is phosphorylated by the GSK3β complex. Phosphorylated β-catenin is specifically recognised by β-transducin repeats-containing proteins (β-TrCP1) (14). CAFs activate the Wnt/β-catenin pathway and induce epithelial to mesenchymal transition (EMT) and cisplatin resistance in ovarian cancer cells (15).
In the present study, the role of LRRC15 in TNBC was explored. The aim of this study was to identify the combined role of LRRC15 and Wnt/β-catenin signalling pathway in the development of TNBC.
Materials and methods
Cell culture
CAFs and normal fibroblasts (NFs) were isolated from the TNBC and adjacent normal tissues of three female patients (age, 41–59 years) undergoing surgery at Dongying People's Hospital (Dongying, China) between April 2017 and March 2019. The adjacent tissues were >2 cm away from the cancer tissue. The patients were women who were diagnosed as TNBC. Those who had received preoperative radiotherapy or chemotherapy were excluded. The experimental protocols were approved by the Ethics Committee of Dongying People's Hospital (approval no. DYYX-2020-023). Written informed consent was obtained from all patients. The TNBC and adjacent tissues were cut into small pieces and placed in 0.1% type I liberase (Sigma-Aldrich; Merck KGaA) for enzymolysis at 37°C for 6 h. The digested cell mixture was filtered through a cell strainer (Corning, Inc.) and then centrifuged at 198 g for 5 min at 4°C. The cell pellet was resuspended in Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.). The supernatant containing the fibroblasts was further centrifuged at 198 g for 9 min at 4°C. NFs and CAFs were identified in the presence of CAF-specific markers [α-smooth muscle actin (α-SMA)]. The final pellet was resuspended in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) containing 20% foetal bovine serum (FBS; HyClone; Cytiva), 100 IU/ml penicillin, and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.). TNBC cell lines (MDA-MB-231 and MDA-MB-468 cells) were purchased from the American Type Culture Collection and cultured in DMEM supplemented with 10% FBS, 100 IU/ml penicillin, and 100 µg/ml streptomycin. All cells were maintained in 5% CO2 at 37°C.
Construction of LRRC15 overexpression and knockdown fibroblasts
The CAFs were seeded in a six-well plate until they reached 80% confluence. LRRC15-targeting small interfering RNAs (siRNAs) and pcDNA3.1-LRRC15 plasmids were designed and synthesised by Guangzhou RiboBio Co., Ltd. The sequences of the siRNAs are listed in Table SI. CAFs cells (1.5×106 cells/ml) were seeded into 6-well plates and transfected with 45 nM siRNA and plasmid using Lipofectamine® 2000 transfection reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Non-targeting siRNA (siNC) and empty vector were used as negative controls. After 48 h, the CAFs transfected with the pcDNA3.1-LRRC15 plasmids or LRRC15-targeting siRNA (siLRRC15) were used to treat TNBC MDA-MB-231 and MDA-MB-468 cells.
Migration and invasion assay
For cell migration, 2×104 MDA-MB-231 and MDA-MB-468 cells in 100 µl serum-free medium were added into the upper chambers (24-well, pore size 8 µm; Corning, Inc.) and CAFs in medium containing 10% FBS were added to the bottom of the insert. After incubation for 24 h at 37°C, the cells were fixed with 4% paraformaldehyde for 5 min at 25°C and stained with 0.5% crystal violet blue for 5 min at 25°C. The migrated cells were photographed and counted using an inverted microscope (Olympus Corporation, magnification, ×100). For the cell invasion assay, Transwell filters were pre-coated with BD Matrigel at 37°C for 60 min (BD Biosciences). 10 µmol/l XAV939 (Wnt/β-catenin pathway inhibitor, MedChemExpress) treated cells for 24 h at 37°C.
Luciferase assay
To evaluate β-catenin/TCF-dependent transcriptional activity, luciferase reporter assay was performed. MDA-MB-231 and MDA-MB-468 cells (5×104) were seeded in 24-well plates and cultured for 24 h with CAFs at a density of 70–80%. The cells were transfected with the β-catenin-responsive Firefly TOP-FLASH or the negative control FOP-FLASH reporter plasmids (VECT75319, Huayueyang Biotech Co., Ltd.) using FuGENE® 6 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Luciferase activity was measured with the Dual-Luciferase Reporter Assay kit (Promega Corporation) 24 h after transfection according to the manufacturer's instructions. Relative Renilla luciferase activity was normalised to Firefly luciferase activity.
Western blotting
Total protein was extracted using a lysis buffer. The Nuclear and Cytoplasmic Isolation Kit (Nanjing KeyGen Biotech Co., Ltd.) was used for cytoplasmic and nuclear protein extraction according to the manufacturer's instructions. Protein concentrations were measured with a BCA protein-assay kit (Beyotime). A total of 30 µg proteins (Total, nuclear, and cytoplasmic proteins) were separated on 10% SDS-PAGE gels. After electrophoresis, the separated protein bands were transferred onto polyvinylidene fluoride membranes (MilliporeSigma) and blocked using 5% non-fat milk for 1 h at room temperature. The membranes were then incubated overnight at 4°C with primary antibodies (1:1,000) against LRRC15 (cat. no. ab151482; Abcam), α-SMA (cat. no. 19245; Cell Signaling Technology, Inc.), MMP2 (cat. no. ab92536; Abcam), MMP7 (cat. no. ab5706; Abcam), MMP9 (cat. no. ab38898; Abcam), β-catenin (cat. no. ab32572; Abcam), phosphorylated (p)-β-catenin (Ser33/37; cat. no. 2009; Cell Signaling Technology, Inc.), β-TrCP (cat. no. 4394; Cell Signaling Technology, Inc.), Axin (cat. no. 2087; Cell Signaling Technology, Inc.), GSK-3β (cat. no. 9315; Cell Signaling Technology, Inc.), p-GSK-3β (Ser9; cat. no. 5558; Cell Signaling Technology, Inc.), cyclin D1 (cat. no. 2922; Cell Signaling Technology, Inc.), c-myc (cat. no. 9402; Cell Signaling Technology, Inc.), β-actin (cat. no. 4970; Cell Signaling Technology, Inc.), Histone H3 (cat. no. 4499; Cell Signaling Technology, Inc.) and GAPDH (cat. no. 5174; Cell Signaling Technology, Inc.). After washing three times, the membranes were incubated with horseradish peroxidase-linked secondary antibodies (1:5,000, cat. no. bs-40295G-HRP, Beijing Biosynthesis Biotechnology Co., Ltd.) at room temperature for 2 h. Finally, the protein bands were visualized using an ECL Kit (Beijing Solarbio Science & Technology Co., Ltd.). Protein expression was quantified using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.).
Bioinformatic analysis
The LRRC15 expression data of 235 patients with TNBC and 208 normal samples were downloaded from The Cancer Genome Atlas (TCGA; portal.gdc.cancer.gov/). Survival curve was generated with the R package ‘survival’ (version 3.6.1, http://www.r-project.org/).
Statistical analyses
Statistical significance was determined using GraphPad Prism 7 (GraphPad Software, Inc.). All experiments were conducted three times. All continuous data are shown as the mean ± SD. Data were statistically analysed using unpaired Student's t-test (two-tailed) and one-way analysis of variance followed by Tukey's test. Survival analysis was performed using the Kaplan-Meier method, the log-rank test was used to determine statistical significance between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
High LRRC15 expression is associated with poor prognosis in patients with TNBC
The LRRC15 expression data of 235 patients with TNBC and 208 normal samples from The Cancer Genome Atlas (TCGA) were analysed and it was found that the TNBC tissues exhibited significantly higher LRRC15 expression compared with normal tissues (Fig. 1A). There was no significant difference in LRRC15 expression between the three TNM stages (Fig. 1B). Kaplan-Meier analysis showed that the patients with TNBC with high LRRC15 expression had poor overall survival (OS; Fig. 1C), disease-specific survival (DSS; Fig. 1D), disease-free interval (DFI; Fig. 1E), and progression-free interval (PFI; Fig. 1F).
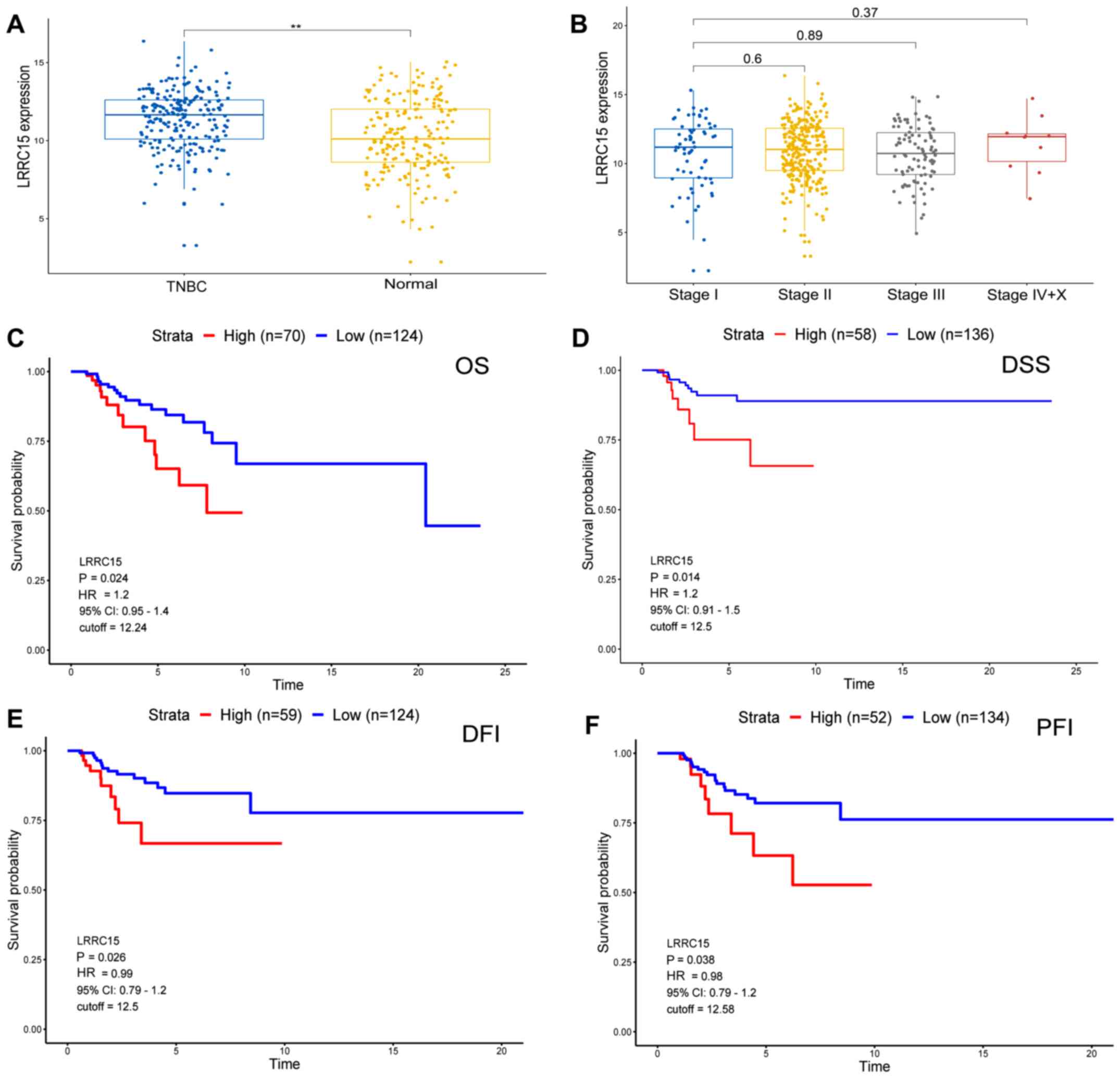 |
Figure 1.
High LRRC15 expression is associated with poor prognosis in patients with TNBC. (A) The LRRC15 expression data of 235 TNBC and 208 normal samples from TCGA database. (B) The LRRC15 expression in the different TNM stages of patients from TCGA database. Kaplan-Meier analysis of (C) OS, (D) DSS, (E) DFI and (F) PFI from TCGA database. **P<0.01. LRRC15, leucine-rich repeat-containing protein 15; TNBC, triple-negative breast cancer; TCGA, The Cancer Genome Atlas; OS, overall survival; DSS, disease-specific survival; DFI, disease-free interval; PFI, progression-free interval; HR, hazard ratio; CI, confidence interval.
|
LRRC15 induces MMPs to mediate migration and invasion
The results of western blotting indicated that the expression of LRRC15 and α-SMA was higher in the CAFs compared with NFs (Fig. 2A and B). To elucidate the mechanism by which LRRC15 regulates migration and invasion as well as its mechanism of action, LRRC15 was knocked down or overexpressed in CAFs (Fig. 2C) and it was found that LRRC15 expression was lower in siLRRC15-2 group than that in siLRRC15-1 group; therefore siLRRC15-2 was used for subsequent experiments. LRRC15 overexpression in CAFs promoted the migration and invasion of MDA-MB-231 and MDA-MB-468 cells, whereas its knockdown inhibited the migration and invasion of both cell lines (Fig. 2D). In agreement with these results, the expression of MMP2, MMP7 and MMP9 in both cell lines were either upregulated or downregulated by LRRC15 overexpression or knockdown, respectively, in CAFs (Fig. 2E).
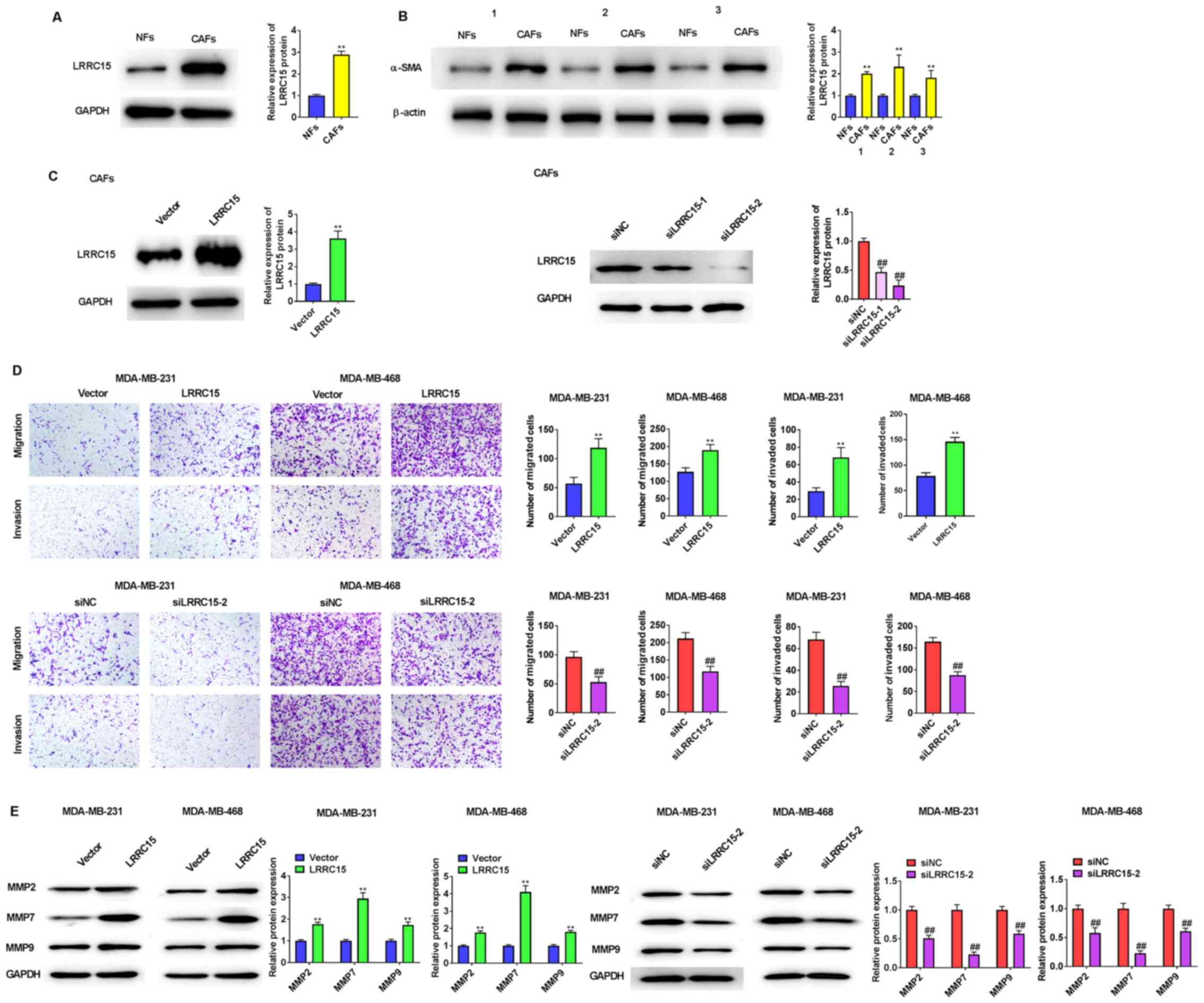 |
Figure 2.
LRRC15 induces MMPs to mediate migration and invasion. (A) Western blot analysis was used to analyse protein expression levels of LRRC15 in CAFs and NFs. (B) Western blot analysis was used to detect protein expression of α-SMA in CAFs and NFs. **P<0.01 vs. NFs. (C) Western blotting showed LRRC15 protein expression after transfection with pcDNA3.1-LRRC15 or siLRRC15 into CAFs. CAFs transfected with pcDNA3.1-LRRC15 plasmids or siLRRC15 were used to treat triple-negative breast cancer MDA-MB-231 and MDA-MB-468 cells. (D) The effect of LRRC15 on the migration and invasion of MDA-MB-231 and MDA-MB-468 cells were detected using Transwell assays. (E) Effect of LRRC15 on the protein expression levels of MMPs was detected by western blotting. **P<0.01 vs. Vector group; ##P<0.01 vs. siNC group. LRRC15, leucine-rich repeat-containing protein 15; CAFs, cancer-associated fibroblasts; NFs, normal fibroblasts; α-SMA, α-smooth muscle actin; si, small interfering RNA; NC, negative control.
|
LRRC15 promotes β-catenin levels and transcriptional activity
The ratio of p-β-catenin/β-catenin was not significantly changed by LRRC15 overexpression or LRRC15 knockdown in CAFs. β-TrCP1 were increased in the MDA-MB-231 and MDA-MB-468 cells by LRRC15 overexpression in CAFs, whereas their levels were decreased by LRRC15 knockdown (Fig. 3A). Compared with that in the vector cells, the amount of β-catenin was increased in nuclear fractions by LRRC15 overexpression, whereas was significantly decreased in cytoplasmic and nuclear fractions by LRRC15 knockdown, respectively (Fig. 3B). To evaluate whether the increased nuclear β-catenin was transcriptionally active, paired TOP-Flash and FOP-Flash control luciferase reporters were used and it was found that the transactivity of β-catenin was increased upon LRRC15 overexpression, but decreased upon LRRC15 knockdown in CAFs (Fig. 3C).
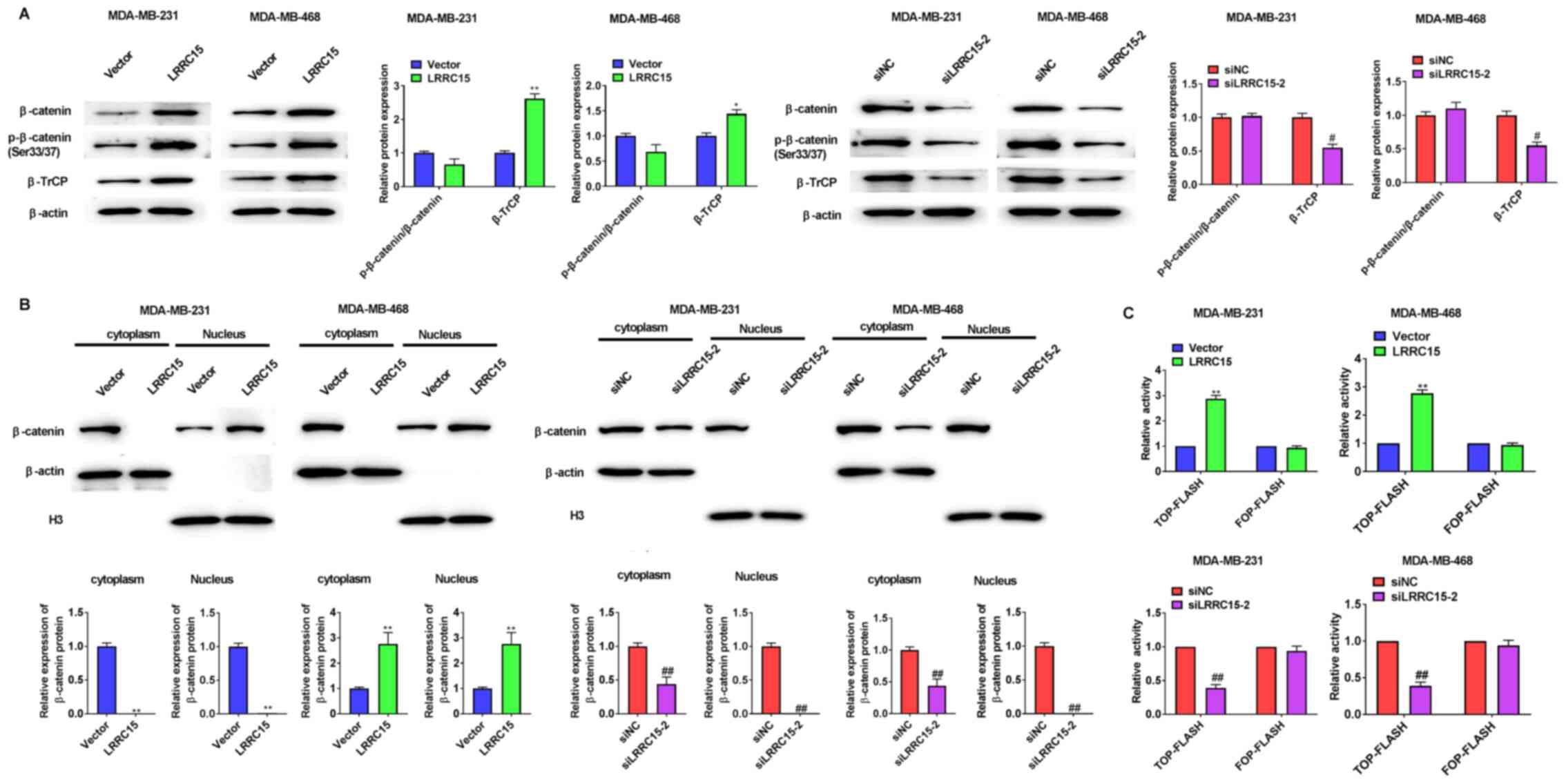 |
Figure 3.
LRRC15 promotes β-catenin levels and transcriptional activity. (A) Effect of LRRC15 on the expression of β-catenin, p-β-catenin (Ser33/37) and β-TrCP protein levels were detected via western blotting. (B) Western blotting results showed that LRRC15 overexpression increased nuclear translocation of β-catenin and knockdown of LRRC15 had the opposite function. (C) Effect of LRRC15 on β-catenin/T cell factor/lymphoid enhancer binding factor-mediated transcriptional activity. *P<0.05, **P<0.01 vs. Vector group; #P<0.05, ##P<0.01 vs. siNC group. LRRC15, leucine-rich repeat-containing protein 15; p-, phosphorylated; β-TrCP, β-transducin repeats-containing proteins; si, small interfering RNA; NC, negative control.
|
LRRC15 promotes β-catenin levels by downregulating the destruction complex protein Axin1
LRRC15 overexpression in CAFs resulted in decreased expression of Axin1 and increased expression levels of GSK3β and p-GSK3β in MDA-MB-231 and MDA-MB-468 cells, whereas LRRC15 knockdown in CAFs showed the opposite results (Fig. 4A). In agreement with these results, the expression of cyclin D1 and c-myc in both cell lines were either upregulated or downregulated by LRRC15 overexpression or knockdown in CAFs, respectively (Fig. 4B).
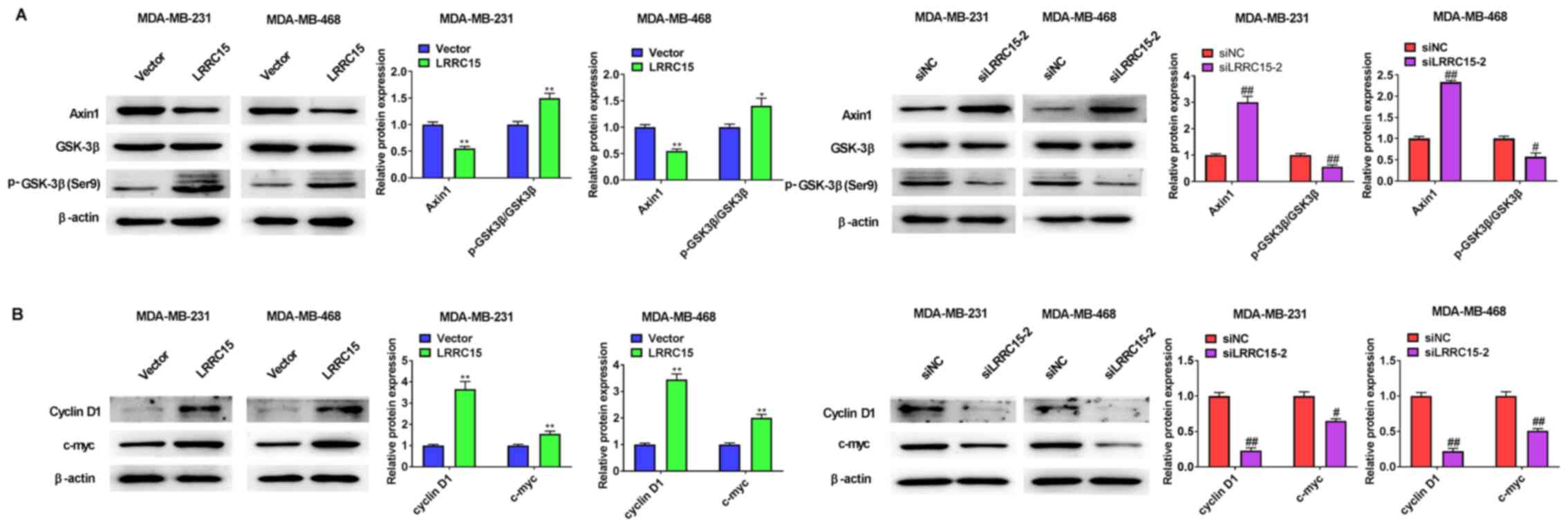 |
Figure 4.
LRRC15 promotes β-catenin levels by downregulating the destruction complex protein Axin1. (A) Effect of LRRC15 on the expression of Axin1, GSK-3β and p-GSK-3β (Ser9) protein expression levels were detected via western blotting. (B) Effect of LRRC15 on the expression of cyclin D1 and c-myc protein expression levels was detected via western blotting. *P<0.05, **P<0.01 vs. Vector group; #P<0.05, ##P<0.01 vs. siNC group. LRRC15, leucine-rich repeat-containing protein 15; p-, phosphorylated; si, small interfering RNA; NC, negative control.
|
LRRC15 induces migration and invasion via Wnt/β-catenin signalling
The migratory and invasive abilities of the two cell lines were observed after the addition of 10 µmol/l XAV939 (Wnt/β-catenin pathway inhibitor) (Fig. 5). It was found that LRRC15 overexpression in CAFs promoted the migration and invasion of MDA-MB-231 and MDA-MB-468 cells, whereas treatment with XAV939 reduced the migration and invasion of both cell lines compared with the LRRC15 overexpression group.
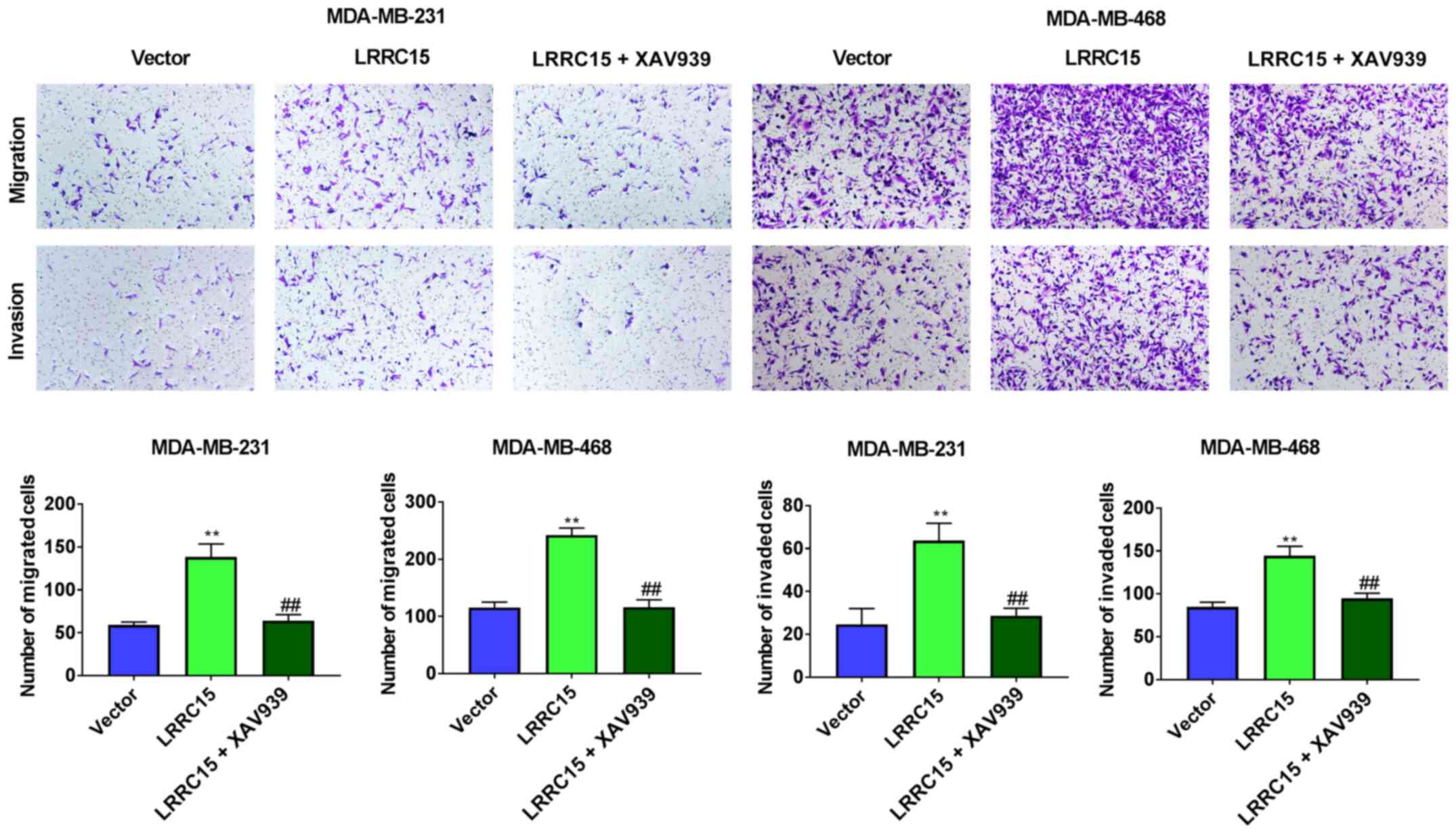 |
Figure 5.
LRRC15 induces migration and invasion via Wnt/β-catenin signalling. The migratory and invasive abilities of MDA-MB-231 and MDA-MB-468 cells were observed after the addition of 10 µmol/l XAV939. The effects of LRRC15 on the migration and invasion of MDA-MB-231 and MDA-MB-468 cells were detected via Transwell assays. **P<0.01 vs. Vector group; ##P<0.01 vs. LRRC15 group. LRRC15, leucine-rich repeat-containing protein 15.
|
Discussion
TNBC is associated with poor long-term outcomes compared with other breast cancer subtypes (3). Therefore, it is essential to discover effective tumour biomarkers to improve the prognosis and treatment for TNBC. LRRC15 is highly expressed in CAFs of multiple solid tumours or is directly expressed in the cancer cells of mesenchymal origin (8). Increased expression of LRRC15 is observed in ovarian cancer with bowel metastasis and knockdown of LRRC15 significantly inhibits tumour progression in mice (16). LRRC15 is also upregulated in bone-specific breast cancer metastasis (17). In this study, it was highly expressed in the CAFs of patients with TNBC. A previous study suggested that the overexpression of LRRC15 is positively correlated with tumour grade and is independently associated with worse metastasis-free survival of patients with soft-tissue sarcomas (9). The present study found that high LRRC15 expression in TNBC tissues was not associated with TNM stage, but with poor prognosis in patients with TNBC.
CAFs play a central role in facilitating tumour progression and metastasis in TNBC (18). They are responsible for the secretion of proteins and cytokines that regulate ECM modifications, tumour cell proliferation and metastasis (19,20). MMPs are zinc-containing endopeptidases that can degrade various components of ECM proteins (21). ECM degradation is the initial step toward tumour cell invasion (22). MMP2, MMP7 and MMP9 have been well-studied as proteins related to TNBC cell migration and invasion. For instance, peptidyl arginine deiminase type 1 inhibition prevents metastasis and decreases MMP2/9 expression in TNBC cells (23). Cadherin-11 knockdown decreases β-catenin, Met, c-Myc and MMP7 expression and attenuates TNBC cell migration and invasion (24). Knockdown of galectin-1 in CAFs notably inhibits CAF-conditioned medium-induced cell migration and invasion, most likely by inhibiting the expression of MMP-9 (25). Consistent with these results, the current study found that LRRC15 overexpression in CAFs induced MMP2, MMP7 and MMP9 expression and promoted the migration and invasion of MDA-MB-231 and MDA-MB-468 cells.
Previous reports have shown that Wnt signalling is associated with metastasis in TNBC. Kwon et al (26) reported that homeobox protein TGIF1 knockdown inhibits Wnt target genes and in vitro cell invasion, suggesting that TGIF1 may inhibit the invasion of TNBC cells. Silencing of paired-related homeobox 1b suppresses the proliferation, migration and invasion of TNBC cells by inhibiting the Wnt/β-catenin signalling pathway (27). In the present study, it was found that LRRC15 promoted the migration and invasion of MDA-MB-231 and MDA-MB-468 cells by regulating the Wnt/β-catenin signalling pathway, and that this function of LRRC15 could be reversed by XAV939.
It is well-established that endogenous β-catenin shuttles between the cytoplasm and nucleus. Additionally, abnormal activation of Wnt signalling often leads to β-catenin nuclear stabilisation and translocation (28) and promotes cyclin D1 gene expression (29). Nuclear β-catenin transcriptionally activates the T cell factor/lymphoid enhancer binding factor family proteins that drive tumour formation (30). Axin, adenomatous polyposis coli, casein kinase Iα and GSK-3β constitute the destruction complex (31). β-catenin stability is controlled by the destruction complex and subsequent binding by the E3 ubiquitin ligase β-TrCP for targeted ubiquitylation and degradation (20). With the knockdown of small ubiquitin-like modifier specific peptidase 7 in mammary epithelial cells, the interaction between Axin1 and β-catenin ceases and β-catenin escapes ubiquitylation-dependent proteasomal degradation (32). Knockdown of Axin1 decreases the association of c-myc with GSK-3β (33). Morrow et al (34) reported that Merlin alters the localisation of β-catenin and significantly reduces the protein levels of β-catenin by targeting it for degradation through the upregulation of Axin1. Consistent with these results, the current results demonstrated that LRRC15 promoted β-catenin expression in both the cytoplasmic and nuclear fractions and that β-catenin could be degraded via GSK-3β-mediated phosphorylation at the residues Ser33/37.
The present study aimed to elucidate the mechanism of LRRC15 in TNBC development, but there are limitations of this study. These findings give an idea of the expression and possible compensatory roles of other members of the LRRC family in the process of TNBC development. However, the expression levels of MMP-2 and MMP-9 were only determined via western blotting. The use of zymography to assess the expression levels would have improved the methodology of this study. In addition, zymography could have been performed to assess the activation status of the enzymes. In summary, these findings showed that high LRRC15 expression was associated with poor prognosis of patients with TNBC. LRRC15 promoted cell migration and invasion of TNBC cells and played a role in the regulation of the Wnt/β-catenin signalling pathway.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YY conceived and designed the present study, and wrote the manuscript. YY, JS and HW performed the experiments. MH, YB and SF were responsible for data analysis and interpretation. YY and HW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocols were approved by the Ethics Committee of Dongying People's Hospital (approval no. DYYX-2020-023). All patients signed written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, et al: American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergin ART and Loi S: Triple-negative breast cancer: Recent treatment advances. F1000Res. 8:F10002019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma P: Biology and management of patients with triple-negative breast cancer. Oncologist. 21:1050–1062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA: Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao H, He G, Yan S, Chen C, Song L, Rosol TJ and Deng X: Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget. 8:1913–1924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pickup MW, Mouw JK and Weaver VM: The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satoh K, Hata M and Yokota H: A novel member of the leucine-rich repeat superfamily induced in rat astrocytes by beta-amyloid. Biochem Biophys Res Commun. 290:756–762. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Purcell JW, Tanlimco SG, Hickson J, Fox M, Sho M, Durkin L, Uziel T, Powers R, Foster K, McGonigal T, et al: LRRC15 Is a novel mesenchymal protein and stromal target for antibody-drug conjugates. Cancer Res. 78:4059–4072. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben-Ami E, Perret R, Huang Y, Courgeon F, Gokhale PC, Laroche-Clary A, Eschle BK, Velasco V, Le Loarer F, Algeo MP, et al: LRRC15 Targeting in Soft-Tissue Sarcomas: Biological and Clinical Implications. Cancers (Basel). 12:122020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schuetz CS, Bonin M, Clare SE, Nieselt K, Sotlar K, Walter M, Fehm T, Solomayer E, Riess O, Wallwiener D, et al: Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res. 66:5278–5286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahman FU, Ali A, Khan IU, Duong HQ, Guo R, Wang H, Li ZT and Zhang DW: Novel phenylenediamine bridged mixed ligands dimetallic square planner Pt(II) complex inhibits MMPs expression via p53 and caspase-dependent signaling and suppress cancer metastasis and invasion. Eur J Med Chem. 125:1064–1075. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eiseler T, Döppler H, Yan IK, Goodison S and Storz P: Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 11:R132009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Liu M, Wang S, He Y, Huo Y, Yang Z and Cao X: Alantolactone induces apoptosis and suppresses migration in MCF 7 human breast cancer cells via the p38 MAPK, NF κB and Nrf2 signaling pathways. Int J Mol Med. 42:1847–1856. 2018.PubMed/NCBI
|
|
14
|
Khan M, Muzumdar D and Shiras A: Attenuation of tumor suppressive function of FBXO16 ubiquitin ligase activates Wnt signaling in glioblastoma. Neoplasia. 21:106–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang F, Cui JY, Gao HF, Yu H, Gao FF, Chen JL and Chen L: Cancer-associated fibroblasts induce epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer via CXCL12/CXCR4 axis. Future Oncol. 16:2619–2633. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mariani A, Wang C, Oberg AL, Riska SM, Torres M, Kumka J, Multinu F, Sagar G, Roy D, Jung DB, et al: Genes associated with bowel metastases in ovarian cancer. Gynecol Oncol. 154:495–504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klein A, Olendrowitz C, Schmutzler R, Hampl J, Schlag PM, Maass N, Arnold N, Wessel R, Ramser J, Meindl A, et al: Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett. 276:212–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Wang XH, Zhao YX, Chen C, Xu XY, Sun Q, Wu HY, Chen M, Sang JF, Su L, et al: Cancer-associated fibroblasts correlate with tumor-associated macrophages infiltration and lymphatic metastasis in triple negative breast cancer patients. J Cancer. 9:4635–4641. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Houthuijzen JM and Jonkers J: Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 37:577–597. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paolillo M and Schinelli S: Extracellular Matrix Alterations in Metastatic Processes. Int J Mol Sci. 20:202019. View Article : Google Scholar
|
|
21
|
Visse R and Nagase H: Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 92:827–839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pittayapruek P, Meephansan J, Prapapan O, Komine M and Ohtsuki M: Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 17:172016. View Article : Google Scholar
|
|
23
|
Qin H, Liu X, Li F, Miao L, Li T, Xu B, An X, Muth A, Thompson PR, Coonrod SA, et al: PAD1 promotes epithelial-mesenchymal transition and metastasis in triple-negative breast cancer cells by regulating MEK1-ERK1/2-MMP2 signaling. Cancer Lett. 409:30–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satriyo PB, Bamodu OA, Chen JH, Aryandono T, Haryana SM, Yeh CT and Chao TY: Cadherin 11 inhibition downregulates β-catenin, deactivates the canonical WNT signalling pathway and suppresses the cancer stem cell-like phenotype of triple negative breast cancer. J Clin Med. 8:82019. View Article : Google Scholar
|
|
25
|
Zhu X, Wang K, Zhang K, Xu F, Yin Y, Zhu L and Zhou F: Galectin-1 knockdown in carcinoma-associated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim Biophys Sin (Shanghai). 48:462–467. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon YJ, Leibovitch BA, Bansal N, Pereira L, Chung CY, Ariztia EV, Zelent A, Farias EF and Waxman S: Targeted interference of SIN3A-TGIF1 function by SID decoy treatment inhibits Wnt signaling and invasion in triple negative breast cancer cells. Oncotarget. 8:88421–88436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv ZD, Yang ZC, Liu XP, Jin LY, Dong Q, Qu HL, Li FN, Kong B, Sun J, Zhao JJ, et al: Silencing of Prrx1b suppresses cellular proliferation, migration, invasion and epithelial-mesenchymal transition in triple-negative breast cancer. J Cell Mol Med. 20:1640–1650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jamieson C, Sharma M and Henderson BR: Targeting the β-catenin nuclear transport pathway in cancer. Semin Cancer Biol. 27:20–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Samimi H, Haghpanah V, Irani S, Arefian E, Sohi AN, Fallah P and Soleimani M: Transcript-level regulation of MALAT1-mediated cell cycle and apoptosis genes using dual MEK/Aurora kinase inhibitor ‘BI-847325’ on anaplastic thyroid carcinoma. Daru. 27:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu FI, Sun YH, Wei CY, Thisse C and Thisse B: Tissue-specific derepression of TCF/LEF controls the activity of the Wnt/β-catenin pathway. Nat Commun. 5:53682014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barua D and Hlavacek WS: Modeling the effect of APC truncation on destruction complex function in colorectal cancer cells. PLOS Comput Biol. 9:e10032172013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karami S, Lin FM, Kumar S, Bahnassy S, Thangavel H, Quttina M, Li Y, Ren J and Bawa-Khalfe T: Novel SUMO-protease SENP7S regulates β-catenin signaling and mammary epithelial cell transformation. Sci Rep. 7:464772017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arnold HK, Zhang X, Daniel CJ, Tibbitts D, Escamilla-Powers J, Farrell A, Tokarz S, Morgan C and Sears RC: The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 28:500–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morrow KA, Das S, Meng E, Menezes ME, Bailey SK, Metge BJ, Buchsbaum DJ, Samant RS and Shevde LA: Loss of tumor suppressor Merlin results in aberrant activation of Wnt/β-catenin signaling in cancer. Oncotarget. 7:17991–18005. 2016. View Article : Google Scholar : PubMed/NCBI
|



















