Introduction
The recombinant adeno-associated virus (rAAV) vector
has been widely used in a number of basic and clinical
investigations (1). Compared to
other viruses, the rAAV vector possesses numerous advantages for
gene delivery, including a low immunogenicity, low genotoxicity,
long-term gene expression, wide tissue tropism and a high
transduction efficiency in vivo (1–3).
In comparison with AAV2, the replication rate of AAV8 has been
shown to be 4–10-fold faster and transgene expression is higher
with AAV8 in mice (4,5). Accordingly, rAAV8 is preferred for
liver gene therapy, with the induction of expression in the
majority of hepatocytes and to a lesser degree in other organs,
including the pancreas, spinal cord and kidney (6).
The key factors affecting the transduction efficacy
of rAAV vectors include vector design, capsid selection, transgene
expression cassette design and drug delivery routes (7). In transgene expression cassette
design, regulatory elements and the cis-acting element play
an important role in regulating transgene expression (8,9).
The promoter is a major cis-acting element in the design of
the expression vector, which dictates the expression, as well as
cell-specificity (5,6). Promoters, with subtle changes, have
a variable impact on overall transgene expression (10). The overall transgene expression
can be increased by up to 90-fold with the cytomegalovirus (CMV)
enhancer (11). The CMV
immediate-early enhancer (CMV–IE)/chicken β-actin (CAG) promoter is
a synthetic promoter (12). The
CAG promoter is widely used in rAAV vectors, and exhibits a potent
and long-term transcriptional activity in rodent livers (13,14). Thyroxine binding globulin (TBG) is
a 54-kDa acidic glycoprotein, which is synthesized primarily in
liver tissues. The TBG promoter is a liver-specific promoter, which
limits transgene expression to hepatic tissues, with a low
distribution in other tissues, including spleen, kidney and large
intestine (15,16). Both the CAG and TBG promoters have
greatly facilitated vector design in liver-targeted gene therapy
(14,16). However, the direct comparison of
CAG and TBG, with routinely used administrations, has not yet been
reported to date, at least to the best of our knowledge.
Delivery methods affect gene transduction efficiency
and the patterns of the vectors. In the process of transduction,
the intravenous (IV) injection of rAAV is the most typically used
administration route (17,18).
However, an IV injection requires higher technical operations with
a low success rate for some investigators. Compared with other
approaches, intraperitoneal (IP) administration provides several
advantages, including simple technology, the minimal induction of
the humoral immune response and the ability to obtain long-term
transgene expression. IP injection can also transduce genes, with
considerable transduction efficiency in the liver (8,19).
The selection of the promoter is the key determinant of transgene
expression intensity and pattern across hepatic lobules (19). However, to date, to the best of
our knowledge, the transduction efficiency between the IV and IP
routes has not been compared for any of the aforementioned
promoters.
In the present study, the transgene expression
efficiency of the CAG, TBG669 and TBG410 promoters in the rAAV8
vector in the liver via IV and IP administrations was compared.
Enhanced green fluorescent protein (EGFP) protein expression was
examined to indicate the working efficiency of the three promoters
and two administration routes.
Materials and methods
Mouse model and viral vector
administration
The animal study was approved by the Institutional
Animal Care and Use Committee (approval no. IACUC 201903-138) of
Ningbo University (Zhejiang, China) in March 2019. The experiment
was performed in January 2020. A total of 70 male ICR mice (age,
6–8 weeks; weight, 30±2 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. The mice were housed at the Animal
Center of Ningbo University and maintained under 12-h light/dark
cycle at 24°C, with a relative humidity of 50–70%. The mice were
provided with free access to commercial rodent chow and pure water.
They were cared for in accordance with the principles of the Guide
for Care and Use of Experimental Animals issued by Ningbo
University.
The mice were randomly divided into seven groups as
follows: The control group (control; n=10), the rAAV8-treated
groups by IV injection (the rAAV8-TBG410-EGFP group, the
rAAV8-TBG669-EGFP group and the rAAV8-CAG-EGFP group; n=10 per
group) and the rAAV8-treated groups by IP injection (the
rAAV8-TBG410-EGFP group, the rAAV8-TBG669-EGFP group and the
rAAV8-CAG-EGFP group; n=10 per group). The vector constructs,
rAAV-TBG410-EGFP, rAAV-TBG669-EGFP and rAAV-CAG-EGFP, encoding EGFP
were designed and purchased from Guangzhou PackGene Biotech Co.
Ltd. The viral particles were diluted in PBS (Thermo Fisher
Scientific, Inc.) at 1×1012 genome copies (GC)/ml
immediately prior to injection and at a total of 1×1011
GC in 100 µl PBS was administered to the mice in the aforementioned
groups via either IV or IP injection. The infusion time for each
mouse was ~30 sec. The mice in the control group were left
untreated.
After the administration of rAAV8, the mental state,
activity, eating, hair state of the mice was observed every day.
When there were significant changes in the mental state, behavior,
sharp decrease in activity, sparse hair, the mice would be
euthanized to avoid greater pain. In the process of the experiment,
no mice showed the aforementioned symptoms and none were found
dead. After 4 weeks, blood was collected from all mice by
retroorbital bleeding. Then, all of the mice were euthanized using
CO2 inhalation at a low flow rate (20% of the volume of
the cage per minute), with ventilation maintained for 1–2 min. The
mice were confirmed dead when no breathing, no corneal reflexes and
body stiffness were examined. The blood samples were centrifuged at
670 × g for 20 min at 4°C and the serum was then stored at −80°C
until further analysis. A section of the freshly isolated liver
tissue was cut and immediately fixed with 4% paraformaldehyde (PFA)
solution (Shanghai Guoyao Reagent Co. Ltd.) at 4°C for 24 h. The
remaining liver tissues were kept at −80°C for future analysis.
Western blot analysis of EGFP
Since only 13 of the 15 instrument lanes were
available for western blot analysis, the livers of four mice in
each group were randomly selected to compare the efficiency between
IV and IP administration. When the transgene expression efficiency
was compared among the three promoters in the IV and IP
administration groups, three of the aforementioned four mouse
livers in each group were used for western blotting. Total protein
was extracted from 20 mg frozen liver tissues, stored at −80°C,
using RIPA lysis buffer, supplemented with 1% protease inhibitors
(both from Beijing Solarbio Science and Technology Co., Ltd.). The
samples were then adequately homogenized at 960 × g for 30 sec at
room temperature using a MagNA Lyser instrument (Roche
Diagnostics). Tissue debris was removed by centrifugation at 2,400
× g at 4°C for 20 min. The protein concentration was determined
using a BCA protein assay kit (Thermo Fisher Scientific, Inc.),
then adjusted to 8 mg/ml. Loading buffer (5X; Beijing Solarbio
Science and Technology Co., Ltd.) was then added to the sample
(volume-volume, 1:4) and heated at 100°C for 5 min. Subsequently, 5
µl/lane of the protein extract was loaded and separated using
SDS-PAGE (10% separating gel; 5% spacer gel) and transferred onto
PVDF membranes following electrophoresis. The membranes were then
blocked with 5% skimmed milk/TBS-0.1% Tween-20 for 3.5 h at room
temperature. This was followed by incubation with EGFP (1:5,000;
cat. no. ab184601) or GAPDH (1:5,000; cat. no. ab181602) (both from
Abcam) primary antibodies overnight at 4°C. After washing with PB,
the membranes were incubated with goat anti-mouse IgG antibody
(1:5,000; cat. no. GAM001) or goat anti-rabbit IgG antibody
(1:5,000; cat. no. GAR007) (both from MultiSciences) for 2 h at
room temperature. Finally, the blotted membranes were exposed to
ECL substrate (Advansta, Inc.), and the chemiluminescence imaging
system, ChemiScope 6100 Touch, was used to capture the images. The
measurement of the protein band density was performed using ImageJ
software (version 1.8.0; National Institutes of Health).
Immunofluorescence
The liver sections were fixed in 4% PFA fix solution
at 4°C for 24 h. They were then sequentially dehydrated in 15 and
30% sucrose solution overnight at 4°C, until the samples sunk. The
samples were embedded in Optimal cutting temperature medium (OCT;
Sakura Finetek Japan Co., Ltd.) and stored at −80°C. The liver
tissues were cut into 10-µm-thick cryosections using a Leica
cryostat (Leica Microsystems GmbH). The sections were then
incubated with mouse monoclonal anti-EGFP antibody (1:100; cat. no.
ab184601; Abcam) overnight at 4°C. The sections were subsequently
incubated with Alexa Fluor® 488 goat anti-mouse IgG
(1:1,000; cat. 4408S; Cell Signaling Technology, Inc.) for 2 h at
room temperature, followed by staining with DAPI (Sigma-Aldrich;
Merck KGaA) for 10 min in the dark. The tissue sections were
visualized using a Leica immunofluorescent confocal microscope
(Leica Microsystems GmbH) at ×200 magnification. The measurement of
the fluorescence intensity was performed using Leica LAS X software
(version 3.4.1; Leica Microsystems GmbH).
Biochemical analysis
To determine whether the treatment induced
hepatotoxicity, the serum samples were thawed at 4°C. The alanine
aminotransferase (ALT), aspartate aminotransferase (AST), total
bile acid (TBA) and total bilirubin (TBIL) activity in the serum
samples was then measured using enzymatic colorimetry with the
Multiskan GO plate reader (Thermo Fisher Scientific, Inc). The
procedures for the analysis were performed according to the
instructions provided by the kits (ALT, cat. no. H001; AST, cat.
no. H002; TBA, cat. no. H101T; TBIL, cat. no. H115; all from Ningbo
Medical System Biotechnology Co., Ltd.).
Histopathological analysis
The formalin-fixed liver tissues were dehydrated in
a gradient ethanol series (70, 80, 90 and 100%) and washed with
xylene. They were then embedded in paraffin at 56°C and cut into
4-µm-thick sections using the Leica RM2235 Manual Rotary Microtome
(Leica Microsystems GmbH) and stained with hematoxylin (cat. no.
G1140) for 3 min and eosin (cat. no. G1100) (both from Beijing
Solarbio Science & Technology Co., Ltd.) for 2 min at room
temperature. The observation of the stained liver tissue sections
was performed using an Olympus BX41 microscope (Olympus
Corporation) at ×100 and ×400 magnifications.
Reverse transcription-quantitative PCR
(RT-qPCR)
The frozen liver tissues (20 mg) of five mice in
each group randomly selected for RT-qPCR analysis. The liver
tissues were lysed with TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.) and homogenized at 960 × g for 30 sec at
room temperature using a MagNA Lyser instrument (Roche
Diagnostics). Pure chloroform was then added for 5 min to extract
total RNA at room temperature. This was followed by centrifugation
at 3, 200 × g for 15 min at 4°C and precipitation with 75% ethanol.
The RNA concentration was quantified using the Multiskan GO plate
reader (Thermo Fisher Scientific, Inc) and its purity was
determined using the OD260/OD280 calculation. Total RNA was reverse
transcribed into cDNA using RT (20 µl; cat. no. CW2569M; CoWin
Biosciences Co., Ltd.) as previously described (20). The RT temperature protocol was as
follows: 25°C for 5 min, 42°C for 1 h, inactivation at 70°C for 5
min and chilling at 4°C for holding. The primer sequences are
presented in Table SI. qPCR was
performed in 96-well plates using a 5-µl system containing 1 µl
total cDNA, 2.2 µl UltraSYBR Mixture (cat. no. CW0957H; CoWin
Biosciences Co., Ltd.), 0.1 µl forward and reverse primer, and 1.6
µl RNase-free water using the LightCycler 480 II system (Roche
Diagnostics). The following thermocycling conditions were used:
Initial denaturation at 95°C for 10 sec, 55°C for 10 sec and 72°C
for 15 sec. The 2−ΔΔCq formula was used to quantify the
expression levels of target genes (21). The measured mRNA abundance was
normalized to 18S rRNA. The expression levels in the control
group were set to 1, and the data of the other six groups were
normalized and expressed as relative expression.
Statistical analysis
The data are presented as the mean ± SEM. Data
analysis was performed using SPSS version 23 software (IBM Corp.)
and column charts were generated using GraphPad Prism software
(version 8.0; GraphPad Software, Inc.). Statistically significant
differences were determined using one-way ANOVA followed by Tukey's
post hoc test for multiple comparisons. When data were not normally
distributed, the Kruskal-Wallis test was used for the determination
of differences among groups and Bonferroni's post hoc test was used
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Schematic presentation of the three
rAAV8 vectors
The schematic presentation of the three different
rAAV8 vectors used in the present study was presented in Fig. 1. rAAV8 vector constructs encoding
EGFP were transduced, driven by the CAG, TBG669 and TBG410
promoters. An equal concentration of 1×1011 GC in 100 µl
PBS was delivered to the ICR mice via either IP or IV injection. At
4 weeks after the injection, the mice were euthanized, and blood
and liver tissues were collected for analysis.
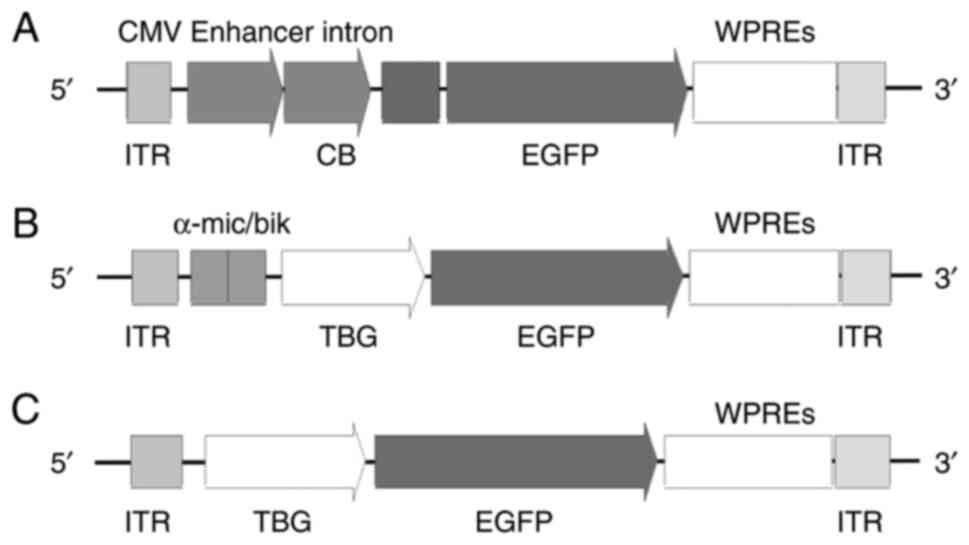 | Figure 1.Schematic representation of the three
rAAV8 constructs. Schematic structure of (A) rAAV8-CAG-EGFP, (B)
rAAV8-TBG669-EGFP and (C) rAAV8-TBG410-EGFP vector. rAAV,
recombinant adeno-associated virus; CMV, cytomegalovirus; CB,
chicken β-actin promoter; α-mic/bik, an enhancer element; ITR,
inverted terminal repeats; EGFP, enhanced green fluorescent
protein; WPRE, woodchuck post-transcriptional regulatory element;
TBG, thyroxine-binding globulin promoter; CAG, cytomegalovirus
immediate-early enhancer/chicken β-actin. |
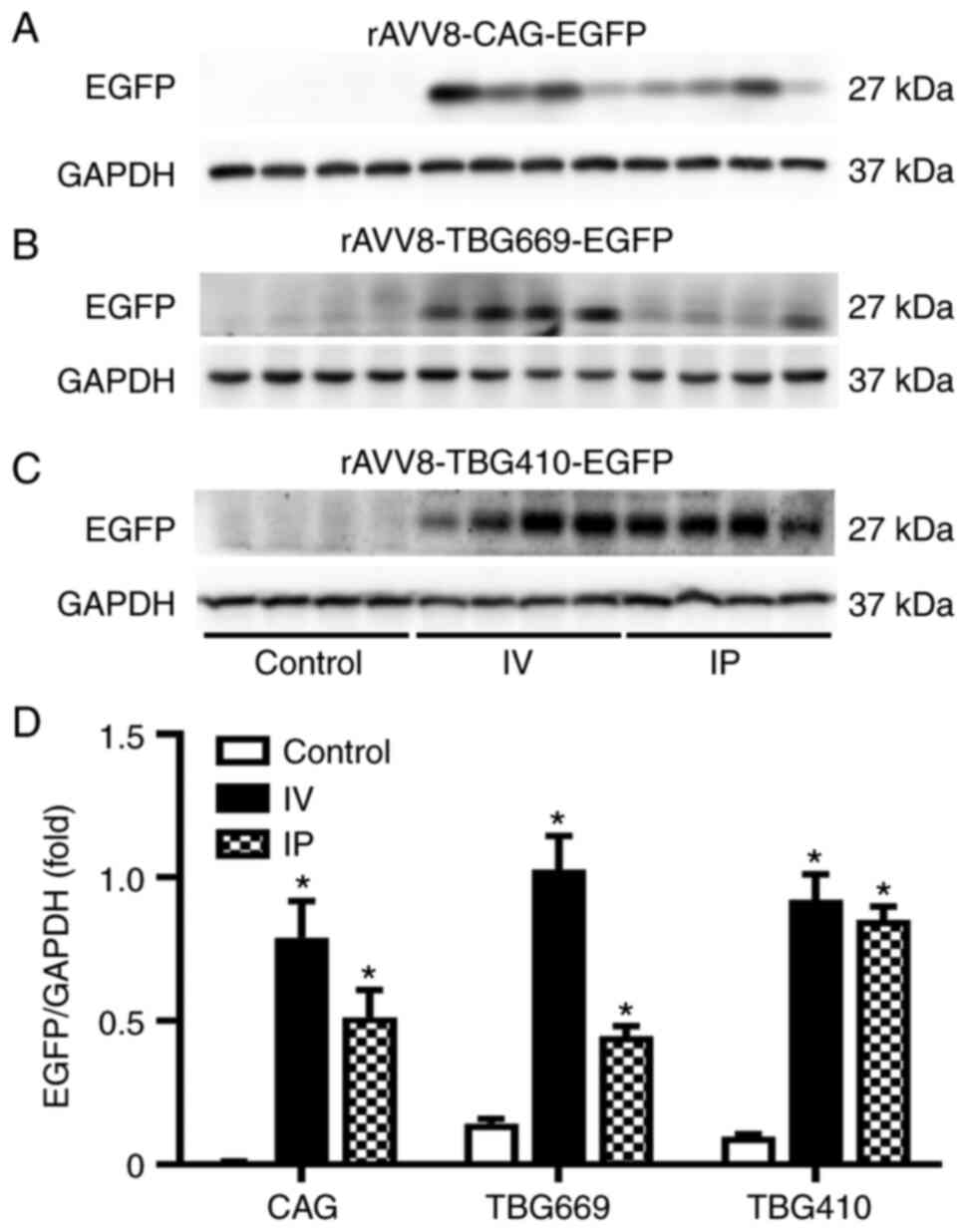
EGFP expression driven by the CAG
promoter is the highest
Western blot analysis was performed to determine the
protein expression level of EGFP among the three promoters, under
the same administration routes. As shown in Fig. 2, the protein expression level of
EGFP was the highest in the rAAV8-CAG-EGFP group, by both
administration routes. With IV administration, significant
differences were observed between the control, TBG410, TBG669 and
CAG groups., EGFP protein expression level induced by the CAG
promoter was 67-fold higher compared with that in the
rAAV8-TBG410-EGFP group and 26-fold higher compared with that in
the TBG669 group. Gene transduction induced by the TBG669 promoter
was almost 3-fold higher than that induced by the TBG410 promoter
(Fig. 2A). Similarly, with the IP
administration, the EGFP expression level in the rAAV8-CAG-EGFP
group was 75-fold higher compared with that in the TBG410 group,
and 41-fold higher compared with that in the TBG669 group (Fig. 2B).
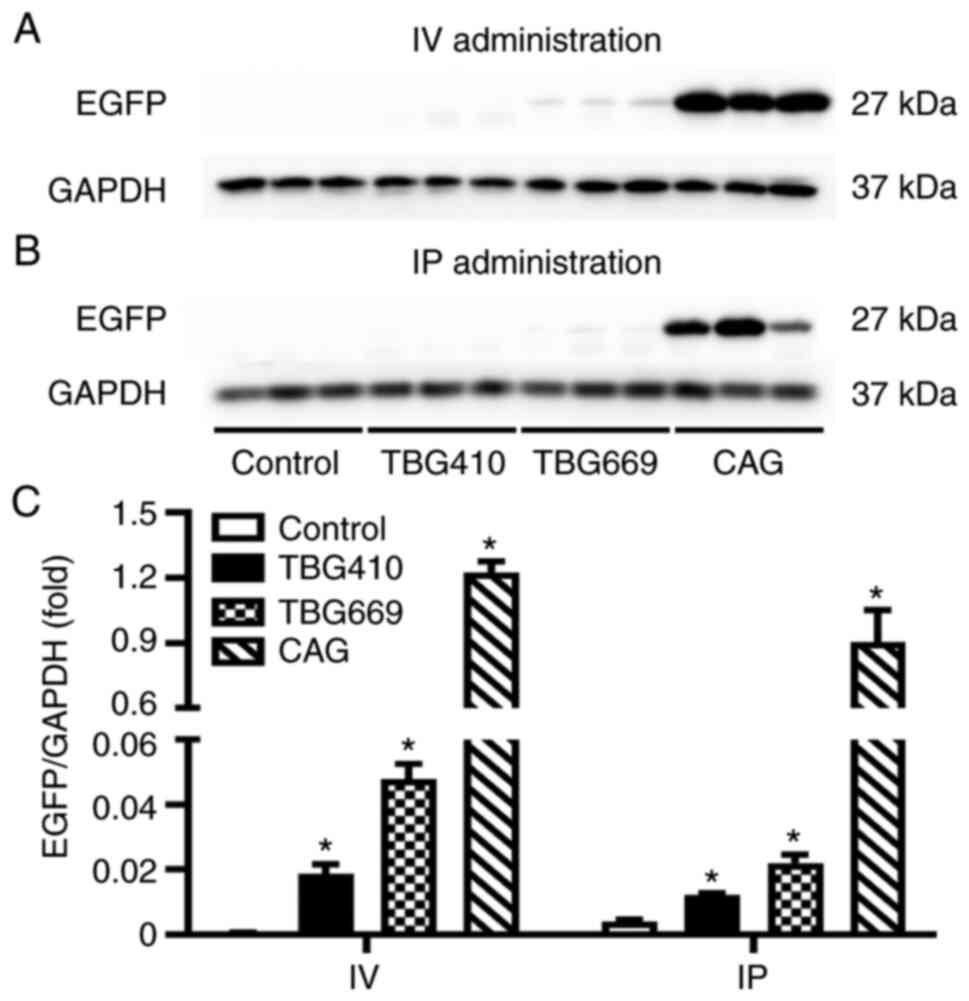 | Figure 2.Comparison of the rAAV8 transduction
efficiency with three different promoters. The EGFP protein
expression level in the liver was analyzed using western blot in
the control, TBG410, TBG669 and CAG promoter groups administered
via (A) IV and (B) IP. (C) Semi-quantification of the western blots
for the control, rAAV8-CAG-EGFP, rAAV8-TBG410-EGFP and
rAAV8-TBG669-EGFP groups. The control group used for IV and IP
groups was the same without any treatment. The livers of three mice
in each group were used for western blot analysis and each lane
represented an individual mouse. The data are presented as the mean
± SEM. n=3. *P<0.05 vs. control. IV, intravenous; IP,
intraperitoneal; CAG, cytomegalovirus immediate-early
enhancer/chicken β-actin; EGFP, enhanced green fluorescent protein;
rAAV, recombinant adeno-associated virus; TBG, thyroxine-binding
globulin promoter. |
IV delivery is more efficient than IP
delivery in the liver
The comparison of the two administration routes also
revealed notable results. Driven by the CAG promoter, the abundance
of EGFP was significantly increased; the ratio of EGFP/GAPDH in the
control, IV and IP groups was 0.008, 0.791 and 0.513, respectively.
The protein expression level of EGFP with the IV and IP injections
was 99- and 64-fold higher compared with that in the control group,
respectively (Fig. 3A). In the
rAAV8-TBG669-EGFP group, the ratio of EGFP/GAPDH in the control, IV
and IP groups was 0.142, 1.027 and 0.446, respectively. EGFP
protein expression with the IV injection was 2-fold higher compared
with that in the IP injection group (Fig. 3B). Generally, EGFP protein
expression via the IV route was superior than that via the IP
route, with all vectors. However, driven by the CAG promoter and
compared with that in the other two promoters, the ratio of
EGFP/GAPDH in the control, IV and IP groups was 0.097, 0.922 and
0.853, respectively. EGFP protein expression was similar between
both delivery routes (Fig.
3C).
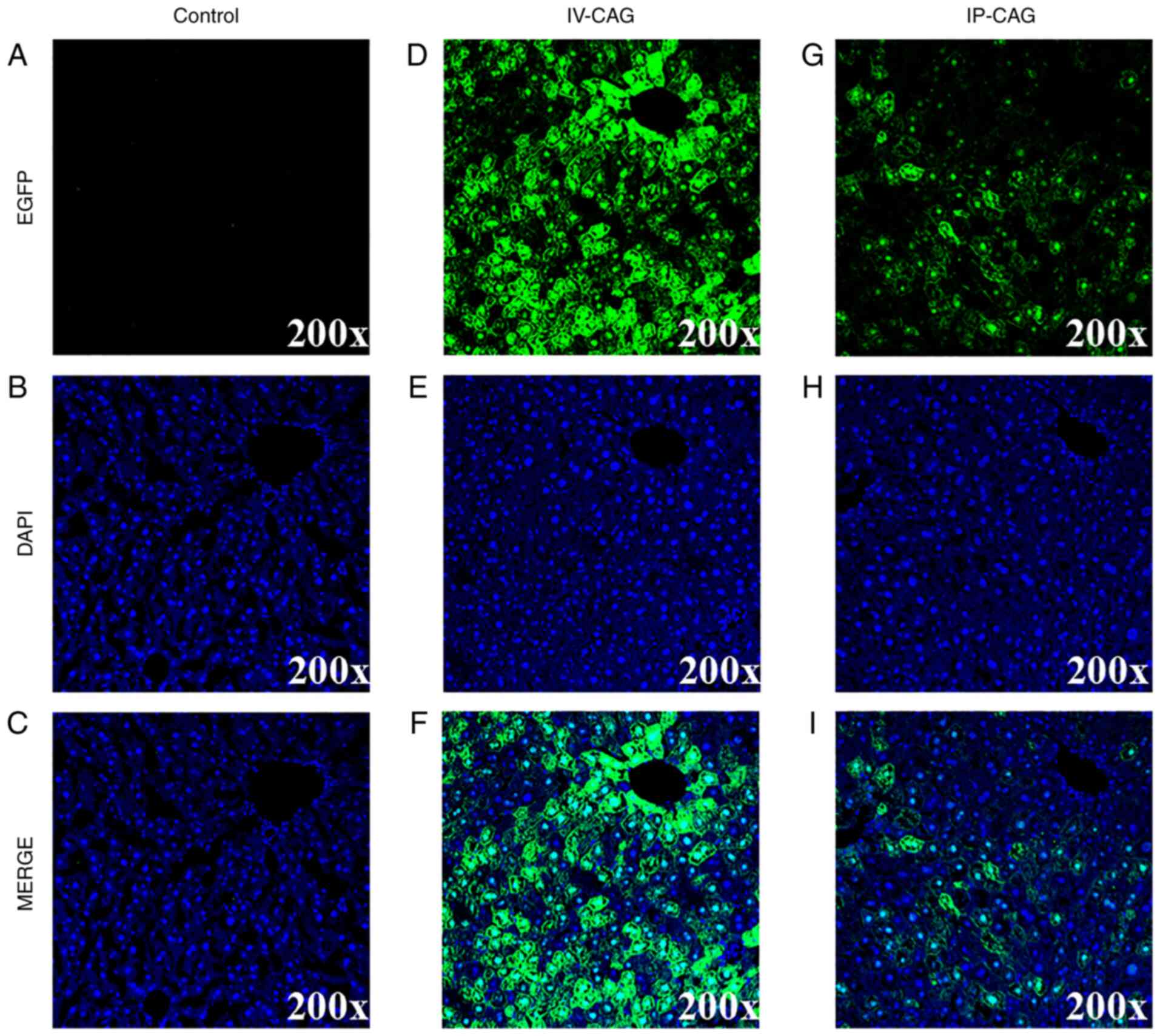
Immunofluorescence analysis of EGFP
expression in the liver
The EGFP expression patterns were assessed using a
fluorescence microscope to confirm the quantification of the
densitometric analysis. The representative fluorescent micrographs
of the CAG promoter with IV and IP administrations are presented in
Fig. 4. In the rAAV8-CAG-EGFP
group, a robust transduction was observed, and gene expression
mainly transduced in the nucleus around the central veins. In
addition, the EGFP intensity was close to 4-fold higher via the IV
injection compared with that via IP injection (Fig. S1). With respect to the TBG669 and
TBG410 promoters, their transgene efficiency was in accordance with
that observed with western blot analysis described above (data not
shown).
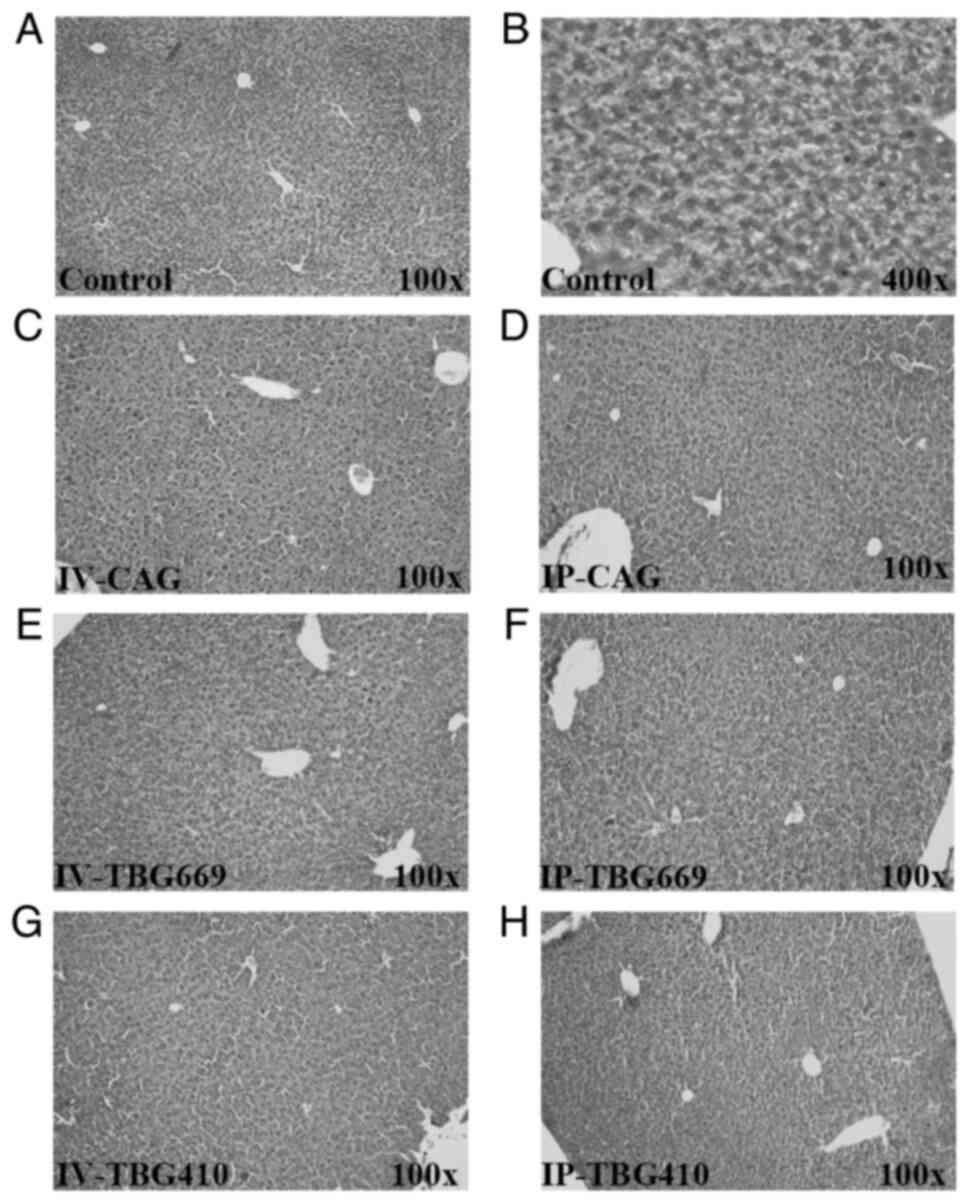
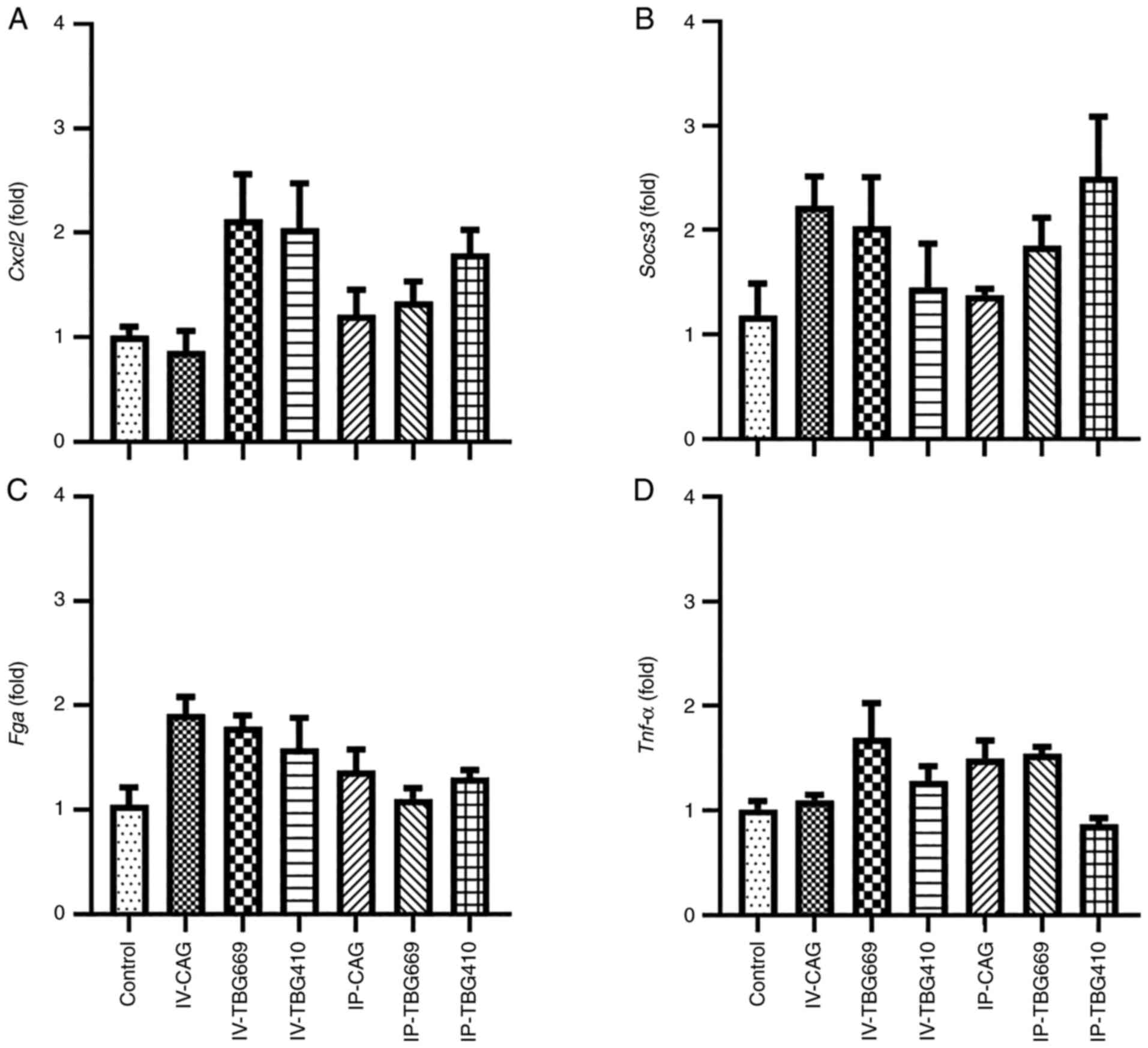
All rAAV8 vectors exhibit sufficient
safety profiles
A total of three different analyses were performed
to evaluate the safety of the three rAAV8 vectors used in the
present study. Biochemically, the serum ALT, AST, TBA and TBIL
levels exhibited no significant changes among the three rAAV8
vectors (Fig. 5). In addition, no
liver injury or inflammation was observed in any of the groups
(Fig. 6). The high magnification
images revealed no evidence of cellular damage and an inflammatory
response (Fig. S2), supporting
the aforementioned and biochemical data. Compared with that in the
control group, the mRNA expression levels of chemokine ligand 2,
Tnf-α, suppressor of cytokine signaling 3 and fibrinogen α
chain in the other six groups exhibited no significant differences
(Fig. 7). These results suggested
that the three rAAV8 vectors did not cause inflammatory
responses.
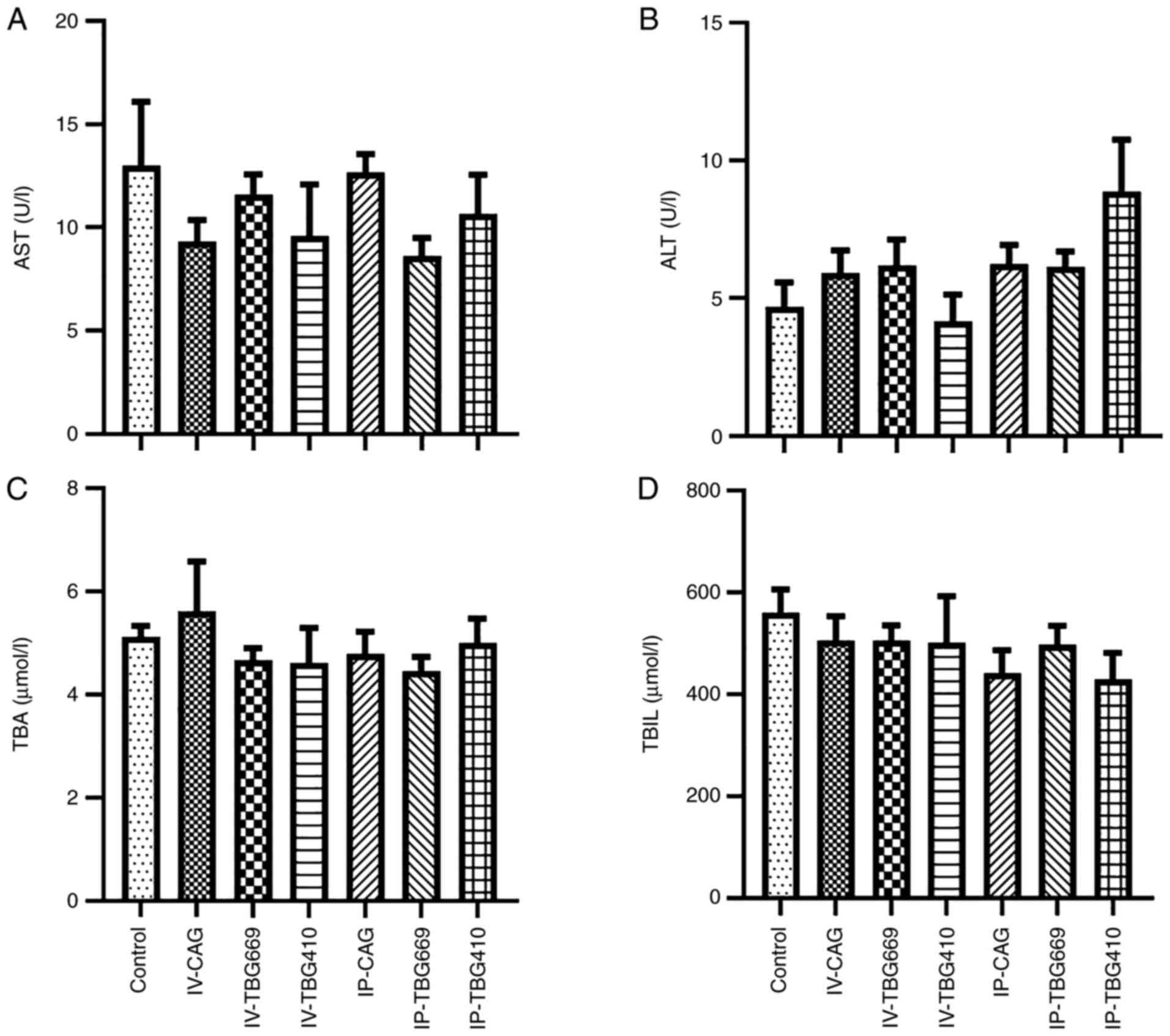 | Figure 5.Hepatic injury biomarkers in mice
treated with rAAV8 vectors did not increase. (A) AST, (B) ALT, (C)
TBA and (D) TBIL levels in seven groups, four weeks after rAAV8
administration. n=5. ALT, alanine aminotransferase; AST, aspartate
aminotransferase; TBA, total bile acid; TBIL, total bilirubin;
rAAV, recombinant adeno-associated virus; IV, intravenous; IP,
intraperitoneal. |
Discussion
rAAV is the most promising gene therapy vehicle, as
the vector itself does not affect gene expression. When a
functional protein is inserted into the rAAV vector, it can
increase or decrease gene expression and affect the organism or the
disease model. How much it will affect the organism or the disease
model depends on the action of the functional protein itself. The
promoter used is crucial for the gene transduction efficiency of
the rAAV vectors (10). The CAG
promoter is a composite promoter, connecting the CMV–IE enhancer
sequence to the chicken β-actin promoter (22). It has been used for >30 years
and a number of gene therapies have made use of this promoter to
achieve a high vector expression (22,23). In previous studies using
AAV-mediated RPE65 transfer to retinal pigment epithelium as gene
therapy for Leber congenital amaurosis, AAV vectors with the CAG
promoter achieved stable RPE expression, and restoration of rod and
cone photoreceptor function for several years (24,25).
The TBG promoter is a liver-specific promoter, which
confers transgene persistently and specific expression to the liver
for up to several months following integration (26). The TBG promoter limits transgene
expression to hepatic tissue with a low distribution in other
tissues. Therefore, this promoter minimizes undesired toxicity or
host immune responses derived from the overexpression of the
transgene outside the liver (15). Quantitively, the efficiency of the
TBG promoter has been reported to be slightly lower than that of
the ubiquitous CMV promoter in driving foreign gene expression
(16). However, in the present
study, with IV administration, a significant induction of EGFP
protein expression occurred in the rAAV8-CAG-EGFP group. EGFP
expression, driven by the CAG promoter, was much higher than those
driven by the TBG410 and the TBG669 promoter (67- and 26-fold
respectively, Fig. 2A).
Similarly, with IP administration, EGFP expression was as high as
those of IV administration (Fig.
2B). With respect to the TBG410 and TBG669 promoters, EGFP
expression was close to 3-fold higher by the TBG669 promoter than
by the TBG410 promoter in the IV group, and 1.8-fold in the IP
group (Fig. 3). This occurred as
an α-mic enhancer was added to the TBG669 promoter.
A number of factors affect rAAV vector transduction
efficiency, such as AAV serotype tropism (1), transgene expression cassette design
(9,27,28), the pattern of administration
(18) and the time of injection
(29,30). In IP administration, rAAVrh.10
showed robust transduction in skeletal muscle, rAAV8 showed
efficient transduction in the pancreas, and rAAV9 and rAAV7 showed
the strongest transduction in the liver (1). The transgene expression cassette
contains an enhancer, promoter, and various pre- and/or
post-regulatory elements. All of them function to achieve a higher
expression of the rAAV (27).
Enhancers were recognized as cis-regulatory DNA elements,
which can increase the expression of target genes in cooperation
with promoters (9). Within an
enhancer sequence, there are multiple transcription factor binding
sites that are required for the regulation of enhancer activity
(28). According to the
administration routes, the transduction efficiency in cirrhotic
livers is lower compared with that in healthy livers, when the
vector is administered via IP injection (18). The injection/infusion rate appears
to be inversely proportionate to the gene transduction efficiency.
A longer infusion time has been found to produce a higher
transduction efficiency than the peak administration concentration.
It was considered to be associated with the time of exposure to the
rAAV vector (29).
The pattern of virus injection is also one of the
factors affecting efficiency. In the present study, transgene
expression with IV injection was more efficient than that with IP
injection in the liver. However, the efficiency of TBG410 was
similar between both delivery routes. When one wants to choose the
administration of a virus, IP injection can also be considered,
especially when IV injection was considered difficult for numerous
investigators.
In a previous study investigating AAV9-CMV-GFP and
AAV9-CBA-GFP vectors, it was found that the gene transfection
efficiency increased in a time-dependent manner (30). The transduction efficiency of
AAV9-CBA-GFP in the liver reached 60% in the second week and
maintained a high level of expression of >80% after the fourth
week. The expression level of GFP reached peak levels at 5 weeks
following virus injection. Certainly, it is interesting to
investigate the dynamic changes of expression efficiency of rAAV
after administration, and to compare them between IV injection and
IP injection. In the present study, the gene transduction
efficiency was compared between three different promoters in two
types of administration routes. The mice are usually sacrificed at
3–4 weeks following the injection to detect gene expression
(31–33). Therefore, in the present study,
the mice were sacrificed at 4 weeks. The choice of sacrificing the
mice at 4 or 5 weeks may not have had a notable impact on the
results.
According to the results obtained, compared with
that in the CAG promoter, the hepatocyte-targeting TBG promoters
led to a lower protein expression level of EGFP. However, the TBG
promoter is still widely used as a hepatocyte-targeting promoter
(26,34,35). When compared with that in the
control, the TBG promoter could still be effectively transduced in
the liver. The efficiency of the TBG669 promoter with the IV
injection was 2-fold higher compared with that for IP injection
(Fig. 3B). The efficiency of the
TBG410 promoter was similar between both delivery routes (Fig. 3C). Therefore, if gene expression
in extrahepatic tissue affects the disease model, the TBG promoter
remains an adequate choice. However, the molecular mechanism is
still unclear as to why the transfection efficiency of the CAG
promoter was higher than that of hepatocyte-targeting TBG promoter
in the liver.
rAAV vectors as gene therapy vectors are widely used
in clinical practice and have exhibited efficacy in a growing
number of clinical trials, with the majority of data suggesting
that they are non-pathogenic (3,36).
However, some experimental studies have reported evidence of the
potential genotoxicity of rAAV vectors (31,36). rAAV may result in cancer
development, with hepatocellular carcinoma (HCC) being the most
likely malignancy (37,38). The AAV vector dose,
enhancer/promoter selection and the timing of gene delivery were
all critical factors for determining HCC incidence after AAV gene
delivery (36). Compared with
that in the healthy adult liver, rAAV gene therapy induces HCC at a
high frequency in mice with chronic liver disease (35). In addition, the transduction of
hepatic tissue by AAV vectors has been reported to be inefficient
in mice with chronic liver disease (39). Extremely high doses
(2×1014 GC/kg) of AAV have been shown to result in acute
toxicity in non-human primates (40). However, this high dose of AAV9 has
been used in patients with spinal muscular atrophy type 1 in a
clinical trial, without any severe treatment-related adverse events
(41). There may be a threshold
for the toxicity of rAAV; however, the doses currently used in the
majority of clinical trials are much lower than this threshold
(42). Furthermore, the
enhancer-promoter may be involved in tumorigenesis in the liver
(43). When compared to other
promoters, AAV vectors with a TBG promoter significantly increase
risk of tumorigenesis (36,44).
In conclusion, the present study demonstrated that
the ubiquitous CAG promoter induced a higher EGFP expression level
than hepatocyte-targeting TBG promoters via both IV and IP
administration. Although less effective than IV administration, the
IP injection exhibited a satisfactory efficiency with a high
success rate of procedure for the three promoters. In particular,
for the TBG410 promoter, the administration route exerted a minimal
effect on the transduction efficiency. These data provided a good
reference for the selection of a suitable rAAV8 promoter for the
gene therapy of liver diseases, as well as a suitable
administration route. However, the molecular mechanism of
differential efficiency between promoter CAG and TBG, as well as
the dynamic comparison remain to be investigated.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Zhejiang Public Welfare
Technology Research Program (grant no. LGD19H070001), the Zhejiang
Provincial Natural Science Foundation of China (grant nos.
LGF19H030008 and LY20H030001), the Ningbo Clinical Medicine
Research Center Project (grant no. 2019A21003) and the Ningbo
Public Welfare Technology Application Research Project (grant no.
202002N3160).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JK and LH performed the experiments, organized the
figures and wrote the manuscript. WZ, JL and XZ collected and
analyzed the data. YS and AL conceived the study, confirmed the
authenticity of all the raw data and revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the institutional animal
care and use committee (approval no. IACUC 201903-138) at Ningbo
University (Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ai J, Li J, Gessler DJ, Su Q, Wei Q, Li H
and Gao G: Adeno-associated virus serotype rh.10 displays strong
muscle tropism following intraperitoneal delivery. Sci Rep.
7:403362017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang D, Zhong L, Nahid MA and Gao G: The
potential of adeno-associated viral vectors for gene delivery to
muscle tissue. Expert Opin Drug Deliv. 11:345–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dismuke DJ, Tenenbaum L and Samulski RJ:
Biosafety of recombinant adeno-associated virus vectors. Curr Gene
Ther. 13:434–452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lotfinia M, Abdollahpour-Alitappeh M,
Hatami B, Zali MR and Karimipoor M: Adeno-associated virus as a
gene therapy vector: Strategies to neutralize the neutralizing
antibodies. Clin Exp Med. 19:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vercauteren K, Hoffman BE, Zolotukhin I,
Keeler GD, Xiao JW, Basner-Tschakarjan E, High KA, Ertl HC, Rice
CM, Srivastava A, et al: Superior in vivo transduction of human
hepatocytes using engineered AAV3 capsid. Mol Ther. 24:1042–1049.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Bell P, Somanathan S, Wang Q, He
Z, Yu H, McMenamin D, Goode T, Calcedo R and Wilson JM: Comparative
study of liver gene transfer with AAV vectors based on natural and
engineered AAV capsids. Mol Ther. 23:1877–1887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naso MF, Tomkowicz B, Perry WL and Strohl
WR: Adeno-Associated Virus (AAV) as a vector for gene therapy.
BioDrugs. 31:317–334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bates R, Huang W and Cao L: Adipose
tissue: An emerging target for adeno-associated viral vectors. Mol
Ther Methods Clin Dev. 19:236–249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mushimiyimana I, Niskanen H, Beter M,
Laakkonen JP, Kaikkonen MU, Ylä-Herttuala S and Laham-Karam N:
Characterization of a functional endothelial super-enhancer that
regulates ADAMTS18 and angiogenesis. Nucleic Acids Res.
49:8078–8096. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pham PL, Kamen A and Durocher Y:
Large-scale transfection of mammalian cells for the fast production
of recombinant protein. Mol Biotechnol. 34:225–237. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Powell SK, Rivera-Soto R and Gray SJ:
Viral expression cassette elements to enhance transgene target
specificity and expression in gene therapy. Discov Med. 19:49–57.
2015.PubMed/NCBI
|
|
12
|
Lee LR, Peacock L, Lisowski L, Little DG,
Munns CF and Schindeler A: Targeting Adeno-Associated Virus vectors
for local delivery to fractures and systemic delivery to the
skeleton. Mol Ther Methods Clin Dev. 15:101–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daly TM, Okuyama T, Vogler C, Haskins ME,
Muzyczka N and Sands MS: Neonatal intramuscular injection with
recombinant adeno-associated virus results in prolonged
beta-glucuronidase expression in situ and correction of liver
pathology in mucopolysaccharidosis type VII mice. Hum Gene Ther.
10:85–94. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kosuga M, Enosawa S, Li XK, Suzuki S,
Matsuo N, Yamada M, Roy-Chowdhury J, Koiwai O and Okuyama T:
Strong, long-term transgene expression in rat liver using chicken
beta-actin promoter associated with cytomegalovirus immediate-early
enhancer (CAG promoter). Cell Transplant. 9:675–680. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SJ, Sanmiguel J, Lock M, McMenamin D,
Draper C, Limberis MP, Kassim SH, Somanathan S, Bell P, Johnston
JC, et al: Biodistribution of AAV8 vectors expressing human
low-density lipoprotein receptor in a mouse model of homozygous
familial hypercholesterolemia. Hum Gene Ther Clin Dev. 24:154–160.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan Z, Yan H and Ou H: Human thyroxine
binding globulin (TBG) promoter directs efficient and sustaining
transgene expression in liver-specific pattern. Gene. 506:289–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Zhu T, Rehman KK, Bertera S, Zhang
J, Chen C, Papworth G, Watkins S, Trucco M, Robbins PD and Xiao X:
Widespread and stable pancreatic gene transfer by adeno-associated
virus vectors via different routes. Diabetes. 55:875–884. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sobrevals L, Enguita M, Rodriguez C,
Gonzalez-Rojas J, Alzaguren P, Razquin N, Prieto J and Fortes P:
AAV vectors transduce hepatocytes in vivo as efficiently in
cirrhotic as in healthy rat livers. Gene Ther. 19:411–417. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cunningham SC, Dane AP, Spinoulas A and
Alexander IE: Gene delivery to the juvenile mouse liver using
AAV2/8 vectors. Mol Ther. 16:1081–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu G, Dai M, Zheng X, Lin H, Liu A and
Yang J: Cholestatic models induced by lithocholic acid and
α-naphthylisothiocyanate: Different etiological mechanisms for
liver injury but shared JNK/STAT3 signaling. Mol Med Rep.
22:1583–1593. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niwa H, Yamamura K and Miyazaki J:
Efficient selection for high-expression transfectants with a novel
eukaryotic vector. Gene. 108:193–199. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buck TM and Wijnholds J: Recombinant
Adeno-Associated Viral Vectors (rAAV)-vector elements in ocular
gene therapy clinical trials and transgene expression and
bioactivity assays. Int J Mol Sci. 21:41972020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Acland GM, Aguirre GD, Bennett J, Aleman
TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE,
Maguire AM, Palczewski K, et al: Long-term restoration of rod and
cone vision by single dose rAAV-mediated gene transfer to the
retina in a canine model of childhood blindness. Mol Ther.
12:1072–1082. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gardiner KL, Cideciyan AV, Swider M,
Dufour VL, Sumaroka A, Komáromy AM, Hauswirth WW, Iwabe S, Jacobson
SG, Beltran WA and Aguirre GD: Long-term structural outcomes of
late-stage RPE65 gene therapy. Mol Ther. 28:266–278. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carrillo-Carrasco N, Chandler RJ,
Chandrasekaran S and Venditti CP: Liver-directed recombinant
adeno-associated viral gene delivery rescues a lethal mouse model
of methylmalonic acidemia and provides long-term phenotypic
correction. Hum Gene Ther. 21:1147–1154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baruteau J, Waddington SN, Alexander IE
and Gissen P: Gene therapy for monogenic liver diseases: Clinical
successes, current challenges and future prospects. J Inherit Metab
Dis. 40:497–517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erokhin M, Vassetzky Y, Georgiev P and
Chetverina D: Eukaryotic enhancers: Common features, regulation,
and participation in diseases. Cell Mol Life Sci. 72:2361–2375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greig JA, Nordin JM, Bote E, Makaron L,
Garnett ME, Kattenhorn LM, Bell P, Goode T and Wilson JM: Impact of
intravenous infusion time on AAV8 vector pharmacokinetics, safety,
and liver transduction in cynomolgus macaques. Mol Ther Methods
Clin Dev. 3:160792016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen BD, He CH, Chen XC, Pan S, Liu F, Ma
X, Li XM, Gai MT, Tao J, Ma YT, et al: Targeting transgene to the
heart and liver with AAV9 by different promoters. Clin Exp
Pharmacol Physiol. 42:1108–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dalwadi DA, Torrens L, Abril-Fornaguera J,
Pinyol R, Willoughby C, Posey J, Llovet JM, Lanciault C, Russell
DW, Grompe M and Naugler WE: Liver injury increases the incidence
of HCC following AAV gene therapy in mice. Mol Ther. 29:680–690.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu DL, Chow N and Wootton SK: JSRV
intragenic enhancer element increases expression from a
heterologous promoter and promotes high level AAV-mediated
transgene expression in the lung and liver of mice. Viruses.
12:12662020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Ling C, Zhong L, Li M, Su Q, He R,
Tang Q, Greiner DL, Shultz LD, Brehm MA, et al: Efficient and
targeted transduction of nonhuman primate liver with systemically
delivered optimized AAV3B vectors. Mol Ther. 23:1867–1876. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greig JA, Calcedo R, Kuri-Cervantes L,
Nordin JM, Albrecht J, Bote E, Goode T, Chroscinski EA, Bell P,
Richman LK, et al: AAV8 gene therapy for crigler-najjar syndrome in
macaques elicited transgene T Cell responses that are resident to
the liver. Mol Ther Methods Clin Dev. 11:191–201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sabatino DE, Lange AM, Altynova ES, Sarkar
R, Zhou S, Merricks EP, Franck HG, Nichols TC, Arruda VR and
Kazazian HH: Efficacy and safety of long-term prophylaxis in severe
hemophilia A dogs following liver gene therapy using AAV vectors.
Mol Ther. 19:442–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chandler RJ, LaFave MC, Varshney GK,
Trivedi NS, Carrillo-Carrasco N, Senac JS, Wu W, Hoffmann V,
Elkahloun AG, Burgess SM and Venditti CP: Vector design influences
hepatic genotoxicity after adeno-associated virus gene therapy. J
Clin Invest. 125:870–880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nault JC, Datta S, Imbeaud S, Franconi A,
Mallet M, Couchy G, Letouzé E, Pilati C, Verret B, Blanc JF, et al:
Recurrent AAV2-related insertional mutagenesis in human
hepatocellular carcinomas. Nat Genet. 47:1187–1193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang PR, Xu M, Toffanin S, Li Y, Llovet JM
and Russell DW: Induction of hepatocellular carcinoma by in vivo
gene targeting. Proc Natl Acad Sci USA. 109:11264–11269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith JS, Tian J, Muller J and Byrnes AP:
Unexpected pulmonary uptake of adenovirus vectors in animals with
chronic liver disease. Gene Ther. 11:431–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hinderer C, Katz N, Buza EL, Dyer C, Goode
T, Bell P, Richman LK and Wilson JM: Severe toxicity in nonhuman
primates and piglets following high-dose intravenous administration
of an adeno-associated virus vector expressing human SMN. Hum Gene
Ther. 29:285–298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mendell JR, Al-Zaidy S, Shell R, Arnold
WD, Rodino-Klapac LR, Prior TW, Lowes L, Alfano L, Berry K, Church
K, et al: Single-dose gene-replacement therapy for spinal muscular
atrophy. N Engl J Med. 377:1713–1722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ginocchio VM, Ferla R, Auricchio A and
Brunetti-Pierri N: Current status on clinical development of
adeno-associated virus-mediated liver-directed gene therapy for
inborn errors of metabolism. Hum Gene Ther. 30:1204–1210. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grimm D and Büning H: Small but
increasingly mighty: Latest advances in AAV vector research,
design, and evolution. Hum Gene Ther. 28:1075–1086. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kattenhorn LM, Tipper CH, Stoica L,
Geraghty DS, Wright TL, Clark KR and Wadsworth SC: Adeno-associated
virus gene therapy for liver disease. Hum Gene Ther. 27:947–961.
2016. View Article : Google Scholar : PubMed/NCBI
|