Introduction
Osteosarcoma is a malignant tumor caused by
osteoblastic mesenchymal cells, mostly occurring in children and
young adults (1). The primary
site of growth of osteosarcoma is the epiphyseal region of long
bones (2). Although novel
technologies have enabled early diagnosis of osteosarcoma, and
improvements in surgery and chemotherapy have increased the 5-year
survival rate for osteosarcoma from <20% to 65–75% (3), the local recurrence rate is ~10%,
and the survival rate of patients with metastases or relapses is
only 10% (4). Currently, tumor
metastasis is the primary issue in osteosarcoma treatment (5). Since 80% of patients with
osteosarcoma already exhibit signs of metastasis or
micro-metastasis at the time of diagnosis, almost all patients
undergo multiagent chemotherapy in addition to surgical treatment
(6). Accordingly, understanding
the mechanisms of tumor development and finding reliable biomarkers
and therapeutic targets is crucial for the treatment of
osteosarcoma.
Absent in melanoma 2 (AIM2) is a cytosolic innate
immune receptor with the ability to recognize double-stranded DNA
(dsDNA) (7). AIM2 not only
promotes the development of sterile inflammatory diseases, such as
chronic kidney disease (8) and
cardiovascular diseases (9), but
also participates in regulating the occurrence and development of
certain cancers, serving a dual role of cancer inhibition and
promotion. For instance, data from several studies suggest that
AIM2 expression is reduced in malignant tumors, such as colon
cancer (10), liver cancer
(11), prostate cancer (12) and breast cancer (13), thereby promoting tumorigenesis and
affecting patient prognosis. Conversely, it has previously been
observed that AIM2 expression is upregulated in oral squamous
carcinoma (14), squamous
carcinoma (15) and lung cancer
(16). These studies also
revealed that knockdown of AIM2 inhibits tumor cell proliferation,
promotes apoptosis and reduces cell migration, indicating that AIM2
functions as an oncogene in oral squamous carcinoma (14), squamous carcinoma (15) and lung cancer (16). However, little attention has been
paid to the role served by AIM2 in osteosarcoma and its molecular
mechanisms.
The PI3K/AKT/mTOR signaling pathway has been
demonstrated to be one of the most common abnormalities in the
metastatic behavior of osteosarcoma, as activation of this
signaling pathway speeds up cell metastasis and is associated with
adverse prognosis in osteosarcoma (17). A previous study revealed that AIM2
can regulate the development of colorectal cancer via the PI3K/AKT
signaling pathway (18), which
has later been demonstrated to play a key role in the progression
of osteosarcoma (19).
Furthermore, evidence has suggested that AIM2 expression deficiency
promotes hepatocellular carcinoma progression by activating the
mTOR-ribosomal protein S6 kinase B1 signaling pathway (11). Therefore, it was hypothesized that
AIM2 may regulate the development of osteosarcoma via the
PI3K/AKT/mTOR signaling pathway.
In the present study, the expression levels of AIM2
in several osteosarcoma cell lines were examined. Subsequently, the
effects of AIM2 overexpression on the proliferation, apoptosis,
invasion, migration and epithelial-mesenchymal transition (EMT) of
osteosarcoma cells, as well as potential mechanisms related to the
PI3K/AKT/mTOR signaling pathway, were explored.
Materials and methods
Cell culture and transfection
hFOB1.19 human osteoblast cells were obtained from
American Type Culture Collection and cultured in DMEM/F12
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Sangon Biotech Co., Ltd.) and 1% penicillin-streptomycin.
C396, CAL-72 and MG-63 osteosarcoma cell lines were purchased from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences, and cultured in DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin. All cells were cultured at 37°C in a
suitable incubator containing 95% air and 5% CO2. MG-63
cells were treated with PI3K/AKT/mTOR signaling pathway inhibitor
LY294002 (20 µM; cat. no. A8250; APExBIO Technology LLC) or
activator 740Y-P (20 µM; cat. no. BCP16353; Shanghai BioChemPartner
Co., Ltd.) at 24 h post-transfection for 48 h at 37°C.
For transfection, AIM2 overexpression plasmid
(Oe-AIM2) and the empty vector plasmid (Oe-NC) were purchased from
Sangon Biotech Co., Ltd., by utilizing pcDNA3.1 vector containing
the full-length AIM2 cDNA sequence. Subsequently, these plasmids
were transfected into MG-63 cells using Lipofectamine®
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. After 48 h of
transfection, cells were collected and the transfection efficiency
was assessed via reverse transcription-quantitative PCR (RT-qPCR)
and western blotting, and subsequent experiments were
performed.
RT-qPCR assay
Extraction of total RNA from cells was performed
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The cDNA template in
the reverse transcription reaction was generated using a QuantiTect
Reverse Transcription Kit provided by Qiagen China Co., Ltd.,
according to the manufacturer's protocols. The gene expression
levels were analyzed via qPCR, which was conducted using iTaq™
Universal One-Step iTaq™ Universal SYBR®-Green Supermix
(Bio-Rad Laboratories, Inc.) on an ABI 7500 instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following primer
sequences were used for qPCR: AIM2 forward,
5′-TGGCAAAACGTCTTCAGGAGG-3′ and reverse,
5′-AGCTTGACTTAGTGGCTTTGG-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The quantification of relative gene
expression was performed using the 2−ΔΔCq method
(20). GAPDH was used as an
internal control for normalization.
Western blotting
Cell lysis was performed on ice using RIPA buffer
(Beyotime Institute of Biotechnology) to obtain proteins. The
protein concentration was measured using a BCA protein assay kit
(GlpBio Technology, Inc.). The separation of proteins (30 µg per
lane) was accomplished using 10% SDS-PAGE. Subsequently, the
separated proteins were transferred onto polyvinylidene fluoride
membranes. After 1 h of blocking with 5% skimmed milk at 37°C, the
membranes and primary antibodies were incubated together at 4°C
overnight. After three washes, the membranes were incubated with a
horseradish peroxidase-linked secondary antibody (1:3,000; cat. no.
7074P2; Cell Signaling Technology, Inc.) at room temperature.
Pierce™ enhanced chemiluminescence western blotting substrate
(Pierce; Thermo Fisher Scientific, Inc.) was used for
visualization, according to the manufacturer's instructions, and
ImageJ software (v6; National Institutes of Health) was used to
analyze the protein bands. Parallel blotting of GAPDH was used as
the internal control. Anti-AIM2 (1:1,000; cat. no. 12948S),
anti-Bax (1:1,000; cat. no. 5023T), anti-Bcl-2 (1:1,000; cat. no.
3498T), anti-cleaved caspase-3 (1:1,000; cat. no. 9664T), caspase-3
(1:1,000; cat. no. 14220T), anti-N-cadherin (1:1,000; cat. no.
13116T), anti-Vimentin (1:1,000; cat. no. 5741T), anti-E-cadherin
(1:1,000; cat. no. 3195T), anti-phosphorylated (p)-AKT (1:1,000;
cat. no. 4060T), anti-AKT (1:1,000; cat. no. 4691T), anti-p-mTOR
(1:1,000; cat. no. 5536T), anti-mTOR (1:1,000; cat. no. 2983T) and
anti-GAPDH (1:1,000; cat. no. 5174T) antibodies were provided by
Cell Signaling Technology, Inc. Anti-p-PI3K (1:1,000; cat. no.
ab278545) and anti-PI3K (1:1,000; cat. no. ab32089) antibodies were
obtained from Abcam.
Cell Counting Kit-8 (CCK-8) assay
For the cell viability assay, MG-63 cells were
plated into 96-well plates and cultured at 37°C for 24 h.
Subsequently, 10 µl CCK-8 solution (MedChemExpress) was loaded into
each well and the cells were incubated for 3 h at 37°C. Optical
density was measured using a microplate reader at a wavelength of
450 nm. Each group of experiments was repeated three times.
Colony formation assay
For the detection of colony formation, MG-63 cells
in the logarithmic growth phase were cultured and made into cell
suspensions under specified conditions. The cell suspensions were
diluted and inoculated into 60-mm diameter culture dishes at a
density of 500 cells per dish, which were gently shaken in a cross
direction to ensure the cells were dispersed evenly. The culture
dishes were placed at 37°C in a 5% CO2 environment for 2
weeks. Then, the culture was terminated and the culture medium was
discarded. After washing with PBS twice, cells were fixed with
methanol at room temperature for 15 min and stained with crystal
violet (Beijing Solarbio Science & Technology Co., Ltd.)
staining solution at room temperature for 10 min. The staining
solution was subsequently washed away. Finally, each colony of
>30 cells was counted using a dissection microscope.
Detection of apoptosis
The apoptosis of MG-63 cells was assessed using a
TUNEL assay (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. After 24 h of cell culture,
transfected cells were fixed with 1% paraformaldehyde at room
temperature for 15 min and 50 µl TUNEL was used to incubate cells
for 1 h at 37°C, followed by staining of nuclear DNA with 10 µg/ml
DAPI at 37°C for 2–3 min and mounted in an anti-fade reagent
(Beijing Solarbio Science & Technology Co., Ltd.). The cells
were analyzed from three fields of view using a fluorescence
microscope (magnification, ×200; Olympus Corporation).
Transwell assay
The upper surface of the Transwell chamber was
coated with Matrigel and incubated at 37°C for 30 min.
Subsequently, 3×104 MG-63 cells in 200 µl serum-free
medium were seeded onto the upper surface of the chamber. A total
of 600 µl medium containing 10% FBS was added to the lower chamber.
After incubation at room temperature for 24 h, cells were fixed
with 4% paraformaldehyde for 20 min at 4°C and stained with 0.1%
crystal violet solution (Beijing Solarbio Science & Technology
Co., Ltd.) for 5 min at room temperature. Finally, these cells were
observed under an inverted microscope. Invasive cells were counted
in three random areas under a light microscope (magnification,
×100; Olympus Corporation).
Wound healing assay
For the evaluation of cell migration, MG-63 cells
(4×105 cells/well) were seeded into six-well plates and
cultured for 48 h. A 20-µl pipette tip was utilized to create a
straight scratch and the medium was replaced with serum-free DMEM.
After two washes with PBS, the wound was observed at 0 and 24 h.
The microscopy images of the cells were captured using a light
microscope (magnification, ×100; Olympus Corporation). The cell
migration rate was calculated using the following equation:
(Initial width at 0 h-final width at 24 h)/Initial width at 0
h.
Statistical analysis
Experimental data are presented as the mean ± SD
from three independent experiments and were analyzed using GraphPad
Prism 8.0 software (GraphPad Software, Inc.). The normality of the
data was analyzed using the Shapiro-Wilk test. One-way ANOVA
followed by Tukey's post hoc test and unpaired t-tests were used
for comparisons among multiple groups and between two groups,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Bioinformatics tools
Cancer Cell Line Encyclopedia (CCLE) database
(https://depmap.org/portal/download/?release=CCLE+2019&release=Fusion&release=DNA+Copy+Number;
dataset: ‘CCLE_Expression.Arrays_2013-03-18.tar.gz’) was applied to
investigate AIM2 expression in osteosarcoma.
Results
AIM2 is expressed at low levels in
osteosarcoma cell lines
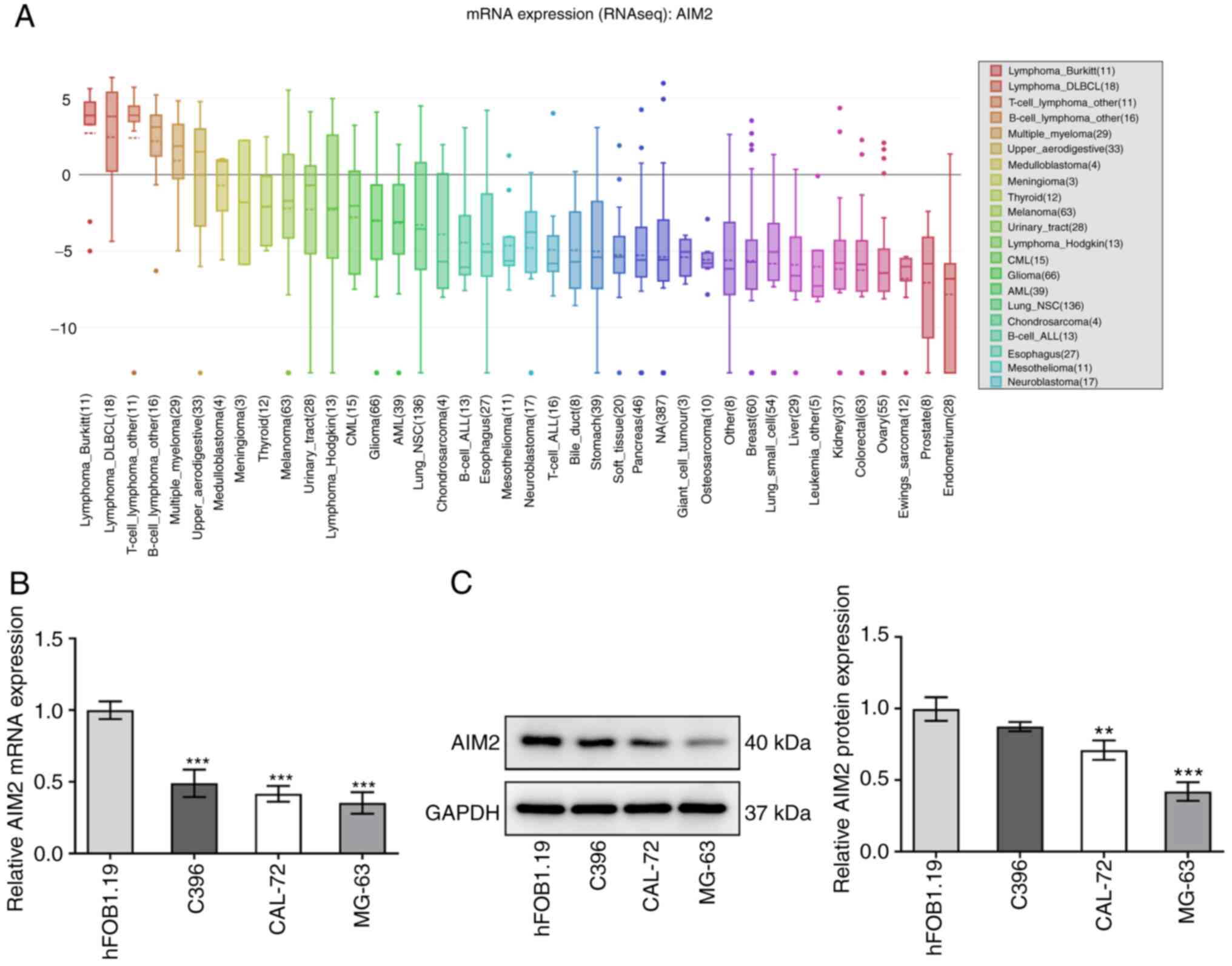
The CCLE database, RT-qPCR and western blotting were
used to gain a deeper insight into AIM2 expression in osteosarcoma
cell lines. As shown in Fig. 1A,
AIM2 was expressed at low levels in osteosarcoma cell lines,
according to the CCLE database. The results in Fig. 1B and C indicated that both mRNA
and protein expression levels of AIM2 were significantly decreased
in C396, CAL-72 and MG-63 cells compared with hFOB1.19 cells. Among
these, MG-63 cells were selected for the subsequent experiments due
to their low expression levels of AIM2. Overall, the results
suggested that AIM2 expression was downregulated in osteosarcoma
cells.
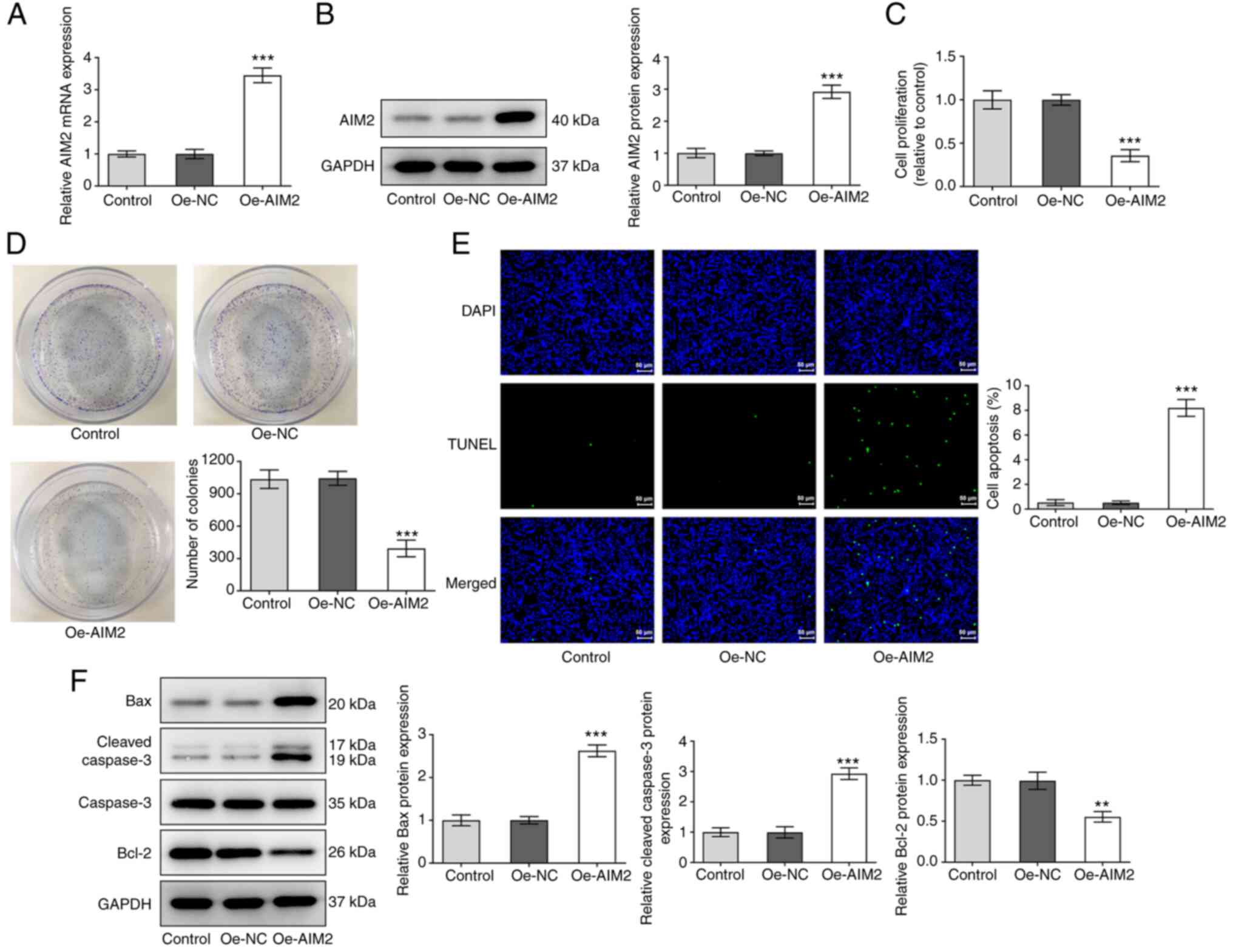
Overexpression of AIM2 inhibits cell
proliferation and promotes apoptosis in osteosarcoma
To gain a detailed understanding of the effect of
AIM2 on cell proliferation and apoptosis in osteosarcoma, MG-63
cells were transfected with an AIM2 overexpression plasmid,
followed by a series of experiments. First, data from RT-qPCR and
western blotting indicated a significant increase in AIM2
expression in the overexpression group (Fig. 2A and B) compared with the empty
vector group. AIM2 overexpression significantly inhibited the
proliferation of osteosarcoma cells according to the CCK-8 and
colony formation assays (Fig. 2C and
D). As shown in Fig. 2E, AIM2
upregulation led to an increase in the number of apoptotic cells
compared with the empty vector group. As indicated in Fig. 2F, the results revealed elevated
expression levels of apoptosis-related proteins Bax and cleaved
caspase-3, as well as decreased expression levels of Bcl-2,
following AIM2 overexpression. In summary, AIM2 overexpression
effectively suppressed proliferation and promoted apoptosis in
osteosarcoma cells.
Overexpression of AIM2 suppresses the
invasion, migration and EMT of osteosarcoma cells
To explore the effect of AIM2 overexpression on the
invasion, migration and EMT of osteosarcoma cells, Transwell, wound
healing and western blotting assays were performed. After AIM2 was
overexpressed, a significant decrease in cell invasion compared
with the Oe-NC group was observed (Fig. 3A). The results in Fig. 3B also revealed a significant
decrease in the migration of MG-63 cells. Additionally, the results
in Fig. 3C revealed a significant
decrease in the expression levels of the EMT-related proteins
N-cadherin and vimentin, but an increase in the expression levels
of E-cadherin, indicating that the EMT process was inhibited
following AIM2 overexpression. These data suggested that AIM2
overexpression exerted an inhibitory effect on the invasion,
migration and EMT of osteosarcoma cells.
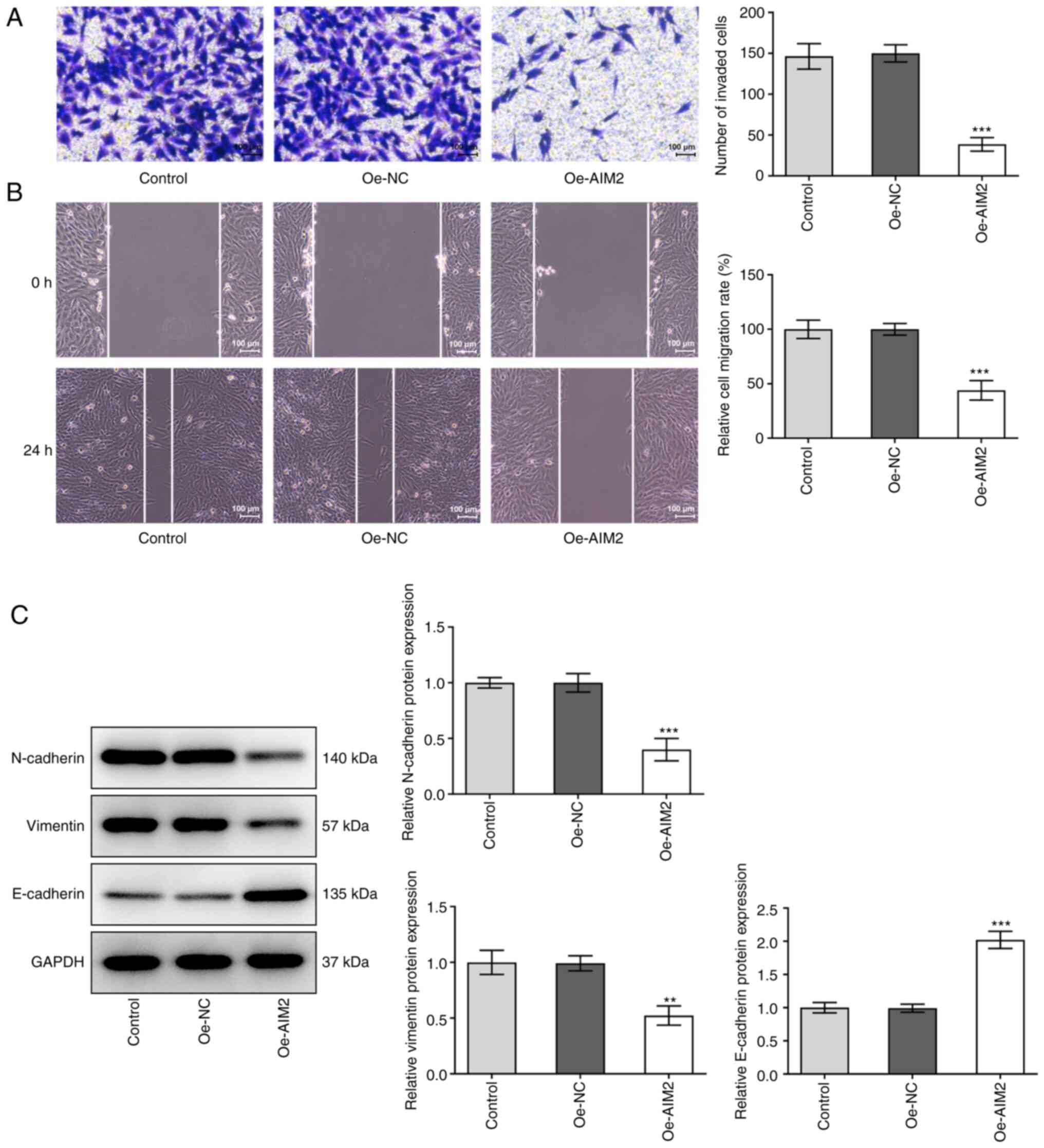 | Figure 3.Overexpression of AIM2 inhibits
osteosarcoma cell invasion, migration and EMT. (A) Cell invasion
was detected using a Transwell assay. Scale bar, 100 µm. (B) Wound
healing assay was utilized to detect cell migration. Scale bar, 100
µm. (C) Western blotting was used to assess the levels of
EMT-related proteins, including N-cadherin, Vimentin and
E-cadherin. Experimental data are presented as the mean ± SD from
three independent experiments. **P<0.01, ***P<0.001 vs.
Oe-NC. EMT, epithelial-mesenchymal transition; AIM2, absent in
melanoma 2; Oe-NC, empty vector plasmid; Oe-AIM2, AIM2
overexpression vector. |
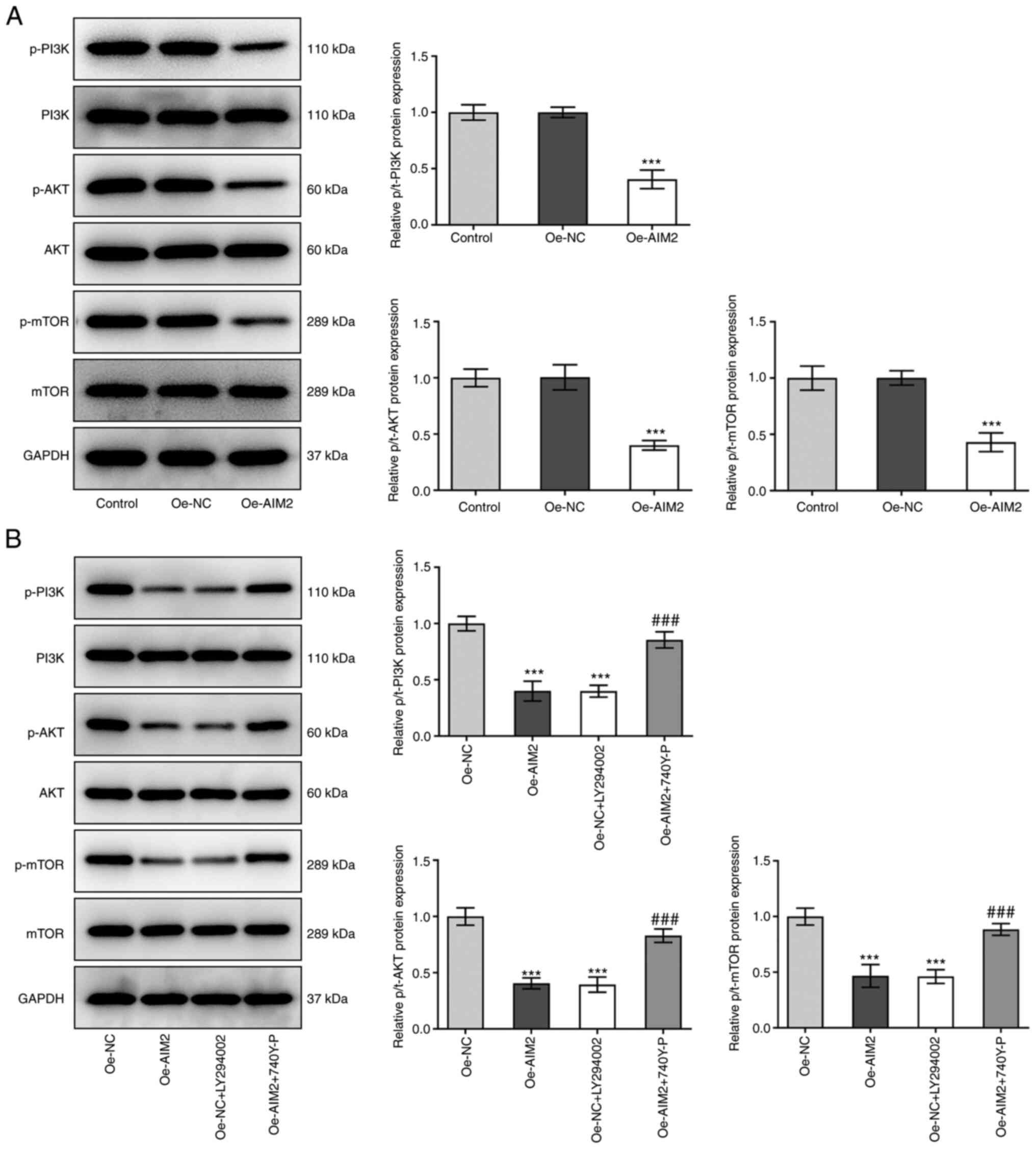
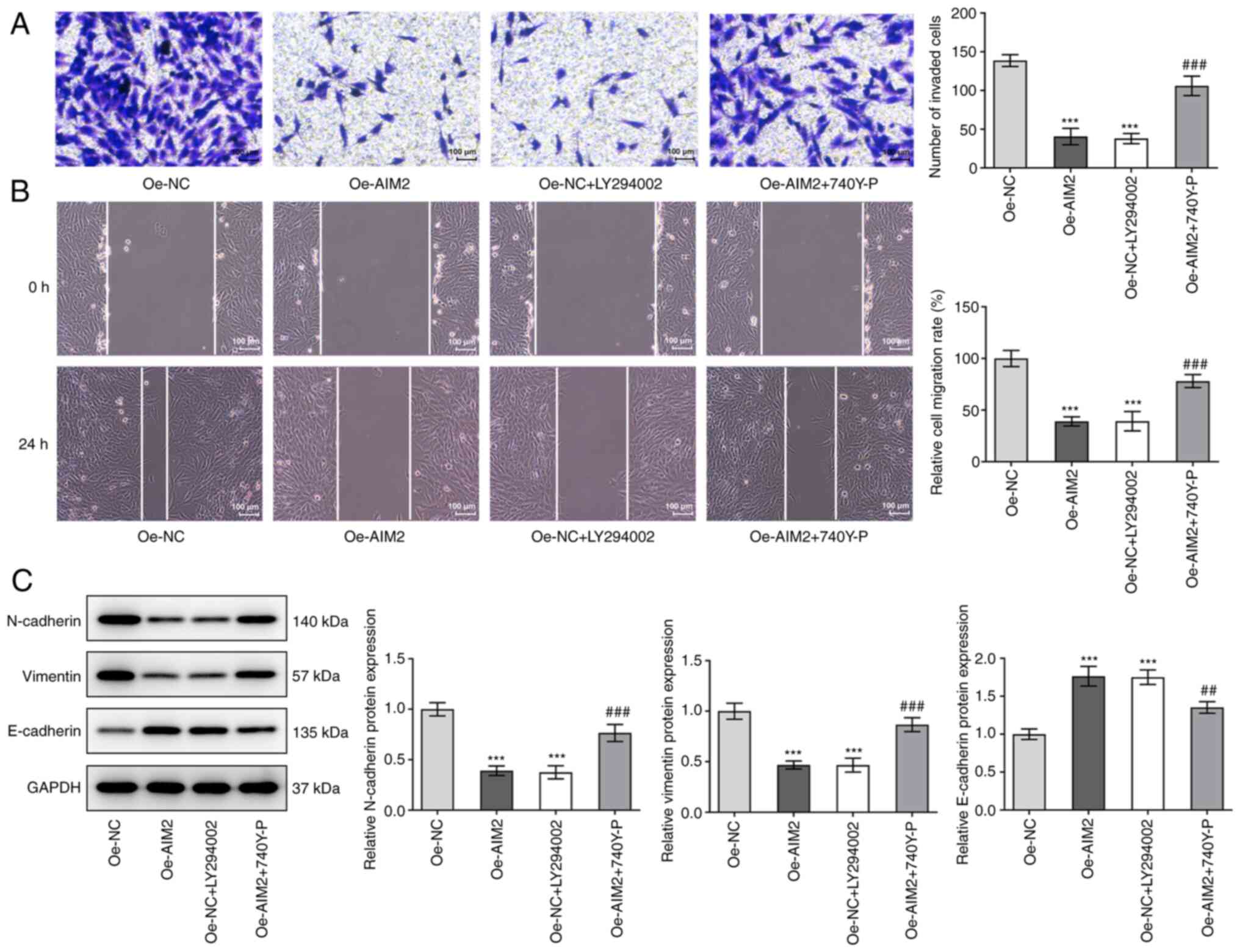
AIM2 overexpression suppresses the
PI3K/AKT/mTOR signaling pathway in osteosarcoma cells
The present study subsequently examined if AIM2
overexpression affected the PI3K/AKT/mTOR signaling pathway
following treatment with the inhibitor LY294002 or the activator
740Y-P. Decreased protein levels of p-P13K, p-AKT and p-mTOR
relative to PI3K, AKT and mTOR were observed in the Oe-AIM2 group
compared with the Oe-NC group (Fig.
4A). In MG-63 cells transfected with Oe-NC, after the addition
of inhibitor LY294002, the levels of p-P13K, p-AKT and p-mTOR were
also significantly downregulated compared with those in the Oe-NC
group (Fig. 4B). By contrast, a
significant increase in the levels of p-P13K, p-AKT and p-mTOR was
observed following addition of 740Y-P in MG-63 cells overexpressing
AIM2. In summary, these results indicated that AIM2 overexpression
could suppress the PI3K/AKT/mTOR signaling pathway in osteosarcoma
cells.
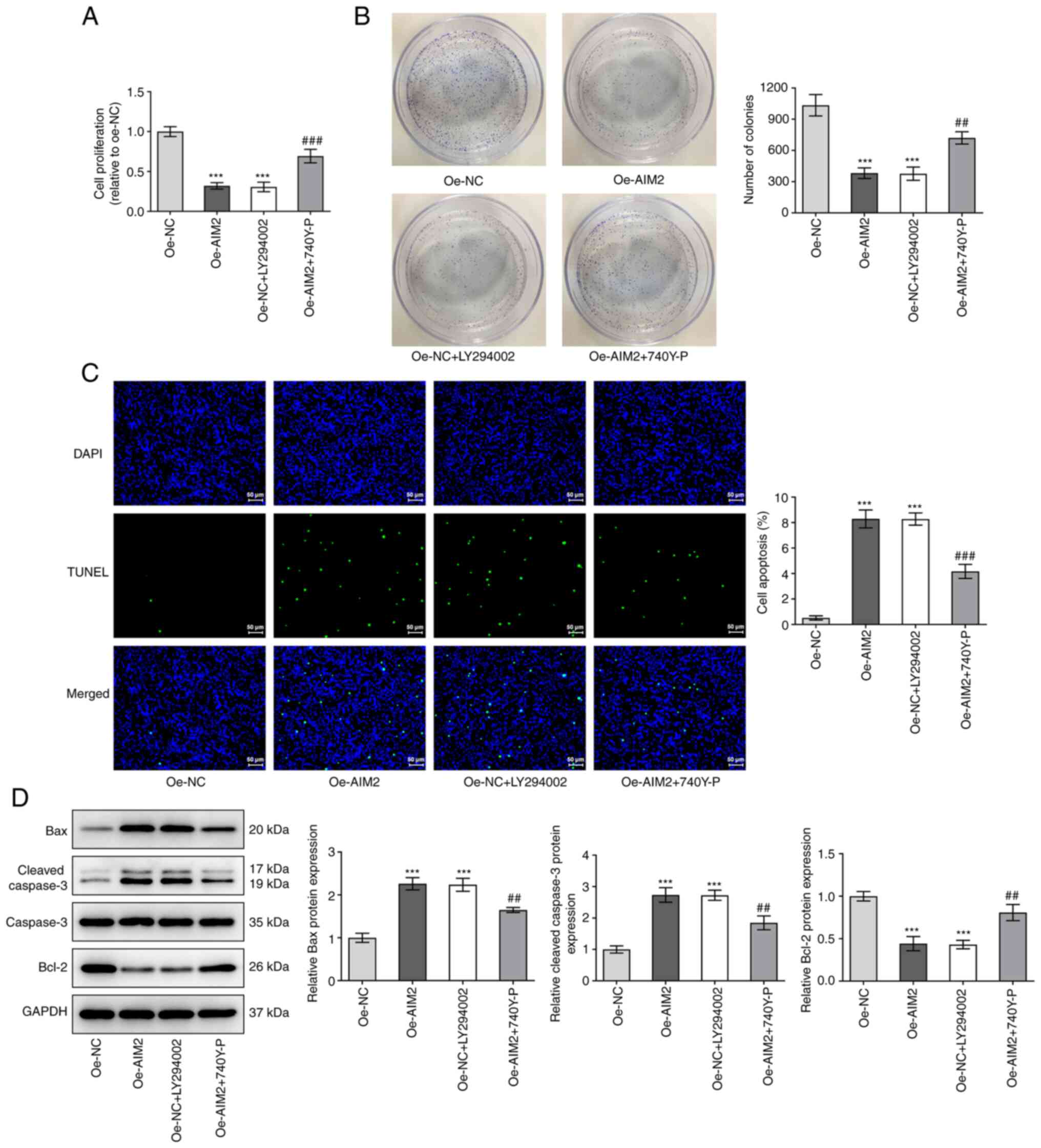
AIM2 overexpression inhibits the
proliferation and promotes the apoptosis of osteosarcoma cells by
inactivating the PI3K/AKT/mTOR signaling pathway
To understand whether the effects of AIM2
overexpression on the progression of osteosarcoma were mediated by
the PI3K/AKT/mTOR signaling pathway, another series of experiment
was conducted. The results in Fig. 5A
and B demonstrated that the inhibitor LY294002 exerted a
significant inhibitory effect on cell proliferation in both the
CCK-8 and colony formation assays, as did AIM2 overexpression,
while the activator 740Y-P reversed the inhibitory effect of AIM2
overexpression. As shown in Fig.
5C, similar to the effect of AIM2 overexpression, the inhibitor
LY294002 promoted apoptosis, but 740Y-P attenuated the effect of
AIM2 overexpression on cell apoptosis. As shown in Fig. 5D, following addition of LY294002,
the protein expression levels of Bax and cleaved caspase-3 were
elevated, but the expression levels of Bcl-2 were decreased
compared with Oe-NC group, and treatment with the activator 740Y-P
had the opposing effect on the levels of these proteins in Oe-AIM2
cells. These results suggested that AIM2 inhibited the
proliferation and promoted the apoptosis of osteosarcoma cells by
inactivating the PI3K/AKT/mTOR signaling pathway.
AIM2 overexpression inhibits the
invasion, migration and EMT of osteosarcoma cells by inactivating
the PI3K/AKT/mTOR signaling pathway
In terms of cell invasion, the results shown in
Fig. 6A revealed that LY294002
effectively suppressed cell invasion compared with the Oe-NC group;
invasion was significantly elevated following addition of 740Y-P in
MG-63 cells transfected with Oe-AIM2. As shown in Fig. 6B, LY294002 had the same inhibitory
effect as AIM2 overexpression on cell migration, while 740Y-P had
the opposite effect. Based on the results in Fig. 6C, the expression levels of
EMT-related proteins N-cadherin and vimentin were decreased but
E-cadherin expression was increased following addition of LY294002
compared with Oe-NC alone. Following overexpression of AIM2 and
addition of the activator 740Y-P, the expression levels of
N-cadherin and vimentin were found to be increased, but the
expression levels of E-cadherin were decreased. This series of
experiments indicated that the inhibitor LY294002 suppressed cell
invasion, migration and EMT, but the activator 740Y-P reversed the
effect of AIM2 overexpression on the invasion, migration and EMT of
osteosarcoma cells.
Discussion
Osteosarcoma is a primary bone malignant tumor with
a high recurrence rate. Although a growing body of research has
demonstrated an association between AIM2 and the progression of
cancer (18,21,22), research into the role of AIM2 in
osteosarcoma has been limited. The present study assessed the role
of AIM2 in osteosarcoma cells and its molecular mechanism, and
demonstrated that AIM2 was expressed at low levels in osteosarcoma
cell lines. Additionally, overexpression of AIM2 inhibited the
proliferation, invasion, migration and EMT, and promoted the
apoptosis of osteosarcoma cells by suppressing the PI3K/AKT/mTOR
signaling pathway.
AIM2 is the prototypical and most well-characterized
member of the AIM2 class of receptors (23). Recognition of dsDNA by AIM2
results in the accumulation of a large multiprotein oligomeric
complex referred to as the inflammasome, which gives rise to the
secretion of bioactive IL-1β and IL-18 (21). In the inflammatory
microenvironment of tumors, caspase-1 and the cytokines it
processes, such as IL-1β, serve an essential role in the occurrence
and development of cancer (24).
Also, caspase-1 functions as a tumor suppressor in breast cancer
(25) and non-small cell lung
cancer (26). Additionally, the
CCLE database revealed that AIM2 expression was downregulated in
osteosarcoma cell lines, which was also validated by RT-qPCR and
western blot assays in the present study. It may be inferred from
these results that AIM2 may contribute to the pathogenesis of
osteosarcoma.
The proliferation, migration and invasion of tumor
cells mark the metastasis of tumors, which in turn causes cancer
recurrence in patients (27,28). AIM2 has been demonstrated to be
closely associated with the progression of cancer in recent studies
(16,18). It has been reported that
overexpression of AIM2 induces cell cycle arrest in colon cancer
cytology and acts as an inhibitor of cell proliferation (29). Furthermore, in organoid cultures,
intestinal stem cells lacking AIM2 proliferate more than wild-type
intestinal stem cells (30). In
addition, AIM2 regulates the viability and apoptosis of human
colorectal cancer cells via the PI3K/AKT signaling pathway
(31). It has been demonstrated
that, in AIM2 knockout mice, the production of pro-inflammatory
cytokines mediated by the inflammasome is reduced, and apoptosis
and pyroptosis are attenuated (32). Furthermore, AIM2 inhibits the
invasion and metastasis of kidney cancer cells by enhancing
autophagy induction (33). AIM2
overexpression inhibits colorectal cancer cell proliferation,
migration and EMT progress (18).
Furthermore, the loss of AIM2 induces the activation of EMT in
hepatocellular carcinoma (34).
Based on the results of the present study, overexpression of AIM2
inhibited osteosarcoma cell proliferation, invasion, migration and
EMT, and promoted apoptosis, which indicated that AIM2 can suppress
the progression of osteosarcoma.
The PI3K/AKT/mTOR signaling pathway serves a
critical role in the regulation of cell proliferation, survival,
protein synthesis and tumor progression (35,36). PI3K phosphorylates and activates
the AKT protein positioned on the plasma membrane, which then
activates its various downstream pathways and consequently mTOR
(37). A considerable body of
evidence on various cancer types has demonstrated that activation
of PI3K/AKT/mTOR signaling induces a variety of malignant
phenotypes, such as cell proliferation, migration and invasion
(38,39). Accumulating evidence has
demonstrated that the PI3K/AKT/mTOR signaling pathway participates
in the occurrence and development of osteosarcoma, and inactivation
of this signaling pathway can block the progression of osteosarcoma
(40,41). Previous studies have demonstrated
that AIM2 regulates the activity and apoptosis of colorectal cancer
cells via PI3K/AKT signaling pathways (29), and attenuates AKT phosphorylation
and mTOR signaling (42). The
present study revealed that AIM2 overexpression suppressed
PI3K/AKT/mTOR signaling. Subsequently, to further elucidate the
effect of AIM2 overexpression on the PI3K/AKT/mTOR signaling
pathway, PI3K/AKT/mTOR pathway inhibitor LY294002 or activator
740Y-P were added. The results revealed that 740Y-P reversed the
effects of AIM2 overexpression on osteosarcoma cell proliferation,
apoptosis, invasion, migration and EMT.
In conclusion, considering the present findings, it
may be concluded that AIM2 can inhibit the development of
osteosarcoma through inactivation of the PI3K/AKT/mTOR signaling
pathway (Fig. 7). These findings
suggested that AIM2 may be a promising therapeutic option for
osteosarcoma and an effective approach to the targeted treatment of
this disease. The lack of investigation into the upstream
mechanisms of AIM2 or inclusion of animal experiments are
limitations of the present study; therefore, comprehensive analysis
is required in the future.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science Foundation of Ningxia
Province (grant nos. NZ16128 and 2019AAC03193).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and CL designed the experimental study and
analyzed the data. JS performed the experiments. KW and XW drafted
the manuscript and interpreted the data. KW and XW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raymond AK and Jaffe N: Osteosarcoma
multidisciplinary approach to the management from the pathologist's
perspective. Cancer Treat Res. 152:63–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cortini M, Avnet S and Baldini N:
Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett.
405:90–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin H, Zheng X, Lu T, Gu Y, Zheng C and
Yan H: The proliferation and invasion of osteosarcoma are inhibited
by miR-101 via targetting ZEB2. Biosci Rep. Feb 8–2019.(Epub ahead
of print). doi: 10.1042/BSR20181283. View Article : Google Scholar
|
|
5
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lugrin J and Martinon F: The AIM2
inflammasome: Sensor of pathogens and cellular perturbations.
Immunol Rev. 281:99–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komada T, Chung H, Lau A, Platnich JM,
Beck PL, Benediktsson H, Duff HJ, Jenne CN and Muruve DA:
Macrophage uptake of necrotic cell DNA activates the AIM2
inflammasome to regulate a proinflammatory phenotype in CKD. J Am
Soc Nephrol. 29:1165–1181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao ZZ, Zheng XL and Jiang ZS: Emerging
roles of absent in melanoma 2 in cardiovascular diseases. Clin Chim
Acta. 511:14–23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson JE, Petrucelli AS, Chen L,
Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y,
Schneider M, et al: Inflammasome-independent role of AIM2 in
suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med.
21:906–913. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma X, Guo P, Qiu Y, Mu K, Zhu L, Zhao W,
Li T and Han L: Loss of AIM2 expression promotes hepatocarcinoma
progression through activation of mTOR-S6K1 pathway. Oncotarget.
7:36185–36197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ponomareva L, Liu H, Duan X, Dickerson E,
Shen H, Panchanathan R and Choubey D: AIM2, an IFN-inducible
cytosolic DNA sensor, in the development of benign prostate
hyperplasia and prostate cancer. Mol Cancer Res. 11:1193–1202.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen IF, Ou-Yang F, Hung JY, Liu JC, Wang
H, Wang SC, Hou MF, Hortobagyi GN and Hung MC: AIM2 suppresses
human breast cancer cell proliferation in vitro and mammary tumor
growth in a mouse model. Mol Cancer Ther. 5:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kondo Y, Nagai K, Nakahata S, Saito Y,
Ichikawa T, Suekane A, Taki T, Iwakawa R, Enari M, Taniwaki M, et
al: Overexpression of the DNA sensor proteins, absent in melanoma 2
and interferon-inducible 16, contributes to tumorigenesis of oral
squamous cell carcinoma with p53 inactivation. Cancer Sci.
103:782–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farshchian M, Nissinen L, Siljamaki E,
Riihilä P, Piipponen M, Kivisaari A, Kallajoki M, Grénman R,
Peltonen J, Peltonen S, et al: Tumor cell-specific AIM2 regulates
growth and invasion of cutaneous squamous cell carcinoma.
Oncotarget. 8:45825–45836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang M, Jin C, Yang Y, Wang K, Zhou Y,
Zhou Y, Wang R, Li T and Hu R: AIM2 promotes non-small-cell lung
cancer cell growth through inflammasome-dependent pathway. J Cell
Physiol. 234:20161–20173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu K, Dai HB and Qiu ZL: mTOR signaling in
osteosarcoma: Oncogenesis and therapeutic aspects (Review). Oncol
Rep. 36:1219–1225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu M, Wang J, Li H, Zhang Z and Cheng Z:
AIM2 inhibits colorectal cancer cell proliferation and migration
through suppression of Gli1. Aging (Albany NY). 13:1017–1031. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Weng Q, Han J and Chen J:
Alantolactone suppresses human osteosarcoma through the PI3K/AKT
signaling pathway. Mol Med Rep. 21:675–684. 2020.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma BR, Karki R and Kanneganti TD: Role
of AIM2 inflammasome in inflammatory diseases, cancer and
infection. Eur J Immunol. 49:1998–2011. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Wang W, Li A, Huang W, Chen S, Han F
and Wang L: Dihydroartemisinin induces pyroptosis by promoting the
AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem Biol
Interact. 340:1094342021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Tian Y and Yin Q: AIM2
inflammasome assembly and signaling. Adv Exp Med Biol.
1172:143–155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin H, Jin X, Cao B and Wang W: Berberine
affects osteosarcoma via downregulating the caspase-1/IL-1β
signaling axis. Oncol Rep. 37:729–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y and Guo Y: Expression of Caspase-1
in breast cancer tissues and its effects on cell proliferation,
apoptosis and invasion. Oncol Lett. 15:6431–6435. 2018.PubMed/NCBI
|
|
26
|
Huang TH, Zhang P, Li W, Zhao T, Zhang Z,
Chen S, Yang Y, Feng Y, Li F, Shirley Liu X, et al: G9A promotes
tumor cell growth and invasion by silencing CASP1 in non-small-cell
lung cancer cells. Cell Death Dis. 8:e27262017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avanzini S and Antal T: Cancer recurrence
times from a branching process model. PLoS Comput Biol.
15:e10074232019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patsos G, Germann A, Gebert J and Dihlmann
S: Restoration of absent in melanoma 2 (AIM2) induces G2/M cell
cycle arrest and promotes invasion of colorectal cancer cells. Int
J Cancer. 126:1838–1849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Man SM, Zhu Q, Zhu L, Liu Z, Karki R,
Malik A, Sharma D, Li L, Malireddi RK, Gurung P, et al: Critical
Role for the DNA Sensor AIM2 in stem cell proliferation and cancer.
Cell. 162:45–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Wang Z and Yu S: AIM2 regulates
viability and apoptosis in human colorectal cancer cells via the
PI3K/Akt pathway. Onco Targets Ther. 10:811–817. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poh L, Fann DY, Wong P, Lim HM, Foo SL,
Kang SW, Rajeev V, Selvaraji S, Iyer VR, Parathy N, et al: AIM2
inflammasome mediates hallmark neuropathological alterations and
cognitive impairment in a mouse model of vascular dementia. Mol
Psychiatry. 26:4544–4560. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chai D, Shan H, Wang G, Li H, Fang L, Song
J, Zhang Q, Bai J and Zheng J: AIM2 is a potential therapeutic
target in human renal carcinoma and suppresses its invasion and
metastasis via enhancing autophagy induction. Exp Cell Res.
370:561–570. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH,
Wang H, Cai SH, Yang X, Xie D, Zhang CZ and Yun JP: HBx-mediated
decrease of AIM2 contributes to hepatocellular carcinoma
metastasis. Mol Oncol. 11:1225–1240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia P and Xu XY: PI3K/Akt/mTOR signaling
pathway in cancer stem cells: From basic research to clinical
application. Am J Cancer Res. 5:1602–1609. 2015.PubMed/NCBI
|
|
36
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ersahin T, Tuncbag N and Cetin-Atalay R:
The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 11:1946–1954.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang H, Zhu D, Zhang G, Luo X and Xie W:
AFAP1-AS1 promotes proliferation of pituitary adenoma cells through
miR-103a-3p to Activate PI3K/AKT signaling pathway. World
Neurosurg. 130:e888–e898. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodrigues Alves AP, Fernandes JC, Fenerich
BA, Coelho-Silva JL, Scheucher PS, Simões BP, Rego EM, Ridley AJ,
Machado-Neto JA and Traina F: IGF1R/IRS1 targeting has cytotoxic
activity and inhibits PI3K/AKT/mTOR and MAPK signaling in acute
lymphoblastic leukemia cells. Cancer Lett. 456:59–68. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang B and Li J: Piceatannol suppresses
the proliferation and induced apoptosis of osteosarcoma cells
through PI3K/AKT/mTOR pathway. Cancer Manag Res. 12:2631–2640.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jin R, Jin YY, Tang YL, Yang HJ, Zhou XQ
and Lei Z: GPNMB silencing suppresses the proliferation and
metastasis of osteosarcoma cells by blocking the PI3K/Akt/mTOR
signaling pathway. Oncol Rep. 39:3034–3040. 2018.PubMed/NCBI
|
|
42
|
Chou WC, Guo Z, Guo H, Chen L, Zhang G,
Liang K, Xie L, Tan X, Gibson SA, Rampanelli E, et al: AIM2 in
regulatory T cells restrains autoimmune diseases. Nature.
591:300–305. 2021. View Article : Google Scholar : PubMed/NCBI
|