Introduction
Ischemic brain injury is a serious neurological
condition and the third most common cause of death worldwide
(1–3). Immediate restoration of blood flow
is recommended as the standard treatment for brain ischemia, which
not only delivers oxygen and transports nutrients to sustain
aerobic metabolism and ATP generation, but also eliminates
accumulated H+ ions to normalize extracellular pH
(4–7). However, ischemia/reperfusion (I/R)
may exacerbate the risk of brain tissue injury (8). Due, in part, to overconsumption of
oxygen, intracellular Ca2+ overload or redistribution
during the first minutes of reperfusion, cerebral I/R contributes
to the destruction of the mitochondrial respiratory chain response,
excessive production of pro-inflammatory mediators in the damaged
areas of the brain tissue, as well as massive deposition of
Ca2+ ions in the pericytes, which further increases the
damage to neurons (5). Despite
significant improvements in timely reperfusion strategies (such as
advanced surgical equipment, as well as safe and effective
antiplatelet and antithrombotic agents), effective therapy for the
prevention of cerebral I/R injury is still lacking (9,10).
Therefore, it is essential to examine the underlying mechanism of
cerebral I/R injury, as this may help the development of prevention
strategies for cerebral I/R injury.
Several microRNA (miRNA/miR) molecules are
considered important regulators of neuronal survival during
cerebral I/R injury (11–15). miR-126 is a family of extensively
studied miRNA molecules known to inhibit inflammatory responses in
various inflammatory-related diseases (16,17). In a Parkinson's disease model,
miR-126 markedly increased neuronal cell proliferation and reduced
apoptosis by targeting Ras-related protein RAB3A interacting
protein (RAB3IP) (18). Moreover,
this miRNA also interacts with various genes, including nuclear
factor erythroid 2 like 2 (Nrf2), to attenuate I/R injury (19,20). Based on the aforementioned
findings, it was hypothesized in the present study that miR-126 may
play a protective role in cerebral I/R injury by targeting RAB3IP.
Therefore, the aim of the present study was to establish an
oxygen-glucose deprivation/reoxygenation (OGD/R) model to simulate
cerebral I/R injury, in order to examine the interaction between
miR-126 and RAB3IP and explore its potential protective effect
during cerebral I/R injury.
Materials and methods
Cell culture
The human SH-SY5Y neuroblastoma cell line was
purchased from the American Type Culture Collection (ATCC; cat. no.
CRL-2266). The authenticity of the cells was confirmed using the
Short Tandem Repeat assay report available at https://www.procell.com.cn/view/1401.html. The cell
line was cultured according to the ATCC protocol. Briefly, cells
were maintained in Eagle's Minimum Essential Medium (EMEM; cat. no.
M4655; MilliporeSigma) supplemented with 10% FBS (cat. no. 12103C;
MilliporeSigma) in a humidified incubator at 37°C with 5%
CO2. The 293T cells were also purchased from the ATCC
and maintained in DMEM (cat. no. D0819; MilliporeSigma)
supplemented with 10% FBS in a humidified incubator at 37°C with 5%
CO2. The 100 U/ml penicillin and 100 µg/ml streptomycin
(cat. no. 10378016; Gibco; Thermo Fisher Scientific, Inc.) were
added in EMEM and DMEM.
Establishment of the OGD/R model
In order to simulate cerebral I/R injury in
vitro, an OGD/R model was established as described previously
(21–24). Briefly, SH-SY5Y cells were seeded
in plates pre-coated with poly-L-lysine (cat. no. P8920;
MilliporeSigma) and incubated in glucose-free EMEM under hypoxic
conditions (1% O2; 5% CO2; 94% N2)
at 37°C for 4 h. The cells were then transferred to normal EMEM and
maintained in normal conditions (5% CO2; 95% air) at
37°C for 48 h (Model group). SH-SY5Y cells continuously maintained
in normal EMEM under normal incubation conditions were used as a
negative control (NC group). All subsequent experiments were
conducted in three replicates.
Transfection
miR-126 mimic (mimic-miR;
5′-CATTATTACTTTTGGTACGCG-3′) and mimic-NC (cat. no. B04001) were
purchased from Shanghai GenePharma Co., Ltd.
Lipofectamine® 3000 (cat. no. L3000075; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to transfect 0.5 pM
mimic-miR and 0.5 pM mimic-NC into 1×106 SH-SY5Y cells
according to the manufacturer's instructions. The transfected cells
(24 h after transfection) were then subjected to OGD/R treatment.
Untransfected SH-SY5Y cells that underwent OGD/R treatment alone
(Model group) were also used as a control. Each assay was only
performed once, but with three wells.
Rescue experiments
RAB3IP overexpression plasmid (OE-RAB3IP) and the
OE-NC plasmid (an empty vector) were generated using the pcDNA3.1
vector purchased from Shanghai GenePharma Co., Ltd. SH-SY5Y cells
(1×106) were co-transfected with 0.8 µg OE-NC or 0.8 µg
OE-RAB3IP vector, together with mimic-NC or mimic-miR using
Lipofectamine® 3000 at 37°C for 24 h. The transfected
cells (24 h after transfection) were subjected to OGD/R treatment.
Thus, the experiment involved the following four groups: i) OE-NC +
mimic-NC; ii) OE-RAB3IP + mimic-NC; iii) OE-NC + mimic-miR; and iv)
OE-RAB3IP + mimic-miR. Each assay was only performed once, but with
three wells.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of cells was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
then reverse transcribed into cDNA using the ReverTra
Ace® qPCR RT kit (cat. no. FSQ-101; Toyobo Life Science)
according to the kit's instruction. qPCR was carried out using
SYBR® Green Realtime PCR Master Mix (cat. no. QPK-20;
Toyobo Life Science). The thermocycling conditions included an
initial denaturation at 95°C for 5 min, followed by 40 cycles of
95°C for 5 sec and 61°C for 30 sec. The primers sequences are
presented in Table I. The
expression levels of miR-126 and RAB3IP were calculated using the
2−ΔΔCq method (25).
GAPDH (for mRNA) and U6 (for miRNA) served as internal references.
Besides, the forward and reverse primers of miR-126 were designed
according to previous studies (26,27). Each assay was only performed once,
but with three wells.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| miR-126 |
ACACTCCAGCTGGGCATTATTACTTTTGGT |
TGTCGTGGAGTCGGCAATTC |
| RAB3IP |
GTGTCATCTACCGGCCACAC |
CCCTCTGAGCTTTTGCGAGT |
| U6 |
CGCTTCGGCAGCACATATACTA |
ATGGAACGCTTCACGAATTTGC |
| GAPDH |
GACCACAGTCCATGCCATCAC |
ACGCCTGCTTCACCACCTT |
Western blot assay
Total protein of cells was extracted using RIPA
lysis buffer (cat. no. R0278; MilliporeSigma). The BCA Assay Kit
for Protein Determination (cat. no. BCA1; MilliporeSigma) was used
to measure the protein concentration. Subsequently, the protein
samples (20 µg) were separated via 4–20% SDS-PAGE and subsequently
transferred to PVDF membranes (cat. no. IPVH00010; MilliporeSigma).
The membranes were then blocked with 5% skimmed milk for 2 h at
37°C and incubated with the primary antibodies (Table II) overnight at 4°C. Following
the primary antibody incubation, the membranes were incubated with
secondary antibodies (Table II)
for 1.5 h at room temperature. The visualization of the protein
bands was performed using Pierce™ ECL Plus Western Blotting
Substrate (cat. no. 32132X3; Thermo Fisher Scientific, Inc.) with
exposure on X-ray film (cat. no. 1753185; Kodak). GAPDH was used as
the internal reference. Each assay was only performed once, but
with three wells. The protein bands were analyzed using ImageJ
1.8.0 software (National Institutes of Health).
 | Table II.Antibodies used in western
blotting. |
Table II.
Antibodies used in western
blotting.
| A, Primary
antibody |
|---|
| Antibody | Supplier | Cat. no. | Dilution |
|---|
| Rabbit polyclonal
against RAB3IP | Invitrogen; Thermo
Fisher Scientific, Inc. | PA5-96927 | 1:500 |
| Rabbit monoclonal
against GAPDH | Abcam | ab181602 | 1:5,000 |
|
| B, Secondary
antibody |
|
| Antibody | Supplier | Cat. no. | Dilution |
|
| HRP-labeled goat
anti-rabbit IgG (H+L) | Abcam | ab6721 | 1:10,000 |
Cell Counting Kit-8 (CCK-8) assay
A volume of 10 µl CCK-8 solution (cat. no. CK04;
Dojindo Laboratories, Inc.) and 90 µl RPMI-1640 medium were added
to the cells in each plate, which were then incubated at 37°C with
5% CO2 for 2 h. The absorbance was measured using a
microplate reader. Each assay was only performed once, but with
three wells.
Cell cycle assay
The Cell Cycle Assay kit (cat. no. C543; Dojindo
Laboratories, Inc.) was used to analyze cell cycle progression. The
cells were harvested, counted and re-suspended in PBS (cat. no.
806552; MilliporeSigma), then fixed with 70% ethanol (cat. no.
A500737; Sangon Biotech Co., Ltd.) at 4°C overnight. After
discarding the ethanol, the cells were stained with working
solution at 4°C for 1 h in the dark. The cells were then analyzed
using a FACSCalibur flow cytometer (BD Biosciences). The data were
analyzed using FlowJo 7.6 software (BD Biosciences). Each assay was
only performed once, but with three wells.
Lactate dehydrogenase activity (LDH)
assay
The LDH assay was performed using the LDH Assay
Kit-WST (cat. no. CK12; Dojindo Laboratories, Inc.). A volume of
100 µl working solution was added and incubated with the cells in
the dark for 20 min. Subsequently, 50 µl working solution was
added. The absorbance was then measured using a microplate reader
at 490 nm. Each assay was only performed once, but with three
wells.
Annexin V (AV)/propidium iodide (PI)
assay
Apoptosis was evaluated using an AV-FITC Apoptosis
Detection kit (cat. no. 4830-01-K; R&D Systems, Inc.). The
cells were resuspended in 100 µl binding buffer, then incubated
with 5 µl AV and 5 µl PI for 15 min at room temperature in the
dark. Apoptotic cells were analyzed using a FACSCalibur flow
cytometer (BD Biosciences). The data were analyzed with FlowJo 7.6
software (BD Biosciences), and the percent of late or early + late
apoptosis cells was assessed. Each assay was only performed once,
but with three wells.
Hoechst/PI assay
Hoechst/PI assay was performed to assess cell
apoptosis. The culture medium was discarded, and PBS was added to
the wells. Subsequently, 1 µl Hoechst 33342 (cat. no. B2261;
MilliporeSigma) and 1 µl PI (cat. no. P4864; MilliporeSigma) were
added to each well and incubated at 4°C for 20 min. An inverted
fluorescence microscope (Olympus Corporation) was used to determine
the percentage of apoptotic cells. Each assay was only performed
once, but with three wells.
Luciferase activity assay
The fragments of the RAB3IP 3′ untranslated region
(UTR) containing the wild-type (WT) miR-126 binding sites
(RAB3IP-WT) or mutant (MT) binding sites (RAB3IP-MT; synthesized by
Sangon Biotech Co., Ltd.) were cloned into the pmirGLO vector (cat.
no. E1330; Promega Corporation). The 293T cells were seeded in
24-well plates (5×104 cells/well) and cultured to 80%
confluence. Subsequently, 100 ng RAB3IP-WT or RAB3IP-MT luciferase
vector and 50 nM mimic-miR or mimic-NC were co-transfected into
293T cells for 48 h using Lipofectamine 3000. The Dual-Luciferase
Reporter Assay system (cat. no. E1910; Promega Corporation) was
used to measure relative luciferase reporter activity. Luciferase
activity was normalized to Renilla luciferase activity. Each
assay was only performed once, but with three wells.
Statistical analysis
SPSS 26.0 (IBM Corp.) was used for data analysis.
The data are presented as the mean ± standard deviation.
Comparisons between two groups were carried out using an unpaired
Student's t-test. Multi-group comparisons were performed using a
one-way ANOVA followed by a Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell viability and miR-126 expression
in the OGD/R model
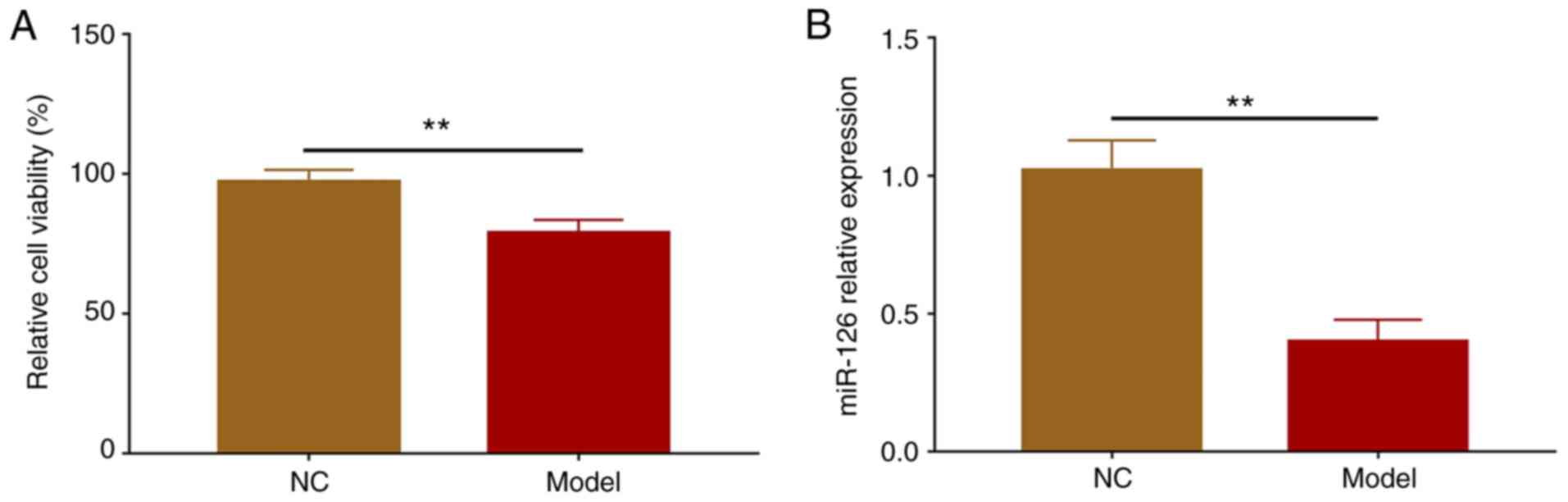
Cell viability was reduced in the Model group
compared with the NC group (P<0.01), suggesting that the
construction of the OGD/R model was successful (Fig. 1A). After the establishment of the
OGD/R model, RT-qPCR was performed to detect miR-126 expression.
The results indicated that miR-126 expression levels were
downregulated in the Model group compared with the NC group
(P<0.01; Fig. 1B).
miR-126 regulates cell viability and
apoptosis during cerebral I/R injury
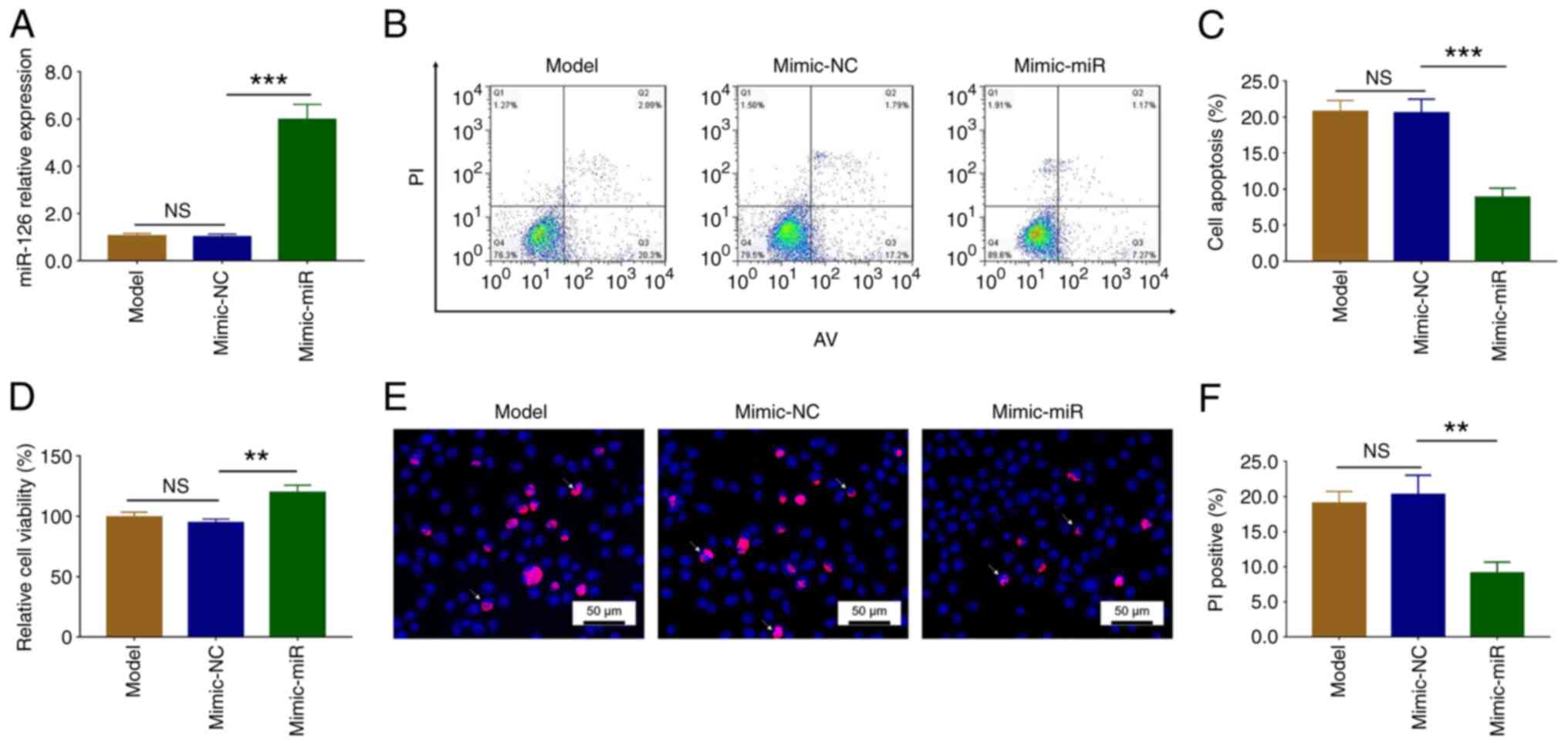
After transfection with the mimic-miR, miR-126
expression increased compared with the mimic-NC group (P<0.001).
There was no difference in miR-126 expression between the Model and
the mimic-NC groups (P>0.05; Fig.
2A). These data demonstrated successful transfection.
AV/PI assays demonstrated that apoptosis was
significantly reduced in the mimic-miR group compared with the
mimic-NC group (P<0.001). There was no difference in apoptosis
between the Model and the mimic-NC groups (P>0.05; Fig. 2B and C). Moreover, cell viability
was significantly increased in the mimic-miR group compared with
the mimic-NC group (P<0.01), but remained similar between the
Model and the mimic-NC groups (P>0.05; Fig. 2D). Additionally, Hoechst/PI assays
confirmed that apoptosis was reduced following mimic-miR
transfection (P<0.01), but was similar in the Model and mimic-NC
groups (P>0.05; Fig. 2E and
F).
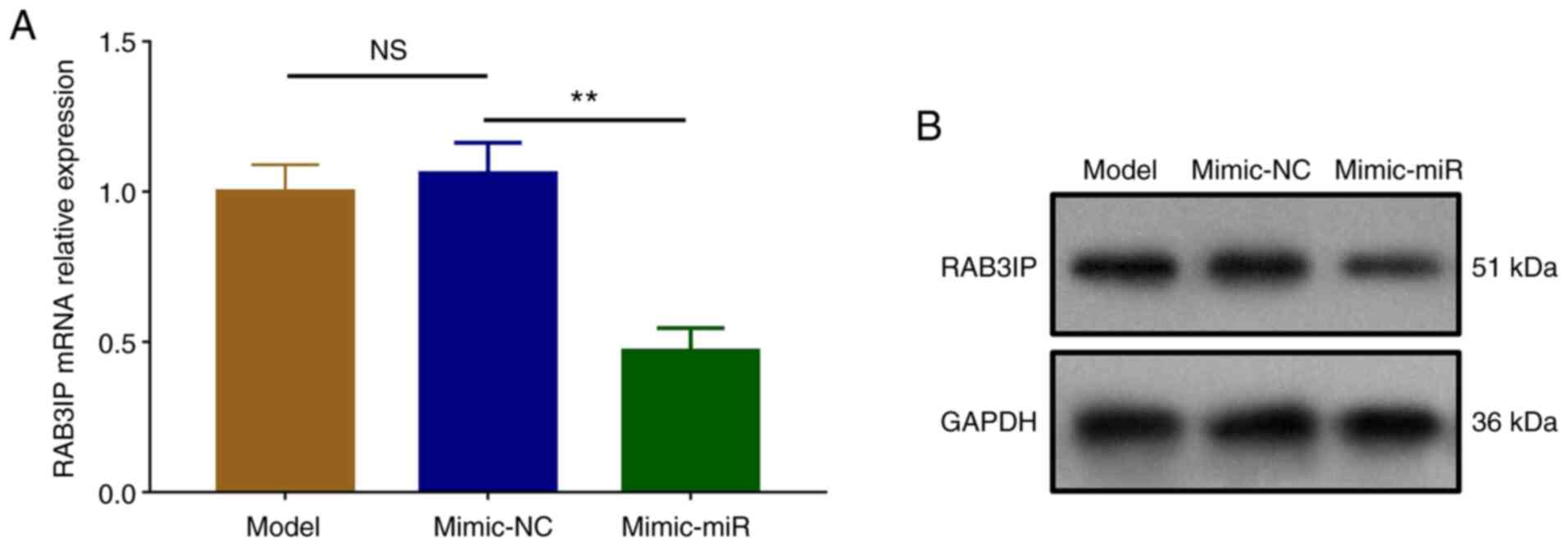
miR-126 downregulates RAB3IP
expression during cerebral I/R injury
The mRNA (P<0.01) and protein expression levels
of RAB3IP were downregulated in the mimic-miR group compared with
the mimic-NC group, but similar between the Model and the mimic-NC
groups (P>0.05; Fig. 3A and
B).
Expression of RAB3IP following
transfection
Following transfection, RAB3IP expression increased
in the OE-RAB3IP group compared with the OE-NC group (P<0.001).
No difference in RAB3IP expression was observed between the Model
and the OE-NC groups (P>0.05; Fig. S1). These data demonstrated
successful transfection.
miR-126 regulates cell viability,
apoptosis and cell cycle progression during cerebral I/R injury via
RAB3IP
In order to determine whether miR-126 exerted
neuroprotective effects during cerebral I/R injury via RAB3IP,
rescue experiments were carried out. The mRNA (P<0.001) and
protein expression levels of RAB3IP were upregulated in the
OE-RAB3IP + mimic-NC group compared with the OE-NC + mimic-NC group
(P<0.001), as well as in the OE-RAB3IP + mimic-miR group
compared with the OE-NC + mimic-miR group (P<0.01; Fig. 4A and B). However, there was no
difference in miR-126 expression between the OE-RAB3IP + mimic-NC
and the OE-NC + mimic-NC groups, nor between the OE-RAB3IP +
mimic-miR and the OE-NC + mimic-miR groups (both P>0.05;
Fig. 4C).
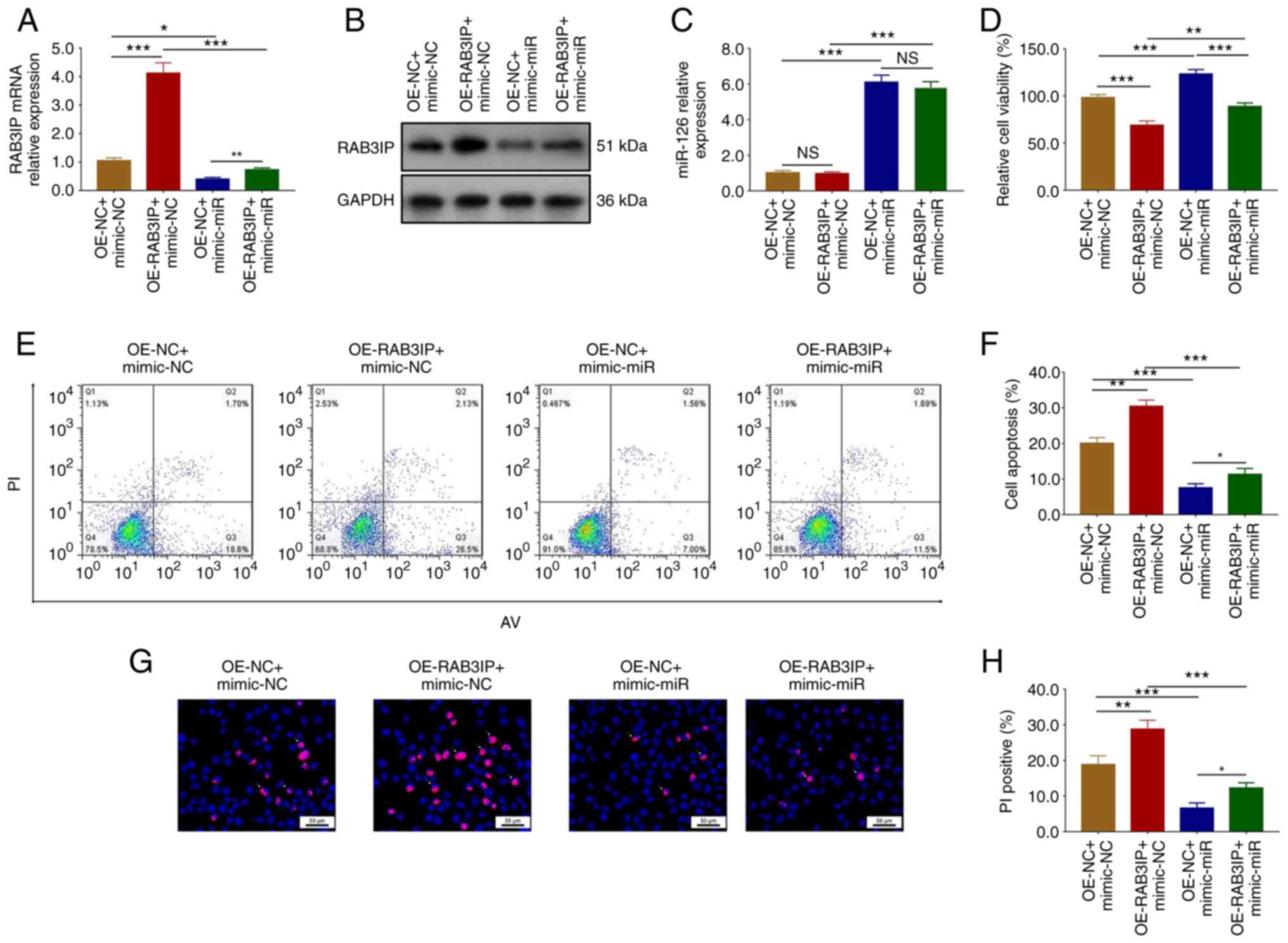 | Figure 4.miR-126 regulates cell viability and
apoptosis during cerebral ischemia/reperfusion injury via RAB3IP.
RAB3IP (A) mRNA and (B) protein expression levels were determined
using reverse transcription-quantitative PCR and western blotting,
respectively. (C) miR-126 expression levels. (D) Cell viability.
(E-H) Apoptosis was determined using AV/PI and Hoechst/PI assays.
n=3. The data were analyzed using one-way ANOVA followed by Tukey's
post hoc test. *P<0.05, **P<0.01, ***P<0.001. NS, not
significant; RAB3IP, Ras-related protein RAB3A interacting protein;
miR, microRNA; OE, overexpression; NC, negative control; AV,
Annexin V; PI, propidium iodide. |
Cell viability was reduced in the OE-RAB3IP +
mimic-NC group compared with the OE-NC + mimic-NC group
(P<0.001), as well as in the OE-RAB3IP + mimic-miR group
compared with the OE-NC + mimic-miR group (P<0.001; Fig. 4D).
Furthermore, AV/PI assays suggested that apoptosis
was significantly increased in the OE-RAB3IP + mimic-NC group
compared with the OE-NC + mimic-NC group (P<0.01), as well as in
the OE-RAB3IP + mimic-miR group compared with the OE-NC + mimic-miR
group (P<0.05; Fig. 4E and F).
Hoechst/PI staining also confirmed these findings (Fig. 4G and H).
Cell cycle progression was also analyzed. The number
of cells in the G0/G1 phase was reduced
following mimic-miR transfection compared with the mimic-NC group
(P<0.05). By contrast, the number of cells in the S phase
increased following transfection with the mimic-miR (P<0.05).
However, there was no difference in the number of cells in the
G2 phase (P>0.05; Fig.
S2A and B). Moreover, the number of cells in the
G0/G1 phase was increased in the OE-RAB3IP +
mimic-NC group compared with the OE-NC + mimic-NC group
(P<0.05), as well as in the OE-RAB3IP + mimic-miR group compared
with the OE-NC + mimic-miR group (P<0.05). The number of cells
in the S phase was reduced following co-transfection with OE-RAB3IP
+ mimic-NC compared with the OE-NC + mimic-NC group (P<0.05).
This was also the case in the OE-RAB3IP + mimic-miR group compared
with the OE-NC + mimic-miR group (P<0.05). There was no
difference in the number of cells in the G2 phase
between these groups (all P>0.05; Fig. S2C and D).
Moreover, LDH assays were also carried out, and the
results demonstrated that LDH release was increased in the Model
group compared with the NC group, suggesting successful
construction of the OGD/R model (P<0.01; Fig. S3A). In addition, LDH release was
reduced in the mimic-miR group compared with the mimic-NC group
(P<0.01), but similar between the Model and mimic-NC groups
(P>0.05; Fig. S3B).
Additionally, LDH release was increased following co-transfection
with OE-RAB3IP + mimic-NC compared with the OE-NC + mimic-NC group
(P<0.01). This was also true for OE-RAB3IP + mimic-miR
transfection compared with the OE-NC + mimic-miR group (P<0.01;
Fig. S3C).
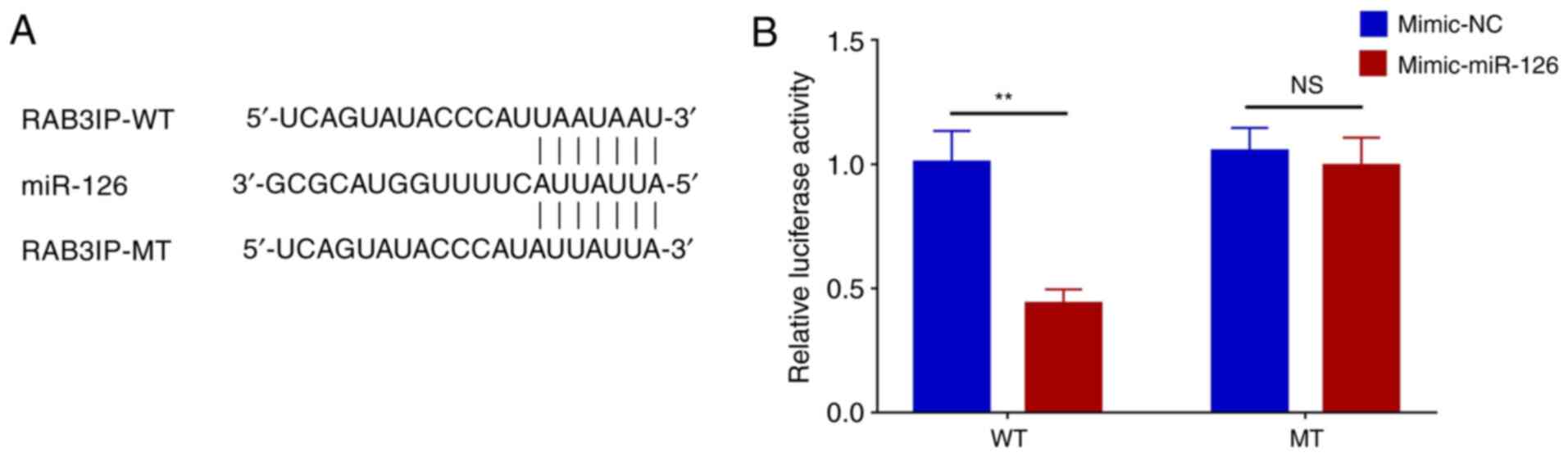
Luciferase reporter assay
The sequences of RAB3IP-WT, RAB3IP-MT and miR-126
are presented in Fig. 5A.
Relative luciferase activity was decreased in the RAB3IP-WT +
mimic-miR group compared with the RAB3IP-WT + mimic-NC group
(P<0.01). However, there was no difference in the relative
luciferase activity between RAB3IP-MT + mimic-miR and the RAB3IP-MT
+ mimic-NC groups (P>0.05; Fig.
5B).
Discussion
To the best of our knowledge, the findings of the
present study demonstrated for the first time that miR-126 could
reduce the effects of OGD/R in SH-SY5Y cells by targeting RAB3IP.
These findings may improve our understanding of the pathogenesis of
cerebral I/R injury and provide novel insights into the treatment
of this condition.
miR-126 is a well-characterized miRNA that plays a
crucial role in a range of pathological processes, including
several inflammatory diseases. For example, this miRNA interacts
with the Akt/Rac family small GTPase 1 signaling pathway to inhibit
inflammatory responses during sepsis (16). Moreover, miR-126 inhibits stromal
cell-derived factor-1 (SDF-1) α and C-C motif chemokine ligand 2
expression to inhibit the recruitment of inflammatory monocytes
into the tumor stroma, thereby suppressing breast cancer metastasis
(17). Furthermore, miR-126
targets TNF receptor associated factor 6 to reduce the expression
of pro-inflammatory cytokines in human gingival fibroblasts under
high-glucose conditions (28). In
addition to its inhibitory function in various inflammatory
diseases, miR-126 also serves a role in neuroregulation in several
nervous system diseases. For example, miR-126 targets RAB3IP to
increase 1-methyl-4-phenylpyridine-induced SH-SY5Y neuronal cell
proliferation and reduce apoptosis in a model of Parkinson's
disease (18). In addition,
miR-126 regulates the SDF-1/C-X-C chemokine receptor (CXCR)7
pathway to promote post-stroke angiogenesis of endothelial
progenitor cell transplantation (29).
Furthermore, miR-126 has emerged as a critical
regulatory molecule during I/R injury. For example, hematopoietic
miR-126 is related to stromal cell-derived factor 1/CXCR4-dependent
vasculogenic progenitor cell mobilization to promote vascular
integrity, thereby protecting against renal I/R injury (30). In addition, miR-126 promotes Nrf2
expression to reduce oxidative stress and renal I/R injury
(19). miR-126 also decreases
oxidative stress and apoptosis from myocardial I/R injury by
targeting epidermal growth factor receptor feedback inhibitor 1
(20). Despite the protective
effect of miR-126 against I/R injury in several organs, limited
evidence is available regarding its role during cerebral I/R
injury. As miR-126 can inhibit inflammation in neuronal cells, it
was hypothesized that miR-126 may exert a protective role in
cerebral I/R injury. In the present study, an OGD/R model was
established in order to simulate cerebral I/R injury in
vitro. miR-126 expression was downregulated in the OGD/R model.
It is possible that miR-16 binds to several genes to decrease
oxidative stress and reduce miR-126 expression. In order to further
explore the effect of miR-126 on cell viability and apoptosis
during cerebral I/R injury, miR-126 mimic transfection was carried
out in SH-SY5Y cells, which were then subjected to OGD/R treatment.
The results demonstrated that miR-126 could promote cell viability
during OGD/R, whilst inhibiting apoptosis. This suggested that
miR-126 could protect SH-SY5Y cells in this model of I/R injury.
The probable explanations were that: i) miR-126 could interact with
multiple genes and pathways to promote cell viability and suppress
apoptosis (19,31); ii) miR-126 increased cell
viability following OGD/R, but decreased apoptosis by targeting
several genes (including RAB31P), which subsequently contributed to
its the inhibitory effect on I/R injury; and iii) miR-126 regulated
several genes and interacted with multiple signaling pathways to
inhibit the inflammatory response, thereby decreasing
inflammatory-induced neuronal injury and exerting neuroprotective
effects during cerebral I/R injury (16,17).
RAB3IP, also known as Rabin 8, is a Rab-specific
guanine nucleotide exchange factor (GEF) and a major activator of
Rab proteins, which is directly regulated by Rab11 and is involved
in multiple biological processes, including neuronal development
(32). For example, a previous
study suggested that RAB3IP regulates nerve growth factor-induced
neurite outgrowth by interacting with Rab8, Rab10, Rab11 or through
a GEF-independent mechanism (33). Another study demonstrated that
RAB3IP was predominantly enriched in the Golgi apparatus in soma
and proximal dendrites, where it regulates Rab8 function to form
and/or deliver post-Golgi vesicles to the dendritic membrane,
thereby promoting synapse development and increasing spine head
diameter (34). In addition,
RAB3IP inhibits cell proliferation, but promotes apoptosis in
Parkinson's disease (21).
Considering the important role of RAB3IP in neuronal development,
it was hypothesized in the present study that miR-126 targeted
RAB3IP to exert beneficial effects on neuronal cells during
cerebral I/R injury.
In order to validate this hypothesis, rescue
experiments were performed, which revealed that miR-126 attenuated
the OGD/R-induced cell apoptosis, possibly via RAB3IP. Moreover, in
a dual-luciferase reporter assay, miR-126 was shown to interact
with RAB3IP. Thus, miR-126 might regulate the protein expression of
RAB3IP by directly binding to RAB3IP mRNA, thereby regulating cell
viability, apoptosis and cell cycle progression in the present
cerebral I/R injury model (Fig.
S4). Although the present study focused on exploring the
underlying mechanism of miR-126/RAB3IP modulation of cellular
function after cerebral I/R injury, additional in vivo
experiments are needed to validate the findings. When detecting
cell viability using CCK-8 and MTT assays, cell proliferation may
affect the accuracy of the results of cell viability. However, an
LDH assay was used to overcome this limitation.
In conclusion, miR-126 protected against I/R-induced
cerebral injury by targeting RAB3IP. These findings offer a novel
perspective for the underlying mechanism of cerebral I/R injury and
may provide valuable insight for the development of potential
prevention strategies against cerebral I/R injury.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and XZ designed the experiments. MZ and JL
performed the experiments and acquired the data. NL, YZ, CC, YG and
QF analyzed the data. ZS and XZ confirm the authenticity of all the
raw data. All authors wrote the manuscript and revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Ischemia/Reperfusion. Compr Physiol. 7:113–170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakai S and Shichita T: Inflammation and
neural repair after ischemic brain injury. Neurochem Int.
130:1043162019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacob B, Stock D, Chan V, Colantonio A and
Cullen N: Predictors of in-hospital mortality following
hypoxic-ischemic brain injury: A population-based study. Brain Inj.
34:178–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galkin A: Brain ischemia/reperfusion
injury and mitochondrial complex I damage. Biochemistry (Mosc).
84:1411–1423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kleindorfer DO, Towfighi A, Chaturvedi S,
Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN,
Kittner SJ, Leira EC, et al: 2021 Guideline for the prevention of
stroke in patients with stroke and transient ischemic attack: A
guideline from the American heart association/American stroke
association. Stroke. 52:e364–e467. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gladstone DJ, Lindsay MP, Douketis J,
Smith EE, Dowlatshahi D, Wein T, Bourgoin A, Cox J, Falconer JB,
Graham BR, et al: Canadian stroke best practice recommendations:
Secondary prevention of stroke update 2020. Can J Neurol Sci. Jun
18–2021.(Epub ahead of print). View Article : Google Scholar
|
|
8
|
Pantazi E, Bejaoui M, Folch-Puy E, Adam R
and Rosello-Catafau J: Advances in treatment strategies for
ischemia reperfusion injury. Expert Opin Pharmacother. 17:169–179.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ibanez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuo ML, Wang AP, Song GL and Yang ZB:
MiR-652 protects rats from cerebral ischemia/reperfusion oxidative
stress injury by directly targeting NOX2. Biomed Pharmacother.
124:1098602020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie YL, Zhang B and Jing L: MiR-125b
blocks Bax/Cytochrome C/Caspase-3 apoptotic signaling pathway in
rat models of cerebral ischemia-reperfusion injury by targeting
p53. Neurol Res. 40:828–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Y, Lei Y, Yu F, Changfeng F, Song W and
Xuming M: MicroRNAs expression and function in cerebral ischemia
reperfusion injury. J Mol Neurosci. 53:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Du L and Zhang J: Febrile
seizure-related miR-148a-3p exerts neuroprotection by promoting the
proliferation of hippocampal neurons in children with temporal lobe
epilepsy. Dev Neurosci. 43:312–320. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu R, Peng Z, Zhang Y, Li R and Wang Y:
Upregulation of miR128 inhibits neuronal cell apoptosis following
spinal cord injury via FasL downregulation by repressing ULK1. Mol
Med Rep. 24:6672021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HF, Wang YQ, Dou L, Gao HM, Wang B,
Luo N and Li Y: Influences of up-regulation of miR-126 on septic
inflammation and prognosis through AKT/Rac1 signaling pathway. Eur
Rev Med Pharmacol Sci. 23:2132–2138. 2019.PubMed/NCBI
|
|
17
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: MiR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin Q, Hou S, Dai Y, Jiang N and Lin Y:
LncRNA HOTAIR targets miR-126-5p to promote the progression of
Parkinson's disease through RAB3IP. Biol Chem. 400:1217–1228. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao B, Chen X and Li H: Protective
effects of miR-126 specifically regulates Nrf2 through ischemic
postconditioning on renal ischemia/reperfusion injury in mice.
Transplant Proc. 52:392–397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Zheng Y, Wang M, Yan M, Jiang J
and Li Z: Exosomes derived miR-126 attenuates oxidative stress and
apoptosis from ischemia and reperfusion injury by targeting ERRFI1.
Gene. 690:75–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang JF, Shi LL, Zhang L, Zhao ZH, Liang
F, Xu X, Zhao LY, Yang PB, Zhang JS and Tian YF: MicroRNA-25
negatively regulates cerebral ischemia/reperfusion injury-induced
cell apoptosis through Fas/FasL pathway. J Mol Neurosci.
58:507–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y and Xu M: MiR-380-5p facilitates
NRF2 and attenuates cerebral ischemia/reperfusion injury-induced
neuronal cell death by directly targeting BACH1. Transl Neurosci.
12:210–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu G, Sun W, Wang W, Le C, Liang D and
Shuai L: Overexpression of microRNA-202-3p in bone marrow
mesenchymal stem cells improves cerebral ischemia-reperfusion
injury by promoting angiogenesis and inhibiting inflammation. Aging
(Albany NY). 13:11877–11888. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Y, Yi Z, Zhao P, Xu Y and Pan P:
MicroRNA-532-5p protects against cerebral ischemia-reperfusion
injury by directly targeting CXCL1. Aging (Albany NY).
13:11528–11541. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kramer MF: Stem-loop RT-qPCR for miRNAs.
Curr Protoc Mol Biol. Chapter 15: Unit 15. 10:2011.PubMed/NCBI
|
|
28
|
Wu Y, Song LT, Li JS, Zhu DW, Jiang SY and
Deng JY: MicroRNA-126 regulates inflammatory cytokine secretion in
human gingival fibroblasts under high glucose via targeting tumor
necrosis factor receptor associated factor 6. J Periodontol.
88:e179–e187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shan C and Ma Y: MicroRNA-126/stromal
cell-derived factor 1/C-X-C chemokine receptor type 7 signaling
pathway promotes post-stroke angiogenesis of endothelial progenitor
cell transplantation. Mol Med Rep. 17:5300–5305. 2018.PubMed/NCBI
|
|
30
|
Bijkerk R, van Solingen C, de Boer HC, van
der Pol P, Khairoun M, de Bruin RG, van Oeveren-Rietdijk AM,
Lievers E, Schlagwein N, van Gijlswijk DJ, et al: Hematopoietic
microRNA-126 protects against renal ischemia/reperfusion injury by
promoting vascular integrity. J Am Soc Nephrol. 25:1710–1722. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao ZH, Wang L, Gan P, He J, Yan BC and
Ding LD: Dynamic Changes in miR-126 expression in the hippocampus
and penumbra following experimental transient global and focal
cerebral ischemia-reperfusion. Neurochem Res. 45:1107–1119. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren H, Xu Z, Guo W, Deng Z and Yu X:
Rab3IP interacts with SSX2 and enhances the invasiveness of gastric
cancer cells. Biochem Biophys Res Commun. 503:2563–2568. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Homma Y and Fukuda M: Rabin8 regulates
neurite outgrowth in both GEF activity-dependent and -independent
manners. Mol Biol Cell. 27:2107–2118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ultanir SK, Hertz NT, Li G, Ge WP,
Burlingame AL, Pleasure SJ, Shokat KM, Jan LY and Jan YN: Chemical
genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8
Uncovers their roles in dendrite arborization and spine
development. Neuron. 73:1127–1142. 2012. View Article : Google Scholar : PubMed/NCBI
|