Introduction
Glioblastoma is the most common malignant primary
tumor of the central nervous system (CNS), accounting for 48.3% of
all malignant primary CNS tumors (1). The 5-year relative survival rate of
patients with glioblastoma is ~6.8% (1). Even with the use of treatments such
as maximal safe surgical resection, radiotherapy and systemic
chemotherapy with temozolomide, patient prognosis remains poor. The
median progression-free and overall survival times from diagnosis
are 6.2-7.5 and 14.6-16.7 months, respectively (2). Research has suggested that
angiogenesis is a contributing factor to the growth and malignancy
of glioblastoma. Angiogenesis, the budding of capillaries from
pre-existing vasculature, occurs in both embryonic development and
certain physiological and pathological conditions, such as
menstruation, wound healing, diabetic retinopathy, rheumatoid
arthritis and psoriasis, as well as in human gliomas (3). In glioblastoma, angiogenesis is the
result of an imbalance between pro- and anti-angiogenic factors
(4). Since 1971 when Folkman
(5) proposed that tumor growth
depended on blood vessel growth, VEGF has been proven to be an
important angiogenic factor affecting health and disease.
Accumulating evidence has indicate that vascular endothelial growth
factor A (VEGFA) is highly upregulated in glioblastoma (6). The expression levels of VEGFA and
its receptors (VEGFR-1 and −2) in glioblastoma are positively
associated with tumor grade and negatively associated with survival
time (7). The Wnt/β-catenin
signaling pathway has also been reported to serve an important role
in the regulation of VEGFA secretion and angiogenesis in
malignancies, such as colon cancer (8–11).
Although the mechanism of VEGFA's role in these malignancies has
been studied extensively, little is known about the role of VEFGA
in glioblastoma angiogenesis.
The frequently rearranged in advanced T-cell
lymphomas-1 (FRAT1) gene, located at the human chromosome 10q24.1
region, encodes a 29-kDa protein comprising 279 amino acids
(12) and is primarily cloned
from mouse T-cell lymphomas (13). FRAT1 positively regulates the
Wnt/β-catenin signaling pathway by inhibiting the activity of
GSK-3β toward β-catenin (14–16). Aberrant activation of FRAT1
promotes Wnt/β-catenin signaling pathway dysregulation and FRAT1 is
highly upregulated in a variety of human cancers (12,17–20). Our previous study demonstrated
that FRAT1 is an important factor for the development of gliomas,
as well as for predicting patient prognosis. Moreover, the
expression of FRAT1 is positively correlated with pathological
grade and angiogenesis in patients with glioma (21). Therefore, the Wnt/β-catenin
signaling pathway may serve as a positive inducer of angiogenesis
in human glioma and FRAT1 may serve a significant role in this
process.
To verify and elucidate this hypothesis in the
present study, the glioblastoma and low-grade glioma (GBMLGG)
RNA-sequencing (RNA-seq) data from The Cancer Genome Atlas (TCGA)
database was used to compare the differences in FRAT1 and VEGFA
expression between tumor tissues and normal samples. The potential
mechanism by which VEGFA and FRAT1 modulated the occurrence and
development of GBMLGG was explored and discussed. To verify this
analysis, the association between FRAT1 and VEGFA was investigated
using reverse transcription-quantitative PCR (RT-qPCR), western
blotting, ELISA and tube formation assays.
Materials and methods
RNA-seq data and bioinformatics
analysis
TCGA project (https://genome-cancer.ucsc.edu/) was used to collect
RNA-seq data (22) and clinical
information from 696 pan-cancer samples and 1,157 normal samples.
According to the expression of VEGFA and FRAT1 in the samples,
samples were divided into high expression and low expression groups
by the median of VEGFA and FRAT1 expression, respectively. The
downloaded data format was level 3 HiSeq-fragments per kilobase per
million, which was converted into transcripts per million format
for subsequent analysis. All procedures performed in the present
study were in accordance with the Declaration of Helsinki (23).
Enrichment analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG;
http://www.genome.jp/kegg/pathway.html), WikiPathway
(WP; http://www.wikipathways.org/index.php/WikiPathways)
and Gene Ontology (GO; http://geneontology.org) databases (24) were analyzed with clusterProfiler
(25). Gene Set Enrichment
Analysis (GSEA) was performed using GSEA 2.0 (26).
Cell culture and transfection
Human U251 glioblastoma cell lines and primary
HUVECs were purchased form the American Type Culture Collection and
used for subsequent experimentation. The use of primary HUVECs was
approved by the ethics committee of The First Hospital of Shanxi
Medical University [approval no. (2021) Y10]. U251 cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.). HUVECs were cultured in endothelial cell
basal medium supplemented with a Growth Medium Supplement Pack
(both purchased from PromoCell GmbH). Both cell types were
incubated at 37°C (5% CO2) in a humidified incubator.
The density at transfection was 2×106 cells/ml. The
medium was replaced every 2 days and the cells were passaged twice
weekly.
To silence the expression of FRAT1, U251 cells were
seeded into 6-well culture plates and transfected with siRNAs
directly targeting FRAT1 (siFRAT1), or the corresponding negative
control (NC) siRNA (siNC). Both siFRAT1 and siNC were transfected
into cells using Lipofectamine™ RNAiMAX Reagent (cat. no. 13778030;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The concentration of siRNA and siNC
transfected was 40 nM. The sequence of siNC was
5′-UUCUCCGAACGUGUCACGU-3′. The sequence of siFRAT1 was
5′-GCAGUUACGUGCAAAGCUU-3′. Transfection efficiency was assessed via
RT-qPCR. Untransfected U251 cells were used as a control. The
transfection was performed according to the manufacturer's
instructions. The cells were collected after transfection at 37°C
for 48 h.
VEGFA ELISA
A VEGFA ELISA was performed to quantify the
secretion of VEGFA into the supernatants of non-transfected,
siFRAT1 and siNC U251 cells. Cells were cultured overnight in DMEM
containing 10% FBS to achieve 80–90% confluency. The culture medium
were removed and the cells were washed with DMEM, prior to
culturing in fresh media containing 2% FBS for 48 h. The Human VEGF
ELISA kit (cat. no. EHC108) was purchased from Neobioscience
Technology Co., Ltd. Supernatants were collected and the VEGFA
concentration was determined using the VEGFA ELISA kit according to
manufacturer's protocol.
Tube formation assay
Untransfected, siFRAT1 and siNC U251 cells were
cultured in complete media (10% FBS) at 37°C for 48 h, after which
the media was replaced with serum-free media at 37°C for 24 h.
Subsequently, 2×104 HUVECs (in 100 µl) were seeded into
a 96-well plate precoated (performed at 37°C for 45 min) with
solidified Matrigel (BD Biosciences) and were incubated with the
cell supernatants from U251 cells at 37°C for 6 h. Relative changes
in tube length among the three groups were visualized using the
Cellomics ArrayScan VT1 Readers (Cellomics, Inc.; Thermo Fisher
Scientific, Inc.).
Western blotting
In the present study, non-transfected, siFRAT1 and
siNC U251 cells were harvested and lysed on ice for 30 min using
RIPA lysis buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology, Inc.). The lysed cells were centrifuged at 12,000 ×
g at 4°C for 20 min and the protein concentration was determined
using a BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific,
Inc.). Total protein (20 µl protein/lane) was subjected to SDS-PAGE
using a 12% gel and the separated proteins were transferred onto a
polyvinylidene fluoride membrane (Thermo Fisher Scientific, Inc.).
The membrane was blocked using TBS with 0.1% Tween-20 (TBST; pH
7.5) containing 5% nonfat dry milk for 1 h at room temperature.
Membranes were subsequently incubated overnight at 4°C with the
following primary antibodies: Anti-VEGFA (1:1,000; cat. no.
66828-1-Ig; ProteinTech Group, Inc.) and anti-β-actin (1:1,000;
cat. no. 3700S; Cell Signaling Technology, Inc.). Following the
primary incubation, the membrane was incubated with a
HRP-conjugated secondary antibody (1:10,000; cat. no. sc-2005;
Santa Cruz Biotechnology, Inc.) at room temperature for 2 h. Then,
the membrane was washed three times with TBST. Subsequently,
protein expression levels were visualized using an Novex™ enhanced
chemiluminescence detection solution (cat. no. WP20005; Pierce;
Thermo Fisher Scientific, Inc.).
RT-qPCR
The expression levels of FRAT1 mRNA in U251 cells
were assessed using RT-qPCR. Total RNA was extracted from
non-transfected, siFRAT1 and siNC U251 cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RT-qPCR was performed
using the RNA PCR Kit (avian myeloblastosis virus) version 3.0
(SYBR Green; Takara Bio, Inc.) according to manufacturer's protocol
and the following primers were used for qPCR: Human FRAT1 forward
(F), 5′-GCCCTGTCTAAAGTGTATTTTCAG-3′ and reverse (R),
5′-CGCTTGAGTAGGACTGCAGAG-3′ (27); human VEGFA F,
5′-AGGGCAGAATCATCACGAAGT-3′ and R, 5′-AGGGTCTCGATTGGATGGCA-3′; and
β-actin F, 5′-GAGCTGCGTGTGGCTCCC-3′ and R,
5′-CCAGAGGCGTACAGGGATAGCA-3′. The qPCR thermocycling conditions
were as follows: 95°C for 1 min; 40 cycles at 95°C for 30 sec, 60°C
for 10 sec and 72°C for 20 sec; and final extension at 95°C for 15
sec, 60°C for 15 sec and 95°C for 15 sec. The qPCR products were
quantified using the 2−ΔΔCq method (28) and β-actin was used as the internal
control for mRNA expression.
Statistical analysis
Each experiment was repeated three times.
Statistical analyses were performed using GraphPad Prism 7.0
software (GraphPad Software, Inc.) and data are presented as the
mean ± standard deviation. Statistical differences among ≥3 groups
were determined using a one-way ANOVA followed by a Tukey's post
hoc test. The TCGA data statistical analyses were performed using R
(version 3.6.3) (29). Wilcoxon
rank sum test was used for continuous variables. The χ2
test and Fisher's exact test were used for categorical variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics
The clinical and demographic characteristics of the
participants are summarized in Table
I and Table II. A total of
696 individuals underwent assessment for the study. According to
the expression of mRNA, individuals were divided into high
expression and low expression groups. Overall, it was determined
that certain groups of individuals exhibited statistically
significant differences in terms of demographic or clinical
characteristics between the high expression group and low
expression group (P<0.05). Subsequently, a multivariate analysis
with the Cox regression model was performed. The results indicated
that FRAT1 and VEGFA expression, World Health Organization grade,
primary therapy outcome and age are significantly independently
associated (Table III).
Multivariate analysis demonstrated that FRAT1 expression levels are
a significant independent risk factor for overall survival in
GBMLGG, but VEGFA expression levels are not statistically
significant.
 | Table I.Clinical and demographic
characteristics of patients with high and low FRAT1 mRNA expression
levels. |
Table I.
Clinical and demographic
characteristics of patients with high and low FRAT1 mRNA expression
levels.
| Characteristic | Low FRAT1
expression levels | High FRAT1
expression levels | P-value |
|---|
| n | 348 | 348 |
|
| WHO grade, n
(%) |
|
| <0.001 |
| G2 | 83 (13.1) | 141 (22.2) |
|
| G3 | 99 (15.6) | 144 (22.7) |
|
| G4 | 147 (23.1) | 21 (3.3) |
|
| IDH status, n
(%) |
|
| <0.001 |
| WT | 202 (29.4) | 44 (6.4) |
|
|
Mut | 138 (20.1) | 302 (44.0) |
|
| 1p/19q codeletion,
n (%) |
|
| <0.001 |
|
codel | 56 (8.1) | 115 (16.7) |
|
|
non-codel | 285 (41.4) | 233 (33.8) |
|
| Primary therapy
outcome, n (%) |
|
| 0.023 |
| PD | 48 (10.4) | 64 (13.9) |
|
| SD | 57 (12.3) | 90 (19.5) |
|
| PR | 13 (2.8) | 51 (11.0) |
|
| CR | 53 (11.5) | 86 (18.6) |
|
| Gender, n (%) |
|
| 0.146 |
|
Female | 139 (20.0) | 159 (22.8) |
|
|
Male | 209 (30.0) | 189 (27.2) |
|
| Race, n (%) |
|
| 0.140 |
|
Asian | 7 (1.0) | 6 (0.9) |
|
| Black
or African American | 22 (3.2) | 11 (1.6) |
|
|
White | 313 (45.8) | 324 (47.4) |
|
| Age, n (%) |
|
| <0.001 |
|
≤60 | 243 (34.9) | 310 (44.5) |
|
|
>60 | 105 (15.1) | 38 (5.5) |
|
| Histological type,
n (%) |
|
| <0.001 |
|
Astrocytoma | 87 (12.5) | 108 (15.5) |
|
|
Glioblastoma | 147 (21.1) | 21 (3.0) |
|
|
Oligoastrocytoma | 43 (6.2) | 91 (13.1) |
|
|
Oligodendroglioma | 71 (10.2) | 128 (18.4) |
|
| OS event, n
(%) |
|
| <0.001 |
|
Alive | 172 (24.7) | 252 (36.2) |
|
|
Dead | 176 (25.3) | 96 (13.8) |
|
| DSS event, n
(%) |
|
| <0.001 |
|
Alive | 174 (25.8) | 257 (38.1) |
|
|
Dead | 161 (23.9) | 83 (12.3) |
|
| PFI event, n
(%) |
|
| <0.001 |
|
Alive | 143 (20.5) | 207 (29.7) |
|
|
Dead | 205 (29.5) | 141 (20.3) |
|
| Age, median
(IQR) | 52 (39, 62) | 39 (32, 51.25) | <0.001 |
 | Table II.Clinical and demographic
characteristics of patients with high and low VEGFA mRNA expression
levels. |
Table II.
Clinical and demographic
characteristics of patients with high and low VEGFA mRNA expression
levels.
| Characteristic | Low VEGFA
expression levels | High VEGFA
expression levels | P-value |
|---|
| n | 348 | 348 |
|
| WHO grade, n
(%) |
|
| <0.001 |
| G2 | 177 (27.9) | 47 (7.4) |
|
| G3 | 128 (20.2) | 115 (18.1) |
|
| G4 | 9 (1.4) | 159 (25.0) |
|
| IDH status, n
(%) |
|
| <0.001 |
| WT | 39 (5.7) | 207 (30.2) |
|
|
Mut | 307 (44.8) | 133 (19.4) |
|
| 1p/19q codeletion,
n (%) |
|
| <0.001 |
|
Codel | 108 (15.7) | 63 (9.1) |
|
|
Non-codel | 240 (34.8) | 278 (40.3) |
|
| Primary therapy
outcome, n (%) |
|
| 0.013 |
| PD | 60 (13.0) | 52 (11.3) |
|
| SD | 104 (22.5) | 43 (9.3) |
|
| PR | 38 (8.2) | 26 (5.6) |
|
| CR | 97 (21.0) | 42 (9.1) |
|
| Gender, n (%) |
|
| 0.592 |
|
Female | 153 (22.0) | 145 (20.8) |
|
|
Male | 195 (28.0) | 203 (29.2) |
|
| Race, n (%) |
|
| 0.072 |
|
Asian | 3 (0.4) | 10 (1.5) |
|
| Black
or African American | 13 (1.9) | 20 (2.9) |
|
|
White | 322 (47.1) | 315 (46.1) |
|
| Age, n (%) |
|
| <0.001 |
|
≤60 | 313 (45) | 240 (34.5) |
|
|
>60 | 35 (5.0) | 108 (15.5) |
|
| Histological type,
n (%) |
|
| <0.001 |
|
Astrocytoma | 125 (18.0) | 70 (10.1) |
|
|
Glioblastoma | 9 (1.3) | 159 (22.8) |
|
|
Oligoastrocytoma | 95 (13.6) | 39 (5.6) |
|
|
Oligodendroglioma | 119 (17.1) | 80 (11.5) |
|
| OS event, n
(%) |
|
| <0.001 |
|
Alive | 270 (38.8) | 154 (22.1) |
|
|
Dead | 78 (11.2) | 194 (27.9) |
|
| DSS event, n
(%) |
|
| <0.001 |
|
Alive | 271 (40.1) | 160 (23.7) |
|
|
Dead | 71 (10.5) | 173 (25.6) |
|
| PFI event, n
(%) |
|
| <0.001 |
|
Alive | 221 (31.8) | 129 (18.5) |
|
|
Dead | 127 (18.2) | 219 (31.5) |
|
| Age, median
(IQR) | 39.5 (32.0,
50.0) | 53 (38.0,
63.0) | <0.001 |
 | Table III.Univariate and multivariate Cox
regression model of prognosis for VEGFA and FRAT1 in patients with
GBMLGG. |
Table III.
Univariate and multivariate Cox
regression model of prognosis for VEGFA and FRAT1 in patients with
GBMLGG.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Characteristic | Total (n) | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| WHO grade (G3 and
G4 vs. G2) | 612 | 5.893
(4.015-8.648) | <0.001 | 2.608
(1.600-4.251) | <0.001 |
| 1p/19q codeletion
(non-codel vs. codel) | 663 | 4.635
(2.963-7.251) | <0.001 | 1.424
(0.776-2.612) | 0.254 |
| Primary therapy
outcome (PR and CR | 443 | 0.204
(0.114-0.365) | <0.001 | 0.232
(0.124-0.436) | <0.001 |
| vs. PD and SD) |
|
|
|
|
|
| IDH status (Mut vs.
WT) | 660 | 0.102
(0.077-0.135) | <0.001 | 0.457
(0.256-0.816) | 0.008 |
| Gender (male vs.
female) | 669 | 1.230
(0.955-1.585) | 0.109 |
|
|
| Age (>60 vs.
≤60) | 669 | 4.716
(3.609-6.161) | <0.001 | 3.467
(2.109-5.698) | <0.001 |
| Histological type
(glioblastoma, oligoastrocytoma and oligodendroglioma vs.
astrocytoma) | 669 | 1.470
(1.096-1.971) | 0.010 | 0.928
(0.592-1.454) | 0.744 |
| FRAT1 (high vs. low
expression) | 669 | 0.287
(0.219-0.374) | <0.001 | 0.388
(0.242-0.621) | <0.001 |
| VEGFA (high vs. low
expression) | 669 | 4.488
(3.392-5.940) | <0.001 | 1.291
(0.793-2.102) | 0.304 |
mRNA expression status of FRAT1 and
VEGFA
As evaluated by the Wilcoxon rank-sum test, the
expression levels of FRAT1 and VEGFA were significantly higher in
tumor tissues compared with normal tissues (Fig. 1A and B), especially for GBMLGG
(Fig. 1C and D;
P<0.001). These results therefore indicated that the
expression of FRAT1 and VEGFA was associated.
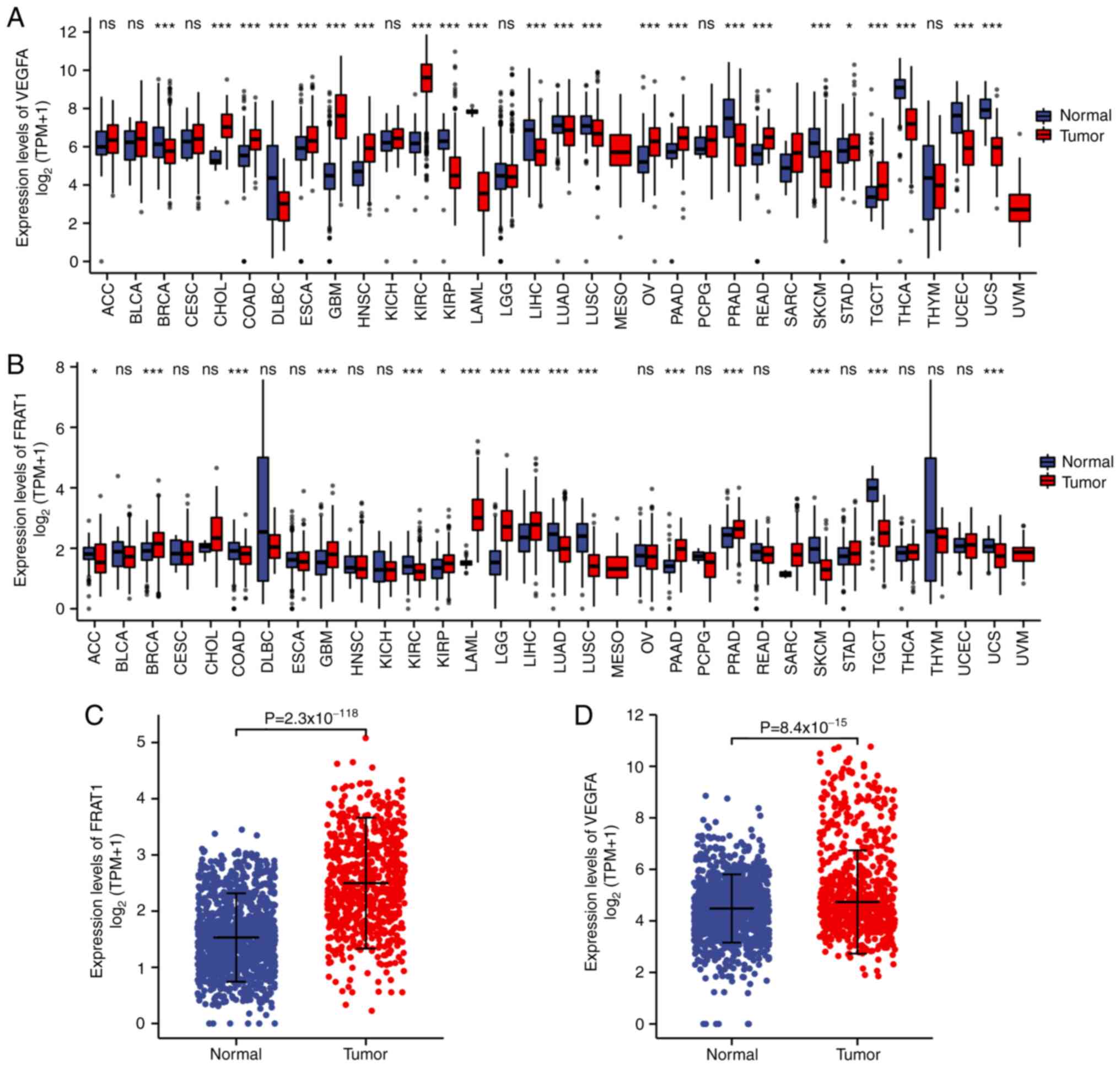 | Figure 1.mRNA expression status of FRAT1 and
VEGFA. (A and B) Wilcoxon rank sum test was used to analyze the
differences in expression of FRAT1 and VEGFA in normal and tumor
samples using the TCGA pan-cancer database. The expression levels
of FRAT1 and VEGFA were demonstrated to be significantly higher in
most tumor tissues compared with normal tissues, especially in GBM,
although a small number of cancer tissues did display lower
expression levels than in the normal tissues. (C and D) FRAT1 and
VEGFA expression levels in normal and tumor samples of TCGA in
GBMLGG. FRAT1, frequently rearranged in advanced T-cell
lymphomas-1; TCGA, The Cancer Genome Atlas; GBMLGG, glioblastoma
and low-grade glioma; ACC, adrenocortical carcinoma; BLCA, bladder
urothelial carcinoma; BRCA, breast invasive carcinoma; CESC,
cervical squamous cell carcinoma and endocervical adenocarcinoma;
CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC,
lymphoid neoplasm diffuse large b-cell lymphoma; ESCA, esophageal
carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck
squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney
renal clear cell carcinoma; KIRP, kidney renal papillary cell
carcinoma; LAML, acute myeloid leukemia; LGG, brain lower grade
glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung
adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO,
mesothelioma; OV, pvarian serous cystadenocarcinoma; PAAD,
pancreatic adenocarcinoma; PCPG, pheochromocytoma and
paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum
adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD,
stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA,
thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma; ns,
not significant. *P<0.05 and ***P<0.001. |
FRAT1 and VEGFA-related functional
enrichment analysis
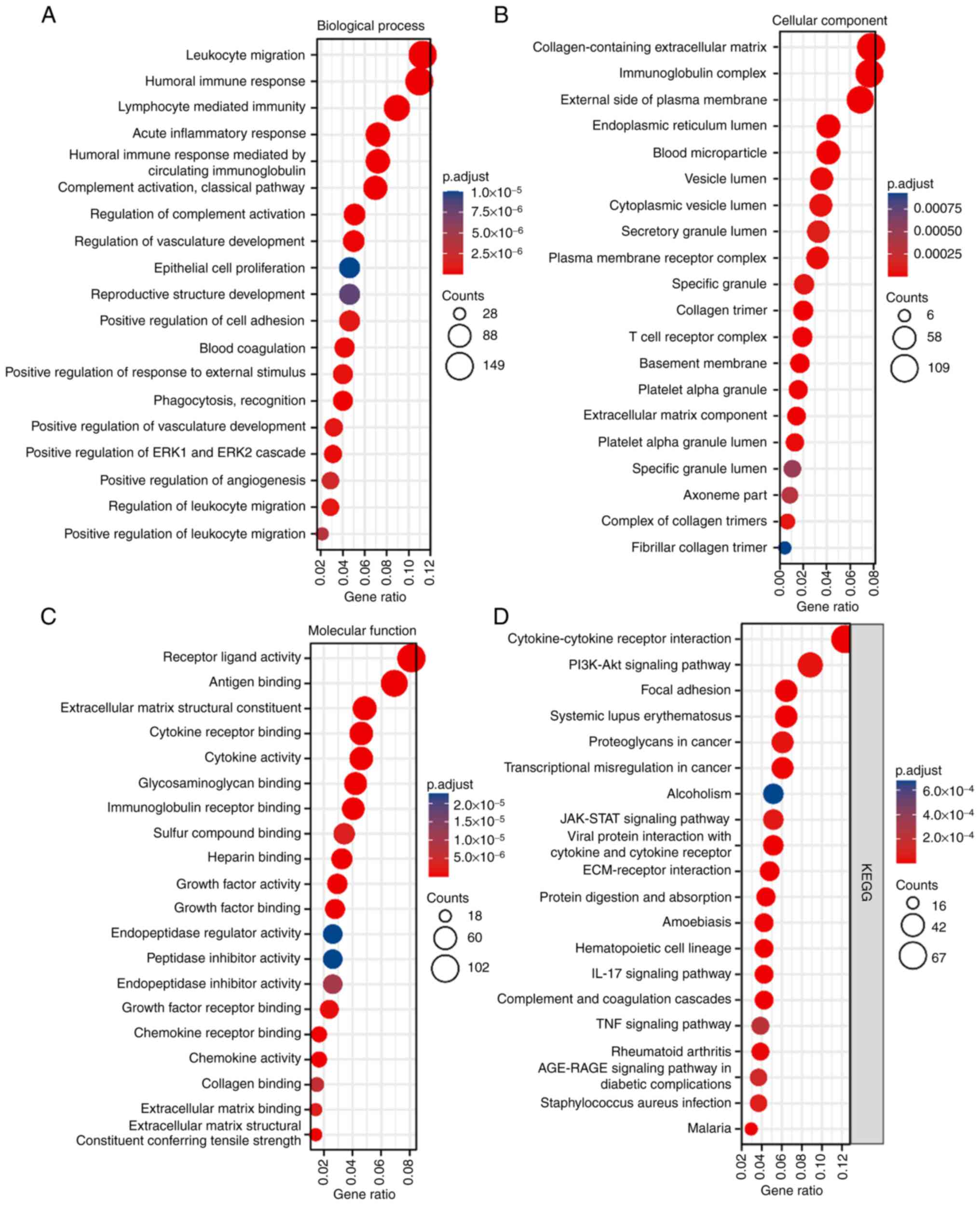
GO and KEGG enrichment analysis of genes with
associated expression revealed various overrepresented terms in the
following three main functional groups: ‘cellular component’,
‘biological process’ and ‘molecular function’. Out of the three
categories, FRAT1 expression patterns (Fig. 2) were mainly related to ‘receptor
ligand activity’, ‘antigen binding’, ‘leukocyte migration’,
‘humoral immune response’, ‘collagen-containing extracellular
matrix’, ‘blood microparticle’, ‘immunoglobulin complex’ and
‘extracellular matrix structural constituent’ among others, with
numerous terms being associated with blood cells and angiogenesis.
The KEGG analysis demonstrated that numerous terms, such as
‘cytokine-cytokine receptor interaction’, ‘PI3K-Akt signaling
pathway’ and ‘focal adhesion’ were highly enriched in
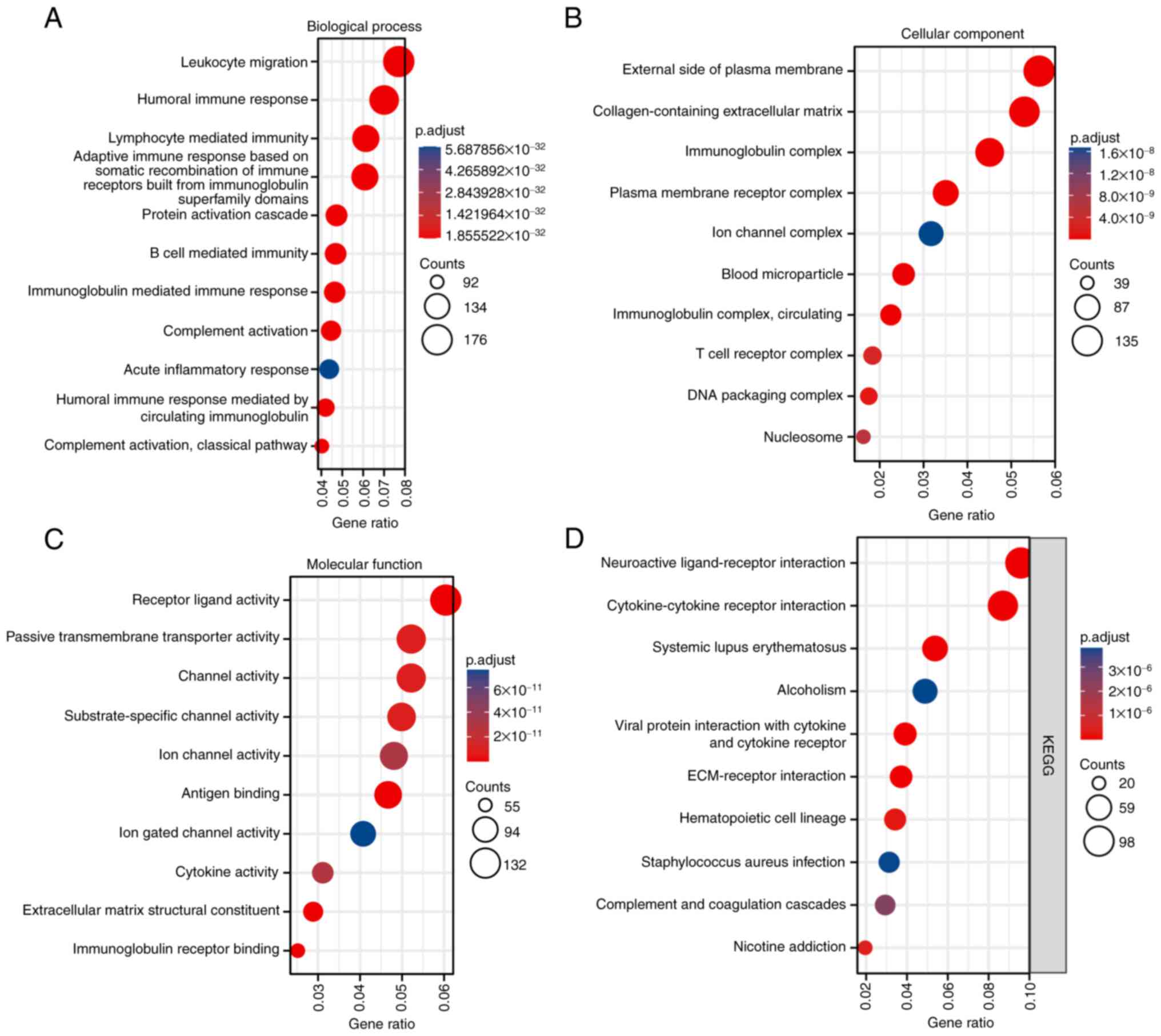
differentially expressed FRAT1. VEGFA expression also enriched
numerous GO terms and KEGG pathways, including ‘leukocyte
migration’, ‘external side of plasma membrane’, ‘receptor ligand
activity’ and ‘hematopoietic cell lineage’, which refer to
angiogenesis and immunity (Fig.
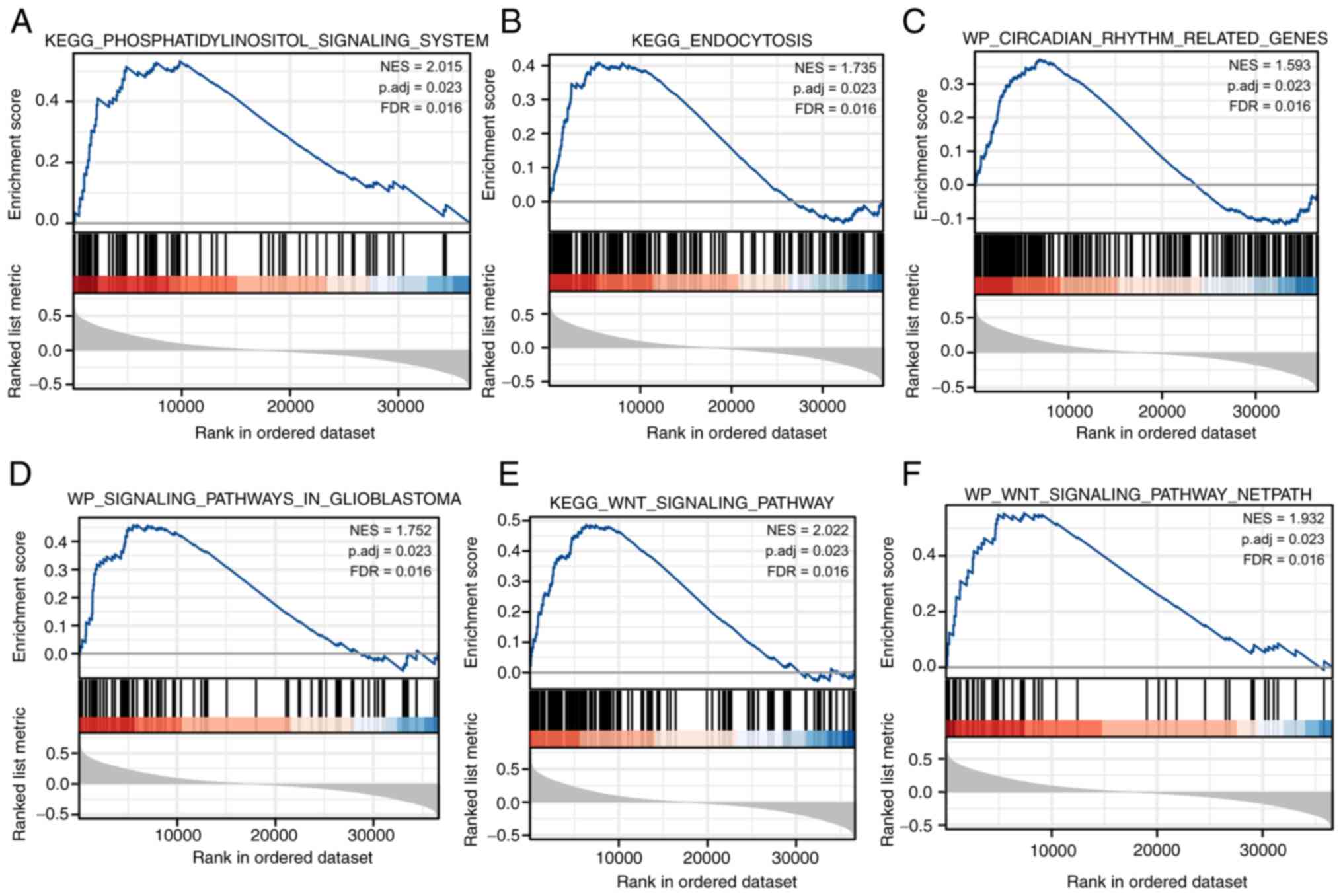
3). GSEA analysis of FRAT1 single gene association further
explored the pathway process involved in FRAT1 expression (Fig. 4). The results demonstrated that
certain pathways, such as ‘WP_SIGNALING_PATHWAYS_IN_GLIOBLASTOMA’,
‘KEGG_ENDOCYTOSIS’, ‘WP_CIRCADIAN_RHYTHM_RELATED_GENES’,
‘KEGG_WNT_SIGNALING_PATHWAY’ and
‘KEGG_PHOSPHATIDYLINOSITOL_SIGNALING_SYSTEM’, were enriched and
related to the regulation of angiogenesis. The aforementioned
results indicated that the functions of FRAT1 and VEGFA overlap in
certain functional groups and that there may be an association
between them.
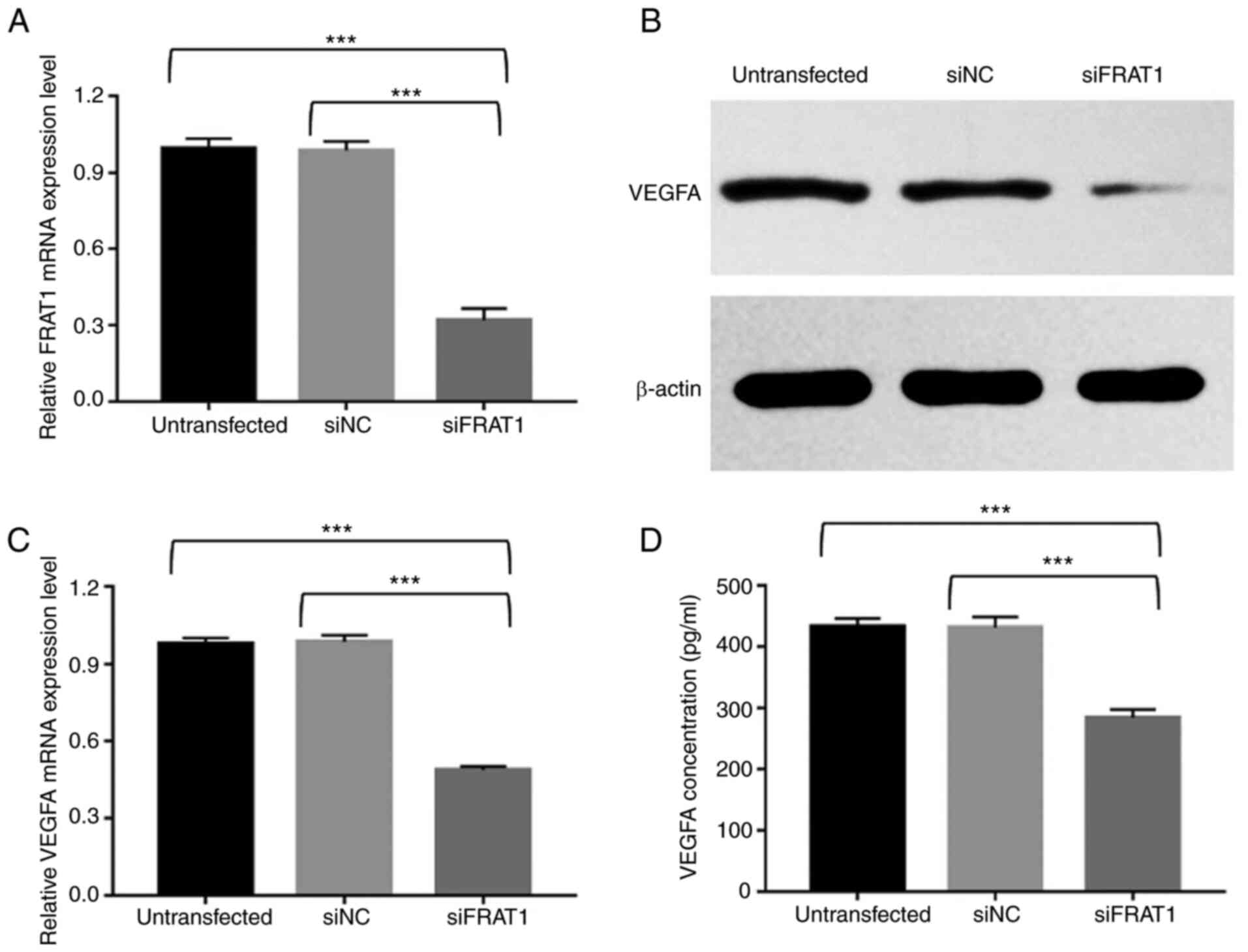
FRAT1 knockdown significantly
downregulates FRAT1 expression levels in human U251 glioblastoma
cells
To further validate the aforementioned results,
following siRNA knockdown, the relative FRAT1 mRNA expression
levels were detected using RT-qPCR. The untransfected group and
siNC group demonstrated no significant difference in FRAT1 mRNA
expression levels. However, following FRAT1 knockdown, FRAT1 was
significantly downregulated in U251 cells compared with the
untransfected and NC groups (Fig.
5A; P<0.001). These results indicated successful silencing
of FRAT1 in U251 cells.
FRAT1 knockdown suppresses VEGFA
expression in human U251 glioblastoma cells
VEGFA is a major pro-angiogenic factor (5). To investigate the relationship
between FRAT1 and VEGFA, the mRNA and protein expression levels of
VEGFA were detected in untransfected, siFRAT1 and siNC U251 cells
and the concentration of VEGFA was measured in the cell
supernatant. VEGFA protein expression levels were detected via
western blotting. The results demonstrated that VEGF protein
expression levels were markedly reduced in siFRAT1 U251 cells
compared with untransfected and siNC U251 cells (Fig. 5B). Subsequently, the effects of
FRAT1 knockdown on the VEGFA mRNA expression level were assessed
via RT-qPCR. The results demonstrated that FRAT1 knockdown
significantly inhibited VEGFA mRNA expression in siFRAT1 U251 cells
compared with the untransfected and siNC U251 cells (Fig. 5C; P<0.001). The effects of
FRAT1 knockdown on VEGFA secretion were further confirmed by ELISA.
Silencing of FRAT1 significantly downregulated VEGFA levels in the
supernatants of siFRAT1 U251 cells compared with those of
untransfected and siNC-U251 cells (Fig. 5D; P<0.001). The ELISA results
were consistent with the results of the RT-qPCR and western
blotting. Furthermore, the aforementioned results displayed no
significant difference between the untransfected and siNC groups.
These results indicated that FRAT1 expression is associated with
VEGFA expression in U251 cells.
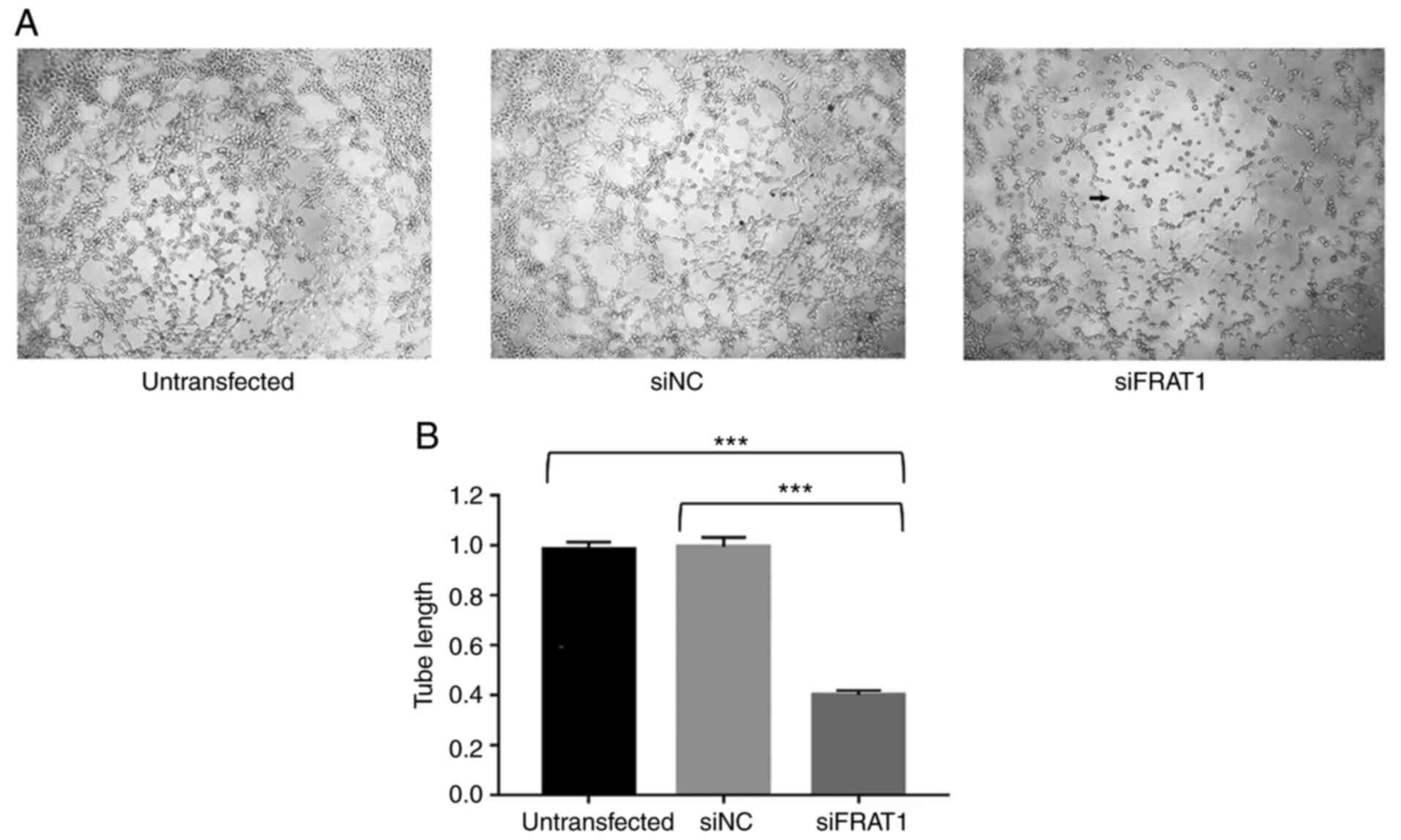
FRAT1 knockdown inhibits angiogenesis
in HUVECs cultured with U251 cell supernatants
Our previous study demonstrated that high FRAT1
expression is positively correlated with angiogenesis in human
glioma tissues (21), which
suggests that FRAT1 knockdown may be a promising method for
inhibiting glioma angiogenesis. However, the associated underlying
mechanism remains unclear. The effects of FRAT1 on angiogenesis
were confirmed using a tube formation assay, to determine whether
FRAT1 knockdown in human U251 glioblastoma cells impaired the
angiogenesis of HUVECs. In the present study, HUVECs were cultured
at 37°C for 6 h with supernatants collected from untransfected,
siFRAT1 and siNC U251 cells, after which tube formation assays were
conducted. For HUVEC angiogenic ability, the untransfected and siNC
groups displayed no significant differences. However, the
supernatants from the siFRAT1 U251 cells markedly impaired HUVEC
tube formation ability (Fig. 6A)
and relative changes in tube length were significantly decreased in
the siFRAT1 group, compared with the untransfected and siNC groups
(Fig. 6B; P<0.001). These
results indicated that FRAT1 knockdown in U251 cells may
significantly inhibit in vitro angiogenesis.
Discussion
Glioblastoma is one of the most highly vascularized
tumors that is characterized by microvascular proliferation and
endothelial cell hyperplasia (30). Patients with glioblastoma have a
poor prognosis and glioblastoma tumor growth and malignancy are
significantly correlated with angiogenesis (30). The postoperative survival rates of
patients with high tumor microvascular density are shorter than
those with low microvascular density (31). Since gliomas exist in a dormant
state when exceeding 1–2 mm3 without neovascularization
(32), angiogenesis is a
prerequisite to overcoming nutrient and oxygen deficiency and
removing waste products. Notably, these newly formed tumor vessels
are tortuous, disorganized, highly permeable and dilated, causing
irregular and inefficient perfusion. Angiogenesis results from an
imbalance between angiogenic stimulators and inhibitors. In
glioblastoma, a plethora of growth factors act as angiogenic
stimulators, including basic fibroblast growth factor,
angiopoietins, platelet-derived growth factor, IL-8 and hepatocyte
growth factor (32). However,
VEGFA is the major promoting stimulator of glioblastoma
angiogenesis and is directly correlated with poor prognosis and the
degree of malignancy (4). VEGFA
is upregulated in, and mainly secreted by, pseudo-palisading glioma
cells of necrotic areas. In a paracrine manner, it then activates
VEGFRs on vascular endothelial cells to promote their activation,
proliferation, migration and tube formation and to inhibit
apoptosis (33,34). Therefore, targeting VEGFA or
VEGFRs is a promising therapeutic approach for the treatment of
glioblastoma. Bevacizumab, a humanized monoclonal anti-VEGFA
antibody, has received approval from the United States Food and
Drug Administration for the treatment of glioblastoma (35). Bevacizumab prolongs
progression-free survival; however, there is no significant benefit
to patient overall survival (35). Therefore, the identification of
novel biomarkers and an increased understanding of VEGFA molecular
signaling pathways are required to enhance the clinical efficacy of
anti-VEGFA therapy.
The Wnt/β-catenin signaling pathway is highly
conserved in metazoan organisms and serves a critical role in
embryonic development and carcinogenesis (36,37). As a positive regulator of
Wnt/β-catenin signaling, FRAT1 is highly upregulated in various
human cancers, such as non-small cell lung cancer (NSCLC) (17,38), chondrosarcoma (39), ovarian cancer (18), colon cancer (19), gastric cancer (12) and esophageal cancer (20). This upregulation commonly leads to
high malignant potential, growth to the point of lethality, and
subsequently, poor patient prognosis in these diseases. However,
the role of FRAT1 in human gliomas remains to be elucidated. Our
previous study indicated that FRAT1 expression levels are highly
upregulated in human glioma tissues, but low or absent in healthy
brain tissues (40). Furthermore,
upregulated FRAT1 expression is closely correlated with
pathological grade, increased proliferation, invasiveness, reduced
apoptosis and poor prognosis in patients with glioma (21,41). Moreover, FRAT1 knockdown inhibits
cellular proliferation, migration and invasiveness and results in
G0/G1 cell cycle arrest in human glioblastoma
U251 cells, as well as suppressing tumorigenesis in nude mice
(23). It can therefore be
hypothesized that FRAT1 acts as an oncogene in human gliomas.
In the Wnt signaling pathway, FRAT1 directly
competes with axin for the same GSK-3β binding sites, resulting in
GSK-3β inactivation and dissociation from the destruction complex,
consisting of adenomatous polyposis coli (APC), axin, casein kinase
I and GSK-3β, thereby inhibiting GSK-3β-mediated phosphorylation of
β-catenin and its degradation (12,14,42,43). Subsequently, stabilized and
dephosphorylated β-catenin accumulates in the nucleus, where it
forms a transcriptional complex with T-cell factor
(TCF)4/lymphoid-enhancing factor 1 to activate oncogenic target
genes, such as cyclin D1 and c-myc (14,44,45). These aforementioned genes serve
important roles in the development and formation of various tumors.
Using immunohistochemistry, the upregulation of FRAT1 was
previously found to be significantly correlated with the aberrant
expression of β-catenin in human glioma tissues (21). Moreover, numerous studies have
reported that the expression levels of FRAT1 and β-catenin are
positively associated in human gastric adenocarcinoma cells, NSCLC,
ovarian cancer, colorectal cancer and esophageal cancer (18,20,44,46,47). Fan et al (48) also reported that knocking down
FRAT1 inhibited β-catenin, cyclin D1 and c-myc expression by
regulating the Wnt/β-catenin signaling pathway in hepatocellular
carcinoma cells. Low FRAT1 expression has also been demonstrated to
reduce β-catenin mRNA and protein expression levels in a glioma
stem cell xenograft mice model, where their expression levels were
positively correlated (49). It
can therefore be hypothesized that FRAT1 acts as an oncogene in
human glioblastoma via activation of the Wnt/β-catenin signaling
pathway.
There is increasing evidence to support the
significant role of the Wnt signaling pathway in angiogenesis in
both physiological and pathological conditions, including cancers.
This signaling pathway influences cellular proliferation, survival,
differentiation, migration and apoptosis (8–11)
and previous studies (50–52)
have reported that certain Wnt antagonists downregulate VEGFA
expression. For example, in hepatocellular carcinoma, blocking the
Wnt/β-catenin signaling pathway using Wnt inhibitory factor 1
(WIF-1) decreased microvessel density (MVD) and VEGFA secretion.
WIF-1 also inhibited the tube formation and migration of human
microvascular endothelial and mouse endothelial progenitor cells
(50). Dickkopf-related protein
1, a Wnt antagonist, has been demonstrated to markedly reduce the
expression levels of VEGFA and β-catenin in the retinal pigment
epithelium of very low-density lipoprotein receptor
(Vldlr)−/− mice, as well as in HUVECs transfected with
Vldlr siRNAs (51). R-spondin3
(Rspo3) is a potent activator of the Wnt/β-catenin signaling
pathway. Rspo3 has been reported to promote VEGFA expression and
in vitro angiogenesis via activation of the Wnt/β-catenin
signaling pathway, whereas disrupting Rspo3 was demonstrated to
induce vascular defects in the placenta and yolk sac (52).
Moreover, components of the Wnt/β-catenin signaling
pathway also regulate VEGFA secretion. β-catenin is the central
regulator of the Wnt/β-catenin pathway and ectopic activation of
β-catenin has been shown to upregulate VEGFA expression in colon
cancer (53,54). The VEGFA gene promoter contains
seven consensus binding sites for β-catenin/TCF and functional
deletion of APC increases VEGFA mRNA and protein expression by
activating β-catenin (54). It
can therefore be hypothesized that VEGFA is a direct target of the
β-catenin gene. Frizzled is a membrane receptor for Wnt ligands.
VEGFA mRNA expression is significantly reduced in the ovarian cells
of Fzd4−/− mice, whereas apoptosis is increased
(55). Furthermore, Wnt1 and
VEGFA protein expression levels are significantly upregulated in
atherosclerotic rats and are positively correlated (56). Moreover, the Wnt target gene c-myc
is a potent inducer of angiogenesis during embryonic development
and tumorigenesis, and in association with hypoxia-inducible
factor-1 (HIF-1), dysregulated c-myc promotes VEGFA expression and
angiogenesis (57). GSK-3β
downregulates β-catenin to inhibit differentiation, migration,
VEGFA expression and angiogenesis in HUVECs (58). Guo et al (59) reported that lithium increases the
levels of GSK-3β phosphorylation and promotes VEGFA secretion in a
dose-dependent manner, in both human brain microvascular
endothelial cells and primary rat cortical astrocytes in
vitro. Zhao et al (60) demonstrated that the overexpression
of GSK-3β not only suppresses the expression of HIF-1a, β-catenin
and VEGFA, but also inhibits proliferation and angiogenesis in
human glioma cells. Furthermore, by regulating HIF-1α expression
under normoxic conditions, aberrant activation of the Wnt/β-catenin
signaling pathway induces various other signaling cascades to
promote VEGFA expression, including the EGFR/PI3K/Akt and STAT3
signaling pathways (61).
Collectively, these findings suggest that VEGFA may be a promising
means of targeting the Wnt/β-catenin signaling cascade in
angiogenesis.
Our previous study demonstrated that the degree of
MVD was significantly associated with FRAT1 expression in human
glioma tissues (21), indicating
that FRAT1 may be a positive angiogenic regulator in human
glioma.
With the development of sequencing technology and
the production of large amounts of sequencing data, tumor gene
expression data analysis was enabled. In the present study, gene
expression analysis in the tumor was performed, which specifically
explored the role of genes in the tumor, from which valuable
biomarkers for diagnosis could be determined. To the best of our
knowledge, the role of FRAT1 and VEGFA in glioma, prognosis and
diagnosis, was investigated for the first time in the present
study, based on using the TCGA database and physiological and
biochemical methods. The results demonstrated that the expression
of FRAT1 and VEGFA in pan-cancer was similar to that seen in
certain previous studies (62,63). Furthermore, FRAT1 and VEGFA mRNA
expression levels were significantly increased in GBMLGG compared
with normal tissues. These results indicated that FRAT1 and VEGFA
are highly associated with the occurrence of GBMLGG.
Key terms identified presented in the differentially
expressed genes in FRAT1 and VEGFA GO enrichment analysis, such as
‘blood microparticle’, ‘immunoglobulin complex’, ‘extracellular
matrix structural constituent’, indicated that FRAT1 may be related
to the process of blood vessel formation. GSEA analysis of FRAT1
single gene correlation further explored the signaling pathway
involved with FRAT1, further supporting the relationship between
angiogenesis and tumorigenesis.
The aforementioned results indicated that FRAT1 may
be a positive angiogenic regulator in human glioma. To confirm this
hypothesis, the effects of FRAT1 knockdown on VEGFA expression and
angiogenesis were analyzed using human U251 glioblastoma cells. The
results demonstrated that FRAT1 knockdown significantly reduced the
mRNA and protein expression levels of VEGFA and decreased VEGFA
concentration in the cell supernatant. FRAT1 knockdown was also
found to markedly inhibit HUVEC angiogenesis, which confirmed the
results of our previous study. These results therefore indicated
that FRAT1 may therefore be an important mediator for glioma
angiogenesis that exerts its effects via VEGFA regulation.
In conclusion, the results of the present study
indicated that FRAT1 may serve an important role in the regulation
of angiogenesis via VEGFA regulation and that suppressing FRAT1 may
inhibit angiogenesis in human glioblastoma U251 cells. Furthermore,
this process may also involve the Wnt/β-catenin signaling pathway.
However, the primary limitation of the present study is the lack of
additional cell lines, including glioblastoma U87 cells, glioma
SHG44 cells and normal human brain cells to determine the effect of
FRAT1 on angiogenesis in glioblastoma. Despite this limitation, the
current findings have provided insights into the role of FRAT1,
which appears to be a novel and valuable biomarker for the
diagnosis and prognosis of human glioblastoma. Moreover, inhibiting
angiogenesis by targeting FRAT1 may be a promising option for
treating human glioblastoma. However, further investigation is
required to elucidate the association between FRAT1 and
angiogenesis in glioblastoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81201991), the China Postdoctoral
Science Foundation (grant no. 2015M571068 and 2016T90115) and the
Beijing Postdoctoral Research Foundation (grant no. 2015ZZ-56 and
2016ZZ-43).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BY, YQR, DL and GG contributed to the conception of
the study and contributed significantly towards data analysis and
manuscript preparation. BY, JPZ and GG performed the experiments.
YQS, XGW, YQW, SLW and SHG analyzed the bioinformatics data, and
participated in the conception and experimental design of the
project. BY and GG confirmed the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The use of primary HUVECs was approved by the ethics
committee of The First Hospital of Shanxi Medical University
[approval no. (2021) Y10].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2012-2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kargiotis O, Rao JS and Kyritsis AP:
Mechanisms of angiogenesis in gliomas. J Neurooncol. 78:281–293.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norden AD, Drappatz J and Wen PY:
Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol.
5:610–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Plate KH, Scholz A and Dumont DJ: Tumor
angiogenesis and anti-angiogenic therapy in malignant gliomas
revisited. Acta Neuropathol. 124:763–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hundsberger T, Reardon DA and Wen PY:
Angiogenesis inhibitors in tackling recurrent glioblastoma. Expert
Rev Anticancer Ther. 17:507–515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masckauchan TN and Kitajewski J:
Wnt/Frizzled signaling in the vasculature: New angiogenic factors
in sight. Physiology (Bethesda). 21:181–188. 2006.PubMed/NCBI
|
|
9
|
Zhang B and Ma JX: Wnt pathway antagonists
and angiogenesis. Protein Cell. 1:898–906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zerlin M, Julius MA and Kitajewski J:
Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 11:63–69.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parmalee NL and Kitajewski J: Wnt
signaling in angiogenesis. Curr Drug Targets. 9:558–564. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saitoh T and Katoh M: FRAT1 and FRAT2,
clustered in human chromosome 10q24.1 region, are up-regulated in
gastric cancer. Int J Oncol. 19:311–315. 2001.PubMed/NCBI
|
|
13
|
Jonkers J, Korswagen HC, Acton D, Breuer M
and Berns A: Activation of a novel proto-oncogene, Frat1,
contributes to progression of mouse T-cell lymphomas. EMBO J.
16:441–450. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hagen T, Cross DA, Culbert AA, West A,
Frame S, Morrice N and Reith AD: FRAT1, a substrate-specific
regulator of glycogen synthase kinase-3 activity, is a cellular
substrate of protein kinase A. J Biol Chem. 281:35021–35029. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferkey DM and Kimelman D: Glycogen
synthase kinase-3 beta mutagenesis identifies a common binding
domain for GBP and Axin. J Biol Chem. 277:16147–16152. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yost C, Farr GH III, Pierce SB, Ferkey DM,
Chen MM and Kimelman D: GBP, an inhibitor of GSK-3, is implicated
in Xenopus development and oncogenesis. Cell. 93:1031–1041. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Yu JH, Lin XY, Miao Y, Han Y, Fan
CF, Dong XJ, Dai SD and Wang EH: Overexpression of Frat1 correlates
with malignant phenotype and advanced stage in human non-small cell
lung cancer. Virchows Arch. 459:255–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Hewitt SM, Liu S, Zhou X, Zhu H,
Zhou C, Zhang G, Quan L, Bai J and Xu N: Tissue microarray analysis
of human FRAT1 expression and its correlation with the subcellular
localisation of beta-catenin in ovarian tumours. Br J Cancer.
94:686–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu K, Guo J, Wang H and Yu W: FRAT1
expression regulates proliferation in colon cancer cells. Oncol
Lett. 12:4761–4766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Liu S, Zhu H, Zhang W, Zhang G,
Zhou X, Zhou C, Quan L, Bai J, Xue L, et al: FRAT1 overexpression
leads to aberrant activation of beta-catenin/TCF pathway in
esophageal squamous cell carcinoma. Int J Cancer. 123:561–568.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo G, Zhong CL, Liu Y, Mao XG, Zhang Z,
Jin J, Liu J, Yang L, Mao JM, Guo YH and Zhao YL: Overexpression of
FRAT1 is associated with malignant phenotype and poor prognosis in
human gliomas. Dis Markers. 2015:2897502015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanzelmann S, Castelo R and Guinney J:
GSVA: Gene set variation analysis for microarray and RNA-Seq data.
BMC Bioinformatics. 14:72013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding J, Liang Z, Feng W, Cai Q and Zhang
Z: Integrated bioinformatics analysis reveals potential pathway
biomarkers and their interactions for clubfoot. Med Sci Monit.
26:e9252492020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Subramanian A, Kuehn H, Gould J, Tamayo P
and Mesirov JP: GSEA-P: A desktop application for gene set
enrichment analysis. Bioinformatics. 23:3251–3253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo G, Kuai D, Cai S, Xue N, Liu Y, Hao J,
Fan Y, Jin J, Mao X, Liu B, et al: Knockdown of FRAT1 expression by
RNA interference inhibits human glioblastoma cell growth, migration
and invasion. PLoS One. 8:e612062013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robinson RL, Sharma A, Bai S, Heneidi S,
Lee TJ, Kodeboyina SK, Patel N and Sharma S: Comparative
STAT3-regulated gene expression profile in renal cell carcinoma
subtypes. Front Oncol. 9:722019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Onishi M, Ichikawa T, Kurozumi K and Date
I: Angiogenesis and invasion in glioma. Brain Tumor Pathol.
28:13–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cea V, Sala C and Verpelli C:
Antiangiogenic therapy for glioma. J Signal Transduct.
2012:4830402012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chi AS, Sorensen AG, Jain RK and Batchelor
TT: Angiogenesis as a therapeutic target in malignant gliomas.
Oncologist. 14:621–636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Irizarry LR, Hambardzumyan D, Nakano I,
Gladson CL and Ahluwalia MS: Therapeutic targeting of VEGF in the
treatment of glioblastoma. Expert Opin Ther Targets. 16:973–984.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Macdonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Han Y, Zheng R, Yu JH, Miao Y,
Wang L and Wang EH: Expression of Frat1 correlates with expression
of beta-catenin and is associated with a poor clinical outcome in
human SCC and AC. Tumour Biol. 33:1437–1444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He L, Yang Z, Zhou J and Wang W: The
clinical pathological significance of FRAT1 and ROR2 expression in
cartilage tumors. Clin Transl Oncol. 17:438–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo G, Mao X, Wang P, Liu B, Zhang X,
Jiang X, Zhong C, Huo J, Jin J and Zhuo Y: The expression profile
of FRAT1 in human gliomas. Brain Res. 1320:152–158. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo G, Liu B, Zhong C, Zhang X, Mao X,
Wang P, Jiang X, Huo J, Jin J, Liu X and Chen X: FRAT1 expression
and its correlation with pathologic grade, proliferation, and
apoptosis in human astrocytomas. Med Oncol. 28:1–6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nager M, Bhardwaj D, Cantí C, Medina L,
Nogués P and Herreros J: β-catenin signalling in glioblastoma
multiforme and glioma-initiating cells. Chemother Res Pract.
2012:1923622012.PubMed/NCBI
|
|
43
|
Dajani R, Fraser E, Roe SM, Yeo M, Good
VM, Thompson V, Dale TC and Pearl LH: Structural basis for
recruitment of glycogen synthase kinase 3beta to the axin-APC
scaffold complex. EMBO J. 22:494–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jamieson C, Sharma M and Henderson BR: Wnt
signaling from membrane to nucleus: β-catenin caught in a loop. Int
J Biochem Cell Biol. 44:847–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sareddy GR, Panigrahi M, Challa S,
Mahadevan A and Babu PP: Activation of Wnt/beta-catenin/Tcf
signaling pathway in human astrocytomas. Neurochem Int. 55:307–317.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu Q, Shang LU, Yu H, Yang Z and Xu D:
Silencing of FRAT1 by siRNA inhibits the proliferation of SGC7901
human gastric adenocarcinoma cells. Biomed Rep. 4:223–226. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M,
Shao Z, Zhang F, Luo Y, Shen Z, et al: Epigenetic silencing of
miR-490-3p promotes development of an aggressive colorectal cancer
phenotype through activation of the Wnt/β-catenin signaling
pathway. Cancer Lett. 376:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fan WH, Du FJ, Liu XJ and Chen N:
Knockdown of FRAT1 inhibits hypoxia-induced
epithelial-to-mesenchymal transition via suppression of the
Wnt/β-catenin pathway in hepatocellular carcinoma cells. Oncol Rep.
36:2999–3004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo G, Liu J, Ren Y, Mao X, Hao Y, Zhong
C, Chen X, Wang X, Wu Y, Lian S, et al: FRAT1 enhances the
proliferation and tumorigenesis of CD133(+)Nestin(+) glioma stem
cells in vitro and in vivo. J Cancer. 11:2421–2430. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu J, Dong A, Fernandez-Ruiz V, Shan J,
Kawa M, Martínez-Ansó E, Prieto J and Qian C: Blockade of Wnt
signaling inhibits angiogenesis and tumor growth in hepatocellular
carcinoma. Cancer Res. 69:6951–6959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Y, Hu Y, Lu K, Flannery JG and Ma JX:
Very low-density lipoprotein receptor, a negative regulator of the
wnt signaling pathway and choroidal neovascularization. J Biol
Chem. 282:34420–34428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kazanskaya O, Ohkawara B, Heroult M, Wu W,
Maltry N, Augustin HG and Niehrs C: The Wnt signaling regulator
R-spondin 3 promotes angioblast and vascular development.
Development. 135:3655–3664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang X, Gaspard JP and Chung DC:
Regulation of vascular endothelial growth factor by the Wnt and
K-ras pathways in colonic neoplasia. Cancer Res. 61:6050–6054.
2001.PubMed/NCBI
|
|
54
|
Easwaran V, Lee SH, Inge L, Guo L,
Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, et
al: beta-Catenin regulates vascular endothelial growth factor
expression in colon cancer. Cancer Res. 63:3145–3153.
2003.PubMed/NCBI
|
|
55
|
Hsieh M, Boerboom D, Shimada M, Lo Y,
Parlow AF, Luhmann UFO, Berger W and Richards JS: Mice null for
frizzled4 (Fzd4-/-) are infertile and exhibit impaired corpora
lutea formation and function. Biol Reprod. 73:1135–1146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Du J and Li J: The role of wnt signaling
pathway in atherosclerosis and its relationship with angiogenesis.
Exp Ther Med. 16:1975–1981. 2018.PubMed/NCBI
|
|
57
|
Kim JW, Gao P, Liu YC, Semenza GL and Dang
CV: Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively
induce vascular endothelial growth factor and metabolic switches
hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol.
27:7381–7393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Skurk C, Maatz H, Rocnik E, Bialik A,
Force T and Walsh K: Glycogen-Synthase Kinase3beta/beta-catenin
axis promotes angiogenesis through activation of vascular
endothelial growth factor signaling in endothelial cells. Circ Res.
96:308–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo S, Arai K, Stins MF, Chuang DM and Lo
EH: Lithium upregulates vascular endothelial growth factor in brain
endothelial cells and astrocytes. Stroke. 40:652–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao P, Li Q, Shi Z, Li C, Wang L, Liu X,
Jiang C, Qian X, You Y, Liu N, et al: GSK-3beta regulates tumor
growth and angiogenesis in human glioma cells. Oncotarget.
6:31901–31915. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vallée A, Guillevin R and Vallée JN:
Vasculogenesis and angiogenesis initiation under normoxic
conditions through Wnt/β-catenin pathway in gliomas. Rev Neurosci.
29:71–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xiao SF, Tang HR, Bai Y, Zou RC, Ren ZF,
Wu XS, Shi ZT, Lan S, Liu W, Wu TG, et al: Swertiamarin suppresses
proliferation, migration, and invasion of hepatocellular carcinoma
cells via negative regulation of FRAT1. Eur J Histochem.
64:271–278. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tian W, Lei N, Guo R, Yuan Z and Chang L:
Long non-coding RNA DANCR promotes cervical cancer growth via
activation of the Wnt/β-catenin signaling pathway. Cancer Cell Int.
20:612020. View Article : Google Scholar : PubMed/NCBI
|