Introduction
Ischemia/reperfusion (I/R) is a serious,
life-threatening disease that can induce heart failure and other
adverse cardiovascular outcomes following myocardial ischemia,
cardiac surgery or circulatory arrest (1). The pathogenesis of I/R involves, at
least in part, inflammation, myocardial necrosis, apoptosis,
intracellular calcium overload and excess reactive oxygen species
production (2–4). However, challenges remain in
clinical practice.
Donepezil is a well-characterized reversible
acetylcholinesterase inhibitor with a protective effect against
neurodegenerative diseases, such as brain injury and Alzheimer's
disease, following cardiac I/R injury (5). Previous studies have demonstrated
that donepezil reduces myocardial I/R injury by balancing
mitochondrial dynamics, mitochondrial phagocytosis and autophagy;
in addition, it also markedly improved the long-term survival of
rats with chronic heart failure after extensive myocardial
infarction (6,7). Moreover, pretreatment with donepezil
counteracts TNF-α-induced endothelial cell permeability (8). However, to the best of our
knowledge, the role of donepezil in cardiac microvascular
endothelial cells (CMECs) has not yet been reported.
Furthermore, the potential role of the
poly(ADP-ribose) polymerase 1 (PARP1) signaling pathway in I/R
injury has been demonstrated in several studies. For instance,
inhibition of PARP1 activation and apoptosis-inducing factor (AIF)
nuclear translocation attenuates caspase-independent cell death in
a rat model of cerebral I/R (9).
Another study has demonstrated the protective effect of modulating
the PARP1/AIF signaling pathway in I/R-induced apoptosis (10). The oxygen-glucose
deprivation/reoxygenation (OGD/R) cell model has been widely used
to investigate the mechanisms of I/R injury (11,12). In addition, it has been suggested
that regulation of the TLR4/PARP1/NF-κB pathway can ameliorate
OGD/R injury (13). However, the
roles of donepezil and the PARP1 signaling pathway remain
unclear.
The aim of the present study was to investigate the
role of donepezil in I/R by establishing a model of OGD/R injury
using CMECs. In addition, the second objective was to determine
whether the protective effects of donepezil are mediated through
the PARP1/NF-κB signaling pathway.
Materials and methods
Blood samples
Blood samples were obtained intravenously from a
total of 30 cases (age range, 16–34; male: female, 2:1) between
April 2015 and January 2017 from the Cardiology Department of The
Rizhao Central Hospital in Shandong Province (Rizhao, China),
including 15 healthy controls (age range, 18–32; male: female, 2:1)
and 15 patients (age range, 16–34; male: female, 2:1) with a
confirmed diagnosis of coronary artery disease. The present study
was approved (approval no. 2020-018) by The Medical Ethics
Committee of Rizhao Central Hospital (Rizhao, China) and written
informed consent was obtained from patients for all samples. The
exclusion criteria was as follows: Patients with other comorbid
syndromes; patients under 18 years of age; or patients who were
unable to cooperate with the research. The samples were left to
stand for 30 min, then centrifuged at 4°C for 20 min at 1,000 × g
to obtain serum.
Cell culture
The human CMECs (cat no. CP-H079) were purchased
from Procell Life Science & Technology Co., Ltd. and cultured
in DMEM supplemented with 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.) and 100 µg/ml penicillin/streptomycin (Beyotime
Institute of Biotechnology) in a 5% CO2 incubator at
37°C. Then, cells were sub-cultured at a ratio of 1:2 when reaching
80–90% confluence and in passages two to three were used in cell
experiments. All the experimental protocol was approved (approval
no. 2020-018) by The Medical Ethics Committee of Rizhao Central
Hospital.
OGD/R injury
CMECs were incubated for 4 h to simulate ischemia by
deprivation of oxygen and glucose in serum/glucose-free DMEM in a
5% CO2, 95% N2 hypoxic chamber at 37°C. After
4 h of incubation, cells were cultured in normal DMEM supplemented
with 10% FBS under normoxic conditions for 12 h at 37°C to recover.
Different concentrations (25, 50 and 100 µM) of donepezil (cat no.
D6821; Sigma-Aldrich; Merck KGaA) were used to treat the cells for
24 h before OGD/R. Control cells were incubated under normoxic
conditions.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 (Shanghai Yusheng Biotechnology Co., Ltd.)
was used to detect cell viability. Briefly, 1×104 cells
were plated into 96-well plates, then 10 µl CCK-8 reagent was added
for 2 h at 37°C. The absorbance at 450 nm was obtained using a
spectrophotometer (Thermo Fisher Scientific, Inc.) as a measure of
cell viability.
Lactate dehydrogenase (LDH) activity
assay
Cytotoxicity was quantified using the LDH assay kit
(cat. no. A020-2-2; Nanjing Jiancheng Bioengineering Institute).
Briefly, the cells were centrifuged at 4°C for 5 min at 600 × g to
collect supernatant. The working solution from this kit was added
to the 96-well plate in this order and the plate was incubated at
37°C for 30 min according to the manufacturer's instructions. The
absorbance values were measured at 450 nm.
Cell transfection
PARP1 overexpression vector (Ov-PARP1; 50 nM) and
negative control vector (Ov-NC; 50 nM) were designed and amplified
by Shanghai GenePharma Co., Ltd. CMECs were seeded into 6-well
plates at a density of 1×106 cells/well, then
transfected with Ov-PARP1 or Ov-NC using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h at 37°C
according to the manufacturer's protocols. Successful transfection
was determined using reverse transcription-quantitative PCR
(RT-qPCR). Subsequent experiments were completed within 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was then reverse transcribed into cDNA using the Revert
Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. qPCR was performed
with 2 µg cDNA using iTaq™ Universal SYBR®
Green Supermix (Bio-Rad Laboratories, Inc.) on an ABI PRISM 7500
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used for qPCR:
Initial denaturation at 95°C for 5 min; followed by 40 cycles at
95°C for 10 sec, 55°C for 20 sec and 72°C for 20 sec, and final
extension step at 72°C for 2 min. The sequences of the PARP1 and
GAPDH primers were as follows: PARP1 forward,
5′-GGCGATCTTGGACCGAGTAG-3′ and reverse, 5′-AGCTTCCCGAGAGTCAGGAT-3′;
and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The mRNA levels of PARP1 were
normalized to those of endogenous control GAPDH and were calculated
using the 2−ΔΔCq method (14).
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.)
with 1% phenylmethanesulfonyl fluoride on ice, and the
concentration was determined using a BCA kit (cat. no. P0012S;
Beyotime Institute of Biotechnology). A mass of 30 µg of protein
was loaded per lane and separated using 6, 10 or 15% SDS-PAGE, and
then transferred to PVDF membranes. The membranes were then blocked
with 5% skimmed milk for 2 h at room temperature. Subsequently, the
membranes were incubated overnight at 4°C with primary antibodies
as follows: Anti-Bcl-2 (cat. no. 15071), anti-Bax (cat. no. 2774),
anti-caspase-3 (cat. no. 14220), anti-cleaved caspase-3 (cat. no.
9661), anti-zona occludens-1 (ZO-1; cat. no. 13663), anti-occludin
(cat. no. 91131), anti-vascular endothelial cadherin (VE-cadherin;
cat. no. 2158), anti-claudin-5 (1:3,000; cat. no. ab131259; Abcam),
anti-PARP1 (cat. no. ab191217; Abcam), NF-κB p65 (cat. no. 8242),
phosphorylated (p)-NF-κB p65 (p-NF-κB p65; cat. no. 3033) and
anti-GAPDH (cat. no. 5174). Following primary incubation, the
membranes were incubated with HRP-conjugated goat anti-mouse IgG2c
(cat. no. 56970) and goat anti-rabbit IgG2c (cat. no. 7074)
secondary antibodies for 1–2 h at room temperature. All antibodies
were purchased from Cell Signaling Technology, Inc. and used at 1
in 1,000 dilution unless otherwise indicated. The membranes were
visualized with enhanced chemiluminescence reagent (ECL System;
MilliporeSigma) and ImageJ software (version 1.8.0; National
Institutes of Health) was used to quantify the grayscale values.
Protein levels were normalized to those of GAPDH.
TUNEL assay
Apoptosis of CMECs was detected by a One Step TUNEL
assay kit (cat. no. C1088; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Briefly, following
fixation with 4% paraformaldehyde at room temperature for 15 min,
cells at the density of 1×106 cells/well were washed
twice with PBS in 24-well plates, then incubated with 50 µl TUNEL
reaction buffer for 1 h at 37°C in the dark. Subsequently, 1 µg/ml
DAPI was used for counterstaining in the dark at room temperature
for 15 min and cells were mounted using DAPI-containing mounting
medium (Vector Laboratories, Inc.). Images were captured using a
fluorescence microscope (magnification, ×200; Olympus Corporation),
and cells were counted in five randomly selected microscopic
fields.
Caspase-3 activity assay
Caspase-3 activity was measured in CMECs using a
caspase-3 activity assay kit (cat. no. C1116; Beyotime Institute of
Biotechnology). The cells were lysed on ice for 15 min and
centrifuged at 16,000 × g for 15 min at 4°C, then added to a
reaction buffer mixture containing the caspase-3-specific substrate
Ac-DEVD-pNA in 96-well plates to incubate for 1–2 h at 37°C. The
absorbance value was measured at a wavelength of 405 nm using a
spectrophotometer (Thermo Fisher Scientific, Inc.).
Human CMEC permeability assay
CMECs (1×104 cells/well) were seeded in
the upper chamber of a Transwell (Corning, Inc.) with 8-µm pores,
then incubated with 0.5 mg/ml fluorochrome-conjugated dextran (cat.
no. D1830; Thermo Fischer Scientific, Inc.) for 30 min at 37°C. 1
ml complete medium was added to the lower chamber. Fluorescence was
measured in the lower chamber at 595 and 615 nm using a plate
reader (Packard Bioscience Company).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 software (GraphPad Software, Inc.). One-way ANOVA
followed by Tukey's post hoc was used to examine the differences
between multiple groups. P<0.05 was considered to indicate a
statistically significant difference. The data are presented as the
mean ± SD of at least three independent experiments.
Results
Donepezil inhibits OGD/R-induced CMEC
injury
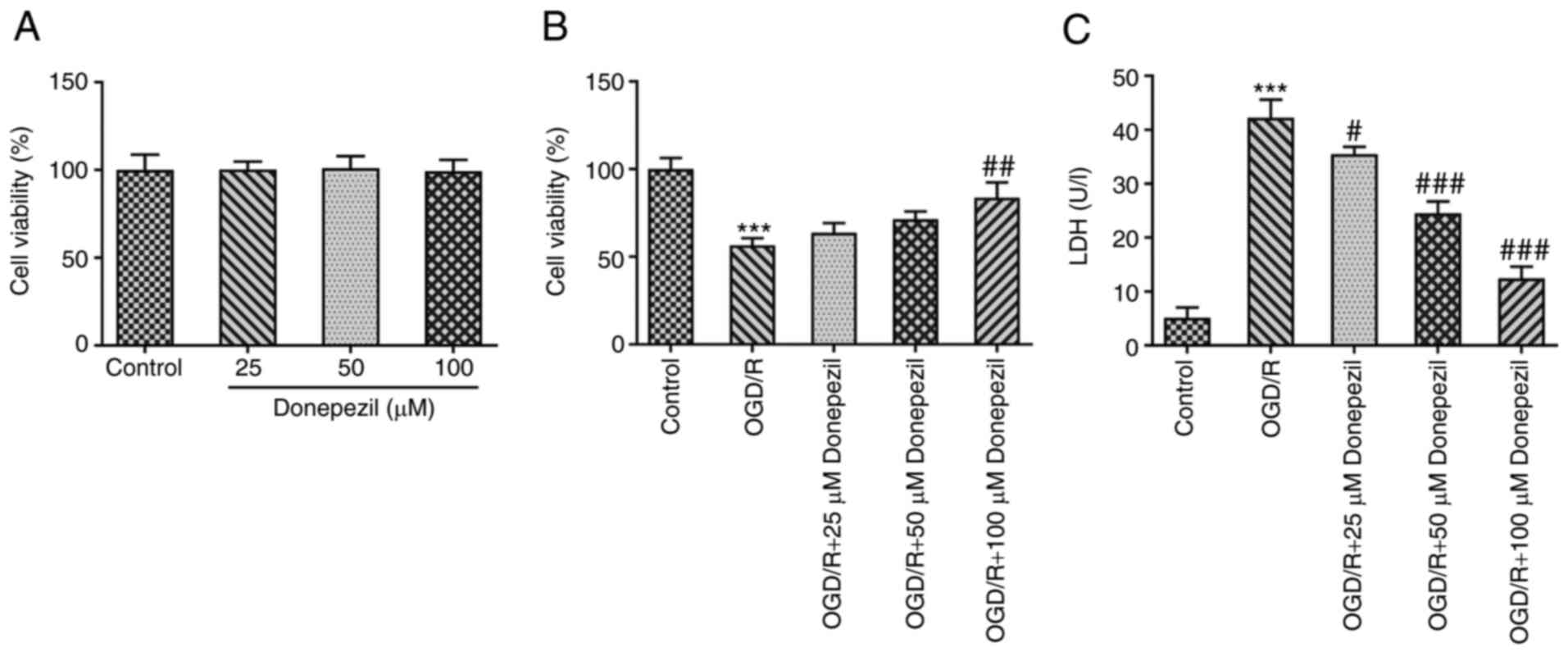
CMEC viability was detected using CCK-8 assays. A
gradient concentration of donepezil (25, 50 and 100 µM) was used to
pre-treat CMECs before OGD/R intervention. The results demonstrated
that no significant difference was present in cell viability
between each concentration alone (Fig. 1A). However, cell viability was
significantly reduced following OGD/R treatment compared with the
untreated group and a concentration-dependent increase was observed
after donepezil treatment compared with the OGD/R group (Fig. 1B). Furthermore, as shown in
Fig. 1C, LDH release (a measure
of cytotoxicity) was significantly increased following OGD/R
injury. In addition, treatment with different concentrations of
donepezil gradually reduced the release of LDH. As demonstrated in
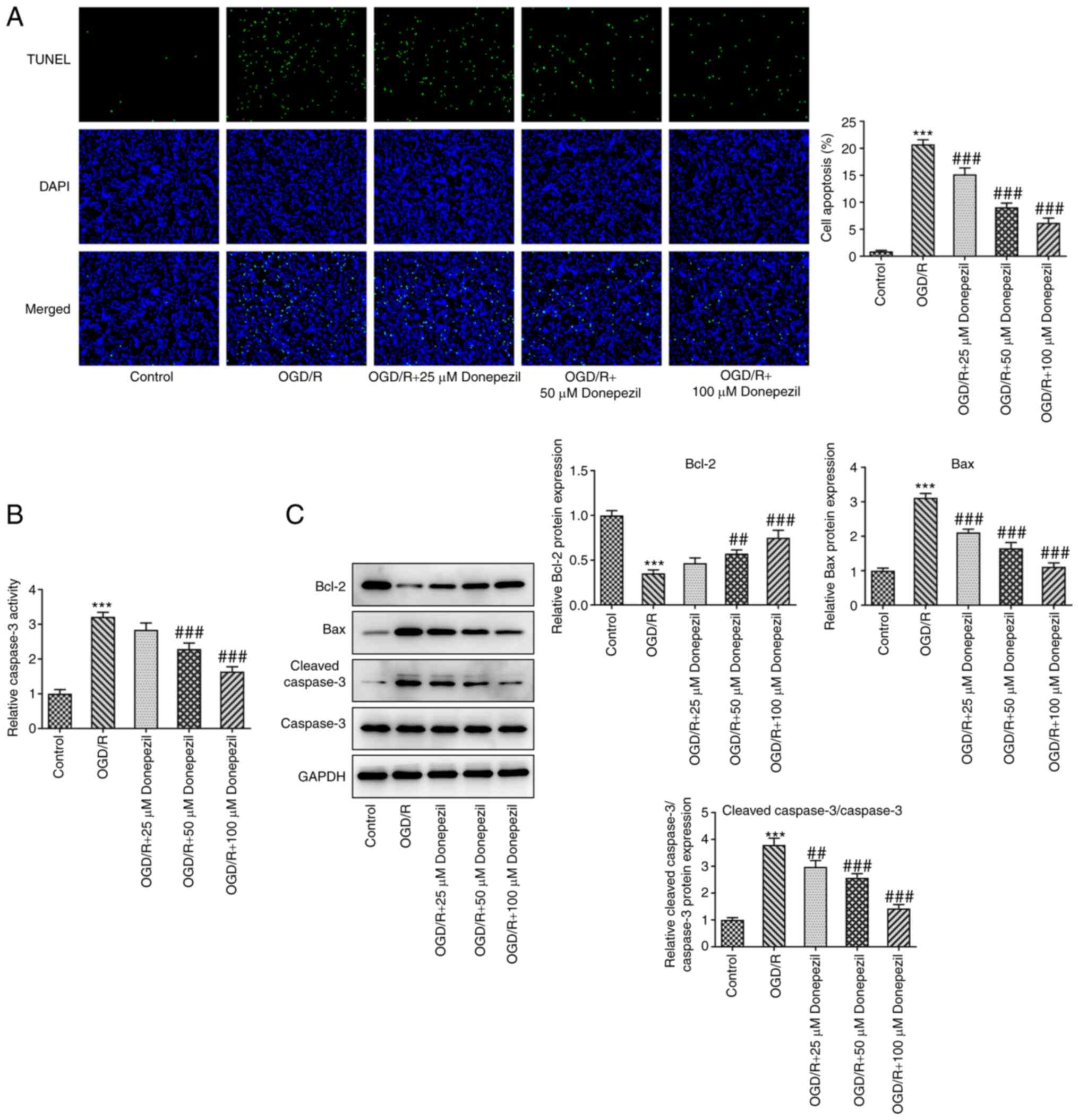
Fig. 2A and B, the apoptotic rate
and caspase-3 activity were both inhibited following donepezil
treatment compared with OGD/R alone. Moreover, donepezil
intervention significantly attenuated the expression of Bax and
cleaved caspase-3/caspase-3 proteins, which was accompanied by
upregulation of the Bcl-2 anti-apoptotic protein (Fig. 2C).
Donepezil ameliorates OGD/R-induced
dysfunction in CMECs
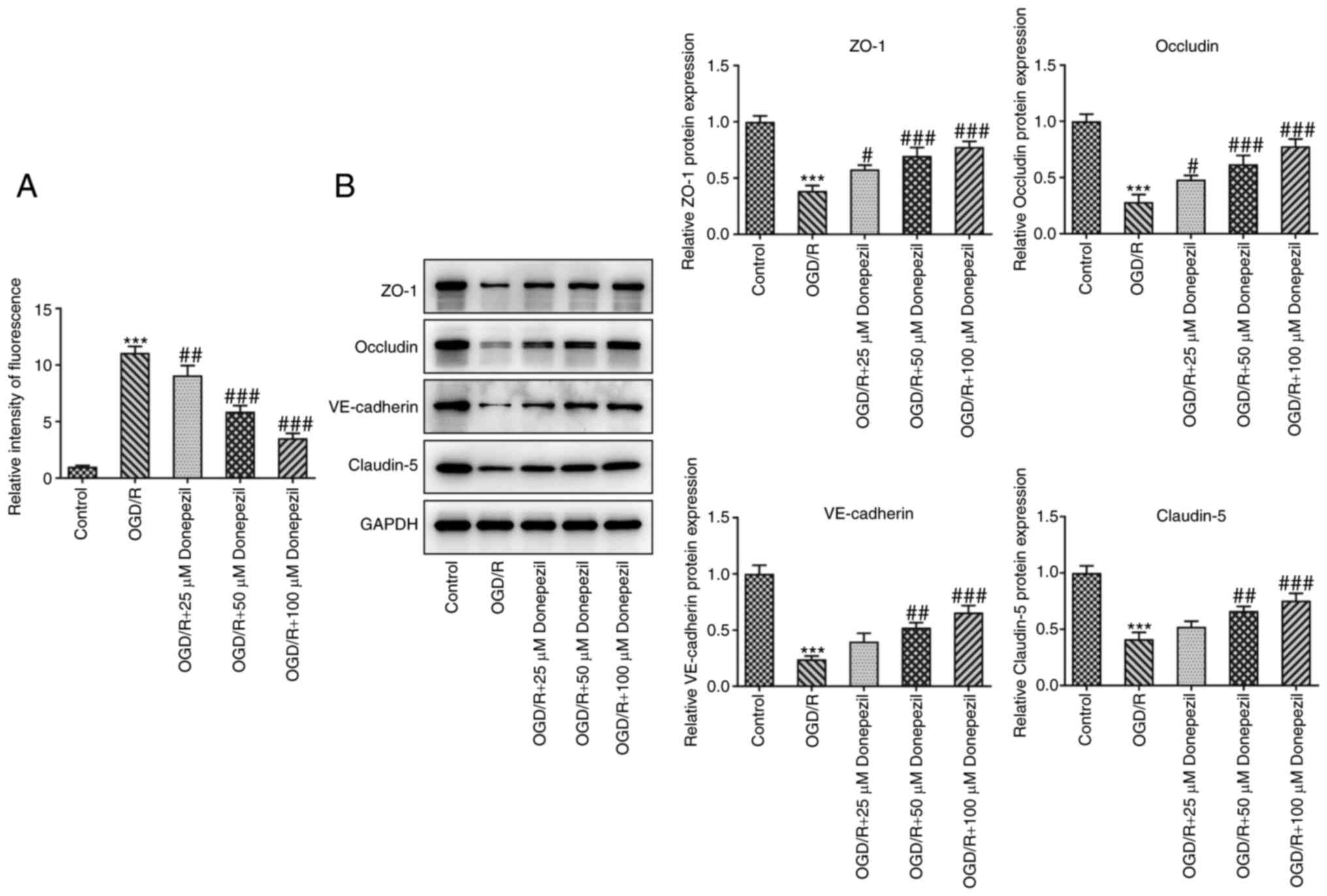
Cell permeability and the expression of tight
junction-associated proteins were analyzed to examine the function
of CMECs. As presented in Fig.
3A, treatment with donepezil reduced the increase in
permeability induced by OGD/R in a concentration-dependent manner,
indicating restoration of endothelial cell barrier function.
Similarly, the levels of tight junction-associated proteins,
including ZO-1, occludin, VE-cadherin and claudin-5, were
significantly reduced in OGD/R-treated cells compared with the
control group. However, the levels of these aforementioned proteins
were significantly increased following donepezil treatment
(Fig. 3B). These results
suggested that donepezil restored CMEC function.
Donepezil ameliorates OGD/R-induced
CMEC dysfunction via the PARP1/NF-κB signaling pathway
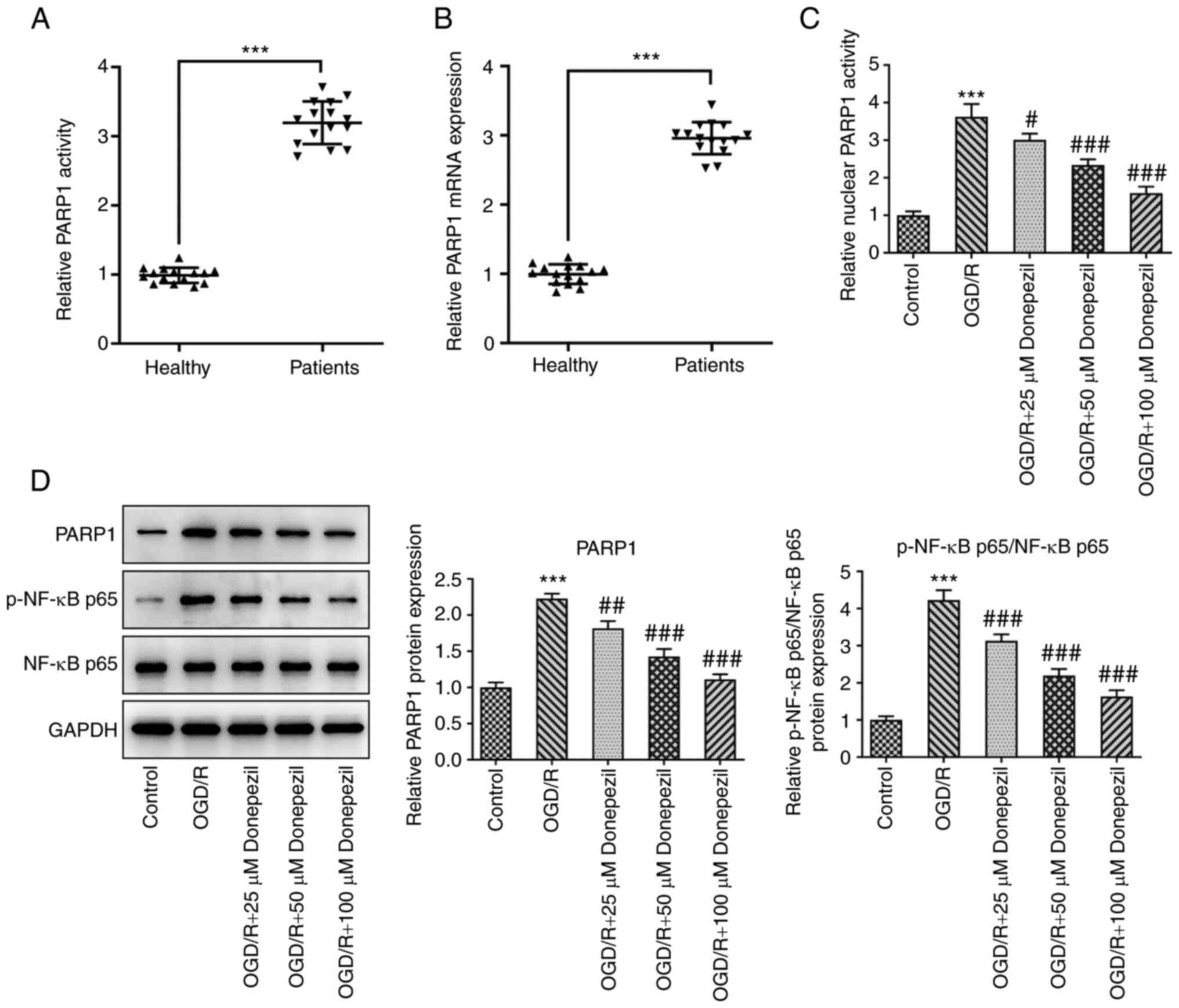
PARP1 expression levels were significantly higher in
serum from patients with myocardial infarction than in healthy
individuals (Fig. 4A and B).
Furthermore, the PARP1 expression levels and p-NF-κB p65/NF-κB p65
protein levels were significantly reduced in OGD/R-exposed CMECs by
donepezil compared with the OGD/R group (Fig. 4C and D), suggesting a potential
role for PARP1/NF-κB signaling. To further investigate the
mechanism of action of donepezil, 100 µM donepezil was used for
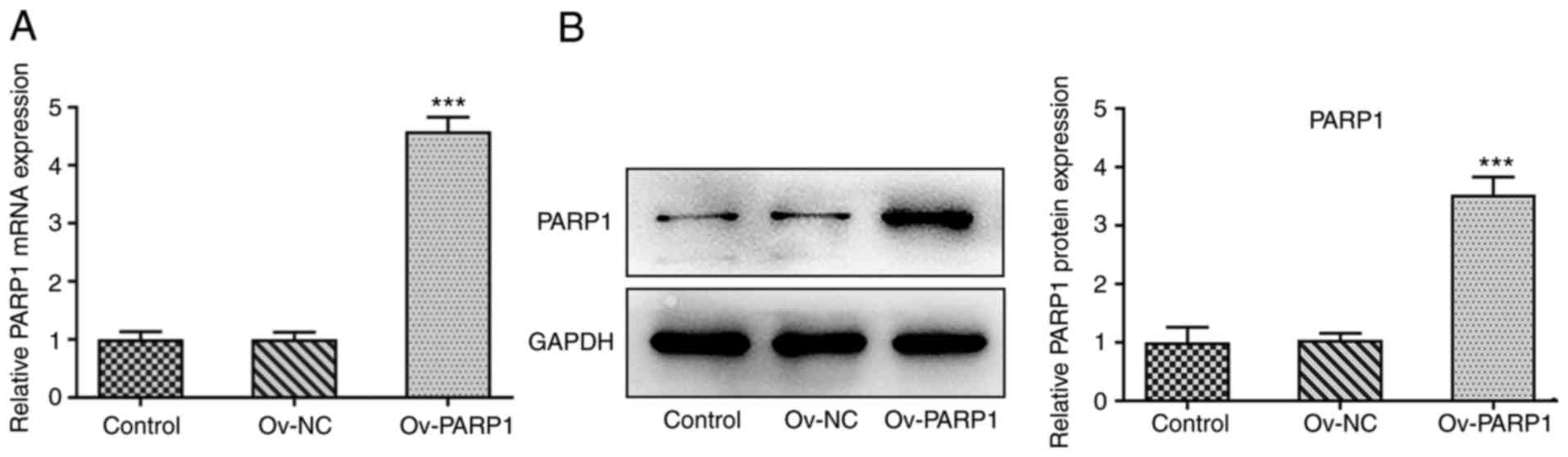
subsequent experiments. Ov-PARP1 was transfected into CMECs, which
led to PARP1 upregulation compared with the Ov-NC group, as
demonstrated by RT-qPCR and western blotting (Fig. 5). In addition, the protective
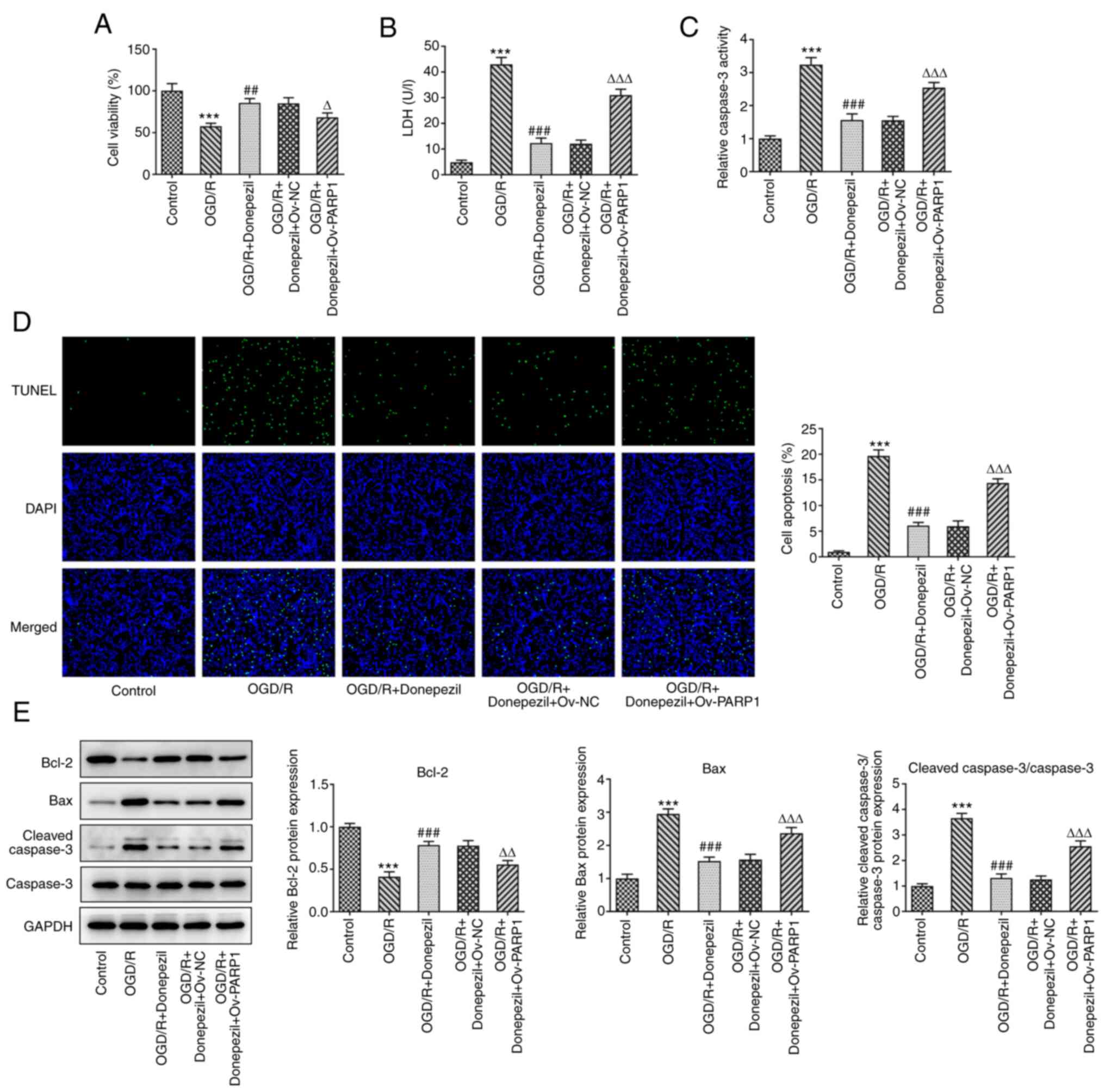
effect of donepezil on OGD/R-induced cell viability was reversed
following PARP1 overexpression (Fig.
6A). Treatment with donepezil in Ov-PARP1-transfected cells
significantly increased LDH release in CMECs compared with the
OGD/R + donepezil group (Fig.
6B). Similarly, apoptosis and caspase-3 activity were also
increased (Fig. 6C and D). In
addition, Bax and cleaved caspase-3 protein levels were
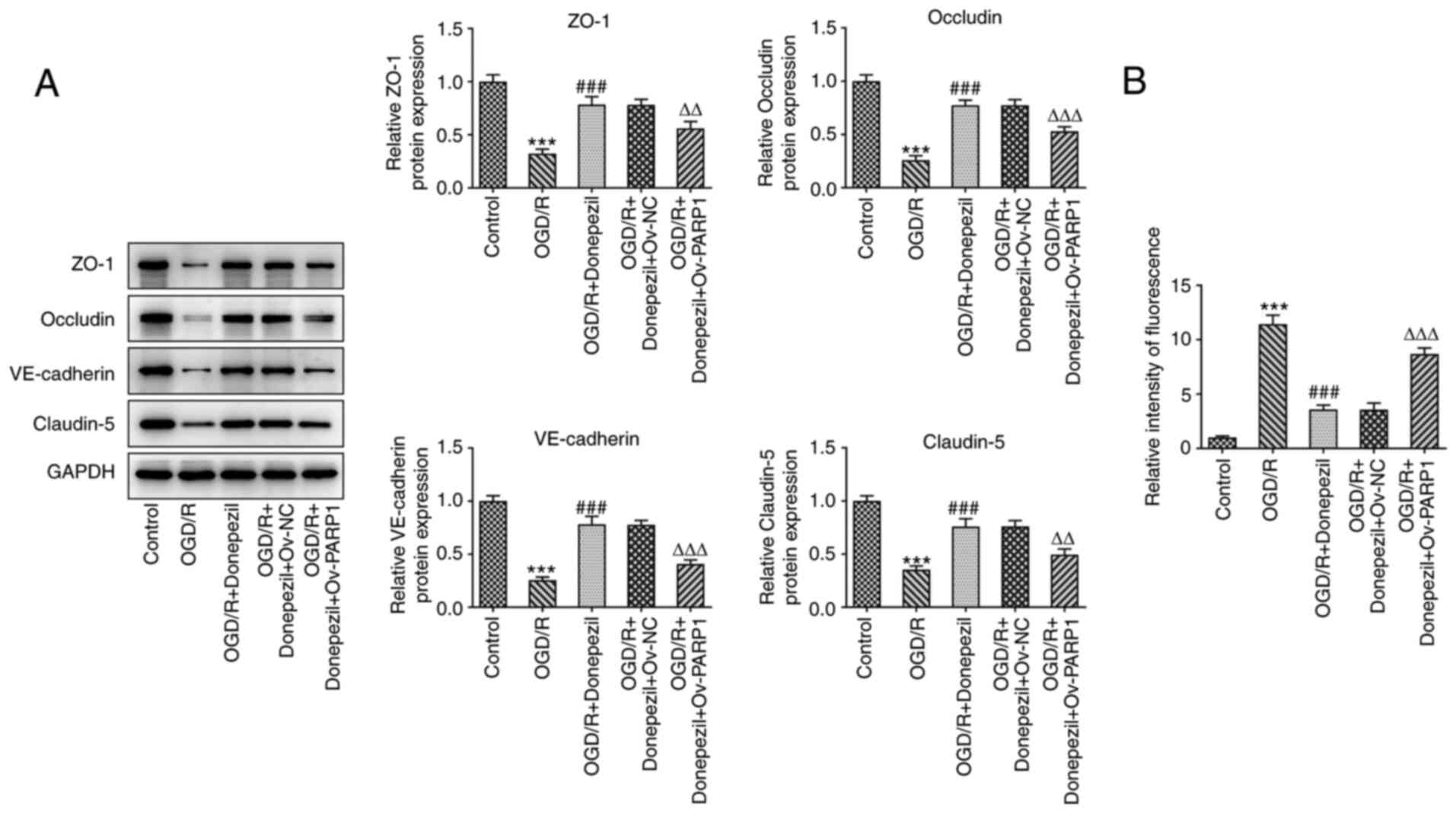
upregulated, while those of Bcl-2 were reduced (Fig. 6E). The expression levels of tight
junction-associated proteins were also significantly reduced after
Ov-PARP1 transfection, which was accompanied by a notable increase
in cell permeability. The barrier function of CMECs was disrupted
following PARP1 overexpression (Fig.
7). This suggested that donepezil protects CMECs from OGD/R
damage through the PARP1/NF-κB pathway.
Discussion
Myocardial I/R injury is a common public health
concern worldwide and numerous studies have demonstrated that
I/R-induced cardiac injury can be reduced following inhibition of
apoptosis (15,16). Furthermore, OGD/R-treated cells
have been studied in vitro to attenuate I/R-induced
apoptosis (17). In the present
study, the apoptotic rate and TUNEL-stained images were revealed to
be significantly increased following OGD/R, which was accompanied
by changes in the expression of apoptosis-associated proteins and
caspase-3 activity.
Donepezil, an acetylcholinesterase inhibitor,
enhances cholinergic neurotransmission by reversibly binding to
acetylcholinesterase enzyme and blocking acetylcholine hydrolysis
(18). Donepezil is approved for
the treatment of Alzheimer's disease (19,20). However, a large body of studies
have also reported a role for donepezil in ischemic or
cardiovascular diseases. For instance, a previous clinical trial
illustrated that treatment of acute ischemic stroke with donepezil
enhanced recovery (21).
Donepezil has also been shown to reduce I/R-induced brain damage
through inhibition of Ca2+ overload and antioxidation
(22), to inhibit apoptosis and
protect I/R renal function in mice (23), and to improve long-term survival
in rats with chronic heart failure after extensive myocardial
infarction (6). Moreover,
donepezil was demonstrated to protect rat primary cerebral cortical
neurons against OGD/R-induced injury (24). In addition, it has been documented
that donepezil plays a protective role against endothelial cell
injury (8,25,26). Notably, it was revealed for the
first time in the present study, to the best of our knowledge, that
donepezil had a protective effect against OGD/R-induced apoptosis
in a dose-dependent manner. Meanwhile, after donepezil pretreatment
in the presence of OGD/R, the permeability of CMECs was markedly
reduced and tight junction protein expression was increased,
suggesting a protective effect of donepezil on the barrier function
of CMECs.
PARP1 is upregulated following I/R injury (27). Furthermore, inhibition of the
PARP1 signaling pathway has been reported to serve a protective
role against I/R injury (28). A
previous study showed that hypoxic preconditioning protects human
brain endothelial cells from ischemic apoptosis (29). Donepezil may work in OGD/R-induced
CMEC by interfering with PARP1/NF-κB signaling. Consistent with the
expected results, OGD/R-induced PARP1 and p-NF-κB p65 expression
levels were found to be reduced following donepezil treatment. More
importantly, overexpression of PARP1 significantly reversed the
effects of donepezil on cell viability, apoptosis and cell barrier
function. Thus, the present study provided the first evidence that
donepezil affected cell function via the PARP1/NF-κB signaling
pathway, which was the highlight of the study.
In summary, the present findings suggested that
donepezil effectively protects against OGD/R injury by inhibiting
apoptosis and maintaining cell function via PARP1/NF-κB signaling
in CMECs. These results provided insight into the mechanisms
underlying I/R-induced microvascular endothelial cell disorders.
However, future studies are required to further demonstrate this
mechanism in an in vivo animal model to exclude the existing
limitations of in vitro studies, including the possible
effects of donepezil on processes such as cellular senescence.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Qingdao Pharmaceutical
Research Guidance Program (grant no. 2019-WJZD184).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and CY conceived and designed the study. YL, XS,
JZ and CY performed the experiments. XS, JZ and JW collected the
data and reviewed the manuscript. YL and XS drafted the manuscript.
YL, XS, JW and CY were responsible for analyzing the data. All
authors read and approved the final manuscript. YL, XS, JW and CY
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
2020-018) by The Medical Ethics Committee of Rizhao Central
Hospital (Rizhao, China) and written informed consent was obtained
from patients for all samples.
Patient consent for publication
The patients consented to the publication of their
data in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American heart association. Circulation.
131:e29–e322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frank A, Bonney M, Bonney S, Weitzel L,
Koeppen M and Eckle T: Myocardial ischemia reperfusion injury: From
basic science to clinical bedside. Semin Cardiothorac Vasc Anesth.
16:123–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toldo S, Mauro AG, Cutter Z and Abbate A:
Inflammasome, pyroptosis, and cytokines in myocardial
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
315:H1553–H1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ongnok B, Khuanjing T, Chunchai T,
Kerdphoo S, Jaiwongkam T, Chattipakorn N and Chattipakorn SC:
Donepezil provides neuroprotective effects against brain injury and
Alzheimer's pathology under conditions of cardiac
ischemia/reperfusion injury. Biochim Biophys Acta Mol Basis Dis.
1867:1659752021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li M, Zheng C, Kawada T, Inagaki M, Uemura
K, Shishido T and Sugimachi M: Donepezil markedly improves
long-term survival in rats with chronic heart failure after
extensive myocardial infarction. Circ J. 77:2519–2525. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khuanjing T, Palee S, Kerdphoo S,
Jaiwongkam T, Anomasiri A, Chattipakorn SC and Chattipakorn N:
Donepezil attenuated cardiac ischemia/reperfusion injury through
balancing mitochondrial dynamics, mitophagy, and autophagy. Transl
Res. 230:82–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang X, Di X and Liu Y: Protective effects
of donepezil against endothelial permeability. Eur J Pharmacol.
811:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li WH, Yang YL, Cheng X, Liu M, Zhang SS,
Wang YH and Du GH: Baicalein attenuates caspase-independent cells
death via inhibiting PARP-1 activation and AIF nuclear
translocation in cerebral ischemia/reperfusion rats. Apoptosis.
25:354–369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nan L, Xie Q, Chen Z, Zhang Y, Chen Y, Li
H, Lai W, Chen Y and Huang M: Involvement of PARP-1/AIF signaling
pathway in protective effects of gualou guizhi decoction against
ischemia-reperfusion injury-induced apoptosis. Neurochem Res.
45:278–294. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong L, Tang Y, An R, Lin M, Chen L and Du
J: RTN1-C mediates cerebral ischemia/reperfusion injury via ER
stress and mitochondria-associated apoptosis pathways. Cell Death
Dis. 8:e30802017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Wang T, Zhang Y, Li F, Yu B and
Kou J: Schizandrin protects against OGD/R-induced neuronal injury
by suppressing autophagy: Involvement of the AMPK/mTOR pathway.
Molecules. 24:36242019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Yang F, Li X, Wu T, Liu L and Song
H: Swertiamarin protects neuronal cells from oxygen glucose
deprivation/reoxygenation via TLR4/PARP1/NF-κB pathway. Pharmazie.
74:481–484. 2019.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun S and Wang P: Coptisine alleviates
ischemia/reperfusion-induced myocardial damage by regulating
apoptosis-related proteins. Tissue Cell. 66:1013922020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Tang C, Tan S, Duan J, Tian H and
Yang Y: Cardioprotective effect of isorhamnetin against myocardial
ischemia reperfusion (I/R) injury in isolated rat heart through
attenuation of apoptosis. J Cell Mol Med. 24:6253–6262. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ge L, Cai Y, Ying F, Liu H, Zhang D, He Y,
Pang L, Yan D, Xu A, Ma H and Xia Z: miR-181c-5p exacerbates
hypoxia/reoxygenation-induced cardiomyocyte apoptosis via targeting
PTPN4. Oxid Med Cell Longev. 2019:19579202019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seltzer B: Donepezil: An update. Expert
Opin Pharmacother. 8:1011–1023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adlimoghaddam A, Neuendorff M, Roy B and
Albensi BC: A review of clinical treatment considerations of
donepezil in severe Alzheimer's disease. CNS Neurosci Ther.
24:876–888. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Birks JS and Harvey RJ: Donepezil for
dementia due to Alzheimer's disease. Cochrane Database Syst Rev.
6:CD0011902018.PubMed/NCBI
|
|
21
|
Barrett KM, Brott TG, Brown RD Jr, Carter
RE, Geske JR, Graff-Radford NR, McNeil RB and Meschia JF; Mayo
Acute Stroke Trial for Enhancing Recovery (MASTER) Study Group, :
Enhancing recovery after acute ischemic stroke with donepezil as an
adjuvant therapy to standard medical care: Results of a phase IIA
clinical trial. J Stroke Cerebrovasc Dis. 20:177–182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang T, Lv P, Jin W, Zhang H, Lang J and
Fan M: Protective effect of donepezil hydrochloride on cerebral
ischemia/reperfusion injury in mice. Mol Med Rep. 9:509–514. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye W, Gong X, Xie J, Wu J and Zhang X,
Ouyang Q, Zhao X, Shi Y and Zhang X: AChE deficiency or inhibition
decreases apoptosis and p53 expression and protects renal function
after ischemia/reperfusion. Apoptosis. 15:474–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akasofu S, Kosasa T, Kimura M and Kubota
A: Protective effect of donepezil in a primary culture of rat
cortical neurons exposed to oxygen-glucose deprivation. Eur J
Pharmacol. 472:57–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kakinuma Y, Furihata M, Akiyama T, Arikawa
M, Handa T, Katare RG and Sato T: Donepezil, an
acetylcholinesterase inhibitor against Alzheimer's dementia,
promotes angiogenesis in an ischemic hindlimb model. J Mol Cell
Cardiol. 48:680–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou S, Li Z, Liu P, Wang S, Zhao J and
Zhang G: Donepezil prevents ox-LDL-induced attachment of THP-1
monocytes to human aortic endothelial cells (HAECs). Chem Res
Toxicol. 33:975–981. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagai W, Okita N, Matsumoto H, Okado H,
Oku M and Higami Y: Reversible induction of PARP1 degradation by
p53-inducible cis-imidazoline compounds. Biochem Biophys Res
Commun. 421:15–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen B, Mei M, Pu Y, Zhang H, Liu H, Tang
M, Pan Q, He Y, Wu X and Zhao H: Necrostatin-1 attenuates renal
ischemia and reperfusion injury via meditation of
HIF-1α/mir-26a/TRPC6/PARP1 signaling. Mol Ther Nucleic Acids.
17:701–713. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Park TS and Gidday JM: Hypoxic
preconditioning protects human brain endothelium from ischemic
apoptosis by Akt-dependent survivin activation. Am J Physiol Heart
Circ Physiol. 292:H2573–H2581. 2007. View Article : Google Scholar : PubMed/NCBI
|