Introduction
Breast cancer is the most common type of malignant
tumor in women. At present, advanced breast cancer can be treated
with different drug approaches, including chemotherapy drugs
(paclitaxel and anthracyclines), targeted therapy (trastuzumab) and
immunotherapy using immune checkpoint inhibitors (1–4).
However, multi-course chemotherapy can induce resistance of tumor
cells to chemotherapy drugs, leading to treatment failure and tumor
progression, which seriously affects the quality of life and
long-term survival of patients (5,6).
Research on the function and mechanism of non-coding
RNA (ncRNA) has garnered attention in the field of cancer (7). ncRNAs, including microRNAs
(miRNAs/miRs) and long ncRNAs (lncRNAs), are involved in different
levels of gene expression, including chromatin structure,
epigenetic memory, transcription, RNA splicing and translation
(8). It has been reported that
lncRNAs, as competing endogenous RNAs (ceRNAs), adsorb miRNAs
through sponge action, and subsequently regulate the expression of
downstream genes at the translational level, thus serving an
important regulatory role in the occurrence, development and
prognosis of cancer (9). Previous
studies have revealed that lncRNAs function as ceRNAs in breast
cancer cells, and affect invasion, metastasis, cell proliferation
and epithelial-mesenchymal transition (EMT) of breast cancer
(10–12). These findings have improved the
knowledge of the molecular mechanisms underlying the occurrence and
development of breast cancer.
The expression levels of lncRNA DDX11 antisense RNA
1 (DDX11-AS1) in colorectal cancer have been revealed to be
positively associated with lymphatic metastasis and TNM stage, and
to have a marked effect on the overall survival of patients with
colorectal cancer. Conversely, knockdown of DDX11-AS1 has been
shown to inhibit the proliferation, migration and invasion of
colorectal cancer cells, and to stimulate cell apoptosis (13). A previous study demonstrated that
DDX11-AS1 is highly expressed in esophageal cancer tissues, and
knockdown of DDX11-AS1 can inhibit DNA topoisomerase IIα
transcription by inhibiting TATA-box binding protein-associated
factor 1, thus reducing the sensitivity of esophageal cancer cells
to paclitaxel (PTX) and inhibiting tumor growth. Therefore,
DDX11-AS1 knockdown may be a promising therapeutic strategy for
esophageal cancer (14). However,
to the best of our knowledge, the effect of lncRNA DDX11-AS1 on
proliferation, migration and PTX resistance in breast cancer has
yet to be determined.
It has been reported that miR-497 expression is
markedly downregulated in breast cancer tissue samples and cell
lines (15). In other types of
cancer, miR-497 has a tumor-suppressive effect (15,16).
Furthermore, previous studies have shown that miR-497 may serve as
a biomarker for cancer prognosis (17,18).
Notably, the expression levels of miR-497 have been shown to be
associated with the chemoresistance phenotype of ovarian cancer,
and the overexpression of miR-497 may induce sensitivity of
resistant ovarian tumors to cisplatin treatment (19).
In the present study, the expression levels of
lncRNA DDX11-AS1 in breast cancer were detected, and its effects on
the proliferation and migration of breast cancer cells, as well as
PTX resistance, were assessed. Additionally, the present study
discussed the mechanism underlying these effects, in order to
provide a theoretical basis for targeted therapy of chemotherapy
resistance in breast cancer.
Materials and methods
Cell culture
MCF-10A human normal mammary cells, and MCF-7 and
MDA-MB-231 human breast cancer cell lines were obtained from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. The cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
in a humidified atmosphere containing 5% CO2 at 37°C.
The PTX-resistant MCF-7/PTX and MDA-MB-231/PTX cell lines were
generated by an intermittent and stepwise method (20). Briefly, the parental MCF-7 and
MDA-MB-231 cells were initially incubated with PTX [Beyotime
Institute of Biotechnology; the concentration of PTX used was the
IC50: 0.46 µmol/l in MCF-7 cells; 0.53 µmol/l in
MDA-MB-231 cells, as determined using a Cell Counting Kit-8 asay
(CCK-8)] under the same conditions as cell culture for 4 days at
37°C, and then cultured in drug-free medium for 3–4 days until
normal proliferative ability was recovered. PTX treatment was
performed six times during the induction period and the
PTX-resistant clones were then harvested. The MCF-7/PTX and
MDA-MB-231/PTX cell lines were subsequently cultured in complete
medium without PTX.
Datebase
The LncBase website (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex)
was used to indicate the binding site between lncRNA DDX11-AS1 and
miR-497.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cultured cells using
RNAzol® RT (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol. The concentration and purity of RNA were
measured using a Nanodrop 2000 (Nanodrop; Thermo Fisher Scientific,
Inc.). The RevertAid First Strand cDNA Synthesis kit (cat. no.
K1622; Thermo Fisher Scientific, Inc.) was used to synthesize cDNA
according to the manufacturer's protocol. The amplification
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 10 sec and 60°C for 60 sec, according to the
manufacturer's protocol, and then amplified in triplicate via qPCR
(cobas Z 480 system; Roche Diagnostics) using SYBR Green (final
reaction volume, 20 µl; Roche Diagnostics GmbH), according to the
manufacturer's protocol. The primer sequences used for qPCR were
obtained from GenScript. U6 was used as a reference gene for
normalization of DDX11-AS1 and miR-497. GAPDH was used as a
reference gene for normalization. The primer sequences used for
qPCR were as follows: DDX11-AS1 forward, 5′-CTGGCTACTCTTCCTCCTGG-3′
and reverse, 5′-CAGAGGACATGTGGGAGGTT-3′; miR-497 forward,
5′-GTGCAGGGTCCGAGGT-3′ and reverse, 5′-TAGCCTGCAGCACACTGTGGT-3′;
and U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. Fold-changes in lncRNA expression
and miR-497 were calculated using the 2−∆∆Cq method
(21).
Cell transfection
MCF-7, MDA-MB-231, MCF-7/PTX and MDA-MB-231/PTX
cells (5×105 cells/well) were seeded into 12-well
plates, and then transfected with short hairpin RNAs (shRNAs)
contained in a vector targeting DDX11-AS1 (shRNA-DDX11-AS1-1 and
shRNA-DDX11-AS1-2) and negative control (shRNA-NC), miR-497
inhibitors (miR-497 inhibitor-1 and miR-497 inhibitor-2, cat. no.
miR20004768-1-5; Guangzhou RiboBio Co., Ltd.) and scrambled
negative control (NC) sequences all at a concentration of 20 nM
(Shanghai GenePharma Co., Ltd.) for 6 h at 37°C. Cell transfection
was performed using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RT-qPCR was used to detect the transfection efficiency at
48 h after transfection. Cells were divided into control,
shRNA-DDX11-AS1, shRNA-DDX11-AS1 + inhibitor-NC and shRNA-DDX11-AS1
+ miR-497 inhibitor groups.
CCK-8 assay
Cell viability was measured using a CCK-8 assay
(Dojindo Molecular Technologies, Inc.), according to the
manufacturer's instructions. Cells were seeded into 96-well plates,
and trypsinized to prepare a single cell suspension with a density
of 2×103 cells/ml. Following incubation at 37°C for 48
h, 10 µl CCK-8 solution was added to each well, and cells were
further incubated for 2 h at 37°C. The absorbance was measured at
450 nm using a microplate reader (BioTek Instruments, Inc.). The Y
value for 50% suppression (IC50) was calculated
according to a linear formula (22).
MTT assay
Cell proliferation was determined using an MTT
assay. The treated cells were plated into 96-well plates
(5×103 cells/well) and incubated with 0.5 mg/ml MTT
(Sigma-Aldrich; Merck KGaA) for 3 h at 37°C. Subsequently, formazan
was dissolved with 600 µl DMSO for 15 min. The optical density was
measured using an enzyme-linked detector at a wavelength of 490
nm.
Colony formation assay
Cells were seeded into six-well plates at a density
of 200 cells/well and cultured at 37°C for 21 days until visible
colonies appeared. Subsequently, cells were washed with PBS three
times, fixed with methanol for 15 min at room temperature and
stained with 0.1% (w/v) crystal violet. Megascopic cell colonies
(>10 cells) were counted using Image-Pro Plus 5.0 software
(Media Cybernetics, Inc.).
Wound-healing assay
Cell migration was determined using a wound-healing
assay. Briefly, transfected cells were plated in 12-well plates at
a density of 1×105 cells/well. Once cells reached 80%
confluence, the medium was replaced with serum-free DMEM and cells
were incubated at 37°C overnight before initiating the experiment.
Subsequently, a wound was created on the surface of the cell
monolayer using a 200-µl pipette tip. The cells were then rinsed
twice with serum-free medium in order to remove free-floating cells
and debris. An inverted light microscope (magnification, ×20; BX51;
Olympus Corporation) was used to monitor cells at the edges of the
scratch. The percentage of wound closure was determined according
to the following equation: [(Ai-At)/Ai] ×100, where Ai represents
the initial area of the wound at 0 h and At represents the area of
the wound after 24 h.
Transwell assay
The 24-well Transwell chambers (pore size, 0.1 µm;
Costar; Corning, Inc.) precoated with 50 µl Matrigel (BD
Biosciences) at 37°C for 30 min were used to detect cell invasion.
Prior to the experiments, the upper and lower chambers were filled
with serum-free DMEM and with DMEM supplemented with 10% FBS,
respectively. Cells (1.0×105 cells/well) were seeded
into the upper chamber and were allowed to invade through the
membrane for 48 h at 37°C in an atmosphere containing 5%
CO2. Finally, the cells on the upper surface of the
membranes (non-invaded cells) were removed and the Transwell
filters were fixed with 10% cold methanol at room temperature for
15 min prior to staining with crystal violet (0.1% in 20% ethanol)
for 30 min at room temperature. Invaded cells were directly counted
using an Olympus light microscope (magnification, ×100; Olympus
BX51; Olympus Corporation) and the average cell counts in five
random fields were calculated.
Western blotting
Total protein was extracted from cells with RIPA
lysis buffer (Thermo Fisher Scientific, Inc.). Total protein was
quantified using a BCA protein assay kit (Bio-Rad Laboratories,
Inc.). Equal amounts (30 µg) of protein samples were separated by
SDS-PAGE on 10% gels and then electrophoretically transferred onto
PVDF membranes (EMD Millipore). The PVDF membranes were incubated
with primary antibodies overnight at 4°C after blocking with 10%
non-fat milk for 1 h at room temperature. Subsequently, the PVDF
membranes were incubated with HRP-conjugated secondary antibody
(1:5,000; cat. no. ab150077; Abcam) at room temperature for 2 h.
Protein signals were detected using a chemiluminescence detection
kit (Amersham; Cytiva) and a semi-quantitative analysis was
conducted using ImageJ software (version 1.8.0; National Institutes
of Health). The following primary antibodies were purchased from
Abcam: Anti-matrix metalloproteinase (MMP)2 (1:1,000; cat. no.
ab92536), anti-MMP9 (1:1,000; cat. no. ab76003), anti-N-cadherin
(1:1,000; cat. no. ab76011) and anti-GAPDH (dilution, 1:1,000; cat.
no. ab181602) antibodies.
Immunofluorescence
For staining of E-cadherin protein, the cells were
fixed in 4% paraformaldehyde/PBS for 20 min and permeabilized with
0.1% Triton X-100/PBS for 3 min at room temperature. Subsequently,
cells were blocked in 2% goat serum plus 1% BSA (both from Thermo
Fisher Scientific, Inc.) in PBS for 30 min at room temperature,
incubated with primary antibody at 4°C overnight, and then
incubated with secondary antibody conjugated to Alexa Fluor 488
(cat. no. A32731; Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature for 2 h. The nuclei were counterstained with DAPI.
The primary antibody used was anti-E-cadherin (1:200; cat. no.
ab40772; Abcam). Images were acquired using a light Leica DM5500B
upright microscope (Leica Microsystems GmbH) with a Retiga SRV
Cooled CCD camera (Teledyne Photometrics) and ImagePro Plus
software (version 6.0; Media Cybernetics, Inc.).
Luciferase reporter gene assay
The interaction between DDX11-AS1 and miR-497 was
predicted using LncBase (version 5.0; http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex).
The interaction was validated using a dual-luciferase reporter
system (cat. no. D0010; Beijing Solarbio Science & Technology
Co., Ltd.). The luciferase vectors containing wild-type (WT) and
mutant (MUT) binding sites of DDX11-AS1 were constructed using the
pGL3 luciferase vector (Promega Corporation). miR-497 mimic and
mimic negative control were co-transfected with 20 nM DDX11-AS1-WT
or DDX11-AS1-Mut into MCF-10A cells (1.0×105 cells/well)
using Lipofectamine 2000 reagent according to the manufacturer's
protocol. After transfection for 48 h, the relative luciferase
activity was measured using a microplate reader (BD Biosciences)
and normalized to Renilla luciferase activity, which was
measured using a Renilla luciferase activity kit (pRL-TK;
Invitrogen; Thermo Fisher Scientific Inc.). The mimic sequences
were as follows: Mimic negative control, sense
UUCUCCGAACGUGUCACGUTT, antisense ACGUGACACGUUCGGAGAATT; miR-497
mimic, sense CAGCAGCACACUGUGGUUUGU, antisense
AAACCACAGUGUGCUGCUGUU.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS v22.0 statistical software (IBM Corp.) was used for all
statistical analyses. Comparisons among groups were analyzed by
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference. Each
experiment was repeated three times.
Results
Expression levels of DDX11-AS1 in
PTX-resistant breast cancer cell lines
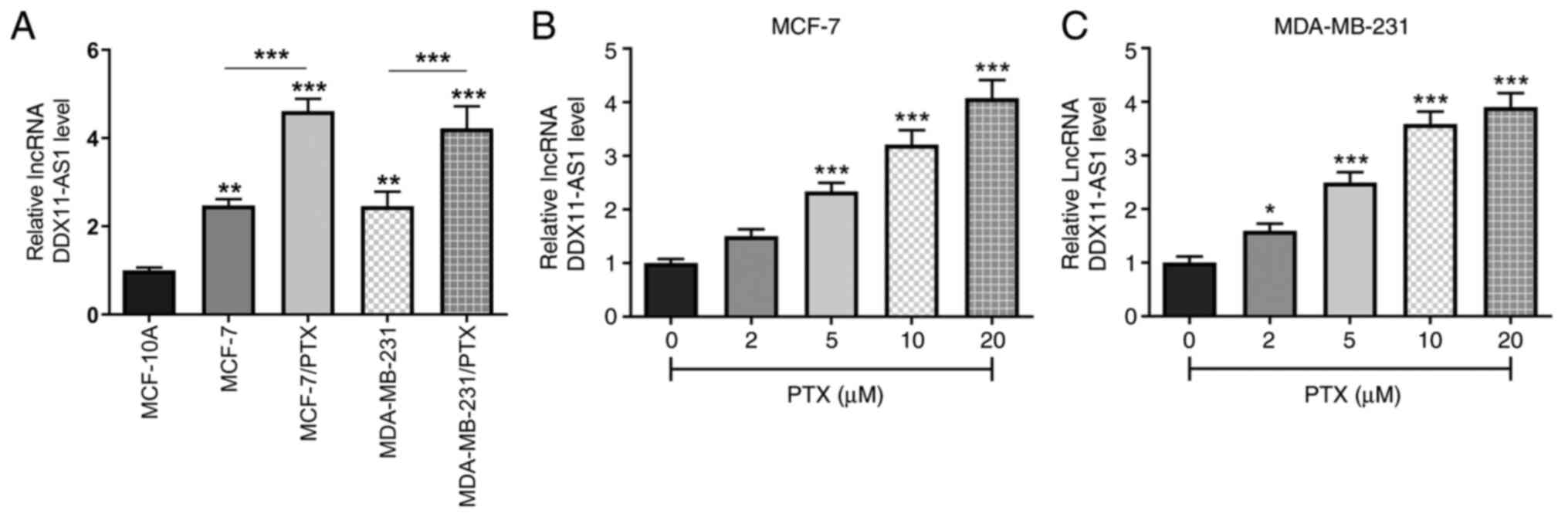
The expression levels of DDX11-AS1 in MCF-10A,
MCF-7, MDA-MB-231, and PTX-resistant MCF-7/PTX and MDA-MB-231/PTX
cell lines were detected by RT-qPCR (Fig. 1A). It was revealed that DDX11-AS1
expression was markedly increased in breast cancer cell lines
compared with that in MCF-10A cells. Compared with those in MCF-7
cells, the expression levels of DDX11-AS1 in MCF-7/PTX cells were
further increased, which was consistent with the expression levels
detected in MDA-MB-231/PTX cells. Subsequently, the expression
levels of DDX11-AS1 in MCF-7 and MDA-MB-231 cells treated with
different concentrations of PTX were detected by RT-qPCR, and it
was revealed that with increasing PTX concentration, the expression
levels of DDX11-AS1 in breast cancer cells were increased (Fig. 1B and C).
Knockdown of DDX11-AS1 reduces the
sensitivity of drug-resistant breast cancer cells to PTX
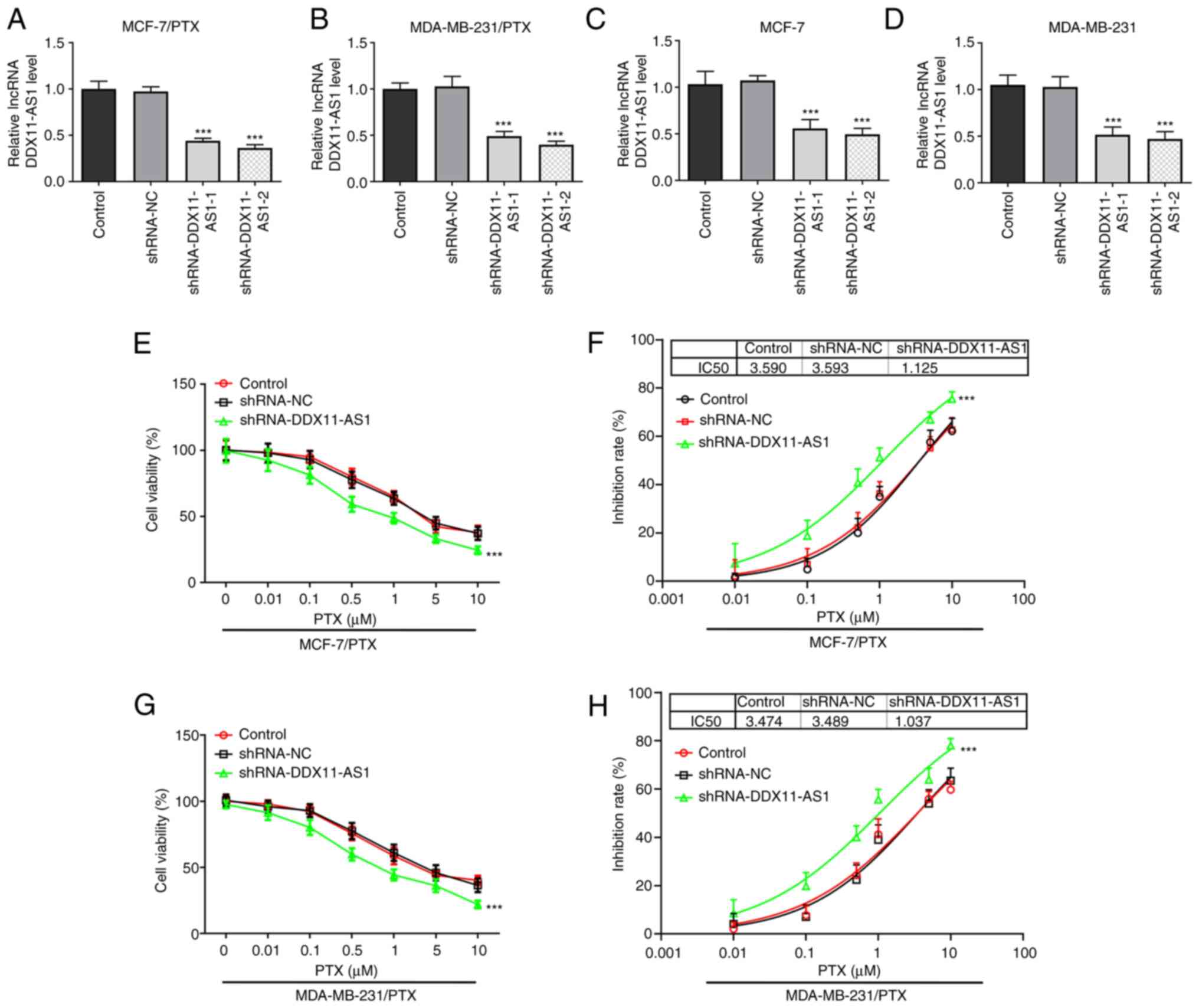
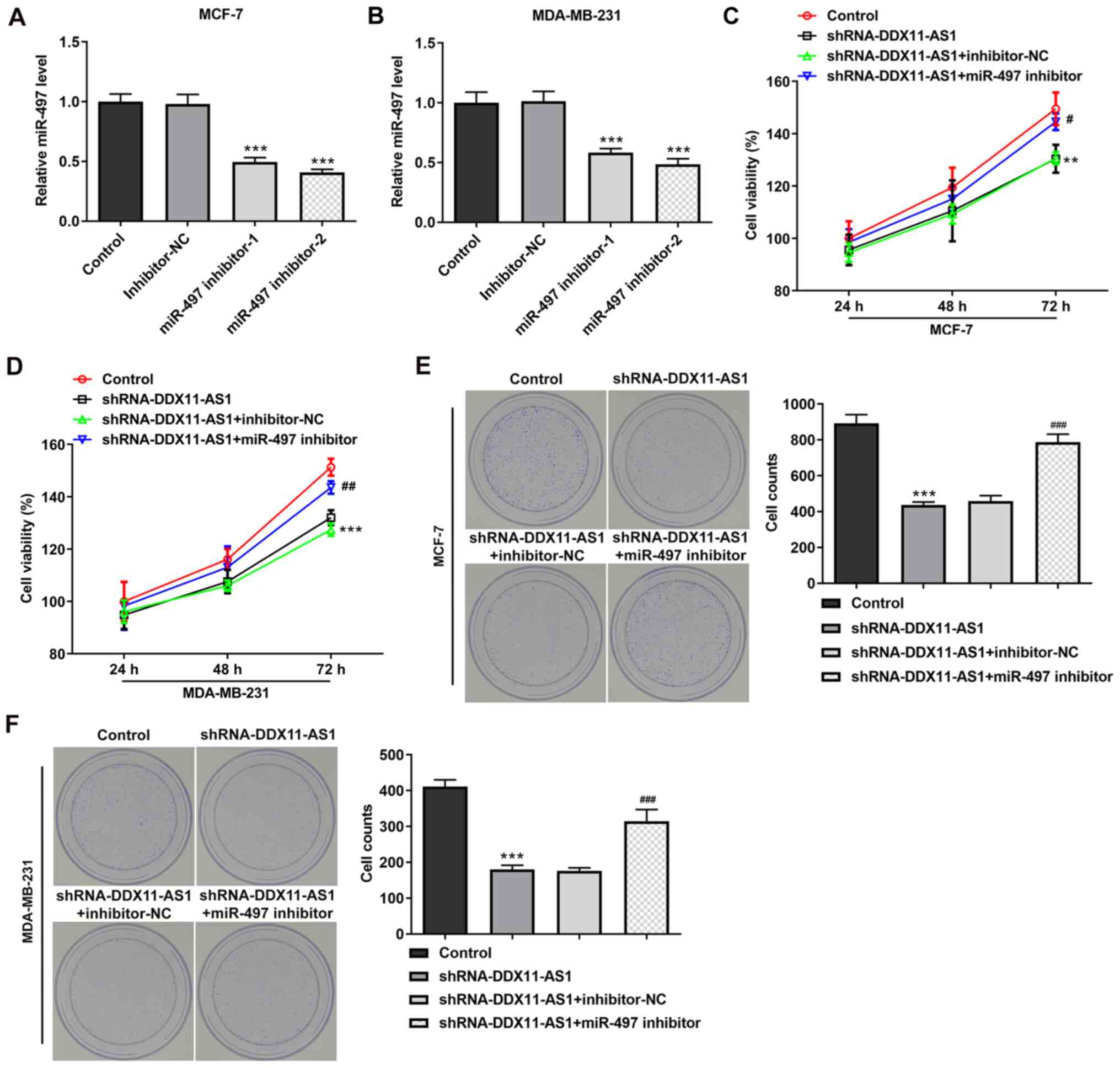
Cell transfection was used to knockdown DDX11-AS1
expression in two PTX-resistant cell lines, and MCF-7 and
MDA-MB-231 cells. Cell transfection efficiency was detected by
RT-qPCR (Fig. 2A-D).
shRNA-DDX11-AS1-2 was selected for the subsequent experiments. The
cells were divided into control, shRNA-NC and shRNA-DDX11-AS1
groups. Notably, a 3-fold reduction in PTX IC50 was
observed in the shRNA-DDX11-AS1 groups compared with that in the
shRNA-NC groups in MCF-7/PTX (Fig. 2E
and F) and MDA-MB-231/PTX (Fig. 2G
and H) cells.
Knockdown of DDX11-AS1 inhibits the
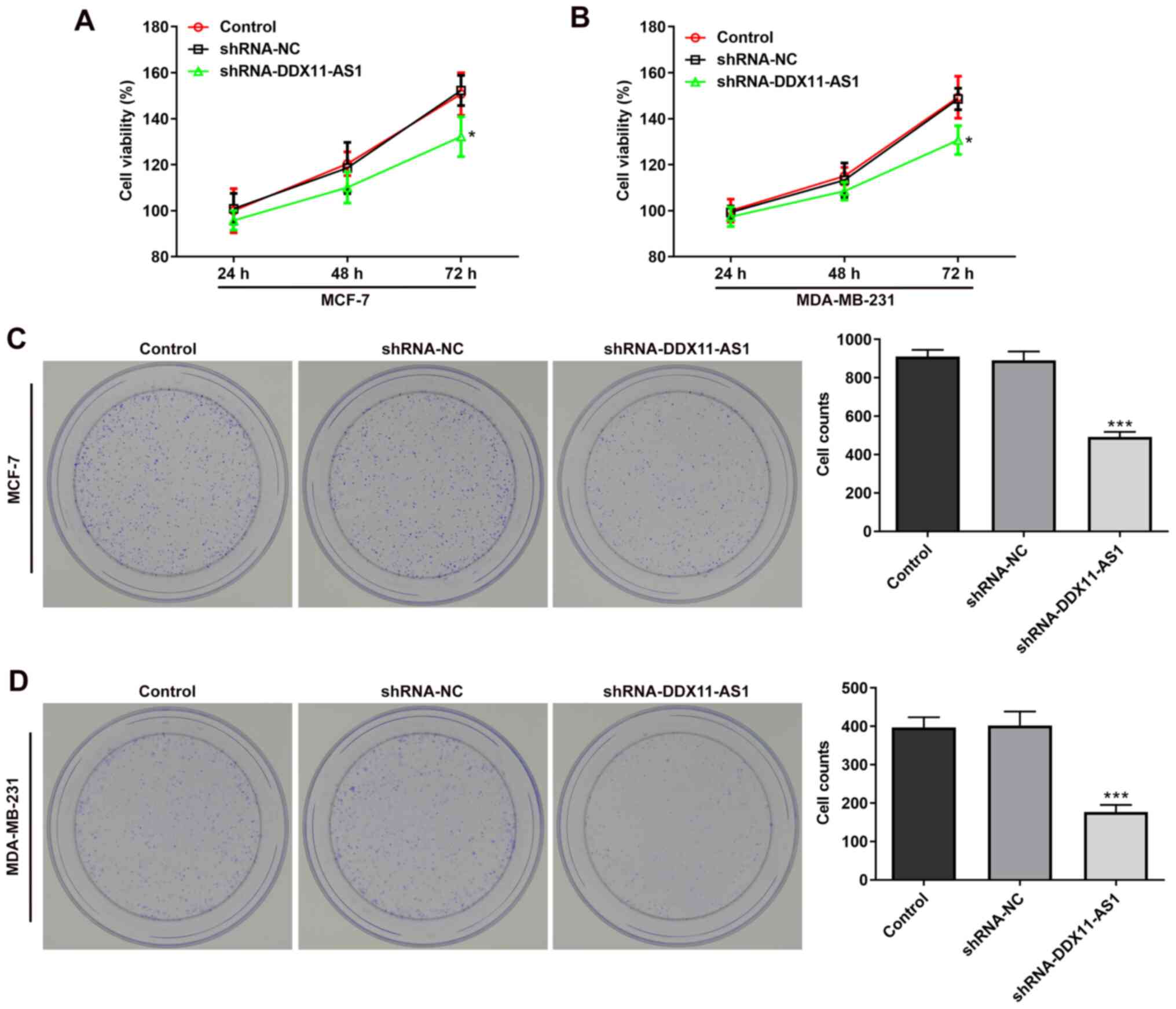
proliferation of breast cancer cell lines
MTT (Fig. 3A and B)
and colony formation (Fig. 3C and
D) assays demonstrated that the viability of cells in the
shRNA-DDX11-AS1 group was markedly decreased compared with that of
parental cells in the shRNA-NC group. These results demonstrated
that knockdown of DDX11-AS1 inhibited the proliferation of breast
cancer cells.
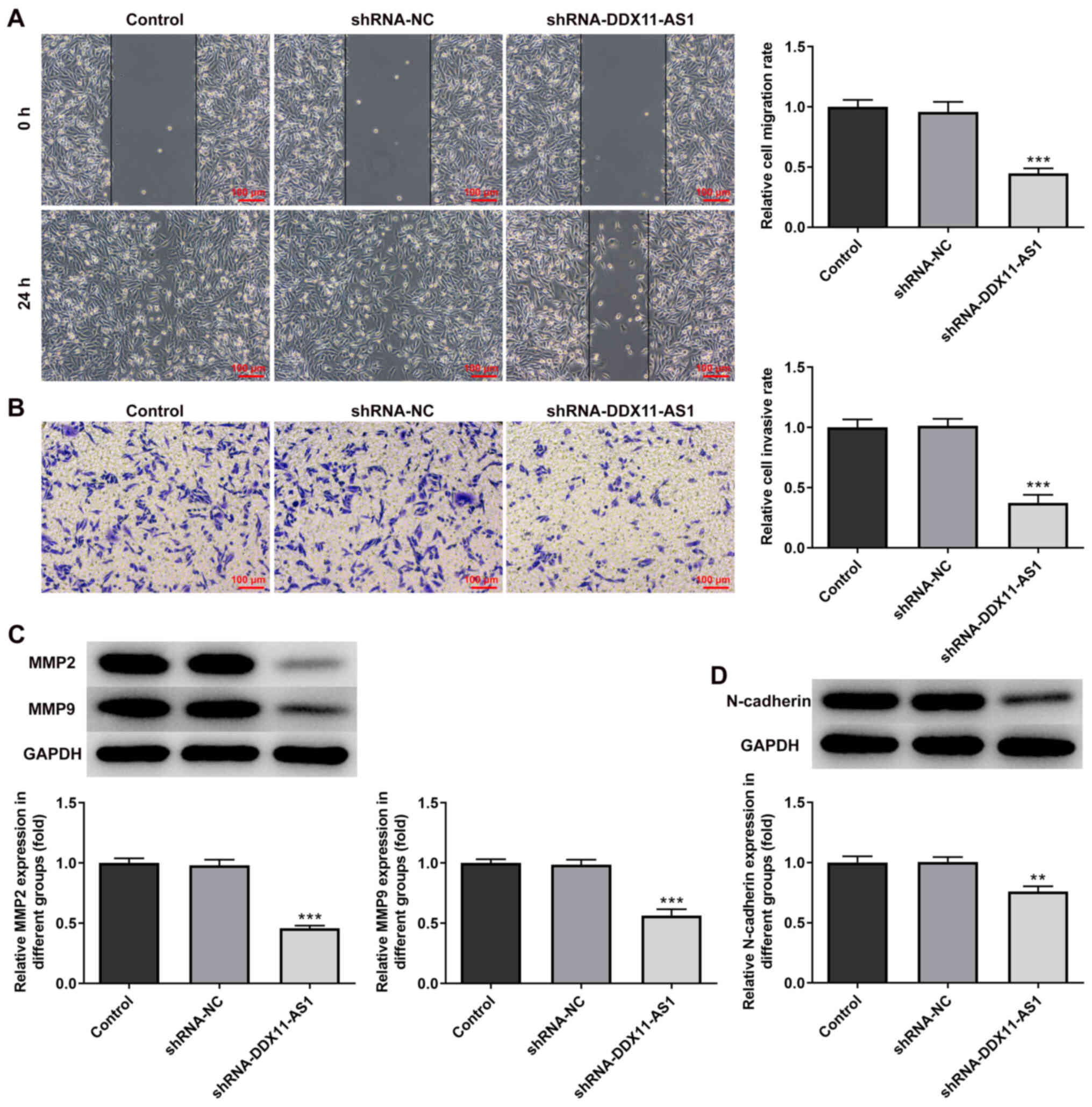
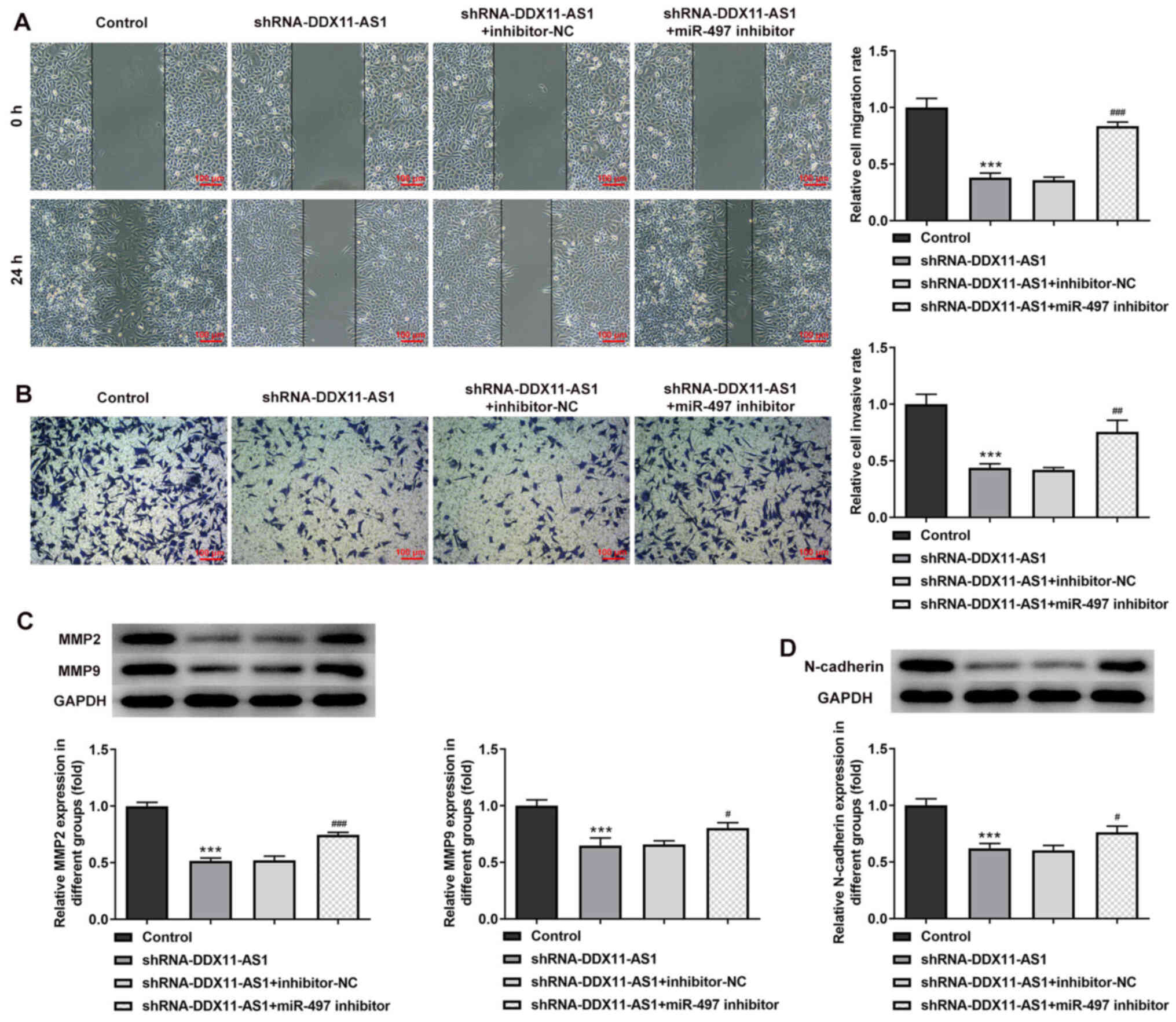
Knockdown of DDX11-AS1 inhibits the
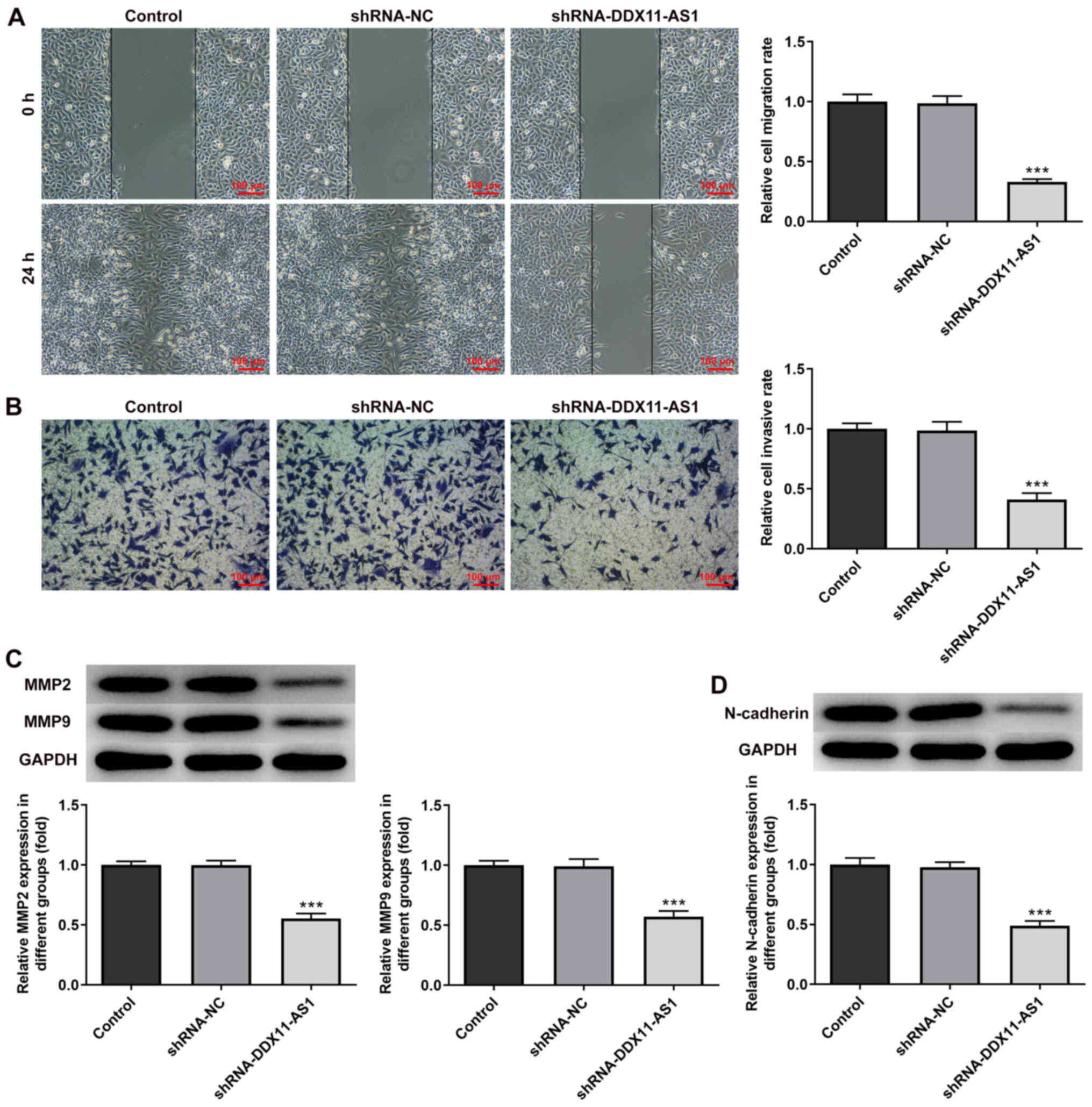
invasion and migration of breast cancer cells
The present study used wound-healing and Transwell
assays to detect the levels of cell migration and invasion. In
MCF-7 cells, the results of the wound-healing assay revealed that
compared with that of the shRNA-NC group, the cell migration
ability of the shRNA-DDX11-AS1 group was markedly decreased
(Fig. 4A). The results of the
Transwell assay revealed that the invasion ability of cells in the
shRNA-DDX11-AS1 group was markedly decreased compared with that of
cells in the shRNA-NC group (Fig.
4B). Western blotting was used to detect the expression levels
of migration-related proteins (MMP2 and MMP9); their trend was
consistent with that of Transwell and wound-healing experiments
(Fig. 4C). Subsequently, N-cadherin
expression was detected by western blotting. The results revealed
that N-cadherin expression was markedly decreased following
DDX11-AS1 knockdown (Fig. 4D). In
MDA-MB-231 cells, the effects of DDX11-AS1 knockdown on invasion
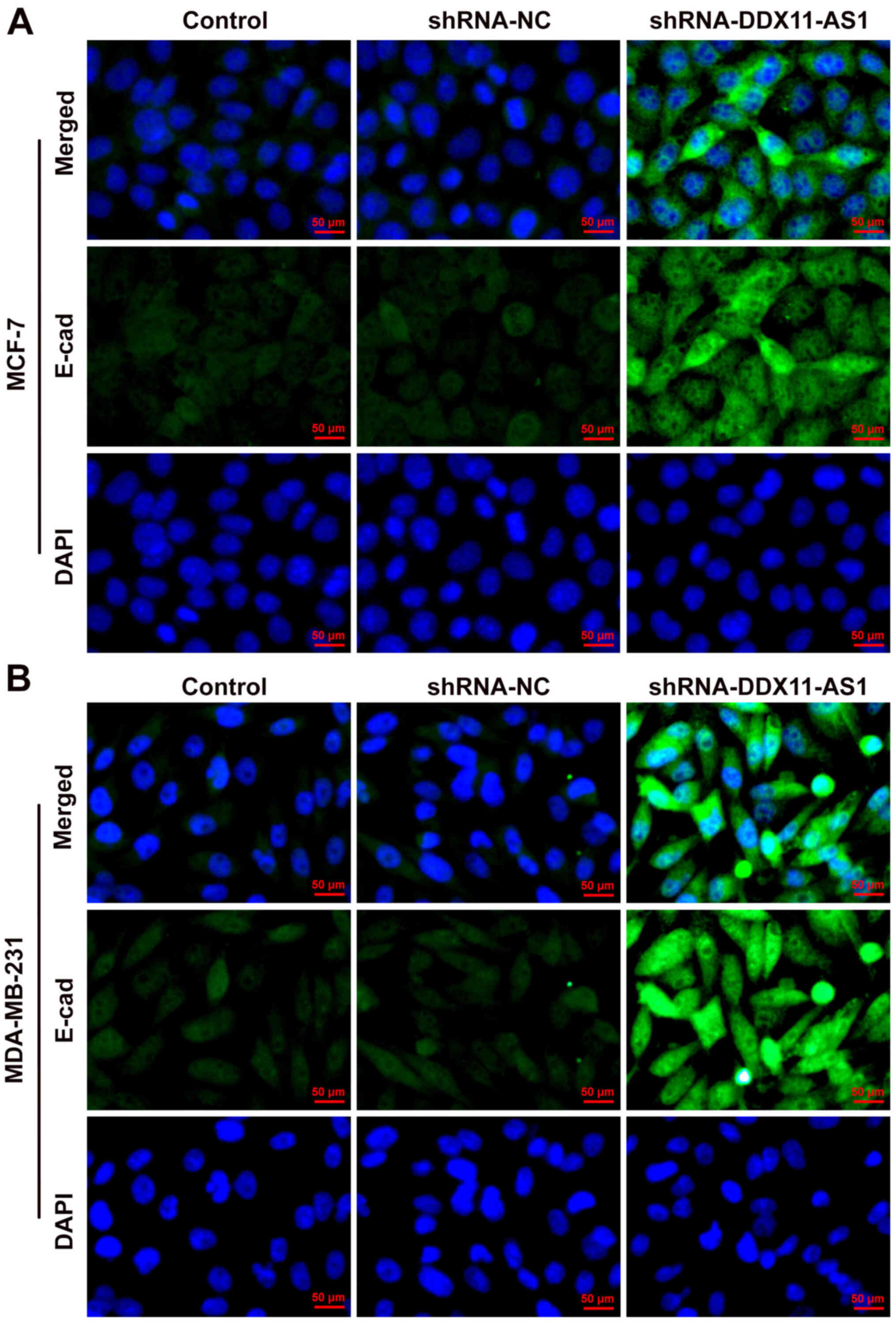
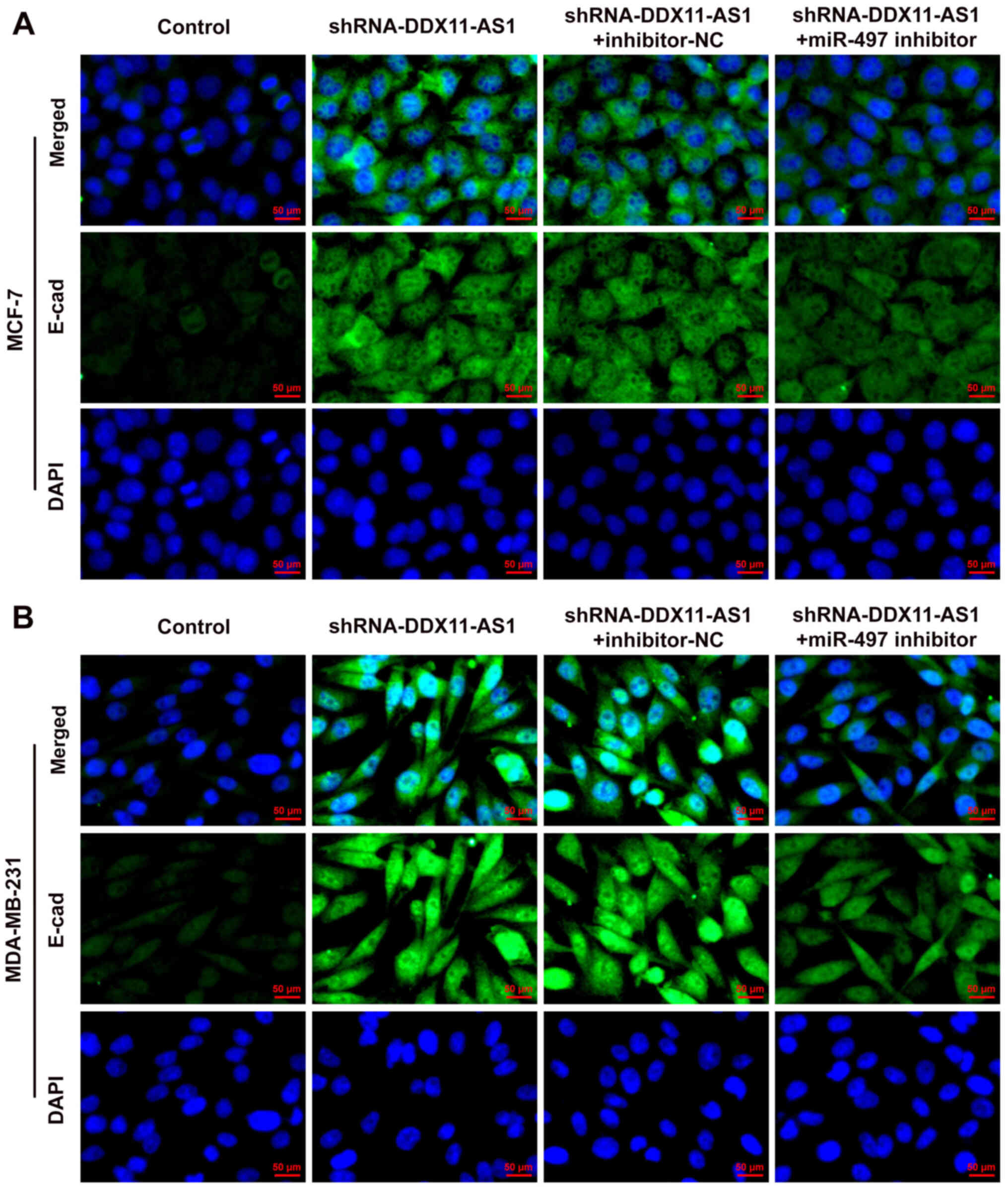
and migration were consistent with those in MCF-7 cells (Fig. 5A-D). Subsequently, the expression of
the EMT-related protein E-cadherin was detected by
immunofluorescence; the results revealed that E-cadherin expression
was markedly increased after DDX11-AS1 knockdown in MCF-7 and
MDA-MB-231 cells (Fig. 6A and
B).
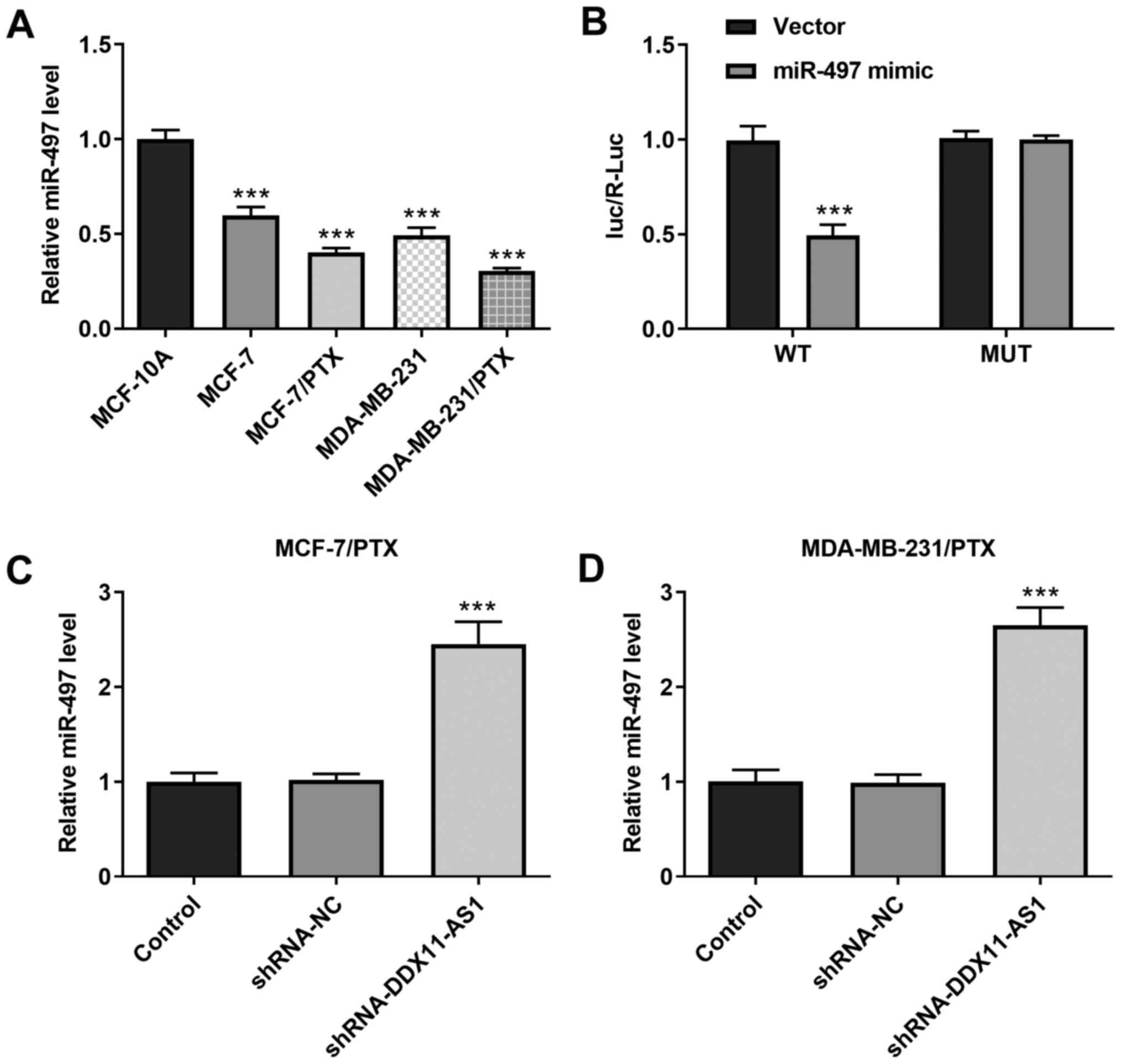
Knockdown of DDX11-AS1 upregulates the
expression levels of miR-497
It was demonstrated that the expression levels of
miR-497 in PTX-resistant cell lines were markedly decreased
(Fig. 7A). In addition, the
targeted binding of DDX11-AS1 and miR-497 was verified using a
luciferase reporter gene assay (Fig.
7B). Furthermore, the expression levels of miR-497 in
PTX-resistant cell lines were markedly increased following
DDX11-AS1 interference (Fig. 7C and
D). These results suggested that knockdown of DDX11-AS1 may
upregulate miR-497 expression.
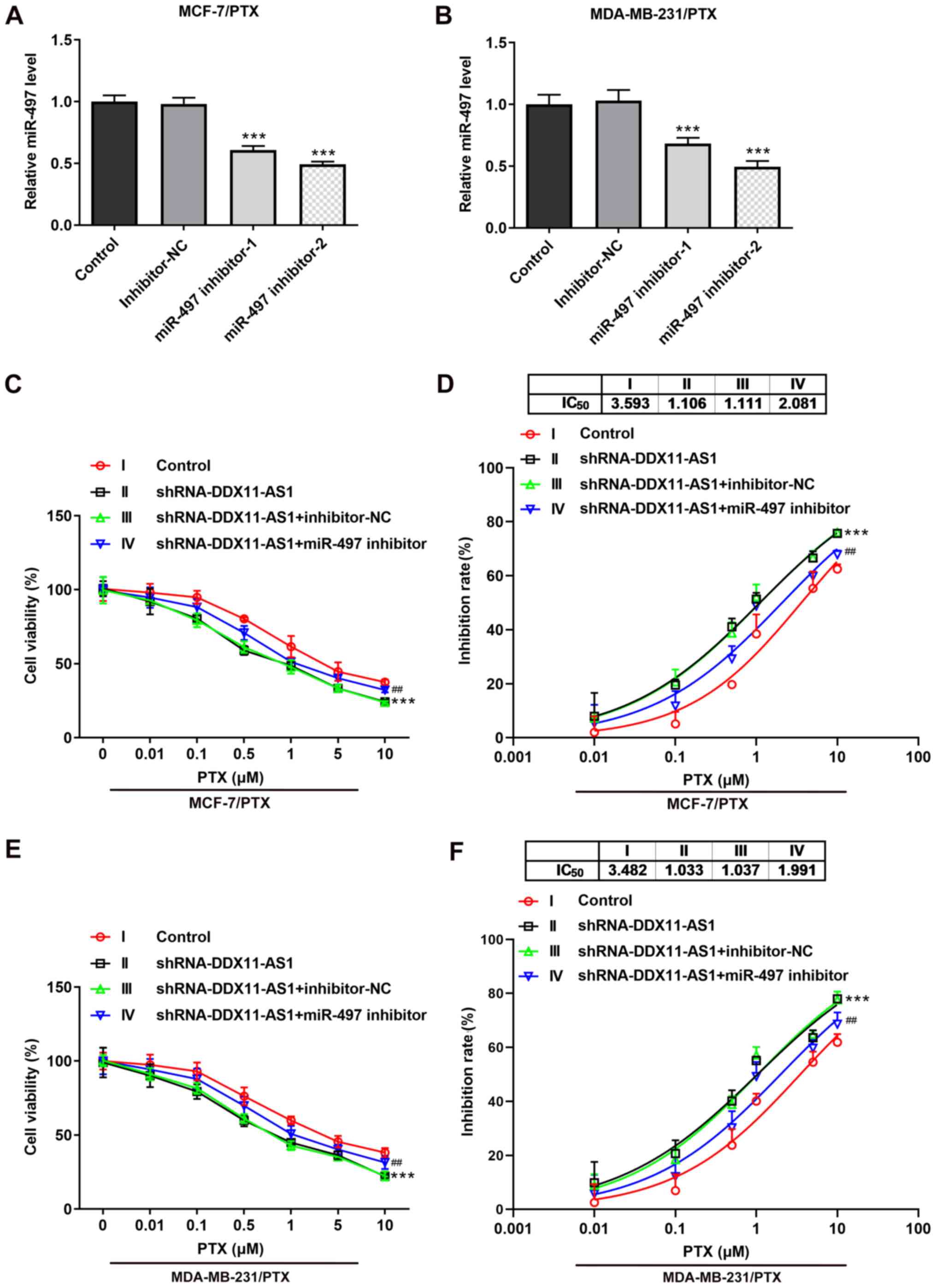
Knockdown of DDX11-AS1 reduces the
sensitivity of drug-resistant breast cancer cells to PTX via
upregulation of miR-497 expression
Transfection was used to knockdown miR-497
expression in PTX-resistant cell lines, and transfection efficiency
was detected by RT-qPCR (Fig. 8A and
B). miR-497 inhibitor-2 was selected for subsequent
experiments. Cells were divided into control, shRNA-DDX11-AS1,
shRNA-DDX11-AS1 + inhibitor-NC and shRNA-DDX11-AS1 + miR-497
inhibitor groups. The results of the CCK-8 assay demonstrated that
the IC50 value of the shRNA-DDX11-AS1 + miR-497
inhibitor group was markedly increased compared with that of the
shRNA-DDX11-AS1 + inhibitor-NC group in MCF-7/PTX (Fig. 8C and D) and MDA-MB-231/PTX (Fig. 8E and F) cells.
Knockdown of DDX11-AS1 inhibits the
proliferation, invasion and migration of breast cancer cells via
upregulation of miR-497
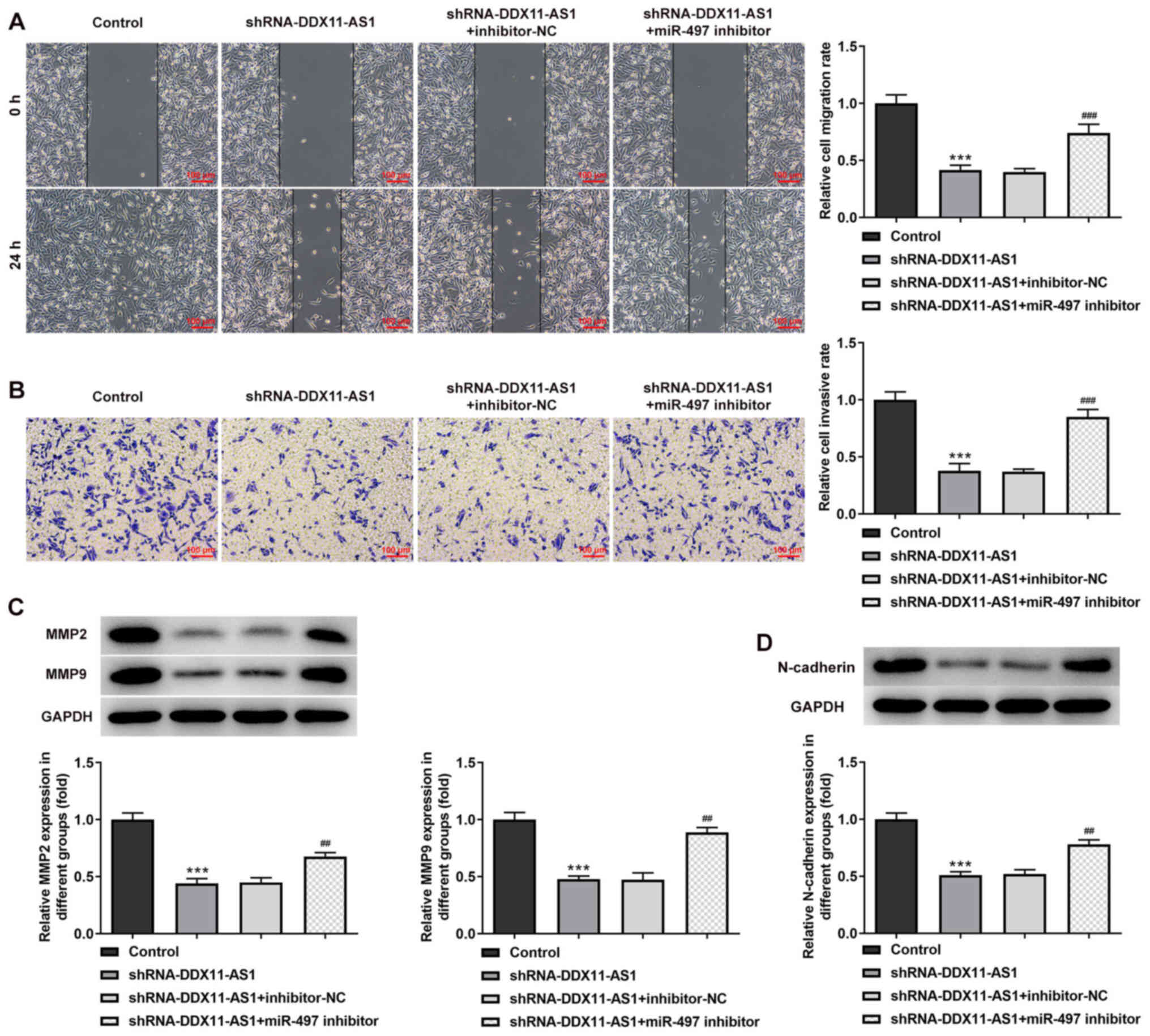
Cell transfection was used to interfere with miR-497
expression in breast cancer parental cells, and the transfection
efficiency was detected by RT-qPCR (Fig. 9A and B). miR-497 inhibitor-2 was
selected for subsequent experiments. The cells were divided into
control, shRNA-DDX11-AS1, shRNA-DDX11-AS1 + inhibitor-NC and
shRNA-DDX11-AS1 + miR-497 inhibitor groups. The results of MTT
(Fig. 9C and D) and colony
formation (Fig. 9E and F) assays
demonstrated that the proliferative ability of cells in the
shRNA-DDX11-AS1 + miR-497 inhibitor group was increased compared
with that of cells in the shRNA-DDX11-AS1 + inhibitor-NC group. In
addition, in MCF-7 cells, the invasion (Fig. 10A) and migration (Fig. 10B) of cells in the shRNA-DDX11-AS1
+ miR-497 inhibitor group were markedly increased compared with
those of cells in the shRNA-DDX11-AS1 + inhibitor-NC group, and
MMP2 and MMP9 expression was increased (Fig. 10C). Finally, the expression levels
of EMT-related proteins (E-cadherin and N-cadherin) were detected.
The expression levels of N-cadherin (Fig. 10D) in MCF-7 cells in the
shRNA-DDX11-AS1 + miR-497 inhibitor group were also increased
compared with those in cells in the shRNA-DDX11-AS1 + inhibitor-NC
group. In MDA-MB-231 cells, the trend of invasion and migration was
consistent with that in MCF-7 cells (Fig. 11A-D). Subsequently, the expression
levels of the EMT-related protein E-cadherin were detected by
immunofluorescence, and the results revealed that the expression
levels of E-cadherin in cells in the shRNA-DDX11-AS1 + miR-497
inhibitor group were decreased compared with those in cells in the
shRNA-DDX11-AS1 + inhibitor-NC group (Fig. 12A and B). These results suggested
that knockdown of DDX11-AS1 inhibited the proliferation, invasion
and migration of breast cancer cells via upregulation of miR-497
expression.
Discussion
Chemotherapy has gradually become the main method of
breast cancer treatment. Cisplatin, carboplatin, docetaxel,
gemcitabine, PTX, tamoxifen and other chemotherapy drugs are able
to improve the quality of life and overall survival of patients to
a certain extent (23). However,
chemotherapy resistance greatly reduces the efficacy of targeted
therapy for breast cancer, and causes the phenomenon of drug
resistance in breast cancer cells (24). Research has demonstrated that due to
drug resistance, the 5-year survival rate of patients with breast
cancer is still <20% worldwide (25). Therefore, it is necessary to study
the drug resistance markers and related mechanisms of breast cancer
in order to reverse chemotherapy drug resistance.
The newly discovered lncRNA DDX11-AS1 has been
reported to be abnormally highly expressed in a number of malignant
tumors, such as liver cancer (26),
colorectal cancer (13) and bladder
cancer (27). These findings
suggested that DDX11-AS1 may be a tumor marker or therapeutic
target. In addition, it has been reported that the elimination of
DDX11-AS1 reduced the resistance of esophageal cancer cells to PTX
(14). This finding indicated that
the drug resistance of tumor cells may be overcome by regulating
DDX11-AS1.
The present study revealed that lncRNA DDX11-AS1
expression was not only abnormally increased in breast cancer
cells, but also markedly increased in PTX-resistant breast cancer
cells. The present experimental results were consistent with those
of Zhang et al (14).
Interfering with DDX11-AS1 inhibited the PTX resistance of breast
cancer cells, and suppressed the proliferation, invasion and
migration of breast cancer cells.
In terms of the underlying mechanism, it was
revealed that DDX11-AS1 could regulate miR-497 expression. The
present study revealed that lncRNA DDX11-AS1 could target miR-497
through the LncBase website. Subsequently, the binding relationship
between the two was verified using the luciferase reporter gene
assay. To the best of our knowledge, there has been no previous
study on the relationship between lncRNA DDX11-AS1 and miR-497. To
the best of our knowledge, the present study is the first to verify
the relationship between lncRNA DDX11-AS1 and miR-497, and study
the regulatory effects of the two on the proliferation, invasion
and drug resistance of breast cancer cells. miR-497 has been shown
to be abnormally expressed in osteosarcoma (28), melanoma (29), squamous cell carcinoma (30) and may be involved in regulation of
the proliferation, invasion and apoptosis of cancer cells. miR-497
expression was previously revealed to be markedly downregulated in
breast cancer tissue samples and cell lines (31,32).
Furthermore, a previous study demonstrated that miR-497 reduced
cisplatin resistance of ovarian cancer cells by targeting
mTOR/P70S6K1 (19). In addition,
upregulation of miR-497 induced resistance of human glioma cells to
temozolomide by targeting mTOR/Bcl-2 (33), which showed opposite results
compared with the current study. These findings suggested that
miR-497 may serve an important role in tumor resistance. Therefore,
it was hypothesized that lncRNA DDX11-AS1 may affect breast cancer
cells and drug resistance by regulating miR-497. The present study
revealed that knockdown of DDX11-AS1 enhanced the sensitivity of
drug-resistant breast cancer cells to PTX, and inhibited the
proliferation, invasion and migration of breast cancer cells via
upregulation of miR-497 expression.
In conclusion, knockdown of lncRNA DDX11-AS1 may
inhibit the proliferation, migration and PTX resistance of breast
cancer cells by upregulating miR-497 expression. The current study
provides a foundation for clinical breast cancer treatment and
targeted therapy of breast cancer resistance.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and BZ wrote the manuscript and analyzed the
data. MW and JJ performed the experiments and supervised the study.
ML searched the literature and revised the manuscript for important
intellectual content. ML and BZ confirmed the authenticity of all
the raw data. All authors read and approved the final manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bevers TB, Helvie M, Bonaccio E, Calhoun
KE, Daly MB, Farrar WB, Garber JE, Gray R, Greenberg CC, Greenup R,
et al: Breast cancer screening and diagnosis, version 3.2018, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
16:1362–1389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falzone L, Salomone S and Libra M:
Evolution of cancer pharmacological treatments at the turn of the
third millennium. Front Pharmacol. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christofi T, Baritaki S, Falzone L, Libra
M and Zaravinos A: Current perspectives in cancer immunotherapy.
Cancers (Basel). 11:14722019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tong CWS, Wu M, Cho WCS and To KKW: Recent
advances in the treatment of breast cancer. Front Oncol. 8:2272018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mustacchi G and De Laurentiis M: The role
of taxanes in triple-negative breast cancer: Literature review.
Drug Des Devel Ther. 9:4303–4318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Telli M: Evolving treatment strategies for
triple-negative breast cancer. J Natl Compr Canc Netw. 13 (Suppl
5):S652–S654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Wu KJ, Jia QJ and Ding XF: Roles of
miRNA and lncRNA in triple-negative breast cancer. J Zhejiang Univ
Sci B. 21:673–689. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:Spec No: 1. R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polo A, Crispo A, Cerino P, Falzone L,
Candido S, Giudice A, Petro GD, Ciliberto G, Montella M, Budillon A
and Costantini S: Environment and bladder cancer: Molecular
analysis by interaction networks. Oncotarget. 8:65240–65252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falzone L, Grimaldi M, Celentano E,
Augustin LSA and Libra M: Identification of modulated MicroRNAs
associated with breast cancer, diet, and physical activity. Cancers
(Basel). 12:25552020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soudyab M, Iranpour M and Ghafouri-Fard S:
The role of long non-coding RNAs in breast cancer. Arch Iran Med.
19:508–517. 2016.PubMed/NCBI
|
|
13
|
Tian JB, Cao L and Dong GL: Long noncoding
RNA DDX11-AS1 induced by YY1 accelerates colorectal cancer
progression through targeting miR-873/CLDN7 axis. Eur Rev Med
Pharmacol Sci. 23:5714–5729. 2019.PubMed/NCBI
|
|
14
|
Zhang S, Jiang H, Xu Z, Jiang Y, She Y,
Huang X, Feng S, Chen W, Chen S, Chen Y, et al: The resistance of
esophageal cancer cells to paclitaxel can be reduced by the
knockdown of long noncoding RNA DDX11-AS1 through TAF1/TOP2A
inhibition. Am J Cancer Res. 9:2233–2248. 2019.PubMed/NCBI
|
|
15
|
Wu Z, Cai X, Huang C, Xu J and Liu A:
miR-497 suppresses angiogenesis in breast carcinoma by targeting
HIF-1α. Oncol Rep. 35:1696–1702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian F, Zhan Y, Zhu W, Li J, Tang M, Chen
X and Jiang J: MicroRNA-497 inhibits multiple myeloma growth and
increases susceptibility to bortezomib by targeting Bcl-2. Int J
Mol Med. 43:1058–1066. 2019.PubMed/NCBI
|
|
17
|
Liu Z, Wu S, Wang L, Kang S, Zhao B, He F,
Liu X, Zeng Y and Liu J: Prognostic value of microRNA-497 in
various cancers: A systematic review and meta-analysis. Dis
Markers. 2019:24912912019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo G, He K, Xia Z, Liu S, Liu H and Xiang
G: Regulation of microRNA-497 expression in human cancer. Oncol
Lett. 21:232021.PubMed/NCBI
|
|
19
|
Xu S, Fu GB, Tao Z, OuYang J, Kong F,
Jiang BH, Wan X and Chen K: miR-497 decreases cisplatin resistance
in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget.
6:26457–26471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panayotopoulou EG, Müller AK, Borries M,
Busch H, Hu G and Lev S: Targeting of apoptotic pathways by SMAC or
BH3 mimetics distinctly sensitizes paclitaxel-resistant triple
negative breast cancer cells. Oncotarget. 8:45088–45104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Z, Lin T, Liao X, Li Z, Lin R, Qi X,
Chen G, Sun L and Lin L: Network pharmacology based research into
the effect and mechanism of Yinchenhao Decoction against
Cholangiocarcinoma. Chin Med. 16:132021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hassan MS, Ansari J, Spooner D and Hussain
SA: Chemotherapy for breast cancer (Review). Oncol Rep.
24:1121–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chun KH, Park JH and Fan S: Predicting and
overcoming chemotherapeutic resistance in breast cancer. Adv Exp
Med Biol. 1026:59–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malhotra A, Jain M, Prakash H, Vasquez KM
and Jain A: The regulatory roles of long non-coding RNAs in the
development of chemoresistance in breast cancer. Oncotarget.
8:110671–110684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Zhuang W, Huang M and Li X: Long
noncoding RNA DDX11-AS1 epigenetically represses LATS2 by
interacting with EZH2 and DNMT1 in hepatocellular carcinoma.
Biochem Biophys Res Commun. 514:1051–1057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen D, Chen J, Gao J, Zhang Y, Ma Y, Wei
W and Wei Y: LncRNA DDX11-AS1 promotes bladder cancer occurrence
via protecting LAMB3 from downregulation by sponging miR-2355-5p.
Cancer Biother Radiopharm. 35:319–328. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pang PC, Shi XY, Huang WL and Sun K:
miR-497 as a potential serum biomarker for the diagnosis and
prognosis of osteosarcoma. Eur Rev Med Pharmacol Sci. 20:3765–3769.
2016.PubMed/NCBI
|
|
29
|
Chai L, Kang XJ, Sun ZZ, Zeng MF, Yu SR,
Ding Y, Liang JQ, Li TT and Zhao J: miR-497-5p, miR-195-5p and
miR-455-3p function as tumor suppressors by targeting hTERT in
melanoma A375 cells. Cancer Manag Res. 10:989–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei XH, Gu XL, Zhou XT, Ma M and Lou CX:
miR-497 promotes the progression of cutaneous squamous cell
carcinoma through FAM114A2. Eur Rev Med Pharmacol Sci.
22:7348–7355. 2018.PubMed/NCBI
|
|
31
|
Zhong H, Yang J, Zhang B, Wang X, Pei L,
Zhang L, Lin Z, Wang Y and Wang C: LncRNA GACAT3 predicts poor
prognosis and promotes cell proliferation in breast cancer through
regulation of miR-497/CCND2. Cancer Biomark. 22:787–797. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai J, Zhao WY, Li WJ, Ying ZW and Jiang
DQ: Long noncoding RNA LINC00473 indicates a poor prognosis of
breast cancer and accelerates tumor carcinogenesis by competing
endogenous sponging miR-497. Eur Rev Med Pharmacol Sci.
23:3410–3420. 2019.PubMed/NCBI
|
|
33
|
Zhu D, Tu M, Zeng B, Cai L, Zheng W, Su Z
and Yu Z: Up-regulation of miR-497 confers resistance to
temozolomide in human glioma cells by targeting mTOR/Bcl-2. Cancer
Med. 6:452–462. 2017. View
Article : Google Scholar : PubMed/NCBI
|