Introduction
Pseudomonas aeruginosa (PA) is the pathogen
most commonly associated with the use of contact lenses (1). PA keratitis is a rapidly developing
and destructive ophthalmic disease, which can lead to ulcer,
corneal perforation and even severe vision loss (2). Despite the rapid progress of modern
medicine, there is still a lack of effective treatment for PA
keratitis (3). It is necessary to
identify novel therapeutic targets for PA keratitis. Therefore,
keeping the immune homeostasis and regulating the inflammatory
immune response of the eyes are the theme of the present study.
Ubiquitination and de-ubiquitination processes have
been reported to maintain the stability of a large number of
critical proteins and internal environment and participate in the
regulation of important physiological processes (4). When the ubiquitination chain of lysine
63 is formed, the activity and other functions of the substrates
are changed (5). If the substrate
is modified by lysine 48 (K48) ubiquitin, the target protein is
degraded by proteasome system (6).
Recently, studies have demonstrated that the ubiquitination process
is crucial for the regulation of inflammatory responses in
PA-induced keratitis (7,8).
Deubiquitinating enzymes (DUBs) are proteases that
process ubiquitin or ubiquitin-like gene products and reverse the
modification of proteins by a single ubiquitin (9). Ubiquitin-specific proteases (USPs) are
the largest subclass of DUBs with specific targets (10). Ubiquitin-specific protease 22
(USP22) is a member of USPs in mammals and contains an N-terminal
zinc-finger domain for substrate interaction and a C-terminal
ubiquitin-specific peptidase domain for protein deubiquitination
(11). Its expression level is
related to tumor metastasis, drug resistance and cell cycle
progress and is crucial in the process of tumor oncogenesis and
development; it is therefore considered a biomarker and treatment
target of tumors (12). However, no
studies, to the best of the authors' knowledge, have documented the
role of USP22 in PA-induced keratitis.
The present study demonstrated that the expression
of USP22 was increased by PA infection. Silencing of USP22
significantly delayed the disease progression of PA-mediated
keratitis and attenuated pro-inflammatory cytokines production
induced by PA infection. Knockdown of USP22 expression suppressed
NF-κB activation and enhanced K48-linked polyubiquitination level
of TRAF6. These results indicated that USP22 is a positive
regulator of pro-inflammatory responses in PA-induced
keratitis.
Materials and methods
Cell culture
Mouse macrophage cell line RAW264.7 was obtained
from American Type Culture Collection and the cells grown in DMEM
medium (HyClone; Cytiva) containing 10% FBS (HyClone; Cytiva), 100
U/ml penicillin (HyClone; Cytiva) and 100 µg/ml streptomycin
(HyClone; Cytiva) at 37°C under 5% CO2.
Experimental infection with PA
Wild-type (WT) 8-week-old female C57BL/6J mice
(18–22 g, n=90) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. The mice were maintained in a specific
pathogen-free grade animal facility at room temperature and
humidity (50–60%) under a 12-h light/dark cycle and were allowed
free access to standard mouse chow and water. The experiments were
carried out according to the National Institutes of Health Guide
for the Care and Use of Laboratory Animals (NIH publication, no.
85–23, revised 2011) (13),
approved by the Animal Ethics Committee of the Scientific
Investigation Board of Shandong First Medical University and
performed as previous reported (7).
Mice were intubated and anaesthetized with mechanical ventilation
using 2–3% isoflurane. Anesthesia was maintained by inhalation of
1–2% isoflurane in 100% oxygen. The adequacy of anaesthesia and
mortality of mice were monitored by measuring heart rate and the
response to tail stimulation. In order to maintain the body
temperature of mice at 37°C, a layer of water circulating at
constant temperature was arranged on the experimental platform.
Following anesthesia with isoflurane, three 1-mm incisions were
made on the left cornea with sterile 25 gauge needle. A bacterial
suspension (5 µl) containing 1×106 colony-forming units
(CFUs) of PA ATCC strain 19660 was used locally on the ocular
surface. The eyes were examined at 24 h or other time points
following the infection to ensure that the mice were infected and
to monitor the disease. For euthanasia, the mice were treated with
pentobarbital (150 mg/kg, administered intraperitoneally;
Sigma-Aldrich; Merck KGaA) followed by cervical dislocation in
accordance with NIH guidelines for the humane treatment of animals.
All efforts were made to minimize suffering. In the experiment,
there was no mortality of mice due to human operations. The corneal
disease was graded as follows: 0, clear or slight opacity partially
or fully covering the pupil; +1, slight opacity partially or fully
covering the anterior segment; +2, dense opacity partially or fully
covering the pupil; +3, dense opacity covering the entire anterior
segment; and +4, corneal perforation or phthisis.
Bacterial plate counts
Subsequently, 5 days after infection, the corneas
(n=5/group/time point) were collected and the number of viable
bacteria was counted as previously reported (14). In brief, the cornea was homogenized
in sterile water containing 0.85% (w/v) NaCl and 0.25% BSA
(Sigma-Aldrich; Merck KGaA). The 10-fold diluent of the sample was
coated on the Pseudomonas Isolation Agar (Difco; Becton, Dickinson
and Company) for three times followed by the incubation overnight
at 37°C. The data were reported as 105 CFU per cornea ± standard
deviation.
Histology
Eyes were collected and fixed in 10% neutral
buffered formalin for 24 h at room temperature. (Sigma-Aldrich;
Merck KGaA) 5 days after PA infection. The eye tissues were
embedded in paraffin and anterior sections (8 µm) of the corneal
epithelium were cut serially, mounted on adhesive glass slides and
stained by hematoxylin (cat. no. C0105M-1; Beyotime Institute of
Biotechnology; 3 min) and eosin (cat. no. C0105M-2; Beyotime
Institute of Biotechnology; 15 sec) at room temperature. All
sections were visualized with a laser scanning confocal microscope
(LSM700; Carl Zeiss AG).
Lentivirus preparation and
infection
Lentivirus (BLOCK-iT™ Lentiviral RNAi Expression
System; cat. no K4944-00; Invitrogen; Thermo Fisher Scientific,
Inc.) containing control plasmid (sequence:
5′-AUUGUCAUCACCUUUGCAGTT −3′) or short hairpin (sh)RNA targeting
USP22 (sequence: 5′-CGUCAAAGGUGAUGACAAUTT-3′) constructed by MDL
Biotech, was used according to the manufacturer's protocols. shRNA
lentivirus was packaged and titered in 293T cells (obtained from
American Type Culture Collection) and the enriched lentivirus
particles were used for cell infection at 50 multiplicities of
infection in the presence of polybrene. For in vivo
infection, the protocol was performed as previously described
(8). Lentiviruses were
subconjunctivally injected into the left eye of C57BL/6J mice (5
µl/mouse at a viral titer of 1×108) once a week for
three times before PA infection.
Reverse transcription-quantitative
(RT-q) PCR
Cells (1×104 cells/well) were cultured to
90% confluence in 6-well plates before RNA extraction. Total RNA
was extracted using the TRIzol® reagent according to the
manufacturer's instructions (Thermo Fisher Scientific, Inc.). Equal
volumes of RNA samples (1.0 µg) were collected and the first strand
of cDNAs was synthesized using TaqManTM Reverse
Transcription Reagents (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. A LightCycler (ABI PRISM 7000;
Applied Biosciences) and a SYBR RT-PCR kit (Takara Biotechnology
Co., Ltd.) were used for RT-qPCR according to the manufacturer's
instructions (25 µl reaction volume). GAPDH was used as the
internal control and the thermocycling conditions were 1 cycle
(95°C for 5 min) and 40 cycles (95°C for 15 sec, 56°C for 30 sec
and 72°C for 30 sec). The 2−ΔΔCq method was used to
evaluate the relative quantities of each amplified product in the
samples (15). Primer sequences
used in qPCR are given in Table I.
The data are representative of three biological replicates.
 | Table I.List of primers used. |
Table I.
List of primers used.
| Gene | Sequence (5′-3′) |
|---|
| USP22 | F:
CCTGCACGTTTTCGTGGAAC |
|
| R:
TCTCCACGATGTTGGTGAGC |
| TNF-α | F:
GCCACCACGCTCTTCTGTCT |
|
| R:
TGAGGGTCTGGGCCATAGAAC |
| IL-1β | F:
ACCTTCCAGGATGAGGACATGA |
|
| R:
AACGTCACACACCAGCAGGTTA |
| IL-6 | F:
ACAACCACGGCCTTCCCTAC |
|
| R:
CATTTCCACGATTTCCCAGA |
| GAPDH | F:
AATGACCCCTTCATTGAC |
|
| R:
TCCACGACGTACTCAGCGC |
ELISA analysis
For in vitro experiments, the supernatants
were collected following PA infection for ELISA analysis. For in
vivo experiments, the corneas were individually collected
following PA infection for 5 days and then homogenized in 0.5 ml of
PBS with 0.1% Tween-20. The levels of IL-6 (cat no. M6000B), TNF-α
(cat no. MTA00B) and matrix metallo-proteinase 1 (cat no. DY901B)
were measured by ELISA kits (R&D Systems) in accordance with
the manufacturer's instructions.
Western blot analysis
The protocols were performed as described previously
(8). The cells or corneas were
lysed using RIPA buffer (MDL Biotech) and total protein in the
supernatants was quantified using a Bio-Rad quantification assay
(Bio-Rad Laboratories, Inc.). Equal amounts of protein (25 µg) was
loaded on 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and then transferred onto a PVDF membrane (EMD
Millipore) followed by blocking with 2.5% nonfat dry milk for 1 h
at room temperature. Antibodies for USP22 (1:1,000; cat. no.
ab195289; Abcam), K48-linked ubiquitin (linkage-specific K48,
1:800; cat. no. ab140601; Abcam), TRAF6 (1:1,000; cat. no. ab33915;
Abcam) and the antibodies specific for p65 (1:800; cat. no. 8242;
Cell Signaling Technology Inc.), phosphorylated (p-)p65 (1:800;
cat. no. 3033, Cell Signaling Technology Inc.), IκBα (1:800; cat.
no. 4814; Cell Signaling Technology Inc.), p-IκBα (1:800; cat. no.
2859; Cell Signaling Technology Inc.) and β-actin (1:2,000; cat.
no. sc58673; Santa Cruz Biotechnology, Inc.) were added and the
membrane incubated overnight at 4°C. Subsequently, the membrane was
incubated with the corresponding horseradish peroxidase-conjugated
secondary antibody (goat anti-rabbit IgG; cat. no. sc-2004;
1:2,000; Santa Cruz Biotechnology, Inc. and goat anti-mouse IgG;
cat. no. sc-2005; 1:2,000; Santa Cruz Biotechnology, Inc.) and
detected with enhanced chemiluminescence (Thermo Fisher Scientific,
Inc.). Protein brands were detected and with Bio-Rad
ChemiDocTM XRS+ System (Bio-Rad Laboratories, Inc.) and
analyzed with Image Lab software (v4.1; Bio-Rad Laboratories,
Inc.).
Immunoprecipitation and
ubiquitination
Following PA infection for the indicated time
points, RAW264.7 cells were lysed with co-IP lysis buffer [150 mM
NaCl, 20 mM Tris-HCl, pH 7.4, 1% Triton X-100 and 1 mM EDTA
supplemented with protease inhibitor cocktail (cat. no.
04693132001, Roche Applied Sciences)]. The cell lysates (5 mg
protein) were subjected to immunoprecipitation with the appropriate
antibodies (TRAF6; 1:500; cat. no. ab33915; Abcam and IgG; 1:500;
cat. no. ab6708; Abcam) overnight at 4°C and then incubated with
protein A/G Plus-Agarose (cat. no. sc-2003; Santa Cruz
Biotechnology, Inc.) for 12 h at 4°C. The beads were washed with
the lysis buffer 3 times by centrifugation at 300 × g for 10 min at
4°C. The immunoprecipitated proteins were separated by 10% SDS-PAGE
followed by immunoblotting with the appropriate antibodies (USP22;
1:1,000; cat. no. ab195289; Abcam, TRAF6; 1:1,000; cat. no.
ab33915; Abcam, β-actin; 1:2,000; cat. no. sc58673; Santa Cruz
Biotechnology, Inc.). The ubiquitination of TRAF6 was detected as
described previously (8). Briefly,
following treatment, the cells were harvested and lysed with buffer
(50 mM Tris, 140 mM NaCl, 1% SDS). Samples were boiled for 5 min
and then diluted 10-fold with co-IP lysis buffer. Following
centrifugation (300 × g for 10 min at 4°C), the supernatants were
incubated with anti-TRAF6 antibody (1:500; cat. no. ab33915; Abcam)
overnight at 4°C and then incubated with protein A/G Plus-Agarose
(cat. no. sc-2003; Santa Cruz Biotechnology, Inc.) for 12 h at 4°C,
followed by immunoprecipitation assay and western blot
analysis.
Statistical analysis
The differences in clinical score between lentivirus
treated corneas were tested by the Mann-Whitney U test at indicated
days following infection. One-way ANOVA was performed to compare
three or more groups. If the ANOVA analysis was significant, the
Tukey's post-hoc test was applied for comparison between each two
groups. The other assays were determined by an unpaired, two-tailed
Student's t test. P<0.05 was considered to indicate a
statistically significant difference.
Results
USP22 expression is increased in mouse
corneas and in in vitro cultured macrophages following PA
infection
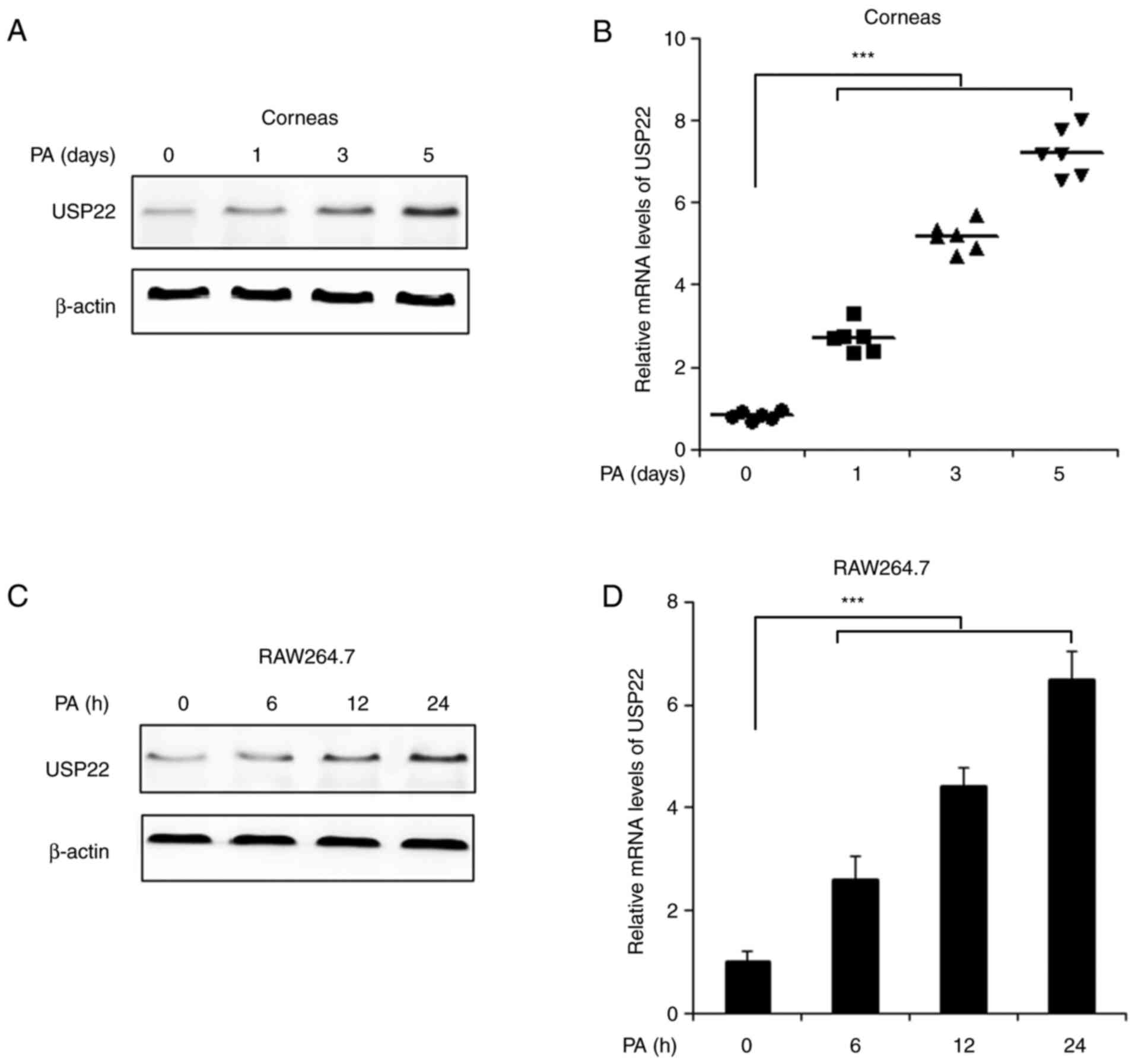
In order to illustrate the function of USP22 in
PA-induced keratitis, the expression of USP22 following PA
infection was first examined. As demonstrated in Fig. 1A and B, the mRNA and protein levels
of USP22 were increased in mice corneas following PA stimulation.
Following PA infection in corneas, macrophages and other
inflammatory cells would infiltrate in the corneal stroma to attack
the bacteria (16). The level of
USP22 in cultured RAW264.7 cells was detected. Consistently, mRNA
and protein levels of USP22 were significantly upregulated by PA
stimulation (Fig. 1C and D).
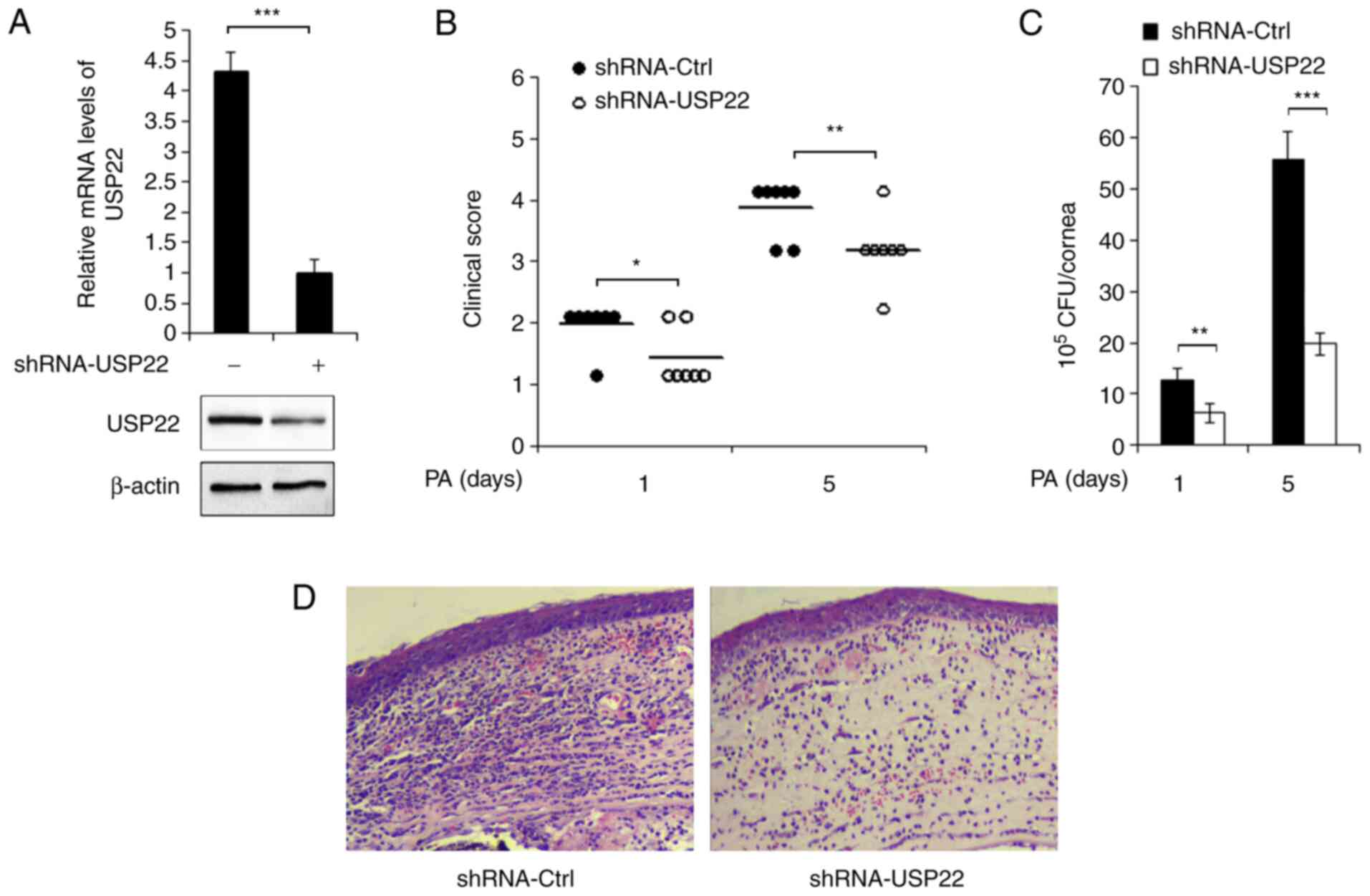
Silencing of USP22 delays the disease
progression of PA-induced keratitis
Lentivirus containing USP22 shRNA plasmid was used
to knockdown the expression of USP22. C57BL/6 mice were
subconjunctivally injected with shRNA-control lentivirus or
shRNA-USP22 lentivirus, followed by PA infection. The decreased
expression of USP22 in mice corneas was confirmed by RT-qPCR and
western blotting (Fig. 2A). The
clinical scores of PA-infected corneas were next examined and it
was found that knocking down the expression of USP22 significantly
attenuated PA-induced disease severity (Fig. 2B). In accordance, as demonstrated in
Fig. 2C, the bacterial load was
markedly decreased in USP22-silenced mice corneas following PA
infection. In addition, hematoxylin and eosin staining results
demonstrated alleviated inflammation and less infiltration of
immune cells in USP22-silenced corneas (Fig. 2D).
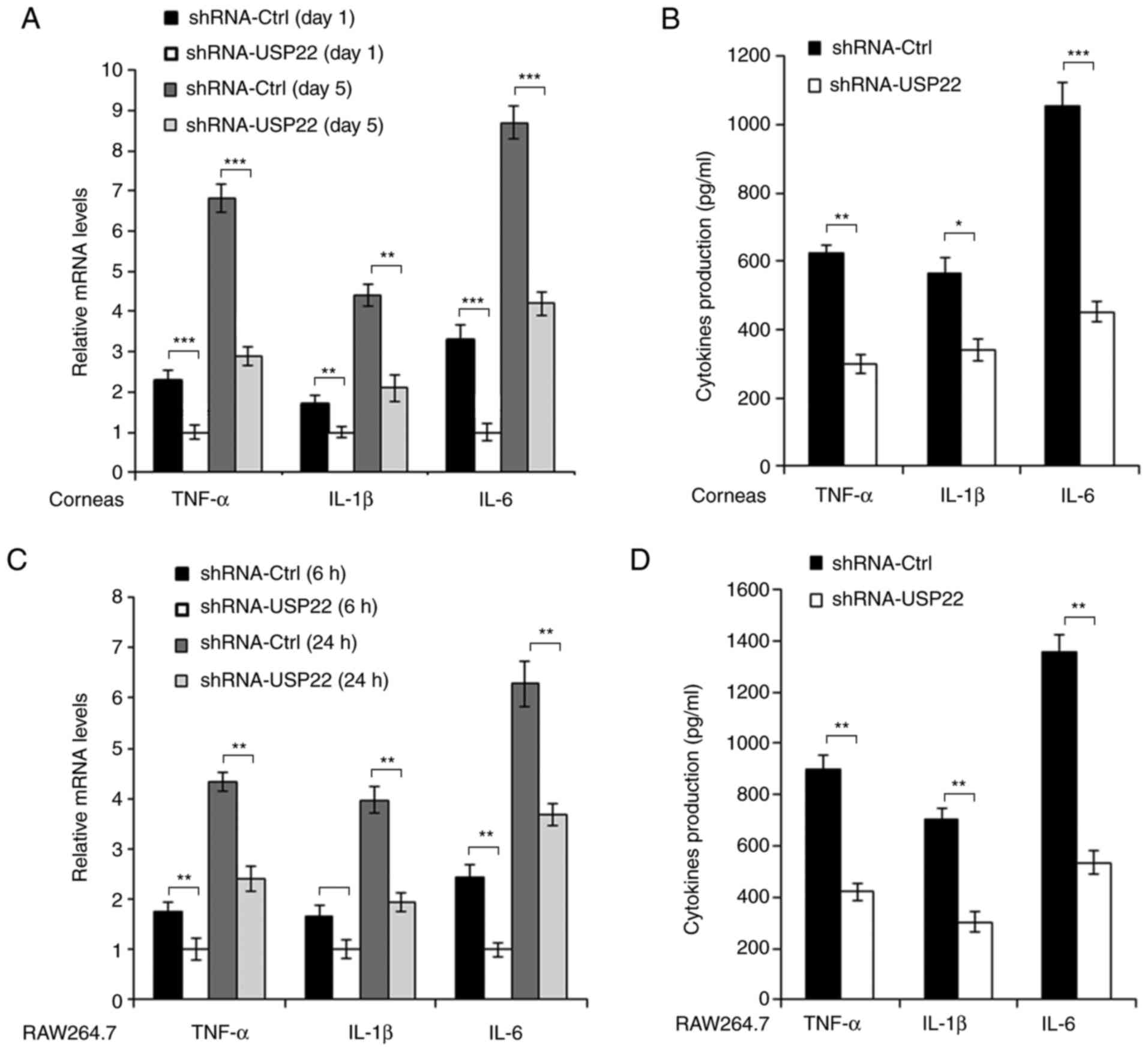
Silencing of USP22 suppresses
PA-induced pro-inflammatory cytokines production
Inflammatory reaction and pro-inflammatory cytokines
production are critical processes in response to PA infection in
corneas. Therefore the pro-inflammatory cytokines production in
mice corneas and RAW264.7 cells was detected following PA
stimulation. As demonstrated in Fig. 3A
and B, mRNA and protein levels of pro-inflammatory cytokines
such as TNF-α, IL-1β and IL-6 were decreased in USP22-shRNA-treated
mice corneas following PA infection. Similar results were also
observed in RAW264.7 cells (Fig. 3C and
D).
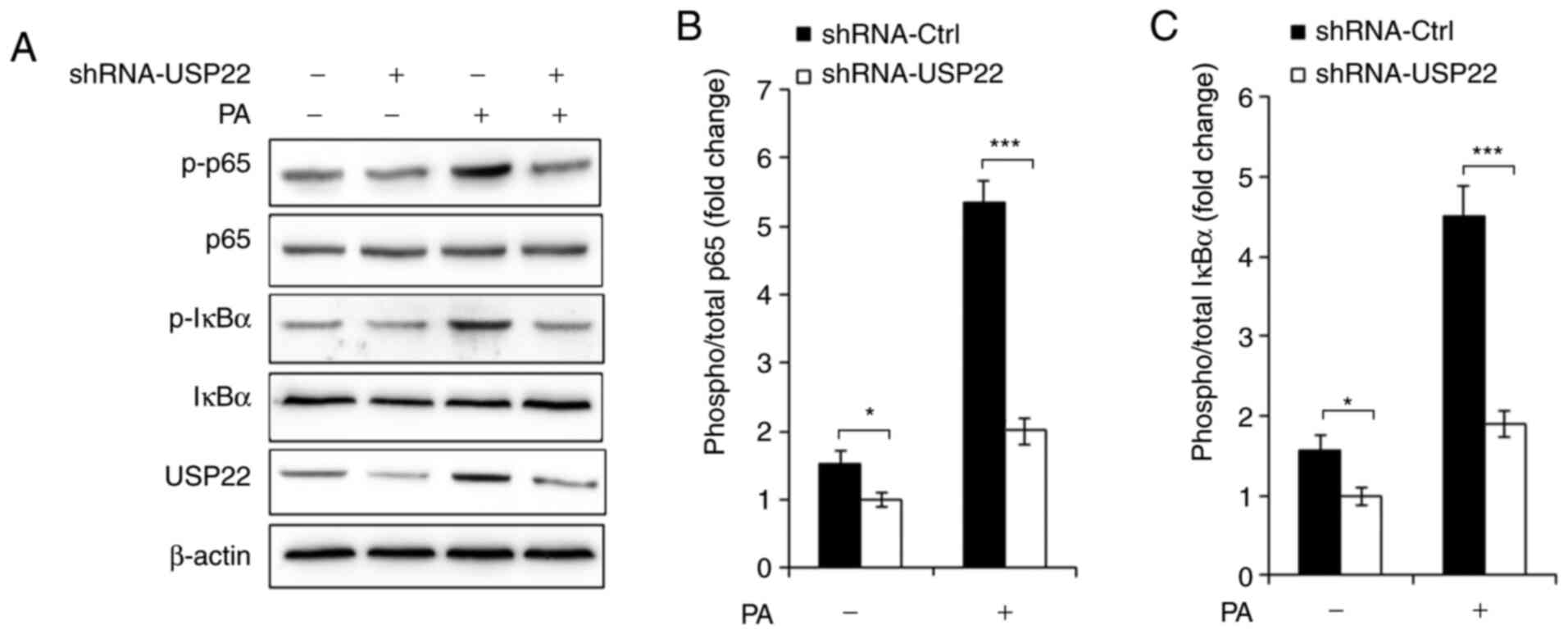
Silencing of USP22 inhibits NF-κB
activation
Production of pro-inflammatory cytokines depends
mainly on the NF-κB activation in response to PA infection, so it
was hypothesized that USP22 could affect NF-κB activation in
PA-infected macrophages. As demonstrated in Fig. 4A-C, the present study found that
silencing of USP22 greatly suppressed phosphorylation of p65 and
IκBα in RAW264.7 cells infected with shRNA-USP22 lentivirus.
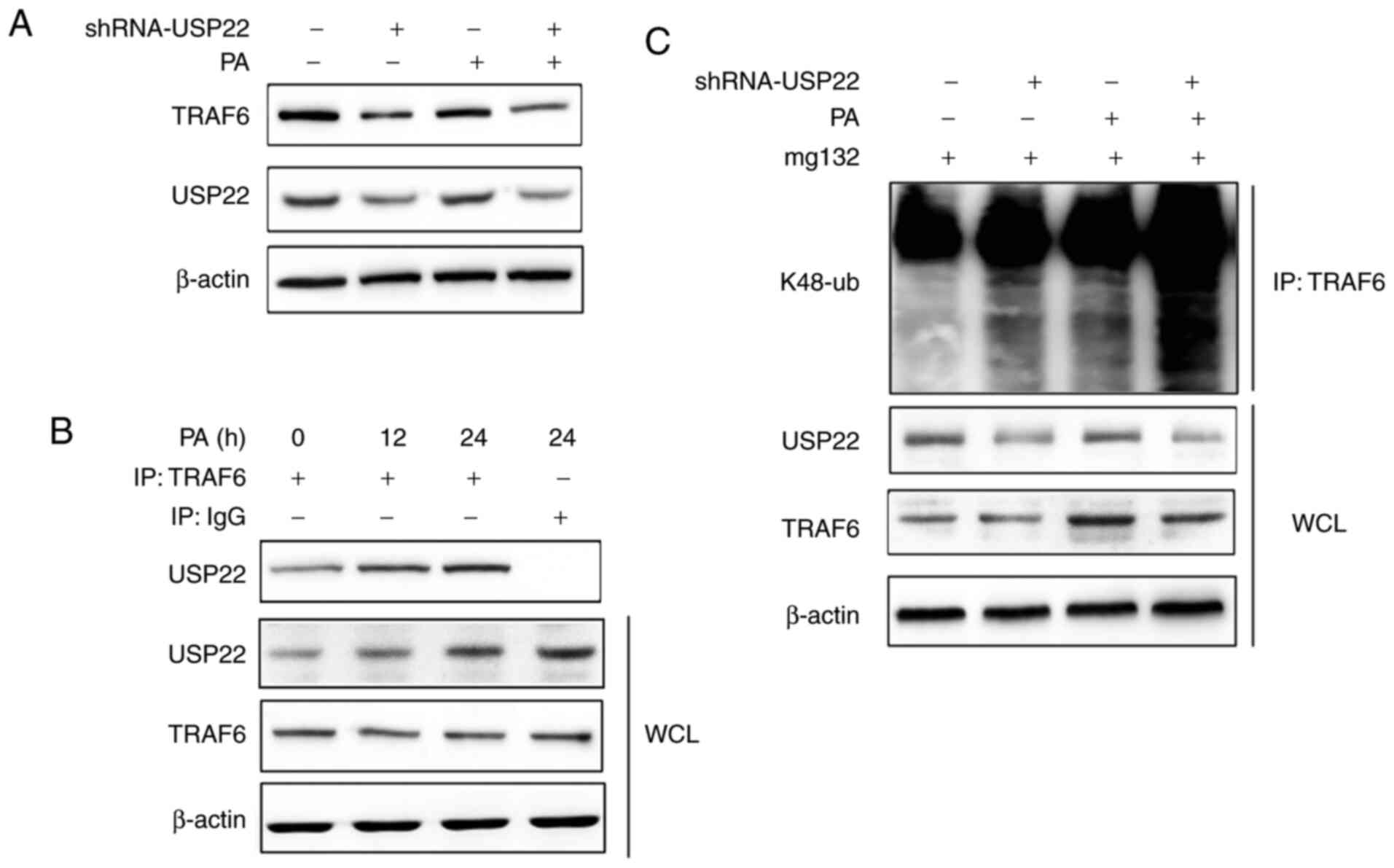
Silencing of USP22 aggravates
K48-linked polyubiquitination of TRAF6
TRAF6 is reported to be an important adaptor of the
NF-κB signaling pathway (17), so
the present study investigated the relationship between USP22 and
TRAF6 by examining the protein level of TRAF6 in USP22-silenced
RAW264.7 cells following PA infection. It was found that knockdown
of USP22 expression decreased TRAF6 expression in control and
PA-treated macrophages (Fig. 5A).
Furthermore, the interaction between USP22 and TRAF6 was observed
following PA infection (Fig. 5B).
Notably, silencing of USP22 enhanced K48-linked polyubiquitination
of TRAF6, especially in PA infected RAW264.7 cells (Fig. 5C).
Discussion
The current study demonstrated the expression of
USP22 and its role in PA-induced keratitis. To the best of the
authors' knowledge, this is the first report on the relationship
between USP22 and PA keratitis.
PA keratitis accounts for ~75% of reported cases of
contact lens-associated diseases (14). The pathogenesis of PA keratitis is
complex and has a number of factors including bacterial factors and
host components. For example, PA can produce a variety of toxic
factors, such as exotoxin A, lipopolysaccharide endotoxin and
exoenzyme ExoU to induce host cell death (18). In addition, as the critical cells of
host immune response, macrophages and monocytes accumulate in the
infected area during PA infection (19). The pro-inflammatory cytokines, such
as TNF-α, IL-1β and IL-6, are produced by macrophages and monocytes
to clear up the bacterial infection, but if not properly
controlled, these inflammatory cytokines can aggravate tissue
damage, even leading to corneal perforation (20). The current study demonstrated that
knocking down the expression of USP22 could delay PA keratitis
progression and decrease PA bacterial load, indicating that USP22
is a positive regulator of PA keratitis. As the present study
demonstrated, USP22 expression is markedly increased by PA
infection in mice corneas and in vitro cultured RAW264.7
cells, which suggested that USP22 expression was regulated by PA
and that PA keratitis tends to be aggravated with the accumulation
of USP22. It was found that silencing of USP22 suppressed
production of pro-inflammatory cytokines in PA-infected RAW264.7
cells; these data suggested USP22 as a pro-inflammatory regulator
following PA infection.
The NF-κB signaling pathway is widely studied as a
paradigm for signal transduction and pro-inflammatory cytokines
production (21). Previous studies
have reported that the NF-κB signaling pathway serves a crucial
role in the development of bacterial keratitis (22–24).
The current study identified that silencing of USP22 suppressed
phosphorylation levels of p65 and IκBα, which indicated that USP22
could promote NF-κB activation, thereby increasing the expression
of pro-inflammatory cytokines. Ubiquitination modification has been
reported to serve crucial roles in NF-κB activation and previous
studies have demonstrated that ubiquitination regulation is
essential for the regulation of progression of PA keratitis
(7,8). In addition, the present study found
that USP22 could remove the K48-linked polyubiquitination chain of
TRAF6, which is the key adaptor of the NF-κB signaling pathway,
leading to the stability of TRAF6 and thereby the promotion of
NF-κB activation and the production of downstream pro-inflammatory
cytokines.
USP22 is a novel deubiquitinating enzyme and is
considered to be important in a number of physiological and
pathological processes such as cell cycle, cell proliferation and
tumor invasion (25–27). However, the function of USP22 in PA
keratitis remains to be elucidated. The present study detected the
level of USP22 in PA-infected mice corneas and RAW264.7 cells and
identified that USP22 expression was induced by PA infection.
Furthermore, USP22 promoted disease progression of PA keratitis,
together with the increased production of pro-inflammatory
cytokines. USP22 enhanced PA-induced NF-κB activation and
stabilized TRAF6 expression by removing K48-linked
polyubiquitination of TRAF6. These findings extended our
understanding of the physiological function of USP22 and suggested
USP22 is a possible medical target for the treatment of PA
keratitis.
However, there were still some limitations in this
study. For example, the detection of NF-κB and TRAF6 activity in
animal models is still insufficient and the use of human cornea for
experiments remains lacking. This will be improved in future
studies and the functional research of USP22 in human tissues
expanded.
Acknowledgements
Not applicable.
Funding
The present study was supported by Project of Shandong Province
Higher Educational Science and Technology Program (grant no.
J05L08), Key Research and Development Program of Shandong Province
(grant no. 2017GGX201010), Natural Science Foundation of Shandong
Province (grant no. ZR2016HM73). JQ was supported by the Taishan
Scholars Program of Shandong Province (grant no. TS201712065).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC and YW designed the present study. DC, DS and JQ
performed the experiments, collecting data and drafted the
manuscript. YM and WL performed the cell experiments and analyzed
the data. DC and YW confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments were carried out according to the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals, approved by the Animal Ethics Committee of the
Scientific Investigation Board of Shandong First Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hilliam Y, Kaye S and Winstanley C:
Pseudomonas aeruginosa and microbial keratitis. J Med
Microbiol. 69:3–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Callaghan R, Caballero A, Tang A and
Bierdeman M: Pseudomonas aeruginosa Keratitis: Protease IV
and PASP as Corneal Virulence Mediators. Microorganisms.
7:E2812019. View Article : Google Scholar
|
|
3
|
Deng QC, Deng CT, Li WS, Shu SW, Zhou MR
and Kuang WB: NLRP12 promotes host resistance against
Pseudomonas aeruginosa keratitis inflammatory responses
through the negative regulation of NF-κB signaling. Eur Rev Med
Pharmacol Sci. 22:8063–8075. 2018.PubMed/NCBI
|
|
4
|
Jang HH: Regulation of protein degradation
by proteasomes in cancer. J Cancer Prev. 23:153–161. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen ZJ and Sun LJ: Nonproteolytic
functions of ubiquitin in cell signaling. Mol Cell. 33:275–286.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu P, Duong DM, Seyfried NT, Cheng D, Xie
Y, Robert J, Rush J, Hochstrasser M, Finley D and Peng J:
Quantitative proteomics reveals the function of unconventional
ubiquitin chains in proteasomal degradation. Cell. 137:133–145.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo L, Kong Q, Dong Z, Dong W, Fu X, Su L
and Tan X: NLRC3 promotes host resistance against Pseudomonas
aeruginosa-induced keratitis by promoting the degradation of
IRAK1. Int J Mol Med. 40:898–906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo L, Dong W, Fu X, Lin J, Dong Z, Tan X
and Zhang T: Tripartite motif 8 (TRIM8) positively regulates
pro-inflammatory responses in Pseudomonas aeruginosa-induced
keratitis through promoting K63-linked polyubiquitination of TAK1
protein. Inflammation. 40:454–463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reyes-Turcu FE, Ventii KH and Wilkinson
KD: Regulation and cellular roles of ubiquitin-specific
deubiquitinating enzymes. Annu Rev Biochem. 78:363–397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Love KR, Catic A, Schlieker C and Ploegh
HL: Mechanisms, biology and inhibitors of deubiquitinating enzymes.
Nat Chem Biol. 3:697–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melo-Cardenas J, Zhang Y, Zhang DD and
Fang D: Ubiquitin-specific peptidase 22 functions and its
involvement in disease. Oncotarget. 7:44848–44856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang M, Liu YD, Wang YY, Liu TB, Ge TT and
Lou G: Ubiquitin-specific protease 22: A novel molecular biomarker
in cervical cancer prognosis and therapeutics. Tumour Biol.
35:929–934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council Committee for
the Update of the Guide for the C and Use of Laboratory A, . The
National Academies Collection: Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals. National Academies Press. (US) Copyright© 2011,
National Academy of Sciences. Washington (DC): 2011, PubMed/NCBI
|
|
14
|
Chen K, Yin L, Nie X, Deng Q, Wu Y, Zhu M,
Li D, Li M, Wu M and Huang X: β-catenin promotes host resistance
against Pseudomonas aeruginosa keratitis. J Infect.
67:584–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hazlett LD: Pathogenic mechanisms of P.
aeruginosa keratitis: A review of the role of T cells,
Langerhans cells, PMN, and cytokines. DNA Cell Biol. 21:383–390.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Lenardo MJ and Baltimore D: 30
Years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berger EA, McClellan SA, Barrett RP and
Hazlett LD: VIP promotes resistance in the Pseudomonas
aeruginosa-infected cornea by modulating adhesion molecule
expression. Invest Ophthalmol Vis Sci. 51:5776–5782. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ciornei CD, Novikov A, Beloin C, Fitting
C, Caroff M, Ghigo JM, Cavaillon JM and Adib-Conquy M:
Biofilm-forming Pseudomonas aeruginosa bacteria undergo
lipopolysaccharide structural modifications and induce enhanced
inflammatory cytokine response in human monocytes. Innate Immun.
16:288–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen K, Fu Q, Liang S, Liu Y, Qu W, Wu Y,
Wu X, Wei L, Wang Y, Xiong Y, et al: Stimulator of interferon genes
promotes host resistance against Pseudomonas aeruginosa
keratitis. Front Immunol. 9:12252018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh JY, Choi H, Lee RH, Roddy GW, Ylöstalo
JH, Wawrousek E and Prockop DJ: Identification of the
HSPB4/TLR2/NF-κB axis in macrophage as a therapeutic target for
sterile inflammation of the cornea. EMBO Mol Med. 4:435–448. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang F, Jiang Z, Li Y, He X, Zhao J, Yang
X, Zhu L, Yin Z, Li X, Wang X, et al: Shigella flexneri T3SS
effector IpaH4.5 modulates the host inflammatory response via
interaction with NF-κB p65 protein. Cell Microbiol. 15:474–485.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin J, Huang Z, Xia Y, Ma F, Zhang LJ, Ma
HH and Li Wang L: Lornoxicam suppresses recurrent herpetic stromal
keratitis through down-regulation of nuclear factor-kappaB: An
experimental study in mice. Mol Vis. 15:1252–1259. 2009.PubMed/NCBI
|
|
25
|
Glinsky GV, Berezovska O and Glinskii AB:
Microarray analysis identifies a death-from-cancer signature
predicting therapy failure in patients with multiple types of
cancer. J Clin Invest. 115:1503–1521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glinsky GV: Genomic models of metastatic
cancer: Functional analysis of death-from-cancer signature genes
reveals aneuploid, anoikis-resistant, metastasis-enabling phenotype
with altered cell cycle control and activated Polycomb Group (PcG)
protein chromatin silencing pathway. Cell Cycle. 5:1208–1216. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y, Lang G, Ito S, Bonnet J, Metzger
E, Sawatsubashi S, Suzuki E, Le Guezennec X, Stunnenberg HG,
Krasnov A, et al: A TFTC/STAGA module mediates histone H2A and H2B
deubiquitination, coactivates nuclear receptors, and counteracts
heterochromatin silencing. Mol Cell. 29:92–101. 2008. View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|