Introduction
Autism is a lifelong neurodevelopmental disorder and
one of the most severe mental health disorders in childhood. In
2013, the diagnosis of autism and several other disease categories
were incorporated into the singular diagnostic category of autism
spectrum disorder (ASD) (1,2).
Autism has a poor prognosis and a high disability rate, which
greatly increases family and social burdens (3). Currently, there is no cure available
for ASD, and its aetiology comprises environmental and genetic
variables. Rodent models based on genetic factors have been widely
used in preclinical research, such as the Fmr1-knockout mouse
model, the BTBR T + tf/J mouse model, the Nlgn-3 and Nlgn-4-mutant
mouse model, the Shank-mutant mouse model and the
Tsc2+/− mouse model (4). While ASD has a strong genetic
component, a wide range of environmental factors, including
prenatal exposure to intrauterine infections; drugs, such as
valproic acid (VPA) and thalidomide; or toxicants, such as
organophosphate insecticides or heavy metals, are also thought to
confer ASD susceptibility (4–7).
VPA, a broad-spectrum antiepileptic drug, has been used worldwide
to treat certain types of epileptic encephalopathy, as well as
bipolar disorder and migraine; exposure to VPA during pregnancy has
been demonstrated to increase the likelihood of autism in children
(5–8). The VPA mouse model, which has stable
face, construct and predictive validity, is a robust model of ASD
that has been used extensively to increase understanding of the
neurobiology underlying autistic behaviour and to screen novel
drugs for autism treatment (5,8–10).
Nevertheless, the underlying biological mechanism of ASD remains
undetermined, and consequently, no specific therapeutic strategy
has yet been established. There are currently a few pharmacological
treatments available, such as risperidone and aripiprazole, which
help relieve some symptoms, such as aggression, self-injurious
behaviour and anxiety, but do not address the core characteristics
of autism, such as impaired social interaction and restrictive
interests (8,11–13).
There is much evidence that the γ-aminobutyric acid
type B (GABA)ergic system is a potential therapeutic target for
core ASD symptoms (13,14). GABAergic system dysregulation is
common in clinical cases of ASD and has been proposed as a cause of
excitation-inhibition (E-I) imbalance (15–21). GABAergic system dysregulation and
E-I imbalance are commonly observed in rodent models of autism, and
correction of these disorders by pharmacological interventions can
normalize core autistic-like phenotypes in these animals (22,23). Notably, previous studies have
suggested that VPA rodent models exhibit GABAergic signalling
dysfunction, extensive alterations in neuronal morphology and local
neocortical microcircuit disorder (24–33). The GABAB receptor (GABABR2)
agonist STX209 is an exploratory drug comprising the single, active
R-enantiomer of baclofen (R-baclofen) and has the advantage of
being a biologically defined and active enantiomer (34,35). The potential of STX209 to treat
autism symptoms in VPA model mice has attracted our attention.
Previous studies have suggested that the exploratory
medicine STX209 (arbaclofen), a selective agonist of GABABR2, may
improve autism-associated behaviours in rodent models with certain
genetic defects (36–40); for example, intraperitoneal/oral
administration of STX209 was shown to exhibit favourable effects on
Fmr1-knockout mice (36,41). Although the results of the
majority of clinical trials have supported the therapeutic effects
of STX209 (42–45), the phase III clinical trial of
R-baclofen for the treatment of fragile X syndrome with the ASD
phenotype was prematurely terminated due to lack of efficacy
(46). These findings indicated
that GABABR2 agonists may be effective for some subgroups of
patients with ASD but not for all; therefore, STX209 may have the
potential to improve autism-like symptoms in some unknown subgroups
of patients with ASD. The present study focused on children with
ASD caused by environmental factors, such as VPA, have attracted
our attention. To the best of our knowledge, there are no relevant
reports of STX209 treatment in VPA-exposed mice.
The present study hypothesized that GABABR2 may
participate in the occurrence of the core behavioural symptoms of
ASD and that chronic administration of STX209 could reverse
autism-like behaviour in an animal model of autism induced by
prenatal exposure to VPA. A series of behavioural experiments was
designed to evaluate the therapeutic potential of STX209 on
autism-relevant behavioural phenotypes in VPA-exposed mice and the
possible mechanism was evaluated.
Materials and methods
Ethics approval
The present study was a preclinical study using a
rodent model. All experimental operations and tests were performed
at the Key Laboratory of Craniocerebral Disease, Ningxia Medical
University (Yinchuan, China). All mice were handled according to
protocols approved by the Institutional Animal Care and Use
Committee of Ningxia Medical University (IACUC Animal Use
Certificate no. 2019-152). All efforts were made to minimize the
number of animals used and their suffering.
Animals
Breeding pairs of C57BL/6J mice (age, 10 weeks;
weight of female mice, 20–25 g; weight of male mice, 22–25 g) were
purchased from Ningxia Medical University Laboratory Animal Center
and housed in a conventional mouse vivarium at the Feeding Unit of
Ningxia Medical University Craniocerebral Laboratory. The total
number of mice purchased for mating was 30 (10 males and 20
female). Each male mouse was housed in a single cage and female
mice were housed in pairs(random allocation). Standard rodent chow
and tap water were available ad libitum. All mice were
maintained under standard laboratory conditions at 22±2°C with
50±10% relative humidity and under a 12-h light/dark cycle. A total
of 1 week after entering the feeding unit, precontact between male
and female mice was conducted for 3 days to regulate the fertility
cycle; when the female mice were in a proestrus state, the animals
were allowed to mate overnight (5 p.m. to 8 a.m. the next day).
Detection of a vaginal plug in female mice was designated as half a
day of pregnancy. The pregnant mice were then housed separately and
divided into vehicle- and VPA-treated groups. Because of precontact
(male and female mice were housed in the same cage, but separated
by isolation nets to prevent mating), female pregnancy and pup
birth could be concentrated within a 3-day period. In the present
study, 10 male mice and 20 female mice were used for mating. After
the offspring were weaned (postnatal day 21, P21), the parent mice
were euthanized by CO2 using a Laboratory Animal
Asphyxiator (SMQ-II; Shanghai Minly Lab Hi-tech Development Co.,
Ltd.) (47). The following model
of general euthanasia was used: Increased CO2
concentration from 0–90%/5–6 min; the CO2 replacement
rate was 60% CO2/min in a chamber, according to the AVMA
Guidelines for the Euthanasia of Animals, 2020 (48). Death was confirmed by the absence
of a heartbeat and breathing.
Prenatal VPA exposure
VPA (MilliporeSigma) was dissolved in 0.9% normal
saline (NS) at a 10 mg/ml concentration. Prenatal VPA exposure was
induced according to a novel method first described by Zheng et
al (49); female mice in the
VPA-treated group received two doses of 300 mg/kg VPA on embryonic
day 10 (E10) and E12 (Fig. 1A);
female mice in the control group were injected with the same amount
of NS on these days. Prior to initiation of this experiment,
several relevant studies were consulted; most reports of this
rodent model administered a single injection of VPA at doses
ranging from 300 to 800 mg/kg between E9-12.5, and 500 mg/kg was
the usual dose (8,24,50). We were cautious about reducing the
dose, as this may increase the risk of an imperfect phenotype of
VPA model mice and it was unclear whether this would affect their
autism-like behaviour. Increased doses may result in an increase in
the rate of miscarriage; this does not conform to animal
theory.
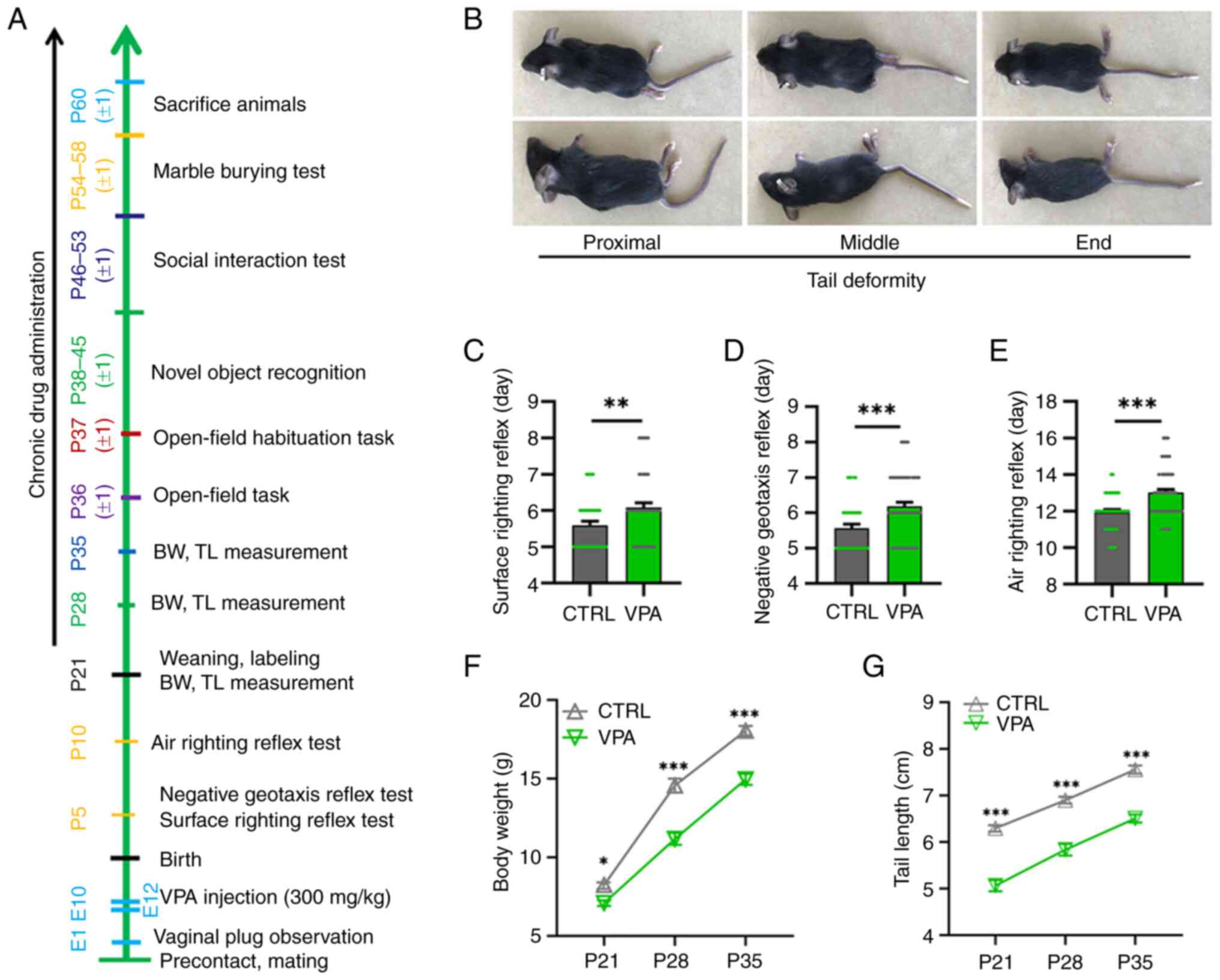 | Figure 1.VPA model mice exhibit delayed
nervous reflex development, delayed growth and tail malformations.
(A) A representative timeline of the experimental approach. Mice
were sacrificed ~60 days after birth for Golgi staining and western
blotting. (B) Images of the prone and lateral positions of three
VPA model mice with deformities in different parts of the tail. All
VPA model mice exhibited tail malformation to different extents.
VPA model pups (n=61, male pups and female pups) exhibited
significantly longer latencies, including latencies to the first
appearance of the (C) surface righting reflex, (D) negative
geotaxis reflex and (E) air righting reflex, than CTRL pups (n=39,
male pups and female pups) in the behavioural tests. **P<0.01,
***P<0.001 (Student's unpaired t-test). (F) BW and (G) TL of VPA
model mice (n=28, only male offspring, contained unpublished data)
were lower than those of CTRL mice from P21 to P35 (n=23, only male
offspring, contained unpublished data). *P<0.05, ***P<0.001
(two-way mixed ANOVA followed by Bonferroni post hoc test). All
data are presented as the mean ± SEM. BW, body weight; CTRL,
control; E, embryonic day; P, postnatal day; TL, tail length; VPA,
valproic acid. |
The new method of administration is closer to the
pathological process that induces ASD after repeated administration
of VPA in the clinic as previously described (49). Foetal mice receive double
administration (double blow) during the critical period of brain
development, and the blows last longer and are more stable.
Compared with the traditional method, this will obviously cause
more serious damage to the brain development of the offspring, and
because low doses of VPA are applied at intervals, the new method
is gentler on the pregnant mice (49). Clinically, the maximum dose of VPA
medication for patients is 30 mg/kg, which is completely absorbed
1–4 h after medication, and reaches a peak value. It is mainly
metabolized by the liver and excreted by the kidneys (49). According to the new method
protocol, after birth, female mice raised their litters. Male
offspring were weaned on P21 and were labelled with ear tags. The
ASD-related behaviour of male VPA model mice has been reported to
be more stable than that of female VPA model mice (5,10,51); therefore, only male offspring (all
mice, n=56; prenatal VPA exposure groups contained VPA group and
VPA + STX209 group, n=28; no prenatal VPA exposure groups contained
CTRL group and CTRL + STX209 group, n=28; n=14/group) were used for
subsequent experiments. The male offspring were administered
isoflurane anaesthesia (4%) at approximately P60 and once it was
confirmed they had been satisfactorily anaesthetized, they were
sacrificed by decapitation (Fig.
1A). Female offspring did not enter the experimental process
and were euthanized by CO2 using a Laboratory Animal
Asphyxiator after they were weaned (P21). The following model of
general euthanasia was used: Increased CO2 concentration
from 0–90%/5–6 min. the CO2 replacement rate was 60%
CO2/min in a chamber as aforementioned. Death was
confirmed by the absence of a heartbeat and breathing. All male
offspring from the same litter were equally distributed to the
STX209 administration groups (CTRL+STX20 group, n=14; VPA+STX209
group, n=14) and the no administration groups (CTRL group, n=14;
VPA group, n=14) to eliminate the litter effect (52).
Drug administration
STX209 (MedChemExpress) was administered
intraperitoneally to CTRL+STX20 and VPA+STX209 group mice at a dose
of 0.6 mg/kg in 0.9% NS twice a day from weaning (P21) until the
end of the experiment (approximately P60) (Fig. 1A). The STX209 dose was selected
based on the effective dose in neuropathy and children with ASD in
experimental animal studies and clinical trials (36,41,42). Injections were given at 8:00 a.m.
and 8:00 p.m.
Neurodevelopmental behaviour
tests
To assess the success and efficiency of the modified
method in generating the mouse model of VPA-induced autism, a
battery of tests was designed and utilized to evaluate
developmental milestones (24,53,54). The sequence and timing of the
tests are shown in Fig. 1A. The
day of onset was recorded and positive reflexes were confirmed the
following day. Because sex of pups was hard to distinguish, we
tested all pups in the VPA (n=61) and CTRL group offspring
(n=39).
Surface righting reflex
Beginning on P5, all new-born mice were placed in
the supine position on a board, and the first appearance of the
surface righting reflex was defined as the ability of the pups to
turn over to the prone position and stand with all four paws in
contact with the board within 10 sec. All pups were checked and
recorded every day until they all reached the standard.
Negative geotaxis reflex
Beginning on P5, pups were placed facing downwards
on a rough wooden slope with a 30° incline, held there for 3 sec
and then released. The ability of the pups to turn to face upwards
(rotate 180°) within 30 sec was assessed. The first day the animals
appeared to successfully accomplish the task was recorded.
Air righting reflex
Pups were dropped naturally from the supine position
onto soft bedding (30 cm high). A mouse was considered to exhibit a
positive reflex if it landed in a normal prone position (belly
facing downwards against the bedding). The test was started on
P10.
Growth and development
The body weight (BW) and tail length (TL) of each
mouse pup (male VPA pups: n=28, contained unpublished data; male
CTRL pups: n=23, contained unpublished data) were recorded on days
21, 28 and 35 after birth. BW was measured by putting the mouse on
a balance and reading the measurement after the mouse rested for 2
sec. Pups were weighed twice and the mean was calculated. Pup TL
measurement started from the root of the tail and the length of the
tail was measured in a straight state. The crooked tail of mice was
observed easily after P10 in the VPA group (Fig. 1B).
Autism-related behaviour tests
The sequence and timing of the tests are shown in
Fig. 1A. All behaviours of the
animals were recorded using a computerized video tracking system
(SMART 3.0; Panlab).
Social interaction test
Mice were tested in a three-chambered social
approach apparatus as previously described with slight
modifications (cages placed diagonally across chambers) (37,38). The test had two tested phases: i)
The sociability phase (scene 1), in which an unfamiliar mouse
(stranger 1) was placed inside a plastic cage in one of the side
chambers, an empty cage was placed in the other chamber, and the
test mouse was allowed to freely explore the apparatus for 5 min;
and ii) the preference for social novelty phase (scene 2), in which
a second unfamiliar mouse (stranger 2) was placed inside the cage
in the opposite chamber and the test mouse was allowed to freely
explore the apparatus for 5 min. The total time spent in each
region and the time spent sniffing the stranger mouse and the empty
cage were recorded. The social preference index (SPI) was
calculated as follows: SPI=(time spent in the region containing
stranger 2)/(time spent in the region containing stranger 1 + time
spent in the region containing stranger 2) in scene 2. The test
started at ~9:30 AM.
Novel object recognition task
The novel object recognition task was performed as
previously reported (40,55) with slight modifications (clear
distinction between white plastic bottle and black glass bottle,
which were used as familiar and novel object, respectively). The
animals were placed in a box containing two identical objects
(white plastic bottle) and allowed to explore for 5 min (scene 1).
Subsequently, one object was replaced with a novel object (black
glass bottle) and the animals were allowed to explore the objects
for 5 min (scene 2) after an interval of 30 sec. The discrimination
index (DI) was calculated as follows: DI=(time spent exploring the
novel object)/(time spent exploring the novel object + time spent
exploring the familiar object). The task started at ~9:30 AM.
Open-field task and open-field
habituation task
The open-field boxes used for this assessment were
made of wood (50.0×50.0×40.0 cm) and an overhead camera was used
for automatic tracking of animal behaviours using SMART 3.0. The
box was divided into two zones: An ‘inner’ zone (a 30×30
cm2 central square) and an ‘outer’ zone (10 cm from the
walls). The duration of the test was 10 min.
Inspired by a previous study (40), the open-field test was repeated at
24-h intervals and the same indexes [time travelled in the inner
area, the distance travelled in the inner area and open-field
exploration index (OFEI)] were measured for every mouse. OFEI was
calculated as follows: OFEI=distance travelled in the inner
zone/the total distance travelled. The task started at ~9:30
a.m.
Marble burying test
The marble burying test was performed as previously
reported (36,37,50) with slight modifications. The
tested mouse was placed in a black cage containing 16 marbles
arranged in a 4×4 grid on clean rice husk bedding <5 cm in
height. A mouse was placed in a standard mouse cage containing 16
marbles arranged in a 4×4 grid on clean rice husk bedding. The
duration of the test was 10 min. Marbles with >75% of their
surface buried in the bedding were counted and recorded. Digital
images and videos of the marbles were captured during the test
period. The numbers of buried marbles and burying actions (strong
and obvious digging or burial movement) were counted from the
digital images and videos by trained investigators (n=3) who were
blinded to the group allocations and the mean result was rounded.
The test started at approximately 09:30 p.m.
Golgi-Cox staining
Golgi staining is used to provide valuable
information regarding the neural morphology and quantitative
assessments such as dendritic spine number, dendritic length
measurements (33). We used the
Golgi-Cox staining method to observe the neuronal dendritic length
and spine number of CA1, DG region of mice. After deep anesthesia
was induced with isoflurane as aforementioned, the mice were
decapitated, and the brains were removed and soaked in mixed AB
liquid immediately (FD Rapid GolgiStain™ kit,
NeuroTechnologies). After 3 weeks, brain tissues that were
completely infiltrated with mixed AB liquid were sliced with a
vibrating slicer (VT1000S; Leica, GmbH) and then brain slices were
placed on the glass dish, soaked in liquid C. The thickness of each
slice was 100 µm. After 5 days, slices were stained with mixed DE
liquid (solution D:solution E:distilled water; 1:1:2) for 10 min,
after which the slices were rinsed with distilled water, dehydrated
with an ascending series of ethanol solution and cleared in xylene
for >2 h. Finally, the slices were sealed on slides with neutral
resin and dried in the dark.
Neurolucida 360 imaging and Sholl
analysis
Z-stack images of neurons were captured using a
Leica DM6 fluorescence microscope (Leica Microsystems GmbH). 3D
reconstruction of neurons from the images and Sholl analysis were
performed with Neurolucida 360 software (MBF Biosciences) on the
Public Technology Platform of Zhejiang University (Hangzhou,
China).
Dendritic spine analysis
Images of spines were obtained with the Extended
Depth of Focus module of a Nikon orthotopic light microscope (Nikon
Corporation). 3D dendritic spine images were combined into a plan
view and the manual counting mode of ImageJ analysis software
(National Institutes of Health, 2020 version) was used to evaluate
the images. Spines were classified into subtypes based on the width
and length of the spine by three investigators who were blinded to
the group allocations and mean values were calculated.
Western blotting
Total proteins were extracted from the hippocampus
of mice using the Protein Extraction Kit (cat. no. KGP2100; Nanjing
KeyGen Biotech Co., Ltd.) and the protein concentration was
measured using the BCA Protein Assay Kit (cat. no. KGP902; Nanjing
KeyGen Biotech Co., Ltd.). Equal amounts of protein (60–80 µg/lane)
were separated by SDS-PAGE on 8 or 10% gels and were then
transferred to PVDF membranes. When the protein transfer was
completed, membranes were blocked with 5% non-fat milk for 1 h in
room temperature, followed by incubation for ~20 h at 4°C with
rabbit anti-GABABR2 (1:500; cat. no. ab230136), anti-glutamic acid
decarboxylase (GAD)65/67 (1:500 cat. no. ab183999) and anti-GAPDH
(1:1,000; cat. no. ab181602) antibodies. Subsequently, the
membranes were washed with TBS-Tween (0.5%) three times (5
min/wash) and were further incubated with the corresponding goat
anti-rabbit IgG secondary antibody (1:1,000; LI-COR Biosciences
cat. no. P/N: 926-32211) for 2 h in room temperature. GAPDH served
as an internal reference. Semi-quantification of bands was
performed from optical density values using the Odyssey CLX
instrument system (LI-COR Biosciences).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical
analysis was performed using GraphPad Prism 8.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference. The BW and TL data were analysed using
two-way mixed ANOVA [independent variables: Treatment (VPA or
control) and time]. Neurodevelopmental behaviour test data were
analysed using unpaired Student's t-test. Social interaction test,
novel object recognition task, Golgi-Cox staining and western
blotting data were analysed using two-way ANOVA [independent
variables: Drug (STX209 or control) and treatment (VPA or
control)]. Open-field test (task 1) and open-field habituation test
(task 2) data were analysed using paired Student's t-test for
comparing the differences between different tasks in the same group
of mice and three-way mixed ANOVA for comparing the differences
among groups of mice in the same task.independent variables: Drug
(STX209 or control), treatment (VPA or control) and scene (1 or
2)]. Marble burying test data were analysed using Pearson
correlation, linear regression analysis or two-way ANOVA
[independent variables: Drug (STX209 or control) and treatment (VPA
or control)]. All two-way and three-way mixed ANOVAs were followed
by Bonferroni post hoc test to compare the differences among the
groups.
Results
Tail malformations in VPA model
mice
Neural tube defects (NTDs) can result from genetic
mutations, malnutrition or exposure to teratogens during gestation
(56). VPA is a known inducer of
NTDs and causes a crooked tail phenotype (a mild NTD sign), which
is often used as a sign of successful modelling in VPA rodent
models of ASD (5,50,57). The crooked tail phenotype was
observed in all VPA model mice in the present study, indicating
successful induction of an ASD model (Fig. 1B).
VPA model mice exhibit delayed nervous
reflex development
As shown in Fig.
1C-E, prenatal VPA-exposed mice exhibited significantly longer
latencies in behavioural ontogeny compared with control mice,
including the first appearance of the surface righting reflex
(P=0.0084; Fig. 1C), negative
geotaxis reflex (P=0.0007; Fig.
1D) and air righting reflex (P<0.0001; Fig. 1E). These results indicated that
prenatal VPA exposure had a neurotoxic effect on offspring mice and
that VPA model mice exhibited nervous reflex developmental defects
in the present experiment.
Growth retardation in VPA model
mice
The BW and TL of pups were measured every week
between P21 and P35 (Fig. 1A).
The BW of VPA mice was significantly smaller than that of control
mice at P21-P35 (BW: P21, P=0.0429; P28, P<0.0001; P35,
P<0.0001; Fig. 1F). The TL of
VPA mice was also significantly shorter than that of control mice
at P21–35 (P21, P<0.0001; P28, P<0.0001; P35, P<0.0001;
Fig. 1G). These results indicated
that VPA mice exhibited severe growth retardation postnatally.
STX209 ameliorates the sociability
deficits of VPA model mice
During scene 1, sociability is defined as the
propensity to spend time in the cage containing stranger 1 compared
with time spent alone in the identical but empty opposite cage
(4). The session indicates the
interest in social cues of the tested mouse (4,58,59). The analysis results of the trace
images and heat images showed (Fig.
2A), mice in the VPA group spent less time in the cage
containing stranger 1 than mice in the control group (P=0.0044) and
VPA + STX209 group (P=0.0280) in scene 1 (Fig. 2B). By contrast, in scene 1, mice
in the VPA group spent more time in the empty cage region (control
vs. VPA, P=0.0012; VPA vs. VPA + STX209, P=0.0323; Fig. 2C). These results revealed that VPA
mice exhibited obvious sociability deficits and that STX209
ameliorated these deficits.
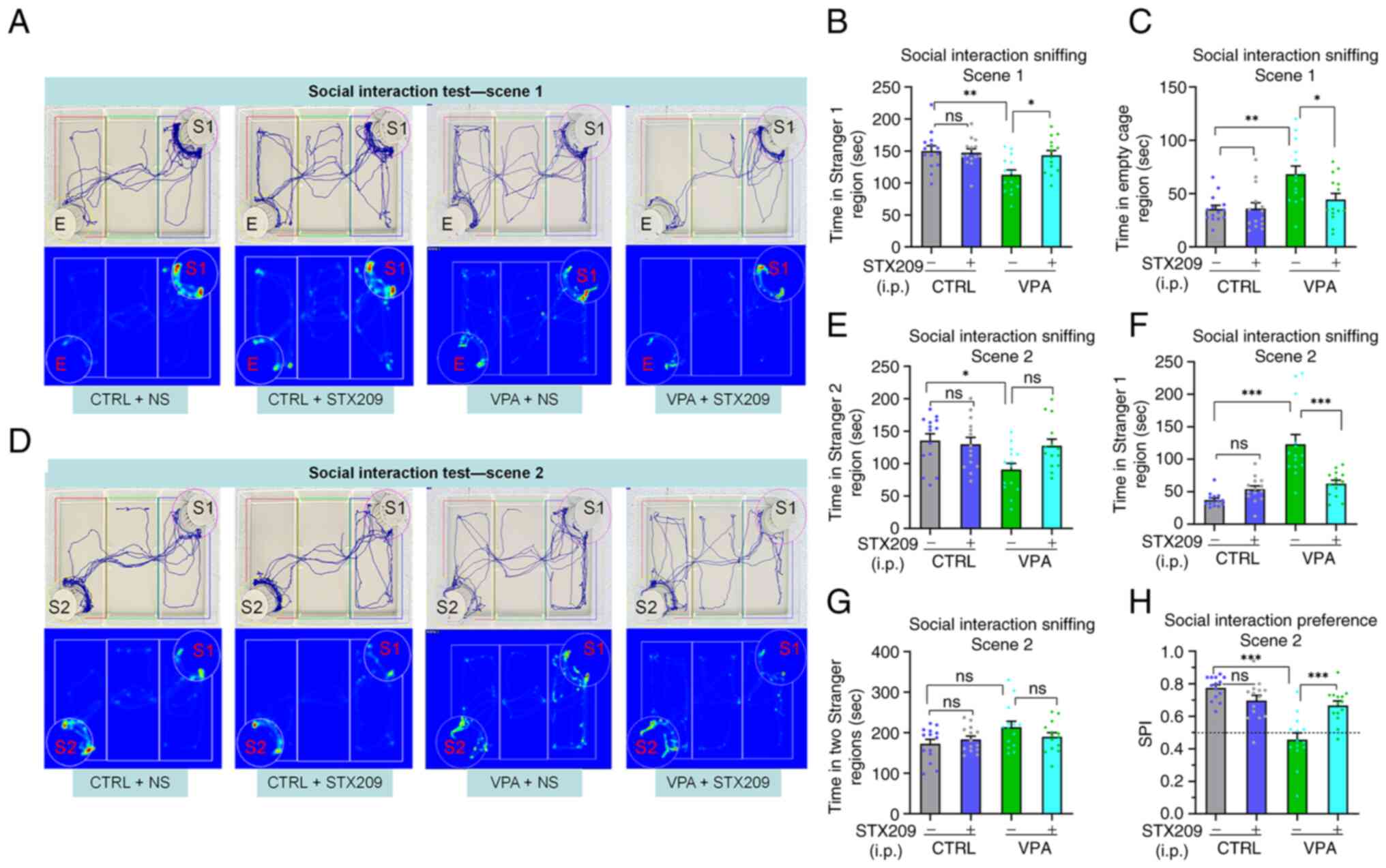 | Figure 2.Treatment with STX209 ameliorates
sociability deficits and preference for social novelty deficits in
VPA model mice. (A) Representative trace and heatmap images from
tested mice in scene 1 in the social interaction test (E, empty;
S1, stranger 1). (B) Time that tested mice entered the region
containing stranger 1 for sniffing in scene 1. (C) Time that tested
mice entered the empty cage region for sniffing in scene 1. (D)
Representative trace and heatmap images from tested mice in scene 2
in the social interaction test (E, empty; S1, stranger 1; S2,
stranger 2). (E) Time that tested mice entered the region
containing stranger 2 for sniffing in scene 2. (F) Time that tested
mice entered the region containing stranger 1 for sniffing in scene
2. (G) Total time that tested mice entered the region containing
stranger 2 and stranger 1 for sniffing in scene 2. (H) SPI of
tested mice in scene 2. *P<0.05, **P<0.01, ***P<0.001
(two-way ANOVA followed by Bonferroni post hoc test). All data are
presented as the mean ± SEM. Each group had 14 mice. CTRL, control;
NS, normal saline; SPI, social preference index; VPA, valproic
acid; ns, no significance. |
STX209 ameliorates the preference for
social novelty deficits of VPA model mice
During scene 2, preference for social novelty is
defined as the propensity to spend time with a new stimulus rather
than with the same stimulus encountered in scene 1 (4,58,59). The session indicates the interest
in new social cues of the tested mouse.
In scene 2 (Fig.
2D), VPA model mice spent less time in the cage containing
stranger 2 than mice in the control group (P=0.0175; Fig. 2E); in addition, the average time
the VPA group spent in the cage containing stranger 2 was less than
that of the VPA + STX209 group, but the difference was not
significant (P=0.0803; Fig. 2E).
By contrast, in scene 2, mice in the VPA group spent more time in
the cage containing stranger 1 than mice in the control group
(P<0.0001) and VPA + STX209 group (P<0.0001) (Fig. 2F). Notably, there were no
significant differences among the mice of the four groups in scene
2 with regard to total time in both cages (Fig. 2G).
Moreover, the SPI of VPA model mice was lower than
that of mice in the control group (P<0.0001); after STX209
treatment, the SPI of VPA model mice was significantly increased
(P<0.0001) (Fig. 2H). These
results revealed that VPA mice exhibited social novelty deficits,
whereas STX209 treatment ameliorated the deficits of VPA mice.
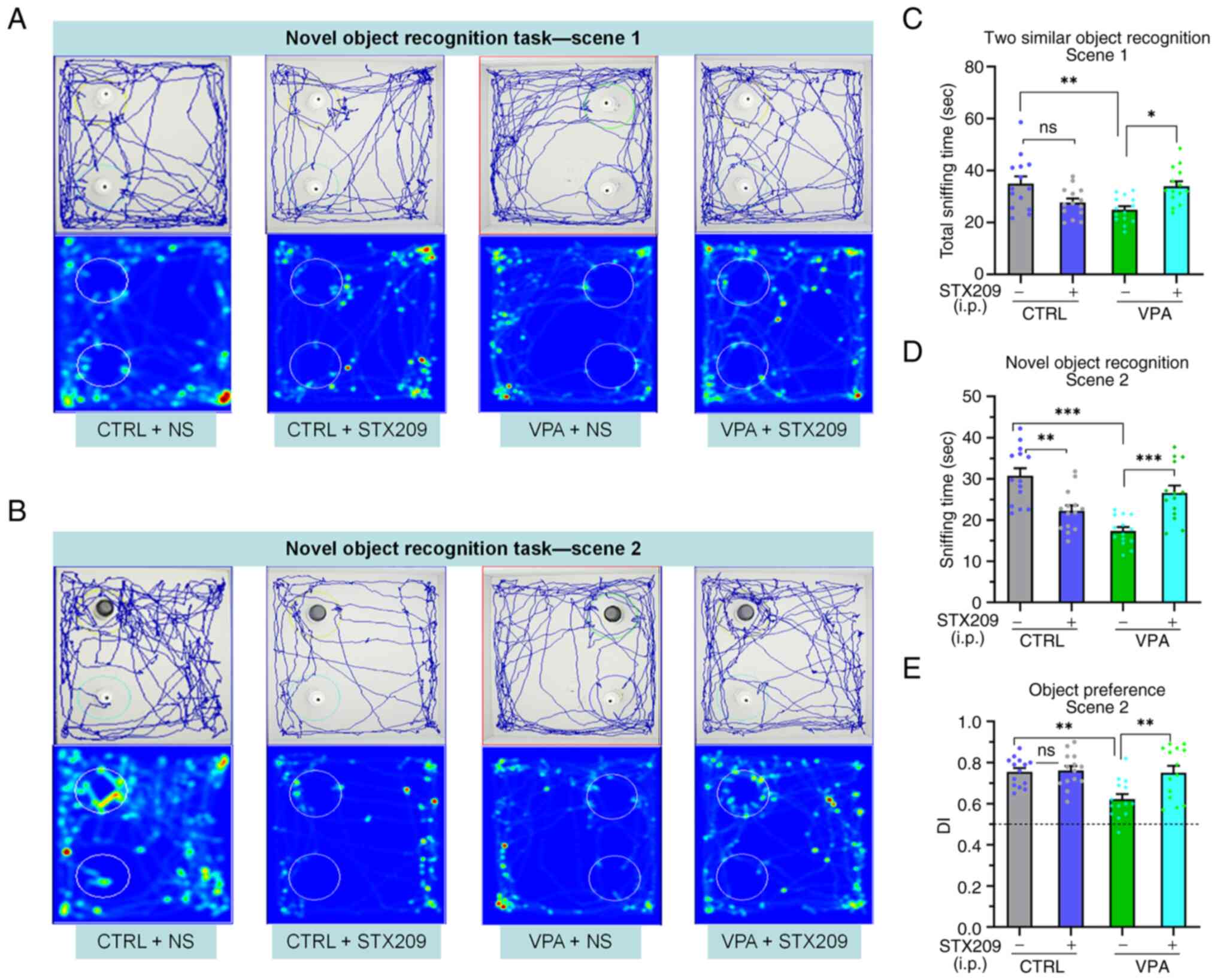
STX209 ameliorates the novelty
recognition deficits of VPA model mice
Similar to previous reports of other ASD model mice
(40), VPA model mice exhibited
novel object preference deficits compared with the control mice, as
demonstrated by VPA model mice spending less time in regions
containing the familiar objects in scene 1 and the novel object in
scene 2; scene 1, P=0.0042; scene 2, P<0.0001; Fig. 3C and D). Chronic STX209 treatment
increased the amount of time VPA model mice spent in the region
containing the novel object (scene 1, P=0.0125; scene 2, P=0.0005;
Fig. 3A-D). The DI is a valuable
index that reflects object recognition memory and preference for
novel objects. The results revealed that the DI of the VPA group
mice was lower than that of the control group mice (P=0.0030) and
VPA + STX209 group mice (P=0.0043) (Fig. 3E). These results revealed that
STX209 treatment ameliorated the novel object preference deficits
of VPA model mice.
In addition, STX209 caused a reduction in the time
control mice spent exploring the novel object in scene 2 (control
vs. control + STX209, P=0.0015; Fig.
3D), but it did not significantly affect the DI (P>0.9999;
Fig. 3E), indicating that STX209
affected only the sniffing time in control mice but had no effect
on the preference for novel objects.
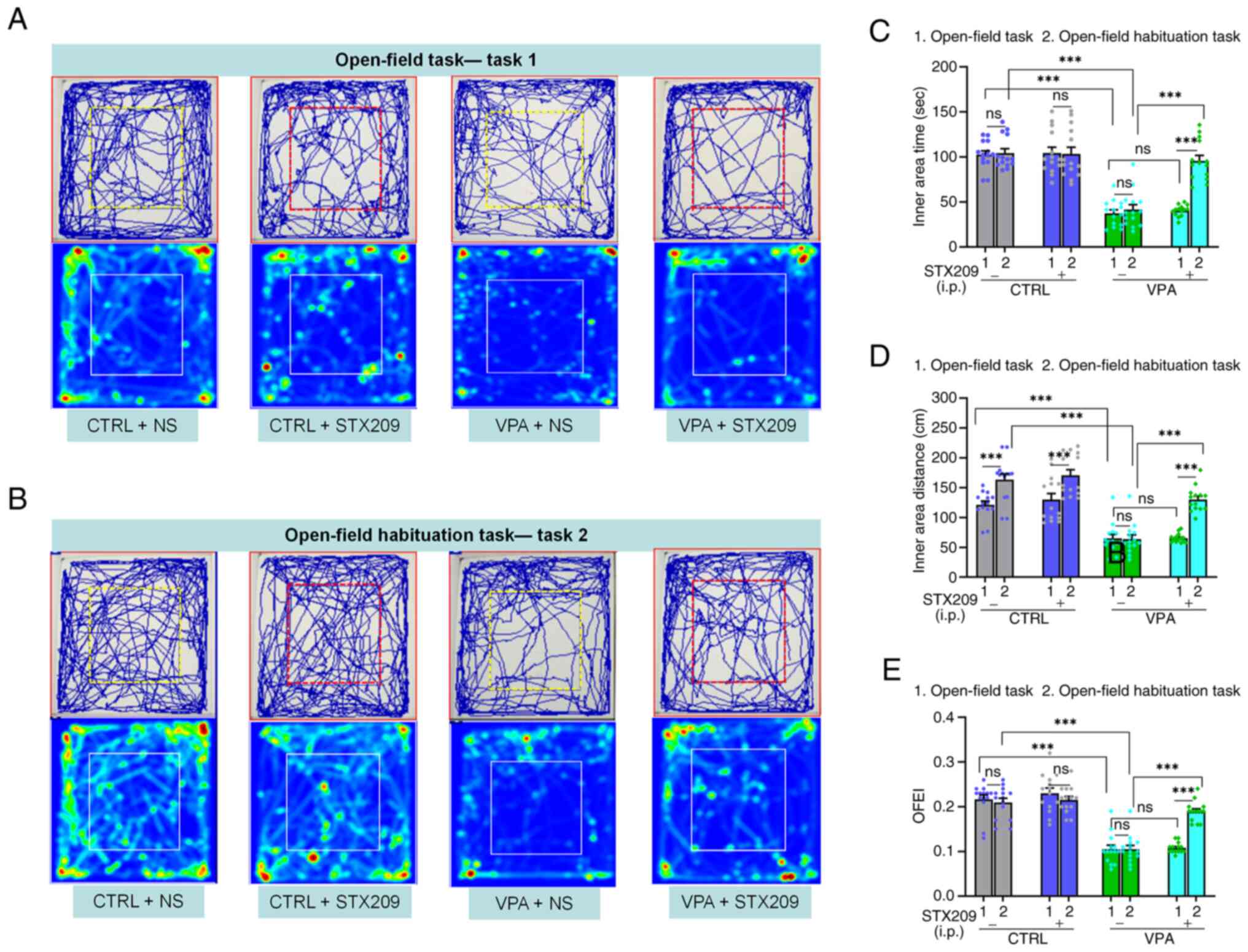
STX209 ameliorates the locomotion and
exploration activity deficits of VPA model mice
In the open-field task (task 1), VPA model mice
exhibited reduced locomotion and exploratory behaviour compared
with control mice, as determined by decreases in the time travelled
in the inner area (P<0.0001; Fig.
4A and C), the distance travelled in the inner area
(P<0.0001; Fig. 4D) and the
OFEI (P<0.0001; Fig. 4E).
STX209 did not exhibit a positive effect on ameliorating locomotion
or exploratory behaviour in VPA mice (P>0.9999, Fig. 4C; P>0.9999, Fig. 4D; P>0.9999, Fig. 4E).
Inspired by a previous study (40), the open-field habituation task
(task 2; two tasks spaced 24 h apart) was redesigned to further
evaluate the therapeutic effect of STX209. As Fig. 4B showed, in contrast to task 1,
mice in the control group and VPA + STX209 group exhibited an
increase in locomotion behaviour in task 2 (control group,
P<0.0001; VPA + STX209 group, P<0.0001; Fig. 4D), although VPA model mice did not
(P=0.9217; Fig. 4D). In addition,
in task 2, STX209 treatment had a positive role in ameliorating the
exploratory activity deficits of VPA model mice (P<0.0001,
Fig. 4C; P<0.0001, Fig. 4D; P<0.0001, Fig. 4E).
The difference in the results of the two tasks may
have been related to a decrease in anxiety and fear, which may have
been caused by previous experience exploring the same apparatus and
familiarity with the environment. Therefore, treatment with STX209
markedly ameliorated the deficit of the VPA model mice in
recognizing a familiar environment in the open-field habituation
task.
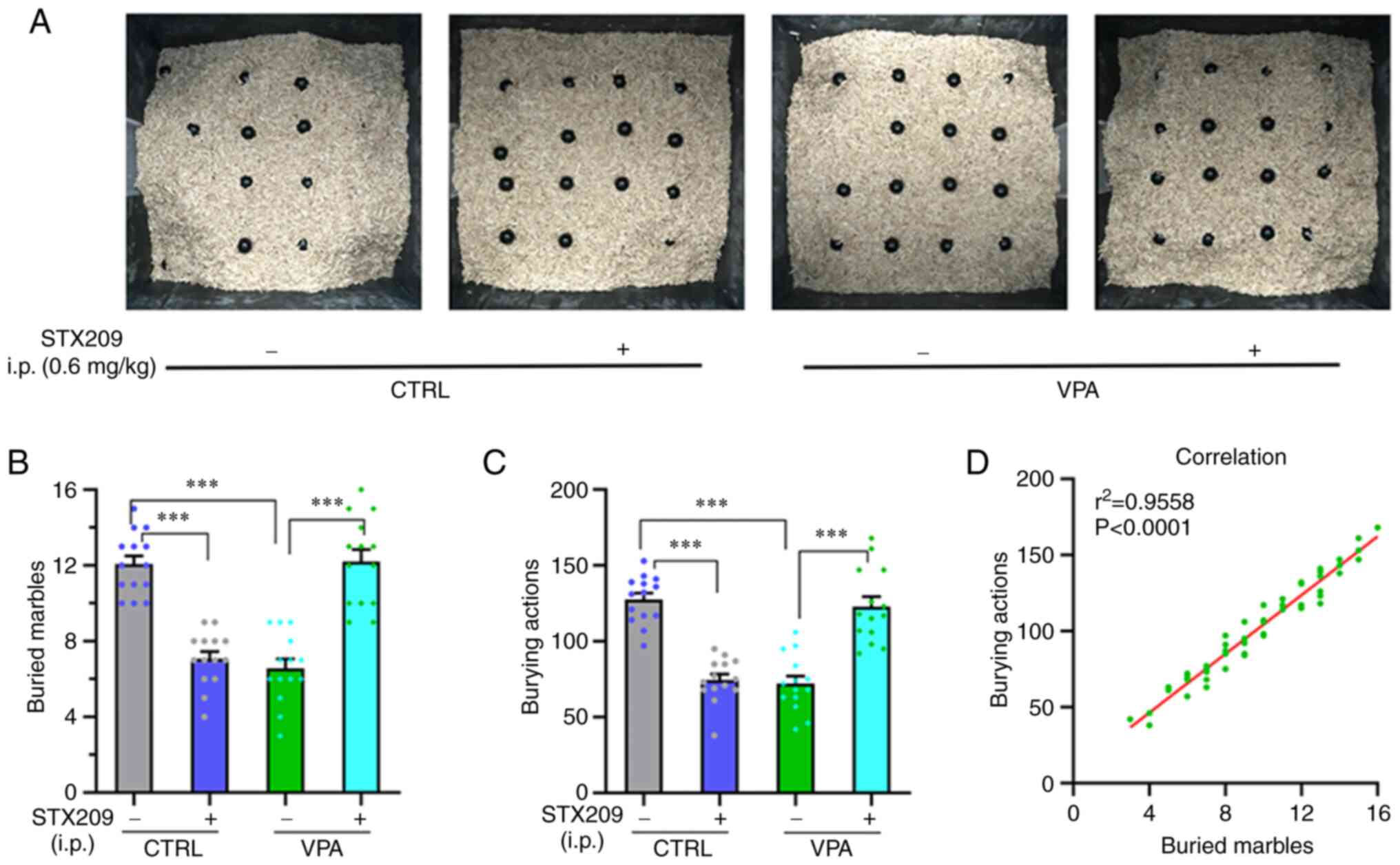
Treatment with STX209 ameliorates the
marble-burying deficits in VPA model mice
As shown in Fig.
5A, the numbers of buried marbles and burying actions were
smaller in the VPA group compared with in the control group
(P<0.0001, Fig. 5B;
P<0.0001, Fig. 5C), indicating
that VPA model mice have marble-burying deficits. Chronic STX209
treatment increased these parameters in the VPA group (P<0.0001,
Fig. 5B; P<0.0001, Fig. 5C), indicating that STX209 had a
certain therapeutic effect on the marble-burying deficit of VPA
model mice.
In addition, STX209 inhibited marble burying in
control mice (control group vs. control + STX209 group,
P<0.0001, Fig. 5B;
P<0.0001, Fig. 5C). This
result may be related to the fact that GABABR2 agonists are
anti-anxiety drugs and that selective inhibition of burying
behaviour in rodents is thought to be an effect of anti-anxiety
drugs, which is consistent with a previous study (36).
Linear correlation between the number
of buried marbles and the number of burying actions
The number of marbles buried and the burying actions
of all groups of mice were counted and linear regression between
them was determined (P<0.0001, r2=0.9558/y=9.653 * ×
+ 7.735; Fig. 5D). This showed
that the marble burying actions in the test were effective, and the
time of test is appropriate.
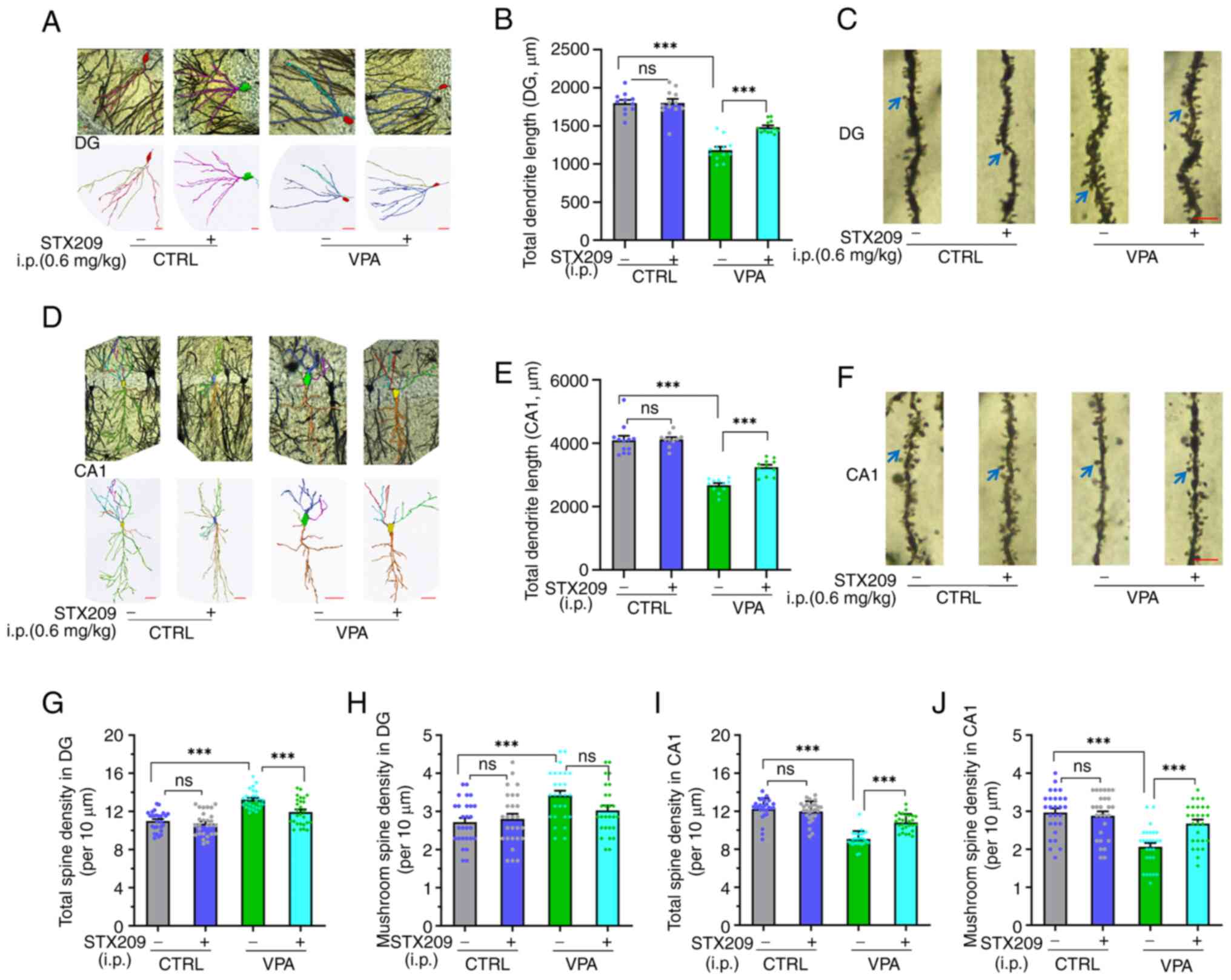
Prenatal VPA exposure causes
rearrangement of neuronal dendrites and spines in the hippocampi of
VPA model mice, and STX209 ameliorates these neuronal structural
defects
Compared with in the control group, Sholl analysis
indicated that the total dendritic length of neurons in the dentate
gyrus (DG) region was reduced in the VPA model group (P<0.0001;
Fig. 6A and B), indicating
neuronal development defects in VPA model mice. Chronic
administration of STX209 ameliorated dendritic length (P<0.0001;
Fig. 6B). Quantitative
morphological analysis of showed that compared with the control
group, density of total spines and mushroom spines at the basal
dendritic terminals of pyramidal neurons in the hippocampal DG
region were increased in the VPA model group (P<0.0001, Fig. 6C and G; P=0.0008, Fig. 6H), indicating that neuronal
connectivity in DG region of VPA model mice was abnormally
increased. Chronic administration of STX209 ameliorated the defects
in total spine density (P=0.0006; Fig. 6G) in VPA model mice; however,
STX209 did not exhibit a significant difference in ameliorating the
defects of mushroom spine density (P=0.1679; Fig. 6H).
Similar to neuronal dendrites in the DG region, as
Fig. 6D showed, the total
dendritic length of neurons in the CA1 region in the VPA model
group was also reduced compared with in the control group
(P<0.0001; Fig. 6E). Chronic
administration of STX209 ameliorated the reduction in dendritic
length (P=0.0009; Fig. 6E) in VPA
model mice. Unlike that in the DG region (Fig. 6F), compared with in the CTRL
group, density of total spines and mushroom spines at the basal
dendritic terminals of pyramidal neurons in the hippocampal CA1
region were decreased in the VPA group (P<0.0001, Fig. 6I; P<0.0001, Fig. 6J), indicating that the neuronal
connectivity in CA1 region of VPA model mice was abnormally
decreased. Chronic administration of STX209 also ameliorated this
defect (P<0.0001, Fig. 6J;
P=0.0007, Fig. 6K) in VPA model
mice.
In conclusion, examination of hippocampal morphology
in this animal model revealed that VPA exposure in utero
altered the dendritic morphology and dendritic spine number of
neurons in the CA1 and DG regions of the hippocampus in offspring.
The dendritic arbors of neurons in the CA1 and DG regions in the
VPA model mice were dysplastic, and the total dendritic length was
abnormal. Chronic administration of STX209 ameliorated these
neuronal structural defects in VPA model mice.
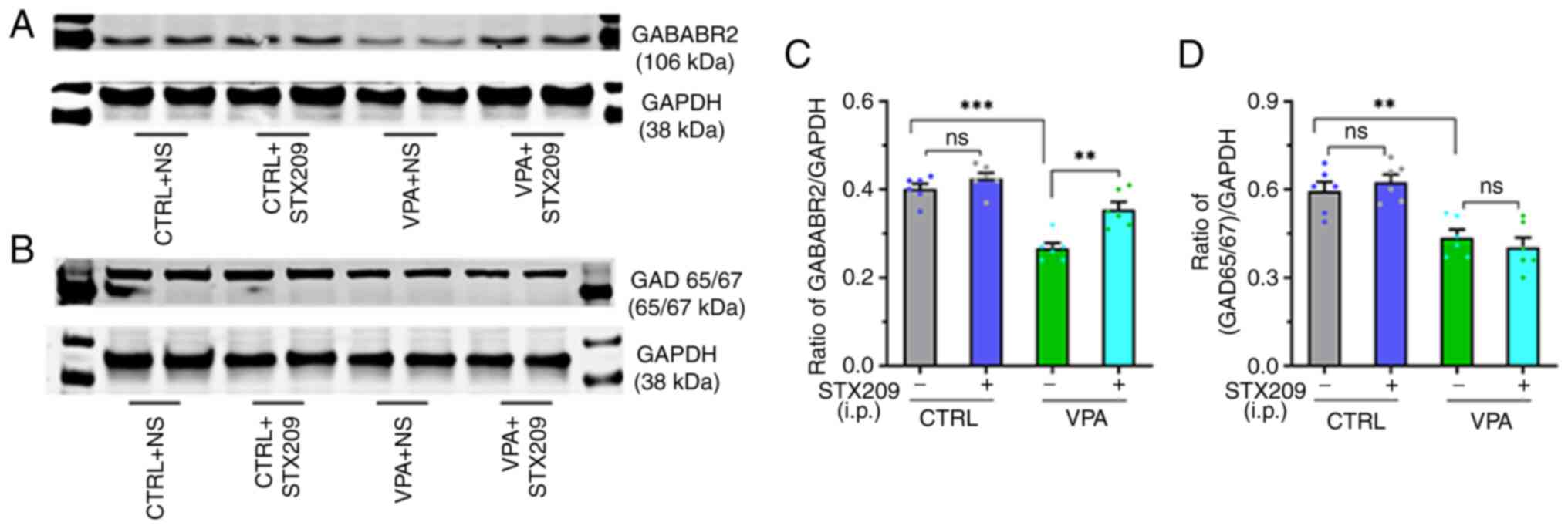
STX209 ameliorates GABAergic system
impairment in VPA model mice
According to the results of western blot analysis
(Fig. 7A and B), the protein
expression levels of GAD65/67 and GABABR2 were decreased in the
hippocampus of VPA model mice compared with those in control mice
(GABABR2, P<0.0001, Fig. 7C;
GAD65/67, P=0.0071, Fig. 7D). By
contrast, STX209 treatment increased the expression levels of
GABABR2 in VPA model mice (P=0.0010, Fig. 7C), although it did not increase
the expression levels of GAD65/67 (P>0.9999, Fig. 7D). These results suggested that
prenatal VPA exposure may lead to dysregulation of the hippocampal
GABAergic system in the brains of offspring mice. After chronic
administration of STX209, the ability of the hippocampal GABAergic
system to produce GABA did not improve, but the GABAergic pathway
transduction ability was enhanced.
Discussion
To the best of our knowledge, the present
preclinical study is the first to focus on the potential use of the
GABABR2 agonist STX209 as a treatment for VPA model mice; this
model is used for simulating children with ASD who were exposed to
VPA in utero. In the present study, STX209 significantly
ameliorated core autism-like behaviours in VPA model mice. The
possible mechanism underlying the behavioural improvement in VPA
model mice may have involved the amelioration of alterations in the
structure and function of the hippocampus, including promotion of
neuronal growth and remodelling, synapse formation, and pruning and
improvement of GABAergic pathway function.
Based on the multifactorial aetiology and complex
pathophysiology of ASD, there is no animal model that can capture,
at once, all of the molecular, cellular and behavioural features of
ASD. Moreover, ASD is defined by specific behavioural
characteristics, and an important method in its research is to
focus on animal behaviours related to the core diagnostic symptoms
of the disease to study both the underlying neural mechanisms and
potential pharmacological targets to ameliorate autism type
(4,32). The robustness of the behavioural
alterations and the neural/molecular changes revealed support the
VPA model mice as a reliable tool for investigating the neural
basis of social disorders (4,5,8,9),
which has been widely used in preclinical studies of ASD (8). In the present study, a modified
method was used to generate VPA model mice (49). The modelling success rate was
satisfactory (the failed pregnancy was only 10.5%) and the mice had
obvious phenotypes, such as delayed weight development, a 100% tail
deformity rate and neurodevelopmental delay (24,49,53). Furthermore, the behavioural tests
conducted confirmed that the model mice exhibited obvious
autism-like behaviour. Regarding the dosage of STX209, according to
the principle of selecting the minimum effective dose, we selected
the minimum dose supported by the reference (36,41,42) that can fully activate the gabab
receptor. A previous study indicated that administration of STX209
in the drinking water at 0.5 mg/ml could provide average brain
exposure equivalent to 6 mg/kg administered by intraperitoneal
injection twice a day, which was sufficient to engage GABABR2 and
reduce excessive protein synthesis in Fmr1−/y mice and
to correct the excess dendritic spine phenotype of Fmr1-deficient
mice (36). This previous study
also demonstrated that acute intraperitoneal administration of
STX209 significantly reduced the percentage of mice displaying
seizures (seizure incidence), with a minimum effective dose of 1.5
mg/kg (36). Another study
revealed that STX209 could suppress audiogenic seizures in
Fmr1-knockout mice on a seizure-resistant C57BL/6 background with
an effective dose of 1.0 mg/kg (single doses) (41). A clinical trial in patients with
fragile X syndrome also showed a benefit of STX209 in children aged
6–11 (~0.66 mg/kg/day) (42).
Therefore, a minimum dosage at which the drug can fully stimulate
the GABABR2 (0.6 mg/kg, intraperitoneal administration, twice a
day; total dose, 1.2 mg/day) was chosen for the present study.
The social interaction test is a widely used,
standardized test for assessing social interaction deficits, which
is often used to evaluate general sociability in rodents (4,8,58,59). The other behavioural tests used
for sociability include the youth play behaviour test and social
(play) behaviour test (40).
Based on the procedures of these simple tests, the behaviour of
tested mice are easily affected by the other mice during
interaction and the various quantitative parameters make their
evaluation highly subjective. Therefore only the three-chamber test
was used for evaluating the sociability of tested mice in the
present study. The results indicated that STX209 treatment
ameliorated sociability deficits and preference for social novelty
deficits in VPA model mice.
Restrictive interest is another core symptom of
ASD, and the novel object recognition task revealed that STX209
ameliorated object recognition memory and preference for the novel
object in VPA model mice. Characteristics associated with ASD
include anxiety, epileptic seizures and cognitive impairment,
anxiety is one of the most common comorbid conditions in patients
(8,9). Furthermore, in the present study,
VPA model mice exhibited obvious locomotion and exploratory
behavioural deficits in the open-field task and open-field
habituation task, which could be improved by STX209. Following
STX209 treatment, VPA mice exhibited reduced anxiety and stronger
exploratory characteristic behaviour in the open-field habituation
task.
Repetitive behaviour is another characteristic
phenotype of VPA model mice and a defining symptom of ASD. The
marble burying test is regarded as an assessment of
perseverative/repetitive behaviour in mice. Previous studies
assessing marble burying or digging bouts have confirmed increased
stereotyped, perseverative/repetitive behaviours in VPA-exposed
animals (50,60). The original purpose of designing
this test was to observe the perseverative/repetitive behaviour of
VPA model mice; however, the results of the present study
demonstrated that VPA model mice exhibited fewer digging and
burying actions, and buried fewer marbles than control mice.
Notably, STX209 improved these phenotypes. These unanticipated
results may be because the marble burying test itself is a robust
test model for identifying anxiolytics (61–63) in the anxiolytic model, glass
marbles are seen as an unfamiliar and potentially threatening
object, and the burying of marbles by the mice to remove the threat
is considered a sign of an instinctive anxiety-like response. This
behavior is called ‘conditioned defensive burying’, which is an
adaptation to a new complex environment (61–63). The results of present study
further support the suggestion that burying marbles is a
manifestation of an instinctive anxiety-like response. STX209
rescued the defect of conditioned defensive burial in VPA model
mice, which may be associated with beneficial effects of long-term
STX209 administration on nervous system development in VPA
mice.
After demonstrating that STX209 was able to improve
autism-like behaviour in VPA model mice, the present study focused
on hippocampal structure. The Golgi-Cox impregnation method is a
powerful neuromorphological staining tool that has been widely used
to obtain valuable information regarding neural morphology and to
quantitatively assess parameters, such as dendritic spine number,
dendritic length and branching complexity. The present study
evaluated neuronal morphology and dendritic spine development in
the hippocampus of the mice by Golgi staining to assess their
synaptic plasticity status. Consistent with previous reports and
the aforementioned behavioural data, neuronal development was
disrupted in the hippocampal CA1 regions in mice exposed to VPA
in utero (33,64,65). Quantitative morphological analysis
of dendritic spine density revealed that the total spine and
mushroom spine density at the end of the basal dendrites of
pyramidal neurons in the CA1 region of the hippocampus were
decreased, but that the density of spines at the end of dendrites
in the DG region was increased. These results suggested that VPA
model mice had hypoconnectivity in the CA1 region and
hyperconnectivity in the DG region. In addition, the results
revealed that STX209 altered the morphology, total spine and
mushroom spine density (although this was not statistically
significant) of neurons in CA1 and the morphology, total spine
density of neurons in DG regions in the VPA model hippocampus so
that they were more similar to those in the control group. The CA1
and DG regions are the main components of the classic trisynaptic
circuit (entorhinal cortex→DG→CA3→CA1→subiculum), which has been
reported to be closely related to memory and to be an important
basis of hippocampal function (66,67). In VPA model mice, the abnormal
dendritic morphology and hypoconnectivity in the CA1 region of the
hippocampus, as well as the morphological defects and local
hyperconnectivity in the DG region, may cause trisynaptic circuit
disorder and E-I imbalance, thus affecting the cognitive processes
of learning and memory associated with navigation, exploration and
locomotion. According to this finding, it was hypothesized that
activation of GABABR2 by STX209 may ameliorate neuronal
developmental defects in the CA1 and DG regions of the hippocampus
in VPA model mice, normalize signal transduction in the hippocampus
and contribute to achieving E-I rebalance. This conclusion is
supported by several mouse models of E-I dysfunction (22,68).
To further explore the mechanisms involved,
GAD65/67 and GABABR2 protein expression levels in the hippocampus
in each group were assessed, and it was revealed that the
expression levels of both proteins were decreased in VPA model
mice. STX209 increased GABABR2 protein expression levels, but did
not increase GAD65/67 protein expression in the hippocampus.
GAD65/67 are the key enzymes in GABA biosynthesis, and GABABR2 is
the site of action of STX209 and GABA. Furthermore, GAD65/67 are
joint nodes of the GABAergic system (69,70). The present findings revealed that
exposure to VPA in utero caused hippocampal GABAergic system
functional defects in offspring mice and that chronic STX209
administration did not improve GABA production capacity in VPA
model mice but did increase GABAergic pathway function. GABABR is
indispensable for regulating the development of neural networks.
Studies have reported that GABA and its receptors serve a classic
role in neurodevelopment and synaptic plasticity, and can regulate
almost all of the key steps of neuronal network formation, and
GABABR dysfunction may cause behavioural defects in ASD (71,72). Chronic administration of a low
dose of STX209 during the growth phase in VPA model pups most
likely activated GABABR, thus compensating for the lack of GABA,
and then produced a series of physiological responses, such as
promotion of hippocampal neuron survival, migration and dendritic
development in VPA model pups, and correction of the formation and
pruning of dendritic spines. This compensatory mechanism may
contribute to maintaining the stability and functionality of neural
circuits in the face of challenges posed by developmental events in
this animal model, thereby ameliorating the ASD-related behaviours
of mice exposed to VPA in utero.
In conclusion, the present study provided strong
evidence that pharmacologically enhancing GABA signalling may be a
promising strategy for the treatment of VPA model mice. While the
experimental results are encouraging, the limitations of the study
are that it is impossible to replicate the complex genetic and
environmental conditions of children with ASD due to the
homogeneity between experimental animals and the same experimental
environment. Furthermore, the present study only used the
exploratory drug, STX209, and lacked the use of a known GABABR2
agonist to test as a positive control, and also lacked measurement
of GABABR signalling activity. Furthermore, STX209 cannot
completely cross the blood-brain barrier; therefore, this is
another limitation of this drug. However, the results indicated the
possibility of early intervention with STX209, an exploratory
GABABR2 agonist, for the treatment of children with ASD. In
addition, the results of the present study supported that GABABR is
a promising drug target for the treatment of neuropsychiatric
disorders and developmental disorders, which may be an important
direction for the development of novel drugs for ASD treatment.
Acknowledgements
The authors would like to thank the Ningxia Key
Laboratory of Cerebrocranial Disease for technical support (special
thanks to Mrs. Zhang Chun for her help in cryopreservation of mouse
brain tissue); Mr. Zhang Xian and Mr. Hao Xiaoyan (Ningxia Medical
University) for their assistance during the experiment; and Mrs.
Liu Li and Mrs. Yang Dan (Neuroscience of the Public Technology
Platform of School of Medicine, Zhejiang University) for their help
in analysing the results of the Golgi-Cox staining.
Funding
This study was supported by the National Natural Science
Foundation of China (NSFC) (grant no. 82060261), the Key Research
Project of Ningxia (grant no. 2018YBZD04917) and the Ningxia Hui
Autonomous Region ‘13th Five-Year Plan’ Major Science and
Technology Projects (Ningxia Brain Project) (grant no.
2016BZ07).
Availability of data and materials
The datasets used and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
All authors contributed to the study conception and
design. Senior authors FW and TS are accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. SJ, LX and YS made major contributions to the
conception, design and the acquisition, analysis, or interpretation
of data for the work. Material preparation, experiments and data
collection were performed by MH, CG, CZ, HC, JD, WL and YW. The
first draft of the manuscript was written by SJ and all authors
commented on previous versions of the manuscript. All authors read
and approved the final manuscript. SJ and FW confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All mice were handled according to protocols
approved by the Institutional Animal Care and Use Committee of
Ningxia Medical University (IACUC Animal Use Certificate No.
2019-152). All efforts were made to minimize the number of animals
used and their suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Psychiatric Association:
Diagnostic and statistical manual of mental disorders:
DSM-5™. (5th edition). American Psychiatric Publushing;
Washington, DC: 2013, View Article : Google Scholar
|
|
2
|
Volkmar FR, Woodbury-Smith M, Macari SL
and Øien RA: Seeing the forest and the trees: Disentangling autism
phenotypes in the age of DSM-5. Dev Psychopathol. 33:625–633. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christensen DL, Maenner MJ, Bilder D,
Constantino JN, Daniels J, Durkin MS, Fitzgerald RT,
Kurzius-Spencer M, Pettygrove SD, Robinson C, et al: Prevalence and
characteristics of autism spectrum disorder among children aged 4
years-early autism and developmental disabilities monitoring
network, seven sites, United States, 2010, 2012, and 2014. MMWR
Surveill Summ. 68:1–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Servadio M, Vanderschuren LJ and Trezza V:
Modeling autism-relevant behavioral phenotypes in rats and mice: Do
‘autistic’ rodents exist? Behav Pharmacol. 26:522–540. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roullet FI, Lai JK and Foster JA: In utero
exposure to valproic acid and autism-a current review of clinical
and animal studies. Neurotoxicol Teratol. 36:47–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taleb A, Lin W, Xu X, Zhang G, Zhou QG,
Naveed M, Meng F, Fukunaga K and Han F: Emerging mechanisms of
valproic acid-induced neurotoxic events in autism and its
implications for pharmacological treatment. Biomed Pharmacother.
137:1113222021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaste P and Leboyer M: Autism risk
factors: Genes, environment, and gene-environment interactions.
Dialogues Clin Neurosci. 14:281–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tartaglione AM, Schiavi S, Calamandrei G
and Trezza V: Prenatal valproate in rodents as a tool to understand
the neural underpinnings of social dysfunctions in autism spectrum
disorder. Neuropharmacology. 159:1074772019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicolini C and Fahnestock M: The valproic
acid-induced rodent model of autism. Exp Neurol. 299:217–227. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneider T, Roman A, Basta-Kaim A, Kubera
M, Budziszewska B, Schneider K and Przewłocki R: Gender-specific
behavioral and immunological alterations in an animal model of
autism induced by prenatal exposure to valproic acid.
Psychoneuroendocrinology. 33:728–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McPheeters ML, Warren Z, Sathe N, Bruzek
JL, Krishnaswami S, Jerome RN and Veenstra-Vanderweele J: A
systematic review of medical treatments for children with autism
spectrum disorders. Pediatrics. 127:e1312–e1321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Owen R, Sikich L, Marcus RN, Corey-Lisle
P, Manos G, McQuade RD, Carson WH and Findling RL: Aripiprazole in
the treatment of irritability in children and adolescents with
autistic disorder. Pediatrics. 124:1533–1540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eissa N, Al-Houqani M, Sadeq A, Ojha SK,
Sasse A and Sadek B: Current enlightenment about etiology and
pharmacological treatment of autism spectrum disorder. Front
Neurosci. 12:3042018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canitano R: New experimental treatments
for core social domain in autism spectrum disorders. Front Pediatr.
2:612014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashemi E, Ariza J, Rogers H, Noctor SC
and Martínez-Cerdeño V: The number of parvalbumin-expressing
interneurons is decreased in the prefrontal cortex in autism. Cereb
Cortex. 27:1931–1943. 2017.PubMed/NCBI
|
|
16
|
Ariza J, Rogers H, Hashemi E, Noctor SC
and Martínez-Cerdeño V: The number of chandelier and basket cells
are differentially decreased in prefrontal cortex in autism. Cereb
Cortex. 28:411–420. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fatemi SH, Folsom TD, Reutiman TJ and
Thuras PD: Expression of GABA(B) receptors is altered in brains of
subjects with autism. Cerebellum. 8:64–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fatemi SH, Folsom TD and Thuras PD:
Deficits in GABA(B) receptor system in schizophrenia and mood
disorders: A postmortem study. Schizophr Res. 128:37–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fatemi SH, Halt AR, Stary JM, Kanodia R,
Schulz SC and Realmuto GR: Glutamic acid decarboxylase 65 and 67
kDa proteins are reduced in autistic parietal and cerebellar
cortices. Biol Psychiatry. 52:805–810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oblak AL, Gibbs TT and Blatt GJ: Reduced
GABAA receptors and benzodiazepine binding sites in the posterior
cingulate cortex and fusiform gyrus in autism. Brain Res.
1380:218–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oblak AL, Gibbs TT and Blatt GJ: Decreased
GABA(B) receptors in the cingulate cortex and fusiform gyrus in
autism. J Neurochem. 114:1414–1423. 2010.PubMed/NCBI
|
|
22
|
Han S, Tai C, Westenbroek RE, Yu FH, Cheah
CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO and
Catterall WA: Autistic-like behaviour in Scn1a+/-mice and rescue by
enhanced GABA-mediated neurotransmission. Nature. 489:385–390.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee E, Lee J and Kim E:
Excitation/inhibition imbalance in animal models of autism spectrum
disorders. Biol Psychiatry. 81:838–347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou Q, Wang Y, Li Y, Chen D, Yang F and
Wang S: A developmental study of abnormal behaviors and altered
GABAergic signaling in the VPA-treated rat model of autism. Front
Behav Neurosci. 12:1822018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banerjee A, García-Oscos F, Roychowdhury
S, Galindo LC, Hall S, Kilgard MP and Atzori M: Impairment of
cortical GABAergic synaptic transmission in an environmental rat
model of autism. Int J Neuropsychopharmacol. 16:1309–1318. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chau DK, Choi AY, Yang W, Leung WN and
Chan CW: Downregulation of glutamatergic and GABAergic proteins in
valproric acid associated social impairment during adolescence in
mice. Behav Brain Res. 316:255–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rinaldi T, Silberberg G and Markram H:
Hyperconnectivity of local neocortical microcircuitry induced by
prenatal exposure to valproic acid. Cereb Cortex. 18:763–770. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rinaldi T, Perrodin C and Markram H:
Hyper-connectivity and hyper-plasticity in the medial prefrontal
cortex in the valproic acid animal model of autism. Front Neural
Circuits. 2:42008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lenart J, Augustyniak J, Lazarewicz JW and
Zieminska E: Altered expression of glutamatergic and GABAergic
genes in the valproic acid-induced rat model of autism: A screening
test. Toxicology. 440:1525002020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Norton SA, Gifford JJ, Pawlak AP, Derbaly
A, Sherman SL, Zhang H, Wagner GC and Kusnecov AW: Long-lasting
behavioral and neuroanatomical effects of postnatal valproic acid
treatment. Neuroscience. 434:8–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schiavi S, Iezzi D, Manduca A, Leone S,
Melancia F, Carbone C, Petrella M, Mannaioni G, Masi A and Trezza
V: Reward-related behavioral, neurochemical and
electrophysiological changes in a rat model of autism based on
prenatal exposure to valproic acid. Front Cell Neurosci.
13:4792019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crawley JN: Translational animal models of
autism and neurodevelopmental disorders. Dialogues Clin Neurosci.
14:293–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bringas ME, Carvajal-Flores FN,
López-Ramírez TA, Atzori M and Flores G: Rearrangement of the
dendritic morphology in limbic regions and altered exploratory
behavior in a rat model of autism spectrum disorder. Neuroscience.
241:170–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lal R, Sukbuntherng J, Tai EH, Upadhyay S,
Yao F, Warren MS, Luo W, Bu L, Nguyen S, Zamora J, et al:
Arbaclofen placarbil, a novel R-baclofen prodrug: Improved
absorption, distribution, metabolism, and elimination properties
compared with R-baclofen. J Pharmacol Exp Ther. 330:911–921. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sanchez-Ponce R, Wang LQ, Lu W, von Hehn
J, Cherubini M and Rush R: Metabolic and pharmacokinetic
differentiation of STX209 and racemic baclofen in humans.
Metabolites. 2:596–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henderson C, Wijetunge L, Kinoshita MN,
Shumway M, Hammond RS, Postma FR, Brynczka C, Rush R, Thomas A,
Paylor R, et al: Reversal of disease-related pathologies in the
fragile X mouse model by selective activation of GABAB receptors
with arbaclofen. Sci Transl Med. 4:152ra1282012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Silverman JL, Pride MC, Hayes JE, Puhger
KR, Butler-Struben HM, Baker S and Crawley JN: GABAB receptor
agonist R-baclofen reverses social deficits and reduces repetitive
behavior in two mouse models of autism. Neuropsychopharmacology.
40:2228–2239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin M, Huang T, Kader M, Krych L, Xia Z,
Burlin T, Zeidler Z, Zhao T and Smith CB: R-baclofen reverses a
social behavior deficit and elevated protein synthesis in a mouse
model of fragile X syndrome. Int J Neuropsychopharmacol.
18:pyv0342015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sinclair D, Featherstone R, Naschek M, Nam
J, Du A, Wright S, Pance K, Melnychenko O, Weger R, Akuzawa S, et
al: GABA-B agonist baclofen normalizes auditory-evoked neural
oscillations and behavioral deficits in the Fmr1 knockout mouse
model of fragile X syndrome. eNeuro. 4:ENEURO.0380–16.2017. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stoppel LJ, Kazdoba TM, Schaffler MD,
Preza AR, Heynen A, Crawley JN and Bear MF: R-baclofen reverses
cognitive deficits and improves social interactions in two lines of
16p11.2 deletion mice. Neuropsychopharmacology. 43:513–524. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pacey LK, Tharmalingam S and Hampson DR:
Subchronic administration and combination metabotropic glutamate
and GABAB receptor drug therapy in fragile X syndrome. J Pharmacol
Exp Ther. 338:897–905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Berry-Kravis EM, Hessl D, Rathmell B,
Zarevics P, Cherubini M, Walton-Bowen K, Mu Y, Nguyen DV,
Gonzalez-Heydrich J, Wang PP, et al: Effects of STX209 (arbaclofen)
on neurobehavioral function in children and adults with fragile X
syndrome: A randomized, controlled, phase 2 trial. Sci Transl Med.
4:152ra1272012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Erickson CA, Veenstra-Vanderweele JM,
Melmed RD, McCracken JT, Ginsberg LD, Sikich L, Scahill L,
Cherubini M, Zarevics P, Walton-Bowen K, et al: STX209 (arbaclofen)
for autism spectrum disorders: An 8-week open-label study. J Autism
Dev Disord. 44:958–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Veenstra-VanderWeele J, Cook EH, King BH,
Zarevics P, Cherubini M, Walton-Bowen K, Bear MF, Wang PP and
Carpenter RL: Arbaclofen in children and adolescents with autism
spectrum disorder: A randomized, controlled, phase 2 trial.
Neuropsychopharmacology. 42:1390–1398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frye RE: Clinical potential, safety, and
tolerability of arbaclofen in the treatment of autism spectrum
disorder. Drug Healthc Patient Saf. 6:69–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Berry-Kravis E, Hagerman R, Visootsak J,
Budimirovic D, Kaufmann WE, Cherubini M, Zarevics P, Walton-Bowen
K, Wang P, Bear MF and Carpenter RL: Arbaclofen in fragile X
syndrome: Results of phase 3 trials. J Neurodev Disord. 9:32017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boivin GP, Hickman DL, Creamer-Hente MA,
Pritchett-Corning KR and Bratcher NA: Review of CO2 as a
euthanasia agent for laboratory rats and mice. J Am Assoc Lab Anim
Sci. 56:491–499. 2017.PubMed/NCBI
|
|
48
|
American Veterinary Medical Association, .
AVMA guidelines for the Euthanasia of animals. 2020 edition.
2020
|
|
49
|
Zheng W, Hu Y, Chen D, Li Y and Wang S:
Improvement of a mouse model of valproic acid-induced autism. Nan
Fang Yi Ke Da Xue Xue Bao. 39:718–723. 2019.(In Chinese).
PubMed/NCBI
|
|
50
|
Choi CS, Gonzales EL, Kim KC, Yang SM, Kim
JW, Mabunga DF, Cheong JH, Han SH, Bahn GH and Shin CY: The
transgenerational inheritance of autism-like phenotypes in mice
exposed to valproic acid during pregnancy. Sci Rep. 6:362502016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kazlauskas N, Seiffe A, Campolongo M,
Zappala C and Depino AM: Sex-specific effects of prenatal valproic
acid exposure on sociability and neuroinflammation: Relevance for
susceptibility and resilience in autism. Psychoneuroendocrinology.
110:1044412019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiménez JA and Zylka MJ: Controlling
litter effects to enhance rigor and reproducibility with rodent
models of neurodevelopmental disorders. J Neurodev Disord.
13:22021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang R, Tan J, Guo J, Zheng Y, Han Q, So
KF, Yu J and Zhang L: Aberrant development and synaptic
transmission of cerebellar cortex in a VPA induced mouse autism
model. Front Cell Neurosci. 12:5002018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Heyser CJ: Assessment of developmental
milestones in rodents. Curr Protoc Neurosci. Chapter 8: Unit 8.18.
2004.PubMed/NCBI
|
|
55
|
Antunes M and Biala G: The novel object
recognition memory: neurobiology, test procedure, and its
modifications. Cogn Process. 13:93–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Modabbernia A, Velthorst E and Reichenberg
A: Environmental risk factors for autism: An evidence-based review
of systematic reviews and meta-analyses. Mol Autism. 8:132017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Binkerd PE, Rowland JM, Nau H and
Hendrickx AG: Evaluation of valproic acid (VPA) developmental
toxicity and pharmacokinetics in Sprague-Dawley rats. Fundam Appl
Toxicol. 11:485–493. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang M, Silverman JL and Crawley JN:
Automated three-chambered social approach task for mice. Curr
Protoc Neurosci. Chapter 8: Unit 8.26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rein B, Ma K and Yan Z: A standardized
social preference protocol for measuring social deficits in mouse
models of autism. Nat Protoc. 15:3464–3477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim KC, Kim P, Go HS, Choi CS, Yang SI,
Cheong JH, Shin CY and Ko KH: The critical period of valproate
exposure to induce autistic symptoms in Sprague-Dawley rats.
Toxicol Lett. 201:137–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dallas T, Pinel JP and Fibiger HC:
Conditioned defensive burying: A new paradigm for the study of
anxiolytic agents. Pharmacol Biochem Behav. 15:619–626. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kung'u N and Handley SL: Evaluation of
marble-burying behavior as a model of anxiety. Pharmacol Biochem
Behav. 38:63–67. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Langer E, Einat H and Stukalin Y:
Similarities and dissimilarities in the effects of benzodiazepines
and specific serotonin reuptake inhibitors (SSRIs) in the defensive
marble burying test: A systematic review and meta-analysis. Eur
Neuropsychopharmacol. 36:38–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yamaguchi H, Hara Y, Ago Y, Takano E,
Hasebe S, Nakazawa T, Hashimoto H, Matsuda T and Takuma K:
Environmental enrichment attenuates behavioral abnormalities in
valproic acid-exposed autism model mice. Behav Brain Res.
333:67–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hara Y, Ago Y, Taruta A, Katashiba K,
Hasebe S, Takano E, Onaka Y, Hashimoto H, Matsuda T and Takuma K:
Improvement by methylphenidate and atomoxetine of social
interaction deficits and recognition memory impairment in a mouse
model of valproic acid-induced autism. Autism Res. 9:926–939. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stepan J, Dine J and Eder M: Functional
optical probing of the hippocampal trisynaptic circuit in vitro:
Network dynamics, filter properties, and polysynaptic induction of
CA1 LTP. Front Neurosci. 9:1602015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Naber PA, Witter MP and Lopes Silva FH:
Networks of the hippocampal memory system of the rat. The pivotal
role of the subiculum. Ann N Y Acad Sci. 911:392–403. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gandal MJ, Sisti J, Klook K, Ortinski PI,
Leitman V, Liang Y, Thieu T, Anderson R, Pierce RC, Jonak G, et al:
GABAB-mediated rescue of altered excitatory-inhibitory balance,
gamma synchrony and behavioral deficits following constitutive
NMDAR-hypofunction. Transl Psychiatry. 2:e1422012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee B, Zhang Y, Kim Y, Kim S, Lee Y and
Han K: Age-dependent decrease of GAD65/67 mRNAs but normal
densities of GABAergic interneurons in the brain regions of
Shank3-overexpressing manic mouse model. Neurosci Lett. 649:48–54.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ribak CE and Roberts RC: GABAergic
synapses in the brain identified with antisera to GABA and its
synthesizing enzyme, glutamate decarboxylase. J Electron Microsc
Tech. 15:34–48. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gaiarsa JL and Porcher C: Emerging
neurotrophic role of GABAB receptors in neuronal circuit
development. Front Cell Neurosci. 7:2062013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Heaney CF and Kinney JW: Role of GABA(B)
receptors in learning and memory and neurological disorders.
Neurosci Biobehav Rev. 63:1–28. 2016. View Article : Google Scholar : PubMed/NCBI
|