Introduction
Systemic lupus erythematosus (SLE) is a chronic and
multisystem autoimmune disease that predominantly affects women,
especially between puberty and menopause (1,2).
However, the mechanisms of SLE are complex and undeciphered.
Although B cells play a central role in adaptive immunity, recent
studies on SLE suggest both T and B cells are involved in the
progression of SLE (3–5). Fas (CD95) is a member of the tumor
necrosis factor receptor family and interacts with Fas ligand
(FasL) after T cell receptor (TCR) activation to initiate apoptosis
(6). Fas-deficiency in
MRL/lpr mice leads to CD4− and CD8−
double-negative (DN) T cell accumulation in MRL/lpr mice,
resulting in lymphadenectasis and splenomegaly (7,8).
DN T cells have been demonstrated to play an important role in the
development of SLE (3,9,10).
Studies have shown that DN T cells in MRL/lpr mice are
strongly cytotoxic (6) and
overexpression of FasL on hyperactivated cytolytic DN T cells
results in an autoimmune disease that attacks tissues that express
low levels of the Fas receptor (6). Recent studies have also observed an
accumulation of DN T cells during lupus nephritis, which induces or
exacerbates tissue injury (3,11).
However, the mechanism that results in the accumulation of DN T
cells remains to be deciphered (12–20). Interestingly, recent studies have
found that the majority of DN T cells also express CD138 in
MRL/lpr lupus mice (21–23). Importantly, our recent study
demonstrated that CD138 expression in CD3+ T cells could
dramatically prevent CD3+ T cell apoptosis and
significantly contribute to the accumulation of DN T cells (Xie T,
Liu X and Li P; unpublished data).
Syndecan-1/CD138 is a marker of plasma cells in
lymphocytes that are believed to originate from B cells (24,25). CD138+ T cells, which
express both CD3 and CD138, were identified in murine systemic
lupus erythematosus (SLE) models (21–23). These abnormal CD138+
cells have also been reported recently to be plasmablastic B-cell
neoplasms as observed in clinical cases (26). These results indicate that CD138
could be expressed on CD3+ T cells of both humans and
mice. However, CD138+ T cells constitute only a small
fraction of cells in the spleen of non-lupus-prone mice (21,23). The majority of the
CD138+ T cells in MRL/lpr mice are also CD4 and
CD8 double-negative (21–23). Previous studies have indicated
that CD138+ T cells play a key role in the progression
of lupus in MRL/lpr mice. The accumulation of
CD138+ T cells in the spleen of MRL/lpr mice has
been observed and progressively increase with the development of
the disease (21). Studies have
also demonstrated that CD138+ T cells significantly
contribute to the production of anti-double-stranded (ds)DNA
antibodies both in vivo and in vitro, which in turn
promote the development of lupus (21,27).
Female MRL/lpr mice have been used
extensively as animal models for SLE. These mice show signs of
lymphadenopathy and splenomegaly, lupus nephritis, and in
vivo inflammation with increased levels of multiple cytokines.
Furthermore, autoantibodies such as anti-dsDNA and anti-SM which
are detected in SLE patients were also observed in MRL/lpr
mouse models (21,28,29). Glucocorticoid treatment is the
first-line treatment option and has shown a significant therapeutic
effect for the clinical treatment of SLE (30–33). Glucocorticoids have been
demonstrated to have a significant therapeutic effect for both SLE
patients and SLE murine models by reducing in vivo
autoantibody secretion, including anti-dsDNA antibodies (30–34). In the present study, we further
investigated the underlying mechanism of glucocorticoid for the
treatment of SLE. We investigated whether glucocorticoid could
prevent CD138+ T cell accumulation and suppress CD138
expression in DN T cells to alleviate DN T cells accumulation in
MRL/lpr mice.
Materials and methods
Animals
A total of 8 female MRL/MPJ mice and 16 female
MRL/lpr lupus mice were purchased from the Slac Laboratory
(Shanghai, China). Mice were housed at 22±1°C with a relative
humidity of 50–60% with a 12-h light/dark cycle. All animal
experiments were approved by the Institutional Animal Care and Use
Committee (IACUC) of the Beijing Institute of Chinese Medicine and
were performed in accordance with Animal Research protocols for
reporting of In Vivo Experiments (ARRIVE) guidelines
(35,36) and institutional regulations.
Methods
The 4-week-old female MRL/MPJ mice (25–30 g) and
4-week-old female MRL/lpr lupus mice (25–30 g) were
acclimatized for one week. Eight female MRL/MPJ mice were used as
negative controls (ddH2O, n=8), while the remaining 16
MRL/lpr lupus mice were randomly divided into two groups,
i.e., the vehicle group (ddH2O, n=8) and the prednisone
(PNS) 5.0 mg/kg/day group (n=8). Oral administration was performed
daily from 9 to 16 weeks of age. Body weight was recorded every
week during the study (Fig. 1A).
At the 17–18th week of age, mice were anesthetized using 1% sodium
pentobarbital (80 mg/kg) for serum collection, and then euthanized
by cervical dislocation under anesthesia. The following tissues
were harvested: lymph nodes and spleen (isolated and weighed), and
kidneys (for histology).
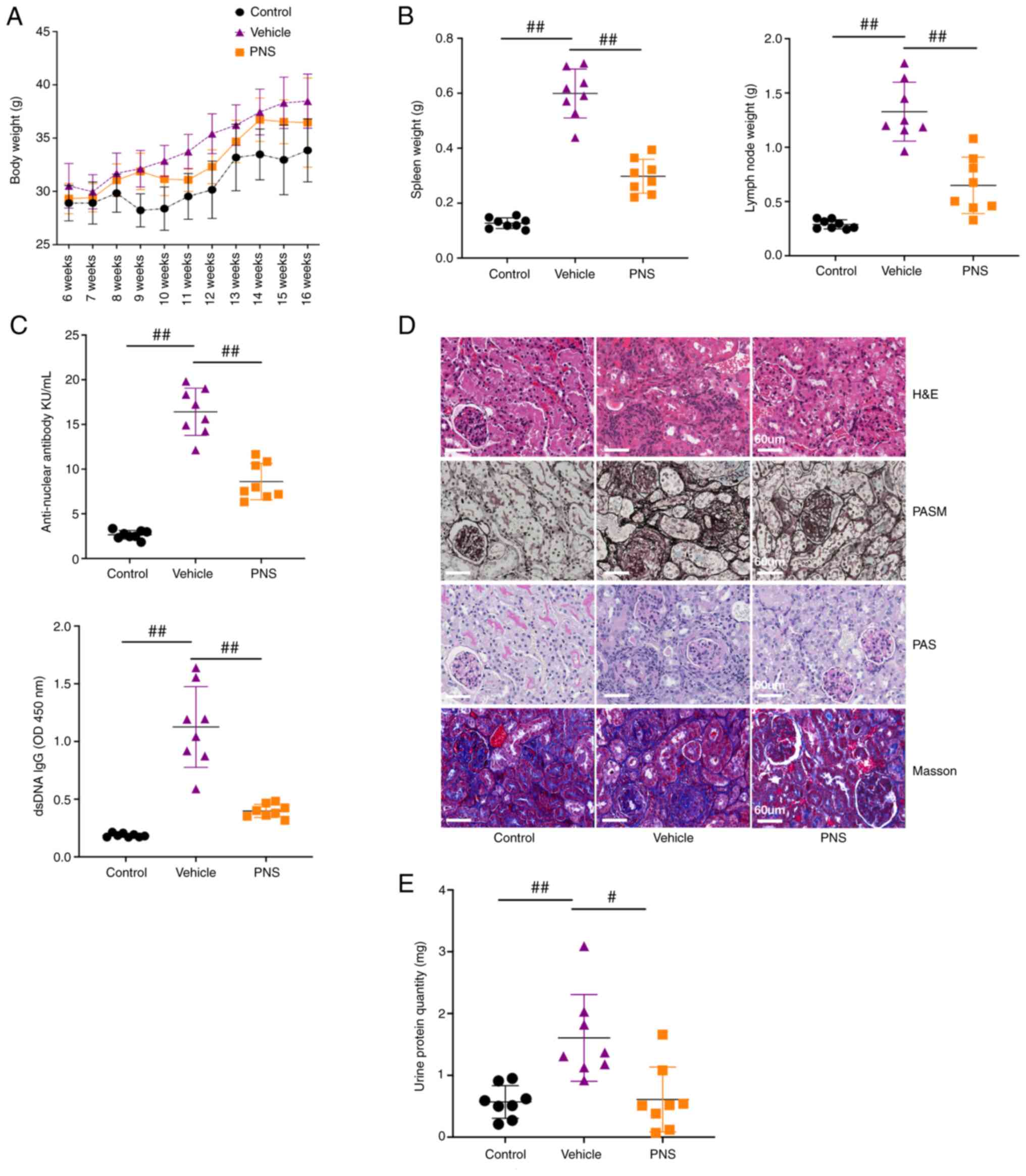 | Figure 1.(A) Body weight of the mice; n=8 per
group from two independent experiments. (B) Scatter plots of spleen
weight, and lymph node weight; n=8 per group from two independent
experiments. (C) Scatter plots showing serum ANA levels and
anti-dsDNA IgG antibody levels; n=8 per group from two independent
experiments. (D) Representative paraffin kidney sections stained
with H&E, PASM, PAS and Masson. Samples from the three groups
were used for H&E, PASM, and PAS staining and were different
from the samples used to perform Masson staining; original
magnification, ×400; scale bars, 60 µm; n=5 per group from two
independent experiments. (E) Scatter plots of protein levels in
urine samples collected over 24 h from 16-week old mice; 24-h
urinary protein quantity=urinary protein levels per liter ×24 h
urinary volume; n=8 per group from two independent experiments;
Data are presented as mean ± SD; #P<0.05,
##P<0.01, by one-way analysis of variance, vs. the
vehicle-treated mice. ANS, anti-nuclear antibody; anti-dsDNA,
anti-double-stranded DNA; PNS, prednisone; H&E, hematoxylin and
eosin; PAS, periodic acid Schiff; PASM, periodic acid-silver
metheramine. |
Histology
To observe renal pathologic changes in lupus-prone
mice, the kidneys were harvested at 17–18 weeks of age and
snap-frozen in OCT compound for frozen tissue sections or fixed in
4% paraformaldehyde. Paraformaldehyde-fixed kidneys were embedded
in paraffin and then sectioned at 4-µm thickness. Hematoxylin and
eosin (H&E), periodic acid schiff (PAS), periodic acid-silver
metheramine (PASM), and Masson trichrome staining was performed on
the paraffin sections.
Measurement of total IgG, anti-dsDNA
IgG, and anti-nuclear antibodies in the serum
Serum levels of total IgG, anti-dsDNA IgG, and
anti-nuclear antibody (ANA) were measured using ELISA (total IgG
ELISA kit, cat. no. 88-50400-22; Thermo Fisher Scientific, Inc.;
anti-dsDNA IgG, cat. no. 5120 and ANA ELISA kit, cat. no. 5210;
Alpha Diagnostic International), and were performed according to
the manufacturers' instructions. Diluted serum samples were added
onto coated 96-well plates and incubated for 1 h at room
temperature. Afterward, the plates were washed with wash buffer and
then incubated with anti-mouse IgG-HRP conjugate for 30 min at room
temperature. Then, TMB solution was added and incubated at room
temperature for 15 min. The reaction was terminated with a stop
solution and the plates were read at 450 nm absorbance using a
microplate reader.
Measurement of antibody isotypes in
serum using the Luminex platform
Serum levels of multiple antibody subtypes were
measured using the Luminex assay kits (Thermo Fisher Scientific,
Inc.; cat. no. EPX070-20815-901). Measurements were performed
according to the manufacturer's instructions. Diluted serum samples
were added onto 96-well plates coated with magnetic beads and
incubated for 120 min after vortexing. The beads were then washed,
and the detection antibody mixture was added and incubated for 30
min at room temperature. After incubation and plate washing, the
samples were analyzed on the Luminex™ platform (Thermo Fisher
Scientific, Inc.).
Measurement of urine protein
levels
Urine samples from individual 16-week-old mice were
collected for 24 h. The concentration of proteins in the urine was
determined using the Coomassie brilliant blue dye-binding assay kit
(Biokits Tech. Inc.; cat. no. BCBU-027) and was performed according
to the manufacturer's instructions (Biokits Tech. Inc.).
Flow cytometry
After harvesting the spleen, single-cell suspensions
of splenocytes were obtained by filtering through a 70-µm cell
strainer. Splenocytes were incubated on ice with CD16/CD32
monoclonal antibody (eBiosience/Thermo Fisher Scientific, Inc.;
ready to use; cat. no. 14-0161-85) for 15 min, and then red blood
cells were lysed using lysis buffer (BD Biosciences). The
splenocytes were then fixed and permeabilized
(Fixation/Permeabilization solution; BD Biosciences) before
intracellular staining was performed. Cells were stained with the
following ready to use antibodies for flow cytometry analysis:
anti-CD3 PE-cy7 (eBiosience; Thermo Fisher Scientific, Inc.; cat.
no. 25-0032-82), anti-CD4 FITC (eBiosience; Thermo Fisher
Scientific, Inc.; cat. no. 11-0041-82), anti-CD8 APC (eBiosience;
Thermo Fisher Scientific, Inc.; cat. no. 17-0081-82), anti-CD19
APC-cy7 (eBiosience; Thermo Fisher Scientific, Inc.; cat. no.
47-0193-82), anti-CD138 PE (Biolegend Inc.; cat. no. 142504), and
anti-CD69 PE (Biolegend Inc.; cat. no. 104507). FACs data were
analyzed using Flowjo software version 10.6 for PC (Tree Star,
Inc.).
Cellular stimulation
CpGC (1 µM) or combined with 50 ng/ml phorbol
12-myristate 13-acetate (PMA) and 1 µg/ml ionomycin were used to
stimulate and activate the cultured splenocytes from the mice.
Statistical analysis
Data from all experiments are expressed as mean ± SD
and were analyzed using SPSS software 17.0 (SPSS, Inc.).
Comparisons between the groups were performed for statistical
significance using Mann-Whitney U test between two groups or
one-way analysis of variance followed by Tukey's post hoc test for
multiple group comparisons. Differences with P-values <0.05 were
considered statistically significant.
Results
Successful construction of the murine
SLE model and the significant therapeutic effects of
prednisone
Compared to the control mice, significant
enlargement of the spleen and lymph nodes, increased serum levels
of anti-nuclear antibody (ANA) and anti-dsDNA IgG antibodies, and
elevated urine protein levels in our murine lupus model were
observed (Fig. 1B, C and E).
Additionally, obvious renal injuries in the vehicle-treated lupus
mice were observed and were characterized by hyaline deposits,
interstitial and perivascular cellular inflammation infiltration,
cellular crescent formation, glomerular fibrosis,
glomerulosclerosis, and tubular cell necrosis (Fig. 1D). These results indicate that the
murine lupus model was successfully constructed. As expected,
prednisone (PNS) treatment exhibited significant therapeutic
effects on lupus mice by significantly alleviating the enlargement
of the spleen and lymph nodes, decreasing serum levels of ANA and
anti-dsDNA IgG antibodies, reducing urine protein levels, and
improving histopathological injuries in the renal tissues (Fig. 1).
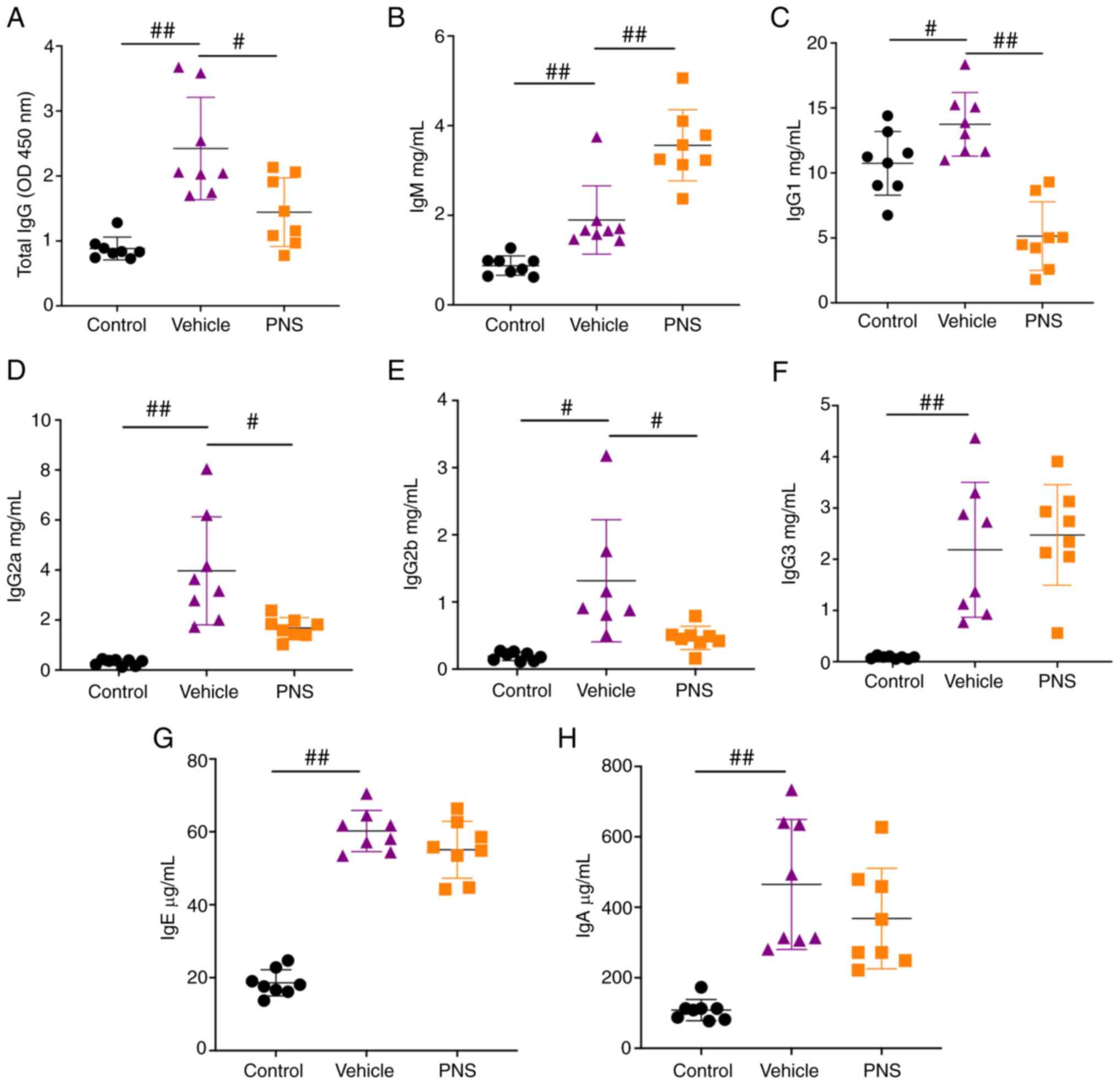
Prednisone reduces total IgG, IgG1,
and IgG2a levels in the serum of the lupus mice, while
simultaneously increasing IgM levels
We found that the levels of total IgG, IgG1, IgG2a,
IgG2b, IgG3, IgM, IgE, and IgA in the serum were significantly
increased in lupus mice compared to the control mice (Fig. 2A-H). However, the levels of total
IgG, IgG1, IgG2a, and IgG2b were significantly reduced in the
MRL/lpr mice treated with PNS (Fig. 2A and C-E). In addition, we
observed a further increase in IgM levels in the serum of
MRL/lpr mice (Fig. 2B).
Our results indicate that PNS reduced the production of total IgG,
IgG1, IgG2a, and IgG2b in the serum of the MRL/lpr mice, and
simultaneously increased serum IgM levels in the MRL/lpr
mice.
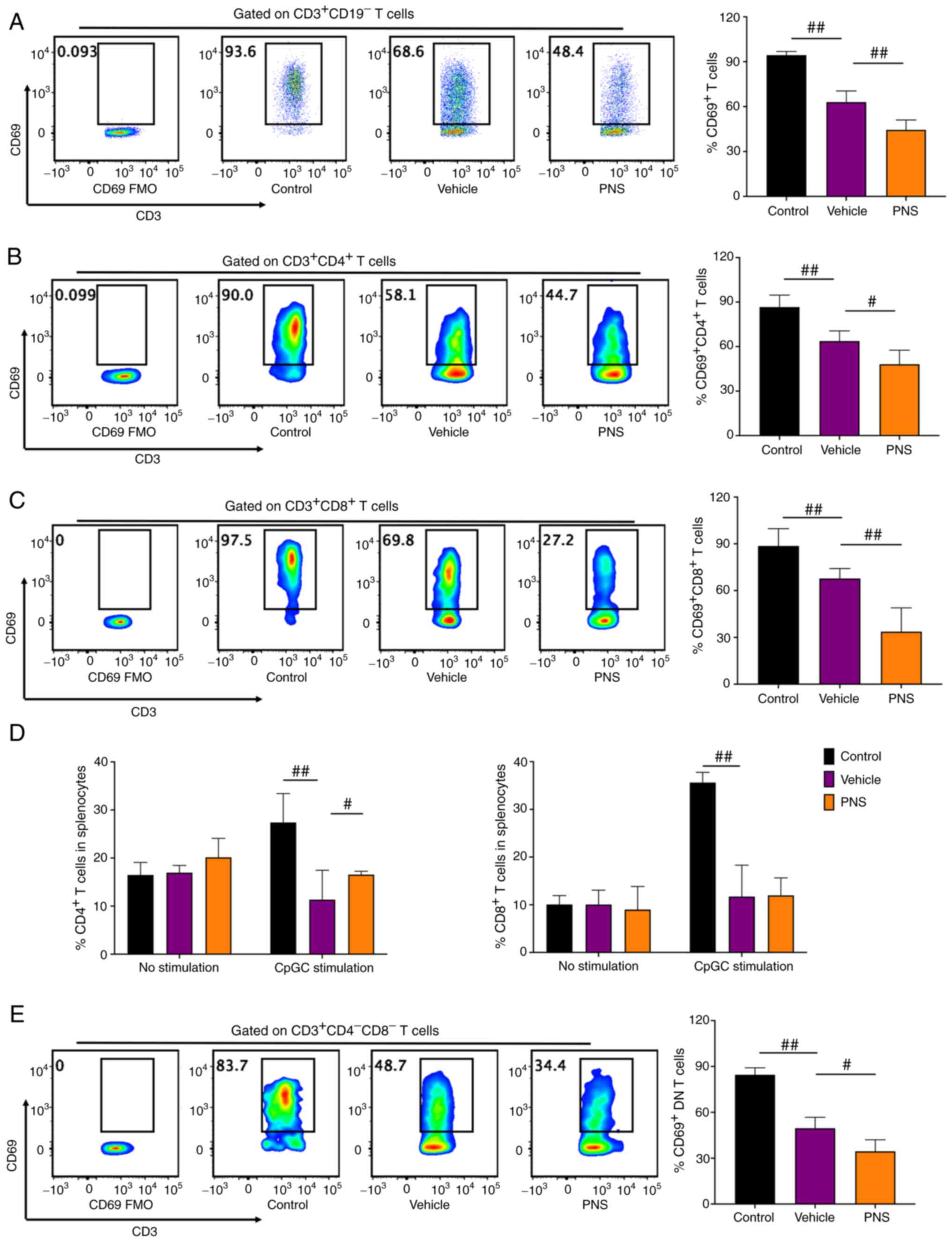
Prednisone prevents activation of
CD3+ T cells in the MRL/lpr mice
We stimulated and activated splenocytes using PMA
and ionomycin for 5 h. Activated T lymphocytes were stained with
CD69 (34,37). Compared to the vehicle-treated
MRL/lpr mice, T cells and T cell subsets including
CD4+, CD8+ and DN T cells in the MRL/MPJ mice
had significantly higher frequencies of CD69+ cells
after 5 h of stimulation (Fig. 3A-C
and E), suggesting that T cells and their subsets in MRL/MPJ
mice were more easily activated. Consistent with these results, we
observed that both CD4+ and CD8+ T cell
frequencies in splenocytes of MRL/MPJ and MRL/lpr mice were
equal before in vitro stimulation (Fig. 3D). But both CD4+ and
CD8+ T cell frequencies in splenocytes of the MRL/MPJ
mice were significantly higher than the frequencies in splenocytes
of the MRL/lpr mice after 24 h of in vitro CpGC
stimulation of splenocytes (Fig.
3D).
PNS showed significant effects on activation of T
cells and T cell subsets. PNS significantly decreased
CD69+ cell frequencies in CD3+ T cells and
its subsets including CD4+, CD8+ and DN T
cells in splenocytes of the MRL/lpr mice (Fig. 3A-C and E). The decrease in
CD69+ cell frequency in CD8+ T cells of the
PNS-treated mice was more significant compared with that in the
CD4+ and DN T cells. However, PNS did not significantly
reduce CD8+ T cell frequency in the splenocytes of the
MRL/lpr mice (Fig. 3D).
Contrarily, CD4+ T cell frequency in the splenocytes of
the MRL/lpr mice with PNS treatment was significantly
increased (Fig. 3D).
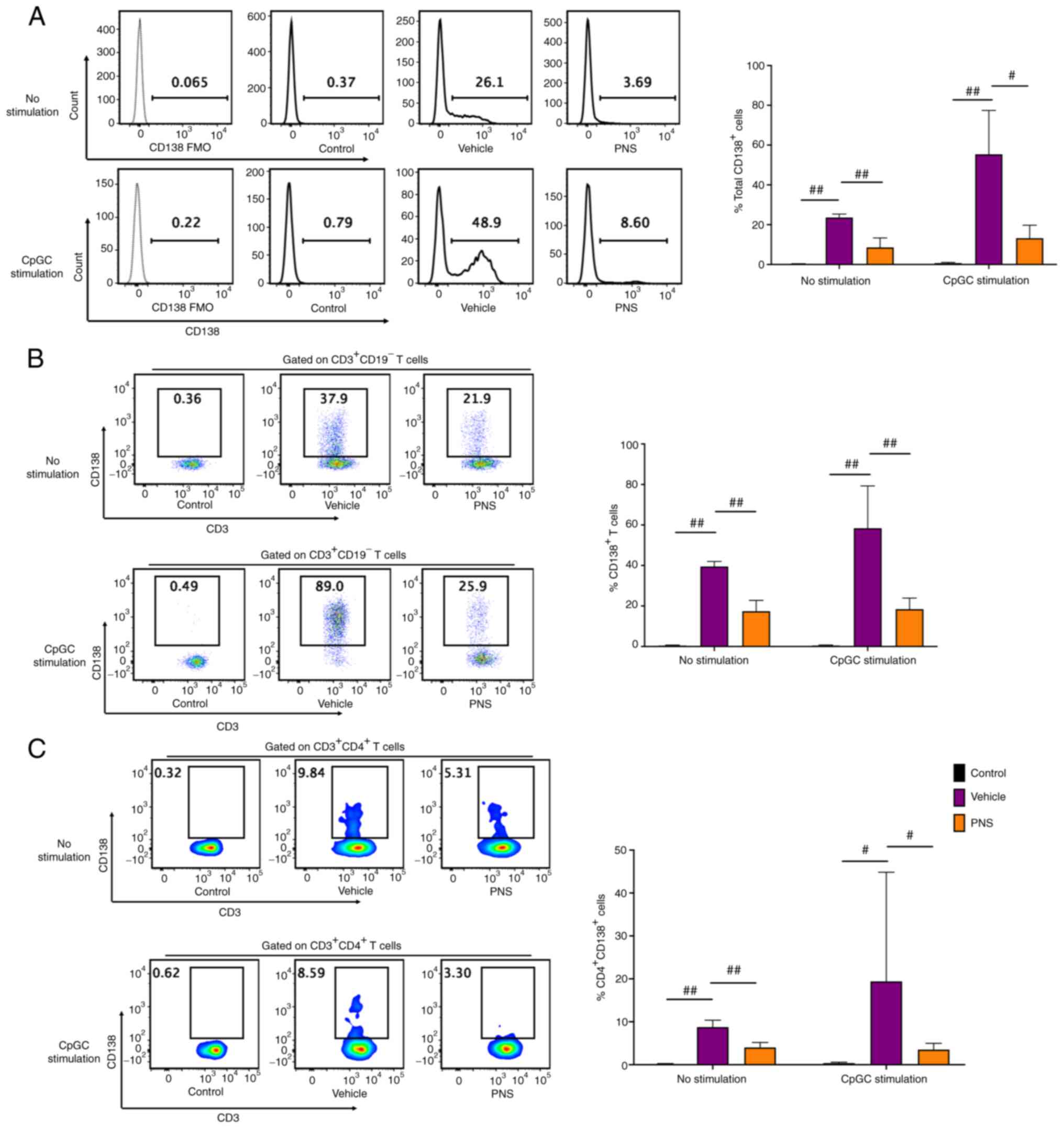
Prednisone prevents CD138 expression
in T cells of MRL/lpr mice
Isolated splenocytes from the MRL/MPJ mice without
stimulation had a near absence of CD138+ cells, whereas
the splenocytes of Fas-deficiency MRL/lpr mice had a
significant increase in CD138+ cell frequency compared
with those of the MRL/MPJ mice (Fig.
4A). We next co-cultured and stimulated splenocytes with CpGC
for 24 h. We also observed that CD138+ cells were
accumulated in splenocytes of the MRL/lpr mice but not in
the MRL/MPJ mice (Fig. 4A).
Compared to the vehicle-treated lupus mice, the CD138+
cell frequencies in splenocytes of the PNS-treated lupus mice were
significantly reduced both with and without 24 h of CpGC
stimulation (Fig. 4A).
The frequencies of CD138+ cells in
CD3+ T cells of the MRL/MPJ mice were still negligible;
however, CD138 was abundantly expressed on CD3+ T cells
in the MRL/lpr mice (Fig.
4B) both before and after 24 h of CpGC stimulation of
splenocytes in vitro. CD138+ T cell frequencies
in CD3+ T cells of the MRL/lpr mice with oral
administration of PNS were significantly decreased compared to the
vehicle-treated MRL/lpr mice both before and after 24 h of
CpGC stimulation of splenocytes in vitro (Fig. 4B).
In addition, we observed that CD138 was also
expressed in the CD4+ T cells in the MRL/lpr mice
but not in the MRL/MPJ mice both with and without 24 h of CpGC
stimulation of splenocytes in vitro (Fig. 4C). PNS also significantly
prevented CD138 expression in CD4+ T cells. The
CD138+ cell frequencies in the CD4+ T cells
were significantly decreased in the MRL/lpr mice after
prednisone treatment compared to the vehicle-treated mice both
before and after CpGC stimulation of splenocytes in vitro
(Fig. 4C).
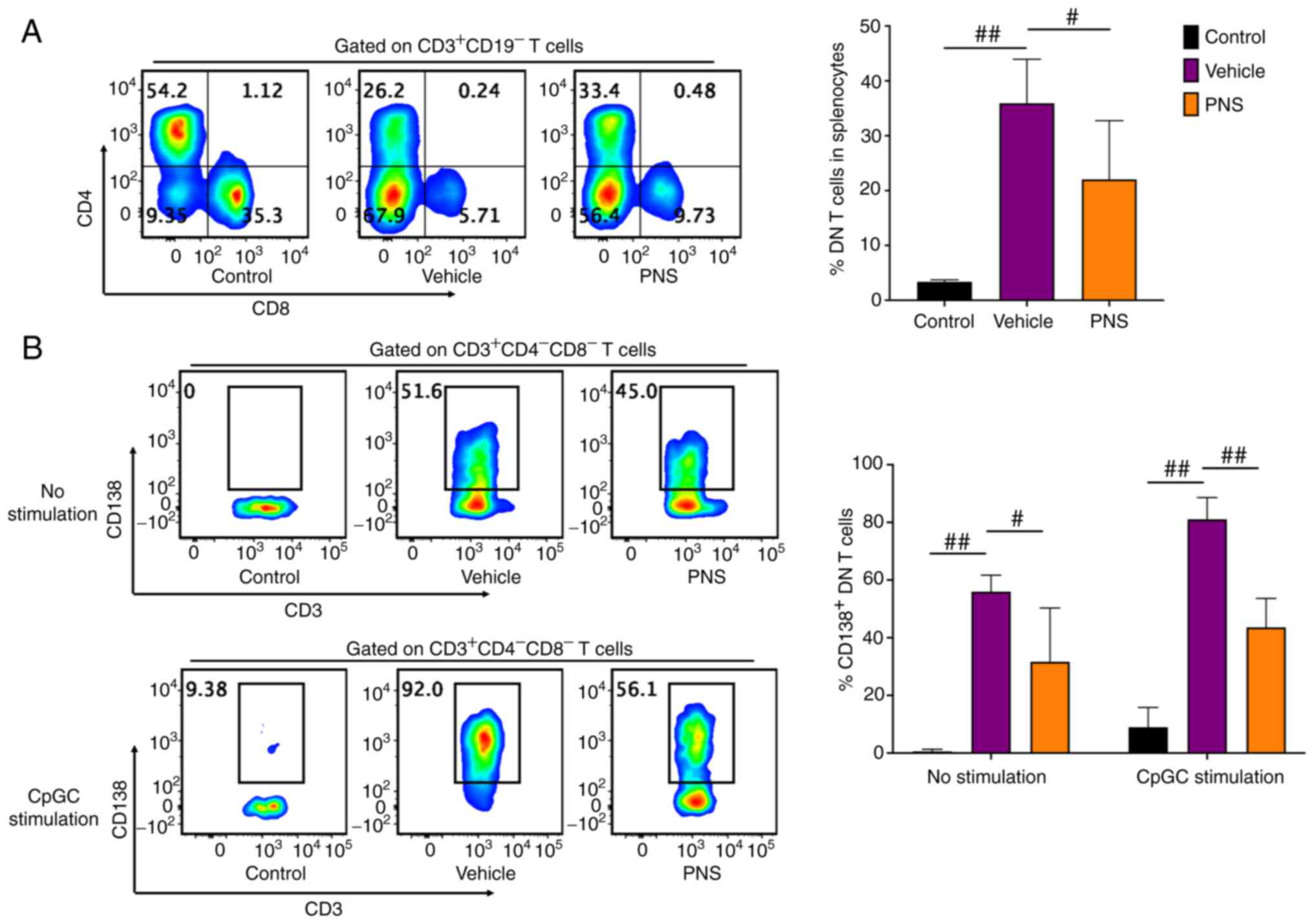
Prednisone prevents DN T cell
accumulation and CD138 expression in DN T cells of MRL/lpr
mice
DN T cells strikingly accumulated in the splenocytes
of the Fas-deficiency MRL/lpr mice but not in the MRL/MPJ
mice (Fig. 5A). PNS significantly
relieved DN T cell accumulation in the splenocytes of the
MRL/lpr mice (Fig. 5A).
Importantly, DN T cells in the MRL/lpr mice but not in the
MRL/MPJ mice commonly expressed CD138 (Fig. 5B). However, PNS also prevented
CD138 expression in DN T cells of the MRL/lpr mice. The
frequency of CD138+ cells in DN T cells of the
PNS-treated lupus mice was significantly reduced compared to the
vehicle-treated mice (Fig. 5B).
Furthermore, even after 24 h of CpGC stimulation of splenocytes
in vitro, the CD138+ cell frequency in DN T cells
of the PNS-treated MRL/lpr mice also showed a significant
reduction compared to the vehicle-treated mice (Fig. 5B).
Discussion
The present study results demonstrated that
prednisone (PNS) significantly relieved systemic lupus
erythematosus (SLE) symptoms in MRL/lpr mice by alleviating
enlargement of the spleen and lymph nodes, reducing the production
of autoantibody in the serum including anti-double-stranded
(anti-ds)DNA antibodies IgG antibody and anti-nuclear antibody
(ANA), and ameliorating renal tissue injury and simultaneously
preventing the accumulation of double negative (DN) T cells in
splenocytes of MRL/lpr mice. In addition, it was
demonstrated that PNS had a significant effect on CD138 expression
in CD3+ T cells of the MRL/lpr mice. PNS
prevented CD138+ T cell accumulation in the
MRL/lpr mice and inhibited CD138 expression in both
CD4+ and DN T cells of the MRL/lpr mice. In
addition, PNS played a protective role by significantly increasing
levels of IgM secretion in the serum of the MRL/lpr mice.
The results showed new insights into the mechanisms of
glucocorticoid on SLE treatment.
PNS, a glucocorticoid drug, exhibited significant
therapeutic effects on MRL/lpr lupus mice in our study. We
observed that PNS was able to significantly reduce the production
of total IgG and multiple IgG antibody subsets such as IgG1, IgG2a,
and IgG2b, and simultaneously increased IgM production in the serum
of the MRL/lpr mice. Previous studies have demonstrated that
in humans, IgA deficiency is associated with autoimmunity. However,
this differs between SLE humans and SLE mice. IgM antibody has been
demonstrated to be a protective antibody isotype in MRL/lpr
mice (38–41). IgM deficiency was previously found
to significantly contribute to accelerated development of lupus and
elevated levels of IgG autoantibody secretion in MRL/lpr
mice (38–41). This indicated that PNS could
ameliorate lupus in MRL/lpr mice by increasing the
production of IgM. This suggests that glucocorticoid ameliorates
SLE by increasing the production of protective antibody subsets.
Furthermore, PNS significantly decreased autoantibody production,
including anti-dsDNA antibody in the MRL/lpr mice. Our results also
showed that PNS significantly reduced
CD4+CD138+ T cell frequency in
CD4+ T cells of the MRL/lpr mice. A previous
study demonstrated that CD138+ T cells contributed to
the production of anti-dsDNA antibodies both in vivo and
in vitro by a CD4 receptor-dependent mechanism. This
suggested that CD4+CD138+ T cells were the
autoreactive CD4+ T cells that promoted anti-dsDNA
antibody production (21). This
indicated that the decline in CD138 expression in CD4+ T
cells induced by PNS prevented anti-dsDNA antibody production,
which resulted in PNS decreasing anti-dsDNA autoantibody levels in
the serum.
DN T cells have been shown to accumulate in the
peripheral blood of SLE patients and the spleen of Fas-deficiency
lupus mice (3,9,10).
Our results showed that PNS significantly prevented DN T cell
accumulation in splenocytes of the MRL/lpr mice. Previous
research has demonstrated that DN T cells are involved in the
development of systemic inflammation and tissue damage in lupus
patients (3). Recent research has
demonstrated that the adoptive transfer of DN T cells aggravates
the pathology in young lupus mice, while significant infiltration
of DN T cells was observed in both adult and pediatric lupus
kidneys (3). However, our results
showed that the majority of DN T cells in splenocytes of the
MRL/lpr mice were CD138 positive. Previous research also
showed that CD138+ T cells were associated with the
production of anti-dsDNA antibodies both in vivo and in
vitro, and demonstrated adoptive transfer of CD138+
T cells significantly contributed to renal tissue injuries in
MRL/lpr lupus mice (21).
These results demonstrated that CD138+ T cells are also
strongly associated with the progression of lupus in MRL/lpr
mice. Our recent study demonstrated that CD138 expression in
CD3+ T cells strikingly prevented the apoptosis of
CD3+ T cells and strikingly contributed to the
accumulation of DN T cells in T cells of MRL/lpr mice (Xie
T, Liu X and Li P; unpublished data). In the present study, our
results also showed that DN T cells in the MRL/lpr mice had
a high level of CD138 expression. PNS treatment significantly
decreased CD138+ cell frequency in DN T cells of lupus
mice. Our results demonstrated that reduced frequency of
CD138+ cells in DN T cells contributed to reducing the
accumulation of DN T cells in MRL/lpr mice after PNS
administration. However, information and data regarding CD138
expression in T cells in MRL/lpr mice is still limited. The
specific mechanism and signaling pathways involved in CD138
expression in CD3+ T cells of MRL/lpr mice are
yet to be deciphered and have not been consistent between studies.
Hence, more studies and data are needed to demonstrate the
underlying mechanism and signaling pathways in the
glucocorticoid-mediated prevention of CD138 expression in T cells
of MRL/lpr mice.
CpG DNA, which includes CpGA, CpGB, and CpGC, are
Toll-like receptor (TLR) agonists (42) that activate and induce interferon
(IFN)-α secretion in plasmacytoid dendritic cells (pDCs) (43). CpGC stimulation was used in this
study to mimic in vivo conditions in SLE patients (44), i.e., to induce the secretion of
IFN-α to promote SLE development and aggravate tissue injury
(43,45). Our present study showed that the
frequencies of CD4+ and CD8+ T cells in fresh
splenocytes of the control mice were nearly equal to the
frequencies in the MRL/lpr mice. However, when splenocytes
were stimulated by CpGC, CD4+ and CD8+ T cell
frequencies in the control mice were significantly higher than the
frequencies in the Fas-deficiency MRL/lpr mice. Furthermore,
T cells in the control mice including CD4+ T cells and
CD8+ T cells, were more likely to be activated in
response to T cell stimulation compared to lupus mice. Our results
indicated that both CD4+ and CD8+ T cells in
the Fas-deficiency MRL/lpr mice had defective proliferation
and activation.
In summary, PNS had a significant therapeutic
effect on MRL/lpr lupus mice. PNS significantly prevented
CD138+ T cell accumulation in the MRL/lpr mice.
CD138 expression in DN T cells of the MRL/lpr mice was
inhibited by PNS which contributed to PNS alleviating DN T cell
accumulation. PNS also prevented CD138 expression in
CD4+ T cells which significantly resulted in reduced
anti-dsDNA antibody production in MRL/lpr mice after PNS
treatment. In addition, PNS also decreased IgG and IgG subset
production and simultaneously promoted IgM secretion which plays a
preventive role in the progression of SLE treatment. Our results
provide new insights into the therapeutic effects and mechanisms of
glucocorticoid treatment for SLE.
Acknowledgements
The authors would like to acknowledge Dr Sabry
Hamza (Tessa Therapeutics Ltd., Singapore) for editing this
manuscript.
Funding
This study was funded by the Beijing Postdoctoral Research
Foundation (grant no. ZZ2019-23) and the MiaoPu Research Foundation
of the Beijing Institute of Chinese Medicine (grant no.
MP-2020-45).
Availability of data and materials
The data generated in the present study are
available from the corresponding author on reasonable request.
Authors' contributions
TX conceived the study and wrote the manuscript. TX
and PL designed the experiments. TX and HL performed the laboratory
work. TX and HL performed the data analysis. PL revised and edited
the manuscript. TX and PL confirm the authenticity of all the raw
data. All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work (including the data
presented) are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of the Beijing
Institute of Chinese Medicine and were performed in accordance with
Animal Research protocols for reporting of In Vivo
Experiments (ARRIVE) guidelines and institutional regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Kaul A, Gordon C, Crow MK, Touma Z,
Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G and Hughes G:
Systemic lupus erythematosus. Nat Rev Dis Primers. 2:160392016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dörner T and Furie R: Novel paradigms in
systemic lupus erythematosus. Lancet. 393:2344–2358. 2019.
View Article : Google Scholar
|
|
3
|
Alexander JJ, Jacob A, Chang A, Quigg RJ
and Jarvis JN: Double negative T cells, a potential biomarker for
systemic lupus erythematosus. Precis Clin Med. 3:34–43. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chesnutt MS, Finck BK, Killeen N, Connolly
MK, Goodman H and Wofsy D: Enhanced lymphoproliferation and
diminished autoimmunity in CD4-deficient MRL/lpr mice. Clin Immunol
Immunopathol. 87:23–32. 1998. View Article : Google Scholar
|
|
5
|
Nagasu A, Mukai T, Iseki M, Kawahara K,
Tsuji S, Nagasu H, Ueki Y, Ishihara K, Kashihara N and Morita Y:
Sh3bp2 Gain-Of-function mutation ameliorates lupus phenotypes in
B6.MRL-Faslpr Mice. Cells. 8:4022019. View Article : Google Scholar
|
|
6
|
Benihoud K, Bonardelle D, Bobé P and Kiger
N: MRL/lpr CD4-CD8− and CD8+ T cells,
respectively, mediate Fas-dependent and perforin cytotoxic
pathways. Eur J Immunol. 27:415–420. 1997. View Article : Google Scholar
|
|
7
|
Martina MN, Noel S, Saxena A, Rabb H and
Hamad AR: Double negative (DN) αβ T cells: Misperception and
overdue recognition. Immunol Cell Biol. 93:305–310. 2015.
View Article : Google Scholar
|
|
8
|
Corneth OBJ, Schaper F, Luk F, Asmawidjaja
PS, Mus AMC, Horst G, Heeringa P, Hendriks RW, Westra J and
Lubberts E: Lack of IL-17 Receptor A signaling aggravates
lymphoproliferation in C57BL/6 lpr mice. Sci Rep. 9:40322019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaltout AS, Sayed D, Badary MS, Nafee AM,
El Zohri MH, Bakry R and Ahmed SH: Effect of IL6 and IL23 on double
negative T cells and anti ds-DNA in systemic lupus erythematosus
patients. Hum Immunol. 77:937–943. 2016. View Article : Google Scholar
|
|
10
|
Brandt D and Hedrich CM: TCRαβ
+ CD3 + CD4−CD8−
(double negative) T cells in autoimmunity. Autoimmun Rev.
17:422–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Getachew Y, Cusimano FA, James LP and
Thiele DL: The role of intrahepatic
CD3+/CD4−/CD8-double negative T (DN T) cells
in enhanced acetaminophen toxicity. Toxicol Appl Pharmacol.
280:264–271. 2014. View Article : Google Scholar
|
|
12
|
Hedrich CM, Rauen T, Crispin JC, Koga T,
Ioannidis C, Zajdel M, Kyttaris VC and Tsokos GC: cAMP-responsive
element modulator α (CREMα) trans-represses the transmembrane
glycoprotein CD8 and contributes to the generation of CD3+CD4-CD8-T
cells in health and disease. J Biol Chem. 288:31880–31887. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hedrich CM, Crispín JC, Rauen T, Ioannidis
C, Koga T, Rodriguez Rodriguez N, Apostolidis SA, Kyttaris VC and
Tsokos GC: cAMP responsive element modulator (CREM) α mediates
chromatin remodeling of CD8 during the generation of CD3+CD4-CD8-T
cells. J Biol Chem. 289:2361–2370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Merino R, Fossati L, Iwamoto M, Takahashi
S, Lemoine R, Ibnou-Zekri N, Pugliatti L, Merino J and Izui S:
Effect of long-term anti-CD4 or anti-CD8 treatment on the
development of lpr CD4-CD8-double negative T cells and of the
autoimmune syndrome in MRL-lpr/lpr mice. J Autoimmun. 8:33–45.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohteki T, Iwamoto M, Izui S and MacDonald
HR: Reduced development of CD4-8-B220+ T cells but normal
autoantibody production in lpr/lpr mice lacking major
histocompatibility complex class I molecules. Eur J Immunol.
25:37–41. 1995. View Article : Google Scholar
|
|
16
|
Trimble LA, Prince KA, Pestano GA, Daley J
and Cantor H: Fas-dependent elimination of nonselected CD8 cells
and lpr disease. J Immunol. 168:4960–4967. 2002. View Article : Google Scholar
|
|
17
|
Koh DR, Ho A, Rahemtulla A, Fung-Leung WP,
Griesser H and Mak TW: Murine lupus in MRL/lpr mice lacking CD4 or
CD8 T cells. Eur J Immunol. 25:2558–2562. 1995. View Article : Google Scholar
|
|
18
|
Ezine S, Lucas B, Vicari A, Dautigny N,
Vasseur F and Penit C: A novel CD45RA+CD4+
transient thymic subpopulation in MRL-lpr/lpr mice: Its relation to
non-proliferating CD4-CD8-CD45RA+ tumor cells. Int
Immunol. 5:89–96. 1993. View Article : Google Scholar
|
|
19
|
Grishkan IV, Ntranos A, Calabresi PA and
Gocke AR: Helper T cells down-regulate CD4 expression upon chronic
stimulation giving rise to double-negative T cells. Cell Immunol.
284:68–74. 2013. View Article : Google Scholar
|
|
20
|
Zhang D, Yang W, Degauque N, Tian Y,
Mikita A and Zheng XX: New differentiation pathway for
double-negative regulatory T cells that regulates the magnitude of
immune responses. Blood. 109:4071–4079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Takeda K and Akkoyunlu M: Disease
stage-specific pathogenicity of CD138 (Syndecan 1)-expressing T
cells in systemic lupus erythematosus. Front Immunol. 11:15692020.
View Article : Google Scholar
|
|
22
|
Seagal J, Leider N, Wildbaum G, Karin N
and Melamed D: Increased plasma cell frequency and accumulation of
abnormal syndecan-1plus T-cells in Igmu-deficient/lpr mice. Int
Immunol. 15:1045–1052. 2003. View Article : Google Scholar
|
|
23
|
Mohamood AS, Bargatze D, Xiao Z, Jie C,
Yagita H, Ruben D, Watson J, Chakravarti S, Schneck JP and Hamad
AR: Fas-mediated apoptosis regulates the composition of peripheral
alphabeta T Cell repertoire by constitutively purging out double
negative T cells. PLoS One. 3:e34652008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu LD, Stump KL, Wallace NH, Dobrzanski P,
Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Mason JL,
et al: Depletion of autoreactive plasma cells and treatment of
lupus nephritis in mice using CEP-33779, a novel, orally active,
selective inhibitor of JAK2. J Immunol. 187:3840–3853. 2011.
View Article : Google Scholar
|
|
25
|
Calame KL: Plasma cells: Finding new light
at the end of B cell development. Nat Immunol. 2:1103–1108. 2001.
View Article : Google Scholar
|
|
26
|
Pan Z, Chen M, Zhang Q, Wang E, Yin L, Xu
Y, Huang Q, Yuan Y, Zhang X, Zheng G and Yuan J: CD3-positive
plasmablastic B-cell neoplasms: A diagnostic pitfall. Mod Pathol.
31:718–731. 2018. View Article : Google Scholar
|
|
27
|
Tsokos GC, Lo MS, Costa Reis P and
Sullivan KE: New insights into the immunopathogenesis of systemic
lupus erythematosus. Nat Rev Rheumatol. 12:716–730. 2016.
View Article : Google Scholar
|
|
28
|
Eisenberg RA, Craven SY, Warren RW and
Cohen PL: Stochastic control of anti-Sm autoantibodies in
MRL/Mp-lpr/lpr mice. J Clin Invest. 80:691–697. 1987. View Article : Google Scholar
|
|
29
|
Eisenberg RA, Craven SY and Cohen PL:
Isotype progression and clonality of anti-Sm autoantibodies in
MRL/Mp-lpr/lpr mice. J Immunol. 139:728–733. 1987.
|
|
30
|
Pinheiro SVB, Dias RF, Fabiano RCG, Araujo
SA and Silva ACSE: Pediatric lupus nephritis. J Bras Nefrol.
41:252–265. 2018.(In English, Portuguese). View Article : Google Scholar
|
|
31
|
Wilhelmus S, Bajema IM, Bertsias GK,
Boumpas DT, Gordon C, Lightstone L, Tesar V and Jayne DR: Lupus
nephritis management guidelines compared. Nephrol Dial Transplant.
31:904–913. 2016. View Article : Google Scholar
|
|
32
|
Mok CC, Yap DY, Navarra SV, Liu ZH, Zhao
MH, Lu L, Takeuchi T, Avihingsanon Y, Yu XQ, Lapid EA, et al:
Overview of lupus nephritis management guidelines and perspective
from Asia. Int J Rheum Dis. 16:625–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dammacco R: Systemic lupus erythematosus
and ocular involvement: An overview. Clin Exp Med. 18:135–149.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y, He S, Bai B, Zhang L, Xue L, Lin Z,
Yang X, Zhu F, He P, Tang W and Zuo J: Therapeutic effects of the
artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition
of TLR-triggered B-cell activation and plasma cell formation. Cell
Mol Immunol. 13:379–390. 2016. View Article : Google Scholar
|
|
35
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG; NC3Rs Reporting Guidelines Working Group, : Animal
research: Reporting in vivo experiments: The ARRIVE guidelines. Br
J Pharmacol. 160:1577–1579. 2010. View Article : Google Scholar
|
|
36
|
Zhang P, Su L, Ma F, Ji X, Su Y, Yue Q,
Zhao C, Zhang S, Sun X and Zhao L: Weilan gum oligosaccharide
ameliorates dextran sulfate sodium-induced experimental ulcerative
colitis. Mol Med Rep. 25:522022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tilstra JS, Avery L, Menk AV, Gordon RA,
Smita S, Kane LP, Chikina M, Delgoffe GM and Shlomchik MJ:
Kidney-infiltrating T cells in murine lupus nephritis are
metabolically and functionally exhausted. J Clin Invest.
128:4884–4879. 2018. View Article : Google Scholar
|
|
38
|
Ehrenstein MR, Cook HT and Neuberger MS:
Deficiency in serum immunoglobulin (Ig)M predisposes to development
of IgG autoantibodies. J Exp Med. 191:1253–1258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carroll MC: A protective role for innate
immunity in systemic lupus erythematosus. Nat Rev Immunol.
4:825–831. 2004. View Article : Google Scholar
|
|
40
|
Yoshizawa Y, Honda S and Shibuya A:
Involvement of Fcα/µR (CD351) in autoantibody production. Mol
Immunol. 57:216–219. 2014. View Article : Google Scholar
|
|
41
|
Boes M, Schmidt T, Linkemann K, Beaudette
BC, Marshak-Rothstein A and Chen J: Accelerated development of IgG
autoantibodies and autoimmune disease in the absence of secreted
IgM. Proc Natl Acad Sci USA. 97:1184–1189. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Deriaud E, Jiao X, Braun D,
Leclerc C and Lo-Man R: Type I interferons protect neonates from
acute inflammation through interleukin 10-producing B cells. J Exp
Med. 204:1107–1118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Menon M, Blair PA, Isenberg DA and Mauri
C: A regulatory feedback between plasmacytoid dendritic cells and
regulatory B cells Is aberrant in systemic lupus erythematosus.
Immunity. 44:683–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu HM, Wang J, Zhang B, Fang L, Xu K and
Liu RY: CpG-ODN promotes phagocytosis and autophagy through JNK/P38
signal pathway in Staphylococcus aureus-stimulated macrophage. Life
Sci. 161:51–59. 2016. View Article : Google Scholar
|
|
45
|
Akiyama C, Tsumiyama K, Uchimura C, Honda
E, Miyazaki Y, Sakurai K, Miura Y, Hashiramoto A, Felsher DW and
Shiozawa S: Conditional upregulation of IFN-α alone Is sufficient
to induce systemic lupus erythematosus. J Immunol. 203:835–843.
2019. View Article : Google Scholar
|