Introduction
The side effects of orthodontic tooth movement
include orthodontic-induced external apical root resorption (EARR),
periodontal inflammation, and tooth demineralization and loosening,
among which EARR is the most common side effect (1). The mechanical factors noted in
specific treatment techniques, such as the duration of force
application and force magnitude and direction, are associated with
the degree of EARR (2,3). It has been shown that periodontal
ligament cells (PDLc), which are compressed by forces applied on
teeth, secrete osteoclastogenic cytokines to stimulate bone
resorption in the direction of the orthodontic force vector
(4). EARR is associated with the
cellular activity of over-compressed PDLc and cementum resorption
(5).
In contrast to these findings, recent studies have
shown an alternative consideration regarding the role of
cementocytes in the mechanotransduction and in the biological
response during tooth movement. Α previous ex vivo study by
the authors indicated that cementocytes were sensitive to
mechanical loading via the upregulation of sclerostin (SOST)
expression, the increase in the receptor activator of nuclear
factor-κB ligand (RANKL)/osteoprotegerin (OPG) ratio,
and the downregulation of the expression levels of osteocalcin
(6). Lira Dos Santos et al
(7) demonstrated that
cementocytes exhibited increased nuclear size and proportion of
euchromatin under orthodontic loading, and reported a significant
downregulation in the expression levels of type IV collagen
following orthodontic tooth movement. In addition, cementocytes are
also involved in cellular cementum apposition in mice and are
associated with changes in cellular ultrastructure and in the
proteomic profile of the cementum (8). These findings indicate that similar
to PDLc, cementocytes play important roles in the biological events
of the orthodontic tooth movement and the associated EARR.
Heavy and prolonged forces induce over-compression
of PDLc and consequent cementum resorption, as aforementioned.
However, the effect of force magnitude on cementocytes remains
largely unknown. In the present study, the response of cementocytes
was examined with regard to the intrusion forces of different
magnitudes, and the variations in the expression levels of
RANKL/OPG, and SOST were assessed in cementocytes
grown under orthodontic forces.
A variety of signaling pathways and cytokines have
been shown to be associated with tooth movement (9). The key regulators among them include
the SOST, OPG, and RANKL proteins, which are capable of regulating
the balance of osteoclastogenic and osteogenic differentiation
(10). Previous in vitro
studies have detected these gene expression profiles in
cementocytes (6,11,12); therefore, in the present study, it
was hypothesized that cementocytes may respond to the in
vivo orthodontic force via regulation of expression of these
genes.
The aim of the present study was to investigate the
regulatory roles of cementocytes in the biological response to
orthodontic intrusion forces, evaluate their contributions to the
microenvironment homeostasis, and clarify the effect of force
magnitude on the cementocyte behavior during orthodontic tooth
intrusion.
Materials and methods
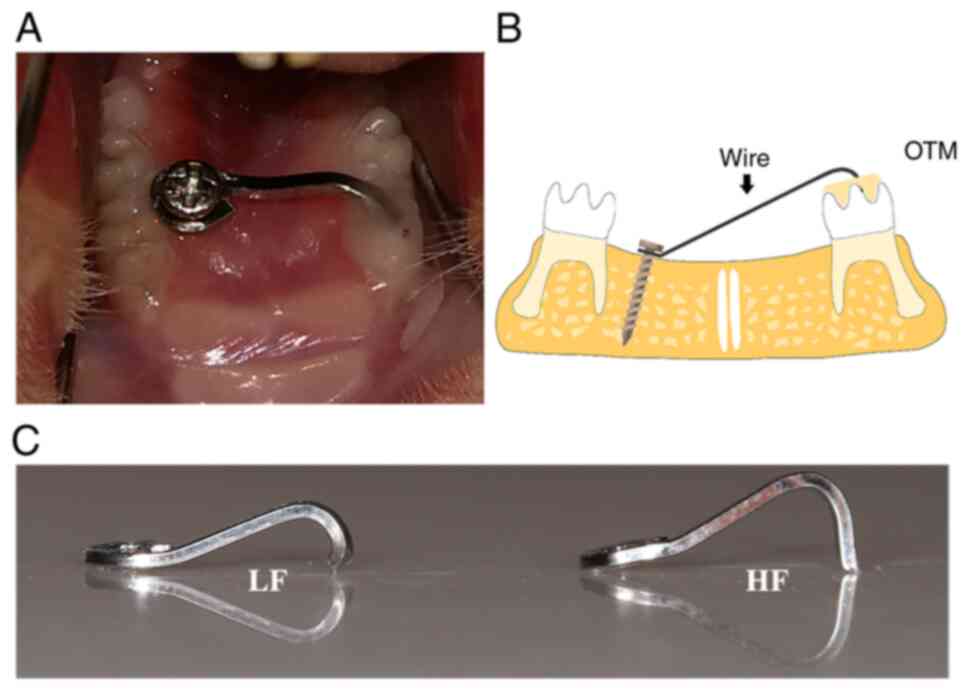
Intrusion loading in a rat model
The animal experiments were approved by the Animal
Ethics Committee of Shanghai Ninth People's Hospital (approval no.
SH9H-2020-A298-1) and the protocols were performed in accordance
with the corresponding guidelines. Sprague Dawley rats (n=90; eight
weeks old; weight, 289.1±37.21 g; 45 males and 45 females of
specific pathogen-free grade) were randomly assigned to three
groups as follows: Control group (n=30), low force group (n=30),
and high force group (n=30). They were housed at 22±2°C at 40–80%
humidity and under a 12-h light/dark cycle with commercial feed and
water ad libitum. Following anesthesia (1% pentobarbital
sodium; 50 mg/kg body weight; intraperitoneal injection), a
sterilized stainless-steel screw (1 mm in diameter and 4 mm in
length) was implanted in the left palatal bone as an anchorage for
loading. A sterilized 0.016×0.022 inch stainless steel wire (3M
Unitek), which was bent into a specially designed ‘L’ loop was
fixed between the screw and right maxillary first molar, followed
by resin adhesion. The elasticity of the wire was adjusted by the
length of the buckling part and the subsequent compressive force
was adjusted to 10 g (low force group) and 50 g (high force group)
using a dynamometer. The rats in the control group received a resin
adhesion without intrusion force loading. None of the intrusion
appliances were removed during treatment. At each time-point, the
rats were sacrificed by overdose of anesthesia (1% pentobarbital
sodium; 100 mg/kg body weight; intraperitoneal injection) and
cervical dislocation if needed, and their vital signs were
confirmed by heartbeat and pupillary response to light, following
the American Veterinary Medical Association guidelines (13). The left first molar and the
surrounding alveolar bone were harvested.
Micro-computed tomography (µ-CT)
scanning
A total of 45 rats were randomly selected in each
group for µ-CT scanning. Three rats were randomly selected each on
days 1, 2, 4, 7 and 14. Their samples were fixed in 70% ethanol for
48 h at room temperature and scanned by a high-solution µ-CT
(Scanco Medical AG). The following conditions were used: Resolution
of 10 µm voxel size, voltage of 45 kV, current of 177 µA, and
integration time of 200 msec. The data were reconstructed and the
serial images were generated using the supporting analyzing
software provided by the manufacturer. The distance of tooth
movement was calculated as previously described (14,15). In short, the tooth movement was
defined by the movement of the mesial buccal occlusal tip of the
right maxillary first molar, and was measured by the deviation of
the tip from the occlusal plane.
Bone histometric analysis
The quantitative analysis of the alveolar bone
changes was conducted using serial images. The region of interest
was set as a square area located in the intra-root alveolar bone of
the right maxillary first molar, as previously described (15). The superior boundary was defined
as the inferior plane of the root furcation. The transverse
boundaries extended distally by the distal buccal root and mesially
by the mesial root of the right maxillary first molar. Bone volume
to tissue volume (BV/TV, 100%), bone mineral density
(g/cm3), trabecular number (per mm), trabecular
thickness (µm), and trabecular separation (mm) were analyzed within
the region.
Tartrate-resistant acid phosphatase
(TRAP) staining
The samples were fixed in 4% paraformaldehyde for 48
h at room temperature, decalcified in 14% EDTA (pH 7.1) for 8
weeks, dehydrated and embedded in paraffin. The specimens were cut
into 4-µm sections along the sagittal axis of the right maxillary
first molar. For TRAP staining, deparaffinized sections were
treated by an acid phosphatase kit (cat. no. 387A-1KT;
MilliporeSigma) for 1 h in a 37°C water bath protected from light,
according to the manufacturer's instructions and counterstained 2
min at room temperature using Harris' hematoxylin solution. Images
were obtained with an Olympus light microscope system (Olympus
Corporation) at an objective magnification of ×200. The region of
interest (ROI) used for TRAP-positive cell quantification was
located on the apex of the mesial root (600 µm width and 400 µm
length). The contour of the cellular cementum and corresponding
alveolar bone in this region were depicted. Osteoclast cells, which
were TRAP-positive multinucleated cells near the linear surfaces of
the cellular cementum and alveolar bone, were quantified
separately. The data were normalized as the mean number of cells
per millimeter of cellular cementum and alveolar bone surface. The
root resorption of the mesial root of the first molar at each
time-point was evaluated by root resorption scores as previously
described (16). In short, the
ROI on the magnified (×200) image was divided into 10×10 mm grids.
Root resorption scores were determined by dividing the number of
grids with resorption lacunae by the total number of grids along
the root surface.
Immunohistochemical staining
Paraffin-embedded tissue sections were used
following deparaffinization with xylene and rehydration through a
series of experimental steps, including alcohol washes, 0.5% pepsin
antigen retrieval, and blocking steps (10% hydrogen peroxide for 15
min at room temperature to quench endogenous peroxidase activity
and 10% normal donkey serum for 30 min at 4°C to block non-specific
binding). The primary antibodies were polyclonal rabbit anti-OPG
(1:400; cat. no. 183910; Abcam) and monoclonal mouse anti-RANKL
(1:100; cat. no. sc-52950; Santa Cruz Biotechnology, Inc.). The
primary antibody was omitted in the negative control and no
labeling was observed in any case. Donkey anti-rabbit and
anti-mouse secondary antibodies (Jackson Immuno Research, Inc.)
were used respectively at 1:200 dilutions. Following incubation
with the primary antibodies at 4°C overnight, secondary antibodies
were used respectively at 1:200 dilutions for 2 h at room
temperature. Sections were incubated using a DAB substrate kit
(Thermo Fisher Scientific, Inc.) for 2 min at room temperature and
then counterstained with Harris' hematoxylin (for RANKL, 2 min,
room temperature) or 0.5% methyl green (for OPG, 1 min, room
temperature). Images were obtained with an Olympus light microscope
system (Olympus Corporation) at an objective magnification of
×200.
Immunofluorescence staining
Paraffin-embedded tissue sections were treated the
same way as the sections used for immunohistochemical staining. The
primary antibody was a polyclonal goat anti-SOST (cat. no. AF1589;
R&D Systems, Inc.) used at 1:50 dilutions. PBS was used as the
control antibody in the negative control sample. Donkey anti-goat
Alexa Fluor secondary antibody (cat. no. A-11058; Invitrogen;
Thermo Fisher Scientific, Inc.) was used at a 1:200 dilution.
Following incubation with the primary antibodies at 4°C overnight,
the secondary antibody was used respectively at 1:200 dilutions for
2 h at room temperature. The sections were subsequently stained
with 1 µg/ml DAPI for 10 min at 4°C (Invitrogen; Thermo Fisher
Scientific, Inc.). The images were obtained using an Olympus
fluorescence microscope system (Olympus Corporation) at a
magnification of ×400. Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc.) was used to assess the integrated optical
density (IOD) value and area of the section. Three random fields
from the cellular cementum and the surrounding alveolar bone were
selected separately to calculate the mean density of each area.
Reverse transcription-quantitative PCR
(RT-qPCR)
To quantitatively compare the gene expression levels
of the cementocytes and osteocytes following orthodontic intrusion,
the cellular cementum and the alveolar bone were separately
collected using a previously described method (17). A total of 15 rats in each group
were sacrificed to collect samples for RT-qPCR. Three rats were
randomly selected for each time-point (n=3). Briefly, the cementum
was detached from the apical region of the mesial root of the
intruded molar and the alveolar bone was isolated from the
periapical area of the same root. The tissues were dissected in
three cycles of serial digestion with collagenase A (300 U/l;
MilliporeSigma) and EDTA (5 mM, in 0.1% bovine serum albumin;
MilliporeSigma) for 20 min each at 37°C for elimination of
periodontal contamination. Total RNA was extracted from each
digested sample using a MiniBEST Universal RNA Extraction kit (cat.
no. 9767; Takara Bio, Inc.) according to the manufacturer's
instructions. The RNA samples were reverse transcribed to cDNA
using a PrimeScript RT reagent Kit (cat. no. RR036A; Takara Bio,
Inc.) according to the manufacturer's instructions. SYBR Premix Ex
Taq (cat. no. RR420A; Takara Bio, Inc.) was used as a probe. The
2−ΔΔCq method was used to estimate the relative RNA
levels, which were normalized to those of GAPDH, as
previously described (18). The
primers were synthesized (Shanghai Sangong Pharmaceutical Co.,
Ltd.) and the sequences are shown in Table I.
 | Table I.List of primer sequences used in
reverse transcription-quantitative PCR. |
Table I.
List of primer sequences used in
reverse transcription-quantitative PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| GAPDH |
TGATGGGTGTGAACCACGAG |
TACTTGGCAGGTTTCTCCAGG |
| OPG |
TGTCCGGATGGGTTCTTCTCA |
GCACAGGGTGACATCTATTCCA |
| RANKL |
CAGCATCGCTCTGTTCCTGTA |
CTGCGTTTTCATGGAGTCTCA |
| SOST |
TACATGCAGCCTTCGTTGCT |
CTCGGACACGTCTTTGGTGT |
Statistical analysis
Data are presented as the mean ± SEM. The data used
for each parameter from µ-CT, TRAP staining, and RT-qPCR were
analyzed and compared using SPSS 17.0 software (SPSS, Inc.).
Multiple groups were compared using one-way ANOVA, followed by
Bonferroni post hoc test to measure variances, while comparisons
between two groups comparisons were performed using an unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Orthodontic forces induce tooth
intrusion and alveolar bone remodeling
To investigate the effects of the intrusion force on
the tooth and alveolar bone, a tooth intrusion model was
established. The forces applied were controlled by the wire length
and measured using a force dynamometer (Fig. 1). The measurements of the forces
were 9.12±1.37 g in the low force group and 54.97±4.10 g in the
high force group. No significant differences were noted with regard
to these parameters between male and female subjects (P>0.05;
data not shown). A significant increase in tooth movement was
detected in the low force group from day 1 to 7 (P<0.01); on day
14, tooth movement was not significantly increased compared with
that of day 7 (P>0.05). In the high force group, the tooth
movement was accelerated on day 4 (340.04±43.42 µm), which was
significantly higher than that noted on day 1 (P<0.05). No
significant increase was noted on day 7 compared with day 4, and on
day 14 compared with day 7 (P>0.05). No significant difference
was noted in the tooth movement between the low and high force
groups detected at the time-points examined in the study
(P>0.05). Quantitative bone histometric analysis revealed the
significant differences noted between each group in the following
parameters: BV/TV, bone mineral density, and trabecular numbers
(P<0.05). The values of these parameters were reduced in the low
force group compared with those noted in the control group, and
were even lower in the high force group. The trabecular was
significantly separated in both the low and high force groups
compared with the control group (P<0.001); however, no
significant difference was noted within them. In addition, the
craters were observed in the alveolar bone in the high force group
(Fig. 2).
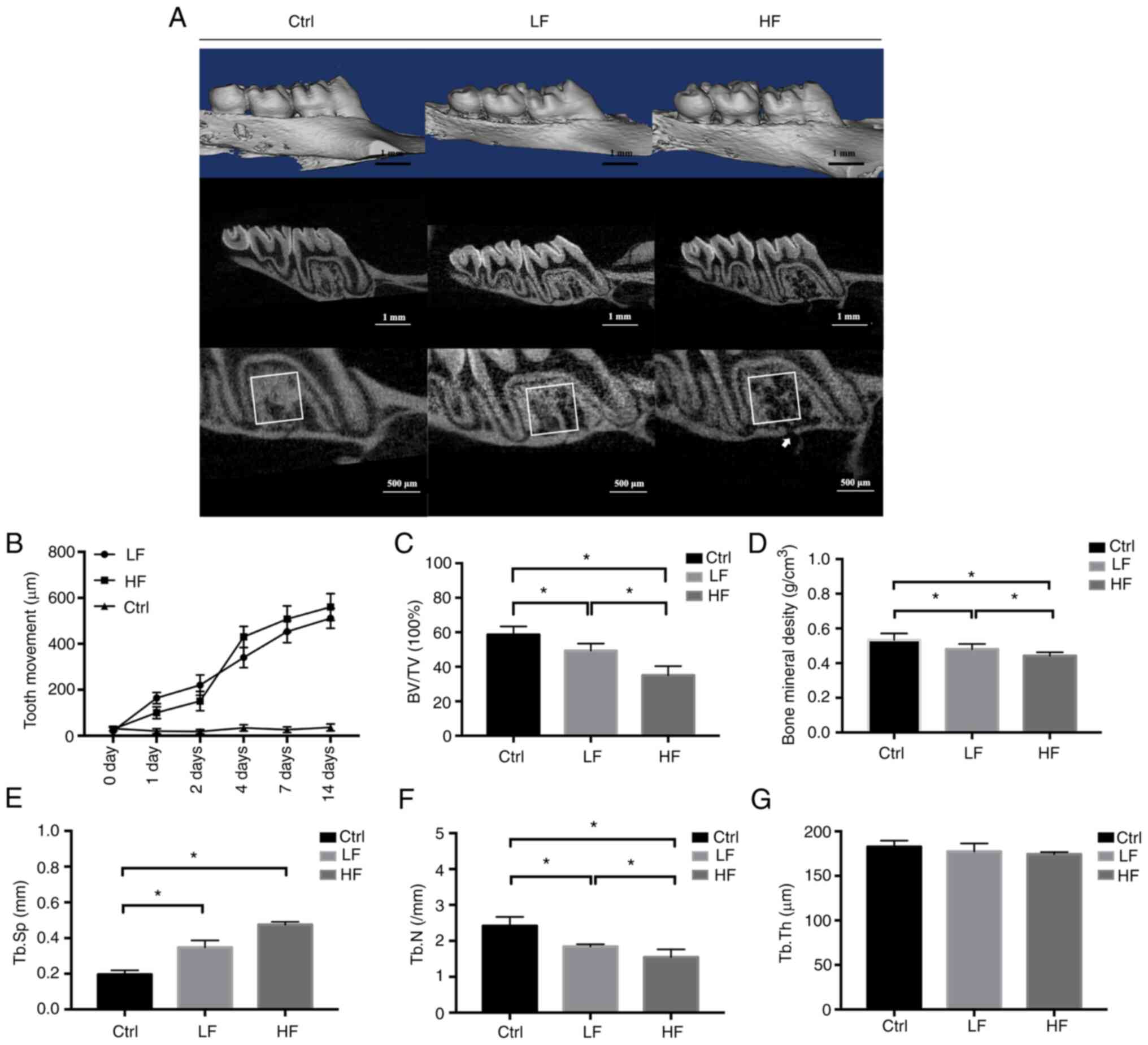 | Figure 2.µ-CT images and bone histometric
analysis. (A) The µ-CT scan and the reconstruction of the
experimental maxillary in Ctrl, LF, and HF groups on day 14. A ROI
was indicated by a square area located in the intra-root alveolar
bone of right maxillary first molar. Histometric analysis was
conducted with the ROI in each group. The white arrow indicated a
small crater on the alveolar bone induced by loading. (B) Linear
measurements of the experimental tooth movement. Data were
presented by the distance of the tip from the occlusal plane (µm)
over time. (C) BV/TV measured within the ROI in the Ctrl, LF and HF
groups on day 14. (D) Bone mineral density analysis on day 14. (E)
Tb.Sp on day 14. (F) Tb.N analysis on day 14. (G) Tb.Th on day 14.
*P<0.05. µ-CT, micro-computed tomography; ROI, region of
interest; BV/TV, bone volume to tissue volume; Ctrl, control; LF,
low force; HF, high force; Tb.Sp, trabecular separation; Tb.N,
trabecular number; Tb.Th, trabecular thickness. |
Osteoclast formation is induced by
compressive force
The resorption of the cellular cementum was detected
under high force loading (Fig.
3). To evaluate the difference of osteoclast formation on the
surface of the cellular cementum with the alveolar bone, the number
of TRAP-positive osteoclasts on either side were quantified
(Fig. 3). On the bone side, a
significant increase in osteoclast numbers was detected after 6 h
(low force, 6.77±1.07 cells/mm; high force, 5.63±1.11 cells/mm;
P<0.01), and the number continued to increase over time. No
significant differences were noted between the low and high force
groups at any time-point. On the cementum side, the osteoclast
number was significantly increased in the high force group at 24 h
(5.18±0.65 cells/mm; P<0.01), but not in the low force group
(0.57±0.49 cells/mm; P>0.05). Therefore, the number of
osteoclasts on the cementum side of the high force group was
considerably higher than that of the low force group when comparing
between 24 h and 14 days (P<0.01). The number of osteoclasts was
significantly higher on the bone side compared with that noted on
the cementum side in the low force group when comparing between 6 h
and 14 days (P<0.05). In the high force group, the differences
were noted at 6 h (P<0.01), 24 h (P<0.05), and 14 days
(P<0.05), when the bone side significantly surpassed the
cementum side. As for the root resorption scores, significant
increases were detected after 24 h in the high force group
(P<0.01), but after 7 days in the low force group (P<0.05).
From 24 h to 14 days, the root resorption scores of the high force
group were significantly higher than those of the low force group
(P<0.05).
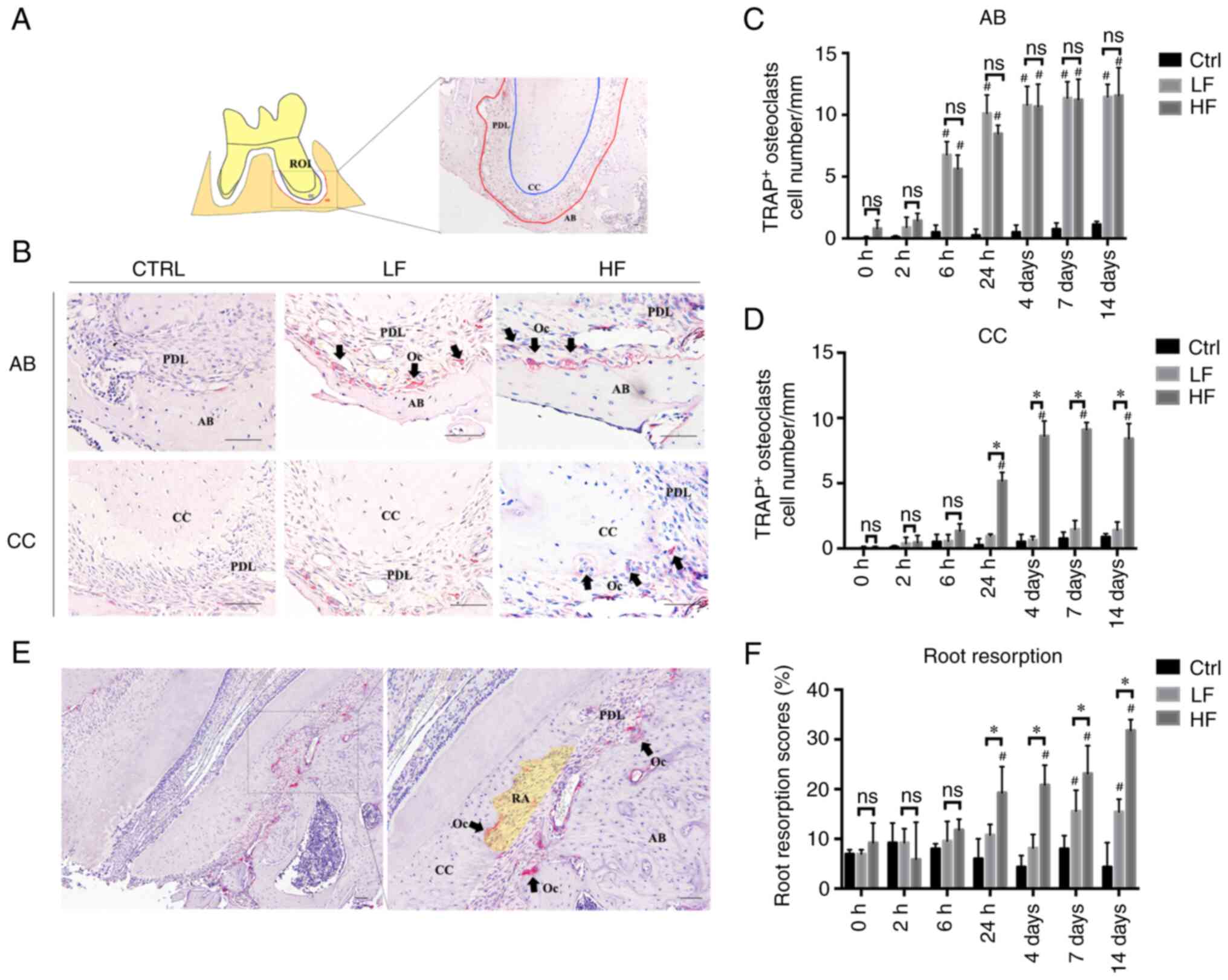 | Figure 3.TRAP staining and osteoclast
quantification. (A) Illustration of the apical area of the intruded
molar. The ROI was 600 µm in width and 400 µm in length. The
contour of CC (blue) and corresponding AB (red) are depicted. (B)
The TRAP staining of CC and AB in the Ctrl, LF and HF groups on day
14. The TRAP-positive osteoclasts (black arrows) were stained in
red. (C) The quantification of the TRAP-positive osteoclasts on
surfaces of AB at different time-points. The TRAP-positive
osteoclasts were quantified and normalized by the length of the
alveolar contour. (D) The quantification of the TRAP-positive
osteoclasts on surfaces of CC. The number was normalized by the
length of cementum contour. (E) Representative TRAP staining image
of cementum resorption under high compression force. Resorption
area was illustrated as a yellow translucent area. (F) Evaluation
of root resorption. Root resorption scores=(the number of grids
with resorption lacunae/the total number of grids along the root
surface) ×100. Scale bar, 50 µm. *P<0.05 compared between LF and
HF at the same time-point; #P<0.05 compared with the
control group. TRAP, tartrate-resistant acid phosphatase; ROI,
region of interest; CC, cellular cementum; AB, alveolar bone; Ctrl,
control; LF, low force; HF, high force; RA, resorption area; Oc,
osteoclasts; PDL, periodontal ligament; ns, not significant. |
Gene expression in the cellular
cementum and the alveolar bone under compression
To investigate the role of cementocyte
mechanotransduction during tooth intrusion, the cellular cementum
mRNA expression profiles of OPG, RANKL and SOST were
assessed. The surrounding alveolar bone was used for comparison
(Fig. 4). In the low force group,
the expression levels of SOST in both the cellular cementum
and the alveolar bone were evaluated over time. The results
indicated increased and decreased expression patterns. The
expression levels in the cellular cementum were slightly increased
at 24 h (1.26±0.56; P>0.05) and significantly decreased on day 7
compared with those of the control group (0.02±0.02; P<0.01).
The expression levels of SOST mRNA in the bone side were
significantly increased from 24 h (3.81±0.59; P<0.01) to 4 days
(3.75±0.89, P<0.01) compared with those noted on day 0. From day
7 to day 14, the expression levels were significantly reduced. From
24 h to 14 days, the alveolar bone expressed significantly higher
levels of SOST mRNA than those of the cellular cementum. In
the high force group, the difference in the expression levels of
SOST between the cellular cementum and the alveolar bone was
only noted on days 4 and 7. At these time-points, SOST
expression levels were significantly higher in the cellular
cementum than those noted in the alveolar bone, indicating opposite
findings to those noted at the same time-points in the low force
group.
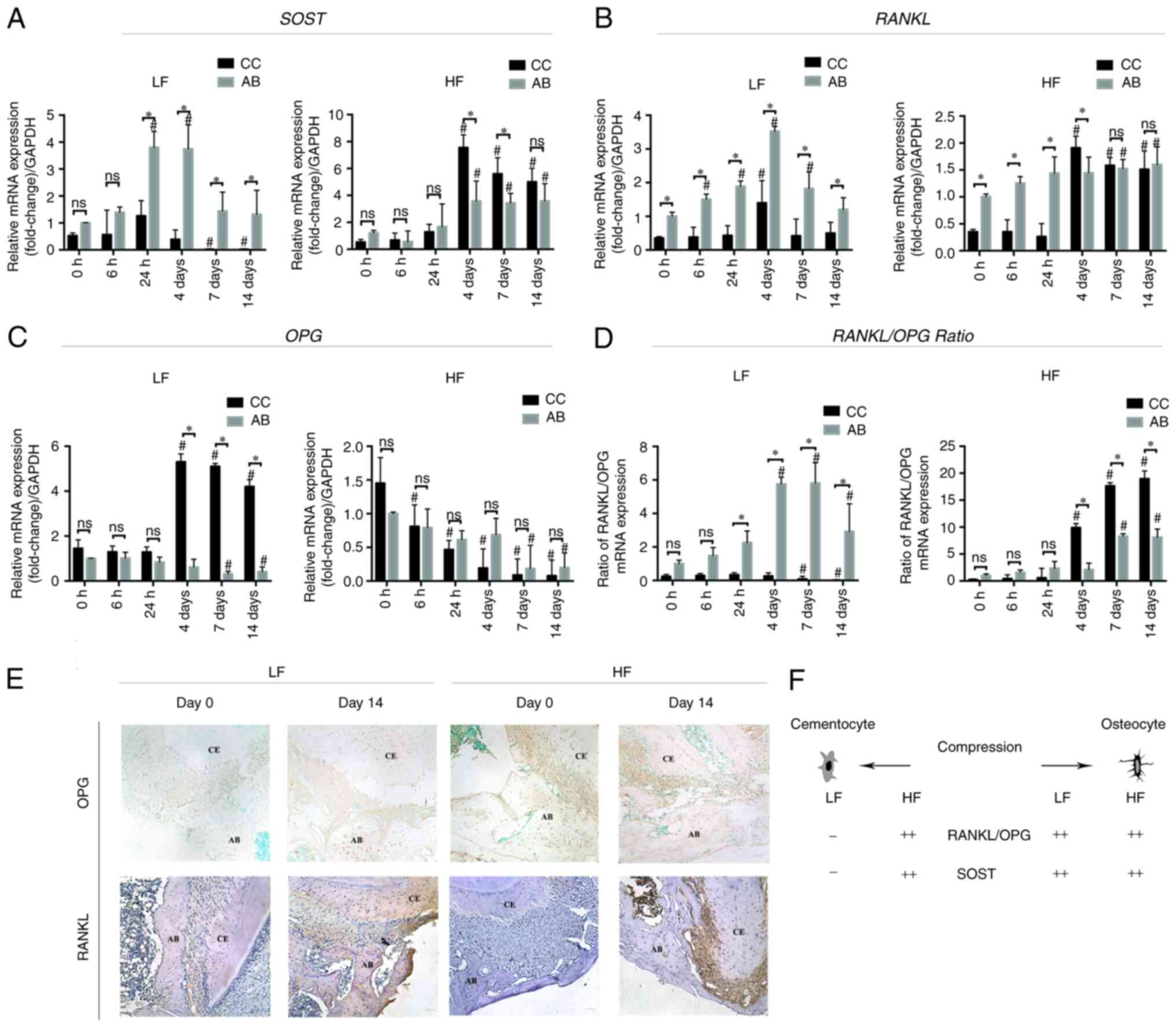 | Figure 4.Reverse transcription-quantitative
PCR analysis. (A) Relative SOST mRNA expression to
GAPDH in the CC low and high force groups. AB was used for
comparison. (B) Relative RANKL mRNA expression. (C) Relative
OPG mRNA expression. (D) Relative ratio of RANKL and
OPG mRNA expression. *P<0.05 compared between CC and AB
at the same time-point; #P<0.05 compared with 0 h.
(E) Immunohistochemical staining (magnification, ×200) of OPG and
RANKL. Scale bar, 50 µm. (F) Illustration of the changes of the
expression profile of cementocytes and osteocytes under orthodontic
compression force. SOST, sclerostin; CC, cellular cementum;
AB, alveolar bone; RANKL, receptor activator of nuclear
factor-κB ligand; OPG, osteoprotegerin; LF, low force; HF,
high force; ns, not significant. |
The expression levels of OPG were
significantly increased in the cellular cementum on days 4
(5.31±0.35), 7 (5.10±0.13), and 14 (4.20±0.31) in the low force
group, while in the high force group, the expression levels were
reduced over time. In addition, the expression levels in the
alveolar bone were significantly decreased from days 7 to 14
compared with those of day 0 in both low and high force groups.
Significant differences between the cellular cementum and the
alveolar bone were only noted in the low force group on days 4, 7
and 14 (P<0.01). The cellular cementum expressed approximately
8.67-(day 4), 16.29-(day 7), and 10.22-(day 14) fold higher levels
of OPG than those noted in the alveolar bone.
Immunohistochemistry identified intense OPG immunolocalization in
the cellular cementum and cementocyte-like cells on day 14 in the
low force group, while the alveolar bone OPG labeling was similar.
In the high force group, OPG labeling in both cellular cementum and
alveolar bone was not found.
RANKL expression was elevated in the alveolar
bone in the low force group from 6 h to 4 days and decreased
thereafter. Upregulation of RANKL expression was noted in
the cellular cementum on day 4 (1.40±0.66). Its expression levels
were significantly lower than those of the alveolar bone
(3.53±0.14) at the same time-point (P<0.05). In the low force
group, the expression levels of the alveolar bone were
significantly higher than those of the cellular cementum at every
time-point observed. In the high force group, RANKL
expression in both cementum and alveolar side was not significantly
elevated with time from 0 to 24 h. Furthermore, the expression in
the alveolar bone side was significantly higher than in the
cementum side at these time-points (0 to 24 h). However, on day 4,
RANKL expression in the cellular cementum surpassed that of
the alveolar bone (1.91±0.21 compared with 1.45±0.29; P<0.05).
Intense RANKL immunolocalization was found in both cementum and
alveolar bone after 14 days of compression, either in the low force
group and high force group. In the high force group, the
periodontal ligament RANKL labeling was also detected.
In the low force group, the RANKL/OPG ratio
in the cementum decreased over time and it was significantly lower
than that of the bone from the time period between 24 h and 14
days. Moreover, the ratio of the alveolar bone was increased over
time and peaked on day 7. In contrast to these findings, in the
high force group, both the cellular cementum and the alveolar bone
demonstrated increased ratios over time. The increase in the
RANKL/OPG ratio was higher in the cellular cementum
than that of the alveolar bone from day 4.
Immunofluorescence staining of the
cementocytes and osteocytes under compression conditions
Immunostaining of SOST protein was performed to
assess its dynamic expression in the cellular cementum and the
alveolar bone. SOST expression (labeled red) increased in
osteocytes incubated under both low and high force conditions at
days 4, 7 and 14 (Fig. 5A and D).
In contrast to these findings, the cementocyte-like cells of the
low force group exhibited a slightly increased SOST protein
expression at day 4, and significant lower expression at days 7 and
14 compared to day 0, while those of the high force group exhibited
an increased trend in SOST protein expression (Fig. 5B and C). The alveolar bone also
exhibited an up-and down expression pattern, but the expression
levels on days 7 and 14 were significantly higher than that on day
0. In the high force group, differences in sclerostin labelling
between cementum and alveolar bone were not found to be associated
with the force magnitude at any time-point. But in the low force
group, higher SOST expression was detected on the compressive bone
side compared to the tooth side at days 4, 7 and 14.
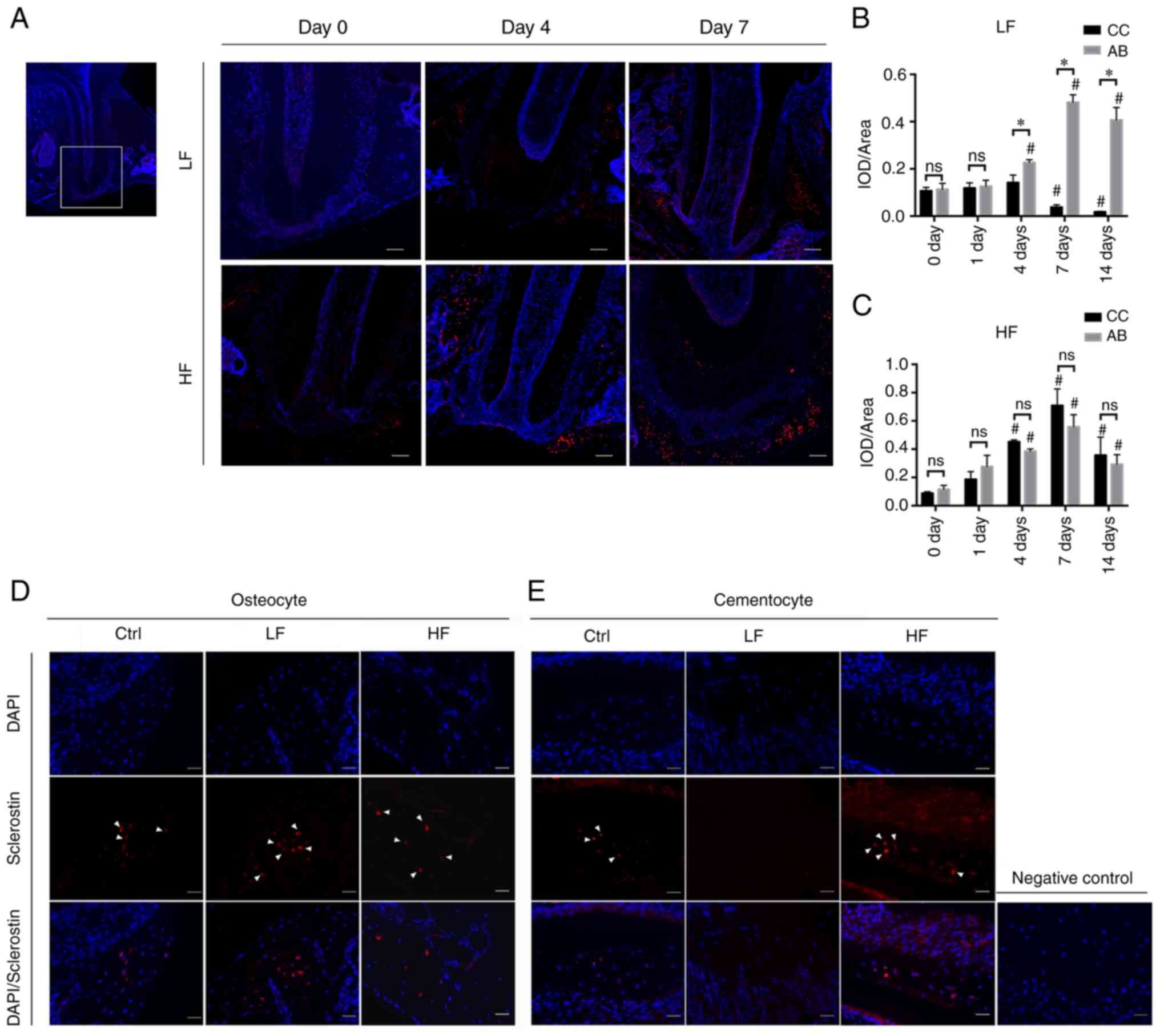 | Figure 5.Immunostaining images of sclerostin.
(A) representative images (magnification, ×100) of the ROI, the
mesial root of the left first molar on days 0, 4 and 7. Scale bar,
50 µm. (B and C) IOD value per area in the ROI region. The mean
density of sclerostin labelling in the (B) light force group and
(C) high force group were presented at each time-point. *P<0.05
compared between CC and AB at the same time-point;
#P<0.05 compared with 0 days. (D) Immunostaining
images (magnification, ×400) of sclerostin on day 14. Sclerostin
expression in the osteocytes in each group. The nuclei of
osteocytes were labeled by DAPI (blue). Sclerostin was labeled red
and indicated by white arrows. (E) Sclerostin (red) and DAPI (blue)
immunostaining of the cementocytes (scale bar, 20 µm). The image on
the right is the negative control. ROI, region of interest; IOD,
integrated optical density; CC, cellular cementum; AB, alveolar
bone; ns, not significant. |
Discussion
The present study established a molar intrusion rat
model and demonstrated that the cementocytes were sensitive to
in vivo orthodontic force by inducing SOST, OPG, and
RANKL expression. The results indicated that cementocytes
contributed to the root protection during tooth intrusion under
light force conditions, while under heavy orthodontic force
conditions, the expression profiles of these markers in the
cementocytes were altered.
A limited number of studies have been performed to
assess the biological behavior of the cementocytes following
mechanical loading. Rodents are considered optimal experimental
models since they possess similar anatomical features to that of
humans (19). The direction of
the orthodontic forces leads to alternative results. Odagaki et
al (20) demonstrated a
different pattern of SOST modulation on the tension and compression
sides of the tooth movement. The mesial traction model of the rat
molar is widely used. This model allows easy access to the
simultaneous tension and compression force. However, since the
molar is a multi-root irregular structure, the traction often leads
to teeth tilting and uneven distribution of stress, notably on the
root tip covered by the cellular cementum. Since the compression
area is often associated with bone remodeling and root resorption,
the current model was established in an attempt to apply
compression forces with single direction and investigate the
intrusion-induced tooth movement and the associated root
resorption. The force direction was designed as parallel to the
long axis of the tooth by the wire shape and position adjustment.
µ-CT indicated tooth intrusion along the long axis of the
tooth.
Force-induced tooth movement is initiated by
instantaneous tooth movement within the socket (21), which is followed by strain
induction in the matrix of the periodontal ligament (22) and a fluid flow shear stress in the
cementum and bone tissue (23).
The tissue strains and fluid flow stress are directly and
indirectly transferred to the cells, activating a variety of
signaling pathways, such as the integrin and the Wnt/b-catenin
pathways, leading to mediator release and the activation of
specific cells (24,25). Several markers which cementocytes
express may play an important role in the process, including E11,
which functions in dendrite development, DMP1, which is a secreted
ECM phosphoprotein that may regulate mineralization, and
sclerostin, which is a negative Wnt signaling regulator (26). A recent study has also
demonstrated the roles and mechanisms of the YAP/TAZ pathway in the
orthodontic force transduction. YAP and TAZ are capable of reading
mechanical cues, such as shear stress, cell shape, and
extracellular matrix rigidity, and stimulate downstream cellular
activity (27).
The macrophage colony-stimulating factor family
stimulates the osteoclast differentiation and RANKL binds to RANK
on the osteoclast precursors initiating differentiation (28). OPG is the competitive antagonist
of RANKL. The binding of OPG to RANK inhibits the terminal stage of
osteoclast differentiation (29).
SOST is a critical regulator of bone remodeling and metabolism and
is a selective marker of mature osteocytes (30). SOST acts as an antagonist of
lipoprotein receptor 5. Downregulation of the expression levels of
SOST and Dickkopf WNT signaling pathway inhibitor 1
(DKK1) is essential for the release of the Wnt protein,
which can activate the Wnt pathway (31). The expression profiles of these
markers regulate the balance of osteoclast and osteoblast
activation and differentiation. Osteocytes have been shown to
express these cytokines and are considered key regulators of bone
remodeling (32).
However, studies that have utilized cementocytes are
limited. The results of the present study indicated that under
light orthodontic forces, the cementocytes exhibited inhibitory
effects on osteoclast differentiation (reduced recruitment of
osteoclasts, downregulation of the expression levels of SOST,
RANKL, and decreased RANKL/OPG ratio, upregulation of
OPG mRNA levels and decrease in SOST protein expression).
Reduced osteoclast activity on the surface of the cementum
contributes to the homeostasis of the tooth root in the tooth
movement. Moreover, the alveolar bone exhibited an increase in
osteoclastogenesis (higher level of osteoclast recruitment,
increased SOST and RANKL expression levels, increased
RANKL/OPG ratio and upregulation of OPG mRNA levels,
all of which accelerated bone remodeling as demonstrated by µ-CT
analysis). These findings may explain the continuous tooth movement
by a relatively steady rate from day 0 to day 7.
Under light force conditions, osteoclast
infiltration was detected at 6 h on the bone side and was
significantly increased over time. Upregulation of RANKL
expression in the alveolar bone was also detected after 6 h. It has
been reported that in the early stage of osteoclast activation,
recruitment of adaptor molecules, such as tumor necrosis factor
receptor-associated factor 6, can mediate RANKL signaling and
induce activation of mitogen-activated protein kinases, nuclear
factor-κB and activator protein-1 (33). Induction of RANKL
expression in the cementocytes was noted by light forces only on
day 4. The temporal increase of the expression of this marker may
be due to the stress change following tooth movement, the occlusal
force interference, and a possible periodontal contamination. The
relative ratio of RANKL/OPG is essential to maintain
osteoclast differentiation, thereby playing a vital role in the
regulation of bone remodeling (34). The results of the present study
indicated that under light force conditions, the cellular cementum
maintained steady levels of the RANKL/OPG ratio in the early
stages, whereas a decrease was noted from days 7 to 14, which
paralleled SOST expression, indicating an osteoclast
inhibitory effect and a potential osteogenesis role in the early
and late stages of mechanical loading, respectively.
Other studies have also reported the expression of
these genes in the cementocytes. Jäger et al (35) demonstrated localized SOST
expression in the cementocytes of mouse and human tissues. In
addition, SOST-deficient mice indicated deposition of a
thicker layer of the cellular cementum together with thicker
alveolar bone (36), and
increased mineral density (37).
Cementocytes have also been reported to express RANKL in response
to endodontic infection in mice (38).
Following the application of heavy forces on teeth,
the expression profiles of both cementocytes and osteocytes were
altered. Heavy forces induced higher SOST and RANKL
expression levels, reduced OPG expression levels and higher
RANKL/OPG ratio compared with the corresponding levels of
these markers noted in osteocytes. The robust effect was initially
detected on day 4 (SOST and RANKL). The expression
levels noted in cementocytes were higher than those noted in the
osteocytes, leading to a higher capacity of osteoclast induction by
the cementocytes. This is a possible reason of root resorption.
Furthermore, µ-CT demonstrated a sudden increase in tooth movement,
indicating that the latter was a possible result of root resorption
rather than bone remodeling. The extent of osteoclast
quantification further indicated that the force magnitude did not
increase the number of osteoclasts in the alveolar bone, while the
increase of the force magnitude caused a significant increase in
the osteoclast number on the cementum surfaces.
The magnitude of the applied force is usually
considered directly proportional to osteoclastogenesis. Overloading
leads to severe bone resorption, the development of pathological
lesions in periodontal tissues, and external root resorption.
Previous studies have reported that significant root resorption
occurs with a loading ≥50 g compared with unloading or loading with
10 g (3). In addition, a 40-g
force induces a higher level of cementocyte death (39). The results of the present study
demonstrated that high force induced more bone remodeling, periodic
tooth movement and more osteoclast distribution on the cementum
side compared with the low force. Additional root resorption under
high force due to the cementocyte behavior may be related to the
small amount of cementocytes, the presence of micro-cracks in the
matrix, the hypoxic environment, as well as the inflammatory
conditions noted in the periodontal cells (40). Further investigations are required
to verify these findings.
Periodontal cells have been demonstrated to play key
roles in stimulating mechanotransduction (41,42). The contamination of periodontal
cells may impact this process. In the present study, various
efforts were made to avoid periodontal contamination. The tissues
were dissected thoroughly in three cycles of serial digestion. The
presented results paralleled with the in vitro study on
cementocytes indicating that the mechanical loading altered the
expression pattern of the cementocytes (6). However, the ability of the loading
to affect the cells in a periodontal-dependent way requires further
assessment. The crosstalk of periodontal cells and cementocytes
should be investigated further.
Other limitations of the present study include lack
of discussion regarding tension. As aforementioned, the cells may
act differently following different force direction. Further
investigations of the cellular response to different force types
need to be performed.
In conclusion, the present study established an
experimental tooth movement model by compressive loading and
presented evidence that the cementocytes participate in the
mechanotransduction pathway leading to tooth movement via
modulation of the expression levels of SOST, RANKL, and
OPG. High forces are not essential to accelerate tooth
movement and induce more osteoclast differentiation on the surfaces
of the cementum. The cementocytes act differently from the
osteocytes by alternative expression patterns of cytokines, leading
to different distribution of the activated osteoclasts when
subjected to low force; therefore they play a protective role in
tooth root homeostasis. Excessive forces may alter this role and
induce higher levels of osteoclast differentiation. Further
investigations, such as the use of gene profiling can potentially
provide relevant targets focusing on the biochemical and
biomechanical roles of the cementocytes. These targets can be
exploited therapeutically for tooth root protection and
regeneration therapy. These findings add to our understanding of
the biological mechanisms of the force-induced tooth movement.
Acknowledgements
The authors would like to thank Dr Quan Yu (Shanghai
Ninth People's Hospital, Shanghai Jiao Tong University School of
Medicine, Shanghai, China) for advice on methodology and
visualization. The authors would also like to thank Dr Zhifeng Yu
(Shanghai Ninth People's Hospital, Shanghai Jiao Tong University
School of Medicine, Shanghai, China) for guidance on micro-computed
tomography scanning.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant nos. 81771104 and 82171011).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW performed the experiments and drafted the
manuscript. ZS contributed to data analysis and interpretation, and
critically revised the manuscript. XW contributed to the
statistical analysis and drafted the manuscript. NZ contributed to
the conception of the study and interpretation of the data, and
critically revised the manuscript. GS contributed to the conception
and design of the study, and critically revised the manuscript. NZ
and GS confirm the authenticity of the raw data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were approved (approval no.
SH9H-2020-A298-1) by the Animal Ethics Committee of Shanghai Ninth
People's Hospital (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EARR
|
external apical root resorption
|
|
RANKL
|
receptor of receptor activator of
nuclear factor-κB ligand
|
|
µ-CT
|
micro-computed tomography
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
BV/TV
|
bone volume to tissue volume
|
References
|
1
|
Feller L, Khammissa RA, Thomadakis G,
Fourie J and Lemmer J: Apical external root resorption and repair
in orthodontic tooth movement: Biological events. Biomed Res Int.
2016:48641952016. View Article : Google Scholar
|
|
2
|
Ozkalayci N, Karadeniz EI, Elekdag-Turk S,
Turk T, Cheng LL and Darendeliler MA: Effect of continuous versus
intermittent orthodontic forces on root resorption: A microcomputed
tomography study. Angle Orthod. 88:733–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gonzales C, Hotokezaka H, Yoshimatsu M,
Yozgatian JH, Darendeliler MA and Yoshida N: Force magnitude and
duration effects on amount of tooth movement and root resorption in
the rat molar. Angle Orthod. 78:502–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueda M, Kuroishi KN, Gunjigake KK, Ikeda E
and Kawamoto T: Expression of SOST/sclerostin in compressed
periodontal ligament cells. J Dent Sci. 11:272–278. 2016.
View Article : Google Scholar
|
|
5
|
Li Y, Zhan Q, Bao M, Yi J and Li Y:
Biomechanical and biological responses of periodontium in
orthodontic tooth movement: Up-date in a new decade. Int J Oral
Sci. 13:202021. View Article : Google Scholar
|
|
6
|
Wei T, Xie Y, Wen X, Zhao N and Shen G:
Establishment of in vitro three-dimensional cementocyte
differentiation scaffolds to study orthodontic root resorption. Exp
Ther Med. 20:3174–3184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lira Dos Santos EJ, de Almeida AB, Chavez
MB, Salmon CR, Mofatto LS, Camara-Souza MB, Tan MH, Kolli TN,
Mohamed FF, Chu EY, et al: Orthodontic tooth movement alters
cementocyte ultrastructure and cellular cementum proteome
signature. Bone. 153:1161392021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lira Dos Santos EJ, Salmon CR, Chavez MB,
de Almeida AB, Tan MH, Chu EY, Sallum EA, Casati MZ, Ruiz KGS,
Kantovitz KR, et al: Cementocyte alterations associated with
experimentally induced cellular cementum apposition in Hyp mice. J
Periodontol. 92:116–127. 2021. View Article : Google Scholar
|
|
9
|
Krishnan V and Davidovitch Z: On a path to
unfolding the biological mechanisms of orthodontic tooth movement.
J Dent Res. 88:597–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borges de Castilhos B, Machado de Souza C,
Simas Netta Fontana ML, Pereira FA, Tanaka OM and Trevilatto PC:
Association of clinical variables and polymorphisms in RANKL, RANK,
and OPG genes with external apical root resorption. Am J Orthod
Dentofacial Orthop. 155:529–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weivoda MM, Youssef SJ and Oursler MJ:
Sclerostin expression and functions beyond the osteocyte. Bone.
96:45–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim WH, Liu B, Hunter DJ, Cheng D, Mah SJ
and Helms JA: Downregulation of Wnt causes root resorption. Am J
Orthod Dentofacial Orthop. 146:337–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Underwood W and Anthony R: AVMA Guidelines
for the Euthanasia of Animals. American Veterinary Medical
Association; Schaumburg: 2020
|
|
14
|
Gonzales C, Hotokezaka H, Arai Y, Ninomiya
T, Tominaga J, Jang I, Hotokezaka Y, Tanaka M and Yoshida N: An in
vivo 3D micro-CT evaluation of tooth movement after the application
of different force magnitudes in rat molar. Angle Orthod.
79:703–714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolf M, Ao M, Chavez MB, Kolli TN,
Thumbigere-Math V, Becker K, Chu EY, Jäger A, Somerman MJ and
Foster BL: Reduced orthodontic tooth movement in Enpp1 mutant mice
with hypercementosis. J Dent Res. 97:937–945. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu LH, Lee K, Imoto S, Kyomen S and Tanne
K: Histological and histochemical quantification of root resorption
incident to the application of intrusive force to rat molars. Eur J
Orthod. 21:57–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao N, Nociti FH Jr, Duan P, Prideaux M,
Zhao H, Foster BL, Somerman MJ and Bonewald LF: Isolation and
functional analysis of an immortalized murine cementocyte cell
line, IDG-CM6. J Bone Miner Res. 31:430–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto T, Domon T, Takahashi S, Islam MN
and Suzuki R: The fibrillar structure of cementum and dentin at the
cemento-dentinal junction in rat molars. Ann Anat. 182:499–503.
2000. View Article : Google Scholar
|
|
20
|
Odagaki N, Ishihara Y, Wang Z, Ei Hsu
Hlaing E, Nakamura M, Hoshijima M, Hayano S, Kawanabe N and Kamioka
H: Role of osteocyte-PDL crosstalk in tooth movement via
SOST/Sclerostin. J Dent Res. 97:1374–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rygh P: Ultrastructural changes in
pressure zones of human periodontium incident to orthodontic tooth
movement. Acta Odontol Scand. 31:109–122. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Binderman I, Bahar H and Yaffe A: Strain
relaxation of fibroblasts in the marginal periodontium is the
common trigger for alveolar bone resorption: A novel hypothesis. J
Periodontol. 73:1210–1215. 2002. View Article : Google Scholar
|
|
23
|
Harter LV, Hruska KA and Duncan RL: Human
osteoblast-like cells respond to mechanical strain with increased
bone matrix protein production independent of hormonal regulation.
Endocrinology. 136:528–535. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du Y, Ling J, Wei X, Ning Y, Xie N, Gu H
and Yang F: Wnt/β-catenin signaling participates in
cementoblast/osteoblast differentiation of dental follicle cells.
Connect Tissue Res. 53:390–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldmann WH: Mechanical aspects of cell
shape regulation and signaling. Cell Biol Int. 26:313–317. 2002.
View Article : Google Scholar
|
|
26
|
Zhao N, Foster BL and Bonewald LF: The
cementocyte-an osteocyte relative? J Dent Res. 95:734–741. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng L, Chen Y, Guo J, Han X and Guo Y:
Roles and mechanisms of YAP/TAZ in orthodontic tooth movement. J
Cell Physiol. 236:7792–7800. 2021. View Article : Google Scholar
|
|
28
|
Nakano Y, Yamaguchi M, Fujita S, Asano M,
Saito K and Kasai K: Expressions of RANKL/RANK and M-CSF/c-fms in
root resorption lacunae in rat molar by heavy orthodontic force.
Eur J Orthod. 33:335–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silva I and Branco JC: Rank/Rankl/opg:
Literature review. Acta Reumatol Port. 36:209–218. 2011.
|
|
30
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu M, Kurimoto P, Zhang J, Niu QT,
Stolina M, Dechow PC, Feng JQ, Hesterman J, Silva MD, Ominsky MS,
et al: Sclerostin and DKK1 inhibition preserves and augments
alveolar bone volume and architecture in rats with alveolar bone
loss. J Dent Res. 97:1031–1038. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JH, Lee NK and Lee SY: Current
understanding of RANK signaling in osteoclast differentiation and
maturation. Mol Cells. 40:706–713. 2017.PubMed/NCBI
|
|
34
|
Yamaguchi M: RANK/RANKL/OPG during
orthodontic tooth movement. Orthod Craniofac Res. 12:113–119. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jäger A, Götz W, Lossdörfer S and
Rath-Deschner B: Localization of SOST/sclerostin in cementocytes in
vivo and in mineralizing periodontal ligament cells in vitro. J
Periodontal Res. 45:246–254. 2010. View Article : Google Scholar
|
|
36
|
Kuchler U, Schwarze UY, Dobsak T, Heimel
P, Bosshardt DD, Kneissel M and Gruber R: Dental and periodontal
phenotype in sclerostin knockout mice. Int J Oral Sci. 6:70–76.
2014. View Article : Google Scholar
|
|
37
|
Li X, Ominsky MS, Niu QT, Sun N, Daugherty
B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, et al: Targeted
deletion of the sclerostin gene in mice results in increased bone
formation and bone strength. J Bone Miner Res. 23:860–869. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Rossi A, Fukada SY, De Rossi M, da
Silva RA, Queiroz AM, Nelson-Filho P and da Silva LA: Cementocytes
express receptor activator of the nuclear factor Kappa-B ligand in
response to endodontic infection in mice. J Endod. 42:1251–1257.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuzawa H, Toriya N, Nakao Y,
Konno-Nagasaka M, Arakawa T, Okayama M and Mizoguchi I: Cementocyte
cell death occurs in rat cellular cementum during orthodontic tooth
movement. Angle Orthod. 87:416–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Diercke K, Kohl A, Lux CJ and Erber R:
Compression of human primary cementoblasts leads to apoptosis: A
possible cause of dental root resorption? J OrofacOrthop.
75:430–445. 2014.
|
|
41
|
Lee SY, Moon JS, Yang DW, Yoo HI, Jung JY,
Kim OS, Kim MS, Koh JT, Chung HJ and Kim SH: SLPI in periodontal
Ligament is not sleepy during biophysical force-induced tooth
movement. J Clin Periodontol. 48:528–540. 2021. View Article : Google Scholar
|
|
42
|
Moon JS, Lee SY, Kim JH, Choi YH, Yang DW,
Kang JH, Ko HM, Cho JH, Koh JT, Kim WJ, et al: Synergistic alveolar
bone resorption by diabetic advanced glycation end products and
mechanical forces. J Periodontol. 90:1457–1469. 2019. View Article : Google Scholar
|