Introduction
Ovarian cancer (OC) is a common malignant tumor of
the female reproductive system. The pathological classification of
OC can be divided into four types: Epithelial tumor, germ cell
tumor, sex cord stromal tumor and other types (1). Among them, epithelial OC accounts
for 90% of OC, and serous OC is the most common subtype with the
highest malignant degree among epithelial OC subtypes (2). Serous OC accounts for 70–80% of OC
deaths, and overall survival (OS) rates have not changed
significantly over the past few decades, remaining at ~30%
(3). In the present study, OC
refers to serous OC. OC is insidious, and often displays no obvious
symptoms or signs before metastasis (4). Furthermore, ~70% of patients with OC
are already at an advanced stage when diagnosed with OC, and
consequently the mortality rate of OC is the highest among the
various types of cancer of the female reproductive system (5). The recurrence and metastasis of OC
present great obstacles to its effective treatment, and greatly
contribute towards the high mortality rate of OC (6). Therefore, it is imperative to fully
elucidate the mechanisms underlying the invasion and metastasis in
OC, and to develop specific targeted drugs for the treatment of
OC.
SIX homeobox 4 (SIX4), a member of the homeobox
family, subfamily SIX, was first found to regulate the expression
of Na+/K+-ATPase subunit (7). Previous studies have demonstrated
that the gene expression levels of SIX4 are closely associated with
the occurrence, development, invasion, metastasis and prognosis of
esophageal squamous cell carcinoma (8), breast cancer (9), bladder cancer (10), non-small cell lung cancer
(11) and other tumor types,
suggesting that the SIX4 gene may function as an oncogene in the
development of a wide variety of tumors. In addition, a previous
study has revealed that SIX4 is involved in the differentiation of
epithelial follicular stem cells in the ovaries of
Drosophila (12).
Therefore, it was possible to hypothesize that the role of SIX4 in
OC may also be that of an oncogene.
Insulin-like growth factor 2 mRNA binding protein 3
(IGF2BP3) has been reported to promote the invasion and metastasis
of tumor cells through the local translation of IGF2BP3-bound
transcripts (13). In addition, a
previous study revealed that high expression levels of IGF2BP3 may
serve as an oncogenic marker in clear cell carcinoma of the ovary
(14). Upregulation of the
RNA-binding proteins lin-28 homolog B and IGF2BP3 is associated
with chemoresistance and adverse disease outcomes in OC (15). Therefore, it may be hypothesized
that IGF2BP3 exerts a role in SKOV3 cells by binding to the protein
SIX4.
The aim of the present study was to investigate the
role of SIX4 in OC, and the mechanism through which it is involved
in the regulation of OC cell proliferation, metastasis and
angiogenesis, in order to provide a theoretical basis for targeted
treatment of OC.
Materials and methods
Databases
The Gene Expression Profiling Interactive Analysis
(GEPIA) database (gepia.cancer-pku.cn) was used to detect the
expression levels of SIX4 in OC tissues (16). In addition, the ENCORI
(starbase.sysu.edu.cn) database was used to detect the association
between the expression levels of SIX4 and the OS rate in patients
with OC (17).
Cell culture
IOSE-80, A2780, OVCAR3 and SKOV3 cells were obtained
from BeNa Culture Collection; Beijing Beina Chunglian Institute of
Biotechnology while human umbilical vein endothelial cells (HUVECs)
were acquired from Procell Life Science & Technology Co., Ltd.
All cells were cultured in HyClone® DMEM (Thermo Fisher
Scientific, Inc.) supplemented with 10% HyClone® FBS and
1% penicillin and streptomycin (Thermo Fisher Scientific, Inc.) at
37°C in an atmosphere of 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Then, reverse
transcription was conducted according to the manufacturer's
instructions of the HIFiscript cDNA Synthesis kit (CoWin
Biosciences) and the system for RT-qPCR was established using SYBR
Green Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.).
GAPDH was used as an internal control. The thermocycling conditions
for RT-qPCR were as follows: 95°C in a 20-µl reaction volume for 10
min, followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and
72°C for 30 sec. The data were analyzed using the 2−ΔΔCq
method (18). The primer
sequences used were as follows: SIX4 forward,
5′-CGAGCTCTACAGCATCCTCG-3′ and reverse, 5′-CGGTACTTGTCTACGGCTCC-3′;
IGF2BP3 forward, 5′-CAAGCAGAAACCATGTGATTTG-3′ and reverse,
5′-AGAGGTGCCTTCAGGAGTAGAG-3′; and GAPDH forward,
5′-GAGCCCGCAGCCTCCCGCTT-3′ and reverse,
5′-CCCGCGGCCATCACGCCACAG-3′.
Western blot analysis
Total protein was extracted from SKOV3 cells in RIPA
lysis buffer (MilliporeSigma) and the protein concentration was
detected using a BCA protein assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. Equal
amounts of protein (30 µg per lane) were separated by 12% SDS-PAGE
and transferred onto PVDF membranes (MilliporeSigma). Subsequently,
the membranes were blocked with 5% skimmed milk powder for 1.5 h at
room temperature, and then incubated with primary antibodies,
including anti-SIX4 (1:500 dilution; cat. no. LS-C101744),
anti-IGF2BP3 (1:1,000 dilution; cat. no. 57145S), anti-E-cadherin
(1:1,000 dilution; cat. no. 14472S), anti-N-cadherin (1:1,000
dilution; cat. no. 13116S), anti-Snail (1:1,000 dilution; cat. no.
3879S), anti-VEGF (1:1,000 dilution; cat. no. 65373S) and
anti-GAPDH (1:1,000 dilution; cat.no. 5174S) at 4°C overnight
(anti-SIX4 antibody was purchased from LifeSpan BioSciences, Inc.;
all the other primary antibodies were purchased from Cell Signaling
Technology, Inc.). The next day, after washing with PBS with 0.1%
Tween-20, the membranes were incubated with a secondary antibody
conjugated to HRP (1:1,000 dilution; cat. no. BS13278; Bioworld
Technology, Inc.) for 1 h at room temperature. The expression
levels of the different proteins were detected using enhanced
chemiluminescence reagent (Bio-Rad Laboratories, Inc.). Proteins
bands were visualized using enhanced chemiluminescence (Thermo
Fisher Scientific, Inc.). The data were analyzed using ImageJ 1.52
k software (version 1.46; National Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
Cells were seeded into 96-well plates (density,
5×104 cells/ml) and a CCK-8 kit (cat. no. G021-1-1;
Beyotime Institute of Biotechnology) was used to detect the cell
viability. Following incubation, 10 µl CCK-8 solution was added to
the cells in each well at 37°C for 3 h. Cell viability was measured
at 450 nm absorbance (optical density) with a microplate reader
(Bio-Rad Laboratories, Inc.).
Cell transfection
Short hairpin RNAs (shRNAs/sh) were cloned into the
lentiviral vector pLKO.01 from Sangon Biotech Co., Ltd using the
restriction enzyme pair AgeI/EcoRI using the 3rd
generation system. The molar ratio used for the lentivirus,
packaging and envelope plasmids was 1:1:1. A total of 2 µg
pLKO-sh-SIX4 (#1 and 2) and pLKO-sh-IGF2BP3 (#1 and 2), or
pLKO-scramble (sh-NC), pCMV-DR8.9 and pCMV–VSVG plasmids, were
transfected into 293T cells (ATCC) to produce lentiviruses. Cell
culture media containing lentiviral particles were collected 48 h
after transfection and the lentiviruses were harvested and used to
infect SKOV3 cells at a multiplicity of infection of 40 with
Invitrogen® Lipofectamine™ 3000 reagent
(Thermo Fisher Scientific, Inc.) at 37°C for 48 h. At 48 h
post-infection, puromycin (2 µg/ml) was used to select stably
infected cells for 3–5 days. RT-qPCR and western blot analyses were
employed to determine the cell transfection efficiency. The
sequences of primers were as follows: sh-SIX4#1,
5′-CCTCCTCATTAGTTAATGTAT-3′; sh-SIX4#2,
5′-CCTCAGCCTTTCCAGTCATAT-3′; sh-IGF2BP3#1,
5′-GCCTCATTCTTATTTCAAGAT-3′; sh-IGF2BP3#2,
5′-CGGTGAATGAACTTCAGAATT-3′; and sh-NC,
5′-CAACAAGATGAAGAGCACCAA-3′.
For the transient IGF2BP3 overexpression
experiments, the cells were transfected either with 2 µg
pCMV-N-Flag-IGF2BP3 (Ov-IGF2BP3) or with pCMV-N-Flag control vector
(Ov-NC) from Beyotime Institute of Biotechnology using
Invitrogen® Lipofectamine™ 3000 reagent
(Thermo Fisher Scientific, Inc.) at 37°C for 48 h. RT-qPCR and
western blot analyses were subsequently used to assess the cell
transfection efficiency at 48 h post-infection.
Colony formation assay
Following cell transfection, SKOV3 cells were seeded
(1.5×103 cells/well) into a 6-well plate. After 14 days,
the medium was discarded, and the clones were fixed with methanol
for 15 min at room temperature. Subsequently, crystal violet was
used to stain the cells for 20 min at room temperature. The numbers
of clones with >10 cells were counted manually under a light
microscope. The number of clones: (cell number >10 cells/colony)
×100%.
Wound healing assay
SKOV3 cells were seeded onto 6-well plates (density,
1×106 cells/ml) and cultured to 80% confluence. After
cell transfection, a scratch-wound assay was performed using a
10-µl pipette tip. The medium was replaced by DMEM without serum,
and 1 µM 5-fluouracil (MilliporeSigma) was added to block cell
proliferation after wounding. The widths of the wounds were
measured after 0 and 24 h with a light microscope (magnification,
×200). The cell migration rate was calculated as follows: (Initial
width-final width)/Initial width.
Transwell assay
The upper compartment surface of the bottom membrane
of the Transwell chamber was coated with Matrigel™ (50 mg/l; 1:8
diluted solution; BD Biosciences) at room temperature for 24 h and
air-dried at 4°C. The culture in the culture plate was removed, and
serum-free DMEM containing 10 mg/ml BSA (Shanghai Aladdin
Biochemical Technology Co., Ltd.) was added to each well at 37°C
for 30 min. Subsequently, the treated cells suspended in 200 µl
serum-free DMEM were seeded onto the upper chamber membranes of
24-well 8-µm pore Transwell insert (Costar; Corning, Inc.) at a
density of 1×104 cells/ml. DMEM supplemented with 10%
FBS was added to the lower chamber. After incubation for 24 h at
37°C, the membrane was fixed with 4% paraformaldehyde for 15 min
and sequentially stained with 0.1% crystal violet solution for 30
min (all at room temperature). The inside of the membrane was
gently wiped with a cotton swab. Finally, the numbers of cells were
counted under a light microscope.
Tube formation assay
Matrigel™ (BD Biosciences) was used to detect the
tube formation of human umbilical vein endothelial cells (HUVECs).
A total of 5×104 HUVECs were plated and co-cultured with
the same amount of SKOV3 cells transfected with sh-SIX4#2 and
Ov-IGF2BP3 on a 96-well plate precoated with Matrigel™ (50 µl/well)
for 30 min at 37°C. After 12 h, the enclosed networks of tubes from
six random high-power microscope fields were examined under a light
microscope. The mean value of 10 cumulative total lengths per well
represented an experimental point. The number of tubes was assessed
using ImageJ 1.52 k software (version 1.46; National Institutes of
Health).
RNA immunoprecipitation (RIP)
assay
A RIP RNA-Binding Protein Immunoprecipitation Kit
(MilliporeSigma) was used to conduct RIP assays. Cells were lysed
in Invitrogen RIP buffer (Thermo Fisher Scientific, Inc.) after
being collected by centrifugation at 200 × g for 5 min at 4°C.
Then, 100 µl cell lysate was pre-cleared with 50 ul protein A/G
magnetic beads (MilliporeSigma) which were conjugated to 5 µg
anti-SIX4 antibody (1:500 dilution; cat. no LS-C101744; LifeSpan
BioSciences, Inc.) or 5 µg anti-IgG antibody (1:50 dilution; cat.
no ab172730; Abcam). A protein-RNA complex was captured and
digested with 0.5 mg/ml proteinase K containing 0.1% SDS to extract
RNA. The magnetic beads were repeatedly washed with RIP washing
buffer to remove non-specific adsorption. Finally, the expression
levels of SIX4 were determined using an RT-qPCR assay as described
above.
Actinomycin D treatment
Following transfection, the cells were treated with
2 mg/ml actinomycin D (MedChemExpress) for 0, 3, 6, 9 or 12 h at
37°C. The mRNA levels of SIX4 following actinomycin treatment were
subsequently measured using an RT-qPCR assay as described
above.
Statistical analysis
All experiments were repeated independently three
times. SPSS 21.0 (IBM Corp.) was employed for statistical analysis.
The data are presented as the mean ± SD. Comparisons between two
groups were performed using unpaired Student's t-test, whereas
comparisons among multiple groups were performed with one-way ANOVA
followed by a Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
SIX4 is highly expressed in OC
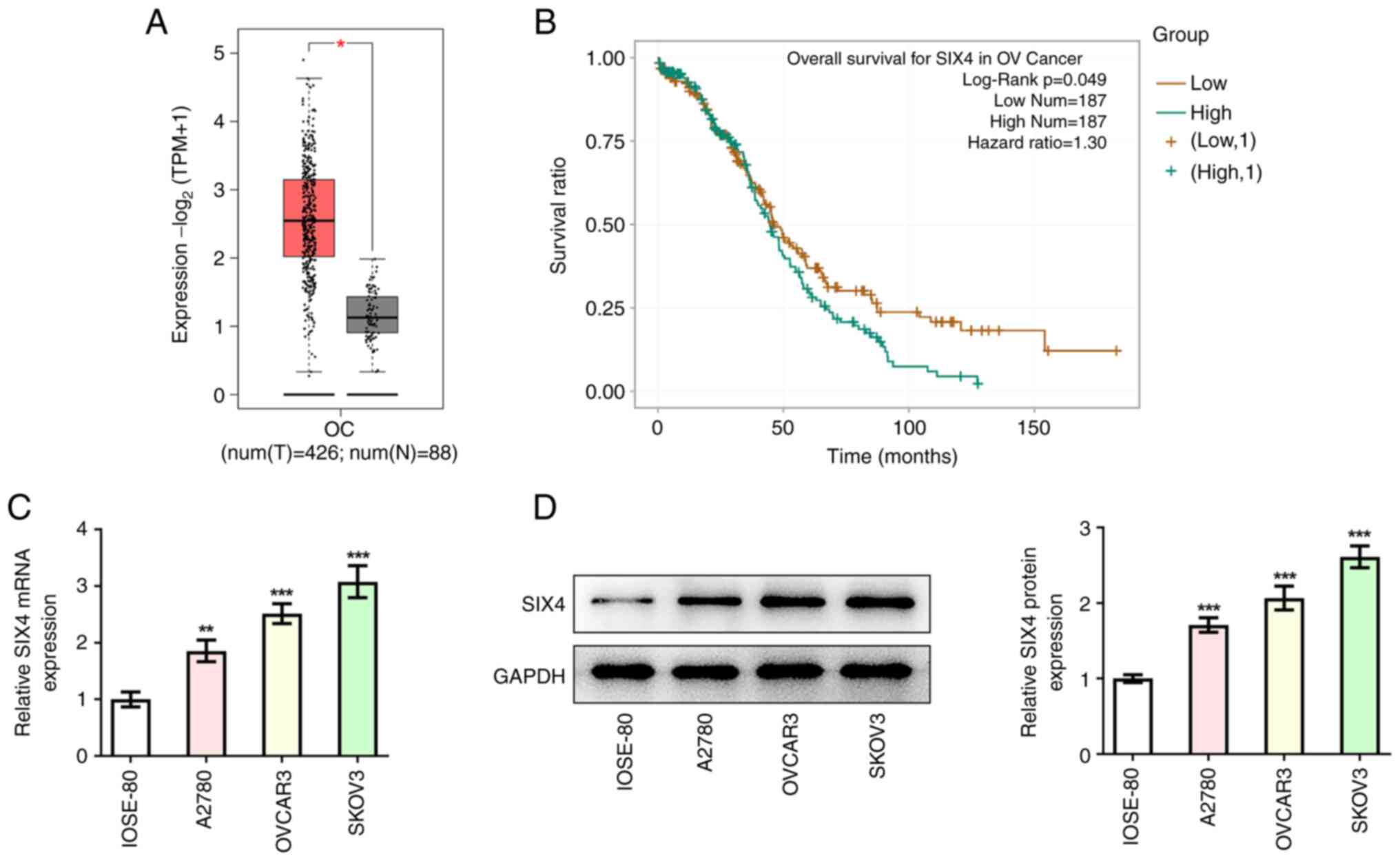
The GEPIA database analysis revealed that SIX4 was
highly expressed in OC tissues compared with tissues from healthy
subjects (Fig. 1A). Subsequently,
the ENCORI database was employed to reveal that high expression
levels of SIX4 were associated with low OS rates in patients with
OC (Fig. 1B). The human normal
ovarian epithelial cell line (IOSE-80) and the OC cell lines
(A2780, OVCAR3 and SKOV3) were selected for subsequent experiments
as previously described (19).
Western blot and RT-qPCR analyses were used to detect the
expression levels of SIX4 in cells. The results obtained revealed
that, compared with those in the IOSE-80 cells, the expression
levels of SIX4 were significantly increased in the A2780, OVCAR3
and SKOV3 cells (Fig. 1C and D).
Furthermore, SIX4 displayed the highest expression in SKOV3 cells
among all selected OC cells; therefore, SKOV3 cells were selected
for the follow-up experiments.
Interference with SIX4 inhibits the
proliferation of SKOV3 cells
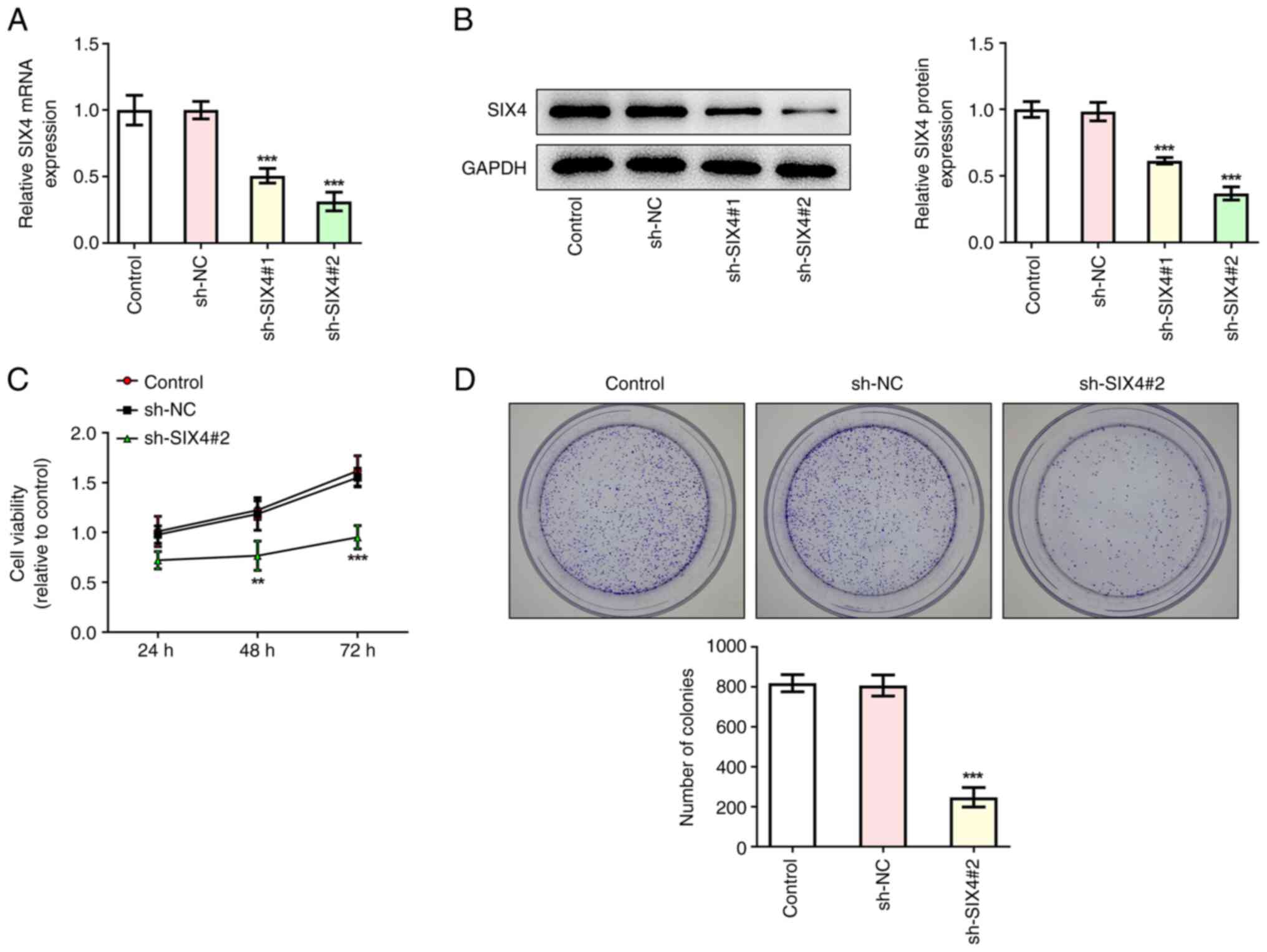
Transfection was used to interfere with SIX4
expression in SKOV3 cells, and the transfection efficiency was
assessed by RT-qPCR and western blot analyses. The results
demonstrated that, compared with those in the sh-NC control group,
the expression levels of SIX4 in the sh-SIX4#1 and sh-SIX4#2 groups
were significantly decreased, indicating successful transfection
(Fig. 2A and B). The sh-SIX4#2
group was demonstrated to exhibit a better interference efficiency.
Therefore, this group was selected for follow-up experiments.
A CCK-8 assay was then used to assess the viability
of the cells following interference with SIX4. The results revealed
that, compared with the sh-NC control group, the cell survival rate
of the sh-SIX4#2 group was significantly decreased at 48 and 72 h
(Fig. 2C). A colony formation
assay subsequently demonstrated that cell proliferation was
significantly decreased in the sh-SIX4#2 group compared with the
sh-NC group (Fig. 2D).
Interference with SIX4 expression
inhibits the migration, invasion and tube formation of SKOV3
cells
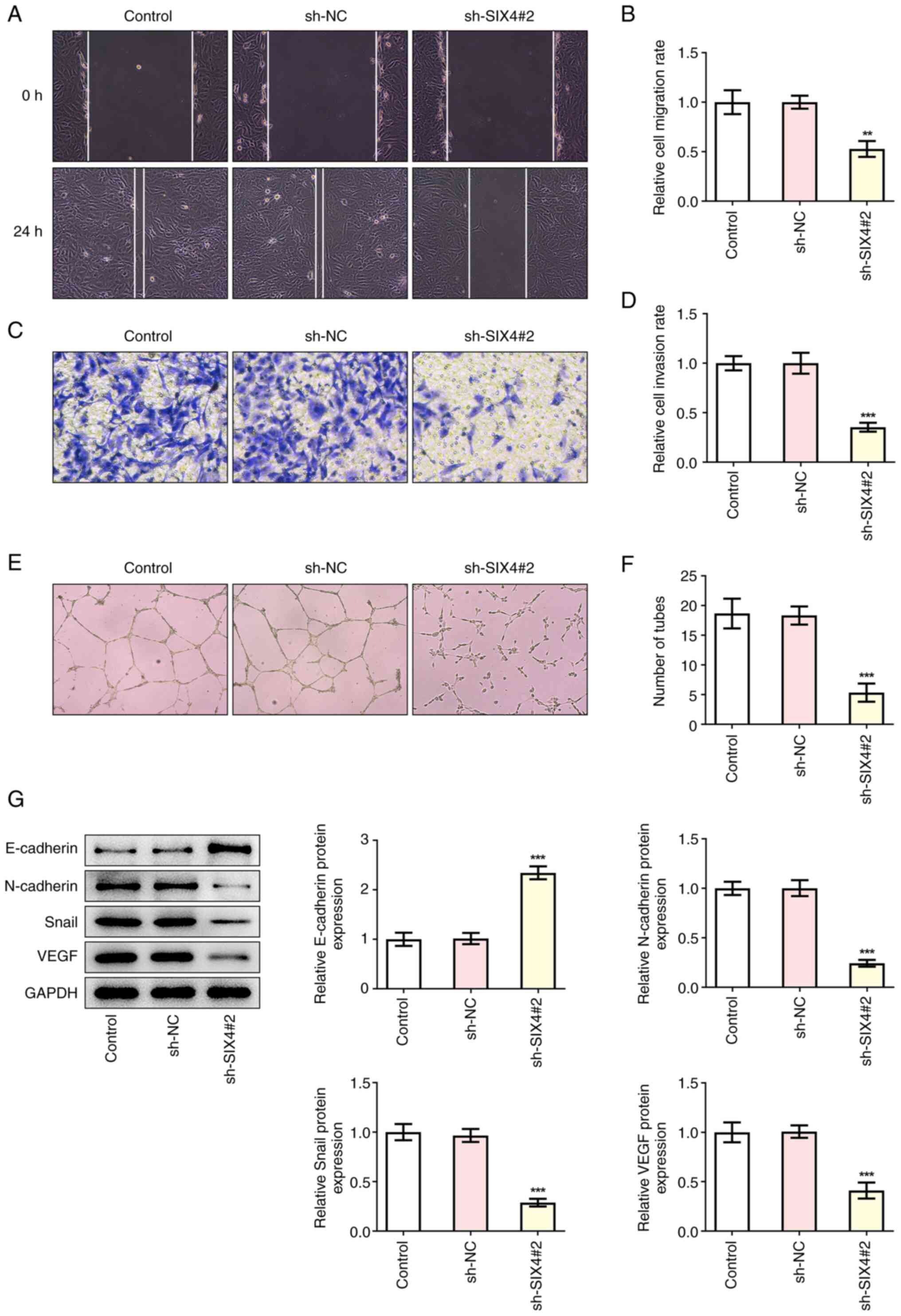
Subsequently, the effects of interference with SIX4
expression on the migration, invasion and tube formation of SKOV3
cells were examined, and it was found that the invasion and
migration of the sh-SIX4#2 group were significantly reduced
compared with those of the sh-NC control group (Fig. 3A-D). Subsequently tube formation
assay was performed to detect the tube formation of the HUVECs. The
results obtained revealed that, compared with the sh-NC group, the
tube formation of the sh-SIX4#2 group was significantly decreased
(Fig. 3E and F). Western blot
analysis was then used to investigate the expression levels of the
metastasis- and angiogenesis-associated proteins E-cadherin,
N-cadherin and Snail, as well as VEGF. The results demonstrated
that, the expression levels of E-cadherin in the sh-SIX4#2 group
were significantly increased, whereas the expression levels of
N-cadherin, Snail and VEGF were significantly decreased compared
with the sh-NC group (Fig. 3G),
suggesting that interference with SIX4 expression could lead to an
inhibition of the metastasis and angiogenesis of SKOV3 cells.
IGF2BP3 increases the stability of
SIX4 mRNA
The GEPIA database analysis revealed that IGF2BP3
was highly expressed in OC tissues (Fig. 4A). In addition, a statistically
significant positive correlation between the expression levels of
IGF2BP3 and SIX4 in OC tissues was also identified (Fig. 4B). Western blot and RT-qPCR
analyses revealed that IGF2BP3 expression in SKOV3 cells was
abnormally elevated compared with that in IOSE-80 cells (Fig. 4C and D). Subsequently, a RIP assay
was used to assess the binding ability of IGF2BP3 and SIX4 mRNA,
and the results revealed that, compared with the control group, the
expression levels of SIX4 in the IGF2BP3 group were significantly
increased, indicating the binding of IGF2BP3 and SIX4 (Fig. 4E). Subsequently, transfection was
used to interfere with IGF2BP3 expression in SKOV3 cells, and the
transfection efficiency was determined by western blot and RT-qPCR
analyses (Fig. 4F and G). Since
the shRNA sh-IGF2BP3#2 was demonstrated to have the best
interference efficiency, sh-IGF2BP3#2 was selected for subsequent
experiments. Following actinomycin D treatment, the stability of
SIX4 mRNA was detected by an RT-qPCR assay. The results
demonstrated that, compared with the sh-NC group, the stability of
SIX4 decreased sharply upon inhibiting IGF2BP3 expression (Fig. 4H), indicating that IGF2BP3 could
stabilize SIX4 expression. In addition, it was found that the
expression levels of SIX4 were significantly decreased following
the inhibition of IGF2BP3 expression in comparison with the sh-NC
group, with lowest SIX4 expression when treated with Actinomycin D
at 12 h (Fig. 4I and J). These
results suggested that IGF2BP3 could improve the stability of SIX4
mRNA in SKOV3 cells.
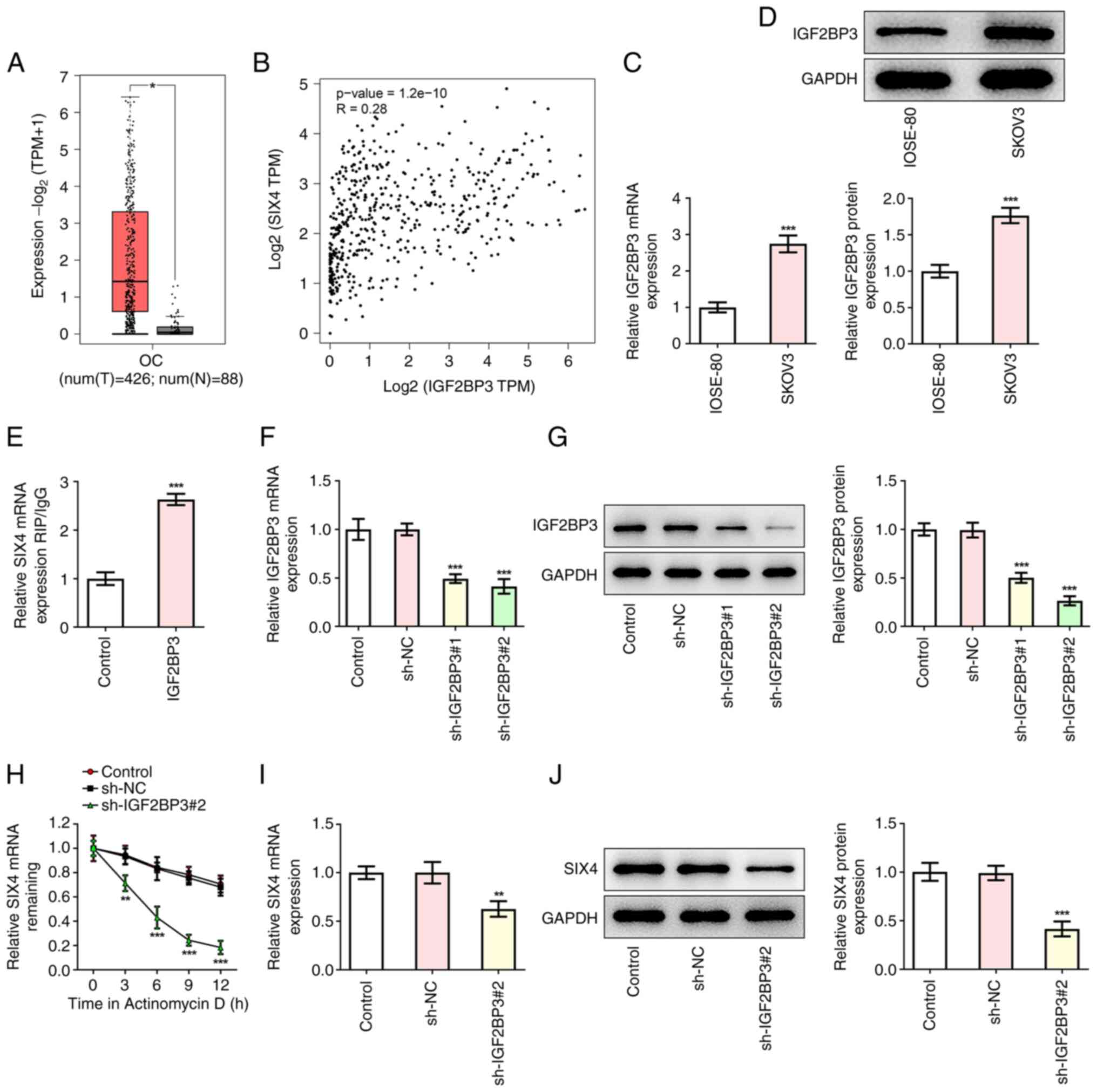 | Figure 4.IGF2BP3 increases the stability of
SIX4 mRNA. (A) GEPIA database analysis predicted the high
expression levels of IGF2BP3 in samples from patients with OC.
Orange box corresponds to OC. Grey box corresponds to healthy
samples. *P<0.05 vs. normal. (B) GEPIA database analysis
predicted that SIX4 expression was correlated with IGF2BP3
expression in patients with OC. (C) RT-qPCR was used to detect the
mRNA levels of IGF2BP3. ***P<0.001 vs. IOSE-80. (D) Western
blotting was used to detect the protein levels of IGF2BP3.
***P<0.001 vs. IOSE-80. (E) RIP assay detecting the binding
ability of IGF2BP3 to SIX4 mRNA. ***P<0.001 vs. Control. (F)
RT-qPCR was used to detect the mRNA levels of IGF2BP3 after
inhibition of IGF2BP3. (G) Western blotting was used to detect the
protein levels of IGF2BP3 after inhibition of IGF2BP3.
***P<0.001 vs. sh-NC. (H) After actinomycin D treatment, the
stability of SIX4 mRNA was detected by RT-qPCR. (I) RT-qPCR was
preformed to detect the mRNA levels of SIX4 after inhibition of
IGF2BP3. (J) Western blotting was performed to detect the protein
levels of SIX4 after inhibition of IGF2BP3. ***P<0.001 vs.
sh-NC. GEPIA, Gene Expression Profiling Interactive Analysis;
IGF2BP3, insulin-like growth factor 2 mRNA binding protein 3; NC,
negative control; OC, ovarian cancer; RIP, RNA immunoprecipitation;
RT-qPCR, reverse transcription-quantitative PCR; sh, short hairpin
RNA; SIX4, SIX homeobox 4; TPM, transcripts per million. |
IGF2BP3-stabilized SIX4 promotes the
proliferation, migration, invasion and tube formation of SKOV3
cells
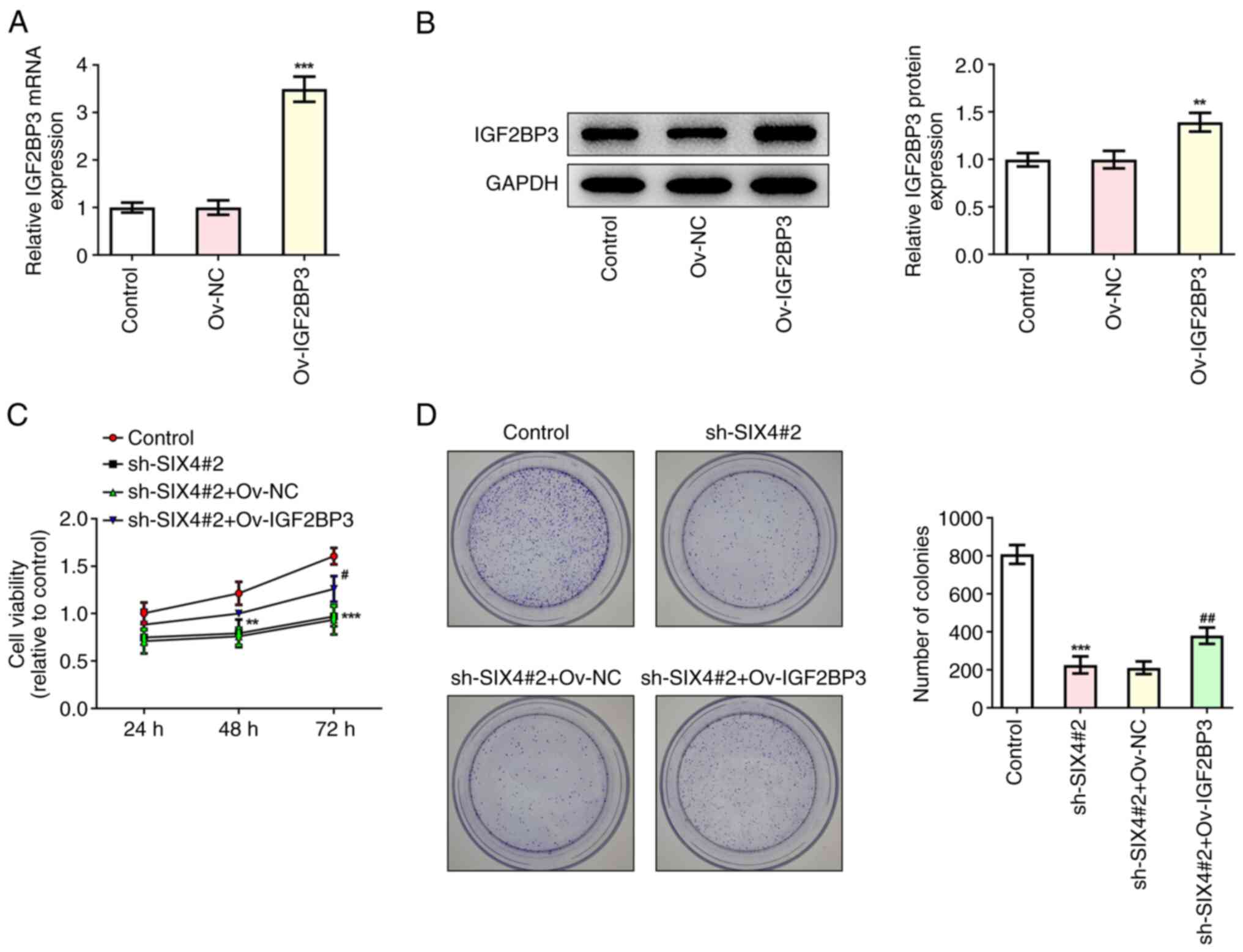
To further explore the regulatory role of
IGF2BP3/SIX4 in SKOV3 cells, IGF2BP3 was overexpressed (Fig. 5A and B), and the cells were
divided into the control, sh-SIX4#2, sh-SIX4#2+Ov-NC and
sh-SIX4#2+Ov-IGF2BP3 treatment groups. The results obtained from
the CCK-8 and colony formation assays demonstrated that, compared
with the sh-SIX4#2+Ov-NC group, the cell viability of the
sh-SIX4#2+Ov-IGF2BP3 group was significantly increased at 72 h
(Fig. 5C), and the impeded cell
proliferation caused by SIX4 silencing was restored by IGF2BP3
overexpression (Fig. 5D). In
addition, the wound healing and Transwell assay results revealed
that, compared with the sh-SIX4#2+Ov-NC group, the invasion and
migration rates of the cells in the sh-SIX4#2+Ov-IGF2BP3 group were
significantly increased (Fig.
6A-D). Compared with the sh-SIX4#2+Ov-NC group, the tube
formation of the sh-SIX4#2+Ov-IGF2BP3 group was significantly
increased (Fig. 6E and F).
Western blotting was subsequently used to detect the protein
expression levels of E-cadherin, N-cadherin, Snail and VEGF, and
the results obtained demonstrated that overexpression of IGF2BP3
could reverse the regulation of these metastasis- and
angiogenesis-associated proteins following the inhibition of SIX4
(Fig. 6G). Collectively, these
results suggested that IGF2BP3-stabilized SIX4 could promote the
proliferation, migration, invasion and tube formation of SKOV3
cells.
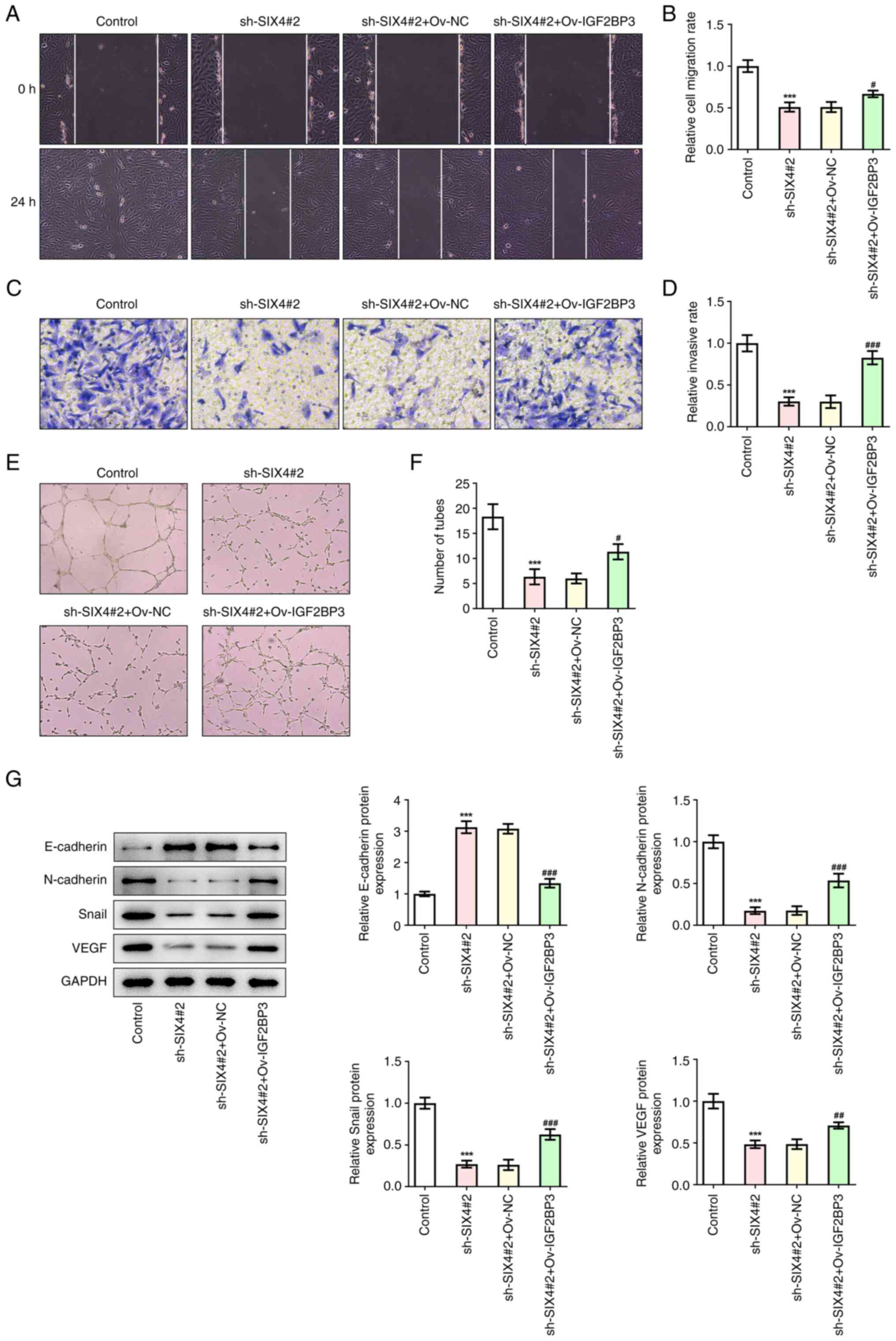 | Figure 6.IGF2BP3-stabilized SIX4 promotes the
migration, invasion and tube formation of SKOV3 cells. (A) Wound
healing assay (magnification, ×100) detecting the cell migration
after cell transfection. (B) Statistical analysis of cell
migration. (C) Transwell assay (magnification, ×100) detecting the
cell invasion. (D) Statistical analysis of cell invasion. (E) A
tube formation assay (magnification, ×400) was used to detect the
tube formation of human umbilical vein endothelial cells. (F)
Statistical analysis of tube formation. (G) Western blotting was
used to detect the metastasis- and angiogenesis-associated proteins
after cell transfection. ***P<0.001 vs. control.
#P<0.05, ##P<0.01,
###P<0.001 vs. sh-SIX4+Ov-NC. IGF2BP3, insulin-like
growth factor 2 mRNA binding protein 3; NC, negative control; Ov,
overexpression; sh, short hairpin RNA; SIX4, SIX homeobox 4. |
Discussion
The pathogenesis and treatment of OC have for a long
time provided a focus for biomedical research. In the present
study, the effects of SIX4 on OC cell proliferation, metastasis and
angiogenesis were studied, and the underlying mechanism was
discussed.
Solid tumor growth is dependent upon continuous and
extensive tumor angiogenesis, which provides nutrients and oxygen
to tumor tissue and also the means for tumor tissue metastasis
(20,21). OC has the highest mortality rate
among the different types of gynecological tumors, and exhibits
clear characteristics of easy metastasis (22,23). The invasion, growth and metastasis
of OC are largely dependent on the formation of an abundant blood
supply (24). Folkman (25) first proposed treating tumors via
inhibiting tumor angiogenesis in 1971. In recent years, several
studies have demonstrated that inhibition of tumor angiogenesis can
inhibit the proliferation and metastasis of tumor cells, thereby
inhibiting the occurrence and development of tumors (21,26,27). Therefore, the focus of the present
study was to explore the possibility of a novel type of OC therapy
via inhibiting the angiogenesis of OC.
SIX4 fulfills an important role in the development
of multiple tumors. A previous study indicated that the
upregulation of SIX4 is an indicator of poor clinical prognosis of
esophageal squamous cell carcinoma, and that this leads to the
promotion of tumor growth and cell metastasis (8). SIX4 is able to promote
hepatocellular carcinoma metastasis via upregulation of the
proteins Yes associated transcriptional regulator 1 and C-Met
(28). Notably, in colorectal
cancer cells, SIX4 increases the expression levels of VEGF-A in
conjunction with hypoxia-inducible factor-1α and upregulation of
SIX4 has been demonstrated to promote tumor growth and angiogenesis
(29). These results suggested
that SIX4 has an important role in cancer metastasis and
angiogenesis. The GEPIA database analysis revealed that SIX4 is
highly expressed in OC tissues. High expression levels of SIX4 were
associated with low OS rates in patients with OC. Cell experiments
in the present study also demonstrated that SIX4 expression is
abnormally elevated in OC cells. Interference with the expression
of SIX4 can therefore lead to inhibition of OC cell proliferation,
migration, invasion and tube formation.
Subsequently, the present study provided novel
insights into the regulatory mechanism of SIX4. The expression
levels of IGF2BP3 and SIX4 were positively correlated according to
GEPIA database analysis. The m6A reader IGF2BP3 has an important
role in the regulation of angiogenesis in gastric cancer cells. In
colon cancer cells, IGF2BP3 has been demonstrated to bind to the
m6A-modified region of VEGF mRNA to regulate the expression and
stability of VEGF mRNA, thereby inhibiting the angiogenesis of
colon cancer (30). In addition,
studies have reported that high expression level of IGF2BP3 is a
marker of poor prognosis of clear cell OC (31,32). In the present study, GEPIA
database analysis revealed that IGF2BP3 was highly expressed in
tissues from patients with OC. IGF2BP3 was also highly expressed in
OC cell lines. Furthermore, the experiments performed in the
present study demonstrated that IGF2BP3 can improve the stability
of SIX4 mRNA. Subsequently, the associated mechanism was further
investigated by inhibiting the expression of SIX4 and
overexpressing IGF2BP3. These experiments revealed that the
overexpression of IGF2BP3 could reverse the inhibitory effect of
SIX4 on the malignant progression of SKOV3 cells, suggesting that
IGF2BP3 regulates the function of SIX4 during the malignant
progression of OC.
To the best of our knowledge, the present study was
the first to investigate the role of SIX4 and IGF2BP3 in SKOV3 OC
cells and the regulatory relationship between them. In the present
study, the mechanism of OC was explored through the combination of
bioinformatics and experiments. However, the present study also had
certain limitations. First of all, the existing conclusions were
not further verified in clinical samples and animal experiments,
and our research group will further verify them in future
experiments. In addition, in the mechanism experiments, only
experiments on interference of SIX4 were conducted, while no
experiments on overexpression of SIX4 in cells were conducted. The
mechanism will be further investigated through overexpression of
SIX4 in future experiments.
In conclusion, it may be determined from the present
study that IGF2BP3-stabilized SIX4 is able to promote the
proliferation, migration, invasion and tube formation of SKOV3
cells. Furthermore, the present study also provides a theoretical
basis for the improved understanding of the mechanism of OC, which
will hopefully facilitate the treatment of OC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH designed and performed experiments. XH analyzed
the data and wrote the manuscript. JH edited the manuscript. JH and
XH confirm the authenticity of all the raw data. Both authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cook DP and Vanderhyden BC: Ovarian cancer
and the evolution of subtype classifications using transcriptional
profilingdagger. Biol Reprod. 101:645–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroeger PT Jr and Drapkin R: Pathogenesis
and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol.
29:26–34. 2017. View Article : Google Scholar
|
|
3
|
Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ,
Bast RC Jr, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et
al: Rethinking ovarian cancer II: Reducing mortality from
high-grade serous ovarian cancer. Nat Rev Cancer. 15:668–679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koutsaki M, Libra M, Spandidos DA and
Zaravinos A: The miR-200 family in ovarian cancer. Oncotarget.
8:66629–66640. 2017. View Article : Google Scholar
|
|
5
|
Yang WL, Lu Z and Bast RC Jr: The role of
biomarkers in the management of epithelial ovarian cancer. Expert
Rev Mol Diagn. 17:577–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colombo N, Sessa C, du Bois A, Ledermann
J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I,
Vergote I, et al: ESMO-ESGO consensus conference recommendations on
ovarian cancer: Pathology and molecular biology, early and advanced
stages, borderline tumours and recurrent diseasedagger. Ann Oncol.
30:672–705. 2019. View Article : Google Scholar
|
|
7
|
Zhang J, Jiang TY, Jiang BG, Yang C, Tan
YX, Yang N, Pan YF, Ding ZW, Yang GZ, Wu MC, et al: RMP predicts
survival and adjuvant TACE response in hepatocellular carcinoma.
Oncotarget. 6:3432–3442. 2015. View Article : Google Scholar
|
|
8
|
Li Y, Jiang X, Yan X and Wang Y:
Upregulation of SIX4 indicates poor clinical outcome and promotes
tumor growth and cell metastasis in esophageal squamous cell
carcinoma. Thorac Cancer. 12:752–759. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun X, Ma J, Chen Q, Hou Z, Luo X, Wang G,
Wang J, Hu J and Cao Z: SIX4 promotes metastasis through STAT3
activation in breast cancer. Am J Cancer Res. 10:224–236.
2020.PubMed/NCBI
|
|
10
|
Na XY, Shang XS, Zhao Y, Ren PP and Hu XQ:
miR-203a functions as a tumor suppressor in bladder cancer by
targeting SIX4. Neoplasma. 66:211–221. 2019. View Article : Google Scholar
|
|
11
|
Tang X, Yang Y, Song X, Liu X, Wang X,
Huang F, Li Y, Chen F and Wan H: SIX4 acts as a master regulator of
oncogenes that promotes tumorigenesis in non-small-cell lung cancer
cells. Biochem Biophys Res Commun. 516:851–857. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnston MJ, Bar-Cohen S, Paroush Z and
Nystul TG: Phosphorylated Groucho delays differentiation in the
follicle stem cell lineage by providing a molecular memory of EGFR
signaling in the niche. Development. 143:4631–4642. 2016.
|
|
13
|
Taniuchi K, Furihata M, Hanazaki K, Saito
M and Saibara T: IGF2BP3-mediated translation in cell protrusions
promotes cell invasiveness and metastasis of pancreatic cancer.
Oncotarget. 5:6832–6845. 2014. View Article : Google Scholar
|
|
14
|
Liu H, Zeng Z, Afsharpad M, Lin C, Wang S,
Yang H, Liu S, Kelemen LE, Xu W, Ma W, et al: Overexpression of
IGF2BP3 as a potential oncogene in ovarian clear cell carcinoma.
Front Oncol. 9:15702019. View Article : Google Scholar
|
|
15
|
Hsu KF, Shen MR, Huang YF, Cheng YM, Lin
SH, Chow NH, Cheng SW, Chou CY and Ho CL: Overexpression of the
RNA-binding proteins Lin28B and IGF2BP3 (IMP3) is associated with
chemoresistance and poor disease outcome in ovarian cancer. Br J
Cancer. 113:414–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Tang Z, Zhang W, Ye Z and Liu F:
GEPIA2021: Integrating multiple deconvolution-based analysis into
GEPIA. Nucleic Acids Res. 49:W242–W246. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao H, Zheng Y, Chen J and Shen H:
miR-198 inhibits proliferation, invasion and migration of ovarian
cancer cells by regulating the PI3K/Akt signaling pathway. Acta
Biochim Pol. 68:673–677. 2021.
|
|
20
|
Viallard C and Larrivee B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Unterleuthner D, Neuhold P, Schwarz K,
Janker L, Neuditschko B, Nivarthi H, Crncec I, Kramer N, Unger C,
Hengstschläger M, et al: Cancer-associated fibroblast-derived WNT2
increases tumor angiogenesis in colon cancer. Angiogenesis.
23:159–177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grunewald T and Ledermann JA: Targeted
therapies for ovarian cancer. Best Pract Res Clin Obstet Gynaecol.
41:139–152. 2017. View Article : Google Scholar
|
|
23
|
Yousefi M, Dehghani S, Nosrati R, Ghanei
M, Salmaninejad A, Rajaie S, Hasanzadeh M and Pasdar A: Current
insights into the metastasis of epithelial ovarian cancer-hopes and
hurdles. Cell Oncol (Dordr). 43:515–538. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang
J and Wu X: Ovarian cancer cell-secreted exosomal miR-205 promotes
metastasis by inducing angiogenesis. Theranostics. 9:8206–8220.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao
HL, Li H, Zhang SR, Xu JZ, Qi ZH, et al: Angiogenesis in pancreatic
cancer: Current research status and clinical implications.
Angiogenesis. 22:15–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Q, Lin Z, Wang Z, Huang W, Tian D, Liu
M and Xia L: SIX4 promotes hepatocellular carcinoma metastasis
through upregulating YAP1 and c-MET. Oncogene. 39:7279–7295. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun X, Hu F, Hou Z, Chen Q, Lan J, Luo X,
Wang G, Hu J and Cao Z: SIX4 activates Akt and promotes tumor
angiogenesis. Exp Cell Res. 383:1114952019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Wang T, Wu D, Min Z, Tan J and Yu
B: RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and
angiogenesis in colon cancer. J Exp Clin Cancer Res. 39:2032020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobel M, Xu H, Bourne PA, Spaulding BO,
Shih IeM, Mao TL, Soslow RA, Ewanowich CA, Kalloger SE, Mehl E, et
al: IGF2BP3 (IMP3) expression is a marker of unfavorable prognosis
in ovarian carcinoma of clear cell subtype. Mod Pathol. 22:469–475.
2009. View Article : Google Scholar
|
|
32
|
Noske A, Faggad A, Wirtz R, Darb-Esfahani
S, Sehouli J, Sinn B, Nielsen FC, Weichert W, Buckendahl AC, Röske
A, et al: IMP3 expression in human ovarian cancer is associated
with improved survival. Int J Gynecol Pathol. 28:203–210. 2009.
View Article : Google Scholar
|