Introduction
Kidney stones are one of the most common urinary
diseases in the world, causing heavy social and economic burden to
mankind (1). The occurrence and
development of kidney stones are closely related to the increased
risk of chronic kidney disease (CKD) (2). calcium oxalate (CaOx) stones account
for 70–80% of renal stones, 35% of patients with CaOx stones were
accompanied by renal injury, and 29% were accompanied by CKD
(3). The prevention and treatment
of CaOx stones focuses on the causes of CaOx stones and surgical
improvement. The treatment of patients with CaOx stones progressing
to CKD has not attracted enough attention. Renal injury induced by
CaOx crystals cannot be completely repaired and progresses to
fibrosis (4). Therefore, the
mechanism of fibrosis after CaOx crystal renal injury should be
urgently clarified, and the progression of patients with CaOx
stones to CKD should be delayed.
The formation process of CaOx stones includes the
precipitation of CaOx crystals when oxalate (Ox) and calcium (Ca)
ions are in a relatively supersaturated state. CaOx crystals adhere
to tubular epithelial cells and interact with other cells. The
crystals stay in the kidney in various ways and finally form kidney
stones (5). The adhesion of CaOx
crystals to renal tubular epithelial cells is the initial process
of stone formation. CaOx crystals can directly produce cytotoxic
damage (6). Renal tubular
epithelial cells undergo apoptosis after injury, resulting in
inflammatory response and a fibrotic environment (7). The molecular mechanism in which CaOx
crystals induce renal tubular epithelial cell injury and
interstitial fibrosis should be explored for the clinical
prevention and treatment of CaOx stone formation and the
progression of patients with CaOx stones to CKD.
Ferroptosis is characterized by intracellular iron
accumulation and lipid peroxidation during cell death. Ferroptosis
can be induced by the downregulation of system XCT activity,
inhibition of glutathione peroxidase 4 (GPX4), and increase of
reactive oxygen species (ROS) (8). System XCT is an important
intracellular antioxidant system, which takes up cystine and
excretes glutamate. Inhibiting the activity of system XCT affects
the synthesis of glutathione (GSH) by inhibiting the absorption of
cystine, which leads to the occurrence of oxidative damage and
ferroptosis. Solute carrier family 7 member 11 (SLC7A11) and GPX4
are the central regulators of ferroptosis, and the reduced levels
of GPX4 and SLC7A11 are always regarded as markers of ferroptosis.
Ferroptosis is involved in tumor development (9), neurodegenerative diseases (10), ischemia-reperfusion injury
(11), and other pathological
processes, and the targeted regulation of ferroptosis and its
signaling pathways have achieved beneficial results. Although the
interactions between ferroptosis and acute kidney injury (AKI) have
been continuously explored (12),
limited studies have focused on ferroptosis and CKD, especially the
progression of patients with CaOx stones to CKD. By high-throughput
screening of small molecule libraries, studies have independently
reported that ferrostatin-1 (Fer-1) acts as a potent inhibitor of
ferroptosis (13,14). In the present study, the role and
mechanism of Fer-1 in Ox-induced renal tubular epithelial cell
injury, fibrosis, and CaOx stone formation were evaluated.
Materials and methods
Animals
A total of 18 C57BL/6J mice (male; 6–8 weeks;
weighing 24–28 g) were purchased from Hubei Provincial Centers for
Disease Control and Prevention. All animal treatments were approved
(approval no. WDRM-20200604) by the Laboratory Animal Welfare and
Ethics Committee of Renmin Hospital of Wuhan University (Wuhan,
China). The mice were kept under specific pathogen-free conditions
and in a steady temperature (22±2°C) and humidity (40–70%) barrier
system with a 12-h light/dark cycle, and food and water
available.
Study design
The 18 mice were divided randomly into three groups
(n=6 per group), namely, the control (Con), CaOx stone, and CaOx
stone + Fer-1 group (CaOx + Fer-1). The CaOx stone model in the
kidneys of mice was established with intraperitoneal injection of
80 mg/kg glyoxylic acid (Sigma-Aldrich; Merck KGaA) for 14 days
(15); 5 mg/kg Fer-1 (inhibitor
of ferroptosis; MedChemExpress) was injected once daily for 3
consecutive days before glyoxylic acid treatment and seventh after
modeling (16). Mice in the
control group received an intraperitoneal injection of saline
solution. The experimental mice were anesthetized by
intraperitoneal injection of pentobarbital (50 mg/kg) for renal
tissue and blood sampling. The blood was centrifuged (4,000 × g)
for 15 min at 4°C to obtain the serum, and the kidneys were
harvested and then stored at −80°C or fixed in paraformaldehyde
solution. All animals were sacrificed with pentobarbital (100
mg/kg) after surgery.
Cell culture
Human proximal tubular cells (HK-2 cells; cat. no.
SCSP-511) were obtained from the Cell Bank of the Chinese Academy
of Sciences, maintained in complete DMEM/F12 with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin and
streptomycin and cultured at 37°C in an incubator with 5%
CO2. Ox (Sigma-Aldrich; Merck KGaA) was added to the
serum-free DMEM/F12 to prepare the Ox intervention solution. When
cell confluence reached 80%, the cells were exposed to 2 mM Ox
intervention solution for 24 h prior to analysis. Subsequently, the
HK-2 cells were treated with Fer-1 (100 µM) with or without Ox
intervention solution (2 mM) for 24 h at 37°C (17).
Iron measurements
Cells (2×106) were rapidly homogenized in
iron assay buffer using an Iron Assay kit (product no. MAK025;
Sigma Aldrich; Merck KGaA). Briefly, iron is released by the
addition of an acidic buffer. Samples were assessed to measure
total iron. Released iron could react with the iron probe resulting
in a colorimetric (593 nm) product, proportional to the iron
present. The solution was then centrifuged at 13,000 × g for 10 min
at 4°C to remove insoluble material and was measured at 593 nm with
a microplate fluorometer (Bio-Rad Laboratories, Inc.).
Cell viability assay
The cell viability after the use of Fer-1 and Ox was
measured by Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology) assay. Cells (3×103/well) were seeded
into 96-well culture plates at 37°C for 24 h. The cells were then
co-treated with different concentrations of Fer-1 (0, 2, 4, 8, 16,
32, and 64 µM) and oxalate (2 mM) in DMEM/F12. A total of 10 µl of
CCK-8 solution was added to each well and incubated at 37°C for
another 2 h. The optical density at 450 nm was determined using a
microplate reader (Bio-Rad Laboratories, Inc.).
Hematoxylin-eosin (H&E)
staining
The kidney tissue (fixed with 4% paraformaldehyde
solution at 26°C) was dehydrated with gradient ethanol, embedded in
paraffin, cut into 4-µM sections, subjected to xylene dewaxing and
gradient ethanol debenzylation, and washed with distilled water.
The tissues were stained with hematoxylin for 5 min and eosin for 1
min at room temperature using the H&E staining kit (cat. no.
C0105; Beyotime Institute of Biotechnology) and injuries were
scored as follows: 0, no tubular injury; 1, <10% tubular damage;
2, 10–25% tubular damage; 3, 26–50% tubular damage; 4, 51–74%
tubular damage; and 5, >75% tubular damage.
Immunofluorescence staining for kidney
tissue
Renal tissues were fixed in 4% paraformaldehyde at
4°C for 24 h, embedded in paraffin and cut into 4-µm sections. The
sample was then washed with pure water, and the slices were
transferred into 0.01 M citrate buffer (pH 6.0), heated in a
microwave oven for antigen repair, and repaired with medium and
high temperatures for 2–8 min. The specific time was adjusted
according to the actual situation of the sample. Subsequently, the
sample was cooled to room temperature, and a circle was drawn
around the tissue with a histochemical pen to prevent the
incubation solution from flowing away in the later process. PBS was
used to wash the sample thrice for 5 min each time. The primary
antibody: α-Smooth muscle actin (α-SMA; 1:200; cat. no. BM0002;
Wuhan Boster Biological Technology, Ltd.), fibronectin (1:400; cat.
no. 15613-1-AP; ProteinTech Group, Inc.), E-cadherin (1:500; cat.
no. 40772; Abcam), GPX4, (1:200; cat. no. 6701; Affinity
Biosciences, Ltd.), SLC7A11 (1:200; cat. no. 26864; ProteinTech
Group, Inc.), and superoxide dismutase 2 (SOD2; 1:200; cat. no.
13534; Abcam) was incubated with 5% BSA 4°C overnight. The sample
was reheated and washed thrice with PBS for 5 min each time. An
appropriate amount of secondary antibody (goat anti-rabbit HRP;
1:1,000; cat. no. A-11034; Thermo Fisher Scientific, Inc.) working
solution was added to the slices, and the sample was incubated in a
water bath at 37°C for 30 min in the dark and washed thrice with
PBS for 5 min each time. DAPI was then added dropwise to stain the
nuclei, and the mixture was incubated in the dark at room
temperature for 20–30 min and washed with PBS. Finally, the film
was sealed with anti-fluorescence quenching sealing agent. All
images were observed under a fluorescence microscope (Nikon
Corporation) and figures were analyzed using ImageJ software
(version 1.8.0; National Institutes of Health).
Immunohistochemistry (IHC)
analysis
In brief, 4-µm paraffin-embedded sections were
deparaffinized, subjected to antigen retrieval using citrate buffer
(pH 6.0) at 95°C for 10 min and treated with 0.3%
H2O2 to block their endogenous peroxidase
activity at room temperature for 10 min. Next, the sections were
continuously treated with blocking reagent QuickBlock™
(cat. no. P0260; Beyotime Institute of Biotechnology), then
incubated with the primary antibody for kidney injury molecular 1
(KIM-1; 1:100) at 4°C overnight, and then with the secondary
antibody (1:5,000; cat. no. TA130017; biotinylated goat
anti-rabbit; OriGene Technologies, Inc.) for 1 h at room
temperature. Peroxidase activity was visualized using
3,3′-diaminobenzidine at room temperature for 5 min. The positive
stained areas of KIM-1, based on the unit area (magnification,
×400), were calculated as the percentage of all examined areas
using ImageJ software (version 1.8.0; National Institutes of
Health).
TUNEL assay
A TUNEL assay kit (cat. no. MK1015; Wuhan Boster
Biological Technology, Ltd.) was used to detect apoptotic cells
according to the manufacturer's instructions. Kidney paraffin
sections were incubated overnight at 4°C with anti-neuronal nuclei
antibody (cat. no. BM4354; 1:100; Wuhan Boster Biological
Technology, Ltd.) according to the manufacturer's instructions. The
sections were incubated with TUNEL reaction mixture for 1 h at 37°C
before being rinsed three times with PBS. Images were captured
using an inverted fluorescence microscope at high magnification
(×400). TUNEL-positive neurons in five randomly selected areas
surrounding the injury site were quantified, and the data were
analyzed using ImageJ software (version 1.8.0; National Institutes
of Health).
Hoechst staining
Hoechst 33342/PI double staining was used for
morphological analysis. Cells were plated in replicates at
1×106 cells per well in 6-well plates. The cells were
collected via centrifugation at 300 × g for 5 min at 25°C,
suspended in cell staining buffer, and incubated with 5 µl Hoechst
33342 and 5 µl PI solution (cat. no. CA1120; Beijing Solarbio
Science & Technology Co., Ltd.) at 4°C for 30 min. All images
were observed under a fluorescence microscope (Nikon Corporation)
and figures were analyzed using ImageJ software (version 1.8.0;
National Institutes of Health).
Masson staining
Renal tissues were fixed in 4% paraformaldehyde at
4°C for 24 h, embedded in paraffin and cut into 4-µm sections.
Then, a circle was drawn around the tissue with a histochemical pen
to prevent the incubation solution from flowing away in the later
process. Subsequently, the sample was washed thrice with pure water
for 5 min each time. By using the Masson staining kit (Wuhan
Servicebio Technology Co., Ltd.), solution A was added, and the
solution was soaked at room temperature overnight. The slices
soaked in liquid were placed into the oven at 62°C for 30 min, and
liquids D and F were added for preheating. The sample was then
washed with tap water until the yellow dye on the tissue was no
longer evident. Equal quantities of liquids B and C were added to
dye the samples for 1 min at room temperature, and were then washed
with tap water. Following differentiation with 1% hydrochloric acid
and alcohol for 1 min, the nucleus was gray black, and the
background was almost colorless. The sample was washed with tap
water and dyed with solution D for 6 min, washed with water, and
then dyed with solution E for 1 min at room temperature. Without
washing, the sample was dyed with solution F for 8–15 sec at room
temperature. Finally, 1% glacial acetic acid was differentiated
thrice for several seconds. Renal fibrosis was verified using
Masson trichrome staining (blue area). All images were observed
under a light microscope (Nikon Corporation) and figures were
analyzed using ImageJ software (version 1.8.0; National Institutes
of Health).
Sirius red staining
Renal tissues were fixed in 4% paraformaldehyde at
4°C for 24 h, embedded in paraffin and cut into 4-µm sections.
Then, a circle was drawn around the tissue with a histochemical pen
to prevent the incubation solution from flowing away in the later
process. The sample was washed thrice with pure water for 5 min
each time. Subsequently, Sirius red staining was carried out for 30
min at room temperature. The slices were directly divided into
absolute ethanol for several seconds. Renal fibrosis was verified
using Sirius red staining (red area). All images were observed
under a light microscope (Nikon Corporation) and figures were
analyzed using ImageJ software (version 1.8.0; National Institutes
of Health).
Von Kossa staining
Renal tissues were fixed in 4% paraformaldehyde at
4°C for 24 h, embedded in paraffin and cut into 4-µm sections. The
sections were then placed in 1% silver nitrate solution under
ultraviolet light for 45 min. Subsequently, the sample was washed
with distilled water, treated with 3% sodium thiosulphate for 5 min
at room temperature, and washed with water. Finally, the sample was
counterstained with van Gieson for 5 min at room temperature and
washed with alcohol. The crystal deposition area (black area) was
quantified using ImageJ software (version 1.8.0; National
Institutes of Health).
Western blotting
Renal tissue homogenates and cell lysates were
prepared in RIPA buffer containing phenylmethylsulfonyl fluoride
and a phosphatase inhibitor cocktail (Wuhan Servicebio Technology
Co., Ltd.). The protein content was measured using a bicinchoninic
acid assay (Bio-Rad Laboratories, Inc). After BCA quantification,
30 µg protein samples were obtained from each hole, separated by
10% SDS-PAGE gel electrophoresis, and transferred to polyvinylidene
fluoride membranes. The membrane was sealed with 5% milk for 1 h at
26°C, and then with 0.5% skim. It was then washed thrice using
Tris-buffered saline containing 1% Tween-20 (TBST) for 10 min each
time. The membrane was then incubated with a primary antibody: SOD2
(1:1,000; cat. no. ab137037; Abcam), heme oxygenase 1 (HO-1;
1:2,000; cat. no. ab52947; Abcam), GPX4 (1:1,000; cat. no. DF6701;
Affinity Biosciences, Ltd.), SLC7A11 (1:1,000; cat. no. DF12509;
Affinity Biosciences, Ltd.), caspase-3 (1:2,000; cat. no. AF7022;
Affinity Biosciences, Ltd.), fibronectin (1:1,000; cat. no.
sc-8422; Santa Cruz Biotechnology, Inc.), E-cadherin (1:5,000; cat.
no. 20874-1; ProteinTech Group, Inc.), vimentin (1:5,000; cat. no.
10366-1; ProteinTech Group, Inc.), α-SMA (1:1,000; cat. no. BM0002;
Wuhan Boster Biological Technology, Ltd.) and GAPDH (1:5,000; cat.
no. 60004-1; ProteinTech Group, Inc.) overnight at 4°C. The sample
was then washed thrice with TBST for 10 min each time. The membrane
was removed and incubated with the secondary antibody (1:1,000;
cat. no. SA00001-2; ProteinTech Group, Inc.) at 37°C for 2 h. TBST
was then used to wash the membrane for 10 min each time. Finally,
ECL (cat. no. BL520A; Biosharp Life Sciences) color was developed
with GAPDH as an internal reference to analyze the protein
expression level on the membrane. ImageJ software (version 1.8.0;
National Institutes of Health) was used to semi-quantify protein
expression with GAPDH as the loading control.
Detection of ROS by flow
cytometry
Intracellular ROS production was detected using the
probe DCF-DA (Beyotime Institute of Biotechnology). Cells
(3×106/well) were seeded in a 6-well plate and
stimulated by Ox, followed by incubation with 10 µM DCF-DA for 20
min at 37°C. The cells were then collected and washed with PBS.
After resuspending the pellet in 200 µl PBS, fluorescence was
detected using a BD Accuri C6 Plus flow cytometer and FACSDiva
software (version 6.13) (BD Biosciences).
Transmission electron microscopy
(TEM)
The kidney tissues were fixed for 2 h with 2.5%
glutaraldehyde in a 0.05 M sodium cacodylate buffer at pH 7.2 and
at 25°C, followed by 2 h in 2% OsO4 in a 0.1 M sodium
cacodylate buffer and 18 h in 1% aqueous uranyl acetate. Following
dehydration through an ethanol series, the specimens were embedded
in epoxy resin 618 at 40°C and cut into 60–80 nm ultrathin sections
and then subjected to uranium lead double staining at 4°C for 20
min. TEM (Hitachi, Ltd.) was used to observe renal tubular
epithelial cells and for image acquisition.
Assessment of blood urea nitrogen
(BUN) and (creatinine) Cr
Renal tissues and blood were collected for
biochemical analysis. BUN and Cr serum levels in renal tissues were
assessed using the corresponding detection kits (cat. no. C011-2
and C013-1; Nanjing Jiancheng Bioengineering Institute) in
accordance with the manufacturer's instructions.
Statistical analysis
Statistical analysis of the data was performed using
GraphPad Prism 5.0 (GraphPad Software, Inc.). The data are
presented as the mean ± standard deviation. The unpaired Student's
t-test was used to compare the mean values between two groups.
One-way analysis of variance and Bonferroni's post hoc test were
used to evaluate the differences among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Activation of ferroptosis in the CaOx
kidney stone mouse model
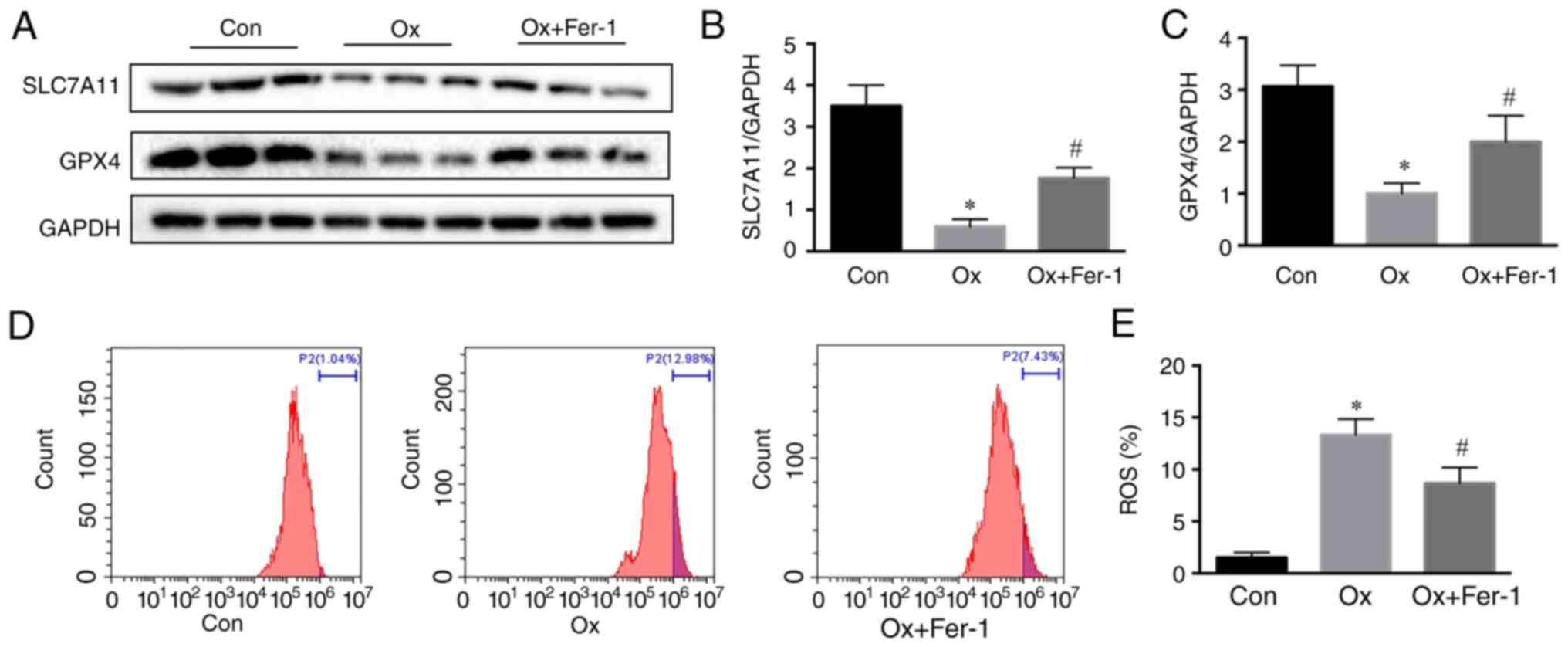
To explore the role of ferroptosis in CaOx stones, a
CaOx kidney stone mouse model was established with intraperitoneal
injection of 80 mg/kg glyoxylic acid for 14 days. Through
immunofluorescence staining of kidney tissue, the levels of SLC7A11
and GPX4 were revealed to be significantly decreased (Fig. 1A-C), and the levels of SOD2 and
HO-1 in renal tissue in the CaOx group were significantly increased
(Fig. 1A, D and E). Through
western blotting of kidney tissue, the levels of SLC7A11 and GPX4
were revealed to be significantly decreased (Fig. 1F-H), and the levels of SOD2 and
HO-1 in renal tissue in the CaOx group were significantly increased
(Fig. 1F, I and J). By using TEM,
it was observed that compared with the control group, the
mitochondrial morphology in the CaOx group exhibited the
characteristic changes of ferroptosis, including the vanishing of
mitochondria cristae and rupture of the outer mitochondrial
membrane (Fig. 1K). Therefore,
ferroptosis occurred in the mouse kidney of CaOx stone.
 | Figure 1.Activation of ferroptosis in mouse
kidneys with CaOx stones. (A) Renal cortex expression of SLC7A11,
GPX4, HO-1 and SOD2 determined by immunofluorescence staining in
mice after glyoxylic acid treatment (magnification, ×400). (B-E)
Quantification of (B) SLC7A11, (C) GPX4, (D) HO-1 and (E) SOD2 in
mouse kidneys assessed using immunofluorescence. (F) Western blot
analysis of SLC7A11, GPX4, HO-1 and SOD2 in renal tissue lysates
from these groups. (G-J) The ratio of the optical density of (G)
SLC7A11, (H) GPX4, (I) HO-1, and (J) SOD2 to GAPDH was
statistically analyzed. (K) Representative images of mitochondrial
injury under glyoxylic acid treatment were observed by TEM. White
triangles represent the injured mitochondria. The data are
presented as the mean ± SD; n=6. *P<0.05 vs. the control group.
CaOx, calcium oxalate; SLC7A11, solute carrier family 7 member 11;
GPX4, glutathione peroxidase 4; HO-1, heme oxygenase 1; SOD2,
superoxide dismutase 2; TEM, transmission electron microscopy. |
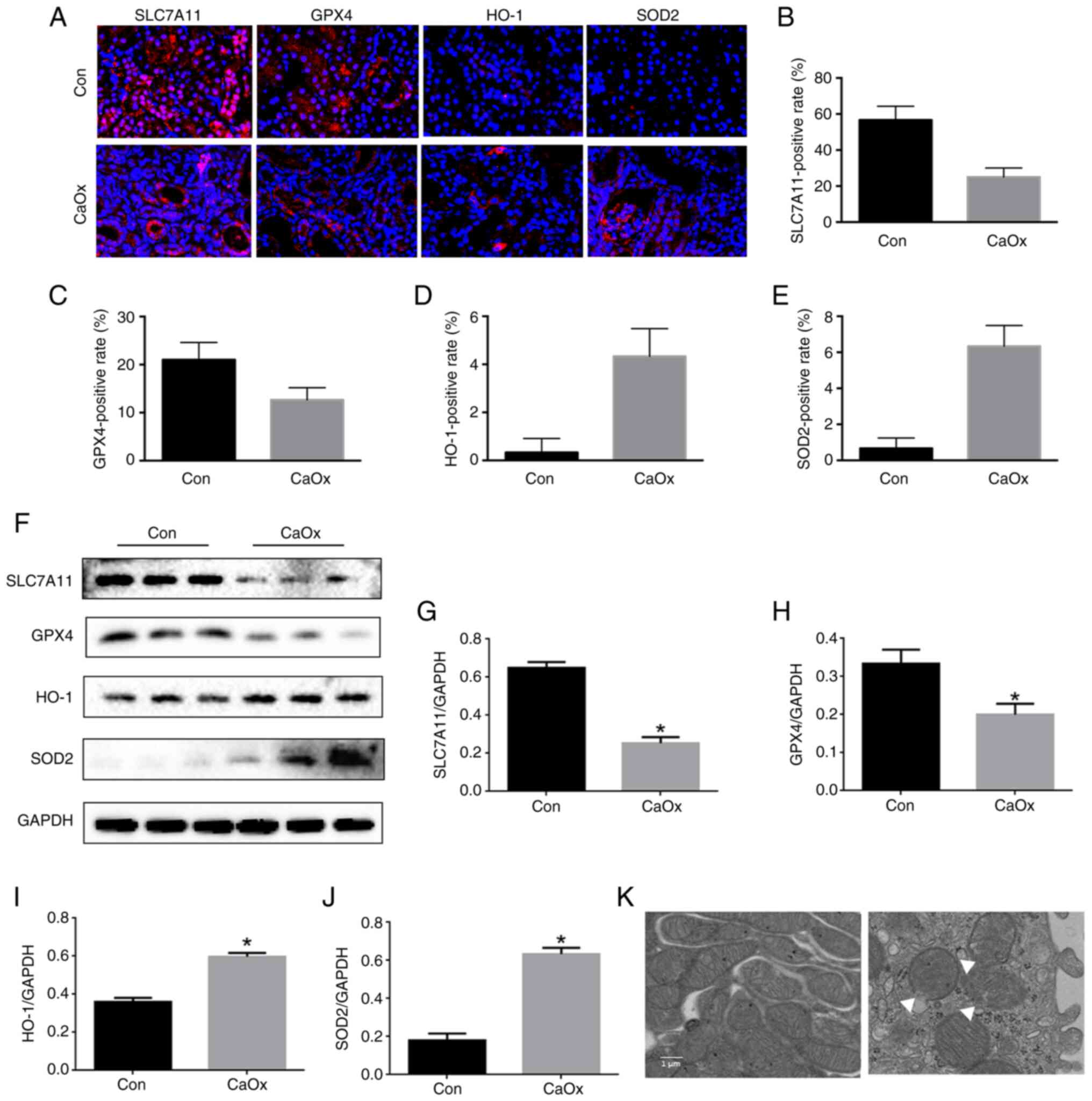
Fer-1 inhibits renal injury induced by
CaOx stones
Fer-1 acts as a specific inhibitor of ferroptosis,
which plays an important role in various diseases. H&E staining
revealed a higher injury score of renal tubules in the CaOx group
than in the control group. Following Fer-1 treatment, renal tubular
injury was decreased compared with the CaOx group (Fig. 2A and B). Through
immunohistochemical staining of kidney tissue, the level of KIM-1
was revealed to be significantly increased compared with the
control group, and the level of KIM-1 in renal tissue in the CaOx +
Fer-1 group was significantly decreased compared with that of the
CaOx stone group (Fig. 2A and C).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling (TUNEL staining) revealed a higher rate of apoptosis of
renal tubules in the CaOx model group compared with the control
group, and renal tubular apoptosis was decreased in the CaOx +
Fer-1 treatment group compared with that of the CaOx stone group
(Fig. 2A and D). Moreover, serum
BUN and Cr levels were increased compared with the control group
and decreased in the CaOx + Fer-1 treatment group compared with
those of the CaOx stone group (Fig.
2E and F).
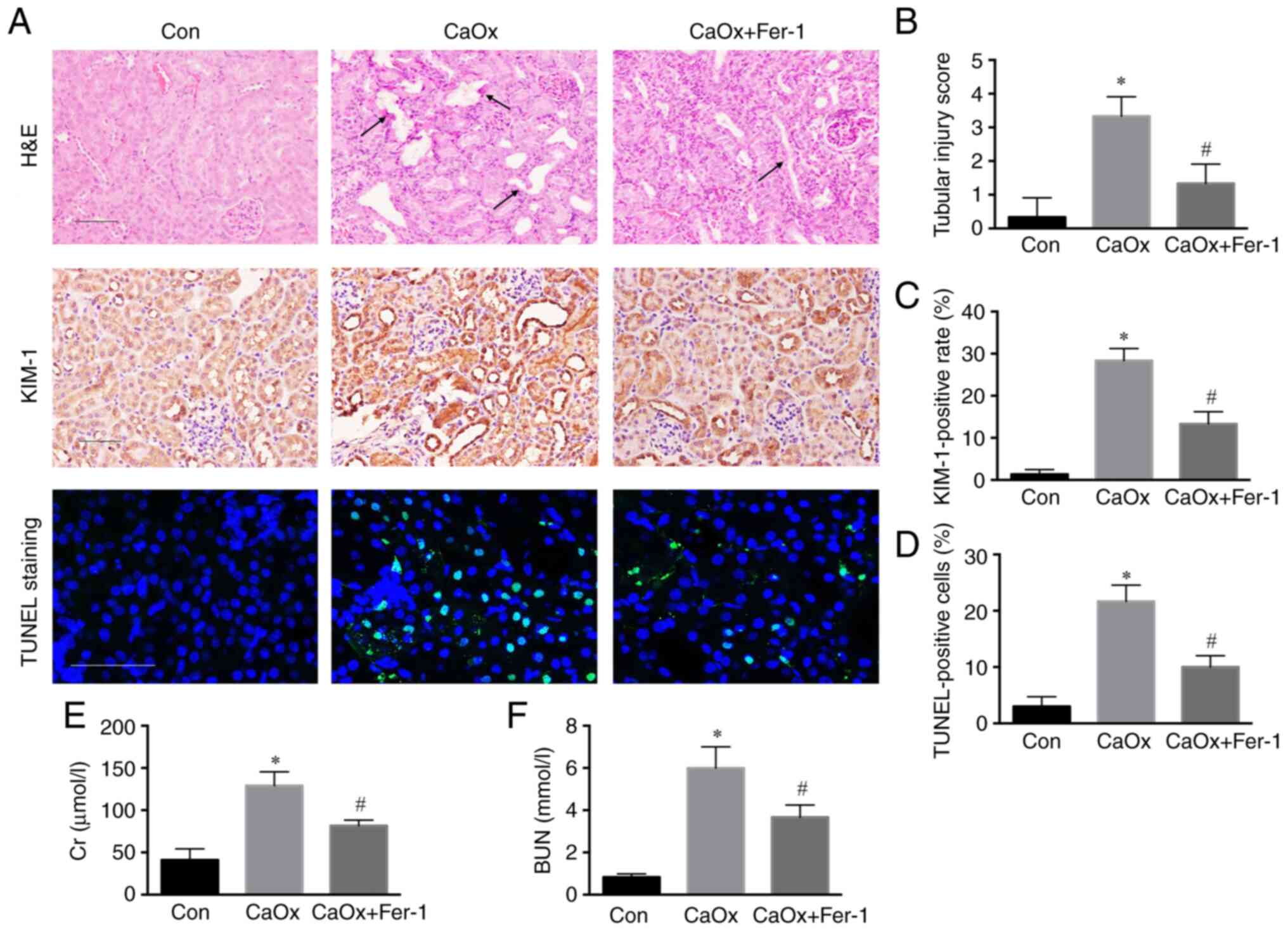 | Figure 2.Fer-1 inhibits renal injury induced
by CaOx stones. (A) Representative images of the renal cortex by
H&E staining, immunohistochemical staining (magnification,
×200) and TUNEL assay (magnification, ×400) following Fer-1
treatment. Black arrows represent the injured renal tubule. (B)
Tissue injury scores in each group were statistically analyzed. (C)
Semi-quantitative statistical analysis of KIM-1. (D) Quantification
of renal apoptosis by TUNEL assay. (E and F) Serum BUN and Cr
levels of three groups following Fer-1 treatment. The data are
presented as the mean ± SD; n=6. *P<0.05 vs. the control group;
#P<0.05 vs. the CaOx group. Fer-1, ferrostatin-1;
CaOx, calcium oxalate; H&E, hematoxylin and eosin; TUNEL,
terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling; KIM-1, kidney injury molecule-1; BUN, blood urea
nitrogen; Cr, creatinine. |
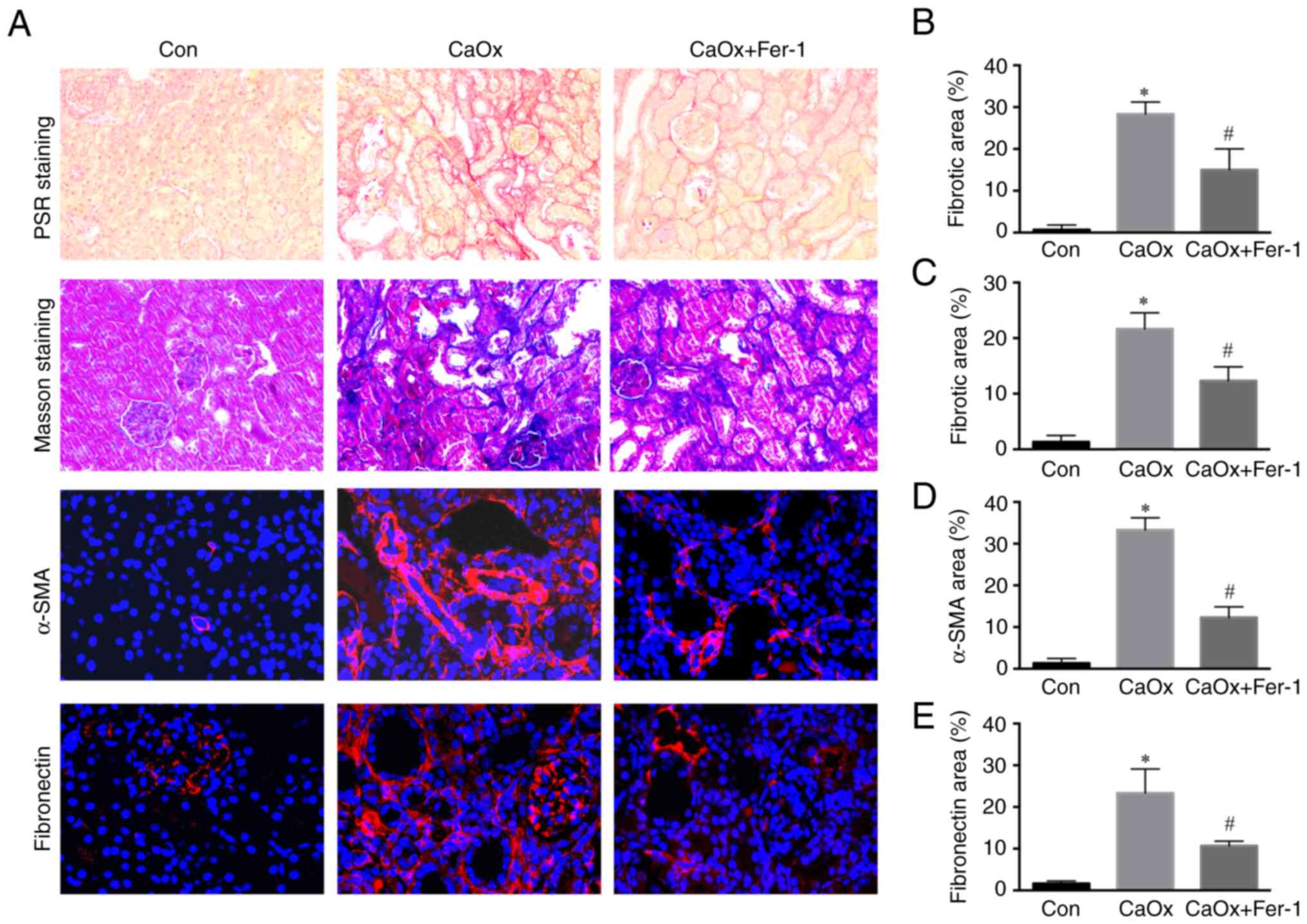
Fer-1 inhibits renal fibrosis after
renal injury induced by CaOx stones
Renal injury is closely associated with the
increased risk of developing CKD, and the risk of CKD incidence is
dependent on the severity of renal injury (18). The results of Picrosirius red
(PSR) staining showed that renal fibrosis in the CaOx group was
increased compared with the control group. Following Fer-1
treatment, renal fibrosis in mice was significantly lower than that
in the CaOx group (Fig. 3A and
B). Similarly, Masson staining revealed that renal fibrosis in
the CaOx group was significantly increased compared with the
control group. Following Fer-1 treatment, renal fibrosis in mice
was significantly lower than that in the CaOx group (Fig. 3A and C). Through
immunofluorescence staining of kidney tissue, the levels of
fibronectin and α-SMA in renal tissue of the CaOx group were
revealed to be significantly increased compared with the control,
whereas the levels of fibronectin and α-SMA were significantly
decreased in the CaOx + Fer-1 treatment group compared with the
levels in the CaOx group (Fig. 3A, D
and E).
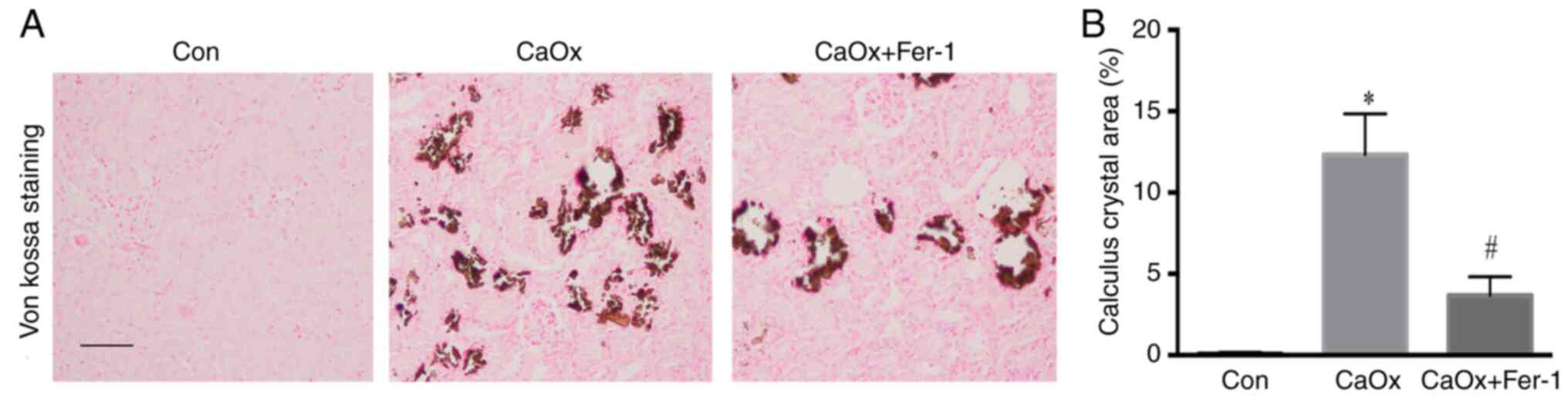
Fer-1 inhibits CaOx stone
formation
Von Kossa staining showed that CaOx crystals were
deposited in the renal interstitium of mice in the CaOx group.
Following Fer-1 treatment, the CaOx crystals in mice were
significantly lower than those in the CaOx group (Fig. 4).
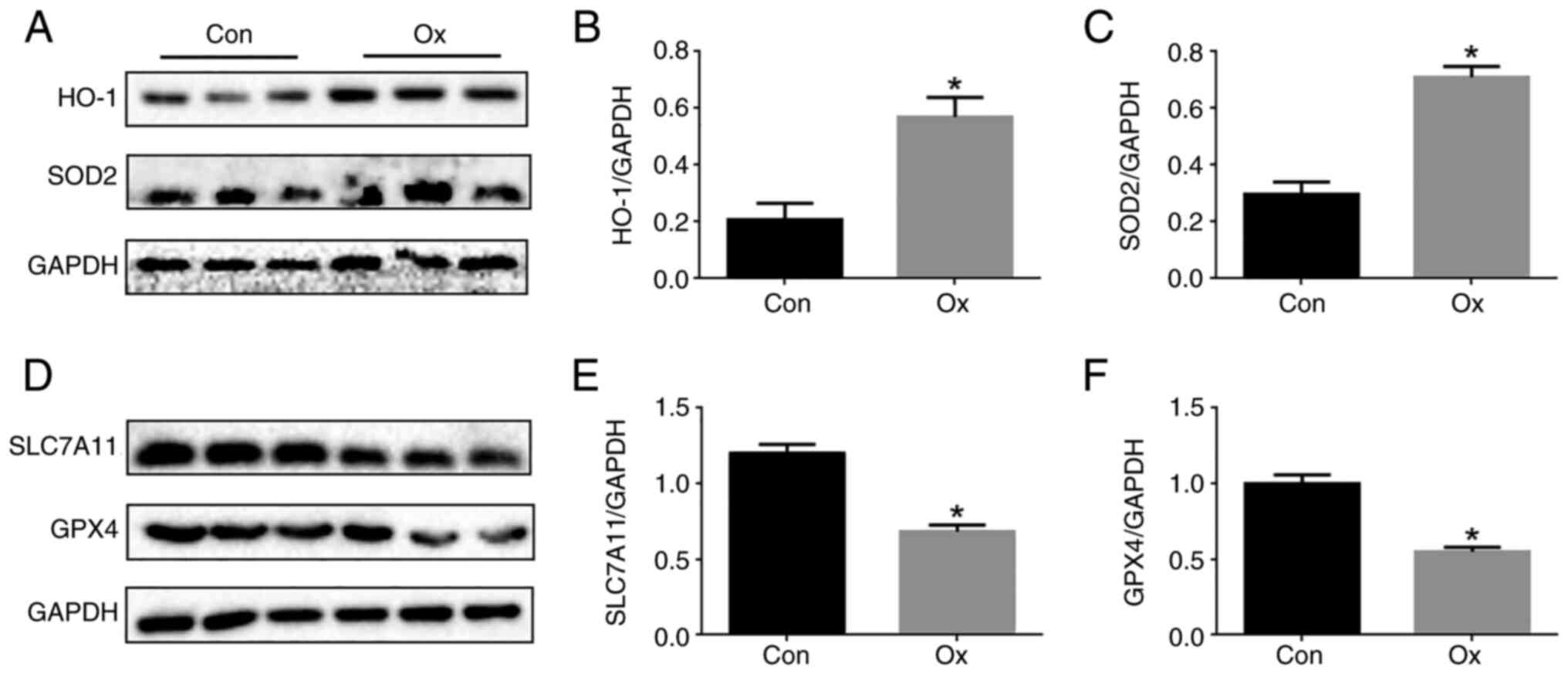
Activation of ferroptosis in Ox
induces injury of renal tubular epithelial cells
This experiment aimed to further explore the role of
ferroptosis in the Ox-induced injury of renal tubular epithelial
cells. Western blot analysis revealed that the levels of HO-1 and
SOD2 in tubular cells in the Ox group were significantly increased
(Fig. 5A-C), and the levels of
GPX4 and SLC7A11 were significantly decreased compared with the
control group (Fig. 5D-F).
Therefore, ferroptosis occurred in the Ox-induced injury of renal
tubular epithelial cells.
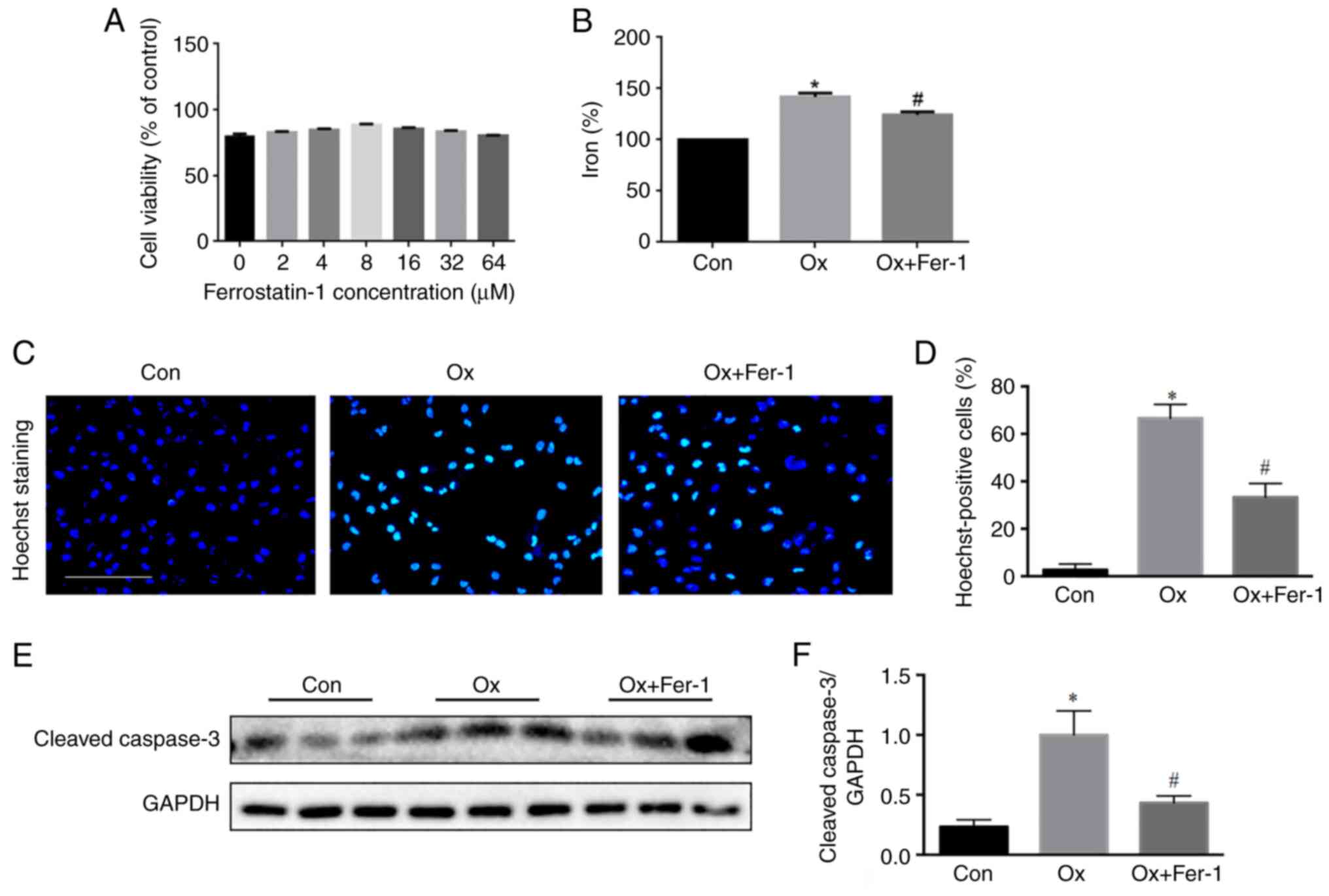
Fer-1 inhibits Ox-induced injury of
renal tubular epithelial cells
Renal tubular epithelial cells were treated with
different concentrations of Fer-1 and 2 mM Ox to explore the
function of Fer-1 in Ox-induced renal tubular epithelial cell
injury. It was determined that the peak protective dose of Fer-1
was 8 µM (P<0.01). Therefore, co-culturing with 8 µM Fer-1 and 2
mM Ox was used in the present study (Fig. 6A). The intracellular iron level
was significantly increased under the stimulation of Ox and
decreased following the Fer-1 treatment (Fig. 6B). Hoechst staining revealed a
higher apoptotic rate of renal tubules in the Ox group than in the
control group. Following Fer-1 treatment, the renal tubular
apoptotic rate was decreased compared with the Ox group (Fig. 6C and D). The results of western
blot analysis of caspase-3 revealed a higher rate of apoptosis of
renal tubules in the Ox group compared with the control group, and
renal tubular apoptosis was decreased in the Ox + Fer-1 group
compared with the Ox group (Fig. 6E
and F).
Fer-1 inhibits epithelial-mesenchymal
transition (EMT) and renal fibrosis following Ox-induced injury of
renal tubular epithelial cells
Through immunofluorescence staining of kidney
tissue, the levels of vimentin and α-SMA were revealed to be
significantly increased in the Ox group while the levels of
E-cadherin were significantly decreased, compared with the control
group. In addition, the levels of vimentin and α-SMA were
significantly decreased, and the levels of E-cadherin were
significantly increased in the Ox + Fer-1 treatment group compared
with the Ox group (Fig. 7A-D).
The results of western blot analysis revealed that the levels of
fibronectin, vimentin and α-SMA were significantly increased and
E-cadherin was decreased in the Ox group, compared with the control
group. Furthermore, the levels of fibronectin, vimentin and α-SMA
were significantly decreased and E-cadherin was increased in the Ox
+ Fer-1 treatment group compared with the Ox group (Fig. 7E-I).
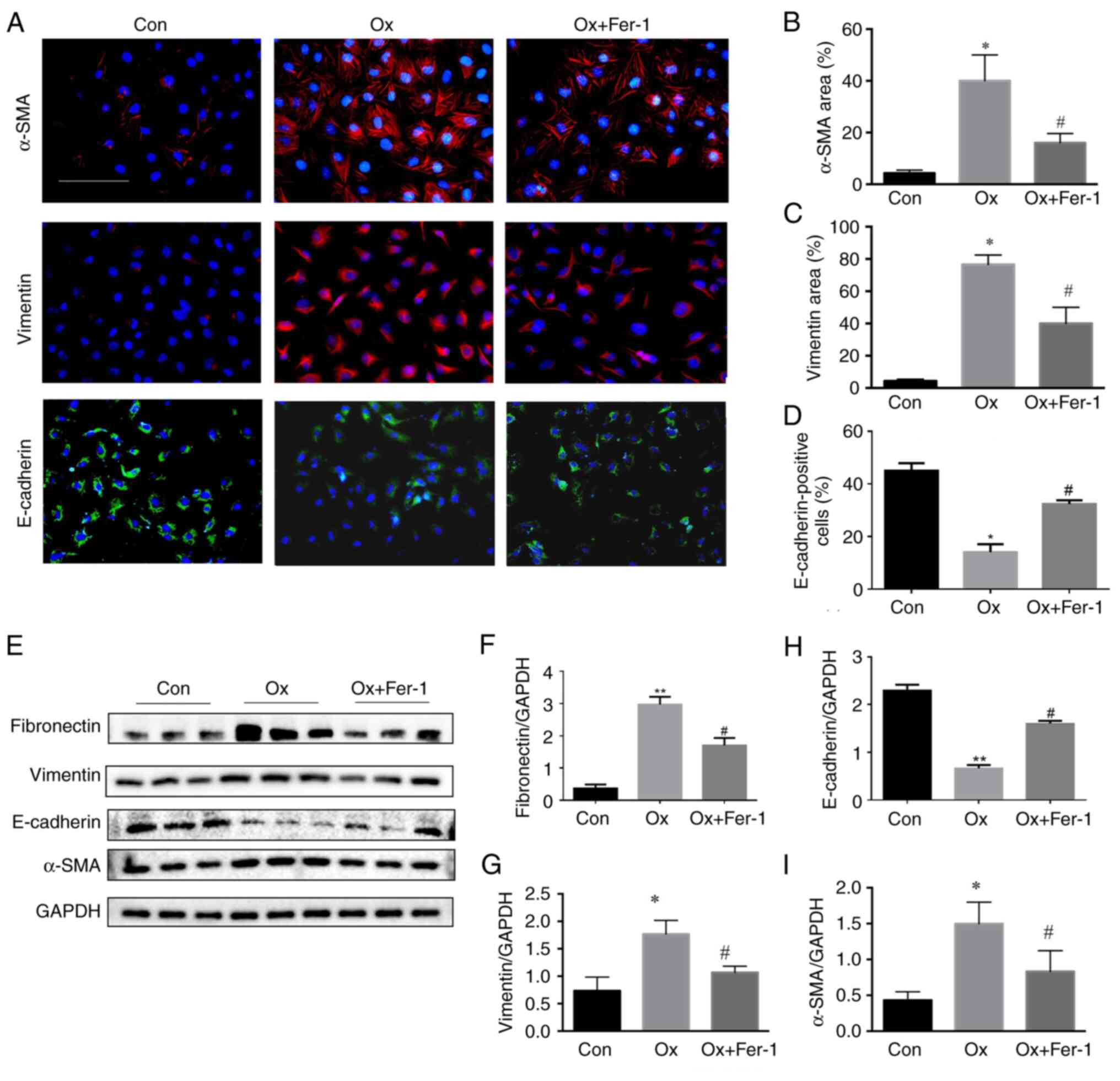 | Figure 7.Fer-1 inhibits EMT and renal fibrosis
following Ox-induced injury of HK-2 cells. Cells were treated with
Fer-1 with or without Ox intervention solution for 24 h. (A)
Representative images of immunofluorescence staining of α-SMA,
vimentin (red) and E-cadherin (green) (magnification, ×400)
following Fer-1 treatment. (B-D) Semi-quantitative statistical
analysis of (B) α-SMA, (C) vimentin and (D) E-cadherin. (E)
Representative images of fibronectin, vimentin, α-SMA and
E-cadherin in HK-2 cells by western blotting. (F-I) Quantification
of (F) fibronectin, (G) vimentin, (H) E-cadherin and (I) α-SMA in
HK-2 cells assessed by western blotting. The data are presented as
the mean ± SD; n=3. *P<0.05 vs. the control group;
#P<0.05 vs. the Ox group. Fer-1, ferrostatin-1; EMT,
epithelial-mesenchymal transition; Ox, oxalate; α-SMA, α-smooth
muscle actin, |
Fer-1 inhibits renal fibrosis after
Ox-induced injury of renal tubular epithelial cells via inhibition
of ferroptosis
The ferroptosis level in renal tubular epithelial
cells was evaluated to analyze the effect of Fer-1. The expression
levels of both SLC7A11 and GPX4 were increased in the Ox + Fer-1
group compared with the Ox group (Fig. 8A-C). Furthermore, the levels of
ROS were highest in the Ox group, followed by the Ox + Fer-1 and
control group (Fig. 8D and E).
Therefore, Fer-1 alleviated Ox-induced injury of renal tubular
epithelial cells by inhibiting ferroptosis, which plays a key role
in renal fibrosis.
Discussion
Ferroptosis is a new type of non-apoptotic
programmed cell death characterized by iron-dependent lipid
peroxidation, which is closely related to the occurrence of blood,
neurological, respiratory, cardiovascular, and reproductive system
diseases and tumors (19–21). However, related studies on the
role of ferroptosis in urinary system diseases have mainly focused
on renal failure and renal tumors (22,23), and to date, limited studies are
available on the topic of urolithiasis formation and the
progression of patients with urolithiasis to CKD. The present
study, to the best of our knowledge, is the first to explore the
pathogenic mechanism of ferroptosis in Ox-induced renal tubular
epithelial cell injury and fibrosis and the role of inhibiting
ferroptosis in improving Ox-induced renal tubular epithelial cell
injury and fibrosis. The mechanism of fibrosis following CaOx stone
renal injury was clarified and the progression of patients with
CaOx stones to CKD was delayed. An in vivo CaOx stone animal
model was established using glyoxylic acid and Ox-induced renal
tubular epithelial cell injury and fibrosis were also established
in vitro. The results of the experiments demonstrated that
Ox promoted lipid peroxidation. Moreover, Ox activated the
ferroptosis-related signaling pathway proteins (HO-1 and SOD2) and
decreased the relative expression levels of SLC7A11 and GPX4. Thus,
renal tubular epithelial cell injury and the degree of ferroptosis
exhibited a positive association with Ox.
To further investigate the association between
ferroptosis and injury to renal tubular epithelial cells induced by
exposure to Ox, the ferroptosis inhibitor, Fer-1, was used. Fer-1
is the first ferroptosis inhibitor, and is widely used in
vitro and in vivo (13,24). The function of Fer-1 against
ferroptosis mainly depends on the inhibition of lipid peroxidation
(25). In the present study,
Fer-1 was applied in both in vitro and in vivo models
and exhibited a marked effect against ferroptosis. Fer-1
significantly decreased the relative expression of the
ferroptosis-promoting signaling pathway proteins, HO-1 and SOD2,
and induced the relative expression of the ferroptosis-inhibiting
signaling pathway proteins, SLC7A11 and GPX4. Ox could induce
ferroptosis, and the increase in ferroptosis levels aggravated
lipid peroxidation levels, leading to an overload of ROS in cells
and an increase in the degree of cell injury. In addition, a
previous study revealed that ferroptosis and apoptosis are closely
linked, that apoptosis can be converted to ferroptosis under
certain conditions, and that ferroptosis promotes the
susceptibility of cells to apoptosis (26). By reducing the degree of
ferroptosis, the damage and death of tubular cells could be
reduced, and this damage to renal tubular epithelial cells plays a
vital role in the formation of CaOx kidney stones (17). Consistent with the results of the
present study, with the increase in the degree of ferroptosis, the
deposition of crystals in the kidneys significantly increased as
was determined by performing Von Kossa staining. These data confirm
the promotive effect of ferroptosis in the formation of
urolithiasis.
Ferroptosis participates in various pathological
models of kidney injury, and in vivo and in vitro
experiments have revealed that ferroptosis inhibitors are effective
against kidney injury (27,28). Mice with GPX4 deletion could
spontaneously develop kidney injury, while GPX4 upregulation
prevented kidney injury (12). In
the kidneys, lipid peroxidation is an important factor that
contributes to the aggravation of kidney injury, particularly in
kidney injury induced by ischemia-reperfusion (29). Hence, classical ferroptosis
inhibitors can interfere with key molecules in the ferroptosis
signaling pathway to resist kidney injury. Notably, ferroptosis
inhibitors are effective against Ox-induced kidney injury in
vivo and in vitro (30,31).
Ferroptosis is involved in multiple pathological
conditions, including renal injury. However, whether ferroptosis is
induced during renal fibrosis and its potential role in renal
fibrosis have not been studied. Repeated and severe episodes of
kidney injury are recognized as major risk factors for the
development of CKD (32). The
inhibition of ferroptosis can mitigate renal fibrosis in CKD rats
by inhibiting TGF-β1/Smad3, inflammation, and oxidative stress
pathways (33). Ferroptosis
played an important role in unilateral ureteral obstruction
(UUO)-induced renal fibrosis, and the ferroptosis inhibitor
attenuated UUO-induced kidney fibrosis by inhibiting
ferroptosis-mediated tubular cell death (34). The ferroptosis-dependent
mechanisms, including GPX4 depletion, lipid peroxidation,
ferritinophagy, and p53, are involved in liver and pulmonary
fibrosis (35,36), and non-targeted agents that
regulate the ferroptosis signaling pathway also exhibit
antifibrotic effects (37).
Therefore, the perspective that ferroptosis is considered a
therapeutic target for organ fibrosis has been gradually accepted.
Current studies focus on the relationship between ferroptosis and
Ox-induced EMT. Ferroptosis inhibitors effectively inhibit
EMT (22,38).
In conclusion, ferroptosis plays an important role
in Ox-induced renal tubular epithelial cell injury, fibrosis and
CaOx stone formation. Moreover, Fer-1 alleviates Ox-induced renal
tubular epithelial cell injury, fibrosis, and CaOx stone formation
via regulating ferroptosis. Therefore, the present study
demonstrated that ferroptosis, a novel form of regulated cell
death, occurred in Ox-induced renal tubular epithelial cell injury,
fibrosis, and CaOx stone formation. Ferroptosis could become a
novel therapeutic target in Ox-induced renal tubular epithelial
cell injury, fibrosis, and CaOx stone formation. Finally, a
ferroptosis inhibitor may be an effective type of drug to delay the
progression of patients with CaOx stones to CKD.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Natural
Science Foundation of China (grant nos. 81800617 and 81870471) and
the Science and Technology Major Project of Hubei Province (grant
no. 2019AEA170).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JX, ZY and XZ conceived and designed the study. LL
and ZY conducted the experiments, analyzed the data and drafted the
manuscript. YX and RY participated in the data acquisition and
analysis. JX and ZY confirm the authenticity of all the raw data.
YR, ZY and XZ provided experimental guidance and participated in
data analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal treatments were approved (approval no.
WDRM-20200604) by the Laboratory Animal Welfare and Ethics
Committee of Renmin Hospital of Wuhan University (Wuhan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thongprayoon C, Krambeck AE and Rule AD:
Determining the true burden of kidney stone disease. Nat Rev
Nephrol. 16:736–746. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buysschaert B, Aydin S, Morelle J, Gillion
V, Jadoul M and Demoulin N: Etiologies, clinical features, and
outcome of oxalate nephropathy. Kidney Int Rep. 5:1503–1509. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lumlertgul N, Siribamrungwong M, Jaber BL
and Susantitaphong P: Secondary oxalate nephropathy: A systematic
review. Kidney Int Rep. 3:1363–1372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steiger S, Grill JF, Ma QY, Bäuerle T,
Jordan J, Smolle M, Böhland C, Lech M and Anders HJ:
Anti-transforming growth factor β IgG elicits a dual effect on CaOx
crystallization and progressive nephrocalcinosis-related chronic
kidney Disease. Front Immunol. 9:6192018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitchell T, Kumar P, Reddy T, Wood KD,
Knight J, Assimos DG and Holmes RP: Dietary oxalate and kidney
stone formation. Am J Physiol Renal Physiol. 316:409–413. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mulay SR and Anders HJ: Crystal
nephropathies: Mechanisms of crystal-induced kidney injury. Nat Rev
Nephrol. 13:226–240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Black LM, Lever JM and Agarwal A: Renal
inflammation and fibrosis: A double-edged sword. J Histochem
Cytochem. 67:663–681. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derry PJ, Hegde ML, Jackson GR, Kayed R,
Tour JM, Tsai AL and Kent TA: Revisiting the intersection of
amyloid, pathologically modified tau and iron in Alzheimer's
disease from a ferroptosis perspective. Prog Neurobiol.
184:1017162020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian
D, Liu D, Zhang F, Ning S, Yao J and Tian X: Ischemia-induced ACSL4
activation contributes to ferroptosis-mediated tissue injury in
intestinal ischemia/reperfusion. Cell Death Differ. 26:2284–2299.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zilka O, Shah R, Li B, Friedmann Angeli
JP, Griesser M, Conrad M and Pratt DA: On the mechanism of
cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of
lipid peroxidation in ferroptotic cell death. ACS Cent Sci.
3:232–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kajarabille N and Latunde-Dada GO:
Programmed cell-death by ferroptosis: Antioxidants as mitigators.
Int J Mol Sci. 20:49682019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin B, Wang Q, Lu Y, Li C, Hu H, Zhang J,
Wang Y, Zhu J, Zhu Y, Xun Y and Wang S: Losartan ameliorates
calcium oxalate-induced elevation of stone-related proteins in
renal tubular cells by inhibiting NADPH oxidase and oxidative
stress. Oxid Med Cell Longev. 2018:12718642018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Zhang H, Yi B, Yang S, Liu J, Hu J,
Wang J, Cao K and Zhang W: VDR activation attenuate cisplatin
induced AKI by inhibiting ferroptosis. Cell Death Dis. 11:732020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song Q, Liao W, Chen X, He Z, Li D, Li B,
Liu J, Liu L, Xiong Y, Song C and Yang S: Oxalate activates
autophagy to induce ferroptosis of renal tubular epithelial cells
and participates in the formation of kidney stones. Oxid Med Cell
Longev. 2021:66303432021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chawla LS and Kimmel PL: Acute kidney
injury and chronic kidney disease: An integrated clinical syndrome.
Kidney Int. 82:516–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Lv H, Zhao B, Zhou L, Wang S, Luo
J, Liu J and Shang P: Iron and leukemia: New insights for future
treatments. J Exp Clin Cancer Res. 38:4062019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masaldan S, Belaidi AA, Ayton S and Bush
AI: Cellular senescence and iron dyshomeostasis in Alzheimer's
disease. Pharmaceuticals (Basel). 12:932019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alvarez SW, Sviderskiy VO, Terzi EM,
Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K
and Possemato R: NFS1 undergoes positive selection in lung tumours
and protects cells from ferroptosis. Nature. 551:639–643. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang S and Xiao X: Ferroptosis and kidney
diseases. Int Urol Nephrol. 52:497–503. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miess H, Dankworth B, Gouw AM, Rosenfeldt
M, Schmitz W, Jiang M, Saunders B, Howell M, Downward J, Felsher
DW, et al: The glutathione redox system is essential to prevent
ferroptosis caused by impaired lipid metabolism in clear cell renal
cell carcinoma. Oncogene. 37:5435–5450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miotto G, Rossetto M, Di Paolo ML, Orian
L, Venerando R, Roveri A, Vučković AM, Bosello Travain V, Zaccarin
M, Zennaro L, et al: Insight into the mechanism of ferroptosis
inhibition by ferrostatin-1. Redox Biol. 28:1013282020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman
M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A and
Stockwell BR: Ferrostatins inhibit oxidative lipid damage and cell
death in diverse disease models. J Am Chem Soc. 136:4551–4556.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan SR: Reactive oxygen species as the
molecular modulators of calcium oxalate kidney stone formation:
Evidence from clinical and experimental investigations. J Urol.
189:803–811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu ZX, Zhang H, Yang SK, Wu XQ, He D, Cao
K and Zhang W: Emerging role of ferroptosis in acute kidney injury.
Oxid Med Cell Longev. 2019:80106142019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Liu Y, Wang Y and Sun L: The
cross-link between ferroptosis and kidney diseases. Oxid Med Cell
Longev. 2021:66548872021.PubMed/NCBI
|
|
29
|
Jiang GP, Liao YJ, Huang LL, Zeng XJ and
Liao XH: Effects and molecular mechanism of pachymic acid on
ferroptosis in renal ischemia reperfusion injury. Mol Med Rep.
23:632021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zou YL and Schreiber SL: Progress in
understanding ferroptosis and challenges in its targeting for
therapeutic benefit. Cell Chem Biol. 27:463–471. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X and Li X: Abnormal iron and lipid
metabolism mediated ferroptosis in kidney diseases and its
therapeutic potential. Metabolites. 12:582022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R,
Cui X, Yang H, Yang Y, Birnbaumer L, et al: Quercetin alleviates
acute kidney injury by inhibiting ferroptosis. J Adv Res.
28:231–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yaito Y, Fujii A, Sawada H, Oboshi M,
Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Hirotani S and Masuyama
T: Association between renal iron accumulation and renal
interstitial fibrosis in a rat model of chronic kidney disease.
Hypertens Res. 38:463–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikeda Y, Ozono I, Tajima S, Imao M,
Horinouchi Y, Izawa-Ishizawa Y, Kihira Y, Miyamoto L, Ishizawa K,
Tsuchiya K and Tamaki T: Iron chelation by deferoxamine prevents
renal interstitial fibrosis in mice with unilateral ureteral
obstruction. PLoS One. 9:e893552014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Zhang Z, Li M, Wang F, Jia Y,
Zhang F, Shao J, Chen A and Zheng S: P53-dependent induction of
ferroptosis is required for artemether to alleviate carbon
tetrachloride-induced liver fibrosis and hepatic stellate cell
activation. IUBMB Life. 71:45–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Z, Yao Z, Wang L, Ding H, Shao J,
Chen A, Zhang F and Zheng S: Activation of ferritinophagy is
required for the RNA-binding protein ELAVL1/HuR to regulate
ferroptosis in hepatic stellate cells. Autophagy. 14:2083–2103.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong Y, Wang N, Liu N and Dong H: Lipid
peroxidation and GPX4 inhibition are common causes for
myofibroblast differentiation and ferroptosis. DNA Cell Biol.
38:725–733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye Z, Xia Y, Zhou X, Li B, Yu W, Ruan Y,
Li H, Ning J, Chen L, Rao T and Cheng F: CXCR4 inhibition
attenuates calcium oxalate crystal deposition-induced renal
fibrosis. Int Immunopharmacol. 107:1086772022. View Article : Google Scholar : PubMed/NCBI
|