COVID-19, caused by novel coronavirus SARS-CoV-2,
has posed a serious threat to human health and public safety
globally with rapid transmission and serious pathogenicity
(1). Novel variants of the
original virus are emerging on a frequent basis. Omicron strain has
rapid speed of transmission and has replaced Delta strain as the
most prevalent currently, causing large scale global infection, it
was first discovered in Johannesburg, South Africa, then identified
in Belgium, Israel, Hong Kong and European countries (2). The Omicron cases in South Africa
peaked at 40,000 per day (Dec 2021), while UK had >100,000 cases
per day (Dec 2021), with Omicron accounting for 90% of all patients
with COVID-19 in London (3,4).
Omicron has been identified in >100 countries and has caused
>1 million daily cases up to 3rd June, 2022 (2).
SARS-CoV-2, a single-stranded RNA β-coronavirus
genus, is enveloped by membrane with polymorphic shape, commonly
round or oval (5). Glycoproteins
on the membrane surface include spike (S) protein, which serves as
a receptor-binding and antigenic sites that trigger cytolysis by
inducing antigenic response (6);
small envelope (E) glycoprotein, which mediates binding to the
envelope; membrane (M) glycoprotein, which is responsible for
nutrient transmembrane transport, budding release of virus and
formation of virus envelope and nucleocapsid (N) protein, which can
be used as a diagnostic antigen and encapsulates the viral genome
(7). As heavily glycosylated S
trimers, S proteins bind to the human angiotensin converting enzyme
2 receptor (ACE2) and mediate viral entry into target human cells,
making S protein the most important in the pathogenesis of
infection (8). SARS-CoV-2 infects
humans by binding to ACE2, which is the same host receptor for
SARS-CoV. SARS-CoV-2 binds ACE2 of respiratory epithelial cells
before multiplying, passing through the airways and finally
entering alveolar epithelial cells (9). Massive viral duplication in the lung
triggers the immune response, which causes aggregation and
accumulation of inflammatory cells, resulting in typical symptoms
of viral pneumonia (10). Acute
pulmonary infection can cause complications, the most severe of
which are acute respiratory distress syndrome (ARDS) and
respiratory failure, which have become the leading cause of death
during the epidemic (11,12). A cohort study of 459 intensive
care units from 50 countries and 5 continents found that the
mortality rate is 26.0-61.5% for patients with ARDS who received
critical care and 65.7-94.0% for patients who received mechanical
ventilation (12).
Similar to other viruses, the novel coronavirus
genome exhibits variations that may alter its biological features.
Changes in the affinity of S protein and ACE-2 may affect viral
invasion of cells, replication and transmission, production of
antibodies during recovery or following vaccination, the
neutralization activity of antibody may be impaired (13). Delta variant (B.1.617.2) exhibits
23 mutations compared with the original strain, 12 of which are in
the novel S protein (14). The
increased number of mutated S proteins make immune recognition and
antibodies attachment more difficult, leading to a higher infection
rate in human cells (15). The
newly reported Omicron strain exhibits a considerable number of
mutations in S protein as well. Among 50 mutations, 23 of them are
in the S protein, preventing antibodies from attaching to S
protein, resulting in increased transmissibility and infectivity
(16).
SARS-CoV-2 appears to be particularly infectious in
crowded places with poor ventilation and the chance of infection
following exposure to SARS-CoV-2 is similar between different age
groups (17), while people appear
to develop a degree of immunity following vaccination or
infection.
Compared with the original strain, variants exhibit
similar epidemiological features. Although there is no conclusive
evidence that Delta variant causes distinct symptoms from Alpha,
patients infected by Delta variant exhibit more rapid onset and
higher viral expression in the respiratory tract (18). It is hypothesized that the
infectiousness of Delta variant is double that of the original
strain, indicating a higher potential infection and death rate
(19). Omicron variant is notably
more contagious compared with Delta as a result of its mutations on
the S protein receptor-binding domain (RBD) (20). Patients infected with Omicron
variant often present with mild symptoms and severe symptoms are
rare (21). In terms of age, it
has a greater impact on young and middle-aged people than previous
variants (22). Omicron also
exhibits greater ability to escape from antibodies, which can lead
to more cases of reinfection and infection following vaccination
(20).
During outbreaks, however, clinical features differ
between age groups. According to a study (23), the incidence of pre-existing
comorbidities such as hypertension, diabetes and cardiovascular
disorder is higher among the elderly (age, >60 years), who may
have more underlying disease and be in poorer physical condition
compared with young and middle-aged groups. Regarding pulmonary
infection and comorbidities, elderly patients have a higher risk of
severe respiratory disease requiring intensive care (24), while younger patients may only
exhibit moderate pneumonia, asymptomatic infection or be less
likely to develop COVID-19 (23).
Additionally, studies have indicated that immunocompromised hosts,
such as patients with HIV or active cancer or receiving high-dose
steroid therapy, may be more likely to develop complications
following infection with SARS-CoV-2 (25,26).
Infected people are the primary source of infection,
as well as asymptomatic patients and patients in latent period
(27). It is hypothesized that
transmission via aerosols is the primary route from infected
patients to non-infected people (28). Patients in latent period without
symptoms also discharge virus particles into the environment at
similar levels to symptomatic patients, which poses a threat to
public safety (29).
Droplets are the primary infective agents of
SARS-CoV-2. When a patient breathes, coughs or sneezes, respiratory
droplets with high viral load are expelled from the mouth and nose
(30). Infected people produce an
aerosol form of SARS-CoV-2 as particles (diameter, <5 µm)
suspended in gas (31). However,
the likelihood of infection depends on the distance between the
source and the susceptible person (32). Short-distance airborne
transmission, including via droplet and aerosol, which is also
known as ‘direct contact’, may serve as the principal pathway of
virus dissemination (33). On the
other hand, ‘indirect contact’ occurs when pathogens exhaled by
carriers of SARS-CoV-2 contaminate objects and infect susceptible
individuals exposed to them (34).
Epidemiological analysis and pathogenesis indicate
the respiratory tract is the primary route of infection (35). ACE2+ cells in the
respiratory tract are viral receptors and may be responsible to
human-to-human transmission (36). Droplets and aerosol carrying the
virus enter the respiratory track of susceptible person, typically
in the form of saliva, sputum and nasal secretion, and begin to
multiply by binding ACE2 (Fig. 1)
(37). Other routes of
transmission such as fecal-oral, mother-to-child and body fluid
transmission are controversial and lack direct, real-word evidence.
Nosocomial infection is a key route of virus transmission. Samples
of item surfaces and air from confirmed patient wards were tested
positive, implying that SARS-CoV-2 can spread in examination rooms
and wards (38), which indicates
the necessity of guarding against infection during clinical
activity requiring open mouth and contact with body fluids, such as
endoscopy, dental treatment and pulmonary function test (39).
Based on an epidemiological survey, the incubation
period for COVID-19 is 1–14 days, with the majority of cases
lasting 3–7 days (40). Fever,
dry cough and exhaustion are reported as the three most prevalent
symptoms, while certain patients exhibit expectoration, nasal
obstruction, runny nose, sore throat, emaciation, hemoptysis,
headache, chest pain, chills, myalgia, gastrointestinal responses
and olfactory and taste disorder (41,42). Vaccinated people or those infected
with Omicron generally present with asymptomatic infection or mild
symptoms, while symptomatic patients primarily manifest with upper
respiratory infection (43).
Dyspnea and hypoxemia are the main manifestations of severely ill
patients and typically develop within one week of symptomatic
presentation (30). Severely ill
patients rapidly develop ARDS, septic shock, coagulopathy,
refractory metabolic acidosis and multiple organ failure. Certain
patients develop central nervous symptom disorder and acral
ischemic necrosis of the extremities (1). Severely or critically ill patients
may present with moderate to low fever, while mild patients present
with slight weariness, odor and taste disturbance, as well as low
fever, but no visible signs or symptoms of pneumonia (44). According to a study in China, mild
cases form the majority of total cases, while severe cases
requiring intensive care and critical cases with life-threatening
emergency complications represented <20% of the study population
(45). As aforementioned, the
risk level of COVID-19 varies by age. In addition, age and male are
risk factors for cardiovascular disease, therefore, elderly men
infected by SARS-CoV-2 may have a higher risk of developing severe
cases with respiratory and circulatory failure, while the young and
middle-aged people may be able to recover in two weeks (44).
In the early phase of COVID-19, peripheral blood
displays lymphopenia and decreased or normal leukocyte count, while
certain patients present with high levels of aspartate
aminotransferase, lactate dehydrogenase, myoglobin, creatine
kinase, troponin and ferritin. In terms of inflammatory indicators,
C-reactive protein and erythrocyte sedimentation are increased in
the majority of patients (9).
Increased expression of D-dimer and decreased peripheral lymphocyte
levels may present in severe cases (46). Hypercytokinemia, characterized by
high expression of cytokines in plasma, is common in severely and
critically ill patients, potentially resulting in death within 16
days of disease onset (47).
Computerized tomography (CT) scanning of the patient
chest commonly reveals bilateral patchy shadow or ground-glass
opacity with subpleural, centrilobular and diffused distribution
(48). The tiny shadows rapidly
expand into a scattered distribution as the disease progresses.
Organizing pneumonia and fibrosis are primarily observed in the
later stages of COVID-19 without pleural effusion (49).
Histological examination shows bilateral diffuse
alveolar injury with mucinous exudation of cellular fibers,
desquamation of pneumocytes and fibrin deposits and hyaline
membrane formation, which indicate the occurrence of ARDS (50). In the course of COVID-19,
inflammatory infiltration of mononuclear cells, primarily
lymphocytes, occurs in alveoli, which indicates that directional
aggregation of lymphocytes may lead to peripheral lymphopenia.
Alveolar cells exhibit large nuclei, double cytoplasmic granules
and obvious nucleoli, indicating cytotoxic alteration, which is
also observed in other organs, such as spleen, hilar lymph node,
bone marrow, heart and blood vessels, liver, gallbladder, kidney,
adrenal gland, alimentary epithelial cells, brain and testicles
(51).
Nucleic acid detection is primarily used for
etiological examination. By serological examination, specific IgM
induced by SARS-CoV-2 infection can be detected; positive results
of IgG antibody may be seen within the first week of onset
(52).
A cohort study of patients recovering from COVID-19
reported symptoms including weariness, muscle discomfort, sleeping
difficulty and psychological problems such as anxiety or depression
6 months after the onset of COVID-19. Severely ill patients
exhibited more obvious symptoms following recovery; in addition to
impaired pulmonary diffusion function and damaged (as revealed by
chest imaging), cardiovascular, nervous, digestive, urinary and
immune symptoms were observed (53,54). Therefore, recovery following
COVID-19 still requires long-term epidemiological
investigation.
During the Omicron epidemic, RT-qPCR nucleic acid
detection was used as the gold standard for diagnosis of COVID-19
(55). For unvaccinated patients,
detection of specific antibodies such as IgM and IgG are used as a
diagnostic reference. However, for those who have been vaccinated
or have a history of previous infection, these antibodies may not
have diagnostic value (56).
Omicron appears to cause more asymptomatic cases and
patients with mild symptoms or in the incubation period can lead to
wide and undetected viral spread (57). Therefore, early identification and
close monitoring of suspected cases are key for public protection
against Omicron. Based on current studies (55,57), symptoms caused by SARS-CoV-2
variants vary between infected individuals, which yields
limitations in common detection methods. Therefore, absence of
respiratory symptoms and pulmonary inflammation or negative PCR
test do not mean the patient is non-infectious, indicating that
early recognition is critical during clinical tests (58). Diagnostic techniques must be
updated to be more sensitive and adaptable to emergence of novel
variants.
RT-qPCR is used for rapid detection of SARS-CoV-2.
Compared with next-generation gene sequencing (NGS), it is faster
and cheaper, provides clear results and has a larger sample
capacity (59). Lower respiratory
tract samples exhibit higher viral load compared with samples from
other sources, with oral and nasopharyngeal swabs being most
convenient and commonly used (60). Negative RT-qPCR results from
respiratory samples have been obtained while positive results were
obtained from intestinal canal and peripheral blood (61), indicating that isolation of live
viruses is key to assess virus reproduction (62). The outcome of RT-qPCR nucleic acid
detection tends to be inadequate due to complicating factors
(57). False negative results can
be due to virus mutations that make primers and probes difficult to
recognize, low viral load in samples caused by viral mutations or
non-standard sampling, different detection reagents and poor
quality control (63,64). Therefore, screening hospital
patients via RT-qPCR may be insufficient and multiple approaches
are needed for confirmation of the novel coronavirus. Specific
primers targeting key mutations in S protein rapidly recognize
variants of concern that may differ from previous Omicron mutations
(65). Metagenomic NGS should
also be used to analyze nucleic acid, especially for patients who
may be infected with a SARS-CoV-19 variant (66). Loop-mediated isothermal
amplification, which is highly specific for mutations, detection
with 6–8 specific primer sequences, may be substitute for a RT-qPCR
diagnosis (67). Contact tracing
is required to avoid the omission of potential transmitters and
help to cut off the transmission route.
Serology can be used for diagnosis along with
RT-qPCR. Antibody-based techniques are not recommended for early
detection owing to the long period for inducing antibody responses,
while antigen-based immunoassays such as ELISA assess immune
response and disease progression by detecting N or S protein on
antibodies (68). However,
serology cannot exclude the effects of cross-reactivity caused by
factors such as muramidase, rheumatoid factors and heterophile
antibodies (69). The intensity
and duration of immune responses may differ between individuals and
disease stage and serological tests exhibit varying sensitivity and
specificity, creating obstacles to their application, while
biosensor technologies may improve the specificity and sensitivity
of diagnosis (70).
Chest X-ray or CT imaging are also used as
diagnostic techniques for the novel coronavirus (71). Bilateral ground-glass opacity is
indicative of SARS-CoV-2. The sensitivity of chest CT has been
proven as it accurately diagnoses infection in the presence of
negative RT-qPCR results (72).
Therefore, chest CT combined with repeated RT-qPCR may be a
reliable technique for suspected cases with negative initial
RT-qPCR detection (73). However,
improper technique and atypical manifestation can result in false
negatives (71).
Recently, artificial intelligence (AI) as an
emerging technology for interpreting chest imaging and quick
diagnosis of COVID-19 has been widely discussed (74–76). AI applications are used in imaging
platforms, region segmentation for lung infection, clinical
assessment and auxiliary diagnosis based on meta-analysis (74). Based on its operational principles
of interpreting chest imaging and quick diagnosis (77), it may also contribute to clinical
and basic research associated with SARS-CoV-2, in addition to
assisting diagnosis in clinical practice. Accurately distinguishing
COVID-19 from other respiratory disease improves the efficiency of
diagnoses and simplifies workflow, which primarily depends on
manual work of radiologists, providing more accurate results and
maintaining safety of medical staff during examination (75). At this stage, AI diagnosis still
needs to improve image acquisition and expand sample capacity,
which is the foundation of segmentation and diagnosis (76). For physicians, AI results of chest
imaging must be considered in light of clinical manifestation and
laboratory examination (78).
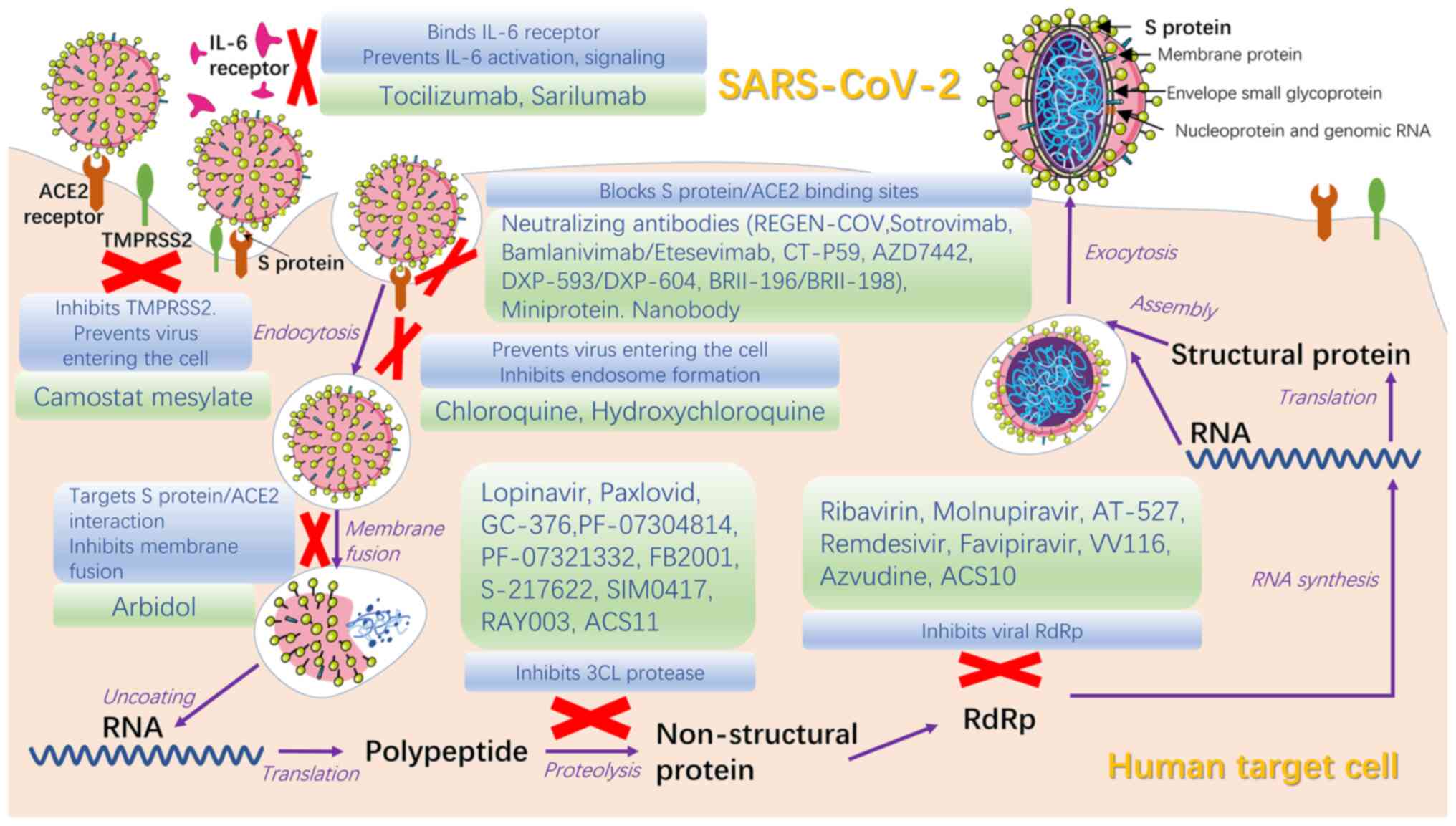
Currently, numerous specific targeted medicines have
passed phase III clinical trials and proven to have therapeutic
efficacy against COVID-19, despite preliminary or controversial
results of clinical trials. Existing antiviral drugs and their
combination are recommended, while extensive tests are needed to
demonstrate the effectiveness and pharmacokinetic and safety
profiles of specific targeted drugs before widespread use as a
therapy for COVID-19 (Table I)
(79).
Antiviral medicines for SARS-CoV-2 can be divided
into two groups based on the molecular mechanism: Drugs targeting
viral protein or RNA and drugs targeting host protein or biological
processes that allow viral entry into cells (80). Antiviral therapies should be used
in the early stage of disease, especially for patients with higher
risk of developing severe illness, as early intervention is more
effective compared with treatment in severe cases (81).
Interferon, which confers congenital immunity to
viruses, induce the expression of antiviral proteins (AVPs) such as
2′-5′A synthase and protein kinase to impede viral replication
(82). Interferon-α has been
demonstrated by studies to be effective against SARS-CoV and may be
more sensitive against SARS-CoV-2 (83). COVID-19 guidelines in China
recommend interferon-α as an antiviral drug (84). Interferon-β has proven useful in
certain trials (85,86) but further studies are needed to
evaluate its effectiveness in high-risk cases.
Protease inhibitors lopinavir and ritonavir were the
first drugs used in clinical trials to target Mpro/3CLpro (87), the primary protease of SARS-CoV-2
that inhibits activation of IFN-α pathway and facilitates natural
immune escape and massive viral replication (88). Although lopinavir/ritonavir had no
significant therapeutic effectiveness for patients with COVID-19,
they are more effective when combined with other drugs such as
ribavirin and interferon (89).
Drugs to inhibit Mpro, such as GC-376, PF-07304814 and PF-07321332,
are in different stages of clinical trials to confirm their
effectiveness and practicability for application worldwide
(90,91). Paxlovid, an oral drug combined
with PF-07321332 and nirmatrelvir, was released by Pfizer, US in
2021; it has a significant effect against COVID-19 and is used to
treat adults and children aged >12 years with mild/moderate
disease, as well as those at high risk of transforming to severe
cases (92). Chloroquine and
hydroxychloroquine, antimalarial drugs, are potential but
controversial drugs in COVID-19 treatment. Biological studies have
proven the effect of hydroxychloroquine on controlling viral load
but clinical trials have reported side effects and no significant
therapeutic benefit (93,94). High-dose chloroquine for COVID-19
treatment is not recommended due to its toxic side effects,
including increased levels of liver enzymes, corrected QT level
prolongation and increased death rate (95,96).
Umifenovir (Arbidol) is a broad-spectrum antiviral
drug for treatment of influenza that targets the interaction
between S protein and ACE2; it has been shown to inhibit membrane
fusion, thereby inhibiting virus diffusion into host cells
(97,98). Clinical data show that it is more
effective compared with lopinavir/ritonavir (89,99), although certain clinical trials
have shown contrary results on patients with mild/moderate COVID-19
(100). GeLactoferrin targets
heparan sulfate proteoglycans to prevent viral attachment to cells
(101). Studies showed that
lactoferrin combined with remdesivir has effects against COVID-19
(102,103), providing a basis further
investigation in the treatment of clinical cases. Camostat
mesylate, developed for treatment of pancreatitis, has been
revealed to block virus entry into lung cells (104).
Inhibitors of viral RNA include remdesivir
(GS-5734), favilavir (T-705), molnupiravir (MK-4482/EIDD-2801),
AT-527, merimepodib and PTC299; their effectiveness for COVID-19
treatment requires investigation (105). Remdesivir, a broad-spectrum
antiviral medicine developed for Ebola virus infection, is the
first drug to be accepted for clinical trials of COVID-19 treatment
(106). The effect of remdesivir
is unknown and the high price and intravenous (IV) route of
administration prevent its widespread use (107). Molnupiravir is the first orally
available drug for COVID-19 that has broad-spectrum anti-RNA virus
activity. Early use of molnupiravir for COVID-19 outpatients
decreases risk of hospitalization or death (108). AT-527 is also an orally
available drug which need further investigation for COVID-19
treatment (109).
By inhibiting host proteins that support viral RNA,
drugs such as plitidepsin, fluvoxamine and ivermectin, may be
potential treatments for COVID-19 (100). Based on biological studies and
mouse experiments (110,111), plitidepsin may exert greater
antiviral effects than remdesivir and its safety has been proven in
a number of cancer clinical trials (112,113). Fluvoxamine, an antidepressant,
was previously suggested to be associated with decreased plasma
levels of certain inflammatory mediators and to prevent viral
infection of epithelial cells (114). Whether ivermectin decreases risk
of SARS-CoV-2 infection is still uncertain and needs further
investigation (115).
Convalescent plasma (CP), a blood-derived product
obtained from patients who have recovered from COVID-19, has been
shown to limit viral expression and modify the inflammatory
response (116). It has proven
to be an effective COVID-19 treatment by randomized controlled
trials and retrospective studies and high-titer CP may have a more
significant effect compared with low-titer CP (117,118). It is more effective in severely
or critically ill patients with rapid progression of illness
(119). The rate of adverse
events is low, but there is still the possibility of enhanced
infection mediated by antibodies and acute lung damage or allergic
reactions associated with transfusion (120).
High-dose intravenous immunoglobulin (IVIg) is a
blood-derived product from patients who have recovered from
COVID-19 and is used as a treatment for severely and critically ill
patients (121). Patients with
ARDS or those on mechanical ventilation support may benefit from
IVIg137 treatment. This therapy is usually not used alone but
combined with other therapies, such as CP and antiviral drugs, to
obtain greater clinical effect (122).
Monoclonal antibodies (mAbs) have been shown to
neutralize COVID-19 infection both in vitro and in
vivo (123,124). Despite the problems of
bioavailability, high cost and limited supply using current
technology, they may have wider clinical applications due to their
ability of self-replicate, which CP does not possess (125). Severely ill patients commonly
present with overexpression of IL-6 and cytokine storms in their
serology profile; therefore, the inflammatory response may be
alleviated by decreasing expression of IL-6 (126). Tocilizumab and sarilumab, high
affinity antibodies for IL-6 receptor that are commonly used to
treat arthritis and cytokine release syndrome, decrease the
inflammatory response in COVID-19 (127). Tocilizumab was found to have no
notable benefit for moderately ill patients in terms of decreased
risk of transition to severe illness or death, while a multi-center
study of critically ill patients revealed that early use of
Tocilzumab may contribute to extended survival period (128). REGEN-COV, a mAb cocktails of
neutralizing antibodies casirivimab and imdevimab, has an effect on
preventing the aggravation of COVID-19 and decreases risk of
hospitalization and death for patients with COVID-19 in high-risk
groups (129). In addition,
subcutaneous injection of REGEN-COV is effective for post-exposure
prophylaxis (130). Clinical
trials have shown that REGEN-COV may have an antagonistic or
synergistic action in combination with anti-inflammatory
medications with diverse mechanisms of action; this requires
further investigation (129).
CT-P59, a fully human anti-SARS-CoV-2 mAb, has high binding
affinity for RBD in S protein and prevents interaction with ACE2,
which is key to prevent the virus from entering human cells
(131). Experiments into the
effect of sarilumab and bevacizumab on COVID-19 are ongoing
(132,133). DXP-593 (based on SARS-CoV
neutralizing mAb) had not meet the endpoint of validity on phase II
trials and its action mechanism remains unknown (134,135).
Nanobodies (alpaca-derived antibodies), miniproteins
(artificially designed proteins), human soluble ACE2 and ACE2
receptor traps can inhibit S protein and have shown potential
therapeutic effects; owing to their diverse biological mechanisms,
their effectiveness in treatment needs to be confirmed by clinical
trials (81).
Corticosteroids relieve the inflammatory response
caused by infection via anti-inflammatory and immunoregulatory
effects (141). However, studies
have shown conflicting results regarding its clinical effects
(142–144). For dexamethasone, certain
studies have shown decreased death rate and notable benefits
especially in severely or critically ill patients receiving
invasive mechanical ventilation (145,146), while other studies showed higher
death rate and multiple organ dysfunction (147,148). In consideration of rebound
phenomena, withdrawal reaction and side effects of corticosteroid
therapy, short-term use (3–7 days) is recommended to begin within
ten days for patients exhibiting rapid disease progression
(144). Attention should also be
paid to the dose of corticosteroids; excessive dose may lengthen
the time of viral elimination owing to its immunosuppressive effect
and induce adverse effects (144).
Active measures, such as prevention and treatment
for complications, treatment for primary illness, prevention of
secondary infection and timely application of organ function
support, which are therapeutic principles for severe and critical
cases, are also required (81).
Airway management is required to improve
humidification of the airway and use of active heating humidifier
and close sputum aspiration are recommended. To promote sputum
drainage and lung rehabilitation, airway clearance treatment should
be performed as early as possible while maintaining stable
oxygenation and hemodynamics (151). Extracorporeal membrane
oxygenation should be applied as soon as possible when meeting the
indications and with no contraindication (152).
Critically ill patients can develop shock as a
complication. Vasoactive medication should be used in addition to
adequate fluid resuscitation. Changes in blood pressure, heart rate
and urine volume, as well as lactic and alkaline residue, must be
closely monitored, and hemodynamic monitoring should be performed
to guide infusion and use of vasoactive drugs to promote tissue
perfusion (149).
Severely and critical patients may be associated
with a prothrombotic state, which increases risk of
life-threatening venous thromboembolism (153). Therefore, prophylactic use of
anticoagulant therapy is recommended for patients with
significantly increased levels of D-dimer with no
contraindications.
Critically ill patients who present with acute
kidney damage may need continuous renal replacement therapy. The
balance of water-electrolyte and acid-base must be closely
monitored for adverse events such as hypoperfusion and medication
(154).
Adsorption, perfusion, plasmapheresis, blood/plasma
filtration and other blood purification systems remove inflammatory
components and minimize the cytokine storm; these serve as an early
or middle-stage therapy for cytokine storm in severe or critical
cases (155).
For COVID-19 child patients with multisystem
inflammatory syndrome, multidisciplinary cooperation of management
is required; treatment for early inflammation, shock, coagulation
dysfunction, organ failure and infection should be administered as
necessary. Patients with COVID-19 with typical or atypical Kawasaki
disease phenotypes are treated similarly to the classic treatment
regimen for Kawasaki disease, with IVIgG, glucocorticoids and oral
aspirin being the most common treatment (156). Intestinal microecological
regulators maintain intestinal microecological balance and prevent
secondary bacterial infection (157).
Traditional Chinese medicine (TCM) is used to treat
and prevent infectious disease, including COVID-19. TCM therapies
have shown therapeutic effects at every stage of the disease with
wide application and no reported cases of exacerbation, even in the
epidemic caused by Omicron (158). In China, TCM therapies such as
decoction, patent medicine and acupuncture are used by >90% of
the population (159). In TCM,
SARS-COV-2 is classified as ‘epidemic disease’ based on its
transmission and clinical features. TCM divides the disease into
medical observation and clinical treatment periods that are
classified as four stages (mild, general, severe, critical)
depending on the severity of disease. Numerous TCM principles and
therapies are recommended in China and are usually combined with
western therapies in clinical treatment to maximize therapeutic
effect (160,161).
Vaccination may be the most effective method of
overall long-term control of the novel coronavirus. Currently,
development of effective vaccines is urgently required to decrease
viral infection and provide protection for public health (162). A total of >100 vaccines have
been developed based on a range of molecular platforms, such as
DNA, mRNA in lipid nanoparticles, inactivated and live attenuated
virus, protein subunits and recombinant vectors (163). A number of vaccines have
exhibited good immunogenic effects in both clinical trials and
real-world data (164,165). To March 2022, ten vaccines have
been added to the World Health Organization Emergency Use Listing
(EUL), while 20 new vaccines are undergoing EUL evaluation and
prequalification (166).
mRNA-based vaccines include mRNA-1273 (Moderna) and
BNT162b2 (BioNTech SE/Pfizer). The number of binding sites on
SARS-CoV-2 S protein is associated with neutralizing antibody
production; protein vaccines exhibit a similar association with
neutralizing antibody response (167). mRNA-1273, a lipid
nanoparticle-formulated mRNA vaccine that targets S protein of the
novel coronavirus, induces a strong neutralizing antibody response
(126). CVnCoV (CureVac) is a
candidate mRNA vaccine that decreases strong T-cell responses and
prevents viral replication in lung of hamsters exposed to wild-type
SARS-CoV-2 (168). However, a
phase IIb/III trial reported an overall efficacy of 48.2% in all
stages of disease and all age groups, which was lower than expected
(169). ARCT-154 (Arcturus
Therapeutics), the first self-amplifying RNA vaccine, is the third
mRNA the third most effective vaccine after Pfizer/BioNTech SE and
Moderna. It uses viral self-replicating behavior to continuously
express viral protein in large quantities. Compared with
conventional mRNA vaccines, ARCT-154 express higher levels of S
protein and induces increased production of neutralizing
antibodies, stronger T cell response and T helper cell 1 and 2
immune responses (170).
However, this self-replication is difficult to control and RNA
interference may be required to inhibit overexpression of viral
protein (162). A clinical trial
in Vietnam showed that ARCT-154 met its immunogenicity primary
endpoint and remains effective against Delta and Omicron with a
protection rate of 95.3% in severe cases (170).
Inactivated virus vaccines, such as BIBP-CorV
(Sinopharm) and CoronaVac (Sinovac Biotech), target the whole
virus, while other types of vaccines use S protein as a target
antigen. A clinical trial in China has shown high neutralizing
antibody production with a low rate of adverse effects induced by
inactivated vaccines WIBP and BIBP and protective efficiency
>72% in a successful phase III trial (171). Recently, a cohort study in
Singapore involving 52,709,899 people double-vaccinated with
mRNA1273 (23% of participants), BNT162b2 (74%), CoronaVac (2%) or
BIBP-CorV (1%) showed that the effectiveness of mRNA vaccines
(mRNA1273 and BNT162b2; 96 and 90% efficiency, respectively) was
higher than that of inactivated virus vaccines (BIBP-CorV and
CoronaVac; 84 and 54% efficiency, respectively) (172).
Against the prevailing variant strains, all vaccines
exhibit notable efficacy against infection with good tolerability
(163). People vaccinated with
BNT162b2 or mRNA-1273 may exhibit the highest efficacy following
full-course inoculation (173,174). A meta-analysis of real-world
data (175) showed that the
observed effectiveness of Pfizer/BioNTech was 91.2%, Moderna was
98.1% and CoronaVac vaccine was 65.7%. CoronaVac. AZD1222 mRNA
vaccine also decreases the rate of severe infection caused by
SARS-CoV variants.
Live-attenuated vaccines merit further investigation
due to their low cost, strong immunogenicity and long-lasting
immune effect. ∆3678 SARS-CoV-2, as a potential candidate for
COVID-19 vaccine, has showed validity to a certain extent in mouse
models (176). The Bacillus
Calmette-Guérin vaccine has showed indirect protection against
COVID-19 and live attenuated Varicella Zoster vaccine has proven to
decrease risk of infection using multivariate logistic regression
analysis (177). DNA vaccines
include AZD1222/ChAdOx1 (Oxford/AstraZeneca),
JNJ-78436735/AD26.COV2.S (Janssen/Johnson & Johnson), Ad5-nCoV
(CanSino Biologics) and ChAdOx1nCoV19 (Covishield) (166). Protein subunit vaccines have
also been investigated; S-Trimer (SCB-2019) may be a candidate as
it induces neutralizing antibody responses and has an acceptable
safety assessment result (178).
ZF2001, a recombinant tandem-repeat dimeric RBD-based protein
subunit vaccine, is well-tolerated and induced a good immune effect
in phase I and II trials and may be a candidate protein subunit
vaccine (179,180).
Adverse effects of vaccines can be considered to
indicate antigenicity and immunogenicity, implying effective
induction of immune responses, and severe adverse events caused by
vaccination are rare (181).
Aside from normal short-term effects such as fever, rash, weakness,
nausea, vomiting, drowsiness, insomnia, pain and induration at
injection site and lymphadenectasis similar to ordinary vaccines
(182,183), the long-term side effects are
unknown due to the short period of monitoring. People with strong
immune responses may be susceptible to higher risk of autoimmune
disease following vaccination, which is similar to other vaccines
(184).
Adherence to hygiene guidelines is still required
following vaccination because there is a delay between vaccination
and optimal level of immunity; this differs between vaccines
(185). Increased asymptomatic
cases and emergence of variants increase risk of infection during
development of vaccine-induced immunity, indicating the necessity
of following hygiene guidelines (20).
Breakthrough infection of fully vaccinated people
occurs in rapid spread of Omicron with high infectivity (186). Studies show that serum
polyclonal antibody responses induced by vaccination or natural
infection may be less effective against Omicron, which may account
for immune failure and high levels of breakthrough infection with
Omicron (187,188). Moreover, vaccine-induced
protection decreases and while patients with breakthrough
infections are more likely to have mild symptoms that do not
require hospitalization compared with unvaccinated patients
(189). Therefore, additional
vaccine doses, changes in vaccine formulation or intervention
should be adopted to when breakthrough infection cases increase, as
well as further studying SARS-CoV-2 variants.
COVID-19 vaccines face challenges. Vaccines limit
viremia and infection-associated syndromes via IgG response but do
not involve IgA response in local mucosa, which is associated with
virus transmission (163).
Therefore, the possibility of transmission via droplets expelled
from asymptomatic vaccinated patients cannot be ruled out and
reinfection following vaccination is also a challenge (20,33). Global strategies are required for
affordable global vaccination. Vaccine uptake presents a challenge
owing to the poor public understanding and trust of vaccines and
regional policies. Ethical and logistical considerations, such as
clinical trials, distribution, prioritization, cultural, religious
and political factors and regulation, are also challenges to
achieving herd immunity (190).
To date, SARS-CoV-2 has been widespread in all
regions with highly infectious Omicron variant posing a novel
threat to global public health. Real-time guidance and
epidemiological analysis should be shared to strengthen global
cooperation against the epidemic. At present, the specific
pathogenicity and response strategies of COVID-19 are uncertain,
requiring further research.
Not applicable.
The present study was supported by The Traditional Chinese
Medicine Science and Technology Development Plan Project of
Shandong Province, China (grant no. 2019-0231).
Not applicable.
ZQ, CH and YC conceived and designed the review,
ZQ, CH, JZ and YS wrote the manuscript. YS and JZ prepared the
figures. LZ and YC performed the literature search. LZ and CH
revised it critically for important intellectual content. All
authors have read and approved the final manuscript. All authors
are responsible for all aspects of the work and approve the
submission in its current form. Data authentication is not
applicable
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scott L, Hsiao NY, Moyo S, Singh L,
Tegally H, Dor G, Maes P, Pybus OG, Kraemer MUG, Semenova E, et al:
Track omicron's spread with molecular data. Science. 374:1454–1455.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dyer O: Covid-19: South Africa's surge in
cases deepens alarm over omicron variant. BMJ. 375:n30132021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Callaway E and Ledford H: How bad is
Omicron? What scientists know so far. Nature. 600:197–199. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giovanetti M, Benedetti F, Campisi G,
Ciccozzi A, Fabris S, Ceccarelli G, Tambone V, Caruso A, Angeletti
S, Zella D and Ciccozzi M: Evolution patterns of SARS-CoV-2:
Snapshot on its genome variants. Biochem Biophys Res Commun.
538:88–91. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S,
Zhang Q, Shi X, Wang Q, Zhang L and Wang X: Structure of the
SARS-CoV-2 spike receptor-binding domain bound to the ACE2
receptor. Nature. 581:215–220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Liu Q and Guo D: Emerging
coronaviruses: Genome structure, replication, and pathogenesis. J
Med Virol. 92:418–423. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ke Z, Oton J, Qu K, Cortese M, Zila V,
McKeane L, Nakane T, Zivanov J, Neufeldt CJ, Cerikan B and Lu JM:
Structures and distributions of SARS-CoV-2 spike proteins on intact
virions. Nature. 588:498–502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta P, McAuley DF, Brown M, Sanchez E,
Tattersall RS and Manson JJ; HLH Across Speciality Collaboration
UK, : COVID-19: Consider cytokine storm syndromes and
immunosuppression. Lancet. 395:1033–1034. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thacker VV, Sharma K, Dhar N, Mancini GF,
Sordet-Dessimoz J and McKinney JD: Rapid endotheliitis and vascular
damage characterize SARS-CoV-2 infection in a human lung-on-chip
model. EMBO Rep. 22:e527442021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Batah SS and Fabro AT: Pulmonary pathology
of ARDS in COVID-19: A pathological review for clinicians. Respir
Med. 176:1062392021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellani G, Laffey JG, Pham T, Fan E,
Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley
DF, et al: Epidemiology, patterns of care, and mortality for
patients with acute respiratory distress syndrome in intensive care
units in 50 countries. JAMA. 315:788–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harvey WT, Carabelli AM, Jackson B, Gupta
RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A; COVID-19
Genomics UK (COG-UK) Consortium, ; et al: SARS-CoV-2 variants,
spike mutations and immune escape. Nat Rev Microbiol. 19:409–424.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bian L, Gao Q, Gao F, Wang Q, He Q, Wu X,
Mao Q, Xu M and Liang Z: Impact of the delta variant on vaccine
efficacy and response strategies. Expert Rev Vaccines.
20:1201–1209. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sternberg A and Naujokat C: Structural
features of coronavirus SARS-CoV-2 spike protein: Targets for
vaccination. Life Sci. 257:1180562020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoffmann M, Krüger N, Schulz S, Cossmann
A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS,
Winkler MS, et al: The omicron variant is highly resistant against
antibody-mediated neutralization: Implications for control of the
COVID-19 pandemic. Cell. 185:447–456.e11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS,
Myoung J, Kim BT and Kim SJ: Current status of epidemiology,
diagnosis, therapeutics, and vaccines for novel coronavirus disease
2019 (COVID-19). J Microbiol Biotechnol. 30:313–324. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiehzadegan S, Alaghemand N, Fox M and
Venketaraman V: Analysis of the delta variant B.1.617.2 COVID-19.
Clin Pract. 11:778–784. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kannan SR, Spratt AN, Cohen AR, Naqvi SH,
Chand HS, Quinn TP, Lorson CL, Byrareddy SN and Singh K:
Evolutionary analysis of the delta and delta plus variants of the
SARS-CoV-2 viruses. J Autoimmun. 124:1027152021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Wang R, Gilby NB and Wei GW:
Omicron variant (B.1.1.529): Infectivity, vaccine breakthrough, and
antibody resistance. J Chem Inf Model. 62:412–422. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Yin XG, Wen Y, Lu J, Zhang RY,
Zhou SH, Liao CM, Wei HW and Guo J: MPLA-adjuvanted liposomes
encapsulating S-trimer or RBD or S1, but not S-ECD, elicit robust
neutralization against SARS-CoV-2 and variants of concern. J Med
Chem. 65:3563–3574. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meo SA, Meo AS, Al-Jassir FF and Klonoff
DC: Omicron SARS-CoV-2 new variant: Global prevalence and
biological and clinical characteristics. Eur Rev Med Pharmacol Sci.
25:8012–8018. 2021.PubMed/NCBI
|
|
23
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al-Shamsi HO, Alhazzani W, Alhuraiji A,
Coomes EA, Chemaly RF, Almuhanna M, Wolff RA, Ibrahim NK, Chua MLK,
Hotte SJ, et al: A practical approach to the management of cancer
patients during the novel coronavirus disease 2019 (COVID-19)
pandemic: An international collaborative group. Oncologist.
25:e936–e945. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grasselli G, Zangrillo A, Zanella A,
Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G,
Fumagalli R, et al: Baseline characteristics and outcomes of 1591
patients infected with SARS-CoV-2 admitted to ICUs of the lombardy
region, Italy. JAMA. 323:1574–1581. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Halpin DMG, Faner R, Sibila O, Badia JR
and Agusti A: Do chronic respiratory diseases or their treatment
affect the risk of SARS-CoV-2 infection? Lancet Respir Med.
8:436–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo C, Yao L, Zhang L, Yao M, Chen X, Wang
Q and Shen H: Possible transmission of severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) in a public bath center in
Huai'an, Jiangsu Province, China. JAMA Netw Open. 3:e2045832020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan JF, Yuan S, Kok KH, To KK, Chu H,
Yang J, Xing F, Liu J, Yip CC, Poon RW, et al: A familial cluster
of pneumonia associated with the 2019 novel coronavirus indicating
person-to-person transmission: A study of a family cluster. Lancet.
395:514–523. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kampf G, Todt D, Pfaender S and Steinmann
E: Persistence of coronaviruses on inanimate surfaces and their
inactivation with biocidal agents. J Hosp Infect. 104:246–251.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Atzrodt CL, Maknojia I, McCarthy RDP,
Oldfield TM, Po J, Ta KTL, Stepp HE and Clements TP: A guide to
COVID-19: A global pandemic caused by the novel coronavirus
SARS-CoV-2. FEBS J. 287:3633–3650. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jayaweera M, Perera H, Gunawardana B and
Manatunge J: Transmission of COVID-19 virus by droplets and
aerosols: A critical review on the unresolved dichotomy. Environ
Res. 188:1098192020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morawska L and Cao J: Airborne
transmission of SARS-CoV-2: The world should face the reality.
Environ Int. 139:1057302020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S
and Ma K: A systematic review of asymptomatic infections with
COVID-19. J Microbiol Immunol Infect. 54:12–16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pereira LJ, Pereira CV, Murata RM, Pardi V
and Pereira-Dourado SM: Biological and social aspects of
coronavirus disease 2019 (COVID-19) related to oral health. Braz
Oral Res. 34:e0412020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jackson CB, Farzan M, Chen B and Choe H:
Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol.
23:3–20. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meyerowitz EA, Richterman A, Gandhi RT and
Sax PE: Transmission of SARS-CoV-2: A review of viral, host, and
environmental factors. Ann Intern Med. 174:69–79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wild PS, Dimmeler S and Eschenhagen T: An
epidemiological study exploring a possible impact of treatment with
ACE inhibitors or angiotensin receptor blockers on ACE2 plasma
concentrations. J Mol Cell Cardiol. 141:108–109. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen
L and Wang M: Presumed asymptomatic carrier transmission of
COVID-19. JAMA. 323:1406–1407. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Soetikno R, Teoh AYB, Kaltenbach T, Lau
JYW, Asokkumar R, Cabral-Prodigalidad P and Shergill A:
Considerations in performing endoscopy during the COVID-19
pandemic. Gastrointest Endosc. 92:176–183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ludwig S and Zarbock A: Coronaviruses and
SARS-CoV-2: A brief overview. Anesth Analg. 131:93–96. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Yang S, Xu Y, Qin X, Liu J, Guo
J, Tian S, Wang S, Liao K, Zhang Y, et al: Epidemiological and
clinical characteristics of imported cases of COVID-19: A
multicenter study. BMC Infect Dis. 21:4062021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giacomelli A, Pezzati L, Conti F,
Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL,
Rizzardini G, et al: Self-reported olfactory and taste disorders in
patients with severe acute respiratory coronavirus 2 infection: A
cross-sectional study. Clin Infect Dis. 71:889–890. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cele S, Jackson L, Khoury DS, Khan K,
Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako DG,
et al: Omicron extensively but incompletely escapes Pfizer BNT162b2
neutralization. Nature. 602:654–656. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wong RSY: The SARS-CoV-2 outbreak: An
epidemiological and clinical perspective. SN Compr Clin Med.
2:1983–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mohamadian M, Chiti H, Shoghli A, Biglari
S, Parsamanesh N and Esmaeilzadeh A: COVID-19: Virology, biology
and novel laboratory diagnosis. J Gene Med. 23:e33032021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen T, Wu D, Chen H, Yan W, Yang D, Chen
G, Ma K, Xu D, Yu H, Wang H, et al: Clinical characteristics of 113
deceased patients with coronavirus disease 2019: Retrospective
study. BMJ. 368:m10912020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fang X, Zhao M, Li S, Yang L and Wu B:
Changes of CT findings in a 2019 novel coronavirus (2019-nCoV)
pneumonia patient. QJM. 113:271–272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
George PM, Wells AU and Jenkins RG:
Pulmonary fibrosis and COVID-19: The potential role for
antifibrotic therapy. Lancet Respir Med. 8:807–815. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma X, Liang M, Ding M, Liu W, Ma H, Zhou X
and Ren H: Extracorporeal membrane oxygenation (ECMO) in critically
Ill patients with coronavirus disease 2019 (COVID-19) pneumonia and
acute respiratory distress syndrome (ARDS). Med Sci Monit.
26:e9253642020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baruah V and Bose S:
Immunoinformatics-aided identification of T cell and B cell
epitopes in the surface glycoprotein of 2019-nCoV. J Med Virol.
92:495–500. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sieker JT, Horowitz C, Hu CK,
Lacombe-Daphnis M, Chirokas B, Pina C, Heger NE, Rabson AR, Zhou M,
Bogen SA and Horowitz GL: Analytic sensitivity of 3 nucleic acid
detection assays in diagnosis of SARS-CoV-2 infection. J Appl Lab
Med. 6:421–428. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang C, Huang L, Wang Y, Li X, Ren L, Gu
X, Kang L, Guo L, Liu M, Zhou X, et al: 6-month consequences of
COVID-19 in patients discharged from hospital: A cohort study.
Lancet. 397:220–232. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chams N, Chams S, Badran R, Shams A, Araji
A, Raad M, Mukhopadhyay S, Stroberg E, Duval EJ, Barton LM and Hajj
Hussein I: COVID-19: A multidisciplinary review. Front Public
Health. 8:3832020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Stang A, Robers J, Schonert B, Jöckel KH,
Spelsberg A, Keil U and Cullen P: The performance of the SARS-CoV-2
RT-PCR test as a tool for detecting SARS-CoV-2 infection in the
population. J Infect. 83:237–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Islam KU and Iqbal J: An update on
molecular diagnostics for COVID-19. Front Cell Infect Microbiol.
10:5606162020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kucirka LM, Lauer SA, Laeyendecker O, Boon
D and Lessler J: Variation in false-negative rate of reverse
transcriptase polymerase chain reaction-based SARS-CoV-2 tests by
time since exposure. Ann Intern Med. 173:262–267. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Harper H, Burridge A, Winfield M, Finn A,
Davidson A, Matthews D, Hutchings S, Vipond B, Jain N; COVID-19
Genomics UK (COG-UK) Consortium, ; et al: Detecting SARS-CoV-2
variants with SNP genotyping. PLoS One. 16:e02431852021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stelzer-Braid S, Walker GJ, Aggarwal A,
Isaacs SR, Yeang M, Naing Z, Ospina Stella A, Turville SG and
Rawlinson WD: Virus isolation of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) for diagnostic and research purposes.
Pathology. 52:760–763. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kleiboeker S, Cowden S, Grantham J, Nutt
J, Tyler A, Berg A and Altrich M: SARS-CoV-2 viral load assessment
in respiratory samples. J Clin Virol. 129:1044392020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang W, Du RH, Li B, Zheng XS, Yang XL,
Hu B, Wang YY, Xiao GF, Yan B, Shi ZL and Zhou P: Molecular and
serological investigation of 2019-nCoV infected patients:
Implication of multiple shedding routes. Emerg Microbes Infect.
9:386–389. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shen M, Zhou Y, Ye J, Abdullah Al-Maskri
AA, Kang Y, Zeng S and Cai S: Recent advances and perspectives of
nucleic acid detection for coronavirus. J Pharm Anal. 10:97–101.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bustin SA and Mueller R: Real-time reverse
transcription PCR (qRT-PCR) and its potential use in clinical
diagnosis. Clin Sci (Lond). 109:365–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
To KK, Tsang OT, Leung WS, Tam AR, Wu TC,
Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, et al: Temporal profiles
of viral load in posterior oropharyngeal saliva samples and serum
antibody responses during infection by SARS-CoV-2: An observational
cohort study. Lancet Infect Dis. 20:565–574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Thomas E, Delabat S, Carattini YL and
Andrews DM: SARS-CoV-2 and variant diagnostic testing approaches in
the United States. Viruses. 13:24922021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Safiabadi Tali SH, LeBlanc JJ, Sadiq Z,
Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM and
Jahanshahi-Anbuhi S: Tools and techniques for severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection.
Clin Microbiol Rev. 34:e00228–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Augustine R, Hasan A, Das S, Ahmed R, Mori
Y, Notomi T, Kevadiya BD and Thakor AS: Loop-mediated isothermal
amplification (LAMP): A rapid, sensitive, specific, and
cost-effective point-of-care test for coronaviruses in the context
of COVID-19 pandemic. Biology (Basel). 9:1822020.PubMed/NCBI
|
|
68
|
Chau CH, Strope JD and Figg WD: COVID-19
clinical diagnostics and testing technology. Pharmacotherapy.
40:857–868. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Alcoba-Florez J, Gil-Campesino H, Artola
DG, González-Montelongo R, Valenzuela-Fernández A, Ciuffreda L and
Flores C: Sensitivity of different RT-qPCR solutions for SARS-CoV-2
detection. Int J Infect Dis. 99:190–192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Giuliani C: The flavonoid quercetin
induces AP-1 activation in FRTL-5 thyroid cells. Antioxidants
(Basel). 8:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ye Z, Zhang Y, Wang Y, Huang Z and Song B:
Chest CT manifestations of new coronavirus disease 2019 (COVID-19):
A pictorial review. Eur Radiol. 30:4381–4389. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kanne JP: Chest CT findings in 2019 novel
coronavirus (2019-nCoV) infections from Wuhan, China: Key points
for the radiologist. Radiology. 295:16–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nivet H, Crombé A, Schuster P, Ayoub T,
Pourriol L, Favard N, Chazot A, Alonzo-Lacroix F, Youssof E, Ben
Cheikh A, et al: The accuracy of teleradiologists in diagnosing
COVID-19 based on a French multicentric emergency cohort. Eur
Radiol. 31:2833–2844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ghose A, Roy S, Vasdev N, Olsburgh J and
Dasgupta P: The emerging role of artificial intelligence in the
fight against COVID-19. Eur Urol. 78:775–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ozsahin I, Sekeroglu B, Musa MS, Mustapha
MT and Uzun Ozsahin D: Review on diagnosis of COVID-19 from chest
CT images using artificial intelligence. Comput Math Methods Med.
2020:97565182020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shi F, Wang J, Shi J, Wu Z, Wang Q, Tang
Z, He K, Shi Y and Shen D: Review of artificial intelligence
techniques in imaging data acquisition, segmentation, and diagnosis
for COVID-19. IEEE Rev Biomed Eng. 14:4–15. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bouchareb Y, Moradi Khaniabadi P, Al Kindi
F, Al Dhuhli H, Shiri I, Zaidi H and Rahmim A: Artificial
intelligence-driven assessment of radiological images for COVID-19.
Comput Biol Med. 136:1046652021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Vaishya R, Javaid M, Khan IH and Haleem A:
Artificial intelligence (AI) applications for COVID-19 pandemic.
Diabetes Metab Syndr. 14:337–339. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Dror AA, Eisenbach N, Taiber S, Morozov
NG, Mizrachi M, Zigron A, Srouji S and Sela E: Vaccine hesitancy:
The next challenge in the fight against COVID-19. Eur J Epidemiol.
35:775–779. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mouffak S, Shubbar Q, Saleh E and El-Awady
R: Recent advances in management of COVID-19: A review. Biomed
Pharmacother. 143:1121072021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gavriatopoulou M, Ntanasis-Stathopoulos I,
Korompoki E, Fotiou D, Migkou M, Tzanninis IG, Psaltopoulou T,
Kastritis E, Terpos E and Dimopoulos MA: Emerging treatment
strategies for COVID-19 infection. Clin Exp Med. 21:167–179. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Richtsmeier WJ: Interferon-present and
future prospects. Crit Rev Clin Lab Sci. 20:57–93. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lee JS and Shin EC: The type I interferon
response in COVID-19: Implications for treatment. Nat Rev Immunol.
20:585–586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hadjadj J, Yatim N, Barnabei L, Corneau A,
Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux
C, et al: Impaired type I interferon activity and inflammatory
responses in severe COVID-19 patients. Science. 369:718–724. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Monk PD, Marsden RJ, Tear VJ, Brookes J,
Batten TN, Mankowski M, Gabbay FJ, Davies DE, Holgate ST, Ho LP, et
al: Safety and efficacy of inhaled nebulised interferon beta-1a
(SNG001) for treatment of SARS-CoV-2 infection: A randomised,
double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med.
9:196–206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rahmani H, Davoudi-Monfared E, Nourian A,
Khalili H, Hajizadeh N, Jalalabadi NZ, Fazeli MR, Ghazaeian M and
Yekaninejad MS: Interferon β-1b in treatment of severe COVID-19: A
randomized clinical trial. Int Immunopharmacol. 88:1069032020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Joseph BA, Dibas M, Evanson KW, Paranjape
G, Vegivinti CTR, Selvan PT, Saravu K, Gupta N, Pulakurthi YS,
Keesari PR, et al: Efficacy and safety of lopinavir/ritonavir in
the treatment of COVID-19: A systematic review. Expert Rev Anti
Infect Ther. 19:679–687. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu Y, Ma L, Zhuang Z, Cai S, Zhao Z, Zhou
L, Zhang J, Wang PH, Zhao J and Cui J: Main protease of SARS-CoV-2
serves as a bifunctional molecule in restricting type I interferon
antiviral signaling. Signal Transduct Target Ther. 5:2212020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
lopinavir-ritonavir in adults hospitalized with severe Covid-19. N
Engl J Med. 382:1787–1799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hu Y, Ma C, Szeto T, Hurst B, Tarbet B and
Wang J: Boceprevir, calpain inhibitors II and XII, and GC-376 have
broad-spectrum antiviral activity against coronaviruses. ACS Infect
Dis. 7:586–597. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Boras B, Jones RM, Anson BJ, Arenson D,
Aschenbrenner L, Bakowski MA, Beutler N, Binder J, Chen E, Eng H,
et al: Preclinical characterization of an intravenous coronavirus
3CL protease inhibitor for the potential treatment of COVID19. Nat
Commun. 12:60552021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wen W, Chen C, Tang J, Wang C, Zhou M,
Cheng Y, Zhou X, Wu Q, Zhang X, Feng Z, et al: Efficacy and safety
of three new oral antiviral treatment (molnupiravir, fluvoxamine
and Paxlovid) for COVID-19: A meta-analysis. Ann Med. 54:516–523.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Colson P, Rolain JM and Raoult D:
Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J
Antimicrob Agents. 55:1059232020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cuadrado-Lavín A, Olmos JM, Cifrian JM,
Gimenez T, Gandarillas MA, García-Saiz M, Rebollo MH,
Martínez-Taboada V, López-Hoyos M, Fariñas MC and Crespo J:
Controlled, double-blind, randomized trial to assess the efficacy
and safety of hydroxychloroquine chemoprophylaxis in SARS CoV2
infection in healthcare personnel in the hospital setting: A
structured summary of a study protocol for a randomised controlled
trial. Trials. 21:4722020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
RECOVERY Collaborative Group, . Horby P,
Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M,
Ustianowski A, Elmahi E, et al: Effect of hydroxychloroquine in
hospitalized patients with Covid-19. N Engl J Med. 383:2030–2040.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56:1059492020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H,
Li Y, Zhao L, Li W, Sun X, et al: The anti-influenza virus drug,
arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell
Discov. 6:282020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li H, Liu R, Zhang R, Zhang S, Wei Y,
Zhang L, Zhou H and Yang C: Protective effect of arbidol against
pulmonary fibrosis and sepsis in mice. Front Pharmacol.
11:6070752020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T,
Lu J and Xue Y: Arbidol monotherapy is superior to
lopinavir/ritonavir in treating COVID-19. J Infect. 81:e21–e23.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Song Y, Zhang M, Yin L, Wang K, Zhou Y,
Zhou M and Lu Y: COVID-19 treatment: close to a cure? A rapid
review of pharmacotherapies for the novel coronavirus (SARS-CoV-2).
Int J Antimicrob Agents. 56:1060802020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang B, Timilsena YP, Blanch E and
Adhikari B: Lactoferrin: Structure, function, denaturation and
digestion. Crit Rev Food Sci Nutr. 59:580–596. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hu Y, Meng X, Zhang F, Xiang Y and Wang J:
The in vitro antiviral activity of lactoferrin against common human
coronaviruses and SARS-CoV-2 is mediated by targeting the heparan
sulfate co-receptor. Emerg Microbes Infect. 10:317–330. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Salaris C, Scarpa M, Elli M, Bertolini A,
Guglielmetti S, Pregliasco F, Blandizzi C, Brun P and Castagliuolo
I: Protective effects of lactoferrin against SARS-CoV-2 infection
in vitro. Nutrients. 13:3282021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tian L, Qiang T, Liang C, Ren X, Jia M,
Zhang J, Li J, Wan M, YuWen X, Li H, et al: RNA-dependent RNA
polymerase (RdRp) inhibitors: The current landscape and repurposing
for the COVID-19 pandemic. Eur J Med Chem. 213:1132012021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cao YC, Deng QX and Dai SX: Remdesivir for
severe acute respiratory syndrome coronavirus 2 causing COVID-19:
An evaluation of the evidence. Travel Med Infect Dis.
35:1016472020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
McDonald S, Turner S, Page MJ and Turner
T: Most published systematic reviews of remdesivir for COVID-19
were redundant and lacked currency. J Clin Epidemiol. 146:22–31.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jayk Bernal A, Gomes da Silva MM,
Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós
A, Caraco Y, Williams-Diaz A, Brown ML, et al: Molnupiravir for
oral treatment of Covid-19 in nonhospitalized patients. N Engl J
Med. 386:509–520. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Good SS, Westover J, Jung KH, Zhou XJ,
Moussa A, La Colla P, Collu G, Canard B and Sommadossi JP: AT-527,
a double prodrug of a guanosine nucleotide analog, is a potent
inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for
treatment of COVID-19. Antimicrob Agents Chemother. 65:e02479–20.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Martinez MA: Plitidepsin: A repurposed
drug for the treatment of COVID-19. Antimicrob Agents Chemother.
65:e00200–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
White KM, Rosales R, Yildiz S, Kehrer T,
Miorin L, Moreno E, Jangra S, Uccellini MB, Rathnasinghe R,
Coughlan L, et al: Plitidepsin has potent preclinical efficacy
against SARS-CoV-2 by targeting the host protein eEF1A. Science.
371:926–931. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rodon J, Muñoz-Basagoiti J, Perez-Zsolt D,
Noguera-Julian M, Paredes R, Mateu L, Quiñones C, Perez C, Erkizia
I, Blanco I, et al: Identification of plitidepsin as potent
inhibitor of SARS-CoV-2-induced cytopathic effect after a drug
repurposing screen. Front Pharmacol. 12:6466762021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Soto-Matos A, Szyldergemajn S, Extremera
S, Miguel-Lillo B, Alfaro V, Coronado C, Lardelli P, Roy E, Corrado
CS and Kahatt C: Plitidepsin has a safe cardiac profile: A
comprehensive analysis. Mar Drugs. 9:1007–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lenze EJ, Mattar C, Zorumski CF, Stevens
A, Schweiger J, Nicol GE, Miller JP, Yang L, Yingling M, Avidan MS
and Reiersen AM: Fluvoxamine vs placebo and clinical deterioration
in outpatients with symptomatic COVID-19: A randomized clinical
trial. JAMA. 324:2292–2300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bartoszko JJ, Siemieniuk RAC, Kum E, Qasim
A, Zeraatkar D, Ge L, Han MA, Sadeghirad B, Agarwal A, Agoritsas T,
et al: Prophylaxis against covid-19: Living systematic review and
network meta-analysis. BMJ. 373:n9492021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J,
Chu Y, Feng Y and Wang Q: Neutralizing antibody responses to severe
acute respiratory syndrome coronavirus 2 in coronavirus disease
2019 inpatients and convalescent patients. Clin Infect Dis.
71:2688–2694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Libster R, Pérez Marc G, Wappner D,
Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C,
Berrueta M, et al: Early high-titer plasma therapy to prevent
severe Covid-19 in older adults. N Engl J Med. 384:610–618. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Abolghasemi H, Eshghi P, Cheraghali AM,
Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, Moeini Maleki
M, Ranjkesh M, Rezapour M, Bahramifar A, et al: Clinical efficacy
of convalescent plasma for treatment of COVID-19 infections:
Results of a multicenter clinical study. Transfus Apher Sci.
59:1028752020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan
J, Wang F, Li D, Yang M, Xing L, et al: Treatment of 5 critically
Ill patients with COVID-19 with convalescent plasma. JAMA.
323:1582–1589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Joyner MJ, Bruno KA, Klassen SA, Kunze KL,
Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge
DO, et al: Safety update: COVID-19 convalescent plasma in 20,000
hospitalized patients. Mayo Clin Proc. 95:1888–1897. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cao W, Liu X, Bai T, Fan H, Hong K, Song
H, Han Y, Lin L, Ruan L and Li T: High-dose intravenous
immunoglobulin as a therapeutic option for deteriorating patients
with coronavirus disease 2019. Open Forum Infect Dis.
7:ofaa1022020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cao W, Liu X, Hong K, Ma Z, Zhang Y, Lin
L, Han Y, Xiong Y, Liu Z, Ruan L and Li T: High-dose intravenous
immunoglobulin in severe coronavirus disease 2019: A multicenter
retrospective study in China. Front Immunol. 12:6278442021.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Scialo F, Daniele A, Amato F, Pastore L,
Matera MG, Cazzola M, Castaldo G and Bianco A: ACE2: The major cell
entry receptor for SARS-CoV-2. Lung. 198:867–877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Shi R, Shan C, Duan X, Chen Z, Liu P, Song
J, Song T, Bi X, Han C, Wu L, et al: A human neutralizing antibody
targets the receptor-binding site of SARS-CoV-2. Nature.
584:120–124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shepard HM, Phillips GL, D Thanos C and
Feldmann M: Developments in therapy with monoclonal antibodies and
related proteins. Clin Med (Lond). 17:220–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jackson LA, Anderson EJ, Rouphael NG,
Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD,
Denison MR, Stevens LJ, et al: An mRNA vaccine against
SARS-CoV-2-preliminary report. N Engl J Med. 383:1920–1931. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Khan FA, Stewart I, Fabbri L, Moss S,
Robinson K, Smyth AR and Jenkins G: Systematic review and
meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab
for COVID-19. Thorax. 76:907–919. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gupta S, Wang W, Hayek SS, Chan L, Mathews
KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J,
et al: Association between early treatment with tocilizumab and
mortality among critically Ill patients with COVID-19. JAMA Intern
Med. 181:41–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Weinreich DM, Sivapalasingam S, Norton T,
Ali S, Gao H, Bhore R, Xiao J, Hooper AT, Hamilton JD, Musser BJ,
et al: REGEN-COV antibody combination and outcomes in outpatients
with Covid-19. N Engl J Med. 385:e812021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
O'Brien MP, Forleo-Neto E, Musser BJ, Isa
F, Chan KC, Sarkar N, Bar KJ, Barnabas RV, Barouch DH, Cohen MS, et
al: Subcutaneous REGEN-COV antibody combination to prevent
Covid-19. N Engl J Med. 385:1184–1195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ryu DK, Kang B, Noh H, Woo SJ, Lee MH,
Nuijten PM, Kim JI, Seo JM, Kim C, Kim M, et al: The in vitro and
in vivo efficacy of CT-P59 against gamma, delta and its associated
variants of SARS-CoV-2. Biochem Biophys Res Commun. 578:91–96.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lescure FX, Honda H, Fowler RA, Lazar JS,
Shi G, Wung P, Patel N and Hagino O; Sarilumab COVID-19 Global
Study Group, : Sarilumab in patients admitted to hospital with
severe or critical COVID-19: A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Respir Med. 9:522–532.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Pang J, Xu F, Aondio G, Li Y, Fumagalli A,
Lu M, Valmadre G, Wei J, Bian Y, Canesi M, et al: Efficacy and
tolerability of bevacizumab in patients with severe Covid-19. Nat
Commun. 12:8142021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tuccori M, Ferraro S, Convertino I,
Cappello E, Valdiserra G, Blandizzi C, Maggi F and Focosi D:
Anti-SARS-CoV-2 neutralizing monoclonal antibodies: Clinical
pipeline. MAbs. 12:18541492020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Dejnirattisai W, Huo J, Zhou D, Zahradník
J, Supasa P, Liu C, Duyvesteyn HME, Ginn HM, Mentzer AJ,
Tuekprakhon A, et al: SARS-CoV-2 omicron-B.1.1.529 leads to
widespread escape from neutralizing antibody responses. Cell.
185:467–484.e15. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Cao Y, Wang J, Jian F, Xiao T, Song W,
Yisimayi A, Huang W, Li Q, Wang P, An R, et al: Omicron escapes the
majority of existing SARS-CoV-2 neutralizing antibodies. Nature.
602:657–663. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mannar D, Saville JW, Zhu X, Srivastava
SS, Berezuk AM, Tuttle KS, Marquez AC, Sekirov I and Subramaniam S:
SARS-CoV-2 omicron variant: Antibody evasion and cryo-EM structure
of spike protein-ACE2 complex. Science. 375:760–764. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Miller NL, Clark T, Raman R and
Sasisekharan R: Insights on the mutational landscape of the
SARS-CoV-2 omicron variant. bioRxiv. doi: 10.1101/2021.12.06.471499
(Preprint).
|
|
139
|
Shah M and Woo HG: Omicron: A heavily
mutated SARS-CoV-2 variant exhibits stronger binding to ACE2 and
potently escapes approved COVID-19 therapeutic antibodies. Front
Immunol. 12:8305272021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Chen RE, Winkler ES, Case JB, Aziati ID,
Bricker TL, Joshi A, Darling TL, Ying B, Errico JM, Shrihari S, et
al: In vivo monoclonal antibody efficacy against SARS-CoV-2 variant
strains. Nature. 596:103–108. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kakoulidis I, Ilias I and Koukkou E:
SARS-CoV-2 infection and glucose homeostasis in pregnancy. What
about antenatal corticosteroids? Diabetes Metab Syndr. 14:519–520.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Bartoletti M, Marconi L, Scudeller L,
Pancaldi L, Tedeschi S, Giannella M, Rinaldi M, Bussini L,
Valentini I, Ferravante AF, et al: Efficacy of corticosteroid
treatment for hospitalized patients with severe COVID-19: A
multicentre study. Clin Microbiol Infect. 27:105–111. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tang X, Feng YM, Ni JX, Zhang JY, Liu LM,
Hu K, Wu XZ, Zhang JX, Chen JW, Zhang JC, et al: Early use of
corticosteroid may prolong SARS-CoV-2 shedding in non-intensive
care unit patients with COVID-19 pneumonia: A multicenter,
single-blind, randomized control trial. Respiration. 100:116–126.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
van Paassen J, Vos JS, Hoekstra EM,
Neumann KMI, Boot PC and Arbous SM: Corticosteroid use in COVID-19
patients: A systematic review and meta-analysis on clinical
outcomes. Crit Care. 24:6962020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
So C, Ro S, Murakami M, Imai R and Jinta
T: High-dose, short-term corticosteroids for ARDS caused by
COVID-19: A case series. Respirol Case Rep. 8:e005962020.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kolilekas L, Loverdos K, Giannakaki S,
Vlassi L, Levounets A, Zervas E and Gaga M: Can steroids reverse
the severe COVID-19 induced ‘cytokine storm’? J Med Virol.
92:2866–2869. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yuan M, Xu X, Xia D, Tao Z, Yin W, Tan W,
Hu Y and Song C: Effects of corticosteroid treatment for non-severe
COVID-19 pneumonia: A propensity score-based analysis. Shock.
54:638–643. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Lu X, Chen T, Wang Y, Wang J and Yan F:
Adjuvant corticosteroid therapy for critically ill patients with
COVID-19. Crit Care. 24:2412020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Rello J, Belliato M, Dimopoulos MA,
Giamarellos-Bourboulis EJ, Jaksic V, Martin-Loeches I, Mporas I,
Pelosi P, Poulakou G, Pournaras S, et al: Update in COVID-19 in the
intensive care unit from the 2020 HELLENIC Athens International
symposium. Anaesth Crit Care Pain Med. 39:723–730. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Lentz S, Roginski MA, Montrief T, Ramzy M,
Gottlieb M and Long B: Initial emergency department mechanical
ventilation strategies for COVID-19 hypoxemic respiratory failure
and ARDS. Am J Emerg Med. 38:2194–2202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wong DJN, El-Boghdadly K, Owen R,
Johnstone C, Neuman MD, Andruszkiewicz P, Baker PA, Biccard BM,
Bryson GL, Chan MTV, et al: Emergency airway management in patients
with COVID-19: A prospective international multicenter cohort
study. Anesthesiology. 135:292–303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Cook TM, El-Boghdadly K, McGuire B,
McNarry AF, Patel A and Higgs A: Consensus guidelines for managing
the airway in patients with COVID-19: Guidelines from the difficult
airway society, the association of anaesthetists the intensive care
society, the faculty of intensive care medicine and the royal
college of anaesthetists. Anaesthesia. 75:785–799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Llitjos JF, Leclerc M, Chochois C,
Monsallier JM, Ramakers M, Auvray M and Merouani K: High incidence
of venous thromboembolic events in anticoagulated severe COVID-19
patients. J Thromb Haemost. 18:1743–1746. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ronco C, Reis T and Husain-Syed F:
Management of acute kidney injury in patients with COVID-19. Lancet
Respir Med. 8:738–742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ronco C, Bagshaw SM, Bellomo R, Clark WR,
Husain-Syed F, Kellum JA, Ricci Z, Rimmelé T, Reis T and Ostermann
M: Extracorporeal blood purification and organ support in the
critically Ill patient during COVID-19 Pandemic: expert review and
recommendation. Blood Purif. 50:17–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kabeerdoss J, Pilania RK, Karkhele R,
Kumar TS, Danda D and Singh S: Severe COVID-19, multisystem
inflammatory syndrome in children, and Kawasaki disease:
Immunological mechanisms, clinical manifestations and management.
Rheumatol Int. 41:19–32. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Megyeri K, Dernovics Á, Al-Luhaibi ZII and
Rosztóczy A: COVID-19-associated diarrhea. World J Gastroenterol.
27:3208–3222. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Lu ZH, Yang CL, Yang GG, Pan WX, Tian LG,
Zheng JX, Lv S, Zhang SY, Zheng PY and Zhang SX: Efficacy of the
combination of modern medicine and traditional Chinese medicine in
pulmonary fibrosis arising as a sequelae in convalescent COVID-19
patients: A randomized multicenter trial. Infect Dis Poverty.
10:312021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhao Z, Li Y, Zhou L, Zhou X, Xie B, Zhang
W and Sun J: Prevention and treatment of COVID-19 using traditional
Chinese medicine: A review. Phytomedicine. 85:1533082021.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ren JL, Zhang AH and Wang XJ: Traditional
Chinese medicine for COVID-19 treatment. Pharmacol Res.
155:1047432020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Ni L, Chen L, Huang X, Han C, Xu J, Zhang
H, Luan X, Zhao Y, Xu J, Yuan W and Chen H: Combating COVID-19 with
integrated traditional Chinese and western medicine in China. Acta
Pharm Sin B. 10:1149–1162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Anand U, Jakhmola S, Indari O, Jha HC,
Chen ZS, Tripathi V and Pérez de la Lastra JM: Potential
therapeutic targets and vaccine development for SARS-CoV-2/COVID-19
pandemic management: A review on the recent update. Front Immunol.
12:6585192021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Tregoning JS, Flight KE, Higham SL, Wang Z
and Pierce BF: Progress of the COVID-19 vaccine effort: Viruses,
vaccines and variants versus efficacy, effectiveness and escape.
Nat Rev Immunol. 21:626–636. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Hou Z, Tong Y, Du F, Lu L, Zhao S, Yu K,
Piatek SJ, Larson HJ and Lin L: Assessing COVID-19 vaccine
hesitancy, confidence, and public engagement: A global social
listening study. J Med Internet Res. 23:e276322021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sharif N, Alzahrani KJ, Ahmed SN and Dey
SK: Efficacy, immunogenicity and safety of COVID-19 vaccines: A
systematic review and meta-analysis. Front Immunol. 12:7141702021.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Francis AI, Ghany S, Gilkes T and
Umakanthan S: Review of COVID-19 vaccine subtypes, efficacy and
geographical distributions. Postgrad Med J. 98:389–394. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Canedo-Marroquín G, Saavedra F, Andrade
CA, Berrios RV, Rodríguez-Guilarte L, Opazo MC, Riedel CA and
Kalergis AM: SARS-CoV-2: Immune response elicited by infection and
development of vaccines and treatments. Front Immunol.
11:5697602020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Rauch S, Roth N, Schwendt K, Fotin-Mleczek
M, Mueller SO and Petsch B: mRNA-based SARS-CoV-2 vaccine candidate
CVnCoV induces high levels of virus-neutralising antibodies and
mediates protection in rodents. NPJ Vaccines. 6:572021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Kremsner PG, Ahuad Guerrero RA, Arana-Arri
E, Aroca Martinez GJ, Bonten M, Chandler R, Corral G, De Block EJL,
Ecker L, Gabor JJ, et al: Efficacy and safety of the CVnCoV
SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and
Latin America (HERALD): A randomised, observer-blinded,
placebo-controlled, phase 2b/3 trial. Lancet Infect Dis.
22:329–340. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Pollock KM, Cheeseman HM, Szubert AJ,
Libri V, Boffito M, Owen D, Bern H, O'Hara J, McFarlane LR, Lemm
NM, et al: Safety and immunogenicity of a self-amplifying RNA
vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial.
EClinical Medicine. 44:1012622022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Al Kaabi N, Zhang Y, Xia S, Yang Y, Al
Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS,
Abdalla J, et al: Effect of 2 inactivated SARS-CoV-2 vaccines on
symptomatic COVID-19 infection in adults: A randomized clinical
trial. JAMA. 326:35–45. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Premikha M, Chiew CJ, Wei WE, Leo YS, Ong
B, Lye DC, Lee VJ and Tan KB: Comparative effectiveness of mRNA and
inactivated whole virus vaccines against COVID-19 infection and
severe disease in Singapore. Clin Infect Dis. Apr 12–2022.(Epub
ahead of print). PubMed/NCBI
|
|
173
|
Zhang Z, Mateus J, Coelho CH, Dan JM,
Moderbacher CR, Gálvez RI, Cortes FH, Grifoni A, Tarke A, Chang J,
et al: Humoral and cellular immune memory to four COVID-19
vaccines. bioRxiv. doi: 10.1101/2022.03.18.484953 (Preprint).
|
|
174
|
Islam N, Sheils NE, Jarvis MS and Cohen K:
Comparative effectiveness over time of the mRNA-1273 (Moderna)
vaccine and the BNT162b2 (Pfizer-BioNTech) vaccine. Nat Commun.
13:23772022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Mohamed K, Rzymski P, Islam MS, Makuku R,
Mushtaq A, Khan A, Ivanovska M, Makka SA, Hashem F, Marquez L, et
al: COVID-19 vaccinations: The unknowns, challenges, and hopes. J
Med Virol. 94:1336–1349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Liu Y, Zhang X, Liu J, Xia H, Zou J,
Muruato AE, Periasamy S, Plante JA, Bopp NE, Kurhade C, et al: A
live-attenuated SARS-CoV-2 vaccine candidate with accessory protein
deletions. bioRxiv. 2022.02.14.480460. 2022.
|
|
177
|
Merzon E, Green I, Somekh E, Vinker S,
Golan-Cohen A, Israel A, Gorohovski A, Frenkel-Morgenstern M and
Stein M: The association of previous vaccination with
live-attenuated varicella zoster vaccine and COVID-19 positivity:
An Israeli population-based study. Vaccines (Basel). 10:742022.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Richmond P, Hatchuel L, Dong M, Ma B, Hu
B, Smolenov I, Li P, Liang P, Han HH, Liang J and Clemens R: Safety
and immunogenicity of S-Trimer (SCB-2019), a protein subunit
vaccine candidate for COVID-19 in healthy adults: A phase 1,
randomised, double-blind, placebo-controlled trial. Lancet.
397:682–694. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Yang S, Li Y, Dai L, Wang J, He P, Li C,
Fang X, Wang C, Zhao X, Huang E, et al: Safety and immunogenicity
of a recombinant tandem-repeat dimeric RBD-based protein subunit
vaccine (ZF2001) against COVID-19 in adults: Two randomised,
double-blind, placebo-controlled, phase 1 and 2 trials. Lancet
Infect Dis. 21:1107–1119. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Jin P, Guo X, Chen W, Ma S, Pan H, Dai L,
Du P, Wang L, Jin L, Chen Y, et al: Safety and immunogenicity of
heterologous boost immunization with an adenovirus type-5-vectored
and protein-subunit-based COVID-19 vaccine (Convidecia/ZF2001): A
randomized, observer-blinded, placebo-controlled trial. PLoS Med.
19:e10039532022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Kuhn M, Campillos M, Letunic I, Jensen LJ
and Bork P: A side effect resource to capture phenotypic effects of
drugs. Mol Syst Biol. 6:3432010. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Menni C, Klaser K, May A, Polidori L,
Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, et
al: Vaccine side-effects and SARS-CoV-2 infection after vaccination
in users of the COVID symptom study app in the UK: A prospective
observational study. Lancet Infect Dis. 21:939–949. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Vetter V, Denizer G, Friedland LR,
Krishnan J and Shapiro M: Understanding modern-day vaccines: What
you need to know. Ann Med. 50:110–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Akinosoglou K, Tzivaki I and Marangos M:
Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon.
Clin Immunol. 226:1087212021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Baden LR, El Sahly HM, Essink B, Kotloff
K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB,
et al: Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N
Engl J Med. 384:403–416. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Lipsitch M, Krammer F, Regev-Yochay G,
Lustig Y and Balicer RD: SARS-CoV-2 breakthrough infections in
vaccinated individuals: Measurement, causes and impact. Nat Rev
Immunol. 22:57–65. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Dejnirattisai W, Shaw RH, Supasa P, Liu C,
Stuart AS, Pollard AJ, Liu X, Lambe T, Crook D, Stuart DI, et al:
Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by
post-immunisation serum. Lancet. 399:234–236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Brown CM, Vostok J, Johnson H, Burns M,
Gharpure R, Sami S, Sabo RT, Hall N, Foreman A, Schubert PL, et al:
Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine
breakthrough infections, associated with large public
gatherings-barnstable county, massachusetts, July 2021. MMWR Morb
Mortal Wkly Rep. 70:1059–1062. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Tay MZ, Wiehe K and Pollara J:
Antibody-dependent cellular phagocytosis in antiviral immune
responses. Front Immunol. 10:3322019. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Forni G and Mantovani A; COVID-19
Commission of Accademia Nazionale dei Lincei Rome, : COVID-19
vaccines: Where we stand and challenges ahead. Cell Death Differ.
28:626–639. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Bagheri A, Moezzi SMI, Mosaddeghi P,
Nadimi Parashkouhi S, Fazel Hoseini SM, Badakhshan F and
Negahdaripour M: Interferon-inducer antivirals: Potential
candidates to combat COVID-19. Int Immunopharmacol. 91:1072452021.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Cáceres CJ, Cardenas-Garcia S, Carnaccini
S, Seibert B, Rajao DS, Wang J and Perez DR: Efficacy of GC-376
against SARS-CoV-2 virus infection in the K18 hACE2 transgenic
mouse model. Sci Rep. 11:96092021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Heilmann E, Costacurta F, Geley S,
Mogadashi SA, Volland A, Rupp B, Harris RS and von Laer D: A
VSV-based assay quantifies coronavirus Mpro/3CLpro/Nsp5 main
protease activity and chemical inhibition. Commun Biol. 5:3912022.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Mahase E: Covid-19: Covid-19: Pfizer's
paxlovid is 89% effective in patients at risk of serious illness,
company reports. BMJ. 375:n27132021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Nojomi M, Yassin Z, Keyvani H, Makiani MJ,
Roham M, Laali A, Dehghan N, Navaei M and Ranjbar M: Effect of
arbidol (Umifenovir) on COVID-19: A randomized controlled trial.
BMC Infect Dis. 20:9542020. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Chang R, Ng TB and Sun WZ: Lactoferrin as
potential preventative and adjunct treatment for COVID-19. Int J
Antimicrob Agents. 56:1061182020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Hoffmann M, Hofmann-Winkler H, Smith JC,
Krüger N, Arora P, Sørensen LK, Søgaard OS, Hasselstrøm JB, Winkler
M, Hempel T, et al: Camostat mesylate inhibits SARS-CoV-2
activation by TMPRSS2-related proteases and its metabolite GBPA
exerts antiviral activity. EBioMedicine. 65:1032552021. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin
Y, Fu S, Gao L, Cheng Z, Lu Q, et al: Remdesivir in adults with
severe COVID-19: A randomised, double-blind, placebo-controlled,
multicentre trial. Lancet. 395:1569–1578. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Udwadia ZF, Singh P, Barkate H, Patil S,
Rangwala S, Pendse A, Kadam J, Wu W, Caracta CF and Tandon M:
Efficacy and safety of favipiravir, an oral RNA-dependent RNA
polymerase inhibitor, in mild-to-moderate COVID-19: A randomized,
comparative, open-label, multicenter, phase 3 clinical trial. Int J
Infect Diss. 103:62–71. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Şimşek-Yavuz S and Komsuoğlu Çelikyurt FI:
An update of anti-viral treatment of COVID-19. Turk J Med Sci.
51:3372–3390. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Wood EM, Estcourt LJ and McQuilten ZK: How
should we use convalescent plasma therapies for the management of
COVID-19? Blood. 137:1573–1581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Schmidt F, Weisblum Y, Rutkowska M, Poston
D, DaSilva J, Zhang F, Bednarski E, Cho A, Schaefer-Babajew DJ,
Gaebler C, et al: High genetic barrier to SARS-CoV-2 polyclonal
neutralizing antibody escape. Nature. 600:512–516. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Case JB, Chen RE, Cao L, Ying B, Winkler
ES, Johnson M, Goreshnik I, Pham MN, Shrihari S, Kafai NM, et al:
Ultrapotent miniproteins targeting the SARS-CoV-2 receptor-binding
domain protect against infection and disease. Cell Host Microbe.
29:1151–1161.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Guo K, Wustoni S, Koklu A, Díaz-Galicia E,
Moser M, Hama A, Alqahtani AA, Ahmad AN, Alhamlan FS, Shuaib M, et
al: Rapid single-molecule detection of COVID-19 and MERS antigens
via nanobody-functionalized organic electrochemical transistors.
Nat Biomed Eng. 5:666–677. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Stone JH, Frigault MJ, Serling-Boyd NJ,
Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R,
Bensaci AM, et al: Efficacy of tocilizumab in patients hospitalized
with Covid-19. N Engl J Med. 383:2333–2344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Gupta A, Gonzalez-Rojas Y, Juarez E,
Crespo Casal M, Moya J, Falci DR, Sarkis E, Solis J, Zheng H, Scott
N, et al: Early treatment for Covid-19 with SARS-CoV-2 neutralizing
antibody sotrovimab. N Engl J Med. 385:1941–1950. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Chen P, Nirula A, Heller B, Gottlieb RL,
Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, et al:
SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with
Covid-19. N Engl J Med. 384:229–237. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Kim JY, Jang YR, Hong JH, Jung JG, Park
JH, Streinu-Cercel A, Streinu-Cercel A, Săndulescu O, Lee SJ, Kim
SH, et al: Safety, virologic efficacy, and pharmacokinetics of
CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike
receptor-binding protein: Two randomized, placebo-controlled, phase
I studies in healthy individuals and patients with mild SARS-CoV-2
infection. Clin Ther. 43:1706–1727. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Levin MJ, Ustianowski A, De Wit S, Launay
O, Avila M, Templeton A, Yuan Y, Seegobin S, Ellery A, Levinson DJ,
et al: Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for
prevention of Covid-19. N Engl J Med. 386:2188–2200. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
ACTIV-3/Therapeutics for Inpatients with
COVID-19 (TICO) Study Group, : Efficacy and safety of two
neutralising monoclonal antibody therapies, sotrovimab and BRII-196
plus BRII-198, for adults hospitalised with COVID-19 (TICO): A
randomised controlled trial. Lancet Infect Dis. 22:622–635. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Weinreich DM, Sivapalasingam S, Norton T,
Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, et al:
REGN-COV2, a neutralizing antibody cocktail, in outpatients with
Covid-19. N Engl J Med. 384:238–251. 2021. View Article : Google Scholar : PubMed/NCBI
|