Introduction
Vogt-Koyanagi-Harada (VKH) disease is an autoimmune
disease that is relatively common in China and is characterized by
ophthalmic, auditory, dermatologic and neurologic effects. The most
common ophthalmic manifestation of VKH disease is granulomatous
intraocular inflammation. Serous retinal detachment (SRD) is the
most common ocular manifestation in patients presenting in the
acute phase of VKH disease (1,2).
The etiology and pathogenesis of VKH disease are
still unknown. One putative mechanism may involve an autoimmune
response to melanocytes, which is mediated through CD4+
T cells (3). Changes in the ratio
of CD4+ lymphocytes and CD4+/CD8+
cells were observed in skin lesions of patients with VKH disease.
Histopathology of skin lesions demonstrated that in addition to
diffuse infiltration of activated T cells in the choroid membrane,
the lesions also contained infiltrations of plasma cells,
multinucleated giant cells and other cells, which suggested that
humoral immunity may also serve a role in the pathogenesis of VKH
disease (4).
Immunoglobulins are globulin proteins with
antibody-like structures that exert important immune antibody
activities. Upon stimulation by an antigen, B cells proliferate and
differentiate into plasma cells, which secrete antigen-relevant Ig
antibodies involved in the regulation of humoral immunity. Igs also
serve immunomodulatory roles by neutralizing specific antigens and
activating the complement system (5). Studies have demonstrated that serum
IgG, IgA and IgM also serve important roles in numerous diseases,
including autoimmune hemolytic anemia, IgA nephropathy and
autoimmune hepatitis (6–8). It has also been reported that the
serum IgG, IgA and IgM levels were significantly increased in
patients with rheumatoid arthritis and the changes in these levels
were associated with disease activity (9). Elevated IgE levels were detected in
patients with other autoimmune diseases, including systemic lupus
erythematosus and bullous pemphigoid, and IgE autoantibodies were
also detected in these patients (10,11). Previous studies have also reported
higher serum total IgE levels in patients with types of autoimmune
uveitis, including acute iridocyclitis, Eales' disease, pars
planitis and multifocal choroiditis compared with normal control
group patients (12,13).
However, few previous studies have assessed Ig
levels in patients with VKH disease or examined the correlation
between Ig levels and retinal structural parameters. Therefore, in
the present study, the medical records of patients admitted to
Shanghai Xuhui Central Hospital with acute VKH disease were
reviewed and the serum Ig levels in these patients were analyzed
according to the extent of SRD. Correlations between Ig levels and
changes in retinal structure in acute VKH disease were also
examined.
Materials and methods
Ethics approval
The present study was approved by the Shanghai Xuhui
Central Hospital Ethics Committee (Shanghai, China; approval no.
2020-179). These protocols followed the tenets of The Declaration
of Helsinki, and written informed consent was obtained from all
patients or their guardian after obtaining ethics approval and
prior to performing the analysis.
Patients
This was a retrospective clinical study. The authors
retrieved the medical records of patients with newly diagnosed
acute VKH who were admitted to Shanghai Xuhui Central Hospital
(Shanghai, China) between August 2015 and June 2020. Patients who
satisfied the diagnostic criteria for acute VKH disease, as
previously described (2), were
eligible for inclusion in the present study. Once identified,
patients were contacted to obtain informed consent. Patients with
corneal disease, glaucoma, eye trauma, a history of eye surgery,
eye developmental abnormalities or genetic diseases were excluded.
Patients with asthma, urticaria, systemic lupus erythematosus,
bullous pemphigoid or other systemic immune diseases were also
excluded. Furthermore, patients who did not cooperate with the
examinations or patients with corneal or lenticular opacity were
excluded. The best-corrected visual acuity (BCVA), which was taken
as the converted logarithm of the minimum angle of resolution
(logMAR), and the interval between the onset of ocular symptoms and
initiation of treatment were retrieved from the patients' medical
records. Optical coherence tomography (OCT) and serum data,
assessed at the same time as BCVA, were also retrieved from the
medical records.
OCT examination of the macular
area
OCT was performed at the time of admission to
hospital. Patients' pupils were dilated with tropicamide (0.5%
tropicamide and 0.5% deoxyepinephrine hydrochloride) eye drops
before the examination. The macular areas were scanned using a
Cirrus HD-OCT 4000 OCT scanner (Carl Zeiss AG) with software
version 4.0. All patients were scanned in both the macular cube
mode and with a five-line raster.
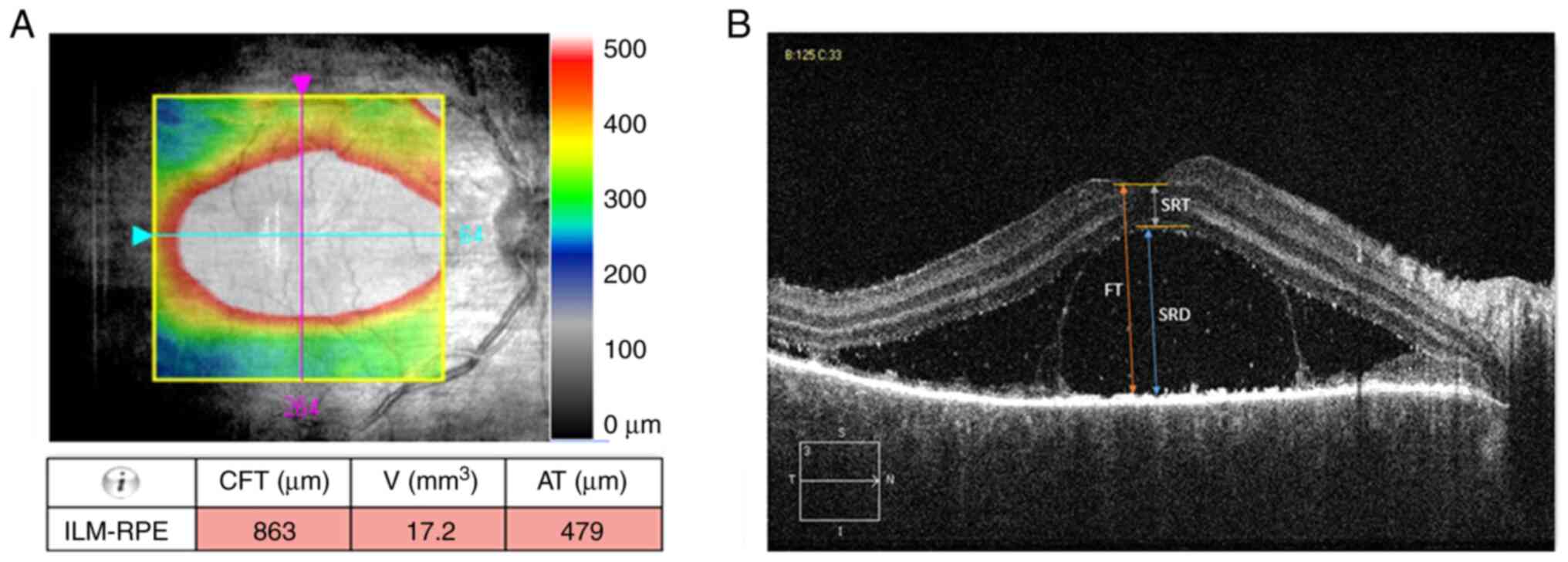
The macular cube 512×128 mode scanned a 6.0×6.0 mm
square region centered on the fovea. The central foveal thickness
(CFT; assessed in the central 1 mm subfield), cube volume (V) and
cube average thickness (AT) were automatically quantified by the
software (Fig. 1A).
In the five-line raster scanning mode, the distance
between each line was 0.25 mm and the transverse scans were
centered on the fovea. Foveal thickness (FT), SRD and sensory
retinal thickness (SRT) were quantified manually using the
software's caliper. FT boundaries were set as the internal limiting
membrane and the inner boundary of the retinal pigment epithelium
(RPE) layer. SRD was defined as the distance from the inner
boundary of the sensory layer to the internal boundary of the RPE
layer in the fovea. SRT was defined as the distance from the inner
boundary membrane in the fovea to the inner boundary of the sensory
layer (Fig. 1B). Each measurement
was repeated twice and the mean value was recorded. The patients
were divided into two groups based on the SRD, a high-detachment
group (>500 µm) and low-detachment group (≤500 µm).
Measurement of serum Igs, CRP and
TNF-α levels
Fasting venous blood samples were obtained at the
time of admission and the sera were separated and stored at −20°C
until testing. Ig levels were quantified by rate-scattering
turbidimetry on a BN II System automatic analyzer (Siemens
Healthineers). CRP and TNF-α levels were assessed using ELISA kits
(R&D Systems, Inc.).
Statistical analysis
SPSS version 15.0 (SPSS, Inc.) was used for
statistical analyses. Data were tested for a normal distribution
using the Kolmogorov-Smirnov test. Data that did not conform to a
normal distribution were presented as the median. The Mann-Whitney
U test was used for comparisons between the two groups. Pearson's
χ2 test was used to test categorical variables. In all
analyses, P<0.05 was considered to indicate a statistically
significant difference.
Results
General clinicopathological
characteristics of patients
The present study included 138 patients, of whom 67
were male (48.55%). The median age was 41.5 years (range, 14–76
years) and the mean interval between the onset of ocular symptoms
and initiation of treatment was 17.71 days (range, 2–90 days). The
mean logMAR BCVA was 0.76±0.56.
General clinicopathological characteristics of
patients in the two groups are presented in Table I. Of the138 patients, 51 were
included in the high-detachment group, 30 of whom were male
(58.82%). The other 87 patients were included in the low-detachment
group, of whom 35 were male (40.23%). In the high-detachment group,
the median age was 42 years [interquartile range (IQR), 28-52] and
the median interval between the onset of ocular symptoms and
initiation of treatment was 10 days (IQR, 7-20). The median logMAR
BCVA for the high-detachment group was 1.0 (IQR, 0.6-1.3). In the
low-detachment group, the median age was 41 years (IQR, 28-50) and
the median interval between the onset of ocular symptoms and
initiation of treatment was 14 days (IQR, 7-21). The median logMAR
BCVA for the low-detachment group was 0.5 (IQR, 0.3-0.7).
 | Table I.General clinicopathological
characteristics of patients with serous retinal detachment. |
Table I.
General clinicopathological
characteristics of patients with serous retinal detachment.
| Clinicopathological
characteristic | High-detachment
group | Low-detachment
group | P-value |
|---|
| n (male; %) | 51.00 (30.00;
58.82) | 87.00 (35.00;
40.23) | 0.035 |
| Age,
yearsa | 42.00 (28.00,
52.00) | 41.00 (28.00,
50.00) | 0.841 |
| Interval between
onset of symptoms and initiation of treatment, daysa | 10.00 (7.00,
20.00) | 14.00 (7.00,
21.00) | 0.535 |
| logMAR
BCVAa | 1.00 (0.60,
1.30) | 0.50 (0.30,
0.70) | <0.001 |
The proportion of males (P=0.035) and the logMAR
BCVA (P<0.001) were significantly different in the
high-detachment group compared with the low-detachment group.
However, there were no significant differences in the age of onset
(P=0.841) or the interval between the onset of ocular symptoms and
initiation of treatment (P=0.535) between the two groups.
Serum Ig, CRP and TNF-α levels
The serum IgA, IgG, IgM, IgE, CRP and TNF-α levels
of the two groups are presented in Table II. There were no significant
differences in the serum levels of IgA (P=0.304), IgG (P=0.208),
IgM (P=0.865), CRP (P=0.082) or TNF-α (P=0.099) between the high-
and the low-detachment groups. However, the serum IgE level was
significantly greater in the high-detachment group compared with
the low-detachment group (P=0.016).
 | Table II.Comparison of serum IgA, IgG, IgM,
IgE, CRP and TNF-α between patients with serous retinal
detachment. |
Table II.
Comparison of serum IgA, IgG, IgM,
IgE, CRP and TNF-α between patients with serous retinal
detachment.
| Serum component | High-detachment
groupa | Low-detachment
groupa | P-value |
|---|
| IgA, g/l | 2.04 (1.77,
2.59) | 2.26 (1.74,
3.08) | 0.304 |
| IgG, g/l | 11.20 (9.74,
13.05) | 11.69 (10.20,
13.76) | 0.208 |
| IgM, g/l | 1.23 (0.92,
1.64) | 1.24 (0.86,
1.67) | 0.865 |
| IgE, U/ml | 83.00 (30.00,
251.00) | 53.00 (32.00,
80.00) | 0.016 |
| CRP, mg/l | 1.04 (0.50,
2.02) | 0.67 (0.30,
3.00) | 0.082 |
| TNF-α, pg/l | 4.60 (4.00,
6.43) | 4.21 (4.00,
5.47) | 0.099 |
Comparison of Oct macular area
morphologic characteristics
The FT, SRD, SRT, CFT, V and AT macular parameters
of the high- and low-detachment groups are presented in Table III. FT (P<0.001), SRD
(P<0.001), CFT (P<0.001), V (P<0.001) and AT (P<0.001)
were significantly higher in the high-detachment group compared
with the low-detachment group. However, although SRT was not
identified as significantly different between the high- and the
low-detachment groups (P=0.052), it was markedly greater in the
high-detachment group.
 | Table III.Comparison of the OCT parameters FT,
SRD, SRT, CFT, V and AT between patients with serous retinal
detachment. |
Table III.
Comparison of the OCT parameters FT,
SRD, SRT, CFT, V and AT between patients with serous retinal
detachment.
| OCT parameter | High-detachment
groupa | Low-detachment
groupa | P-value |
|---|
| FT, µm | 864.00 (661.00,
1247.25) | 324.50 (232.00,
446.25) | <0.001 |
| SRD, µm | 701.00 (421.75,
997.25) | 162.00 (96.00,
282.00) | <0.001 |
| SRT, µm | 141.00 (124.00,
161.25) | 134.00 (118.00,
158.50) | 0.052 |
| CFT, µm | 813.00 (606.75,
1016.25) | 362.00 (268.75,
445.50) | <0.001 |
| V,
mm3 | 17.60 (14.70,
20.38) | 11.80 (10.90,
13.00) | <0.001 |
| AT, µm | 489.50 (408.00,
566.75) | 327.50 (320.75,
360.25) | <0.001 |
Correlations and associations between
IgE, BCVA and OCT macular characteristics
Correlations between IgE or BCVA and OCT assessed
macular characteristics are presented in Table IV. The serum IgE levels were
weakly positively associated with SRD (r=0.136; P=0.024), CFT
(r=0.137; P=0.023) and AT (r=0.125; P=0.038). Moreover, the BCVA
was significantly positively correlated with FT (r=0.644;
P<0.001), SRD (r=0.618; P<0.001), SRT (r=0.160, P=0.008), CFT
(r=0.588; P<0.001), V (r=0.596; P<0.001) and AT (r=0.554;
P<0.001).
 | Table IV.Correlations between IgE, BCVA and
optical coherence tomography macular parameters. |
Table IV.
Correlations between IgE, BCVA and
optical coherence tomography macular parameters.
| Component | IgE | logMAR BCVA | FT | SRD | SRT | CFT | V | AT |
|---|
| IgE |
|
|
|
|
|
|
|
|
|
r-valuea | 1.000 | 0.067 | 0.110 | 0.136 | −0.041 | 0.137 | 0.113 | 0.125 |
|
P-value |
| 0.269 | 0.067 | 0.024 | 0.500 | 0.023 | 0.061 | 0.038 |
| logMAR BCVA |
|
|
|
|
|
|
|
|
|
r-valuea | 0.067 | 1.000 | 0.644 | 0.618 | 0.160 | 0.588 | 0.596 | 0.554 |
|
P-value | 0.269 |
| <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 |
Analysis of risk factors for severe
SRD
Multivariate binary logistic regression was
performed using IgA, IgG, IgM, IgE, CRP and TNF-α as the
independent variables and severity of SRD (SRD >500 µm=1; SRD
≤500 µm=0) as the dependent variable. Age, sex (male=1; female=2)
and interval between ocular symptom onset and initiation of
treatment were also included for adjustment. In this analysis, male
(P=0.049) and serum IgE level (P=0.014) were identified as putative
significant independent risk factors for severe SRD (Table V). The receiver operating
characteristic curve analysis demonstrated that the area under the
curve for IgE as a diagnosis of severe SRD was 0.623 (P=0.016).
 | Table V.Multivariate binary logistic
regression analysis of risk factors for severe serous retinal
detachment. |
Table V.
Multivariate binary logistic
regression analysis of risk factors for severe serous retinal
detachment.
| Item | B-value | Wald-value | P-value | Odds ratio
(95%CI) |
|---|
| Sex | 2.447 | 3.876 | 0.049 | 1.004-5.964 |
| Age | 0.996 | 0.089 | 0.766 | 0.968-1.024 |
| Interval | 1.001 | 0.014 | 0.906 | 0.982-1.020 |
| IgA | 1.284 | 1.146 | 0.284 | 0.812-2.029 |
| IgG | 0.972 | 0.150 | 0.698 | 0.842-1.122 |
| IgM | 0.754 | 0.945 | 0.331 | 0.427-1.333 |
| IgE | 0.997 | 6.034 | 0.014 | 0.995-0.999 |
| CRP | 1.034 | 0.151 | 0.698 | 0.875-1.221 |
| TNF-α | 0.917 | 0.497 | 0.481 | 0.719-1.168 |
Discussion
All of the patients included in the present study
were of Han Chinese ethnicity. The mean age at onset of VKH disease
was 41.65 years (range 14–76 years). The percentage of females
(51.45%) was lower compared with a previous report (3). Possible explanations may include the
differing sample sizes or racial differences (3). However, the proportion of males was
significantly greater in the high-detachment group than in the
low-detachment group and the regression analysis demonstrated that
male sex was a significant risk factor for SRD in this cohort of
patients with VKH disease. These results suggest that, among Han
Chinese, males with acute VKH disease are more likely than females
to present with severe SRD.
VKH disease is a common type of panuveitis. As
choroidal inflammation develops, it first affects the adjacent RPE
layer, causing it to crease. As choroidal vascular permeability
increases, inflammatory choroidal fluid accumulates beneath the
neuroepithelium, which causes neuroepithelial detachment.
Fluorescein and indocyanine green angiography can be used to detect
any leaks from retinal blood vessels that may lead to SRD. SRD is a
common manifestation of VKH disease that indirectly reflects the
severity of inflammation (1).
OCT, a non-invasive imaging modality, can provide clear tomographic
images that demonstrate the microstructure of the retina (14). The extent of SRD can be quantified
using numerous OCT parameters. In the present study, the age at
onset and the interval between ocular symptom onset and initiation
of treatment were not significantly different between the high and
low-detachment groups. However, FT, SRD, CFT, V and AT were
significantly greater in the high-detachment group than in the
low-detachment group, demonstrating that the leakage caused by
inflammation was more severe in the high-detachment group. However,
SRT was not significantly different between the two groups, which
suggested that in the acute stage, the inflammatory process had not
caused marked changes in the sensory layer of the retina. Although
not statistically significant, SRT was generally greater in the
high-detachment group. The BCVA was significantly worse in the
high-detachment compared with the low-detachment group, it was
significantly positively correlated with FT, SRD, SRT, CFT, V and
AT. These findings demonstrated that OCT scans in patients with
acute VKH disease not only depicted changes in the retinal
structure, but also indirectly reflected the patient's disease
severity and BCVA.
The present study demonstrated no significant
differences in the serum IgA, IgG, IgM, CRP or TNF-α levels when
compared between the high- and low-detachment groups. However, the
serum IgE level was significantly greater in the high-detachment
group compared with the low-detachment group. IgE synthesis is
regulated through a number of factors, including T lymphocytes, B
lymphocytes and numerous cytokines (15). The interaction between CD40 on the
surface of B lymphocytes and CD40L expressed by CD4+ T
lymphocytes is crucial for mediating antigen-specific IgE responses
in vivo. The IgE response is dependent on T lymphocytes
because the activation of B lymphocytes requires the additional T
lymphocyte factors IL-4 and IL-3 (16). IgE is highly sensitive to the T
cell-derived cytokine environment because it is regulated by
cytokines secreted from CD4+ T cells (15). The most widely recognized
pathogenesis of VKH disease involves autoimmune inflammation
mediated by CD4+ T cells targeting melanocytes (3). Therefore, it may be hypothesized
that elevated IgE levels may also be involved in the autoimmune
inflammation mediated by CD4+ T cells.
IgE is recognized as the antibody that mediates
parasitic immunity and type I hypersensitivity. Previous studies
have reported that the serum IgE level is elevated in patients with
certain autoimmune diseases; IgE autoantibodies are also detected
in a number of patients (10,12). A retrospective study of 1,583
patients reported that allergic diseases (53.14%) and autoimmune
diseases (47.37%) were the most common disease groups associated
with patients assessed as having elevated serum IgE levels
(17). The correlation between
the serum total IgE level and autoimmune disease severity has been
reported as being the same as that for specific IgE autoantibodies
(10). Moreover, several previous
studies have reported that elevated serum total IgE levels were
closely associated with the activity of diseases, including
systemic lupus erythematosus and bullous pemphigoid (18–20). Permin and Wiik (21) also reported that, although immune
complexes comprised IgE autoantibodies, IgE itself increased
vascular permeability by mediating the release of vasoactive
amines. In the present study, the serum IgE levels in the
high-detachment group was significantly higher compared with that
in the low-detachment group, and it was weakly positively
associated with SRD, CFT and AT. Logistic regression analysis
demonstrated that serum IgE level was an independent risk factor
for severe SRD. We hypothesized that high IgE levels may lead to a
marked increase in vascular permeability, which then progressed to
severe SRD, and that high serum IgE levels may have contributed to
the severe condition of patients with acute VKH disease.
To the best of our knowledge, the present study is
the first to investigate the relationship between SRD and Igs in
patients with acute VKH disease. However, the present study has
certain limitations; owing to the limitations of the Cirrus OCT-HD
4000 scanner, the images of the choroid were unclear and disease
inflammation also made it difficult to quantify the relevant
choroid parameters. Furthermore, the serum total IgE level was
quantified but the IgE autoantibody levels were not assessed in
patients with acute VKH disease.
In conclusion, males with acute VKH disease were
more likely to present with severe SRD. Furthermore, the severity
of SRD was associated with high serum IgE levels, which suggested
that IgE may be involved in the pathogenesis and/or progression of
VKH disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Medical Project of
Shanghai Xuhui Central Hospital (grant no. 2019-019).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ and NZ analyzed and interpreted data and wrote
the manuscript. HJ and MLZ collected and analyzed the data and
confirm the authenticity of all the raw data. JD and MZ were
responsible for the conception and design of the work. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Shanghai Xuhui
Central Hospital Ethics Committee (Shanghai, China; approval no.
2020-179). Written informed consent was obtained from all patients
after obtaining ethics approval and prior to performing the
analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rao NA, Gupta A, Dustin L, Chee SP, Okada
AA, Khairallah M, Bodaghi B, Lehoang P, Accorinti M, Mochizuki M,
et al: Frequency of distinguishing clinical features in
Vogt-Koyanagi-Harada disease. Ophthalmology. 117:591–599.
599.e12010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang P, Zhong Y, Du L, Chi W, Chen L,
Zhang R, Zhang M, Wang H, Lu H, Yang L, et al: Development and
evaluation of diagnostic criteria for Vogt-Koyanagi-Harada disease.
JAMA Ophthalmol. 136:1025–1031. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Keefe GA and Rao NA:
Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 62:1–25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patil YB, Garg R, Rajguru JP, Sirsalmath
M, Bevinakatti VA, Kumar M and Sharma S: Vogt-Koyanagi-Harada (VKH)
syndrome: A new perspective for healthcare professionals. J Family
Med Prim Care. 9:31–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turula H and Wobus CE: The role of the
polymeric immunoglobulin receptor and secretory immunoglobulins
during mucosal infection and immunity. Viruses. 10:2372018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajmi H, Mabrouk S, Hassayoun S, Regaieg H,
Tfifha M, Jalel C, Skouri H, Zouari N and Abroug S: Success of
anti-CD20 monoclonal antibody treatment for severe autoimmune
hemolytic anemia caused by warm reactive immunoglobulin A,
immunoglobulin G, and immunoglobulin M autoantibodies in a child: A
case report. J Med Case Rep. 11:3212017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wyatt RJ and Julian BA: IgA nephropathy. N
Engl J Med. 368:2402–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pape S, Schramm C and Gevers TJ: Clinical
management of autoimmune hepatitis. United European Gastroenterol
J. 7:1156–1163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanjuan MA, Sagar D and Kolbeck R: The
role of IgE in autoimmunity. J Allergy Clin Immunol. 137:1651–1661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Messingham KA, Holahan HM and Fairley JA:
Unraveling the significance of IgE autoantibodies in organ-specific
autoimmunity: Lessons learned from bullous pemphigoid. Immunol Res.
59:273–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Romero MD, Muiño JC, Bianco GA, Ferrero M,
Juarez CP, Luna JD and Rabinovich GA: Circulating anti-galectin-1
antibodies are associated with the severity of ocular disease in
autoimmune and infectious uveitis. Invest Ophthalmol Vis Sci.
47:1550–1556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muiño JC, Juárez CP, Luna JD, Castro CC,
Wolff EG, Ferrero M and Romero-Piffiguer MD: The importance of
specific IgG and IgE autoantibodies to retinal S antigen, total
serum IgE, and sCD23 levels in autoimmune and infectious uveitis. J
Clin Immunol. 19:215–222. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta V, Gupta P, Singh R, Dogra MR and
Gupta A: Spectral-domain Cirrus high-definition optical coherence
tomography is better than time domain stratus optical coherence
tomography for evaluation of macular pathologic features in
uveitis. Am J Ophthalmol. 145:1018–1022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He JS, Narayanan S, Subramaniam S, Ho WQ,
Lafaille JJ and Curotto de Lafaille MA: Biology of IgE production:
IgE cell differentiation and the memory of IgE responses. Curr Top
Microbiol Immunol. 388:1–19. 2015.PubMed/NCBI
|
|
16
|
Turqueti-Neves A, Otte M, Prazeres da
Costa O, Höpken UE, Lipp M, Buch T and Voehringer D:
B-cell-intrinsic STAT6 signaling controls germinal center
formation. Eur J Immunol. 44:2130–2138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Liu N and Zhang M: The detection
results of total IgE in patients with different types of diseases.
Labeled Immunoassays Clin Med. 26:1452–1460. 2019.(In Chinese).
|
|
18
|
Cozzani E, Gasparini G, Di Zenzo G and
Parodi A: Immunoglobulin E and bullous pemphigoid. Eur J Dermatol.
28:440–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Atta AM, Sousa CP, Carvalho EM and
Sousa-Atta MLB: Immunoglobulin E and systemic lupus erythematosus.
Braz J Med Biol Res. 37:1497–1501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lamb PM, Patton T and Deng JS: The
predominance of IgG4 in prodromal bullous pemphigoid. Int J
Dermatol. 47:150–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Permin H and Wiik A: The prevalence of IgE
antinuclear antibodies in rheumatoid arthritis and systemic lupus
erythematosus. Acta Pathol Microbiol Scand C. 86C:245–249.
1978.PubMed/NCBI
|