Introduction
Liver cancer is the second leading cause of
cancer-related death worldwide. Currently, the number of diagnosed
cases of liver cancer in China accounts for ~45% of the global
incidence, and the disease ranks first and second among diverse
types of cancer in rural and urban areas, respectively. At present,
China has the highest incidence of primary liver cancer in the
world (1–4). Although the one-year survival rate
after radical resection of liver cancer has increased from 39 to
87% in recent decades in China, the five-year survival rate after
curative resection has been reported to be 15–40% (5,6).
The lack of effective targeted therapies and high frequency of
metastatic tumors are the main causes of poor prognosis for
patients with primary liver cancer (7,8).
Epidemiological evidence indicated that inflammation
may lead to tumorigenesis and cancer development. It has been
estimated that 20% of malignant tumors were induced or promoted by
inflammation (9,10). Furthermore, for liver cancer,
numerous studies have reported that inflammation is closely
associated with the molecular mechanisms underlying liver cancer.
In addition, inflammation has been indicated to facilitate the
development and metastasis of liver cancer (11,12). Although the molecular mechanisms
of how inflammation may cause malignancies have remained to be
fully elucidated, inflammation is well known to promote the
proliferation, invasion and migration of cancer cells. It has been
demonstrated that overexpression of nuclear factor-κB (NF-κB)-p50
accounts for the inability of tumor-associated macrophages (TAMs)
to mount an effective M1 antitumor response capable of inhibiting
tumor growth (13). Furthermore,
PI3K-γ is able to control the transformation of M1-type to M2-type
macrophages, thereby promoting tumor immunosuppression and
progression.
Macrophages may polarize into pro- or
anti-inflammatory phenotypes (M1-like and M2-like macrophages,
respectively). Cancer-related inflammation in the tumor
microenvironment (TME) is a hallmark of cancer. A study reported
the association of the incidence of liver cancer with TAM. The
M2-type macrophages polarized from TAM were indicated to promote
the migration and epithelial-mesenchymal transition of liver cancer
cells through the Toll-like receptor 4/STAT3 signaling pathway
(14). In addition, microRNA-101
inhibited macrophage-induced growth of liver cancer by targeting
dual-specificity phosphatase 1 (15). In addition, the importance of
M1-like TAM was reported, as they promoted the migration and
invasion of liver cancer cells (16,17). However, the effects of M1-like TAM
on the proliferation and anti-apoptotic ability of liver cancer
cells have remained elusive. Therefore, the present study aimed to
evaluate the role of infiltration of M1-like TAM in the
proliferation and anti-apoptotic ability of liver cancer cells.
Furthermore, it was attempted to investigate the relationship
between M1-like TAM and the efficacy of postoperative transcatheter
arterial chemoembolization (TACE) in patients with liver
cancer.
Materials and methods
Sample collection and ethical
statement
All pathologically confirmed liver cancer samples
were collected from 79 patients who underwent curative resection of
primary liver cancer. He patients consisted of 64 males and 15
females, aged 22–84 years (median, 57 years). The surgical
procedures were undertaken at the Affiliated Hospital of Inner
Mongolia Medical University (Hohhot, China) between January and
December 2018. All patients underwent TACE at 1–2 months
post-operation. After TACE, in accordance with the Guidelines for
the Diagnosis and Treatment of Primary Liver Cancer in China from
2017 (18), a total of 45
patients with liver cancer were assessed for their eligibility to
be included in the study and were assigned into groups of complete
remission (CR) or partial remission (PR). In the CR group, all
visible features of the tumor disappeared after less than one
month. In the PR group, a >50% decrease in the cross-product of
the two largest diameters of the tumor was observed, which lasted
>1 month. In addition, 34 patients with liver cancer were
allocated to the no remission (NR) group, where the cross-product
of the two largest perpendicular diameters of the tumor decreased
by <50.0% or increased after TACE. The study was approved by the
Ethics Committee of the Affiliated Hospital of Inner Mongolia
Medical University (Hohhot, China; approval no. YJ 2020001) and the
study was performed in accordance with the Declaration of Helsinki.
In addition, all participants provided written informed consent
prior to the study commencing.
Immunohistochemistry (IHC)
Paraffin-embedded sections of adjacent non-tumor
liver tissues and tumor tissues were analyzed by IHC. IHC was used
to detect the expression levels of CD68, human leukocyte antigen
(HLA)-DR and phosphorylated (p)-p65 of NF-κB proteins. The sections
were washed with PBS and the proteins were detected with the
Vectastain Elite ABC kit (Vector Laboratories, Inc.). Staining for
CD68 and HLA-DR was performed with a double-staining kit (catalog
no. SP-900; ZSGB-Bio, Inc.) according to the manufacturer's
protocol. In brief, 3.5-µm paraffin-embedded sections were placed
in an autoclave, the contained distilled water was heated to the
boil and the samples were maintained for 3 min for antigen
retrieval. The paraffin-embedded sections were incubated at 60°C
for 2 h, deparaffinized and hydrated with xylene and ethanol. This
was followed by washing with PBS and double-distilled water to
retrieve the nuclear antigen, and finally by staining. Primary
antibodies were as follows: Anti-NF-κB p65 (dilution, 1:500; cat.
no. ab86299; Abcam), anti-p-NF-κB p65 (dilution, 1:100; cat. no.
ab86299; Abcam), anti-CD68 (dilution, 1:400; cat. no. ZM-0464;
ZSGB-Bio) and anti-HLA-DRα (dilution, 1:200; cat. no. 2741-1;
Epitomics, Inc.). Secondary antibodies were as follows: Goat
anti-mouse IgG (H&L; dilution, 1:2,000; cat. no. ab205719;
Abcam), goat F(ab')2 anti-rabbit IgG H&L (AP) (dilution, 1:500;
cat. no. ab6015; Abcam) and goat anti-rabbit IgG (H&L;
dilution, 1:2,000; cat. no. ab6702; Abcam). Primary antibodies were
incubated overnight at 4°C and secondary antibodies were incubated
for 2 h at 37°C. Finally, the samples were observed under a TCS SP5
microscope (Leica Microsystems GmbH) and the images acquired with
512×512 pixels were processed by LAS AF Lite 2.6.0 software (Leica
Microsystems GmbH). In the present study, CD68-, HLA-DRα- and
p-p65-positive cells were stained red, brown and brown,
respectively.
Cell lines and culture
HepG2 [HB-8065; American Type Culture Collection
(ATCC)], SNU-182 (CRL-2235; ATCC) and THP-1 (TIB-202; ATCC) cells
were authenticated by short tandem repeat profiling and were
cultured in a Dulbecco's modified Eagle's medium (DMEM; 12491-15;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere with
constant 5% CO2. Subsequently, 5 µM JSH-23 (HY-P13982;
MCE) was added to the medium, followed by incubation for 6 h. The
medium was then replaced with a conditioned medium (CM)
(M1-TAM-CM). Next, Adriamycin (ADM; 10 mg/ml) was added to the
medium, followed by incubation for 24 h. In another experiment, to
assess the effects of radiation, the medium was exposed to 4 Gy for
24 h without adding ADM using a 43885D X-ray machine (Faxition
Bioptics, LLC) with the energy of 50 keV and a distance of ~0.3 m
from the surface of the sample. The cells were irradiated with a
dose rate of 1.084 Gy/min at room temperature and the final dose of
irradiation reached 4.0 Gy.
Preparation of M1-TAM-CM
M1 macrophages were derived from THP-1 (TIB-202;
ATCC) as previously described (19,20). M1 macrophages were cultured in
regular medium (RM) to obtain a CM. In brief, 5×105
cells were seeded into 12-well plates and were incubated with 320
nM phorbol myristate acetate (PMA; cat. no. 16561-29-8;
MilliporeSigma) for 6 h. Samples were then cultured with the
addition of 100 ng/ml lipopolysaccharide (LPS; 297-473-0;
MilliporeSigma) and 20 ng/ml interferon-γ (IFN-γ; SRP3058;
MilliporeSigma) for another 18 h. Next, THP-1 cells were washed
thrice with PBS and cultured in RM at 37°C with 5% CO2
for 24 h. Finally, the media were collected to obtain the CM.
Cell proliferation assay
The HepG2 or SNU-182 cells were seeded into
96-(5,000 cells/well) or 6-(200 cells/well) well plates. In the
96-well plates, the MTT Cell Proliferation and Cytotoxicity Assay
Kit (cat. no. C0009; Beyotime Institute of Biotechnology) was used
to evaluate the cell proliferation ability following the
manufacturer's protocol. In the 6-well plates, the culture medium
was changed every 3 days and the cultivation was stopped when
visible clones were observed. After that, the cells were washed
thrice with PBS and fixed with 4% formaldehyde for 15 min at room
temperature. Supernatants were removed, stained with 0.25% crystal
violet for 25 min at room temperature and were then slowly rinsed
with ultrapure water. The cell proliferation ability was assessed
by measuring absorbance at a wavelength of 595 nm (Aurora-600;
Hangzhou Haipei Instrument Co., Ltd.).
Cell cycle analysis
Liver cancer cells were cultured in RM or CM for 24
h and were then collected. The Cell Cycle and Apoptosis Analysis
Kit (cat. no. 40301ES50; Yeasen Biotechnology Co., Ltd.) was used
to assess the cell cycle with the MACSQuant® Analyzer 10
(Miltenyi Biotec GmbH) in accordance with the manufacturer's
instructions.
Cell apoptosis assay
The Annexin V-FITC/PI kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to prepare cells for flow cytometry. The
CytoFLEX flow cytometer (Beckman Coulter, Inc.) was utilized to
analyze the apoptosis of cells.
Western blot analysis
RIPA lysis buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.) was used to extract the total
protein via a BCA Protein Assay Kit (cat. no. P0010; Beyotime
Institute of Biotechnology). A standardized amount of 40 µg total
protein per sample was used for separation of proteins by 10%
SDS-PAGE at 90 mA for 2 h. Samples were then transferred onto
polyvinylidene fluoride (PVDF) membranes (IB24001; Invitrogen;
Thermo Fisher Scientific, Inc.) at 400 mA for 1 h. The PVDF
membranes were then blocked with 5% fat-free milk powder dissolved
in PBS (blocking solution) for 1 h at room temperature.
Subsequently, the membranes were incubated with the primary
antibodies diluted in the blocking solution at 4°C overnight. Next,
the membranes were incubated with the secondary antibodies at room
temperature for 1 h after three washes with PBS. Finally, Western
Lightning Plus-ECL (PerkinElmer, Inc.) was used to visualize the
protein bands and ImageJ 1.8.0 software (National Institutes of
Health) was employed for image processing. Primary antibodies were
as follows: Anti-NF-κB p65 (dilution, 1:1500; cat. no. ab86299;
Abcam), anti-p-NF-κB p65 (dilution, 1:1,000; cat. no. ab86299;
Abcam), anti-CDK1 (dilution, 1:2,000; cat. no. ab201008; Abcam),
anti-CDK2 (dilution, 1:1,000; cat. no. ab33147; Abcam), anti-cyclin
D1 (dilution, 1:1,000; cat. no. ab1663; Abcam), anti-p21 (dilution,
1:1,500; cat. no. ab109199; Abcam), anti-Bax (dilution, 1:1,000;
cat. no. ab32503; Abcam), anti-Bcl-2 (dilution, 1:1,000; cat. no.
ab32124; Abcam), anti-caspase-3 (dilution, 1:1,000; cat. no.
ab184787; Abcam) and anti-cleaved caspase-3 (dilution, 1:1,000;
cat. no. ab32042; Abcam). Secondary antibodies were as follows:
Goat anti-mouse IgG (H&L; dilution, 1:5,000; cat. no. ab205719;
Abcam) and goat anti-rabbit IgG (H&L; dilution, 1:5,000; cat.
no. ab6702; Abcam). Primary antibodies were incubated overnight at
4°C and secondary antibodies were incubated for 2 h at 37°C.
Statistical analysis
SPSS 20.0 software (IBM Corporation) was utilized
for data analysis. Differences between the two treatment groups
were analyzed by the Student's t-test or the Chi-square test.
Differences among multiple groups were analyzed using one-way ANOVA
and Duncan's post-hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Infiltration of M1-TAM negatively
influences the efficacy of postoperative TACE for patients with
liver cancer
Liver cancer tissues derived from 79 patients were
pathologically examined prior to the patients undergoing TACE to
evaluate the significance of the infiltration of M1-TAM. The
significance of the infiltration of M1-TAM was assessed by
detecting the expression levels of CD68 and HLA-DR via IHC
(Fig. 1A-C). As presented in
Fig. 1B and C, CD68 and HLA-DR
were expressed on the same cell, as is clearly indicated by a red
and brown overlap in Fig. 1C. As
for Fig. 1B, due to the low
expression level, individual expression was observed on certain
cells without any overlap. All patients underwent TACE at 1–2
months post-operation. The abundance of M1-TAM in liver cancer
tissues in the CR+PR group was significantly lower than that in the
NR group (Fig. 1D).
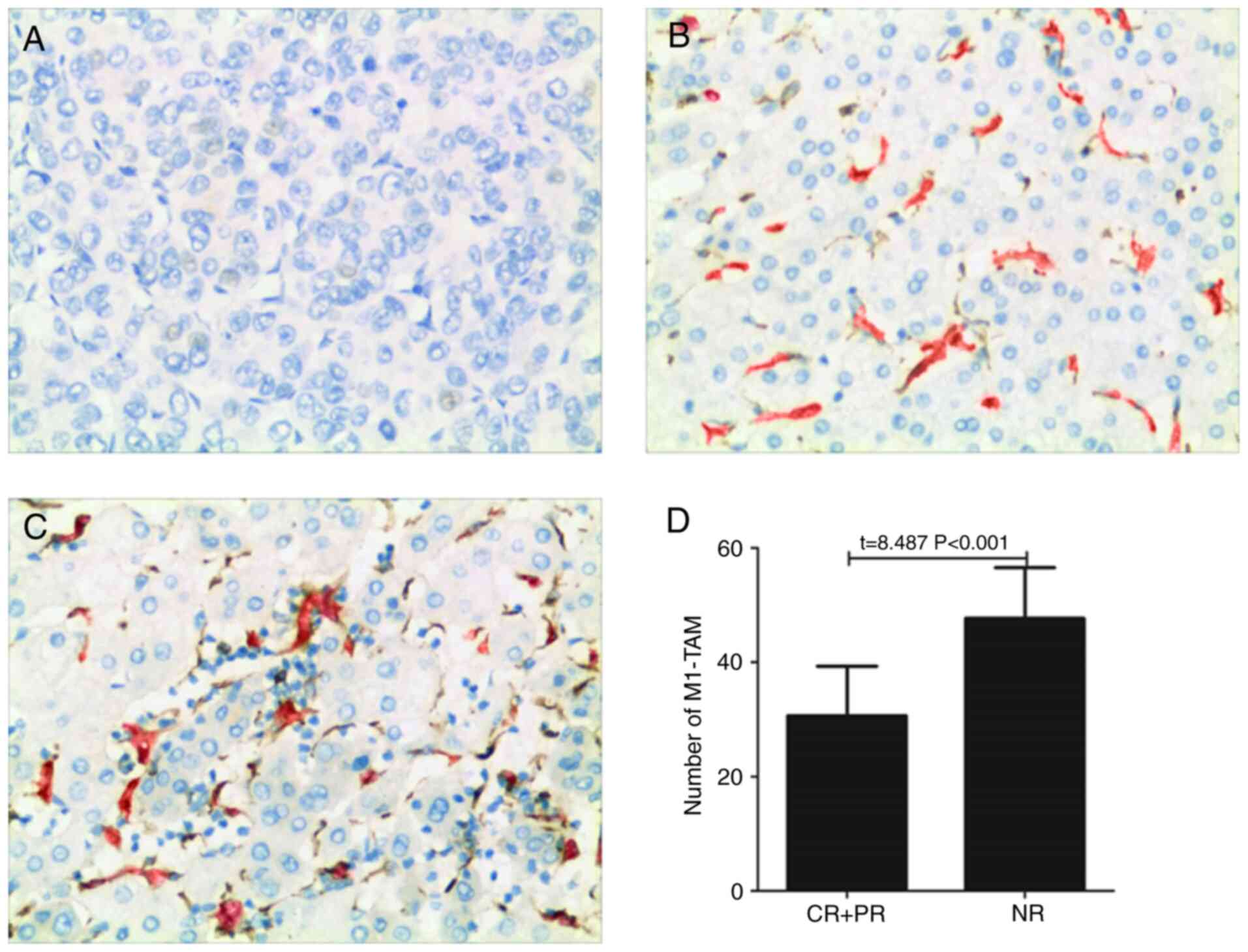 | Figure 1.Infiltration of M1-TAM in human
hepatoma tissue is associated with the therapeutic effects in
patients with liver cancer. (A-C) Immunohistochemistry was used to
detect the expression levels of CD68 (red) and HLA-DR (brown) in a
hepatoma tissue, and representative images of immunohistochemical
detection of CD68+HLA-DR+-labeled M1-like TAM in case of (A) no
expression in a male patient (age, 53 years) with therapeutic
efficacy rated as CR; (B) low expression in a male patient (age, 49
years) with therapeutic efficacy rated as PR; and (C) high
expression in a male patient (age, 58 years) with therapeutic
efficacy rated as NR (magnification, ×200). (D) Number of
CD68+HLA-DR+ (M1-TAM) cells in the hepatoma tissue of patients with
liver cancer. HLA, human leukocyte antigen; TAM, tumor-associated
macrophages; CR, complete remission; PR, partial remission; NR, no
remission. |
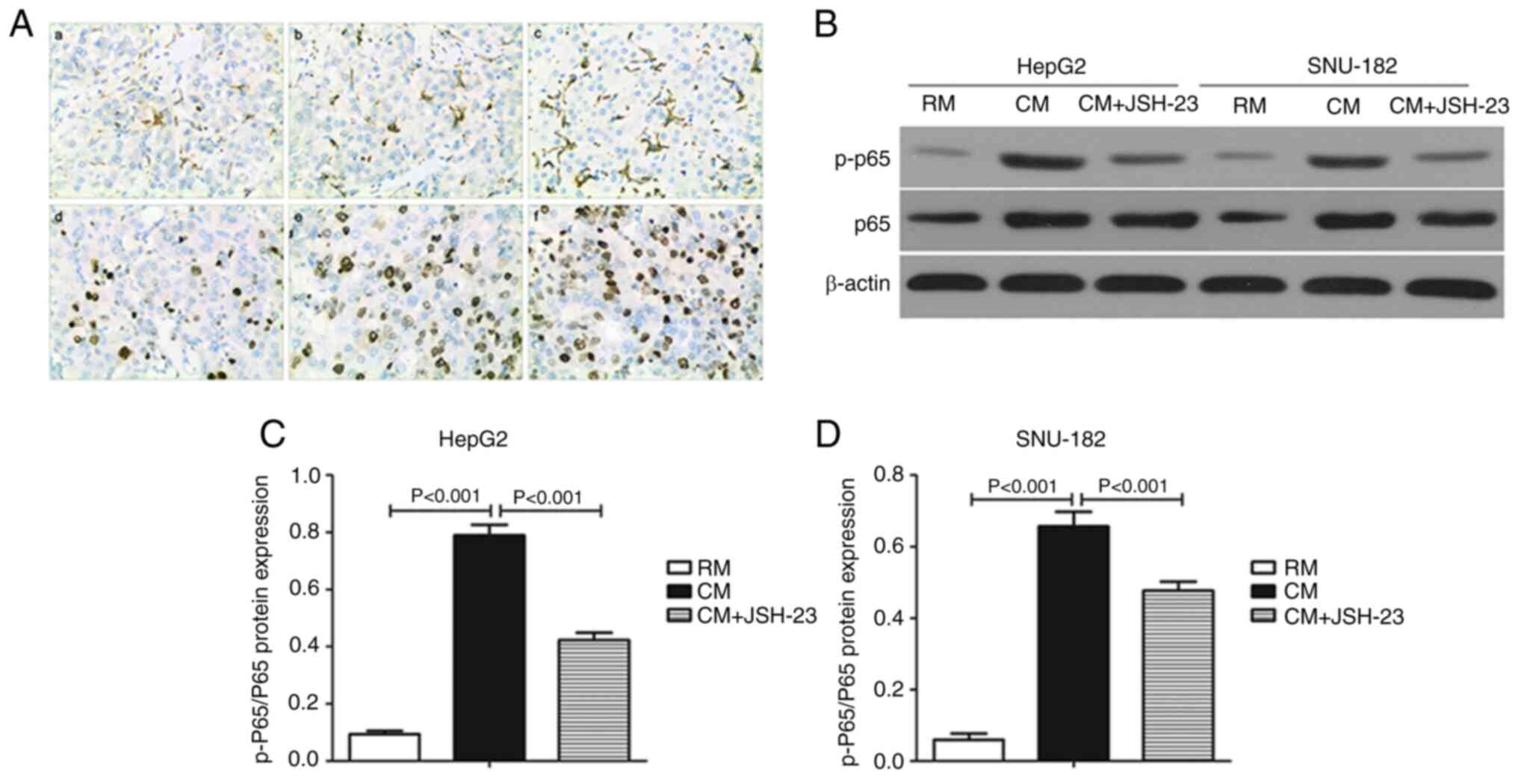
Infiltration of M1-TAM activates the
NF-κB pathway in liver cancer tissues
The level of p-p65 was upregulated as the
infiltration of M1-TAM increased in the liver cancer tissues
(Fig. 2A). In addition, the
p-p65/p65 ratio in both HepG2 (Fig.
2B and C) and SNU-182 (Fig. 2B
and D) cells was significantly higher in the CM than that in
the RM group. Furthermore, JSH-23, an inhibitor that prevents p65
from entering the nucleus, significantly decreased the p-p65/p65
ratio in both HepG2 (Fig. 2B and
C) and SNU-182 cells (Fig. 2B and
D) grown in CM compared to those cultured in RM.
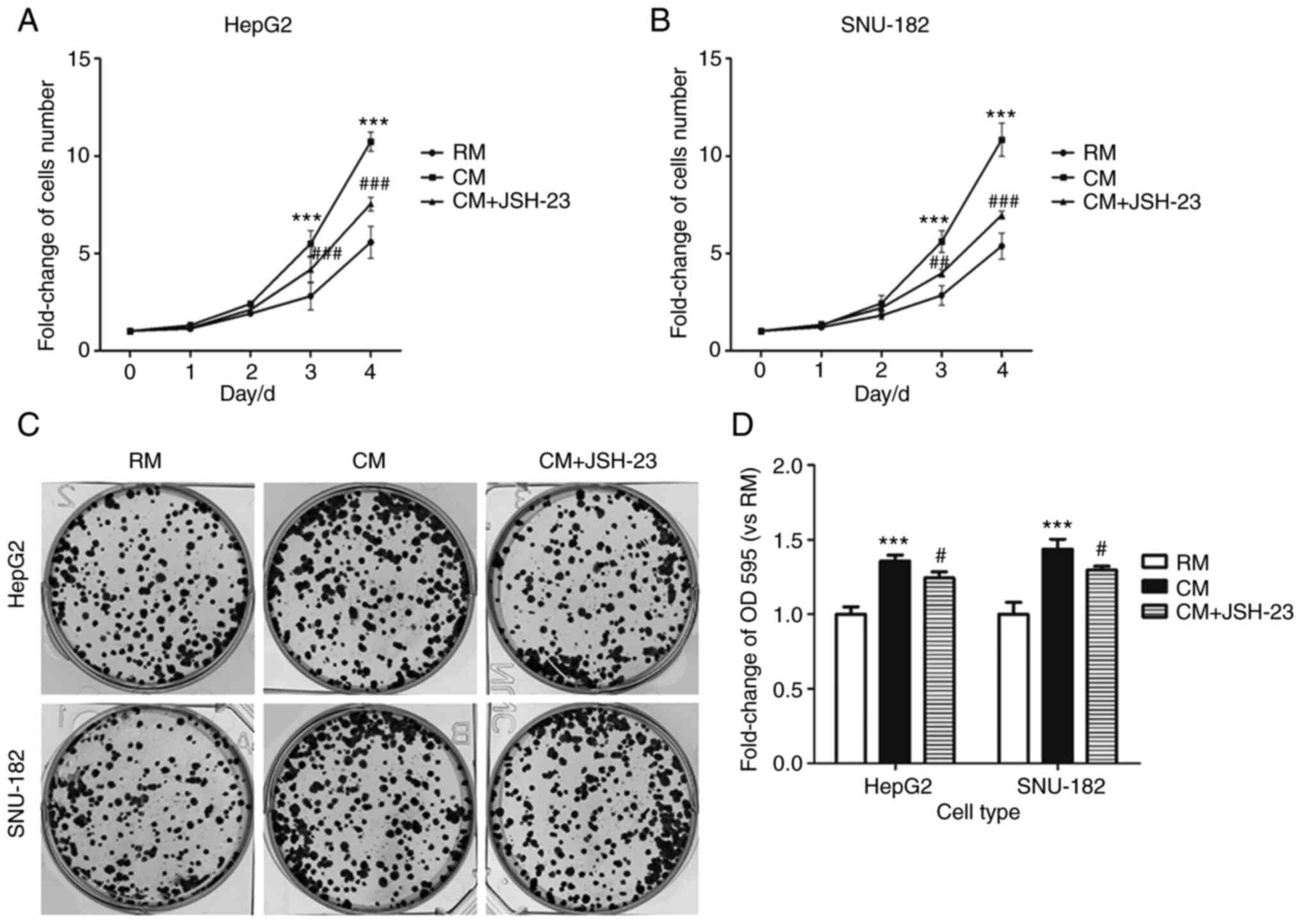
M1 macrophages increase the
proliferation of liver cancer cells
Liver cancer cells were cultured in different media
and after the third day of culture, the number of liver cancer
cells in the CM was markedly higher than that in the RM (Fig. 3A and B). Of note, JSH-23
significantly decreased the number of liver cancer cells in the CM.
In addition, the number of cell clones in the CM group was
significantly higher than that in the RM group and JSH-23 markedly
decreased the number of cell clones in the CM compared with that in
the RM group (Fig. 3C and D).
Furthermore, M1-TAM-CM significantly decreased the ratio of liver
cancer cells in the G1/G0 cell cycle phase, while it markedly
increased the ratio of liver cancer cells in the S and G2/M phases.
However, JSH-23 was able to reverse these changes (Fig. 4). The expression levels of cell
cycle-related proteins, such as CDK1, CDK2, Cyclin D1 and p21, were
different in liver cancer cells in different media. Of note, the
expression levels of CDK1, CDK2 and cyclin D1 in the RM were
markedly lower than those in the CM; however, the expression level
of p21 was significantly higher in the RM than that in the CM.
Collectively, M1 macrophages stimulated liver cancer cell
proliferation, which was reversed by the administration of JSH-23
(an inhibitor of NF-kB), i.e. this effect was mediated via
NF-κB.
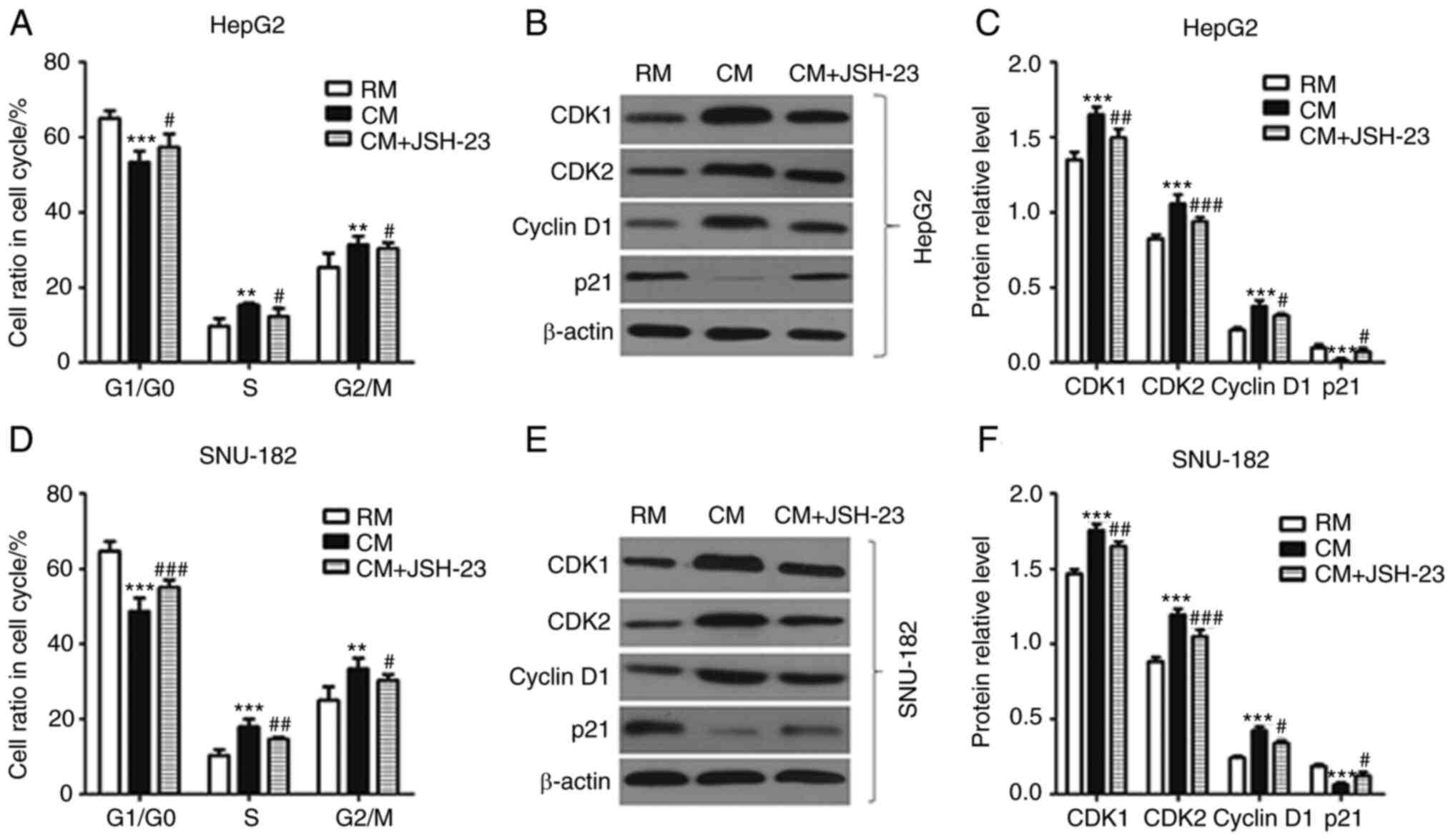 | Figure 4.Effects of M1 macrophages on the cell
cycle of liver cancer cells via activating the NF-κB pathway. (A)
The cell cycle of HepG2 cells was detected by flow cytometry. (B
and C) The expression levels of CDK1, CDK2, cyclin D1 and P21 in
HepG2 cells were detected by western blot analysis. (B)
Representative western blot image and (C) quantified results. (D)
The cell cycle of SNU-182 cells was detected by flow cytometry. (E
and F) The expression levels of CDK1, CDK2, cyclin D1 and P21 in
SNU-182 cells were detected by western blot analysis. (E)
Representative western blot image and (F) quantified results. All
experiments were performed in triplicate. **P<0.01,
***P<0.001 vs. RM group; #P<0.05,
##P<0.01, ###P<0.001 vs. CM group. RM,
regular medium; CM, conditioned medium; CDK, cyclin-dependent
kinase. |
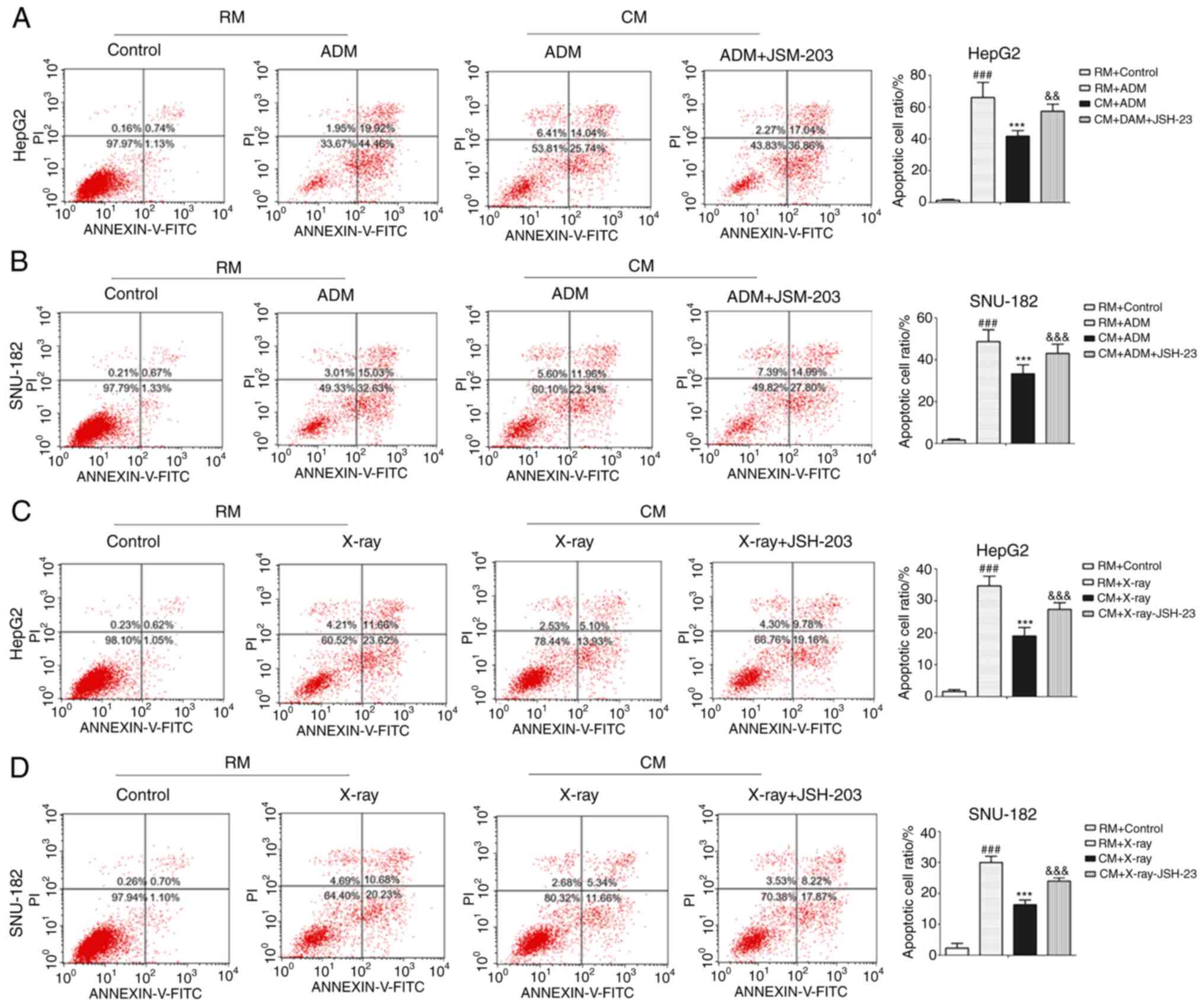
M1 macrophages enhance the
anti-apoptotic ability of liver cancer cells
ADM is frequently used for the treatment of liver
cancer and may induce apoptosis of liver cancer cells (21). In the present study, it was
indicated that ADM significantly increased the number of apoptotic
liver cancer cells in the RM (Fig. 5A
and B). In addition, with ADM administration, the number of
apoptotic liver cancer cells in the CM was significantly lower than
that in the RM. The present results indicated that JSH-23 markedly
increased the number of apoptotic liver cancer cells in the CM.
X-ray irradiation was also used to induce apoptosis of liver cancer
cells similar to ADM and the X-rays significantly increased the
number of apoptotic liver cancer cells in the RM (Fig. 5C and D). However, the number of
apoptotic liver cancer cells in the CM was significantly lower than
that in the RM following exposure to X-rays. It was also observed
that JSH-23 significantly increased the number of apoptotic liver
cancer cells in samples subjected to X-ray in the CM. Furthermore,
the expression levels of apoptosis-related proteins, such as Bax,
Bcl-2 and caspase-3, were detected by western blot analysis. It was
observed that after AMD treatment, the expression levels of Bax and
caspase-3 in the RM were significantly higher than those in the CM
(Fig. 6). In addition, the
expression level of Bcl-2 in the RM was significantly lower than
that in the CM after treatment with AMD. Taken together, M1
macrophages had anti-apoptotic effects on liver cancer cells, which
were reversed by the administration of JSH-23 (an inhibitor of
NF-kB), i.e. this effect was mediated via NF-κB.
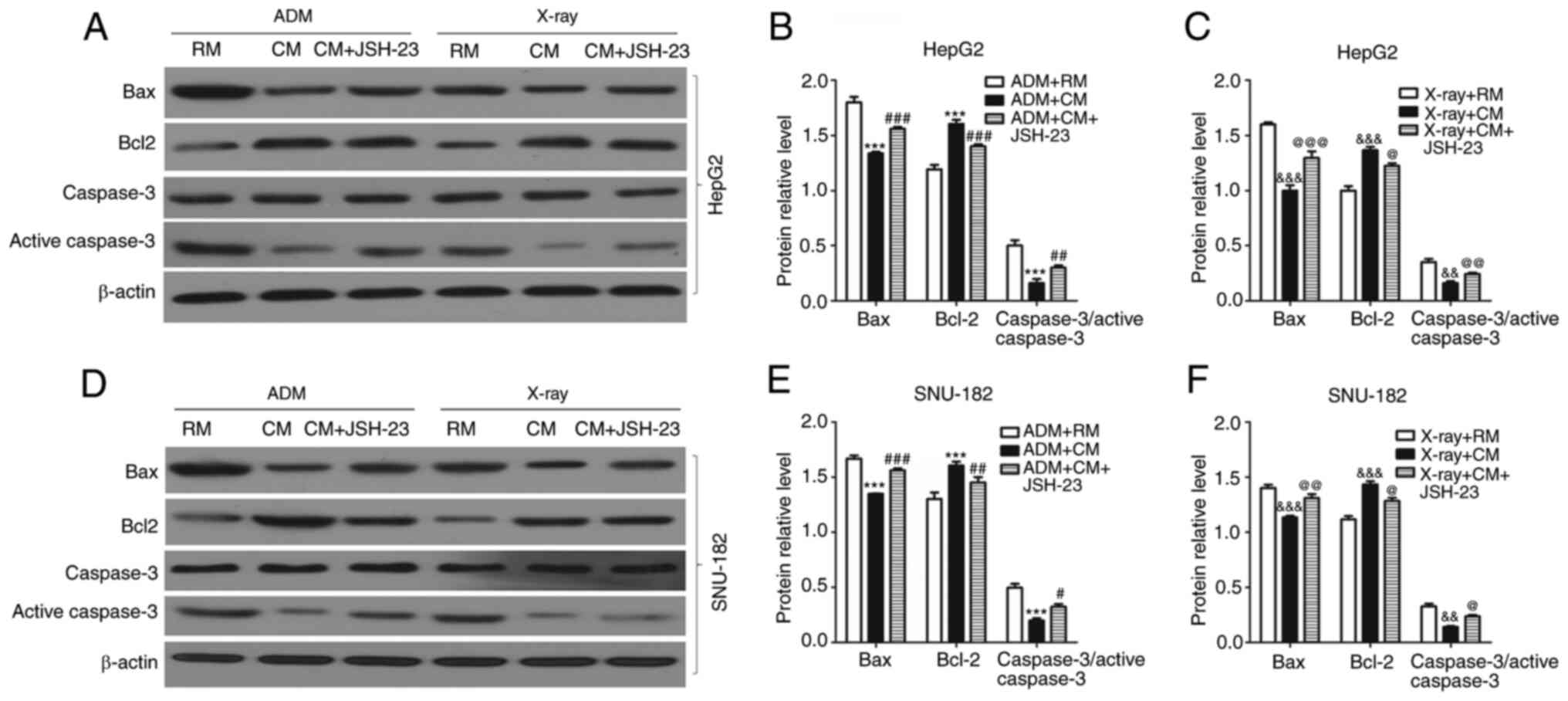 | Figure 6.Effects of M1 macrophages on the
expression levels of apoptosis-related proteins in liver cancer
cells via activating the NF-κB signaling pathway. (A-C) Western
blot analysis was used to detect the expression levels of Bax,
Bcl-2 and caspase-3 in HepG2 cells in RM or CM after treating with
or without ADM or treating with or without X-rays. (A)
Representative western blot image and quantified expression levels
in (B) the ADM experiment and (C) the X-ray experiment. (D-F)
Western blot analysis was employed to detect the expression levels
of Bax, Bcl-2 and caspase-3 in SNU-182 cells in RM or CM after
treating with or without ADM or treating with or without X-rays.
(D) Representative western blot image and quantified expression
levels in (E) the ADM experiment and (F) the X-ray experiment. All
experiments were performed in triplicate. ***P<0.001 vs. ADM+RM
group; #P<0.05, ##P<0.001,
###P<0.001 vs. CM+ADM group;
&&P<0.01,
&&&P<0.001 vs. X-ray + RM group;
@P<0.05, @@P<0.01,
@@@P<0.001 vs. X-ray + CM group. RM, regular medium;
CM, conditioned medium; ADM, Adriamycin. |
Discussion
Tumor-associated macrophages are a well-known
component of the TME and may have pivotal roles in the onset and
development of liver cancer, as well as in the regulation of
inflammation in the TME (22).
However, the association between the infiltration of macrophages
and their effects on the effectiveness of liver cancer treatment
has remained elusive. Concerning post-resection therapy for
patients with liver cancer, TACE is able to limit blood supply to
tumorous tissues and significantly inhibit tumor progression in
comparison with other popular treatments (23,24). Thus, the approach of the present
study is highly significant, as it was observed that a high number
of CD68+HLA-DR+ M1-like TAM in tumorous tissues was negatively
associated with the therapeutic efficacy of TACE. It should also be
pointed out that the therapeutic effect of TACE on liver cancer
depends on not only the sensitivity to a drug (ADM) but also on the
degree of embolization by lipiodol and gelatin sponges.
Consistently with this, in the present study, it was determined
that infiltration of M1-like TAM was associated with the level of
p-p65 in liver cancer tissues. In general, TAMs are reported to
closely resemble M2 macrophages (25,26), but in the present study, M2
macrophages were not detected. In addition, it was observed that
M1-like TAM were able to activate the NF-κB signaling pathway in
liver cancer cells. Therefore, it was concluded that the
infiltration of M1-like TAM in liver cancer tissues may activate
the NF-κB signaling pathway in liver cancer cells.
The NF-κB signaling pathway is also important, as it
is a crucial mediator of inflammation-associated tumors (27,28). The continuous activation of the
NF-κB pathway has been confirmed to have roles in the initiation,
development and progression of inflammation-associated liver cancer
and it is an important documented link between hepatitis and liver
cancer (29,30). The activation of the NF-κB pathway
is associated with the onset of liver cancer, increased expression
of the related genes in the liver, as well as the regulation of the
expression of anti-apoptotic and pro-apoptotic genes [e.g.,
Bcl-2/Bax and Bcl-2/Bcl-2-associated death promoter (Bad)]
(31). In addition, several
studies have concluded that the NF-κB signaling pathway mainly
participates in the proliferation and anti-apoptotic regulation of
liver cancer cells through the following pathways: i) Promoting
transformation of cells from G1/G0 to S phase via regulating the
expression of cyclin D1, thereby leading to excessive cell
proliferation (32,33); ii) inducing the expression of
various inhibitors of apoptosis, such as cellular inhibitor of
apoptosis protein 1 (c-IAP1) and c-IAP2, thereby increasing the
resistance of cancer cells to apoptosis (34,35); iii) inhibiting cell autophagy
(36,37); and iv) inducing the expression of
genes essential for survival (38,39). Consequently, the infiltration of
M1-like TAM in liver cancer tissues may participate in the
molecular mechanisms underlying the regulation of proliferation and
apoptosis of liver cancer cells via stimulating the NF-κB signaling
pathway.
Cell proliferation assays were employed in the
present study to indicate whether infiltration of M1-like TAM into
liver cancer tissues was able to promote the proliferation of liver
cancer cells by activating the NF-κB signaling pathway. As
expected, M1-like TAM promoted the proliferation of liver cancer
cells in vitro. However, JSH-23 attenuated the proliferation
of liver cancer cells. Notably, the present study indicated that
M1-like TAM promoted the transition of liver cancer cells from
G1/G0 to S and G2/M phases, while JSH-23 reversed these effects.
Regarding the molecular mechanisms, it was determined that M1-like
TAM upregulated the expression levels of CDK1, CDK2 and cyclin D1,
whereas it decreased the expression levels of p21 in liver cancer
cells. CDKs and CDK inhibitors (CDKIs) have substantial roles in
regulating the transition from G1 to S phase of the cell cycle
(40,41). Furthermore, p21 is a member of the
CDKI family and is a negative regulator of the cell cycle with
diverse effects on tumorigenesis according to its localization
within different subcellular compartments. The CDKIs, through
binding to cyclin, CDK or cyclin-CDK, consequently cause cell cycle
arrest and block cell proliferation (42,43). The present results regarding the
role of p21 are significant, as p21 is an important downstream gene
of the p53 gene and it has a specific binding site for the p53
protein. When cells are subjected to various types of damage in
vivo and in vitro, p53 protein acts upon the p21 gene to
promote the expression level of p21 in a relatively fast manner.
Consequently, the p21 protein binds to almost all cyclin-CDK
complexes and inhibits them. This includes cyclin D1-CDK4, cyclin
E-CDK2 and cyclin A-CDK2, while p21 is able to weakly inhibit
cyclin B-related complexes. Furthermore, p21 inhibits the
activities of cyclin D1-CDK4 and cyclin E-CDK2 to block the
phosphorylation of the Rb protein and cause the release of E2F
protein, thereby causing cell cycle arrest in G1 phase. It may be
concluded that M1-like TAM regulate the expression of cell
cycle-associated proteins by activating the NF-κB signaling
pathway. Thus, M1-like TAM may stimulate the transformation of
liver cancer cells from G1/G0 to S phase and promote the
proliferation of liver cancer cells.
ADM is widely used for the treatment of liver
cancer, as it may induce apoptosis of liver cancer cells.
Radiotherapies are frequently combined with TACE to treat patients
with liver cancer, as they may induce apoptosis of cancer cells.
The sensitivity of tumors to radiotherapies has been indicated to
be consistent with the sensitivity of tumor cells to apoptosis
(44,45). In the present study, ADM and X-ray
irradiation were used as external factors to induce apoptosis of
liver cancer cells. It was indicated that the number of apoptotic
liver cancer cells in the CM was significantly lower than that in
the RM after treatment with either ADM or X-rays. However, the
addition of JSH-23 to CM significantly increased the amount of ADM
and X-ray-induced apoptosis of liver cancer cells. Furthermore, the
results of the present study revealed that M1-like TAM
significantly decreased both ADM- and X-ray-induced apoptosis of
liver cancer cells. In addition, M1-like TAM increased the
expression levels of Bax and caspase-3 proteins, while it decreased
Bcl-2 expression in liver cancer cells. However, JSH-23 was
observed to reverse the above-mentioned effects. It is also
noteworthy that M1-TAM may be directly affected by ADM and
irradiation. Previous studies reported that the anti-apoptotic
function of NF-κB family proteins may be a result of consequential
unbalancing of the expression levels of apoptotic and
anti-apoptotic proteins, as well as their direct interactions with
tumor suppressor proteins (35,46). In an animal-based study, increased
activity of the NF-κB signaling pathway promoted the incidence of
hepatocarcinoma and stimulated the survival of liver cancer cells
by significantly enhancing the levels of anti-apoptotic proteins,
such as Bcl-2/Bax and Bcl-2/Bad, compared with those of
pro-apoptotic proteins (31). In
the present study, the CM of THP-1 cells induced by PMA, LPS and
IFN-γ was used to evaluate the effects of M1-like TAM on liver
cancer cells. Although the constituents of the CM were not
determined, previous studies suggested that the constituents
accounting for the regulatory effects of M1-like TAM on the
proliferation and apoptosis of liver cancer cells may be
inflammatory cytokines and growth factors (47,48). However, further studies are still
required to verify the present results.
Regarding limitations of the present study, it
should be pointed out that only CD68 and HLA-DR were assessed to
detect M1 macrophages. The present results did not fully reflect
the polarization of diverse macrophages in the TME in vivo.
In addition, Ki-67 expression was not detected in clinical
specimens.
In conclusion, the present study suggested that
infiltration of CD68+HLA-DR+ M1-like TAM were negatively associated
with the efficacy of postoperative TACE for patients with liver
cancer. M1-like TAM may promote the proliferation of liver cancer
cells and enhance their anti-apoptotic ability by activating the
NF-κB signaling pathway.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Doctoral Program of
Inner Mongolia Natural Science Foundation (grant no. 2018BS08004),
the Natural Science Foundation of Inner Mongolia (grant no.
2019LH08028) and the Science and Technology Million Project of
Inner Mongolia Medical University [grant no.
YKD2017KJBW(LH)002].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GS and DZ designed the study, prepared the figures
and drafted the manuscript. GS, HC and XH performed the
experiments. GS, JM and DZ contributed to the conceptualization of
the research, drafted the manuscript and revised it critically. GS
and DZ confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Inner Mongolia Medical University
(Hohhot, China; approval no. YJ 2020001) and the study was
performed in accordance with the Declaration of Helsinki. In
addition, all participants provided written informed consent prior
to enrolment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: Past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu XQ and Baade P: RE: Cancer incidence
and mortality in China, 2013 by Chen et al. Cancer Lett.
401:72–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shalapour S, Lin XJ, Bastian IN, Brain J,
Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, et
al: Inflammation-induced IgA+ cells dismantle anti-liver cancer
immunity. Nature. 561:340–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang YM, Kim SY and Seki E: Inflammation
and liver cancer: Molecular mechanisms and therapeutic targets.
Semin Liver Dis. 39:26–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W: Cancer statistics: Updated cancer
burden in China. Chin J Cancer Res. 27:12015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Ni W, Qu L, Cui X, Lin Z, Liu Q,
Zhou H and Ni R: Decreased expression of EHD2 promotes tumor
metastasis and indicates poor prognosis in hepatocellular
carcinoma. Dig Dis Sci. 61:2554–2567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yokoo T, Patel AD, Levcohain N, Singal AG,
Yopp AC and Pedrosa I: Extrahepatic metastasis risk of
hepatocellular carcinoma based on α-fetoprotein and tumor staging
parameters at cross-sectional imaging. Cancer Manag Res. 9:503–511.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aggarwal BB, Sung B and Gupta SC: In
inflammation and cancer. Advances in Experimental Medicine and
Biology. Vol 816. Springer LLC; New York, NY: pp. pp52014
|
|
11
|
Gunassekaran GR, Poongkavithai Vadevoo SM,
Baek MC and Lee B: M1 macrophage exosomes engineered to foster M1
polarization and target the IL-4 receptor inhibit tumor growth by
reprogramming tumor-associated macrophages into M1-like
macrophages. Biomaterials. 278:1211372021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barberi T, Martin A, Suresh R, Barakat DJ,
Harris-Bookman S, Drake CG, Lim M and Friedman AD: Absence of host
NF-κB p50 induces murine glioblastoma tumor regression, increases
survival, and decreases T-cell induction of tumor-associated
macrophage M2 polarization. Cancer Immunol Immunother.
67:1491–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saccani A, Schioppa T, Porta C, Biswas SK,
Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A and Sica A:
p50 nuclear factor-kappaB overexpression in tumor-associated
macrophages inhibits M1 inflammatory responses and antitumor
resistance. Cancer Res. 66:11432–11440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao RR, Li JH, Zhang R, Chen RX and Wang
YH: M2-polarized tumor-associated macrophages facilitated migration
and epithelial-mesenchymal transition of HCC cells via the
TLR4/STAT3 signaling pathway. World J Surg Oncol. 16:92018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei X, Tang C, Lu X, Liu R, Zhou M, He D,
Zheng D, Sun C and Wu Z: MiR-101 targets DUSP1 to regulate the
TGF-β secretion in sorafenib inhibits macrophage-induced growth of
hepatocarcinoma. Oncotarget. 6:18389–18405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arvanitakis K, Koletsa T, Mitroulis I and
Germanidis G: Tumor-associated macrophages in hepatocellular
carcinoma pathogenesis, prognosis and therapy. Cancers (Basel).
14:2262022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zong Z, Zou J, Mao R, Ma C, Li N, Wang J,
Wang X, Zhou H, Zhang L and Shi Y: M1 macrophages induce PD-L1
expression in hepatocellular carcinoma cells through IL-1β
signaling. Front Immunol. 10:16432019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medical Administration and Hospital
Administration Bureau of Health and Family Planning Commission of
the People's Republic of China, . Standard for diagnosis and
treatment of primary liver cancer (2017 edition). Chin J Dig Surg.
16:132017.
|
|
19
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwende H, Fitzke E, Ambs P and Dieter P:
Differences in the state of differentiation of THP-1 cells induced
by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol.
59:555–561. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Z, Guo J, Hu M, Gao Y and Huang L:
Icaritin exacerbates mitophagy and synergizes with doxorubicin to
induce immunogenic cell death in hepatocellular carcinoma. ACS
Nano. 14:4816–4828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Lai X, Zhao Y, Zhang Y, Li M, Li D,
Kong J, Zhang Y, Jing P, Li H, et al: Loss of NDRG2 in liver
microenvironment inhibits cancer liver metastasis by regulating
tumor associate macrophages polarization. Cell Death Dis.
9:2482018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu H, Liu S, Zheng J, Ji G, Han J and Xie
Y: Transcatheter arterial chemoembolization (TACE) for lymph node
metastases in patients with hepatocellular carcinoma. J Surg Oncol.
112:372–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barone M, Ettorre GC, Ladisa R,
Schiavariello M, Santoro C, Francioso G, Vinciguerra V and
Francavilla A: Transcatheter arterial chemoembolization (TACE) in
treatment of hepatocellular carcinoma. Hepatogastroenterology.
50:183–187. 2003.PubMed/NCBI
|
|
25
|
Zeng XY, Xie H, Yuan J, Jiang XY, Yong JH,
Zeng D, Dou YY and Xiao SS: M2-like tumor-associated
macrophages-secreted EGF promotes epithelial ovarian cancer
metastasis via activating EGFR-ERK signaling and suppressing lncRNA
LIMT expression. Cancer Biol Ther. 20:956–966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee C, Jeong H, Bae Y, Shin K, Kang S, Kim
H, Oh J and Bae H: Targeting of M2-like tumor-associated
macrophages with a melittin-based pro-apoptotic peptide. J
Immunother Cancer. 7:1472019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Archiv. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu H, Aravindan N, Xu J and Natarajan M:
Inter- and intra-cellular mechanism of NF-kB-dependent survival
advantage and clonal expansion of radio-resistant cancer cells.
Cell Signal. 31:105–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang W, Yang S, Zhang M, Gao D, He T and
Guo M: ZNF545 suppresses human hepatocellular carcinoma growth by
inhibiting NF-kB signaling. Genes Cancer. 8:528–535. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Q, Song G, Yao L, Liu Y, Liu M, Li S
and Tang H: miR-3928v is induced by HBx via NF-κB/EGR1 and
contributes to hepatocellular carcinoma malignancy by
down-regulating VDAC3. J Exp Clin Cancer Res. 37:142018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park SG, Lee T, Kang HY, Park K, Cho KH
and Jung G: The influence of the signal dynamics of activated form
of IKK on NF-kappaB and anti-apoptotic gene expressions: A systems
biology approach. FEBS Lett. 580:822–830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malhotra U, Hittelman WN, Wu TT, Luthra R,
Swisher S, Luthra M, Correa A, Aggarwal BB, Ajani J and Izzo JG:
Association of activated NF-κB, altered cyclin D1 and poor outcome
in esophageal adenocarcinoma. Cancer Res. 65 (9
Suppl):S5482005.
|
|
33
|
Ozeki M, Hamajima Y, Feng L, Ondrey FG,
Schlentz E and Lin J: Id1 induces the proliferation of cochlear
sensory epithelial cells via the nuclear factor-κB/cyclin D1
pathway in vitro. J Neurosci Res. 90:22252012. View Article : Google Scholar
|
|
34
|
Varfolomeev E, Goncharov T, Fedorova AV,
Dynek JN, Zobel K, Deshayes K, Fairbrother WJ and Vucic D: c-IAP1
and c-IAP2 are critical mediators of tumor necrosis factor alpha
(TNFalpha)-induced NF-kappaB activation. J Biol Chem.
283:24295–24299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vázquez-Franco JE, Reyes-Maldonado E,
Vela-Ojeda J, Domínguez-López ML and Lezama RA: Src, Akt, NF-κB,
BCL-2 and c-IAP1 may be involved in an anti-apoptotic effect in
patients with BCR-ABL positive and BCR-ABL negative acute
lymphoblastic leukemia. Leuk Res. 36:862–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu CC, Kuo YH and Hsieh SL: 345 Sedanolide
induces human liver tumor cell autophagy through regulation of
NF-kB pathway. Eur J Cancer. 48 (Suppl 5):S842012. View Article : Google Scholar
|
|
37
|
Zhang H, Chen Z, Miranda RN, Medeiros LJ
and McCarty N: TG2 and NF-κB signaling coordinates the survival of
mantle cell lymphoma cells via IL-6-mediated autophagy. Cancer Res.
76:6410–6423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim YS, Schwabe RF, Qian T, Lemasters JJ
and Brenner DA: TRAIL-mediated apoptosis requires NF-kappaB
inhibition and the mitochondrial permeability transition in human
hepatoma cells. Hepatology. 36:1498–1508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morotti A, Cilloni D, Pautasso M, Messa F,
Arruga F, Defilippi I, Carturan S, Catalano R, Rosso V, Chiarenza
A, et al: NF-κB inhibition as a strategy to enhance
etoposide-induced apoptosis in K562 cell line. Am J Hematol.
81:938–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kent LN and Leone G: The broken cycle: E2F
dysfunction in cancer. Nat Rev Cancer. 19:326–338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ocker M, Bitar SA, Monteiro AC,
Gali-Muhtasib H and Schneider-Stock R: Epigenetic regulation of
p21cip1/waf1 in human cancer. Cancers (Basel).
11:13432019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim EM, Jung CH, Kim J, Hwang SG, Park J
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leszczynska κB, Foskolou IP, Abraham AG,
Anbalagan S, Tellier C, Haider S, Span PN, O'Neill EE, Buffa FM and
Hammond EM: Hypoxia-induced p53 modulates both apoptosis and
radiosensitivity via AKT. J Clin Invest. 125:2385–2398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Olive PL and Durand RE: Apoptosis: An
indicator of radiosensitivity in vitro? Int J Radiat Biol.
71:695–707. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Papademetrio DL, Lompardía SL, Simunovich
T, Costantino S, Mihalez CY, Cavaliere V and Álvarez É: Inhibition
of survival pathways MAPK and NF-kB triggers apoptosis in
pancreatic ductal adenocarcinoma cells via suppression of
autophagy. Target Oncol. 11:183–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh R, Shankar BS and Sainis KB:
TGF-β1-ROS-ATM-CREB signaling axis in macrophage mediated migration
of human breast cancer MCF7 cells. Cell Signal. 26:1604–1615. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S
and Jian Z: IL-6/STAT3 pathway intermediates M1/M2 macrophage
polarization during the development of hepatocellular carcinoma. J
Cell Biochem. 119:9419–9432. 2018. View Article : Google Scholar : PubMed/NCBI
|