Introduction
The SARS-CoV-2 virus, responsible for COVID-19
disease, is characterized by a broad spectrum of clinical symptoms;
these can be separated into mild symptoms, such as fever, dyspnea,
coughing, loss of taste and smell, and into more severe symptoms,
especially in elderly people with concomitant pathological
conditions, including respiratory and severe alveolar insufficiency
(1,2). In extremely severe forms of
COVID-19, rapidly progressive multi-organ insufficiency may take
place, a condition that usually manifests as a series of
complications including shock, acute heart damage, disseminated
intravascular coagulopathy, Acute Respiratory Distress Syndrome
(ARDS) and acute renal impairment (1). Recent studies have shown that
respiratory failure from COVID-19 disease is not only due to ARDS,
but may also be due to macro- and microvascular involvement
(3), in which case vascular
endothelial damage plays a central role (4). Recent observations suggest that
COVID-19 is an endothelial disease, responsible for the observed
manifestations of inflammation, cytokine storm, oxidative stress
and coagulopathy (5). This
hypothesis is also supported by the fact that patients with
endothelial dysfunction due to various concomitant pathologies
(obesity, hypertension and type 2 diabetes mellitus), develop more
severe forms of COVID-19, as evidenced by additional pathological
changes of the already dysfunctional vascular endothelium (4). The endothelial tissue produces a
wide variety of molecules that are associated with homeostasis in
the human body. Some of the regulatory mechanisms responsible for
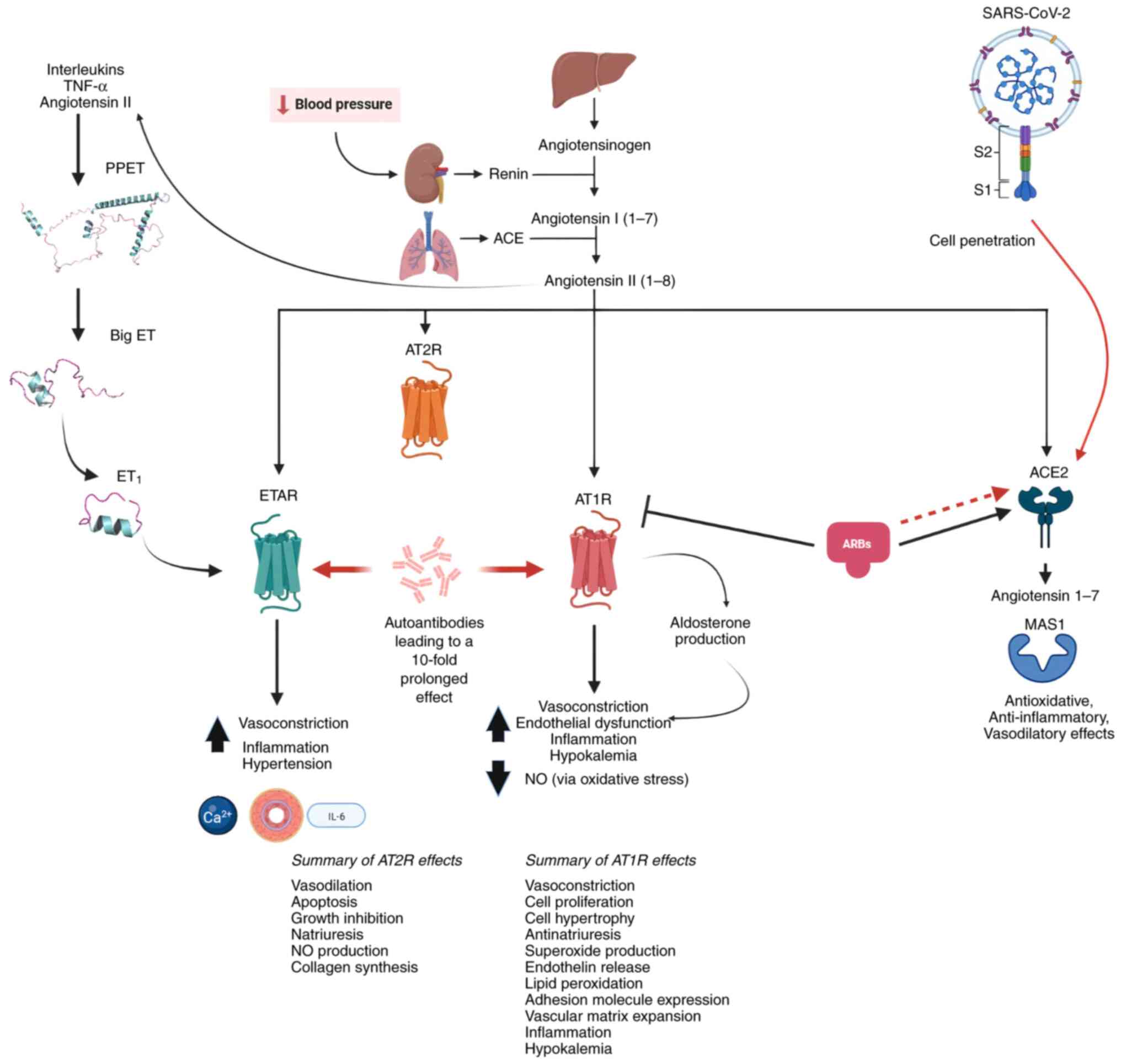
vascular homeostasis are antagonistic and are shown in Fig. 1.
Viral entry of SARS-COV2 and other coronaviruses
into the host (human) cells is facilitated through the angiotensin
converting enzyme 2 (ACE2) acting as a receptor that binds to the
spike protein on the viral envelope. ACE2 is a vital component of
the renin angiotensin aldosterone system (RAAS) that is responsible
for normal cardiovascular function (6). During SARS-CoV-2 infection, in
addition to RAAS, the endothelin (ET) 1 pathway, which is known to
have vasoconstrictive power, is also affected. The present review
provided a thorough discussion of how these systems are implicated
in COVID-19 disease and described a novel approach for
predicting/assessing SARS-CoV-2 infection severity; the latter
includes a detailed description of the functional auto-antibodies
against the systems regulating normal vascular activity and which
exhibit similar functions to the natural ligands. Lastly, the
action of nitric oxide (NO) and ARBs are discussed and their
implication in COVID-19 infections, as well as their use as a
supplement treatment and possible cure.
How does vasoconstriction occur?
Contraction of vascular smooth muscles is triggered
through stimulation of G-protein coupled receptors via ET1 and Ang
II, which causes a Ca2+ influx through the
receptor-operated Ca2+ channels of the plasma membrane
and initiates phospholipase C to produce inositol-trisphosphate
(IP3) and diacylglycerol (DAG). IP3 then
initiates calcium release from the sarcoplasmic reticulum stores
via IP3 and ryanodine receptors. The increase of
intracellular calcium then activates the myosin light chain kinase,
leading to myosin phosphorylation, thereby resulting in smooth
muscle contraction (7).
Furthermore, DAG initiates PKC activation, which induces the
phosphorylation and subsequent activation of the protein
phosphatase type 1 inhibitor protein CPI-17, causing inhibition of
myosin light chain phosphatase (8). This inhibition causes a prolonged
myosin phosphorylation for a given Ca2+ concentration
(7).
Endothelin and endothelin receptors
The endothelin family consists of the ET1, ET2 and
ET3 vasoconstrictor peptides which, depending on their expression
location, are coded by three different genes, with ET1 being the
main isoform (9). The promoter of
the EDN1 gene, which codes for the ET1 protein, possesses
several regulatory elements that facilitate transcription via
different environmental and hormonal stimuli such as ILs, TNFα and
Ang II. In humans, the gene product consists of a 212 amino acid
(aa) long protein termed preproendothelin-1, which undergoes
proteolytic cleavage and gives a 38-aa-long ‘big endothelin-1’ (big
ET-1) (10). This 38-aa peptide
can be converted into the active 21-aa form of ET-1 through the
actions of endothelin converting enzymes 1, 2 and 3 respectively,
even though several other enzymes have also been found to convert
ET-1 into its active form (11,12). In addition, even though big ET-1
is relatively inactive, it still possesses low receptor affinity
and can protect the final, active ET-1, from proteolysis (13).
Of the two endothelin receptors, ETAR (endothelin
type A receptor) is located on vascular smooth muscle cells and
ETBR (endothelin type B receptor) is located on both vascular
smooth muscle cells and endothelial cells (14). The ETAR has the highest affinity
for the ET1 protein, whereas the ETBR has the same affinity for all
three ETs, with the two receptors exhibiting the same affinity for
ET1 (15). Depending on tissue
type, the receptors may have synergistic or opposing functions. For
example, each receptor behaves differently upon ET1 binding. When
ET1 binds to ETAR, the effect is long lasting due to slow
dissociation of ET1 from the receptor (16). On the other hand, when ET1 binds
to ETBR, the receptor is targeted to the lysosomes for destruction,
suggesting that these receptors play a main role in the clearing of
ET1 from the circulation (17).
Macrophages also possess ETARs, which release cytokines upon
induction (18). By contrast, the
activation of ETBR by ET1 induces NO synthesis through endothelial
nitric oxide synthase (eNOS), which in turn causes the stimulation
of soluble guanylate cyclase acid [cyclic guanosine monophosphate
(cGMP)] synthesis, leading to reduced intracellular Ca2+
concentration and ultimately to vasodilation (19). Furthermore, impairment of the ETBR
has been associated with impaired clearance of ET-1, leading to
increased blood pressure (20).
This further stratifies the roles of the two receptors and their
possible implication in COVID-19 infected patients. Improved
understanding of the functions and actions of each receptor and how
these may vary depending on the location and tissue type, may help
in the introduction of novel treatment approaches with the
potential to relieve the severity of COVID-19 (21).
RAAS
The RAAS constitutes a natural defense mechanism for
maintaining normal blood circulation. Renin release from the
juxtaglomerular apparatus is stimulated by renal hypoperfusion. The
role of renin is to convert angiotensinogen (Ang) to Ang I, which
is further hydrolyzed by angiotensin-converting enzyme (ACE) to Ang
II (22). Furthermore, Ang II
stimulates the production of aldosterone via the Ang II type 1
receptor (AT1R), resulting in retention of sodium, water
reabsorption and ultimately in vasoconstriction (23). ACE2 maintains a balance by
converting Ang II to Ang 1–7, which induces antioxidant,
anti-inflammatory and vasodilatory effects through binding to the
mitochondrial assembly protein 1 (MAS1) receptor (24). In cases where there is
insufficient expression of ACE2, Ang II predominantly binds to AT1R
and exhibits vasoconstrictive and pro-inflammatory effects
(25).
The implication of RAAS in COVID-19 disease has been
observed from the very beginning of the pandemic, due to the fact
that SARS-CoV-2 enters human cells via the angiotensin-converting
enzyme 2 (ACE2) receptor, which is expressed mainly in the
respiratory epithelium and vascular endothelium (24). In addition, viral cell entry has
been suspected to utilize several proteases, such as transmembrane
protease serine subtype 2, turin, cathepsin B or L, and basigin
(CD147) (26).
ACE2 plays an essential role in the
renin-angiotensin system (RAS), being responsible for
cardiovascular homeostasis and for the generation of Ang 1–9 and
Ang 1–7 through carboxypeptidase activity (27). In addition to being a direct route
for viral cell entry, ACE2 function might also be implicated in the
manifestation of disease severity variations (28). A previous study has shown that
RAAS-modulating drugs can also modulate ACE2 expression and
activity in different ways, specifically, certain ACE inhibitors
(ACEIs) have been shown to downregulate ACE2 expression, while
angiotensin II receptor blockers (ARBs) and mineralocorticoid
receptor antagonists (MRAs) seem to increase the activity of ACE2
(29).
In the alveolar epithelial cells, binding of the
viral spike protein to ACE2 reduces the receptor's expression,
blocking Ang II conversion to Ang 1–7 and causing Ang II to bind to
AT1R, which in turn induces hypokalemia, hyperaldosteronism,
proliferation of inflammatory cells, as well as vasoconstriction
and fibrosis in severe cases of the disease (30). Notably, loss of ACE2 expression
has been shown to result in impaired lung function in mice, via
increased vascular permeability, pulmonary edema and neutrophil
accumulation (31).
Another possible consequence of COVID-19 infection
is thought to be viral neurotropism. Neurons in the
circumventricular organs that are implicated in respiratory and
cardiovascular regulation express ACE2 receptors and have almost no
protection through the blood brain barrier (32). This provides an opportunity to the
virus to infect a region representing the central nervous system
and to further disrupt the regulation of respiratory and
cardiovascular events (33).
Excessive Ang II has also been associated with poor outcomes in the
COVID-19 setting. Significantly higher Ang II plasma concentrations
have been observed in COVID-19 patients, as compared with healthy
subjects, and Ang II levels in patients have been associated with
viral load and lung damage (34).
In addition to severe inflammation and hypoxemia in
COVID-19 patients through vasoconstriction in small pulmonary
vessels, Ang II induces the expression of the plasminogen
activator-1 (PAI-1) inhibitor in endothelial cells through binding
to the AT1R (35). Fibrin
deposits in the alveoli of the patients lead to PAI-1 expression
(36). In addition, excessive Ang
II can be metabolized to angiotensin IV, which enhances the
development of thrombosis, as hypercoagulation is observed in
highly severe cases, suggesting that a decrease in ACE2 expression
possibly contributes to an increase in thrombotic risk (36).
As ACE2 is known to facilitate viral entry into host
cells, the question that has been raised is whether RAAS modulators
(ACEIs and ARBs) can increase the risk of developing severe viral
infection. This hypothesis is based on the results of studies on
animal models that demonstrate increased levels of ACE2 expression
following intravenous infusion of ACEI and ARB (37). To determine whether RAAS
modulators increase the risk for severe disease, the activity of
both ARBs and ACEIs was compared in patients with COVID-19 and
concomitant hypertension; it was deduced that those receiving ARBs
were less likely to develop severe COVID-19, while those being
administered ACEIs did not exhibit similar outcomes to those
receiving ARBs, as ACEIs inhibit the AGT hydrolysis step in the
RAAS pathway, preventing the formation of Ang 1–7 that induces
vasodilatory effects (38).
Based on the significant association of ACE2
expression with COVID-19 infection, several potential therapeutic
approaches have been proposed. The majority of these strategies are
based on results from animal models or in vitro studies,
which however require additional and more in-depth research before
therapies can be tested in humans and become available to the
general public (39,40).
NO
Hypertension is believed to arise from remodeling of
the vessel walls and from abnormal vascular volume, salt regulation
and vascular tone (41). These
abnormalities are in turn thought to be the result of an imbalance
between NO and Ang II, as disruption of either pathway can lead to
heart failure, renal failure, vascular and cerebrovascular disease
(42). NO synthesis takes place
in the endothelium through the actions of endothelial nitric oxide
synthase (eNOS), which converts L-arginine to NO and citrulline
(43). NO then initiates a
cascade through the actions of cGMP, which activates protein kinase
G (PKG) I and II, with PKG I being the kinase responsible for
vasodilation and inhibition of platelet aggregation (44). The role of NO in the RAAS system
mainly lies in antagonizing the effects of angiotensin II via
downregulation of ACE and AT1R, thereby positively balancing
cardiovascular activity overall (41). On the other hand, if there is
inhibition of NO or if Ang II predominates over it, there is a
disruption of homeostasis and increased production of aldosterone
and superoxide anions (O2−), resulting in
vasoconstriction and persistent hypertension (45,46). Furthermore, superoxide anions
(O2−) cause oxidative stress and subsequently
a reduction in NO with concomitant increase of ACE2, hence
increasing the severity of COVID-19 infection (47).
NO and Ang II both interact with other vasoactive
regulators. Ang II can induce the release of ET-1 from endothelial
cells, which leads to more severe Ang II-induced vasoconstriction
and hypertension (48,49). On the other hand, as
aforementioned, the activation of ETb causes eNOS to synthesize NO,
and this leads to a reduction in the ETAR calcium-induced
vasoconstriction due to an ET-1 inhibiting function that is
facilitated via a cGMP-dependent mechanism (50).
Upon SARS-CoV-2 infection, the virus enters the cell
through the ACE2 receptor, which decreases its availability,
leading to reduced conversion of Ang II to Ang 1–7 (51). Hence, this results in increased
Ang II concentration and AT1R activity, inducing higher generation
of reactive oxygen species (ROS), limiting the bioavailability of
NO (52). This is proportional
and correlates with the severity of the disease. Furthermore,
reduced NO bioavailability can in part lead to endothelial
dysfunction, by predisposing the vasculature towards prothrombotic
and pro-inflammatory state expressing adhesion molecules and
pro-inflammatory cytokines (53).
These data further signify the vital role which NO plays in
COVID-19 severity, along with being of immense importance in
cardiovascular regulation (51).
AT1R and ETAR auto-antibodies
Autoantibodies (AAs) against AT1R and ETAR are
functional agonists able to activate both receptors (54). They have similar binding abilities
and functions to the natural ligands, including remodeling of the
extracellular matrix, vasoconstriction and inflammation (55). AAs differ in their ability to
dissociate; binding to the AT1R results in a 10-fold longer
vasoconstriction as compared with Ang II (55,56), as shown in Fig. 1. Furthermore, the autoantibodies
only bind to AT1R, which is responsible for inducing
vasoconstriction inflammation and is actively involved in the
production of ROS, reducing NO (57). Even though Ang II has the same
binding affinity for both AT1R and AT2R, the latter has an opposing
effect to the former, resulting in reduced vasoconstriction and
inflammation and therefore to a positive regulation of AT1R
activity (55). Similar to the
AT1R AAs, the AAs against ETAR have the same effect, with the only
difference in that they induce vasoconstriction and inflammation
without utilizing ETBR (55). The
presence of AAs has been observed in systemic sclerosis, where it
has been associated with increased mortality and more severe
disease, graft injury and loss in transplantation (58,59), as well as in more severe COVID-19
infections and end-stage cystic fibrosis (60,61). In general, the presence of these
AAs is regarded as an independent risk factor, significantly
related to adverse outcomes and death (62).
The mechanism behind AA generation is still not
fully understood. A previous study speculated that they arise from
damaged endothelium that is characterized by an accumulation of
extracellular AT1Rs and ETARs (63). Additional investigations including
more control groups will possibly shed more light into this
field.
Conclusions
Taking all of the above systems and factors into
consideration, it can be assumed that the abnormal interaction of
RAAS, ET receptors, NO and AAs is a necessary prerequisite for
severe manifestation of COVID-19 disease and increased mortality.
Even in healthy individuals, disturbance of the aforementioned
systems and their respective interactions can lead to
cardiovascular disease and chronic inflammation, and also
contribute to the appearance of related pathologies.
So far, the presence of AT1R and ETAR AAs, even at
low concentrations, has been associated with more severe forms of
the disease, drastically reducing the time to mortality (62). Nonetheless, further investigations
are warranted to confirm the precise mechanism of action and effect
of AAs, and to facilitate the testing of more specialized therapies
for COVID-19 in clinical trials.
The implication of ARBs in SARS-CoV-2 infection is
still controversial (64).
Initially they were developed to treat hypertension by blocking
AT1R, inducing higher concentrations of ACE2 and converting Ang II
into Ang 1–7, which results in MAS1 activation. The latter is
regarded a major system protective pathway, which reduces
inflammation organ fibrosis and downregulates several signaling
pathways (65). ARBs also seem to
be an optimal therapeutic strategy for patients with severe
critical disorders (such as inflammatory lung disease, pneumonia,
influenza, sepsis and Ebola), by maintaining insulin sensitivity,
energy and lipid metabolism, protecting mitochondrial function and
regulating the coagulation cascade (66–68). Despite the numerous clinical
benefits conferred by ARBs to non-COVID patients, it is still not
clear whether a similar effect would be achieved in COVID-19
patients, or whether higher ACE2 expression is responsible for a
more severe disease. To this end, certain studies have hypothesized
that COVID-19 patients with cardiovascular problems, diabetes and
kidney disorders might have progressed to more severe disease and
mortality following administration of ARBs (69,70). Although no harmful effects of ARBs
has been observed on COVID-19 susceptibility, severity and
mortality, the benefits are still uncertain and further research is
warranted (71).
Another potential treatment and possible preventive
measure against COVID-19 is the administration of NO. The latter
has an antiviral action by inhibiting SARS-CoV-1 and SARS-CoV-2
replication (72). There are
several different ways of supplementing NO, the most effective of
which is thought to be the inhalation method, currently being
investigated in clinical trials (including NCT04312243, NCT04338828
and NCT04305457) (72). Other
methods of administration include supplementation of NO donor
molecules such as citrulline, arginine and nitro glycerin,
phosphodiesterase inhibitors such as Viagra and a high intake in
nitrate-rich food such as beetroots, spices and leafy green
vegetables (73,74). So far, inhalation of NO is the
method that mostly targets the respiratory tract, while observed
benefits include improved arterial oxygenation, reduced
hypertension, as well as spread and density of pulmonary
infiltrates (75). Furthermore,
as NO is constantly produced by the epithelial cells of the
nasopharynx and the paranasal sinuses, it activates the secretion
of mucus and initiates ciliary movement which removes viral
particles and foreign molecules from the respiratory tract
(76–78). Another essential activity of NO is
the capacity to prevent pulmonary infections by inducing
antimicrobial/antiviral effects, a property that is facilitated via
the modification and inactivation of nucleic acids and proteins
that are vital for viral replication (79–81).
Last but not least, the presence of AAs could
represent a potential diagnostic marker of the severity of COVID-19
disease. This could further direct the risk stratification of
patients and guide more optimal treatment decisions. Preliminary
data from ongoing clinical studies appear quite promising and hold
much hope for a more effective diagnosis and treatment of this
disease in the near future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
TK, IT and KD conceptualized the study. TK and KD
prepared the first draft of the manuscript. KD, IT, RC and NS wrote
the manuscript. All authors were involved in the revision of the
draft manuscript. KD and IT provided expertise in genomics. MA, DAS
and VZ provided expertise in proteomics. RC, NS and VM provided
expertise in clinical medicine. VM, RH, VZ and DAS supervised the
study. KD and IT visualized data. TK, RH, MA, VZ, DAS and VM
reviewed and edited the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H,
Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
A single-centered, retrospective, observational study. Lancet
Respir Med. 8:475–481. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zoumpourlis V, Goulielmaki M, Rizos E,
Baliou S and Spandidos DA: [Comment] The COVID-19 pandemic as a
scientific and social challenge in the 21st century. Mol Med Rep.
22:3035–3048. 2020.PubMed/NCBI
|
|
3
|
Magro C, Mulvey JJ, Berlin D, Nuovo G,
Salvatore S, Harp J, Baxter-Stoltzfus A and Laurence J: Complement
associated microvascular injury and thrombosis in the pathogenesis
of severe COVID-19 infection: A report of five cases. Transl Res.
220:1–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amraei R and Rahimi N: COVID-19,
renin-angiotensin system and endothelial dysfunction. Cells.
9:16522020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Libby P and Lüscher T: COVID-19 is, in the
end, an endothelial disease. Eur Heart J. 41:3038–3044. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan Y, Shang J, Graham R, Baric RS and Li
F: Receptor recognition by the novel coronavirus from Wuhan: An
analysis based on decade-long structural studies of SARS
coronavirus. J Virol. 94:e00127–e00120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brassington K, Selemidis S, Bozinovski S
and Vlahos R: Chronic obstructive pulmonary disease and
atherosclerosis: Common mechanisms and novel therapeutics. Clin Sci
(Lond). 136:405–423. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Somlyo AV: New roads leading to Ca2+
sensitization. Circ Res. 91:83–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kostov K: The causal relationship between
endothelin-1 and hypertension: Focusing on endothelial dysfunction,
arterial stiffness, vascular remodeling, and blood pressure
regulation. Life (Basel). 11:9862021.PubMed/NCBI
|
|
10
|
Kumar A, Choudhury M, Batra SD, Sikri K
and Gupta A: In vivo assessment of a single adenine mutation in
5′UTR of endothelin-1 gene in paediatric cases with severe
pulmonary hypertension: An observational study. BMC Res Notes.
14:1942021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasegawa H, Hiki K, Sawamura T, Aoyama T,
Okamoto Y, Miwa S, Shimohama S, Kimura J and Masaki T: Purification
of a novel endothelin-converting enzyme specific for big
endothelin-3. FEBS Lett. 428:304–308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Orléans-Juste P, Plante M, Honoré JC,
Carrier E and Labonté J: Synthesis and degradation of endothelin-1.
Can J Physiol Pharmacol. 81:503–510. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida T, Matsuura K, Goya S, Ma D,
Shimada K, Kitpipatkun P, Namiki R, Uemura A, Suzuki K and Tanaka
R: Metformin prevents the development of monocrotaline-induced
pulmonary hypertension by decreasing serum levels of big
endothelin-1. Exp Ther Med. 20:1492020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner OF, Christ G, Wojta J, Vierhapper
H, Parzer S, Nowotny PJ, Schneider B, Waldhäusl W and Binder BR:
Polar secretion of endothelin-1 by cultured endothelial cells. J
Biol Chem. 267:16066–16068. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watts SW: Endothelin receptors: What's new
and what do we need to know? Am J Physiol Regul Integr Comp
Physiol. 298:R254–R260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Wang L and Yan F: Understanding
the molecular mechanism of endothelin ETA receptor selecting
isopeptides endothelin-1 and −3. Biophys J. 121:2490–2502. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ergul A: Endothelin-1 and endothelin
receptor antagonists as potential cardiovascular therapeutic
agents. Pharmacotherapy. 22:54–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruetten H and Thiemermann C: Endothelin-1
stimulates the biosynthesis of tumour necrosis factor in
macrophages: ET-receptors, signal transduction and inhibition by
dexamethasone. J Physiol Pharmacol. 48:675–688. 1997.PubMed/NCBI
|
|
19
|
Stencel MG, VerMeer M, Giles J and Tran
QK: Endothelial regulation of calmodulin expression and
eNOS-calmodulin interaction in vascular smooth muscle. Mol Cell
Biochem. 477:1489–1498. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barinda AJ, Arozal W, Sandhiutami NMD,
Louisa M, Arfian N, Sandora N and Yusuf M: Curcumin prevents
epithelial-to mesenchymal transition-mediated ovarian cancer
progression through NRF2/ETBR/ET-1 axis and preserves mitochondria
biogenesis in kidney after cisplatin administration. Adv Pharm
Bull. 12:128–141. 2022.PubMed/NCBI
|
|
21
|
Nabeh OA, Matter LM, Khattab MA and
Menshawey E: The possible implication of endothelin in the
pathology of COVID-19-induced pulmonary hypertension. Pulm
Pharmacol Ther. 71:1020822021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chow JH, Mazzeffi MA and McCurdy MT:
Angiotensin II for the treatment of COVID-19-related vasodilatory
shock. Anesth Analg. 131:102–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deshotels MR, Xia H, Sriramula S,
Lazartigues E and Filipeanu CM: Angiotensin II mediates angiotensin
converting enzyme type 2 internalization and degradation through an
angiotensin II type I receptor-dependent mechanism. Hypertension.
64:1368–1375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rahman MM, Hasan M and Ahmed A: Potential
detrimental role of soluble ACE2 in severe COVID-19 comorbid
patients. Rev Med Virol. 31:e22132021. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henry BM, Vikse J, Benoit S, Favaloro EJ
and Lippi G: Hyperinflammation and derangement of
renin-angiotensin-aldosterone system in COVID-19: A novel
hypothesis for clinically suspected hypercoagulopathy and
microvascular immunothrombosis. Clin Chim Acta. 507:167–173. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konrath EL, Berger M, Lopes da Rosa R and
Beys-da-Silva WO: Acmella oleracea is a medicinal plant that
decreases chymase activity, oxidative stress, and inflammation:
Possible role in the adjuvant treatment of COVID-19. J Med Food.
24:1243–1244. 2021.PubMed/NCBI
|
|
28
|
Gómez J, Albaiceta GM, García-Clemente M,
López-Larrea C, Amado-Rodríguez L, Lopez-Alonso I, Hermida T,
Enriquez AI, Herrero P, Melón S, et al: Angiotensin-converting
enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene.
762:1451022020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carà GA, Pasin L, Alborino E, Zarbock A,
Bellomo R and Landoni G: Angiotensin II - A brief review and role
in severe SARS-COV-2 sepsis. J Cardiothorac Vasc Anesth [Internet].
2022 Jul 22;[cited 2022 Sep 16]; Available from:. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9304073/
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ravarotto V, Bertoldi G, Stefanelli LF,
Nalesso F and Calò LA: Gitelman's and Bartter's Syndromes: From
genetics to the molecular basis of hypertension and more. Kidney
Blood Press Res. 20:1–9. 2022.PubMed/NCBI
|
|
31
|
Kuba K, Imai Y, Rao S, Jiang C and
Penninger JM: Lessons from SARS: Control of acute lung failure by
the SARS receptor ACE2. J Mol Med (Berl). 84:814–820. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steardo L, Steardo L and Verkhratsky A:
Psychiatric face of COVID-19. Transl Psychiatry. 10:2612020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giannopoulou I, Galinaki S, Kollintza E,
Adamaki M, Kympouropoulos S, Alevyzakis E, Tsamakis K, Tsangaris I,
Spandidos DA, Siafakas N, et al: COVID-19 and post-traumatic stress
disorder: The perfect ‘storm’ for mental health (Review). Exp Ther
Med. 22:11622021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Y, Yang Y, Zhang C, Huang F, Wang F,
Yuan J, Wang Z, Li J, Li J, Feng C, et al: Clinical and biochemical
indexes from 2019-nCoV infected patients linked to viral loads and
lung injury. Sci China Life Sci. 63:364–374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seo JW, Kim DY, Yun N and Kim DM:
Coronavirus disease 2019-associated coagulopathy. Microorganisms.
10:15562022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwaan HC and Lindholm PF: The central role
of fibrinolytic response in COVID-19-a hematologist's perspective.
Int J Mol Sci. 22:12832021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferrario CM, Jessup J, Chappell MC,
Averill DB, Brosnihan KB, Tallant EA, Diz DI and Gallagher PE:
Effect of angiotensin-converting enzyme inhibition and angiotensin
II receptor blockers on cardiac angiotensin-converting enzyme 2.
Circulation. 111:2605–2610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shyh GI, Nawarskas JJ and Cheng-Lai A:
Angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers in patients with coronavirus disease 2019: Friend or foe?
Cardiol Rev. 28:213–216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Batlle D, Wysocki J and Satchell K:
Soluble angiotensin-converting enzyme 2: A potential approach for
coronavirus infection therapy? Clin Sci (Lond). 134:543–545. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H, Penninger JM, Li Y, Zhong N and
Slutsky AS: Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2
receptor: Molecular mechanisms and potential therapeutic target.
Intensive Care Med. 46:586–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou MS, Schulman IH and Raij L: Nitric
oxide, angiotensin II, and hypertension. Semin Nephrol. 24:366–378.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raij L: Nitric oxide, salt sensitivity,
and cardiorenal injury in hypertension. Semin Nephrol. 19:296–303.
1999.PubMed/NCBI
|
|
43
|
Li H, Brodsky S, Basco M, Romanov V, De
Angelis DA and Goligorsky MS: Nitric oxide attenuates signal
transduction: Possible role in dissociating caveolin-1 scaffold.
Circ Res. 88:229–236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Massberg S, Sausbier M, Klatt P, Bauer M,
Pfeifer A, Siess W, Fässler R, Ruth P, Krombach F and Hofmann F:
Increased adhesion and aggregation of platelets lacking cyclic
guanosine 3′,5′-monophosphate kinase I. J Exp Med. 189:1255–1264.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Takemoto M, Egashira K, Usui M, Numaguchi
K, Tomita H, Tsutsui H, Shimokawa H, Sueishi K and Takeshita A:
Important role of tissue angiotensin-converting enzyme activity in
the pathogenesis of coronary vascular and myocardial structural
changes induced by long-term blockade of nitric oxide synthesis in
rats. J Clin Invest. 99:278–287. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Katoh M, Egashira K, Usui M, Ichiki T,
Tomita H, Shimokawa H, Rakugi H and Takeshita A: Cardiac
angiotensin II receptors are upregulated by long-term inhibition of
nitric oxide synthesis in rats. Circ Res. 83:743–751. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hati S and Bhattacharyya S: Impact of
thiol-disulfide balance on the binding of covid-19 spike protein
with angiotensin-converting enzyme 2 receptor. ACS Omega.
5:16292–16298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sasser JM, Pollock JS and Pollock DM:
Renal endothelin in chronic angiotensin II hypertension. Am J
Physiol Regul Integr Comp Physiol. 283:R243–R248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ortiz MC, Sanabria E, Manriquez MC, Romero
JC and Juncos LA: Role of endothelin and isoprostanes in slow
pressor responses to angiotensin II. Hypertension. 37((2 Pt 2)):
505–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boulanger CM and Lüscher TF: Differential
effect of cyclic GMP on the release of endothelin-1 from cultured
endothelial cells and intact porcine aorta. J Cardiovasc Pharmacol.
17 (Suppl 7):S264–S266. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Montiel V, Lobysheva I, Gérard L,
Vermeersch M, Perez-Morga D, Castelein T, Mesland JB, Hantson P,
Collienne C, Gruson D, et al: Oxidative stress-induced endothelial
dysfunction and decreased vascular nitric oxide in COVID-19
patients. EBioMedicine. 77:1038932022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: Physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vanhoutte PM: Endothelium and control of
vascular function. State of the Art lecture. Hypertension.
13:658–667. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Philogene MC, Johnson T, Vaught AJ,
Zakaria S and Fedarko N: Antibodies against angiotensin II type 1
and endothelin A receptors: Relevance and pathogenicity. Hum
Immunol. 80:561–567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lukitsch I, Kehr J, Chaykovska L, Wallukat
G, Nieminen-Kelhä M, Batuman V, Dragun D and Gollasch M: Renal
ischemia and transplantation predispose to vascular constriction
mediated by angiotensin II type 1 receptor-activating antibodies.
Transplantation. 94:8–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang S, Zheng R, Yang L, Zhang X, Zuo L,
Yang X, Bai K, Song L, Tian J, Yang J and Liu H: Angiotensin type 1
receptor autoantibody from preeclamptic patients induces human
fetoplacental vasoconstriction. J Cell Physiol. 228:142–148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Papola F, Biancofiore V, Angeletti C,
Grimaldi A, Carucci AC, Cofini V, Necozione S, Rosciano A,
Marinangeli F and Cervelli C: Anti-AT1R autoantibodies and
prediction of the severity of Covid-19. Human Immunol. 83:130–133.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ohe H, Uchida Y, Yoshizawa A, Hirao H,
Taniguchi M, Maruya E, Yurugi K, Hishida R, Maekawa T, Uemoto S and
Terasaki PI: Association of anti-human leukocyte antigen and
anti-angiotensin II type 1 receptor antibodies with liver allograft
fibrosis after immunosuppression withdrawal. Transplantation.
98:1105–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
O'Leary JG, Demetris AJ, Philippe A,
Freeman R, Cai J, Heidecke H, Smith C, Hart B, Jennings LW, Catar
R, et al: Non-HLA antibodies impact on C4d staining, stellate cell
activation and fibrosis in liver allografts. Transplantation.
101:2399–2409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Budding K, van de Graaf EA, Hoefnagel T,
Kwakkel-van Erp JM, van Kessel DA, Dragun D, Hack CE and Otten HG:
Anti-ETAR and anti-AT1R autoantibodies are elevated in patients
with endstage cystic fibrosis. J Cyst Fibros. 14:42–45. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cabral-Marques O, Halpert G, Schimke LF,
Ostrinski Y, Vojdani A, Baiocchi GC, Freire PP, Filgueiras IS,
Zyskind I, Lattin MT, et al: Autoantibodies targeting GPCRs and
RAS-related molecules associate with COVID-19 severity. Nat Commun.
13:12202022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Abadir PM, Jain A, Powell LJ, Xue QL, Tian
J, Hamilton RG, Bennett DA, Finucane T, Walston JD and Fedarko NS:
Discovery and validation of agonistic angiotensin receptor
autoantibodies as biomarkers of adverse outcomes. Circulation.
135:449–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Q and Reed EF: The importance of
non-HLA antibodies in transplantation. Nat Rev Nephrol. 12:484–495.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Saavedra JM: Angiotensin receptor blockers
and COVID-19. Pharmacol Res. 156:1048322020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chung MK, Karnik S, Saef J, Bergmann C,
Barnard J, Lederman MM, Tilton J, Cheng F, Harding CV, Young JB, et
al: SARS-CoV-2 and ACE2: The biology and clinical data settling the
ARB and ACEI controversy. EBioMedicine. 58:1029072020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jia H: Pulmonary angiotensin-converting
enzyme 2 (ACE2) and inflammatory lung disease. Shock. 46:239–248.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gurwitz D: Angiotensin receptor blockers
as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 81:537–540.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fedson DS: Treating the host response to
emerging virus diseases: Lessons learned from sepsis, pneumonia,
influenza and Ebola. Ann Transl Med. 4:4212016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fang L, Karakiulakis G and Roth M: Are
patients with hypertension and diabetes mellitus at increased risk
for COVID-19 infection? Lancet Respir Med. 8:e212020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Diaz JH: Hypothesis:
Angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers may increase the risk of severe COVID-19. J Travel Med.
27:taaa0412020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Matsuzawa Y, Kimura K, Ogawa H and Tamura
K: Impact of renin-angiotensin-aldosterone system inhibitors on
COVID-19. Hypertens Res. 45:1147–1153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Martel J, Ko YF, Young JD and Ojcius DM:
Could nasal nitric oxide help to mitigate the severity of COVID-19?
Microbes Infect. 22:168–171. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lundberg JO, Weitzberg E and Gladwin MT:
The nitrate-nitrite-nitric oxide pathway in physiology and
therapeutics. Nat Rev Drug Discov. 7:156–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lundberg JO, Carlström M and Weitzberg E:
Metabolic effects of dietary nitrate in health and disease. Cell
Metabolism. 28:9–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen L, Liu P, Gao H, Sun B, Chao D, Wang
F, Zhu Y, Hedenstierna G and Wang CG: Inhalation of nitric oxide in
the treatment of severe acute respiratory syndrome: A rescue trial
in Beijing. Clin Infect Dis. 39:1531–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lundberg JO, Farkas-Szallasi T, Weitzberg
E, Rinder J, Lidholm J, Anggåard A, Hökfelt T, Lundberg JM and
Alving K: High nitric oxide production in human paranasal sinuses.
Nat Med. 1:370–373. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Runer T, Cervin E, Lindberg S and Uddman
R: Nitric oxide is a regulator of mucociliary activity in the upper
respiratory tract. Otolaryngol Head Neck Surg. 119:278–287. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nagaki M, Shimura S, Irokawa T, Sasaki T
and Shirato K: Nitric oxide regulation of glycoconjugate secretion
from feline and human airways in vitro. Respir Physiol. 102:89–95.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xu W, Zheng S, Dweik RA and Erzurum SC:
Role of epithelial nitric oxide in airway viral infection. Free
Radic Biol Med. 41:19–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Keyaerts E, Vijgen L, Chen L, Maes P,
Hedenstierna G and Van Ranst M: Inhibition of SARS-coronavirus
infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric
oxide donor compound. Int J Infect Dis. 8:223–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Åkerström S, Gunalan V, Keng CT, Tan YJ
and Mirazimi A: Dual effect of nitric oxide on SARS-CoV
replication: Viral RNA production and palmitoylation of the S
protein are affected. Virology. 395:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|