Introduction
Osteoblasts and osteoclasts are the major cells of
bone that function in bone formation and bone resorption,
respectively. Osteocytes are embedded in the mineralized bone
matrix and responsible for the regeneration of adult bone cells
(1). Osteocytes continue to
increase with age and bone size. Because of the limited access to
the osteocytes in the bone matrix, knowledge of its functions
remained unclear. Due to their particular morphology, they have
multiple functions and communication features (2). Kato et al developed MLO-Y4
cells, which are remarkably close to primary osteocytes (3) and this cell line, which has high
expression of osteocalcin, connexin 43 but low alkaline
phosphatase, was obtained from a transgenic mouse. Ca2+
and phosphate metabolism is regulated by the osteocyte
lacuno-canalicular, which contains multiple proteins such as
Phosphate regulating neutral endopeptidase on chromosome X (PHEX),
Matrix extracellular phosphoglycoprotein (MEPE), sclerostin, and
Dentin matrix protein 1 (DMP1) that can affect systemic mineral
homeostasis (4). Osteocytes also
play a role in the regulation of calcium and phosphate metabolism
which contains multiple proteins such as phosphate regulating
neutral endopeptidase on chromosome X (PHEX), matrix extracellular
phosphoglycoprotein (MEPE), sclerostin, and dentin matrix protein 1
(DMP1) that can affect systemic mineral homeostasis.
These proteins are secreted by osteocytes and have
an essential role in bone modeling and remodeling (5). DMP1 is highly expressed in
osteocytes and has a function in bone mineralization. MEPE is a
bone mineralization regulator that modifies mineralization within
the osteocyte microenvironment in response to mechanical loading.
Additionally, MEPE and DMP1 belong to the same SIBLING family
(6). Another osteocyte specific
protein is PHEX which is related to biomineralization and phosphate
homeostasis and sclerostin inhibits osteoblastic bone formation and
is expressed and released in osteocytes and other terminally
differentiated cell types embedded inside mineralized matrices
(7).
Previous studies have shown that these proteins also
have a function in the extracellular matrix as a transcription
factor (8,9). Ling et al showed that bone
mineralization was decreased in DMP1 null mice (10). MEPE protein expression was shown
in human bone osteocytes and bone marrow by Rowe et al
(11). Over-expression of MEPE
protein in bone causes a mineralization defect in a murine mice
model (12). Lu et al
reported that MEPE protein was produced by both osteoblasts and
osteocytes during skeletogenesis as early two days of the
post-natal period (13). It is
therefore clear that PHEX, MEPE and DMP1 are secreted by
osteocytes. Ca2+ is an important intracellular secondary
messenger and it is stored in the skeletal system in a form that is
linked to carbonated apatite crystals. Ca2+, on the
other hand, is found in soluble form in the extracellular domain
(14). In osteoblast cells,
increased extracellular Ca2+ has been shown to trigger
intracellular Ca2+ signaling (15). In osteoprogenitor cells, increased
Ca2+ concentration has also been shown to boost
proliferation, differentiation, protein matrix production, and
mineralized nodule formation in a dose-dependent manner (16). Ca2+ and phosphate in
the cell culture medium are often analyzed and imaged, especially
to quantify mineral deposition rates (17). The measurement of Ca/P ratios used
for the identification of the mineralization in the medium can be
compared to the ratio in tissue. Similarly, just demonstrating that
the culture's Ca2+ or phosphate concentration increases
over time is insufficient because numerous anionic matrix molecules
can bind Ca2+, and the culture's development typically
entails changes in matrix protein phosphorylation (18). The cells must produce a collagen
matrix on which the mineral will deposit, and that matrix must
contain proteins and peptides that support mineralization (19). The addition of serum used in cell
culture (which increases Ca2+ concentration) provides
both mineralization inhibitors and beneficial growth factors
(20,21).
There are limited number of studies investigating
the Ca2+ induced expression levels of bone
mineralization proteins in osteocytes. Our study was designed to
test the hypothesis, if such biological changes occur as a result
of changes in Ca2+ concentration, there would be changes
in the expression levels of PHEX, MEPE and DMP1 which play an
important role in bone formation while osteocytes induced by
different Ca2+ concentrations. On the other hand, we
have shown the apoptosis by evaluating caspase-3 levels and
oxidative stress-related enzymes CAT, SOD, GSH, NOX and TAS/TOS
levels of MLO-Y4 cell in the presence of different concentrations
of Ca2+.
Materials and methods
Cell culture
MLO-Y4 cells were purchased from the Kerafast
Company. Cells were grown to confluence in type I rat tail
collagen-coated cell culture dishes at 37°C in a humidified
atmosphere containing 5% CO2 air in 5% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., 16000044)
supplemented α-MEM medium (Gibco; Thermo Fisher Scientific, Inc.,
12571063), 5% calf serum (HyClone, SH30401) and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.,
15140122).
Live/dead cells assay
The cell viability of MLO-Y4 cell lines was tested
using a live/dead assay (Sigma-Aldrich; Merck KGaA, CBA415) at
various Ca2+ (Sigma-Aldrich; Merck KGaA, 10043-52-4)
concentrations (1.8, 6, 12, 18, 24 and 50 mM). After MLO-Y4
osteocytes were seeded 5×103 cells/cm2 in 48
collagen-coated well plates and incubated for 72 h. The cells were
treated with Ca2+ at 1.8, 6, 12, 18, 24 and 50 mM
concentrations for 15, 60 min and 24 h. After the treatment of
MLO-Y4 cells with different Ca2+ concentrations for 15
min, 60 min and 24 h, all media were removed and live/dead assay
was performed at the end of the time period. The pictures were
captured from the same region of interest for each group. At the
same time, the percentages of live and dead cells were
calculated.
CAT, SOD, GSH and NOX
measurements
To detect cellular oxidative stress, the activity of
SOD (YLA0115RA), CAT (YLA0123RA), GSH (YLA0121RA) and NOX
(YLA1501RA) enzymes were analyzed by using colorimetric diagnostic
kits (Shanghai YL Biotech, China). MLO-Y4 cells (1×106
cells) were seeded in collagen-coated 6 well plates and incubated
for 72 h. After the incubation period, the cells were treated with
the different concentrations of Ca2+ (1.8, 6, 12, 18, 24
and 50 mM) containing media for 15 and 60 min. High concentrations
of calcium led to elevated cell death in 24 h and it was difficult
to measure the oxidant-antioxidant enzymes SOD, CAT, GSH, NOX
levels in dead cells. So we determined the enzyme levels in 15 and
60th min. At the end of each time point, cells were washed by PBS
three times and lysed with the ultrasonic waves to collect. Samples
were centrifugated at 2×103 g for 5 min, the
supernatants were collected and added to ELISA plate wells with
HRP-conjugate reagent and incubated at 37°C for 60 min. After the
incubation, the ELISA plate well was washed five times with the
wash buffer. Then, chromogen solution was added to each well and
avoided the light for 15 min at 37°C. After that, stop solution was
added to each well. OD value of each well was measured at 450 nm
and the activities were calculated according to the standard
curve.
Determination of the total
antioxidant, oxidant status and oxidative stress index
To determine antioxidant status and oxidant status
according to the different concentrations of Ca2+ in
MLO-Y4 cells, Rel Assay Diagnostic Total Antioxidant Status (TAS)
and Total Oxidant Status (TOS) kits (Rel Assay Diagnostic) were
utilized. Erel's method was used for the calculations (22). Oxidative Stress Index (OSI) was
measured as: OSI=[(TOS, µmol H2O2
equivalent/l)/(TAS, mmol Trolox equivalent/l) ×10].
Immunoblotting for PHEX, MEPE, DMP1
and GAPDH
Cell lysates were prepared in ice-cold RIPA buffer
(Cell Signaling Technology, Inc.) after MLO-Y4 cell lines were
treated with six different Ca2+ concentrations for 24 h.
Cell lysates were spun to remove cellular debris by centrifuge at
12×103 g for 10 min at +4°C. Equal amounts of proteins
were subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) on 10% polyacrylamide gels. The gels
were transferred on PVDF membrane by using a wet transfer system
overnight and were labeled to perform immunoblot analysis with PHEX
(Thermo Fisher Scientific, Inc., bs-12313R), MEPE (bioss,
bs-8689R), DMP1 (biorbyt, orb255063), GAPDH (Cell Signaling
Technology, Inc.) and Horseradish peroxidase-labeled antirabbit
secondary antibodies (Cell Signaling Technology, Inc.). Proteins
were visualized by using Image Studio Lite Ver 4.0 in LI-COR.
Protein-content determination was measured by the method described
by Bradford.
Caspase-3 analysis
The activity of the caspase-3 was measured using a
colorimetric assay (Elabscience). Centrifugation at
1×103 g for 10 min was used to collect cells that had
been exposed to six different concentrations of Ca2+ for
15 and 60 min. Lysis buffer was used to lyse the pelleted cells.
Then, the cell lysates were incubated on ice for 10 min and were
centrifuged at 14×103 g for 1 min. Following the
centrifugation, supernatants were transferred to new tubes. For
measuring caspase-3 enzyme activity, 100 µl of the samples were
added to the wells, after the incubation period 100 µl Biotinylated
Detection Antibody working solution, 100 µl HRP conjugate working
solution, 90 µl Substrate Reagent and 50 µl Stop Solution applied
to each well respectively and incubated for 2 h at 37°C in
CO2 incubator. Absorbances of the samples were read
under 450 nm via the ELISA reader (Biotek).
Statistical analysis
Data are expressed as mean ± standard deviation of
three independent determinations. The non-parametric approach was
preferred and the Kruskal Wallis analysis of variance was used for
comparison between groups. When a significant difference was
detected as a result of the Kruskal Wallis test, the post hoc
Dunn's test was used. In the Kruskal Wallis analysis of variance
results, P<0.05 was accepted as significant. All analyses were
performed with SPSS 25.0 (IBM SPSS Statistics 25 software (IBM
Corp.) package program.
Results
The effects of Ca2+
concentration cell viability in MLO-Y4 cells
Since Ca2+ has a critical role in bone
mineralization, we have designed to investigate the effect of
Ca2+ concentration on cell viability in an experiment
that consists of six different Ca2+ concentrations and
three-time points. After MLO-Y4 cells were treated with 1.8, 6, 12,
18, 24 and 50 mM Ca2+ for 15 min, cell viability was
determined by live/death assay. After Ca2+ treatment at
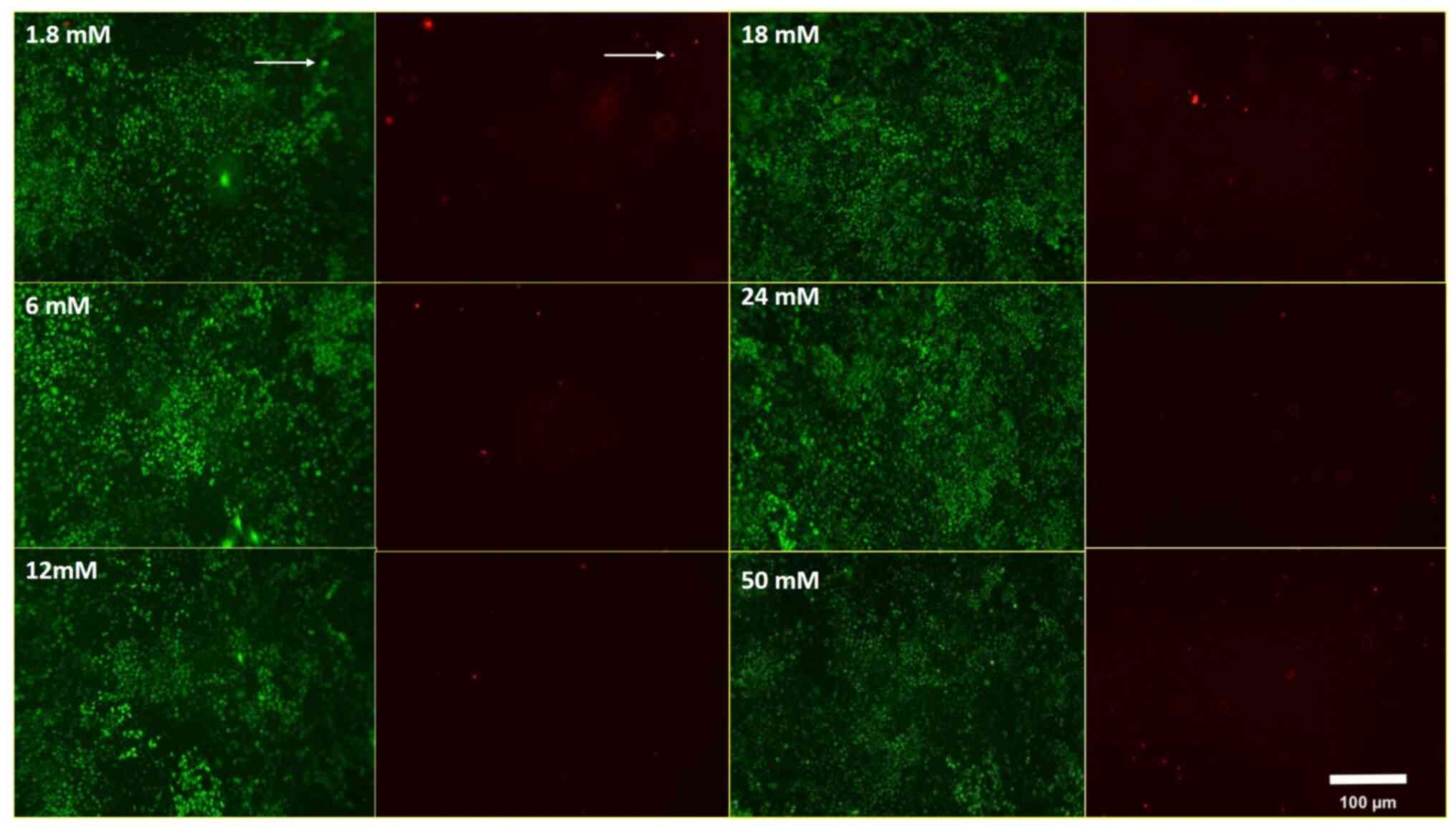
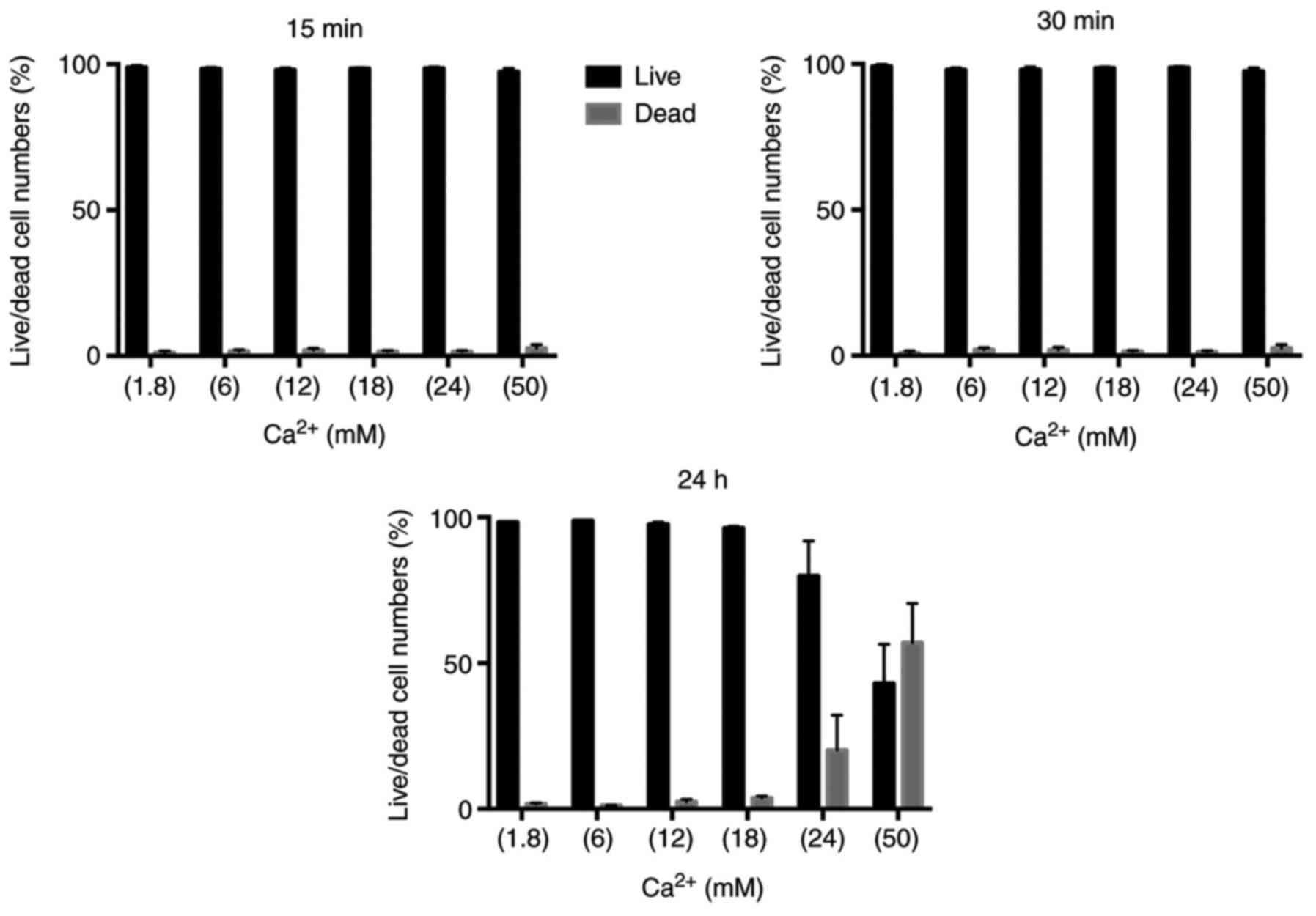
the 15th min, there were much more live MLO-Y4 cells than dead
MLO-Y4 cells in all Ca2+ concentrations (Fig. 1). Based on the above results, we
questioned whether there was a correlation between the incubation
period of Ca2+ and cell viability. Therefore; we set up
two experiments as 60 min and 24 h at the same Ca2+
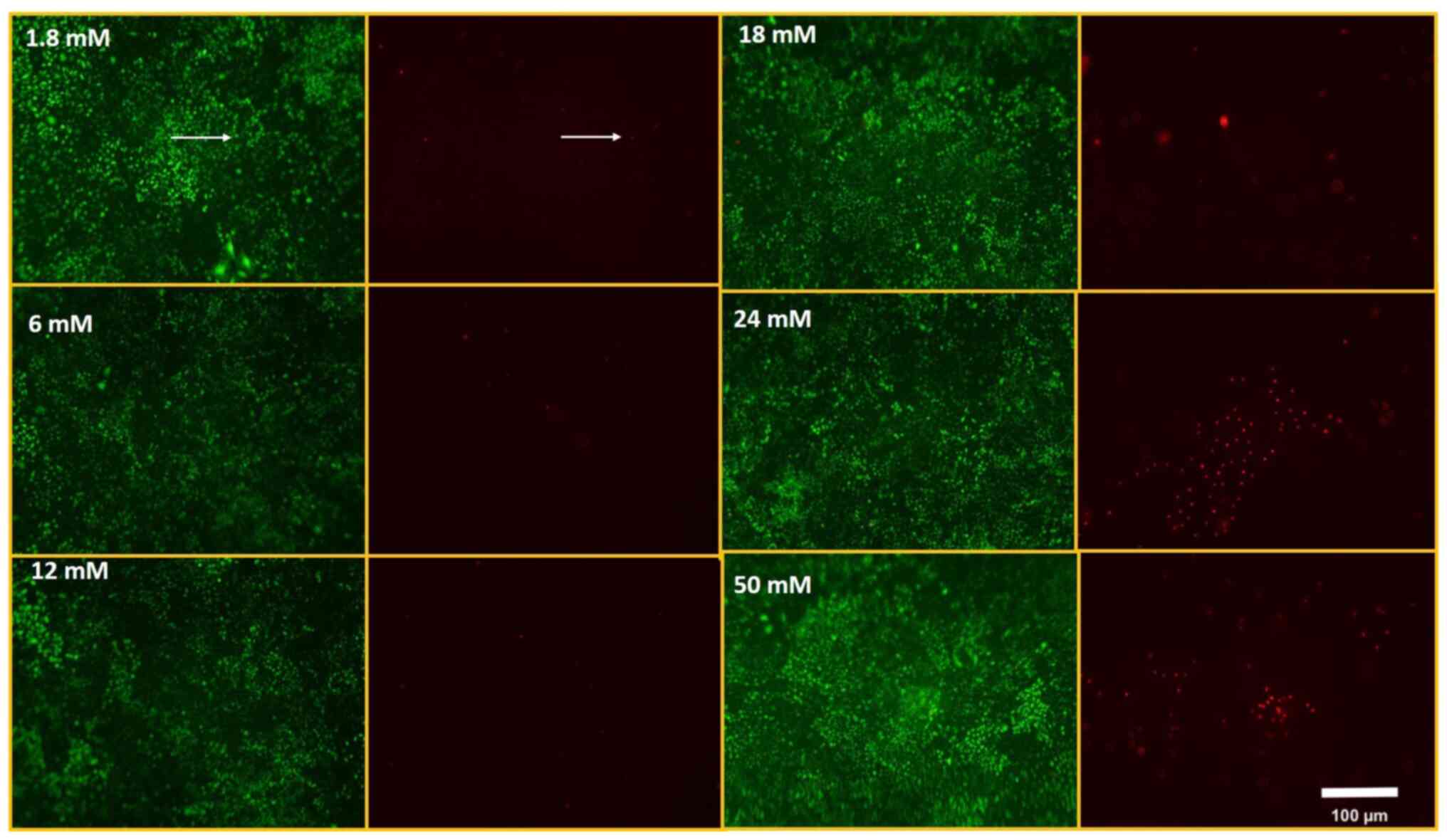
concentrations. Dead cells were increased at 24 and 50 mM
Ca2+ concentrations in 60 min (Fig. 2). In 24 h of Ca2+
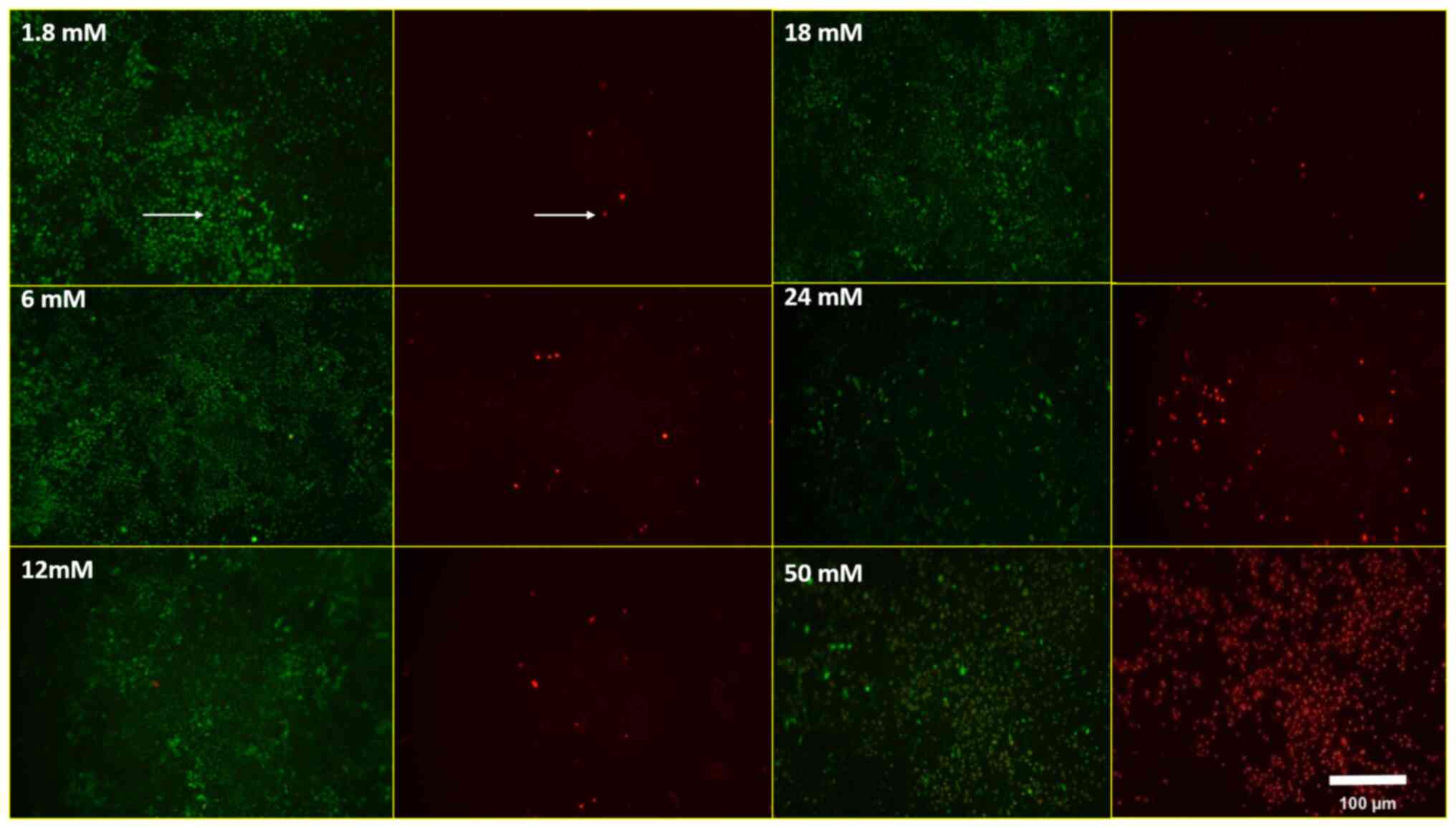
treatment, there were much more dead cells than live MLO-Y4 cells
on 50 mM Ca2+ treatment (Fig. 3). The percentage of live/dead
cells were shown in Fig. 4.
The Ca2+ increases CAT,
SOD, GSH and NOX levels in MLO-Y4 cell lines
Ca2+ has important role between the free
radical and enzymatic antioxidant capacity in bone metabolism.
Therefore, we aimed to show how Ca2+ stimulation
regulates the CAT, SOD, GSH and NOX levels. After MLO-Y4 cells were
treated with Ca2+ (1.8, 6, 12, 18, 24 and 50 mM) for 15
and 60 min, CAT, SOD, GSH and NOX levels were determined by ELISA
assay.
SOD and CAT increased and reached the maximum levels
in 12 mM Ca2+ in 15 min (Fig. 5A and B). GSH levels in different
Ca2+ inductions did not differ significantly (Fig. 5C). In 15 min, the higher
Ca2+ levels steadily raised NOX levels (Fig. 5D). 18, 24 and 50 mM
Ca2+ concentrations led to the depletion of antioxidant
enzymes such as CAT and SOD. The significantly increased NOX
activity was observed in 18, 24 and 50 mM Ca2+
concentrations (P<0.05) (Fig.
5D). 24 mM Ca2+ concentration led to a significant
increase in CAT levels within 60 min of treatment (Fig. 6A). SOD activity gradually
increased by the elevated concentrations of Ca2+ in 60
min. The statistically significant difference in SOD was observed
in 24 and 50 mM Ca2+ concentrations compared to that of
1.8 mM Ca2+ treatment in 60 min (Fig. 6B). There was no significant change
in GSH activity in different Ca2+ points (Fig. 6C). NOX activity was at the highest
level in 24 and 50 mM compared to that of 1.8 mM Ca2+
concentrations in 15 and 60 min (Fig.
6D). Our results clearly show that 12 mM Ca2+
concentration augments CAT and SOD levels, but from the beginning
of 12 mM Ca2+ application NOX enzyme levels
significantly increased in dose dependent on manner in 15 and 60
min. 24 mM Ca2+ concentration significantly increased
SOD and CAT levels in 60 min. GSH levels of MLO-Y4 cells were
stable by the induction of different Ca2+ concentrations
and different time points. NOX activity gradually increased
concentration and time.
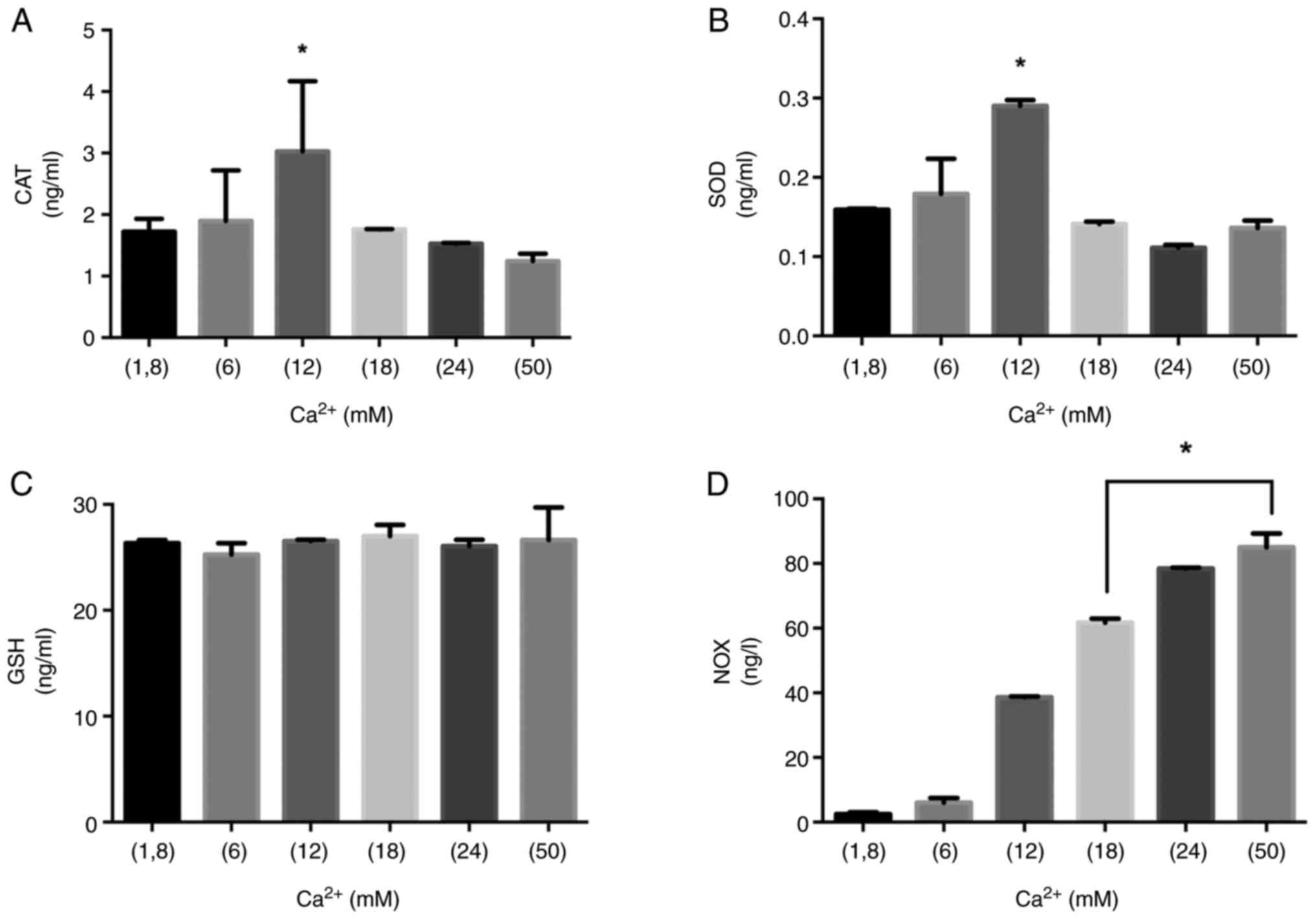 | Figure 5.CAT, SOD, GSH and NOX levels at 15
min. The role of the Ca2+ on oxidant/antioxidant balance
in MLO-Y4 at the incubation of 15 min. (A) CAT, (B) SOD, (C) GSH
and (D) NOX levels were measured in MLO-Y4 cells treated with
Ca2+ (1.8, 6, 12, 18, 24 and 50 mM) for 15 min.
*P<0.05 vs. 1.8 mM Ca2+ group. CAT, catalase; SOD,
superoxide dismutase; GSH, glutathione; NOX, NADPH oxidase. |
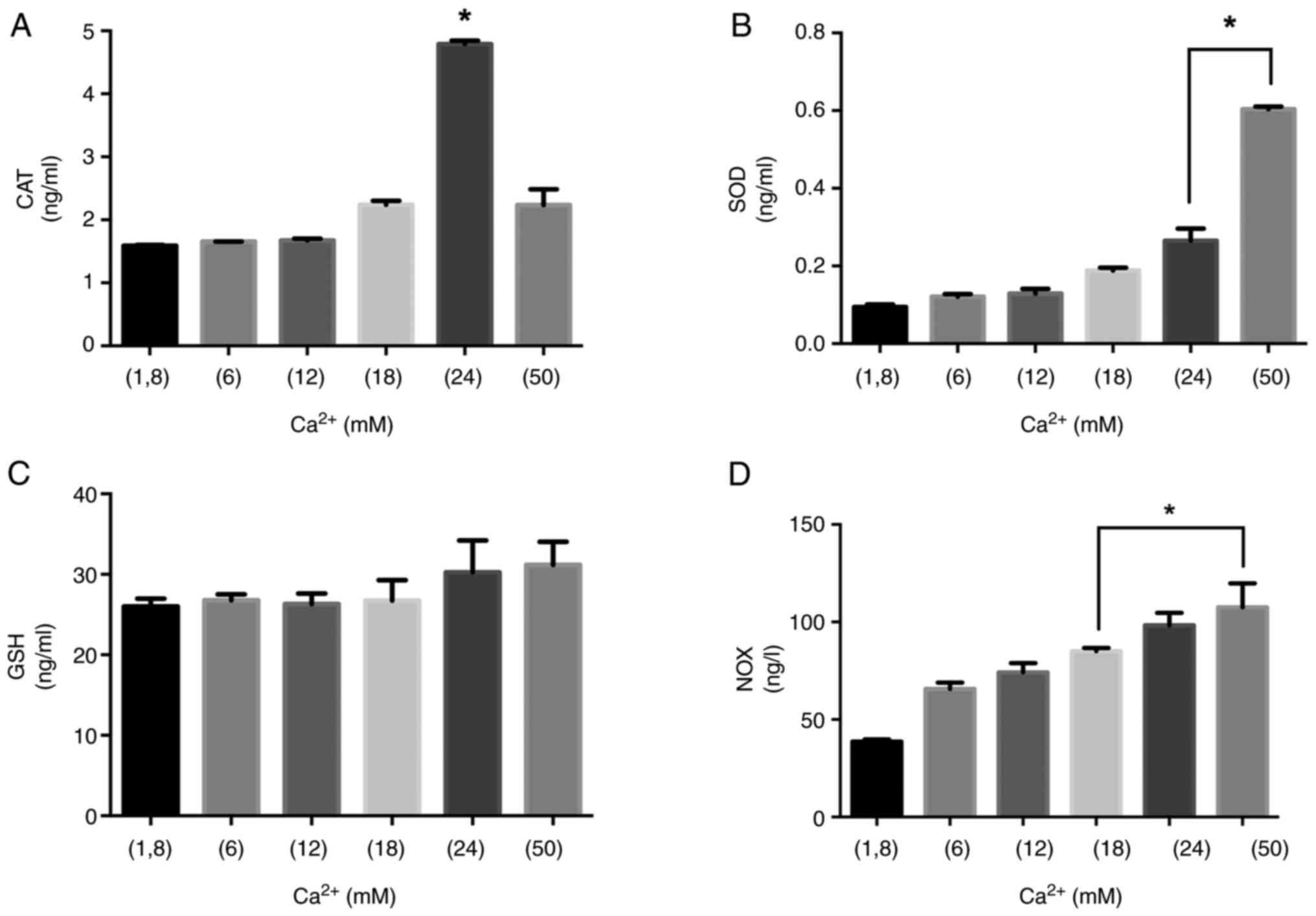 | Figure 6.CAT, SOD, GSH and NOX levels at 60
min. The role of the Ca2+ on oxidant/antioxidant balance
in MLO-Y4 at the incubation of 60 min. (A) CAT, (B) SOD, (C) GSH
and (D) NOX levels were measured in MLO-Y4 cells treated with
Ca2+ (1.8, 6, 12, 18, 24 and 50 mM) for 60 min.
*P<0.05 vs. 1.8 mM Ca2+ group. CAT, catalase; SOD,
superoxide dismutase; GSH, glutathione; NOX, NADPH oxidase. |
Ca2+ induces bone
mineralization by triggering crosstalk between ROS dependent
activation and PHEX, MEPE, DMP1
Previous studies have shown that ROS and/or
antioxidant systems are related to the pathogenesis of bone loss.
In our study; TAS and TOS levels were measured at the end of the 24
h of different concentrations of Ca2+ application, and
OSI was calculated (Table I).
While the amount of Ca2+ increased, total oxidant status
and OSI were significantly increased at 12, 18, 24 and 50 mM
Ca2+ (P<0.05 compared to 1.8 mM Ca2+).
 | Table I.TAS, TOS and OSI were measured in
MLO-Y4 cells after 24 h of Ca2+ application. |
Table I.
TAS, TOS and OSI were measured in
MLO-Y4 cells after 24 h of Ca2+ application.
| Experimental
group | TAS (mmol/l) | TOS (µmol/l) | OSI (arbitrary
unit) |
|---|
| 1.8 mM Ca | 0.366 |
4.580 |
1.250 |
| 6 mM Ca | 0.180 |
4.580 |
2.544a |
| 12 mM Ca | 0.252 | 17.328a |
6.877a |
| 18 mM Ca | 0.383 | 18.244a |
4.758a |
| 24 mM Ca |
0.149a | 21.374a | 14.376a |
| 50 mM Ca | 0.390 | 27.786a | 7.130a |
Ca2+ upregulates crosstalk
between PHEX, MEPE, DMP1 expression and bone mineralization
PHEX, MEPE and DMP1 play an important role in bone
remodeling. Therefore, to assess the expression levels of PHEX,
MEPE and DMP1 in MLO-Y4 cells, we performed Western Blot analysis.
Immunoblot analysis revealed that Ca2+ upregulates
expressions of PHEX, MEPE and DMP1 in a dose dependent manner. The
highest expression levels of PHEX, MEPE and DMP1 were seen at 24 mM
Ca2+ treatment. We also observed a depletion/feedback in
the expression of PHEX, MEPE and DMP1 at 50 mM Ca2+
concentration. The expression of PHEX, a pro-mineralization gene,
was notably increased at the 24 mM Ca2+ but was
suppressed at 50 mM Ca2+; the highest tested
concentration of Ca2+. Increased exposure to
Ca2+ appeared to alter the interaction of other
osteoblast mineralization-associated genes. Our data show that
optimum Ca2+ concentration induces bone mineralization
while high Ca2+ concentration cause depletion of PHEX,
MEPE and DMP1 expression (Fig.
7).
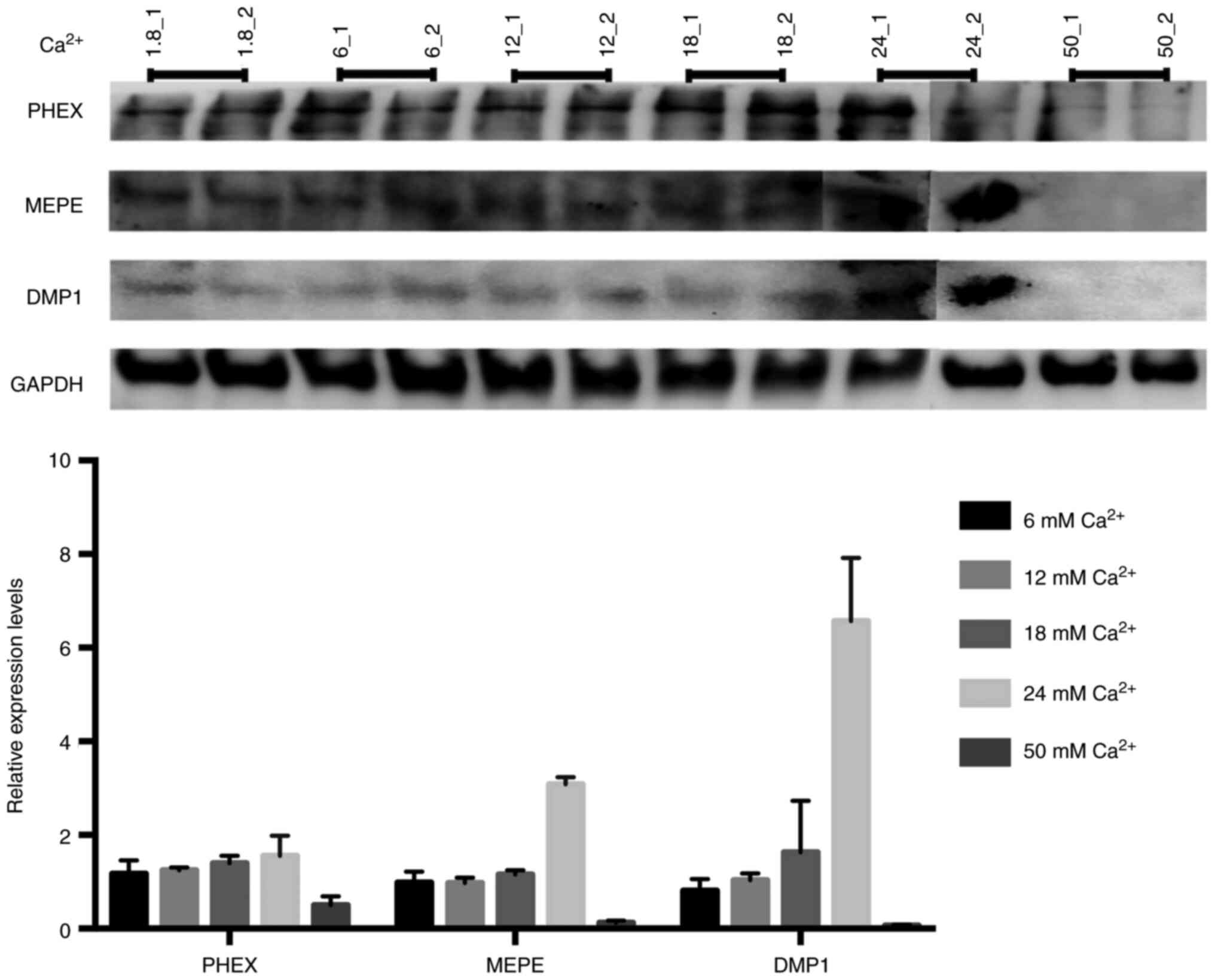 | Figure 7.PHEX, DMP1 and MEPE expression
levels. The Ca2+ promotes the PHEX, DMP1/MEPE axis.
Western blotting analysis of PHEX, MEPE, DMP1 and GAPDH levels in
MLO-Y4 cells treated with different concentrations of
Ca2+. Each Ca2+ concentration was analyzed
densitometrically according to its GAPDH (n=2), (_1 is sample one,
_2 is sample two of each Ca2+ concentration). Two
sibling gels were prepared, run and transferred in the same
electrophoresis equipment. 24_2, 50_1 and 50_2 samples were loaded
to the sibling gel. PHEX, phosphate-regulating neutral
endopeptidase on chromosome X; DMP1, dentin matrix protein 1; MEPE,
matrix extracellular phosphoglycoprotein. |
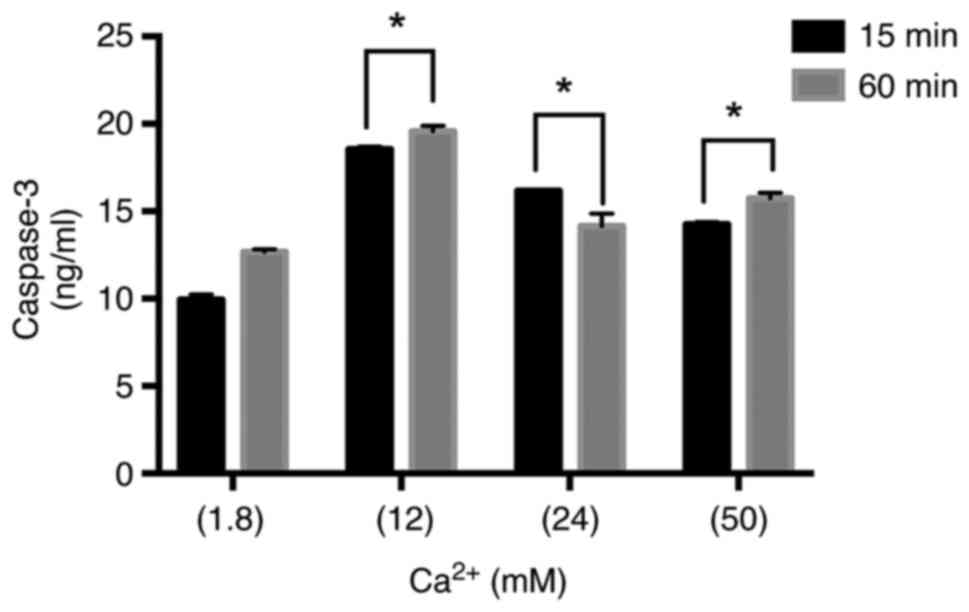
Caspase-3 analysis
Caspase-3 levels were studied in MLYO-4 cells grown
in complete media supplemented with different concentrations of
Ca2+ for 15 and 60 min. Caspase-3 levels were
significantly elevated by the 12, 24 and 50 mM Ca2+ when
compared to 1.8 mM Ca2+ treated control cells in both
timelines. The rate of rising was most visible in 12 mM compared to
other groups in 15 and 60 min. Incubation of MLO-Y4 cells with 1.8,
12, 24 and 50 mM of Ca2+ for 60 min led to increased
caspase-3 levels compared to the 15 min treatment for each
(Fig. 8). The data obtained from
our results show all (12, 24 and 50 mM) Ca2+
concentrations stimulated the caspase-3 activity which is the key
factor for the initiation of the apoptotic pathway.
Discussion
Calcium plays an important role in many basic
biological processes like the muscle system, neural system, enzyme
activity, hormone metabolism, and membrane permeability. Moreover,
Ca2+ is an important structural component of the
skeleton. Additionally, control of Ca2+ ion in the
extracellular fluid is vital for many metabolic activities and some
endocrine control systems have developed to maintain constant
Ca2+ concentration (23). Additionally, the breakdown of
mesoporous bioactive glass scaffold releases active substances like
calcium that have the potential to increase gene expression and
cause osteoblast development and they also suggested that
electrical stimulation could encourage cellular differentiation by
controlling calcium ionic pathways and increasing calcium ion
inflow into cells (24,25). Total calcium levels in healthy
persons are normally kept within the range of 2.2-2.6 mM, while
ionized calcium levels are usually maintained between 1.1-1.3 mM.
However, soluble Ca2+ levels in the bone interstitial
fluid may be higher, since previous studies demonstrated that the
levels of Ca2+ ranged from 8 to 40 mM at the nearest
area of the resorbing osteoclasts (26,27). Ca2+concentrations can
range from 8 to 40 mM in the microenvironment of the resorbing
osteoclast. Osteocytes can also release considerable quantities of
Ca2+, as seen during lactation, implying that they are
exposed to high extracellular Ca2+ concentrations
(27). Elevated serum free
calcium concentrations may have an impact on the maximal calcium
concentration in the interstitial fluid of the bone that is closely
next to osteoclasts. According to Sugimoto et al, bone
remodeling depends on the presence of mononuclear cells and a high
calcium concentration at the resorptive site (28).
Our results indicate that Ca2+
concentration significantly inhibits cell viability in a dose and
time-dependent manner (Fig. 1,
Fig. 2, Fig. 3). Changes in Ca2+
concentration have been shown to promote the function and
cooperation of the osteocytes and osteoblasts (29). It has also been shown that many
biological activities such as muscle contraction and nerve
conduction are regulated as a result of these changes in
Ca2+ concentration, which occurs outside the cell,
especially in the presence of mechanical stress. Osteocytes are
derived from the mature osteoblasts that have a more differentiated
morphology during the bone formation process and are embedded in
the matrix (30). The osteocytes
contact with adjacent cells and communicate via dendritic processes
in the canaliculi in the mineralized matrix of the bone.
Furthermore, osteocytes can communicate with other cells by
secreting factors into the bone marrow space or straight into the
bloodstream (31,32). Cellular oxidative damage is a
basic general mechanism of cell damage and is mainly caused by
reactive oxygen species (ROS). While ROS is overproduced, it can
cause oxidative stress which is characterized as an imbalance
between ROS formation and antioxidant defense systems (33) and NADPH oxidases (NOX) produce ROS
in a controlled to specifically regulate signal transduction
(34). Oxidative stress can
affect age-related diseases, including osteoporosis and
significantly affect osteocytes. Increasing concentrations of
cytoplasmic Ca2+ causes Ca2+ influx into
mitochondria and Ca2+ disrupts metabolism of the cell.
Additionally, Ca2+ in the cytoplasm can regulate
phosphorylation/dephosphorylation of proteins (35). The lack of an impact of Ca channel
inhibitors on expression levels of PHEX, MEPE and DMP1 proteins,
SOD, CAT, GSH, NOX and Caspase-3 levels and total antioxidant and
oxidant status is a limitation of our work. A further in
vivo examination is necessary to determine the effect of high
serum calcium concentrations on bone remodeling, which is another
limitation of our work.
Cellular oxidative stress disrupts bone remodeling
and leads to an imbalance between osteoclast and osteoblast
activity. Previous studies have shown that ROS and/or antioxidant
systems are related to the pathogenesis of bone loss (36). Osteoporosis is a metabolic bone
disease characterized by low bone mineral density and a loss in
bone mass, making the bone fragile and prone to fracture (37). According to a recent review
article by Domazetovic et al, few data have shown how the
molecular mechanism changes with apoptosis and oxidative status
(38) but, recently, more studies
have been aiming to explain the details.
In the present study, antioxidant-oxidant (CAT, SOD,
GSH and NOX) enzyme levels were measured after 15 and 60 min of
Ca2+ administration in MLO-Y4 cells (Figs. 5 and 6). Additionally, ROS induces the
apoptosis of osteoblasts and supports osteoclastogenesis (39). Advanced oxidation products had
been shown to inhibit the proliferation and differentiation of rat
osteoblastic cells and rat mesenchymal stem cells (40) but osteocytes have been reported as
a major source of the cytokine receptor activator of nuclear factor
kappa-B ligand (RANKL), its functions as a key factor for
osteoclast differentiation and activation (41). Yu et al reported that the
treatment with advanced oxidation protein products significantly
triggered apoptosis of MLO-Y4 cells and induced the phosphorylation
of c-Jun N-terminal kinases (JNK) and p38 mitogen-activated protein
kinases. Their findings cumulatively suggest that the advanced
oxidation protein products induced activation of JNK/p38 MAPK is
reactive oxygen species (ROS)-dependent (42). Superoxide is formed when oxygen
acquires an additional electron and oxygen is generally converted
by the superoxide dismutase (SOD) which is the first enzymatic
structure of the antioxidant defense system (43). Kobayashi et al revealed
that mitochondrial superoxide dismutase 2 (Sod2) depletion
in osteocytes improved the production of cellular superoxide and
Sod2 loss decreased the number of live osteocytes inside the
lucano-canalicular networks (44). To estimate oxidative and
antioxidative status in cells, TOS and TAS are generally used.
While Ca2+ concentration was increased, TOS and the OSI
values also increased in our findings (Table I). Glutathione (GSH) is a
tripeptide in living organisms and it acts as an antioxidant either
directly by interacting with ROS (45). There was no significant difference
between GSH levels of different Ca2+ concentrations. CAT
is the one of the most important antioxidant enzymes which break
down two H2O2 molecules into one molecule of
oxygen and two molecules of water (46). Increased concentrations of
Ca2+ in 15 min treatments led to elevated CAT activity.
Previous studies suggested that concentrations of Ca2+
in the range of 10–15 mM demonstrated a moderate negative effect on
cell survival in calvarial cells of the newborn mouse (26). Consistent with these findings, our
study demonstrates that 12, 24 and 50 mM Ca2+
concentrations result in elevated caspase-3 levels osteocytes are
sensitive to the extracellular concentration of Ca2+, up
to around 12 mM in 15 and 60 min of induction (Fig. 7). PHEX, MEPE and DMP1 are
essential proteins released from osteocytes and play a significant
role in bone mineralization. Topically administered Ca2+
to osteocytes in varying amounts could have a variety of effects.
The goal of our study was to reveal the changes in bone
mineralization-related protein levels in different Ca2+
concentrations and at different time points. Our results showed
that PHEX, MEPE, and DMP1 expression levels correlated to the
increased concentrations of Ca2+ (6, 12, 18 mM). The
highest increase in PHEX, MEPE and DMP1 expressions was observed at
a Ca2+ concentration of 24 mM (Fig. 7). However, the expression levels
of the proteins decreased at 50 mM Ca2+ concentration
because of the enhanced dead cell ratio.
Some important clinical problems arise in deficiency
or inactivation of PHEX and MEPE and it is important to understand
the metabolism of these proteins. The inactivation of PHEX or/and
DMP1 has been shown to result in an increased fibroblast growth
factor 23 (FGF23) (47), and they
play a key role in the progression of hypophosphatemic conditions
such as tumor-induced osteomalacia and X-linked hypophosphatemic
rickets/osteomalacia. PHEX deficiency results in the overproduction
of fibroblast growth factor 23 (FGF23) in osteocytes (48), which leads to hypophosphatemia and
impaired vitamin D metabolism (47). Miyagawa et al noted that
DMP1 expression in osteocytic cells was increased after the 24 h
treatment with 10 mM phosphate (47). Both PHEX and DMP1 are also
important proteins on phosphate homeostasis. Because another role
of osteocytes is to regulate phosphate (Pi) homeostasis through the
release of several molecules such as PHEX, MEPE, DMP1 and FGF23
(49). DMP1 and PHEX inhibit the
expression of FGF23, whereas MEPE increases it, although the
mechanisms are still unknown (50,51). Both DMP1 and PHEX are excessively
expressed in osteocytes and might regulate the maturation of the
osteocyte cells via FGF23 regulation of phosphate homeostasis
(8). Magne et al cultured
M2H4 cells and supplemented the media with 3 mM Pi from day 8 to 20
(20). They have shown that M2H4
cells had more mineralized the dentin extracellular matrix than
control conditions (1 mM Pi) and medium supplemented 3 mM Pi
accumulate Ca2+ in cell layers (20). In our study, both expressions of
PHEX and MEPE increased at 24 mM Ca2+ induced.
Increased levels of both PHEX and MEPE in the
treatment of 24 mM Ca2+ are important for osteocytes
metabolism and they might be used in bone treatment in the future.
PHEX, MEPE, and DMP1 have also been demonstrated to be controlled
during loading and unloading settings in previous investigations
(52–54). These proteins involved in bone
construction are expected to be upregulated in response to anabolic
loading, and they are responsible for absorption (55). Gluhak-Heinrich et al
(52) revealed that alveolar
osteocytes showed high basal levels of MEPE that decreased during
the first day of loading compared to that of DMP1. Kulkarni et
al (56) have shown that
mechanical loading upregulated gene expression of MEPE but not PHEX
in MLO-Y4 cells during 1 and 6 h pulsating fluid flow. RANKL plays
an important role during the loading/unloading mechanism because
unloading increases RANKL which is an essential promoter for
osteocytes (57). Osteocytes has
dendritic process, which is associated with mechanical stimulation,
and this process is regulated by E11 which is the earliest
osteocytes protein increased by mechanical loading by osteocytes
(58). Moreover, E11, MEPE and
DMP1 have higher levels of expression in basal bone than in
alveolar bone (59). During the
skeleton is exposed to loading, an increase in these proteins
secreted from osteocytes can be expected, which is very important
in the mineralization of bone tissue. A new strategy for bone
treatment can be designed by giving the appropriate Ca2+
concentration to increase the release of these proteins from
osteocytes.
In a summary, our results suggest that high
Ca2+ concentrations can perturb osteocyte cell
hemostasis which leads to tissue damage and apoptosis of the bone
and result in metabolic bone diseases such as osteoporosis. 24 mM
Ca2+ concentration can trigger bone mineralization
markers such as PHEX, MEPE and DMP1 can be a convenient dosage for
the treatment of bone damage.
Acknowledgements
Not applicable.
Funding
This study was supported by Pamukkale University (grant nos.
2019BSP006 and 2019BSP002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BOD and OA designed all experimental procedures of
the study, and BOD conducted cell culture and the live/dead cells
assay. ACD and ERK conducted CAT, SOD, GSH and NOX measurements,
determination of TAS and TOS, immunoblotting for PHEX, MEPE, DMP1
and GAPDH, and caspase-3 analysis. ACD and ERK confirm the
authenticity of all the raw data. JC calculated the percentage of
live and dead cells. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Palumbo C and Ferretti M: The osteocyte:
From ‘Prisoner’ to ‘Orchestrator’. J Funct Morphol Kinesiol.
6:282021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Florencio-Silva R, da Silva Sasso GR,
Sasso-Cerri E, Simões J and Cerri PS: Biology of bone tissue:
Structure, function, and factors that influence bone cells. Biomed
Res Int. 2015:4217462015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato Y, Windle JJ, Koop BA, Mundy GR and
Bonewald LF: Establishment of an osteocyte-like cell line, MLO-Y4.
J Bone Miner Res. 12:2014–2023. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robling AG and LF, . Bonewald the
osteocyte: New insights. Annu Rev Physiol. 82:485–506. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kao RS, Abbott MJ, Louie A, O'Carroll D,
Lu W and Nissenson R: Constitutive protein kinase A activity in
osteocytes and late osteoblasts produces an anabolic effect on
bone. Bone. 55:277–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fisher LW and Fedarko NS: Six genes
expressed in bones and teeth encode the current members of the
SIBLING family of proteins. Connect Tissue Res. 44 (Suppl
1):S33–S40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo D, Keightley A, Guthrie J, Veno PA,
Harris SE and Bonewald LF: Identification of osteocyte-selective
proteins. Proteomics. 10:3688–3698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas
SL, McKee MD, Drezner MK, Bonewald LF and Feng JQ: The biological
function of DMP-1 in osteocyte maturation is mediated by its 57-kDa
C-terminal fragment. J Bone Miner Res. 26:331–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siyam A, Wang S, Qin C, Mues G, Stevens R,
D'Souza RN and Lu Y: Nuclear localization of DMP1 proteins suggests
a role in intracellular signaling. Biochem Biophys Res Commun.
424:641–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ
and Boskey AL: DMP1 depletion decreases bone mineralization in
vivo: An FTIR imaging analysis. J Bone Miner Res. 20:2169–2177.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rowe PS, de Zoysa PA, Dong R, Wang HR,
White KE, Econs MJ and Oudet CL: MEPE, a new gene expressed in bone
marrow and tumors causing osteomalacia. Genomics. 67:54–68. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
David V, Martin A, Hedge AM and Rowe PSN:
Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal
hormone and vascularization modulator. Endocrinology.
150:4012–4023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu C, Huang S, Miclau T, Helms JA and
Colnot C: Mepe is expressed during skeletal development and
regeneration. Histochem Cell Biol. 121:493–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung H, Mbimba T, Unal M and Akkus O:
Repetitive short-span application of extracellular calcium is
osteopromotive to osteoprogenitor cells. J Tissue Eng Regen Med.
12:e1349–e1359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung H, Best M and Akkus O: Microdamage
induced calcium efflux from bone matrix activates intracellular
calcium signaling in osteoblasts via L-type and T-type
voltage-gated calcium channels. Bone. 76:88–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamauchi M, Yamaguchi T, Kaji H, Sugimoto
T and Chihara K: Involvement of calcium-sensing receptor in
osteoblastic differentiation of mouse MC3T3-E1 cells. Am J Physiol
Endocrinol Metab. 288:E608–E616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boskey AL and Roy R: Cell culture systems
for studies of bone and tooth mineralization. Chem Rev.
108:4716–4733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonewald LF, Harris SE, Rosser J, Dallas
MR, Dallas SL, Camacho NP, Boyan B and Boskey A: Von Kossa staining
alone is not sufficient to confirm that mineralization in vitro
represents bone formation. Calcif Tissue Int. 72:537–547. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Wu T, Dong X, Wang Q and Shen J:
Adsorption mechanism of BMP-7 on hydroxyapatite (001) surfaces.
Biochem Biophys Res Commun. 361:91–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magne D, Bluteau G, Lopez-Cazaux S, Weiss
P, Pilet P, Ritchie HH, Daculsi G and Guicheux J: Development of an
odontoblast in vitro model to study dentin mineralization. Connect
Tissue Res. 45:101–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Price PA, June HH, Hamlin NJ and
Williamson MK: Evidence for a serum factor that initiates the
re-calcification of demineralized bone. J Biol Chem.
279:19169–19180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erel O: A new automated colorimetric
method for measuring total oxidant status. Clin Biochem.
38:1103–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheweita SA and Khoshhal KI: Calcium
metabolism and oxidative stress in bone fractures: Role of
antioxidants. Curr Drug Metab. 8:519–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shuai C, Liu G, Yang Y, Qi F, Peng S, Yang
W, He C, Wang G and Qian G: A strawberry-like Ag-decorated barium
titanate enhances piezoelectric and antibacterial activities of
polymer scaffold. Nano Energy. 74:1048252000. View Article : Google Scholar
|
|
25
|
Shuaia C, Xu Y, Feng P, Wang G, Xiong S
and Peng S: Antibacterial polymer scaffold based on mesoporous
bioactive glass loaded with in situ grown silver. Chemical
Engineering J. 374:304–345. 2019. View Article : Google Scholar
|
|
26
|
Maeno S, Niki Y, Matsumoto H, Morioka H,
Yatabe T, Funayama A, Toyama Y, Taguchi T and Tanaka J: The effect
of calcium ion concentration on osteoblast viability, proliferation
and differentiation in monolayer and 3D culture. Biomaterials.
26:4847–4855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Welldon KJ, Findlay DM, Evdokiou A, Ormsby
RT and Atkins GJ: Calcium induces pro-anabolic effects on human
primary osteoblasts associated with acquisition of mature osteocyte
markers. Mol Cell Endocrinol. 376:85–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugimoto T, Kanatani M, Kano J, Kaji H,
Tsukamoto T, Yamaguchi T, Fukase M and Chihara K: Effects of high
calcium concentration on the functions and interactions of
osteoblastic cells and monocytes and on the formation of
osteoclast-like cells. J Bone Miner Res. 8:1445–1452. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dvorak MM, Siddiqua A, Ward DT, Carter DH,
Dallas SL, Nemeth EF and Riccardi D: Physiological changes in
extracellular calcium concentration directly control osteoblast
function in the absence of calciotropic hormones. Proc Natl Acad
Sci USA. 101:5140–5145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mullen CA, Haugh MG, Schaffler MB, Majeska
RG and McNamara LM: Osteocyte differentiation is regulated by
extracellular matrix stiffness and intercellular separation. J Mech
Behav Biomed Mater. 28:183–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fulzele K, Lai F, Dedic C, Saini V, Uda Y,
Shi C, Tuck P, Aronson JL, Liu X, Spatz JM, et al:
Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes
to Beige Adipogenesis in Peripheral Fat Depots. J Bone Miner Res.
32:373–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uda Y, Azab E, Sun N, Shi C and Pajevic
PD: Osteocyte mechanobiology. Curr Osteoporos Rep. 15:318–325.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarban S, Kocyigit A, Yazar M and Isikan
UE: Plasma total antioxidant capacity, lipid peroxidation, and
erythrocyte antioxidant enzyme activities in patients with
rheumatoid arthritis and osteoarthritis. Clin Biochem. 38:981–986.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schroder K: NADPH oxidases in bone
homeostasis and osteoporosis. Free Radic Biol Med. 132:67–72. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ermak G and Davies KJ: Calcium and
oxidative stress: From cell signaling to cell death. Mol Immunol.
38:713–721. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ostman B, Michaëlsson K, Helmersson J,
Byberg L, Gedeborg R, Melhus H and Basu S: Oxidative stress and
bone mineral density in elderly men: Antioxidant activity of
alpha-tocopherol. Free Radic Biol Med. 47:668–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Banfi G, Iorio EL and Corsi MM: Oxidative
stress, free radicals and bone remodeling. Clin Chem Lab Med.
46:1550–1555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Domazetovic V, Marcucci G, Iantomasi T,
Brandi ML and Vincenzini MT: Oxidative stress in bone remodeling:
Role of antioxidants. Clin Cases Miner Bone Metab. 14:209–216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jilka RL, Noble B and Weinstein RS:
Osteocyte apoptosis. Bone. 54:264–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhong ZM, Bai L and Chen JT: Advanced
oxidation protein products inhibit proliferation and
differentiation of rat osteoblast-like cells via NF-kappaB pathway.
Cell Physiol Biochem. 24:105–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakashima T, Hayashi M, Fukunaga T, Kurata
K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et
al: Evidence for osteocyte regulation of bone homeostasis through
RANKL expression. Nat Med. 17:1231–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu C, Huang D, Wang K, Lin B, Liu Y, Liu
S, Wu W and Zhang H: Advanced oxidation protein products induce
apoptosis, and upregulate sclerostin and RANKL expression, in
osteocytic MLO-Y4 cells via JNK/p38 MAPK activation. Mol Med Rep.
15:543–550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Branicky R, Noë A and Hekimi S:
Superoxide dismutases: Dual roles in controlling ROS damage and
regulating ROS signaling. J Cell Biol. 217:1915–1928. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kobayashi K, Nojiri H, Saita Y, Morikawa
D, Ozawa Y, Watanabe K, Koike M, Asou Y, Shirasawa T, Yokote K, et
al: Mitochondrial superoxide in osteocytes perturbs canalicular
networks in the setting of age-related osteoporosis. Sci Rep.
5:91482015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lushchak VI: Glutathione homeostasis and
functions: Potential targets for medical interventions. J Amino
Acids. 2012:7368372012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nandi A, Yan LY, Jana CK and Das N: Role
of catalase in oxidative stress- and age-associated degenerative
diseases. Oxid Med Cell Longev. 2019:96130902019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miyagawa K, Yamazaki M, Kawai M, Nishino
J, Koshimizu T, Ohata Y, Tachikawa K, Mikuni-Takagaki Y, Kogo M,
Ozono K and Michigami T: Dysregulated gene expression in the
primary osteoblasts and osteocytes isolated from hypophosphatemic
Hyp mice. PLoS One. 9:e938402014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Wang S, Li C, Gao T, Liu Y,
Rangiani A, Sun Y, Hao J, George A, Lu Y, et al: Inactivation of a
novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in
mice. PLoS Genet. 8:e10027082012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bellido T: Osteocyte-driven bone
remodeling. Calcif Tissue Int. 94:25–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Martin A, Liu S, David V, Li H, Karydis A,
Feng JQ and Quarles LD: Bone proteins PHEX and DMP1 regulate
fibroblastic growth factor Fgf23 expression in osteocytes through a
common pathway involving FGF receptor (FGFR) signaling. FASEB J.
25:2551–2562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lavi-Moshayoff V, Wasserman G, Meir T,
Silver J and Naveh-Many T: PTH increases FGF23 gene expression and
mediates the high-FGF23 levels of experimental kidney failure: A
bone parathyroid feedback loop. Am J Physiol Renal Physiol.
299:F882–F889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gluhak-Heinrich J, Pavlin D, Yang W,
MacDougall M and Harris SE: MEPE expression in osteocytes during
orthodontic tooth movement. Arch Oral Biol. 52:684–690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang W, Lu Y, Kalajzic I, Guo D, Harris
MA, Gluhak-Heinrich J, Kotha S, Bonewald LF, Feng JQ, Rowe DW, et
al: Dentin matrix protein 1 gene cis-regulation: use in osteocytes
to characterize local responses to mechanical loading in vitro and
in vivo. J Biol Chem. 280:20680–20690. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng
JQ, MacDougall M, Harris SE and Pavlin D: Mechanical loading
stimulates dentin matrix protein 1 (DMP1) expression in osteocytes
in vivo. J Bone Miner Res. 18:807–817. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bonewald LF: The role of the osteocyte in
bone and nonbone disease. Endocrinol Metab Clin North Am. 46:1–18.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kulkarni RN, Bakker AD, Everts V and
Klein-Nulend J: Inhibition of osteoclastogenesis by mechanically
loaded osteocytes: involvement of MEPE. Calcif Tissue Int.
87:461–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xiong J, Onal M, Jilka RL, Weinstein RS,
Manolagas SC and O'Brien CA: Matrix-embedded cells control
osteoclast formation. Nat Med. 17:1235–1241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang K, Barragan-Adjemian C, Ye L, Kotha
S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ and
Bonewald LF: E11/gp38 selective expression in osteocytes:
Regulation by mechanical strain and role in dendrite elongation.
Mol Cell Biol. 26:4539–4552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zakhary I, Alotibi F, Lewis J, ElSalanty
M, Wenger K, Sharawy M and Messer RLW: Inherent physical
characteristics and gene expression differences between alveolar
and basal bones. Oral Surg Oral Med Oral Pathol Oral Radiol.
122:35–42. 2016. View Article : Google Scholar : PubMed/NCBI
|