Introduction
Irritable bowel syndrome (IBS) is a functional bowel
disorder characterized by symptoms of chronic abdominal pain and
changes in dietary habits (1).
Worldwide, the incidence of IBS is 11.2%, whereas the incidence in
China ranges from 5–6% (2). The
etiology of IBS has been well described, and it has been attributed
to genetic factors, diet, exercise, drugs, stress, cognitive
behavior, infectious enteritis and dysregulation of the gut-brain
axis (3).
More than 10% of patients with infectious enteritis
(notably protozoal enteritis, including that caused by
Trichinella spiralis larvae) will develop IBS (4). The pathogenesis of post-infectious
IBS (PI–IBS) is characterized by low-grade intestinal inflammation,
increased intestinal permeability, muscle hypercontractility and
visceral hypersensitivity (4,5).
Treatment of IBS is often targeted towards management of the
symptoms, rather than the underlying pathophysiology (3). Consequently, treatments are not
sufficiently effective and the natural progression of the disorder
remains unaltered by the majority of therapeutic interventions
(4). The molecular mechanisms,
which underpin PI–IBS, are complex and not fully understood.
Increasing evidence has indicated that oxidative
stress and inflammation are involved in the pathophysiology of
PI–IBS (6). The roles of nuclear
factor erythroid 2-related factor 2 (Nrf2) and nuclear factor
erythroid 2-related factor 2 (NLRP3) activation in PI–IBS have
previously been demonstrated (7).
Under normal conditions, Nrf2 is sequestered by Kelch-like
ECH-associated protein 1 (Keap1) in the cytoplasm. When this
Keap1/Nrf2 complex is disrupted, Nrf2 translocates to the nucleus
where it binds to the antioxidant response element sequences and
activates the transcription of its downstream targets, including
heme oxygenase-1 (HO-1). It has been reported that activation of
the Nrf2 signaling pathway confers protective effects against
PI–IBS (8). Moreover, previous
studies have demonstrated that NLRP3 overactivation during PI–IBS
is associated with intestinal dysfunction (9,10).
NLRP3 binds to apoptosis-associated speck-like protein containing a
CARD (ASC) and recruits pro-caspase-1, which leads to the
maturation of caspase-1. Caspase-1 cleaves pro-IL-1β into IL-1β and
pro-IL-18 into IL-18 (11).
Crosstalk between the Nrf2 and NLRP3 signaling pathways may exist
in PI–IBS (12,13).
Coptis chinensis, a traditional Chinese
medicine, has been used for thousands of years to treat type 2
diabetes mellitus, and cardiovascular, hepatic and renal disorders
(14). The main constituents
responsible for its bioactive properties are the isoquinoline
alkaloids (15). Coptisine is the
second most abundant isoquinoline alkaloid in C. chinensis.
Coptisine exerts diverse beneficial effects, including anticancer,
anti-inflammatory and antibacterial properties (16). Recently, coptisine was reported to
exert suppressive effects on NLRP3 inflammasome activation in in
vivo models (17). Coptisine
has also been reported to reduce the degree of diarrhea and mucosal
injury to the ileum via modulation of the IκBα/NF-κB signaling
pathway (17). Therefore, it was
hypothesized that coptisine may potentially exert protective
effects against PI–IBS via Nrf2-dependent inhibition of the NLRP3
inflammasome. The present study aimed to assess the effect of
coptisine on a rat model of IBS and investigate the underlying
mechanisms.
Materials and methods
Reagents
Coptisine (cat. no. HY-N0430) was purchased from
MedChemExpress. The Nrf2 inhibitor ML385 (cat. no. SML1833) was
purchased from Sigma-Aldrich (Merck KGaA). The following
enzyme-linked immunosorbent assay (ELISA) kits were purchased from
Abcam: Protein Carbonyl ELISA Kit (cat. no. ab238536),
8-hydroxy-2-deoxyguanosine (8-OHdG) ELISA Kit (cat. no. ab201734),
Rat TNF-α ELISA Kit (cat. no. ab236712), Rat IL-1β ELISA Kit (cat.
no. ab255730), Rat IL-18 ELISA Kit (cat. no. ab213909), and the
Lipid Peroxidation 4-hydroxynonenal (4-HNE) Assay Kit (cat. no.
ab238538). Primary antibodies, including anti-Nrf2 antibody (cat.
no. ab92946), recombinant anti-HO-1 antibody (cat. no. ab68477),
recombinant anti-NLRP3 antibody (cat. no. ab263899), anti-ASC
antibody (cat. no. ab180799), recombinant anti-pro-caspase-1 and
cleaved caspase-1 antibody (cat. no. ab179515), anti-histone H3
antibody (cat. no. ab1791) and anti-β-actin antibody (cat. no.
ab8226) were all purchased from Abcam. The secondary antibodies
goat anti-mouse IgG heavy chain and light chain (H&L) (HRP;
cat. no. ab6789) and goat anti-rabbit IgG H&L (HRP; cat. no.
ab6721) were also purchased from Abcam. Protein extraction kits
were purchased from Santa Cruz Biotechnology, Inc. The
bicinchoninic acid (BCA) assay and Pierce™ ECL Plus Western
Blotting Substrate were purchased from Pierce (Thermo Fisher
Scientific, Inc.).
Animals and ethics
The protocol used in the present study was approved
by the Ethics Committee for Animal Use of The First Affiliated
Hospital of Shenzhen University, The Second People's Hospital of
Shenzhen (approval no. 20190902002). Male, Sprague-Dawley rats
(n=80; 250±10 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. and housed in a specific pathogen-free
laboratory environment (temperature, 22±2°C; relative humidity,
55±15%; 12 h light/dark cycle) with ad libitum access to
food and water.
Animal treatment
After 7 days of environmental adaptation, 80 rats
were randomly divided into the following four groups (Fig. 1A): i) Control; ii) PI–IBS model;
iii) coptisine treatment; and iv) coptisine + ML385 treatment. A
total of 20 animals were used in each group. The PI–IBS rat model
was established as previously described (9). Trichinella spiralis larvae
were obtained from the Institute of Pathogen Biology, Chinese
Academy of Medical Sciences and Peking Union Medical College. In
brief, the rats were intragastrically administered 350–400
Trichinella spiralis larvae (diluted in 0.2 ml saline). On
day 14 post-infection, abdominal withdrawal reflex (AWR) scores
were assessed to determine whether the model was successfully
established. The rats in the coptisine group were administered
coptisine (50 mg/kg) intragastrically for 14 consecutive days. The
rats in the coptisine + ML385 group were injected with ML385 (30
mg/kg) intraperitoneally and treated with coptisine (50 mg/kg)
intragastrically 30 min later for 14 consecutive days. The rats in
the control and PI–IBS model groups were administered
intraperitoneally and intragastrically an equivalent volume of
saline. On day 14 post-infection, the body weight and fecal water
content of all rats were assessed. On day 14 post-infection, 10
rats from each group were randomly selected for the
gastrointestinal motility assay. The remaining 10 rats from each
group were used for the assessment of AWR. After gastrointestinal
mobility or AWR tests, 14 rats were randomly selected and were
anesthetized using isoflurane/oxygen (induction, 5% isoflurane;
maintenance, 2% isoflurane) and a Matrix VIP 3000 calibrated
vaporizer (Midmark Corporation). Rats were subsequently decapitated
individually with a rodent guillotine and then transcardially
perfused with saline. The proximal colon tissues were collected for
ELISA (n=8/group), western blotting (n=3/group) or fixed in
glutaraldehyde for transmission electron microscopy (n=3/group).
The remaining rats (n=6/group) were anesthetized with isoflurane
(induction, 5%; maintenance, 2%) and decapitated individually with
a rodent guillotine prior to perfusion with 100 ml of cold (4°C) 4%
paraformaldehyde following saline perfusion. The proximal colon
tissues were collected for hematoxylin and eosin (H&E)
staining.
Gastrointestinal motility
On day 14 post-infection, gastrointestinal motility
was assessed by feeding the rats charcoal meals and determining the
distance traveled by the charcoal in a fixed amount of time. Prior
to the measurement of gastrointestinal motility, the rats
(n=10/group) were fasted overnight and were subsequently
administered 0.2 ml 10% charcoal in 5% acacia gum via oral gavage.
The rats were euthanized 30 min later and the intestinal tracts
were carefully removed. The total length from the pylorus to the
cecum was measured. Gastrointestinal motility was expressed as the
percentage of the total intestinal length traversed by the
charcoal. At least three separate experiments were performed.
AWR score
Visceral sensory functions in rats were evaluated
via AWR scores on day 14 post-infection. Briefly, the rats
(n=10/group) were fasted for 24 h and anesthetized with
isoflurane/oxygen (induction, 5%; maintenance, 2%). A
Vaseline-coated latex, double-lumen catheter attached to a balloon
dilator (6 Fr; external diameter, 2 mm; RWD Life Science Co., Ltd.)
was slowly inserted into the descending colon. Colorectal
distention was performed following adaptation. The rats were fixed
on a table and provided 20, 40, 60 and 80 mmHg pressure stimulation
every 5 min. These conditions were maintained for 20 sec. The
balloon was deflated and withdrawn following the assessment of the
AWR score. AWR scoring was a double-blind procedure completed by
the operator and the observer. The AWR scoring criteria were as
follows: 0, no behavioral response; 1, the body remained immobile
with a simple head movement; 2, abdominal contraction of muscles
that did not leave the ground; 3, abdominal muscle contraction was
accompanied by the lifting of the abdomen; and 4, arching of the
body along with the lifting of the pelvis and scrotum. The same
pressure stimulations were performed three times and the median
score was calculated.
ELISA
Excised colons were homogenized in RIPA buffer
(Takara Bio, Inc.) containing 1% phosphatase inhibitors and
complete protease inhibitor cocktail (Roche Diagnostics). The
homogenates were subsequently centrifuged at 3,000 × g for 20 min
at 4°C and the protein concentrations in the supernatant of the
homogenates were determined using a BCA assay kit. The levels of
the specific oxidative stress markers, 4-HNE, protein carbonyl and
8-OHdG, and the proinflammatory cytokines TNF-α, IL-1β and IL-18 in
the colon homogenates were assessed using ELISA and specific assay
kits according to manufacturer's protocol. At least three separate
experiments were performed.
H&E staining
The colon tissues were fixed with 4%
paraformaldehyde at 4°C for 48 h, and then embedded in paraffin and
dissected into 5-µm coronal sections. The sections were dewaxed in
xylene and then rehydrated using a graded alcohol series (100, 90,
80 and 70%) at room temperature for 5 min. Rehydrated sections were
stained using H&E. The slides were scanned using a PANNORAMIC
MIDI scanner (3DHISTECH, Ltd.) and blind evaluated by two
histopathologists.
Transmission electron microscopy
analysis
Colon tissues were fixed using 1% glutaraldehyde in
0.1 M sodium cacodylate buffer (pH 7.2) overnight at room
temperature. Following fixation, the colons were treated with
reduced 1% osmium tetroxide, followed by 1% tannic acid in 0.1 M
sodium cacodylate buffer at room temperature for 1 h. The colons
were subsequently stained using a 2% aqueous solution of uranyl
acetate at room temperature for 30 min, dehydrated in a graded
ethanol series and processed for embedding in PolyBed
(Polysciences, Inc.). The blocks were sectioned at a 90-nm
thickness, post-stained with Venable's lead citrate at room
temperature for 5 min and imaged using a transmission electron
microscope (JEOL, Ltd.) by observers who were blinded to the
experimental groups.
Western blotting
Fresh colon tissues were homogenized and lysed in
RIPA buffer (Takara Bio, Inc.) containing 1% phosphatase inhibitors
and complete protease inhibitor cocktail (Roche Diagnostics). The
total cytosolic and nuclear protein extract were prepared using
cell nuclear protein extraction kits (Thermo Fisher Scientific,
Inc.). The samples were subsequently centrifuged at 3,000 × g for
20 min at 4°C and the protein concentrations in the supernatant of
the homogenates were determined using a BCA assay kit. An equal
amount of protein (20 µg) was separated by SDS-PAGE on 8–12% gels
and transferred to polyvinylidene difluoride membranes. The
membranes were blocked at 4°C using 5% non-fat milk solution in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h and
incubated overnight at 4°C with anti-Nrf2 (1:1,000), anti-HO-1
(1:1,000), anti-NLRP3 (1:1,000), anti-ASC (1:1,000),
anti-pro-caspase-1 and cleaved caspase-1 (1:1,000), Histone H3
(1:2,000) and anti-β-actin (1:2,000) antibodies. After washing with
TBST, the membranes were then incubated with goat anti-rabbit IgG
H&L (HRP; 1:2,000) or goat anti-mouse IgG H&L (HRP;
1:2,000) antibodies at room temperature for 1 h. The protein bands
were developed using enhanced chemiluminescence kits and were
visualized using the ChemiDoc Touch Imaging System (Bio-Rad
Laboratories, Inc.). The bands were semi-quantified via Image Lab
Software (version 6.1; Bio-Rad Laboratories, Inc.). The protein
band density of cytosolic protein was normalized compared with that
of β-actin. The protein band density of nuclear protein was
normalized compared with that of Histone H3. At least three
separate experiments were performed.
Statistical analysis
Data were analyzed using SPSS (version 20; IBM
Corp.). AWR scores are presented as the median and range, and were
analyzed using Kruskal-Wallis and Dunn's post hoc test. Continuous
data are presented as the mean ± SD and were analyzed using one-way
ANOVA followed by Tukey's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Coptisine prevents intestinal
dysmotility in PI–IBS rats
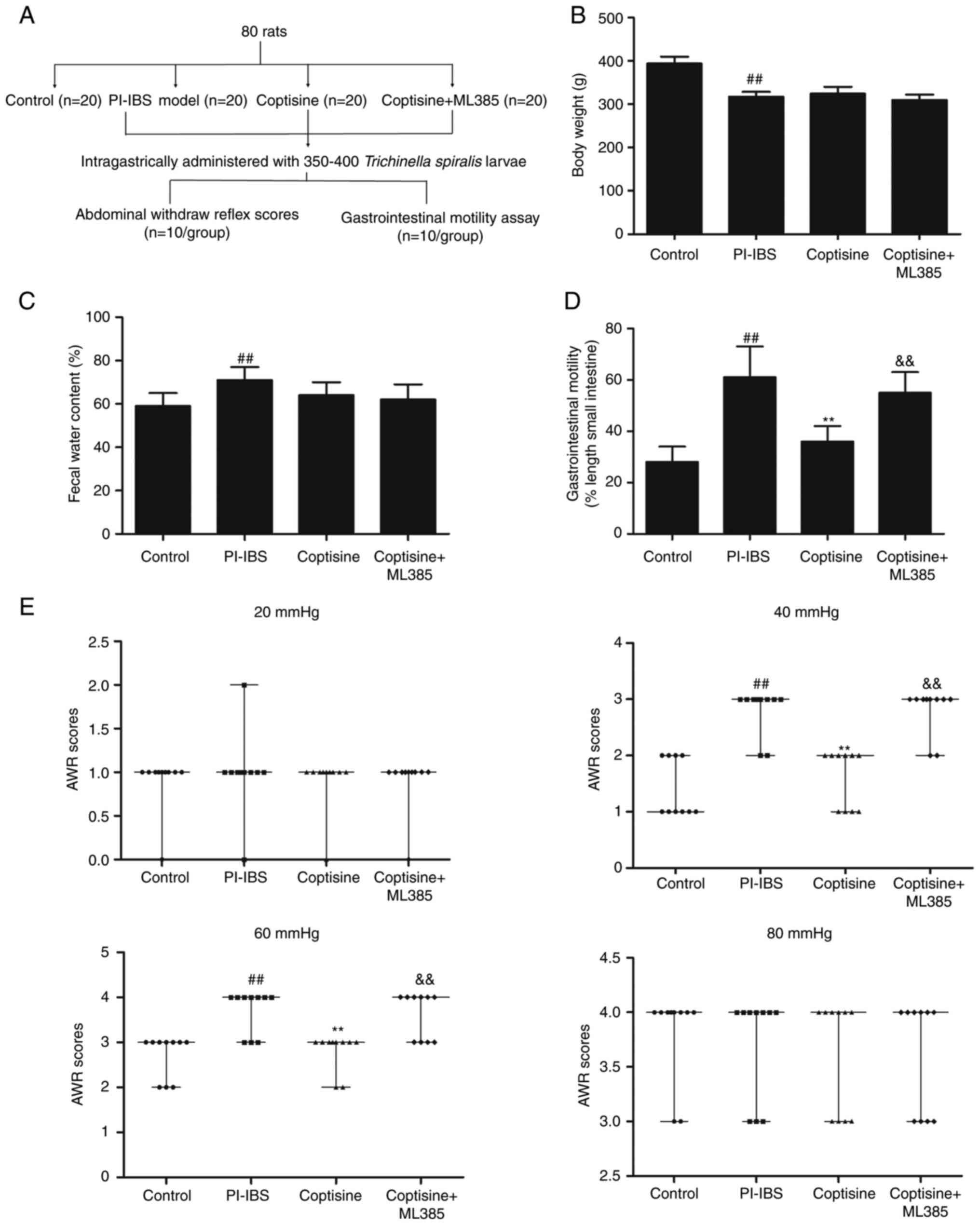
A diagram of the establishment and grouping of
animal models is shown in Figure
1A. The body weight (Fig. 1B)
was significantly decreased, whereas fecal water content (Fig. 1C) was significantly increased in
PI–IBS rats compared with those in the control group (P<0.01).
Treatment with coptisine (50 mg/kg) and co-treatment with ML385 for
14 days did not significantly affect the body weight and fecal
water content of PI–IBS rats (P>0.05). Gastrointestinal motility
was assessed by feeding rats charcoal meals and determining the
distance traveled by the charcoal in a fixed amount of time.
Gastrointestinal motility was significantly increased in PI–IBS
rats compared with the that in the control group (P<0.01;
Fig. 1D). Treatment of the rats
with coptisine (50 mg/kg) for 14 days significantly decreased
gastrointestinal motility compared with that in the PI–IBS model
group (P<0.01). The visceral sensitivity of colorectal
distention in rats was assessed using the AWR scores. The PI–IBS
group exhibited significantly higher AWR scores compared with those
in the control group following pressure stimulation at 40 and 60
mmHg (P<0.01; Fig. 1E). The
AWR scores of the PI–IBS group at 20 and 80 mmHg were comparable
with the control group, which suggested that too much or too little
pressure could affect the AWR scores. However, the
coptisine-treated rats demonstrated significantly lower AWR scores
compared with those in the PI–IBS group following pressure
stimulation at 40 and 60 mmHg (P<0.01). The decreased
gastrointestinal motility and the lower AWR scores following
pressure stimulation at 40 and 60 mmHg mediated by coptisine were
abolished by ML385 treatment. These results indicated that
coptisine significantly alleviated gut hypersensitivity of rats in
the PI–IBS rats.
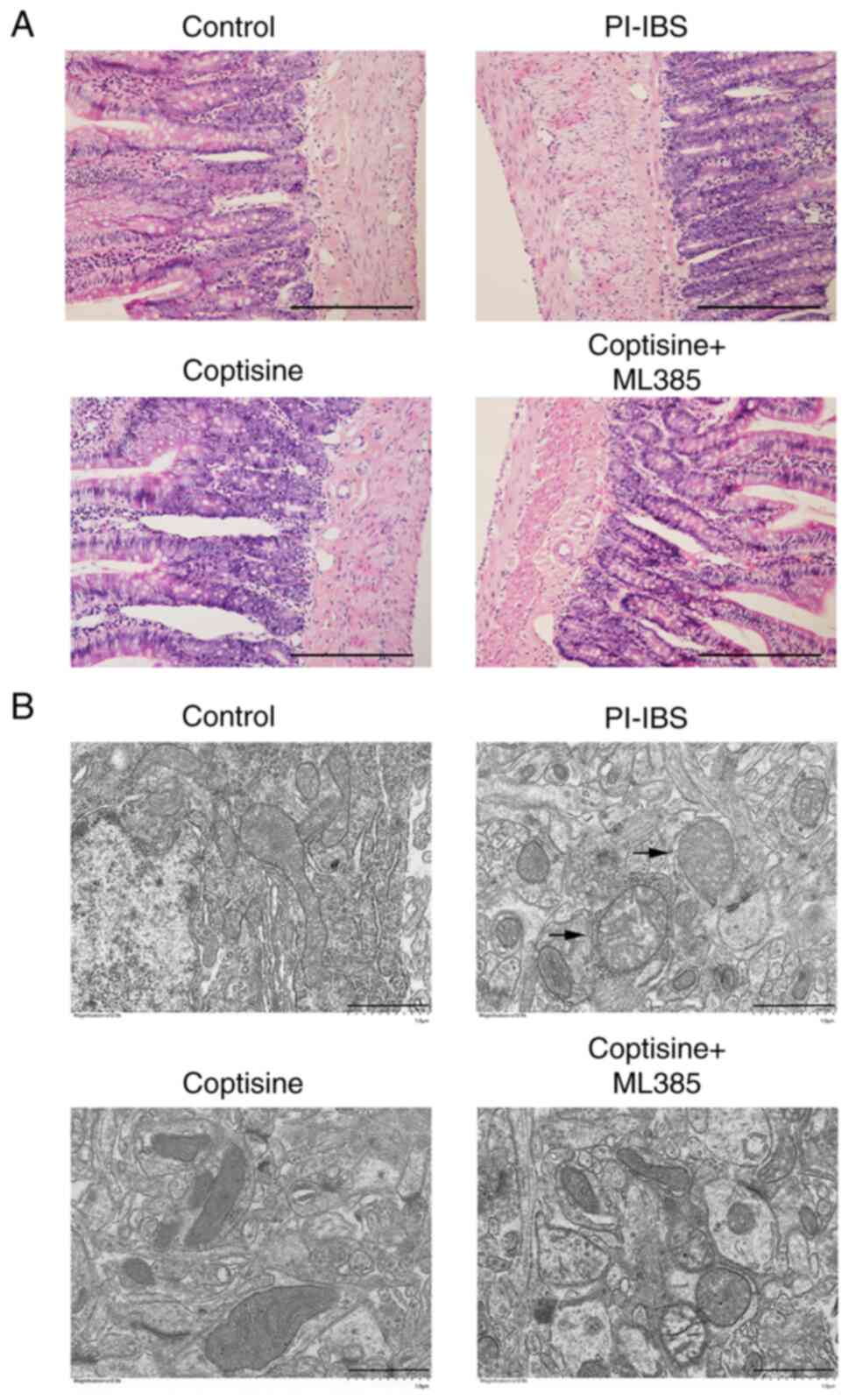
Coptisine improves colonic
ultrastructure in PI–IBS rats
No significant pathomorphological changes were noted
in the colon tissues of rats in the PI–IBS and coptisine-treatment
groups compared with in the control (Fig. 2A). The colonic ultra-structures of
the rats were assessed via transmission electron microscopy. The
mitochondria in the control rats were oval-shaped with clear and
regular cristae (Fig. 2B).
However, the mitochondria became swollen with larger sizes and
blurred inner crests in the colons of the PI–IBS rats. Coptisine
treatment markedly reversed the aforementioned changes in the
colonic ultrastructure of PI–IBS rats. The improvements in colonic
ultrastructure mediated by coptisine were abolished by ML385
treatment. These results suggested that coptisine significantly
improved colonic ultrastructure in PI–IBS rats.
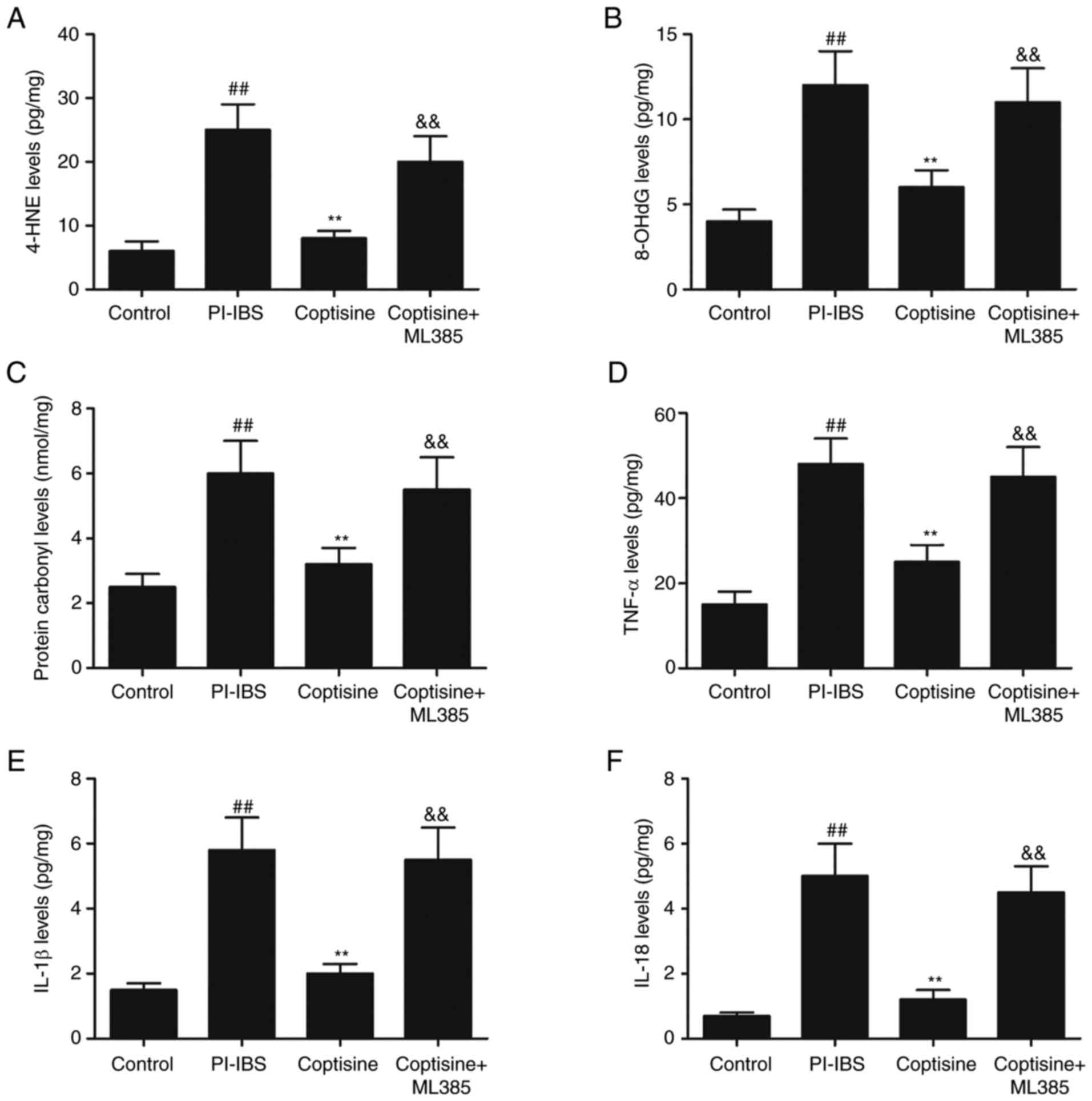
Coptisine suppresses oxidative stress
and inflammation in PI–IBS rats
Oxidative stress and inflammation serve pivotal
roles in the occurrence and persistence of PI–IBS symptoms
(7,9). In the present study, the levels of
oxidative stress and inflammation markers were detected using ELISA
in colon tissue lysates from the different treatment groups. The
PI–IBS group exhibited significantly increased levels of 4-HNE
(Fig. 3A), 8-OHdG (Fig. 3B) and protein carbonyl (Fig. 3C) compared with those in the
control group (P<0.01). However, the coptisine treatment group
exhibited significantly lower levels of 4-HNE, protein carbonyl and
8-OHdG levels compared with those in the PI–IBS group (P<0.01).
Furthermore, T. spiralis infection caused a significant
increase in the levels of proinflammatory cytokines (TNF-α, IL-1β
and IL-18) in the colon compared with those in the control group
(P<0.01; Fig. 3D-F). Treatment
with coptisine significantly reduced the levels of TNF-α (Fig. 3D), IL-1β (Fig. 3E) and IL-18 levels (Fig. 3F) compared with those in the
PI–IBS group (P<0.01). The decreased levels of 4-HNE, 8-OHdG,
protein carbonyl, TNF-α, IL-1β and IL-18 mediated by coptisine were
abolished by ML385. These results indicated that coptisine
significantly suppressed oxidative stress and inflammation in
PI–IBS rats.
Coptisine attenuates NLRP3
inflammasome activation via Nrf2 signaling
The protein expression levels of nuclear Nrf2 and
HO-1 were significantly decreased, whereas the protein expression
level of cytoplasm Nrf2 was significantly increased in the colon
tissues of the PI–IBS group compared with those in the control
group (Fig. 4A; P<0.01).
Treatment of the rats with coptisine significantly increased the
protein expression levels of nuclear Nrf2 and HO-1 and
significantly decreased cytoplasm Nrf2 compared with those in the
PI–IBS group (P<0.01). In addition, he protein expression levels
of NLRP3 and ASC were significantly increased in the PI–IBS group
compared with those in the control group (Fig. 4B; P<0.01). Coptisine treatment
significantly decreased the protein expression levels of NLRP3 and
ASC compared with those in the PI–IBS group. T. spiralis
infection significantly increased the level of cleaved caspase-1,
which was markedly decreased by coptisine treatment (P<0.01).
The increased levels of nuclear Nrf2 and HO-1, and the decreased
levels of cytoplasm Nrf2, NLRP3, ASC and cleaved caspase-1, that
were mediated by coptisine, were abolished following the treatment
of rats with ML385. These results suggested that coptisine
significantly attenuated NLRP3 inflammasome activation via Nrf2
signaling.
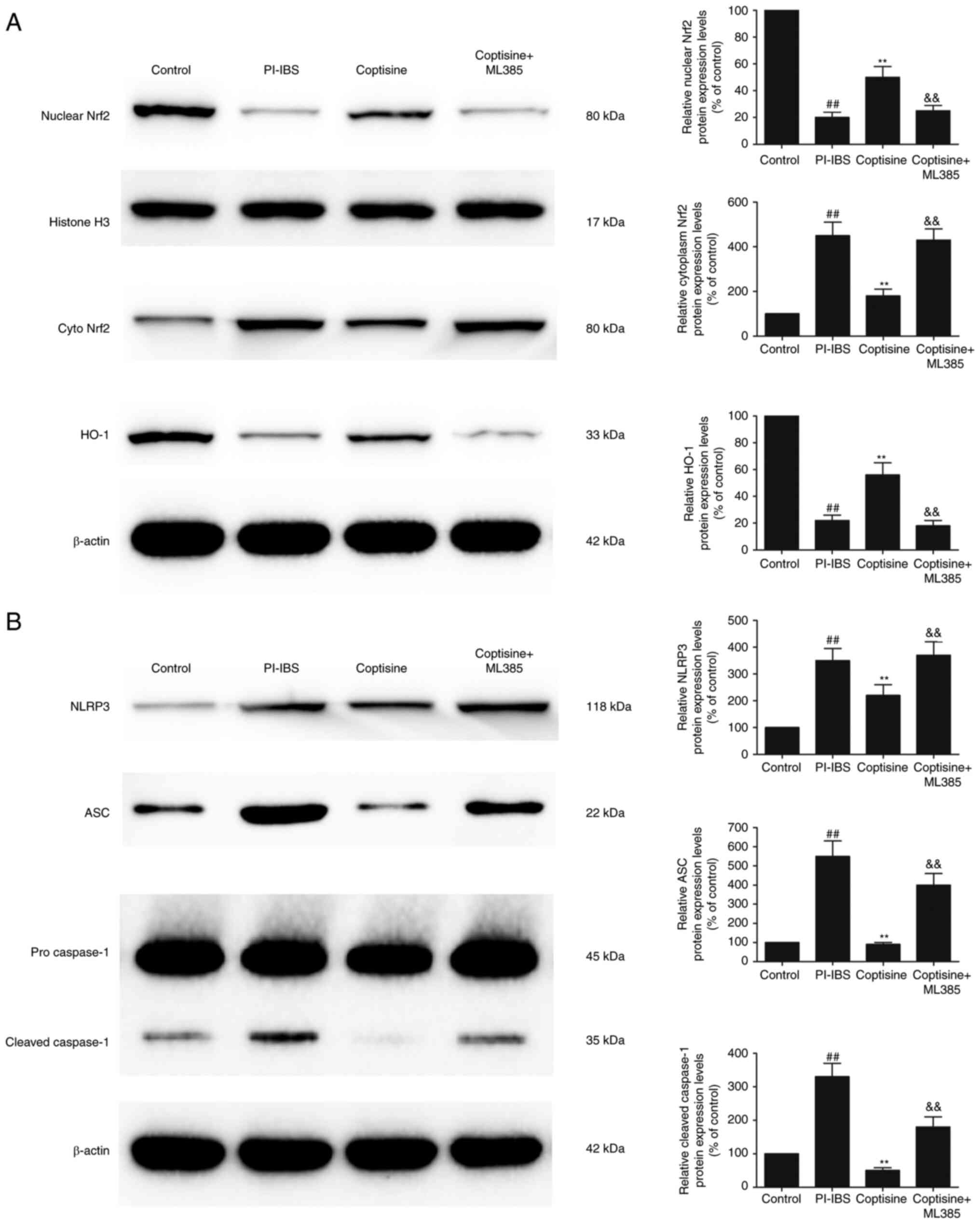 | Figure 4.Coptisine attenuates NLRP3
inflammasome activation via Nrf2 signaling. Protein expression
levels of (A) nuclear Nrf2, cyto Nrf2 and HO-1, and (B) NLRP3, ASC,
pro-caspase-1 and cleaved caspase-1 in the colon tissues of the
rats (normalized to the control). ##P<0.01 vs.
control; **P<0.01 vs. PI–IBS; &&P<0.01 vs.
coptisine. NLRP3, NLR family pyrin domain-containing 3; Nrf2,
nuclear factor erythroid 2-related factor 2; cyto, cytoplasmic;
HO-1, heme oxygenase-1; ASC, apoptosis-associated speck-like
protein containing a CARD; PI–IBS, post-infectious irritable bowel
syndrome. |
Discussion
PI–IBS is a common, chronic and multifactorial
gastrointestinal disorder associated with recurrent abdominal pain
(18), the pathogenesis of which
has not been fully characterized. Intestinal dysmotility symptoms,
such as abdominal pain, diarrhea and tenesmus, are frequently
reported in patients with PI–IBS (19). In the present study, T.
spiralis-infected rats were used as a PI–IBS animal model and
developed symptoms similar to those of patients with PI–IBS,
including gut hypersensitivity. Gastrointestinal motility and AWR
scores were significantly increased in PI–IBS rats. However,
treatment of the rats with coptisine significantly decreased both
gastrointestinal motility and AWR scores.
It has previously been demonstrated that oxidative
stress and inflammation are important in the development of PI–IBS
(8,20). Oxidative stress results from a
disturbance of redox homeostasis, in which the production of
reactive oxygen species is beyond the capability of the endogenous
antioxidant enzyme system to manage, which results in lipid
peroxidation, protein carbonylation and DNA oxidation (21). HO-1, a potent anti-oxidative
enzyme, is primarily regulated by Nrf2 (9). Under normal conditions, Nrf2 is
sequestered in the cytoplasm by Kelch-like ECH associating protein
1 (Keap1). Nrf2, exposed to various insults and stresses, including
drugs, free radicals and ROS, dissociates from Keap1 and
translocates to the nucleus where it induces HO-1 expression
(22). Overexpression of HO-1 can
also suppress inflammation. Induction of the inflammatory cascade
can cause damage to intestinal epithelial cells and the formation
of crypt abscesses, followed by an increase in intestinal
permeability (7). Excessive
inflammation in the intestine is due to disruption of the
intestinal epithelial barrier causing deterioration of the colonic
mucus layer, which is recurrent in patients with intestinal
disorders (9). It has been
reported that coptisine exhibits multiple pharmacological effects,
including antioxidative and anti-inflammatory activities (23,24). The LD50 value of
coptisine has previously been reported as 880.10 mg/kg (25), which indicates that the maximum
dose used in the present study included a wide margin of safety. To
the best of our knowledge, the present study is the first to
demonstrate that coptisine attenuated PI–IBS. The precise
mechanisms by which coptisine regulates this process requires
further investigation.
The NLR inflammasome is involved in the intestinal
inflammation characteristic of PI–IBS (2). NLRP3 is one of the members of the
NLR family, which forms the inflammasome following binding to ASC
and caspase-1 (26). The
activation of caspase-1 promotes the release of proinflammatory
cytokines, such as IL-1β, TNF-α and IL-18. In the present study, it
was demonstrated that coptisine treatment significantly reduced the
protein expression levels of NLRP3, ASC and cleaved caspase-1.
Furthermore, coptisine significantly increased the nuclear
translocation of Nrf2, which potentially significantly induced the
upregulation of HO-1 protein expression. All of these
coptisine-mediated effects were significantly abolished by the
selective Nrf2 inhibitor ML385, which suggested that coptisine
potentially blocked the signal transduction of the NLRP3 signaling
pathway via the activation of Nrf2. The modulation of the
inflammasome pathway via coptisine markedly reduced the
pathological indications of IBS. Therefore, this treatment strategy
can be considered a valuable strategy to reduce the development of
PI–IBS.
A limitation of the present study was the lack of
investigation of the toxicity of coptisine, although no obvious
abnormalities were observed in the rats administered with coptisine
(50 mg/kg) for 14 days. Another limitation of the present study is
that an ML385-only treatment group was not included.
In conclusion, the present study indicated that
coptisine potentially exhibited a protective effect on PI–IBS via
Nrf2-dependent inhibition of the NLPR3 inflammasome. The resultant
protective effects indicated that coptisine may merit consideration
for its use as a therapeutic agent in PI–IBS. Furthermore, the
results of the present study proposed that coptisine should be
explored further for its use as a prophylactic treatment of PI–IBS
in humans.
Acknowledgements
Not applicable.
Funding
This work was supported by the International Scientific and
Technological Cooperation Projects of Shenzhen Collaborative
Innovation Technology Plan (grant no. GJHZ20150316141713255), the
Science and Technology Project of Shenzhen (grant nos.
JCYJ20180228163012046 and JCYJ20210324103605014) and the Shenzhen
Health and Family Planning Commission Scientific Research Project
(grant no. 201601021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and LZ contributed to the conception and the
design of the study. HW, CC, LJ, JZ and YT contributed to the
acquisition, analysis and interpretation of the data. YX
contributed to drafting the manuscript. LZ contributed to critical
revisions of the intellectual content. YX and LZ confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The protocol used in the present study was approved
by the Ethics Committee for Animal Use of The First Affiliated
Hospital of Shenzhen University, The Second People's Hospital of
Shenzhen (approval no. 20190902002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aziz I and Simrén M: The overlap between
irritable bowel syndrome and organic gastrointestinal diseases.
Lancet Gastroenterol Hepatol. 6:139–148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao CH, Wang CY, Li GN, Yan YL, Wang D,
Jin XM, Wu LY, Liu HR, Wang XM, Shi Z and Wu HG: Effect of mild
moxibustion on intestinal microbiota and NLRP6 inflammasome
signaling in rats with post-inflammatory irritable bowel syndrome.
World J Gastroenterol. 25:4696–4714. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Black CJ and Ford AC: Global burden of
irritable bowel syndrome: Trends, predictions and risk factors. Nat
Rev Gastroenterol Hepatol. 17:473–486. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klem F, Wadhwa A, Prokop LJ, Sundt WJ,
Farrugia G, Camilleri M, Singh S and Grover M: Prevalence, risk
factors, and outcomes of irritable bowel syndrome after infectious
enteritis: A systematic review and meta-analysis. Gastroenterology.
152:1042–1054.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian Z, Zhuang X, Luo M, Yin W and Xiong
L: The propionic acid and butyric acid in serum but not in feces
are increased in patients with diarrhea-predominant irritable bowel
syndrome. BMC Gastroenterol. 20:732020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu ZC, Cen YX, Wu BH, Wei C, Xiong F, Li
DF, Liu TT, Luo MH, Guo LL, Li YX, et al: Berberine prevents
stress-induced gut inflammation and visceral hypersensitivity and
reduces intestinal motility in rats. World J Gastroenterol.
25:3956–3971. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scuderi SA, Casili G, Lanza M, Filippone
A, Paterniti I, Esposito E and Campolo M: Modulation of NLRP3
inflammasome attenuated inflammatory response associated to
diarrhea-predominant irritable bowel syndrome. Biomedicines.
8:5192020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng L, Li K, Wei H, Hu J, Jiao L, Yu S
and Xiong Y: A Novel EphA2 inhibitor exerts beneficial effects in
PI–IBS in vivo and in vitro models via Nrf2 and NF-κB signaling
pathways. Front Pharmacol. 9:2722018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu QY, Zhang J and Feng YC: Role of NLRP3
inflammasome in Bifidobacterium longum-regulated visceral
hypersensitivity of postinfectious irritable bowel syndrome. Artif
Cells Nanomed Biotechnol. 44:1933–1937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao L, Schneider KM, Galvez EJC, Frissen
M, Marschall HU, Su H, Hatting M, Wahlström A, Haybaeck J, Puchas
P, et al: Intestinal dysbiosis augments liver disease progression
via NLRP3 in a murine model of primary sclerosing cholangitis. Gut.
68:1477–1492. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hennig P, Garstkiewicz M, Grossi S, Di
Filippo M, French LE and Beer HD: The Crosstalk between Nrf2 and
Inflammasomes. Int J Mol Sci. 19:5622018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arioz BI, Tastan B, Tarakcioglu E, Tufekci
KU, Olcum M, Ersoy N, Bagriyanik A, Genc K and Genc S: Melatonin
attenuates LPS-Induced acute depressive-like behaviors and
microglial NLRP3 Inflammasome activation through the SIRT1/Nrf2
pathway. Front Immunol. 10:15112019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Vong CT, Zeng S, Gao C, Chen Z, Fu
C, Wang S, Zou L, Wang A and Wang Y: Tracking evidences of Coptis
chinensis for the treatment of inflammatory bowel disease from
pharmacological, pharmacokinetic to clinical studies. J
Ethnopharmacol. 268:1135732021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song D, Hao J and Fan D: Biological
properties and clinical applications of berberine. Front Med.
14:564–582. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Luo Y, Deng D, Su S, Li S, Xiang L,
Hu Y, Wang P and Meng X: Coptisine from Coptis chinensis exerts
diverse beneficial properties: A concise review. J Cell Mol Med.
23:7946–7960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Luo Y, Jiang Q, Li S, Huang W, Xiang
L, Liu D, Hu Y, Wang P, Lu X, et al: Coptisine from Coptis
chinensis blocks NLRP3 inflammasome activation by inhibiting
caspase-1. Pharmacol Res. 147:1043482019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adriani A, Ribaldone DG, Astegiano M,
Durazzo M, Saracco GM and Pellicano R: Irritable bowel syndrome:
The clinical approach. Panminerva Med. 60:213–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghoshal UC and Gwee KA: Post-infectious
IBS, tropical sprue and small intestinal bacterial overgrowth: The
missing link. Nat Rev Gastroenterol Hepatol. 14:435–441. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong Y, Li KX, Wei H, Jiao L, Yu SY and
Zeng L: Eph/ephrin signalling serves a bidirectional role in
lipopolysaccharide-induced intestinal injury. Mol Med Rep.
18:2171–2181. 2018.PubMed/NCBI
|
|
21
|
Forman HJ and Zhang H: Targeting oxidative
stress in disease: Promise and limitations of antioxidant therapy.
Nat Rev Drug Discov. 20:689–709. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puentes-Pardo JD, Moreno-SanJuan S, Carazo
Á and León J: Heme Oxygenase-1 in gastrointestinal tract health and
disease. Antioxidants (Basel). 9:12142020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng M, Kong SZ, Wang ZX, He K, Zou ZY, Hu
YR, Ma H, Li XG and Ye XL: The protective effect of coptisine on
experimental atherosclerosis ApoE−/− mice is mediated by
MAPK/NF-κB-dependent pathway. Biomed Pharmacother. 93:721–729.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu YR, Ma H, Zou ZY, He K, Xiao YB, Wang
Y, Feng M, Ye XL and Li XG: Activation of Akt and JNK/Nrf2/NQO1
pathway contributes to the protective effect of coptisine against
AAPH-induced oxidative stress. Biomed Pharmacother. 85:313–322.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He K, Ye X, Wu H, Wang Y, Zou Z, Ning N,
Hu Y, Chen B, Fang X and Li X: The safety and
anti-hypercholesterolemic effect of coptisine in Syrian golden
hamsters. Lipids. 50:185–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mangan MSJ, Olhava EJ, Roush WR, Seidel
HM, Glick GD and Latz E: Targeting the NLRP3 inflammasome in
inflammatory diseases. Nat Rev Drug Discov. 17:588–606. 2018.
View Article : Google Scholar : PubMed/NCBI
|