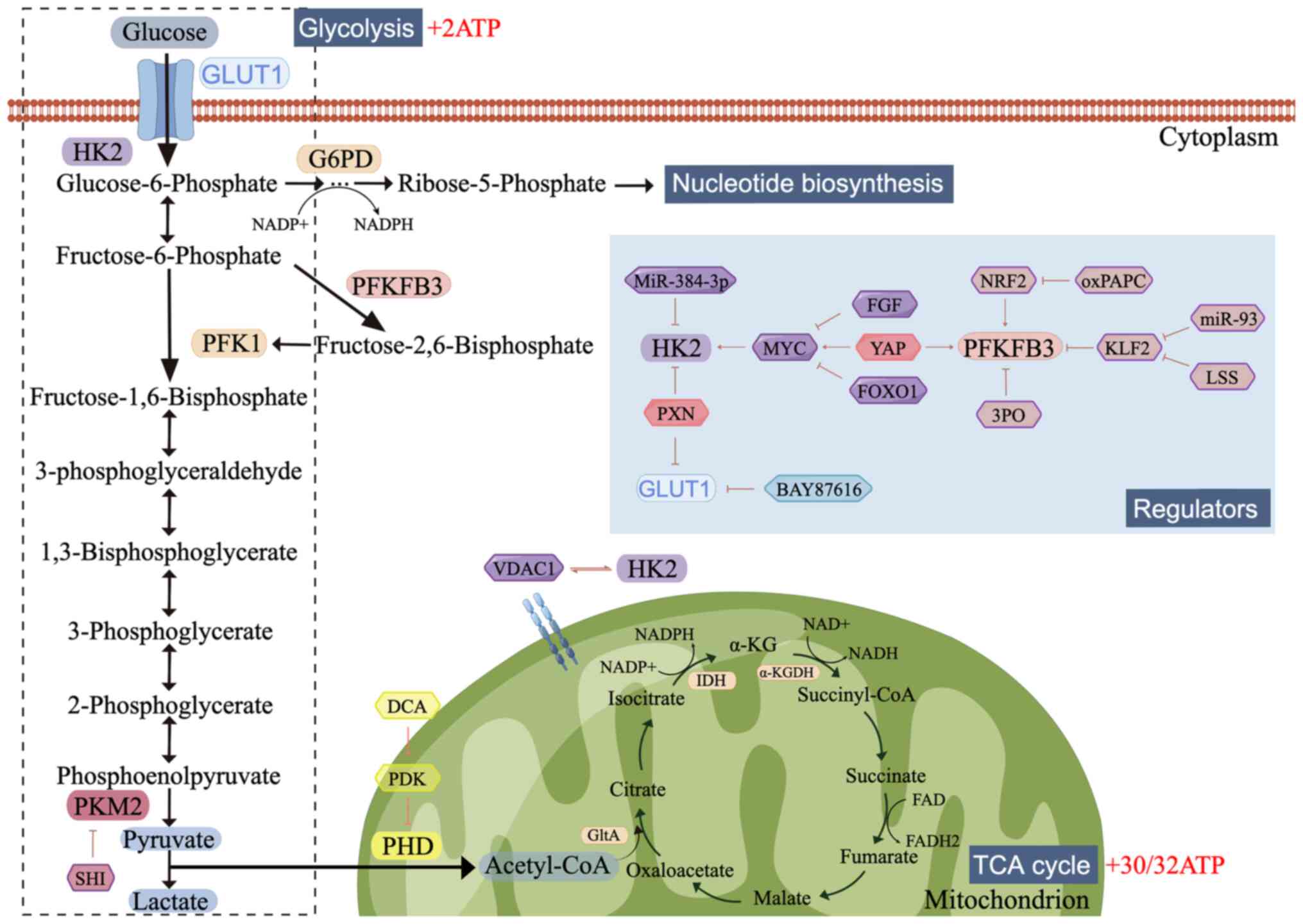
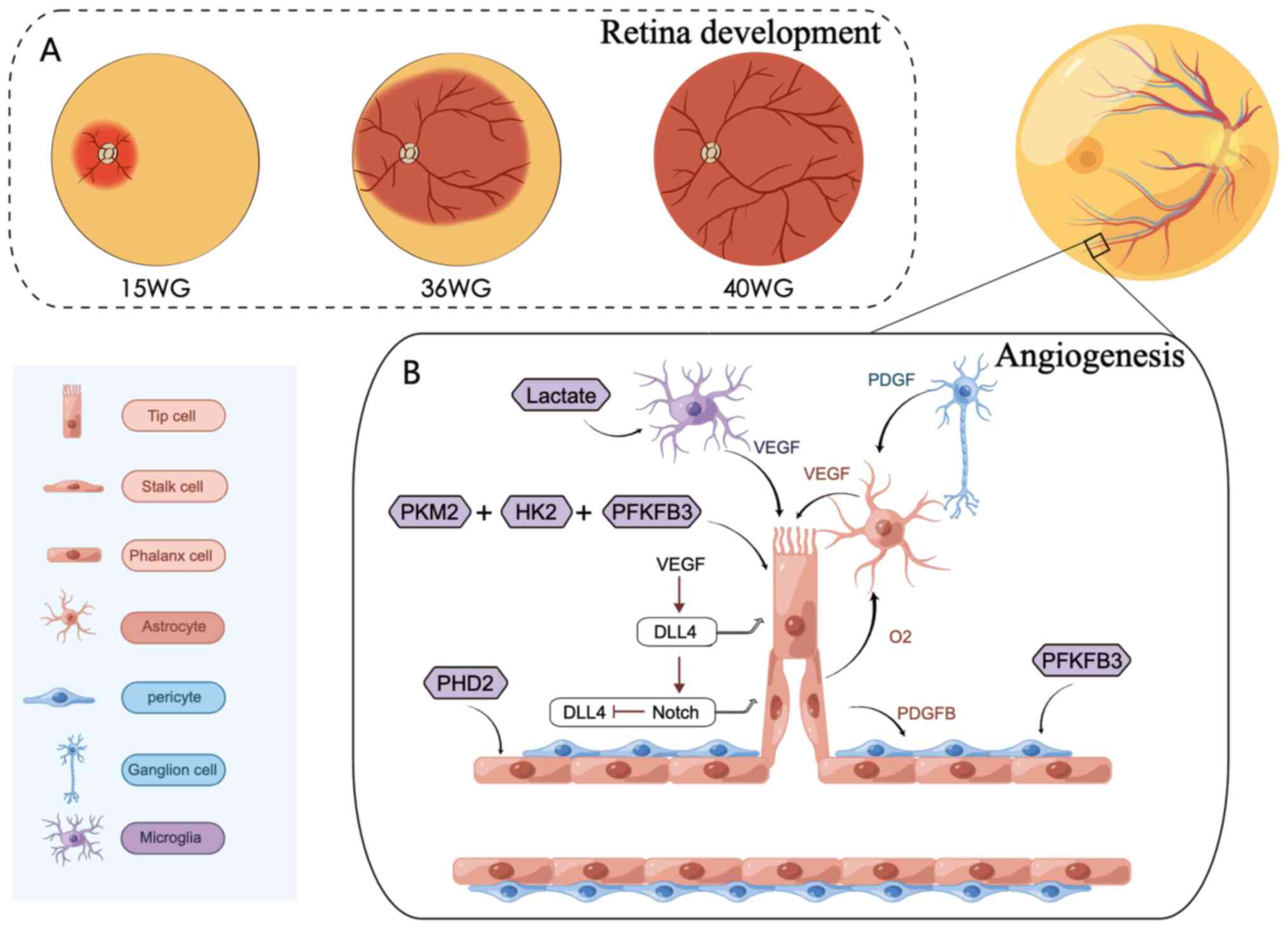
|
1
|
Adamis AP, Aiello LP and D'Amato RA:
Angiogenesis and ophthalmic disease. Angiogenesis. 3:9–14. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y and Smith LEH: Retinal vasculature
in development and diseases. Annu Rev Vis Sci. 4:101–122. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selvam S, Kumar T and Fruttiger M: Retinal
vasculature development in health and disease. Prog Retin Eye Res.
63:1–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Theodorou K and Boon RA: Endothelial cell
metabolism in atherosclerosis. Front Cell Dev Biol. 6:822018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geudens I and Gerhardt H: Coordinating
cell behaviour during blood vessel formation. Development.
138:4569–4583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebos JM and Kerbel RS: Antiangiogenic
therapy: Impact on invasion, disease progression, and metastasis.
Nat Rev Clin Oncol. 8:210–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X and Carmeliet P: Targeting angiogenic
metabolism in disease. Science. 359:1335–1336. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du W, Ren L, Hamblin MH and Fan Y:
Endothelial cell glucose metabolism and angiogenesis. Biomedicines.
9:1472021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doddaballapur A, Michalik KM, Manavski Y,
Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M,
Dimmeler S and Boon RA: Laminar shear stress inhibits endothelial
cell metabolism via KLF2-mediated repression of PFKFB3.
Arterioscler Thromb Vasc Biol. 35:137–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eelen G, de Zeeuw P, Simons M and
Carmeliet P: Endothelial cell metabolism in normal and diseased
vasculature. Circ Res. 116:1231–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Xu J, Ma Q, Zhang X, Yang Q, Wang
L, Cao Y, Xu Z, Tawfik A, Sun Y, et al: Glycolysis links reciprocal
activation of myeloid cells and endothelial cells in the retinal
angiogenic niche. Sci Transl Med. 12:eaay13712020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Bock K, Georgiadou M, Schoors S,
Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B,
Cauwenberghs S, Eelen G, et al: Role of PFKFB3-driven glycolysis in
vessel sprouting. Cell. 154:651–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krützfeldt A: Metabolism of exogenous
substrates by coronary endothelial cells in culture. Journal of
Molecular and Cellular Cardiology. 22:1393–1404. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilhelm K, Happel K, Eelen G, Schoors S,
Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger
T, et al: FOXO1 couples metabolic activity and growth state in the
vascular endothelium. Nature. 529:216–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vizan P, Sanchez-Tena S, Alcarraz-Vizan G,
Soler M, Messeguer R, Pujol MD, Lee WN and Cascante M:
Characterization of the metabolic changes underlying growth factor
angiogenic activation: Identification of new potential therapeutic
targets. Carcinogenesis. 30:946–952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu P, Wilhelm K, Dubrac A, Tung JK, Alves
TC, Fang JS, Xie Y, Zhu J, Chen Z, De Smet F, et al: FGF-dependent
metabolic control of vascular development. Nature. 545:224–228.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eelen G, de Zeeuw P, Treps L, Harjes U,
Wong BW and Carmeliet P: Endothelial Cell Metabolism. Physiol Rev.
98:3–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Guo Y, Ge W, Zhou X and Pan M:
High glucose induces apoptosis of HUVECs in a
mitochondria-dependent manner by suppressing hexokinase 2
expression. Exp Ther Med. 18:621–629. 2019.PubMed/NCBI
|
|
19
|
Bouche C, Serdy S, Kahn CR and Goldfine
AB: The cellular fate of glucose and its relevance in type 2
diabetes. Endocr Rev. 25:807–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agathocleous M, Love NK, Randlett O,
Harris JJ, Liu J, Murray AJ and Harris WA: Metabolic
differentiation in the embryonic retina. Nat Cell Biol. 14:859–864.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Romano AH and Conway T: Evolution of
carbohydrate metabolic pathways. Res Microbiol. 147:448–455. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan T, Sun G, Sun X, Zhao L, Zhong R and
Peng Y: Tumor energy metabolism and potential of 3-Bromopyruvate as
an inhibitor of aerobic glycolysis: Implications in tumor
treatment. Cancers (Basel). 11:3172019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DeBerardinis RJ and Cheng T: Q's next: The
diverse functions of glutamine in metabolism, cell biology and
cancer. Oncogene. 29:313–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Kumar A and Carmeliet P: Metabolic
pathways fueling the endothelial cell drive. Annu Rev Physiol.
81:483–503. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Groschner LN, Waldeck-Weiermair M, Malli R
and Graier WF: Endothelial mitochondria-less respiration, more
integration. Pflugers Arch. 464:63–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Bock K, Georgiadou M and Carmeliet P:
Role of endothelial cell metabolism in vessel sprouting. Cell
Metab. 18:634–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong BW, Marsch E, Treps L, Baes M and
Carmeliet P: Endothelial cell metabolism in health and disease:
impact of hypoxia. EMBO J. 36:2187–2203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan C, Cen HF, Cui X, Tian DY, Tadesse D
and Zhang YW: Proline improves switchgrass growth and development
by reduced lignin biosynthesis. Sci Rep. 9:201172019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thakur C and Chen F: Connections between
metabolism and epigenetics in cancers. Semin Cancer Biol. 57:52–58.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hassell KN: Histone deacetylases and their
inhibitors in cancer epigenetics. Diseases. 7:572019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma U and Rando OJ: Metabolic inputs
into the epigenome. Cell Metab. 25:544–558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Racey LA and Byvoet P: Histone
acetyltransferase in chromatin. Evidence for in vitro enzymatic
transfer of acetate from acetyl-coenzyme A to histones. Exp Cell
Res. 64:366–370. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McBrian MA, Behbahan IS, Ferrari R, Su T,
Huang TW, Li K, Hong CS, Christofk HR, Vogelauer M, Seligson DB and
Kurdistani SK: Histone acetylation regulates intracellular pH. Mol
Cell. 49:310–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goel A, Mathupala SP and Pedersen PL:
Glucose metabolism in cancer. Evidence that demethylation events
play a role in activating type II hexokinase gene expression. J
Biol Chem. 278:15333–15340. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Provis J: Development of the primate
retinal vasculature. Prog Retin Eye Res. 20:799–821. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gariano R: Cellular mechanisms in retinal
vascular development. Prog Retin Eye Res. 22:295–306. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kolb H, Fernandez E and Nelson R:
Webvision: The Organization of the Retina and Visual System
[Internet]. University of Utah Health Sciences Center Copyright;
Salt Lake City, UT: 1995
|
|
41
|
Chase J: The evolution of retinal
vascularization in mammals. Ophthalmology. 89:1518–1525. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baba T, McLeod DS, Edwards MM, Merges C,
Sen T, Sinha D and Lutty GA: VEGF 165 b in the developing
vasculatures of the fetal human eye. Dev Dyn. 241:595–607. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saint-Geniez M and D'Amore PA: Development
and pathology of the hyaloid, choroidal and retinal vasculature.
Int J Dev Biol. 48:1045–1058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu M, Madigan MC, van Driel D, Maslim J,
Billson FA, Provis JM and Penfold PL: The human hyaloid system:
Cell death and vascular regression. Exp Eye Res. 70:767–776. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gariano RF and Gardner TW: Retinal
angiogenesis in development and disease. Nature. 438:960–966. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
West H, Richardson WD and Fruttiger M:
Stabilization of the retinal vascular network by reciprocal
feedback between blood vessels and astrocytes. Development.
132:1855–1862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen W, Xia P, Wang H, Tu J, Liang X,
Zhang X and Li L: The endothelial tip-stalk cell selection and
shuffling during angiogenesis. J Cell Commun Signal. 13:291–301.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gerhardt H, Golding M, Fruttiger M,
Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C,
Alitalo K, Shima D and Betsholtz C: VEGF guides angiogenic
sprouting utilizing endothelial tip cell filopodia. J Cell Biol.
161:1163–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Benedito R, Roca C, Sorensen I, Adams S,
Gossler A, Fruttiger M and Adams RH: The notch ligands Dll4 and
Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suchting S, Freitas C, le Noble F,
Benedito R, Bréant C, Duarte A and Eichmann A: The Notch ligand
Delta-like 4 negatively regulates endothelial tip cell formation
and vessel branching. Proc Natl Acad Sci USA. 104:3225–3230. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fraisl P, Mazzone M, Schmidt T and
Carmeliet P: Regulation of angiogenesis by oxygen and metabolism.
Dev Cell. 16:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Trost A, Lange S, Schroedl F, Bruckner D,
Motloch KA, Bogner B, Kaser-Eichberger A, Strohmaier C, Runge C,
Aigner L, et al: Brain and retinal pericytes: Origin, function and
role. Front Cell Neurosci. 10:202016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gerhardt H and Betsholtz C:
Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res.
314:15–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lindahl P, Johansson BR, Leveen P and
Betsholtz C: Pericyte loss and microaneurysm formation in
PDGF-B-deficient mice. Science. 277:242–245. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hellstrom M, Gerhardt H, Kalén M, Li X,
Eriksson U, Wolburg H and Betsholtz C: Lack of pericytes leads to
endothelial hyperplasia and abnormal vascular morphogenesis. J Cell
Biol. 153:543–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cantelmo AR, Conradi LC, Brajic A, Goveia
J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen
LA, et al: Inhibition of the Glycolytic activator PFKFB3 in
endothelium induces tumor vessel normalization, impairs metastasis,
and improves chemotherapy. Cancer Cell. 30:968–985. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rangasamy S, Monickaraj F, Legendre C,
Cabrera AP, Llaci L, Bilagody C, McGuire P and Das A:
Transcriptomics analysis of pericytes from retinas of diabetic
animals reveals novel genes and molecular pathways relevant to
blood-retinal barrier alterations in diabetic retinopathy. Exp Eye
Res. 195:1080432020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao J, Ha Y, Liou GI, Gonsalvez GB, Smith
SB and Bollinger KE: Sigma receptor ligand, (+)-pentazocine,
suppresses inflammatory responses of retinal microglia. Invest
Ophthalmol Vis Sci. 55:3375–3384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Langston PK, Shibata M and Horng T:
Metabolism supports macrophage activation. Front Immunol. 8:612017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhou Y, Yoshida S, Nakao S, Yoshimura T,
Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K,
et al: M2 macrophages enhance pathological neovascularization in
the mouse model of oxygen-induced retinopathy. Invest Ophthalmol
Vis Sci. 56:4767–4777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lutty GA, Hasegawa T, Baba T, Grebe R,
Bhutto I and McLeod DS: Development of the human choriocapillaris.
Eye (Lond). 24:408–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hasegawa T, McLeod DS, Bhutto IA, Prow T,
Merges CA, Grebe R and Lutty GA: The embryonic human
choriocapillaris develops by hemo-vasculogenesis. Dev Dyn.
236:2089–2100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Baba T, Grebe R, Hasegawa T, Bhutto I,
Merges C, McLeod DS and Lutty GA: Maturation of the fetal human
choriocapillaris. Invest Ophthalmol Vis Sci. 50:3503–3511. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vitale G, Cozzolino A, Malandrino P,
Minotta R, Puliani G, Saronni D, Faggiano A and Colao A: Role of
FGF system in neuroendocrine neoplasms: Potential therapeutic
applications. Front Endocrinol (Lausanne). 12:6656312021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Stine ZE, Walton ZE, Altman BJ, Hsieh AL
and Dang CV: MYC, metabolism, and cancer. Cancer Discov.
5:1024–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Van Schaftingen E, Lederer B, Bartrons R
and Hers HG: A kinetic study of pyrophosphate: Fructose-6-phosphate
phosphotransferase from potato tubers. Application to a microassay
of fructose 2,6-bisphosphate. Eur J Biochem. 129:191–195. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schoors S, De Bock K, Cantelmo AR,
Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW,
Quaegebeur A, Goveia J, et al: Partial and transient reduction of
glycolysis by PFKFB3 blockade reduces pathological angiogenesis.
Cell Metab. 19:37–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lee S, Birukov KG, Romanoski CE,
Springstead JR, Lusis AJ and Berliner JA: Role of phospholipid
oxidation products in atherosclerosis. Circ Res. 111:778–799. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jyrkkanen HK, Kansanen E, Inkala M, Kivelä
AM, Hurttila H, Heinonen SE, Goldsteins G, Jauhiainen S, Tiainen S,
Makkonen H, et al: Nrf2 regulates antioxidant gene expression
evoked by oxidized phospholipids in endothelial cells and murine
arteries in vivo. Circ Res. 103:e1–e9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kuosmanen SM, Kansanen E, Kaikkonen MU,
Sihvola V, Pulkkinen K, Jyrkkänen HK, Tuoresmäki P, Hartikainen J,
Hippeläinen M, Kokki H, et al: NRF2 regulates endothelial
glycolysis and proliferation with miR-93 and mediates the effects
of oxidized phospholipids on endothelial activation. Nucleic Acids
Res. 46:1124–1138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cecchi E, Giglioli C, Valente S, Lazzeri
C, Gensini GF, Abbate R and Mannini L: Role of hemodynamic shear
stress in cardiovascular disease. Atherosclerosis. 214:249–256.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Guo FX, Hu YW, Zheng L and Wang Q: Shear
stress in autophagy and its possible mechanisms in the process of
atherosclerosis. DNA Cell Biol. 36:335–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gomez-Escudero J, Clemente C, Garcia-Weber
D, Acín-Pérez R, Millán J, Enríquez JA, Bentley K, Carmeliet P and
Arroyo AG: PKM2 regulates endothelial cell junction dynamics and
angiogenesis via ATP production. Sci Rep. 9:150222019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim B, Jang C, Dharaneeswaran H, Li J,
Bhide M, Yang S, Li K and Arany Z: Endothelial pyruvate kinase M2
maintains vascular integrity. J Clin Invest. 128:4543–4556. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Azoitei N, Becher A, Steinestel K, Rouhi
A, Diepold K, Genze F, Simmet T and Seufferlein T: PKM2 promotes
tumor angiogenesis by regulating HIF-1α through NF-κB activation.
Mol Cancer. 15:32016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Veys K, Fan Z, Ghobrial M, Bouché A,
García-Caballero M, Vriens K, Conchinha NV, Seuwen A, Schlegel F,
Gorski T, et al: Role of the GLUT1 glucose transporter in postnatal
cns angiogenesis and blood-brain barrier integrity. Circ Res.
127:466–482. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yeh WL, Lin CJ and Fu WM: Enhancement of
glucose transporter expression of brain endothelial cells by
vascular endothelial growth factor derived from glioma exposed to
hypoxia. Mol Pharmacol. 73:170–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hellström A, Smith LEH and Dammann O:
Retinopathy of prematurity. Lancet. 382:1445–1457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hartnett ME and Penn JS: Mechanisms and
management of retinopathy of prematurity. N Engl J Med.
367:2515–2526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pierce EA, Foley ED and Smith LE:
Regulation of vascular endothelial growth factor by oxygen in a
model of retinopathy of prematurity. Arch Ophthalmol.
114:1219–1228. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hoppe G, Yoon S, Gopalan B, Savage AR,
Brown R, Case K, Vasanji A, Chan ER, Silver RB and Sears JE:
Comparative systems pharmacology of HIF stabilization in the
prevention of retinopathy of prematurity. Proc Natl Acad Sci USA.
113:E2516–E2525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ogurtsova K, da Rocha Fernandes JD, Huang
Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE and
Makaroff LE: IDF diabetes atlas: Global estimates for the
prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract.
128:40–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Antonetti DA, Klein R and Gardner TW:
Diabetic retinopathy. N Engl J Med. 366:1227–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Smith LE, Wesolowski E, McLellan A, Kostyk
SK, D'Amato R, Sullivan R and D'Amore PA: Oxygen-induced
retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–111.
1994.PubMed/NCBI
|
|
88
|
Bai Y, Bai X, Wang Z, Zhang X, Ruan C and
Miao J: MicroRNA-126 inhibits ischemia-induced retinal
neovascularization via regulating angiogenic growth factors. Exp
Mol Pathol. 91:471–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xia F, Sun JJ, Jiang YQ and Li CF:
MicroRNA-384-3p inhibits retinal neovascularization through
targeting hexokinase 2 in mice with diabetic retinopathy. J Cell
Physiol. 234:721–730. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Schoors S, Cantelmo AR, Georgiadou M,
Stapor P, Wang X, Quaegebeur A, Cauwenberghs S, Wong BW, Bifari F,
Decimo I, et al: Incomplete and transitory decrease of glycolysis:
a new paradigm for anti-angiogenic therapy? Cell Cycle. 13:16–22.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu
J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim J, Kim YH, Kim J, Park DY, Bae H, Lee
DH, Kim KH, Hong SP, Jang SP, Kubota Y, et al: YAP/TAZ regulates
sprouting angiogenesis and vascular barrier maturation. J Clin
Invest. 127:3441–3461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Feng Y, Zou R, Zhang X, Shen M, Chen X,
Wang J, Niu W, Yuan Y and Yuan F: YAP promotes ocular
neovascularization by modifying PFKFB3-driven endothelial
glycolysis. Angiogenesis. 24:489–504. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen JF, Eltzschig HK and Fredholm BB:
Adenosine receptors as drug targets-what are the challenges? Nat
Rev Drug Discov. 12:265–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lutty GA, Merges C and McLeod DS:
5′nucleotidase and adenosine during retinal vasculogenesis and
oxygen-induced retinopathy. Investigative Ophthalmol Visual Sci.
41:218–229. 2000.PubMed/NCBI
|
|
97
|
Liu Z, Yan S, Wang J, Xu Y, Wang Y, Zhang
S, Xu X, Yang Q, Zeng X, Zhou Y, Gu X, et al: Endothelial adenosine
A2a receptor-mediated glycolysis is essential for pathological
retinal angiogenesis. Nat Commun. 8:5842017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Drake CJ and Fleming PA: Vasculogenesis in
the day 6.5 to 9.5 mouse embryo. Blood. 95:1671–1679. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
van Lookeren Campagne M, LeCouter J,
Yaspan BL and Ye W: Mechanisms of age-related macular degeneration
and therapeutic opportunities. J Pathol. 232:151–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lambert V, Lecomte J, Hansen S, Blacher S,
Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart
JM, et al: Laser-induced choroidal neovascularization model to
study age-related macular degeneration in mice. Nat Protoc.
8:2197–2211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Draoui N and Feron O: Lactate shuttles at
a glance: From physiological paradigms to anti-cancer treatments.
Dis Model Mech. 4:727–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Song J, Lee K, Park SW, Chung H, Jung D,
Na YR, Quan H, Cho CS, Che JH, Kim JH, et al: Lactic acid
upregulates VEGF expression in macrophages and facilitates
choroidal neovascularization. Invest Ophthalmol Vis Sci.
59:3747–3754. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lambert V, Hansen S, Schoumacher M,
Lecomte J, Leenders J, Hubert P, Herfs M, Blacher S, Carnet O, Yip
C, et al: Pyruvate dehydrogenase kinase/lactate axis: A therapeutic
target for neovascular age-related macular degeneration identified
by metabolomics. J Mol Med (Berl). 98:1737–1751. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Vallée A, Lecarpentier Y, Guillevin R and
Vallée JN: Aerobic glycolysis hypothesis through WNT/beta-catenin
pathway in exudative age-related macular degeneration. J Mol
Neurosci. 62:368–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yokosako K, Mimura T, Funatsu H, Noma H,
Goto M, Kamei Y, Kondo A and Matsubara M: Glycolysis in patients
with age-related macular degeneration. Open Ophthalmol J. 8:39–47.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Han G, Wei P, He M and Teng H: Glucose
metabolic characterization of human aqueous humor in relation to
wet age-related macular degeneration. Invest Ophthalmol Vis Sci.
61:492020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Joyal JS, Gantner ML and Smith LEH:
Retinal energy demands control vascular supply of the retina in
development and disease: The role of neuronal lipid and glucose
metabolism. Prog Retin Eye Res. 64:131–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Vidal E, Lalarme E, Maire MA, Febvret V,
Grégoire S, Gambert S, Acar N and Bretillon L: Early impairments in
the retina of rats fed with high fructose/high fat diet are
associated with glucose metabolism deregulation but not
dyslipidaemia. Sci Rep. 9:59972019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang Y, Xie L, Zhu M, Guo Y, Tu Y, Zhon Y,
Zeng J, Zhu L, Du S, Wang Z, et al: Shikonin alleviates choroidal
neovascularization by inhibiting proangiogenic factor production
from infiltrating macrophages. Exp Eye Res. 213:1088232021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nicholas MP and Mysore N: Corneal
neovascularization. Exp Eye Res. 202:1083632021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Clements JL and Dana R: Inflammatory
corneal neovascularization: Etiopathogenesis. Semin Ophthalmol.
26:235–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yang W, Yang Y, Wan S, Xu Y, Li J, Zhang
L, Guo W, Zheng Y, Xiang Y and Xing Y: Exploring the mechanism of
the miRNA-145/paxillin axis in cell metabolism during
VEGF-A-induced corneal angiogenesis. Invest Ophthalmol Vis Sci.
62:252021. View Article : Google Scholar
|
|
113
|
Liu G, Chen L, Cai Q, Wu H, Chen Z, Zhang
X and Lu P: Streptozotocin induced diabetic mice exhibit reduced
experimental choroidal neovascularization but not corneal
neovascularization. Mol Med Rep. 18:4388–4398. 2018.PubMed/NCBI
|