Introduction
Glucose homeostasis is regulated by hormonal and
neuronal pathways and is important for the maintenance of energy
metabolism (1). Chronic disorders
of these regulatory pathways may result in the development of
obesity, diabetes and/or arteriosclerotic myocardial infarction
(2). Insulin, which is a master
regulator of glucose homeostasis, is secreted by pancreatic β cells
in response to increased peripheral blood glucose concentrations
and activates insulin receptors in various tissues. In muscle and
adipocytes, glucose uptake is enhanced via the glucose
transporter on the cell surface. In liver and muscle, glucose is
converted to glycogen and stored and, as a result, blood glucose
concentrations quickly return to normal (3). Glucagon-like peptide (GLP)-1 is an
incretin that is secreted by ileal L cells in response to nutrient
ingestion, enhances insulin secretion through the GLP-1 receptor on
β cells and serves a major role in stimulating insulin secretion in
healthy subjects.
In patients with type 2 diabetes, decreased GLP-1
plasma concentrations have been observed and GLP-1 function is
impaired. However, the reactivity of GLP-1 receptors is preserved
and GLP-1 receptor agonists, such as liraglutide, significantly
reduce plasma glucose and improve glycemic control in these
patients. Due to this pharmacological advantage, the use of GLP-1
receptor agonists is well established for the treatment of type 2
diabetes (4). However,
therapeutic agents that promote secretion of endogenous GLP-1 have
not yet been developed.
Nesfatin-1 is an 82-amino acid peptide that was
originally identified in the hypothalamus as the N-terminal product
of the nucleobindin (NUCB)-2 protein (5). Nesfatin-1 is secreted by neurons in
the hypothalamus and spinal cord (6) and peripheral tissues (7–11).
A previous study reported that centrally administered nesfatin-1 by
continuous intracerebroventricular injection decreases food intake
and causes body weight loss in rats (5). Nesfatin-1 administered via
intracerebroventricular infusion increases insulin sensitivity in
rats fed a high-fat diet through the activation of the insulin
receptor/insulin receptor substrate-1/AMP-dependent protein
kinase/AKT kinase target of rapamycin complex 2 phosphorylation
pathway in the hypothalamus (12). The hypothalamus serves a pivotal
role in controlling food intake and energy metabolism (13–16). Peripherally administered
nesfatin-1 by intraperitoneal or intravenous injection reduces
blood glucose concentrations in db/db mice, which is a
leptin receptor-deficient model of type 2 diabetes mellitus
presenting with hyperglycemia and obesity, and in
streptozotocin-induced C57BL/6J mice, which is a model of type 1
diabetes (17). Subcutaneous
infusion of nesfatin-1 increases insulin concentrations during oral
glucose tolerance tests and decreases glucose concentrations during
insulin tolerance tests in rats (18). Li et al (19) demonstrate that continuous
subcutaneous infusion of nesfatin-1 improves glucose metabolism
using the oral glucose tolerance test and insulin sensitivity using
the insulin tolerance test in normal and high-fat diet-fed mice. In
addition, nesfatin-1 increases insulin-stimulated phosphorylation
of AKT in skeletal muscle, adipose tissue and liver and increases
glucose transporter 4 membrane translocation in skeletal muscle and
adipose tissue in mice fed a normal diet (19). In vitro studies demonstrate
that nesfatin-1 increases insulin-stimulated glucose uptake in L6
skeletal muscle myoblasts and primary adipocytes (18) and accelerates glucose-dependent
insulin release from islets isolated from rats and the rat
insulinoma line INS-1 832/13 (20). In a human study, the level of
NUCB2 mRNA expression in islets from patients with type 2
diabetes was lower compared with that of control donors (20). However, plasma nesfatin-1
concentrations in diabetes is a controversial issue. Li et
al (21) report that plasma
nesfatin-1 levels in patients with type 2 diabetes are lower than
those in healthy subjects and in patients with type 1 diabetes.
Zhang et al (22) and Guo
et al (23) report
elevated plasma nesfatin-1 concentrations in patients with newly
diagnosed type 2 diabetes.
Nesfatin-1 has been shown to directly stimulate
insulin secretion from mouse pancreatic β cells by accelerating
Ca2+ influx into the β cells through L-type channels
(24) and inhibiting
voltage-gated K+ channels that function as a brake on
Ca2+ influx (25).
Furthermore, nesfatin-1 was shown to stimulate GLP-1 secretion from
the enteroendocrine STC-1 cell line (26). However, whether peripheral
administration of exogenous nesfatin-1 stimulates GLP-1 secretion
in vivo remains to be elucidated. Therefore, the aim of the
present study was to investigate the effects of nesfatin-1 on the
secretion of GLP-1 followed by insulin release using fasted mice.
It demonstrated for the first time that nesfatin-1 promotes GLP-1
secretion in mice in vivo.
Materials and methods
Synthesis of mouse nesfatin-1-related
peptides
Mouse nesfatin-1 was synthesized by solid-phase
methodology with 9-fluorenylmethoxycarbonyl using an automated
peptide synthesizer (Model Pioneer; Thermo Fisher Scientific,
Inc.). The crude peptide was purified by reverse-phase
high-performance liquid chromatography (HPLC; Delta 600 HPLC
system; Waters Corporation) using a Develosil 300 ODS-HG-5 column
(2×25 cm; Nomura Chemical Co., Ltd.). Mouse C-terminal nesfatin-1
Cys-(48–82) and mouse N-terminal
nesfatin-1 (1–35)-RRC were also synthesized in a
manner similar to that described above. The purity of the synthetic
peptides was confirmed by analytical HPLC, matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry and amino
acid analysis.
Production of antiserum against
nesfatin-1
Immunization and antiserum production were performed
by Yanaihara Institute Inc. Briefly, three Japanese white rabbits
(12 weeks old, male, body weight: 2.0-2.5 kg; SLC Japan Inc.) were
individually housed in hutches (W 330 mm × L 480 mm × H 360 mm)
placed on an automatic washing machine stand. The rabbits had been
bred in an environment with 12-h light/dark cycles at 23±2°C and
55±5% humidity with free access to food and water. Immunization
using synthetic mouse C-terminal nesfatin-1 Cys-(48–82) as the immunogen was performed as
previously described (27). After
the sixth immunization, pentobarbital sodium (45 mg/kg) was
administered through the ear vein and then the animals were
sacrificed by whole blood collection from the carotid artery under
subsequent anesthesia. Mortality of rabbits used for antiserum
production was confirmed by cardiac arrest, respiratory arrest and
dilated pupils. After separating the serum from collected blood,
one of the three rabbits had a high titer antiserum against
nesfatin-1 Cys-(48–82).
Similarly, a high titer antiserum against mouse N-terminal
nesfatin-1 (1–35)-RRC was prepared.
Animals
Male C57BL/6J mice (8 weeks old; weight, 22–26 g)
were obtained from SLC Japan Inc. A total of 74 mice were used in
the present study (dose-response study: 24 mice, time-course study:
30 mice, blocking study with specific antiserum: 20 mice). Mice
were maintained in the pathogen-free animal facility at Kobe
Pharmaceutical University under standard conditions at 23±1°C and
55±5% humidity with a 12-h light/dark cycle (light-dark phase
reversal: dark phase from 7:00 AM to 7:00 PM) with ad
libitum access to sterile standard chow (CE-2; CLEA Japan Inc.)
and water. Studies were performed in accordance with the Guide for
the Care and Use of Laboratory Animals adopted and promulgated by
the National Institutes of Health (https://www.ncbi.nlm.nih.gov/books/NBK54050/). All
animal protocols for this study were approved by the Kobe
Pharmaceutical University Committee for Animal Experiments. The
animal study was reviewed and approved by Kobe Pharmaceutical
University Committee for Animal Experiments (approval no.
2017-046). Measured values which were far above or below other
measurements within the same group were disregarded.
Experimental protocol
After a resting period of 1 week, mice (21–25 g body
weight) were randomly assigned to treatment groups for the
experiments. In dose-response studies, nesfatin-1 (0.63, 1.25 and
2.5 µmol/kg) or saline (vehicle) was administered to six mice/group
and the mice were sacrificed after 30 min. Similarly, in time
course studies, nesfatin-1 (0.63 µmol/kg) or vehicle was
administered to five mice/group and the mice were sacrificed at 30,
60 or 90 min. Nesfatin-1 was dissolved in 0.1 ml of physiological
saline and intraperitoneally administered to mice after 18 h of
food deprivation. At 30 min post-injection, peripheral blood
samples were collected in tubes that contained 500 KIU/ml aprotinin
(cat. no. 010-11834; FUJIFILM Wako Pure Chemical Corporation). In
time course experiments, peripheral blood samples were collected at
30, 60 and 90 min after intraperitoneal administration of 1.25
µmol/kg nesfatin-1 or saline from the orbital vein under inhalation
anesthesia with 2.5% isoflurane (cat. no. 099-06571; FUJIFILM Wako
Pure Chemical Corporation). After blood sampling at experimental
time points, the mice were promptly sacrificed by exsanguination
under inhalation anesthesia with 2.5% isoflurane and pancreatic
tissue and a 1-cm length of terminal ileum were obtained. Using the
collected blood samples, plasma was immediately separated and
transferred into tubes containing the dipeptidyl peptidase (DPP) IV
inhibitor 1c hydrochloride (cat. no. 2783; Tocris Bioscience). The
plasma and tissue samples were stored at −80°C until analysis.
Measurements of blood glucose and
plasma GLP-1, insulin, glucagon and nesfatin-1 concentrations
Concentrations of GLP-1, insulin and glucagon were
measured using ELISA kits in accordance with the manufacturer's
instructions [GLP-1: cat. no. AKMGP-011; Levis GLP-1 (Active);
Shibayagi Co., Ltd.; insulin: cat. no. 10-1249-01; Ultrasensitive
Mouse Insulin ELISA; Mecrcodia AB; glucagon: cat. no. YK090;
Glucagon EIA kit; Yanaihara Institute Inc. or cat. no. 10-1271-01;
Glucagon ELISA; Mecrcodia AB]. Glucose concentrations were measured
using a Glucocard meter (Arkray, Inc.). Plasma nesfatin-1
concentrations were measured by Kobe pharmaceutical university
medical biochemistry laboratory-made two-site sandwich ELISA
(measurable range: 0.24–25 ng/ml). The coefficients of variation of
within- and between-assays were <3.8 and <7.8%, respectively
(see Data S1 for the assay
procedure and Fig. S1 for the
calibration curve).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from isolated pancreatic
tissues using ISOGEN reagent (Nippon Gene Co., Ltd.) and cDNA was
synthesized using a ReverTra Ace qPCR RT Master Mix kit (Toyobo
Life Science) in accordance with the manufacturer's protocol. The
RT-qPCR analysis was performed with a KOD SYBR qPCR Mix kit (Toyobo
Life Science) following the manufacturer's protocol. PCR
amplification was performed on PCR machine LightCycler 96 System
(Roche Diagnostics GmbH). qPCR was performed using the following
thermocycling conditions: Initial denaturation at 98°C for 120 sec;
then 40 cycles were performed at 98°C for 10 sec, 68°C for 10 sec
and 60°C for 30 sec; finally, the dissolution process was carried
out at 95°C, 65°C and 97°C for 10, 60 and 1 sec, respectively.
Expression levels of mRNA were analyzed using the comparative
threshold cycle method (28) and
normalized to β-actin or glyceraldehyde 3-phosphate dehydrogenase.
The primers used in the RT-qPCR are shown in Table I.
 | Table I.Primer sequences for the reverse
transcription-quantitative PCR analysis. |
Table I.
Primer sequences for the reverse
transcription-quantitative PCR analysis.
| Gene | NCBI reference
sequence | Forward (5′-3′)
Reverse (5′-3′) | Size (base
pair) |
|---|
| Insulin 1 | NM_008386 |
AGGACCCACAAGTGGAACAA | 132 |
|
|
|
GCTGGTAGAGGGAGCAGATG |
|
| Preproglucagon | NM_008100 |
TGAAGACAAACGCCACTCAC | 132 |
|
|
|
TGACGTTTGGCAATGTTGTT |
|
| β-actin | NM_007393 |
AGATCAAGATCATTGCTCCTCCTG | 174 |
|
|
|
ACGCAGCTCAGTAACAGTCC |
|
| GAPDH | NM_008084 |
GGTTGTCTCCTGCGACTTCA | 118 |
|
|
|
GCCGTATTCATTGTCATACCAGG |
|
Treatment with antiserum against GLP-1
or nesfatin-1
Rabbit anti-mouse GLP-1 serum and normal rabbit
serum were purchased from Yanaihara Institute Inc. Antiserum
against GLP-1, nesfatin-1 (RK-IK-10), or normal serum was
administered intraperitoneally (0.1 ml/mouse) 30 min before
intraperitoneal administration of 1.25 µmol/kg nesfatin-1.
Peripheral blood and pancreatic and ileal tissues were collected at
60 min after intraperitoneal administration of nesfatin-1 and
stored as described above.
Data analysis
The data are presented as the mean ± standard error
of the mean (SEM). Comparisons between two groups were performed
using two-sample t-tests. One-way analysis of variance (ANOVA)
followed by Tukey's multiple comparison test was used to compare
three or more groups. All statistical analyses were performed using
StatFlex ver. 6 (Artech Co., Ltd.). P<0.05 was considered to
indicate a statistically significant difference.
Results
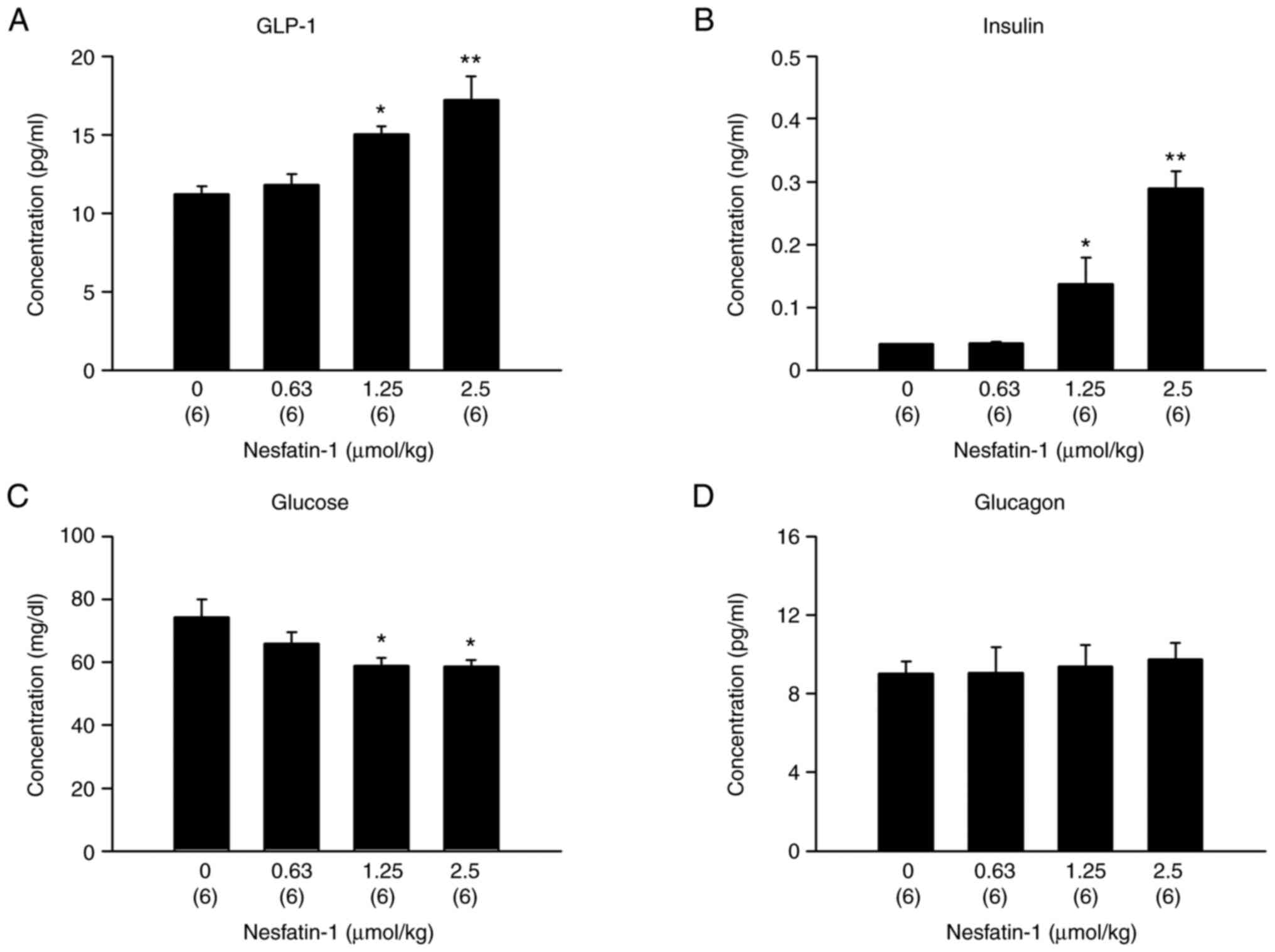
Intraperitoneal administration of
nesfatin-1 increases GLP-1 and insulin concentrations and decreases
glucose concentrations in the blood
To investigate the effect of peripheral nesfatin-1
on GLP-1 and insulin secretion in vivo, nesfatin-1 (0, 0.63,
1.25 or 2.5 µmol/kg) was intraperitoneally administered to healthy
mice that were fasted overnight. These doses were chosen according
to a previous study (29). The
2.5 µmol/kg dose of nesfatin-1 in mice resulted in significantly
higher GLP-1 and insulin concentrations than those in
vehicle-treated (controls) mice (Fig.
1A; Fbetween-group variation: 3, residual variation:
20=9.665, P=0.0004 and B; F3,20=21.578,
P<0.0001; one-way ANOVA). However, the 1.25 and 2.5 µmol/kg
nesfatin-1 doses resulted in significantly lower glucose
concentrations than those in controls (Fig. 1C; F3,20=3.765;
P=0.0272; one-way ANOVA). Glucagon concentrations were not altered
by nesfatin-1 treatment (Fig. 1D;
F3,20=0.117; P=0.9492; one-way ANOVA).
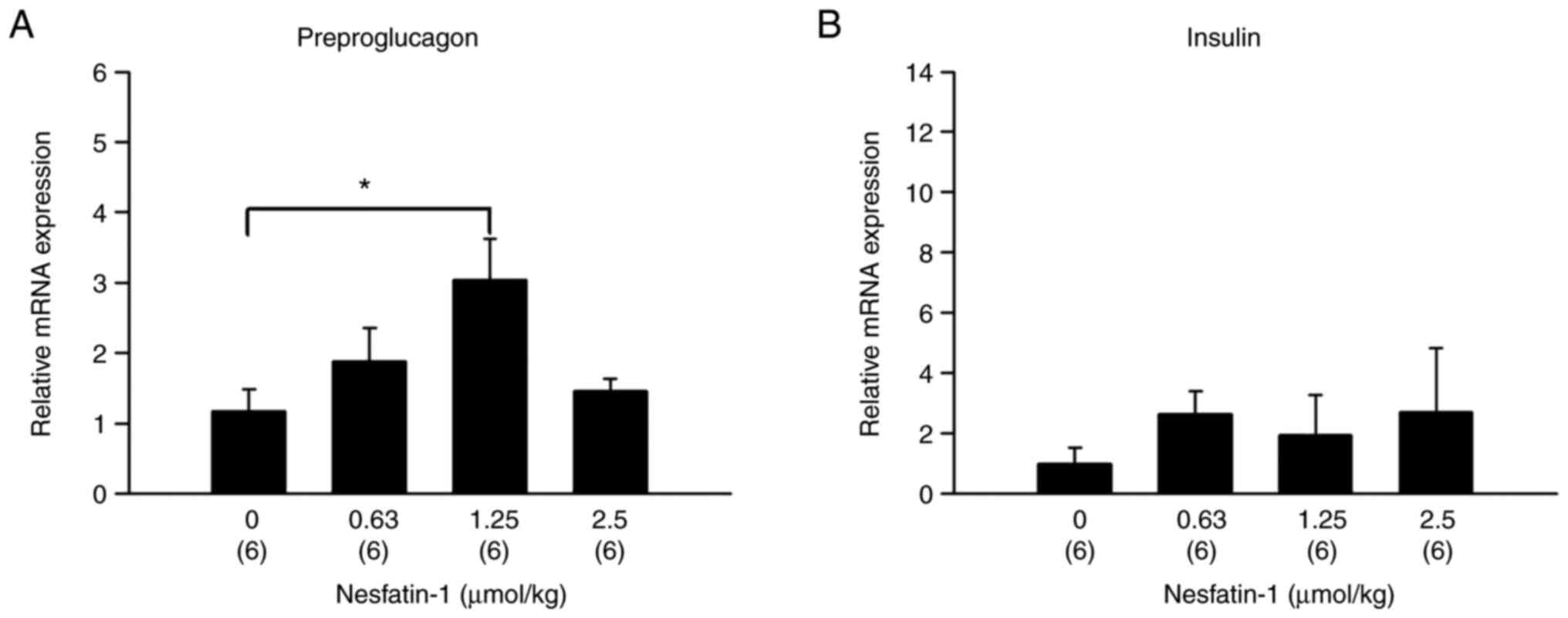
Intraperitoneal administration of
nesfatin-1 increases mRNA expression of preproglucagon but not
insulin
Nesfatin-1 treatment (1.25 µmol/kg) in mice resulted
in significantly higher mRNA expression of preproglucagon compared
with vehicle-treated mice in ileal tissue (Fig. 2A; F3,20=3.800;
P=0.0263; one-way ANOVA). However, nesfatin-1 did not alter mRNA
expression of insulin in the pancreas (Fig. 2B; F3,20=0.347,
P=0.7916).
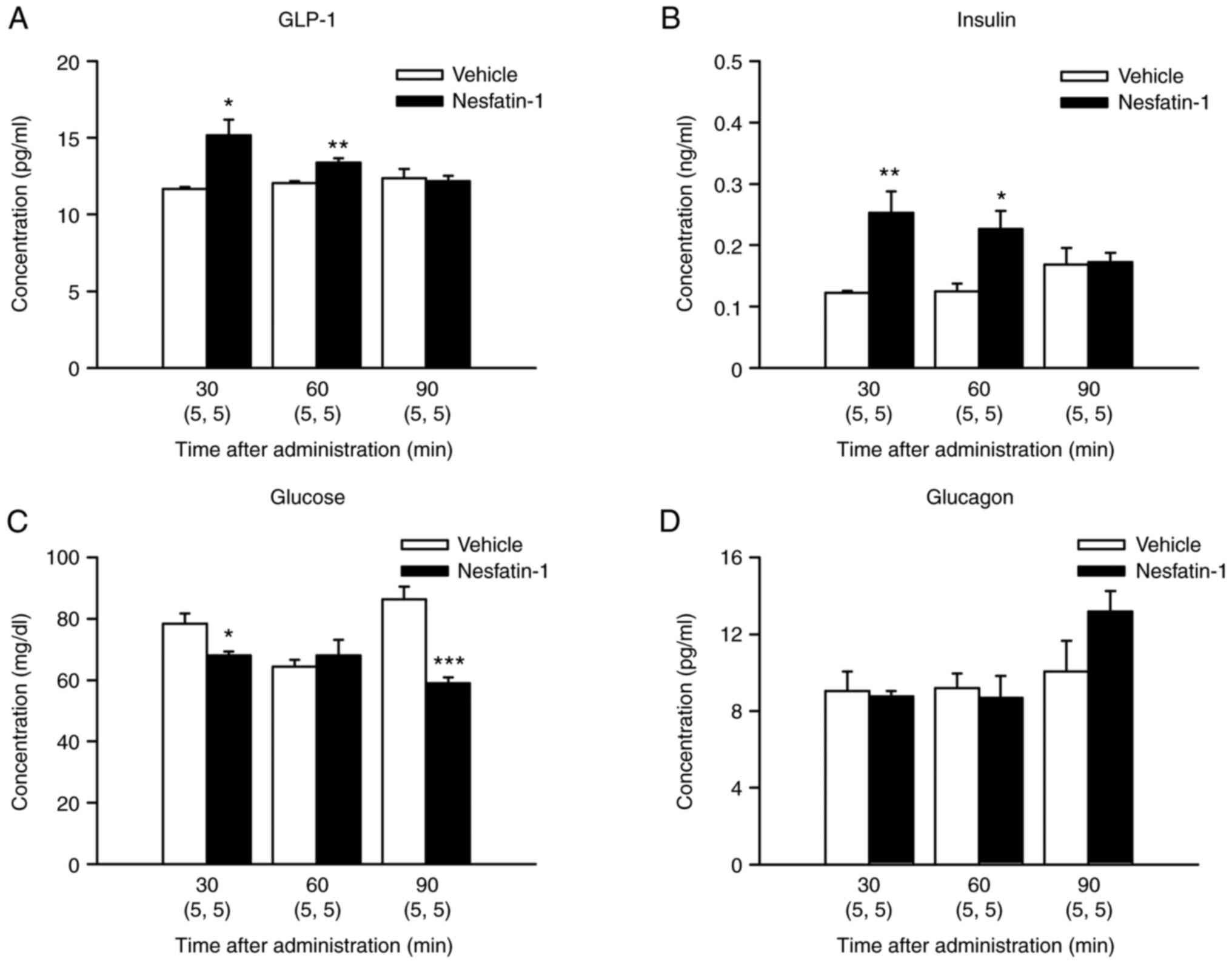
Intraperitoneal administration of
nesfatin-1 increases blood concentrations of GLP-1 at 30 and 60 min
and those of insulin at 30 and 60 min after injection
GLP-1 concentrations in 1.25 µmol/kg
nesfatin-1-treated mice were significantly higher than those in
vehicle-treated mice at 30 and 60 min after injection (Fig. 3A; t(8)=−3.3553; P=0.0100 and
t(8)=−4.2537; P=0.0028, respectively). Insulin concentrations in
nesfatin-1-treated mice were significantly higher than those in
vehicle-treated mice at 30 and 60 min after injection (Fig. 3B; t(8)=−3.6619; P=0.0064 and
t(8)=−3.1476; P=0.0136, respectively). However, glucose
concentrations in nesfatin-1-treated mice were significantly lower
than those in vehicle-treated mice at 30 and 90 min after injection
(Fig. 3C; t(8)=2.8669; P=0.0209
and t(8)=5.9707; P=0.0003, respectively). Control glucose
concentrations at 90 min appeared higher than those at 0 min
because different mice were killed at each time (Fig. 3C). Nesfatin-1 did not
significantly alter glucagon concentrations at any time point
(Fig. 3D).
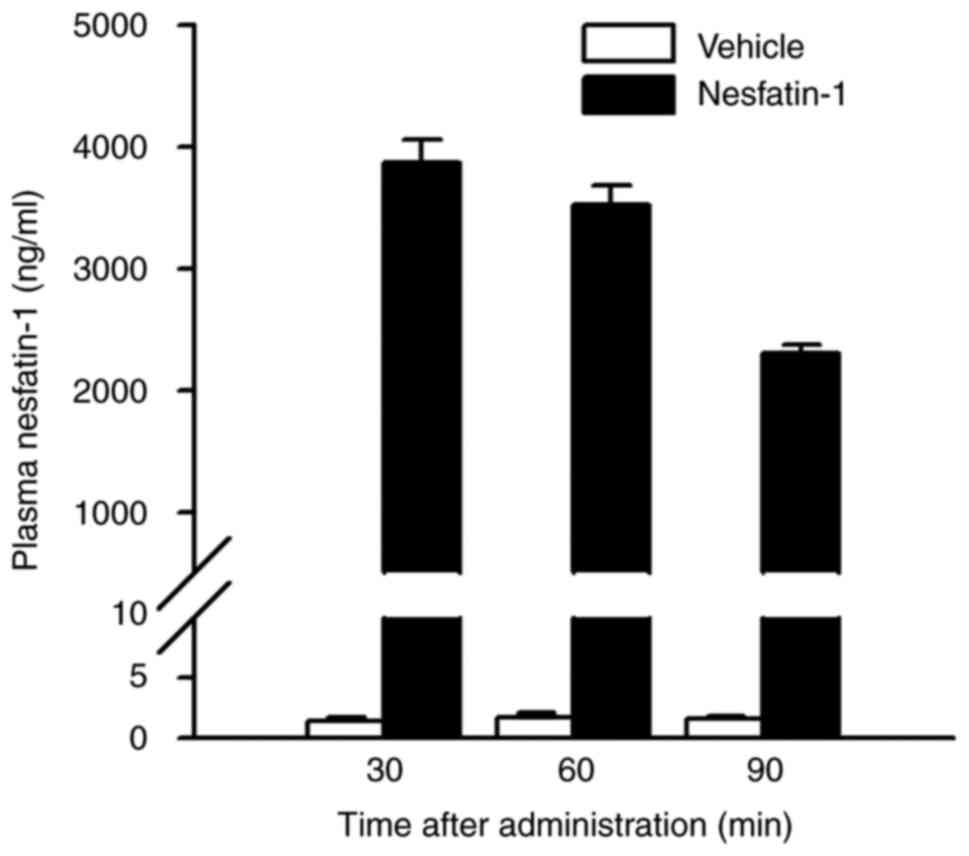
Plasma concentrations of nesfatin-1
following nesfatin-1 injection
Plasma concentrations of nesfatin-1 in
nesfatin-1-treated mice during the study period are shown in
Fig. 4. At 30 min after
administration, the plasma concentration of nesfatin-1 peaked at
~2,800 times that of the non-administered group. It then decreased
gradually, but remained ~1,500 times higher than in the
non-administered group, even after 90 min. There was no obvious
difference in the appearance or behavior of nesfatin-1-treated mice
compared with controls throughout the experiment.
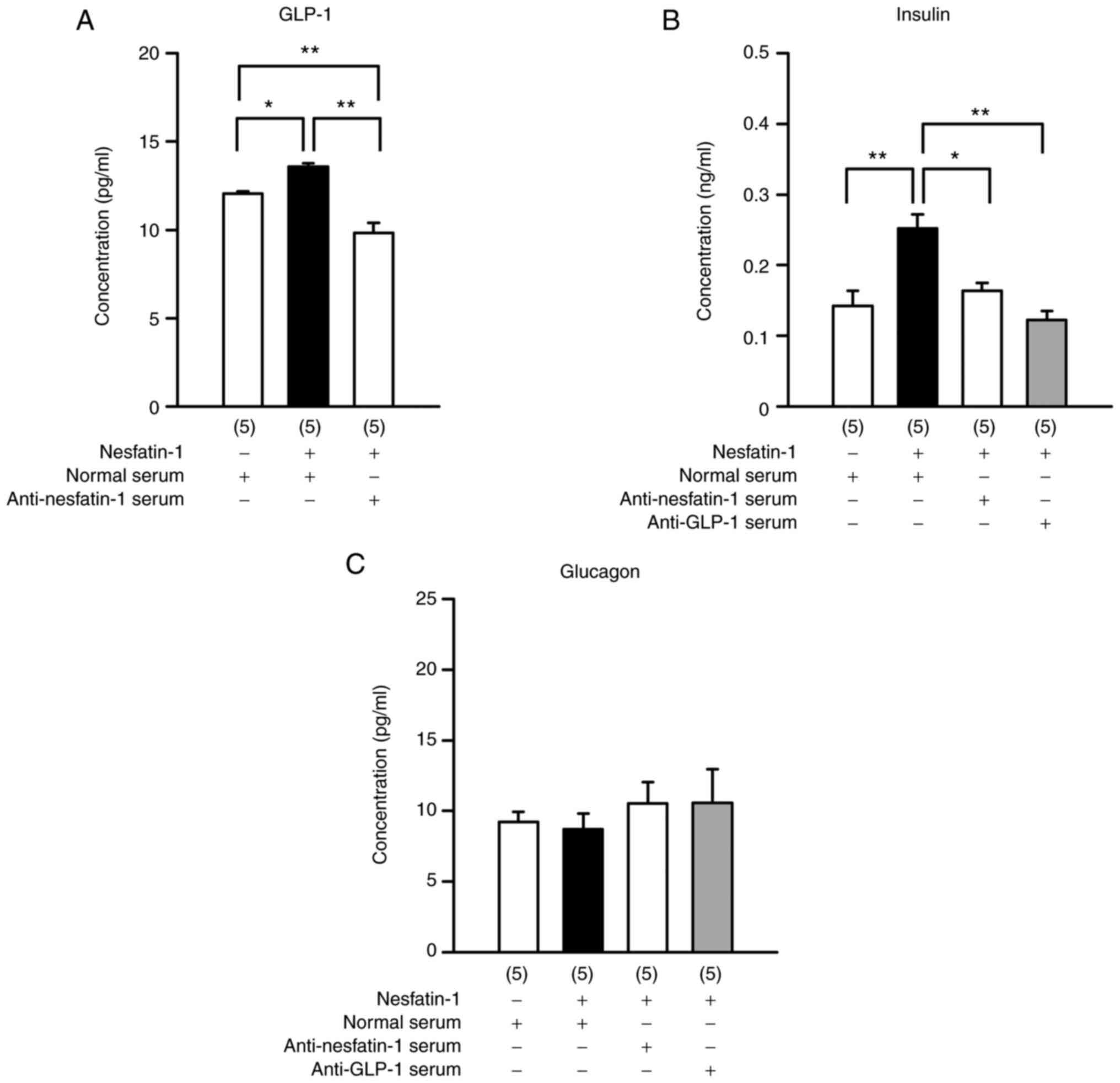
Anti-nesfatin-1 and anti-GLP-1 sera
block the effects of nesfatin-1
Pre-administration of anti-nesfatin-1 serum blocked
nesfatin-1-induced GLP-1 production in peripheral blood (Fig. 5A; F3,16=32.771;
P<0.0001; one-way ANOVA). Anti-GLP-1 serum blocked insulin
production induced by nesfatin-1 treatment (Fig. 5B; F3,16=11.061;
P=0.0004; one-way ANOVA). However, neither of these antiserums
altered glucagon concentrations (Fig.
5C; F3,16=0.359; P=0.7832; one-way ANOVA).
Discussion
Blood concentrations of nesfatin-1 in humans
(21–23,30) and NUCB2 mRNA expression in human
islets (20) suggest that
nesfatin-1 is associated with metabolic syndrome and type 2
diabetes (30) The results of
previous in vivo studies (12,17–20) have indicated that the
anti-hyperglycemic effect of peripheral nesfatin-1 is dependent on
insulin or glucose. Additionally, in vitro studies (24,25) suggest the possibility of direct
effects of nesfatin-1 on insulin release from pancreatic islets.
However, the underlying mechanism of the release of insulin by
nesfatin-1 in vivo remains to be elucidated. To clarify the
mechanism of the insulinotropic action of nesfatin-1 in
vivo, the present study examined whether peripheral nesfatin-1
promotes basal insulin secretion from the pancreas by GLP-1 release
from the intestine under fasting conditions.
To the to the best of the authors' knowledge, this
is the first time that peripheral nesfatin-1 has promoted GLP-1
secretion in vivo. The present study found that
intraperitoneal administration of nesfatin-1 elevated plasma GLP-1
concentrations, increased plasma insulin concentrations and
decreased blood glucose concentrations in overnight-fasted mice.
Moreover, the increase in plasma insulin concentrations were
diminished by the pre-administration of anti-GLP-1 serum. The
results suggested that nesfatin-1 stimulated GLP-1 secretion
followed by insulin release and that nesfatin-1 promoted GLP-1
secretion at basal glucose and insulin concentrations. This
GLP-1-releasing effect of nesfatin-1 may be glucose- or
insulin-independent. However, these findings were obtained from
experiments on mice and cannot be directly applied to humans.
Nesfatin-1 increased GLP-1 concentrations in a
dose-dependent manner (Fig. 1A)
and the changes in preproglucagon mRNA expression showed a
bell-shaped dose response (Fig.
2A). Transcriptional activity of preproglucagon mRNA may
increase before peptide synthesis and preproglucagon mRNA
expression might be affected by plasma GLP-1 concentrations
(31,32). However, the biochemical nature and
implications of these possibilities remain to be assessed.
Nesfatin-1 might affect not only GLP-1-producing L cells in the
ileum, but also those in the jejunum and colon. In the present
study, changes in preproinsulin mRNA expression (Fig. 2B) were not associated with a
significant increase in insulin concentrations (Fig. 1B). At 30 min following nesfatin-1
administration, insulin released from insulin granules to the
extracellular space may be promoted in pancreatic β cells,
resulting in a marked increase in blood insulin concentrations.
However, preproinsulin transcriptional levels in cells might not be
enhanced at this time.
Plasma concentrations of GLP-1 are 5–10 pmol/l in
the fasted state and increase rapidly to 15–50 pmol/l in healthy
human subjects after eating (33–35). In a previous study on patients
with type 2 diabetes, plasma concentrations of GLP-1 after eating
were 15–20 pmol/l without incretin treatment and 30–35 pmol/l with
incretin treatment, the latter of which resulted in decreased blood
glucose concentrations (36).
Additionally, GLP-1 concentrations prior to eating were ~5 pmol/l
in both patient groups. The GLP-1-insulin system is a therapeutic
target for type 2 diabetes (4).
GLP-1 secretion from L cells into the circulation is promptly
inactivated by DPP-4, an enzyme presents in peripheral blood. GLP-1
receptor agonists and DPP-4 inhibitors have been successful as
treatment strategies for type 2 diabetes (37,38). In addition, various substances
that accelerate GLP-1 release have been reported, including fats,
protein, bile acids, L-arginine, curcumin, glutamine,
lipopolysaccharide and berberin (39). G protein-coupled receptor agonists
and D-allulose promote GLP-1 secretion in a glucose-independent
manner from enteroendocrine cell lines and intestinal L cells,
respectively (40,41). In the present study, nesfatin-1
increased plasma GLP-1 concentrations in the fasted state. The
mechanism of nesfatin-1 action may be similar to that of G
protein-coupled receptor agonists, which promote GLP-1 secretion
(40). Mechanistic studies on how
nesfatin-1 stimulates GLP-1 secretion in vivo are required.
Several studies have shown that the effects of nesfatin-1 involve
the AKT pathway (42–47). Although various studies have been
conducted on nesfatin-1 (48,49), the nesfatin-1 receptor has not yet
been identified. To clarify the mechanism by which nesfatin-1
promotes GLP-1 secretion, it is necessary to continue examining the
involvement of GPR119 in enteroendocrine cells (40), vagal afferent signaling in animals
(41) and Akt/AMP-activated
protein kinase/target of rapamycin complex-2 pathways in the brain
(12).
The present study selected the doses of nesfatin-1
based on the fact that the intraperitoneal administration of
0.25-1.25 µmol/kg nesfatin-1 previously significantly decre-ased
the 3-h food intake of mice (29). The doses of nesfatin-1 in the
present study were higher than those in previous studies of this
insulin secretagogue (18–20,50).
However, in those studies, nesfatin-1 was administered as a single
intravenous injection or continuous subcutaneous infusion in mice
or rats. Mouse islet β cells require 10–100 times higher plasma
concentrations of nesfatin-1 to potentiate glucose-induced insulin
secretion by promoting Ca2+ influx through L-type
Ca2+ channels (24).
To maintain high tissue concentrations of target molecules, plasma
concentrations of these molecules should be further elevated. For
example, if target molecule blood vessel concentrations need to be
10 times higher than those in tissues and if those in tissues need
to be maintained at 100 times higher than normal plasma ranges,
blood vessel levels will reach 1,000 times higher than normal
plasma ranges. In the present study, nesfatin-1 plasma
concentrations were ~2,800 times higher than those before its
administration. Therefore, it was hypothesized that 1.25 µmol/kg of
nesfatin-1 would be necessary to induce increased GLP-1
secretion.
The present study used normal healthy mice in
experiments and showed that nesfatin-1 stimulated GLP-1 secretion
in vivo. Further studies in hyperglycemic mice to mimic the
type 2 diabetes model are required because GLP-1, which is released
by nesfatin-1, is considered a promising therapeutic approach for
type 2 diabetes. In addition, future studies are necessary to
examine the validity of this action of GLP-1 by repeated or
continuous administration of nesfatin-1 in the long term.
In recent years, GLP-1 receptor agonists have been
used as anti-obesity drugs. By continuously acting on specific
nerve cells in the hypothalamus that control appetite and eating
behavior, this medication is thought to prevent postprandial
hyperglycemia, promote visceral fat burning and improve basal
metabolism (51). Therefore, the
administration of nesfatin-1 may stimulate the secretion of GLP-1
and cause beneficial effects similar to those of GLP-1 receptor
agonists as aforementioned. However, because endogenous GLP-1 has a
short half-life in the blood, nesfatin-1 is likely to be less
effective than GLP-1 receptor agonists as an antidiabetic agent at
present. However, the present study indicated that nesfatin-1
promoted endogenous GLP-1 secretion and it might become a novel
antidiabetic drug for stimulating GLP-1 release.
The biological effects of nesfatin-1 need to be
evaluated. Previous studies have already reported that nesfatin-1
stimulates insulin secretion in vitro (20,24,25) and in vivo (12,17–20). Another study has also reported
that nesfatin-1 promotes GLP-1 secretion in vitro (26). These studies support the
hypothesis of the present study that the significant differences
found in this study reflect biological relevance.
In conclusion, the present study showed that
intraperitoneal administration of nesfatin-1 stimulated GLP-1
release even under low glucose conditions, such as after fasting.
The findings provided the first in vivo evidence that
peripheral nesfatin-1 increased endogenous GLP-1 secretion. The
present study also suggested that nesfatin-1 promoted insulin
production via an increase in GLP-1 concentrations. The present
study hypothesized that nesfatin-1 is a GLP-1 secretagogue that may
be useful as a therapeutic strategy for type 2 diabetes. Further
studies are required to clarify the mechanism by which nesfatin-1
promotes GLP-1 secretion and its biological significance in energy
homeostasis.
Supplementary Material
Supporting Data
Acknowledgment
Not applicable.
Funding
IK received grants-in-aid for the Center for Advanced Research
and Technology of Kobe Pharmaceutical University from the
Association of Private Universities of Japan.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AA and IK designed the study. NT, HO and HM
conducted the experiments. NT, AA and IK wrote the paper. NT, HO
and HM analyzed the data. NT and IK confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal study was reviewed and approved by Kobe
Pharmaceutical University Committee for Animal Experiments
(approval no. 2017-046).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Holst JJ, Gribble F, Horowitz M and Rayner
CK: Roles of the gut in glucose homeostasis. Diabetes Care.
39:884–892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galicia-Garcia U, Benito-Vicente A, Jebari
S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H and Martin C:
Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci.
21:62752020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prentki M, Matschinsky FM and Madiraju SR:
Metabolic signaling in fuel-induced insulin secretion. Cell Metab.
18:162–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nauck MA and Meier JJ: Incretin hormones:
Their role in health and disease. Diabetes Obes Metab. 20 (Suppl
1):S5–S21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh-I S, Shimizu H, Satoh T, Okada S,
Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et
al: Identification of nesfatin-1 as a satiety molecule in the
hypothalamus. Nature. 443:709–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konczol K, Pinter O, Ferenczi S, Varga J,
Kovacs K, Palkovits M, Zelena D and Toth ZE: Nesfatin-1 exerts
long-term effect on food intake and body temperature. Int J Obes
(Lond). 36:1514–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonzalez R, Tiwari A and Unniappan S:
Pancreatic beta cells colocalize insulin and pronesfatin
immunoreactivity in rodents. Biochem Biophys Res Commun.
381:643–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramanjaneya M, Chen J, Brown JE, Tripathi
G, Hallschmid M, Patel S, Kern W, Hillhouse EW, Lehnert H, Tan BK
and Randeva HS: Identification of nesfatin-1 in human and murine
adipose tissue: A novel depot-specific adipokine with increased
levels in obesity. Endocrinology. 151:3169–3180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stengel A, Goebel M, Yakubov I, Wang L,
Witcher D, Coskun T, Tache Y, Sachs G and Lambrecht NW:
Identification and characterization of nesfatin-1 immunoreactivity
in endocrine cell types of the rat gastric oxyntic mucosa.
Endocrinology. 150:232–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osaki A, Shimizu H, Ishizuka N, Suzuki Y,
Mori M and Inoue S: Enhanced expression of nesfatin/nucleobindin-2
in white adipose tissue of ventromedial hypothalamus-lesioned rats.
Neurosci Lett. 521:46–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang AQ, Li XL, Jiang CY, Lin L, Shi RH,
Chen JD and Oomura Y: Expression of nesfatin-1/NUCB2 in rodent
digestive system. World J Gastroenterol. 16:1735–1741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang M, Zhang Z, Wang C, Li K, Li S, Boden
G, Li L and Yang G: Nesfatin-1 action in the brain increases
insulin sensitivity through Akt/AMPK/TORC2 pathway in diet-induced
insulin resistance. Diabetes. 61:1959–1968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marraudino M, Bonaldo B, Farinetti A,
Panzica G, Ponti G and Gotti S: Metabolism disrupting chemicals and
alteration of neuroendocrine circuits controlling food intake and
energy metabolism. Front Endocrinol (Lausanne). 9:7662018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drougard A, Fournel A, Valet P and Knauf
C: Impact of hypothalamic reactive oxygen species in the regulation
of energy metabolism and food intake. Front Neurosci. 9:562015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stanley SA, Kelly L, Latcha KN, Schmidt
SF, Yu X, Nectow AR, Sauer J, Dyke JP, Dordick JS and Friedman JM:
Bidirectional electromagnetic control of the hypothalamus regulates
feeding and metabolism. Nature. 531:647–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adriaenssens AE, Biggs EK, Darwish T,
Tadross J, Sukthankar T, Girish M, Polex-Wolf J, Lam BY, Zvetkova
I, Pan W, et al: Glucose-dependent insulinotropic polypeptide
receptor-expressing cells in the hypothalamus regulate food intake.
Cell Metab. 30:987–996. e62019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su Y, Zhang J, Tang Y, Bi F and Liu JN:
The novel function of nesfatin-1: Anti-hyperglycemia. Biochem
Biophys Res Commun. 391:1039–1042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gonzalez R, Perry RL, Gao X, Gaidhu MP,
Tsushima RG, Ceddia RB and Unniappan S: Nutrient responsive
nesfatin-1 regulates energy balance and induces glucose-stimulated
insulin secretion in rats. Endocrinology. 152:3628–3637. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Gao L, Tang H, Yin Y, Xiang X, Li Y,
Zhao J, Mulholland M and Zhang W: Peripheral effects of nesfatin-1
on glucose homeostasis. PLoS One. 8:e715132013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riva M, Nitert MD, Voss U, Sathanoori R,
Lindqvist A, Ling C and Wierup N: Nesfatin-1 stimulates glucagon
and insulin secretion and beta cell NUCB2 is reduced in human type
2 diabetic subjects. Cell Tissue Res. 346:393–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li QC, Wang HY, Chen X, Guan HZ and Jiang
ZY: Fasting plasma levels of nesfatin-1 in patients with type 1 and
type 2 diabetes mellitus and the nutrient-related fluctuation of
nesfatin-1 level in normal humans. Regul Pept. 159:72–77. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Li L, Yang M, Liu H, Boden G and
Yang G: Increased plasma levels of nesfatin-1 in patients with
newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol
Diabetes. 120:91–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo Y, Liao Y, Fang G, Dong J and Li Z:
Increased nucleobindin-2 (NUCB2) transcriptional activity links the
regulation of insulin sensitivity in type 2 diabetes mellitus. J
Endocrinol Invest. 36:883–888. 2013.PubMed/NCBI
|
|
24
|
Nakata M, Manaka K, Yamamoto S, Mori M and
Yada T: Nesfatin-1 enhances glucose-induced insulin secretion by
promoting Ca2+ influx through L-type channels in mouse
islet beta-cells. Endocr J. 58:305–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maejima Y, Horita S, Kobayashi D, Aoki M,
O'Hashi R, Imai R, Sakamoto K, Mori M, Takasu K, Ogawa K, et al:
Nesfatin-1 inhibits voltage gated K+ channels in
pancreatic beta cells. Peptides. 95:10–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramesh N, Mortazavi S and Unniappan S:
Nesfatin-1 stimulates glucagon-like peptide-1 and glucose-dependent
insulinotropic polypeptide secretion from STC-1 cells in vitro.
Biochem Biophys Res Commun. 462:124–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizutani M, Atsuchi K, Asakawa A, Matsuda
N, Fujimura M, Inui A, Kato I and Fujimiya M: Localization of acyl
ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat
stomach and their responses to intragastric pH. Am J Physiol
Gastrointest Liver Physiol. 297:G974–G980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimizu H, Oh IS, Hashimoto K, Nakata M,
Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, et al:
Peripheral administration of nesfatin-1 reduces food intake in
mice: The leptin-independent mechanism. Endocrinology. 150:662–671.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tekin T, Cicek B and Konyaligil N:
Regulatory peptide nesfatin-1 and its relationship with metabolic
syndrome. Eurasian J Med. 51:280–284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dumonteil E, Magnan C, Ritz-Laser B, Meda
P, Dussoix P, Gilbert M, Ktorza A and Philippe J: Insulin, but not
glucose lowering corrects the hyperglucagonemia and increased
proglucagon messenger ribonucleic acid levels observed in
insulinopenic diabetes. Endocrinology. 139:4540–4546. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
da Silva Xavier G, Farhan H, Kim H,
Caxaria S, Johnson P, Hughes S, Bugliani M, Marselli L, Marchetti
P, Birzele F, et al: Per-arnt-sim (PAS) domain-containing protein
kinase is downregulated in human islets in type 2 diabetes and
regulates glucagon secretion. Diabetologia. 54:819–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oben J, Morgan L, Fletcher J and Marks V:
Effect of the entero-pancreatic hormones, gastric inhibitory
polypeptide and glucagon-like polypeptide-1(7–36) amide, on fatty
acid synthesis in explants of rat adipose tissue. J Endocrinol.
130:267–272. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Orskov C, Wettergren A and Holst JJ:
Biological effects and metabolic rates of glucagonlike peptide-1
7–36 amide and glucagonlike peptide-1 7–37 in healthy subjects are
indistinguishable. Diabetes. 42:658–661. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drucker DJ and Nauck MA: The incretin
system: Glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aoki K, Kamiyama H, Yoshimura K, Shibuya
M, Masuda K and Terauchi Y: Miglitol administered before breakfast
increased plasma active glucagon-like peptide-1 (GLP-1) levels
after lunch in patients with type 2 diabetes treated with
sitagliptin. Acta Diabetol. 49:225–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brunton SA and Wysham CH: GLP-1 receptor
agonists in the treatment of type 2 diabetes: role and clinical
experience to date. Postgrad Med. 132:3–14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahren B: DPP-4 Inhibition and the path to
clinical proof. Front Endocrinol (Lausanne). 10:3762019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tolhurst G, Reimann F and Gribble FM:
Nutritional regulation of glucagon-like peptide-1 secretion. J
Physiol. 587:27–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lan H, Lin HV, Wang CF, Wright MJ, Xu S,
Kang L, Juhl K, Hedrick JA and Kowalski TJ: Agonists at GPR119
mediate secretion of GLP-1 from mouse enteroendocrine cells through
glucose-independent pathways. Br J Pharmacol. 165:2799–2807. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iwasaki Y, Sendo M, Dezaki K, Hira T, Sato
T, Nakata M, Goswami C, Aok R, Arai T, Kumari P, et al: GLP-1
release and vagal afferent activation mediate the beneficial
metabolic and chronotherapeutic effects of D-allulose. Nat Commun.
9:1132018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feijoo-Bandin S, Rodriguez-Penas D,
Garcia-Rua V, Mosquera-Leal A, Otero MF, Pereira E, Rubio J,
Martinez I, Seoane LM, Gualillo O, et al: Nesfatin-1 in human and
murine cardiomyocytes: Synthesis, secretion and mobilization of
GLUT-4. Endocrinology. 154:4757–4767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu D, Yang M, Chen Y, Jia Y, Ma ZA, Boden
G, Li L and Yang G: Hypothalamic nesfatin-1/NUCB2 knockdown
augments hepatic gluconeogenesis that is correlated with inhibition
of mTOR-STAT3 signaling pathway in rats. Diabetes. 63:1234–1247.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tasatargil A, Kuscu N, Dalaklioglu S,
Adiguzel D, Celik-Ozenci C and Ozdem S, Barutcigil A and Ozdem S:
Cardioprotective effect of nesfatin-1 against isoproterenol-induced
myocardial infarction in rats: Role of the Akt/GSK-3beta pathway.
Peptides. 95:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fan XT, Tian Z, Li SZ, Zhai T, Liu JL,
Wang R, Zhang CS, Wang LX, Yuan JH, Zhou Y and Dong J: Ghrelin
receptor is required for the effect of nesfatin-1 on glucose
metabolism. Front Endocrinol (Lausanne). 9:6332018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li T, Wei S, Fan C, Tang D and Luo D:
Nesfatin-1 promotes proliferation, migration and invasion of
HTR-8/SVneo trophoblast cells and inhibits oxidative stress via
activation of PI3K/AKT/mTOR and AKT/GSK3beta pathway. Reprod Sci.
28:550–561. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Su RY, Geng XY, Yang Y and Yin HS:
Nesfatin-1 inhibits myocardial ischaemia/reperfusion injury through
activating Akt/ERK pathway-dependent attenuation of endoplasmic
reticulum stress. J Cell Mol Med. 25:5050–5059. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Prinz P, Goebel-Stengel M, Teuffel P, Rose
M, Klapp BF and Stengel A: Peripheral and central localization of
the nesfatin-1 receptor using autoradiography in rats. Biochem
Biophys Res Commun. 470:521–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rupp SK, Wolk E and Stengel A: Nesfatin-1
receptor: Distribution, signaling and increasing evidence for a G
protein-coupled receptor-A systematic review. Front Endocrinol.
12:7401742021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dong J, Xu H, Wang PF, Cai GJ, Song HF,
Wang CC, Dong ZT, Ju YJ and Jiang ZY: Nesfatin-1 stimulates
fatty-acid oxidation by activating AMP-activated protein kinase in
STZ-induced type 2 diabetic mice. PLoS One. 8:e833972013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gabery S, Salinas CG, Paulsen SJ,
Ahnfelt-Ronne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E,
Fekete C, Frederiksen KS, et al: Semaglutide lowers body weight in
rodents via distributed neural pathways. JCI insight.
5:e1334292020. View Article : Google Scholar : PubMed/NCBI
|