Introduction
Cancer is one of the leading causes of deaths
worldwide, and chemotherapy is the main treatment approach for a
majority of human cancers (1,2).
Epidemiological studies suggest that patients with cancer achieve a
substantially longer survival time after treatment with
chemotherapeutic agents, such as 5-fluorouracil, paclitaxel,
doxorubicin, and cisplatin (CP) (3–5).
There have been reports on the potential side effects of
chemotherapeutic agents that adversely affect the quality of life
of patients with cancer, including oral mucositis, ototoxicity,
immunotoxicity, neurotoxicity, hepatotoxicity, and cardiotoxicity
(6–10). Thus, it is necessary to
investigate potential strategies for effective adjuvant therapy to
reduce and prevent the incidence of side effects from
chemotherapeutic agents.
Natural products have shown promising potential as
supplements for the prevention of chemotherapy-induced side effects
(11,12). Polysaccharides derived from
vegetables, fruits, and plants, have attractive properties,
including low toxicity, high efficacy, and a wide range of sources.
Accordingly, the use of active polysaccharides has been discussed
in the fields of medicine, functional food, and molecular biology
(13). For example,
polysaccharides from Ganoderma atrum have shown preventative
effects against cyclophosphamide-induced myelosuppression and
oxidative stress (14).
Polysaccharides from Astragalus alleviate the
paclitaxel-induced cytotoxicity by reversing the changes in cell
cycle and apoptosis (15).
Recently, we had reported that polysaccharides from the Cudrania
tricuspidata fruit play an important role in the alleviation of
CP-induced cytotoxicity in macrophages and a mouse model (16). These findings suggest that further
research is needed on polysaccharides to assess their role in
preventing chemotherapy-induced toxic side effects.
Annona muricata (also known as graviola) has
extensively been used as a source of traditional medicine, with a
long history of treating various diseases, such as cancer,
inflammation, and hypertension (17–19). Our studies have also shown that
galactose (68.4%) is the major monosaccharide among the A.
muricata leaf polysaccharides (ALPS), followed by glucose
(24.37%), mannose (9.81%), and other sugars. ALPS exert protective
effects against oxidative stress-induced cellular damage and
radiation-induced skin injury (20,21). However, there is no evidence
supporting the cytoprotective role of ALPS against
chemotherapy-induced toxicity.
Therefore, this study aimed to evaluate the
cytoprotective effects of ALPS in CP-treated macrophages and to
explore the potential use of ALPS as a supplement to reduce
immunotoxicity during chemotherapy.
Materials and methods
Preparation of ALPS
Leaves of A. muricata were purchased from
Todam (Cheonan, Korea). ALPS were prepared following a previously
described method (22). Briefly,
50 g of A. muricata leaf powder were extracted with 500 ml
of deionized water at 100°C for 2 h. The extract was filtered
through Whatman no. 4 filter paper then incubated with five volumes
of 70% ethanol overnight at 4°C. The ethanol phase was centrifuged
at 1,700 × g for 20 min to collect the precipitated
polysaccharides, which were lyophilized (Hanil, Gwangju, Korea) and
dissolved in sterile deionized water.
Reagents and antibodies (Abs)
CP and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
fluorescein isothiocyanate (FITC)-conjugated annexin V/propidium
iodide (PI) kit was purchased from BD Biosciences (San Diego, CA,
USA). Monoclonal primary Abs against cleaved caspase-3 (#9661),
cleaved caspase-8 (#9496), cleaved caspase-9 (#9501), cleaved poly
(ADP-ribose) polymerase (PARP, #9541), B-cell lymphoma 2 (Bcl-2,
#2870), Bcl-2-associated X protein (BAX, #2772), cytochrome c
(#12959), and β-actin (#4970), and horseradish
peroxidase-conjugated goat anti-rabbit (#7074) and anti-mouse
secondary Abs (#91196) were obtained from Cell Signaling Technology
(Danvers, MA, USA). 2′,7′-Dichlorodihydrofluorescein diacetate
(H2DCFDA) and 3,3′-dihexyloxacarbocyanine
(DiOC6) were purchased from Thermo Fisher Scientific
(Waltham, MA, USA).
Cell culture
Human lung cancer cell lines (A549 and H460) and a
murine macrophage cell line (RAW 264.7) were obtained from the
Korea Cell Line Bank (Seoul, Korea). Cells were cultured in
complete Dulbecco's modified Eagle's medium (DMEM; Gibco BRL,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum and 100
U/ml penicillin/streptomycin (Gibco BRL), and maintained in a
humidified chamber at 37°C with 5% CO2.
Measurement of cell viability
A549, H460, and RAW 264.7 cells were seeded in
complete DMEM into 96-well plates and allowed to grow to
approximately 70–80% confluency at 37°C with 5% CO2. The
cells were treated with 0–1,000 µg/ml ALPS for 2 h and then
incubated with CP (0, 10, 15, and 20 µM) for 24 h. Subsequently,
the medium was replaced with MTT solution (0.5 mg/ml in complete
DMEM) and incubation continued for 2 h. The solution was aspirated,
and the formed formazan crystals were dissolved in 150 µl of
dimethyl sulfoxide (Sigma-Aldrich) per well. Absorbance was
measured at 570 nm using an Epoch microplate reader (BioTek
Instruments, Winooski, VT, USA).
Flow cytometric analysis of
apoptosis
The extent of apoptosis was determined by flow
cytometry using a FITC-conjugated annexin V/PI kit and a terminal
deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay.
RAW 264.7 cells were incubated in a 48-well plate for 12 h and then
treated with CP in the presence or absence of ALPS for 24 h. The
cells were harvested and washed with phosphate-buffered saline
(PBS; Gibco BRL), resuspended in the binding buffer, and stained
with annexin V-FITC and PI for 15 min. The stained cells were
analyzed using a FACSVerse™ flow cytometer (BD Biosciences). The
TUNEL assay was performed using the DeadEnd™ fluorometric TUNEL
system (Promega, Madison, WI, USA) following the manufacturer's
instructions. Briefly, the cells were fixed with a 4% formaldehyde
solution in PBS for 25 min, then permeabilized with 0.2% Triton
X-100 in PBS for 5 min. After washing with PBS, the samples were
incubated in the reaction buffer from the staining kit for 60 min.
TUNEL-positive cells were analyzed using a FACSVerse™ flow
cytometer and FlowJo software (version 10, BD Biosciences).
TUNEL assay using confocal
microscopy
RAW 264.7 cells were cultured on glass slides for 12
h, then treated with CP in the presence or absence of ALPS for 24
h. The cells were fixed in 4% paraformaldehyde in PBS for 30 min,
then permeabilized in 0.2% Triton X-100/PBS (Sigma-Aldrich,
Darmstadt, Germany) for 5 min. After washing the slides twice using
PBS and adding 100 µl of the equilibration buffer for 10 min at
4°C, the samples were incubated in 50 µl of the TdT reaction
mixture for 1 h at 37°C in a humidified chamber in the dark. To
stop the reaction, the glass ± diamidino-2-phenylindole was added
in the mounting medium and TUNEL-positive cells were analyzed using
a LSM510 confocal laser scanning microscope (Carl Zeiss, Jena,
Germany).
Isolation of bone marrow-derived
macrophages (BMDMs)
Five, seven-week-old (18±2 g) female C57BL/6 mice
were purchased from Orient Bio (Seoul, Korea). They were acclimated
to the temperature (25±2°C) and humidity (55±5%) of the housing
unit and fed a sterile commercial mouse diet and water ad
libitum. BMDMs were isolated from these mice following an
established protocol (23).
Specifically, after sacrifice by cervical dislocation, bone marrow
cells were isolated from the femur and tibia. Erythrocytes were
lysed using a red blood cell lysing buffer (Sigma-Aldrich).
Thereafter, BMDMs were plated in a petri dish and differentiated
for six days in complete DMEM containing 10 ng/ml macrophage
colony-stimulating factor (R&D Systems, Minneapolis, MN, USA).
On day 3 of differentiation, 10 ml of the macrophage complete
medium were added. After 6 days of differentiation, the cells were
harvested and the F4/80+ and CD11b+ BMDM
populations (>90% purity) were isolated with a FACSverse using
anti-F4/80 and anti-CD11b Abs (BD Bioscience). The animal
experiment was approved by the Institutional Animal Care and Use
Committee of the Korea Atomic Energy Research Institute
(KAERI–IACUC-2020-005).
Analysis of BMDM apoptosis
On day 7 of differentiation, adherent BMDMs were
harvested using a 0.25% trypsin-EDTA solution (Gibco BRL) and
plated into 48-well plates in complete DMEM at a density of 10,000
cells/well. The cells were treated with CP in the presence or
absence of ALPS for 24 h, then stained with annexin V/PI as
described above.
Western blotting
RAW 264.7 cells or BMDMs were seeded into 6-well
plates and treated with CP in the presence or absence of ALPS. The
cells were lysed in RIPA buffer (Pierce) containing a protease
inhibitor cocktail and 1 mM phenylmethylsulfonyl fluoride
(Sigma-Aldrich). After centrifugation at 16,000 × g for 20 min at
4°C, the total protein concentration was determined in the
supernatant using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific). The cell lysates (20 µg of protein) were
resolved by 10–12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and the separated proteins were electrotransferred
to polyvinylidene difluoride membranes. The membranes were blocked
with 5% nonfat milk and incubated with primary Abs (diluted
1:1,000) against cleaved caspases-3, −8, and −9, cleaved PARP, BAX,
Bcl-2, cytochrome c, and β-actin overnight at 4°C. After washing,
the membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit or anti-mouse secondary Abs (diluted 1:5,000) for
1 h. Protein bands were visualized using the Pierce ECL western
blotting substrate (Thermo Fisher Scientific).
Measurement of reactive oxygen species
(ROS)
Intracellular ROS levels were measured using the
H2DCFDA assay (24). After
incubation with CP and ALPS, 10 µM H2DCFDA was added to the cells
for 30 min at 37°C in the dark, and the cells were detached from
the plates. After the cells were washed twice with PBS, the
fluorescence intensity of the oxidized DCF was detected using a
FACSVerse™ flow cytometer and FlowJo software.
Measurement of the mitochondrial
transmembrane potential (MTP)
Loss of the MTP was analyzed using DiOC6.
Cells were incubated with 10 nM DiOC6 in fresh medium
for 30 min at 37°C in the dark, washed, and resuspended in PBS. The
fluorescence intensity of DiOC6 was detected using a FACSVerse™
flow cytometer and FlowJo software.
Statistical analyses
All experiments were repeated three times using
triplicate wells. Statistical significance was analyzed by a one
and two-way analysis of variance followed by Tukey's test using
Prism version 8.0 software (GraphPad Software, San Diego, CA, USA).
The results are expressed as means ± standard deviation. Values of
*P<0.05, **P<0.01 and ***P<0.001 were considered
statistically significant.
Results
Effects of ALPS on CP-induced toxicity
in lung cancer cells and RAW 264.7 macrophages
To identify the mitigating effect of ALPS in
CP-induced cytotoxicity, RAW 264.7 macrophages- and human lung
cancer cell lines (A549 and H460) were used to demonstrate the
effect of ALPS on normal and cancer cells. To determine the
appropriate concentration of ALPS, ALPS cytotoxicity was first
evaluated against RAW 264.7 macrophages. As shown in Fig. 1A, ALPS did not exert cytotoxicity
at the concentration range of 15.6–500 µg/ml in RAW 264.7
macrophages. Next, we investigated whether ALPS affected the
viability of CP-treated lung cancer (A549 and H460) cells and RAW
264.7 macrophages. In A549 and H460 cells, both CP alone (10, 15,
and 20 µM) and in combination with ALPS (15.6, 31.25, and 62.5
µg/ml) resulted in a concentration-dependent inhibition of tumor
cell growth compared with that in the control (CP-untreated) group.
These results were in agreement with supplement effect of ALPS on
CP-induced ROS production and MTP loss in lung cancer cells
(Figs. S1 and S2). Meanwhile, the CP-induced
cytotoxicity was effectively suppressed by treatment of RAW 264.7
macrophages with ALPS at various concentrations (15.6, 31.25, and
62.5 µg/ml) compared with that in the CP alone group (Fig. 1B). Furthermore, at concentrations
of ALPS higher than 62.5 µg/ml, the cytoprotective effect was
similar to the concentration at 62.5 µg/ml of ALPS in
cisplatin-treated RAW 264.7 macrophage (data not shown). Based on
the results obtained, the appropriate concentrations for ALPS
(31.25 and 62.5 µg/ml) and CP (15 µM) were determined and used in
all subsequent experiments.
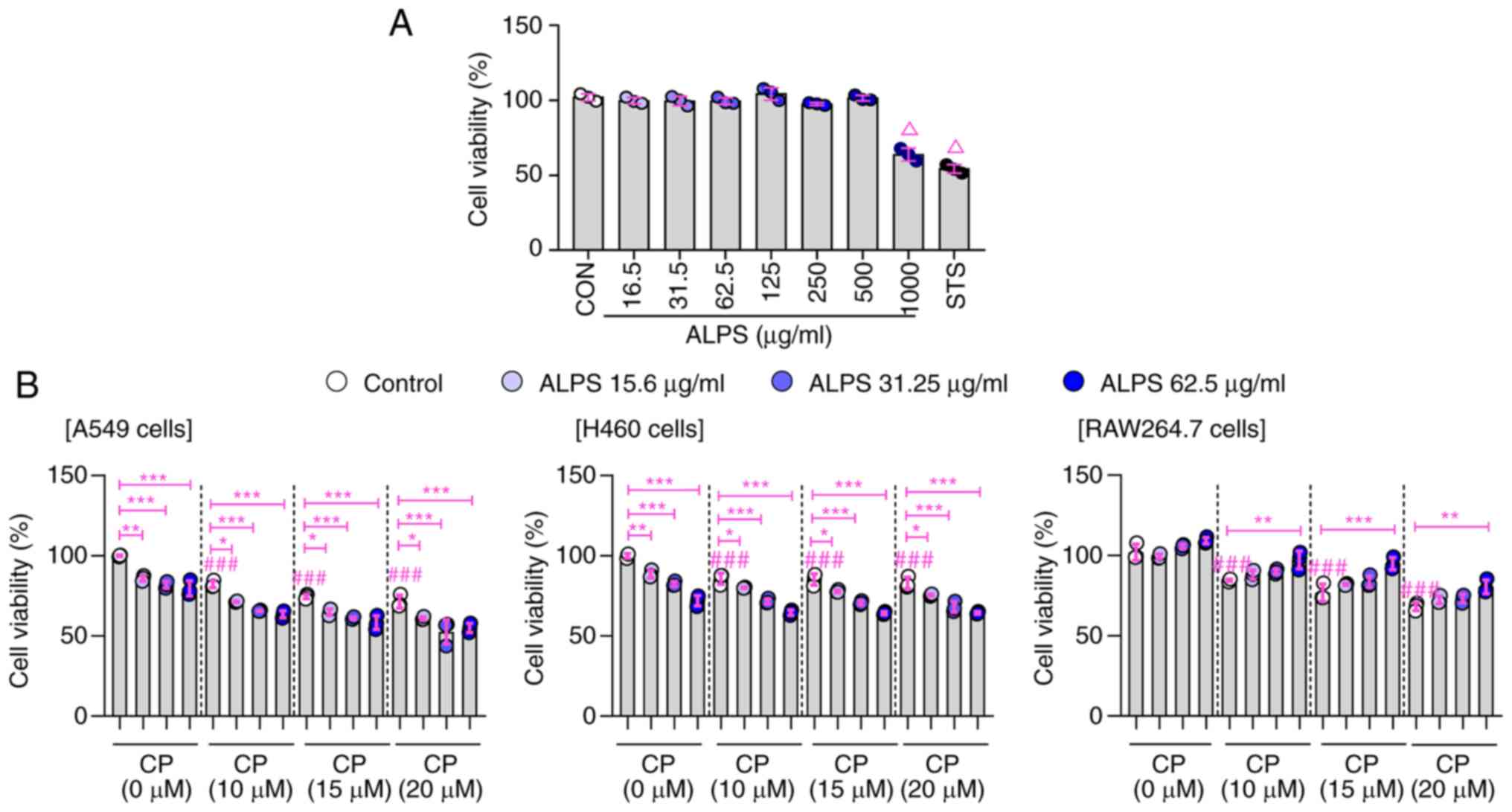 | Figure 1.Effects of ALPS on the viability of
CP-treated lung cancer cells (A549 and H460) and RAW 264.7
macrophage. (A) Viability of RAW 264.7 cells incubated with various
concentrations of ALPS (16.5-1,000 µg/ml) for 24 h. (B) After
pre-stimulation of ALPS for 2 h, the cellular viability was
assessed in the presence of CP (10, 15 and 20 µM) for 24 h.
ΔP<0.001 vs. control group (one-way ANOVA, followed by Tukey's
post-hoc test); ###P<0.001 vs. CP (0 µM) treated control group
(two-way ANOVA, followed by Tukey's post-hoc test); *P<0.05,
**P<0.01 and ***P<0.001 vs. CP-only treated group (one-way
ANOVA, followed by Tukey's post-hoc test). ALPS, Annona
muricata leaf polysaccharides; CP, cisplatin; CON, control. |
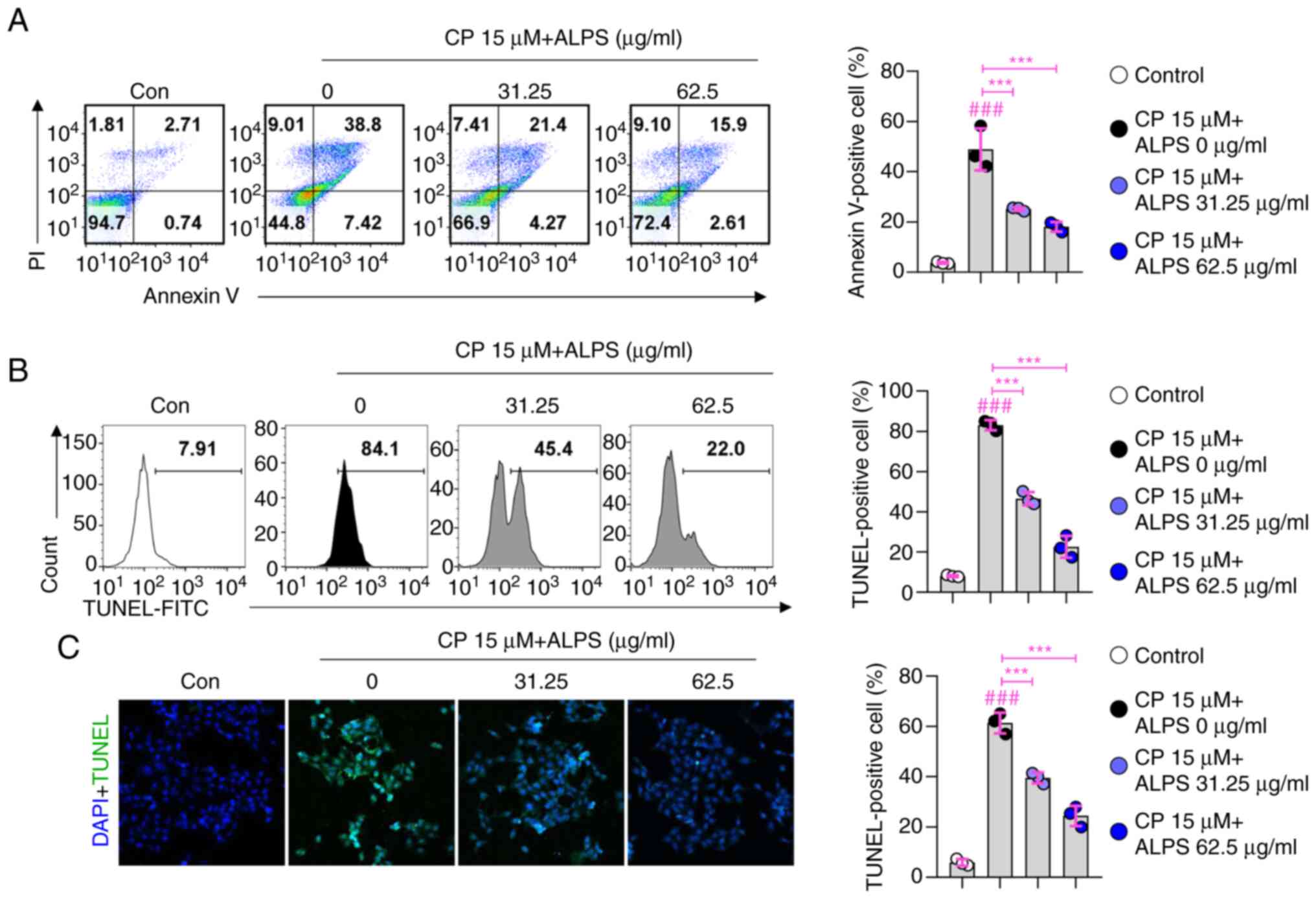
Inhibitory effects of ALPS on
CP-induced apoptotic cell death in RAW 264.7 macrophages
To further confirm that treatment with ALPS resulted
in a concentration-dependent increase in the viability of
CP-treated RAW 264.7 macrophage cells, the cytoprotective effects
of ALPS against CP-induced apoptosis were evaluated using annexin
V/PI or TUNEL staining. As shown in Fig. 2A, pretreatment with ALPS (31.25
and 62.5 µg/ml) resulted in a significant increase in cell
viability compared with that in the CP only group (P<0.001).
Furthermore, the CP only treated group showed an increased number
of TUNEL-positive cells compared with that in the CP-untreated
group, whereas the number was significantly (P<0.001) reduced by
pretreatment with ALPS (31.25 and 62.5 µg/ml) in CP-treated RAW
264.7 macrophages (Fig. 2B).
These results were consistent with the confocal microscopic
analysis via TUNEL staining (Fig.
2C). Collectively, these results demonstrated that ALPS
attenuated the apoptotic cell death of CP-treated RAW 264.7
macrophages.
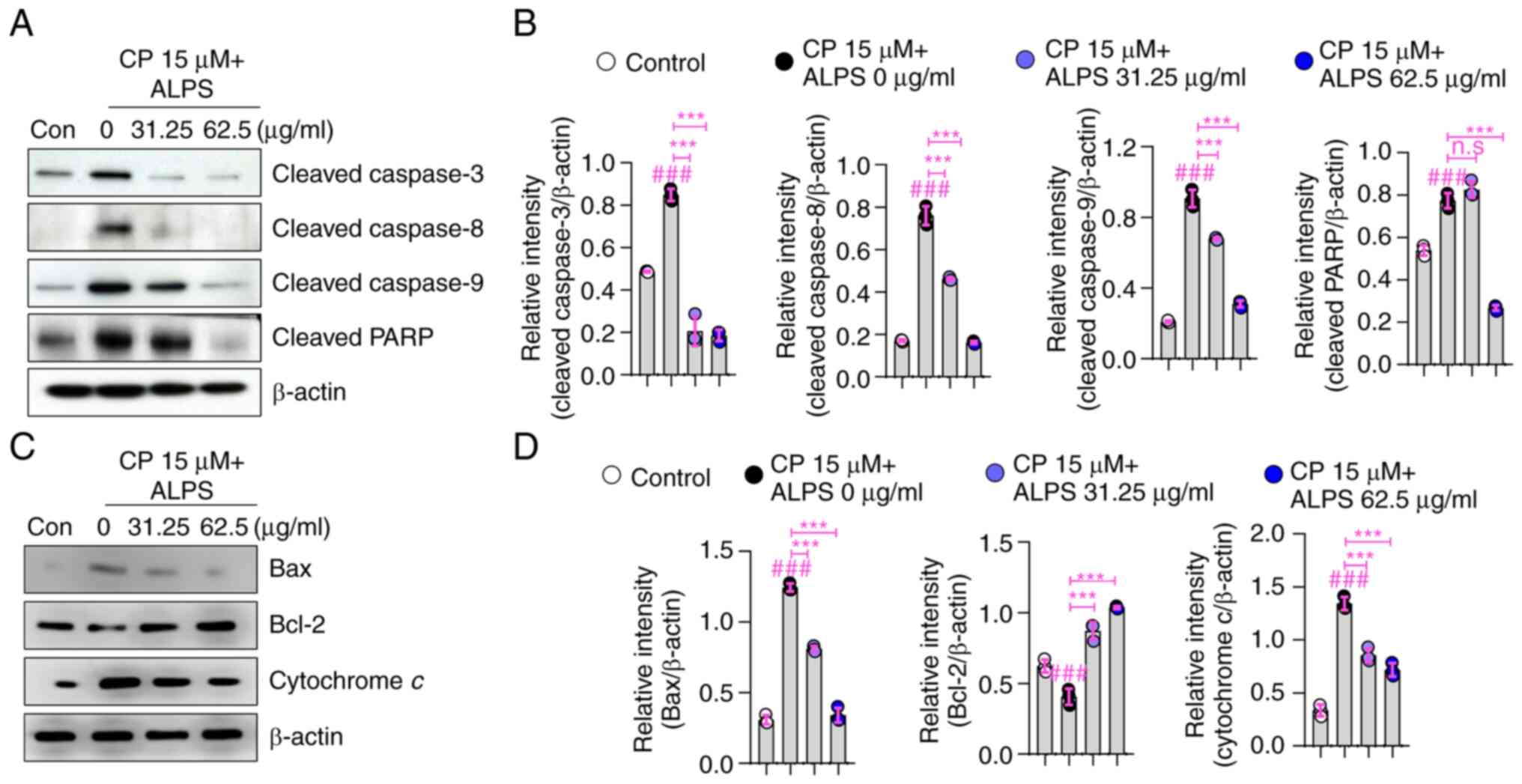
Inhibitory effects of ALPS on the
CP-induced apoptotic signaling pathways in RAW 264.7
macrophages
To elucidate the possible molecular pathways
involved in the cytoprotective action of ALPS against CP-induced
apoptosis, we examined the expression of the proapoptotic BAX and
antiapoptotic Bcl-2 proteins, as well as that of PARP, cytochrome
c, caspases-3, −8, and −9, in the CP-treated RAW 264.7 macrophages.
As shown in Fig. 3A and B,
exposure to CP resulted in increased levels of cleaved caspases-3,
−8, and −9 in RAW 264.7 macrophages. In contrast, the levels of
cleaved caspases-3, −8, and −9 were noticeably reduced in the
CP-treated cells after pretreatment with ALPS (31.25 and 62.5
µg/ml) (P<0.001). Furthermore, PARP cleavage was higher in the
CP-treated group than in the control group, whereas pretreatment
with ALPS significantly (P<0.001) suppressed PARP cleavage in
CP-treated RAW 264.7 macrophages.
Next, we investigated the involvement of BAX, Bcl-2,
and cytosolic cytochrome c in the cytotoxic effects observed in
CP-treated RAW 264.7 macrophages. The CP only treated group showed
upregulation of BAX and cytosolic cytochrome c and downregulation
of Bcl-2, whereas pretreatment with ALPS (31.25 and 62.5 µg/ml)
significantly (P<0.001) reduced the expression of BAX and
cytosolic cytochrome c and increased Bcl-2 expression (Fig. 3C and D). These findings suggested
that ALPS inhibited the apoptotic cascade involved in CP-induced
cell death, thereby promoting macrophage survival through a
cytoprotective action.
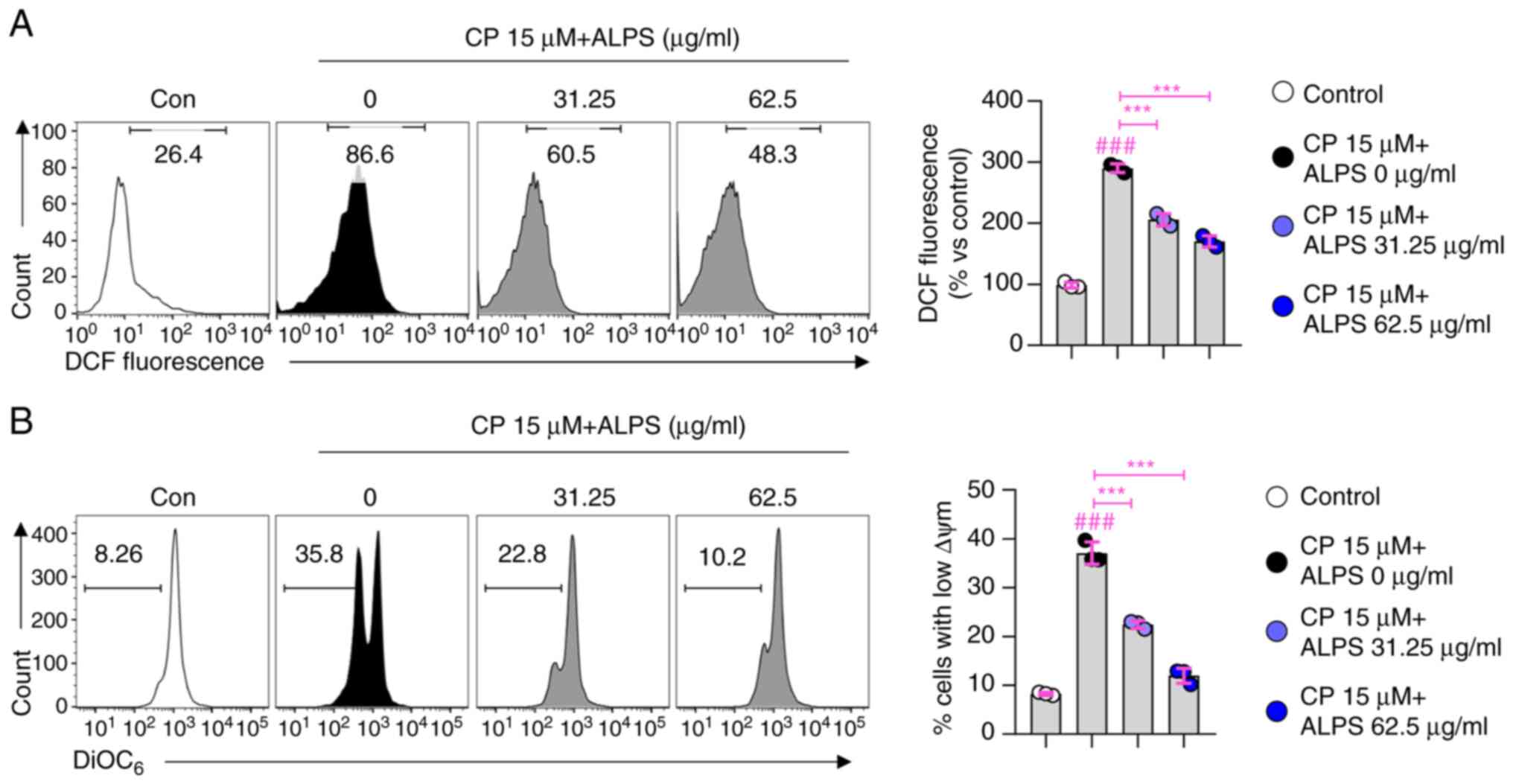
Effects of ALPS on ROS production and
MTP loss in CP-treated RAW 264.7 macrophages
Next, we examined whether the cytoprotective action
of ALPS (31.25 and 62.5 µg/ml) is associated with ROS generation
and MTP loss in CP-treated macrophages. As shown in Fig. 4A, treatment of macrophages with CP
(15 µM) resulted in an increase in the ROS levels compared with
those in the control group, whereas this increase was significantly
attenuated by pretreatment of macrophages with ALPS (31.25 and 62.5
µg/ml) (P<0.001). In addition, a significant MTP loss was
observed in CP-treated RAW 264.7 macrophages (Fig. 4B), which was attenuated by
pretreatment with ALPS (31.25 and 62.5 µg/ml) (P<0.001). These
findings suggested that the ALPS-induced cytoprotective effect was
due to the inhibition of the apoptotic cascade by reducing
CP-induced ROS production and MTP loss.
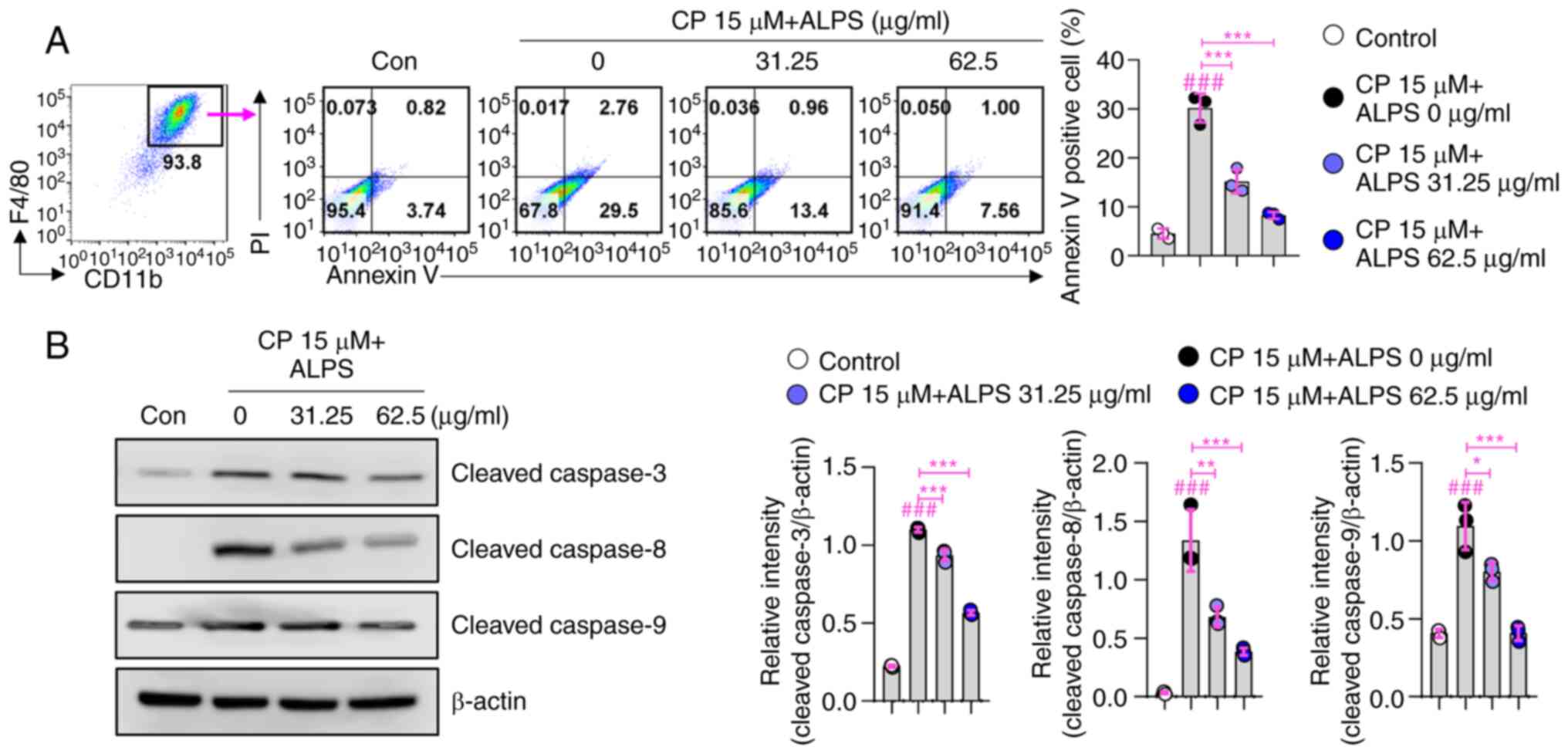
Protective effects of ALPS against
CP-induced apoptotic cell death of BMDMs
The cytoprotective effects of ALPS against
CP-induced apoptosis were further elucidated using normal primary
BMDMs. Consistent with the results obtained using RAW 264.7
macrophages, pretreatment with ALPS (31.25 and 62.5 µg/ml) induced
a significant increase in BMDM viability compared to that of BMDMs
treated with CP alone (P<0.001; Fig. 5A). Additionally, pretreatment with
ALPS (31.25 and 62.5 µg/ml) significantly inhibited the
CP-triggered activation of caspases-3 (P<0.001), −8 (P<0.01),
and −9 (P<0.05) in BMDMs, thereby attenuating the apoptotic cell
death (Fig. 5B). These findings
suggest that ALPS might act as an effective adjuvant therapy
against the toxic side effects induced by the chemotherapeutic
agents.
Discussion
CP, a DNA targeting agent that forms toxic platinum
DNA adducts, is one of the most effective and widely used
anticancer agents (25). CP also
induces direct damage to the mitochondrial DNA, resulting in
oxidative stress by increasing the intracellular ROS level
(26). CP-induced oxidative
stress contributes to a higher toxicity in tumors and causes damage
to the normal tissues owing to its non-selectivity (27). CP induces various side effects,
such as myelosuppression, hepatotoxicity, nephrotoxicity, and
immunotoxicity (28,29). As these adverse effects can reduce
the efficiency of chemotherapy, combination therapy using natural
products could be a novel strategy against CP-induced side effects
(30). Herein, we showed that the
treatment with CP alone and in combination with ALPS resulted in a
concentration-dependent inhibition of the growth of tumor cells,
whereas the CP-induced cytotoxicity was effectively alleviated by
pretreatment of RAW 264.7 macrophages with ALPS.
CP induces ROS generation to activate the
pro-apoptotic proteins and induce the translocation of BAX to the
mitochondrial outer membrane, thus releasing cytochrome c into the
cytosol (31,32). The apoptosis signal from BAX then
initiates the activation of caspase-9 and stimulates the activation
of the downstream caspase-3 causing apoptosis by cleavage of PARP,
which acts as a DNA repair agent (33). Therefore, we investigated whether
ALPS could alleviate the immune cell toxicity by reducing the
CP-induced oxidative stress. First, we observed that ALPS
effectively protected the macrophages from the CP-induced apoptosis
without loss of toxicity against the lung cancer cell lines. Next,
we revealed that ALPS significantly suppressed the apoptotic
cascade in the CP-treated RAW 264.7 cells via the upregulation of
BAX, cytosolic cytochrome c, and caspases-3, −8, and −9, as well as
PARP cleavage and downregulation of Bcl-2.
The induction of the apoptotic signaling pathways is
closely associated with the mitochondrial function and ROS
production, which are related to various pathological processes
such as cellular apoptosis (34–36). Consistently, our results showed
that the cytoprotective activity of ALPS was associated with the
suppression of the apoptotic signaling pathways via reduction of
MTP loss and ROS production in the CP-treated RAW 264.7 cells. In
addition, ALPS exerted the cytoprotective effects in the BMDMs via
suppressing the caspase signaling pathway. These findings suggest
that ALPS may be a potential supplement to alleviate the adverse
effects of the chemotherapeutic drugs.
In conclusion, the present study provides strong
evidence that ALPS exert cytoprotective effects against CP-induced
cytotoxicity in macrophages and could be considered as a potential
candidate for combination chemotherapy with CP. In the future, we
aim to identify the physicochemical properties of ALPS, as its
function can vary depending on the composition and structure of the
polysaccharides. We also aim to elucidate the chemoprotective roles
of ALPS in the CP-mouse model.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research Foundation of
Korea (NRF) funded by Ministry of Science and
ICT(RS-2022-00164733), (NRF2022R1A2C4001251), and
(NRF-2022R1F1A1063850), the internal R&D program of KAERI
(523210) funded by Ministry of Science and ICT (MIST), and a
research grant from Kongju National University in 2022 (grant
number 2022-0306-01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH wrote the paper and analyzed the data. HS, KK and
SP acquired the data. WP analyzed the data. EBB designed the study.
EHB wrote the paper and designed the study. EBB and EHB confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experimental procedure for the animal study was
approved by the Institutional Animal Care and Use Committee of the
Korea Atomic Energy Research Institute (approval no.
KAERI–IACUC-2020-002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akazawa H: Cardiotoxicity of cancer
chemotherapy-mechanisms and therapeutic approach. Gan To Kagaku
Ryoho. 44:2058–2063. 2017.(In Japanese). PubMed/NCBI
|
|
2
|
Vineis P and Wild CP: Global cancer
patterns: Causes and prevention. Lancet. 383:549–557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aiba K: Chemotherapy. Gan To Kagaku Ryoho.
31:706–711. 2004.(In Japanese). PubMed/NCBI
|
|
4
|
Rivankar S: An overview of doxorubicin
formulations in cancer therapy. J Cancer Res Ther. 10:853–858.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knezevic CE and Clarke W: Cancer
chemotherapy: The case for therapeutic drug monitoring. Ther Drug
Monit. 42:6–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong BY, Sobue T, Choquette L, Dupuy AK,
Thompson A, Burleson JA, Salner AL, Schauer PK, Joshi P, Fox E, et
al: Chemotherapy-induced oral mucositis is associated with
detrimental bacterial dysbiosis. Microbiome. 7:662019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen RL, Wang Z, Huang P, Sun CH, Yu WY,
Zhang HH, Yu CH and He JQ: Isovitexin potentiated the antitumor
activity of cisplatin by inhibiting the glucose metabolism of lung
cancer cells and reduced cisplatin-induced immunotoxicity in mice.
Int Immunopharmacol. 94:1073572021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu H, Luo H, Zhang W, Shen Z, Hu X and
Zhu X: Molecular mechanisms of cisplatin resistance in cervical
cancer. Drug Des Devel Ther. 10:1885–1895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Z, Xu L, Liu D, Zhang X, Di S, Li W,
Zhang J, Reiter RJ, Han J, Li X and Yan X: Utilizing melatonin to
alleviate side effects of chemotherapy: A potentially good partner
for treating cancer with ageing. Oxid Med Cell Longev.
2020:68415812020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pearce A, Haas M, Viney R, Pearson SA,
Haywood P, Brown C and Ward R: Incidence and severity of
self-reported chemotherapy side effects in routine care: A
prospective cohort study. PLoS One. 12:e01843602017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palipoch S, Punsawad C, Koomhin P and
Suwannalert P: Hepatoprotective effect of curcumin and
alpha-tocopherol against cisplatin-induced oxidative stress. BMC
Complement Altern Med. 14:1112014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Huang P, Law S, Tian H, Leung W and
Xu C: Preventive effect of curcumin against chemotherapy-induced
side-effects. Front Pharmacol. 9:13742018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng T, Yang X, Kong Q and Lu J:
Editorial: Food bioactive polysaccharides and their health
functions. Front Nutr. 8:7462552021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Q, Nie SP, Wang JQ, Liu XZ, Yin PF,
Huang DF, Li WJ, Gong DM and Xie MY: Chemoprotective effects of
Ganoderma atrum polysaccharide in cyclophosphamide-induced mice.
Int J Biol Macromol. 64:395–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bao WR, Li ZP, Zhang QW, Li LF, Liu HB, Ma
DL, Leung CH, Lu AP, Bian ZX and Han QB: Astragalus polysaccharide
RAP selectively attenuates paclitaxel-induced cytotoxicity toward
RAW 264.7 cells by reversing cell cycle arrest and apoptosis. Front
Pharmacol. 9:15802018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Byun EB, Song HY, Kim WS, Han JM, Seo HS,
Park SH, Kim K and Byun EH: Protective effect of polysaccharides
extracted from Cudrania tricuspidata fruit against
cisplatin-induced cytotoxicity in macrophages and a mouse model.
Int J Mol Sci. 22:75122021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paul J, Gnanam R, Jayadeepa RM and Arul L:
Anti cancer activity on graviola, an exciting medicinal plant
extract vs various cancer cell lines and a detailed computational
study on its potent anti-cancerous leads. Curr Top Med Chem.
13:1666–1673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hajdu Z and Hohmann J: An
ethnopharmacological survey of the traditional medicine utilized in
the community of Porvenir, Bajo Paraguá Indian Reservation,
Bolivia. J Ethnopharmacol. 139:838–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oliveira AP, Sá I, Pereira DM, Gonçalves
RF, Andrade PB and Valentão P: Exploratory studies on the in vitro
anti-inflammatory potential of two herbal teas (Annona muricata L.
and Jasminum grandiflorum L.), and relation with their phenolic
composition. Chem Biodivers. 14:e17000022017. View Article : Google Scholar
|
|
20
|
Kim WS, Kim YE, Cho EJ, Byun EB, Park WY,
Song HY, Kim K, Park SH and Byun EH: Neuroprotective effect of
Annona muricata-derived polysaccharides in neuronal HT22 cell
damage induced by hydrogen peroxide. Biosci Biotechnol Biochem.
84:1001–1012. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Byun EB, Song HY and Kim WS:
Polysaccharides from Annona muricata leaves protect normal human
epidermal keratinocytes and mice skin from radiation-induced
injuries. Radiat Phys Chem. 170:1086722020. View Article : Google Scholar
|
|
22
|
Kim WS, Han JM, Song HY, Byun EH, Lim ST
and Byun EB: Annona muricata L.-derived polysaccharides as a
potential adjuvant to a dendritic cell-based vaccine in a
thymoma-bearing model. Nutrients. 12:16022020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Goncalves R and Mosser DM: The
isolation and characterization of murine macrophages. Curr Protoc
Immunol. Chapter 14: Unit 14.1. 2008. View Article : Google Scholar
|
|
24
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Methods Mol Biol. 594:57–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Makovec T: Cisplatin and beyond: Molecular
mechanisms of action and drug resistance development in cancer
chemotherapy. Radiol Oncol. 53:148–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marullo R, Werner E, Degtyareva N, Moore
B, Altavilla G, Ramalingam SS and Doetsch PW: Cisplatin induces a
mitochondrial-ROS response that contributes to cytotoxicity
depending on mitochondrial redox status and bioenergetic functions.
PLoS One. 8:e811622013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Liu H, Liu F and Dong Z:
Mitochondrial dysregulation and protection in cisplatin
nephrotoxicity. Arch Toxicol. 88:1249–1256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hassan I, Chibber S and Naseem I:
Ameliorative effect of riboflavin on the cisplatin induced
nephrotoxicity and hepatotoxicity under photoillumination. Food
Chem Toxicol. 48:2052–2058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khalaf AA, Hussein S, Tohamy AF, Marouf S,
Yassa HD, Zaki AR and Bishayee A: Protective effect of Echinacea
purpurea (Immulant) against cisplatin-induced immunotoxicity in
rats. Daru. 27:233–241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hussain Y, Islam L, Khan H, Filosa R,
Aschner M and Javed S: Curcumin-cisplatin chemotherapy: A novel
strategy in promoting chemotherapy efficacy and reducing side
effects. Phytother Res. 35:6514–6529. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar S and Tchounwou PB: Molecular
mechanisms of cisplatin cytotoxicity in acute promyelocytic
leukemia cells. Oncotarget. 6:40734–40746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu S, Gong LS, Li NF, Pan YF and Zhang L:
Galangin (GG) combined with cisplatin (DDP) to suppress human lung
cancer by inhibition of STAT3-regulated NF-κB and Bcl-2/Bax
signaling pathways. Biomed Pharmacother. 97:213–224. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, Xiang H, Bai X, Fei N, Huang Y,
Song X, Zhang H, Zhang L and Tong D: Porcine parvovirus infection
activates mitochondria-mediated apoptotic signaling pathway by
inducing ROS accumulation. Virol J. 13:262016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oyinloye BE, Adenowo AF and Kappo AP:
Reactive oxygen species, apoptosis, antimicrobial peptides and
human inflammatory diseases. Pharmaceuticals (Basel). 8:151–175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kleih M, Böpple K, Dong M, Gaißler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis. 10:8512019.
View Article : Google Scholar : PubMed/NCBI
|