Introduction
Currently, revascularization has become the most
important therapy for patients with myocardial ischemia (1). Although the timely recovery of
myocardial perfusion is related to long-term improved cardiac
function (2), certain patients
may suffer from continuous cardiac dysfunction despite of effective
revascularization therapy, which is currently considered to be
caused by the ischemia and reperfusion (I/R) injury (3). The pathogenesis of this disease is
complicated and several mechanisms are involved, including
conventional mechanisms of inflammatory response, oxidative stress,
calcium overload, myocardial hibernation and stunning (4). Previous studies have shown that
ras-related C3 botulinum toxin substrate (RAC) 1, a member of the
RAC family of guanosine triphosphate phosphohydrolases, is a key
regulator of myocardial I/R injury (5). Physiologically, RAC1 maintains the
constitutive sarcomere reorganization (6) and the antioxidative efficacy of
nicotinamide adenine dinucleotide phosphate oxidases (7) in cardiomyocytes. During I/R, RAC1 is
overexpressed in cardiomyocytes and this induces I/R injury by
stimulating reactive oxygen species (ROS)-mediated myocardial
apoptosis via the inhibition of RAC1-driven processes (8,9).
However, the potential regulatory mechanisms for the role of RAC1
during I/R remain to be elucidated.
MicroRNAs (miRs) are a cluster of small non-coding
ribonucleotides (~22 nucleotides in length) which
post-transcriptionally target mRNAs, suppress protein synthesis or
increase mRNA degradation, thereby regulating important cellular
processes, including myocardial I/R injury (10,11). It is notable that the
post-translational modification of RAC1 has been highlighted to
participate in several cellular processes (12), such as carcinogenesis and
metastasis (13,14). Previous studies have also shown
that the interactions of miRs with RAC1 may be important for the
development of hypoxia (15) and
hyperglycemia-induced injury of cardiomyocytes (16). Therefore, it was hypothesized that
the interactions of miRs with RAC1 may also be involved in the
pathogenesis of myocardial I/R injury. Accordingly, the present
study validated the changes in the expression levels of RAC1 during
hypoxia and reoxygenation (H/R) in H9C2 cells by using isobaric
tags used for relative and absolute quantification (iTRAQ)-based
proteomic analysis (17).
Moreover, bioinformatic analysis was used to identify the potential
miRs targeting RAC1 in this process and to examine the associated
molecular mechanisms.
Materials and methods
H9C2 cell culture and induction of H/R
injury
The rat embryonic cardiac cell line H9C2 (Wuhan
GeneCreate Biological Engineering Co. Ltd.), which was preserved in
our institution, was used in the present study. The cells were
routinely cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
penicillin/streptomycin and 4 mM L-glutamine and maintained in a
CO2 incubator (Shellab) at 37°C. The medium was changed
every 2–3 days and the cells were subcultured and passaged at
70–80% confluency. For subsequent experiments, the cells in the
logarithmic phase of growth were selected. Following digestion with
trypsin, the cells were harvested and seeded into 12-well plates
for modeling of the H/R injury and subsequent molecular biological
analyses. The processes of the induction of H/R injury in H9C2
cells were performed as previously reported (18). Briefly, prior to the incubation
under hypoxic conditions, the regular medium was removed and
replaced with DMEM under hypoxic conditions, which lacked glucose
(pH 6.8). Subsequently, the cells were moved to a hypoxia chamber
with 94% (v/v) N2, 5% (v/v) CO2 and 1% (v/v)
O2 for 6 h at 37°C. Following incubation under hypoxic
conditions, the medium was removed and changed to regular DMEM with
4.5 mM glucose (pH 7.4); the cells were maintained under normoxic
conditions (5% CO2 and 95% air) for 24 h.
iTRAQ-based proteomic analysis
Total protein was extracted by extraction buffer [7
M Urea/2 M Thiourea/4% SDS/40 mM Tris-HCl (pH 8.5)/1 mM PMSF/2 mM
EDTA] from H9C2 cells of the control and the H/R injury groups for
subsequent analyses. The concentrations of the proteins were
determined with the Bradford assay according to the manufacturer's
instructions (Abcam). For each sample, an aliquot of 100 µg protein
was further digested with trypsin solution for 12 h at 37°C to
obtain the tryptic digested peptides, which were desalted, dried
and reconstituted in a triethylammonium bicarbonate solution (0.5
M). Subsequently, an iTRAQ reagent kit (SCIEX) was used and the
peptides were labeled according to the manufacturer's protocol. For
nano-liquid chromatography (LC)-mass spectrometry (MS)/MS analysis,
the iTRAQ-labeled plasma peptide mixtures were diluted in 20 mM
ammonium formate (pH 10) and loaded onto a reverse phase column
using an Eksigent ultra-performance liquid chromatography system.
Subsequently, a 50 min linear gradient elution with 80%
acetonitrile and 20% 20 mM ammonium formate (pH 10) was performed
at a flow rate of 800 µl/min, leading to a collection of 50
separate fractions per min. These fractions were pooled, desalted,
dried and stored at −80°C for subsequent analyses as previously
described (19). The Triple-time
of flight (TOF) 5600 system (SCIEX) was used for MS and MS/MS
analysis of the LC eluent. The key parameters and conditions for
the working protocol of the Triple-TOF 5600 were set according to
the manufacturer's instructions and previously published reports
(20). Briefly, the peptides were
labeled according to the ITRAQ-8 standard Kit instructions (SCIEX)
and mixed. Next, these labeled peptides were segregated via the
Ultimate 3000 HPLC system (Dionex; Thermo Fisher Scientific, Inc.).
After labeling, the peptides were analyzed by 2D LC MS/MS analysis.
The identification and quantification analysis of the significantly
differentially expressed proteins detected in H9C2 cells was
achieved between the H/R and the control groups following input of
the iTRAQ-based proteomics data into the ProteinPilot software v4.5
(SCIEX) with the SwissProt Homo sapiens database (https://www.ebi.ac.uk/uniprot/) (release May
2022, containing 23,752 sequences) for bioinformatic analyses.
Subsequently, the iTRAQ ratios and P values were estimated via the
ProteinPilot software. The levels of significance were set at a
ratio of >1.2 or <0.83 and P<0.05 was considered to
indicate a statistically significant difference in the expression
levels of the cellular proteins of the H/R and the control H9C2
cells. To further clarify the functional classifications of the
differentially expressed proteins, the online DAVID tool
(http://david.abcc.ncifcrf.gov) was
searched for the Gene Ontology annotation. Moreover, the
pathway-related information of the identified proteins with
different expression levels was obtained with the Kyoto
Encyclopedia of Genes and Genomes database (http://www.genome.jp/kegg/). Subsequent analysis of
the potential network of the protein-protein interactions was
performed using the online tool of STRING 10.5 (http://string-db.org) as previously described
(20).
Identification of potential miRs which
interact with RAC1 in rat and humans: TargetScan and miRDB
The miRs that potentially regulate RAC1 expression
in rats and humans were identified by TargetScan 7.2 (www.targetscan.org). Subsequently, the putative
binding sites were also predicted by miRDB (http://mirdb.org/miRDB/). The analysis was performed
to identify common miRs between humans and rats, which may
potentially interact with RAC1. It was considered that these
candidate miRs are potential regulators of RAC1 protein expression
by interacting with the 3′-untranslated region (UTR) of RAC1
mRNA.
Transfection of mimics and inhibitors
of miR-194-5p and overexpression of RAC1 in H9C2
cardiomyocytes
Exogenous mimics, inhibitors of miR-194-5p and their
controls were synthesized by the Wuhan GeneCreate Biological
Engineering co. Ltd. The sequences are shown in Table I. Overexpression of the RAC1
proteins in H9C2 cells was achieved via the transfection of the
plasmid vectors (6 µg) containing the cDNA of RAC1 (pcDNA3.1-GAPs),
which was also constructed and obtained from the Wuhan GeneCreate
Biological Engineering co. Ltd. Empty vector was used as the
negative control. The transfection of 10 pmol mimics, inhibitors of
miR-194-5p and their controls was performed with the
Lipofectamine® RNAi MAX transfection medium (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Following transfection at 37°C for 20 min, the cells were cultured
for 48 h prior to further experiments.
 | Table I.Sequences of miR-194-5p mimics and
controls. |
Table I.
Sequences of miR-194-5p mimics and
controls.
| Group | Sequence (5′-3′) |
|---|
| miR-194-5p
mimics |
UGUAACAGCAACUCCAUGUGGA |
| Control mimics |
UUGUACUACACAAAAGUACUG |
| miR-194-5p
inhibitor |
UCCACAUGGAGUUGCUGUUACA |
| Inhibitor
control |
CAGUACUUUUGUGUAGUACAA |
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from 5×106 H9C2c
cells using total TRIzol® RNA extraction kit (AxyPrep;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Subsequently, the removal of the genomic DNA and the
reverse transcription reactions were performed using the gDNA
Eraser and the PrimeScript RT reagent kit (Takara Bio, Inc.)
according to the instructions provided by the manufacturer.
Following measurement of the concentrations of the obtained cDNA,
the products were dispensed and stored at −20°C for subsequent use.
GAPDH was used as an internal control for the detection of the mRNA
levels of miR-194-5p and RAC1. The process of the quantitative
real-time PCR was performed with the PrimeScript RT reagent kit
(Takara Bio, Inc.) with a total of 20 µl reaction mixture. The
conditions for the quantitative real-time PCR were set as follows:
60 sec at 95°C, followed by 40 cycles of 95°C for 15 sec, 60°C for
15 sec and 72°C for 45 sec. The 2−ΔΔCq method was used
to estimate the relative mRNA expression levels (21). The experiment was repeated three
times. The sequences of the forward and reverse primers were shown
in Table II.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-194-5p |
ACACTCCAGCTGGGTGTAACAGCAACTCCA |
TGGTGTCGTGGAGTCG |
| RAC1 |
CTCTCCTACCCGCAAACAGA |
TCAAGCTTCGTCCCCACTAG |
| GAPDH |
GCAAGTTCAACGGCACAG |
GCCAGTAGACTCCACGACAT |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
Western blot analysis
To detect the protein expression levels of RAC1
compared with the internal control of GAPDH, western blot analysis
was performed with an antibody against RAC1 (Abcam). Briefly,
following the specific treatment in each group of H9C2 cells, the
buffer of the radioimmunoprecipitation assay (Beyotime Institute of
Biotechnology) was used to obtain the total protein of each group
and the concentrations of the total proteins were determined by the
bicinchoninic protein assay kit (Beyotime Institute of
Biotechnology) as instructed by the manufacturer's protocol. The
samples (15 µl/lane) were separated on 15% gels using SDS-PAGE,
transferred to polyvinylidene fluoride membranes, incubated with 5%
non-fat milk for 2 h at room temperature, washed with TBS plus 0.1%
Tween 20 (TBST) and incubated with the primary rabbit antibodies
against RAC1 (1:1,000; cat. no. ab155938; Abcam) and GAPDH
(1,10,000; cat. no. ab37168; Abcam) overnight at 4°C. The following
day, the solution containing the primary antibody was removed and
the membrane was washed with TBST six times (5 min each).
Subsequently, the membranes were incubated with the HRP goat
anti-rabbit secondary antibody (1:10,000; cat. no. AS1107; Wuhan
Aspen Biotechnology Co., Ltd.) for 2 h at room temperature.
Finally, the intensity levels of the bands corresponding to each
protein were visualized using an ECL detection system (Amersham;
Cytiva). The intensity levels of the bands corresponding to each
protein of interest were analyzed using the Image Q analysis system
(Storm Optical Scanner; Molecular Dynamics).
Immunofluorescence and
cytochemistry
The cells in the exponential phase were cultured for
48 h, the culture medium removed, washed three times with PBS, then
an appropriate amount of Fluo-4 AM (cat. no. S1060; Beyotime
Institute of Biotechnology) original solution was diluted to 0.5–5
µM working solution with PBS. The Fluo-4 AM working solution to
completely cover the cells and incubated at 20–37°C for 10–60 min
to load the fluorescent probe. After washing with PBS for three
times, the fluorescence of Fluo-4 was observed in nine random
fields using a fluorescence microscope (Olympus Corporation) to
determine the change of intracellular calcium concentration.
Luciferase assay
The constructs of pGL3-RAC1 (RAC1-WT) or
pGL3-RAC1-MUTANT (RAC1-MUT) (Wuhan GeneCreate Biological
Engineering Co. Ltd.) were transfected into H9C2 cells with or
without miR-194-5p mimics or control mimics by
Lipofectamine® 2000 (cat. no. 11668019; Invitrogen;
Thermo Fisher Scientific, Inc.) prior to the H/R culture.
Subsequently, the luciferase activity levels were measured by
dividing the relative light unit (RLU) of firefly luciferase by the
RLU of sea kidney luciferase (to represent the activation degree of
the target report gene) at 6 h following transfection using the
dual luciferase assay kit (cat. no. E1910; Promega Corporation) in
accordance with the instructions of the manufacturer.
Apoptosis detection
The induction of H9C2 cell apoptosis was evaluated
in each group by an Annexin-fluorescein isothiocyanate (FITC)
apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd.). The
samples were analyzed with fluorescence-activated cell sorting
(FACS) and flow cytometry. H9C2 cells were harvested from each
group and washed with PBS twice. The cells were resuspended in 500
µl buffer and subsequently stained and incubated with Annexin
V-FITC (5 µl) and propidium iodide (5 µl) at room temperature in
the dark for 15 min. Subsequently, the cells were transferred into
specific tubes for flow cytometry analysis using a BD Accuri C6
flow cytometer (BD Biosciences) and BD Accuri C6 Plus software
(version C6; BD Biosciences). The apoptotic rate included the
percentage of early and late apoptotic cells. A total of 10,000
cells were analyzed for each group.
ROS assay
To reflect the intracellular ROS levels in the H9C2
cells of each group, the cells were stained with
2,7-dichlorofluorescein diacetate (DCFH-DA; Nanjing Jiancheng
Bioengineering Institute). In brief, the cells from each group were
washed twice with PBS and incubated in DMEM, which contained 10 µM
DCFH-DA, for 20 min at 37°C in the dark. Subsequently, the cells
were washed again, collected following trypsin treatment (0.25%
trypsin-EDTA), centrifuged at 225 × g for 3 min at 37°C and
resuspended in 500 µl PBS. FACS was used with an excitation
wavelength of 488 nm and an emission wavelength of 521 nm.
Statistical analysis
The data are presented as mean ± SEM and compared
with one-way analysis of variance among the groups. Post-hoc
analysis with Fisher's least significant difference test, Dunnett's
test or unpaired Student's t-test were used to compare the
differences between the groups as appropriate with the SPSS
software 20.0 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Upregulation of RAC1 expression in
H9C2 cardiomyocytes undergoing H/R
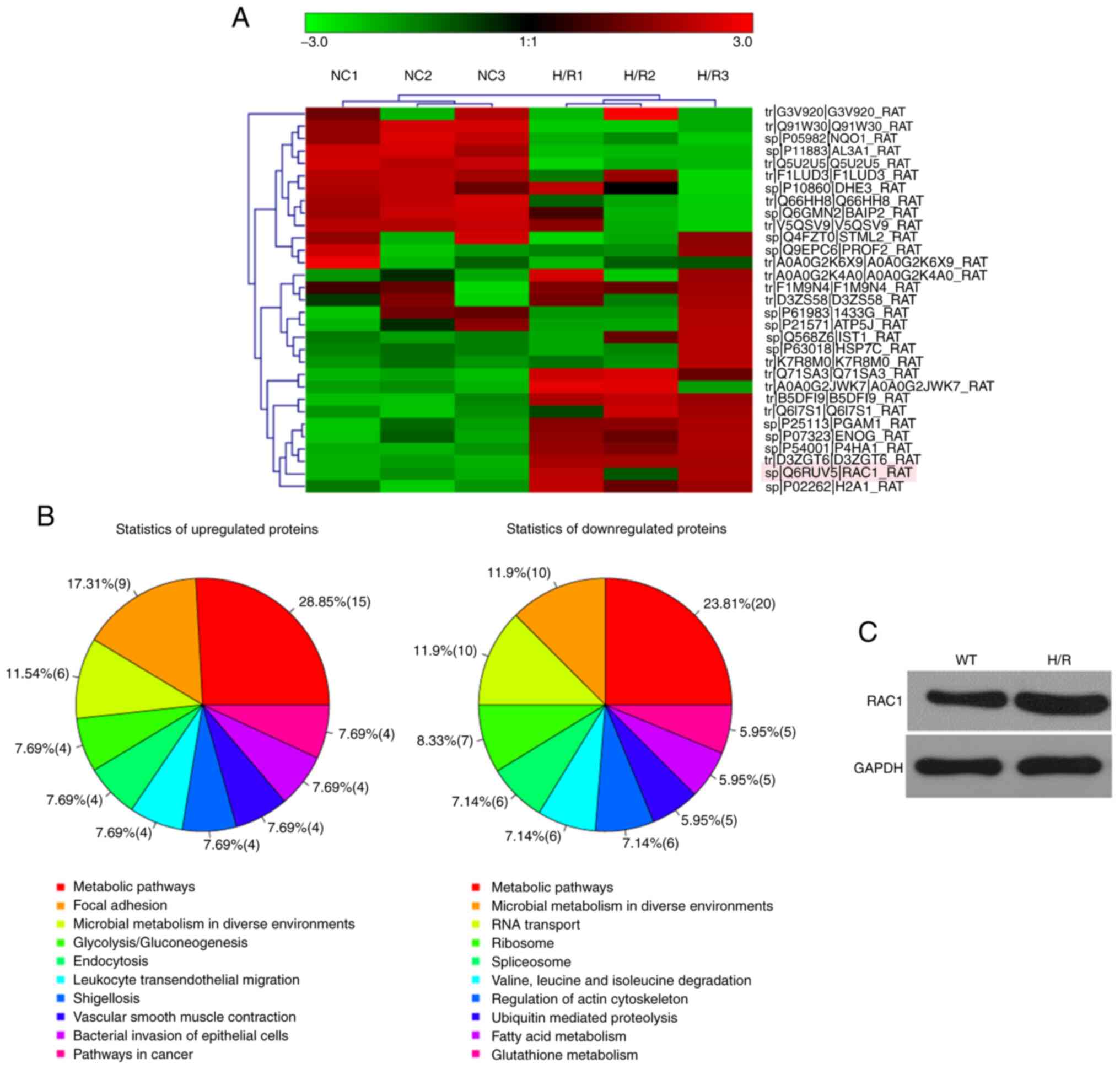
The relative fold-changes in the expression levels
of the top 30 proteins, which were differentially expressed in H9C2
cardiomyocytes undergoing H/R compared with the control cells, were
detected by the iTRAQ proteomic analysis (Fig. 1A). Subsequent pathway analyses for
the 199 proteins that were differentially expressed between H9C2
cardiomyocytes undergoing H/R and those cultured under normoxic
conditions indicated that several proteins were involved in
pathways regulating cellular metabolism and communication (Fig. 1B). The protein levels of RAC1 were
significantly higher in H9C2 cardiomyocytes undergoing H/R compared
with those of the control cells cultured under normoxic conditions;
these results were further validated by western blot analysis
(Fig. 1C).
Identification of miR-194-5p as a
regulator of RAC1 in H/R
Previous studies indicated that the activation of
RAC1 is involved in H/R-induced cardiomyocyte injury by mediating
sarcomere dysfunction and ROS accumulation (6,7).
Since miRs have been involved in the pathogenesis of myocardial I/R
injury at least partially via the regulation of the ROS pathway,
the present study further explored using bioinformatic analysis the
miRs, which may interact with RAC1 during the induction of H/R in
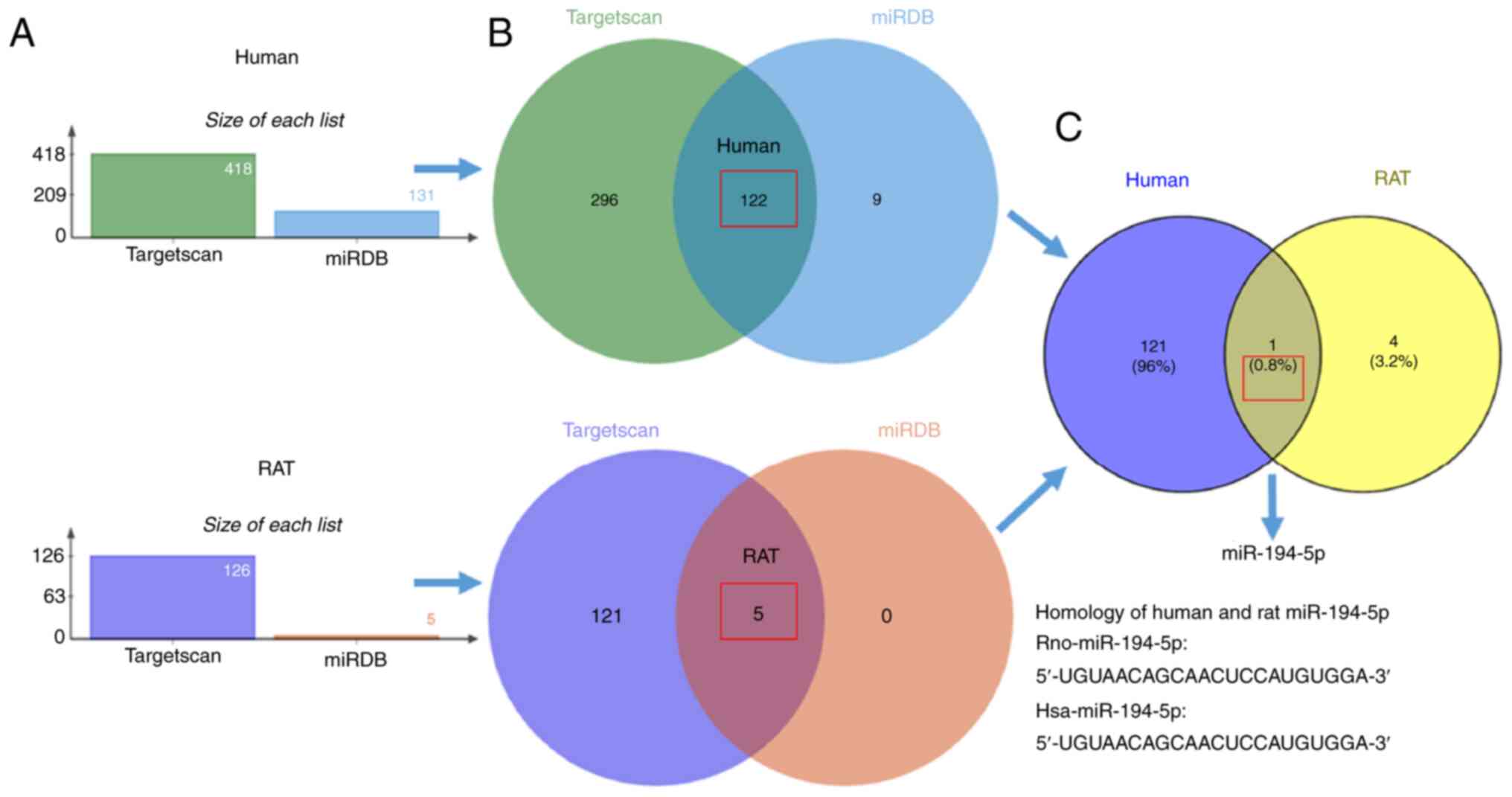
cardiomyocytes. Firstly, TargetScan and miRDB database analyses
were used to predict a cluster of miRs, which may potentially
interact with RAC1 in humans and rats (Fig. 2A). Subsequent analysis identified
the miRs, which were common between humans and rats and may
potentially interact with RAC1 (Fig.
2B). Finally, sequence homogeneity analyses of miRs and RAC1
indicated that the miR-194-5p in humans and rats share similar
sequences (Fig. 2C) and was
likely to be the miR that regulated the expression of RAC1 in
cardiomyocytes.
Effects of miR-194-5p on RAC1
expression in H9C2 cardiomyocytes
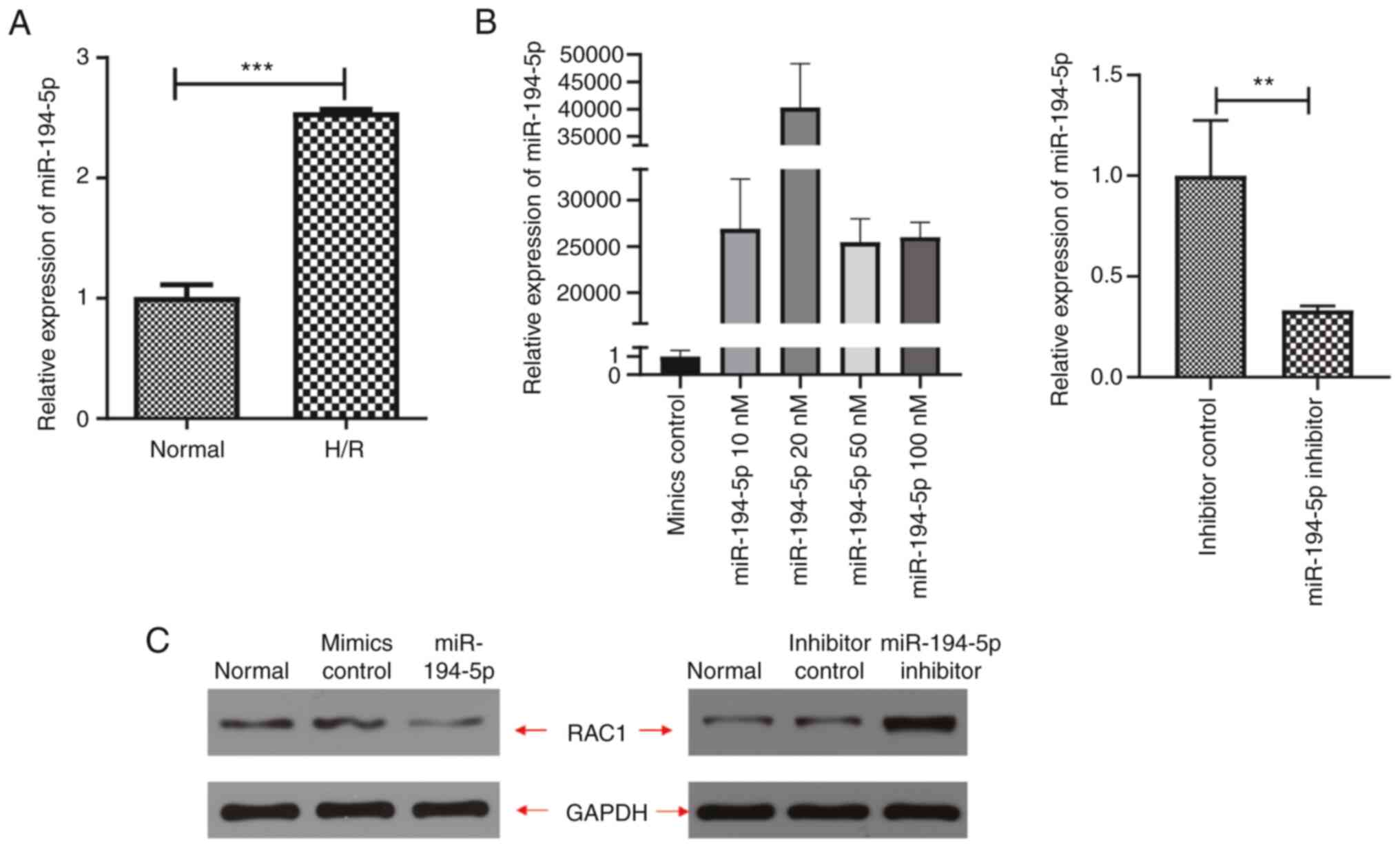
The changes in the expression levels of miR-194-5p
in H9C2 cardiomyocytes during H/R were evaluated using qPCR
analyses. The results indicated that the expression levels of
miR-194-5p were significantly higher in H9C2 cardiomyocytes
cultured under H/R conditions compared with those of the control
cells cultured under normoxic conditions (P<0.001, Fig. 3A). Subsequently, exogenous
miR-194-5p mimics and inhibitors were administered to evaluate the
potential effects on the expression levels of RAC1. The results
indicated that administration of 20 nM miR-194-5p mimics was
associated with the most effective overexpression of cellular
miR-194-5p (P<0.001, Fig. 3B),
which caused inhibition of RAC1 expression. Accordingly, 20 nM
miR-194-5p mimics was used as the optimal concentration for
subsequent studies. Higher concentrations of miR-194-5p mimics may
cause cellular injury, overactivated oxidative stress and
upregulation of RAC1 expression (Fig.
3C). These results were further validated by western blot
analysis, which indicated that administration of miR-194-5p mimics
(20 nM) was associated with significant inhibition of RAC protein
expression levels compared with the administration of mimics
control; however, administration of miR-194-5p inhibitors
significantly increased the expression levels of the RAC1 protein
compared with the expression levels of RAC1 noted following
administration of inhibitor controls (Fig. 3C).
To further clarify the targeting sequence of RAC1
mRNA for miR-194-5p, the luciferase activity assay was performed.
Administration of miR-194 suppressed the relative luciferase
activity of the constructs encompassing the binding sites of RAC1
3′-UTR (P<0.001), while administration of miR-194-5p did not
significantly affect the relative luciferase activity of the
constructs encompassing the mutant binding sites of the RAC1
3′-UTR, suggesting that miR-194-5p may inhibit RAC1 expression
targeting this sequence (Fig.
4).
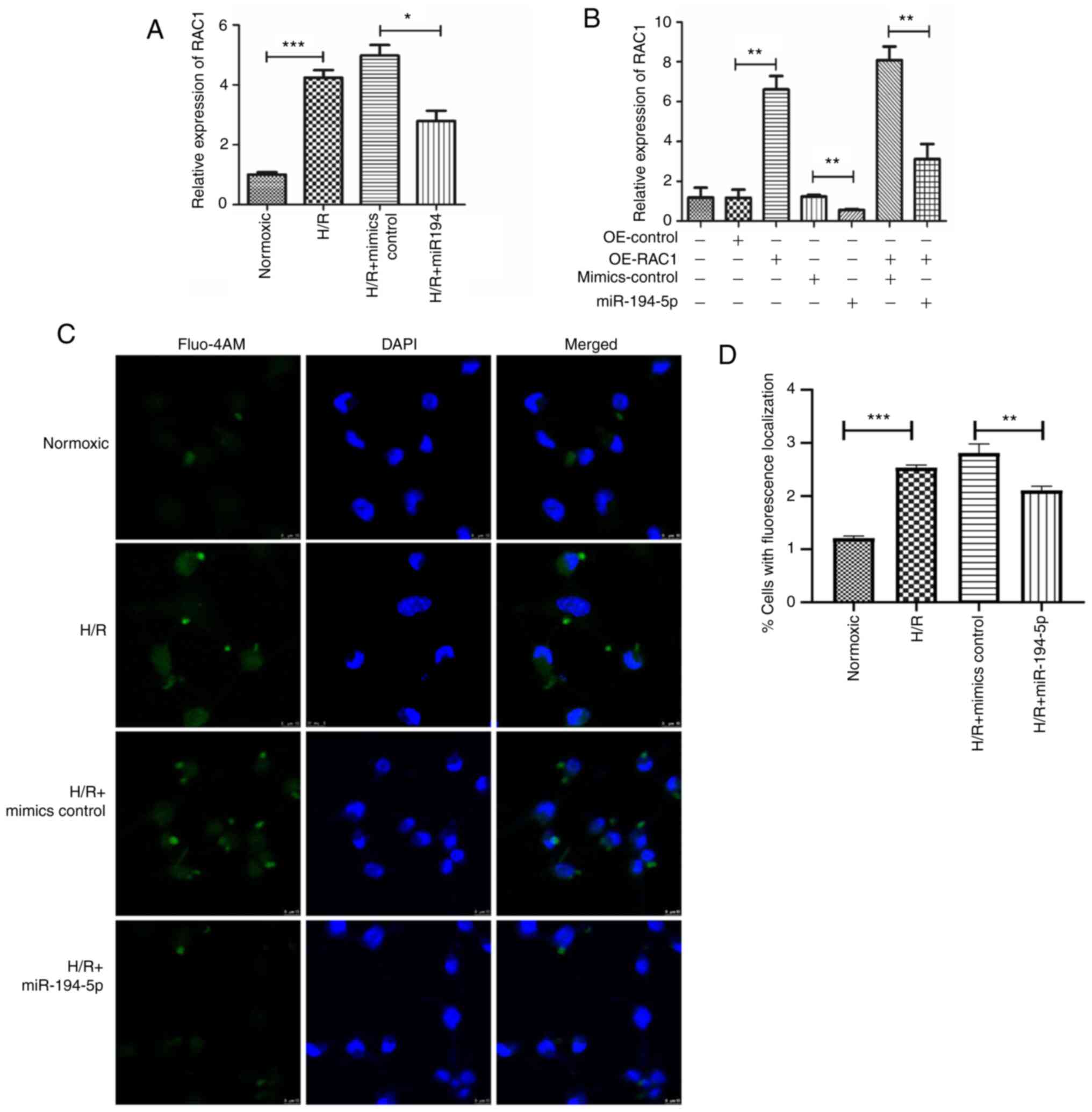
To further validate the interactions between
miR-194-5p and RAC1 in H9C2 cardiomyocytes undergoing H/R, specific
plasmids were constructed to overexpress RAC1 (OE-RAC1) and
exogenous miR-194-5p (miR-194 mimics) were transfected into H9C2
cardiomyocytes. The results of the qPCR analysis indicated a
significant induction in RAC1 expression of H9C2 cardiomyocytes
cultured under H/R conditions compared with the control cells
cultured under normoxic conditions (P<0.001; Fig. 5A). However, administration of
exogenous miR-194-5p significantly attenuated the upregulation of
RAC1 expression in H9C2 cardiomyocytes cultured under H/R
conditions (P<0.05; Fig. 5A).
Further studies using qPCR indicated that exogenous expression of
miR-194-5p significantly attenuated the upregulation of RAC1
expression in H9C2 cardiomyocytes cultured under H/R conditions and
transfected with OE-RAC1 plasmids (P<0.01; Fig. 5B). In addition, the results of the
immunofluorescence and immunocytochemistry analyses indicated that
H/R significantly induced the cellular calcium concentration in
H9C2 cardiomyocytes compared with the control cells cultured under
normoxic conditions (P<0.01), while administration of exogenous
miR-194-5p significantly attenuated the HR-induced upregulation in
the concentration levels of cellular calcium in H9C2 cardiomyocytes
(P<0.01; Fig. 5C).
Taken together, these results indicated that both
miR-194-5p and RAC1 expressions were increased during H/R in
cardiomyocytes and that miR-194-5p could attenuate the
overexpression of RAC1 by targeting the 3′-UTR of RAC1 mRNA.
Effects of miR-194-5p on cardiomyocyte
apoptosis and ROS accumulation in H9C2 cells during H/R
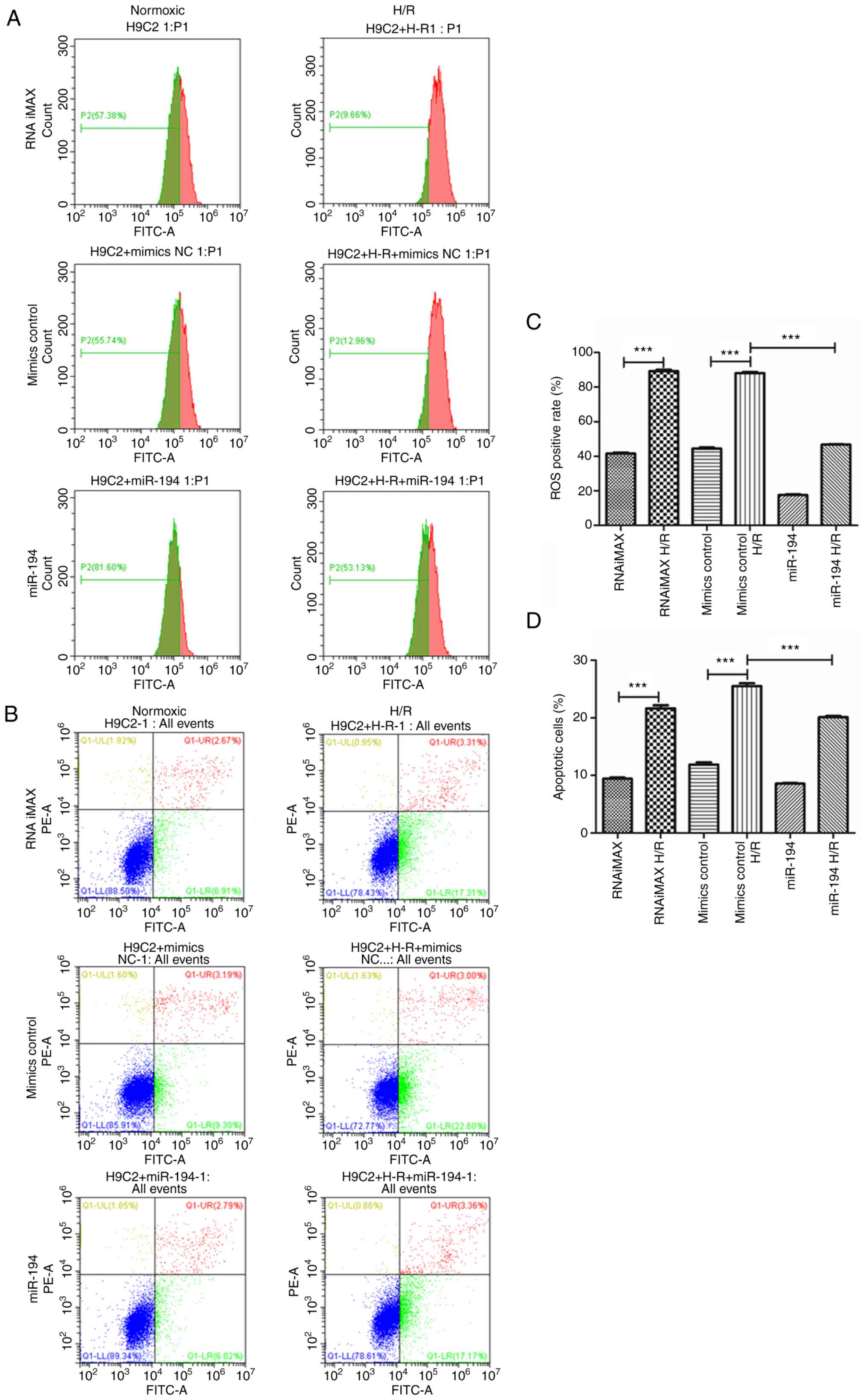
Compared with H9C2 cardiomyocytes cultured under
normoxic conditions, H/R significantly increased the concentration
levels of cellular ROS in these cells (P<0.001), while exogenous
administration of miR-194 significantly reduced the cellular levels
compared with those of the H9C2 cardiomyocytes cultured under H/R
conditions and transfected with control mimics (P<0.001;
Fig. 6A). Moreover, the results
of the flow cytometry analyses indicated that H/R significantly
induced apoptosis of H9C2 cardiomyocytes compared with the control
cells cultured under normoxic conditions (P<0.001), while
exogenous administration of miR-194 significantly reduced the
apoptotic rate in H9C2 cardiomyocytes cultured under H/R conditions
and transfected with control mimics (P<0.001; Fig. 6B). Subsequent quantitative
analyses indicated similar results (Fig. 6C and D). These results indicated
that miR-194-5p attenuated H/R-induced apoptosis in H9C2
cardiomyocytes by alleviating cellular ROS accumulation.
Role of RAC1 in the potential
protective effect of miR-194-5p on H/R-induced apoptosis
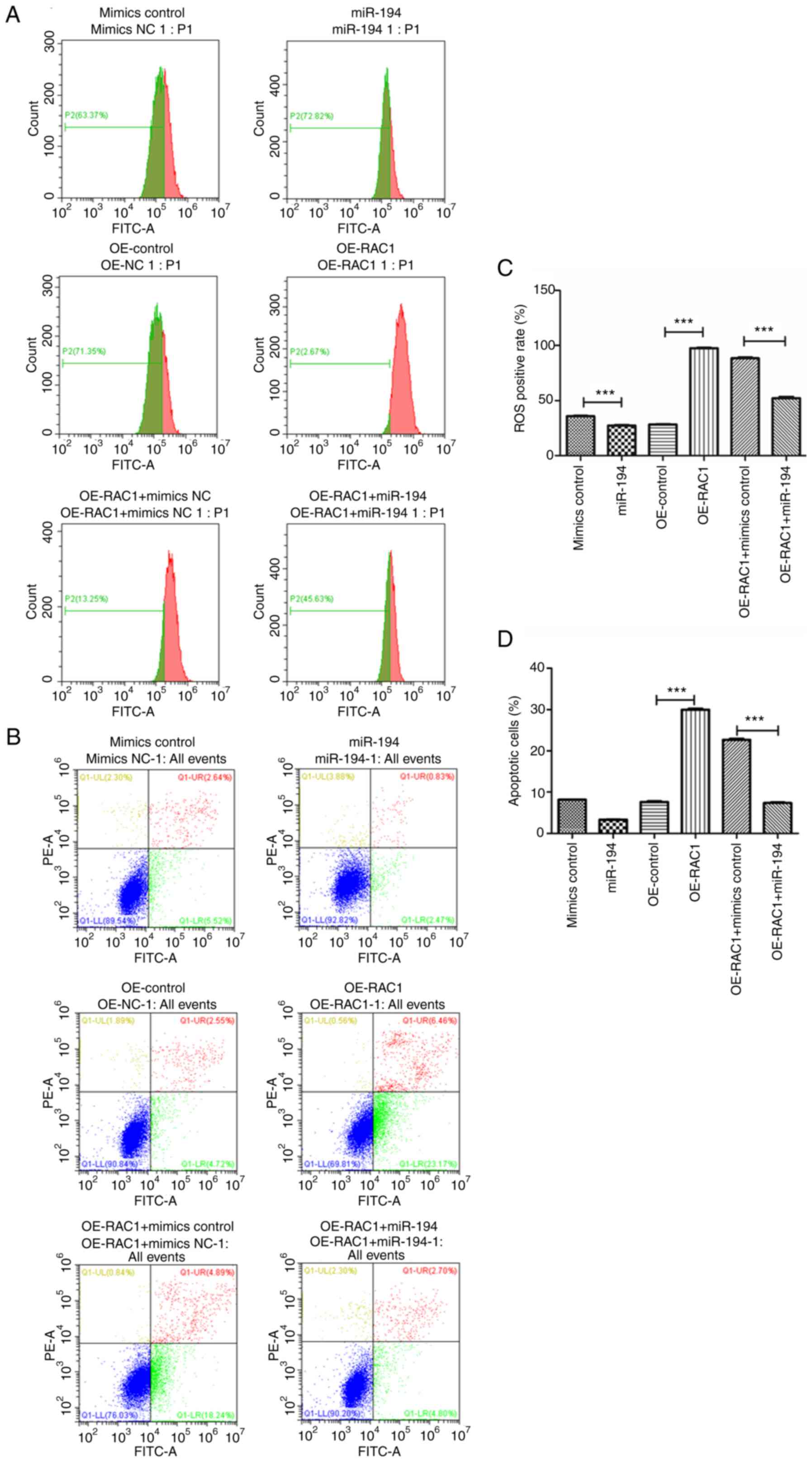
The results of the flow cytometry analysis indicated
that exogenous administration of miR-194 significantly reduced the
cellular ROS levels compared with those of the H9C2 cardiomyocytes
cultured under H/R conditions and transfected with control mimics
(P<0.001), while overexpression of RAC1 dramatically increased
the cellular levels compared with those of H/R-cultured H9C2
cardiomyocytes transfected with OE-control. Moreover, exogenous
administration of miR-194 significantly reduced the cellular ROS
levels induced by H/R and reduced the overexpression of RAC1 in
H9C2 cardiomyocytes (Fig. 7A).
Similarly, exogenous administration of miR-194 significantly
reduced the apoptotic rate of the cells compared with that of the
H/R-cultured H9C2 cardiomyocytes transfected with control mimics
(P<0.001), while overexpression of RAC1 dramatically increased
the apoptotic rate of the cells compared with that of the
H/R-cultured H9C2 cardiomyocytes transfected with OE-control.
Moreover, exogenous administration of miR-194-5p significantly
reduced the apoptotic rate of H9C2 cells cultured under H/R
conditions and transfected with RAC1 overexpressing plasmids
(Fig. 7B). Subsequent
quantitative analyses indicated similar results (Fig. 7C and D). Taken together, these
results demonstrated that overexpression of RAC1 may mediate
H/R-induced apoptosis in H9C2 cardiomyocytes by enhancing cellular
ROS accumulation, whereas upregulation of miR-194-5p expression may
function as a negative regulator of the aforementioned
pathophysiological process.
Discussion
In the present study, an in vitro cultured
H9C2 cardiomyocyte model was established under H/R conditions. The
data indicated that RAC1 was significantly overexpressed. This
effect facilitated the induction of apoptosis in cardiomyocytes by
enhancing cellular accumulation of ROS. It is notable that
bioinformatic and luciferase analyses confirmed that miR-194-5p
could inhibit the translation of RAC1 in H9C2 cells. miR-194-5p
expression was upregulated under H/R conditions in H9C2
cardiomyocytes, which subsequently ameliorated cellular ROS levels
and the induction of apoptosis via inhibition of RAC1
overexpression. Taken together, these results indicated that the
upregulated expression levels of miR-194-5p may function as a
self-regulated cardioprotective response against RAC1-mediated ROS
accumulation and cardiomyocyte apoptosis. Exogenous administration
of miR-194-5p may be a novel target to ameliorate I/R-induced
injury related to myocardial apoptosis.
Previous studies have indicated that constitutive
expression of RAC1 in cardiomyocytes is essential for specific
physiological functions including cytoskeletal formation, ROS
clearance and energy biogenesis (8,22,23). However, overexpression of RAC1 in
a mouse model of myocardial I/R injury has been attributed to
causing exacerbation of myocardial injury and deteriorated cardiac
function during follow-up (9).
The results of the present study provided further information on
the pathological role of RAC1 overexpression in the pathogenesis of
myocardial I/R injury by indicating that H/R enhanced the protein
levels of RAC1 in H9C2 cardiomyocytes and thereby led to cellular
ROS accumulation and induction of cardiomyocyte apoptosis. Since
myocardial apoptosis causes direct loss of functional
cardiomyocytes, it has been shown to be a major factor of
deteriorated cardiac function in an animal model and in patients
with ischemic heart disease (24,25). These results further confirmed
that overexpression of RAC1 in cardiomyocytes cultured under H/R
conditions, is a key molecular pathway underlying the pathogenesis
of I/R-mediated myocardial dysfunction.
Although previous studies have shown that miRs may
interact with RAC1, the majority of these studies are focused on
cancer (12–14). To the to the best of the authors'
knowledge, a few studies have evaluated the potential interaction
between miRs and RAC1 proteins in the pathogenesis of
cardiovascular diseases. In an early study, which used
streptozotocin-induced diabetic mice, the changes in the miR
expression profiles and their potentially targeted proteins were
identified (26). The
miR-mediated upregulation of RAC1 expression was proposed to be
involved in the pathogenesis of diabetic cardiomyopathy (26). An additional study was performed
in H9C2 cells cultured under persistent hypoxia and the results
indicated that upregulation of miR-145 expression was associated
with overexpressed RAC1; moreover, silencing of miR-145 expression
protected H9C2 cells against hypoxia-induced injury by targeting
RAC1 (15). Transfection of the
cells with miR-182 mimics was shown to attenuate
hyperglycemia-induced hypertrophy of cardiomyocytes via targeting
the overactivated RAC1 protein (16). The present study, used a model
with the mimics and the inhibitors of miR-194-5p and a luciferase
reporter assay. The data indicated that the H/R-induced
upregulation of miR-194-5p expression may be a self-protective
mechanism which can target RAC1 overexpression and related ROS
accumulation and apoptosis of H9C2 cardiomyocytes. Exogenous
administration of miR-194-5p was shown to reduce the overactivated
RAC1 protein and subsequently ameliorate the H/R-induced ROS
accumulation and apoptosis of H9C2 cardiomyocytes. Future studies
are required to determine the potential therapeutic efficacy of
miR-194-5p upregulation on myocardial injury and cardiac function
in animal model of I/R injury.
In conclusion, the present study indicated that
upregulation of miR-194-5p may function as a self-regulated
cardioprotective response against RAC1-mediated ROS accumulation
and cardiomyocyte apoptosis, although animal and clinical
experiments are needed to further confirm these results. Exogenous
administration of miR-194-5p may be a novel target to ameliorate
I/R injury-mediated myocardial apoptosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Scientific
Research Fund Project of Yunnan Education Department (grant no.
2019J1308), the National Natural Science Foundation of China (grant
no. 82060062), the Joint special fund of Applied Fundamental
Research of Kunming Medical University granted by Science and
Technology Office of Yunnan (grant nos. 202001AY070001-097 and
202001AY070001-167). The funders had no role in the study design,
data collection, data analysis, decision to publish, or preparation
of the manuscript.
Availability of data and materials
The mass spectrometry proteomics data have been
deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via
the iProX partner repository with the dataset identifier
PXD015991.
Authors' contributions
Material preparation, data collection and analysis
were performed by CL, YaL, YiL, YW, YT and YH. All authors also
contributed to the study conception and design. The manuscript was
written by CL and YinL and all authors commented on the previous
versions of the manuscript. All authors read and approved the final
version of the manuscript. YT and YH confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Katritsis DG, Mark DB and Gersh BJ:
Revascularization in stable coronary disease: Evidence and
uncertainties. Nat Rev Cardiol. 15:408–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benjamin EJ, Muntner P, Alonso A,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Das SR, et al: Heart disease and stroke statistics-2019
update: A report from the American heart association. Circulation.
139:e56–e528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Araszkiewicz A, Grygier M, Lesiak M and
Grajek S: The impact of ischemia-reperfusion injury on the
effectiveness of primary angioplasty in ST-segment elevation
myocardial infarction. Postepy Kardiol Interwencyjnej. 9:275–281.
2013.PubMed/NCBI
|
|
4
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marei H and Malliri A: Rac1 in human
diseases: The therapeutic potential of targeting Rac1 signaling
regulatory mechanisms. Small GTPases. 8:139–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sussman MA, Welch S, Walker A, Klevitsky
R, Hewett TE, Price RL, Schaefer E and Yager K: Altered focal
adhesion regulation correlates with cardiomyopathy in mice
expressing constitutively active rac1. J Clin Invest. 105:875–886.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hordijk PL: Regulation of NADPH oxidases:
The role of Rac proteins. Circ Res. 98:453–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elnakish MT, Awad MM, Hassona MD, Alhaj
MA, Kulkarni A, Citro LA, Sayyid M, Abouelnaga ZA, El-Sayed O,
Kuppusamy P, et al: Cardiac remodeling caused by transgenic
overexpression of a corn Rac gene. Am J Physiol Heart Circ Physiol.
301:H868–H880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Talukder MA, Elnakish MT, Yang F,
Nishijima Y, Alhaj MA, Velayutham M, Hassanain HH and Zweier JL:
Cardiomyocyte-specific overexpression of an active form of Rac
predisposes the heart to increased myocardial stunning and
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
304:H294–H302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu S and Li G: MicroRNA expression and
function in cardiac ischemic injury. J Cardiovasc Transl Res.
3:241–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye Y, Perez-Polo JR, Qian J and Birnbaum
Y: The role of microRNA in modulating myocardial
ischemia-reperfusion injury. Physiol Genomics. 43:534–542. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdrabou A and Wang Z: Post-translational
modification and subcellular distribution of Rac1: An Update.
Cells. 7:2632018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang ZB, Ma BQ, Liu SG, Li J, Yang GM,
Hou YB, Si RH, Gao P and Yan HT: miR-365 regulates liver cancer
stem cells via RAC1 pathway. Mol Carcinog. 58:55–65. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao X, Xu W, Lu T, Zhou J, Ge X and Hua D:
MicroRNA-142-3p promotes cellular invasion of colorectal cancer
cells by activation of RAC1. Technol Cancer Res Treat.
17:15330338187905082018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Zhang Y, Wang H, Zhao G and Fa X:
MicroRNA-145 aggravates hypoxia-induced injury by targeting Rac1 in
H9c2 cells. Cell Physiol Biochem. 43:1974–1986. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng Z, Wang Y, Lin Y, Nan S, Xu W, Hu B
and Shen E: MicroRNA-182 modulates high glucose-induced
cardiomyocyte hypertrophy via targeting Rac1. Zhonghua Xin Xue Guan
Bing Za Zhi. 43:619–624. 2015.(In Chinese). PubMed/NCBI
|
|
17
|
Moulder R, Bhosale SD, Goodlett DR and
Lahesmaa R: Analysis of the plasma proteome using iTRAQ and
TMT-based Isobaric labeling. Mass Spectrom Rev. 37:583–606. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao XB, Jiang ZH, Dong L, Zheng Y and Li
Y: Effects of modulation of ion channel currents by salidroside in
H9C2 myocardial cells in hypoxia and reoxygenation. Evid Based
Complement Alternat Med. 2019:82128682019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang P, Zhu S, Zhao M, Zhao P, Zhao H,
Deng J and Li J: Identification of plasma biomarkers for diffuse
axonal injury in rats by iTRAQ-coupled LC-MS/MS and bioinformatics
analysis. Brain Res Bull. 142:224–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Liu F, Ma X, Adi D, Gai MT, Jin X,
Yang YN, Huang Y, Xie X, Li XM, et al: iTRAQ analysis of a mouse
acute myocardial infarction model reveals that vitamin D binding
protein promotes cardiomyocyte apoptosis after hypoxia. Oncotarget.
9:1969–1979. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abo A, Pick E, Hall A, Totty N, Teahan CG
and Segal AW: Activation of the NADPH oxidase involves the small
GTP-binding protein p21rac1. Nature. 353:668–670. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elnakish MT, Moldovan L, Khan M, Hassanain
HH and Janssen PML: Myocardial Rac1 exhibits partial involvement in
thyroxin-induced cardiomyocyte hypertrophy and its inhibition is
not sufficient to improve cardiac dysfunction or contractile
abnormalities in mouse papillary muscles. J Cardiovasc Pharmacol.
61:536–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Ponnusamy M, Dong Y, Zhang L, Wang
K and Li P: Effects of miRNAs on myocardial apoptosis by modulating
mitochondria related proteins. Clin Exp Pharmacol Physiol.
44:431–440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao ZQ: Oxidative stress-elicited
myocardial apoptosis during reperfusion. Curr Opin Pharmacol.
4:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diao X, Shen E, Wang X and Hu B:
Differentially expressed microRNAs and their target genes in the
hearts of streptozotocin-induced diabetic mice. Mol Med Rep.
4:633–640. 2011.PubMed/NCBI
|