Introduction
Coronary atherosclerotic heart disease (CAHD)
represents the leading cause of death in the global population
(1). The incidence and prevalence
of heart disease is expected to rise due to the combination of the
rise in obesity and predicted worsening of other cardiovascular
risk factors in the general population (2). With recent advancements in
revascularization techniques and technologies, percutaneous
coronary intervention (PCI) and stenting have become the most
commonly performed procedures for the treatment of CAHD (3). First generation drug-eluting stents
(DESs) such as the Cypher™ sirolimus eluting stent and
Taxus™ paclitaxel eluting stent have led to a radical
reduction in restenosis (4).
However, in-stent restenosis (ISR) remains a significant burden for
patients undergoing coronary intervention (5). Although the introduction of DESs has
reduced the rate of target lesion revascularization (TLR) when
compared with bare metal stents (BMSs), 7–10% of patients continue
to require further procedures within 5 years of treatment, and ~20%
of patients need TLR within 10 years (6,7).
ISR is characterized by platelet aggregation, growth
factor release, inflammatory cell infiltration, medial smooth
muscle cell proliferation and migration, and extracellular matrix
remodeling (8). The vascular
response to injury is dependent on the type of cells within the
vessels and is also mediated by circulating cells derived from the
bone marrow (9). Understanding the
molecular mechanisms underlying the physiological healing process
and the response to pathological restenosis has become the focus
for extensive research.
Vascular smooth muscle cells (VSMCs) retain marked
levels of plasticity during postnatal development and can undergo
dedifferentiation into a synthetic phenotype (10). This process is considered to
provide a survival advantage, as it permits the efficient repair of
the vasculature following injury (11). Similar to numerous evolutionarily
conserved processes, these properties can also be considered a
disadvantage and predispose patients to various abnormal responses
after injury, which contribute to restenosis (8). In a previous study, Chen (12) reported that the recruitment of
smooth muscle cells into the intima of the vessel wall was a
significant contributor to atherosclerotic plaque progression.
Moreover, VSMCs within the medial layer of the vessel wall were
then activated to migrate and proliferate in response to ISR
(12). A previous study suggested
that the Kruppel-like factor (KLF) family serves a vital role in
homeostasis maintenance within the body, including in the immune,
digestive, respiratory, hematopoietic and cardiovascular systems
(13). KLF members demonstrate
variable biological functions and distinct phenotypes in different
diseases, which mainly result from their N-terminal sequences,
which provide unique protein interaction motifs and
post-translational modification sites (14). KLF4 is expressed in the vascular
wall, which includes endothelial cells (ECs) and VSMCs, and has
been reported to serve a critical role in vascular wall biology.
KLF4 confers an anti-inflammatory and vasoprotective phenotype on
ECs by the inhibition of NF-κB activation (15). More importantly, it has been
reported that KLF4 inhibits angiogenesis and endothelial
proliferation by mediating microRNAs (miRNAs/miRs) in VSMCs
(14,16). miRNAs are a class of novel
endogenous regulators of gene expression that act at the
post-transcriptional level. Furthermore, miRNAs serve essential
roles in the regulation of numerous cellular events, including
cellular proliferation, differentiation and apoptosis (17).
miR-92a, a member of the miR17-92 cluster, has been
reported to be highly expressed in ECs (18). miR-92a has also been reported to
serve a potential functional role in VSMCs by providing protection
against apoptosis induced by oxidative stress (19). However, little is known about the
role of miR-92a and its associated mechanism of action in the
phenotypic modulation of VSMCs. In the present study, miR-92a
targeting of KLF4 and promotion of VSMC proliferation were
evaluated.
Materials and methods
Aortic VSMC culture
Human aortic VSMCs (ZQ0491) were purchased from
Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd. The cells were
3rd generation cells and were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin in a
humidified atmosphere containing 5% CO2 at 37°C. Cells
were grown to 80–90% confluence and passaged at a ratio of 1:3. The
cells used in the experiments were passaged three to five times
before use. Kenpaullone (HY-12302) was used to treat VSMCs at a
concentration of 5 µM for 6 h.
Cell transfection
The miR-92a mimic, negative control (NC) mimic,
miR-92a inhibitor and NC inhibitor were purchased from Guangzhou
RiboBio Co., Ltd., and were transfected into cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Briefly, cells were
seeded in 6-well plates and transfected to ~80% confluence. The
related mimic or inhibitor (50 nM) were transfected into cells
using Lipofectamine 2000 in OPTI-MEM (Gibco; Thermo Fisher
Scientific, Inc.). The cells were cultured for 6–8 h in an
incubator at 37°C, then the transfection medium was discarded and
cells were cultured with normal medium for 24 h at 37°C before use.
The sequences of the miRNAs used were as follows: miR-92a mimic
forward (F), 5′-UAUUGCACUUGUCCCGGCCUGU-3′ and reverse (R),
5′-AGGCCGGGACAAGUGCAAUAUU-3′; mimics NC F,
5′-UUCUCCGAACGUGUCACGUTT-3′ and R, 5′-ACGUGACACGUUCGGAGAATT-3′;
miR-92a inhibitor, 5′-ACAGGCCGGGACAAGUGCAAU-3′; and miRNA inhibitor
NC, 5′-CAGUACUUUUGUGUAGUACAA−3′.
Patient sample collection and
inclusion criteria
The Second Affiliated Hospital of Jiaxing University
(Jiaxing, China) provided blood samples from patients with CAHD
after PCI. The details of the patients (age range, 45–79 years)
[ISR (n=20) and non-ISR (n=20)] are presented in Table I. The blood samples were frozen at
−80°C and stored until RNA extraction. Written informed consent was
provided by each patient. All experimental protocols were approved
by The Second Affiliated Hospital of Jiaxing University
(institutional review board protocol number: JXEY-2021JX083).
Patients who visited the Second Affiliated Hospital of Jiaxing
University between January 1, 2021, and June 31, 2022, were
included. The inclusion criteria were as follows: i) An age of
18–80 years; ii) patient received a DES during the first PCI; iii)
patient received chlorine treatment with 75 mg/day clopidogrel and
100 mg/day aspirin; iv) coronary angiography was followed up for
10–12 months; v) arterial embolism or acute myocardial infarction
were excluded in patients with complete occlusion; and vi) cardiac
function grades I–III. Exclusion criteria were as follows: i) Age
<18 or >80 years; ii) myocardial infarction during treatment;
iii) failure to take medication regularly for a long time (≥3
months); and iv) cardiac function grade IV. ISR was defined as
stenosis of ≥50% in the stent segment and ≥5 mm at the stent edge
during follow-up coronary angiography after PCI; non-ISR was
defined as <5 mm in the stent segment and <5 mm at the edge
of the stent during follow-up coronary angiography, with diameter
stenosis <50%.
 | Table I.Baseline characteristics of the study
subjects. |
Table I.
Baseline characteristics of the study
subjects.
| Characteristic | Non-ISR (n=20) | ISR (n=20) | P-value |
|---|
| Age,
yearsa | 61.75±8.43 | 66.32±8.42 | 0.099 |
| Sex (male), n | 15 | 15 | - |
| BMI,
kg/m2a | 23.55±1.43 | 23.94±1.28 | 0.374 |
| History of smoking,
n | 9 | 10 | - |
| Drinking history,
n | 6 | 7 | - |
| Diabetes (type 2),
n | 6 | 6 | - |
| Hypertension,
n | 11 | 12 | - |
| Hyperlipidemia,
n | 3 | 4 | - |
| TC,
mg/dla | 70.75±31.17 | 78.65±24.37 | 0.385 |
| TG,
mg/dla | 29.24±6.53 | 30.11±7.19 | 0.694 |
| HDL-C,
mg/dla | 19.66±6.06 | 21.02±5.40 | 0.465 |
| LDL-C,
mg/dla | 34.63±14.68 | 38.23±15.31 | 0.459 |
Western blotting
RIPA buffer containing protease inhibitors (Beyotime
Institute of Biotechnology) was used to extract the total protein
from cells. For each sample, 30 µg protein (measured with a
bicinchoninic acid protein assay kit) was added per lane, separated
on 10% gels using SDS-PAGE and transferred onto a 0.45-µm PVDF
membrane. After blocking with 5% non-fat milk at 37°C for 2 h, the
membrane was incubated separately at 4°C overnight with antibodies
as follows: SMMHC antibody (1:500; cat. no. 18569-1-AP), α-SMA
antibody (1:1,500; cat. no. 14395-1-AP), osteopontin (OPN)
polyclonal antibody (1:500 dilution; cat. no. 25715-1-AP) and GAPDH
polyclonal antibody (1:8,000, cat. no. HRP-60004) (all ProteinTech
Group, Inc.). The following day, the PVDF membrane was washed three
times with 0.1% Tween 20 (TBST) containing 5% bovine serum albumin
and incubated with goat anti-rabbit HRP (1:2,000; cat. no.
SAB90200; Frdbio Bioscience & Technology) and goat anti-mouse
HRP (1:8,000; cat. no. SAB90100, Frdbio Bioscience &
Technology) secondary antibodies for 2 h at room temperature. The
blots were visualized using ECL reagents (cat. no. P0018S; Beyotime
Institute of Biotechnology). The semi-quantitative analysis of
protein bands was performed using ImageJ software V1.8.0 (National
Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA from patients' blood was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcription was performed at 42°C for 2 min,
37°C for 15 min and 85°C for 5 sec to obtain the first strand of
complementary DNA using a PrimeScript® RT reagent kit
(Takara Biotechnology Co., Ltd.). qPCR was performed using 2X SYBR
Green Master Mix (cat. no. S2014; US Everbright Inc.). The
thermocycling conditions were as follows: 95°C for 30 sec, 15 sec
at 95°C and 60 sec at 60°C for 45 cycles. All mRNA and miRNA
expression levels were normalized to GAPDH or U6, respectively,
using the 2−∆∆Cq method (20). The primer sequences used were as
follows: α-SMA F, 3′-TGTTCCAGCCGTCCTTCATC-5′ and R,
3′-GGGAGCCAAAGCAGTGATCT-5′; SMMHC F, 3′-CGAAGGGCTTGAATGAGGAGT-5′
and R, 3′-GCTTCCTCCCAAGGAGCTGTAT-5′; OPN F,
3′-GAGGAAAAGGAGACCCTTCCA-5′ and R, 3′-TGAAAACTTCGGTTGCTGGC-5′; KLF4
F, 3′-ATGCTCACCCCACCTTCTTC-5′ and R, 3′-CTTCCCCTCTTTGGCTTGGG-5′;
miR-92a, 3′-TATATCTATTGCACTTGTCCCG-5′; miR-125b,
3′-TCCCTGAGACCCTAACTTGTGA-5′; miR-26a,
3′-GCCGAGTTCAAGTAATCCAGGA-5’; miR-214,
3′-TGCCTGTCTACACTTGCTGTGC-5′; miR-199a,
3′-CCCAGTGTTCAGACTACCTGTTC-5′; miR-9,
3′-GCCGAGTCTTTGGTTATCTAGCT-5′; miR-559,
3′-TCGGCAGGTAAAGTAAATATG-5′; miR-100, 3′-AACCCGTAGATCCGAACTTGTG-5′;
U6 F, 3′-CTCGCTTCGGCAGCACA-5′ and R, 3′-AACGCTTCACGAATTTGCGT-5′;
and GAPDH F, 3′-ACAGTCAGCCGCATCTTCTT-5′ and R,
3′-GACTCCGACCTTCACCTTCC-5′. The miR-125b, miR-26a, miR-214,
miR-199a, miR-559 and miR-100 downstream primers were universal
primers provided in a customized kit [General Biology (Anhui) Co.,
Ltd.].
Dual-luciferase reporter assay
The relative luciferase activity was detected using
a Dual-Luciferase Assay Kit (cat. no E190; Promega Corporation). A
bioinformatics analysis performed using TargetScan 7.1 (https://www.targetscan.org/vert_71/) demonstrated
that KLF4 contained an miR-92a binding site at the 3′-untranslated
region (3′-UTR). The mutant 3′-UTR of KLF4 was amplified using the
pGL3/Luc-KLF4 3′-UTR as a template and cloned downstream of the
pGL3/Luc vector (Shanghai GenePharma Co., Ltd.). VSMCs were seeded
into 96-well plates and co-transfected with either 100 nM miR-92a
mimic or NC and 2 µg pGL3/Luc-KLF4 3′-UTR or mutant 3′-UTR using
Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The relative luciferase
activity was determined 48 h later based on the ratio of the
luciferase activity of firefly luciferase to that of Renilla
luciferase. Luciferase intensity was assessed using the Dual
Luciferase Reporter 1000 Assay System (Promega Corporation).
MTT assay
VSMCs transfected with miR-92a mimic or inhibitor
were seeded into 96-well plates with an adjusted density of 7,000
cells per well and cultured at 37°C under 5% CO2 for 24,
48, 72 or 96 h. Next, MTT (MilliporeSigma) diluted in DMEM was
added to the medium, with a final concentration of 5 mg/ml in each
well for a further 4 h. The supernatant was carefully removed and
dimethyl sulfoxide (200 µl) was added to each well. The suspension
was placed in the dark for 2 h at room temperature, after which the
absorbance was quantified at 570 nm using an absorbance reader
(Thermo Fisher Scientific, Inc.).
Transwell migration assays
Cellular migration was assessed using Transwell
chambers with a pore size of 8 µm (Corning, Inc.). Briefly,
following transfection with an miR-92a mimic or miR-92a inhibitor,
1×105 VSMCs were added to the upper chamber in
serum-free DMEM medium and the low chamber was filled with culture
medium containing 10% FBS and incubated at 37°C. After 0, 24, 48,
72 or 96 h, the insert membranes were fixed using chilled methanol
and stained using 0.5% crystal violet at room temperature. The
number of invading cells was counted under an inverted light
microscope and imaged.
Statistical analysis
Results are presented as the mean ± standard
deviation or n data. Statistical analysis was performed using SPSS
version 13.0 (SPSS, Inc.) and GraphPad Prism 8.0 (GraphPad
Software, Inc.). ImageJ software 6.0 (National Institutes of
Health) was used to perform image analysis. The statistical
significance of the differences between groups was calculated using
one-way analysis of variance followed by Tukey's post hoc test, or
using a two-tailed unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Bioinformatics (http://starbase.sysu.edu.cn/index.php) was used to
analyze the relationship between miR-92a and KLF4. Pearson's
correlation was used to analyze the linear relationship between
miR-92a and KLF4. All experiments were repeated three times unless
otherwise stated.
Results
Association between miR-92a and KLF4
in patients with ISR
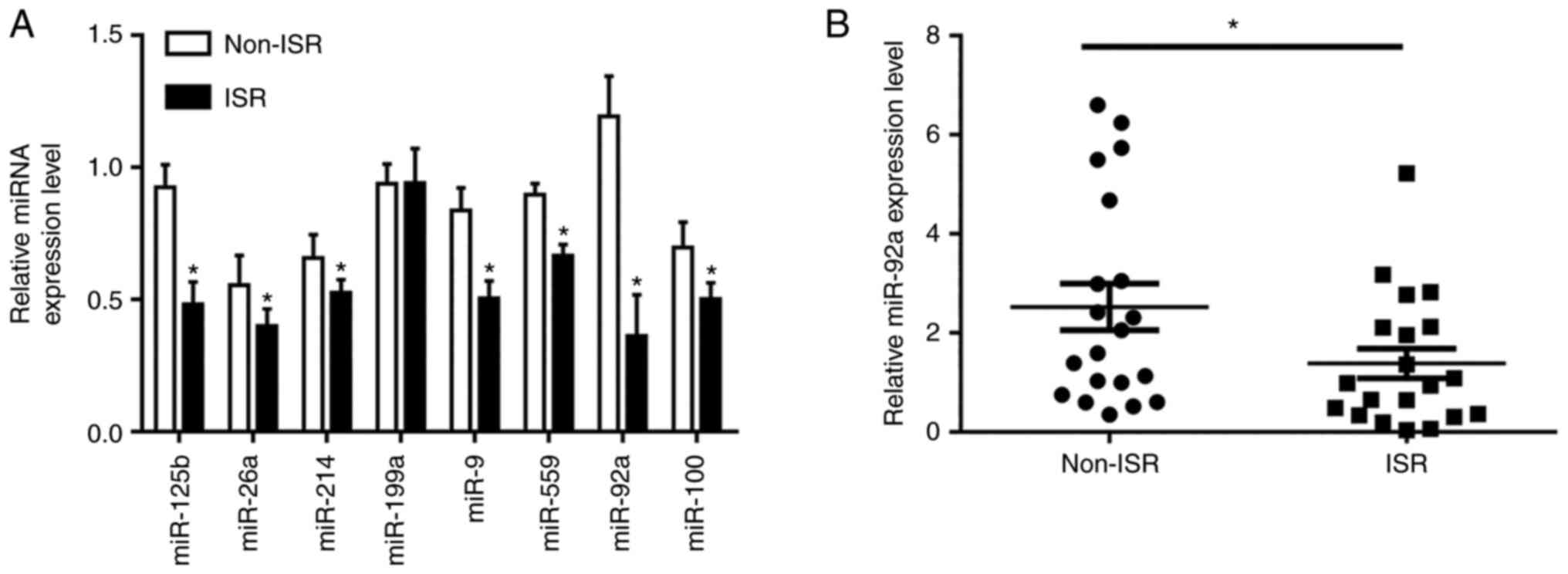
To assess the miRNA expression levels in ISR and
non-ISR patients, RT-qPCR was used to assess the expression levels
of certain serum miRNAs of interest in blood samples from ISR and
non-ISR patients. In the preliminary experiment, certain existing
miRNAs were used to assess the difference between ISR and non-ISR
groups, which demonstrated that the expression levels of miR-125b,
miR-26a, miR-214, miR-9, miR-559, miR92a and miR-100 were
significantly different. The results demonstrated that miR-92a was
the miRNA with the greatest difference between the ISR and non-ISR
groups (Fig. 1A); therefore, it
was used in subsequent experiments. Further experiments
demonstrated that the expression levels of miR-92a were
significantly lower in the patients with ISR (n=20) compared with
the control (non-ISR) patients (n=20) (Fig. 1B). However, as presented in
Table I, the comorbidities and
lipid level combined indices in non-ISR and ISR groups were not
significantly different. A schematic representation of the current
study is shown in Fig. S1. These
results indicated that miR-92a was downregulated in ISR.
miR-92a promotes KLF4 expression by
targeting its 3′-UTR
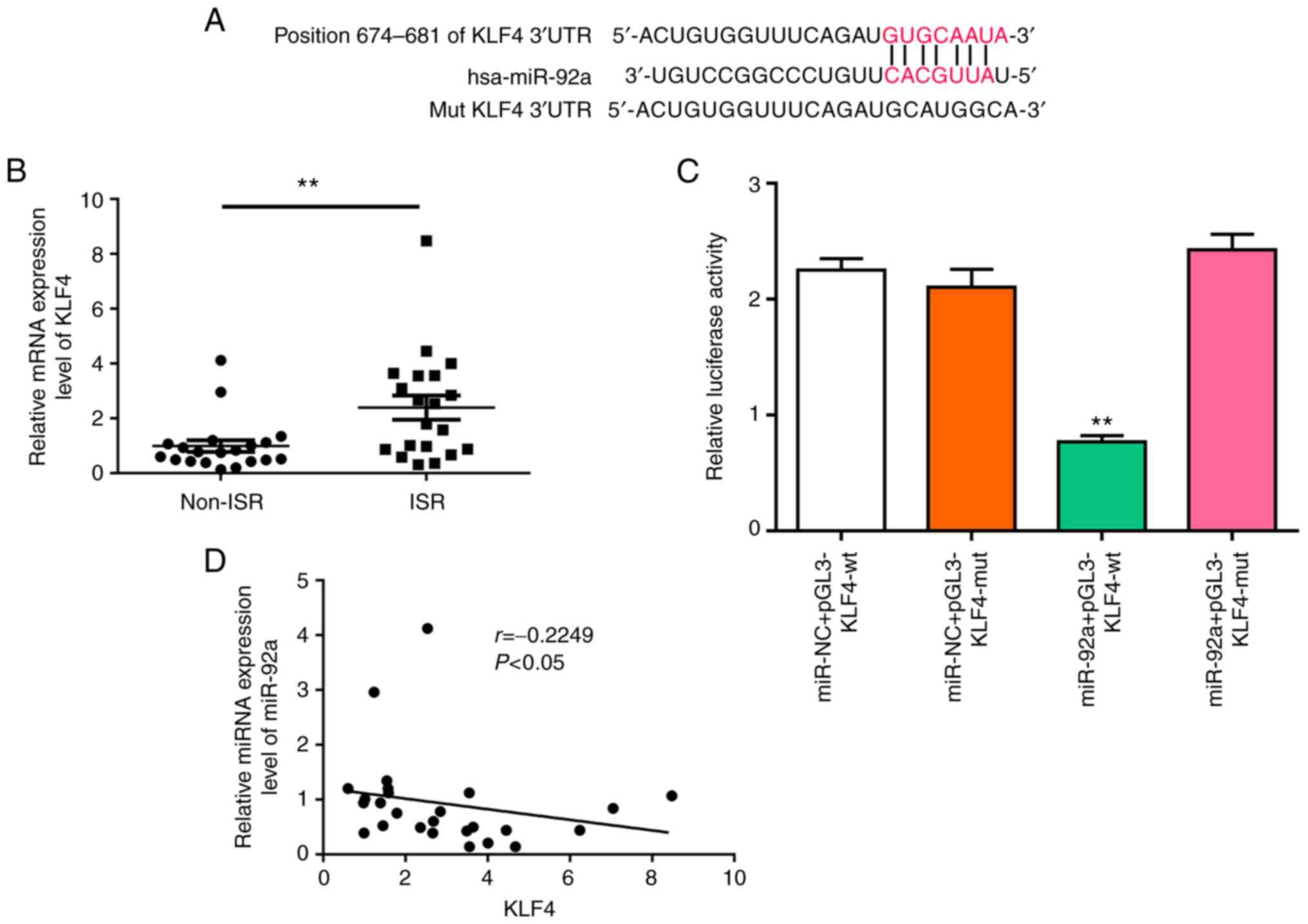
Previous studies have reported that KLF4 is an
antiproliferative regulator of VSMCs (21). In the present study, to elucidate
whether KLF4 was a direct target of miR-92a, a bioinformatics
approach was used to search for a potential miR-92a matching site
in the KLF4 3′-UTR (Fig. 2A). The
level of KLF4 was upregulated in the ISR group (Fig. 2B). A luciferase reporter assay was
used to determine the interaction between KLF4 and miR455-5p
(Fig. 2C). It was also
demonstrated that miR-92a levels were significantly inversely
associated with the mRNA expression level of KLF4 in patients with
ISR, as demonstrated using a Pearson's correlation model (Fig. 2D). This suggested that this miRNA
served an important role in the regulation of gene expression.
miR-92a contributes to cerebral VSMC
synthetic phenotype switching
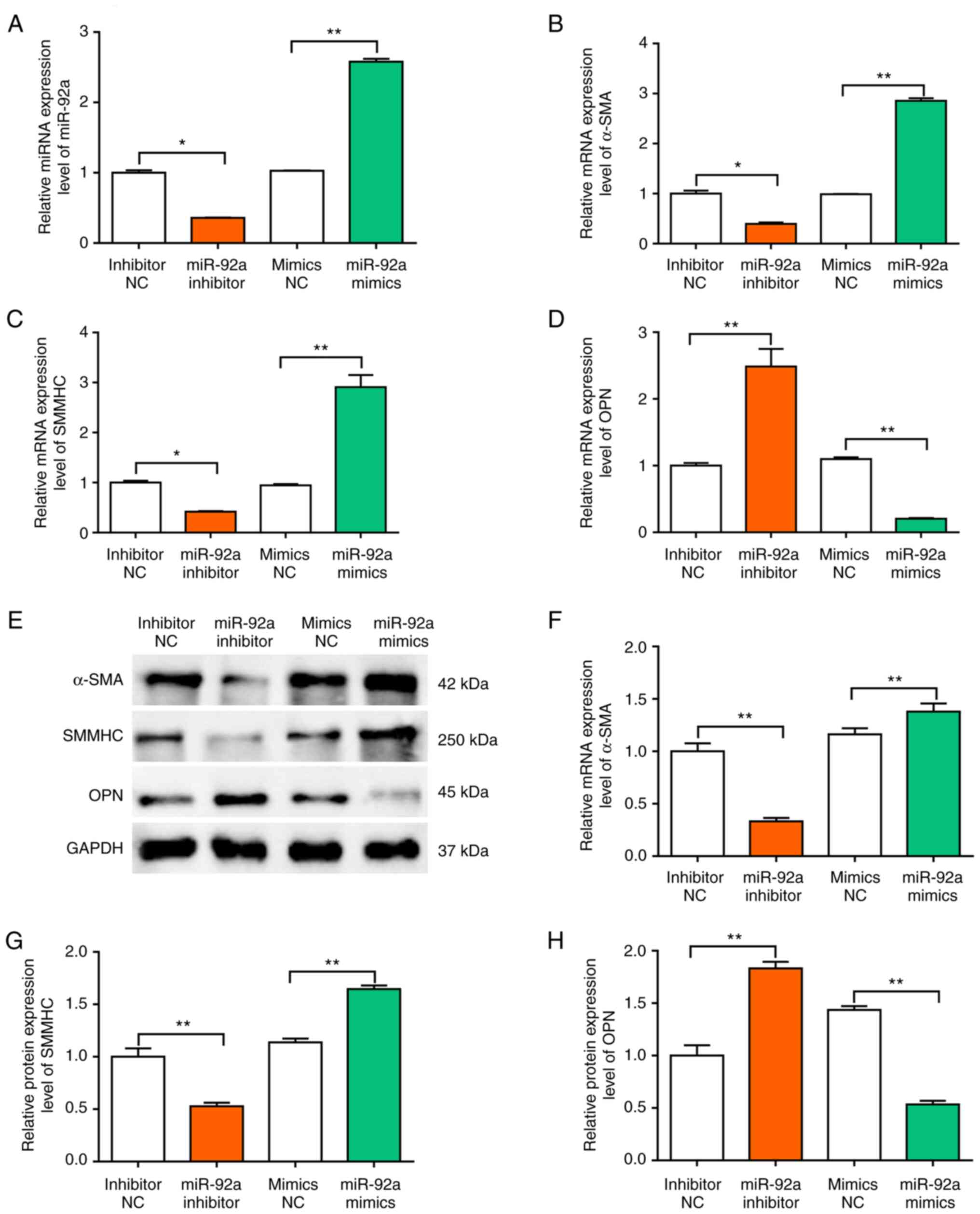
To evaluate whether miR-92a modulated the VSMC
phenotype, the mRNA and protein expression levels of contractile
and synthetic protein markers in cerebral VSMCs were assessed in
the present study. In both mature and normal blood vessels, VSMCs
possess a highly quiescent and contractile phenotype that is
associated with high levels of contractile marker proteins, such as
α-smooth muscle actin (α-SMA) and smooth muscle myosin heavy chain
(SMMHC) (22). In atherosclerosis
and arterial restenosis, VSMCs can change to a dedifferentiated,
proliferative and migratory phenotype via the downregulation of the
gene expression of VSMC contractile markers and via the
upregulation of OPN synthetic protein expression (23,24).
First, the level of miR-92a was confirmed after transfection with
miR-92a mimic or inhibitor (Fig.
3A). Compared with those in the NC group, the mRNA and protein
expression levels of α-SMA and SMMHC were significantly lower in
the miR-92a inhibitor group (Fig. 3B,
C and E-G). However, the mRNA and protein expression levels of
OPN were significantly higher in the miR-92a inhibitor group
compared with those in the NC (Fig. 3D
and H). Collectively, these results demonstrated that miR-92a
could promote VSMC phenotypic modulation.
miR-92a promotes VSMC phenotypic
modulation
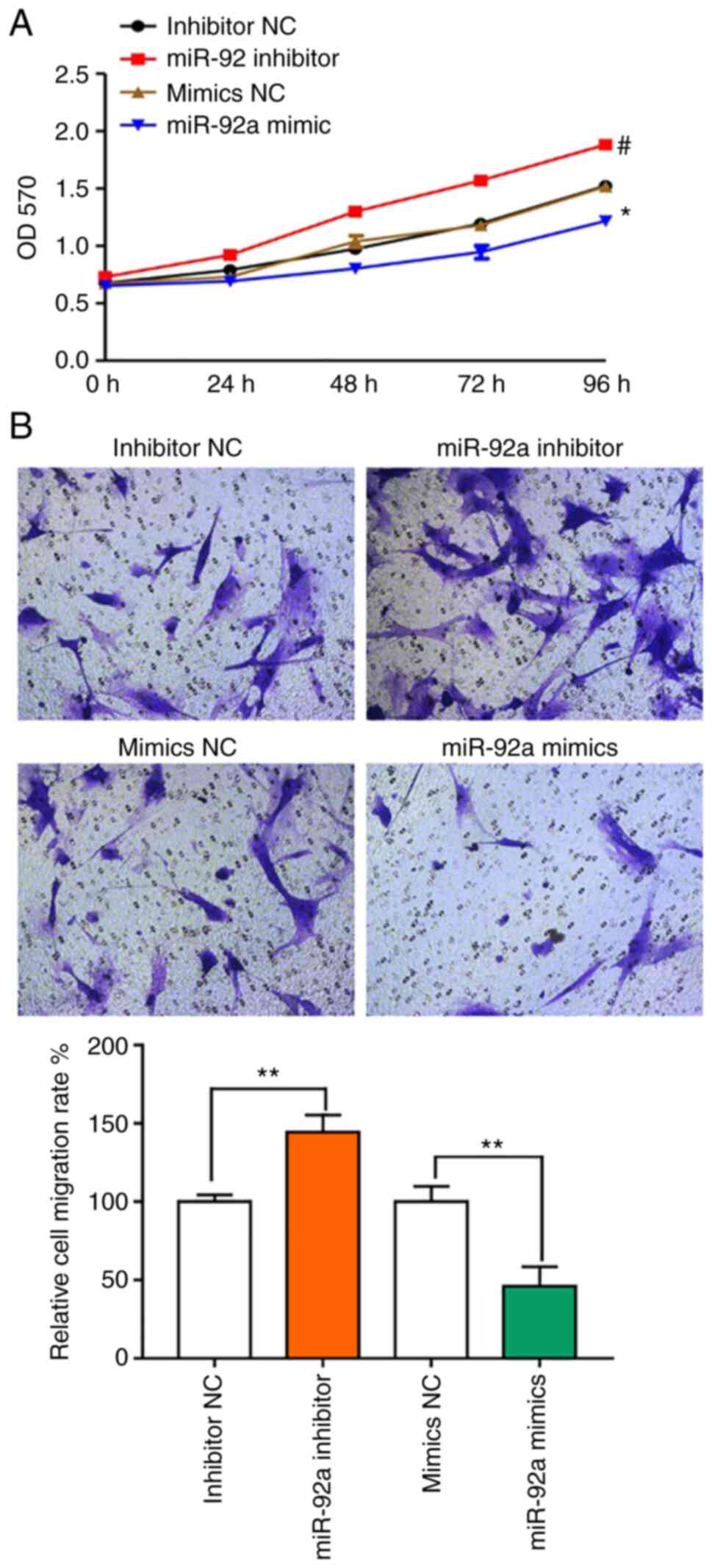
To evaluate the functional effects of miR-92a, VSMCs
were transfected with an miR-92a mimic or inhibitor for 24 h and
assessed using MTT and Transwell migration analysis. The results
demonstrated that compared with the NC, treatment with an miR-92a
inhibitor could significantly increase cell proliferation, whereas
the miR-92a mimic significantly reduced proliferation compared with
the NC (Fig. 4A). Furthermore, the
number of migrating cells was markedly increased after treatment
with the miR-92a inhibitor compared with the number in the NC group
(Fig. 4B).
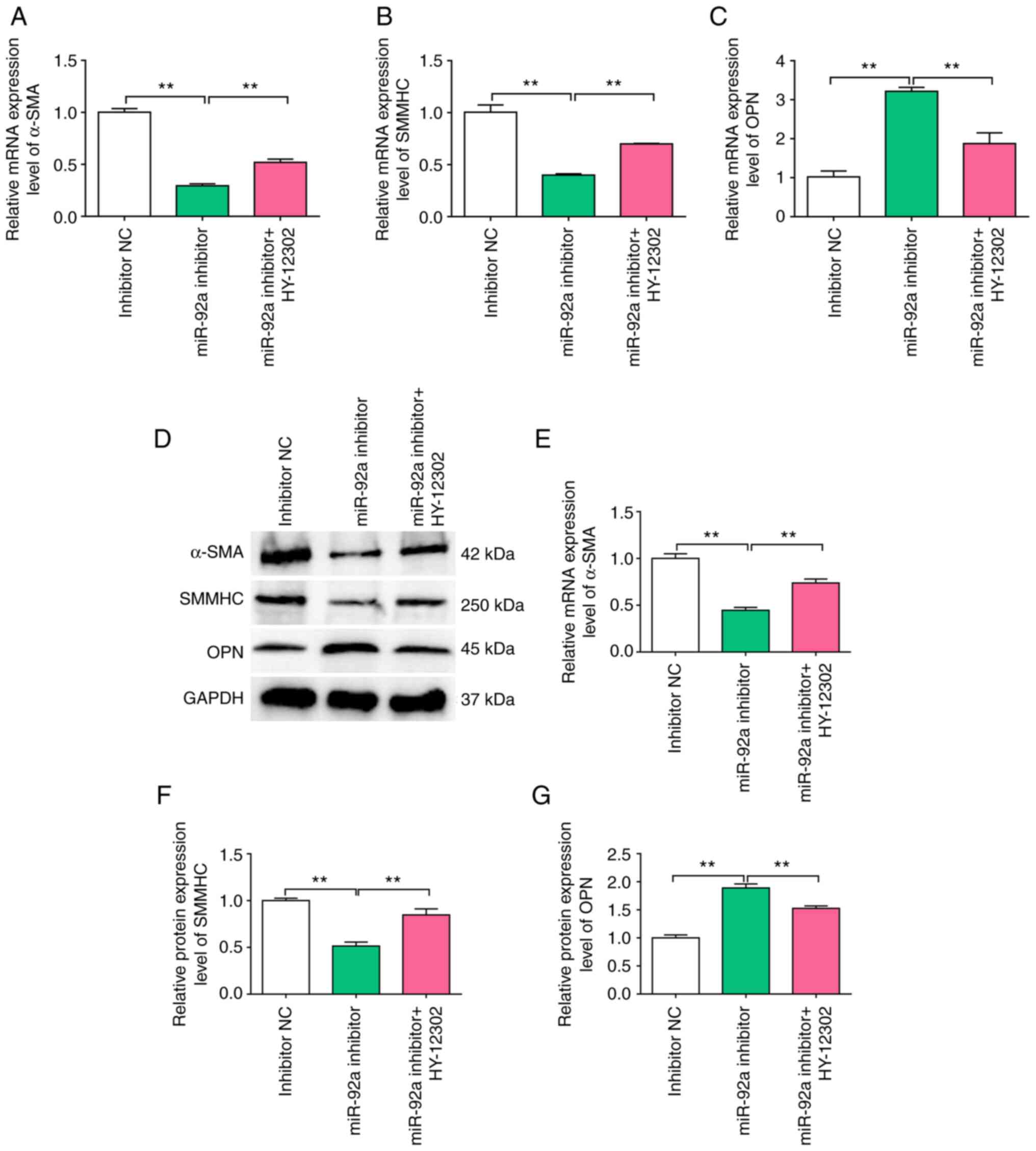
Kenpaullone (HY-12302) promotes
synthetic phenotype switching in cerebral VSMCs
We hypothesized that KLF4 may partially mediate the
effects of miR-92a on VSMCs. HY-12302 is a small molecule inhibitor
of KLF4, which is used in conjunction with miR-92a inhibitors in
functional assays involving VSMCs. In the present study, VSMCs
demonstrated significantly increased expression of the synthetic
gene, OPN, following transfection with an miR-92a inhibitor, and
significantly decreased expression of the contractile genes (α-SMA
and SMMHC) at both the mRNA (Fig.
5A-C) and protein (Fig. 5D-G)
expression levels compared with the NC. These results demonstrated
that treatment with the miR-92a inhibitor promoted a phenotypic
alteration in VSMCs from differentiated to de-differentiated cells.
Moreover, treatment with the KLF4 inhibitor, HY-12302,
significantly antagonized the de-differentiation response of VSMCs
to the miR-92a inhibitor at the mRNA and protein expression levels
(Fig. 5).
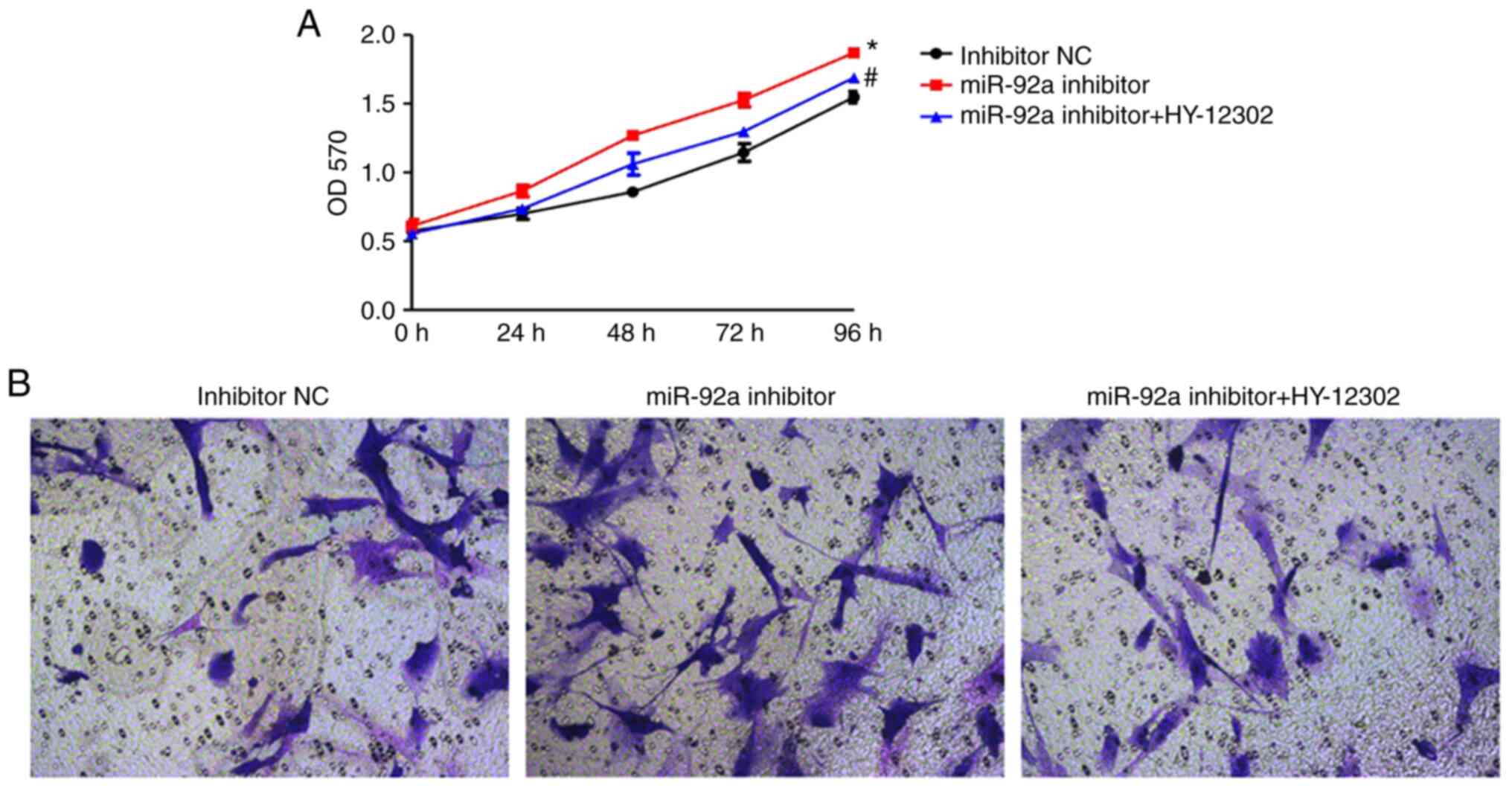
HY-12302 partially abrogates the
migration and proliferation of VSMCs
The MTT and Transwell migration assays were used to
evaluate the VSMC cell proliferation and migration capabilities of
each group. The KLF4 inhibitor, HY-12302, significantly abrogated
the accelerated cellular proliferation induced by treatment with
the miR-92a inhibitor. Furthermore, treatment with HY-12302
combined with the miR-92a inhibitor was demonstrated to markedly
reduce cellular migration compared with the miR-92a inhibitor group
(Fig. 6).
Discussion
Revascularization remains the cornerstone for the
management of patients with CAHD. Although PCI and metal scaffold
insertion, known as stenting, has become the preferred method for
the restoration of vessel patency, up to 30% of patients will
gradually experience a re-narrowing of the lumen caused by
neointima formation, which results in a condition known as ISR
(25). Descriptive histological
studies reported that ISR involved an influx of inflammatory cells
subsequent to the appearance of synthetic SMCs and myofibroblasts
that produced an abundant extracellular matrix (ECM) (26,27).
In response to vascular injury, growth factors and inflammatory
stimuli, VSMCs can switch from a quiescent ‘contractile’ phenotype
into an active ‘synthetic’ phenotype (28). Fully differentiated or mature VSMCs
have been reported to exhibit the contractile phenotype, with
characteristics of a very low rate of proliferation and the
expression of certain contractile proteins, such as SMMHC, α-SMA
and calponin (29). Moreover,
while the proteins are all necessary for contractile functionality,
upon vascular injury (such as caused by angioplasty or bypass
surgery), VSMCs can de-differentiate from the contractile phenotype
to a highly synthetic phenotype (30). This new phenotype is characterized
by higher proliferation and migration rates, enhanced ECM component
production and decreased VSMC-specific markers (31). Synthetic VSMCs migrate and
proliferate, and their accumulation over time leads to the
formation of the neointima (32,33).
As such, the inhibition of inflammation in VSMCs may represent an
important step in limiting vascular injury-triggered stenosis in a
clinical scenario. Consistent with this hypothesis, the present
study demonstrated significantly increased mRNA and protein
expression levels of VSMC synthetic proteins and proliferation in
the presence of miR-92a mimics. These results demonstrated that
miR-92a was a positive regulator of the VSMC synthetic phenotype
in vitro. The first limitation of the present study was that
certain existing miRNAs previously purchased by the research group
were used to assess the difference between ISR and non-ISR, and
that these 8 miRNA had a marked difference in ISR and non-ISR
samples; therefore, the effect of miR-92a in ISR was demonstrated
accidentally.
KLF4 serves an important role in a number of
vascular diseases and is expressed by a range of cell types
involved in vascular disease development, including VSMCs (34). One previous study reported KLF4 as
a transcriptional target that could modulate VSMC differentiation
(35). Although KLF4 is not
typically expressed in differentiated VSMCs in vivo, it is
transiently induced in VSMCs following vascular injury (36). The present study demonstrated that
the protein expression level of KLF4 expression was significantly
higher in ISR patients, whereas the levels of miR-92a expression
were significantly lower. Other information about the comorbidities
and the lipid level combined indices in non-ISR and ISR was
evaluated; however, there were no significant differences between
these two groups (Table I). Qiu
and Sun (37) reported that the
number of lesions, MALAT1 expression level, diabetes, N-terminal
pro-brain natriuretic peptide and high sensitivity C-reactive
protein were independent risk factors for ISR, in 95 patients with
coronary heart disease who presented to The Second Hospital of
Shandong University (Jinan, China); however, the data from the
present study only demonstrated that miR-92a and KLF4 expression
were associated with ISR. It could be hypothesized that this
difference may be due to the lack of different geographical cases.
Furthermore, the evaluation of more cases from different areas
could more comprehensively reflect the differences in relevant
indicators between ISR patients and non-ISR patients, which was the
second limitation of the present study.
The present study demonstrated that the miRNA
expression level of miR-92a was significantly negatively associated
with the KLF4 protein expression level. Collectively, the data from
the present study indicated that KLF4 was a direct target of
miR-92a. This suggested that specific mediation of the
VSMC-miR-92a-KLF4 pathway may represent an effective therapeutic
option by which ISR can be mitigated in the clinic. miRNAs are a
class of non-coding small RNA molecules that serve an important
role in regulating post-transcriptional gene expression. In the
majority of cases, miRNAs interact with the 3′-UTR of target mRNAs
to inhibit gene translation or induce mRNA degradation (38). Moreover, miR-92a has been reported
to be an oncogene in numerous different forms of tumors (39). It was also reported that miR-92a
was a negative regulator of endothelial function and angiogenesis
(40); however, data from the
present study demonstrated that miR-92a was a positive regulator of
the synthetic phenotype in VSMCs, functioning by modulation of
KLF4. The third limitation of the present study was that the
relevant disease model was not established in animal experiments
in vivo to further investigate the effect of the
miR-92A-KLF4 pathway on ISR treatment, which is something that
should be evaluated in the future.
In conclusion, the present study demonstrated that
the VSMC-miR-92a-KLF4 pathway could mediate the negative regulation
of VSMC differentiation in ISR. Since vascular injury induces KLF4
expression and activation, miR-92a overexpression or treatment with
a KLF4 inhibitor (HY-12302) could protect against injury-induced
VSMC polarization. These effects may be attributed to increased
contractile protein expression in VSMCs and the inhibition of
proliferation and migration. Given that the expression levels of
miR-92a were significantly reduced in ISR patients, the results of
the present study provide an important mechanistic foundation to
therapeutically target ISR using a KLF4 inhibitor.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiaxing Science and
Technology Project (grant nos. 2021AD30103, 2020AD30119 and
2021AD30090).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJ and LD conceptualized and planned the study. BZ,
XZ and RZ performed the experiments. QL, FS and JX analyzed the
data. LD wrote the manuscript. FJ and LD confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was provided by each
patient. All experimental protocols were approved by The Second
Affiliated Hospital of Jiaxing University (institutional review
board protocol number: JXEY-2021JX083).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malakar AK, Choudhury D, Halder B, Paul P,
Uddin A and Chakraborty S: A review on coronary artery disease, its
risk factors, and therapeutics. J Cell Physiol. 234:16812–16823.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faroux L, Guimaraes L, Wintzer-Wehekind J,
Junquera L, Ferreira-Neto AN, Del Val D, Muntané-Carol G, Mohammadi
S, Paradis JM and Rodés-Cabau J: Coronary artery disease and
transcatheter aortic valve replacement: JACC state-of-the-art
review. J Am Coll Cardiol. 74:362–372. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Lamee RK, Nowbar AN and Francis DP:
Percutaneous coronary intervention for stable coronary artery
disease. Heart. 105:11–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davis AA and Patel VG: The role of PD-L1
expression as a predictive biomarker: An analysis of all US food
and drug administration (FDA) approvals of immune checkpoint
inhibitors. J Immunother Cancer. 7:2782019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ali RM, Abdul Kader MASK, Wan Ahmad WA,
Ong TK, Liew HB, Omar AF, Mahmood Zuhdi AS, Nuruddin AA, Schnorr B
and Scheller B: Treatment of coronary drug-eluting stent restenosis
by a sirolimus- or paclitaxel-coated balloon. JACC Cardiovasc
Interv. 12:558–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lansky A, Grubman D and Scheller B:
Paclitaxel-coated balloons: A safe alternative to drug-eluting
stents for coronary in-stent restenosis. Eur Heart J. 41:3729–3731.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ullrich H, Olschewski M, Münzel T and Gori
T: Coronary in-stent restenosis: Predictors and treatment. Dtsch
Arztebl Int. 118:637–644. 2021.PubMed/NCBI
|
|
8
|
Huynh DTN and Heo KS: Role of
mitochondrial dynamics and mitophagy of vascular smooth muscle cell
proliferation and migration in progression of atherosclerosis. Arch
Pharm Res. 44:1051–1061. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Majesky MW: Vascular development.
Arterioscler Thromb Vasc Biol. 38:e17–e24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Touyz RM, Alves-Lopes R, Rios FJ, Camargo
LL, Anagnostopoulou A, Arner A and Montezano AC: Vascular smooth
muscle contraction in hypertension. Cardiovasc Res. 114:529–539.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Nanda V, Direnzo D, Ye J, Xiao S,
Kojima Y, Howe KL, Jarr KU, Flores AM, Tsantilas P, et al: Clonally
expanding smooth muscle cells promote atherosclerosis by escaping
efferocytosis and activating the complement cascade. Proc Natl Acad
Sci USA. 117:15818–15826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen MF: The role of calmodulin and
calmodulin-dependent protein kinases in the pathogenesis of
atherosclerosis. Tzu Chi Med J. 34:160–168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Y, Lu H, Liang W, Hu W, Zhang J and
Chen YE: Krüppel-like factors and vascular wall homeostasis. J Mol
Cell Biol. 9:352–363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Xu H, Lyu W, Xu Q, Fan S, Chen H,
Wang D, Chen J and Dai J: KLF4 regulates TERT expression in
alveolar epithelial cells in pulmonary fibrosis. Cell Death Dis.
13:4352022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rane MJ, Zhao Y and Cai L: Krϋppel-like
factors (KLFs) in renal physiology and disease. EBioMedicine.
40:743–750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YZ, Wen L, Wei X, Wang QR, Xu LW, Zhang
HM and Liu WC: Inhibition of miR-7 promotes angiogenesis in human
umbilical vein endothelial cells by upregulating VEGF via KLF4.
Oncol Rep. 36:1569–1575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du M, Espinosa-Diez C, Liu M, Ahmed IA,
Mahan S, Wei J, Handen AL, Chan SY and Gomez D: miRNA/mRNA
co-profiling identifies the miR-200 family as a central regulator
of SMC quiescence. iScience. 25:1041692022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sand M, Hessam S, Amur S, Skrygan M,
Bromba M, Stockfleth E, Gambichler T and Bechara FG: Expression of
oncogenic miR-17-92 and tumor suppressive miR-143-145 clusters in
basal cell carcinoma and cutaneous squamous cell carcinoma. J
Dermatol Sci. 86:142–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Zhou M, Wang Y, Huang W, Qin G,
Weintraub NL and Tang Y: miR-92a inhibits vascular smooth muscle
cell apoptosis: Role of the MKK4-JNK pathway. Apoptosis.
19:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Chen F, Guo R, Jia S, Li W and Zhang
B: Tanshinone IIA inhibits homocysteine-induced proliferation of
vascular smooth muscle cells via miR-145/CD40 signaling. Biochem
Biophys Res Commun. 522:157–163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang HX, Qin XP and Li J: Role of the
signal transducer and activator of transcription 3 protein in the
proliferation of vascular smooth muscle cells. Vascular.
28:821–828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in atherosclerosis. Circ Res. 118:692–702.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY,
Liao X and Sun HJ: Chicoric acid prevents PDGF-BB-induced VSMC
dedifferentiation, proliferation and migration by suppressing
ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 14:656–668.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura Y, Izumiya Y, Araki S, Yamamura S,
Hanatani S, Onoue Y, Ishida T, Arima Y, Nakamura T, Yamamoto E, et
al: Sirt7 deficiency attenuates neointimal formation following
vascular injury by modulating vascular smooth muscle cell
proliferation. Circ J. 85:2232–2240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang DS, Ganaha F, Kao EY, Lee J, Elkins
CJ, Waugh JM and Dake MD: Local stent-based release of transforming
growth factor-β1 limits arterial in-stent restenosis. J Lab Autom.
21:305–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osherov AB, Gotha L, Cheema AN, Qiang B
and Strauss BH: Proteins mediating collagen biosynthesis and
accumulation in arterial repair: Novel targets for anti-restenosis
therapy. Cardiovasc Res. 91:16–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sorokin V, Vickneson K, Kofidis T, Woo CC,
Lin XY, Foo R and Shanahan CM: Role of vascular smooth muscle cell
plasticity and interactions in vessel wall inflammation. Front
Immunol. 11:5994152020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan D, Liu G, Li B, Jiang J, Chen W, Li W,
Zhang L, Hu Y, Xie S and Yang H: MicroRNA-1246 regulates
proliferation, invasion, and differentiation in human vascular
smooth muscle cells by targeting cystic fibrosis transmembrane
conductance regulator (CFTR). Pflugers Arch. 473:231–240. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu M, Liu W, Huang H, Chen Z, Chen Y,
Zhong Y, Jin Z, Liu X and Zou L: PVT1/miR-145-5p/HK2 modulates
vascular smooth muscle cells phenotype switch via glycolysis: The
new perspective on the spiral artery remodeling. Placenta.
130:25–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi J, Yang Y, Cheng A, Xu G and He F:
Metabolism of vascular smooth muscle cells in vascular diseases. Am
J Physiol Heart Circ Physiol. 319:H613–H631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu JH, Zhou YF, Hong CD, Chen AQ, Luo Y,
Mao L, Xia YP, He QW, Jin HJ, Huang M, et al: Semaphorin-3A
protects against neointimal hyperplasia after vascular injury.
EBioMedicine. 39:95–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu W, Zhang W, Choi M, Zhao J, Gao P, Xue
M, Singer HA, Jourd'heuil D and Long X: Vascular smooth
muscle-MAPK14 is required for neointimal hyperplasia by suppressing
VSMC differentiation and inducing proliferation and inflammation.
Redox Biol. 22:1011372019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wen Y, Lu X, Ren J, Privratsky JR, Yang B,
Rudemiller NP, Zhang J, Griffiths R, Jain MK, Nedospasov SA, et al:
KLF4 in macrophages attenuates TNFα-mediated kidney injury and
fibrosis. J Am Soc Nephrol. 30:1925–1938. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yap C, Mieremet A, de Vries CJM, Micha D
and de Waard V: Six shades of vascular smooth muscle cells
illuminated by KLF4 (Krüppel-like factor 4). Arterioscler Thromb
Vasc Biol. 41:2693–2707. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghaleb AM and Yang VW: Krüppel-like factor
4 (KLF4): What we currently know. Gene. 611:27–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu S and Sun J: lncRNA-MALAT1 expression
in patients with coronary atherosclerosis and its predictive value
for in-stent restenosis. Exp Ther Med. 20:1292020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9:4022018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li M, Peng J, Shi Y and Sun P: miR-92a
promotes progesterone resistance in endometriosis through PTEN/AKT
pathway. Life Sci. 242:1171902020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu PJ, Ye YX, Wang YX, Du JX, Pan YH and
Fang XB: MiRNA-92a promotes cell proliferation and invasion through
binding to KLF4 in glioma. Eur Rev Med Pharmacol Sci. 23:6612–6620.
2019.PubMed/NCBI
|