Introduction
Obesity is a major risk factor for type 2 diabetes,
metabolic syndrome, cancer, and cardiovascular diseases (1–3). At
the cellular level, obesity is characterized by an increase in the
number and size of adipocytes differentiated from pre-adipocytes in
adipose tissues. In addition, adipose tissues regulate energy
homeostasis. An excessive accumulation of adipose tissue results
from increased adipogenesis and adipocyte differentiation, leading
to the conversion of pre-adipocytes into adipocytes. Adipogenesis
and the differentiation of pre-adipocytes into adipocytes are
regulated by the expression and/or activation of
adipogenesis/lipolysis-related factors (4,5).
These pre-adipocytes are used to study the molecular mechanisms of
adipogenesis and lipogenesis. Mouse fibroblast 3T3-L1 cells are an
established model for obesity research (6). Many studies using 3T3-L1 cells have
demonstrated that various compounds suppress cell differentiation
into adipocytes and downregulate CCAAT enhancer-binding protein α
(C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ).
The action of these adipogenesis-related factors during the
differentiation of 3T3-L1 cells into adipocytes results in the
suppression of lipid droplet accumulation in these cells (7,8).
Raspberry ketone (4-(4-hydroxyphenyl)-2-butanone;
RK) is one of the major natural phenolic ketone compounds present
in European red raspberry (Rubus idaeus L.) (9,10),
and may possess lipolytic and anti-obesity effects (Fig. 1). Several studies in mice have
reported that RK prevents increases in body weight and the weight
of the liver and visceral adipose tissues (epididymal,
retroperitoneal, and mesenteric) induced by a high-fat diet
(11–13). Rhododendrol
(4-(4-hydroxyphenyl)-2-butanol; ROH) is present in Betula
platyphylla and Acer nikoense Maximowicz (14,15).
When administered orally to mammals, RK is metabolized via several
pathways (16). ROH, formed by the
reduction of the ketone group of RK, is excreted as a major
metabolite after a single oral administration in rats, guinea pigs,
and rabbits (17), suggesting that
RK is reductively metabolized in the small intestine or liver
(first-pass effect) (Fig. 1).
Carbonyl compounds, such as aldehydes and ketones,
are converted into their corresponding alcohol metabolites in
vivo. Interestingly, many xenobiotic carbonyl compounds are
metabolized into active reductive metabolites. For example,
loxoprofen sodium, an anti-inflammatory drug, is reduced to its
active metabolite (18).
Sennoside, a natural product, is reduced to rhein anthrone, an
active metabolite produced by intestinal bacteria in mice (19). We also found that ketone-containing
medicines, such as metyrapone, acetohexamide, and befunolol, were
reduced to their active metabolites in mammals (20–23).
Recently, Zhao et al reported that RK is rapidly absorbed
and metabolized in mice. They also found that the total
bioavailability (AUC0-12 h) of RK and its metabolites,
including ROH, as well as their accumulation in white adipose
tissue, were higher in obese mice than in control mice when RK was
administered orally (24). This
suggests that the metabolism of RK, including its reduction, may
differ depending on the pathophysiological stage of transition from
non-obesity to obesity. These experimental results imply that the
anti-obesity effect of RK may be an additive effect of ROH, as RK
is easily eliminated by the mammalian body. However, no information
currently exists on the reductive metabolism of RK in humans,
taking it as a supplement, or on the anti-obesity effect of ROH.
Therefore, it is important to examine unmetabolized RK as well as
RK metabolism and the anti-obesity effects of its metabolites.
In this study, we clarified that RK is reductively
metabolized to ROH in humans and investigated whether ROH and RK
have anti-obesity effects in 3T3-L1 cells. We also investigated the
effects of ROH on the expression of C/EBPα, PPARγ, and
adipogenesis-related genes.
Materials and methods
Chemicals
RK and isoproterenol were obtained from Tokyo
Chemical Industry Co. Ltd. (Tokyo, Japan), and
3-Isobutyl-1-methylxanthine was purchased from FUJIFILM Wako Pure
Chemical Co. (Tokyo, Japan). Insulin, dexamethasone, Dulbecco's
modified Eagle's medium (DMEM), and a penicillin/streptomycin
solution were purchased from Sigma-Aldrich (St. Louis, MO, USA). A
glycerol assay kit (Cat. ab133130) was purchased from Abcam
(Cambridge, UK). A pool of 150-donor mixed-gender human liver
microsomes (Cat. 45215) and cytosol (Cat. 452117) was obtained from
Corning Gentest (Corning, NY, USA). A CytoTox 96 non-radioactive
cytotoxicity assay kit (Cat. G1780) was purchased from Promega
(Madison, WI, USA). A lipid assay kit (Cat. AK09F) was purchased
from Cosmo Bio Co. Ltd. (Tokyo, Japan). The reduced form of
β-nicotinamide adenine dinucleotide phosphate (NADPH) was purchased
from Oriental Yeast Co. Ltd. (Tokyo, Japan). HyClone™ fetal bovine
serum (FBS) was purchased from Cytiva (Tokyo, Japan). Monoclonal
antibodies against mouse C/EBPα (Cat. #8187) and horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Cell Signaling Technology (Danvers, MA, USA). Monoclonal
antibodies against mouse PPARγ (Cat. sc-7273) were purchased from
Santa Cruz Biotechnology Inc. (Dallas, TX, USA). HRP-conjugated
anti-mouse β-actin antibody (Cat. A3854) was purchased from
Sigma-Aldrich.
Procedure for synthesis of ROH. All commercial
chemicals and solvents were of reagent grade and used without
further purification. ROH was synthesized according to the method
described by Kitayama et al (25). A mixture of RK (12 mmol) and sodium
borohydride (48 mmol) in methanol (50 ml) was stirred at 22°C for 3
h. The reaction progress was monitored by thin-layer chromatography
(TLC) using commercially prepared silica gel 60 F254
glass-backed plates. After the solvent had evaporated, the residue
was added to 10% hydrochloric acid (50 ml). The mixture was
extracted three times with ethyl acetate (50 ml). The organic layer
was washed with H2O and brine, dried over anhydrous
MgSO4, and evaporated under reduced pressure. The
residue was purified using column chromatography on silica gel
(n-hexane/ethyl acetate). ROH was obtained as a white powder
after recrystallization from n-hexane/ethyl acetate with a
yield of 99%. The melting point of the purified ROH was determined
using a Yanagimoto micromelting point apparatus (ANATEC YANACO Co.,
Kyoto, Japan). Infrared (IR) spectra were recorded using an
FTIR-8400S spectrometer (Shimadzu Co., Kyoto, Japan). The
1H nuclear magnetic resonance (1H NMR)
spectrum was obtained using a JNM-ECA500 spectrometer (JEOL Ltd.,
Tokyo, Japan). Proton chemical shifts were referenced to a
tetramethylsilane internal standard. The J values are given
in hertz. High-resolution mass spectrometry (HRMS) was performed
using a JMS-T100GCv spectrometer (JEOL Ltd.). Elemental analysis
was performed using a CE-440 CHN/O/S elemental analyzer (Exeter
Analytical Inc., MA, USA), and the results were within ± 0.3% of
the theoretical values: m.p. 71–72°C. IR (KBr) cm−1:
3350, 3036 (OH). 1H NMR (500 MHz,
CDCl3-d) δ: 7.01 (d, 2H, J=8.6 Hz,
ArH), 6.73 (d, 2H, J=8.6 Hz, ArH), 3.81 (m,
1H, -CH(OH)-), 2.97 (brs, 1H, -CH(OH)-), 2.60 (m, 2H,
-CH2CH2-), 1.72 (m, 2H,
-CH2CH2-), 1.21 (d, 3H, J=6.3 Hz,
-CH3). HRMS (EI) m/z: [M]+ Calcd for
C10H14O2 166.0994; Found 166.0991.
Anal. Calcd for C10H14O2: C,
72.26; H, 8.49; O, 19.25. Found: C, 72. 21; H, 8. 58; O, 19.
21.
Assay for RK-reductase activity
The reaction mixture consisted of 1.0 mM RK, 10 mM
NADPH and 0.2 mg protein/ml human pooled liver microsomes or
cytosol in 0.1 M potassium/sodium-phosphate buffer (pH 7.4) at a
final volume of 2 ml. The reaction was performed at 37°C for 20
min. After incubation, the mixture was extracted twice with 5 ml
ethyl acetate containing 1 µM ethyl 4-hydroxybenzoate (an internal
standard). The extraction mixture was centrifuged, and the organic
layer was collected and evaporated to dryness. The residue was
dissolved in 0.2 ml acetonitrile, and a 20 µl sample was subjected
to high-performance liquid chromatography (HPLC) analysis using a
GL-7450 Hitachi chromatograph equipped with a CAPCELLPAK C8 UG120
column (Shiseido Co., Ltd., Tokyo, Japan) and 5 µm (4.6×250 mm).
The mobile phase was acetonitrile:0.1% acetic acid (3:7). The
chromatograph was operated at a flow rate of 1 ml/min at 40°C, with
detection at 280 nm. The amount of ROH formed was determined from
the peak areas. The RK reductive activity was expressed as ROH
nmol/min/mg protein.
3T3-L1 cell culture and
differentiation
Mouse 3T3-L1 pre-adipocytes were obtained from the
Japanese Collection of Research Resources Cell Bank (Osaka, Japan).
The 3T3-L1 pre-adipocytes were cultured and maintained in DMEM
containing 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin
(10% FBS-DMEM) (maintenance Medium; MM) at 37°C in 5%
CO2. The cells were seeded in 24-well plates at a
density of 5×104 cells/well and cultured in MM for 2
days until they reached semi-confluence. Differentiation to
adipocytes was initiated by replacing the previous medium with MM
containing 0.5 mM 3-isobutyl-1-methylxanthine, 1 µM dexamethasone,
and 1 mg/ml insulin (differentiation medium; DM) (day 0) and
incubating for 48 h (days 0–2). The DM was then replaced with MM
containing 1 mg/ml insulin (adipocyte maintenance medium; AMM) and
incubated for 48 h (days 2–4). The cells were then maintained, and
the MM was replaced every 2 days until day 8 (days 4–8). The
pre-adipocytes and adipocytes were incubated with DM, AMM, or MM in
the absence or presence of ROH or RK (20–100 µM) from days 0 to 8.
The cells differentiated by incubation with DM/AMM/MM without ROH
or RK on day 8 were used as mature adipocytes.
Cell viability assay
The cytotoxic effects of ROH and RK on 3T3-L1 cells
were estimated using a CytoTox 96 non-radioactive cytotoxicity
assay kit. Briefly, cells were seeded in 24-well plates at a
density of 5×104 cells/well. After 2 days, the cells
were incubated with 10% FBS-DMEM in the absence or presence of ROH
or RK (10–200 µM). After treatment for 24 h, lactate dehydrogenase
(LDH) concentrations in the cell lysates were measured using the
CytoTox 96 non-radioactive cytotoxicity assay kit following the
manufacturer's instructions.
Oil Red O staining
Following differentiation, the culture medium was
removed, and the cells were washed with phosphate buffered saline
(−) [PBS (−)]. Oil Red O staining was performed using a lipid assay
kit. Briefly, the cells were fixed with 10% formalin for 15 min at
22°C and then stained with Oil Red O-staining solution (60%
isopropanol solution). This solution was prepared by diluting the
Oil Red O stock solution to 60% with distilled water for 30 min at
22°C. The cells were washed with 60% isopropanol and distilled
water. Images were captured using an Olympus IX71 inverted
microscope (Olympus Co., Tokyo, Japan). The stained lipid droplets
were dissolved in isopropanol and quantified by spectrophotometry
at a wavelength of 540 nm.
Glycerol release assay
Mature 3T3-L1 adipocytes were incubated with DMEM
containing 2% (w/v) fatty acid-free bovine serum albumin in the
absence or presence of various concentrations of RK or ROH (20–100
µM). After incubation for 24 h, the culture medium was collected,
and glycerol release activity was measured using a glycerol assay
kit (Abcam PLC, Cambridge, UK) according to the manufacturer's
instructions.
RNA isolation and quantitative
polymerase chain reaction (qPCR)
Total mRNA was isolated using ISOGEN II (Nippon Gene
Co. Ltd., Tokyo, Japan) following the manufacturer's instructions.
RNA was quantified using a Multiskan Sky High Microplate
Spectrophotometer with a µdrop plate (Thermo Fisher Scientific
Inc., Waltham, MA, USA), and 1 µg of RNA was reverse-transcribed
using the ReverTra Ace qPCR RT primary mix (Toyobo Co., Ltd.,
Osaka, Japan). PCR amplification of mouse leptin, C/EBPα, PPARγ,
and β-actin was performed using Thunderbird SYBR qPCR Mix (Toyobo
Co., Ltd., Osaka, Japan) and a StepOne™ real-time PCR system
(Thermo Fisher Scientific Inc., Waltham, MA, USA). The following
thermocycling conditions for qPCR were used: initial denaturation
at 95°C for 60 sec; and 40 cycles of denaturation at 95°C for 15
sec, and annealing/extension at 60°C for 60 sec. The primer
sequences used for PCR were as follows: leptin forward
5′-CAGGATCAATGACATTTCACACA-3′; leptin reverse
5′-GCTGGTGAGGACCTGTTGAT-3′; C/EBPα forward
5′-GCAGGAGGAAGATACAGGAAG-3′; C/EBPα reverse
5′-ACAGACTCAAATCCCCAACA-3′; PPARγ forward
5′-GTGCTCCAGAAGATGACAGAC-3′; PPARγ reverse
5′-GGTGGGACTTTCCTGCTAA-3′; β-actin forward
5′-TGGAATCCTGTGGCATCCATGAAAC-3′, and β-actin reverse
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. C/EBPα and PPARγ mRNA levels were
calculated using the 2−ΔΔCq method and normalized
against the expression level of β-actin as an internal standard
(26). All quantifications were
performed independently three times.
Western blot analysis
The mature 3T3-L1 adipocytes were collected on day
8, washed twice with PBS (−), and lysed in radioimmunoprecipitation
assay (RIPA) buffer (50 mM Tris-HCl, pH 8.0, 150 mM sodium
chloride, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium
dodecyl sulfate) containing protease inhibitor cocktail set I
(FUJIFILM Wako Pure Chemical Co., Tokyo, Japan) on ice for 10 min.
Cell lysates were centrifuged at 12,000 × g for 15 min at 4°C. The
supernatant was collected from the lysates and protein
concentrations were determined using the bicinchoninic acid (BCA)
method, with bovine serum albumin as the standard (27). Subsequently, 20 µg protein per lane
was separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred to a polyvinylidene difluoride membrane (Millipore
Sigma, Bedford, MA, USA). The membranes were incubated with
Tris-buffered saline (20 mM Tris-HCl, pH 7.4, 150 mM sodium
chloride) containing 0.05% Tween 20 and 2% skim milk as blocking
solution for 2 h at 22°C. Membranes were then incubated with the
indicated primary antibodies (mouse monoclonal anti-mouse C/EBPα,
1:2,000; rabbit monoclonal anti-mouse PPARγ, 1:2,000;
HRP-conjugated anti-mouse β-actin, 1:200,000) for 2 h at 22°C,
further incubated with the HRP-conjugated secondary antibodies
(1:5,000) for 1 h at 22°C and visualized using an enhanced
ImmunoStar LD or ImmunoStar Zeta (both FUJIFILM Wako Pure Chemical
Co.) with a LuminoGraph I (ATTO, Tokyo, Japan). Densitometric
analysis was performed using the CS Analyzer 4 software (ATTO,
Tokyo, Japan) and normalized against the expression level of
β-actin as an internal standard.
Data analyses and statistics
All data are expressed as mean ± standard deviation
of the mean (SD). Statistical analyses were performed by using the
unpaired Student's t-test or one-way ANOVA followed by
Dunnett's post hoc test for multiple group comparisons.
Calculations were performed using R (R Development Core Team).
Statistical significance was set at P<0.05.
Results
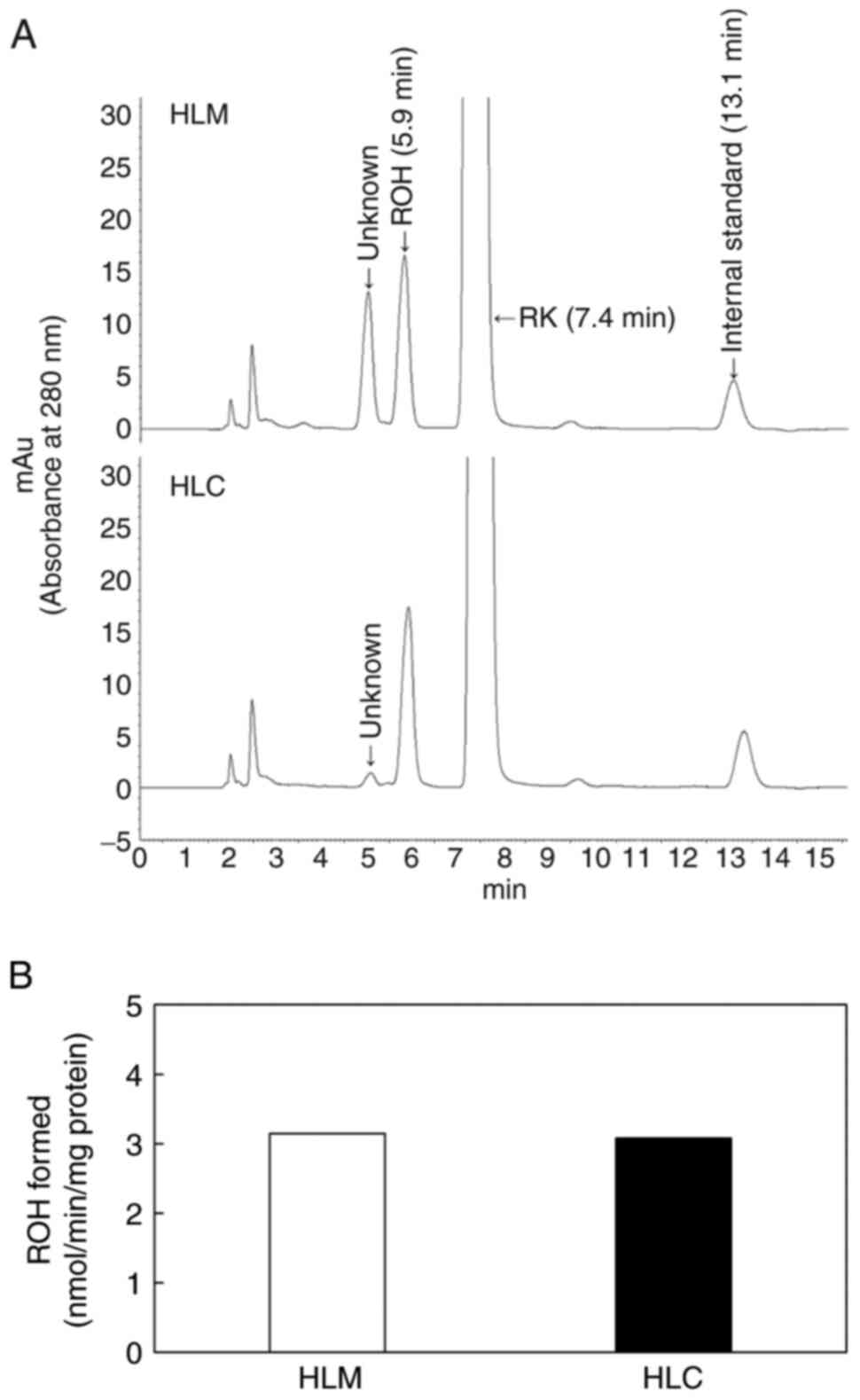
RK reduction to ROH in human liver
microsomes or cytosol
To elucidate the metabolism of RK in humans, we
examined the reduction of RK to ROH in human liver microsomes and
cytosol. RK was incubated with human liver microsomes or the
cytosol in the presence of NADPH. An HPLC chromatogram of the
extract from the incubation mixtures containing liver microsomes
revealed two peaks with retention times of 5.0 and 5.9 min, which
differed from those of RK (7.4 min) and the internal standard (13.1
min). However, the peak at 5.0 min was slight in the incubation
mixtures containing human liver cytosol (Fig. 2A). When RK was incubated with a
cofactor and boiled human liver microsomes or cytosol, two peaks
(5.0 and 5.9 min) were not detected (data not shown). The peak at
5.9 min, but not that at 5.0 min, was identified as ROH based on
the retention time of the synthesized ROH (Fig. 2A). Both the microsomes and cytosol
showed RK reduction activity in the presence of NADPH (Fig. 2B). These results suggest that RK is
reduced in humans as well as in other mammals.
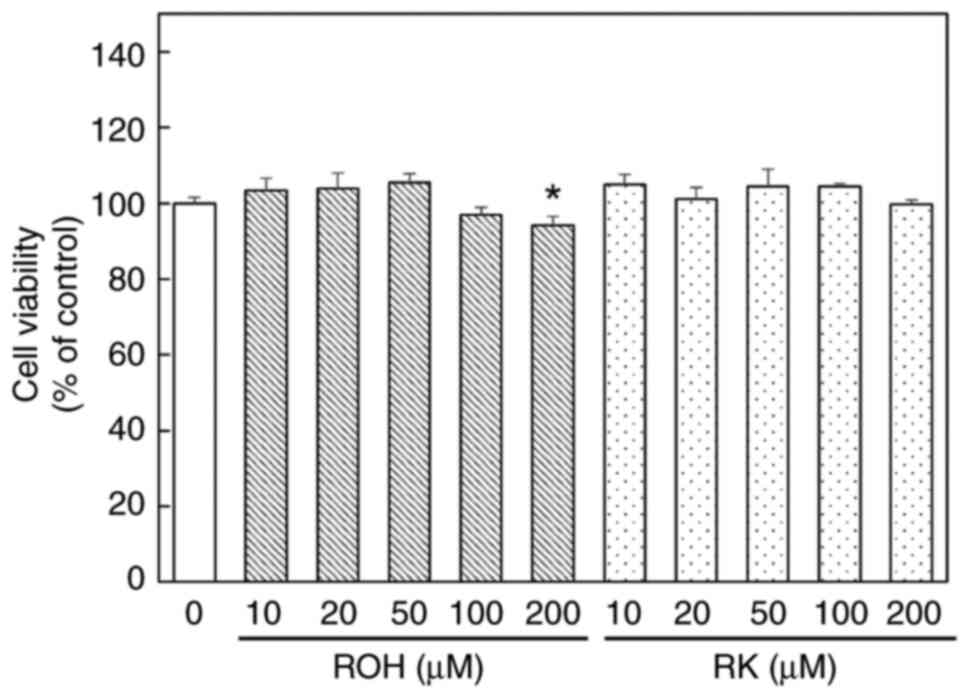
Effects of ROH and RK on cell
viability
The anti-adipogenic effect of RK at each maximum
concentration of 10–300 µM has been determined (28–31).
Here, 3T3-L1 cells were treated with various concentrations of ROH
or RK (10–200 µM) for 24 h (Fig.
3) to evaluate cell viability. Cell viability at 24 h after
treatment with 10–100 µM ROH or RK was similar to that of the
untreated controls. However, treatment with 200 µM ROH resulted in
only slight cytotoxicity. These results indicated that ROH or RK at
concentrations up to 100 µM did not have a significant cytotoxic
effect on these cells. Hence, all experiments were performed with
ROH or RK at concentrations up to 100 µM.
Effects of ROH and RK on adipocyte
differentiation in 3T3-L1 cells
Leptin is predominantly expressed in adipose tissue
and is considered an adipocyte marker protein (32,33).
We measured leptin mRNA levels as adipocyte marker to confirm the
formation of adipocytes from 3T3-L1 pre-adipocytes. Treatment of
pre-adipocyte 3T3-L1 cells with DM/AMM/MM for 8 days significantly
increased leptin mRNA levels compared with untreated pre-adipocytes
(Fig. 4A). Therefore, we
determined that pre-adipocyte 3T3-L1 cells were differentiated into
mature adipocytes following DM/AMM/MM treatment for 8 days. The
effects of ROH and RK on lipid accumulation during the
differentiation of 3T3-L1 cells into adipocytes were compared. Oil
Red O staining revealed that the addition of ROH (20–100 µM) to the
culture medium during differentiation (days 0–8) decreased the
number of lipid droplets in the cells in a dose-dependent manner,
and had effects similar to those of RK (Fig. 4B). Moreover, the significant
inhibitory effect of ROH, which was equivalent to that of RK, on
lipid accumulation was confirmed by measuring Oil Red O levels in
the cells (Fig. 4C). These results
suggest that ROH, a metabolite of RK, is an active metabolite that
can inhibit lipid droplet accumulation in 3T3-L1 cells during their
differentiation into adipocytes.
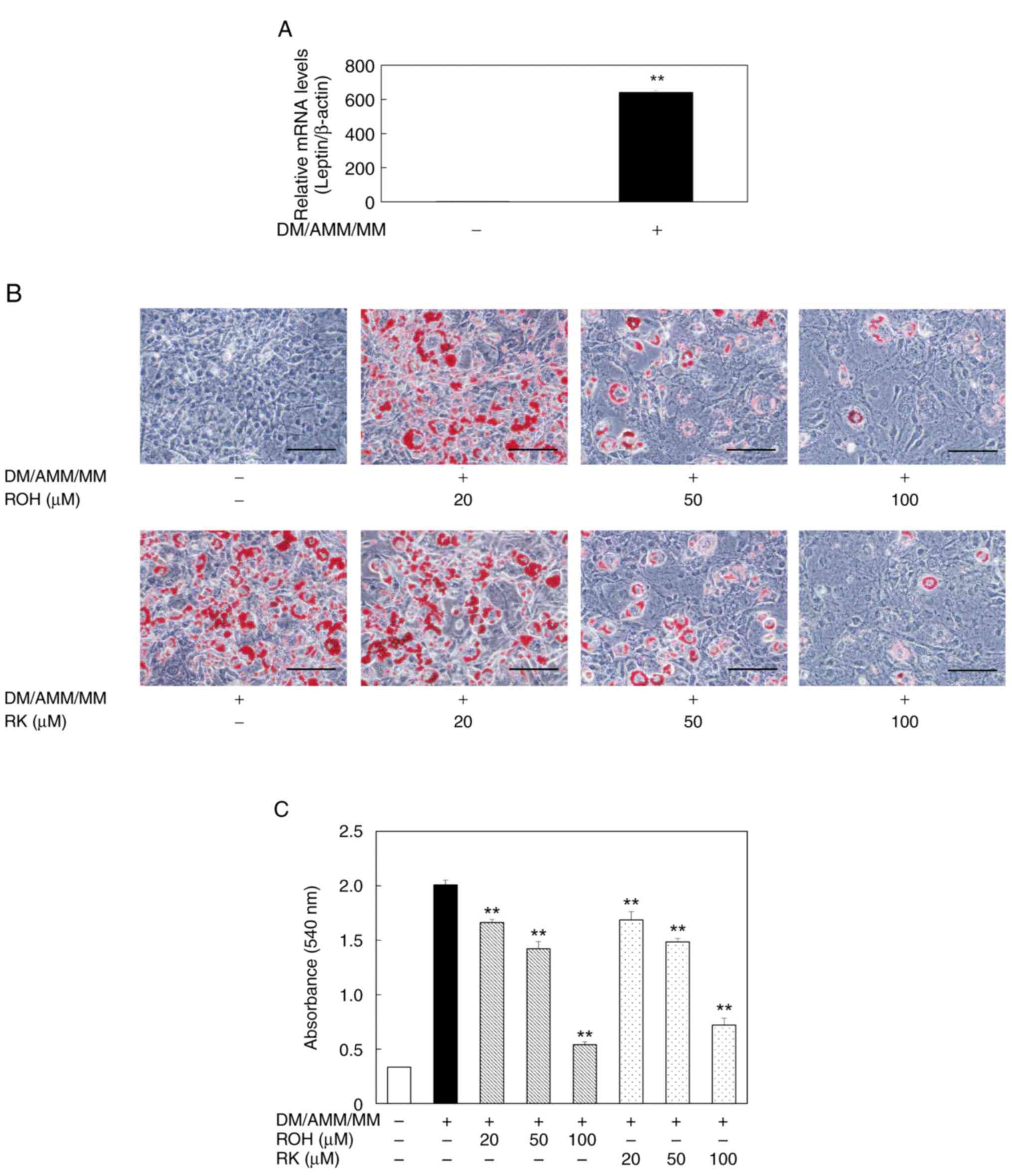 | Figure 4.Effects of ROH and RK on lipid
accumulation during differentiation of 3T3-L1 cells to adipocytes.
(A) During differentiation of 3T3-L1, cells were treated with or
without DM/AMM/MM for 8 days. Leptin mRNA levels in mature
adipocytes (day 8) were examined by qPCR as an adipocyte marker.
Each bar represents the mean ± SD of three independent experiments.
**P<0.01 vs. without DM/AMM/MM. (B) During the differentiation
of 3T3-L1, cells were treated with or without ROH or RK at various
concentrations (20–100 µM) for 8 days. Representative
photomicrographs (magnification: ×200, scale bar: 100 µm) are shown
for each treatment group. Oil Red O reagent-stained lipid droplets
in the mature 3T3-L1 adipocytes (day 8). (C) The amount of lipid
accumulated in mature 3T3-L1 adipocytes was quantified by measuring
absorbance at 540 nm. Each bar represents the mean ± SD of three
independent experiments. **P<0.01 vs. DM/AMM/MM alone. AMM,
adipocyte maintenance medium; DM, differentiation medium; MM,
maintenance medium; qPCR, quantitative PCR; ROH, rhododendrol; RK,
raspberry ketone; SD, standard deviation. |
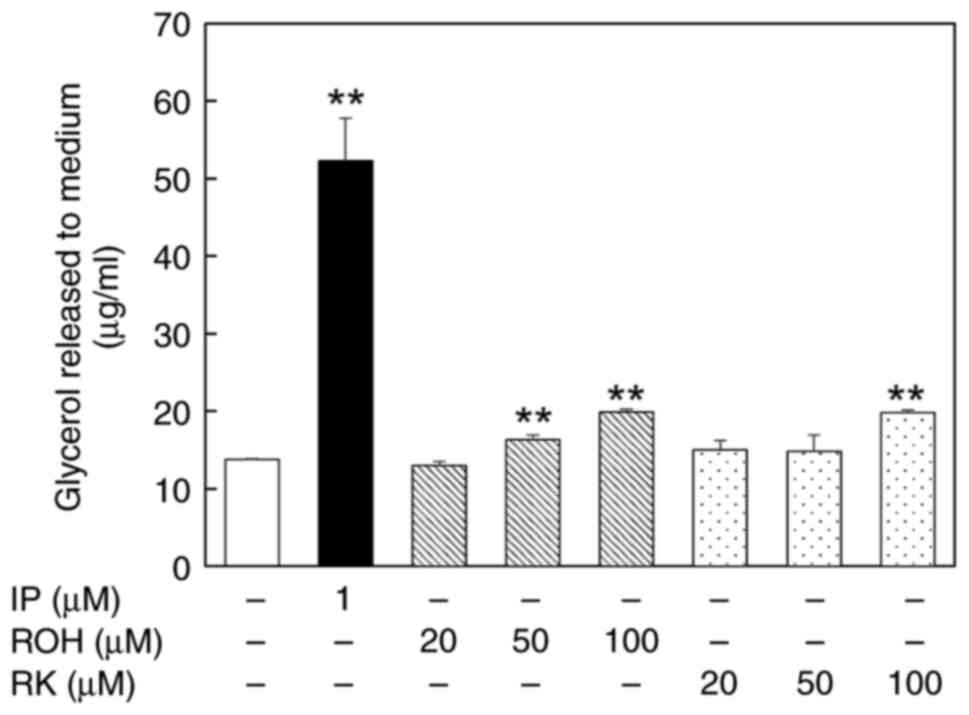
Effects of ROH and RK on glycerol
release from mature 3T3-L1 adipocytes
We examined the release of glycerol into the culture
medium after treatment with ROH or RK (20–100 µM) for 24 h to
assess whether ROH promoted lipolysis in mature 3T3-L1 adipocytes.
Treatment of the cells with 50 µM ROH, 100 µM ROH, or RK increased
glycerol release into the medium by 1.18-fold, 1.44-fold and
1.43-fold, respectively, compared with untreated cells (Fig. 5). Therefore, ROH shows a
lipolysis-promoting effect, similar to that of RK.
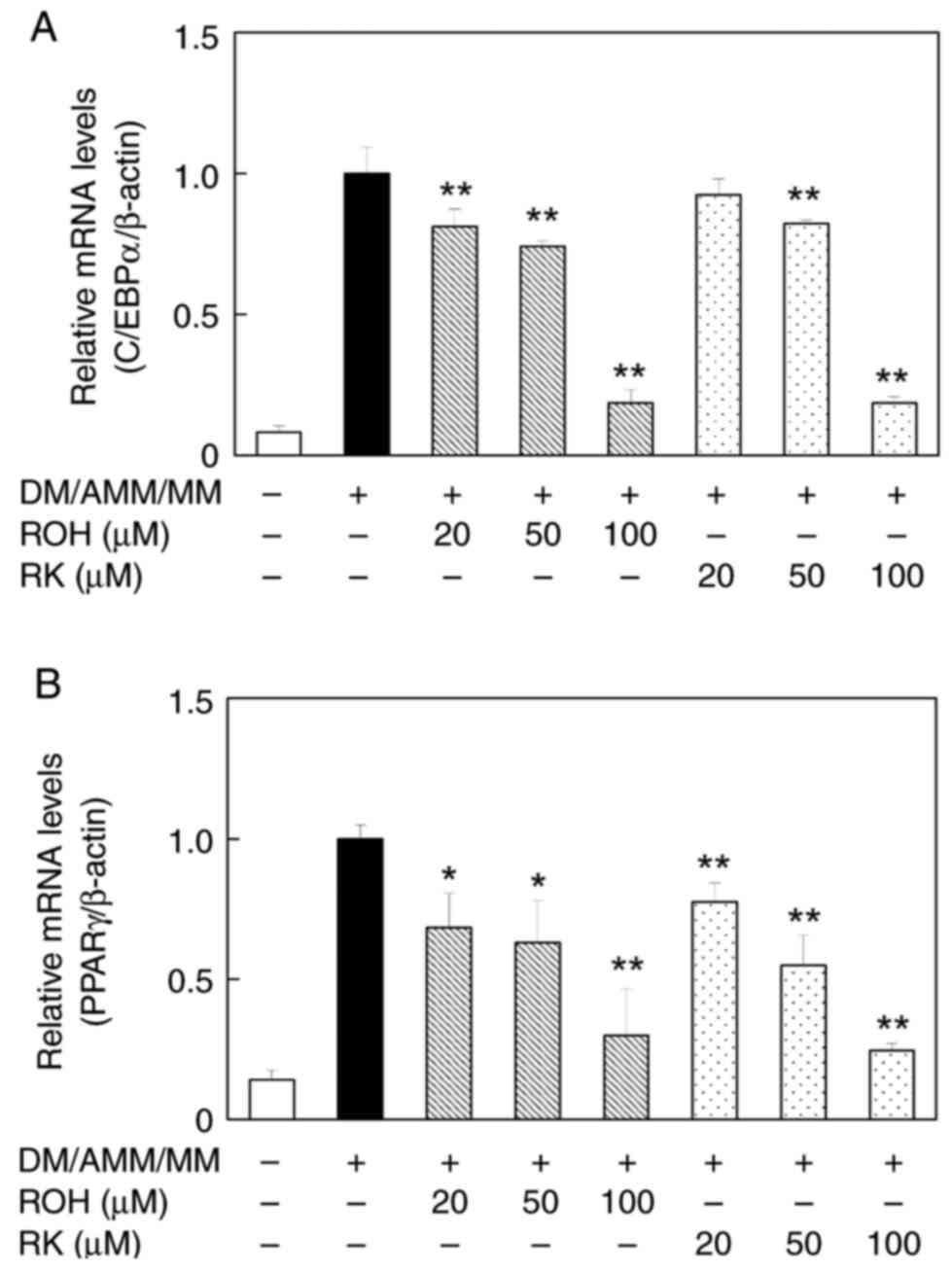
Effects of ROH and RK on C/EBPα and
PPARγ mRNA and protein levels in 3T3-L1 adipocytes
As ROH showed an inhibitory effect on lipid
accumulation, we focused on how it might affect the regulation of
the expression of adipogenesis-related genes such as C/EBPα and
PPARγ. The effects of ROH on C/EBPα and PPARγ mRNA levels in mature
adipocytes were investigated and compared with those of RK. When
3T3-L1 pre-adipocytes were treated with ROH (20–100 µM) during
their differentiation into adipocytes over 8 days, C/EBPα and PPARγ
mRNA levels decreased in a concentration-dependent manner.
Treatment with RK produced similar results (Figs. 6A and B). Furthermore, the
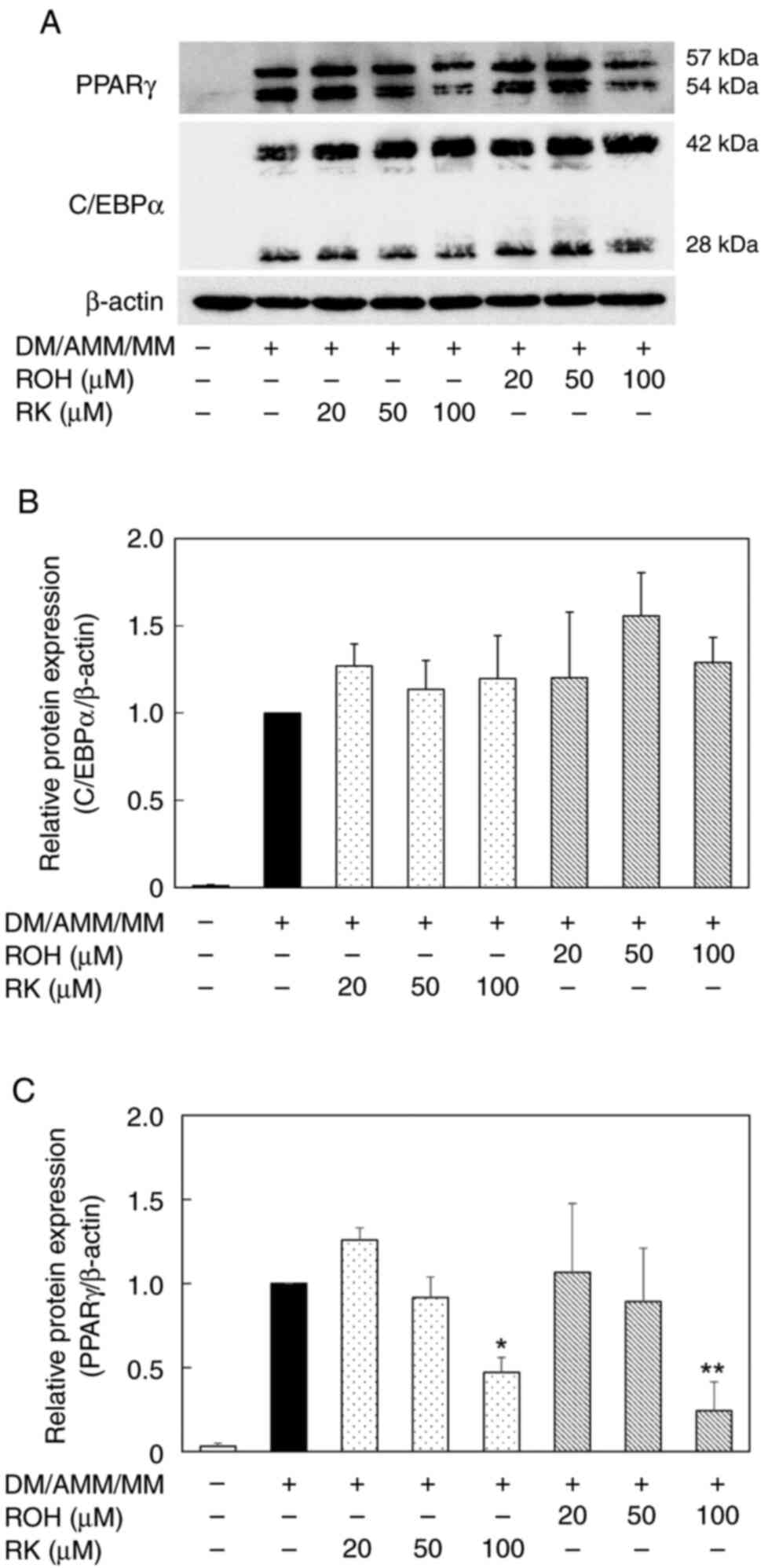
expression levels of C/EBPα and PPARγ proteins were monitored using
western blot analysis (Fig. 7A).
The treatment of the pre-adipocyte 3T3-L1 cells with ROH and RK
(20–100 µM) during differentiation downregulated PPARγ protein
expression in mature 3T3-L1 adipocytes on day 8 in a
concentration-dependent manner (Fig.
7C). However, the levels of C/EBPα protein remained unchanged
(Fig. 7B). The anti-adipogenic
effects of ROH and RK may be attributed to their PPARγ lowering
effects; however, it remains unclear as to why the lowering effects
of ROH and RK on C/EBPα mRNA levels were not reflected in their
protein levels.
Discussion
This study demonstrated that RK was reduced to ROH
in human liver microsomes and cytosol. Because the reduction of RK
occurs in both human liver microsomes and the cytosol in the
presence of NADPH, and other carbonyl compounds, such as warfarin
and nabumetone, are also metabolized in this manner, multiple
enzymes are thought to be involved in this reaction (34,35).
Additionally, our data showed a new peak for the RK metabolite with
a retention time of 5.0 min after incubation with human liver
microsomes. In addition to ROH, the metabolites of RK include
4-(3,4-Dihydroxyphenyl) butanone, which is generated by the
hydroxylation of RK, and 4-(2-Hydroxyethyl) phenol (tyrosol), which
is generated by decarboxylation of ROH (24). However, when ROH was incubated with
human liver microsomes in the presence of NADPH, no unknown
metabolites were detected, with a retention time of 5 min (data not
shown). Therefore, the metabolite with a retention time of 5 min in
the chromatogram was not 4-(2-hydroxyethyl) phenol (tyrosol). We
are also interested in metabolites other than ROH, and further
studies should focus on the metabolic mechanisms that produce these
molecules and how they affect the suppression of fat
accumulation.
RK and various other compounds suppress the
differentiation of 3T3-L1 cells into adipocytes (7,8,30,31).
Our data revealed that RK suppressed the lipid accumulation-induced
differentiation of 3T3-L1 pre-adipocytes in a dose-dependent
manner, which is consistent with previous reports. In addition,
this study showed that ROH, a reductive metabolite of RK,
suppressed lipid accumulation during the differentiation of
pre-adipocytes into adipocytes, suggesting that ROH is an active
metabolite. Recently, a pharmacokinetic study of orally
administered RK demonstrated that the accumulation of RK and its
metabolites in the white adipose tissue of obese mice was higher
than that in normal mice (24).
Therefore, ROH produced when RK is orally administered during the
development of obesity may act additively with RK in pre-adipose
and adipose tissues to produce anti-obesity effects. A dietary
supplement mixture containing RK, capsaicin, caffeine, garlic, and
Citrus aurantium reduced the body weight and fat in overweight
adults (36), but there is no
information on their effects after oral administration of RK alone
to human or human adipocytes. The present study will be a useful
basis for investigating the anti-obesity effects ROH and RK in
humans. Studies of foods containing active ingredients with
anti-obesity effects, such as health foods and supplements, have
examined the ingredients that are ingested. The results of this
study suggest that metabolites produced after oral ingestion may
also exhibit anti-adipogenic and lipolysis-promoting activities.
Our findings may aid in the development of more effective
anti-obesity drugs and the prevention of visceral fatty obesity and
fatty liver disease.
The regulation of the expression of
adipogenesis-related factors, including C/EBPα and PPARγ,
contributes to the differentiation of pre-adipocytes into
adipocytes (4,5). Regulation of C/EBPα and PPARγ gene
expression is involved in adipogenesis, and their downregulation is
related to adipogenesis suppression (37). In addition, activation
(phosphorylation) of AMP-activated protein kinase (AMPK), which
exerts anti-obesity effects via regulation of the expression and
activation of enzymes involved in lipid metabolism, is a critical
event in lipolysis (38,39). Previous studies have demonstrated
that several natural flavonoids suppress adipogenesis by activating
AMPK and downregulating the C/EBPα and PPARγ genes (40,41).
The suppressive effect of RK on 3T3-L1 cell differentiation into
adipocytes seemed to be caused by the downregulation of the mRNA
levels of C/EBPα and PPARγ. In the present study, we also found
that, similar to RK, ROH suppressed the differentiation of cells
and decreased the mRNA levels of C/EBPα and PPARγ and protein
expression of PPARγ, but not C/EBPα protein. Our data suggest that
the downregulation of PPARγ by ROH and RK contributed to their
anti-adipogenic effects. PPARγ plays an important role in adipose
differentiation through the regulation of the expression of
adipocyte-specific genes, such as adipocyte fatty acid-binding
protein-2 (aP2) and fatty acid synthase (FASN) (42). The ROH- or RK-suppressed PPARγ
expression may affect the regulation of their adipocyte-related
genes. Further study remains on the effects of ROH and RK against
the genes regulated by PPARγ.
Park demonstrated that RK has a lipolysis-promoting
effect on glycerol release from 3T3-L1 adipocytes (30). Our data support this finding and
suggest that ROH has a lipolysis-promoting effect similar to that
of RK. Therefore, the suppressive effects of ROH and RK on 3T3-L1
cell differentiation into adipocytes may be caused by the
downregulation of mRNA and protein expression of
adipogenesis-related factors and lipolysis. If lipolysis is not
involved, glycerol released from the cells may have a similar
effect. Further studies are required to elucidate the mechanisms by
which ROH and RK promote lipolysis.
The chemical structure of RK is similar to those of
capsaicin, 6-gingerol, and synephrine, which are the principal
components of hot red pepper, ginger, and citrus plants,
respectively. These compounds have been shown to suppress lipid
accumulation (43–45). The anti-adipogenic effects of ROH
and RK may be due to their structural similarities to these
compounds. Whether there exists a structure-activity relationship
that contributes to the anti-obesity effects of RK, capsaicin,
6-gingerol, synephrine, and their metabolites is of considerable
interest; studies to determine this are now underway.
In conclusion, we showed that ROH, a reductive
metabolite of RK, has an anti-adipogenic effect similar to that of
RK in the differentiation of 3T3-L1 cells into adipocytes. These
results imply that both RK and ROH might contribute to the
anti-obesity effects of orally ingested RK. Our results suggest
that the biological effects of natural compounds, including their
anti-obesity effects, may be due to their metabolites. Therefore,
we propose that it is important to evaluate the biological activity
of all detectable metabolites and consider their
pharmacokinetics.
Acknowledgements
Not applicable.
Funding
This work was supported in part by JSPS KAKENHI (grant no.
JP21K11599).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
NU and TH contributed to experimental design. NU,
AK, MO and TH performed the experiments. NU, MO and TH wrote the
manuscript. NU, AK, MO and TH confirm the authenticity of all raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RK
|
raspberry ketone
|
|
ROH
|
rhododendrol
|
References
|
1
|
Ding J, Reynolds LM, Zeller T, Müller C,
Lohman K, Nicklas BJ, Kritchevsky SB, Huang Z, de la Fuente A,
Soranzo N, et al: Alterations of a cellular cholesterol metabolism
network are a molecular feature of obesity-related type 2 diabetes
and cardiovascular disease. Diabetes. 64:3464–3474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garg SK, Maurer H, Reed K and Selagamsetty
R: Diabetes and cancer: Two diseases with obesity as a common risk
factor. Diabetes Obes Metab. 16:97–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao Z, Umek RM and McKnight SL: Regulated
expression of three C/EBP isoforms during adipose conversion of
3T3-L1 cells. Genes Dev. 5:1538–1552. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrison S and McGee SL: 3T3-L1 adipocytes
display phenotypic characteristics of multiple adipocyte lineages.
Adipocyte. 4:295–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EJ, Kang MJ, Seo YB, Nam SW and Kim
GD: Acer okamotoanum Nakai leaf extract inhibits adipogenesis via
suppressing expression of PPAR γ and C/EBP α in 3T3-L1 cells. J
Microbiol Biotechnol. 28:1645–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee MS, Kim CT, Kim IH and Kim Y:
Inhibitory effects of green tea catechin on the lipid accumulation
in 3T3-L1 adipocytes. Phytother Res. 23:1088–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gallois A: Quantitative evaluation of
raspberry ketone using thin-layer chromatography. Sci Aliments.
2:99–106. 1982.
|
|
10
|
Larsen M, Poll L, Callesen O and Lewis M:
Relations between the content of aroma compounds and the sensory
evaluation of 10 raspberry varieties (Rubus idaeus L). Acta Agric
Scand. 41:447–454. 1991. View Article : Google Scholar
|
|
11
|
Mehanna ET, Barakat BM, El Sayed MH and
Tawfik MK: An optimized dose of raspberry ketone controls
hyperlipidemia and insulin resistance in male obese rats; Effect on
adipose tissue expression of adipocytokines and aquaporin 7. Eur J
Pharmacol. 832:81–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Meng X and Zhang F: Raspberry
ketone protects rats fed high-fat diets against nonalcoholic
steatohepatitis. J Med Food. 15:495–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morimoto C, Satoh Y, Hara M, Inoue S,
Tsujita T and Okuda H: Anti-obese action of raspberry ketone. Life
Sci. 77:194–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuchino H, Konishi S, Satoh T, Yagi A,
Saitsu K, Tatsumi T and Tanaka N: Chemical evaluation of Betula
species in Japan. II. Constituents of Betula platyphylla var
japonica. Chem Pharm Bull. 44:1033–1038. 1996. View Article : Google Scholar
|
|
15
|
Inoue T, Ishidate Y, Fujita M, Kubo M,
Fukushima M and Nagai M: Studies on the constituents of Aceraceae
plants. I. Constituents in the leaves and the stem bark of Acer
nikoense Maxim (author's transl). Yakugaku Zasshi. 98:41–46.
1978.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Wei T, Wu M, Chen F, Zhang P, Deng
ZY and Luo T: Potential metabolic activities of raspberry ketone. J
Food Biochem. 46:e140182022.PubMed/NCBI
|
|
17
|
Sporstøl S and Scheline RR: The metabolism
of 4-(4-hydroxyphenyl)butan-2-one (raspberry ketone) in rats,
guinea-pigs and rabbits. Xenobiotica. 12:249–257. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka Y, Nishikawa Y, Matsuda K, Yamazaki
M and Hayashi R: Purification and some properties of ketone
reductase forming an active metabolite of sodium
2-[4-(2-oxocyclopentylmethyl)-phenyl]propionate dihydrate
(loxoprofen sodium), a new anti-inflammatory agent, in rabbit liver
cytosol. Chem Pharm Bull (Tokyo). 32:1040–1048. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasaki K, Yamauchi K and Kuwano S:
Metabolic activation of sennoside A in mice. Planta Med.
37:370–378. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murata H, Higuchi T and Otagiri M: Oral
pharmacokinetics and in-vitro metabolism of metyrapone in male
rats. J Pharm Pharmacol. 68:970–979. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imamura Y, Iwamoto K, Yanachi Y, Higuchi T
and Otagiri M: Postnatal development, sex-related difference and
hormonal regulation of acetohexamide reductase activities in rat
liver and kidney. J Pharmacol Exp Ther. 264:166–171.
1993.PubMed/NCBI
|
|
22
|
Higuchi T, Imamura Y and Otagiri M:
Kinetic studies on the reduction of acetohexamide catalyzed by
carbonyl reductase from rabbit kidney. Biochim Biophys Acta.
1158:23–28. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imamura Y, Nozaki Y, Higuchi T and Otagiri
M: Reactivity for prostaglandins and inhibition by nonsteroidal
anti-inflammatory drugs of rabbit liver Befunolol reductase. Res
Commun Chem Pathol Pharmacol. 71:49–57. 1991.PubMed/NCBI
|
|
24
|
Zhao D, Yuan B, Kshatriya D, Polyak A,
Simon JE, Bello NT and Wu Q: Influence of diet-induced obesity on
the bioavailability and metabolism of raspberry ketone
(4-(4-hydroxyphenyl)-2-butanone) in mice. Mol Nutr Food Res.
64:e19009072020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitayama T, Isomori S and Nakamura K:
Asymmetric synthesis of enantiomerically pure zingerols by
lipase-catalyzed transesterification and efficient synthesis of
their analogues. Tetrahedron Asymmetry. 24:621–627. 2013.
View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith PK, Krohn RI, Hermanson GT, Mallia
AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ and
Klenk DC: Measurement of protein using bicinchoninic acid. Anal
Biochem. 150:76–85. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leu SY, Chen YC, Tsai YC, Hung YW, Hsu CH,
Lee YM and Cheng PY: Raspberry ketone reduced lipid accumulation in
3T3-L1 cells and ovariectomy-induced obesity in Wistar rats by
regulating autophagy mechanisms. J Agric Food Chem. 65:10907–10914.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsai YC, Chen JH, Lee YM, Yen MH and Cheng
PY: Raspberry ketone promotes FNDC5 protein expression via HO-1
upregulation in 3T3-L1 adipocytes. Chin J Physiol. 65:80–86. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park KS: Raspberry ketone increases both
lipolysis and fatty acid oxidation in 3T3-L1 adipocytes. Planta
Med. 76:1654–1658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park KS: Raspberry ketone, a naturally
occurring phenolic compound, inhibits adipogenic and lipogenic gene
expression in 3T3-L1 adipocytes. Pharm Biol. 53:870–875. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Halaas JL, Gajiwala KS, Maffei M, Cohen
SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK and Friedman JM:
Weight-reducing effects of the plasma protein encoded by the obese
gene. Science. 269:543–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Malátková P, Sokolová S, Chocholoušová
Havlíková LC and Wsól V: Carbonyl reduction of warfarin:
Identification and characterization of human warfarin reductases.
Biochem Pharmacol. 109:83–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumoto K, Hasegawa T, Koyanagi J,
Takahashi T, Akimoto M and Sugibayashi K: Reductive metabolism of
nabumetone by human liver microsomal and cytosolic fractions:
Exploratory prediction using inhibitors and substrates as marker
probes. Eur J Drug Metab Pharmacokinet. 40:127–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arent SM, Walker AJ, Pellegrino JK,
Sanders DJ, McFadden BA, Ziegenfuss TN and Lopez HL: The combined
effects of exercise, diet, and a multi-ingredient dietary
supplement on body composition and adipokine changes in overweight
Adults. J Am Coll Nutr. 37:111–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
White UA and Stephens JM: Transcriptional
factors that promote formation of white adipose tissue. Mol Cell
Endocrinol. 318:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang BB, Zhou G and Li C: AMPK: An
emerging drug target for diabetes and the metabolic syndrome. Cell
Metab. 9:407–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ceddia RB: The role of AMP-activated
protein kinase in regulating white adipose tissue metabolism. Mol
Cell Endocrinol. 366:194–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Piao GC, Liu GC, Jin XJ, Jin D and Yuan
HD: Tetrahydropalmatine inhibits lipid accumulation through AMPK
signaling pathway in 3T3-L1 adipocytes. Mol Med Rep. 15:3912–3918.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang G, Wu B, Xu W, Jin X, Wang K and Wang
H: The inhibitory effects of Juglanin on adipogenesis in 3T3-L1
adipocytes. Drug Des Dev Ther. 14:5349–5357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jefcoate CR, Wang S and Liu X: Methods
that resolve different contributions of clonal expansion to
adipogenesis in 3T3-L1 and C3H10T1/2 cells. Methods Mol Biol.
456:173–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee MS, Kim CT, Kim IH and Kim Y: Effects
of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother
Res. 25:935–939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tzeng TF and Liu IM: 6-gingerol prevents
adipogenesis and the accumulation of cytoplasmic lipid droplets in
3T3-L1 cells. Phytomedicine. 20:481–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo LX, Chen G, Yin ZY, Zhang YH and Zheng
XX: p-Synephrine exhibits anti-adipogenic activity by activating
the Akt/GSK3β signaling pathway in 3T3-L1 adipocytes. J Food
Biochem. 43:e130332019. View Article : Google Scholar : PubMed/NCBI
|