Introduction
Atherosclerosis (AS) is a multifactorial chronic
inflammatory disease with high morbidity and mortality rates
worldwide (1). Cell death and
inflammation are closely related to the pathogenesis mechanisms
underlying AS (2,3). The former mainly occurs via apoptosis
and necrosis, but a novel type of programmed cell death, that is,
pyroptosis, was recently reported (4–6). The
classical pyroptosis pathway depends on the activation of
caspase-1. Cleaved caspase-1 is a cysteine-dependent protease that
serves a vital role in the maturation and secretion of gasdermin D
(GSDMD), IL-1β, IL-18 and apoptosis-associated speck-like protein
containing a CARD (ASC) (7). In
the late stage of pyroptosis, GSDMD is cleaved to generate the
N-terminus of GSDMD (GSDMD-N) and then forms a transmembrane pore
for IL-1β and IL-18 release (8).
Numerous studies have reported that pyroptosis is related to the
inflammatory process of AS (4,5,9). It
has been reported that histone deacetylase 11 (HDAC11) promotes AS
in high-fat diet (HFD)-fed ApoE-/- mice and that HDAC11 deletion
alleviates tumor necrosis factor-α (TNF-α)-induced pyroptosis in
human umbilical vein endothelial cells (HUVECs) by suppressing, NLR
family pyrin domain containing 3 (NLRP3)/caspase-1/GSDMD and
caspase-3/gasdermin E signaling pathways (10). Moreover, hydroxytyrosol acetate
inhibits HFD-fed ApoE-/- mice AS by suppressing TNF-α-induced
pyroptosis of HUVECs via negative regulation of the HDAC11 related
signaling pathway (11). Another
study reported that sirtuin 6 reduced TNF-α-induced pyroptosis of
vascular endothelial cells by negatively regulating the
Lin28b/let-7 signaling pathway in AS (12). Therefore, targeted regulation of
pyroptotic endothelial cells is of significance to prevent AS.
Previous studies have reported that lncRNAs and
micro RNAs (miRNAs) serve vital roles in cell pyroptosis (13). LncRNAs are composed of more than
200 nucleotides, and a member of the lncRNAs family, MALAT1, has
been reported to be associated with pyroptosis in cardiovascular
disease. For instance, MALAT1 promoted high glucose-induced
pyroptosis of H9c2 cells by competitively binding to miR-141-3p
(14). Moreover, up-regulation of
MALAT1 aggravates NLRP3-mediated cell pyroptosis in diabetic
atherosclerosis (15,16). MALAT1 knockdown significantly
decreases high-glucose- induced endothelial and epithelial cell
pyroptosis (16,17). However, the effect of MALAT1 on
TNF-α-induced pyroptosis of rat aortic endothelial cells (RAOECs)
and the underlying mechanisms have been inadequately investigated.
miRNAs are composed of 20–24 nucleotides, and their main function
is to participate in the regulation of post-transcriptional gene
expression (18). Numerous studies
have reported that dysregulation of microRNAs, such as miR-30c-5p,
leads to the development of AS. Ceolotto et al (19) reported the decreased level of
miR-30c-5p in microparticles accelerated AS. Moreover, Li et
al (20) reported that a
miR-30c-5p mimic decreased NLRP3-induced endothelial cells
pyroptosis by inhibiting forkhead box O3 (FOXO3) expression.
However, the effects and precise molecular mechanisms of the
MALAT1/miR-30c-5p axis in TNF-α-mediated RAOEC pyroptosis remain to
be elucidated.
Connexin 43 (Cx43) is an abundant component in
numerous cells of the cardiovascular system. It has been reported
that the development of AS is correlated with Cx43 expression
(21). Furthermore, a previous
study reported that miRNAs serve vital roles in the
post-transcriptional regulation of Cx43 expression (22). Yang et al (23) reported that miR-1 aggravated
arrhythmogenesis via regulation of Cx43. Interestingly, miR-130a
also results in cardiac arrhythmias through the downregulation of
Cx43 (24). Although MALAT1,
miR-30c-5p and Cx43 have been reported to be involved in AS, the
mechanisms and interactions still need to be elucidated.
The present study was designed to investigate the
functional roles of the MALAT1/miR-30c-5p/Cx43 axis in
TNF-α-induced RAOEC pyroptosis and its underlying mechanisms, which
could provide new insights into AS treatment.
Materials and methods
Cell culture and treatment
RAOECs (Cell Applications Inc.) and 293T cells
(American Type Culture Collection) were cultured in Dulbecco's
Modified Eagle Medium (Beijing Solarbio Science & Technology
Co., Ltd.) supplemented with 10% fetal bovine serum (Invitrogen;
Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and
streptomycin (100 µg/ml) mixture (Beijing Solarbio Science &
Technology Co., Ltd.) in a humidified incubator containing 5%
CO2 at 37°C. RAOECs were exposed to TNF-α (PeproTech,
Inc.) at 10 ng/ml for 24 h to induce cell pyroptosis in a
humidified incubator containing 5% CO2 at 37°C.
The sense and anti-sense sequences for MALAT1, Cx43
and the negative controls were designed by Biomics Biopharma. The
sequences for miR-30c-5p mimic (cat. no. miR10000804), miR-30c-5p
inhibitor (cat. no. miR20000804), mimic negative control (NC) (cat.
no. miR1N0000001-1-1) and inhibitor NC (cat. no. miR2N0000001-1-1)
were designed and synthesized by Guangzhou RiboBio Co., Ltd. RAOECs
were seeded in six-well plates with 1×106 cells/well and
then incubated overnight in a humidified incubator containing 5%
CO2 at 37°C. 50 nM short interfering RNA (siRNA), 30 nM
miR-30c-5p mimic or 100 nM miR-30c-5p inhibitor and mimic NC or
inhibitor NC were transfected into RAOECs using riboFECT™ CP
Reagent (Guangzhou RiboBio Co., Ltd.) for 24 h followed by 10 ng/ml
TNF-α for 24 h in a humidified incubator containing 5%
CO2 at 37°C. The sequences of purchased siRNA, mimic and
inhibitor were presented in Table
I.
 | Table I.Sequences of siRNAs. |
Table I.
Sequences of siRNAs.
| Target | Sequence
(5′-3′) |
|---|
| siNC | Sense:
UUCUCCGAACGUGUCACGU |
|
| Anti-sense:
UUUAAGCUGUUUAAGUCAC |
| siMALAT1-1 | Sense:
GUGACUUAAACAGCUUAAA |
|
| Anti-sense:
UUUAAGCUGUUUAAGUCAC |
| siMALAT1-2 | Sense:
GGUAGGUCUGGGUUUACUA |
|
| Anti-sense:
UAGUAAACCCAGACCUACC |
| siMALAT1-3 | Sense:
GAUUAGUAGUCAAAGCAAA |
|
| Anti-sense:
UUUGCUUUGACUACUAAUC |
| siCx43 | Sense:
GCAUCGAGCUGUCGAUUAU |
|
| Anti-sense:
AUAAUCGACAGCUCGAUGC |
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from RAOECs using
TRIzol® reagent (Takara Biotechnology Co., Ltd.). The
thermocycling conditions used in RT-qPCR were set as follows: The
cDNA synthesis reaction mix was incubated at 25°C for 5 min and
42°C for 30 min followed by 85°C for 5 sec. Then the reaction was
terminated at 4°C. The qPCR reaction mix was incubated at 95°C for
30 sec for 1 cycle), at 95°C for 15 sec and at 60°C for 30 sec for
40 cycles), followed by dissociation. RT-qPCR was performed using
Servicebio™ 2×SYBRGreen qPCR Master Mix (Wuhan Servicebio
Technology Co., Ltd.) using a CFX Connect™ system (Bio-Rad
Laboratories, Inc.). All experiments were performed three times,
and the relative gene expression was calculated using the
2−ΔΔCq method (25). U6
was used as an internal control for miR-30c-5p, and GAPDH was used
as the internal control for other targets. In the present study,
the stem ring method was used for the design of primers for the
detection of miR-30c-5p, as the length of mature miRNA is about 20
bp, and the appropriate detection length of RT-qPCR is 80–150 bp.
Therefore, it was necessary to add stem-ring structures in the
original sequence to extend the miRNA and the miRNA reverse primer
was located in in the stem ring, not the miRNA. The primers for
miR-30c-5p, MALAT1, U6 and GAPDH were designed and synthetized by
Sangon Biotech Co., Ltd. The gap junction protein alpha 1 (GJA1)
gene encodes the protein Cx43 and the primer for GJA1 was designed
and synthetized by TsingKe Biological Technology. The sequences of
the primers were presented in Table
II.
 | Table II.Sequences of primers used for reverse
transcription-quantitative PCR |
Table II.
Sequences of primers used for reverse
transcription-quantitative PCR
| Gene | Sequence
(5′-3′) |
|---|
| MALAT1 | F:
CTCACTAAAGGCACCGAAGG |
|
| R:
GGCAGAGAAGTTGCTTGTGG |
| miR-30c-5p | F:
GCGCGTGTAAACATCCTACACT |
|
| R:
AGTGCAGGGTCCGAGGTATT |
| Cx43 | F:
TCTGCCTTTCGCTGTAACACT |
|
| R:
GGGCACAGACACGAATATGAT |
| GAPDH | F:
GTCATCAACGGGAAACCCAT |
|
| R:
ACGCCAGTAGACTCCACGACAT |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
Western blotting
Following experimental treatment, RAOECs were lysed
using RIPA lysis buffer (Beyotime Institute of Biotechnology) mixed
with phenylmethanesulfonyl fluoride (Beijing Solarbio Science &
Technology Co., Ltd.) at a ratio of 1:100. The protein
concentration was assessed using a BCA protein assay kit (Applygen
Technologies, Inc.). Protein samples of 20 µg/well were loaded and
separated using 10 or 12% SDS-PAGE and then transferred onto PVDF
membranes (MilliporeSigma). Subsequently, the membranes were
blocked using 5% bovine serum albumin (Shanghai Yeasen
Biotechnology Co., Ltd.) for 2 h at room temperature, and then
incubated with primary antibodies against NLRP3 (1:500; cat. no.
27458-1-AP; Proteintech Group, Inc.), cleaved caspase-1 (1:500;
cat. no. sc-398715; Santa Cruz Biotechnology, Inc.), IL-1β (1:500;
cat. no. sc-515598; Santa Cruz Biotechnology, Inc.), Cx43 (1:1,000;
cat. no. sc-271837; Santa Cruz Biotechnology, Inc.), GSDMD-N
(1:1,000; cat. no. YT7991; ImmunoWay Biotechnology Company), ASC
(1:1,000; cat. no. YT0365; Immunoway Biotechnology Company) and
GAPDH (1:1,000 cat. no. TA802519; OriGene Technologies, Inc.) and
β-actin (1:5,000; cat. no. 66009-1-Ig; Proteintech Group, Inc.)
overnight at 4°C. After washing 3 times with TBST containing 0.05%
Tween-20, the membranes were incubated with peroxidase-conjugated
goat anti-rabbit IgG(H+L) (1:8,000; cat. no. 33101ES60; Shanghai
Yeasen Biotechnology Co., Ltd.) or peroxidase-conjugated goat
anti-mouse IgG(H+L) (1:8,000; cat. no. 33201ES60; Shanghai Yeasen
Biotechnology Co., Ltd.) secondary antibodies for 2 h at room
temperature. Protein signaling was detected using an enhanced
chemiluminescence kit (Abbkine Scientific Co., Ltd.) and analyzed
using ImageJ software v1.8.0 (National Institutes of Health). GAPDH
was used as the internal control. Each experiment was performed
three times.
Lactate dehydrogenase (LDH) release
assay
An LDH assay kit (cat. no. C0017; Beyotime Institute
of Biotechnology) was used to determine the LDH activity of RAOECs
in each group according to the manufacturer's protocols. Briefly,
120 µl cell supernatant, collected by centrifugation at 400 × g for
5 min at room temperature and 60 µl reaction mixture (20 µl
lactate, 20 µl 2-p-iodophenyl-3-nitrophenyl tetrazolium chloride
and 20 µl diaphorase) were mixed and incubated for 0.5 h at room
temperature. A Bio-Rad 680 spectrophotometric microplate reader
(Bio-Rad Laboratories, Inc.) was used to quantify the absorbance at
490 nm.
Hoechst 33342/propidium iodide (PI)
staining
RAOECs (1×105 cells/well in 12-well
plates) were treated with TNF-α or transfected with different
constructs. After washing with PBS, the cells were incubated with
2.5 µl Hoechst 33342 solution and PI (Beyotime Institute of
Biotechnology) for 0.5 h in the dark at 4°C. After washing 3 times
with PBS, the stained cells were imaged using a DMi8 inverted
fluorescence microscope at 200× magnification (Leica Microsystems
GmbH).
Dual-luciferase reporter assay
The targets of MALAT1 were predicted using the
StarBase (http://starbase.sysu.edu.cn) and BiBiServ2 (http://bibiserv.techfak.uni-bielefeld.de/) databases.
Briefly, the gene sequences of MALAT1 (human sequence used as no
entry for rat on NCBI) and miR-30c-5p were acquired from NCBI
(https://www.ncbi.nlm.nih.gov/) and
searched using standard conditions on the BiBiServ2 website. The
targets of miR-30c-5p in humans were searched using TargetScan8.0
online. The dual-luciferase reporter assay was performed to
evaluate the binding of MALAT1 and miR-30c-5p as well as that of
miR-30c-5p and Cx43. Partial sequences of MALAT1 or Cx43 carrying
putative wild-type (WT) miR-30c-5p binding sites or mutant (MUT)
sites were cloned into the GV272 luciferase vector (Shanghai
GeneChem Co., Ltd.) and named MALAT1-WT, MALAT1-MUT, Cx43-WT and
Cx43-MUT. Similarly, the CV045 vector containing the renilla
luciferase reporter gene was also purchased from Shanghai GeneChem
Co., Ltd. The 293T cell line, a powerful tool cell for expressing
exogenous genes, is competent to replicate vectors carrying the
SV40 region of replication, which has been widely used for
retroviral production, gene expression and protein production
(26). So 293T cells were
co-transfected with 20 pmol miR-30c-5p mimic or mimic NC and the
constructed vectors (0.5 µg of GV272 plasmid and 50 ng of CV045
plasmid) using Hieff Trans™ Liposomal Transfection Reagent
(Shanghai Yeasen Biotechnology Co., Ltd.). After 48 h, firefly and
Renilla luciferase activities were measured using the
dual-luciferase reporter assay system (Shanghai Yeasen
Biotechnology Co., Ltd.) according to the manufacturer's
protocols.
Statistical analysis
All the experiments in the present study were
performed independently at least three times. The data were
presented as the mean ± SD. GraphPad Prism software version 8.3.0
(GraphPad Software; Dotmatics) was used for statistical analysis.
For comparisons between two groups, the unpaired Student's t-test
was performed. For comparisons among multiple groups, one way
analysis of variance with Tukey's post hoc test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of MALAT1 mRNA and Cx43
protein are elevated but miR-30c-5p expression is decreased in
TNF-α-mediated RAOEC pyroptosis
To evaluate whether MALAT1 mRNA, and miR-30c-5p and
Cx43 protein expression were related to TNF-α-induced RAOEC
pyroptosis, first, RAOECs were incubated with 10 ng/ml TNF-α for 0,
6, 12, 24 or 48 h. RAOEC morphology was characterized by large
bubbles, which emerged from the plasma membrane at 12 h, and
especially at 24 and 48 h (Fig.
1A). Consistently, western blotting results demonstrated that
compared with control groups, the protein expression levels of
GSDMD-N and ASC were significantly upregulated after RAOEC
treatment with TNF-α for 24 and 48 h; however, there was no
significant difference between the 24 and 48 h groups (P<0.05,
Fig. 1B). Therefore, the RAOEC
pyroptosis model which used 10 ng/ml TNF-α for 24 h was used in the
subsequent experiments. Next, cell damage was evaluated by
assessing LDH release, which indicated that TNF-α treatment
significantly increased LDH release (t=6.031, P=0.0038, Fig. 1C). Furthermore, western blotting
was performed to assess the protein expression levels of NLRP3,
cleaved caspase-1 and IL-1β in TNF-α-stimulated RAOECs, and the
results indicated that NLRP3 (t=7.967, P=0.0013), cleaved caspase-1
(t=6.482, P=0.0029) and IL-1β (t=5.149, P=0.0067) were all
significantly upregulated compared with the control (Fig. 1D). Accordingly, it was demonstrated
that the percentage of PI-positive cells was significantly elevated
in TNF-α-treated RAOECs compared with the control (t=11.35,
P=0.0003, Fig. 1E). Interestingly,
it was also demonstrated that TNF-α treatment significantly
increased the mRNA expression level of MALAT1 (t=5.801, P=0.0044,
Fig. 1F) and protein expression
level of Cx43 (t=6.512, P=0.0029, Fig.
1G), but significantly reduced the RNA expression level of
miR-30c-5p (t=7.352, P=0.0018, Fig.
1H). The aforementioned results indicated that MALAT1,
miR-30c-5p and Cx43 could be associated with TNF-α-induced RAOEC
pyroptosis.
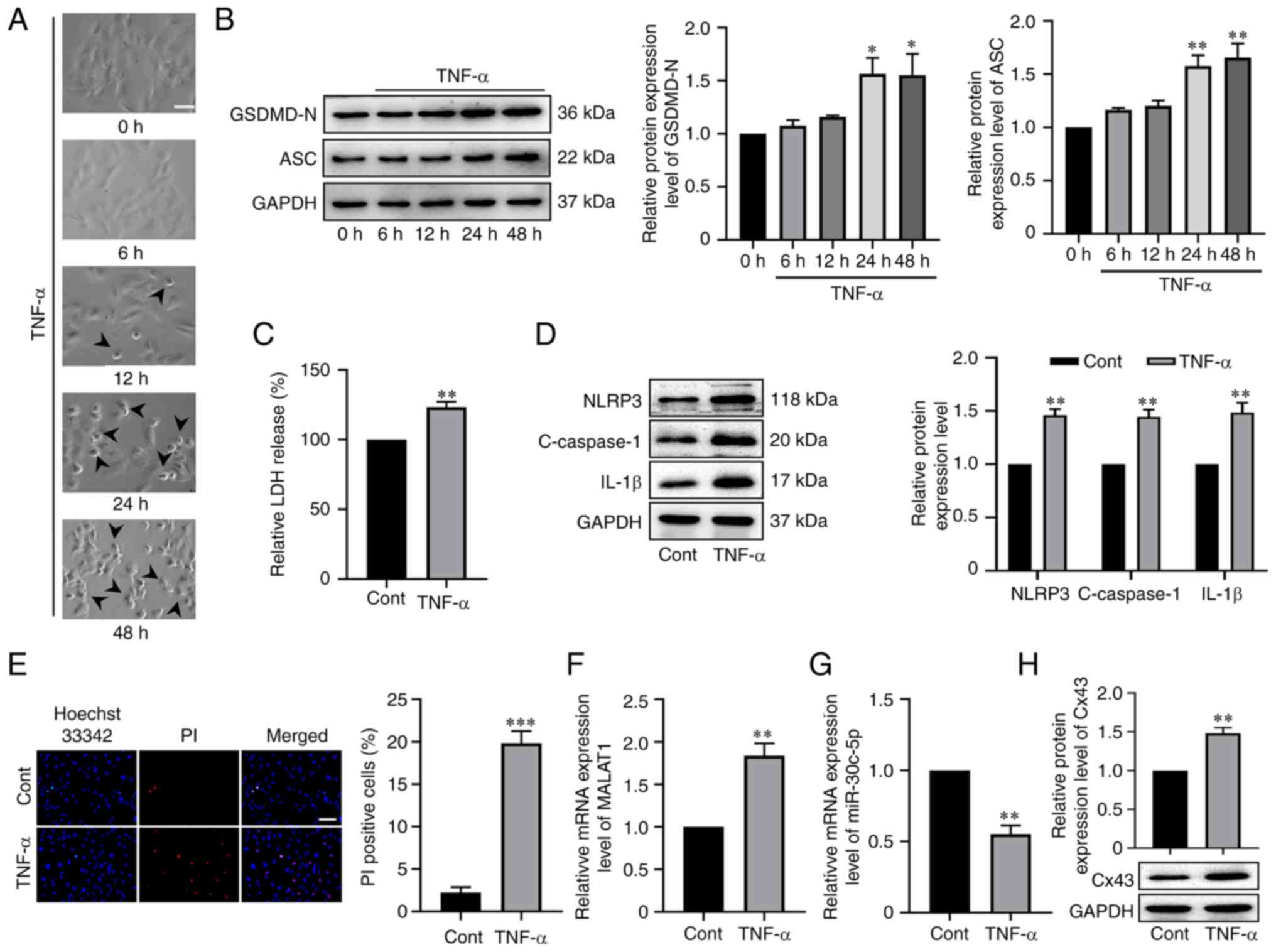 | Figure 1.MALAT1 and Cx43 were up-regulated but
miR-30c-5p was down-regulated in TNF-α-induced RAOEC pyroptosis.
(A) Representative bright-field microscopic images of RAOEC treated
with 10 ng/ml TNF-α for 0, 6, 12, 24 and 48 h. The arrowheads
(black) indicate pyroptotic cells (scale bar=50 µm). (B) Western
blotting was performed to assess the protein expression levels of
GSDMD-N and ASC in RAOEC treated with 10 ng/ml TNF-α for 0, 6, 12,
24 and 48 h. (C) A LDH assay kit was used to assess the LDH release
of RAOECs. (D) Western blotting was performed to assess the protein
expression levels of NLRP3, C-caspase-1 and IL-1β. (E) Hoechst
33342 (blue) and PI (red) double-fluorescent staining of RAOECs,
after different treatments, was used to assess pyroptotic cell
death (scale bar=100 µm). The mRNA expression levels of (F) MALAT1
and (G) miR-30c-5p and protein expression levels of (H) Cx43 in
RAOECs were evaluated using reverse transcription-quantitative PCR
and western blotting, respectively. All data are presented as the
mean ± SD (n=3). *P<0.05, **P<0.01 and ***P<0.001 vs.
Cont. MALAT1, metastasis associated lung adenocarcinoma transcript
1; Cx43, connexin 43; miR, micro RNA; TNF-α, tumor necrosis
factor-α; RAOECs, rat aorta endothelial cells; GSDMD-N, N-terminus
of gasdermin D; ASC, apoptosis-associated speck-like protein
containing a CARD; Cont, control; C-caspase-1, cleaved caspase-1;
PI, propidium iodide. |
Knockdown of MALAT1 alleviates
TNF-α-mediated RAOEC pyroptosis
To further assess the effect of MALAT1 on
TNF-α-mediated RAOEC pyroptosis, three specific siRNAs targeting
MALAT1 were designed, and the mRNA expression level of MALAT1 was
assessed using RT-qPCR. The results demonstrated a significant
reduction in MALAT1 expression in the siMALAT1-1 (P<0.0001) and
siMALAT1-3 (P=0.0013) groups compared with the negative control,
and siMALAT1-1 was considered the best and so used for subsequent
experiments (Fig. 2A). Next, it
was demonstrated that knockdown of MALAT1 significantly reversed
the elevated mRNA expression level of MALAT1 caused by TNF-α
treatment in RAOECs (Fig. 2B).
Subsequently, the influence of silencing MALAT1 on TNF-α-induced
pyroptosis was assessed using LDH release, pyroptosis-related
protein expression levels and the percentage of PI-positive cell
numbers. The results demonstrated that the increase in LDH release
in TNF-α-stimulated RAOECs was significantly attenuated by MALAT1
knockdown (P=0.0007, Fig. 2C).
Moreover, MALAT1 knockdown significantly reversed the up-regulation
of NLRP3 (P=0.0161), GSDMD-N (P=0.0007), ASC (P=0.0159), cleaved
caspase-1 (P=0.0396) and IL-1β (P=0.0267) protein expression levels
in TNF-α-stimulated RAOECs compared with the TNF-α-stimulated
negative control (Fig. 2D).
Furthermore, the elevation of the number of PI-positive cells
induced by TNF-α was significantly suppressed by MALAT1 knockdown
(P=0.0135, Fig. 2E). These data
indicated that MALAT1 knockdown was effective in inhibiting
TNF-α-induced RAOEC pyroptosis.
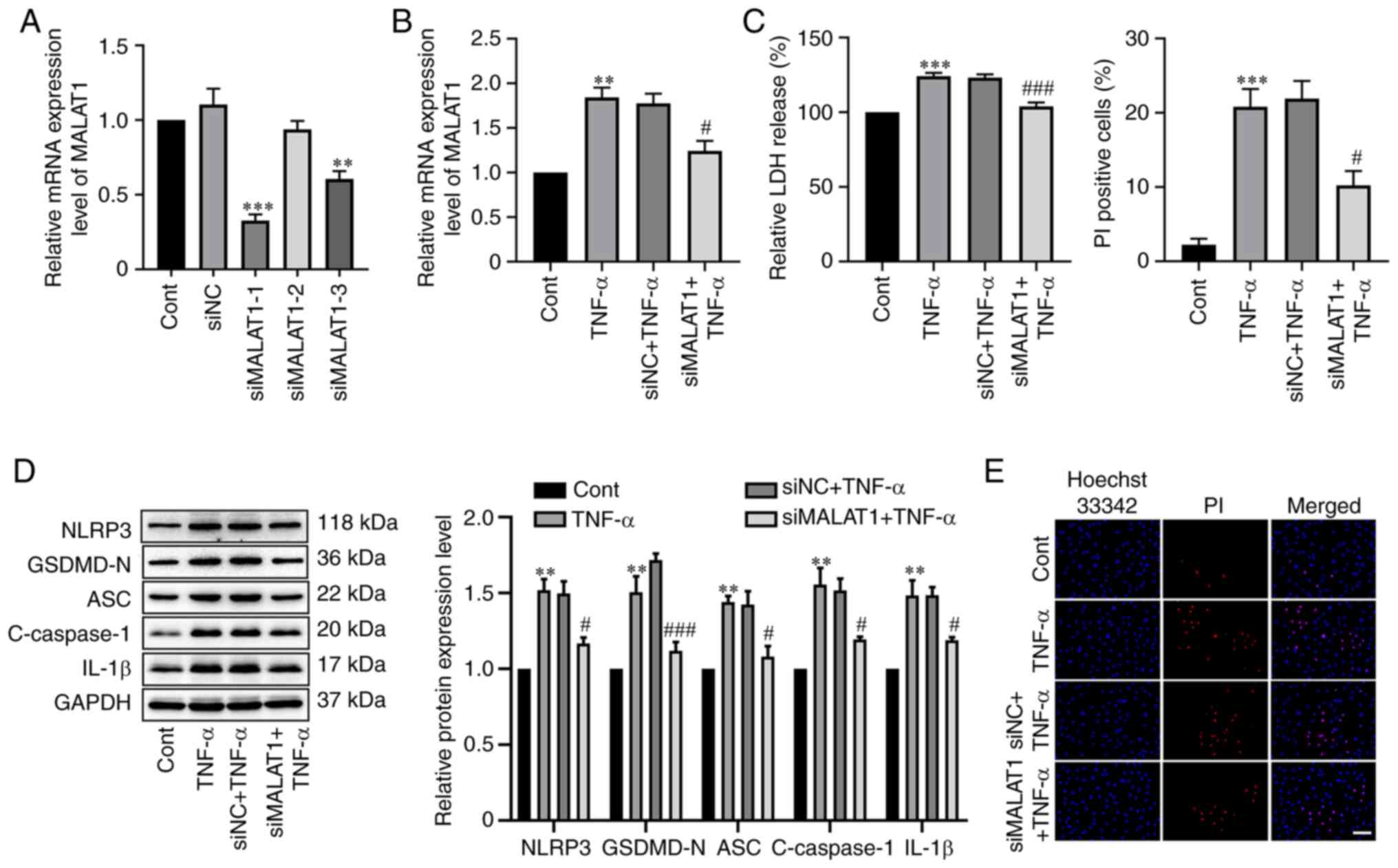 | Figure 2.MALAT1 knockdown inhibited
TNF-α-induced RAOEC pyroptosis. (A) The mRNA expression level of
MALAT1 was evaluated using RT-qPCR in RAOEC with Cont, siNC, or
specific siRNAs (siMALAT1-1, siMALAT1-2, siMALAT1-3). **P<0.01
and ***P<0.001 vs. siNC. (B) The mRNA expression level of MALAT1
in RAOECs transfected with siMALAT1 for 24 h and then treated with
TNF-α for 24 h was evaluated using RT-qPCR analysis. (C) A LDH
assay kit was used to assess LDH release. (D) Western blotting was
performed to assess the protein expression levels of NLRP3,
GSDMD-N, ASC, C-caspase-1 and IL-1β. (E) Hoechst 33342 (blue) and
PI (red) double-fluorescent staining of RAOECs, after different
treatments, was used to indicate pyroptotic cell death (scale
bar=100 µm). All data are presented as the mean ± SD (n=3).
**P<0.01 and ***P<0.001 vs. Cont. #P<0.05 and
###P<0.001 vs. siNC + TNF-α. MALAT1, metastasis
associated lung adenocarcinoma transcript 1; TNF-α, tumor necrosis
factor-α; RAOECs, rat aorta endothelial cells; siRNA, short
interfering RNA; Cont, control; NC, negative control; RT-qPCR,
reverse transcription-quantitative PCR; GSDMD-N, N-terminus of
gasdermin D; NLRP3, NLR family pyrin domain containing 3; ASC,
apoptosis-associated speck-like protein containing a CARD; Cont,
control; C-caspase-1, cleaved caspase-1; PI, propidium iodide. |
miR-30c-5p overexpression attenuates
TNF-α-mediated RAOEC pyroptosis
To elucidate the effect of miR-30c-5p in
TNF-α-mediated RAOEC pyroptosis, miR-30c-5p overexpression was
successfully established which significantly increased miR30c-5p
RNA expression levels compared with the negative control
(P<0.0001, Fig. 3A). In the
present study, it was also demonstrated that the RNA expression
level of miR-30c-5p in RAOECs transfected with 100 nM miR-30c-5p
inhibitor for 48 h was significantly decreased (P=0.0030). Next,
the cells were transfected with miR-30c-5p mimic before treatment
with TNF-α. RT-qPCR analysis demonstrated that in the miR-30c-5p
mimic + TNF-α group, the level of miR-30c-5p was significantly
increased compared with the mimic NC + TNF-α group (P=0.0009,
Fig. 3B). Furthermore, compared
with the mimic NC + TNF-α group, the miR-30c-5p mimic significantly
attenuated LDH release (P=0.0074, Fig.
3C), significantly reduced the increased protein expression
levels of NLRP3 (P=0.0408), GSDMD-N (P=0.0016), ASC (P=0.0199),
cleaved caspase-1 (P=0.0255) and IL-1β (P=0.0029) (Fig. 3D), and significantly reduced the
proportion of PI-positive cells (P=0.0125, Fig. 3E). These results indicated that the
overexpression of miR-30c-5p could inhibit TNF-α-mediated RAOEC
pyroptosis.
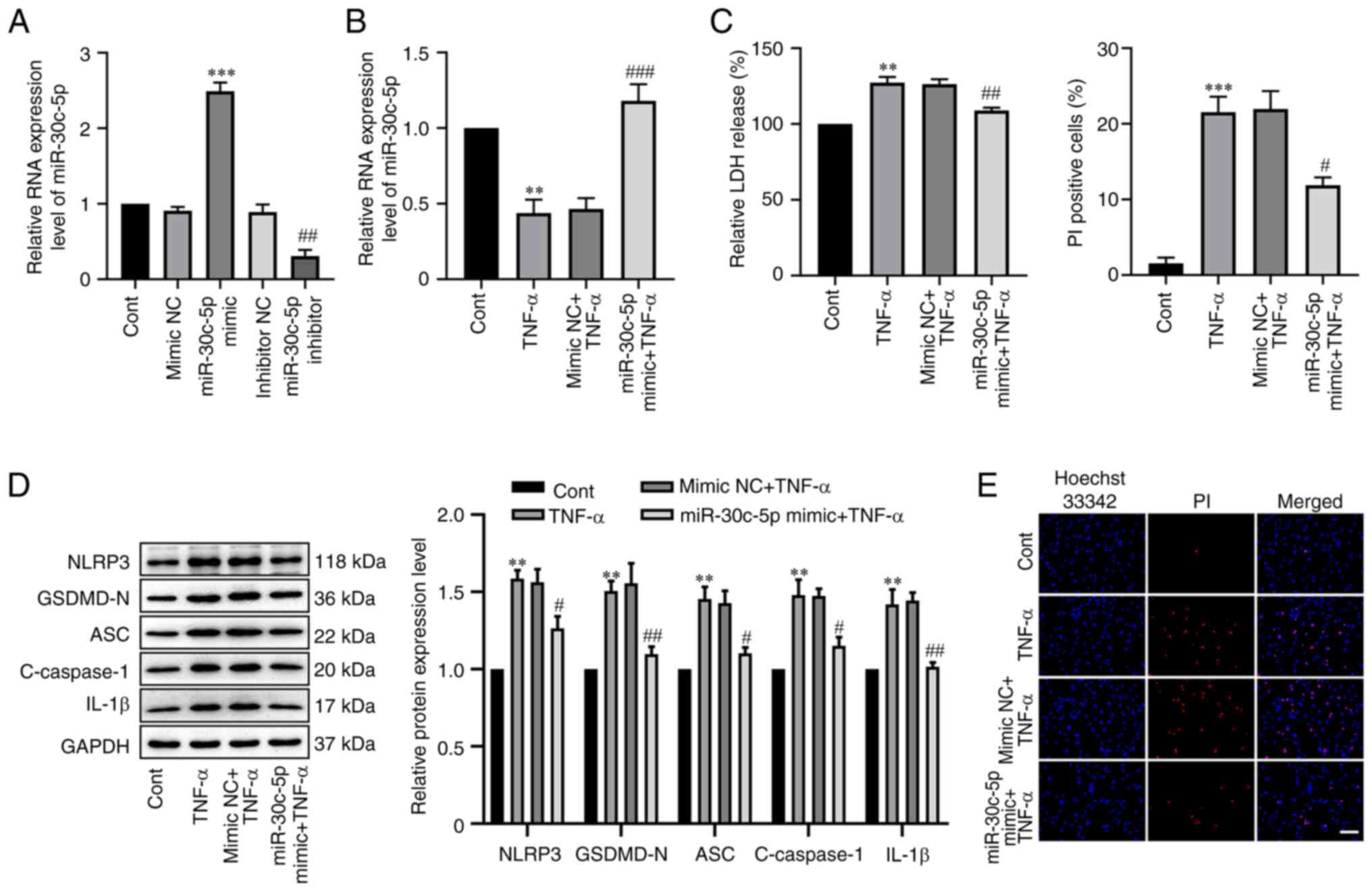 | Figure 3.Overexpression of miR-30c-5p reduced
TNF-α-mediated RAOEC pyroptosis. (A) The RNA expression level of
miR-30c-5p in RAOEC transfected with 30 nM miR-30c-5p mimic or 100
nM miR-30c-5p inhibitor for 48 h was determined using RT-qPCR. (B)
The RNA expression level of miR-30c-5p in RAOECs transfected with
miR-30c-5p mimic for 24 h and then treated with TNF-α for 24 h was
assessed using RT-qPCR. (C) A LDH assay kit was used to assess LDH
release. (D) Western blotting was performed to assess the protein
expression levels of NLRP3, GSDMD-N, ASC, C-caspase-1 and IL-1β.
(E) Hoechst 33342 (blue) and PI (red) double-fluorescent staining
of RAOECs, after different treatments, was used to indicate
pyroptotic cell death (scale bar=100 µm). All data are presented as
the mean ± SD (n=3). **P<0.01 and ***P<0.001 vs. Cont.
#P<0.05, ##P<0.01 and
###P<0.001 vs. mimic NC + TNF-α. RAOECs, rat aorta
endothelial cells; TNF-α, tumor necrosis factor-α; miR, micro RNA;
RT-qPCR, reverse transcription-quantitative PCR; GSDMD-N,
N-terminus of gasdermin D; NLRP3, NLR family pyrin domain
containing 3; ASC, apoptosis-associated speck-like protein
containing a CARD; Cont, control; C-caspase-1, cleaved caspase-1;
PI, propidium iodide. |
miR-30c-5p is a target of MALAT1 and
can target Cx43
Previous studies have reported that lncRNAs function
as a competitive endogenous RNAs (ceRNAs) of certain miRNAs to
regulate the expression and function of target mRNAs (27). To further elucidate the mechanism
by which MALAT1 was involved in TNF-α-induced RAOEC pyroptosis a
bioinformatic approach was used to screen the potential targets of
MALAT1. Using the StarBase (http://starbase.sysu.edu.cn/) and BiBiServ (http://bibiserv.techfak.uni–bielefeld.de/)
database, miR-30c-5p was identified as a potential target gene of
MALAT1 (Fig. 4A). Next, to verify
the predicted binding sites, a dual-luciferase reporter vector with
either the wild-type MALAT1 fragment or the mutant MALAT1 fragment
was constructed. The dual-luciferase reporter gene analysis results
demonstrated that miR-30c-5p overexpression significantly
attenuated the luciferase activity of the MALAT1-WT reporter
(P=0.0047), whereas the luciferase activity of MALAT1-MUT reporter
demonstrated no apparent change (Fig.
4B), which indicated a specific interaction between miR-30c-5p
and MALAT1. Furthermore, the RNA expression level of miR-30c-5p was
significantly elevated by MALAT1 knockdown compared with the
negative control as shown using RT-qPCR analysis (P=0.0430,
Fig. 4C). These demonstrated that
miR-30c-5p was a target of MALAT1 and was negatively regulated by
MALAT1.
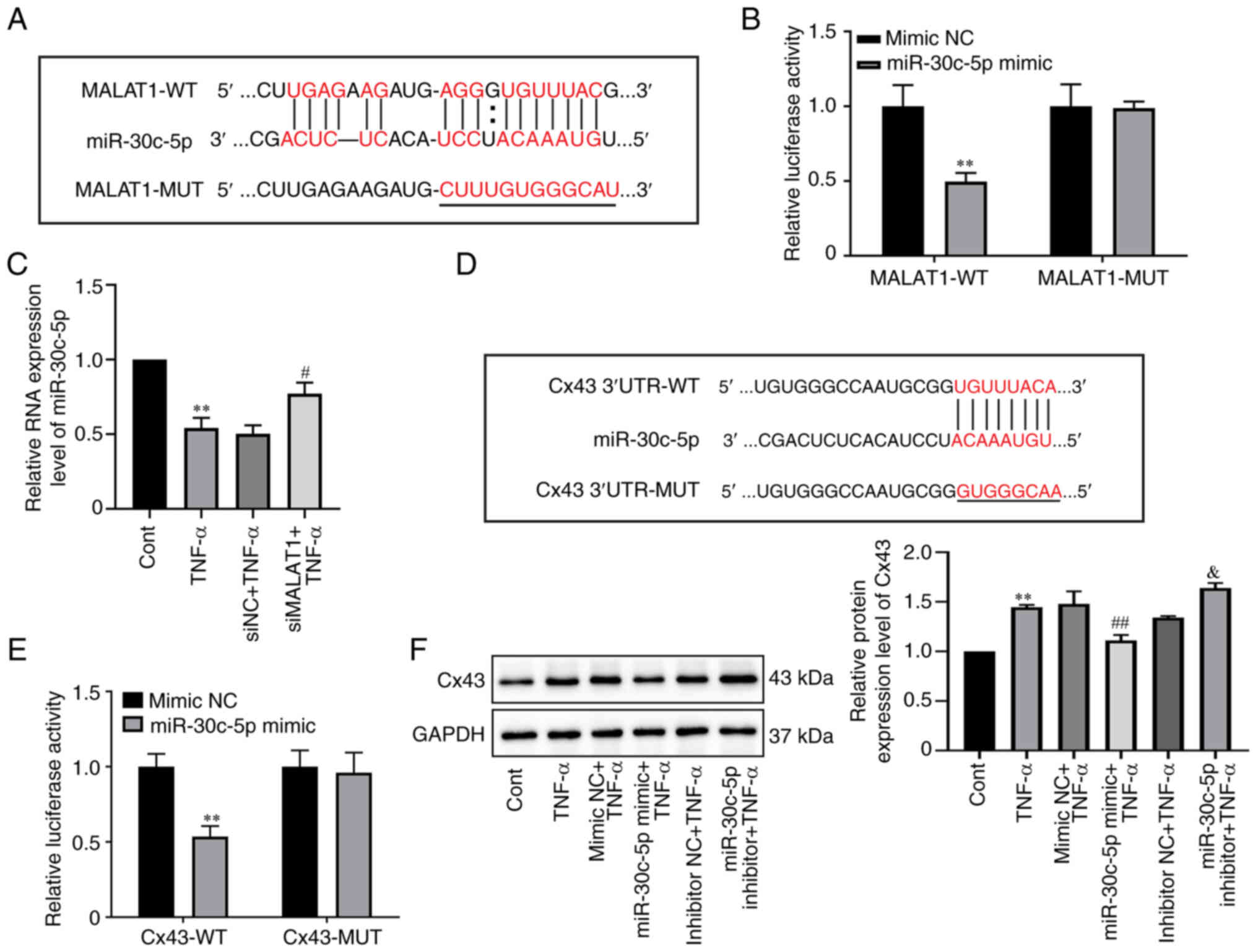 | Figure 4.miR-30c-5p is a target of MALAT1 and
can target Cx43 in RAOEC. (A) Predicted miR-30c-5p binding sites in
the MALAT1-WT and corresponding MALAT1-MUT. The red indicates the
binding sites. (B) Luciferase activity in each group was assessed
in 293T cells co-transfected with MALAT1-WT or MALAT1-MUT vector
and mimic NC or miR-30c-5p mimic. (C) The RNA expression level of
miR-30c-5p was assessed in RAOECs transfected with siMALAT1 and
then treated with TNF-α, using RT-qPCR. (D) Putative miR-30c-5p
binding sites in the 3′UTR of Cx43-WT mRNA and corresponding
Cx43-MUT. The red indicates the binding sites. (E) Luciferase
activity was evaluated in 293T cells co-transfected with WT or MUT
Cx43 reporter and mimic NC or miR-30c-5p mimic. (F) Western
blotting was performed to assess the protein expression level of
Cx43 in RAOECs transfected with miR-30c-5p mimic or miR-30c-5p
inhibitor and then treated with TNF-α. **P<0.01 vs. Cont or
mimic NC. #P<0.05 and ##P<0.01 vs. siNC
+ TNF-α or mimic NC + TNF-α. &P<0.05 vs.
inhibitor NC + TNF-α. All data are presented as the mean ± SD
(n=3). RAOECs, rat aorta endothelial cells; Cx43, connexin 43;
TNF-α, tumor necrosis factor-α; miR, micro RNA; siRNA, short
interfering RNA; RT-qPCR, reverse transcription-quantitative PCR;
NC, negative control; WT, wild-type; MUT, mutant. |
Similarly, it was demonstrated that the 3′UTR of
Cx43 contained certain miR-30c-5p binding sites according to the
TargetScan online software (Fig.
4D). To further confirm the predicted binding sites, a
dual-luciferase reporter vector with either the wild-type 3′UTR or
mutant 3′UTR of Cx43 was constructed. The results demonstrated that
the miR-30c-5p mimic significantly decreased the luciferase
activity of the Cx43-WT reporter (P=0.0019) in 293T cells, but had
no significant effect on the Cx43-MUT reporter (Fig. 4E). Moreover, compared with the
mimic NC + TNF-α groups, the protein expression level of Cx43 was
significantly decreased in the miR-30c-5p mimic + TNF-α groups in
RAOECs (P=0.0099), whereas compared with the inhibitor NC + TNF-α
groups, the protein expression level of Cx43 was significantly
increased in the miR-30c-5p inhibitor + TNF-α group (P=0.0393,
Fig. 4F). The above results
indicated the negative regulatory effect of miR-30c-5p on Cx43
expression in TNF-α-treated RAOECs.
Cx43 downregulation attenuated
TNF-α-mediated RAOEC pyroptosis
To further analyze the effect of Cx43 on
TNF-α-induced RAOEC pyroptosis, RAOECs were transfected with 50 nM
Cx43 siRNA and then stimulated using TNF-α. RT-qPCR and western
blotting analysis demonstrated that Cx43 siRNA transfection
significantly suppressed the mRNA (P=0.0149) and protein (P=0.0297)
expression levels of Cx43 in TNF-α-treated RAOECs compared with the
negative control (Fig. 5A and B).
Moreover, compared with the siNC + TNF-α group, silencing of Cx43
significantly reduced LDH release (P=0.0265, Fig. 5C). Furthermore, the significantly
increased levels of NLRP3 (P=0.0044), GSDMD-N (P=0.0310), ASC
(P=0.0129), cleaved caspase-1 (P=0.0279) and IL-1β (P=0.0493)
induced by TNF-α were significantly reduced by Cx43 knockdown
(Fig. 5D). Downregulation of Cx43
significantly reduced the proportion of PI-positive cells
stimulated by TNF-α compared with the negative control (P=0.0221,
Fig. 5E). These results
demonstrated that knockdown of Cx43 attenuated TNF-α-induced RAOEC
pyroptosis.
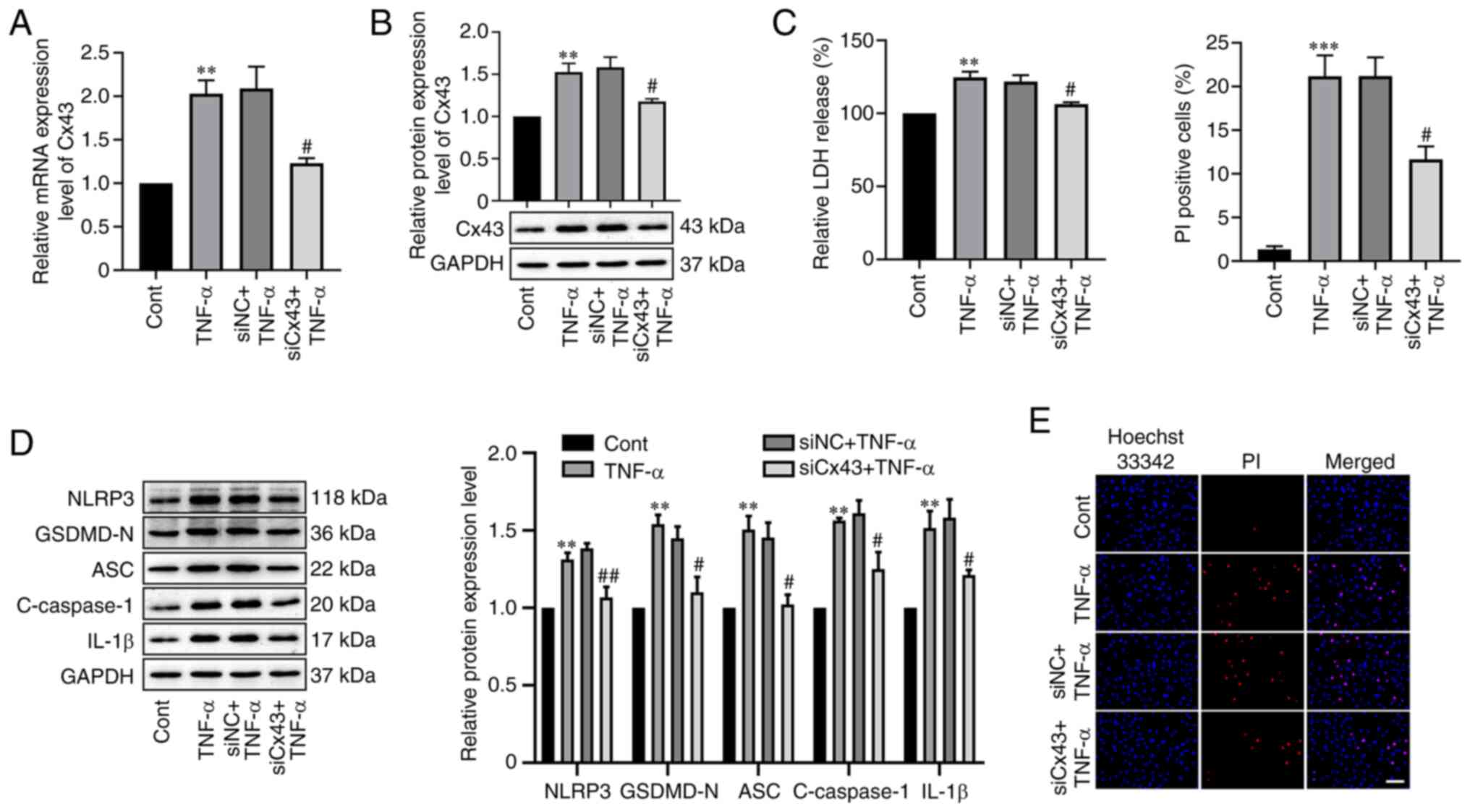 | Figure 5.Cx43 knockdown alleviated
TNF-α-induced RAOEC pyroptosis. (A) The mRNA expression level of
Cx43 in RAOECs transfected with siCx43 for 24 h and then treated
with TNF-α for 24 h was assessed using RT-qPCR. (B) Western
blotting was performed to assess the protein expression level of
Cx43 in RAOEC transfected with siMALAT1 for 24 h and then treated
with TNF-α for 24 h. (C) A LDH assay kit was used to assess LDH
release. (D) Western blotting was performed to assess the protein
expression levels of NLRP3, GSDMD-N, ASC, C-caspase-1 and IL-1β.
(E) Hoechst 33342 (blue) and PI (red) double-fluorescent staining
of RAOECs, after different treatments, was used to assess
pyroptotic cell death (scale bar=100 µm). All data are presented as
the mean ± SD (n=3). **P<0.01 and ***P<0.001 vs. Cont.
#P<0.05 and ##P<0.01 vs. siNC + TNF-α.
RAOECs, rat aorta endothelial cells; Cx43, connexin 43; TNF-α,
tumor necrosis factor-α; siRNA, short interfering RNA; RT-qPCR,
reverse transcription-quantitative PCR; NC, negative control;
GSDMD-N, N-terminus of gasdermin D; NLRP3, NLR family pyrin domain
containing 3; ASC, apoptosis-associated speck-like protein
containing a CARD; Cont, control; C-caspase-1, cleaved caspase-1;
PI, propidium iodide. |
MALAT1 knockdown inhibited
TNF-α-induced RAOEC pyroptosis via miR-30c-5p/Cx43 axis
To further elucidate whether MALAT1 could inhibit
TNF-α-induced RAOEC pyroptosis through the miR-30c-5p/Cx43 axis.
RAOECs were transfected with siMALAT1 (or siNC) alone or together
with miR-30c-5p inhibitor (or inhibitor NC) before treatment with
TNF-α. Western blotting demonstrated that the protein expression
level of Cx43 was significantly decreased by siMALAT1 transfection
in TNF-α-induced RAOECs (P=0.0374), but this effect was
significantly weakened by co-transfection of siMALAT1 with
miR-30c-5p inhibitor (P=0.0456, Fig.
6A). Similarly, MALAT1 knockdown significantly decreased LDH
release in TNF-α-induced RAOECs (P=0.0011), which was significantly
reversed by co-transfection of siMALAT1 and miR-30c-5p inhibitor
(P=0.0229, Fig. 6B). Furthermore,
it was demonstrated that siMALAT1 significantly decreased the
protein expression levels of NLRP3 (P=0.0100), GSDMD-N (P=0.0066),
ASC (P=0.0115), cleaved caspase-1 (P=0.0041) and IL-1β (P=0.0304)
(Fig. 6C), and the percentage of
PI-positive cells (P=0.0008, Fig.
6D) compared with the negative control. However,
co-transfection of siMALAT1 and miR-30c-5p inhibitor significantly
attenuated the decrease in the protein expression levels of NLRP3
(P=0.0232), GSDMD-N (P=0.0421), ASC (P=0.0342), cleaved caspase-1
(P=0.0157) and IL-1β (P=0.0363), and the percentage of PI-positive
cells (P=0.0174). The aforementioned results strongly indicated
that miR-30c-5p inhibitor could reverse the inhibitory effects of
siMALAT1 on TNF-α-induced RAOEC pyroptosis. Hence, it was concluded
that MALAT1 served a vital role in TNF-α-induced RAOEC pyroptosis
by the regulation of Cx43 expression by sponging miR-30c-5p.
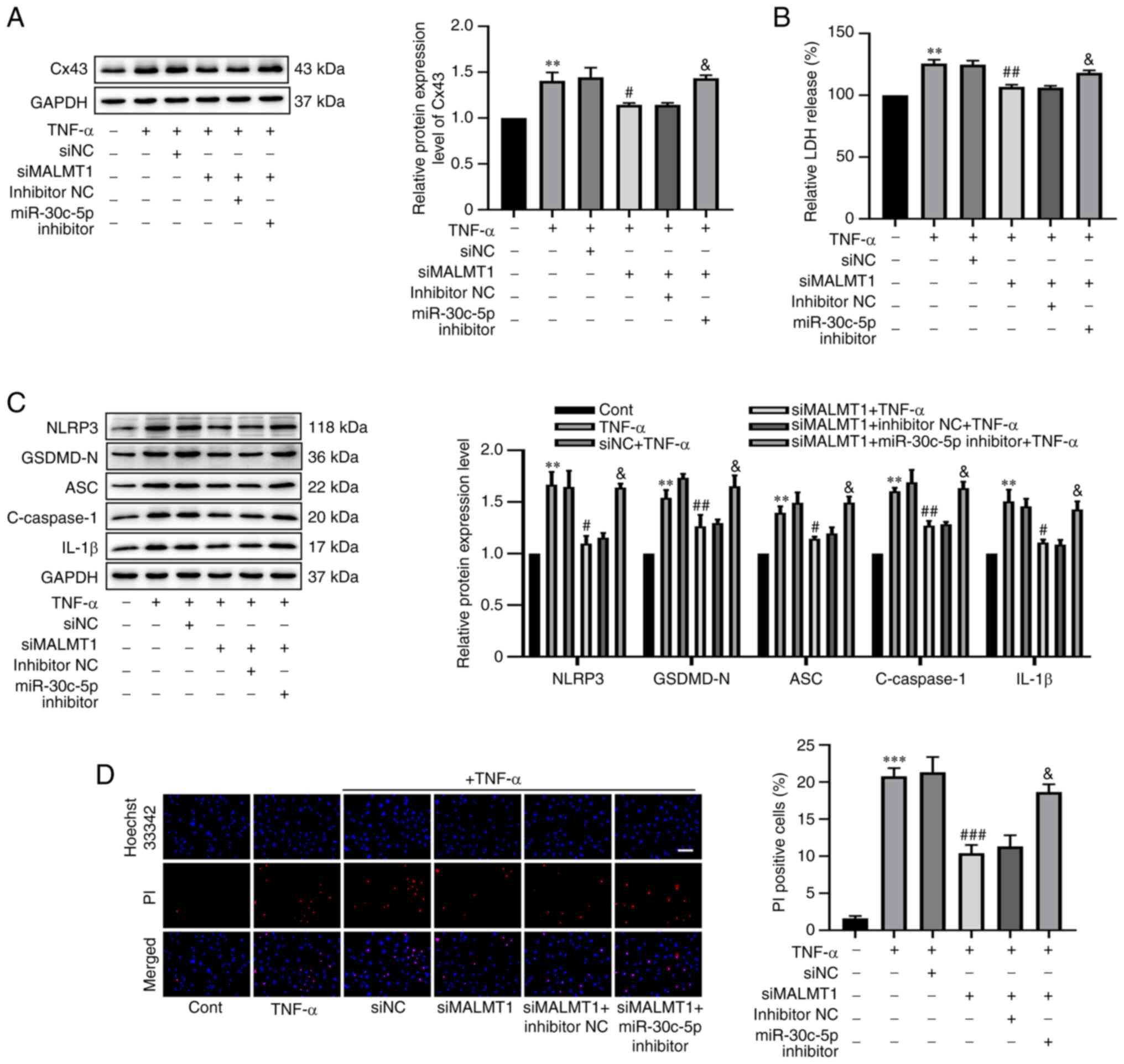 | Figure 6.Knockdown of MALAT1 reduced Cx43
expression through targeting miR-30c-5p to decrease TNF-α-induced
RAOEC pyroptosis. RAOECs were transfected with siNC, siMALAT1,
siMALAT1 + inhibitor NC or siMALAT1 + miR-30c-5p inhibitor and then
treated with TNF-α. (A) The protein expression level of Cx43 was
assessed using western blotting. (B) A LDH assay kit was used to
assess LDH release. (C) Western blotting was performed to assess
the protein expression levels of NLRP3, GSDMD-N, ASC, C-caspase-1
and IL-1β. (D) Hoechst 33342 (blue) and PI (red) double-fluorescent
staining of RAOECs, after different treatments, was used to assess
pyroptotic cell death (scale bar=100 µm). All data are presented as
mean ± SD (n=3). **P<0.01 and ***P<0.001 vs. Cont.
#P<0.05, ##P<0.01 and
###P<0.001 vs. siNC + TNF-α.
&P<0.05 vs. siMALAT1 + inhibitor NC + TNF-α.
MALAT1, metastasis associated lung adenocarcinoma transcript 1;
RAOECs, rat aorta endothelial cells; Cx43, connexin 43; TNF-α,
tumor necrosis factor-α; siRNA, short interfering RNA; RT-qPCR,
reverse transcription-quantitative PCR; NC, negative control;
GSDMD-N, N-terminus of gasdermin D; NLRP3, NLR family pyrin domain
containing 3; ASC, apoptosis-associated speck-like protein
containing a CARD; Cont, control; C-caspase-1, cleaved caspase-1;
PI, propidium iodide. |
Discussion
Endothelial dysfunction is considered to be an early
step in the development of AS (28). In recent decades, anti-inflammatory
agents have become an important therapeutic approach for AS
(29–31). Pyroptosis is the result of
inflammasome activation and has been reported to be closely
involved in AS. However, the molecular mechanism has not yet been
fully elucidated. Therefore, the present study assessed the
upstream regulatory mechanism of pyroptosis. Previous studies have
reported that cells incubated with certain concentrations of TNF-α
for different time points will exhibit varied effects, including
pyroptosis, apoptosis and exhaustion (32–34).
Furthermore, accumulated evidence has indicated that TNF-α is a
critical inflammatory factor that can increase pyroptosis-related
protein expression (32). For
instance, Wang et al (35)
reported that a decrease in TNF-α could attenuate the process of
pyroptosis. Moreover, ghrelin reduces pyroptosis, apoptosis and
autophagy in human hepatocytes treated with TNF-α (32). In the present study, it was
demonstrated that treatment with 10 ng/ml TNF-α for 24 h resulted
in a typical pyroptotic morphology and increase in LDH release,
pyroptosis-related protein expression and the proportion of
PI-positive cells in RAOECs, which suggested that TNF-α induced
RAOEC pyroptosis. However, whether treatment with 10 ng/ml TNF-α
for 24 h impacts cells exhaustion and apoptosis requires further
evaluation.
Recent studies have reported that MALAT1 is of
significance in the regulation of pyroptosis in numerous cell types
through direct or indirect pathways. For example, MALAT1 is
considered a ceRNA that regulates the level of NLRP3 by
downregulating miR-22 in high glucose-induced human endothelial
cell pyroptosis (17). Similarly,
knockdown of MALAT1 up-regulated miR-558 by decreasing GSDMD to
inhibit inflammation, apoptosis and pyroptosis of chondrocytes in
ankylosing spondylitis (36).
Furthermore, MALAT1 knockdown significantly decreased the
inflammation and pyroptosis of macrophages (37). In agreement with the aforementioned
previous studies, in the present study, MALAT1 was significantly
upregulated in TNF-α-induced RAOEC pyroptosis, whereas knockdown of
MALAT1 significantly attenuated this effect. The demonstrated that
MALAT1 was involved in TNF-α-induced RAOEC pyroptosis was an
innovation of the present study. LncRNAs are reported to function
as a ‘sponge’ or ‘ceRNA’, thus regulating miRNA expression
(38). In the present study, it
was demonstrated that TNF-α treatment significantly decreased the
miR-30c-5p expression level in RAOECs. Previous studies have
indicated that miR-30 family members, especially miR-30c-5p, act as
protective factors in numerous solid tumors, such as non-small cell
lung (39), pancreatic (40) and liver (41) tumors. Recently, miR-30c-5p has been
reported to be closely associated with the development of
cardiovascular diseases, including AS (19), diabetic nephropathy (42) and myocardial ischemia-reperfusion
injury (43). Furthermore, Li
et al (20) reported that
the level of miR-30c-5p was decreased in ox-LDL-mediated
endothelial cells, and that overexpression of miR-30c-5p suppressed
the endothelial cell pyroptosis triggered by ox-LDL. Consistent
with these findings, the present study also demonstrated that
overexpression of miR-30c-5p attenuated TNF-α-induced RAOEC
pyroptosis. Huntzinger et al (44) reported that microRNAs could induce
the translational repression of targeted mRNA (relatively common in
plants) or the degradation of targeted mRNA (relatively common in
mammals). Subsequently, by performing a bioinformatic analysis,
Pubmed search and dual-luciferase reporter assay, the potential
interaction between MALAT1 and miR-30c-5p was demonstrated.
Similarly, based on prediction using the TargetScan website, it was
hypothesized that Cx43 might be a target gene for miR-30c-5p, which
was involved in TNF-α-induced RAOEC pyroptosis. Furthermore, the
dual-luciferase reporter assays significantly demonstrated that
Cx43 was a direct target of miR-30c-5p, and was negatively
regulated by miR-30c-5p, in accordance with the results that TNF-α
treatment downregulated miR-30c-5p and upregulated Cx43 expression.
Cx43 is a transmembrane protein whose main function is to form a
gap junction that allows the exchange of molecules that are smaller
than 1.2 kDa, such as small molecules (ATP, GTP, etc.), ions
(K+, Ca2+, etc.) and secondary messengers
(cAMP, cGMP, etc.) (45). Cx43 can
function in various forms, namely, in gap junctions, in
hemichannels or by itself, and it is involved in all cell cycle
stages, such as growth, differentiation and apoptosis (46). Increasing evidence indicates that
Cx43 is also closely associated with the development of
cardiovascular diseases, especially AS (47,48).
Nevertheless, the underlying mechanisms are not clearly defined.
Morel et al (49) reported
that decreased levels of Cx43 served a protective role in the
development of atherosclerotic lesions in mice. Moreover,
accumulating research has demonstrated that Cx43 is closely related
to inflammasome activation and Cx43 gap junctions or hemichannels
allowing communication between the intracellular and extracellular
milieu (50,51). However, Cx43 can regulate immune
cell activation, which is influenced by inflammasome activation
(52). Similarly, Cx43 is
associated with NLRP3 inflammasome activation in nerve pain
(53), retinal disease (54) and chronic kidney disease (55). Zhang et al (56) reported that bioactive glass
suppressed endothelial cell pyroptosis via decrease of the levels
of Cx43 and reactive oxygen species. Blocking Cx43 alleviated renal
fibrosis by decreasing the pyroptosis of macrophages (57). Consistently, the results of the
present study demonstrated that Cx43 expression was markedly
elevated in TNF-α-induced RAOECs and knockdown of Cx43 attenuated
TNF-α-induced RAOEC pyroptosis, which indicated that Cx43 was
related to the TNF-α-induced RAOEC pyroptosis.
The results of the present study demonstrated that
MALAT1 could indirectly regulate the level of Cx43 by targeting
miR-30c-5p. Western blotting analysis demonstrated that MALAT1
knockdown significantly reduced Cx43 expression, while miR-30c-5p
inhibition significantly reversed this effect. Furthermore,
miR-30c-5p inhibition suppressed the MALAT1 knockdown-induced
inhibitory effect on pyroptosis in TNF-α-induced RAOEC. The present
study demonstrated that MALAT1 could regulate Cx43 expression in
TNF-α-induced RAOEC pyroptosis by sponging miR-30c-5p.
To the best of our knowledge, the present study is
the first to demonstrate that MALAT1 regulates TNF-α-induced RAOEC
pyroptosis via the miR-30c-5p/Cx43 axis, which further elucidates
the pathogenesis of AS. However, certain limitations due to the
complexity and diversity of the regulation between molecules in the
cells used in the present study need to be taken into consideration
in the future. For example, the effects of the
MALAT1/miR-30c-5p/Cx43 axis in RAOEC pyroptosis in AS need to be
further assessed in vivo. Secondly, in the present study,
bioinformatic analyses demonstrated that miR-30c-5p has a broadly
conserved binding site with lncRNA MALAT1, and it is known that
miR-30c-5p is closely related to endothelial cells injury in AS
(20). Furthermore, previous
studies have reported that miR-30c-5p was a target gene of MALAT1
(58–60). However, the relationship between
miRNA and lncRNA is very complex, and it is not currently possible
to rule out other possible targets of MALAT1. Therefore, more
molecules, such as miR-24, miR-204-5p and miR-26b-5p, need to be
further evaluated in future. Finally, the present study was at the
early stage and mainly assessed the role and underlying mechanisms
of MALAT1 in TNF-α-mediated endothelial cell pyroptosis. Further
studies are required to elucidate if manipulation of these genes
impacted tube formation and angiogenesis of endothelial cells in
vitro and in vivo.
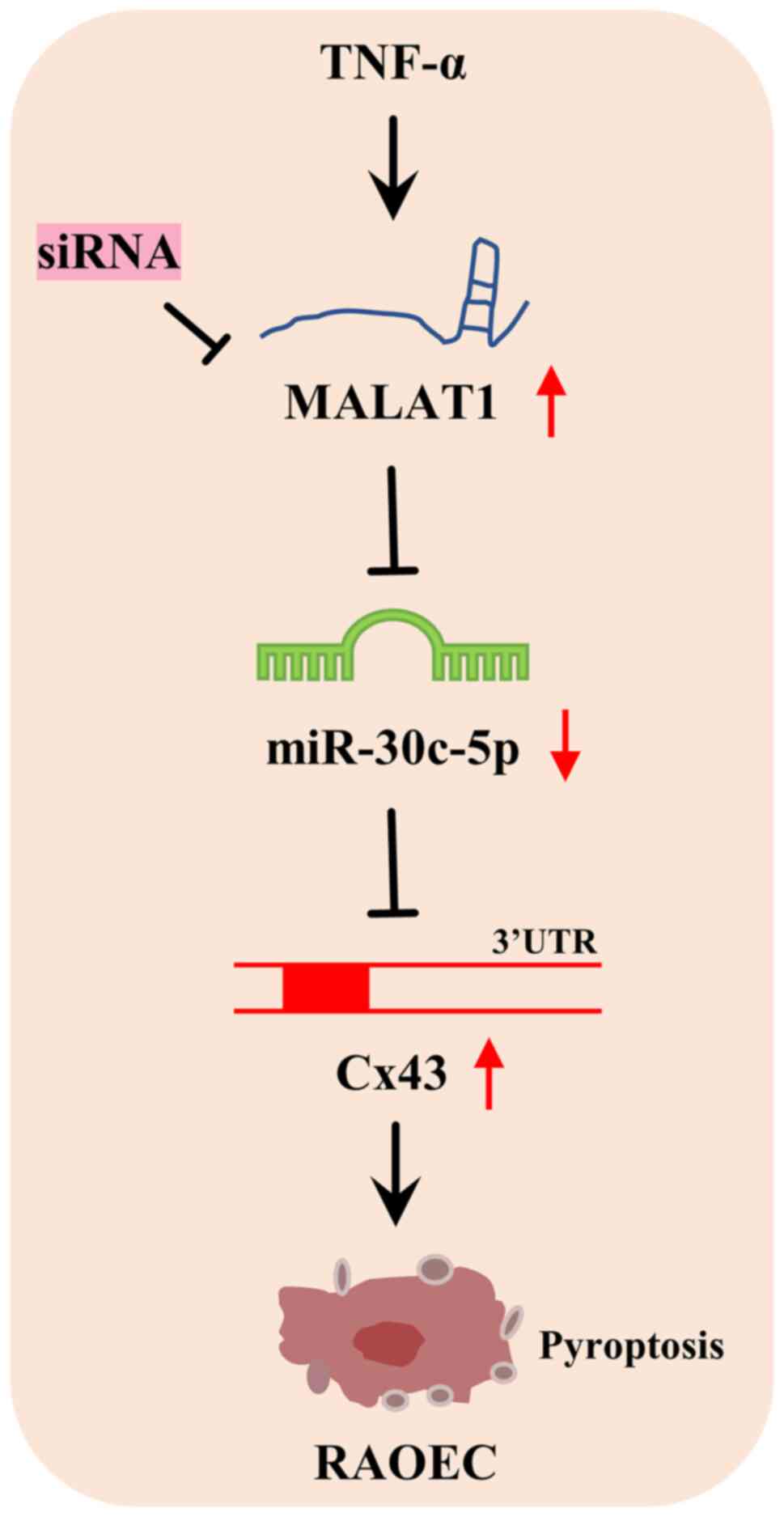
In summary, MALAT1 was demonstrated to be
significantly elevated in TNF-α-treated RAOECs and targeted
miR-30c-5p expression. As a target gene of miR-30c-5p, Cx43 was
significantly elevated in TNF-α-induced RAOECs. Knockdown of MALAT1
could inhibit the TNF-α-induced pyroptosis of RAOECs by indirectly
downregulating Cx43 via the targeting of miR-30c-5p expression
(Fig. 7), which suggested that
MALAT1 may be a potential therapeutic target for the treatment of
AS.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 82160686), the Key Program of the
Natural Science Foundation of Jiangxi Province (grant no.
20202ACB206001), the Key Research and Development Program of
Jiangxi Province (grant no. 20192BBG70012), and the Research Fund
for Key Laboratory of Drug Targets and Drug Screening of Jiangxi
Province (grant no. 20171BCD40007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZJY and RL were responsible for conceptualization,
the methodology, data curation and writing the original draft of
the manuscript. XJH was responsible for conceptualization, formal
analysis and reviewing the manuscript. CLQ and LHL were responsible
for the methodology and data analysis. GLD, ZQL and YL were
responsible for the methodology and data interpretation. LPJ was
responsible for conceptualization, the methodology, formal
analysis, reviewing the manuscript, funding acquisition and
provided supervision. Z-JY and RL confirm the authenticity of all
the raw data.
Ethics approval and consent for
publication
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LncRNAs
|
long non-coding RNAs
|
|
AS
|
atherosclerosis
|
|
MALAT1
|
metastasis associated lung
adenocarcinoma transcript 1
|
|
TNF-α
|
tumor necrosis factor-α
|
|
RAOEC
|
rat aorta endothelial cell
|
|
miR
|
microRNA
|
|
Cx43
|
connexin 43
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
GSDMD
|
gasdermin D
|
|
ASC
|
apoptosis-associated speck-like
protein containing a CARD
|
|
GSDMD-N
|
N-terminus of gasdermin D
|
|
HDAC11
|
histone deacetylase 11
|
|
HFD
|
high-fat diet
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
NLRP3
|
NLR family pyrin domain containing
3
|
|
miRNAs
|
micro RNAs
|
|
FOXO3
|
forkhead box O3
|
|
siRNA
|
short interfering RNA
|
|
GJA1
|
gap junction protein alpha 1
|
|
PI
|
propidium iodide
|
|
NC
|
negative control
|
|
ceRNAs
|
competitive endogenous RNAs
|
|
LDH
|
lactate dehydrogenase
|
|
WT
|
wild-type
|
|
MUT
|
mutant
|
|
Cont
|
control
|
|
C-caspase-1
|
cleaved caspase-1
|
|
F
|
forward
|
|
R
|
reverse
|
References
|
1
|
Li Y, Zhang L, Ren P, Yang Y, Li S, Qin X,
Zhang M, Zhou M and Liu W: Qing-Xue-Xiao-Zhi formula attenuates
atherosclerosis by inhibiting macrophage lipid accumulation and
inflammatory response via TLR4/MyD88/NF-κB pathway regulation.
Phytomedicine. 93:1538122021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Libby P: Inflammation during the life
cycle of the atherosclerotic plaque. Cardiovasc Res. 117:2525–2536.
2021.PubMed/NCBI
|
|
3
|
Li M, Wang ZW, Fang LJ, Cheng SQ, Wang X
and Liu NF: Programmed cell death in atherosclerosis and vascular
calcification. Cell Death Dis. 13:4672022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qian Z, Zhao Y, Wan C, Deng Y, Zhuang Y,
Xu Y, Zhu Y, Lu S and Bao Z: Pyroptosis in the initiation and
progression of atherosclerosis. Front Pharmacol. 12:6529632021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He X, Fan X, Bai B, Lu N, Zhang S and
Zhang L: Pyroptosis is a critical immune-inflammatory response
involved in atherosclerosis. Pharmacol Res. 165:1054472021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, Wu J, Zeng Y, Chen K, Wang C, Yang
S, Sun N, Chen H, Duan K and Zeng G: Pyroptosis: A pro-inflammatory
type of cell death in cardiovascular disease. Clin Chim Acta.
510:62–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Jiao Y, Li X, Gao S, Zhou N, Duan
J and Zhang M: Pyroptosis: A new insight into eye disease therapy.
Front Pharmacol. 12:7971102021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burdette BE, Esparza AN, Zhu H and Wang S:
Gasdermin D in pyroptosis. Acta Pharm Sin B. 11:2768–2782. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He B, Nie Q, Wang F, Han Y, Yang B, Sun M,
Fan X, Ye Z, Liu P and Wen J: Role of pyroptosis in atherosclerosis
and its therapeutic implications. J Cell Physiol. 236:7159–7175.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao F, Jin Z, Zheng Z, Lv X, Ren L, Yang
J, Chen D, Wang B, Yang W, Chen L, et al: HDAC11 promotes both
NLRP3/caspase-1/GSDMD and caspase-3/GSDME pathways causing
pyroptosis via ERG in vascular endothelial cells. Cell Death
Discov. 8:1122022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao F, Jin Z, Lv X, Zheng Z, Gao H, Deng
Y, Liu Y, Chen L, Wang W, He J, et al: Hydroxytyrosol acetate
inhibits vascular endothelial cell pyroptosis via the HDAC11
signaling pathway in atherosclerosis. Front Pharmacol.
12:6562722021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao F, Lv X, Jin Z, Chen D, Zheng Z, Yang
J, Ren L, Wang B, Wang W, He J, et al: Sirt6 inhibits vascular
endothelial cell pyroptosis by regulation of the Lin28b/let-7
pathway in atherosclerosis. Int Immunopharmacol. 110:1090562022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao J, Chen X, Wei P, Wang Y, Li P and
Shao K: Regulation of pyroptosis in cardiovascular pathologies:
Role of noncoding RNAs. Mol Ther Nucleic Acids. 25:220–236. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu A, Sun W and Mou F: lncRNA-MALAT1
promotes high glucose-induced H9C2 cardiomyocyte pyroptosis by
downregulating miR-141-3p expression. Mol Med Rep. 23:2592021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han Y, Qiu H, Pei X, Fan Y, Tian H and
Geng J: Low-dose sinapic acid abates the pyroptosis of macrophages
by downregulation of lncRNA-MALAT1 in rats with diabetic
atherosclerosis. J Cardiovasc Pharmacol. 71:104–112. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Zeng L, Cao C, Lu C, Lian W, Han J,
Zhang X, Zhang J, Tang T and Li M: Long noncoding RNA MALAT1
regulates renal tubular epithelial pyroptosis by modulated miR-23c
targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res.
350:327–335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song Y, Yang L, Guo R, Lu N, Shi Y and
Wang X: Long noncoding RNA MALAT1 promotes high glucose-induced
human endothelial cells pyroptosis by affecting NLRP3 expression
through competitively binding miR-22. Biochem Biophys Res Commun.
509:359–366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rahimian P and He JJ: HIV-1 tat-shortened
neurite outgrowth through regulation of microRNA-132 and its target
gene expression. J Neuroinflammation. 13:2472016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ceolotto G, Giannella A, Albiero M,
Kuppusamy M, Radu C, Simioni P, Garlaschelli K, Baragetti A,
Catapano AL, Iori E, et al: miR-30c-5p regulates
macrophage-mediated inflammation and pro-atherosclerosis pathways.
Cardiovasc Res. 114:19082018. View Article : Google Scholar
|
|
20
|
Li P, Zhong X, Li J, Liu H, Ma X, He R and
Zhao Y: MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated
endothelial cell pyroptosis through FOXO3 down-regulation in
atherosclerosis. Biochem Biophys Res Commun. 503:2833–2840. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morel S, Burnier L and Kwak BR: Connexins
participate in the initiation and progression of atherosclerosis.
Semin Immunopathol. 31:49–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klotz LO: Posttranscriptional regulation
of connexin-43 expression. Arch Biochem Biophys. 524:23–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B,
Zhang Y, Xu C, Bai Y, Wang H, et al: The muscle-specific microRNA
miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1
and KCNJ2. Nat Med. 13:486–491. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Osbourne A, Calway T, Broman M, McSharry
S, Earley J and Kim GH: Downregulation of connexin43 by
microRNA-130a in cardiomyocytes results in cardiac arrhythmias. J
Mol Cell Cardiol. 74:53–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reus JB, Trivino-Soto GS, Wu LI, Kokott K
and Lim ES: SV40 large T antigen is not responsible for the Loss of
STING in 293T cells but can inhibit cGAS-STING interferon
induction. Viruses. 12:1372020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Zeng X, Wang N, Zhao W, Zhang X,
Teng S, Zhang Y and Lu Z: Long noncoding RNA DANCR, working as a
competitive endogenous RNA, promotes ROCK1-mediated proliferation
and metastasis via decoying of miR-335-5p and miR-1972 in
osteosarcoma. Mol Cancer. 17:892018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Milutinović A, Šuput D and Zorc-Pleskovič
R: Pathogenesis of atherosclerosis in the tunica intima, media, and
adventitia of coronary arteries: An updated review. Bosn J Basic
Med Sci. 20:21–30. 2020.PubMed/NCBI
|
|
29
|
Sitia S, Tomasoni L, Atzeni F, Ambrosio G,
Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P,
Camici P, et al: From endothelial dysfunction to atherosclerosis.
Autoimmun Rev. 9:830–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu S, Ilyas I, Little PJ, Li H, Kamato D,
Zheng X, Luo S, Li Z, Liu P, Han J, et al: Endothelial dysfunction
in atherosclerotic cardiovascular diseases and beyond: From
mechanism to pharmacotherapies. Pharmacol Rev. 73:924–967. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raggi P, Genest J, Giles JT, Rayner KJ,
Dwivedi G, Beanlands RS and Gupta M: Role of inflammation in the
pathogenesis of atherosclerosis and therapeutic interventions.
Atherosclerosis. 276:98–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ezquerro S, Mocha F, Frühbeck G,
Guzmán-Ruiz R, Valentí V, Mugueta C, Becerril S, Catalán V,
Gómez-Ambrosi J, Silva C, et al: Ghrelin reduces TNF-α-induced
human hepatocyte apoptosis, autophagy, and pyroptosis: Role in
obesity-associated NAFLD. J Clin Endocrinol Metab. 104:21–37.
2019.PubMed/NCBI
|
|
33
|
Liu Y and Tie L: Apolipoprotein M and
sphingosine-1-phosphate complex alleviates TNF-α-induced
endothelial cell injury and inflammation through PI3K/AKT signaling
pathway. BMC Cardiovasc Disord. 19:2792019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jing ZT, Liu W, Xue CR, Wu SX, Chen WN,
Lin XJ and Lin X: AKT activator SC79 protects hepatocytes from
TNF-α-mediated apoptosis and alleviates d-Gal/LPS-induced liver
injury. Am J Physiol Gastrointest Liver Physiol. 316:G387–G396.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Zhang H, Chen Q, Jiao F, Shi C,
Pei M, Lv J, Zhang H, Wang L and Gong Z: TNF-α/HMGB1 inflammation
signalling pathway regulates pyroptosis during liver failure and
acute kidney injury. Cell Prolif. 53:e128292020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen W, Wang F, Wang J, Chen F and Chen T:
The molecular mechanism of long non-coding RNA MALAT1-mediated
regulation of chondrocyte pyroptosis in ankylosing spondylitis. Mol
Cells. 45:365–375. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shu B, Zhou YX, Li H, Zhang RZ, He C and
Yang X: The METTL3/MALAT1/PTBP1/USP8/TAK1 axis promotes pyroptosis
and M1 polarization of macrophages and contributes to liver
fibrosis. Cell Death Discov. 7:3682021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kato M, Wang M, Chen Z, Bhatt K, Oh HJ,
Lanting L, Deshpande S, Jia Y, Lai JY, O'Connor CL, et al: An
endoplasmic reticulum stress-regulated lncRNA hosting a microRNA
megacluster induces early features of diabetic nephropathy. Nat
Commun. 7:128642016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Shi H, Du Y, Zhao G, Wang X, Li Q,
Liu J, Ye L, Shen Z, Guo Y and Huang Y: lncRNA DLEU2 modulates cell
proliferation and invasion of non-small cell lung cancer by
regulating miR-30c-5p/SOX9 axis. Aging (Albany NY). 11:7386–7401.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka T, Okada R, Hozaka Y, Wada M,
Moriya S, Satake S, Idichi T, Kurahara H, Ohtsuka T and Seki N:
Molecular pathogenesis of pancreatic ductal adenocarcinoma: Impact
of miR-30c-5p and miR-30c-2-3p regulation on oncogenic genes.
Cancers (Basel). 12:27312020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He Z, Tian M and Fu X: Reduced expression
of miR-30c-5p promotes hepatocellular carcinoma progression by
targeting RAB32. Mol Ther Nucleic Acids. 26:603–612. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao BH, Wu H, Wang X, Ji LL and Chen C:
MiR-30c-5p inhibits high glucose-induced EMT and renal fibrogenesis
by down-regulation of JAK1 in diabetic nephropathy. Eur Rev Med
Pharmacol Sci. 24:1338–1349. 2020.PubMed/NCBI
|
|
43
|
Wang L, Chen X and Wang Y, Zhao L, Zhao X
and Wang Y: MiR-30c-5p mediates the effects of panax notoginseng
saponins in myocardial ischemia reperfusion injury by inhibiting
oxidative stress-induced cell damage. Biomed Pharmacother.
125:1099632020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martins-Marques T, Ribeiro-Rodrigues T,
Batista-Almeida D, Aasen T, Kwak BR and Girao H: Biological
functions of connexin43 beyond intercellular communication. Trends
Cell Biol. 29:835–847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li C, Tian M, Gou Q, Jia YR and Su X:
Connexin43 modulates X-ray-induced pyroptosis in human umbilical
vein endothelial cells. Biomed Environ Sci. 32:177–188.
2019.PubMed/NCBI
|
|
47
|
Ji H, Qiu R, Gao X, Zhang R, Li X, Hei Z
and Yuan D: Propofol attenuates monocyte-endothelial adhesion via
modulating connexin43 expression in monocytes. Life Sci.
232:1166242019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meghwani H and Berk BC: MST1
kinase-Cx43-YAP/TAZ pathway mediates disturbed flow endothelial
dysfunction. Circ Res. 131:765–767. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Morel S, Chanson M, Nguyen TD, Glass AM,
Sarieddine MZ, Meens MJ, Burnier L, Kwak BR and Taffet SM:
Titration of the gap junction protein Connexin43 reduces
atherogenesis. Thromb Haemost. 112:390–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yin X, Feng L, Ma D, Yin P, Wang X, Hou S,
Hao Y, Zhang J, Xin M and Feng J: Roles of astrocytic connexin-43,
hemichannels, and gap junctions in oxygen-glucose
deprivation/reperfusion injury induced neuroinflammation and the
possible regulatory mechanisms of salvianolic acid B and
carbenoxolone. J Neuroinflammation. 15:972018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu Y, Chen X, Lu Y, Fan S, Yang Y, Chen
Q, Huang Q, Xia L, Wei Y, Zheng J and Liu X: Diphenyleneiodonium
enhances P2X7 dependent non-opsonized phagocytosis and suppresses
inflammasome activation via blocking CX43-mediated ATP leakage.
Pharmacol Res. 166:1054702021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang Y, Mao Z, Zhang Z, Obata F, Yang X,
Zhang X, Huang Y, Mitsui T, Fan J, Takeda M and Yao J: Connexin43
contributes to inflammasome activation and
lipopolysaccharide-initiated acute renal injury via modulation of
intracellular oxidative status. Antioxid Redox Signal.
31:1194–1212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tonkin RS, Bowles C, Perera CJ, Keating
BA, Makker PGS, Duffy SS, Lees JG, Tran C, Don AS, Fath T, et al:
Attenuation of mechanical pain hypersensitivity by treatment with
Peptide5, a connexin-43 mimetic peptide, involves inhibition of
NLRP3 inflammasome in nerve-injured mice. Exp Neurol. 300:1–12.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lyon H, Shome A, Rupenthal ID, Green CR
and Mugisho OO: Tonabersat inhibits connexin43 hemichannel opening
and inflammasome activation in an in vitro retinal epithelial cell
model of diabetic retinopathy. Int J Mol Sci. 22:2982020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Price GW, Chadjichristos CE, Kavvadas P,
Tang SCW, Yiu WH, Green CR, Potter JA, Siamantouras E, Squires PE
and Hills CE: Blocking connexin-43 mediated hemichannel activity
protects against early tubular injury in experimental chronic
kidney disease. Cell Commun Signal. 18:792020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang K, Chai B, Ji H, Chen L, Ma Y, Zhu
L, Xu J, Wu Y, Lan Y, Li H, et al: Bioglass promotes wound healing
by inhibiting endothelial cell pyroptosis through regulation of the
connexin 43/reactive oxygen species (ROS) signaling pathway. Lab
Invest. 102:90–101. 2022. View Article : Google Scholar
|
|
57
|
Xu H, Wang M, Li Y, Shi M, Wang Z, Cao C,
Hong Y, Hu B, Zhu H, Zhao Z, et al: Blocking connexin 43 and its
promotion of ATP release from renal tubular epithelial cells
ameliorates renal fibrosis. Cell Death Dis. 13:5112022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xia J, Tian Y, Shao Z, Li C, Ding M, Qi Y,
Xu X, Dai K, Wu C, Yao W and Hao C: MALAT1-miR-30c-5p-CTGF/ATG5
axis regulates silica-induced experimental silicosis by mediating
EMT in alveolar epithelial cells. Ecotoxicol Environ Saf.
249:1143922023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jiang T, Cai Z, Ji Z, Zou J, Liang Z,
Zhang G, Liang Y, Lin H and Tan M: The lncRNA MALAT1/miR-30/spastin
axis regulates hippocampal neurite outgrowth. Front Cell Neurosci.
14:5557472020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yi J, Liu D and Xiao J: LncRNA MALAT1
sponges miR-30 to promote osteoblast differentiation of
adipose-derived mesenchymal stem cells by promotion of Runx2
expression. Cell Tissue Res. 376:113–121. 2019. View Article : Google Scholar : PubMed/NCBI
|