Introduction
Glioblastoma (GBM; World Health Organization grade
IV) is the most common malignant primary cancer of the central
nervous system, and is characterized by aggressive invasiveness and
proliferation (1). The incidence
of glioblastoma ranges from 0.59 to 5 per 100,000 persons and keeps
rising in certain countries, such as Korea and England (2), possibly as a consequence of an aging
population, increasing ease of access to neuroimaging, exposure to
ionizing radiation, radiofrequency electromagnetic fields and air
pollution (3–6). Although progress has been made in
diagnostic and prognostic stratification, the 5-year survival of
GBM patients is ~5% after initial diagnosis (7) and the median survival is ~14.6 months
(8,9). Therefore, elucidation of the
molecular mechanism of GBM development is very important for
finding new therapeutic strategies.
Vav guanine nucleotide exchange factor 3 (VAV3) is a
member of the VAV gene family. The VAV proteins are guanine
nucleotide exchange factors (GEFs) which regulate the Rho family
GTPases. There are three members of the Vav kinase family: VAV1,
VAV2 and VAV3. VAV1 is mainly expressed in hematopoietic cells,
whereas VAV2 and VAV3 are ubiquitous in numerous cell types
(10,11). VAV family members are highly
homologous at the protein level (50–70%) and share an array of
structural motifs, therefore they have the overlapping and unique
functions (12). Previous studies
have reported that VAV3 is an oncogene and regulates numerous
cellular signaling process, such as cell proliferation and
apoptosis, gene transcription, and cytoskeleton organization
(13,14). VAV3 has been reported to be
overexpressed in numerous human cancers, including prostate cancer,
endometrial cancer, breast cancer and colorectal cancer (10,14–16).
VAV3 can regulate prostate cancer cell growth via activation of the
androgen receptor-mediated signaling axis (15). VAV3 overexpression is directly
correlated with decreased survival in gastric cancer, and knockdown
of VAV3 has been reported to inhibit cancer proliferation both
in vitro and in vivo (17). A study reported that the
Rac-activating GEFs Trio, Ect2 and Vav3 regulated glioblastoma cell
migration and invasion (18).
However, the expression and prognostic significance of VAV3 in
glioblastoma is still unknown and needs to be further assessed.
Materials and methods
Cell lines and cancer stem-like cell
culture
U251 cells (cat no. TCHu58) and U87 glioblastoma of
unknown origin (cat. no. TCHu138) cells were purchased from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences (Shanghai, China) and the cell lines were identified by
STR. Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.). The serum-free medium (SFM) was composed of DMEM/F12 (Gibco;
Thermo Fisher Scientific, Inc.) with 20 µl/ml B27 supplement
(Thermo Fisher Scientific, Inc.), 20 ng/ml basic fibroblast growth
factor (MilliporeSigma) and 20 ng/ml epidermal growth factor
(MilliporeSigma). Glioblastoma stem-like cells (GSCs) were isolated
from U251 cells transfected with shCTRL or shVAV3-#1/2 using the
serum-free medium (SFM). These cells can form neurosphere-like cell
aggregates in <7 days (19).
For the selection of stable glioma cell lines, U251 cells were
cultured in 400 µg/ml neomycin for 14 days after transfection.
RT-qPCR
mRNA expression of VAV3 in human glioblastoma
samples was analyzed by the online database GlioVis
(gliovis.bioinfo.cnio.es/). Total RNA from cells was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The total RNA was subsequently treated with RNase-free DNase
I (Roche Diagnostics). Complementary DNA synthesis was performed
using a BcaBest RNA PCR kit from Takara Bio, Inc. according to the
manufacturer's protocols. qPCR was performed using an iQ™5
Multicolor Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc.) with Realtime PCR Master Mix (SYBR Green, Toyobo Life
Science). The thermocycling conditions used for qPCR were as
follows: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 30 sec, in a total volume of 20 µl. Relative mRNA
expression levels were assessed using the 2−ΔΔCq method
(20). GAPDH was used as the
endogenous control. The PCR primer sequences used were as follows:
VAV3 forward (F): 5′-CTGCCAGCTGCTTAACAACC-3′ and reverse (R),
5′-CAGGCCGTGAGAAATGTCCT-3′; miR-218 RT primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACATGG-3′ (21); miR-218 qPCR F primer,
5′-GTGCAGGGTCCGAGGTATTC-3′, miR-218 qPCR R primer,
5′-TTGATCTAACCATGTGTCGTA-3′; and GAPDH F, 5′-GAAGGTGAAGGTCGGAGTC-3′
and R, 5′-GAAGATGGTGATGGGATTTC-3′.
Western blotting
The glioblastoma cells lysates were prepared using
RIPA lysis and extraction buffer (Thermo Fisher Scientific, Inc.)
with complete protease inhibitor cocktail (Roche Diagnostics). The
protein concentration was determined using a BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). The samples (20 µg/line)
were separated by 12 % SDS-PAGE and transferred to polyvinylidene
fluoride membranes (Roche Diagnostics). The membranes were blocked
using 5% non-fat dry milk in TBS, followed by incubation with the
primary antibodies at 4°C overnight and then HRP-labeled secondary
antibodies at room temperature for an hour (Cat#: 61-6520, 1:5,000,
HRP-labeled, Thermo Fisher Scientific, Inc). The immunolabeled
proteins were detected using an ECL detection system (Wuhan Boster
Biological Technology, Ltd). The primary antibodies used were as
follows: Actin (1:1,000, cat. no. AA128; Beyotime Institute of
Biotechnology), VAV3 (1:1,000, cat. no. 2398s, Cell Signaling
Technology, Inc.), Nestin (1:1,000, cat. no. MAB5326,
MilliporeSigma) and SOX2 (1:1,000, cat. no. 3579s, Cell Signaling
Technology, Inc.).
VAV3 shRNA synthesis and
transfection
Cell transfections with 1 µg nucleic acid were
performed using Lipofectamine™ 2000 transfection reagent (Thermo
Fisher Scientific, Inc) according to the manufacturer's protocol.
For the selection of stable cell lines, the transformed cells were
cultured in 400 ug/ml G418 for 14 days and the expression of VAV3
was assessed using western blotting and RT-qPCR. The sequences of
the shRNAs were as follows: VAV3 shRNA-1#, CGAAGTTGTTGTCTAGCAGAA
and VAV3 shRNA-2#, CGGAACCTAATGCAAGAGATT.
Luciferase assay
The binding site of miR-218 to VAV3 was predicted by
the online database Target scan (targetscan.org/vert_80/; version
8.0). The miR-218 vector and pmirGLO-VAV3-3′UTR,
pmirGLO-VAV3-3′UTR-mutant (mut), were co-transfected into U251
cells using Lipofectamine™ 2000 transfection reagent(Thermo Fisher
Scientific, Inc). The cells were harvested and lysed at 24 h
post-transfection. The luciferase activity was measured using the
dual-luciferase reporter assay system (Promega Corporation)
according to the manufacturer's protocol. A total of 48 h
post-transfection, firefly and Renilla luciferase activities were
detected using the GloMax®-Multi Jr Single Tube
Multimode Reader (Promega, Madison, WI, USA), according to the
Dual-Luciferase Reporter Assay system (Promega, Madison, WI,
USA).
In vitro migration and invasion
assays
Glioblastoma Cells (5×105) were added on
the top side of polycarbonate Transwell filters (without Matrigel
for Transwell migration assays) or plated on the top side of
polycarbonate Transwell filter coated with Matrigel at 37°C for an
hour (for Transwell invasion assay) in the upper chamber of the
QCM™ 24-Well Cell Invasion Assay (Cell Biolabs, Inc.) plates. For
Transwell migration assays, cells were suspended in SFM and SFM was
used in the lower chamber. For the invasion assay, cells were
suspended in SFM and DMEM with fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) was used as a chemoattractant in the lower
chamber. The cells were incubated at 37°C for 8 h (migration assay)
or 48 h (invasion assay). The cells in the top chambers were then
removed using cotton swabs. The migrated and invaded cells on the
lower membrane surface were fixed using 100% methanol for 10 min at
room temperature, air-dried, then stained with DAPI at room
temperature for 10 min and quantified using a fluorescence OLYMPUS
U-HGLGPS microscope and cellSens entry software 1.0 (Olympus,
Japan).
Wound healing assays
U251 cells were seeded in six-well plates and
cultured at 37°C overnight in DMEM medium until 100% confluency. A
wound was then created by manually scraping the cell monolayer with
a 200 µl pipette tip. The cultures were washed twice with PBS to
remove floating cells. The cells were then incubated at 37°C in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 1%
FBS (Gibco; Thermo Fisher Scientific, Inc.). Cell migration into
the wound was assessed at three preselected time points (0, 24 and
48 h) in eight randomly selected fields of view for each condition
and time point. Images were captured using a Nikon DS-5M Camera
System mounted on a phase-contrast Leitz microscope. The distance
traveled by the cells was determined by measuring the wound width
at different time points and then subtracting it from the wound
width at 0 h using Image-Pro Plus 6.0 (Media Cybernetics, Bethesda,
MD).
Limiting dilution assay
A limiting dilution assay was performed as described
previously (19,22). Cancer sphere cells were dissociated
and plated on 96-well micro-well plates in 0.2 ml SFM. Final cell
dilutions ranged from 20 cells/well to 160 cell/well in a total
volume of 0.2 ml. Cultures were added with 0.025 ml of SFM every 2
days until day 7, then the percentage of wells without spheres was
calculated for each cell plating density and plotted against the
number of cells per well. Limiting dilution analysis was performed
using online software ELDA 1.0 (Extreme Limiting Dilution Analysis)
(http://bioinf.wehi.edu.au/software/elda/).
Cell counting kit-8 (CCK8)
U251 cells (3000 cells/well in 100 µl DMEM medium)
were seeded into 96 well tissue culture plate, each group was
repeated 3 times. CCK8 (Selleck Chemicals) regent was add with 10
µl/well at four time points (24, 48, 72 and 96 h), incubated 2 h at
37°C; after shaking the plates for 1 min, the absorbance of samples
was measured using an ELISA reader at 450 nm.
Animal studies
The present study was compliant with all relevant
ethical regulations regarding animal research and was approved by
the Institutional Animal Care and Use Committee of Xi'an Medical
University (No. XYLS2022190). U251 cells stably expressing
VAV3-shRNA-1# or empty vector controls (4.0×106 cells in
200 µl per mouse) were injected subcutaneously in the flanks of
female athymic nude BALB/c nu/nu mice (n=6 per treatment group;
age, ~6 weeks). Mice were housed in a standard environment which
was characterized by 12 h light/dark cycle, 22–25°C and 40–60%
humidity with free access to water and chow. Tumor volumes were
calculated according to the formula: Volume=length ×
width2/2. The experiment lasted for 20 days and no
animals died during the experimental period. Animal health was
monitored every day and the size of the tumor was measured every
four days. A total of six mice per group were used and tumor cells
were injected in both sides to minimize the number of mice used.
The mice were euthanized when a single tumor volume reached 3,000
mm3. The mice were euthanized cervical dislocation and
death was confirmed when mice stopped breathing and had no nerve
reflex.
Statistical analysis
In vitro experiments were performed with at
least three independent biological samples unless otherwise stated.
Data were presented as mean ± standard error. Comparisons between
two groups were performed using the Student's t-test. Comparisons
among multiple groups were performed using one-way ANOVA followed
by Dunnett's post-hoc test. Survival of animals in in vivo
studies was analyzed using Kaplan-Meier analyses and log-rank test.
GraphPad Prism 8.0 for windows (GraphPad Software, Inc.) was used
for data analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
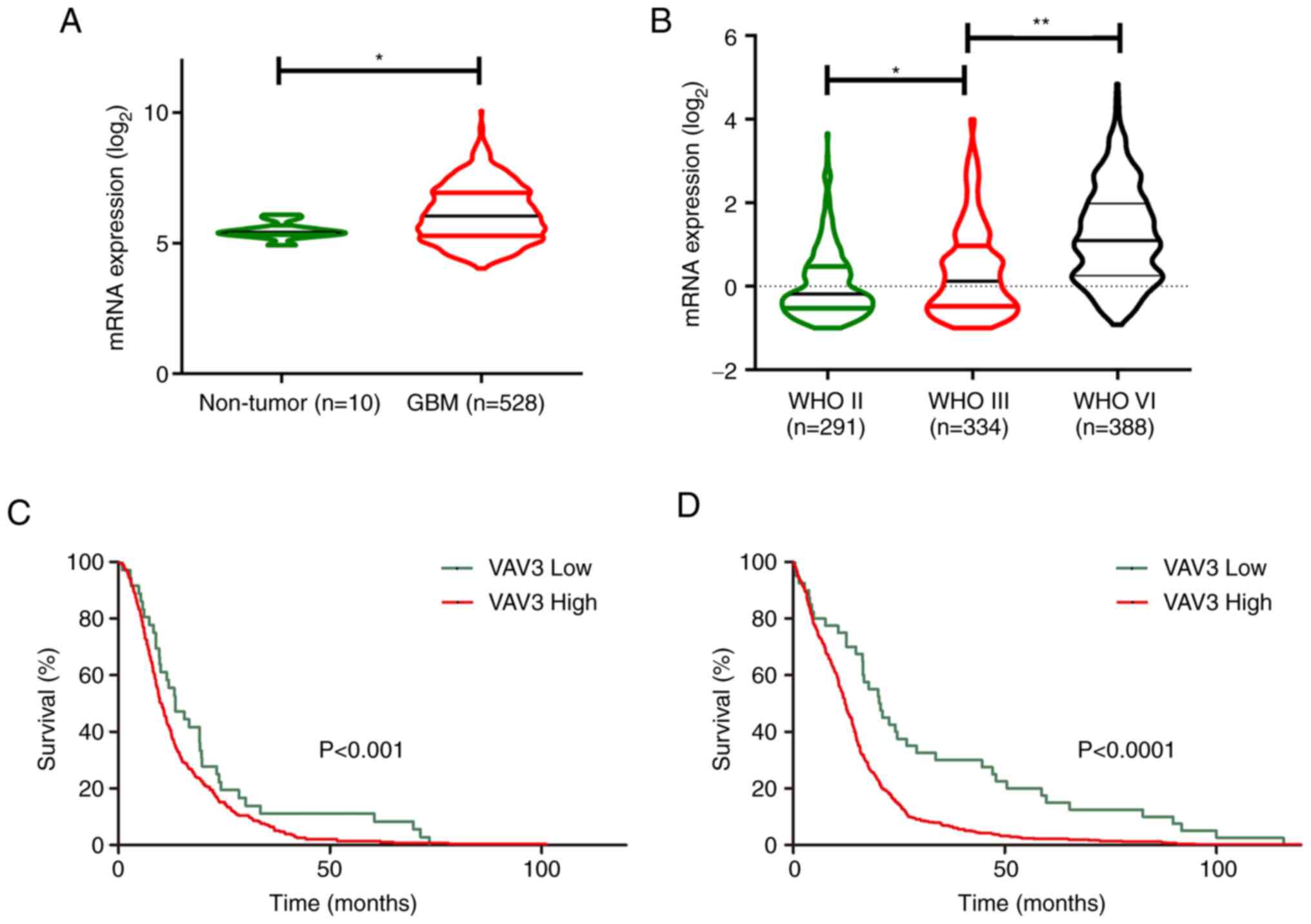
VAV3 oncogene is overexpressed in
human glioblastoma and associated with patient survival
To evaluate whether VAV3 was crucial for human
glioblastoma tumorigenesis, the publicly available data at GlioVis
(23) were assessed. Analysis of
GlioVis by The Cancer Genome Atlas (TCGA) demonstrated that VAV3
mRNA was expressed at a significantly higher level in GBM compared
with normal brain tissues (Fig.
1A). Further analysis of the Chinese Glioma Genome Atlas (CGGA)
(24), demonstrated that the mRNA
expression level of VAV3 was significantly increased with
increasing tumor grade (Fig. 1B),
the levels of VAV3 expression in tumor specimens were associated
with their World Health Organization grades. Kaplan-Meier analysis
using the optimal cutoff demonstrated that mRNA expression levels
of VAV3 in GBM specimens were inversely associated with patients'
survival time based on data from TCGA (Fig. 1C) and CGGA (Fig. 1D). Collectively, these analyses
suggested that VAV3 was an oncogene and could be a potential
therapeutic target for GBM therapy.
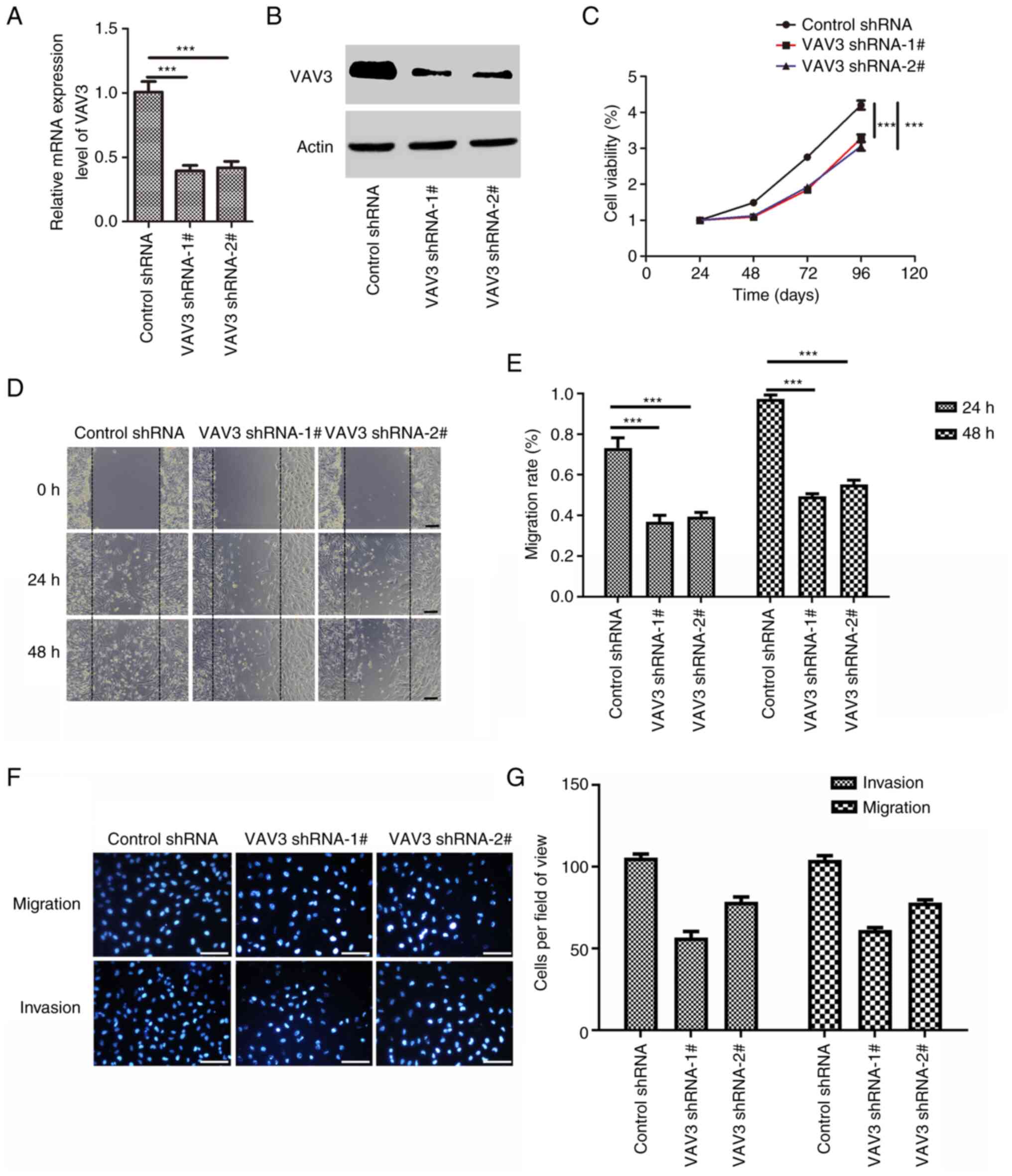
VAV3 knockdown inhibits U251
glioblastoma cell migration, invasion and proliferation
To evaluate the function of VAV3 in glioblastoma
cells, shRNA targeting VAV3 mRNA (shVAV3) was used to suppress the
expression of VAV3 in U251 cells. The knockdown effect of shRNA was
assessed using RT-qPCR and western blotting analysis (Fig. 2A and B). The results demonstrated
that both the VAV3 shRNAs markedly suppressed VAV3 mRNA and protein
expression levels and that VAV3-shRNA-1# was better. CCK-8 cell
viability assays demonstrated that the suppression of the
expression of VAV3 in U251 cells significantly inhibited the
proliferation of glioblastoma cells compared with the control
(Fig. 2C). The wound healing assay
demonstrated that reduced VAV3 expression significantly suppressed
the migration ability of U251 cells compared with the control
(Fig. 2D and E). Furthermore, the
migration and invasive potential of VAV3 shRNA U251 cells was also
markedly reduced compared with the control (Fig. 2F and G). All these suggest that
VAV3 regulate the U251 glioblastoma cell migration, invasion and
proliferation, migration and invasion.
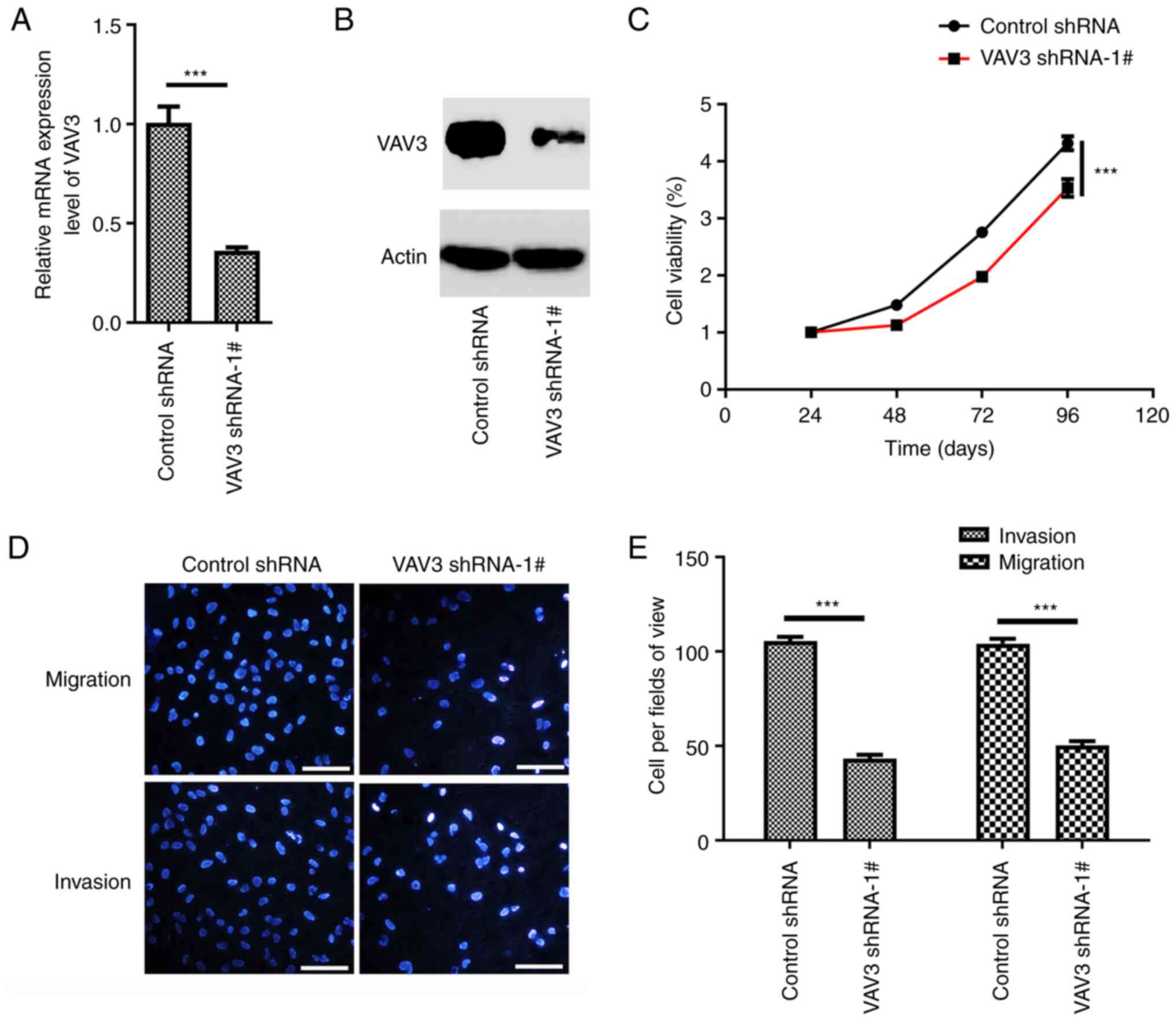
VAV3 knockdown inhibits U87 migration,
invasion and proliferation
To evaluate the role of VAV3 in glioblastoma cells,
VAV3-shRNA-1# was used to specifically suppress the expression of
VAV3 in U87 cells. The knockdown effect of shRNA was assessed using
RT-qPCR and western blotting analysis (Fig. 3A and B). The results demonstrated
that the VAV3 shRNA could markedly suppress VAV3 expression in U87
cells compared with the control. CCK-8 cell viability assays
demonstrated that the suppression of the expression of VAV3 in U87
cells significantly inhibited the proliferation of glioblastoma
cells compared with the control (Fig.
3C). Furthermore, the migration and invasion ability of VAV3
shRNA U87 cells was also significantly reduced compared with the
control (Fig. 3D and E).
Collectively, these analyses suggested that VAV3 regulate the U87
glioblastoma cell migration, invasion and proliferation, migration
and invasion.
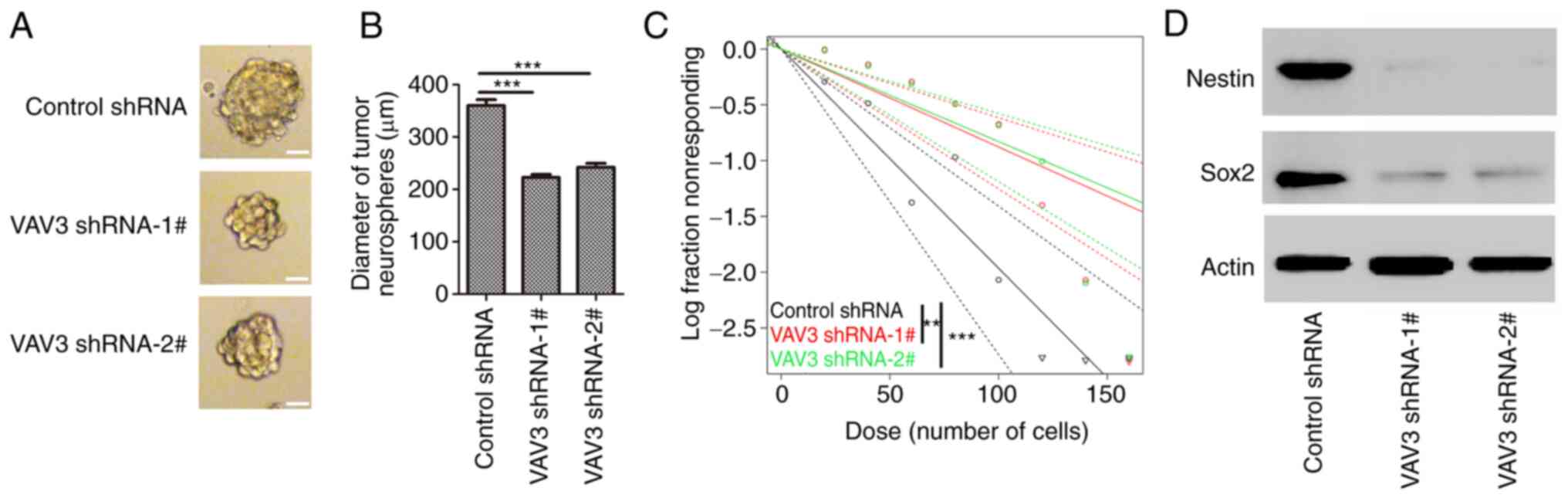
VAV3 knockdown inhibits the
self-renewal capacity of glioblastoma stem-like cells
The effects of VAV3 on glioblastoma stem like cell
self-renewal, U251 cells were cultured in SFM to induce the tumor
sphere formation. A significant decrease in the diameter of U251
neurospheres in VAV3 knockdown groups (VAV3 shRNA-1#, 217.3±9.9 µm;
VAV3 shRNA-2#, 235.2±14.8 µm) was demonstrated compared with that
of the control (311.5±15.4 µm) (Fig.
4A and B). Furthermore, downregulation of VAV3 reduced the
self-renewal capacity of U251 cancer stem-like cells compared with
the control. The number of cells required to generate at least one
tumor sphere/well was 113.9 µm in VAV3 shRNA-1#-U251 cells, 120.6
µm in VAV3 shRNA-2#-U251 cells, and 50.4 µm in control shRNA-U251
cell (Fig. 4C). The protein
expression levels of the stem cell markers Nestin and Sox2 in these
tumor neurospheres was assessed using western blotting, which
demonstrated that the expression of these GSC makers were markedly
decreased in the tumor spheres from VAV3 shRNA-transfected U251
cells compared with control shRNA (Fig. 4D). These data suggested that the
downregulation of VAV3 expression could inhibit the self-renewal
capacity of glioblastoma stem-like cells.
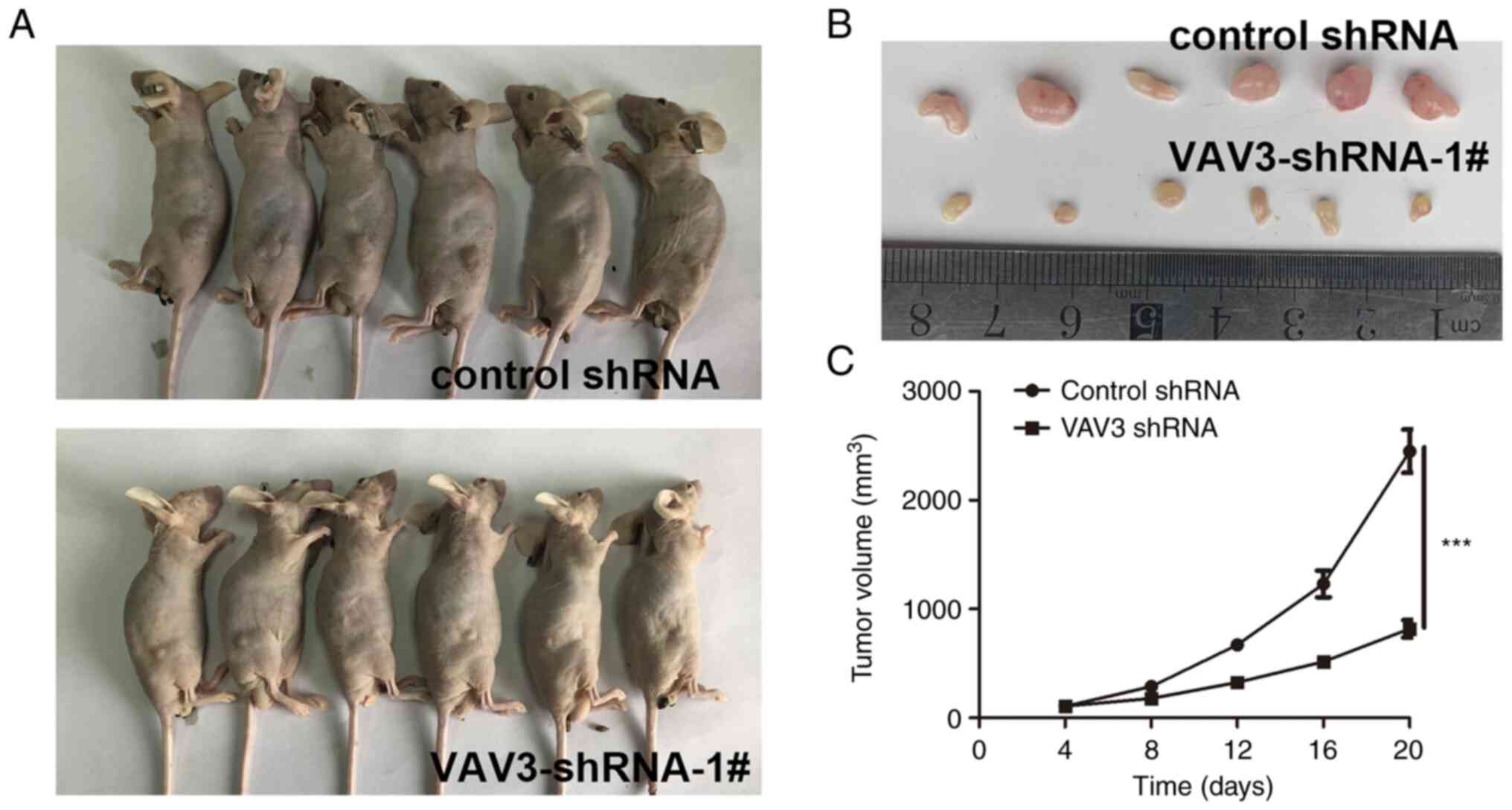
VAV3 inhibits the glioblastoma
progression in vivo
To analyze the role of VAV3 in glioblastoma
carcinogenesis, the effect of VAV3 decrease on tumor growth were
assessed in vivo. VAV3-shRNA-1#-U251 cells and control cells
were implanted into the left and right flanks of nude mice (n=6 per
group) by subcutaneous injection. At 20 days post-injection, the
tumors derived from VAV3-shRNA-1#-U251 cells were significantly
smaller than those derived from control-U251 cells (Fig. 5). Which demonstrated that knockdown
of VAV3 significantly inhibited the proliferation of glioblastoma
cells in vivo.
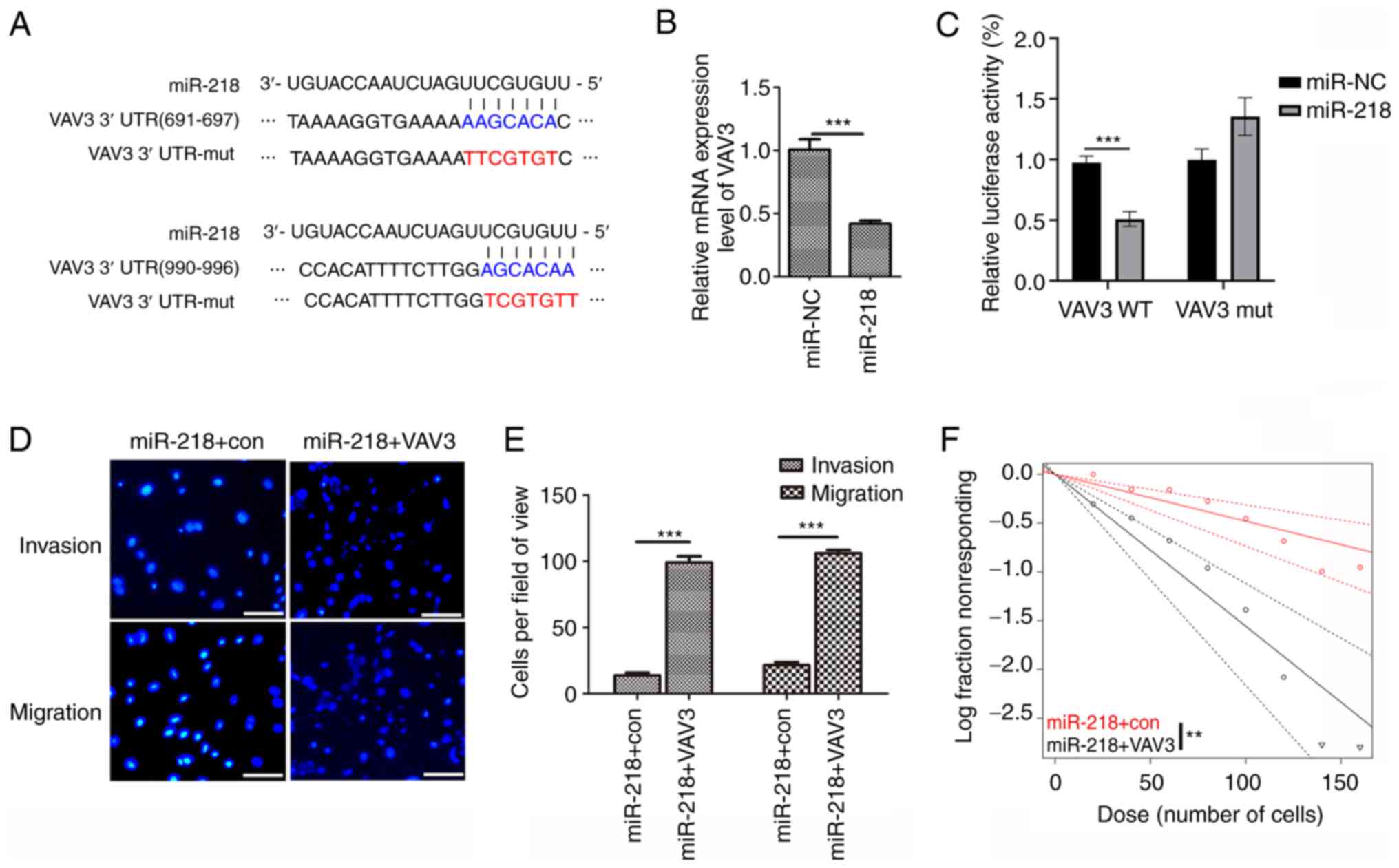
VAV3 is a target gene of miR-218 and
overexpression of VAV3 eliminated the effects of miR-218 on U251
glioblastoma cells
Several computational methods were used to evaluate
the potential targets of miR-218 in humans. The TargetScan program
identified two conserved binding sites for miR-218 in the 3′UTR
region of the VAV3 mRNA) (Fig.
6A). To evaluate if VAV3 was a direct target of miR-218,
RT-qPCR was used to assess VAV3 mRNA expression levels in U251
cells, which demonstrated significantly decreased VAV3 mRNA
expression levels in miR-218 overexpression cells compared with the
control (Figs. 6B and S1). To assess whether miR-218 directly
targeted VAV3, a dual-luciferase reporter system containing the
wild-type (WT) and mut 3′-UTR of VAV3 was used. The luciferase
activity in the miR-218/WT-VAV3-UTR-transfected cells was
significantly decreased compared with the control, whereas the
luciferase activity in the miR-218/mut-VAV3-UTR-transfected cells
demonstrated no significant difference, which suggested that
miR-218 directly targeted the 3′-UTR of VAV3. Furthermore, miR-218
and the VAV3 overexpression vector were co-transfected into U251
cells to assess whether miR-218 performed its tumor suppression
functions via targeting of VAV3. After co-transfection, it was
demonstrated that the overexpression of VAV3 significantly
counteracted the tumor suppressor effect of miR-218 in glioblastoma
cells during migration and invasion (Fig. 6D). Further analysis demonstrated
that VAV3 overexpression markedly reduced the effects of miR-218 on
the self-renewal capacity of glioblastoma stem-like cells (Fig. 6E). These findings further
demonstrated that VAV3 was a target gene of miR-218.
Discussion
Glioblastoma is a lethal brain cancers and VAV3 was
demonstrated to be up-regulated in glioblastoma and inversely
associated with patients' survival time through systematic
bioinformatic analyses. The results of the present study suggested
that VAV3 was an oncogene and may serve an important role in the
regulation of glioblastoma tumorigenesis. Moreover, the results of
the present study demonstrated that knockdown of VAV3 expression by
shRNA markedly suppressed GBM cell migration, invasion,
proliferation, and glioblastoma stem-like cells self-renewal.
Furthermore, knockdown of VAV3 significantly inhibited the
proliferation of two glioblastoma cells in vitro. These data
suggested that VAV3 could be a prognostic marker or therapeutic
target in glioblastoma.
VAV3 has been reported to be highly expressed in
numerous types of cancer (10,14,25).
Salhia et al (18) reported
that the three guanine nucleotide exchange factors trio (trio Rho
guanine nucleotide exchange factor), Ect2 (epithelial cell
transforming 2) and Vav3 were expressed at higher levels in GBM
compared with low-grade glioma in 2008. This was consistent with
the results of the present study, which analyzed the TCGA and CGGA
databases in 2022, with the number of patient samples analyzed
being much higher than in 2008. Furthermore, the results of the
present study demonstrated that knockdown of VAV3 markedly
inhibited the migration and invasion of glioblastoma cells. Uen
et al (14) reported that
VAV3 knockdown could regulate the cell cycle and metastasis-related
molecules via inhibition of the PI3K-AKT signaling pathway in
colorectal cancer cells. Recently, Nayak (26) reported that nuclear VAV3 served an
important role in B-cell lymphoblastic leukemogenesis through
regulation of nuclear Bmi1 phosphorylation and polycomb repression
complex-1 (PRC1) activity. the role of BMI 1 in the regulation of
glioblastoma migration, invasion and stem cell renewal has been
reported by numerous previous studies (27–30).
Therefore, VAV3 could regulate glioblastoma migration, invasion and
stem cell stemness via regulation of the PI3K-AKT signaling pathway
or PRC1 activity.
VAV3 belongs to the GEF family which regulate the
Rho family GTPases. Previous studies have reported that the Rho
family small GTPases serve an important role in the regulation of
the cytoskeleton dynamics, cell proliferation, invasion and
survival of cancer cells (31–33).
Salhia et al (18) reported
that the GEFs factors trio, Ect2 and Vav3 serve an important role
in the regulation of glioblastoma invasion. As these GEFs factors
act on the small GTPase RhoG, the role of RhoG in the regulation of
the invasive behavior of glioblastoma cells has been previously
assessed, a previous study reported that GTPase RhoG mediated
glioblastoma cell invasion and promoted glioblastoma cell survival
(34). The present study also
demonstrated that VAV3 regulated glioblastoma cell migration and
invasion. These data indicated that VAV3 may mediate downstream
effectors such as RhoGases to regulate glioblastoma migration and
invasion.
A previous study reported that miRNAs could regulate
numerous cancer processes (35).
Numerous mRNAs have been reported to serve key roles in
glioblastoma cell migration, invasion, proliferation, apoptosis and
stem cell stemness (36–39). Because of their critical roles in
glioma development, miRNAs have been considered as potential
biomarkers and therapeutic targets (40). miR-218 has been reported to be
down-regulated in glioblastoma and to have inhibited glioma
invasion, migration, proliferation and cancer stem-like cell
self-renewal (21). In the present
study, miRNA-218 target analysis indicated VAV3 as a new direct
target of miR-218. Bioinformatic analysis indicated that miR-218
could bind to the VAV3 3′-UTR at two sites: 691–697 and 990–996 nt.
The dual-luciferase reporter and RT-qPCR assay demonstrated that
miR-218 could directly target VAV3 by recognizing the 3′-UTR of
VAV3 mRNA and significantly reduced VAV3 expression levels.
Moreover, overexpression of VAV3 markedly reversed the tumor
suppressor effect of miR-218 in glioblastoma cells. These results
further demonstrated that VAV3 may be a target gene of miR-218 and
could regulate glioblastoma tumorigenesis.
This study has several limitations. First of all,
the experiment was only carried out in two glioma cell lines, and
more glioma cell lines should be used in future research to verify
the experimental results. Second, we only studied the function of
VAV3 in vitro. In the future, it is necessary to verify the
anti-tumor effect of miR-218 and VAV in clinical samples of
glioblastoma.
In conclusion, the results of the present study
demonstrated that VAV3 could regulate glioblastoma cell migration,
invasion, proliferation and glioblastoma stem-like cell
self-renewal. For the first time, to the best of our knowledge, the
present study demonstrate that VAV3 is a target gene of miR-218
which regulates glioblastoma development by targeting VAV3, which
indicates that VAV3 could be a novel therapeutic target for
suppressing GBM invasion and recurrence.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Natural Science Basic
Research Plan in Shaanxi Province of China (grant no. 2021SF-273),
Scientific Research Program Funded by Shaanxi Provincial Education
Department (grant no. 20JG029) and Shaanxi Province Innovation
capability support plan project (grant no. 2023-CX-PT-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RM and NG performed the experiments and contributed
to the writing of the manuscript. DH, KZ, YL, XZ and YC performed
experiments and contributed to the writing of the article. RM and
NG confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Xi'an Medical University (No.
XYLS2022190).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torrisi F, Alberghina C, D'Aprile S,
Pavone AM, Longhitano L, Giallongo S, Tibullo D, Di Rosa M, Zappalà
A, Cammarata FP, et al: The hallmarks of glioblastoma:
Heterogeneity, intercellular crosstalk and molecular signature of
invasiveness and progression. Biomedicines. 10:8062022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grech N, Dalli T, Mizzi S, Meilak L,
Calleja N and Zrinzo A: Rising incidence of glioblastoma multiforme
in a well-defined population. Cureus. 12:e81952020.PubMed/NCBI
|
|
3
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Non-ionizing radiation, Part 2:
Radiofrequency electromagnetic fields. IARC Monogr Eval Carcinog
Risks Hum. 102:1–460. 2013.PubMed/NCBI
|
|
4
|
Fuks KB, Weinmayr G, Basagana X, Gruzieva
O, Hampel R, Oftedal B, Sørensen M, Wolf K, Aamodt G, Aasvang GM,
et al: Long-term exposure to ambient air pollution and traffic
noise and incident hypertension in seven cohorts of the European
study of cohorts for air pollution effects (ESCAPE). Eur Heart J.
38:983–990. 2017.PubMed/NCBI
|
|
5
|
Smetana K Jr, Lacina L, Szabo P,
Dvorankova B, Broz P and Sedo A: Ageing as an important risk factor
for cancer. Anticancer Res. 36:5009–5017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trylcova J, Busek P, Smetana K Jr,
Balaziova E, Dvorankova B, Mifkova A and Sedo A: Effect of
cancer-associated fibroblasts on the migration of glioma cells in
vitro. Tumour Biol. 36:5873–5879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang K, Zhang B, Guo X, Zong M, Rahman R,
Sanchez D, Winder N, Reardon DA, Zhao B, Wen PY and Huang RY:
Multimodal imaging patterns predict survival in recurrent
glioblastoma patients treated with bevacizumab. Neuro Oncol.
18:1680–1687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C,
Bogdahn U, et al: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. New Eng J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee K, Liu Y, Mo JQ, Zhang J, Dong Z and
Lu S: Vav3 oncogene activates estrogen receptor and its
overexpression may be involved in human breast cancer. BMC Cancer.
8:1582008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahaman SO, Li W and Silverstein RL: Vav
Guanine nucleotide exchange factors regulate atherosclerotic lesion
development in mice. Arterioscler Thromb Vasc Biol. 33:2053–2057.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujikawa K, Inoue Y, Sakai M, Koyama Y,
Nishi S, Funada R, Alt FW and Swat W: Vav3 is regulated during the
cell cycle and effects cell division. Proc Natl Acad Sci USA.
99:4313–4318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hornstein I, Alcover A and Katzav S: Vav
proteins, masters of the world of cytoskeleton organization. Cel
Signal. 16:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uen YH, Fang CL, Hseu YC, Shen PC, Yang
HL, Wen KS, Hung ST, Wang LH and Lin KY: VAV3 oncogene expression
in colorectal cancer: Clinical aspects and functional
characterization. Sci Rep. 5:93602015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lyons LS and Burnstein KL: Vav3, a Rho
GTPase guanine nucleotide exchange factor, increases during
progression to androgen independence in prostate cancer cells and
potentiates androgen receptor transcriptional activity. Mol
Endocrinol. 20:1061–1072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boesch M, Sopper S, Marth C, Fiegl H,
Wiedemair A, Rössler J, Hatina J, Wolf D, Reimer D and Zeimet AG:
Evaluation of Vav3.1 as prognostic marker in endometrial cancer. J
Cancer Res Clin Oncol. 144:2067–2076. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan B, Li Y, Zhao Q, Fan L, Wang D and Liu
Y: Inhibition of gastric cancer cell growth and invasion through
siRNA-mediated knockdown of guanine nucleotide exchange factor
Vav3. Tumour Biol. 35:1481–1488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salhia B, Tran NL, Chan A, Wolf A, Nakada
M, Rutka F, Ennis M, McDonough WS, Berens ME, Symons M and Rutka
JT: The guanine nucleotide exchange factors trio, Ect2, and Vav3
mediate the invasive behavior of glioblastoma. Am J Pathol.
173:1828–1838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB,
Liu W, Qi Q, Lu N, Tao L, Wang XT, et al: Isolation and
characterization of cancer stem like cells in human glioblastoma
cell lines. Cancer Lett. 279:13–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tropepe V, Sibilia M, Ciruna BG, Rossant
J, Wagner EF and van der Kooy D: Distinct neural stem cells
proliferate in response to EGF and FGF in the developing mouse
telencephalon. Dev Biol. 208:166–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bowman RL, Wang Q, Carro A, Verhaak RG and
Squatrito M: GlioVis data portal for visualization and analysis of
brain tumor expression datasets. Neuro Oncol. 19:139–141. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Z, Zhang KN, Wang Q, Li G, Zeng F,
Zhang Y, Wu F, Chai R, Wang Z, Zhang C, et al: Chinese glioma
genome atlas (CGGA): A comprehensive resource with functional
genomic data from chinese glioma patients. Genomics Proteomics
Bioinformatics. 19:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Mo JQ, Hu Q, Boivin G, Levin L and
Lu S, Yang D, Dong Z and Lu S: Targeted overexpression of vav3
oncogene in prostatic epithelium induces nonbacterial prostatitis
and prostate cancer. Cancer Res. 68:6396–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nayak RC, Chang KH, Singh AK, Kotliar M,
Desai M, Wellendorf AM, Wunderlich M, Bartram J, Mizukawa B,
Cuadrado M, et al: Nuclear Vav3 is required for polycomb repression
complex-1 activity in B-cell lymphoblastic leukemogenesis. Nat
Commun. 13:30562022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baxter PA, Lin Q, Mao H, Kogiso M, Zhao X,
Liu Z, Huang Y, Voicu H, Gurusiddappa S, Su JM, et al: Silencing
BMI1 eliminates tumor formation of pediatric glioma CD133+ cells
not by affecting known targets but by down-regulating a novel set
of core genes. Acta Neuropathol Commun. 2:1602014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang J, Wang P, Xie S, Wang W, Zhou X, Hu
J, Shi Q, Zhang X and Yu R: Bmi-1 regulates the migration and
invasion of glioma cells through p16. Cell Biol Int. 39:283–290.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vora P, Seyfrid M, Venugopal C, Qazi MA,
Salim SA, Isserlin R, Subapanditha M, O'Farrell E, Mahendram S,
Singh M, et al: Bmi1 regulates human glioblastoma stem cells
through activation of differential gene networks in CD133+ brain
tumor initiating cells. J Neurooncol. 143:417–428. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Freire-Beneitez V, Pomella N, Millner TO,
Dumas AA, Niklison-Chirou MV, Maniati E, Wang J, Rajeeve V,
Cutillas P and Marino S: Elucidation of the BMI1 interactome
identifies novel regulatory roles in glioblastoma. NAR Cancer.
3:zcab0092021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Crosas-Molist E, Samain R, Kohlhammer L,
Orgaz JL, George SL, Maiques O, Barcelo J and Sanz-Moreno V: Rho
GTPase signaling in cancer progression and dissemination. Physiol
Rev. 102:455–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mosaddeghzadeh N and Ahmadian MR: The RHO
family GTPases: Mechanisms of regulation and signaling. Cells.
10:18312021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karlsson R, Pedersen ED, Wang Z and
Brakebusch C: Rho GTPase function in tumorigenesis. Biochim Biophys
Acta. 1796:91–98. 2009.PubMed/NCBI
|
|
34
|
Kwiatkowska A, Didier S, Fortin S, Chuang
Y, White T, Berens ME, Rushing E, Eschbacher J, Tran NL, Chan A and
Symons M: The small GTPase RhoG mediates glioblastoma cell
invasion. Mol Cancer. 11:652012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Si W, Shen J, Zheng H and Fan W: The role
and mechanisms of action of microRNAs in cancer drug resistance.
Clin Epigenetics. 11:252019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Xu J, Chen H, Bai J, Li S, Zhao Z,
Shao T, Jiang T, Ren H, Kang C and Li X: Comprehensive analysis of
the functional microRNA-mRNA regulatory network identifies miRNA
signatures associated with glioma malignant progression. Nucleic
Acids Res. 41:e2032013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lian S, Shi R, Bai T, Liu Y, Miao W, Wang
H, Liu X and Fan Y: Anti-miRNA-23a oligonucleotide suppresses
glioma cells growth by targeting apoptotic protease activating
factor-1. Curr Pharm Des. 19:6382–6389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun G, Cao Y, Shi L, Sun L, Wang Y, Chen
C, Wan Z, Fu L and You Y: Overexpressed miRNA-137 inhibits human
glioma cells growth by targeting Rac1. Cancer Biother Radiopharm.
28:327–334. 2013.PubMed/NCBI
|
|
39
|
Que T, Song Y, Liu Z, Zheng S, Long H, Li
Z, Liu Y, Wang G, Liu Y, Zhou J, et al: Decreased miRNA-637 is an
unfavorable prognosis marker and promotes glioma cell growth,
migration and invasion via direct targeting Akt1. Oncogene.
34:4952–4963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang JF, Zhang JS, Zhao ZH, Yang PB, Ji
SF, Li N, Shi QD, Tan J, Xu X, Xu CB and Zhao LY: MicroRNA-770
affects proliferation and cell cycle transition by directly
targeting CDK8 in glioma. Cancer Cell Int. 18:1952018. View Article : Google Scholar : PubMed/NCBI
|