Introduction
Periodontitis is an infection-associated chronic
inflammatory disease of the periodontium; it is one of the most
prevalent chronic diseases and a major cause of tooth loss
(1). Interleukin (IL)-1β plays a
significant role in pathogenic host defense; however, excessive
IL-1β causes pathogenicity, resulting in the destruction of
periodontal tissue and thus periodontitis (2,3). The
production of IL-1β involves cytosolic machinery named the
inflammasome (4,5). The components of the inflammasome
consist of NOD-like receptor family pyrin domain-containing protein
(NLRP) and apoptosis-associated speck-like protein containing a
caspase recruitment domain (ASC), which recruits and activates
caspase-1. The activation of caspase-1 causes the cleavage of
pro-IL-1β to produce mature IL-1β. Thus, inflammasome activation is
required for IL-1β production.
A number of inflammasomes have been identified in
humans. They contain different NLRP family proteins, such as NLRP1,
NLRP3 and NLRC4, and respond to different stimuli for activation
(6). The NLRP3 inflammasome is the
inflammasome activated in periodontitis (7). It has been reported that
Porphyromonas gingivalis infection stimulates activation of
the NLRP3 inflammasome and the production of IL-1β (8). Similarly, bacterial endotoxin
lipopolysaccharide (LPS) has been shown to activate the NLRP3
inflammasome and stimulate the production of IL-1β in oral
epithelial cells (9,10). Gingival epithelial cells (GECs)
have been reported to have an important role as a component of
innate host response to periodontal bacteria and significantly
contribute to gingival health (11). Thus, targeting the inflammasome may
be a potential approach for the prevention and treatment of
periodontitis.
The use of stem cell-based therapies has been
reported in different diseases, such as inflammatory bowel
diseases, multiple sclerosis, myocardial infarction and type 1
diabetes (12). Stem cell therapy
exerts beneficial effects predominantly through indirect paracrine
actions, rather than direct differentiation and substitution of
damaged cells (13–15). It is well documented that stem
cells possess immunomodulatory and anti-inflammatory functions
(14,16). Studies have demonstrated that stem
cell-conditioned culture media (SCM) exhibits protective effects
against various pathological conditions (17–19).
Notably, administration of SCM shows similar protective effects to
stem cell transplantation (15,20).
Due to stem cell therapy-associated risks, treatment with SCM has
been considered as a promising alternative (21). Considering the anti-inflammatory
functions that stem cells possess and that LPS induces inflammasome
activation (22–24), it was hypothesized that SCM may
inhibit inflammasome activation and protect against LPS-induced
inflammatory damage in human GECs. To test the hypothesis, the
current study first determined the inhibitory effect of SCM on
LPS-induced inflammasome activation, then measured IL-1β production
and NF-κB activation, and finally, accessed the damage of the GECs.
To the best of our knowledge, the results from the present study
were the first to demonstrate that SCM inhibited LPS-induced NLRP3
inflammasome activation and attenuated LPS-induced inflammatory
damage in human GECs.
Materials and methods
Cell culture and preparation of
SCM
Human GECs were cultured and treated as described
previously (25). Briefly, GECs
(cat. no. 1626; Beijing Yuhengfeng Technology Co., Ltd.) were
cultured in a defined medium (EpiCM; cat. no. 4101; Beijing
Yuhengfeng Technology Co., Ltd.), in which 2% FBS, 1% epithelial
cell growth supplement, penicillin (100 IU/ml) and streptomycin
(100 µg/ml) were supplemented, at 37°C in a humidified atmosphere
containing 5% CO2. Human mesenchymal stem cells (MSCs)
were purchased from Cyagen Biosciences (cat. no. HUXMA-01001;
cryopreserved at second passage) and cultured according to the
manufacturers' instructions using the defined MSC medium (Human MSC
Complete Medium; cat. no. HUXMA-90011; Cyagen Biosciences). After
72 h, the medium was collected and used as SCM. Sixth passage MSCs
were used in the present study. Notably, these MSCs have been
wildly used in various studies, including in the preparation of SCM
(26–34). The control conditioned media (CCM)
was obtained from culturing human fibroblast cells (cat. no. 2621;
Beijing Yuhengfeng Technology Co., Ltd.) for 72 h using the same
medium for the culture of MSCs. The human GECs and fibroblasts were
immortalized cells, and experiments were performed using cells at
passages 4–6 after initial thawing.
Experimental groups
Upon reaching 80% confluence, the GECs were treated
with either vehicle + CCM, LPS (2 µg/ml; cat. no. abx165771;
Beijing Yuhengfeng Technology Co., Ltd.) + CCM or LPS + SCM for 16
h. Subsequently, cells were harvested for the isolation of
proteins. For immunofluorescence imaging, cells were cultured on
glass coverslips. The concentration of LPS was based on previous
studies (9,35–37).
Using a cell viability assay, these studies concluded that higher
concentrations of LPS (>5 µg/ml) were toxic to cells, and that
1, 2 and 2.5 µg/ml LPS did not cause cell death (38,39).
LPS was dissolved in H2O (stock solution 1 mg/ml), 10 µl
H2O (vehicle) or 10 µl LPS stock solution was added into
5 ml culture medium in 60-mm dishes, which gave 2 µg/ml PLS in
working solution.
Western blot analysis for levels of
NLRP3, ASC, caspase-1 and NF-κB (p65)
Cytosolic and nuclear proteins were prepared as
described previously (25,40,41).
Briefly, cells were washed with PBS and homogenized in ice-cold
HEPES buffer (buffer-A) containing 10 mM HEPES (pH 7.9), 1.5 mM
MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), 0.5 mM
phenylmethylsulfonyl fluoride (PMSF) and 10% Nonidet P-40. The
homogenate was centrifuged at 1,000 × g for 5 min at 4°C, and the
supernatant and pellet were collected for cytosolic protein
preparation and nuclear protein isolation, respectively. The
supernatant was centrifuged again at 6,000 × g for 10 min at 4°C
and the resulting cytosolic protein-containing supernatant was used
for western blot analysis of NLRP3, ASC and caspase-1.
For nuclear fraction isolation, the pellets from the
first centrifugation, which contained cell nuclei, were washed with
ice-cold buffer-A, and then incubated on ice with ice-cold HEPES
buffer (buffer-B) containing 5 mM HEPES (pH 7.9), 1.5 mM
MgCl2, 300 mM NaCl, 400 mM KCl, 0.2 mM EDTA, 0.5 mM DTT,
0.5 mM PMSF, and 26% glycerol for 30 min to release nuclear
proteins. Next, the reaction mixtures were centrifuged at 14,000 ×
g for 30 min at 4°C, and the supernatants were collected and stored
at −80°C until they were used as nuclear extracts for western blot
analysis of NF-κB levels.
Western blot analysis was performed as previously
described (42). Briefly, protein
concentration was measured by a BCA Protein Assay Kit (cat. no.
P0010S; Beyotime Institute of Biotechnology), samples (20 µg) were
then separated by SDS-PAGE on 10% gels and transferred onto
nitrocellulose membranes. The membranes were blocked with the
QuickBlock™ blocking buffer (cat. no. P0252; Beyotime Institute of
Biotechnology) for 15 min at room temperature and then incubated
with primary antibodies at 4°C overnight, followed by incubation
with horseradish peroxidase (HRP)-labeled secondary antibodies at
room temperature for 1 h. β-actin and PCNA were used as loading
controls for cytosolic and nuclear fractions, respectively. After
incubation with ECL detection solution (BeyoECL Plus; cat. No.
P0018S, Beyotime Biotechnology), the membranes were visualized
using a chemiluminescence imaging system (BeyoImager Luminometers,
Beyotime). The intensity of the blots was semi-quantified using
ImageJ (1.53e; National Institutes of Health).
Primary antibodies used in the present study
included anti-human NLRP3 (1:500; cat. no. sc-134306; mouse
monoclonal), ASC (1:1,000; cat. no. sc-514414; mouse monoclonal),
caspase-1 (1:3,000; cat. no. sc-1218-R; rabbit polyclonal) and
NF-κB (p65) (1:1,000; cat. no. sc-8008, mouse monoclonal), which
were purchased from Santa Cruz Biotechnology, Inc. Anti-β-actin
(1:3,000, cat. No. sc-47778; mouse; Santa Cruz Biotechnology, Inc.)
and anti-PCNA (1:1,000, cat. no. ab92552; rabbit; Abcam) were used
as loading controls for cytoplasmic and nuclear protein detection,
respectively. Secondary antibodies used in the present study
included HRP-conjugated goat-anti-mouse and -rabbit antibodies
(1:2,000; cat. nos. A0216 and A0208, respectively; Beyotime
Institute of Biotechnology).
Co-immunoprecipitation (Co-IP) of
NLRP3 and ASC
Co-IP was performed using the Capturem Co-IP kit
(cat. no. 635721; Takara Bio, Beijing) according to the
manufacturer's instructions. Briefly, cell lysates (200 µl) were
mixed with an antibody against ASC (1 µg; cat. no. sc-514414;
mouse; Santa Cruz Biotechnology, Inc.), the mixture was then loaded
onto a Protein A column and centrifuged at 1,000 × g for 1 min at
room temperature. The flowthrough was reloaded onto the Protein A
column and centrifuged again at 1,000 × g for 1 min at room
temperature. After washing the Protein A column with 200 µl washing
buffer, 50 µl Elution buffer was added, and the eluted sample was
collected by 1,000 × g centrifugation for 1 min at room temperature
and subjected to western blot analysis with the aforementioned
anti-NLRP3 or anti-ASC antibody. 5% input was loaded as the control
and a normal mouse IgG1 was used as an isotype control
(cat. no. sc-3877; Santa Cruz Biotechnology, Inc.).
Immunofluorescence microscopy
Immunofluorescence staining was performed in cells
cultured on glass coverslips as described previously (25,40).
After fixation with 2% paraformaldehyde for 30 min at room
temperature, the cells were incubated with anti-ASC (1:100; cat.
no. sc-514414; mouse), anti-caspase-1 (1:100; cat. no. sc-1218-R;
rabbit), anti-E-cadherin (1:200; cat. no. sc-21791; mouse) or
anti-NF-κB (1:200; cat. no. sc-8008; mouse) antibodies (all Santa
Cruz Biotechnology, Inc.) at 4°C overnight. After washing, the
slides were incubated with Alexa Fluor 488-labeled goat anti-rabbit
(1:500; cat. no. ab150077; Abcam) or Alexa Fluor 555-labeled
anti-mouse secondary antibody (1:500; cat. no. ab150113; Abcam) for
1 h at room temperature and then subjected to examination using a
Fluoview FV1000 confocal laser scanning microscope (Olympus
Corporation). Images were analyzed and the co-localization
coefficients were calculated using Image-Pro® Plus
(version 7.01; Media Cybernetics, Inc.) (43). Caspase-1 was detected using Alexa
Fluor-488 anti-rabbit IgG secondary antibody and ASC, NF-κB or
E-cadherin using Alexa Fluor-555 anti-mouse secondary antibody. For
the staining of NF-κB, nuclei were counterstained using Nuclear
Green DCS1 (cat. no. ab138905; Abcam).
Measurement of IL-1β in cell culture
media
IL-1β levels in culture media were measured as
described previously (25) using a
Valukine ELISA kit (cat. no. VAL101; R&D Systems, Inc.)
according to the manufacturer's instruction.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism version 9.4.1 (Graphpad Software; Dotmatics). Data are
presented as the mean ± SEM. The significance of differences in
mean values between multiple groups was evaluated using one-way
ANOVA followed by a Tukey's multiple range test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of SCM on the expression levels
of NLRP3, ASC and caspase-1
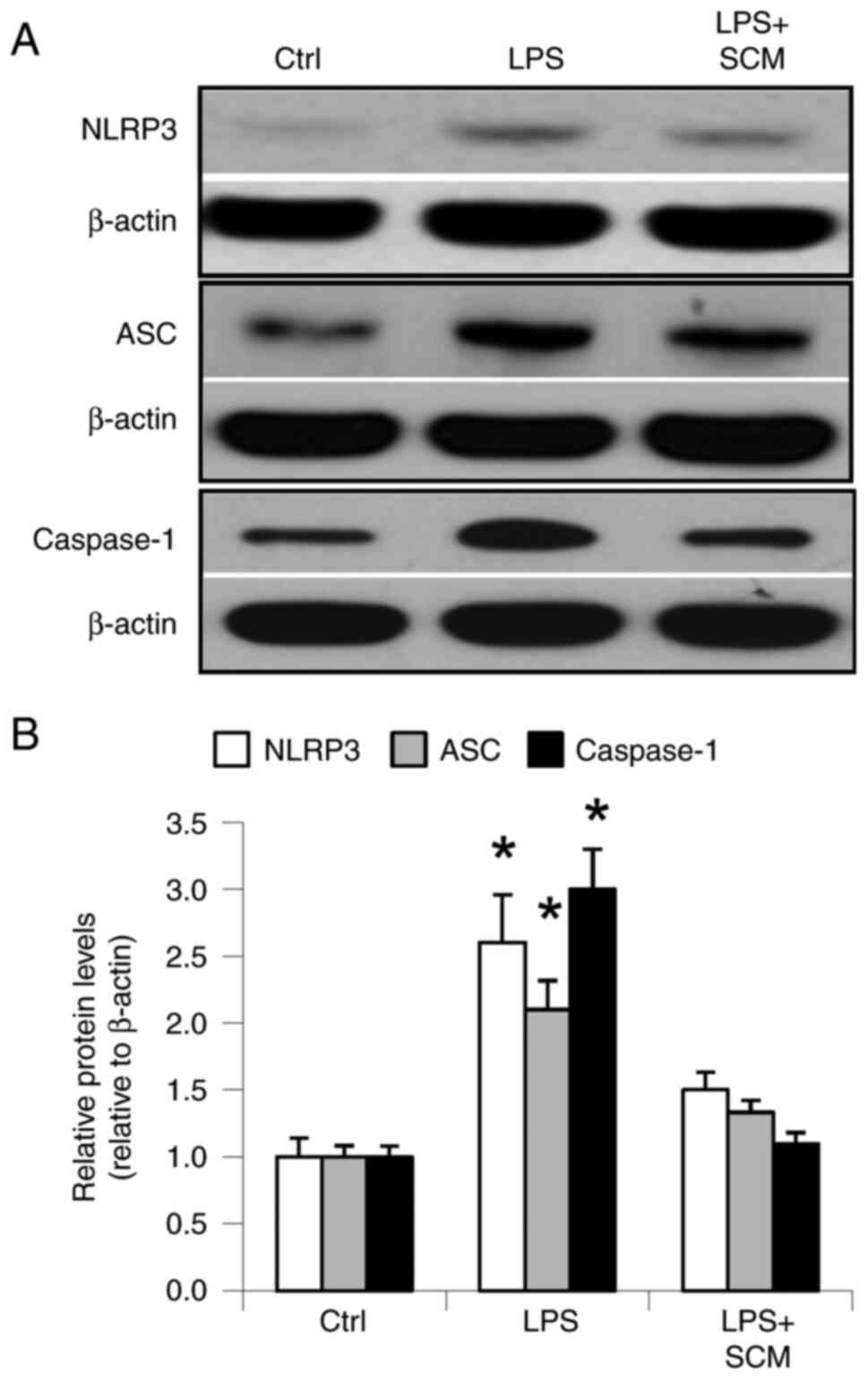
LPS significantly increased the protein expression
levels of inflammasome components, NLRP3, ASC and caspase-1. SCM
inhibited the LPS-induced increases in NLRP3, ASC and caspase-1
expression (Fig. 1). These results
demonstrated that LPS stimulated activation of the inflammasome and
that SCM blocked activation of the inflammasome induced by LPS.
Effect of SCM on inflammasome
assembly
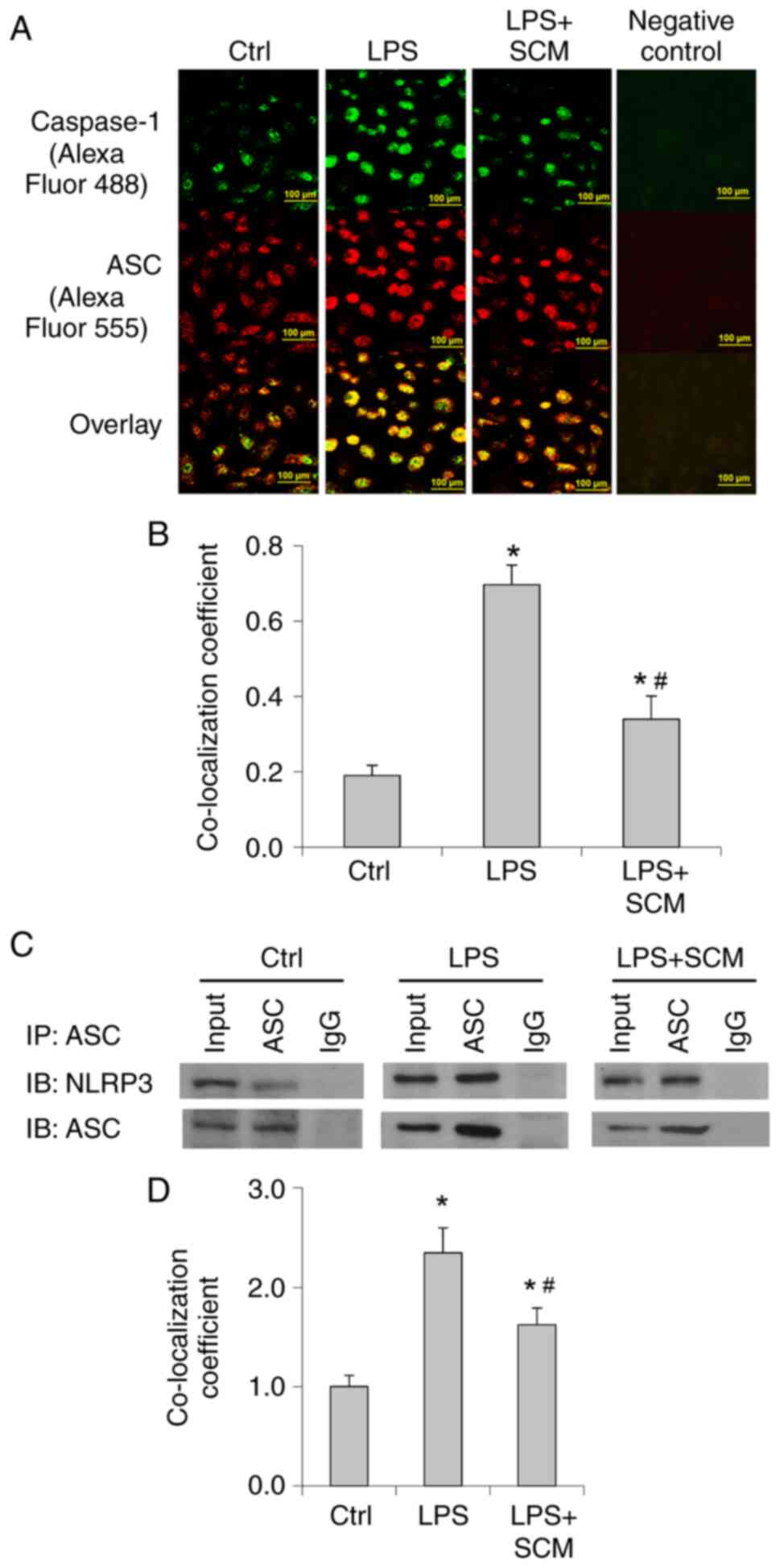
LPS significantly increased the fluorescence
intensity and the colocalization of caspase-1 and ASC
immunostaining (Fig. 2A and B). In
addition, Co-IP analysis suggested that LPS treatment markedly
enhanced the binding of NLRP3 and ASC (Fig. 2C and D). SCM inhibited the increase
in the co-localization of ASC and caspase-1 as well as the binding
of ASC and NLRP3 (Fig. 2). These
data indicated that LPS enhanced formation of the inflammasome and
the recruitment of caspase-1, further suggesting that LPS induced
activation of the inflammasome, and that SCM attenuated LPS-induced
inflammasome activation.
Effect of SCM on the production of
IL-1β
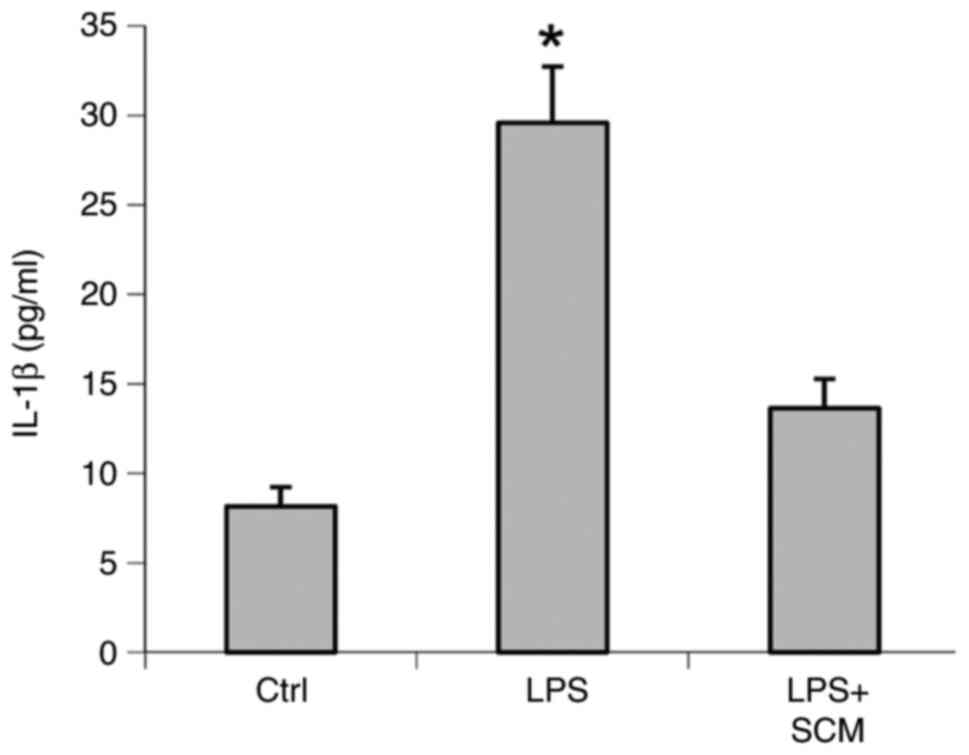
LPS-treated cells exhibited a 3.6-fold increase in
IL-1β levels compared with controls, whereas the LPS-induced
increase in IL-1β level was reduced by 74% in LPS + SCM-treated
cells (Fig. 3). These data
suggested that SCM inhibited activation of the inflammasome and
thereby inhibited the excessive production of IL-1β induced by
LPS.
Effect of SCM on activation of the
inflammatory factor NF-κB
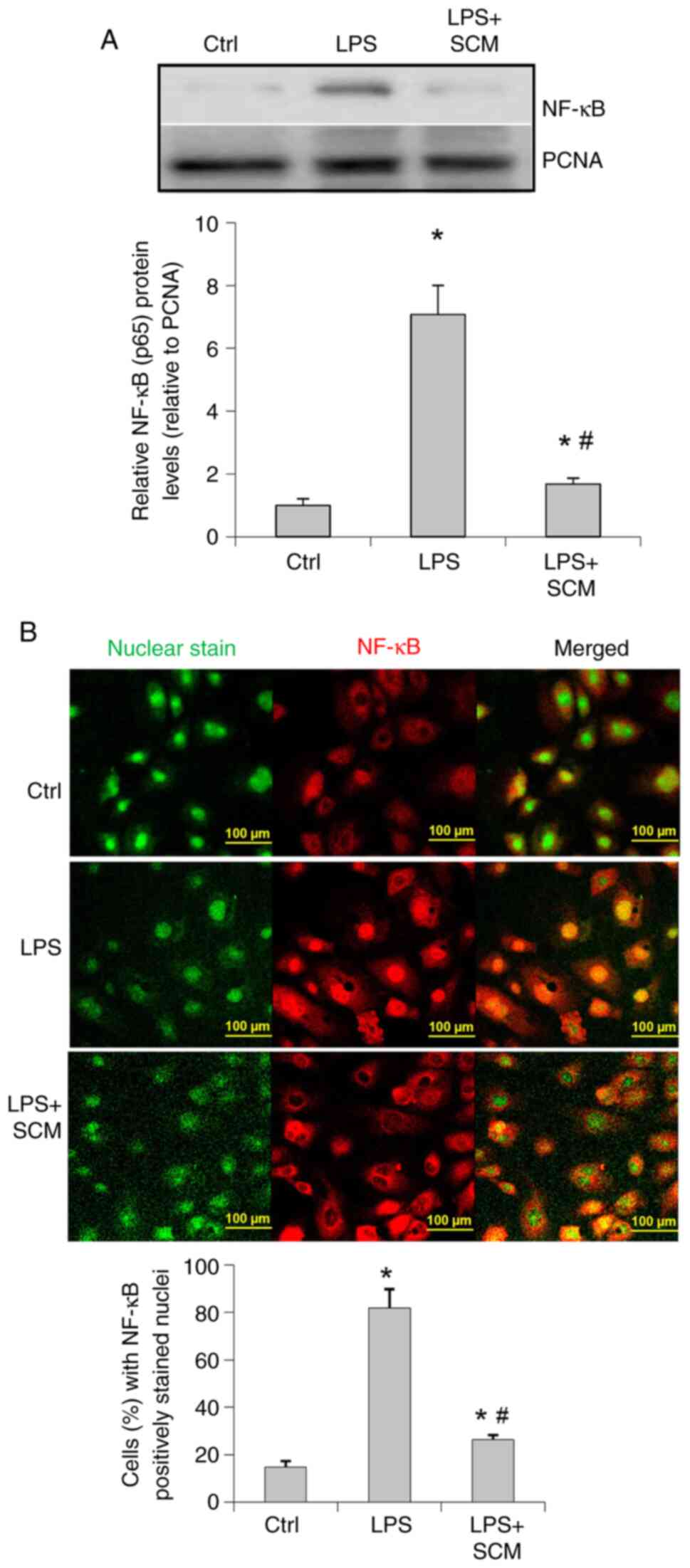
Western blotting results suggested that LPS
significantly upregulated NF-κB levels in the nuclear extracts
(Fig. 4A). Fluorescence staining
demonstrated that the location of NF-κB was predominantly in the
cytoplasm in control cells (Fig.
4B), whereas NF-κB was mainly localized in the nuclear area in
LPS-treated cells, suggesting an enhanced activation of NF-κB
induced by LPS. SCM appeared to block NF-κB translocation into the
nuclei (Fig. 4A and B).
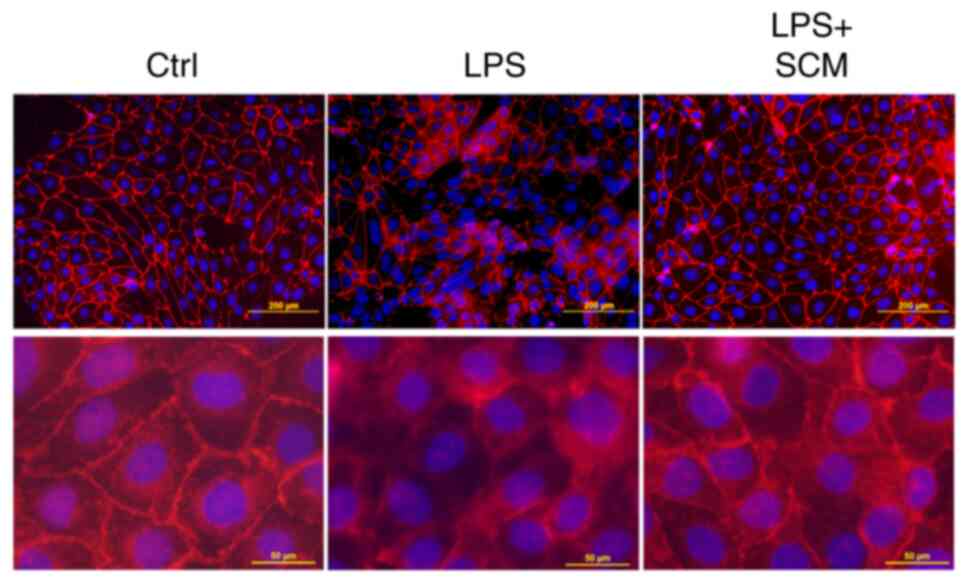
Effect of SCM on cell damage
Immunofluorescence microscopy showed that the
immunostaining pattern of E-cadherin, a cell junction protein, was
disturbed in LPS-treated cells. However, the LPS-induced
disarrangement of E-cadherin was blocked in SCM-treated cells
(Fig. 5), suggesting that SCM
inhibited NLRP3 inflammasome activation, and therefore reduced the
production of its downstream pro-inflammatory effectors, protecting
cells against LPS-induced damage.
Discussion
The results of the present study showed that SCM
reduced the LPS-induced increase in the expression levels of NLRP3,
ASC and caspase-1, inhibited the assembly of inflammasome
components, and blocked IL-1β production, as well as activation of
the pro-inflammatory factor NF-κB. Furthermore, SCM attenuated the
cell damage induced by LPS in cultured human GECs. To the best of
our knowledge, these findings suggested, for the first time, that
SCM can inhibit inflammasome activation and protect human GECs from
LPS-induced inflammatory damage.
Epithelial cells are the first line of defense
against pathogens and danger signals, such as bacterial toxins.
These cells act as a physical barrier to protect other cells, such
as osteoblasts and fibroblasts, from exposure to pathogens. The
present study thus chose to assess GECs. Consistent with previous
reports (23,25), results from the present study
suggested that LPS treatment increased the levels of NLRP3
inflammasome components in GECs. The present results also indicated
that LPS not only increased expression levels of the components of
the NLRP3 inflammasome, but also enhanced assembly of the NLRP3
inflammasome, further suggesting that NLRP3 inflammasome activation
could be induced by LPS. Consequently, LPS increased the production
of IL-1β; however, SCM significantly inhibited LPS-induced
increases in the levels of NLRP3 inflammasome components, assembly
of the inflammasome and the production of IL-1β, suggesting that
SCM inhibits LPS-induced activation of the NLRP3 inflammasome in
GECs.
Previous studies have shown that IL-1β activates
NF-κB (44,45) and that IL-1β mediates the
activation of NF-κB induced by LPS (24). The present study therefore
evaluated the effect of SCM on NF-κB activation. Results from the
present study suggested that LPS enhanced the nuclear translocation
of NF-κB, as indicated by the elevation of NF-κB levels in the
nuclear extract and the increase of NF-κB immunostaining in the
nuclei. The present results are consistent with the literature
showing that LPS induces the increase in the expression levels of
both NF-κB and the NLRP3 inflammasome (46,47).
SCM blocked the nuclear translocation of NF-κB induced by LPS.
These results indicated that SCM blocked the activation of
downstream inflammatory factors associated with the inflammasome.
The present data suggested that SCM inhibits NLRP3 inflammasome
activation and thereby blocks the downstream inflammatory response
induced by LPS.
Inhibition of pro-inflammatory factors IL-1β and
NF-κB by SCM is expected to protect cells from LPS-induced damage.
The present study examined the staining pattern of E-cadherin, a
cell junction protein, to determine epithelial integrity.
Consistent with a previous report (48), the present study showed a
disarrangement of the staining pattern of E-cadherin, suggesting an
LPS-induced disruption of cell junctions. The present results were
supported by previous reports showing that NLRP3 inflammasome
activation was associated with the reduction of E-cadherin
expression (49–51). Notably, SCM blocked the disturbance
in the staining pattern of E-cadherin, suggesting that SCM
protected the cells from LPS-induced inflammatory damage, probably
through inhibition of LPS-induced activation of the NLRP3
inflammasome.
Notably, several studies have shown the inhibition
of NLRP3 inflammasome activation and protection against LPS-induced
damage in human gingival fibroblasts using agents such as the
Vitamin D analog, Eldecalcitol (52), and the antioxidants, Flavocoxid
(9) and Fisetin (53). Although those studies were similar
in design, the current study provided a number of novel
observations compared with the aforementioned studies. First, the
current study suggested a new potential approach, SCM, for the
management of periodontitis. Second, human GECs were used because
GECs are the first line of defense against pathogens and function
as a physical barrier to protect other cells, such as osteoblasts
and fibroblasts, from exposure. Therefore, studies using GECs may
have better clinical relevance and also are in a different cell
type from the previous reports. Third, unlike previous reports, the
present study measured not only the expression of NLRP3
inflammasome components, but also the assembly by double staining
and Co-IP, which better represented activation of the NLRP3
inflammasome. Moreover, the present study examined the nuclear
translocation of NF-κB in addition to the levels of IL-1β, which
mapped the changes of the inflammatory cascade with different
signals. Examination of the immunostaining pattern produced by
E-cadherin showed the morphological integrity of the cells, which
is a more reliable indicator of cell damage than quantifying
molecular signals alone. Overall, the present study suggested a
potential novel approach for the management of periodontitis
supported by observations that have, to the best of our knowledge,
not been revealed in previous studies.
The current study did not attempt to investigate the
mechanisms or molecules produced in SCM that exert the actions
revealed in this study. In this regard, SCM has been shown to
contain a number of growth factors and cytokines that may
contribute to the beneficial effects of SCM (54,55).
Recent studies have also shown that SCM contains microvesicles or
extracellular vesicles (EVs) and that the EVs obtained from MSCs
exert beneficial effects by transferring biological cargo,
including cytoskeleton proteins, signaling proteins, lipids,
enzymes, transcription factors, mRNA, microRNAs, long non-coding
RNAs, DNA and metabolites, to host cells (56,57).
However, the exact factors responsible for the effects observed in
the present study remain unknown and require further investigation
(54). In addition, the findings
in the present in vitro study require confirmation through
in vivo studies in the future.
In summary, the present results demonstrated that
SCM inhibited activation of the NLRP3 inflammasome induced by LPS,
reduced the consequent production of pro-inflammatory factors, and
thereby attenuated LPS-induced damage in human GECs. To conclude,
SCM protects GECs from LPS-induced inflammatory damage, which
provides scientific foundations for testing SCM in vivo and
may assist development of a potential strategy for the prevention
and treatment of chronic periodontitis.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and Technology Innovation
Team Program for Universities of Henan (grant no.
16IRTSTHN066).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, LS and YW conceived, designed and performed the
experiments. HL wrote the paper, LS and YW reviewed and edited the
manuscript. All authors read and approved the final manuscript. LS
and YW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Nazir MA: Prevalence of periodontal
disease, its association with systemic diseases and prevention. Int
J Health Sci (Qassim). 11:72–80. 2017.PubMed/NCBI
|
|
2
|
Bascones A, Noronha S, Gomez M, Mota P,
Moles MA and Dorrego MV: Tissue destruction in periodontitis:
Bacteria or cytokines fault? Quintessence Int. 36:299–306.
2005.PubMed/NCBI
|
|
3
|
Orozco A, Gemmell E, Bickel M and Seymour
GJ: Interleukin-1beta, interleukin-12 and interleukin-18 levels in
gingival fluid and serum of patients with gingivitis and
periodontitis. Oral Microbiol Immunol. 21:256–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pedra JH, Cassel SL and Sutterwala FS:
Sensing pathogens and danger signals by the inflammasome. Curr Opin
Immunol. 21:10–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Broz P and Monack DM: Molecular mechanisms
of inflammasome activation during microbial infections. Immunol
Rev. 243:174–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bostanci N, Emingil G, Saygan B, Turkoglu
O, Atilla G, Curtis MA and Belibasakis GN: Expression and
regulation of the NALP3 inflammasome complex in periodontal
diseases. Clin Exp Immunol. 157:415–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park E, Na HS, Song YR, Shin SY, Kim YM
and Chung J: Activation of NLRP3 and AIM2 inflammasomes by
Porphyromonas gingivalis infection. Infect Immun. 82:112–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Picciolo G, Mannino F, Irrera N, Minutoli
L, Altavilla D, Vaccaro M, Oteri G, Squadrito F and Pallio G:
Reduction of oxidative stress blunts the NLRP3 inflammatory cascade
in LPS stimulated human gingival fibroblasts and oral mucosal
epithelial cells. Biomed Pharmacother. 146:1125252022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang X, Yang X, Ni J, Xie B, Liu Y, Xuan
D and Zhang J: Hyperglucose contributes to periodontitis:
Involvement of the NLRP3 pathway by engaging the innate immunity of
oral gingival epithelium. J Periodontol. 86:327–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yilmaz O: The chronicles of porphyromonas
gingivalis: The microbium, the human oral epithelium and their
interplay. Microbiology (Reading). 154:2897–2903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larijani B, Esfahani EN, Amini P, Nikbin
B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M,
Dowlati Y, et al: Stem cell therapy in treatment of different
diseases. Acta Med Iran. 50:79–96. 2012.PubMed/NCBI
|
|
13
|
Malliaras K and Marban E: Cardiac cell
therapy: Where we've been, where we are, and where we should be
headed. Br Med Bull. 98:161–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Souidi N, Stolk M and Seifert M:
Ischemia-reperfusion injury: Beneficial effects of mesenchymal
stromal cells. Curr Opin Organ Transplant. 18:34–43. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bi B, Schmitt R, Israilova M, Nishio H and
Cantley LG: Stromal cells protect against acute tubular injury via
an endocrine effect. J Am Soc Nephrol. 18:2486–2496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mariani E and Facchini A: Clinical
applications and biosafety of human adult mesenchymal stem cells.
Curr Pharm Des. 18:1821–1845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ionescu L, Byrne RN, van Haaften T,
Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F
and Thébaud B: Stem cell conditioned medium improves acute lung
injury in mice: In vivo evidence for stem cell paracrine action. Am
J Physiol Lung Cell Mol Physiol. 303:L967–L977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walter MN, Wright KT, Fuller HR, MacNeil S
and Johnson WE: Mesenchymal stem cell-conditioned medium
accelerates skin wound healing: An in vitro study of fibroblast and
keratinocyte scratch assays. Exp Cell Res. 316:1271–1281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nguyen BK, Maltais S, Perrault LP, Tanguay
JF, Tardif JC, Stevens LM, Borie M, Harel F, Mansour S and Noiseux
N: Improved function and myocardial repair of infarcted heart by
intracoronary injection of mesenchymal stem cell-derived growth
factors. J Cardiovasc Transl Res. 3:547–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Fang X, Gupta N, Serikov V and
Matthay MA: Allogeneic human mesenchymal stem cells for treatment
of E. coli endotoxin-induced acute lung injury in the ex vivo
perfused human lung. Proc Natl Acad Sci USA. 106:16357–16362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lepperdinger G, Brunauer R, Jamnig A,
Laschober G and Kassem M: Controversial issue: Is it safe to employ
mesenchymal stem cells in cell-based therapies? Exp Gerontol.
43:1018–1023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hua KF, Chou JC, Lam Y, Tasi YL, Chen A,
Ka SM, Fang Z, Liu ML, Yang FL, Yang YL, et al: Polyenylpyrrole
derivatives inhibit NLRP3 inflammasome activation and inflammatory
mediator expression by reducing reactive oxygen species production
and mitogen-activated protein kinase activation. PLoS One.
8:e767542013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yilmaz O, Sater AA, Yao L, Koutouzis T,
Pettengill M and Ojcius DM: ATP-dependent activation of an
inflammasome in primary gingival epithelial cells infected by
Porphyromonas gingivalis. Cell Microbiol. 12:188–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamo N, Ke B, Ghaffari AA, Shen XD,
Busuttil RW, Cheng G and Kupiec-Weglinski JW: ASC/caspase-1/IL-1β
signaling triggers inflammatory responses by promoting HMGB1
induction in liver ischemia/reperfusion injury. Hepatology.
58:351–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H, Zhou X and Zhang J: Induction of
heme oxygenase-1 attenuates lipopolysaccharide-induced inflammasome
activation in human gingival epithelial cells. Int J Mol Med.
34:1039–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
SHEN H, ZHANG D and LIU H: Mesenchymal
stem cell conditioned medium azacytidine, panobinostat and GSK126
alleviate TGF-β-induced EMT in lung cancer. Food Sci Technol.
42:530212021. View Article : Google Scholar
|
|
27
|
Wang L, Li Y, Zhang X, Liu N, Shen S, Sun
S, Jiang Y, Li P, Jin H and Shen L: Paracrine interleukin-8 affects
mesenchymal stem cells through the Akt pathway and enhances human
umbilical vein endothelial cell proliferation and migration. Biosci
Rep. 41:BSR202101982021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Gao Y, Chen P, Zhou Y, Guo S,
Wang L and Chen J: Bone marrow-derived mesenchymal stem cells
(BMSCs)-exosome carrying MiRNA-312 inhibits sevoflurane-induced
cardiomyocyte apoptosis through activation of phosphatidylinositol
3-Kinase/Protein Kinase B (PI3K/AKT) pathway. J Biomaterials Tissue
Engineering. 12:947–952. 2022. View Article : Google Scholar
|
|
29
|
Yu F, Wu F, Li F, Liao X, Wang Y, Li X,
Wang C, Shi Y and Ye L: Wnt7b-induced Sox11 functions enhance
self-renewal and osteogenic commitment of bone marrow mesenchymal
stem cells. Stem Cells. 38:1020–1033. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu MH, Bian BSJ, Cui X, Liu LT, Liu H,
Huang B, Cui YH, Bian XW and Zhou Y: Mesenchymal stem cells
regulate mechanical properties of human degenerated nucleus
pulposus cells through SDF-1/CXCR4/AKT axis. Biochim Biophys Acta.
1863:1961–1968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin H, Zhou Y, Lei Q, Lin D, Chen J and Wu
C: Effect of inorganic phosphate on migration and osteogenic
differentiation of bone marrow mesenchymal stem cells. BMC Dev
Biol. 21:12021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou B, Peng K, Wang G, Chen W and Kang Y:
Polo like kinase 4 (PLK4) impairs human bone marrow mesenchymal
stem cell (BMSC) viability and osteogenic differentiation. Biochem
Biophys Res Commun. 549:221–228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Li S, Xu L, Jiang M, Li X, Zhang
Y, Tighe S, Zhu Y and Li G: Differential gene expression between
limbal niche progenitors and bone marrow derived mesenchymal stem
cells. Int J Med Sci. 17:549–557. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Meng Y, Cui Q, Qin F, Yang H, Chen
Y, Cheng Y, Shi J and Guo Y: MiR-101 Targets the EZH2/Wnt/β-catenin
the pathway to promote the osteogenic differentiation of human bone
marrow-derived mesenchymal stem cells. Sci Rep. 6:369882016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Picciolo G, Mannino F, Irrera N, Altavilla
D, Minutoli L, Vaccaro M, Arcoraci V, Squadrito V, Picciolo G,
Squadrito F and Pallio G: PDRN, a natural bioactive compound,
blunts inflammation and positively reprograms healing genes in an
‘in vitro’ model of oral mucositis. Biomed Pharmacother.
138:1115382021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Ascola A, Irrera N, Ettari R, Bitto A,
Pallio G, Mannino F, Atteritano M, Campo GM, Minutoli L, Arcoraci
V, et al: Exploiting curcumin synergy with natural products using
quantitative analysis of dose-effect relationships in an
experimental in vitro model of osteoarthritis. Front Pharmacol.
10:13472019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Picciolo G, Pallio G, Altavilla D, Vaccaro
M, Oteri G, Irrera N and Squadrito F: β-Caryophyllene reduces the
inflammatory phenotype of periodontal cells by targeting CB2
receptors. Biomedicines. 8:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Yin S, Chen Y, Wu Y, Zheng W, Dong
H, Bai Y, Qin Y, Li J, Feng S and Zhao P: LPS-induced
proinflammatory cytokine expression in human airway epithelial
cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol Med
Rep. 17:5484–5491. 2018.PubMed/NCBI
|
|
39
|
Li J, Qin Y, Chen Y, Zhao P, Liu X, Dong
H, Zheng W, Feng S, Mao X and Li C: Mechanisms of the
lipopolysaccharide-induced inflammatory response in alveolar
epithelial cell/macrophage co-culture. Exp Ther Med. 20:762020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han WQ, Zhu Q, Hu J, Li PL, Zhang F and Li
N: Hypoxia-inducible factor prolyl-hydroxylase-2 mediates
transforming growth factor beta 1-induced epithelial-mesenchymal
transition in renal tubular cells. Biochim Biophys Acta.
1833:1454–1462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Schley G, Turkoglu G, Burzlaff N,
Amann KU, Willam C, Eckardt KU and Bernhardt WM: The protective
effect of prolyl-hydroxylase inhibition against renal ischaemia
requires application prior to ischaemia but is superior to EPO
treatment. Nephrol Dial Transplant. 27:929–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu Q, Wang Z, Xia M, Li PL, Zhang F and
Li N: Overexpression of HIF-1α transgene in the renal medulla
attenuated salt sensitive hypertension in Dahl S rats. Biochim
Biophys Acta. 1822:936–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zinchuk V, Zinchuk O and Okada T:
Quantitative colocalization analysis of multicolor confocal
immunofluorescence microscopy images: Pushing pixels to explore
biological phenomena. Acta Histochem Cytochem. 40:101–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tseng HC, Lee IT, Lin CC, Chi PL, Cheng
SE, Shih RH, Hsiao LD and Yang CM: IL-1β promotes corneal
epithelial cell migration by increasing MMP-9 expression through
NF-kappaB- and AP-1-dependent pathways. PLoS One. 8:e579552013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vinuales C, Gascon S, Barranquero C, Osada
J and Rodriguez-Yoldi MJ: Interleukin-1beta reduces galactose
transport in intestinal epithelial cells in a NF-kB and protein
kinase C-dependent manner. Vet Immunol Immunopathol. 155:171–181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Feng M, Wei S, Zhang S and Yang Y:
Anti-inflammation and anti-pyroptosis activities of mangiferin via
suppressing NF-κB/NLRP3/GSDMD signaling cascades. Int J Mol Sci.
23:101242022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang CH, Wang SC, Chen IC, Chen YT, Liu
PL, Fang SH, Huang SP, Yeh HC, Liu CC and Lee PY: Protective effect
of piplartine against LPS-induced sepsis through attenuating the
MAPKs/NF-κB signaling pathway and NLRP3 inflammasome activation.
Pharmaceuticals (Basel). 14:5882021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He D, Su Y, Usatyuk PV, Spannhake EW,
Kogut P, Solway J, Natarajan V and Zhao Y: Lysophosphatidic acid
enhances pulmonary epithelial barrier integrity and protects
endotoxin-induced epithelial barrier disruption and lung injury. J
Biol Chem. 284:24123–24132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Marandi Y, Hashemzade S, Tayebinia H,
Karimi J, Zamani A and Khodadadi I: NLRP3-inflammasome activation
is associated with epithelial-mesenchymal transition and
progression of colorectal cancer. Iran J Basic Med Sci. 24:483–492.
2021.PubMed/NCBI
|
|
50
|
Tian R, Zhu Y, Yao J, Meng X, Wang J, Xie
H and Wang R: NLRP3 participates in the regulation of EMT in
bleomycin-induced pulmonary fibrosis. Exp Cell Res. 357:328–334.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song S, Qiu D, Luo F, Wei J, Wu M, Wu H,
Du C, Du Y, Ren Y, Chen N, et al: Knockdown of NLRP3 alleviates
high glucose or TGFB1-induced EMT in human renal tubular cells. J
Mol Endocrinol. 61:101–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang C, Zhang C, Yang P, Chao R, Yue Z,
Li C, Guo J and Li M: Eldecalcitol inhibits LPS-induced NLRP3
inflammasome-dependent pyroptosis in human gingival fibroblasts by
activating the Nrf2/HO-1 signaling pathway. Drug Des Devel Ther.
14:4901–4913. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang X, Shen H, Liu Y, Qiu S and Guo Y:
Fisetin attenuates periodontitis through FGFR1/TLR4/NLRP3
inflammasome pathway. Int Immunopharmacol. 95:1075052021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang D, Wang W, Li L, Peng Y, Chen P,
Huang H, Guo Y, Xia X, Wang Y, Wang H, et al: The relative
contribution of paracine effect versus direct differentiation on
adipose-derived stem cell transplantation mediated cardiac repair.
PLoS One. 8:e590202013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pawitan JA: Prospect of stem cell
conditioned medium in regenerative medicine. Biomed Res Int.
2014:9658492014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Taei AA, Khodabakhsh P, Nasoohi S,
Farahmandfar M and Dargahi L: Paracrine effects of mesenchymal stem
cells in ischemic stroke: Opportunities and challenges. Mol
Neurobiol. 59:6281–6306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tan KX, Chang T and Lin X: Secretomes as
an emerging class of bioactive ingredients for enhanced
cosmeceutical applications. Exp Dermatol. 31:674–688. 2022.
View Article : Google Scholar : PubMed/NCBI
|