Introduction
Diabetic retinopathy (DR) is one of the most serious
complications of diabetes mellitus. DR is the primary cause of
vision impairment and blindness in patients with diabetes worldwide
(1). In recent years, it has been
demonstrated that retinal neurodegeneration is found in patients
with diabetes (2) and is involved
in the initiation and progression of DR (3); however, the effects of diabetes on
the retinal pigment epithelium (RPE) have received markedly less
attention (4). The RPE is a
monolayer of pigmented cells located between the neuroretina and
choroids. Cells of the RPE have many biological functions including
transporting nutrients, ions, water and metabolic end products.
Cells of the RPE are also involved in renewing photoreceptor outer
segments, which affect vision and form the outer blood-retina
barrier. Dysfunction of the cells in the RPE has been reported in
numerous inherited and acquired diseases that cause permanent
blindness (5). More specifically,
the breakdown of the RPE barrier is associated with DR (6), and RPE cell dysfunction plays
important roles in DR physiopathology (7). Besides, RPE treated with glucose is
commonly used as an ideal in vitro model for DR research
(8).
The main pathophysiology of DR is various changes
caused by hyperglycemia, which can induce multiple factors and
signaling pathways involved in processes such as oxidative stress
(OS) and autophagy (9). OS is a
phenomenon caused by an imbalance between reactive oxygen species
(ROS) production and the antioxidant system in cells and tissues.
OS can lead to mitochondrial dysfunction which plays a critical
role in the pathogenesis of DR. Certain studies have demonstrated
that OS is involved in the course of DR (10,11).
Therefore, inhibiting ROS generation or scavenging excessive ROS
have been employed as therapeutic strategies for the treatment of
DR (12).
The thioredoxin (Trx) antioxidant system consists of
Trx, Trx reductases, NADPH and peroxiredoxin (13,14).
Trx is the major component of the Trx antioxidant system. It acts
as the natural barrier for cells in the defense of OS,
anti-apoptosis (15) and
regulating autophagy (16). There
are three types of Trx: Trx1 located in the cytoplasm, Trx2 located
in the mitochondria and Trx3 located in sperm.
Extensive research has been reported on the
pathophysiology of DR; however, the exact etiology of this disease
is not fully understood, and little attention has been paid to the
role of RPE in DR. Thus, ARPE19 cells were used to investigate the
effect of Trx1 on high glucose (HG)-induced RPE cell dysfunction
and its related mechanism in vitro, with the aim of finding
and providing evidence for new clinical therapeutic targets of DR
in the future.
Materials and methods
Patients
The present study included 100 patients with
diabetes and 30 healthy individuals as the control group. All
patients (mean age, 63 years; male/female ratio, 1:1) were
recruited (from January 2020 to January 2021) from the Department
of Endocrinology at The Second Hospital of Dalian Medical
University (Dalian, China). The patients with diabetes were divided
into non-DR (NDR), non-proliferative DR (NPDR) and proliferative DR
(PDR) groups. All participants underwent optical coherence
tomography (OCT) and full-field Electroretinogram (ERG) on the same
day. Signed consent forms were obtained from all patients. The
present study was approved (approval no. 2020008; approval date,
January 10, 2020) by the ethics committee of The Second Hospital of
Dalian Medical University (Dalian, China).
Cell culture and treatment
The ARPE19 cell line was obtained from the Institute
of Biochemistry and Cell Biology, Chinese Academy of Sciences and
maintained in DMEM-F12 medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (Biological Industries),
penicillin (100 U/ml) and streptomycin (100 µg/ml) (HyClone;
Cytiva). ARPE19-Trx1 and ARPE19-LacZ were either untreated or
treated with 75 mM glucose and mannitol for 24 h in the
experiment.
Measurement of intracellular ROS
The generation of intracellular ROS was detected by
the DCFH-DA probe method (cat. no. S0033S; Beyotime Institute of
Biotechnology). Briefly, the cells were seeded in 6-well plates at
2×105 cells/well and all groups were treated with or
without high glucose (75 mM) for 24 h. Cells were then washed with
PBS three times, before incubating with a DCFH-DA probe at a final
concentration of 10 µmol/l at 37°C for 30 min. Fluorescence
intensity was measured by a fluorescence microscope.
Measurement of mitochondrial membrane
potential (MMP)
Cells were seeded in a six-well plate (Guangzhou Jet
Bio-Filtration Co., Ltd.) at a density of 2×105
cells/well. Briefly, the cells in all groups were treated with or
without high glucose (75 mM). After washing with PBS, the cells
were incubated with 1 ml cell culture medium and 1 ml JC-1 staining
solution (Beyotime Institute of Biotechnology) at 37°C in a cell
incubator for 20 min. Images were obtained using fluorescence
microscopy (NikonTi-S; Nikon Corporation).
Western blotting
The cells were lysed on ice in buffer containing 50
mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5%
deoxycholate, 0.1% SDS, 1 mM PMSF and 150 U/ml aprotinin. The
concentration of the protein was measured by BCA assay. The same
amount of protein (30 µg/lane) was loaded on a 10% polyacrylamide
gel for each sample. Proteins separated by SDS-PAGE were
electro-transferred to PVDF membranes. The membranes were blocked
with 5% skimmed milk diluted with TBST (0.5% Tween-20) at room
temperature for 1.5 h and then incubated with the following primary
antibodies: β-actin (Santa Cruz Biotechnology Inc.; cat. no.
sc-47778; 1:1,000), cytochrome C (Cyt C; Abcam; cat. no. ab133504;
1:1,000), Bax (Proteintech Group, Inc.; cat. no. 50599-2-Ig;
1:1,000), Bcl-2 (Abcam; cat. no. ab182858; 1:1,000),
apoptosis-inducing factor (AIF; Abcam; cat. no. ab1998; 1:1,000),
Trx1 (Abcam; cat. no. ab133524; 1:5,000), RPE65 (Abcam; cat. no.
ab231782; 1:1,000), Caspase9 (Proteintech Group, Inc.; cat. no.
10380-1-AP; 1:1,000), Caspase3 (Proteintech Group, Inc.; cat. no.
19677-1-AP; 1:1,000) and zonula occludens-1 (ZO-1; Proteintech
Group, Inc.; cat. no. 21773-1-AP; 1:1,000), overnight at 4°C.
Membranes were washed with TTBS three times for 10 min each and
incubated with the appropriate secondary antibody (goat anti-rabbit
IgG; cat. no. SA00001-2; or goat anti-mouse IgG; cat. no.
SA00001-1; both Proteintech Group, Inc.) diluted in blocking buffer
at 1:1,000, for 1 h at room temperature. Protein bands were imaged
using the enhanced chemiluminescence system (Bio-Rad Laboratories,
Inc.). The intensities of the bands were measured using the
LabWorks 4.5 software (Analytik Jena AG).
Generation of stable cell line
The recombinant plasmids (0.4 µg) (16), pIRES2-EGFP-Trx1 and
pIRES2-EGFP-LacZ, were transfected into ARPE19 cells using the
Effectene Transfection Reagent (Qiagen China Co.) according to the
manufacturer's protocol. Transfection complexes were removed and
fresh medium was added to the cells. The cells were then incubated
in their normal growth conditions after transfection at 37°C for 24
h. A fluorescent microscope was used to check for transfection
efficiency. Stable cell lines were prepared by selection in medium
containing 500 µg/ml G418 (Sigma-Aldrich; Merck KGaA). After 4
weeks of selection, several independent clones were picked and were
confirmed by immunofluorescence or western blotting to detect the
target protein level expression.
Immunofluorescence
The cells were seeded onto coverslips, followed by
treatment when the cells reached ~80% confluency. The medium was
removed and the cells were washed twice with PBS. The cells were
then fixed in 4% paraformaldehyde at room temperature for 30 min,
followed by incubation with PBS containing 0.25% Triton X-100
(Sigma-Aldrich; Merck KGaA) for 10 min. The cells were washed in
PBST (containing 0.025% Tween) three times for 5 min, and then
blocked with 10% normal horse serum (Beijing Solarbio Science &
Technology Co., Ltd.) in PBST (containing 0.1% Tween) for 1 h.
Lastly, slides were incubated with ZO-1 primary antibody
(Proteintech Group, Inc.; cat. no. 21773-1-AP; 1:500), Trx1 (Abcam;
cat. no. ab133524; 1:100) at 4°C overnight. The cells were then
washed with PBST three times, followed by incubation with secondary
antibody (Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
A16111/A-31572; 1:2,000) diluted in blocking buffer at room
temperature for 1 h in the dark. DAPI (0.9 µg/ml) was also used to
stain the nucleus. One drop of mounting medium (cat. no. H-1000;
Vector Laboratories, Inc.) was loaded on top of the specimen,
before coverslips were placed on the slides. Fluorescence images
were acquired by a fluorescent microscope (NikonTi-S; Nikon
Corporation).
Flow cytometric analysis
Cell apoptosis was analyzed by staining with Annexin
V/propidium iodide (PI) (Nanjing KeyGen Biotech Co., Ltd.).
Briefly, ARPE19 cells were treated with HG for 24 h. The cells were
washed twice with PBS and resuspended in 500 µl 1X binding buffer
(Nanjing KeyGen Biotech Co., Ltd.), followed by incubation at 25°C
for 15 min in the dark. Fluorescence of PI and Annexin V was
monitored by FACS (ACEA Bio-science, Inc.) at 525 and 630 nm,
respectively. The data was analyzed using BD Accuri C6 software (BD
Biosciences).
Trans-epithelial electrical resistance
(TEER) measurement
ARPE19 cells (5×104 cells/well) were
cultured in Transwell inserts on a 0.4-µm pore polyethylene
terephthalate membrane (6.5 mm diameter; Corning Inc.). The media
were replaced every 2–3 days after the cells adhered to the
membrane. TEER was conducted over a 4-week period using an EVOM 2
epithelial volt ohmmeter and 4-mm STX2 chopstick electrode (World
Precision Instruments). Briefly, the electrode was sterilized in
70% ethanol, rinsed in PBS and equilibrated in pre-warmed culture
medium before being placed into the apical and basal Transwell
compartments. Measurements were recorded from at least five
separate wells per experiment. In each well, three measurements
were recorded to obtain an average value. The TEER value was
calculated using the following formula: TEER (Ω
cm2)=[total resistance-blank resistance (Ω)]x[area
(cm2)].
Transmission electron microscopy
(TEM)
The ARPE19 cells were treated with different
experimental conditions, collected and fixed at 4°C with
glutaraldehyde. PBS (0.1 M) was used to wash the samples three
times for 15 min each time, and then the samples were stained with
osmic anhydride at room temperature for 2 h. The samples were
washed again with 0.1 M PBS three times for 15 min each, and then
dehydrated using an ethanol series. Epoxy propylene was used for 10
min and the sample was placed into Epon12 embedding medium
(GP18010). Next, the samples were heated in a 37°C oven for 24 h, a
45°C oven for 24 h and a 60°C oven for 48 h. Ultrathin slices were
prepared and observed under a transmission electron microscope
(JEM-2000EX; JEOL Ltd.).
Detection of SOD activity
ARPE19 cells (3×105 cells/well) were
seeded in six-well plates. Then cells were treated with 75 mM
glucose for 24 h when the cell confluence reached 50–60%. The SOD
activity in ARPE19 cells was detected by the kit provided by the
Beyotime Institute of Biotechnology (cat. no. S0101S), according to
the manufacturer's instructions. The intracellular SOD activity was
detected by microplate reader at a wavelength of 450 nm.
Statistical analysis
The data were presented as the mean ± SD from three
independent experiments and analyzed by unpaired Student's t-test
(two groups) or one-way ANOVA (three or more groups) followed by
Tukey's or Bonferroni's post hoc test. All statistical analyses
were conducted using GraphPad Prism (version 6.0; Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Diabetes-induced RPE cell degeneration
with vision damage in clinical patients
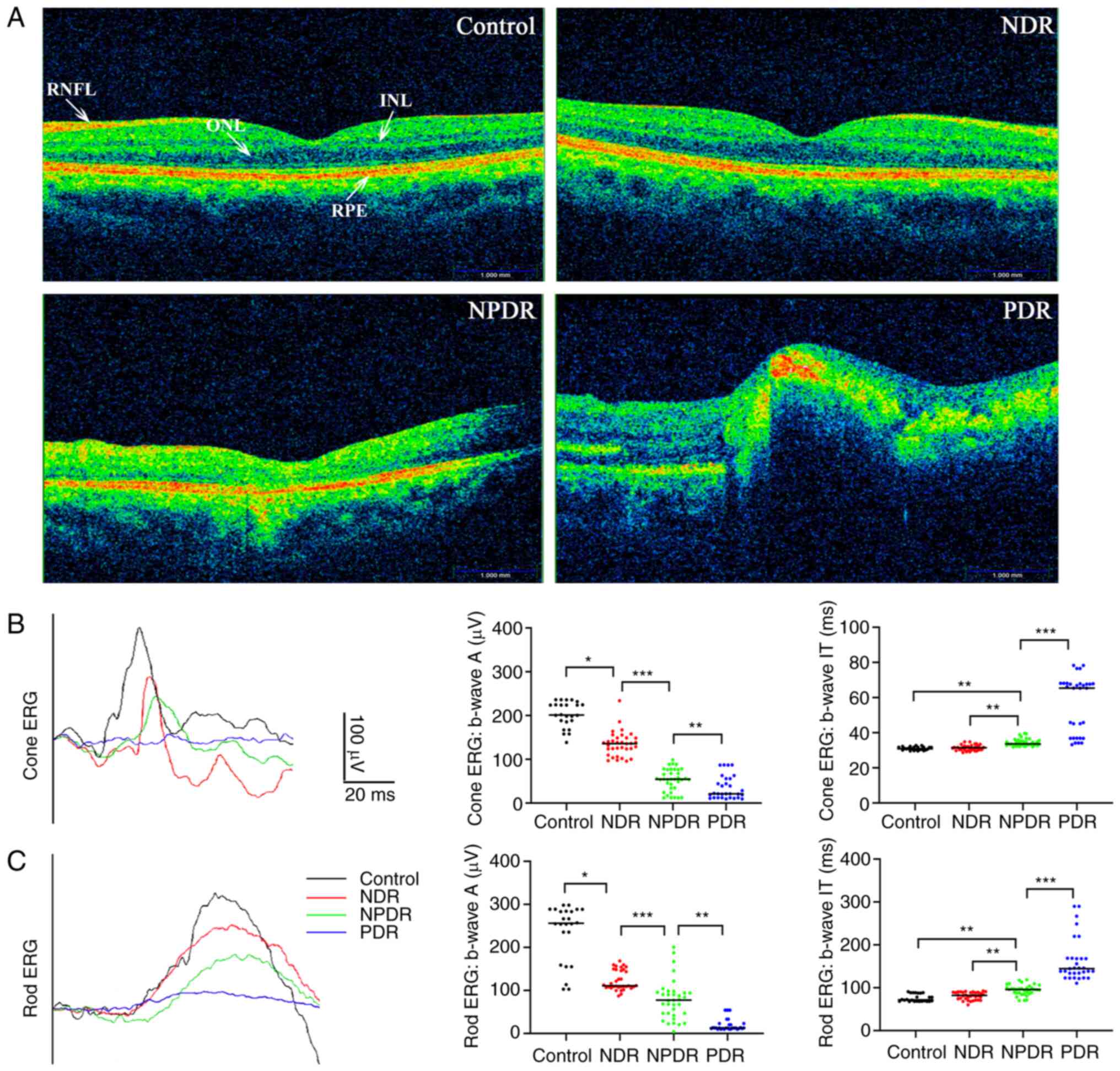
Aiming to explore the association of DR with RPE
cells, data of clinical patients were collected. As shown in the
OCT images in Fig. 1, there was no
difference in the morphology of the RPE layer in patients with
diabetes in the NDR group compared with the control group; however,
the OCT images of the NPDR and PDR groups exhibited an abnormal
morphology of the RPE layer compared with the control group.
Moreover, the ERG results for the patients with diabetes in the
NDR, NPDR and PDR groups demonstrated that the A-wave and B-wave
amplitudes of the rods and cones were decreased compared with the
control group.
Diabetes-induced RPE cell apoptosis in
vitro
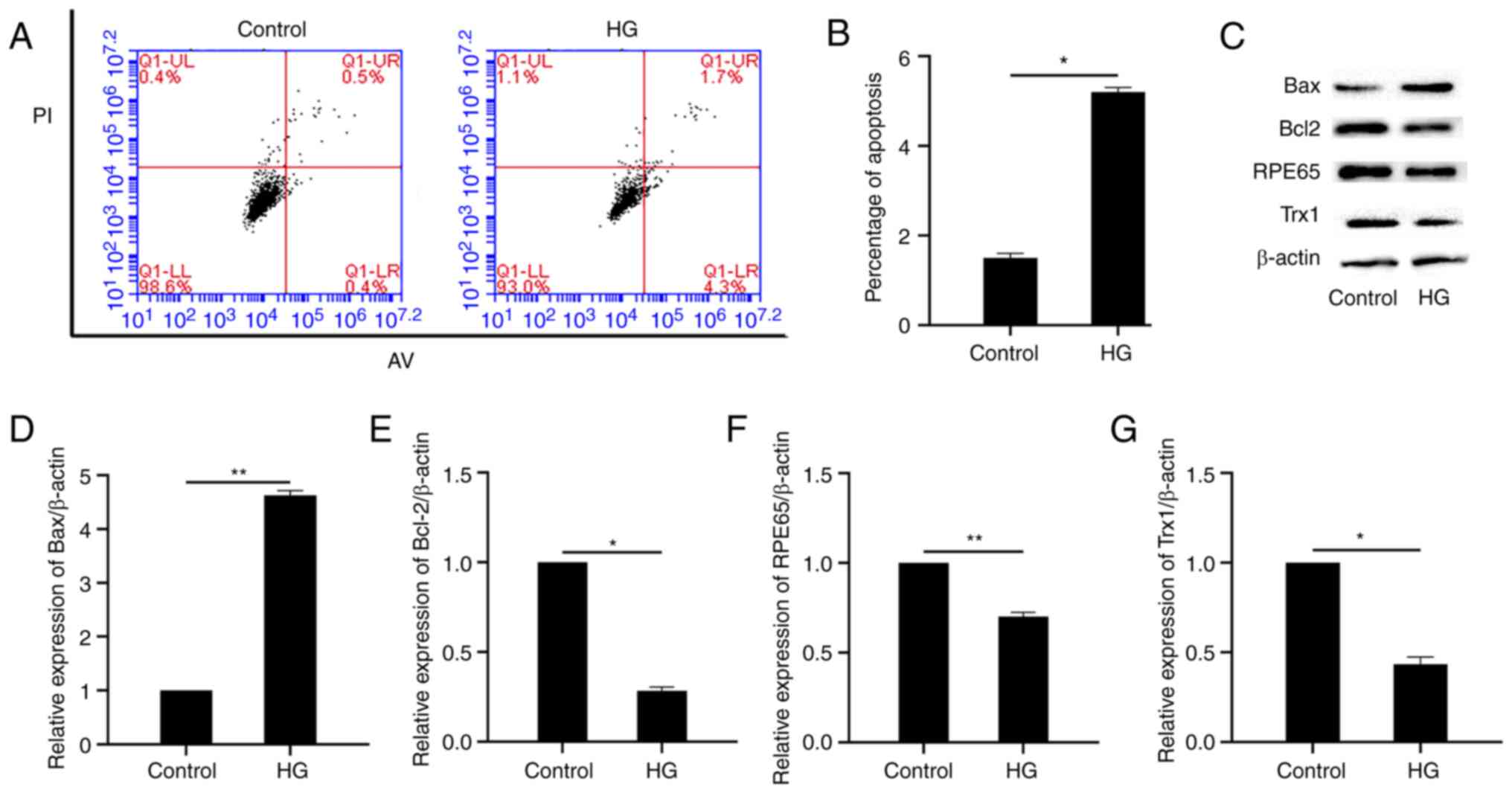
Aiming to explore the effect of glucose-induced high
osmotic pressure as well as HG in RPE cell, a cell viability
experiment was performed using mannitol (equal concentration to the
HG) to explore the effect of osmotic pressure and no effect was
observed (Fig. S1). In vitro cell
apoptosis was analyzed by flow cytometry. As shown in Fig. 2A and B, the percentage of apoptotic
cells increased significantly after HG treatment. The expression of
the pro-apoptotic and anti-apoptotic proteins, Bax and Bcl-2, were
also detected by western blotting. As demonstrated in Fig. 2C-E, the expression of Bax and Bcl-2
increased and decreased, respectively, after HG treatment, compared
with the control group. In addition, the expression of Trx1 and
RPE65 also decreased (Fig. 2C, F and
G).
Overexpression of Trx1 attenuates
diabetes-induced RPE cell apoptosis in vitro
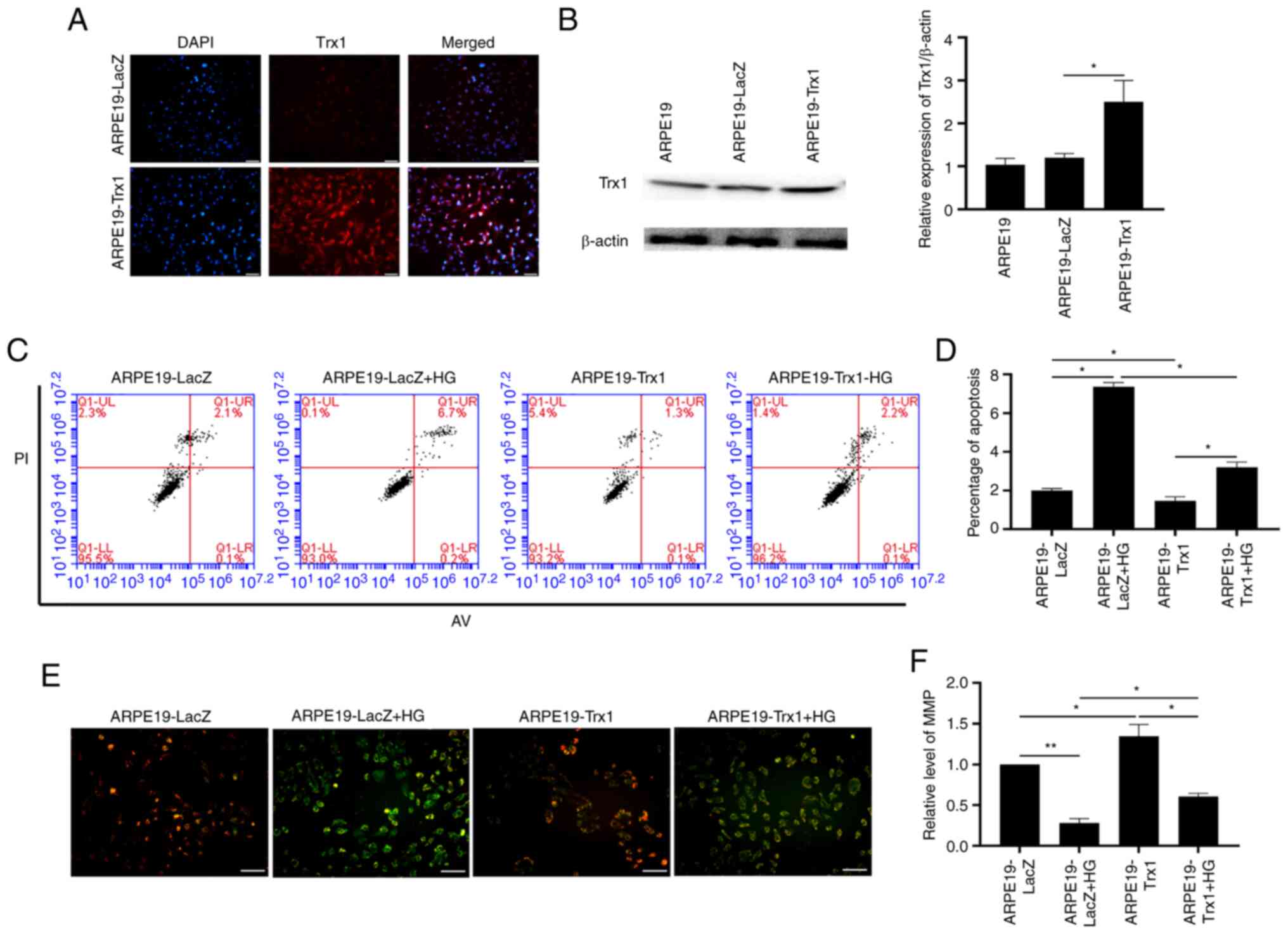
After overexpression of Trx1 and the ARPE19-LacZ
cells as the control, the apoptosis of ARPE19 cells was analyzed by
flow cytometry. The stable overexpression Trx1 ARPE19 cell was
identified by immunofluorescence and western blotting (Fig. 3A and B). As shown in Fig. 3C and D, the percentage of apoptotic
cells increased in the ARPE19-LacZ cells after HG treatment whereas
it decreased in the ARPE19-Trx1 cells. Additionally, the MMP assay
results demonstrated that the red/green fluorescence ratio was
decreased in the ARPE19 cells after HG treatment; however, this was
reversed by overexpression of Trx1 (Fig. 3E and F).
Overexpression of Trx1 attenuates
hyperglycemia-induced RPE cell dysfunction in vitro
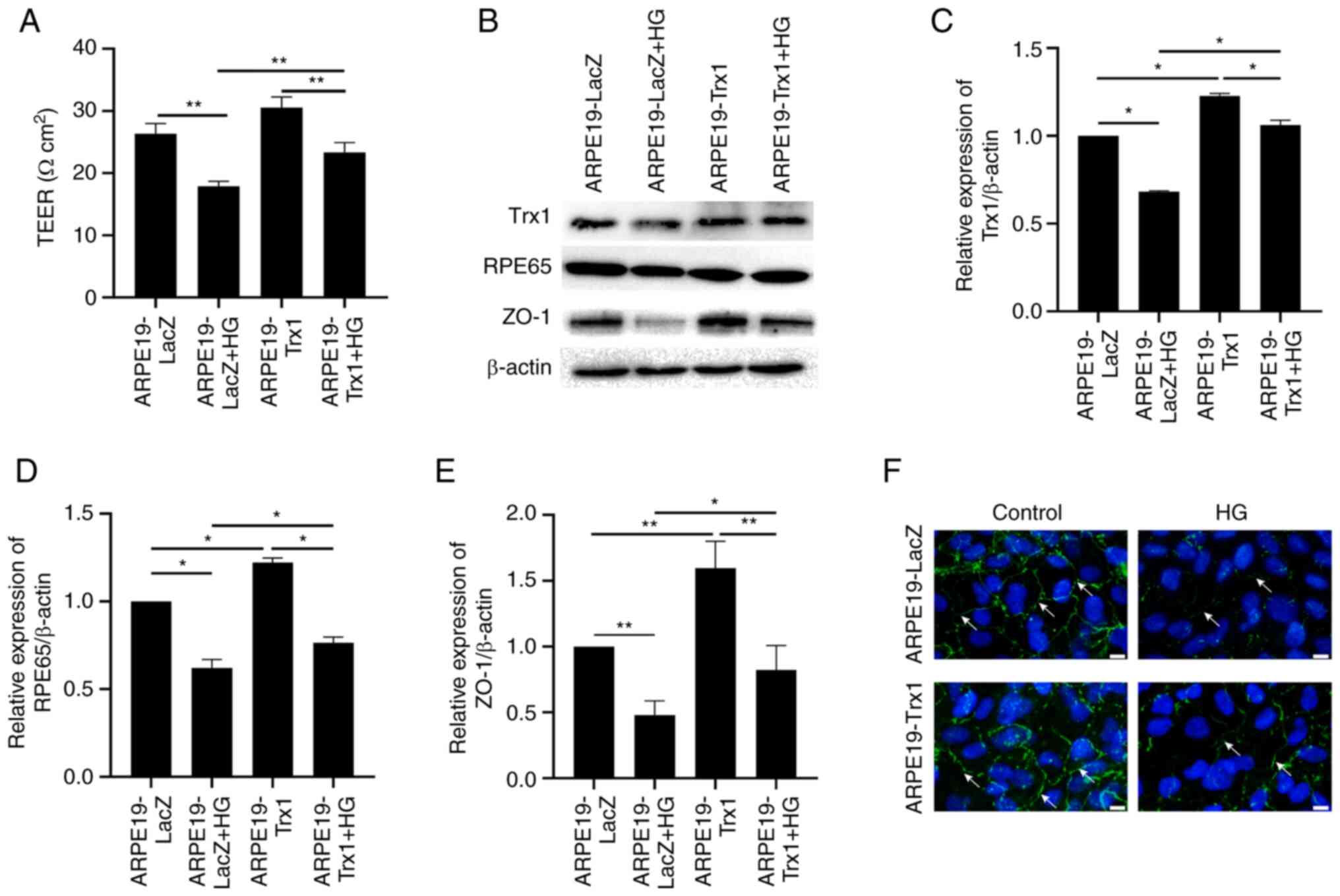
The TEER and the expression of RPE65 and ZO-1 were
measured to present the function of RPE cells after HG treatment
in vitro. After HG treatment for 24 h, the TEER was
decreased significantly both in the ARPE19-Trx1 and ARPE19-LacZ
groups; however, the change was slow in the ARPE19-Trx1 group
compared with the ARPE19-LacZ group (Fig. 4A). The expression of Trx1, RPE65
and ZO-1 decreased in the APRE19-LacZ group after HG treatment;
however, they increased in the APRE19-Trx1 group compared with
APRE19-LacZ group (Fig. 4B-E).
Besides, the immunofluorescent staining for the tight junction
protein, ZO-1, demonstrated that the expression of continuous
junctions between cells in the ARPE19-LacZ + HG group was decreased
compared with the ARPE19-LacZ group, while it increased after Trx1
overexpression (Fig. 4F).
Overexpression of Trx1 attenuates
hyperglycemia-induced RPE cell dysfunction via the
OS/mitochondrial-mediated cell apoptotic pathway in vitro
As demonstrated in Fig.
5A and B, HG induced an increase in ROS formation; however,
overexpression of Trx1 in ARPE19 cells attenuated the process.
Furthermore, SOD activity was also detected to evaluate the level
of OS (Fig. 5C). TEM was used to
observe the effect of Trx1 overexpression on mitochondrial
morphology in ARPE19 cells with or without HG treatment. The
results demonstrated that the mitochondrial shape was abnormal and
the membrane outline was unclear in ARPE19-LacZ cells compared with
the ARPE19-Trx1 cells after HG treatment (Fig. 5D).
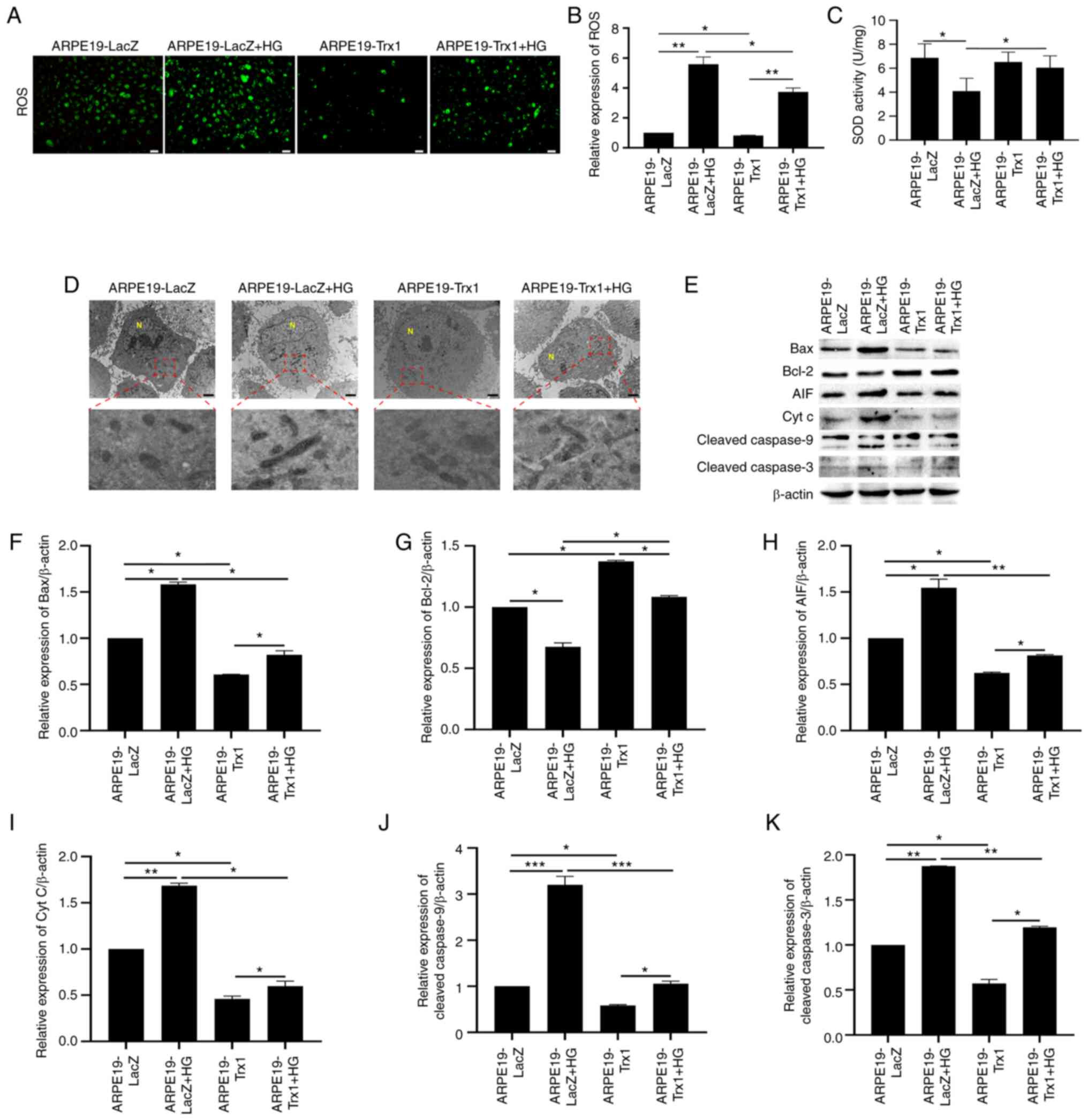 | Figure 5.Overexpression of Trx1 delays
diabetes-induced RPE cell dysfunction via the
ROS/mitochondrial-mediated cell apoptotic pathway in vitro.
(A and B) The effect of Trx1 overexpression on ROS generation in
ARPE19 cells with or without HG treatment (scale bar, 50 µm). (C)
The effect of Trx1 overexpression on SOD activity in ARPE19 cells
with or without HG treatment. (D) The effect of Trx1 overexpression
on mitochondrial morphology in ARPE19 cells after HG treatment
(scale bar, 2 µm). (E) Representative western blotting images
showing the effect of Trx1 overexpression on the expression of Bax,
Bcl-2, AIF, Cyt C, cleaved-Caspase9 and cleaved-Caspase3 in ARPE19
cells with or without HG treatment. Semi-quantitative analysis
showed the effect of Trx1 overexpression on the expression of (F)
Bax, (G) Bcl-2, (H) AIF, (I) Cyt C, (J) cleaved-Caspase9 and (K)
cleaved-Caspase3 in ARPE19 cells with or without HG treatment.
β-actin was used as the internal control. Data are presented as the
mean ± SD; n=3. *P<0.05, **P<0.01 and ***P<0.001. AIF,
apoptosis-inducing factor; Cyt C, cytochrome C; HG, high glucose;
N, nucleus; RPE, retinal pigment epithelium; ROS, reactive oxygen
species; Trx1, thioredoxin1; SOD, superoxide dismutase. |
Moreover, western blotting was conducted to detect
the expression of mitochondrial-mediated cell apoptotic proteins.
The expression of Bax, AIF, cleaved-Caspase9, cleaved-Caspase3 and
Cyt C increased in ARPE19-LacZ cells after HG treatment; however,
the expression of Bcl-2 decreased. Overexpression of Trx1
downregulated the expression of Bax, AIF, cleaved-Caspase9,
cleaved-Caspase3 and Cyt C, and upregulated the expression of Bcl-2
(Fig. 5E-K).
Discussion
DR is a leading cause of visual impairment and
blindness in individuals aged 24–64 years old. Historically, DR was
regarded as a common microvascular complication in diabetes. In the
last few decades, most studies have focused on vascular
abnormalities in DR. Diabetes-induced retinal vasculature
abnormality was considered as the main cause of vision loss and
impairment in patients with diabetes (17). Nevertheless, in recent years,
alterations in the neural retina also have been detected, and their
contribution to vision loss is under study (18). RPE cells are regarded as a
component of the retina and play an important role in maintaining
retina homeostasis, particularly in the maintenance of vision
function; however, there are fewer studies reporting the role of
RPE cells during DR. In the present study, it was found that
hyperglycemia induced RPE cell damage in vitro, which was
related to the downregulation of Trx1 expression. Moreover,
hyperglycemia-induced RPE apoptosis in APRE19 cells was inhibited
by Trx1 overexpression in vitro. These results indicated
that Trx1 overexpression can inhibit ARPE19 cell apoptosis during
DR in vitro.
The RPE cell has many functions, including active
transport of ions and other substances, light absorption,
photopigment renewal, trophic factor secretion, immune modulation
and phagocytosis of the photoreceptor outer segment membranes
(19,20); therefore, the function of ARPE19
cells in the presence or absence of HG treatment was analyzed in
the present study. It was observed that the function of RPE cells
decreased in the presence of HG treatment; however, overexpression
of Trx1 reversed this process. These data suggested that
hyperglycemia-induced RPE cell dysfunction was attenuated by
overexpression of Trx1.
DR is the most common complication of diabetes, and
the exact underlying mechanism is still not fully defined due to
its multi-factorial character. Hyperglycemia, the condition that
sustains abnormally HG levels in the blood, characterizes diabetes,
and is considered to be involved in the initiation and progression
of diabetes and its subsequent complications. Hyperglycemia affects
the cells in multiple ways, including by non-enzymatic based
protein modification, OS, chronic inflammation and the activation
of the protein kinase C pathway (21–24).
OS has been implicated in a host of diseases, including diabetes
and its complications. In the present study, the main focus was on
OS-induced apoptosis after HG treatment in ARPE19 cells. When
ARPE19 cells were treated with HG, the formation of ROS increased
significantly, the percentage of apoptotic ARPE19 cells increased
and the MMP decreased. TEM was also used to observe the
morphological changes of the mitochondria in ARPE19 cells after HG
treatment. The morphology (shape and membrane) of mitochondria was
abnormal after HG treatment. By contrast, the percentage of
apoptotic cells decreased, the MMP increased and the morphology
changes were markedly normal after Trx1 overexpression in ARPE19
cells, compared with the control group after HG treatment. In
addition, the expression of mitochondrial-mediated cell apoptosis
proteins was also detected to illustrate the related mechanism. In
the present study, the expression of Bax, AIF, cleaved-Caspase9,
cleaved-Caspase3 and Cyt C increased in ARPE19-LacZ cells after HG
treatment; however, the expression of Bcl-2 decreased.
Overexpression of Trx1 downregulated the expression of Bax, AIF,
cleaved-Caspase9, cleaved-Caspase3 and Cyt C, and upregulated the
expression of Bcl-2. These data suggested that Trx1 overexpression
may protect ARPE19 cells from HG-induced dysfunction related to the
ROS/mitochondrial-mediated cell apoptotic pathway. Autophagy is
also closely related to DR, and a previous study also confirmed
that autophagy plays an important role in DR (25). Piano et al (26) also observed increased autophagy
levels in the other types of retina cells in STZ-induced diabetic
mice, such as in the retinal ganglion cell layer. Nevertheless,
there is a limitation to the present study; the ARPE19 cell line
was only used as the model. In further research, it would be better
to fully understand the mechanism by other cell lines or primary
cell culture method together.
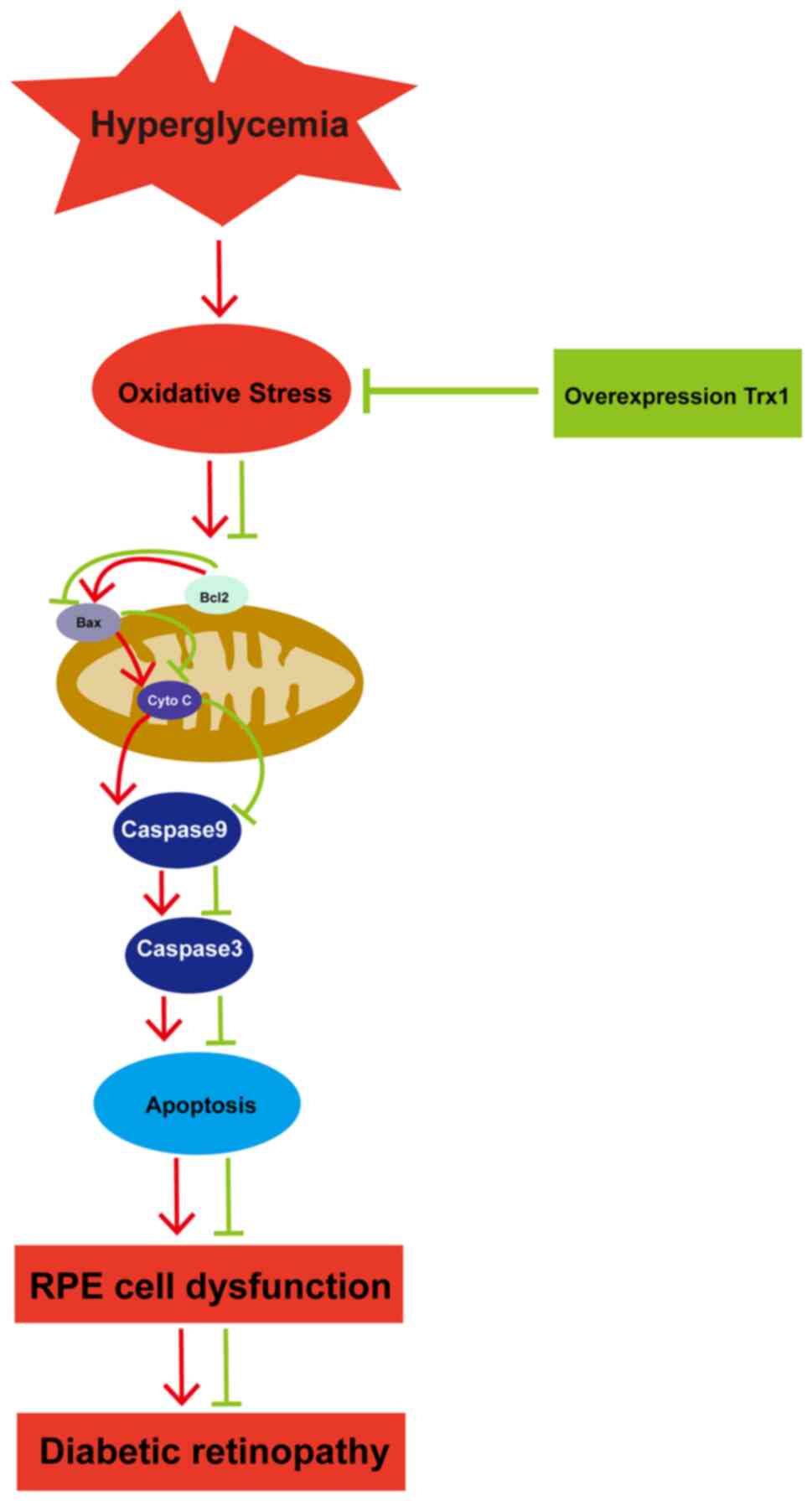
To summarize, it was demonstrated in the present
study that overexpression of Trx1 could increase the function of
ARPE19 cells and protected ARPE19 cells from hyperglycemia-induced
cell dysfunction through regulating the ROS/mitochondria-mediated
apoptosis pathway (Fig. 6).
Furthermore, the relationship between thioredoxin1 and DR in clinic
was also studied. Considering this, targeting Trx1 or its related
signaling pathways may be a candidate therapeutic approach for the
treatment or prevention of DR in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science
Foundation of China (grant no. 31371218) and The National and Local
Joint Engineering Research Center for Mongolian Medicine Research
and Development (grant no. MDK2019075). This project was also
supported by The Liaoning Provincial Program for Top Discipline in
Basic Medical Sciences and The Innovation and Entrepreneurship
Training Program of Dalian Medical University (grant no.
2017161030).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HQ, TL, JL, QT, ZM, CZ, SWa and SWe performed the
experiments and interpreted the experimental results. HQ, XR, HK
and LK conceived the idea and designed the experiments. TL, JL, QT,
XR and ZM participated in the data analysis. XR and HQ wrote the
manuscript. XR revised the manuscript. LK and HK funded the study.
HK and LK confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All participants were recruited from the Second
Hospital of Dalian Medical University. The present study was
approved (approval no. 2020008; approval date, January 10, 2020) by
the ethics committee of The Second Hospital of Dalian Medical
University (Dalian, China). Informed consent was obtained from all
subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hendrick AM, Gibson MV and Kulshreshtha A:
Diabetic retinopathy. Primary Care. 42:451–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mrugacz M, Bryl A and Zorena K: Retinal
vascular endothelial cell dysfunction and neuroretinal degeneration
in diabetic patients. J Clin Med. 10:4582021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rossino MG, Dal Monte M and Casini G:
Relationships between neurodegeneration and vascular damage in
diabetic retinopathy. Front Neurosci. 13:11722019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia T and Rizzolo LJ: Effects of diabetic
retinopathy on the barrier functions of the retinal pigment
epithelium. Vision Res. 139:72–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lakkaraju A, Umapathy A, Tan LX, Daniele
L, Philp NJ, Boesze-Battaglia K and Williams DS: The cell biology
of the retinal pigment epithelium. Prog Retin Eye Res.
1008462020.doi: 10.1016/j.preteyeres.2020.100846 (Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simó R, Villarroel M, Corraliza L,
Hernández C and Garcia-Ramírez M: The retinal pigment epithelium:
Something more than a constituent of the blood-retinal
barrier-implications for the pathogenesis of diabetic retinopathy.
J Biomed Biotechnol. 2010:1907242010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tarchick MJ, Bassiri P, Rohwer RM and
Samuels IS: Early functional and morphologic abnormalities in the
diabetic nyxnob mouse retina. Invest Ophthalmol Vis Sci.
57:3496–3508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DI, Park MJ, Lim SK, Choi JH, Kim JC,
Han HJ, Kundu TK, Park JI, Yoon KC, Park SW, et al:
High-glucose-induced CARM1 expression regulates apoptosis of human
retinal pigment epithelial cells via histone 3 arginine 17
dimethylation: Role in diabetic retinopathy. Arch Biochem Biophys.
560:36–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dehdashtian E, Mehrzadi S, Yousefi B,
Hosseinzadeh A, Reiter RJ, Safa M, Ghaznavi H and Naseripour M:
Diabetic retinopathy pathogenesis and the ameliorating effects of
melatonin; involvement of autophagy, inflammation and oxidative
stress. Life Sci. 193:20–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodríguez ML, Pérez S, Mena-Mollá S, Desco
MC and Ortega ÁL: Oxidative stress and microvascular alterations in
diabetic retinopathy: Future therapies. Oxid Med Cell Longev.
2019:49408252019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang Q and Yang C: Oxidative stress and
diabetic retinopathy: Molecular mechanisms, pathogenetic role and
therapeutic implications. Redox Biol. 37:1017992020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yodoi J, Masutani H and Nakamura H: Redox
regulation by the human thioredoxin system. Biofactors. 15:107–111.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu J and Holmgren A: The thioredoxin
antioxidant system. Free Radical Biol Med. 66:75–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Li X, Han X, Liu R and Fang J:
Targeting the Thioredoxin System for Cancer Therapy. Trends
Pharmacol Sci. 38:794–808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren X, Wang NN, Qi H, Qiu YY, Zhang CH,
Brown E, Kong H and Kong L: Up-regulation thioredoxin inhibits
advanced glycation end products-induced neurodegeneration. Cell
Physiol Biochem. 50:1673–1686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tonade D and Kern TS: Photoreceptor cells
and RPE contribute to the development of diabetic retinopathy. Prog
Retin Eye Res. 83:1009192021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simó R, Stitt AW and Gardner TW:
Neurodegeneration in diabetic retinopathy: Does it really matter?
Diabetologia. 61:1902–1912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonilha VL: Age and disease-related
structural changes in the retinal pigment epithelium. Clin
Ophthalmol. 2:413–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nass N, Bartling B, Navarrete Santos A,
Scheubel RJ, Börgermann J, Silber RE and Simm A: Advanced glycation
end products, diabetes and ageing. Z Gerontol Geriatr. 40:349–356.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Volpe CMO, Villar-Delfino PH, Dos Anjos
PMF and Nogueira-Machado JA: Cellular death, reactive oxygen
species (ROS) and diabetic complications. Cell Death Dis.
9:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koya D and King GL: Protein kinase C
activation and the development of diabetic complications. Diabetes.
47:859–866. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Busik JV, Mohr S and Grant MB:
Hyperglycemia-induced reactive oxygen species toxicity to
endothelial cells is dependent on paracrine mediators. Diabetes.
57:1952–1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu D, Yu JY, Yang S, Wu M, Hammad SM,
Connell AR, Du M, Chen J and Lyons TJ: Survival or death: A dual
role for autophagy in stress-induced pericyte loss in diabetic
retinopathy. Diabetologia. 59:2251–2261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piano I, Novelli E, Della Santina L,
Strettoi E, Cervetto L and Gargini C: Involvement of autophagic
pathway in the progression of retinal degeneration in a mouse model
of diabetes. Front Cell Neurosci. 10:422016. View Article : Google Scholar : PubMed/NCBI
|