Inflammasomes are a group of multiprotein complexes
that recognize both extracellular and cytoplasmic intracellular
pathogens and danger signals. The assembly of the specific
inflammasomes is induced by various pattern-recognition receptors
(PRRs) in response to pathogen associated molecular patterns
(PAMPs) or damage-associated molecular patterns (DAMPs), and
results in the induction of pro-inflammatory cytokines, such as
IL-1β and IL-18 (1–3). In addition, inflammasomes have been
proposed to regulate other key events in inflammation and tissue
repair, such as pyroptosis, a highly inflammatory form of
programmed cell death. Therefore, the inflammasomes play an
important role in a variety of human pathophysiological processes,
including antimicrobial response and development of metabolic
syndromes, cancer, autoimmune and neurodegenerative diseases
(4).
To date, the following five types of PRRs have been
identified: Toll-like receptors (TLRs), retinoic acid-inducible
gene (RIG)-I-like receptors, C-type lectin receptors (CLRs), DNA
sensors [DNA-dependent activator of IFN-regulatory factors
and absent in melanoma 2], and the nucleotide-binding domain (NBD),
leucine-rich repeat-containing (LRR) proteins (NLRs) (1,2).
Several studies have demonstrated that the NLR family includes NLR
proteins (NLRP) 1, 2, 3, 6, 12, the NLR family caspase activation
and recruitment domain (CARD), and the nucleotide-binding
oligomerization domain-containing protein 2 (5–9).
Among them, the NLRP3 inflammasome can be activated by a wide
variety of stimuli, including DAMPs, PAMPs and bacterial toxins. In
the present review, the biological process of the NLRP3
inflammasome assembly, its activation, and the recent developments
of its involvement in viral infection are reviewed. The findings
highlight the research challenges and future directions on this
scientific field.
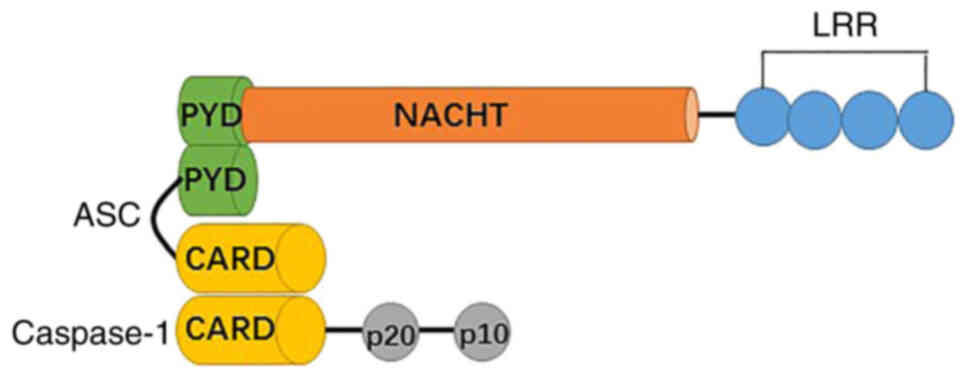
NLRs consist of three separate domains, including an
N-terminal pyrin domain (PYD), a central NBD, and a C-terminal LRR
(Fig. 1). The N terminal domain is
a caspase recruitment domain, a CARD, or a baculovirus inhibitory
repeat domain, which has been applied as a structural
subclassification of the NLR family (1,10).
NLRP3 (NLR family, pyrin domain containing 3), which is also called
cryopyrin, NALP3, CIAS1, CLR1.1, and PYPAF1 contains a PYD, a
central NACHT (nucleotide-binding domain or NAIP, CIITA, HET-E and
TP1) and a C-terminal LRR, which lacks CARD and cannot recruit
pro-caspase-1 in the absence of the adaptor molecule caspase
recruitment domain (ASC) (1). The
NACHT domain is primarily responsible for dNTPase activity and
oligomerization, while the PYD domain mediates downstream signaling
of homotypic protein-protein interactions. LRR has been involved in
ligand-sensing and autoregulation. All three domains are involved
in the protein interaction networks (7).
In addition to the inflammasome sensor molecules,
ASCs are present that connect caspase 1 in the NLRP3 inflammasome
complex. ASC contains two death-fold domains: One PYD and one CARD.
The interaction between the upstream inflammasome sensor molecules
triggers ASC assembly via the pyrin domain into a large protein
complex (11,12). ASC brings procaspase 1 into close
proximity with CARD, which initiates caspase 1 self-cleavage and
activation (13,14).
The cysteine protease caspase 1 is a key player of
the inflammatory response. Procaspase-1 recruitment to the ASC
speck enables its dimerization and autoactivation (1). The CARD domain is separated by a CARD
domain linker from the C-terminal catalytic domain, containing
large (p20) and small (p10) subunits (15). A cleavage product, p33 (CARD +
p20), is generated by the interdomain linker between p20 and p10.
It is confirmed that the active species of caspase 1 in macrophages
is a transient tetramer composed of p33 and p10 subunits (p33/p10).
The complex removes the CARD domain by self-cleavage, leading to
the release of p20/p10 and the loss of enzymatic activity (15).
Active caspase 1 further processes pro-IL-1β and
pro-IL-18 into their mature forms. Serine proteinases including
cathepsin G, elastase, and proteinase 3 can cleave pro-IL-1β except
for caspase-1 (16). Furthermore,
mast cell chymase is able to cleave pro-IL-18 (17). IL-1 family cytokines are key
initiators and regulators of immune responses and inflammation.
IL-1β and IL-18 are significant cytokines that regulate innate and
adaptive immune responses (18).
IL-18 plays a major role in the induction of the Th1 response,
while IL-1β contributes to the T-helper 17 response (19). In various cases, the secretion of
IL-1 cytokines is closely linked to cell death. Previous studies
have suggested that cell lysis is the primary release mechanism for
IL-1β and IL-18 (19,20). However, recently, a common
secretory pathway in the absence of cell death and cell lysis was
identified, which depended on membrane permeability (20). The secretion of IL-1β and IL-18
indicates the important role of the NLRP3 inflammasome in
inflammatory and infectious diseases.
Except for the cytokine release, inflammasomes also
trigger pyroptosis, which is induced by inflammatory caspases
(murine caspase 1 and caspase 11 or human caspase 4 and caspase 5)
(21). Pyroptosis is a necrotic
form of infected cell death that is different from classical
apoptosis or necrosis and is characterized by cell swelling,
osmotic lysis and the subsequent release of intracellular content
(21). Gasdermin D is required for
the induction of pyroptosis and its N-terminal fragment indicates
the intrinsic pyroptosis-inducing activity (22).
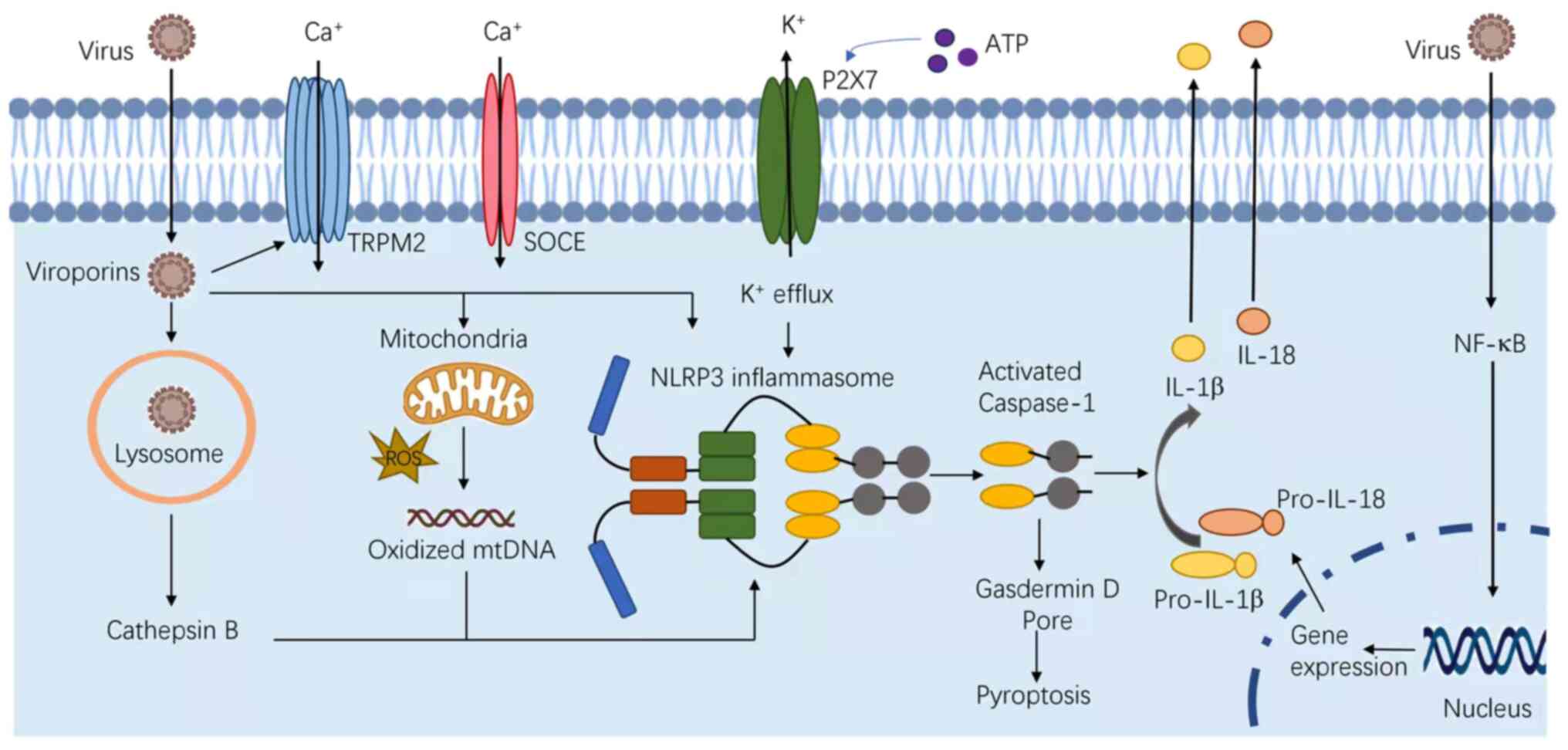
NLRP3 is known to respond to a variety of stimuli,
including PAMPs, DAMPs and bacterial toxins. These PAMPs include
fungi, such as Candida albicans and Saccharomyces
cerevisiae (23); bacteria,
including Staphylococcus aureus (S. aureus), Listeria
monocytogenes and Neisseria gonorrhoeae (24–26);
and viruses, such as Sendai, influenza, encephalomyocarditis
viruses and adenovirus (7,27–29).
In addition, several host-derived molecules, which are indicative
of cellular injury or stress can also activate the NLRP3
inflammasome, including ATP released from necrotic cells,
hyaluronan, glucose, monosodium urate, myeloid-β, skin irritants,
imidazoquinoline compounds, silica, asbestos and alum (Fig. 2) (30). In certain cases, the inflammasome
is activated by individual microbial components, such as, the
α-toxin of S. aureus (24,31–33).
Despite these findings, a universal activation
mechanism for NLRP3 has not been reported. Extracellular ATP
stimulates the P2X purinoceptor 7, triggering efflux of potassium
and inducing the progressive recruitment of the membrane pore
pannexin-1 (34). Under the second
model, phagocytes engulf the inflammasomal activators, such as
silica, monosodium urate, amyloid-β, asbestos, and alum, leading to
lysosomal damage. The cytosolic release of lysosomal contents is
sensed by the NLRP3 inflammasome (35,36).
At last, nearly all NLRP3 agonists induce the generation of
reactive oxygen species (ROS) (37–39).
ROS blockade by chemical scavengers inhibit NLRP3 inflammasome
activation (23,40,41).
In addition, mitochondrial dysfunction induces changes in
intracellular calcium levels, and the release of oxidized
mitochondrial DNA can lead to NLRP3 activation (42).
Inflammasome activation can be triggered by ROS;
however, the exact mechanism remains unclear. A nicotinamide
adenine dinucleotide phosphate (NADPH) oxidase is involved in the
process, since NLRP3 inflammasome is inhibited upon suppression of
NADPH oxidase common p22 subunit (39). A recent study suggests that
thioredoxin-interacting protein (TXNIP/VDUP1) is implicated in
NLRP3 activation. Inflammasome activators induce the ROS-dependent
TXNIP dissociation from thioredoxin. TXNIP further binds to NLRP3
and its deficiency impairs activation of the inflammasome and
subsequent secretion of IL-1β (30).
The oxidized mtDNA from stressed mitochondria is
also suggested to be the cytoplasmic activator of the NLRP3
inflammasome. A previous study conducted by Shimada et al
(43) revealed that oxidized mtDNA
could bind directly and activate the NLRP3 inflammasome, while
macrophages lacking mtDNA severely reduced IL-1β production. In
addition, ROS may also be of mitochondrial origin. However, whether
ROS or oxidized mtDNA acts as a direct activator of the NLRP3
inflammasome or as its cofactor remains unknown.
In complement with the canonical inflammasome
pathway, the non-canonical pathway is mediated by murine caspase 11
and caspase 4 or caspase 5 in human cells in response to
Gram-negative bacteria (44–47).
Lipopolysaccharide (LPS, a component of the Gram-negative bacterial
cell wall) was shown to induce the activation of caspase 11 in mice
and of caspase 4 or caspase 5 in human cells, which was previously
attributed to the LPS receptor TLR4 (45–47).
However, recent studies have shown that host cells have developed
TLR4-independent mechanisms to recognize the cytoplasmic LPS. It
was found that TLR4(−/-) mice primed with the TLR3 agonist
polyinosinic: Polycytidylic acid induced pro-caspase-11 expression,
which could possibly explain this discrepancy (45–47).
The activation of caspases 4, 5 and 11 initiates pyroptosis
similarly to caspase 1, which is not responsible for cleaving
pro-IL-1β or pro-IL-18 (44,48).
Upon activation, these caspases also promote the assembly of the
NLRP3 inflammasome (44,49,50).
In the absence of microbial stimulation, exposure to
TNF-α significantly promotes ATP or silica-mediated caspase 1
activation and IL-1 secretion in macrophages and dendritic cells
(51). Signals provided by NF-κB
activators are not sufficient for NLRP3 activation (52). Certain studies have shown that
NIMA-related kinase 7 is an important component of the NLRP3
complex, which is required for NLRP3 activation, responding to both
canonical and non-canonical stimuli (53–55).
The kinase spleen tyrosine kinase (SYK) is essential for NLRP3
inflammasome activation (23,41).
Over the past decade, numerous studies have
uncovered the role and regulation of inflammasomes during certain
pathophysiological processes. The regulatory mechanisms include
transcriptional, post-transcriptional regulation,
post-translational modifications and regulatory proteins that
target the receptors, ASC or the caspases. Furthermore, several
other studies have highlighted the microbial evolution of
inflammasome inhibitors.
The integration with PRRs or cytokine receptors
highly influences the NLRP3 inflammasome activation and pro-IL-1β
availability (51,52). In response to PRR stimulation,
NLRP3 deubiquitylation, which is mediated by the K63-specific
deubiquitinase BRCA1-BRCA2-containing complex subunit 3, also
induces inflammasome signaling (56,57).
The double-stranded RNA-dependent protein kinase R or eukaryotic
translation initiation factor 2-α kinase 2 was shown to control
NLRP3 inflammasome activity (58).
Selective activation of the NLRP3 inflammasome was promoted by
guanylate binding protein 5, in responses to pathogenic and soluble
bacteria but not in response to crystalline inflammasome priming
substances (59). These data
suggest that small heterodimer partner is a key mediator in
controlling NLRP3 inflammasome activation via mechanisms that
interact with NLRP3 and maintain mitochondrial homeostasis
(60).
The SYK and JNK enzymes phosphorylate the
inflammasome adaptor ASC, which further contributes to the
activation of caspase 1 (61). It
has also been shown that SYK promotes NLRP3-dependent caspase 1
activation during infections with Candida albicans (23). The TGFβ-activated kinase 1 (TAK1 or
MAP3K7)-JNK pathway was activated by ruptured lysosomes and
promoted NLRP3 inflammasome activation, through the oligomerization
of an adapter protein and apoptosis-associated speck-like protein
including ASC (62).
A number of proteins containing a CARD or a PYD have
been proposed to inhibit inflammasome activity by blocking
inflammasome component recruitment. Certain CARD-only proteins
(COPs), such as human CARD16 (also known as COP or PSEUDO-ICE),
CARD17 (also known as INCA), and CARD18 (also known as ICEBERG),
are highly similar to the CARD of caspase 1 (63–65).
They are suggested to suppress inflammasome activation by
sequestering caspase 1. In addition, they may also regulate other
signaling pathways. For instance, CARD16 and CARD18 activate
receptor-interacting serine/threonine protein kinase 2 (63–65).
Caspase 12 is an inhibitor of caspase 1 and its overexpression
appears to abrogate caspase 1 activity (66).
PYD-only proteins (POPs), including pyrin, POP1
(PYDC1), POP2 (PYDC2) and POP3 (PYDC5), regulate inflammasome
signaling at the molecular level of the PYD-PYD interaction
(67–70). It was shown that binding of POP1 to
ASC inhibited ASC-dependent inflammasome assembly by preventing
inflammasome nucleation, and consequently reducing IL-1β release.
Although mice do not have the ortholog of POP1, its transgenic
expression can protect them from PAMP-triggered inflammation.
Moreover, POP1 was regulated by TLR and IL-1 receptor signaling;
therefore, it was proposed that POP1 would provide a regulatory
feedback loop and shut down excessive inflammatory responses
(70). An additional member of
POPs, POP2, binds to ASC and PAN1, and inhibits the formation of
cryopyrin and inflammasomes (68).
In addition, it also inhibits NF-κB activation (67). The other PYD-only protein, POP3 was
identified to compete with ASC for recruitment of liver
regeneration-associated proteins, further reducing inflammasome
activation (69).
The kinase IκB kinase-α, is involved in the negative
regulation of ASC by controlling its subcellular localization in
resting macrophages; however, it is not clear whether this process
is associated with ASC phosphorylation (71).
The NLRP3 inflammasome is critical in the host
anti-viral immune function. Previous studies have shown that
infection from both RNA and DNA viruses can induce activation of
the NLRP3 inflammasome and promote the secretion of IL-1β and IL-18
(72), such as Rift Valley fever
virus, encephalomyocarditis virus (EMCV), foot-and-mouth disease
virus, Mayaro virus (MAYV), hepatitis B and C viruses, Zika virus
(ZIKV), H7N9 influenza A virus, Dengue virus and varicella-zoster
virus (72–80). When viruses invade their hosts, the
NLRP3 inflammasome can be activated by sensing viral components,
including RNA, DNA and proteins. However, the mechanisms of the
NLRP3 inflammasome activation during a viral infection are still
debatable.
Viroporins are a group of low-molecular-weight
proteins, which are important for innate immune responses, notably
for the activation of the NLRP3 inflammasome, such as P7 viroporin
of the hepatitis C virus, viroporin 2B of EMCV, and envelope (E)
protein of severe acute respiratory syndrome coronavirus viroporin
3a (72,81–83).
During the infection, viroporins can increase the cell membrane
permeability, facilitate viral invasion, and cause imbalance of ion
concentration levels, such as Ca2+, which in turn
produces ion fluxes and promotes the activation of NLRP3 (84). Angiotensin converting enzyme 2,
which is expressed in injured type II alveolar epithelial cells may
lead to NLRP3 activation during coronavirus disease (COVID)-19
infection (85).
A subsequent study revealed that certain infections
from RNA viruses could cause potassium efflux, which is a trigger
for the activation of the NLRP3 inflammasome (86). Infection by murine hepatitis viral
strain-3 can induce the quick release of ROS, which may act as a
trigger for NLRP3 inflammasome activation (87). ROS also plays an important role in
human immunodeficiency virus (HIV)-1 infection. HIV-1 will promote
ROS production and induce inflammasome activation (88). MAYV infection can induce ROS
release and potassium efflux, which subsequently triggers the
activation of the NLRP3 inflammasome. Human parainfluenza virus
type 3 infection can induce NLRP3 inflammasome activation via TLR2
activation and potassium efflux (89). The M2 and PB1-F2 proteins of the
influenza virus can activate the NLRP3 inflammasome by regulating
intracellular ionic and mitochondrial ROS production. During this
process, oxidized DNA plays an important role and influenza viruses
can induce oxidized DNA release (90). Influenza viral infection will also
induce lysosomal damage through the lysosomal pathway, further
activating NLRP3 (29). Adenoviral
infections release lysosomal cathepsin B into the cytoplasm, which
can cause the activation of the NLRP3 inflammasome (91).
The RNase L system can sense double-stranded RNAs
and generate RNA cleavage products to activate the NLRP3
inflammasome. DExD/H-box helicase and the mitochondria-associated
molecule mitochondrial antiviral-signaling protein (MAVS)
participate in this process (92).
MAVS can recruit NLRP3 to the mitochondria and promote the
secretion of IL-1β (93).
DEAD/H-box RNA helicases (DDX), DDX33 and DDX19A act as RNA sensors
involved in NLRP3 inflammasome activation (94). The RNA helicase RIG-I acts as a
sensor to certain RNA viruses, such as vesicular stomatitis and
influenza viruses, through the NF-κB pathway to activate the NLRP3
inflammasome (29). Certain RNA
viruses activate NLRP3 via the RIP1-receptor-interacting
serine/threonine-protein kinase 3 (RIP3)-DRP1 pathway. The
RIP1-RIP3 complex is assembled, which induces mitochondrial damage
and activates NLRP3 (95).
Viruses have also evolved multiple mechanisms to
evade the host immune response. Certain viral proteins can suppress
the NLRP3 inflammasome-associated immune response, such as the V
protein of measles virus. Sendai virus can inhibit activation of
the NLRP3 inflammasome and reduce secretion of IL-1β by interacting
with NLRP3 (96,97). The influenza A viral NS1 protein
suppresses ASC ubiquitination and speck formation via the
RIG-I/type I IFN pathway to inhibit NLRP3 inflammasome activation
and reduce the production of IL-1β (98,99).
The PB1-F2 protein of avian influenza A suppresses NLRP3
inflammasome-dependent IL-1β secretion by targeting the MAVS-NLRP3
interaction (100). RNA I of
adenovirus VA inhibits the activation of the NLRP3 inflammasome by
suppressing ASC phosphorylation and oligomerization (101). The non-structural protein NS3 of
ZIKV reduces NLRP3-mediated IL-1β secretion (102). An Epstein-Barr virus microRNA
(miR) can inhibit the NLRP3 inflammasome by targeting the miR-223
site (103). Enterovirus 71 can
cleave NLRP3 by activation of proteases 2A and 3C, which will
inhibit NLRP3 and IL-1β secretion (104). Hepatitis B viral infection can
inhibit NF-κB phosphorylation and ROS production. Hepatitis B
e-antigen suppresses the activation of NLRP3 and IL-1β production
(105). Herpes simplex virus 1
has evolved mechanisms to block the NLRP3 activity (106).
The NLRP3 inflammasome has been widely studied in
innate immune responses. However, the exact mechanisms of NLRP3
inflammasome activation and regulation remain unclear, notably
during the viral infection process. In the present review, the
current knowledge of the NLRP3 assembly process and its regulatory
mechanisms during viral infection were summarized. The findings
indicate that viruses can evade the host immune response by
inhibiting the NLRP3 cytokine storm. As a result, it is of value to
elucidate the immunological balance and the underlying mechanism of
this process. Certain studies have supported the hypothesis that
targeting the NLRP3 pathway can be used in the development of
therapeutic strategies for viral infection, notably for COVID-19.
The development of antiviral therapies requires additional studies
that will explore the molecular pathogenesis of NLRP3 inflammasome
activation in response to viral infection.
Not applicable.
The present study was supported by the Young Scientists Fund of
the National Natural Science Foundation of China (grant no.
81801992), and the Medical and Health Science and Technology
Project of Zhejiang (grant nos. 2018256428 and 2022514972).
Not applicable.
QZ and HC conceived and designed the study. QZ, CH
and QL collected data. QZ, CH and QL prepared the draft of the
manuscript. HC reviewed and edited the manuscript. All authors read
and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fusco R, Siracusa R, Genovese T, Cuzzocrea
S and Di Paola R: Focus on the role of NLRP3 inflammasome in
diseases. Int J Mol Sci. 21:42232020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farag NS, Breitinger U, Breitinger HG and
El Azizi MA: Viroporins and inflammasomes: A key to understand
virus-induced inflammation. Int J Biochem Cell Biol.
122:1057382020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnett KC, Li S, Liang K and Ting JPY: A
360° view of the inflammasome: Mechanisms of activation, cell
death, and diseases. Cell. 186:2288–2312. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chou WC, Jha S, Linhoff MW and Ting JPY:
The NLR gene family: from discovery to present day. Nat Rev
Immunol. Mar 27–2023.(Epub ahead of print). View Article : Google Scholar
|
|
7
|
Chai R, Li Y, Shui L, Ni L and Zhang A:
The role of pyroptosis in inflammatory diseases. Front Cell Dev
Biol. 11:11732352023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhan X, Li Q, Xu G, Xiao X and Bai Z: The
mechanism of NLRP3 inflammasome activation and its pharmacological
inhibitors. Front Immunol. 13:11099382023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agostini L, Martinon F, Burns K, McDermott
MF, Hawkins PN and Tschopp J: NALP3 forms an IL-1beta-processing
inflammasome with increased activity in Muckle-Wells
autoinflammatory disorder. Immunity. 20:319–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song JY, Park YM and Choi SY: Type 2 human
papillomavirus E7 attenuates E-cadherin expression in human
keratinocytes. J Microbiol. 59:616–625. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu TG, Cha JS, Kim G, Sohn YK, Yoo Y, Kim
U, Song JJ, Cho HS and Kim HS: Oligomeric states of ASC specks
regulate inflammatory responses by inflammasome in the
extracellular space. Cell Death Discov. 9:1422023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Proell M, Gerlic M, Mace PD, Reed JC and
Riedl SJ: The CARD plays a critical role in ASC foci formation and
inflammasome signalling. Biochem J. 449:613–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Y, Gu D, Huang T, Li A, Zhou Y, Kang
X, Meng C, Xiong D, Song L, Jiao X and Pan Z: Salmonella
Enteritidis T1SS protein SiiD inhibits NLRP3 inflammasome
activation via repressing the mtROS-ASC dependent pathway. PLoS
Pathog. 19:e10113812023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Souza JG, Starobinas N and Ibañez OCM:
Unknown/enigmatic functions of extracellular ASC. Immunology.
163:377–388. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross C, Chan AH, von Pein JB, Maddugoda
MP, Boucher D and Schroder K: Inflammatory caspases: Toward a
unified model for caspase activation by inflammasomes. Annu Rev
Immunol. 40:249–269. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Triantafilou K: Enigmatic inflammasomes.
Immunology. 162:249–251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Yang T, Xiao J, Xu C, Alippe Y,
Sun K, Kanneganti TD, Monahan JB, Abu-Amer Y, Lieberman J and
Mbalaviele G: NLRP3 inflammasome activation triggers gasdermin
D-independent inflammation. Sci Immunol. 6:eabj38592021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhen Y and Zhang H: NLRP3 inflammasome and
inflammatory bowel disease. Front Immunol. 10:2762019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voet S, Srinivasan S, Lamkanfi M and van
Loo G: Inflammasomes in neuroinflammatory and neurodegenerative
diseases. EMBO Mol Med. 11:e102482019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tapia VS, Daniels MJD, Palazón-Riquelme P,
Dewhurst M, Luheshi NM, Rivers-Auty J, Green J, Redondo-Castro E,
Kaldis P, Lopez-Castejon G and Brough D: The three cytokines IL-1β,
IL-18, and IL-1α share related but distinct secretory routes. J
Biol Chem. 294:8325–8335. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long J, Sun Y, Liu S, Yang S, Chen C,
Zhang Z, Chu S, Yang Y, Pei G, Lin M, et al: Targeting pyroptosis
as a preventive and therapeutic approach for stroke. Cell Death
Discov. 9:1552023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Devant P and Kagan JC: Molecular
mechanisms of gasdermin D pore-forming activity. Nat Immunol. Jun
5–2023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Song W, Tong Y, Zhang X, Zhao J, Gao
X, Yong J and Wang H: Isoliquiritin ameliorates depression by
suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis.
J Neuroinflammation. 18:12021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariathasan S, Weiss DS, Newton K, McBride
J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM and
Dixit VM: Cryopyrin activates the inflammasome in response to
toxins and ATP. Nature. 440:228–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Li L, Xu T, Guo X, Wang C, Li Y,
Yang Y, Yang D, Sun B, Zhao X, et al: HUWE1 mediates inflammasome
activation and promotes host defense against bacterial infection. J
Clin Invest. 130:6301–6316. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Zhang Y, Gan L, Wei F, Chai B, A
Aljaafreh AAH, Liu X, Duan X, Jiang J, Wang X, et al: Progesterone
suppresses Neisseria gonorrhoeae-induced inflammation
through inhibition of NLRP3 inflammasome pathway in THP-1 cells and
murine models. Front Microbiol. 12:5700932021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muruve DA, Pétrilli V, Zaiss AK, White LR,
Clark SA, Ross PJ, Parks RJ and Tschopp J: The inflammasome
recognizes cytosolic microbial and host DNA and triggers an innate
immune response. Nature. 452:103–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allen IC, Scull MA, Moore CB, Holl EK,
McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ and Ting JP:
The NLRP3 inflammasome mediates in vivo innate immunity to
influenza A virus through recognition of viral RNA. Immunity.
30:556–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poeck H, Bscheider M, Gross O, Finger K,
Roth S, Rebsamen M, Hannesschläger N, Schlee M, Rothenfusser S,
Barchet W, et al: Recognition of RNA virus by RIG-I results in
activation of CARD9 and inflammasome signaling for interleukin 1
beta production. Nat Immunol. 11:63–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflammasome activation. Nat Immunol. 11:136–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walev I, Reske K, Palmer M, Valeva A and
Bhakdi S: Potassium-inhibited processing of IL-1 beta in human
monocytes. EMBO J. 14:1607–1614. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gurcel L, Abrami L, Girardin S, Tschopp J
and van der Goot FG: Caspase-1 activation of lipid metabolic
pathways in response to bacterial pore-forming toxins promotes cell
survival. Cell. 126:1135–1145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu R, Liu Y, Liu C, Gao A, Wang L, Tang
H, Wu Q, Wang X, Tian D, Qi Z and Shen Y: NEK7-mediated activation
of NLRP3 inflammasome is coordinated by potassium Efflux/Syk/JNK
signaling during Staphylococcus aureus infection. Front
Immunol. 12:7473702021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pelegrin P: P2X7 receptor and the NLRP3
inflammasome: Partners in crime. Biochem Pharmacol. 187:1143852021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Halle A, Hornung V, Petzold GC, Stewart
CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ and
Golenbock DT: The NALP3 inflammasome is involved in the innate
immune response to amyloid-beta. Nat Immunol. 9:857–865. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hornung V, Bauernfeind F, Halle A, Samstad
EO, Kono H, Rock KL, Fitzgerald KA and Latz E: Silica crystals and
aluminum salts activate the NALP3 inflammasome through phagosomal
destabilization. Nat Immunol. 9:847–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cruz CM, Rinna A, Forman HJ, Ventura ALM,
Persechini PM and Ojcius DM: ATP activates a reactive oxygen
species-dependent oxidative stress response and secretion of
proinflammatory cytokines in macrophages. J Biol Chem.
282:2871–2879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cassel SL, Eisenbarth SC, Iyer SS, Sadler
JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA and
Sutterwala FS: The Nalp3 inflammasome is essential for the
development of silicosis. Proc Natl Acad Sci USA. 105:9035–9040.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dostert C, Pétrilli V, Van Bruggen R,
Steele C, Mossman BT and Tschopp J: Innate immune activation
through Nalp3 inflammasome sensing of asbestos and silica. Science.
320:674–677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koumangoye R: The role of Cl−
and K+ efflux in NLRP3 inflammasome and innate immune
response activation. Am J Physiol Cell Physiol. 322:C645–C652.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shio MT, Eisenbarth SC, Savaria M, Vinet
AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A,
Flavell RA and Olivier M: Malarial hemozoin activates the NLRP3
inflammasome through Lyn and Syk kinases. PLoS Pathog.
5:e10005592009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Latz E, Xiao TS and Stutz A: Activation
and regulation of the inflammasomes. Nat Rev Immunol. 13:397–411.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shimada K, Crother TR, Karlin J, Dagvadorj
J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et
al: Oxidized mitochondrial DNA activates the NLRP3 inflammasome
during apoptosis. Immunity. 36:401–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cabral A, Cabral JE, Wang A, Zhang Y,
Liang H, Nikbakht D, Corona L, Hoffman HM and McNulty R:
Differential binding of NLRP3 to non-oxidized and Ox-mtDNA mediates
NLRP3 inflammasome activation. Commun Biol. 6:5782023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zamyatina A and Heine H:
Lipopolysaccharide recognition in the crossroads of TLR4 and
caspase-4/11 mediated inflammatory pathways. Front Immunol.
11:5851462020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rathinam VAK, Zhao Y and Shao F: Innate
immunity to intracellular LPS. Nat Immunol. 20:527–533. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Downs KP, Nguyen H, Dorfleutner A and
Stehlik C: An overview of the non-canonical inflammasome. Mol
Aspects Med. 76:1009242020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Naseer N, Zhang J, Bauer R, Constant DA,
Nice TJ, Brodsky IE, Rauch I and Shin S: Salmonella enterica
serovar typhimurium induces NAIP/NLRC4- and NLRP3/ASC-independent,
caspase-4-dependent inflammasome activation in human intestinal
epithelial cells. Infect Immun. 90:e00663212022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Baker PJ, Boucher D, Bierschenk D, Tebartz
C, Whitney PG, D'Silva DB, Tanzer MC, Monteleone M, Robertson AA,
Cooper MA, et al: NLRP3 inflammasome activation downstream of
cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J
Immunol. 45:2918–2926. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schmid-Burgk JL, Gaidt MM, Schmidt T,
Ebert TS, Bartok E and Hornung V: Caspase-4 mediates non-canonical
activation of the NLRP3 inflammasome in human myeloid cells. Eur J
Immunol. 45:2911–2917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Franchi L, Eigenbrod T and Núñez G:
Cutting edge: TNF-alpha mediates sensitization to ATP and silica
via the NLRP3 inflammasome in the absence of microbial stimulation.
J Immunol. 183:792–796. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulating NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen X, Liu G, Yuan Y, Wu G, Wang S and
Yuan L: NEK7 interacts with NLRP3 to modulate the pyroptosis in
inflammatory bowel disease via NF-κB signaling. Cell Death Dis.
10:9062019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu G, Chen X, Wang Q and Yuan L: NEK7: A
potential therapy target for NLRP3-related diseases. Biosci Trends.
14:74–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sharif H, Wang L, Wang WL, Magupalli VG,
Andreeva L, Qiao Q, Hauenstein AV, Wu Z, Núñez G, Mao Y and Wu H:
Structural mechanism for NEK7-licensed activation of NLRP3
inflammasome. Nature. 570:338–343. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Juliana C, Fernandes-Alnemri T, Kang S,
Farias A, Qin F and Alnemri ES: Non-transcriptional priming and
deubiquitination regulate NLRP3 inflammasome activation. J Biol
Chem. 287:36617–36622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Py BF, Kim MS, Vakifahmetoglu-Norberg H
and Yuan J: Deubiquitination of NLRP3 by BRCC3 critically regulates
inflammasome activity. Mol Cell. 49:331–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lu B, Nakamura T, Inouye K, Li J, Tang Y,
Lundbäck P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, et al:
Novel role of PKR in inflammasome activation and HMGB1 release.
Nature. 488:670–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li Y, Lin X, Wang W, Wang W, Cheng S,
Huang Y, Zou Y, Ke J and Zhu L: The proinflammatory role of
guanylate-binding protein 5 in inflammatory bowel diseases. Front
Microbiol. 13:9269152022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang CS, Kim JJ, Kim TS, Lee PY, Kim SY,
Lee HM, Shin DM, Nguyen LT, Lee MS, Jin HS, et al: Small
heterodimer partner interacts with NLRP3 and negatively regulates
activation of the NLRP3 inflammasome. Nat Commun. 6:61152015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hara H, Tsuchiya K, Kawamura I, Fang R,
Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E,
Tybulewicz V and Mitsuyama M: Phosphorylation of the adaptor ASC
acts as a molecular switch that controls the formation of
speck-like aggregates and inflammasome activity. Nat Immunol.
14:1247–1255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Okada M, Matsuzawa A, Yoshimura A and
Ichijo H: The lysosome rupture-activated TAK1-JNK pathway regulates
NLRP3 inflammasome activation. J Biol Chem. 289:32926–32936. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Humke EW, Shriver SK, Starovasnik MA,
Fairbrother WJ and Dixit VM: ICEBERG: A novel inhibitor of
interleukin-1beta generation. Cell. 103:99–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Druilhe A, Srinivasula SM, Razmara M,
Ahmad M and Alnemri ES: Regulation of IL-1beta generation by
Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment
domain proteins. Cell Death Differ. 8:649–657. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lamkanfi M, Denecker G, Kalai M, D'hondt
K, Meeus A, Declercq W, Saelens X and Vandenabeele P: INCA, a novel
human caspase recruitment domain protein that inhibits
interleukin-1beta generation. J Biol Chem. 279:51729–51738. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Saleh M, Mathison JC, Wolinski MK,
Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR and
Nicholson DW: Enhanced bacterial clearance and sepsis resistance in
caspase-12-deficient mice. Nature. 440:1064–1068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bedoya F, Sandler LL and Harton JA:
Pyrin-only protein 2 modulates NF-kappaB and disrupts ASC:CLR
interactions. J Immunol. 178:3837–3845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dorfleutner A, Bryan NB, Talbott SJ, Funya
KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC and Stehlik
C: Cellular pyrin domain-only protein 2 is a candidate regulator of
inflammasome activation. Infect Immun. 75:1484–1492. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Khare S, Ratsimandresy RA, de Almeida L,
Cuda CM, Rellick SL, Misharin AV, Wallin MC, Gangopadhyay A, Forte
E, Gottwein E, et al: The PYRIN domain-only protein POP3 inhibits
ALR inflammasomes and regulates responses to infection with DNA
viruses. Nat Immunol. 15:343–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
de Almeida L, Khare S, Misharin AV, Patel
R, Ratsimandresy RA, Wallin MC, Perlman H, Greaves DR, Hoffman HM,
Dorfleutner A and Stehlik C: The PYRIN domain-only protein POP1
inhibits inflammasome assembly and ameliorates inflammatory
disease. Immunity. 43:264–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Martin BN, Wang C, Willette-Brown J,
Herjan T, Gulen MF, Zhou H, Bulek K, Franchi L, Sato T, Alnemri ES,
et al: IKKα negatively regulates ASC-dependent inflammasome
activation. Nat Commun. 5:49772014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ito M, Yanagi Y and Ichinohe T:
Encephalomyocarditis virus viroporin 2B activates NLRP3
inflammasome. PLoS Pathog. 8:e10028572012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ermler ME, Traylor Z, Patel K, Schattgen
SA, Vanaja SK, Fitzgerald KA and Hise AG: Rift Valley fever virus
infection induces activation of the NLRP3 inflammasome. Virology.
449:174–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pinar A, Dowling JK, Bitto NJ, Robertson
AA, Latz E, Stewart CR, Drummond GR, Cooper MA, McAuley JL, Tate MD
and Mansell A: PB1-F2 peptide derived from avian influenza A virus
H7N9 induces inflammation via activation of the NLRP3 inflammasome.
J Biol Chem. 292:826–836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xu J, Zhou Z, Zheng Y, Yang S, Huang K and
Li H: Roles of inflammasomes in viral myocarditis. Front Cell
Infect Microbiol. 13:11499112023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang W, Li G, De Wu, Luo Z, Pan P, Tian M,
Wang Y, Xiao F, Li A, Wu K, et al: Zika virus infection induces
host inflammatory responses by facilitating NLRP3 inflammasome
assembly and interleukin-1β secretion. Nat Commun. 9:1062018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
de Castro-Jorge LA, de Carvalho RVH, Klein
TM, Hiroki CH, Lopes AH, Guimarães RM, Fumagalli MJ, Floriano VG,
Agostinho MR, Slhessarenko RD, et al: The NLRP3 inflammasome is
involved with the pathogenesis of Mayaro virus. PLoS Pathog.
15:e10079342019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shrivastava G, Visoso-Carvajal G,
Garcia-Cordero J, Leon-Juarez M, Chavez-Munguia B, Lopez T, Nava P,
Villegas-Sepulveda N and Cedillo-Barron L: Dengue virus serotype 2
and its non-structural proteins 2A and 2B activate NLRP3
inflammasome. Front Immunol. 11:3522020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xie WH, Ding J, Xie XX, Yang XH, Wu XF,
Chen ZX, Guo QL, Gao WY, Wang XZ and Li D: Hepatitis B virus X
protein promotes liver cell pyroptosis under oxidative stress
through NLRP3 inflammasome activation. Inflamm Res. 69:683–696.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhi X, Zhang Y, Sun S, Zhang Z, Dong H,
Luo X, Wei Y, Lu Z, Dou Y, Wu R, et al: NLRP3 inflammasome
activation by Foot-and-mouth disease virus infection mainly induced
by viral RNA and non-structural protein 2B. RNA Biol. 17:335–349.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhao C and Zhao W: NLRP3 inflammasome-A
key player in antiviral responses. Front Immunol. 11:2112020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harris J and Borg NA: The multifaceted
roles of NLRP3-modulating proteins in virus infection. Front
Immunol. 13:9874532022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen IY, Moriyama M, Chang MF and Ichinohe
T: Severe acute respiratory syndrome coronavirus viroporin 3a
activates the NLRP3 inflammasome. Front Microbiol. 10:502019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Guo HC, Jin Y, Zhi XY, Yan D and Sun SQ:
NLRP3 inflammasome activation by viroporins of animal viruses.
Viruses. 7:3380–3391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Freeman TL and Swartz TH: Targeting the
NLRP3 inflammasome in Severe COVID-19. Front Immunol. 11:15182020.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
da Costa LS, Outlioua A, Anginot A, Akarid
K and Arnoult D: RNA viruses promote activation of the NLRP3
inflammasome through cytopathogenic effect-induced potassium
efflux. Cell Death Dis. 10:3462019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guo S, Yang C, Diao B, Huang X, Jin M,
Chen L, Yan W, Ning Q, Zheng L, Wu Y and Chen Y: The NLRP3
inflammasome and IL-1β accelerate immunologically mediated
pathology in experimental viral fulminant hepatitis. PLoS Pathog.
11:e10051552015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Guo H, Gao J, Taxman DJ, Ting JPY and Su
L: HIV-1 infection induces interleukin-1β production via TLR8
protein-dependent and NLRP3 inflammasome mechanisms in human
monocytes. J Biol Chem. 289:21716–21726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shil NK, Pokharel SM, Banerjee AK, Hoffman
M and Bose S: Inflammasome antagonism by human parainfluenza virus
type 3 c protein. J Virol. 92:e01776–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Moriyama M, Nagai M, Maruzuru Y, Koshiba
T, Kawaguchi Y and Ichinohe T: Influenza virus-induced oxidized DNA
activates inflammasomes. iScience. 23:1012702020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barlan AU, Danthi P and Wiethoff CM:
Lysosomal localization and mechanism of membrane penetration
influence nonenveloped virus activation of the NLRP3 inflammasome.
Virology. 412:306–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chakrabarti A, Banerjee S, Franchi L, Loo
YM, Gale M Jr, Núñez G and Silverman RH: RNase L activates the
NLRP3 inflammasome during viral infections. Cell Host Microbe.
17:466–477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Subramanian N, Natarajan K, Clatworthy MR,
Wang Z and Germain RN: The adaptor MAVS promotes NLRP3
mitochondrial localization and inflammasome activation. Cell.
153:348–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li J, Hu L, Liu Y, Huang L, Mu Y, Cai X
and Weng C: DDX19A senses viral RNA and mediates NLRP3-dependent
inflammasome activation. J Immunol. 195:5732–5749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang X, Jiang W, Yan Y, Gong T, Han J,
Tian Z and Zhou R: RNA viruses promote activation of the NLRP3
inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat
Immunol. 15:1126–1133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Komune N, Ichinohe T, Ito M and Yanagi Y:
Measles virus V protein inhibits NLRP3 inflammasome-mediated
interleukin-1β secretion. J Virol. 85:13019–13026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Komatsu T, Tanaka Y, Kitagawa Y, Koide N,
Naiki Y, Morita N, Gotoh B and Yokochi T: Sendai virus V protein
inhibits the secretion of interleukin-1β by preventing NLRP3
inflammasome assembly. J Virol. 92:e00842–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Pothlichet J, Meunier I, Davis BK, Ting
JP, Skamene E, von Messling V and Vidal SM: Type I IFN triggers
RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A
virus infected cells. PLoS Pathog. 9:e10032562013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Park HS, Liu G, Thulasi Raman SN, Landreth
SL, Liu Q and Zhou Y: NS1 protein of 2009 pandemic influenza A
virus inhibits porcine NLRP3 inflammasome-mediated interleukin-1
beta production by suppressing ASC ubiquitination. J Virol.
92:e00022–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cheung PHH, Ye ZW, Lee TWT, Chen H, Chan
CP and Jin DY: PB1-F2 protein of highly pathogenic influenza A
(H7N9) virus selectively suppresses RNA-induced NLRP3 inflammasome
activation through inhibition of MAVS-NLRP3 interaction. J Leukoc
Biol. 108:1655–1663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Darweesh M, Kamel W, Gavrilin MA,
Akusjärvi G and Svensson C: Adenovirus VA RNAI blocks ASC
oligomerization and inhibits NLRP3 inflammasome activation. Front
Immunol. 10:27912019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gim E, Shim DW, Hwang I, Shin OS and Yu
JW: Zika virus impairs host NLRP3-mediated inflammasome activation
in an NS3-dependent manner. Immune Netw. 19:e402019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Haneklaus M, Gerlic M, Kurowska-Stolarska
M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O'Neill LA and
Masters SL: Cutting edge: miR-223 and EBV miR-BART15 regulate the
NLRP3 inflammasome and IL-1β production. J Immunol. 189:3795–3799.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang H, Lei X, Xiao X, Yang C, Lu W, Huang
Z, Leng Q, Jin Q, He B, Meng G and Wang J: Reciprocal regulation
between enterovirus 71 and the NLRP3 inflammasome. Cell Rep.
12:42–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yu X, Lan P, Hou X, Han Q, Lu N, Li T,
Jiao C, Zhang J, Zhang C and Tian Z: HBV inhibits LPS-induced NLRP3
inflammasome activation and IL-1β production via suppressing the
NF-κB pathway and ROS production. J Hepatol. 66:693–702. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Johnson KE, Chikoti L and Chandran B:
Herpes simplex virus 1 infection induces activation and subsequent
inhibition of the IFI16 and NLRP3 inflammasomes. J Virol.
87:5005–5018. 2013. View Article : Google Scholar : PubMed/NCBI
|