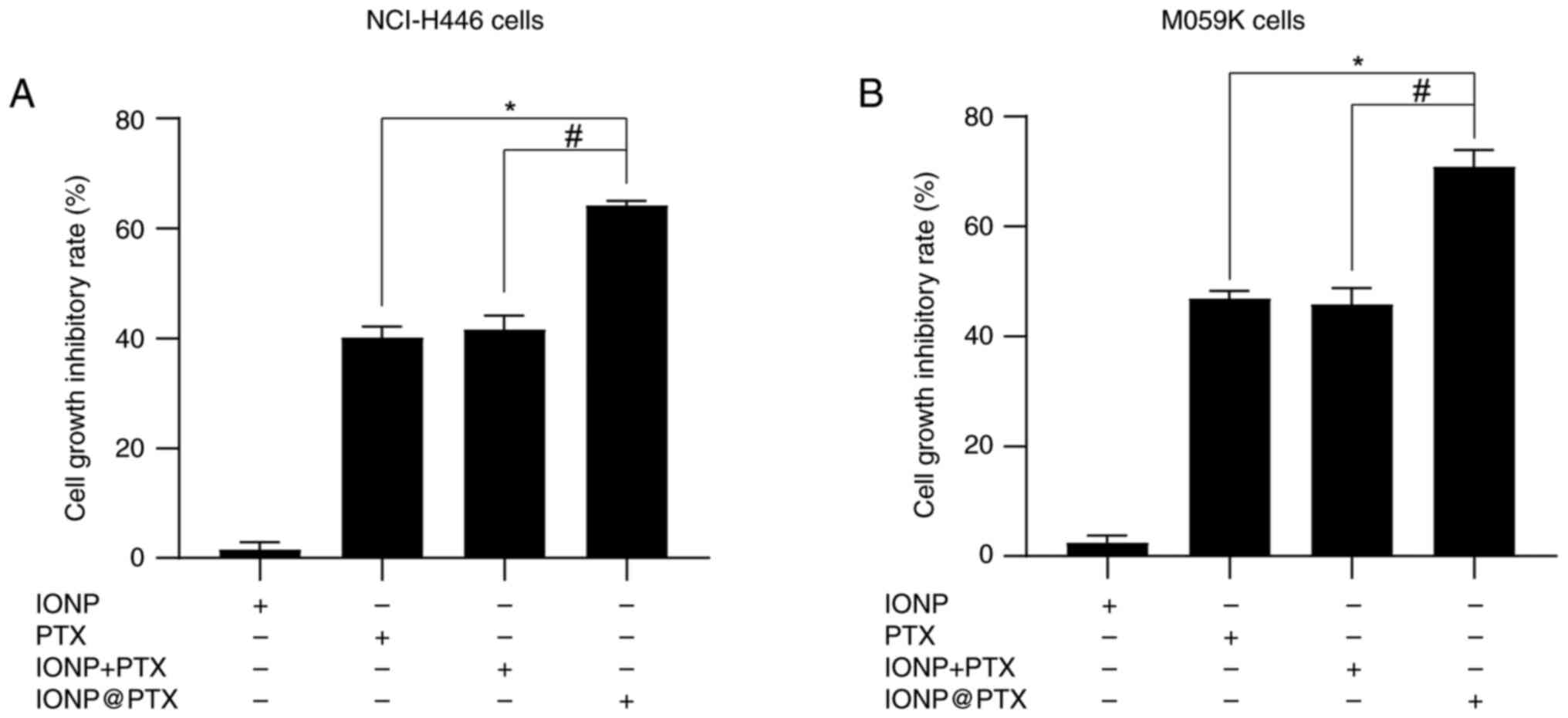
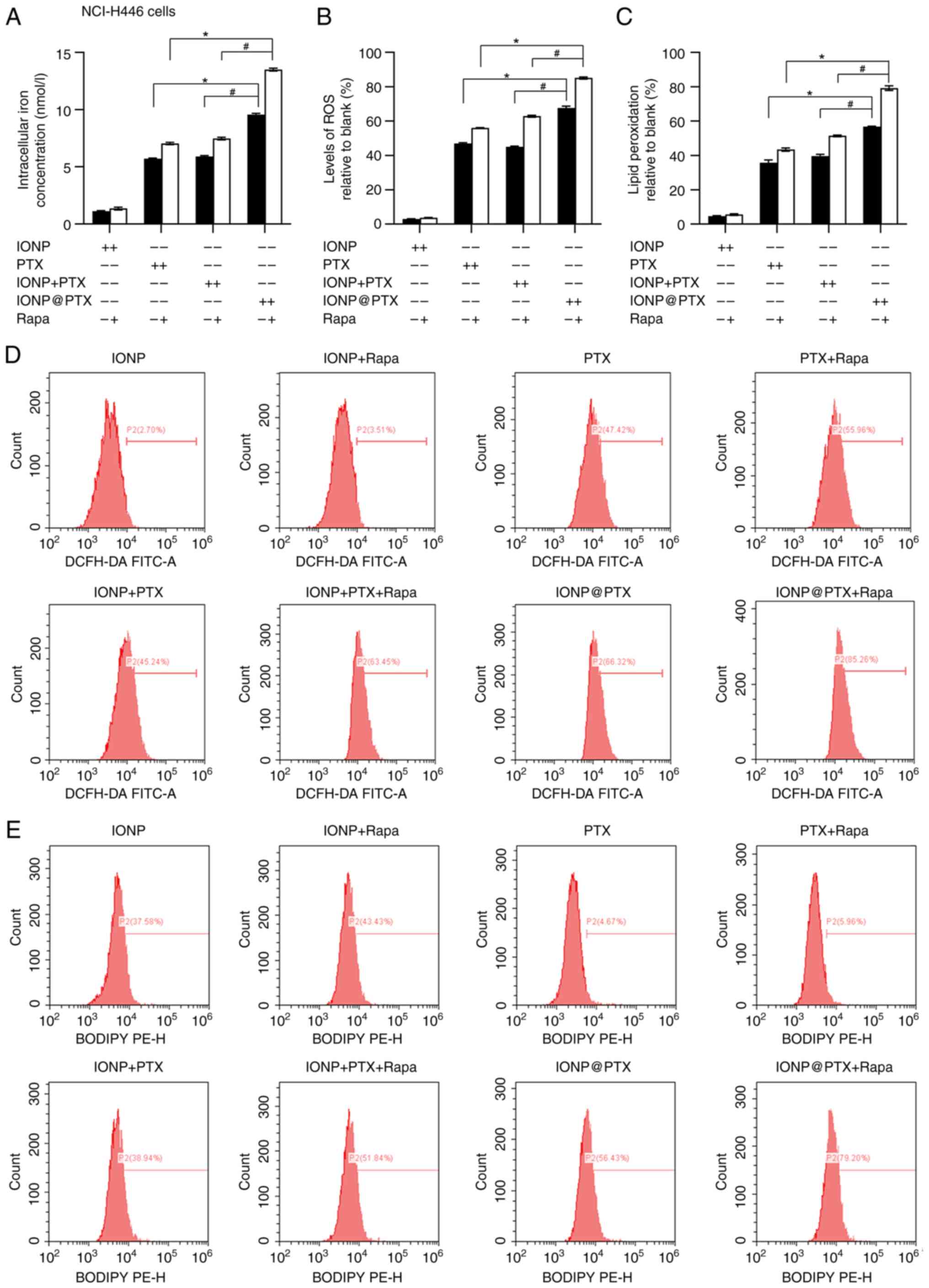
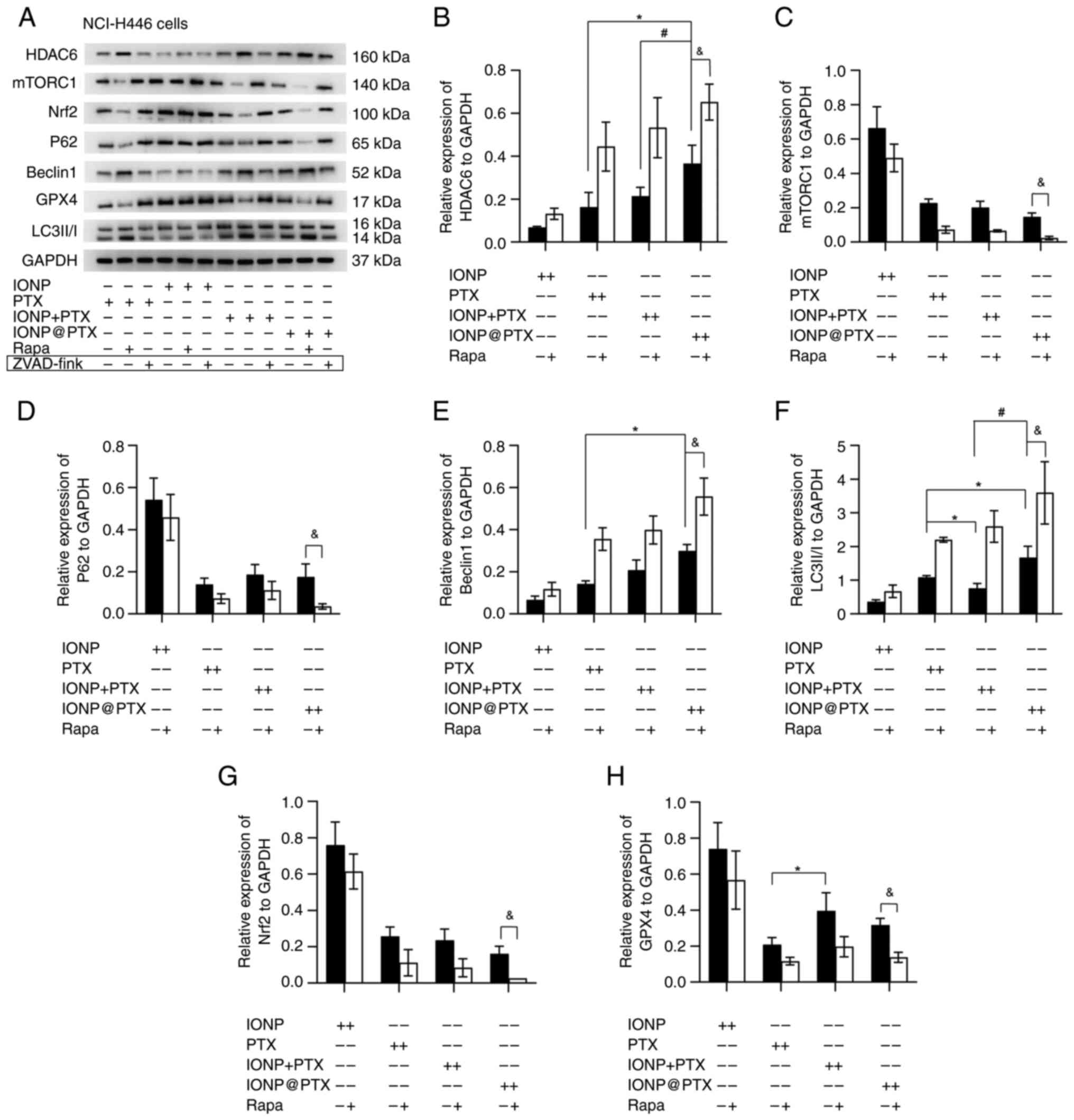
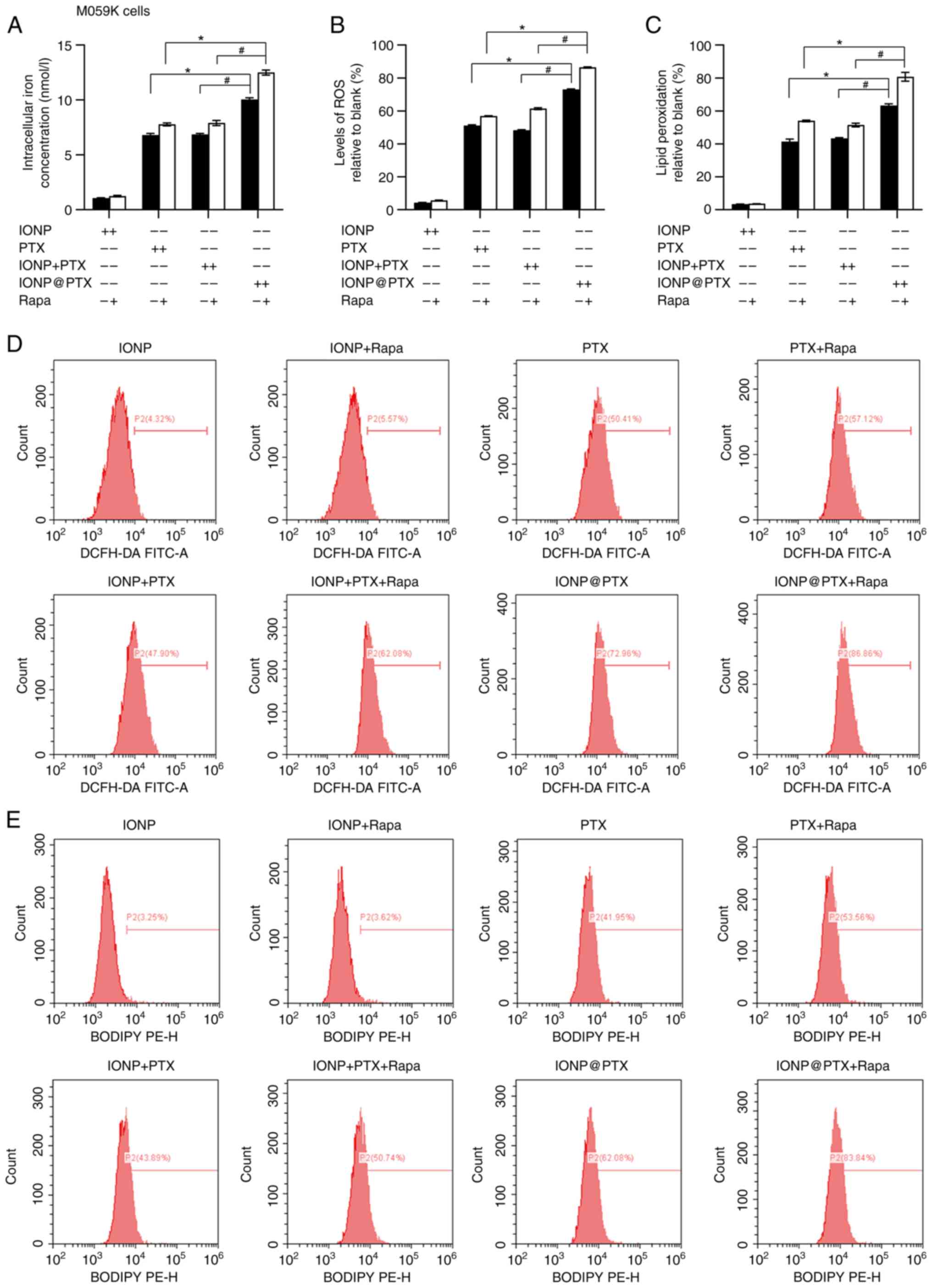
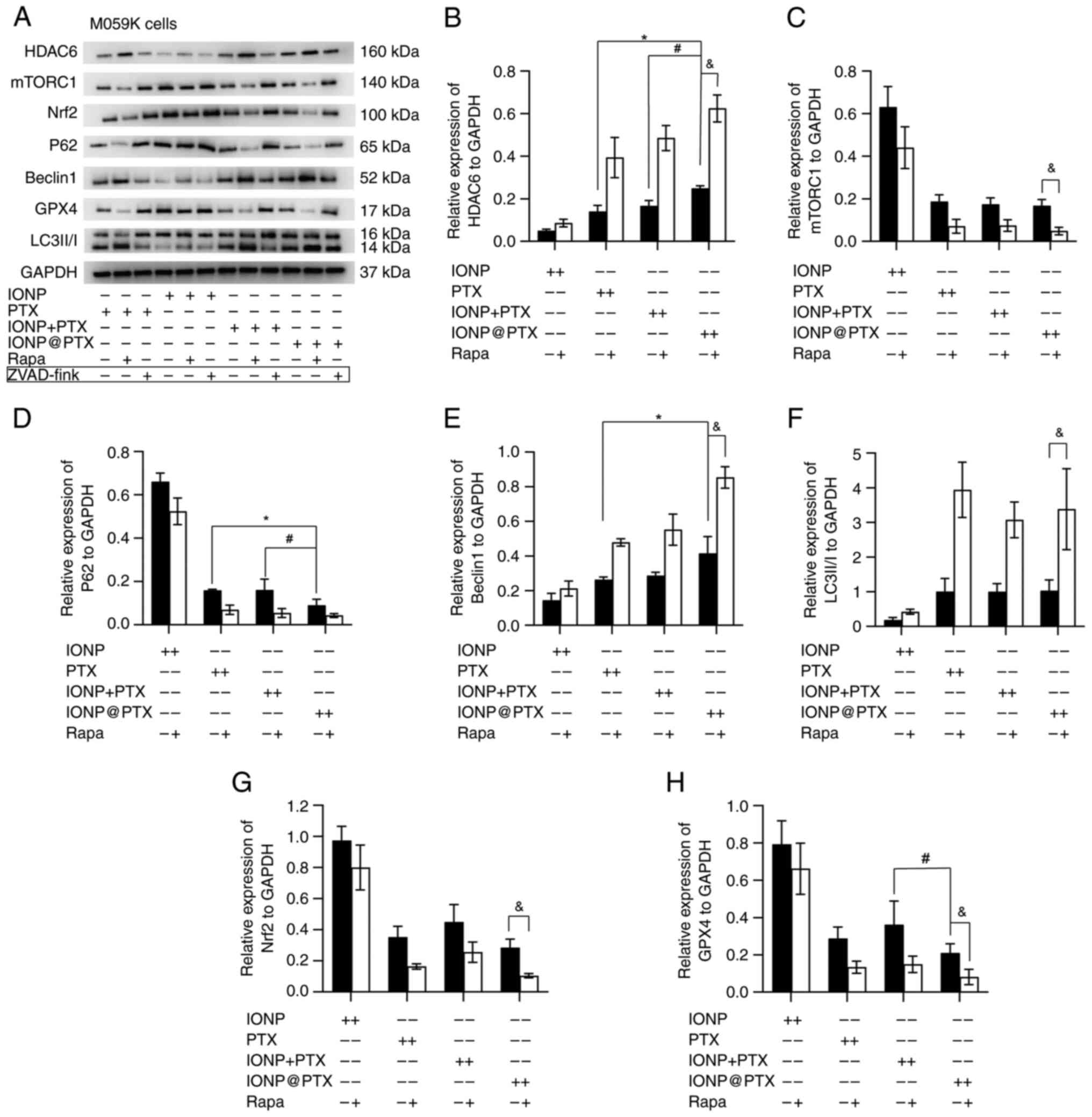
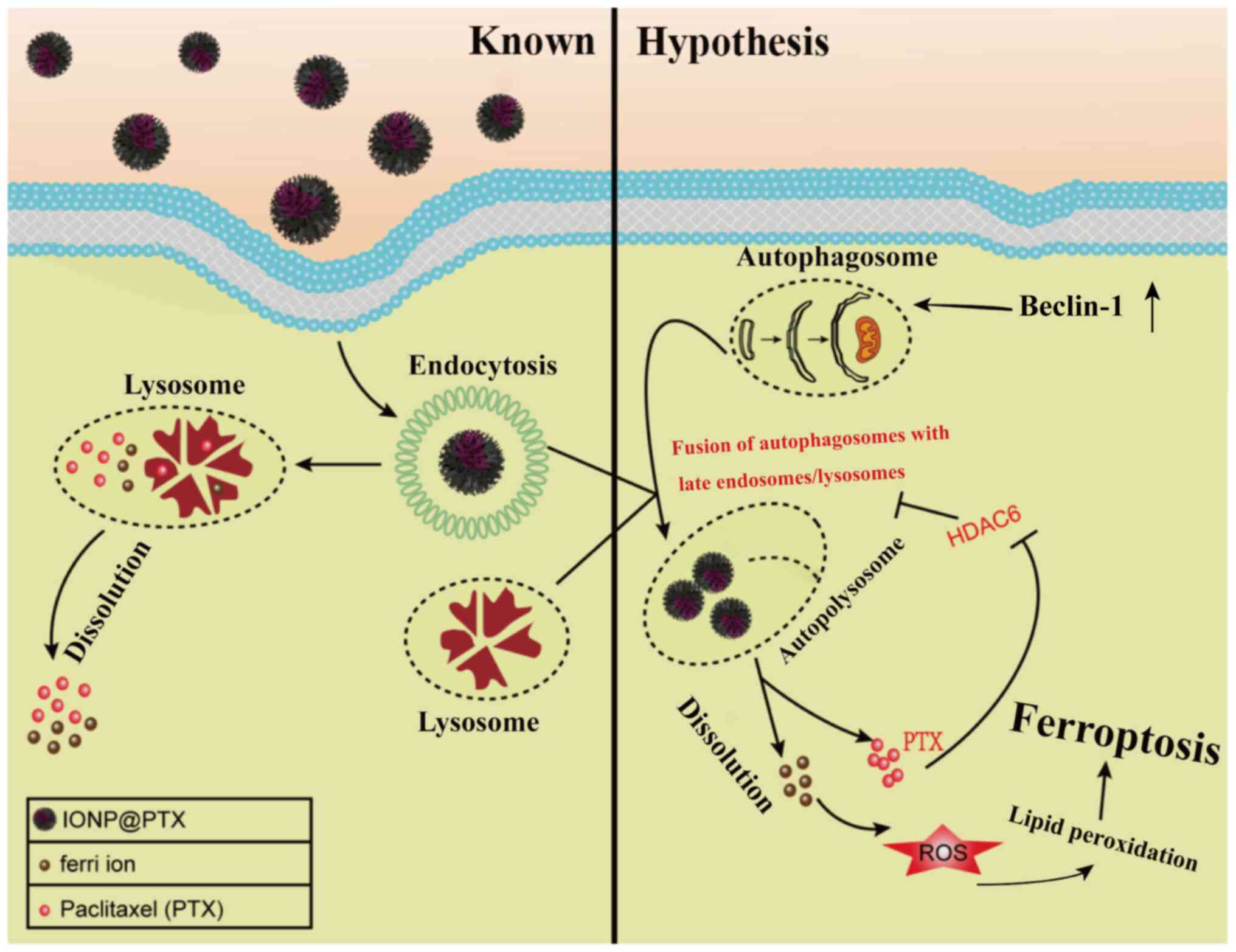
|
1
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caeser R, Egger JV, Chavan S, Socci ND,
Jones CB, Kombak FE, Asher M, Roehrl MH, Shah NS, Allaj V, et al:
Genomic and transcriptomic analysis of a library of small cell lung
cancer patient-derived xenografts. Nat Commun. 13:21442022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan M, Zhao Y, Arkenau HT, Lao T, Chu L
and Xu Q: Signal pathways and precision therapy of small-cell lung
cancer. Signal Transduct Target Ther. 7:1872022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mak DWS, Li S and Minchom A: Challenging
the recalcitrant disease-developing molecularly driven treatments
for small cell lung cancer. Eur J Cancer. 119:132–150. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
George J, Lim JS, Jang SJ, Cun Y, Ozretić
L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al:
Comprehensive genomic profiles of small cell lung cancer. Nature.
524:47–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Low JT, Ostrom QT, Cioffi G, Neff C, Waite
KA, Kruchko C and Barnholtz-Sloan JS: Primary brain and other
central nervous system tumors in the United States (2014–2018): A
summary of the CBTRUS statistical report for clinicians. Neurooncol
Pract. 9:165–182. 2022.PubMed/NCBI
|
|
9
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou YS, Wang W, Chen N, Wang LC and Huang
JB: Research progress of anti-glioma chemotherapeutic drugs
(review). Oncol Rep. 47:1012022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Zhai M, Chen Z, Han X, Yu F, Li
Z, Xie X, Han C, Yu L, Yang Y and Mei X: Dual-modified liposome
codelivery of doxorubicin and vincristine improve targeting and
therapeutic efficacy of glioma. Drug Deliv. 24:1045–1055. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
d'Angelo M, Castelli V, Benedetti E,
Antonosante A, Catanesi M, Dominguez-Benot R, Pitari G, Ippoliti R
and Cimini A: Theranostic nanomedicine for malignant gliomas. Front
Bioeng Biotechnol. 7:3252019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu L and Chen L: Progress in research on
paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 24:402019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng W, Kwan Law BY, Wai Wong VK, Bik Chan
DS, Fai Mok SW, Ying Gao JJ, Yan Ho RK, Liang X, Li JH, Lee MT, et
al: HM30181A, a potent P-glycoprotein inhibitor, potentiates the
absorption and in vivo antitumor efficacy of paclitaxel in an
orthotopic brain tumor model. Cancer Biol Med. 17:9862020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YH, Mao JW and Tan XL: Research
progress on the source, production, and anti-cancer mechanisms of
paclitaxel. Chin J Nat Med. 18:890–897. 2020.PubMed/NCBI
|
|
17
|
Gonzalez-Angulo AM and Hortobagyi GN:
Optimal schedule of paclitaxel: Weekly is better. J Clin Oncol.
26:1585–1587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joos G, Schallier D, Pinson P, Sterckx M
and Van Meerbeeck JP: Paclitaxel (PTX) as second line treatment in
patients (pts) with small cell lung cancer (SCLC) refractory to
carboplatin-etoposide: A multicenter phase II study. J Clin Oncol.
22 (14 Suppl):S72112004. View Article : Google Scholar
|
|
19
|
Nakao M, Fujita K, Suzuki Y, Arakawa S,
Sakai Y, Sato H and Muramatsu H: Nab-paclitaxel monotherapy for
relapsed small cell lung cancer: Retrospective analysis and review.
Anticancer Res. 40:1579–1585. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmed Khalil A, Rauf A, Alhumaydhi FA,
Aljohani ASM, Javed MS, Khan MA, Khan IA, El-Esawi MA, Bawazeer S,
Bouyahya A, et al: Recent developments and anticancer therapeutics
of paclitaxel: An update. Curr Pharm Des. 28:3363–3373. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silva P, Nascimento A, Martinho O, Reis R
and Bousbaa H: Targeting BUB3 in combination with paclitaxel
inhibits proliferation of glioblastoma cells by enhancing cellular
senescence. Sci Lett. 1:12022.
|
|
22
|
Erthal LCS, Shi Y, Sweeney KJ, Gobbo OL
and Ruiz-Hernandez E: Nanocomposite formulation for a sustained
release of free drug and drug-loaded responsive nanoparticles: An
approach for a local therapy of glioblastoma multiforme. Sci Rep.
13:50942023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu HC, Feng Y, Song XY, Song CY, Chen JL,
Wang YC, He XL, Liang RC, Li JH and Tan H: Implantable polyurethane
scaffolds loading with PEG-paclitaxel conjugates for the treatment
of glioblastoma multiforme. Chin J Polym Sci. 40:491–503. 2022.
View Article : Google Scholar
|
|
24
|
Jibodh RA, Lagas JS, Nuijen B, Beijnen JH
and Schellens JH: Taxanes: Old drugs, new oral formulations. Eur J
Pharmacol. 717:40–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharifi-Rad J, Quispe C, Patra JK, Singh
YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, et
al: Paclitaxel: Application in modern oncology and
nanomedicine-based cancer therapy. Oxid Med Cell Longev.
2021:36877002021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mali P and Sherje AP: Cellulose
nanocrystals: Fundamentals and biomedical applications. Carbohydr
Polym. 275:1186682022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kahn B, Collazo J and Kyprianou N:
Androgen receptor as a driver of therapeutic resistance in advanced
prostate cancer. Int J Biol Sci. 10:588–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li K, Zhan W, Chen Y, Jha RK and Chen X:
Docetaxel and doxorubicin codelivery by nanocarriers for
synergistic treatment of prostate cancer. Front Pharmacol.
10:14362019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pulvirenti L, Monforte F, Lo Presti F, Li
Volti G, Carota G, Sinatra F, Bongiorno C, Mannino G, Cambria MT
and Condorelli GG: Synthesis of MIL-modified
Fe3O4 magnetic nanoparticles for enhancing
uptake and efficiency of temozolomide in glioblastoma treatment.
Int J Mol Sci. 23:28742022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernández-Acosta R, Iriarte-Mesa C,
Alvarez-Alminaque D, Hassannia B, Wiernicki B, Díaz-García AM,
Vandenabeele P, Vanden Berghe T and Pardo Andreu GL: Novel iron
oxide nanoparticles induce ferroptosis in a panel of cancer cell
lines. Molecules. 27:39702022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Y, Wu H, Huang J, Qian W, Martinson DE,
Ji B, Li Y, Wang YA, Yang L and Mao H: Probing and enhancing
ligand-mediated active targeting of tumors using sub-5 nm ultrafine
iron oxide nanoparticles. Theranostics. 10:2479–2494. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen H and Wen J: Iron oxide nanoparticles
loaded with paclitaxel inhibits glioblastoma by enhancing
autophagy-dependent ferroptosis pathway. Eur J Pharmacol.
921:1748602022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rakesh R, PriyaDharshini LC, Sakthivel KM
and Rasmi RR: Role and regulation of autophagy in cancer. Biochim
Biophys Acta Mol Basis Dis. 1868:1664002022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Q, Long S, Gan Z, Tettamanti G, Li K
and Tian L: Transcriptional and post-transcriptional regulation of
autophagy. Cells. 11:4412022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Yang KB, Chen W, Mai J, Wu XQ, Sun
T, Wu RY, Jiao L, Li DD, Ji J, et al: CUL3 (cullin 3)-mediated
ubiquitination and degradation of BECN1 (beclin 1) inhibit
autophagy and promote tumor progression. Autophagy. 17:4323–4340.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ciołczyk-Wierzbicka D, Krawczyk A,
Zarzycka M, Zemanek G and Wierzbicki K: Three generations of mTOR
kinase inhibitors in the activation of the apoptosis process in
melanoma cells. J Cell Commun Signal. 17:975–989. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou Z, Tao T, Li H and Zhu X: mTOR
signaling pathway and mTOR inhibitors in cancer: Progress and
challenges. Cell Biosci. 10:312020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Fang Y, Cheng X, Lian Y and Xu H:
Interaction between TRPML1 and p62 in regulating
autophagosome-lysosome fusion and impeding neuroaxonal dystrophy in
Alzheimer's disease. Oxid Med Cell Longev.
2022:80960092022.PubMed/NCBI
|
|
39
|
Hai R, Yang D, Zheng F, Wang W, Han X,
Bode AM and Luo X: The emerging roles of HDACs and their
therapeutic implications in cancer. Eur J Pharmacol.
931:1752162022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang Q, Li X and Wang J: Tubulin
deacetylase NDST3 modulates lysosomal acidification: Implications
in neurological diseases. Bioessays. 44:e22001102022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Yu M, Fu S, Liu D and Tan Y: Role of
selective histone deacetylase 6 inhibitor ACY-1215 in cancer and
other human diseases. Front Pharmacol. 13:9079812022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Johansen T and Lamark T: Selective
autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J
Mol Biol. 432:80–103. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou B, Liu J, Kang R, Klionsky DJ,
Kroemer G and Tang D: Ferroptosis is a type of autophagy-dependent
cell death. Semin Cancer Biol. 66:89–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Ge H, Ma Y, Song L, Ma Y, Tian G,
Wang L, Meng Q and Sun X: Engineered anti-cancer nanomedicine for
synergistic ferroptosis-immunotherapy. Chem Eng J. 455:1406882023.
View Article : Google Scholar
|
|
45
|
Shen Z, Liu T, Li Y, Lau J, Yang Z, Fan W,
Zhou Z, Shi C, Ke C, Bregadze VI, et al:
Fenton-reaction-acceleratable magnetic nanoparticles for
ferroptosis therapy of orthotopic brain tumors. ACS Nano.
12:11355–11365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song X and Long D: Nrf2 and ferroptosis: A
new research direction for neurodegenerative diseases. Front
Neurosci. 14:2672020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mouri A, Yamaguchi O, Miyauchi S, Shiono
A, Utsugi H, Nishihara F, Murayama Y, Kagamu H and Kobayashi K:
Combination therapy with carboplatin and paclitaxel for small cell
lung cancer. Respir Investig. 57:34–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yun T, Kim HT, Han JY, Yoon SJ, Kim HY,
Nam BH and Lee JS: A phase II study of weekly paclitaxel plus
gemcitabine as a second-line therapy in patients with metastatic or
recurrent small cell lung cancer. Cancer Res Treat. 48:465–472.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oi H, Matsuda T, Kimura T, Morise M,
Yamano Y, Yokoyama T, Kataoka K and Kondoh Y: Weekly nanoparticle
albumin-bound paclitaxel and paclitaxel for relapsed small cell
lung cancer: A retrospective observational study. Medicine
(Baltimore). 101:e288632022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mittal S, Ali J and Baboota S: Overcoming
the challenges in the treatment of glioblastoma via
nanocarrier-based drug delivery approach. Curr Pharm Des.
27:4539–4556. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang L, Wang X, Shen L, Alrobaian M, Panda
SK, Almasmoum HA, Ghaith MM, Almaimani RA, Ibrahim IAA, Singh T, et
al: Paclitaxel and naringenin-loaded solid lipid nanoparticles
surface modified with cyclic peptides with improved tumor targeting
ability in glioblastoma multiforme. Biomed Pharmacother.
138:1114612021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang DY, Dmello C, Chen L, Arrieta VA,
Gonzalez-Buendia E, Kane JR, Magnusson LP, Baran A, James CD,
Horbinski C, et al: Ultrasound-mediated delivery of paclitaxel for
glioma: A comparative study of distribution, toxicity, and efficacy
of albumin-bound versus cremophor formulations. Clin Cancer Res.
26:477–486. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu W, Lin Q, Fu Y, Huang S, Guo C, Li L,
Wang L, Zhang Z and Zhang L: Target delivering paclitaxel by
ferritin heavy chain nanocages for glioma treatment. J Control
Release. 323:191–202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen T and Gong T, Zhao T, Liu X, Fu Y,
Zhang Z and Gong T: Paclitaxel loaded phospholipid-based gel as a
drug delivery system for local treatment of glioma. Int J Pharm.
528:127–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang X, Ye L, He W, Teng C, Sun S, Lu H,
Li S, Lv L, Cao X, Yin H, et al: In situ targeting
nanoparticles-hydrogel hybrid system for combined
chemo-immunotherapy of glioma. J Control Release. 345:786–797.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Que W, Li S and Chen J: NS-398 enhances
the efficacy of bortezomib against RPMI8226 human multiple myeloma
cells. Mol Med Rep. 7:1641–1645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wen J, Chen H, Ren Z, Zhang P, Chen J and
Jiang S: Ultrasmall iron oxide nanoparticles induced ferroptosis
via Beclin1/ATG5-dependent autophagy pathway. Nano Converg.
8:102021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sugiyama A, Ohta T, Obata M, Takahashi K,
Seino M and Nagase S: xCT inhibitor sulfasalazine depletes
paclitaxel-resistant tumor cells through ferroptosis in uterine
serous carcinoma. Oncol Lett. 20:2689–2700. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rudin CM, Brambilla E, Faivre-Finn C and
Sage J: Small-cell lung cancer. Nat Rev Dis Primers. 7:32021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chang S, Tang M, Zhang B, Xiang D and Li
F: Ferroptosis in inflammatory arthritis: A promising future. Front
Immunol. 13:9550692022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He H, Du L, Guo H, An Y, Lu L, Chen Y,
Wang Y, Zhong H, Shen J, Wu J and Shuai X: Redox responsive metal
organic framework nanoparticles induces ferroptosis for cancer
therapy. Small. 16:20012512020. View Article : Google Scholar
|
|
64
|
Xue CC, Li MH, Zhao Y, Zhou J, Hu Y, Cai
KY, Zhao Y, Yu SH and Luo Z: Tumor microenvironment-activatable
Fe-doxorubicin preloaded amorphous CaCO3 nanoformulation
triggers ferroptosis in target tumor cells. Sci Adv.
6:eaax13462020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen G, Yang Y, Xu Q, Ling M, Lin H, Ma W,
Sun R, Xu Y, Liu X, Li N, et al: Self-amplification of tumor
oxidative stress with degradable metallic complexes for synergistic
cascade tumor therapy. Nano Lett. 20:8141–8150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Luo S, Ma D, Wei R, Yao W, Pang X, Wang Y,
Xu X, Wei X, Guo Y, Jiang X, et al: A tumor microenvironment
responsive nanoplatform with oxidative stress amplification for
effective MRI-based visual tumor ferroptosis. Acta Biomater.
138:518–527. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Friedmann Angeli JP, Krysko DV and Conrad
M: Ferroptosis at the crossroads of cancer-acquired drug resistance
and immune evasion. Nat Rev Cancer. 19:405–414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Assi M and Kimmelman AC: Impact of
context-dependent autophagy states on tumor progression. Nat
Cancer. 4:596–607. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang X, Sui S, Wang L, Li H, Zhang L, Xu
S and Zheng X: Inhibition of tumor propellant glutathione
peroxidase 4 induces ferroptosis in cancer cells and enhances
anticancer effect of cisplatin. J Cell Physiol. 235:3425–3437.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xia H, Green DR and Zou W: Autophagy in
tumour immunity and therapy. Nat Rev Cancer. 21:281–297. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang F, Du L, Song G, Zong X, Jin X, Yang
X and Qi Z: Rapamycin and 3-methyladenine influence the apoptosis,
senescence, and adipogenesis of human adipose-derived stem cells by
promoting and inhibiting autophagy: An in vitro and in vivo study.
Aesthetic Plast Surg. 45:1294–1309. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ding R, Liu Z, Tan J and Sun B: Advanced
oxidation protein products mediate human keratinocytes apoptosis by
inducing cell autophagy through the mTOR-Beclin-1 pathway. Cell
Biochem Funct. 40:880–887. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rhaman A, Mahmoud E, Abu Alfadl EM,
Mohammed DH and Sheneef A: Expression of autophagy related genes
mTOR, ATG10 and P62 in the peripheral blood mononuclear cells of
systemic lupus erythematosus Egyptian patients. Egypt J Med
Microbiol. 32:133–140. 2023. View Article : Google Scholar
|
|
74
|
Zhang J, Han L, Ma Q, Wang X, Yu J, Xu Y,
Zhang X, Wu X and Deng G: RIP3 impedes Mycobacterium tuberculosis
survival and promotes p62-mediated autophagy. Int Immunopharmacol.
115:1096962023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Schaaf MBE, Keulers TG, Vooijs MA and
Rouschop KMA: LC3/GABARAP family proteins: Autophagy-(un) related
functions. FASEB J. 30:3961–3978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gordon MS, Shapiro GI, Sarantopoulos J,
Juric D, Lu B, Zarotiadou A, Connarn JN, Le Bruchec Y, Dumitru CD
and Harvey RD: Phase Ib study of the histone deacetylase 6
inhibitor citarinostat in combination with paclitaxel in patients
with advanced solid tumors. Front Oncol. 11:7861202022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang N, Sun P, Jin H, Yang Y, Zhao Q,
Zhou L, Guo L, Yang X and Lu L: Chidamide combined with paclitaxel
effectively reverses the expression of histone deacetylase in lung
cancer. Anticancer Drugs. 31:702–708. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yoo J, Jeon YH, Lee DH, Kim GW, Lee SW,
Kim SY, Park J and Kwon SH: HDAC6-selective inhibitors enhance
anticancer effects of paclitaxel in ovarian cancer cells. Oncol
Lett. 21:2012021. View Article : Google Scholar : PubMed/NCBI
|