Introduction
Heat shock proteins (HSPs) are induced by stressful
environments such as high temperature or pathological conditions
(1). HSPs are generally
characterized as molecular chaperones that prevent protein
aggregation and restore protein homeostasis (1). Based on their molecular weight, HSPs
are now divided into seven categories as follows: HSPA family
(HSP70), HSPH family (HSP110), HSPC family (HSP90), HSPD/E family
(HSP60/HSP10), HSPB family (small HSPs), DNAJ (HSP40) and
chaperonin containing tailless complex polypeptide 1 or tailless
complex polypeptide 1 ring complex (1). Among these groups, HSP70 is expressed
in unstressed cells and acts as an ATP-dependent molecular
chaperone (2). HSP70 has been
reported to serve an important role in promoting protein
homeostasis and folding, and survival of cells under stressful
conditions (3). Regarding the
functions of HSP70 in bone metabolism, HSP70 increases both
alkaline phosphatase activity and mineralization of human
mesenchymal stem cells (hMSCs) (4). HSP70 also increases the expression of
osteogenic markers such as runt-related transcription factor 2
(Runx2) and Osterix in hMSCs, which promotes osteoblastic
differentiation (4). In bone
cells, we previously reported that HSP70 was highly expressed in
mouse calvaria-derived MC3T3-E1 osteoblastic cells (5). As for the functions of HSP70, we
showed that TGF-β VEGF release was downregulated by HSP70 in
MC3T3-E1 osteoblastic cells (6).
However, the functions of HSP70 in bone metabolism, especially in
osteoblasts, have not yet been fully elucidated.
Bone is a dynamic organ, in which osteoclasts
reabsorb calcified bone and osteoblasts subsequently form new bone.
These processes, termed bone remodeling, are tightly regulated to
maintain proper bone mass under physiological conditions. Imbalance
of bone formation and resorption leads to metabolic bone disorders,
including osteoporosis (7).
Interleukin-6 (IL-6) is a multifunctional cytokine that is released
from numerous types of cells and serves important roles in
biological processes, such as immunity, hematopoiesis and bone
metabolism (8). During bone
metabolism, IL-6 activates signal transduction pathways in
osteoblasts via the gp130 receptor and induces the expression of
receptor-activator of nuclear factor-κB-ligand (RANKL) (9). RANKL attaches to RANK expressed on
osteoclast precursors and induces differentiation into mature
osteoclasts (9). Therefore, IL-6
has been generally considered as a bone resorptive cytokine. By
contrast, it has been reported that IL-6 is essential in the
process of fracture healing (10,11).
Therefore, IL-6 is known as an osteotropic factor that may modulate
bone formation under bone turnover-accelerating conditions
(12). Basic fibroblast growth
factor (bFGF or FGF-2) is the most abundant FGF and is known as an
angiogenic factor (13). During
bone metabolism, bFGF serves crucial roles in bone regeneration and
fracture repair (12). bFGF
increases vascularization during the early stages of fracture
healing and promotes the proliferation and differentiation of bone
marrow stromal cells (14). We
previously reported that bFGF stimulated IL-6 release through p38
MAPK in mouse MC3T3-E1 osteoblastic cells (15,16).
However, the roles of HSP70 in the bFGF-stimulated IL-6 release in
osteoblasts are still unknown. The present study evaluated whether
HSP70 serves a role in the bFGF-stimulated release of IL-6 and the
mechanism involved in this, using osteoblastic cells.
Materials and methods
Materials
bFGF was purchased from Roche Diagnostics GmbH.
HSP70 inhibitors, VER-155008 and YM-08, were purchased from
MilliporeSigma. SB203580 (cat. no. 559389) were purchased from
Calbiochem; Merck KGaA. Phosphorylated (p)-p38 MAPK (cat. no.
4511S) and p38 MAPK (cat. no. 9212S) antibodies were purchased from
Cell Signaling Technology, Inc. HSP70 antibodies (cat. np.
ADI-SPA-812) were purchased from Enzo Life Sciences, Inc. GAPDH
antibodies (cat. no. sc-25778) were purchased from Santa Cruz
Biotechnology, Inc. Peroxidase-labeled anti-rabbit IgG antibodies
(cat. no. 5220-0336; SeraCare Life Sciences, Inc.) and
peroxidase-labeled anti-mouse IgG antibodies (cat. no. #7076; Cell
Signaling Technology, Inc.) were used as secondary antibodies. An
ECL western blot detection system (cat. no. RPN2106) was purchased
from Cytiva. Mouse (cat. no. M6000B) and human (cat. no. D6050)
IL-6 ELISA kits were purchased from R&D Systems, Inc.
Cell culture
Cloned MC3T3-El osteoblast-like cells established
from neonatal mouse calvaria (17)
were donated by Dr M Kumegawa (Department of Dentistry, Graduate
School of Dentistry, Meikai University, Sakado, Japan), and
maintained in α-minimum essential medium (α-MEM; MilliporeSigma)
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific
Inc.) at 37°C in a humidified atmosphere of 5% CO2−95%
air, as previously described (18). For western blot analyses, the
MC3T3-E1 osteoblastic cells were seeded into 90-mm diameter dish
plates (2×105 cells/dish) in α-MEM supplemented with 10%
FBS. For ELISA and RT-PCR analysis, the cells were seeded into
35-mm diameter dish plates (5×104 cells/dish) in α-MEM
supplemented with 10% FBS. The culture medium was changed for α-MEM
supplemented with 0.3% FBS after 5 days. These cells were used for
the experiments after 48 h.
Normal human osteoblasts, which were originally
derived from human samples from patients who provided informed
consent, were purchased from Cambrex Bio Science Rockland, Ltd.
(cat. no. CC-2538; passage 2) and used in the present study. The
cultured normal human osteoblasts were cultured under the same
conditions as those used for mouse MC3T3-E1 osteoblastic cells.
These normal human osteoblasts were seeded into 35-mm diameter dish
plates (5×104 cells/dish) in α-MEM supplemented with 10%
FBS. The medium was changed to α-MEM supplemented with 0.3% FBS
after 17 days. These cells were used for ELISA experiments after a
further 48 h (19).
Measurement of IL-6
MC3T3-E1 osteoblast-like cells and normal human
osteoblasts were pre-treated with 1, 10 and 30 µM VER-155008 in 1
ml α-MEM supplemented with 0.3% FBS at 37°C for 60 min. These cells
were subsequently treated with 30 ng/ml bFGF or vehicle and
incubated for 48 h without washing away the VER-155008.
Pre-incubation with 10 µM SB203580 was performed for 60 min prior
to the VER-155008 stimulation. The conditioned medium was collected
at the end of the incubation, and the IL-6 concentration in the
medium was assessed using mouse and human IL-6 ELISA kits (16,19).
Reverse transcription-quantitative
(RT-q)PCR analysis
MC3T3-E1 osteoblast-like cells were pre-treated with
30 µM VER-155008 for 60 min, and then stimulated with 30 ng/ml bFGF
in α-MEM supplemented with 0.3% FBS for 3 h. Total RNA was isolated
using TRIzol® reagent (Invitrogen; Thermo Fischer Scientific, Inc.)
and reverse transcribed into cDNA at 37°C for 60 min using an
Omniscript Reverse Transcriptase kit (Qiagen, Inc.). RT-qPCR was
performed using a Light Cycler system with Fast Start DNA Master
SYBR Green I according to the manufacturer's standard protocol
(Roche Diagnostics). Samples were subjected to the following PCR
thermocycling conditions: Initial denaturation at 95°C for 10 min,
followed by 40 cycles of denaturation at 95°C for 1 sec, annealing
at 60°C for 5 sec and elongation at 72°C for 7 sec. IL-6 and GAPDH
primers were purchased from Takara Bio, Inc., with sequences as
follows: IL-6 forward (F), 5′-CCACTTCACAAGTCGGAGGCTTA-3′ and
reverse (R), 5′-GCAAGTGCATCATCGTTGTTCATAC-3′; and GAPDH F,
5′-AACGACCCCTTCATTGAC-3′ and R, 5′-TCCACGACATACTCAGCAC-3′. All
measurements were analyzed using the 2−ΔΔCq method
(20).
Western blotting
MC3T3-E1 cells were pre-incubated with 30 µM of
VER-155008 or 10 and 20 µM YM-08, and then stimulated using 30
ng/ml bFGF in α-MEM containing 0.3% FBS for 10 min. For western
blot analysis, MC3T3-E1 osteoblast-like cells were rinsed twice
with phosphate-buffered saline, and lysed and sonicated in 800 µl
lysis buffer containing 62.5 mM Tris/HCl, 50 mM dithiothreitol, 2%
SDS and 10% glycerol. Proteins were separated on a 10% gel using
SDS-PAGE and transferred to a PVDF membrane (21). Although the mass of protein per
lane was not quantified, the same number of cells (2×105
cells/dish) was seeded in each dish. The days of treatment,
conditions, and lysis buffer dose were also the same for each dish.
Lysates (10 µl) were applied per lane of all SDS-PAGE gels. The
membrane was subsequently incubated at 4°C overnight with primary
antibodies against p38 MAPK, p-p38 MAPK, HSP70 and GAPDH (1:1,000)
followed by incubation with the appropriate secondary antibodies
(1:1,000) at room temperature for 1 h. Following the detection of
specific antibodies, the same membranes were stripped with WB
stripping solution (cat. no. 46430, Thermo Fisher Scientific Inc.)
for 15 min at room temperature and reprobed. An ECL western
blotting kit was used for the detection as previously described
(22).
Densitometric analysis
Densitometric analysis of the western blots was
performed using a scanner and ImageJ analysis software (version
1.48; National Institutes of Health). The phosphorylated protein
levels were calculated as follows: The background subtracted signal
intensity of each phosphorylation signal was normalized to the
respective intensity of total protein and plotted as the fold
increase compared with that in the control cells without
stimulation.
Statistical analysis
All data were analyzed using Mini StatMate (version
2.01; ATMS Co., Ltd.). ANOVA followed by Bonferroni's significant
difference test was used for multiple comparisons. All measurements
were performed in triplicate from dependent cell preparations and
analyzed. Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
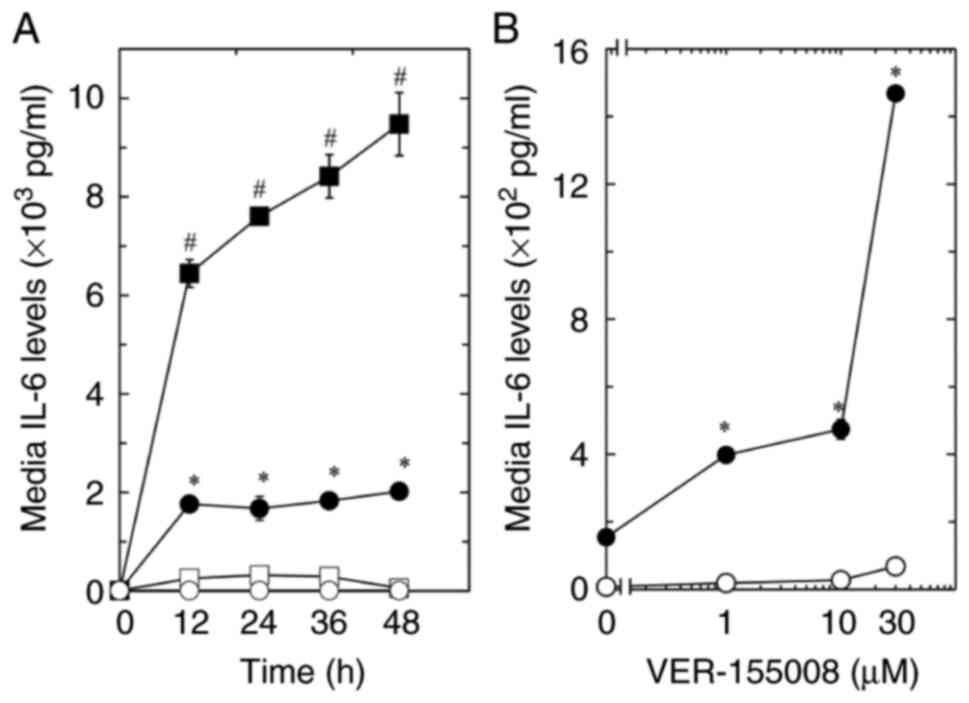
VER-155008 increases the
bFGF-stimulated IL-6 release in MC3T3-E1 osteoblast-like cells and
normal human osteoblasts
We previously reported that HSP70 was highly
expressed in unstimulated MC3T3-E1 osteoblast-like cells (5,23).
In the present study, the roles of HSP70 in bFGF-stimulated IL-6
release using VER-155008, a HSP70 inhibitor (24), were analyzed in MC3T3-E1
osteoblast-like cells. As a result, the bFGF-stimulated release of
IL-6 was significantly increased by VER-155008 at each time point
compared with the respective control (Fig. 1A). By contrast, the IL-6 release
was not affected by treatment with VER-155008 alone. Furthermore,
the effects of VER-155008 on the bFGF-induced release of IL-6
tended to be dose-dependent in the range between 1 and 30 µM
(Fig. 1B).
Normal human osteoblasts were used to analyze the
role of HSP70 inhibitors in different osteoblast types. As a
result, it was confirmed that IL-6 release was significantly
increased by bFGF in normal human osteoblasts compared with the
control. Moreover, bFGF-stimulated release of IL-6 was
significantly enhanced by VER-155008 compared with the bFGF only
group. VER-155008 alone did not significantly affect the release of
IL-6 in normal human osteoblasts (Fig.
2A).
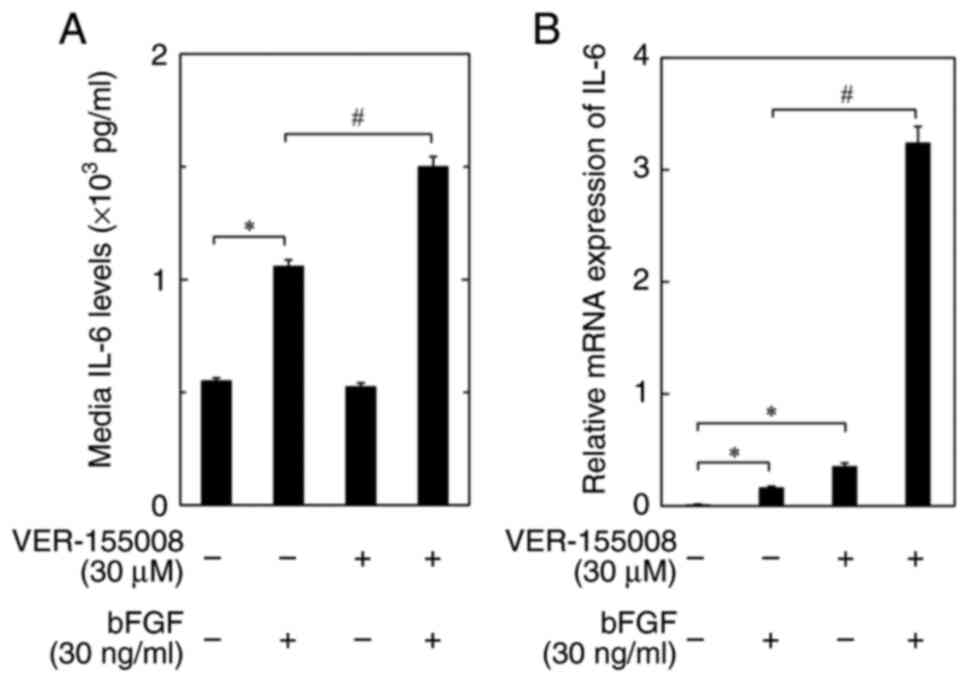
VER-155008 enhances the bFGF-induced
expression levels of IL-6 mRNA in MC3T3-E1 osteoblast-like
cells
To evaluate whether the enhancing effect of the
HSP70 inhibitor on the bFGF-stimulated release of IL-6 in MC3T3-E1
osteoblastic cells was mediated through transcriptional events, the
effect of VER-155008 on the bFGF-induced expression of IL-6 mRNA
was assessed. It was demonstrated that VER-155008 significantly
increased the mRNA expression levels of IL-6 compared with the bFGF
only group (Fig. 2B).
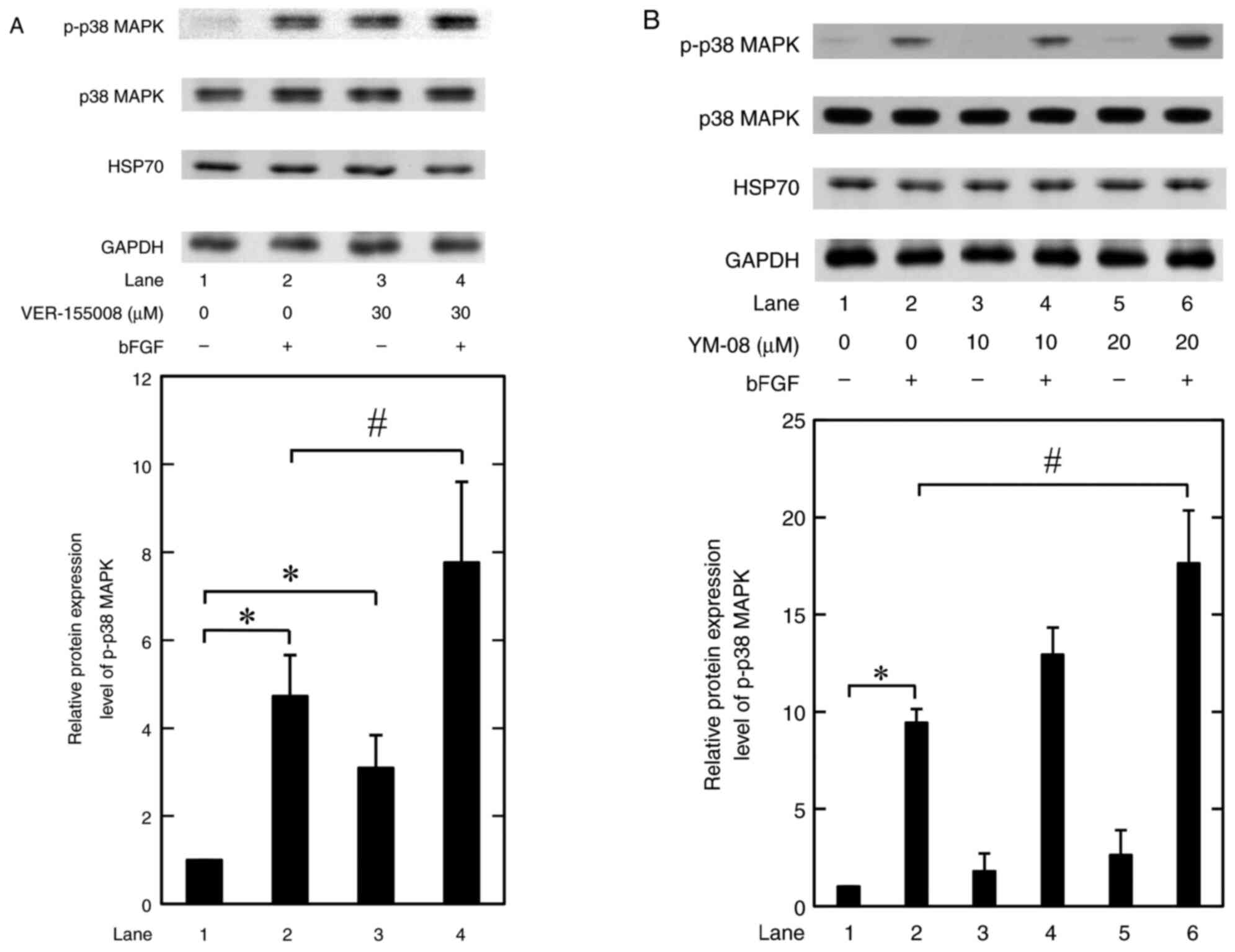
VER-155008 and YM-08 stimulate
bFGF-induced phosphorylation of p38 MAPK in MC3T3-E1
osteoblast-like cells
We have previously reported that P38 MAPK activation
is related to the bFGF-stimulated release of IL-6 in MC3T3-E1
osteoblastic cells (16).
Therefore, the effects of HSP70 inhibitors on the bFGF-induced
phosphorylation of p38 MAPK was assessed in MC3T3-E1 osteoblastic
cells. VER-155008 at 30 mM significantly increased the
phosphorylation levels of p38 MAPK induced by bFGF compared with
the bFGF only group. Furthermore, 30 mM VER-155008 alone
significantly increased the levels of p38 MAPK phosphorylation
compared with the untreated control (Fig. 3A). YM-08, another type of HSP70
inhibitor (25), significantly
increased the bFGF-induced phosphorylation of p38 MAPK, compared
with the bFGF only group, at 20 mM; however it had no significant
effect on the phosphorylation when used alone (Fig. 3B).
Neither VER-155008 nor YM-08 affect
the expression of HSP70 with or without bFGF stimulation in
MC3T3-E1 osteoblast-like cells
We previously reported that HSP70 was highly
expressed in unstimulated MC3T3-E1 osteoblast-like cells (5,23).
Whether VER-155008 and YM-08 with or without bFGF could affect the
HSP70 expression in these cells was assessed in the present study.
VER-155008 and YM-08 did not significantly affect the expression of
HSP70 with or without bFGF stimulation (Fig. 3).
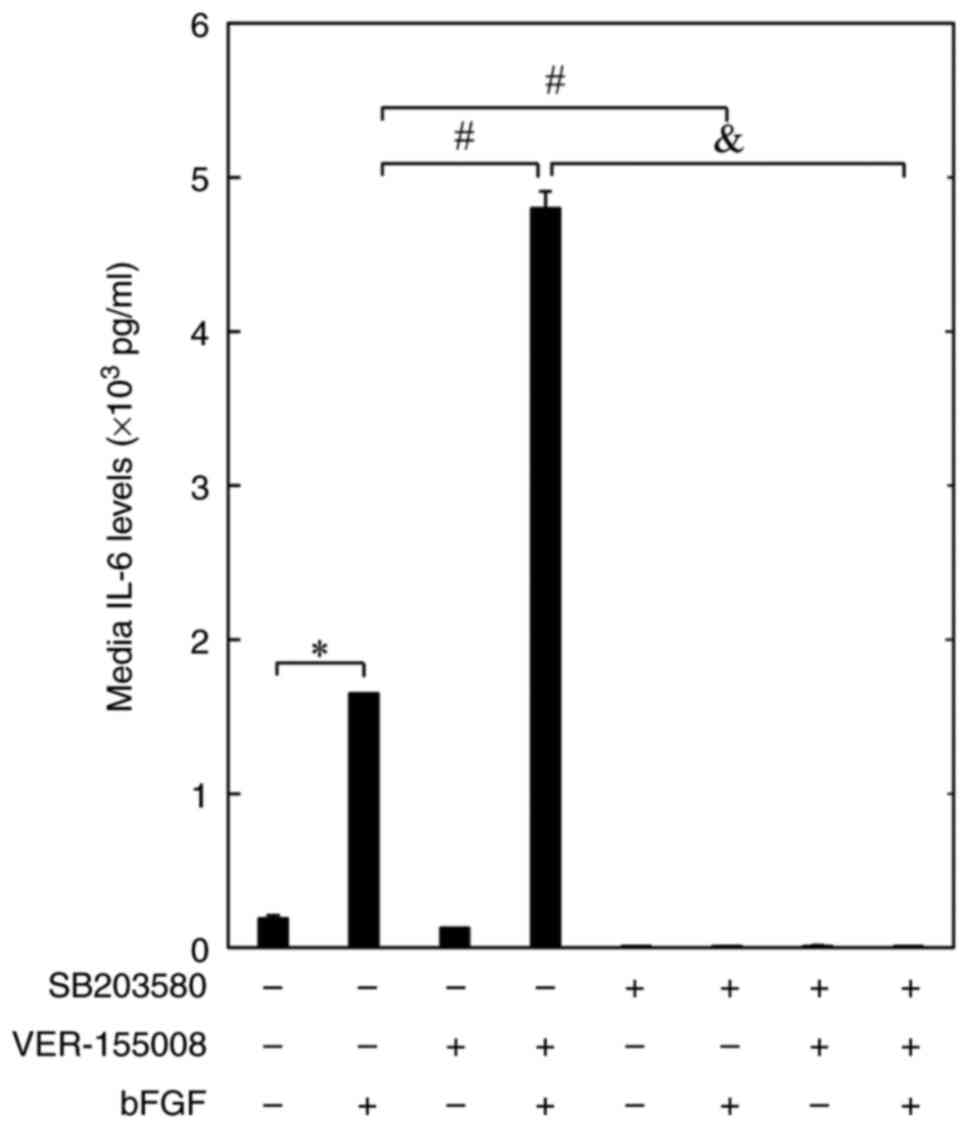
SB203580 inhibits the amplification of
bFGF-elicited IL-6 release by VER-155008 in MC3T3-E1
osteoblast-like cells
Finally, the effect of SB203580, a specific
inhibitor of p38 MAPK (26), on
the amplification of the bFGF-elicited release of IL-6 by
VER-155008 was assessed in MC3T3-E1 osteoblast-like cells. The
results of the present study confirmed those of a previous report
showing that SB203580 significantly inhibited the bFGF-elicited
release of IL-6 (16). SB203580
almost completely inhibited the amplifying effects of VER-155008 on
the bFGF-stimulated release of IL-6 in MC3T3-E1 cells (Fig. 4).
Discussion
The present study demonstrated that the HSP70
inhibitor, VER-155008, significantly increased the bFGF-induced
release of IL-6 in mouse MC3T3-E1 osteoblastic cells and normal
human osteoblasts. Furthermore, VER-155008 significantly increased
the mRNA expression levels of IL-6 induced by bFGF in MC3T3-E1
osteoblastic cells, which suggested that the enhancing effects of
VER-155008 on the bFGF-induced release of IL-6 may be mediated
through a transcriptional event. Our previous study demonstrated
that HSP70 inhibitors, VER-155008 and YM-08, significantly
increased TGF-β-stimulated VEGF release and the TGF-β-induced mRNA
expression levels of VEGF in MC3T3-E1 osteoblast-like cells
(6). These results indicated that
HSP70 inhibitors were involved in VEGF synthesis, as well as IL-6
synthesis, in MC3T3-E1 osteoblastic cells.
Regarding the intracellular signaling exerted by
bFGF in osteoblasts, our previous study demonstrated that bFGF
elicits the synthesis of IL-6 via the activation of p38 MAPK in
MC3T3-E1 osteoblastic cells (16).
In the present study, both VER-155008 and YM-08 significantly
increased the bFGF-elicited phosphorylation of p38 MAPK, which
suggested that HSP70 inhibition upregulated bFGF-elicited
activation of p38 MAPK in these cells. The inhibitors did not
significantly affect the protein expression levels of HSP70, either
with or without bFGF in these cells. Therefore, it is likely that
the upregulation by HSP70 inhibitors of the activation of p38 MAPK
causes the enhancement of IL-6 synthesis induced by bFGF in
MC3T3-E1 osteoblastic cells. Treatment with VER-155008 alone, at 30
µM, caused a significant increase in the phosphorylation of p38
MAPK, which might be due to non-specific effects other than HSP70
inhibition. MAP kinase kinase kinases (MAP3Ks), including apoptosis
signal-regulating kinase-1 (ASK-1) and MEK kinase (MEKK) are
activated in response to numerous inflammatory cytokines. MAP3Ks
activate MAP kinase kinases such as MKK3/6, and MKK3/6 subsequently
activates p38 MAPK (27). HSP70 is
known as a molecular chaperone that interacts with client proteins
and modulates intracellular signal transduction. The inhibition of
HSP70 chaperone function can cause inactivation and degradation of
HSP70-dependent client proteins. The present study demonstrated
that the HSP70 inhibitor VER-155008 (30 µM) significantly enhanced
the phosphorylation of p38 MAPK. Although this remains to be
confirmed as a genuine effect of the inhibitor, this result
indicated that the activation of p38 MAPK could be elicited by
HSP70 silencing. Therefore, HSP70 might downregulate p38 MAPK
signaling through the chaperone effect on the upstream kinase(s)
such as ASK-1, MEKK and MKK3/6 in MC3T3-E1 osteoblast-like
cells.
HSP70 is highly expressed in osteoblasts (5,23).
HSP70 serves a central role in protein homeostasis by folding
proteins into the correct form as a molecular chaperone and also
prevents unfolded protein aggregation by binding to client proteins
(28). In bone cells, it has been
previously reported that HSP70 increases alkaline phosphatase
activity and promotes hMSC mineralization, and that it also
significantly upregulates the expression of Runx2 and Osterix under
osteogenic induction conditions (29). Furthermore, overexpression of HSP
family A member 1A, which encodes the cognate HSP70, stimulates
osteoblastic differentiation of bone marrow stromal cells via the
Wnt/β-catenin signaling pathway (30). Our previous study demonstrated that
HSP70 inhibitors suppress the migration of MC3T3-E1 osteoblast-like
cells induced by insulin-like growth factor-I or epidermal growth
factor (31,32). However, the precise mechanism by
which HSP70, by itself or together with client proteins, influences
bone metabolism has not yet been fully elucidated, and the exact
functions of HSP70 in osteoblasts have not been fully reported. Our
previous study reported that TGF-β-stimulated VEGF synthesis is
upregulated by HSP70 inhibitors through activation of p38 MAPK in
MC3T3-E1 cells (6). Therefore, the
action of HSP70 against p38 MAPK in bFGF-stimulated MC3T3-E1
osteoblastic cells in this study seemed to be similar to the effect
of HSP70 on p38 MAPK in TGF-β-stimulated same cells in our previous
study. Furthermore, it was demonstrated that SB203580 almost
completely inhibited the enhancement by VER-155008 of the
bFGF-stimulated release of IL-6. Our previous study reported that
SB203580 inhibited the enhancement, by VER-155008 and YM-08, of
TGF-β-stimulated VEGF release (6).
These results strongly indicated the involvement of p38 MAPK in the
increased release of IL-6 and VEGF. Taking these findings into
account, it is likely that HSP70 negatively regulates
bFGF-stimulated synthesis of IL-6 as well as the TGF-β-stimulated
synthesis of VEGF in MC3T3-E1 osteoblast-like cells, and that the
effects of HSP70 are exerted by the inhibition of p38 MAPK
activation. Furthermore, our recent study reported that HSP70
inhibitors upregulated prostaglandin E1
(PGE1)-stimulated synthesis of IL-6 through p38 MAPK in
MC3T3-E1 osteoblastic cells (33).
Although their receptors are quite different, the inhibition of
HSP70 can cause upregulation of p38 MAPK in osteoblasts stimulated
by both bFGF and PGE1, which results in an increase in
IL-6 synthesis. The potential mechanisms underlying regulation of
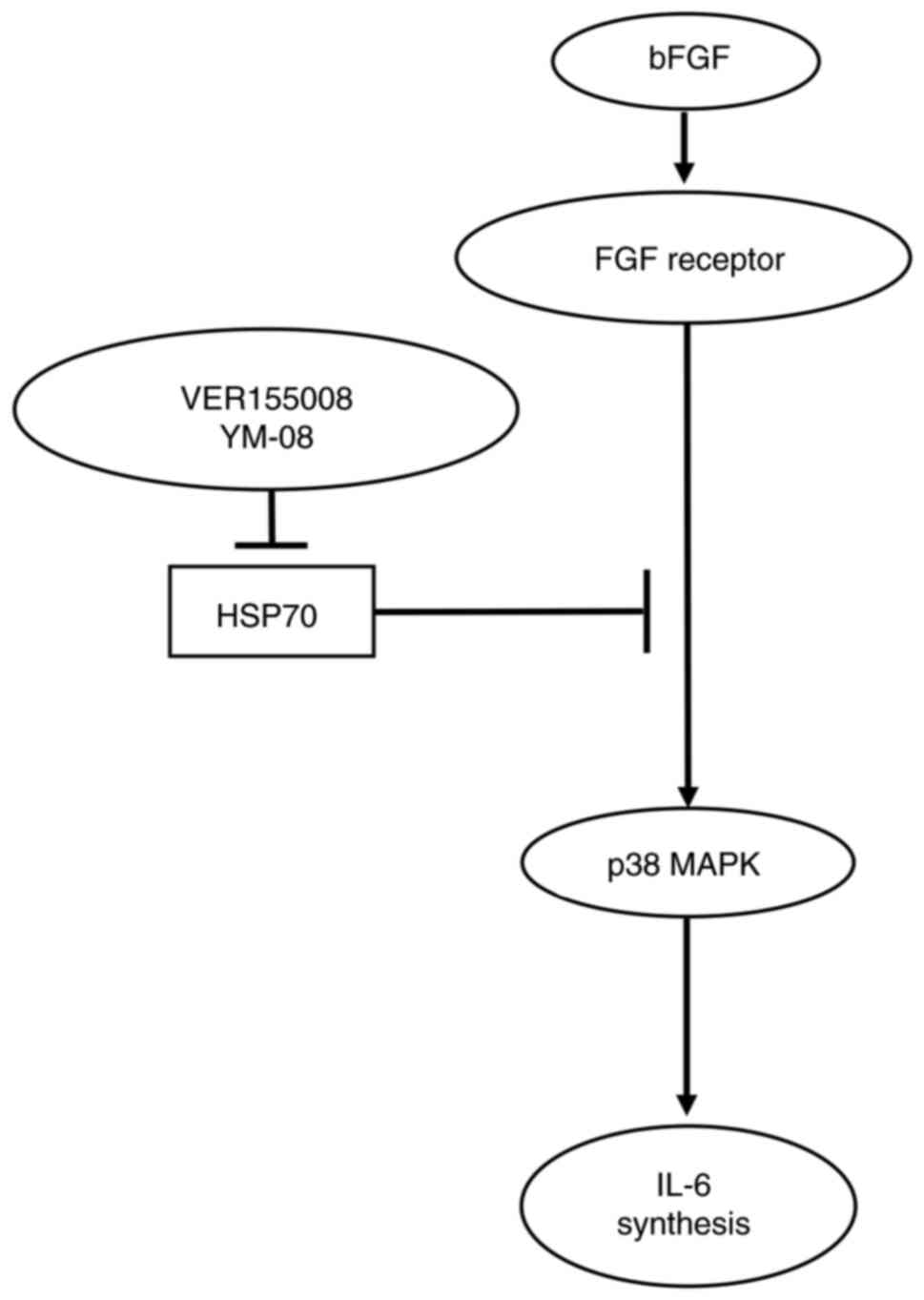
bFGF-stimulated IL-6 release by HSP70 in osteoblasts is presented
in Fig. 5. As HSP70 is an
essential factor for cell survival, the knockdown of HSP70 by small
interfering (si)RNA for >24 h is toxic to MC3T3-E1 cells, which
has been indicated in our previous paper (6), but the results have not been fully
published. The reason why only HSP70 siRNA, but not the HSP70
inhibitors, was toxic to cells was not reported. However, it can be
hypothesized that the siRNA might suppress HSP70 function more
completely than the inhibitors, which were demonstrated in the
present study to not affect the expression of HSP70.
In bone metabolism, the multifunctional cytokine
IL-6 is recognized as an osteotropic factor, which promotes bone
formation under conditions of increased bone turnover, including
during fracture healing (10,11).
bFGF is known to be highly expressed during fracture healing as an
angiogenic factor, which serves a crucial role in bone regeneration
(13). However, HSP70 is
constitutively expressed in non-stressed cells, including
osteoblasts (2). Therefore, HSP70
may play an important role in bone metabolism by regulating the
effects of bFGF and IL-6. The amplifying effects of the HSP70
inhibitors on the bFGF-induced release of IL-6 in osteoblast-like
cells demonstrated in the present study indicated that the
suppression of HSP70 might be a novel method to promote bone
regeneration under conditions such as fracture healing.
In conclusion, the results of the present study
suggested that HSP70 inhibitor amplified the bFGF-induced release
of IL-6 in osteoblasts and that the inhibitory effect of HSP70 was
exerted by inhibition of the activation of p38 MAPK.
Acknowledgements
The authors would like to thank Mrs. Yumiko Kurokawa
(Department of Pharmacology, Gifu University Graduate School of
Medicine, Gifu, Japan) for providing technical assistance. The
authors would also thank Dr Masayoshi Kumegawa (Department of
Dentistry, Graduate School of Dentistry, Meikai University, Sakado,
Japan) for donating cloned MC3T3-El osteoblast-like cells.
Funding
This study was supported by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science
and Technology of Japan (grant nos. 19K09370 and 19K18471) and the
Research Funding for Longevity Sciences from the National Center
for Geriatrics and Gerontology, Japan (grant nos. 20-12 and
21-1).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GK and HT performed data analysis and
interpretation, and wrote the manuscript. GK and HT confirm the
authenticity of all the raw data. TH and RMN collated and analyzed
the data. OK and HT conceived and designed the study, and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hendrick JP and Hartl FU: Molecular
chaperone functions of heat-shock proteins. Annu Rev Biochem.
62:349–384. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartl FU, Bracher A and Hayer-Hartl M:
Molecular chaperones in protein folding and proteostasis. Nature.
475:324–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murphy ME: The HSP70 family and cancer.
Carcinogenesis. 34:1181–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li C, Sunderic K, Nicoll SB and Wang S:
Downregulation of heat shock protein 70 impairs osteogenic and
chondrogenic differentiation in human mesenchymal stem cells. Sci
Rep. 8:5532018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Tokuda H, Hatakeyama D, Hirade K,
Niwa M, Ito H, Kato K and Kozawa O: Mechanism of simvastatin on
induction of heat shock protein in osteoblasts. Arch Biochem
Biophys. 415:6–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakai G, Tokuda H, Fujita K, Kainuma S,
Kawabata T, Matsushima-Nishiwaki R, Kozawa O and Otsuka T: Heat
shock protein 70 negatively regulates TGF-β-stimulated VEGF
synthesis via p38 MAP kinase in osteoblasts. Cell Physiol Biochem.
44:1133–1145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kishimoto T, Akira S, Narazaki M and Taga
T: Interleukin-6 family of cytokines and gp130. Blood.
86:1243–1254. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sims NA: Cell-specific paracrine actions
of IL-6 family cytokines from bone, marrow and muscle that control
bone formation and resorption. Int J Biochem Cell Biol. 79:14–23.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prystaz K, Kaiser K, Kovtun A,
Haffner-Luntzer M, Fischer V, Rapp AE, Liedert A, Strauss G,
Waetzig GH, Rose-John S and Ignatius A: Distinct effects of IL-6
classic and trans-signaling in bone fracture healing. Am J Pathol.
188:474–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franchimont N, Wertz S and Malaise M:
Interleukin-6: An osteotropic factor influencing bone formation?
Bone. 37:601–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roodman GD: Perspectives: Interleukin-6:
An osteotropic factor? J Bone Miner Res. 7:475–478. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Charoenlarp P, Rajendran AK and Iseki S:
Role of fibroblast growth factors in bone regeneration. Inflamm
Regen. 37:102017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luong LN, Ramaswamy J and Kohn DH: Effects
of osteogenic growth factors on bone marrow stromal cell
differentiation in a mineral-based delivery system. Biomaterials.
33:283–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozawa O, Suzuki A and Uematsu T: Basic
fibroblast growth factor induces interleukin-6 synthesis in
osteoblasts: Autoregulation by protein kinase C. Cell Signal.
9:463–468. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kozawa O, Tokuda H, Matsuno H and Uematsu
T: Involvement of p38 mitogen-activated protein kinase in basic
fibroblast growth factor-induced interleukin-6 synthesis in
osteoblasts. J Cell Biochem. 74:479–485. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozawa O, Suzuki A, Tokuda H and Uematsu
T: Prostaglandin F2alpha stimulates interleukin-6 synthesis via
activation of PKC in osteoblast-like cells. Am J Physiol. 272((2 Pt
1)): E208–E211. 1997.PubMed/NCBI
|
|
19
|
Kondo A, Otsuka T, Matsushima-Nishiwaki R,
Kuroyanagi G, Mizutani J, Wada I, Kozawa O and Tokuda H: Inhibition
of SAPK/JNK leads to enhanced IL-1-induced IL-6 synthesis in
osteoblasts. Arch Biochem Biophys. 535:227–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kozawa O, Niwa M, Hatakeyama D, Tokuda H,
Oiso Y, Matsuno H, Kato K and Uematsu T: Specific induction of heat
shock protein 27 by glucocorticoid in osteoblasts. J Cell Biochem.
86:357–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schlecht R, Scholz SR, Dahmen H, Wegener
A, Sirrenberg C, Musil D, Bomke J, Eggenweiler HM, Mayer MP and
Bukau B: Functional analysis of Hsp70 inhibitors. PLoS One.
8:e784432013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyata Y, Li X, Lee HF, Jinwal UK,
Srinivasan SR, Seguin SP, Young ZT, Brodsky JL, Dickey CA, Sun D
and Gestwicki JE: Synthesis and initial evaluation of YM-08, a
blood-brain barrier permeable derivative of the heat shock protein
70 (Hsp70) inhibitor MKT-077, which reduces tau levels. ACS Chem
Neurosci. 4:930–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cuenda A, Rouse J, Doza YN, Meier R, Cohen
P, Gallagher TF, Young PR and Lee JC: SB203580 is a specific
inhibitor of a MAP kinase homologue which is stimulated by cellular
stresses and interleukin-1. FEBS Lett. 364:229–233. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thouverey C and Caverzasio J: Focus on the
p38 MAPK signaling pathway in bone development and maintenance.
Bonekey Rep. 4:7112015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mayer MP and Bukau B: Hsp70 chaperones:
Cellular functions and molecular mechanism. Cell Mol Life Sci.
62:670–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen E, Xue D, Zhang W, Lin F and Pan Z:
Extracellular heat shock protein 70 promotes osteogenesis of human
mesenchymal stem cells through activation of the ERK signaling
pathway. FEBS Lett. 589((24 Pt B)): 4088–4096. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Xue D, Yin H, Wang S, Li C, Chen
E, Hu D, Tao Y, Yu J, Zheng Q, et al: Overexpression of HSPA1A
enhances the osteogenic differentiation of bone marrow mesenchymal
stem cells via activation of the Wnt/β-catenin signaling pathway.
Sci Rep. 6:276222016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawabata T, Tokuda H, Sakai G, Fujita K,
Matsushima-Nishiwaki R, Kuroyanagi G, Otsuka T and Kozawa O: HSP70
inhibitor suppresses IGF-I-stimulated migration of osteoblasts
through p44/p42 MAP kinase. Biomedicines. 6:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawabata T, Otsuka T, Fujita K, Sakai G,
Kim W, Matsushima-Nishiwaki R, Kuroyanagi G, Kozawa O and Tokuda H:
HSP70 inhibitors reduce the osteoblast migration by epidermal
growth factor. Curr Mol Med. 18:486–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuroyanagi G, Tachi J, Fujita K, Kawabata
T, Sakai G, Nakashima D, Kim W, Tanabe K, Matsushima-Nishiwaki R,
Otsuka T, et al: HSP70 inhibitors upregulate prostaglandin
E1-induced synthesis of interleukin-6 in osteoblasts. PLoS One.
17:e02791342022. View Article : Google Scholar : PubMed/NCBI
|