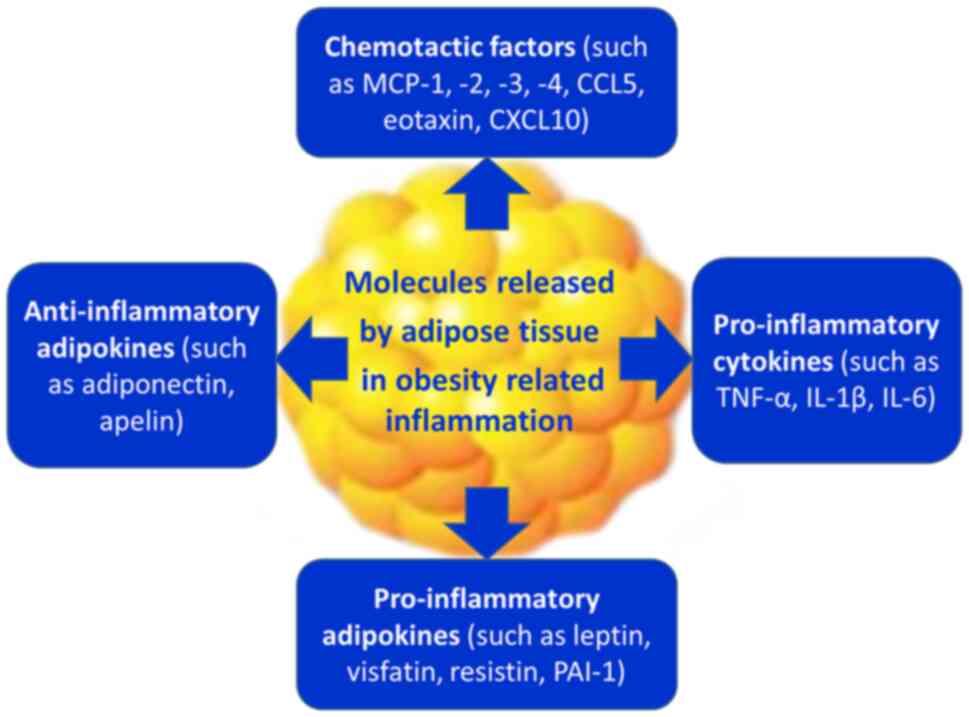
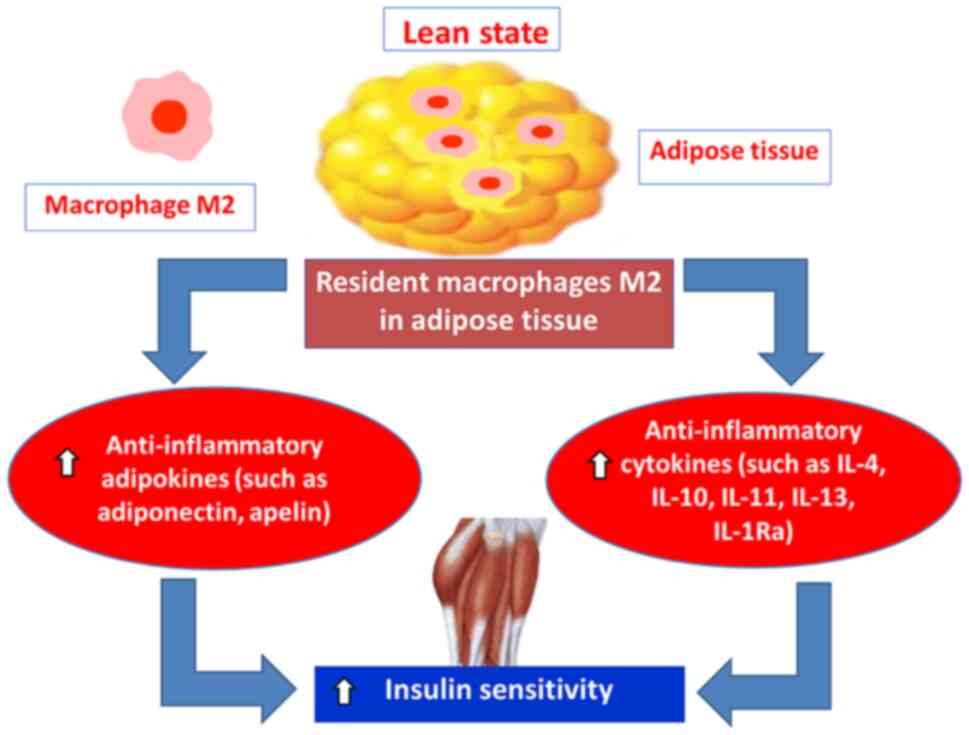
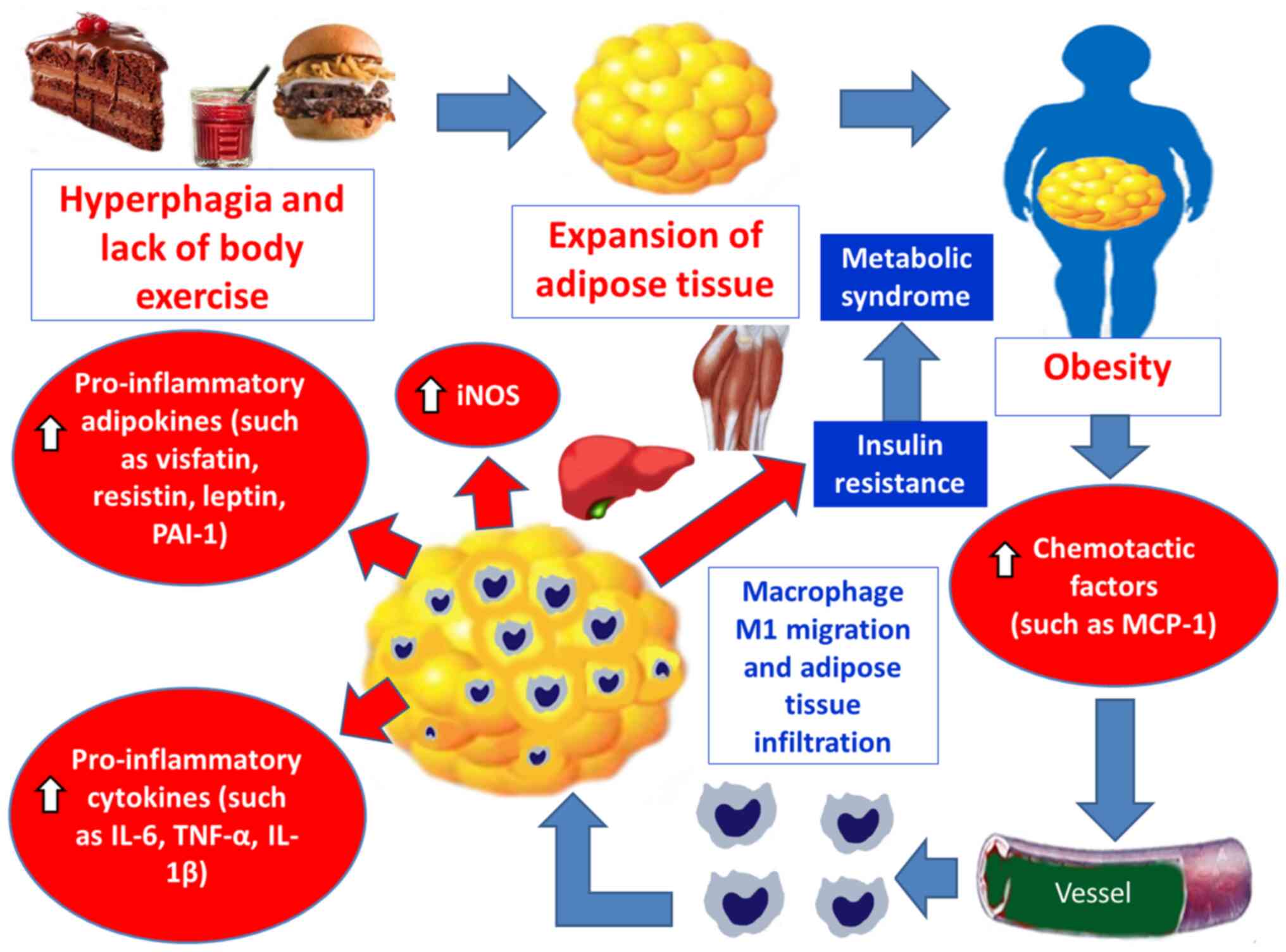
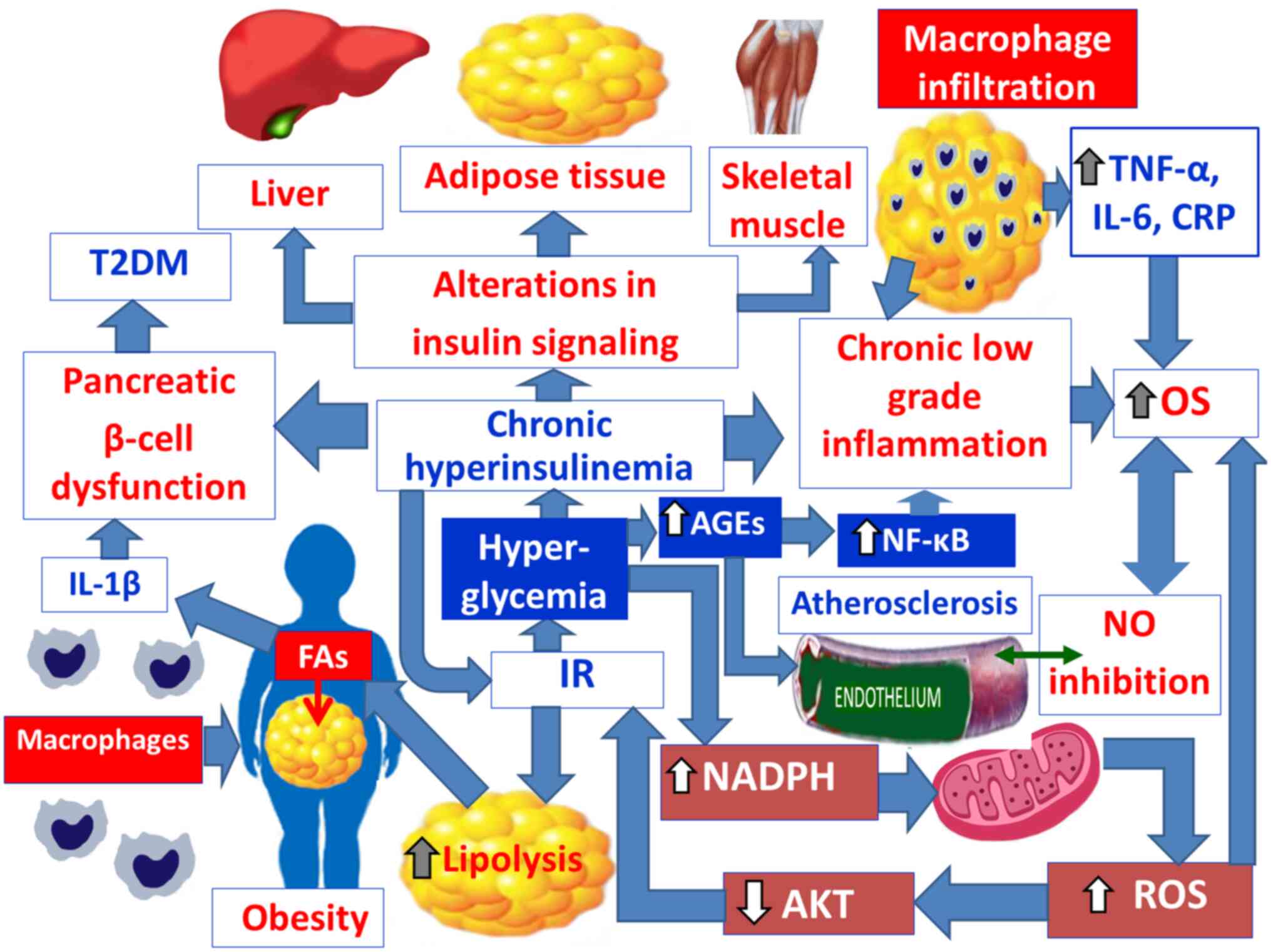
|
1
|
Sethi JK and Vidal-Puig AJ: Thematic
review series: Adipocyte biology. Adipose tissue function and
plasticity orchestrate nutritional adaptation. J Lipid Res.
48:1253–1262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo L and Liu M: Adipose tissue in control
of metabolism. J Endocrinol. 231:R77–R99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung UJ and Choi MS: Obesity and its
metabolic complications: The role of adipokines and the
relationship between obesity, inflammation, insulin resistance,
dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci.
15:6184–6223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Curat CA, Miranville A, Sengenè C, Diehl
M, Tonus C, Busse R and Bouloumié A: From blood monocytes to
adipose tissue-resident macrophages: Induction of diapedesis by
human mature adipocytes. Diabetes. 53:1285–1292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cypess AM, Lehman S, Williams G, Tal I,
Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al:
Identification and importance of brown adipose tissue in adult
humans. N Engl J Med. 360:1509–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nauli AM and Matin S: Why do men
accumulate abdominal visceral fat? Front Physiol. 10:14862019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahn CR, Wang G and Lee KY: Altered
adipose tissue and adipocyte function in the pathogenesis of
metabolic syndrome. J Clin Invest. 129:3990–4000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blüher M: Adipose tissue dysfunction in
obesity. Exp Clin Endocrinol Diabetes. 117:241–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan CY and Vidal-Puig A: Adipose tissue
expandability: The metabolic problems of obesity may arise from the
inability to become more obese. Biochem Soc Trans. 36:935–940.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sebo ZL and Rodeheffer MS: Assembling the
adipose organ: Adipocyte lineage segragation and adipogenesis in
vivo. Development. 146:dev1720982019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lafontan M and Langin D: Lipolysis and
lipid modilization in human adipose tissue. Prog Lipid Res.
48:275–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frayn KN: Adipose tissue as a buffer for
daily lipid flux. Diabetologia. 45:1201–1210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cinti S, Mitchell G, Barbatelli G, Murano
I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS and Obin MS:
Adipocyte death defines macrophage location and function in adipose
tissue of obese mice and humans. J Lipid Res. 46:2347–2355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ellulu MS, Patimah I, Khazaai H, Rahmat A
and Abed Y: Obesity and inflammation: The linking mechanism and the
complications. Arch Med Sci. 13:851–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fantuzzi G: Adipose tissue, adipokines,
and inflammation. J Allergy Clin Immunol. 115:911–919. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weir CB and Jan A: BMI classification
percentile and cut off points. StatPearls Treasure Island, FL:
StatPearls Publishing; 2020
|
|
18
|
Marcadenti A and de Abreu-Silva EO:
Different adipose tissue depots: Metabolic implications and effects
of surgical removal. Endocrinol Nutr. 62:458–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuster JJ, Ouchi N, Gokce N and Walsh K:
Obesity-induced changes in adipose tissue microenvironment and
their impact on cardiovascular disease. Circ Res. 118:1786–1807.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osborn O and Olefsky JM: The cellular and
signaling networks linking the immune system and metabolism in
disease. Nat Med. 18:363–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gustafson B, Hedjazifar S, Gogg S,
Hammarstedt A and Smith U: Insulin resistance and impaired
adipogenesis. Trends Endocrinol Metab. 26:193–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuk JL, Katzmarzyk PT, Nichaman MZ, Church
TS, Blair SN and Ross R: Visceral fat in an independent predictor
of all-cause mortality in men. Obesity (Silver Spring). 14:336–341.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klein J, Permana PA, Owecki M, Chaldakov
GN, Böhm M, Hausman G, Lapière CM, Atanassova P, Sowiński J,
Fasshauer M, et al: What are subcutaneous adipocytes really good
for? Exp Dermatol. 16:45–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cameron AJ, Magliano DJ and Soderberg S: A
systemic review of the impact of including both waist and hip
circumference in risk models for cardiovascular diseases, diabetes
and mortality. Obes Rev. 14:86–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koster A, Murphy RA, Eiriksdottir G,
Aspelund T, Sigurdsson S, Lang TF, Gudnason V, Launer LJ and Harris
TB: Fat distribution and mortality: The AGES-Reykjavik study.
Obesity (Silver Spring). 23:893–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arner P, Andersson DP, Thörne A, Wirén M,
Hoffstedt J, Näskybd E, Thorell A and Rydén M: Variations in the
size of the major omentum are primarily determined by fat cell
number. J Clin Endocrinol Metab. 98:E897–E901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chait A and den Hartigh LJ: Adipose tissue
distribution, inflammation and its metabolic consequences,
including diabetes and cardiovascular disease. Front Cardiovasc
Med. 25:222020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
James WP: Assessing obesity: Are ethnic
differences in body mass index and waist classification criteria
justified? Obes Rev. 6:179–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
James WP, Rigby N and Leach R: Obesity and
the metabolic syndrome: The stress on society. Ann N Y Acad Sci.
1083:1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Sayed AM, Scarborough P and Galea S:
Ethnic inequalities in obesity among children and adults in the UK:
A systematic review of the literature. Obes Rev. 12:e516–e534.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barnett AH, Dixon AN, Bellary S, Hanif MW,
O'Hare JP, Raymond NT and Kumar S: Type 2 diabetes and
cardiovascular risk in the UK south Asian community. Diabetologia.
49:2234–2246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Misra A and Khurana L: Obesity-related
non-communicable diseases: South Asians vs White Caucasians. Int J
Obes (Lond). 35:167–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pi-Sunyer X: The medical risks of obesity.
Postgrad Med. 121:21–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kyrou I, Randeva HS, Tsigos C, Kaltsas G,
Weickert MO, Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos
G, et al: Clinical problems caused by obesity. Endotext [Internet]
South Dartmouth (MA): MDText.com, Inc; 2018
|
|
35
|
Khanna D, Khanna S, Khanna P, Kahar P and
Patel BM: Obesity: A chronic low-grade inflammation and its
markers. Cureus. 14:e227112022.PubMed/NCBI
|
|
36
|
Bobbert T, Rochlitz H, Wegewitz U, Akpulat
S, Mai K, Weickert MO, Möhlig M, Pfeiffer AFH and Spranger J:
Changes of adiponectin oligomer composition by moderate weight
reduction. Diabetes. 54:2712–2719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-α:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mohlig M, Weickert MO, Ghadamgadai E,
Machlitt A, Pfüller B, Arafat AM, Pfeiffer AFH and Schöfl C:
Adipocyte fatty acid-binding protein is associated with marker of
obesity, but is an unlikely link between obesity, insulin
resistance and hyperandrogenism in polycystic ovary syndrome women.
Eur J Endocrinol. 157:195–200. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fonseca-Alaniz MH, Takada J, Alonso-Vale
MI and Lima FB: Adipose tissue as an endocrine organ: From theory
to practice. J Pediatr. 83:192–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maury E and Brichard SM: Adipokine
dysregulation, adipose tissue inflammation and metabolic syndrome.
Mol Cell Endocrinol. 314:1–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gregor MF and Hotamilsigil GS:
Inflammatory mechanisms in obesity. Annu Rev Immunol. 29:415–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weickert MO, Hodges P, Tan BK and Randeva
HS: Neuroendocrine and endocrine dysfunction in the
hyperinsulinemic PCOS patient: The role of metformin. Minerva
Endocrinol. 37:25–40. 2012.PubMed/NCBI
|
|
43
|
Randeva HS, Tan BK, Weickert MO, Lois K,
Nestler JE, Sattar N and Lehnert H: Cardiometabolic aspects of the
polycystic ovary syndrome. Endocr Rev. 33:812–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Makki K, Froguel P and Wolowczuk I:
Adipose tissue in obesity-related inflammation and insulin
resistance. Cells, cytokines and chemokines. ISRN Inflamm.
2013:1392392013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sivakumar K, Bari MF, Adaikalakoteswari A,
Guller S, Weickert MO, Randeva HS, Grammatopoulos DK, Bastie CC and
Vatish M: Elevated fetal adispin/acylation-stimulating protein
(ASP) in obese pregnancy: Novel placental secretion via Hofbauer
cells. J Clin Endocrinol Metab. 98:4113–4122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
von Loeffelholz C, Mohlig M, Arafat AM,
Isken F, Spranger J, Mai K, Randeva HS, Pfeiffer AFH and Weickert
MO: Circulation vaspin is unrelated to insulin sensitivity in a
cohort of nondiabetic humans. Eur J Endocrinol. 162:507–513. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Elmasry SA, Al-Azzawi MA, Ghoneim AH, Nasr
MY and AboZaid MMN: Role of oxidant-antioxidant imbalance in the
pathogenesis of chronic obstructive pulmonary disease. Egypt J
Chest Dis Tuberc. 64:813–820. 2015. View Article : Google Scholar
|
|
48
|
Marseglia L, Manti S, D'Angelo G, Nicotera
A, Parisi E, Di Rosa G, Gitto E and Arrigo T: Oxidative stress in
obesity: A critical component in human diseases. Int J Mol Sci.
16:378–400. 2015. View Article : Google Scholar
|
|
49
|
Reilly SM and Saltiel AR: Adapting to
obesity with adipose tissue inflammation. Nat Rev Endocrinol.
13:633–643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rheinheimer J, de Souza BM, Cardoso NS,
Bauer AC and Crispim D: Current role of the NLRP3 inflammasome on
obesity and insulin resistance: A systematic review. Metabolism.
74:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deshmane SL, Kremley S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Taylor EB: The complex role of adipokines
in obesity, inflammation and autoimmunity. Clin Sci (Lond).
135:731–752. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matsushima K, Larsen CG, DuBois GC and
Oppenheim JJ: Purification and characterization of a novel monocyte
chemotactic and activating factor produced by a human
myolomonocytic cell line. J Exp Med. 169:1485–1490. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rollins BJ: Chemokines. Blood. 90:909–928.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kanda H, Tateya S, Tamori Y, Kotani K,
Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K and
Kasuga M: MCP-1 contributes to macrophage infiltration into adipose
tissue, insulin resistance, and hepatic steatosis in obesity. J
Clin Invest. 116:1494–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Singh S, Anshita D and Ravichandiran V:
MCP-1: Function, regulation, and involvement in disease. Int
Immunopharmacol. 101((PtB)): 1075982021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dietze-Schroeder D, Sell H, Uhlig M,
Koener M and Eckel J: Autocrine action of adiponectin on human fat
cells prevents the release of insulin resistance-inducing factors.
Diabetes. 54:2003–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Christiansen T, Richelsen B and Bruun JM:
Monocyte chemoattractant protein-1 is produced in isolated
adipocytes, associated with adiposity and reduced after weight loss
in morbid obese subjects. Int J Obes (Lond). 29:146–150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cancello R, Henegar C, Viguerie N, Taleb
S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL,
et al: Reduction of macrophage infiltration and chemoattractant
gene expression changes in white adipose tissue of morbidly obese
subjects after surgery-induced weight loss. Diabetes. 54:2277–2286.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Piemonti L, Calori G, Mercalli A, Lattuada
G, Monti P, Garancini MP, Constantio F, Ruotolo G, Luzi L and
Perseglin G: Fasting plasma leptin, tumor necrosis factor-alpha
receptor 2, and monocyte chemoattracting protein 1 concentration in
a population of glucose-tolerant and glucose-intolerant women:
Impact on cardiovascular mortality. Diabetes Care. 26:2883–2889.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Simeoni E, Hoffman MM, Winkelman BR, Ruiz
J, Fleury S, Boehm BO, März W and Vassalli G: Association between
the A-2518G polymorphism in the monocyte chemoattractant protein-1
gene and insulin resistance and type 2 diabetes mellitus.
Diabetologia. 47:1574–1580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Herder C, Baumert J, Thorand B, Koenig W,
de Jager W, Meisinger C, Illig T, Martin S and Kolb H: Chemokines
as risk factors for type 2 diabetes: Results from the MONICA/KORA
Augsburg study, 1984–2002. Diabetologia. 49:921–929. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim CS, Park HS, Kawada T, Kim JH, Lim D,
Hubbard NE, Kwon BS, Rrickson KL and Yu R: Circulating levels of
MCP-1 and IL-8 are elevated in human obese subjects and associated
with obesity-related parameters. Int J Obes (Lond). 30:1347–1355.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shimobayashi M, Albert V, Woelnerhanssen
B, Frei IC, Weissenberger D, Meyer-Gerpach AC, Clement N, Moes S,
Colombi M, Meier JA, et al: Insulin resistance causes inflammation
in adipose tissue. J Clin Invest. 128:1538–1550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhu B, Guo X, Xu H, Jiang B, Li H, Wang Y,
Yin O, Zhou T, Cai JJ, Glaser S, et al: Adipose tissue inflammation
and systemic insulin resistance in mice with diet-induced obesity
is possibly associated with disruption of PFKFB3 in hematopoietic
cells. Lab Invest. 101:328–340. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ylä-Herttuala S, Lipton A, Rosenfeld ME,
Särkioja T, Yoshimura T, Leonard EJ, Witztum JL and Steinberg D:
Expression of monocyte chemoattractant protein 1 in macrophage-rich
areas of human and rabbit atherosclerotic lesions. Proc Natl Acad
Sci USA. 88:5252–5256. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Arakelyan A, Petrkova J, Hermanova Z,
Boyajyan A, Lukl J and Petrek M: Serum levels of the MCP-1
chemokine in patients with ischemic stroke and myocardiac
infarction. Mediators Inflamm. 14:175–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sukumar D, Partridge C, Wang X and Shapses
SA: The high serum monocyte chemoattractant protein-1 in obesity is
influenced by high parathyroid hormone and not adiposity. J Clin
Endocrinol Metab. 296:1852–1858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Takeya M, Yoshimura T, Leonard EJ and
Takahashi K: Detection of monocyte chemoattractant protein-1 in
human atherosclerotic lesions by an anti-monocyte chemoattractant
protein-1 monoclonal antibody. Hum Pathol. 24:534–539. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gu L, Οkada Y, Clinton SK, Gerard C,
Sukhova GK, Libby P and Rollins BJ: Absence of monocyte
chemoattractant protein-1 reduces atherosclerosis in low density
lipoprotein receptor-defined mice. Mol Cell. 2:275–281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Boring M, Gosling J, Cleary M and Charo
IF: Decreased lesion in CCR2-/- mice reveals a role for chemokines
in the initation of atherosclerosis. Nature. 394:894–897. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Deng Y and Scherer PE: Adipokines as novel
biomarkers and regulators of the metabolic syndrome. Ann N Y Acad
Sci. 1212:E1–E19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dutheil F, Gordon BA, Naughton G, Crendal
E, Courteix D, Chaplais E, Thivel D, Lac G and Benson AC:
Cardiovascular risk of adipokines: A review. J Inter Med Res.
46:2082–2095. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Szumilas K, Szumilas P,
Słuczanowska-Głąbowsk S, Zgutka K and Pawlik A: Role of adiponectin
in the pathogenesis of Rheumatoid arthritis. Int J Mol Sci.
21:82652020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Adolph TE, Grander C, Grabherr F and Tilg
H: Adipokines and non-alcoholic fatty liver disease: Multiple
interactions. Int J Mol Sci. 18:16492017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Neumann UH, Chen S, Tam YY, Baker RK,
Covey SD, Dullis PP and Kieffer TJ: IGFBP2 is neither sufficient
nor necessary for the physiological actions of leptin on glucose
homeostasis in male ob/ob mice. Endocrinology. 155:716–725. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zorena K, Jachimowicz-Duda O, Ślęzak D,
Robakowska M and Mrugacz M: Adipokines in obesity. Potential lind
to metabolic disorders and chronic complications. Int J Mol Sci.
21:35702020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kumada M, Kihara S, Ouchi N, Kobayashi H,
Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, et
al: Adiponectin specifically increased tissue inhibitor of
metalloproteinase-1 through interleukin-10 expression in human
macrophages. Circulation. 109:2046–2049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Quedraogo R, Wu X, Xu SQ, Fuchsel L,
Motoshima H, Mahadev K, Hough K, Scalia R and Goldstein BJ:
Adiponectin suppression of high-glucose-induced reactive oxygen
species in vascular endothelial cells: Evidence for involvement of
a cAMP signaling pathway. Diabetes. 55:1840–1846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yuan F, Li YN, Liu YH, Yi B, Tian JW and
Liu FY: Adiponectin inhibits the generation of reactive oxygen
species induced by high glucose and promotes endothelial NO
synthase formation in human mesangial cells. Mol Med Rep.
6:449–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Furukawa S, Fujita T, Shimabukuro M, Iwaki
M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and
Shimomura I: Increased oxidative stress in obesity and its impact
on metabolic syndrome. J Clin Invest. 114:1752–1761. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Castro JR, Grune T and Speckmann B: The
two faces of reactive oxygen species (ROS) in adipocyte function
and dysfunction. Biol Chem. 397:709–724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fujita K, Nishizawa H, Funahashi T,
Shimomura I and Shimabukuro M: Systemic oxidative stress is
associated with visceral fat accumulation and the metabolic
syndrome. Circulation. 70:1437–1442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kanazawa I, Yamaguchi T, Yano S, Yamauchi
M, Yamamoto M and Sugimoto T: Adiponectin and AMP kinase activator
stimulate proliferation, differentiation, and mineralization of
osteoblastic MC3T3-E1 cells. BMC Cell Biol. 8:512007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xie C and Chen Q: Adipokines: New
therapeutic target for osteoarthritis? Curr Reumatol Rep.
21:712020.
|
|
86
|
Gamberi Τ, Μagherini F, Modesti A and
Fiaschi T: Adiponectin signaling pathways in liver diseases.
Biomedicines. 6:522018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ouchi N, Kihara S, Arita Y, Nishida M,
Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K,
Nishizawa H, et al: Adipocyte-derived plasma protein, adiponectin,
suppresses lipid accumulation and class A scavenger receptor
expression in human monocyte-derived macrophages. Circulation.
103:1057–1063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Matsuda M, Shimomura I, Sata M, Arita Y,
Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H,
et al: Role of adiponectin in preventing vascular stenosis. The
missing link of adipo-vascular axis. J Biol Chem. 277:37487–37491.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kadowaki T and Yamauchi T: Adiponectin and
adiponectin receptors. Endocr Rev. 26:439–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zha N, Wu X and Gao P: Adiponectin and its
receptors in diabetic kidney disease: Molecular mechanisms and
clinical potential. Endocrinol. 158:2022–2034. 2017. View Article : Google Scholar
|
|
91
|
Alnaggar ARLR, Sayed M, El-Deena KE, Gomma
M and Hamed Y: Evaluation of serum adiponectin levels in diabetic
nephropathy. Diabetes Metab Syndr. 13:128–131. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ouedraogo R, Gong Y, Berzins B, Wu X,
Mahadev K, Hough K, Chan L, Goldstein BJ and Scalia R: Adiponectin
deficiency increases leukocyte-endothelium interactions via
up-regulation of endothelial cell adhesion molecules in vivo. J
Clin Invest. 117:1718–1761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Abella V, Scotece M, Conde J, López V,
Lazzaro V, Pino J, Gómez-Rein O and Gualillo O: Adipokines,
metabolic syndrome and rheumatic diseases. J Immunol Res.
2014:3437462014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stefan N and Stumvoll M: Adiponectin-its
role in metabolism and beyond. Horm Metab Res. 34:469–474. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Weyer C, Funahashi T, Tanaka S, Hotta K,
Matsuzawa Y, Pratley RE and Tataranni PA: Hypoadiponectinemia in
obesity and type 2 diabetes: Close association with insulin
resistance and hyperinsulinemia. J Clin Endocrinol Metab.
86:1930–1935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Arita Y, Kihara S, Ouchi N, Takahashi M,
Maed K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et
al: Paradoxical decrease of an adipose-specific protein,
adiponectin, in obesity. Biochem Biophys Res Commun. 257:79–83.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Scherer PE: Adipose tissue. From lipid
storage compartment to endocrine organ. Diabetes. 55:1537–1545.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Trujillo ME and Scherer PE: Adipose
tissue-derived factors: Impact on health and disease. Endocr Rev.
27:762–778. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Oh DK, Ciaraldi T and Henry RR:
Adiponectin in health and disease. Diabetes Obes Metab. 9:282–289.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li S, Shin HJ, Ding EL and van Dam RM:
Adiponectin levels and risk of type 2 diabetes: A systematic review
and meta-analysis. JAMA. 302:179–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li C, Cheng H, Adhikari BK, Wang S, Yang
N, Liu W, Sun J and Wang Y: The role of apelin-APJ system in
diabetes and obesity. Front Endocrinol (Lausanne). 2022:132022.
|
|
102
|
Al-Mansoori L, Al-Jaber H, Price MS and
Elrayess MA: Role of inflammatory cytokines, growth factors and
adipokines in adipogenesis and insulin resistance. Inflammation.
45:31–44. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Vykoukal D and Davies MG: Vascular biology
of metabolic syndrome. J Vasc Surg. 54:819–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Than A, He HL, Chua SH, Xu D, Sun L, Leow
MKS and Chen P: Apelin enhances brown adipogenesis and browning of
white adipocytes. J Biol Chem. 290:1469–14691. 2015. View Article : Google Scholar
|
|
105
|
Yamamoto T, Habata Y, Matsumoto Y,
Yasuhara Y, Hashimoto T, Hamajyo H, Anayama H, Fujii R, Fuse H,
Shintani Y and Mori M: Apelin-transgenic mice exhibit a resistance
against diet-induced obesity by increasing vascular mass and
mitochondrial biogenesis in skeletal muscle. Biochim Biophys Acta.
1810:853–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mughal A and O'Rourke ST: Vascular effects
on apelin: Mechanisms and therapeutic potential. Pharmacol Ther.
190:139–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yamazaki S, Sekiguchi A, Uchiyama A,
Fujiwara C, Inoue Y, Yokoyama Y, Ogino S, Torii R, Hosoi M, Akai R,
et al: Apelin/APJ signaling suppresses the pressure ulcer formation
in cutaneous ischemia-perfusion injury mouse model. Sci Rep.
10:13492020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Attané C, Foussal C, Gonidec SL, Benani A,
Daviaud D, Wanecq E, Guzmán-Ruiz R, Dray C, Bezaire V, Rancoule C,
et al: Apelin treatment increases complete fatty acid oxidation,
mitochondrial oxidative capacity and biogenesis in muscle of
insulin-resistant mice. Diabetes. 61:310–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lay SL, Simard G, Martinez MC and
Andriantsitohaina R: Oxidative stress and metabolic pathologies:
From an adipocentric point of view. Oxid Med Cell Longev.
2014:9085392014.PubMed/NCBI
|
|
110
|
Kim S, Kim S, Hwang AR, Choi HC, Lee JY
and Woo CH: Apelin-13 inhibits methylglyoxal-induced unfolded
protein responses and endothelial dysfuction via regulating AMPK
pathway. Int J Mol Sci. 21:40692020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Fibbi B, Marroncini G, Naldi L and Peri A:
The Yin and Yang effects of the apelinergic system in oxidative
stress. Int J Mol Sci. 24:47452023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obes
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Halaas JL, Gajiwala KS, Maffei M, Cohen
SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK and Friedman JM:
Weight-reducing effects of the plasma protein encoded by the obese
gene. Science. 269:543–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Caro JF, Sinha MK, Kolaczynski JW, Zhang
PL and Considine RV: Leptin: The tale of an obesity gene. Diabetes.
45:1455–1462. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fantuzzi G and Faggioni R: Leptin in the
regulation of immunity, inflammation and haematopoiesis. J Leukoc
Biol. 68:437–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Münzberg H and Morrison CD: Structure,
production and signaling of leptin. Metabolism. 64:13–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Trayhurn P, Duncan JS, Hoggard N and
Rayner DV: Regulation of leptin production: A dominant role for the
sympathetic nervous system? Proc Nutr Soc. 57:413–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Dieguez C, Vazquez MJ, Romero A, Lopez M
and Nogueiras R: Hypothalamic control of lipid metabolism: Focus on
leptin, ghrelin and melanocortins. Neuroendocrinology. 94:1–11.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Morton GJ and Schwartz MW: Leptin and the
central nervous system control of glucose metabolism. Physiol Rev.
91:389–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sahu A: Leptin signaling in the
hypothalamus: Emphasis on energy homeostasis and leptin resistance.
Front Neuroendocrinol. 24:225–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Blaszczak AM, Jalilvand A and Hsueh WA:
Adipocytes, innate immunity and obesity: A mini-review. Front
Immunol. 12:6507682021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Obradovic M, Sudar-Milovanovic E, Soskic
S, Essack M, Arya S, Stewart AJ, Gojobori T and Isenovic ER: Leptin
and obesity: Role and clinical implication. Front Endocrinol
(Lausanne). 12:5858872021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Fahed G, Aoun L, Zerdan MB, Allam S,
Zerdan MB, Bouferraa Y and Assi HI: Metabolic syndrome: Updates on
pathophysiology and management in 2021. Int J Mol Sci. 23:7862022.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
van den Hoek AM, Teusink B, Voshol PJ,
Havekes LM, Romijn JA and Piji H: Leptin deficiency per se dictates
body composition and insulin action in ob/ob mice. J
Neuroendocrinol. 20:120–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang H, Xie H, Zhao Q, Xie GQ, Wu XP,
Liao EY and Luo XH: Relationships between serum adiponectin,
apelin, leptin, resistin, visfatin levels and bone mineral density,
and bone biochemical markers in post-menopausal Chinese women. J
Endocrinol Invest. 33:707–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara
K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, et
al: Pathophysiological role of leptin in obesity-related
hypertension. J Clin Investig. 105:1243–1252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ferri C, Desideri G, Valenti M, Bellini C,
Pasin M, Santucci A and De Mattia G: Early up-regulation of
endothelial adhesion molecules in obese hypertensive men.
Hypertension. 34:568–573. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hukshorn CJ, Lindeman JH, Toet KH, Saris
WH, Eilers PH, Westerterp-Plantenga MS and Kooistra T: Leptin and
the proinflammatory state associated with human obesity. J Clin
Endocrinol Metab. 89:1773–1778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kim JE, Kim JS, Jo MJ, Cho E, Ahn SY, Kwon
YJ and Ko GJ: The roles and associated mechanisms of adipokines in
development of metabolic syndrome. Molecules. 27:3342022.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Romacho T, Valencia I, Ramos-González MR,
Vallejo S, López-Esteban M, Lorenzo O, Cannata P, Romero A,
Hipólito-Luengo AS, Gómez-Cerezo JF, et al: Visfatin/eNampt induces
endothelial dysfunction in vivo: A role for toll-like receptor 4
and NLRP3 inflammasome. Sci Rep. 10:53862020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Toussirot E: Mini review: The contribution
of adipokines to joint inflammation in inflammatory rheumatic
diseases. Front Endocrinol (Lausanne). 11:6065602020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Catalán V, Gómez-Ambrosi J, Rodríguez A,
Ramírez B, Silva C, Rotellar F, Cienfuegos JA, Salvador J and
Frühbeck G: Association of increased visfatin/PBEF/NAMPT
circulating concentrations and gene expression levels in peripheral
blood cells with lipid metabolism and fatty liver in human morbid
obesity. Nutr Metab Cardiovasc Dis. 21:245–253. 2011.PubMed/NCBI
|
|
133
|
Chang YH, Chang DM, Lin KC, Shin SJ and
Lee YJ: Visfatin in overweight/obesity, type 2 diabetes mellitus,
insulin resistance, metabolic syndrome and cardiovascular diseases:
A meta-analysis and systemic review. Diabetes Metab Res Rev.
27:515–527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Martos-Moreno GA, Kratzch J, Korner A,
Barrios V, Hawkins F, Kiess W and Argente J: Serum visfatin and
vispin levels in prepubertal childres: Effect of obesity and weitht
loss after beharior modifications on their secretion and
relationship with glucose metabolism. Int J Obes (Lond).
35:1355–1362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Olszanecka-Glinianowicz M, Kocełak P,
Nylec M, Chudek J and Zahorska-Markiewicz B: Circulating visfatin
level and visfatin/insulin ration in obese women with metabolic
syndrome. Arch Med Sci. 8:214–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
de Luis DA, Aller R, Sagrado MG, Conde R,
Izaola O and de la Fuente B: Serum visfatin levels and metabolic
syndrome criteria in obese female subjects. Diabetes Metab Res Rev.
29:576–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Friebe D, Neef M, Kratzch J, Erbs S,
Dittrich K, Garten A, Petzold-Qunque S, Blüher S, Reinehr T,
Stumvoll M, et al: Leucocytes are a major source of circulating
nicotinamide phorsphoribosyltransferase (NAMPT)/pre-B cell colony
(PBEF)/visfatin linking obesity and inflammation in humans.
Diabetologia. 54:1200–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH,
Koo TH, Jang HO, Yun Il, Kim KW, Kwon YG, et al: Visfatin enhances
ICAM-1 and VCAM-1 expression through ROS-dependent NF-κΒ activation
in endothelial cells. Biochim Biophys Acta. 1783:886–895. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Patel SD, Rajala MW, Rossetti L, Scherer
PE and Shapiro L: Disulfide-dependent multimeric assembly of
resistin family hormones. Science. 304:1154–1158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Oki K, Yamane K, Kamei N, Nojima H and
Kohno N: Circulatin visfatin level is correlated with inflammation,
but not with insulin resistance. Clin Endocrinol (Oxf). 67:796–800.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Moschen AR, Kaser A, Enrich B, Mosheimer
B, Theurl M, Niederegger H and Tigl H: Visfatin an adipocytokine
with proinflammatory and immunomodulating properties. J Immunol.
178:1748–1758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Krysiak R, Handzlik-Orlik G and Okopien B:
The role of adipokines in connective tissue diseases. Eur J Nutr.
51:513–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Heo YJ, Choi SE, Jeon JY, Han SJ, Kim DJ,
Kang Y, Lee KW and Kim HJ: Visfatin induces inflammation and
insulin resistance via the NF-κΒ and STAT3 signaling pathways in
hepatocytes. J Diabet Res. 2019:40216232019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Francisco V, Sanz MJ, Real JT, Marques P,
Capuozzo M, Eldjoudi DA and Gualillo O: Adipokines in non-alcoholic
fatty liver disease: Are we on the road toward new biomarkers and
therapeutic targets? Biology (Basel). 11:12372022.PubMed/NCBI
|
|
145
|
Oita RC, Ferdinando D, Wilson S, Bunce C
and Mazzatti DJ: Visfatin induces oxidative stress in
differentiated C2C12 myotubes in an Akt- and MAPK-independent,
NFκΒ-dependent manner. Pflugers Arch. 459:619–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim
J, Lee S, Kim JY, Lee J, Yang HM, et al: Adenylyl
cyclase-associated protein 1 (CAP1) is a receptor for human
resistin and mediated inflammatory actions of human monocytes. Cell
Metab. 19:484–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Li Y, Yang Q, Cai D, Guo H, Fang J, Cui H,
Gou L, Deng J, Wang Z and Zuo Z: Resistin, a novel host defence
peptide of innate immunity. Front Immunol. 12:6998072021.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kawanami D, Maemura K, Takeda N, Harada T,
Nojiri T, Imai Y, Manabe I, Utsunomiya K and Nagai R: Direct
reciprocal effects of resistin and adiponectin on vascular
endothelial cells: A new insight into adipocytokine-endothelial
cell interactions. Biochem Biophys Res Commun. 314:415–419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bokarewa M, Nagaev I, Dahlberg L, Smith U
and Tarkowski A: Resistin, an adipokine with potent proimflammatory
properties. J Immunol. 174:5789–5795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Agaev I, Bokarewa M, Tarkowski A and Smith
U: Human resistin is a systemic immune-derived proinflammatory
cytokine targeting both leukocytes and adipocytes. PLoS One.
1:e312006. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Ebihara T, Matsumoto H, Matsubara T,
Matsuura H, Hirose T, Shimizu K, Ogura H, Kang S, Tanaka T and
Shimazu T: Adipocytokine profile reveals resistin forming a
prognostic-related cytokine network in the acute phase of sepsis.
Shock. 56:718–726. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Heilbronn LK, Rood J, Janderova L, Albu
JB, Kelley DE, Ravussin E and Smith SR: Relationship between serum
resistin concentrations and insulin resistance in nonobese, obese,
and obese diabetic subjects. J Clin Endocrinol Metabol.
89:1844–1848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Steppan CM, Bailey ST, Bhat S, Brown EJ,
Banerjee RR, Wright CM, Patel HR, Ahima RS and Lazar MA: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Vidal-Puig A and O'Rahilly S: Resistin: A
new link between obesity and insulin resistance? Clin Endocrinol
(Oxf). 55:437–438. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
McTernan CL, McTernan PG, Harte AL, Levick
PL, Barnet AH and Kumar S: Resistin, central obesity, and type 2
diabetes. Lancet. 359:46–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Wang H, Chu WS, Hemphill C and Elbein SC:
Hunan resistin gene: Molecular scanning and evaluation of
association with insulin sensitivity and type 2 diabetes in
Caucasians. J Clin Endocrinol Metabol. 87:2520–2524. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Osawa H, Yamada K, Onuma H, Murakami A,
Ochi M, Kawata H, Nishimiya T, Niiya T, Shimizu I, Nishida W, et
al: The G/G genotype of resistin single-nucleotide polymorphism at
−420 increases type 2 diabetes mellitus susceptibility by inducing
promoter activity through specific binding of Sp1/3. Am J Hum
Genet. 75:678–686. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kielstein JT, Becker B, Graf S, Brabant G,
Haller H and Fliser D: Increased resistin blood levels are not
associated with insulin resistance in patients with renal disease.
Am J Kidney Dis. 42:62–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Patel L, Buckels AC, Kinghorn IJ, Mourdock
PR, Holbrook JD, Plumpton C, Macphee CH and Smith SA: Resistin is
expressed in human macrophages and directly regulated by PPAR gamma
activators. Biochem Biophys Res Commun. 300:472–476. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Chen C, Jiang J, Lü JM, Chai H, Wang X,
Lin PH and Yao Q: Resistin descreases expression of endothelial
nitric oxide synthase through oxidative stress in human coronary
artery endothelial cells. Am J Physiol Heart Circ Physiol.
299:H193–H201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Acquarone E, Monacelli F, Borghi R,
Nencioni A and Odetti P: Resistin: A reappraisal. Mech Ageing Dev.
178:46–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Iwaki T, Urano T and Umemura K: PAI-1,
progress in understanding the clinical problem and its aetiology.
Br J Heamatol. 157:291–298. 2012. View Article : Google Scholar
|
|
163
|
Para I, Albu A and Porojan MD: Adipokines
and arterial stiffness in obesity. Medicina (Kaunas). 57:6532021.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Mertens I and Van Gaal LF: Obesity,
haemostasis and the fibrinolytic system. Obes Rev. 3:85–101. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Juhan-Vague I, Alessi MC, Mavri A and
Morange PE: Plasminogen activator inhibitor-1, inflammation,
obesity, insulin resistance and vascular risk. J Thromb Haemost.
1:1575–1579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Tschoner A, Sturm W, Engl J, Kaser S,
Laimer M, Laimer E, Klaus A, Patsch JR and Ebenbichler CF:
Plasminogen activator inhibitor 1 and visceral obesity during
pronounced weight loss after bariatric surgery. Nutr Metab
Cardiovasc Dis. 22:340–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Khoukaz HB, Ji Y, Braet DJ, Vadali M,
Abdelhamid AA, Emal CD, Lawrence DA and Fay WP: Drug targeting of
plasminogen activator inhibitor-1 inhibits metabolic dysfunction
and atherosclerosis in murine model of metabolic syndrome.
Arterioscler Thromb Vasc Biol. 40:1479–1490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Eitzman DT, Westrick RJ, Xu Z, Tyson J and
Grinsburg D: Plasminogen activator inhibitor-1 deficiency protects
against atherosclerosis progression in the mouse carotid artery.
Blood. 96:4212–4215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Alessi MC and Juhan-Vague I: PAI-1 and the
metabolic syndrome: Links, causes and consequences. Arterioscler
Thromb Vasc Biol. 16:2200–2207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ma LJ, Mao SL, Taylor KL, Kanjanabuch T,
Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH,
et al: Prevention of obesity and insulin resistance in mice lacking
plasminogen activator inhibitor 1. Diabetes. 53:336–346. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Gleeson LE, Sheedy FJ, Palsson-McDermott
EM, Triglia D, O'Leary SM, O'Sullivan MP, O'Neill LA and Keane J:
Cytting edge: Mycobacterium tuberculosis induces aerobic glycolysis
in human alveolar macrophages that is required for control of
intracellular bacillary replication. J Immunol. 196:2444–2449.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Shoelson SE, Herrero L and Naaz A:
Obesity, inflammation and insulin resistance. Gastroenterology.
132:2169–2180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Stojsavljević S, Palčić MG, Jukić LV,
Duvnjak LS and Duvnjak M: Adipokines and proinflammatory cytokines,
the key mediators in the pathogenesis of nonalcoholic fatty liver
disease. World J Gastroenterol. 20:18070–18091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Plomgaard P, Bouzakri K, Krogh-Madsen R,
Mittendorfer B, Zierath JR and Pedersen BK: Tumor necrosis
factor-alpha induces skeletal muscle insulin resistance in healthy
human subjects via inhibition of Akt substrate 160 phosphorylation.
Diabetes. 54:2936–2945. 2005. View Article : Google Scholar
|
|
175
|
Ruan H and Lodish HF: Insulin resistance
in adipose tissue: Direct and indirect effects of tumor necrosis
factor-α. Cytokine Growth Factor Rev. 14:447–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Illei GG and Lipsky PE: Novel,
antigen-specific therapeutic approaches to autoimmuneinflammatory
diseses. Curr Opin Immunol. 12:712–718. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Chandel NS, Schumacker PT and Arch RH:
Reactive oxygen species are downstream products of TRAF-mediated
signal trasduction. J Biol Chem. 276:42728–42736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Micheau O and Tschopp J: Induction of TNF
receptor I-mediated apoptosis via two sequential signaling
complexes. Cell. 114:181–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Campbell J, Ciesielski CJ, Hunt AE,
Horwood NJ, Beech JT, Hayes LA, Denys A, Feldmann M, Brennan FM and
Foxwell BMJ: A novel mechanism for TNF-alpha regulation by p38
MAPK: Involvement of NF-kappa B with implications for therapy in
rheumatoid arthritis. J Immunol. 173:6928–6937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Wang B and Trayhurn P: Acute and prolonged
effects of TNF-alpha on the expression and secretion of
inflammation-related adipokines by human adipocytes differentiated
in culture. Pflugers Arch. 452:418–427. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Rider P, Carmi Y, Guttman O, Braiman A,
Cohen I, Voronov E, White MR, Dinarello CA and Apte RN: IL-1α and
IL-1β recruit different myeloid cells and promote different stages
of sterile inflammation. J Immunol. 187:4835–4843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Thornberry NA, Bull HG, Calaycay JR,
Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner
JR and Aunins J: A novel heterodimeric cysteine protease is
required for interleukin-1 beta processing in monocytes. Nature.
356:768–774. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Akdis M, Arab A, Altunbulakli C, Azkur K,
Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R,
et al: Interleukins (from IL-1 to IL-38), interferons, transforming
growth factor β, and TNF-α: Receptors, functions, and roles in
diseases. J Allergy Clin Immunol. 138:984–1010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Ghabari M, Maragheh SM, Aghazadeh A,
Mehrjuyan SR, Hussen BM, Shadbad MA, Dastmalchi N and Safaralizadeh
R: Interleukin-1 in obesity-related low-grade inflammation: From
molecular mechanisms to therapeutic strategies. Int
Immunopharmacol. 96:1077652021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Speaker KJ and Fleshner M: Interleukin-1
beta: A potential link between stress and the development of
visceral obesity. BMC Physiol. 12:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Bruun JM, Pedersen SB, Kristensen K and
Richelsen B: Effects of pro-inflammatory cytokines and chemokines
on leptin production in human adipose tissue in vitro. Mol Cell
Endocrinol. 190:91–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Gonzalez RR and Leavis P: Leptin
upregulates β3-integrin expression and interleukin-1β upregulates
leptin and leptin receptor expression in human endometrial
epithelial cell cultures. Endocrine. 16:21–28. 2021. View Article : Google Scholar
|
|
188
|
Müller G, Ertl J, Gerl M and Preidisch G:
Leptin impairs metabolic actions of insulin in isolated rat
adipocytes. J Biol Chem. 272:10585–10593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Moschen AR, Molnar C, Enrich B, Geiger S,
Ebenbichler CF and Tilg H: Adipose and liver expression of
interleukin (IL)-1 family members in morbid obesity and effects of
weight loss. Mol Med. 17:840–845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Shaul ME, Bennett G, Strissel KJ,
Greenberg AS and Obin MS: Dynamic, M2-like remodeling phenotypes of
CD11c+ adipose tissue macrophages during high-fat diet-induced
obesity in mice. Diabetes. 59:1171–1181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Schoettl T, Fischer IP and Ussar S:
Heterogeneity of adipose tissue in development and metabolic
function. J Exp Biol. 221 (Pt Suppl 1):jeb1629582018. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Buechler C, Krautbauer S and Eisinger K:
Adipose tissue fibrosis. World J Diabetes. 6:548–553. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Shikama Y, Aki N, Hata A, Nishimura M,
Oyadomari S and Funaki M: Palmitate-stimulated monocytes induce
adhesion molecule expression in endothelial cells via IL-1
signaling pathway. J Cell Physiol. 230:732–742. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Miura K, Kodama Y, Inokuchi S, Scnabl B,
Aoyama T, Ohnishi H, Olefsky JM, Brenner DA and Seki E: Toll-like
receptor 9 promotes steatohepatitis by induction of
interleukin-1beta in mice. Gastroenterology. 139:323–334. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Gao D, Madi M, Ding C, Fok M, Steele T,
Ford C, Hunter L and Bing C: Interleukin-1β mediates
macrophage-induced impairemnet of insulin signalin in human primary
adipocytes. Am J Physiol Endocrinol Metab. 307:E289–E304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflammasome activation. Nat Immunol. 11:136–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Calabrese L, Fiocco Z, Satoh TK, Peris K
and French LE: Therapeutic potential of targeting interleukin-1
family cytokines in chronic inflammatory skin diseases. Br J
Dermatol. 186:925–941. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Maedler K, Sergeev P, Ris F, Oberholzer J,
Holler-Jemelka H, Spinas GA, Kaiser N, Halban PA and Donath MY:
Glucose-induced beta cell production of IL-1beta contributes to
glucotoxicity in human pancreatic islets. J Clin Invest.
110:851–860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wang Q, Zhang H, Zhao B and Fei H:
IL-1beta caused pancreatic beta-cells apoptosis is mediated in part
by endoplasmic reticulum stress via the induction of endoplasmic
reticulum Ca2+ release through the c-Jun N-terminal kinase pathway.
Mol Cell Biochem. 324:183–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Brichory FM, Misek DE, Yim AM, Krause MC,
Giordano TJ, Beer DG and Hanash SM: An immune response manifested
by the common occurrence of annexins I and II aytoantibodies and
high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci
USA. 98:9824–9829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
IL6R Genetics Consortium Emerging Risk
Factors and Collaboration, . Sarwar N, Butterworth AS, Freitag DF,
Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, et
al: Interleukin-6 receptor pathways in coronary heart disease: A
collaborative meta-analysis of 82 studies. Lancet. 379:1205–1213.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Elhage R, Clamens S, Besnard S, Mallat Z,
Tedgui A, Arnal J, Maret A and Bayard F: Involvement of
interleukin-6 in atherosclerosis but not in the prevention of fatty
streak formation by 17beta-estradiol in apolipoprotein E-deficient
mice. Atherosclerosis. 156:315–320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Schieffer B, Selle T, Hilfiker A,
Hilfiker-Kleiner D, Grote K, Tietge UJF, Trautwein C, Luchtefeld M,
Schmittkamp C, Heeneman S, et al: Impact of interleukin-6 on plaque
development and morphology in experimental atherosclerosis.
Circulation. 110:3493–3500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Mishra D, Richard JE, Maric I, Porteiro B,
Häring M, Kooijman S, Musovic S, Eerola K, López-Ferreras L, Peris
E, et al: Parabrachial interleukin-6 reduces body weight and food
intake and increases thermogenesis to regulate energy metabolism.
Cell Rep. 26:3011–3026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Rosen ED and Spiegelman BM: What we talk
about when we talk about fat. Cell. 156:20–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Mohamed-Ali V, Goodrick S, Rawesh A, Katz
DR, Miles JM, Yudkin JS, Klein S and Coppack SW: Subcutaneous
adipose tissue related interleukin-6, but not tumor necrosis
factor-alpha, in vivo. J Clin Endocrinol Metab. 82:4196–4200. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Sopasakis VR, Sandqvist M, Gustafson B,
Hammerstedt A, Schmelz M, Yang X, Jansson PA and Smith U: High
local concentrations and effects on differentiation implicate
interleukin-6 as a paracrine regulator. Obes Res. 12:454–460. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Fernandez-Real JM and Ricart W: Insulin
resistance and chronic cardiovascular inflammatory sundrome. Endocr
Rev. 24:278–301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Charles BA, Doumatey A, Huang H, Zhou J,
Chen G, Shriner D, Adeyemo A and Rotimi CN: The roles of IL-6,
IL-10 and IL-1RA in obesity and insulin resistance in
African-Americans. J Clin Endocrinol Metab. 96:E2018–E2022. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Tsigos C, Papanicolaou DA, Kyrou I,
Defensor R, Mitsiadis CS and Chrousos GP: Dose-dependent effects of
recombinant human interleukin-6 on glucose regulation. J Clin
Endocrinol Metab. 82:4167–4170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Pradhan AD, Manson JE, Rifai N, Buring JE
and Ridker PM: C-reactive protein, interleukin 6, and risk of
developing type 2 diabetes mellitus. JAMA. 286:327–334. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Bastard JP, Maachi M, van Nhieu JT, Jardel
C, Bruckert E, Grimaldi A, Robert JJ, Capeau J and Hainque B:
Adipose tissue IL-6 content correlates with resistance to insulin
activation of glucose uptake both in vivo and in vitro. J Clin
Endocrinol Metab. 87:2084–2089. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Kopp HP, Kopp CW, Festa A, Krzyzanowaska
K, Kriwanek S, Minar E, Roka R and Schernthaner G: Impact of weight
loss on inflammatory proteins and their association with the
insulin resistance syndrome in morbidly obese patients.
Arterioscler Throm Vasc Biol. 23:1042–1047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Xu E, Pereira MMA, Karakasilioti I,
Theurich S, Al-Maarri M, Rappl G, Waisman A, Wenderlich FT and
Brüning JC: Temporal and tissue-specific requirements for
T-lympocyte IL-6 signaling in obesity-associated inflammation and
insulin resistance. Nat Commun. 8:148032017. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Wondmkum YT: Obesity, insulin resistance,
and type 2 diabetes: Associations and therapeutic implications.
Diabetes Metab Syndr Obes. 13:3611–3616. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Fu Z, Gilbert ER and Liu D: Regulation of
insulin synthesis and secretion and pancreatic Beta-cell
dysfunction in diabetes. Curr Diabetes Rev. 9:25–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Röder PV, Wu B, Liu Y and Han W:
Pancreatic regulation of glucose homeostasis. Exp Mol Med.
48:e2192016. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Fazakerley DJ, Krycer JR, Kearney AL,
Hocking SL and James DE: Muscle and adipose tissue insulin
resistance: Malady without mechanism? J Lipid Res. 60:1720–1732.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Newsholme P and Krause M: Nutritional
regulation of insulin secretion: Implications for diabetes. Clin
Biochem Rev. 33:35–47. 2012.PubMed/NCBI
|
|
222
|
Dashty M: A quick look at biochemistry:
Carbohydrate metabolism. Clin Biochem. 46:1339–1352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Samuel VT and Shulman GI: The pathogenesis
of insulin resistance: Intergrating signaling pathways and
substrate flux. J Clin Invest. 126:12–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signaling pathways: Insights into insulin action.
Nat Rev Mol Cell Biol. 7:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Merry TL, Hedges CP, Masson SW, Laube B,
Pöhlmann D, Wueest S, Walsh ME, Arnold M, Langhans W, Konrad D, et
al: Partial impairment of insulin receptor expression mimics
fasting to prevent diet-induced fatty liver diseasee. Nat Commun.
11:20802020. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Shanik MH, Xu Y, Skrha J, Dankner R, Zick
Y and Roth J: Insulin resistance and hyperinsulinemia: Is
hyperinsulinemia the cart or the horse? Diabetes Car. 31 (Suppl
2):S262–S268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Braccini L, Ciraolo E, Campa CC, Perino A,
Longo DL, Tibolla G, Pregnolato M, Cao Y, Tassone B, Damilano F, et
al: PI3K-C2γ is a Rab5 effector selectively controlling endosomal
Akt2 activation downstream of insulin signalling. Nat Commun.
6:74002015. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Huang X, Liu G, Guo J and Su Z: The
PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci.
14:1483–1496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Gray SL, Donald C, Jetha A, Covey SD and
Kieffer TJ: Hyperinsulinemia precedes insulin resistance in mice
lacking pancreatic beta-cell leptin signaling. Endocrinology.
151:4178–4186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Smith BW and Adams LA: Nonalcoholic faty
liver disease and diabetes mellitus: Pathogenesis and treatment.
Nat Rev Endocrinol. 7:456–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Willians AJK and Long AE: Following the
fate of the failing β-cell: New insights from first-phase insulin
responses. Diabetes. 62:3990–3992. 2013. View Article : Google Scholar
|
|
233
|
Gariani K, Philippe J and Jornayvaz FR:
Non-alcoholic fatty liver disease and insulin resistance: From
bench to bedside. Diabetes Metab. 39:16–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Zhao J, Wu Y, Rong X, Zheng C and Guo J:
Anti-lipolysis induced by insulin in diverse pathophysiologic
conditions of adipose tissue. Diabetes Metab Syndr Obes.
13:1575–1585. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Griffin ME, Marcucci MJ, Cline GW, Bell K,
Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF and Shulman GI:
Free fatty acid-induced insulin resistance is associated with
activation of protein kinase C theta and alterations in the insulin
signaling cascade. Diabetes. 48:1270–1274. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Boden G and Shulman GI: Free fatty acids
in obesity and type 2 diabetes: Defining their role in the
development of insulin resistance and beta-cell dysfunction. Eur J
Clin Invest. 32:14–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Unger RH and Zhou YT: Lipotoxicity of
beta-cells in obesity and in other causes of fatty acid spillover.
Diabetes. 50 (Suppl 1):S118–S121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Bugianesi E, Gastaldelli A, Vanni E,
Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferranini E and
Rizzetto M: Insulin resistance in non-diabetic patients with
non-alcoholic fatty liver disease: Sites and mechanisms.
Diabeltologia. 48:634–642. 2005. View Article : Google Scholar
|
|
239
|
Perry RJ, Samuel VT, Petersen KF and
Shulman GI: The role of hepatic insulin resistance and type 2
diabetes. Nature. 510:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Brown MS and Goldstein JL: Selective
versus total insulin resistance: A pathogenic paradox. Cell Metab.
7:95–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Kim GT, Kim SJ, Park SH, Lee D and Park
TS: Hepatic expression of the serine palmitoyltansferase subunit
Sptlc2 reduces lipid droplets in the liver by activating VLDL
secretion. J Lipid Atheroscler. 9:291–303. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Hall JE, da Silva AA, do Carmo JM,
Dubinion J, Hamza S, Munusamy S, Smith G and Stec DE:
Obesity-induced hypertension: Role of sympathetic nervous system,
leptin, and melanocortins. J Biol Chem. 285:17271–17276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Weickert MO and Pfeiffer AFH: Signalling
mechanisms linking hepatic glucose and lipid metabolism.
Diabetologia. 4:1732–1741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Murakami T, Michelagnoli S, Longhi R,
Gianfranceschi G, Pazzucconi F, Calabresi L, Sirtori CR and
Franceschini G: Triglycerides are major determinants of cholesterol
esterification/transfer and HDL remodeling in human plasma.
Arterioscler Thromb Vasc Biol. 15:1819–1828. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Eisenberg S, Gavish D, Oschry Y, Fainaru M
and Deckelbaum RJ: Abnormalities in very low, low and high density
lipoproteins in hypertriglyceridemia. Reversal toward normal with
bezafibrate treatment. J Clin Invest. 74:470–482. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Tripathy D, Mohanty P, Dhindsa S, Syed T,
Ghanim H, Aljada A and Dandona P: Elevation of free fatty acids
induces inflammation and impairs vascular reactivity in healthy
subjects. Diabetes. 52:2882–2887. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Esler M, Rumantir M, Wiesner G, Kaye D,
Hastings J and Lambert G: Sympathetic nervous system and insulin
resistance from obesity to diabetes. Am J Hypertens. 14((11 Pt 2)):
304S–309S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Samad F and Ruf W: Inflammation, obesity
and thrombosis. Blood. 122:3415–3422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Ernst E and Resch KL: Fibrinogen as a
cardiovascular risk factor: A meta-analysis and review of the
literature. Ann Intern Med. 118:956–963. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Kannel WB, Wolf PA, Castelli WP and
D'Agostino RB: Fibrionogen and risk of cardiovascular disease. The
Framingham study. JAMA. 258:1183–1186. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Nieuwdorp M, Stroes ES, Meijers JC and
Buller H: Hypercoagulability in the metabolic syndrome. Curr Opin
Pharmacol. 5:155–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Raynaud E, Perez-Martin A, Brun JF,
Aïssa-Benhaddad A, Fédou C and Mercier J: Atherosclerosis.
150:365–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Tabrez S, Jabir NR, Shakil S and Alama MN:
Association of plasma fibrinogen level with insulin resistance in
angiographically confirmed coronary artery disease patients. Crit
Rev Eukaryot Gene Expr. 29:277–285. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Bryk-Wiązania AH and Undas A:
Hypofribrinolysis in type 2 diabetes and its clinical implications:
From mechanisms to pharmacological modulation. Cardiovasc Diabetol.
20:1912021. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Davalos D and Akassoglou K: Fibrinogen as
a key regulator of inflammation in disease. Semin Immunopathol.
34:43–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Nawaz SS and Siddiqui K: Plasminogen
activator inhibitor-1 mediated downregulation of adiponectin in
type 2 diabetic patients with metabolic syndrome. Cytokine X.
4:1000642002. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Chen R, Yan J, Liu P, Wang Z and Wang C:
Plasminogen activator inhibitor links obesity and thrombotic
cerebrovascular diseases: The roles of PAI-1 and obesity on stroke.
Metab Brain Dis. 32:667–673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Matsuzawa Y: The metabolic syndrome and
adipocytokines. FEBS Lett. 580:2917–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Mertens I, Ballaux D, Funahashi T,
Matsuzawa Y, Van der Planken M, Verrijken A, Ruge JB and Gaal LFV:
Inverse relationship between plasminogen activator inhibitor-I
activity and adiponectin in overweight and obese women.
Interrelationship with visceral adipose tissue, insulin resistance,
HDL-chol and inflammation. Thromb Haemost. 94:1190–1195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Shimomura I, Funahashi T, Takahashi M,
Maeda K, Kotani K, Nakamura T, Yamashita S, Miura N, Fukuda Y,
Takemura K, et al: Enhanced expression of PAI-1 in visceral fat:
Possible contributor to vascular disease in obesity. Nat Med.
2:800–803. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Kaji H: Adipose tissue-derived plasminogen
activator inhibitor-1 function and regulation. Compr Physiol.
6:1873–1896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Reaven GM: Banting lecture 1988. Role of
insulin resistance in human disease. Diabetes. 37:1595–1607. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Alberti KG, Zimmet P and Shaw J; IDF
Edipemiology Task Force Consensus Group, : The metabolic syndrome-a
new world-wide definition. Lancet. 366:1059–1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Zafar U, Khaliq S, Ahmad HU, Manzoor S and
Lone KP: Metabolic syndrome: An update on diagnostic criteria,
pathogenesis, and genetic links. Hormones (Athens). 17:299–313.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Mottilo S, Filion KB, Genest J, Joseph L,
Pilote L, Poirier P, Rinfret S, Schiffrin EL and Eisenberg MJ: The
metabolic syndrome and cardiovascular risk a systematic review and
meta-analysis. J Am Coll Cardiol. 56:1113–1132. 2010. View Article : Google Scholar
|
|
267
|
Weiss R, Bremer AA and Lusting RH: What is
metabolic syndrome, and why are children getting it? Ann N Y Acad
Sci. 1281:123–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Grundy SM: Metabolic syndrome pandemic.
Arteroscler Thromb Vasc Biol. 28:629–636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Ford ES, Li C and Zhao G: Prevalence and
correlates of metabolic syndrome based on a harmonious definition
among adults in the US. J Diabetes. 2:180–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Kahn R, Buse J, Ferrannini E and Stern M:
The metabolic syndrome: Time for a critical appraisal. Joint
statement from the Americal diabetes association and the European
association for the study of diabetes. Diabetologia. 48:1684–1699.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Pi-Sunyer X: The metabolic syndrome: How
to approach differing definitions. Med Clin North Am. 91:1025–1040.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Chung G, Jung HS and Kim HJ:
Sociodemographic and health characteristics associated with
metabolic syndrome in men and women aged ≥50 Years. Metab Sundr
Relat Disord. 19:159–166. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Hydrie MZ, Shera AS, Fawwad A, Basit A and
Hussain A: Prevalence of metabolic syndrome in urban Pakistan
(Karachi): Comparison of newly proposed international diabetes
federation and modified adult treatment panel III criteria. Metab
Syndr Relat Disord. 7:119–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Rosenbaum M, Sy M, Pavlovich K, Leibel RL
and Hirsch J: Leptin reverses weight loss-induced changes in
regional neural activity responses to visual food stimuli. J Clin
Invest. 118:2583–2591. 2008.PubMed/NCBI
|
|
275
|
Imai SI: Nicotinamide
phosphoribosyltrasferase (Nampt): A link between NED biology,
metabolism, and disease. Curr Pharm Des. 15:20–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Lago F, Dieguez C, Gomez-Reino G and
Gulillo O: Adipokines as emerging mediators of immune response and
inflammation. Nat Clin Pract Rheumatol. 3:716–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Hopps E, Noto D, Caimi G and Averna MR: A
novel comoponent of the metabolic syndrome: The oxidative stress.
Nutr Metab Cardiovasc Dis. 20:72–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Vona R, Gambardella L, Cittadini C,
Straface E and Pietraforte D: Biomarkers of oxidative stress in
metabolic syndrome and associated dieseases. Oxid Med Cell Longev.
2019:82672342019. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Juan CA, de la Lastra JM, Plou FJ and
Pérez-Lebeña EP: The chemistry of reactive oxygen species (ROS)
revisited: Outlining their role in biological macromolecules (DNA,
lipids and proteins) and induced pathologies. Int J Mol Sci.
22:46422021. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Grattagliano I, Palmieri VO, Portincasa P,
Moschetta A and Palasciano G: Oxidative stress-induced risk factors
associated with the metabolic syndrome: A unifying hypothesis. J
Nutr Biochem. 19:491–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Fernández-Sánchez A, Madrigal-Santillán E,
Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino
C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C and
Morales-González JA: Inflammation, oxidative stress, and obesity.
Int J Mol Sci. 12:3117–3132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Sena CM, Leadro A, Azul L, Seiça R and
Perry G: Vascular oxidative stress: Impact and therapeutic
approaches. Front Physiol. 9:16682018. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Smirne C, Croce E, Di Benedetoo D,
Cantaluppi V, Comi C, Sainaghi PP, Minisini R, Grossini E and
Pirisi M: Oxidative stress in non-alchoholic fatty liver disease.
Livers. 2:30–76. 2022. View Article : Google Scholar
|
|
285
|
Field AE, Coakley EH, Must A, Spadano JL,
Laird N, Dietz WH, Rimm E and Golditz GA: Imact of overweight on
the risk of developing common chronic diseases during a 10-year
period. Arch Intern Med. 161:1581–1586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Rother KI: Diabetes treatment-Bridging the
devide. N Engl J Med. 356:1499–1501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Norhammar A and Schenck-Gustafsson K: Type
2 diabetes and cardiovascular disease in women. Diabetologia.
56:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Chan JM, Rimm EB, Colditz GA, Stampfer MJ
and Willett WC: Obesity, fat distribution, and weight gain as risk
factors for clinical diabetes in men. Diabetes Care. 17:961–969.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Colditz GA, Willett WC, Rotnitzky A and
Manson JE: Weight gain as a risk factor for clinical diabetes
mellitus in women. Ann Intern Med. 122:481–486. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Wannamethee SG and Shaper AG: Weight
change and duration of overweight and obesity in the incidence of
type 2 diabetes. Diabetes Care. 22:1266–1272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Schienkiewitz A, Schulz MB, Hoffmann K,
Kroke A and Boeing H: Body mass index history and risk of type 2
diabetes: Results from the European Prospective Investigation into
cancer nutrition (EPIC)-Potsdam study. Am J Clin Nutr. 84:427–433.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
DeFronzo RA: Pathogenesis of type 2
diabetes mellitus. Med Clin North Am. 88:787–835. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Ferrannini E, Gastaldelli A, Miyazaki Y,
Matsuda M, Mari A and DeFronzo RA: Beta-cell function in subjects
spanning the range from normal glucose tolerance to overt diabetes:
A new analysis. J Clin Endocrinol Metab. 90:493–500. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Panenin F, Castantino S and Cosentino F:
Insulin resistance, diabetes, and cardiovascular risk. Curr
Atheroscler Rep. 16:4192014. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Abdul-Ghami MA and DeFronzo RA:
Phathophysiology of prediabetes. Curr Diab Rep. 9:193–199. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Reaven GM: Insulin resistance: The link
between obesity and cardiovascular disease. Med Clin North Am.
95:875–892. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
DeFronzo RA, Ferrannini E and Simonson DC:
Fasting hyperglycemia in non-insulin-dependent diabetes mellitus:
contributions of excessive hepatic glucose production and impaired
tissue glucose uptake. Metabolism. 38:387–395. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
298
|
Shulman GI, Rothman DL, Jue T, Stein P,
DeFronzo RA and Shulman RG: Quatitation of muscle glycogen
synthesis in normal subjects and subjects with
non-insulin-dependent diabetes by 13C nuclear magnetic resonance
spectroscopy. N Engl J Med. 322:223–228. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
McGarry JD: Banting lecture 2001:
Dysregulation of fatty acid metabolism in the etiology of type 2
diabetes. Diabetes. 51:7–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Kashyap S, Belfort R, Gastaldelli A,
Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Madarino L,
DeFronzo R and Cusi K: A substained increase in plasma free fatty
acids impairs insulin secretion in nondiabetic sujects genetically
predisposed to develop type 2 diabetes. Diabetes. 52:2461–2474.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Lei XG and Vatamaniuk MZ: Two tales of
antioxidant enzymes on β cells and diabetes. Antioxid Redox Signal.
14:489–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Krebs M, Krssaak M, Bernroider E,
Anderwald C, Brehm A, Meyerspeer M, Nowotny P, Roth E, Waldhäusl W
and Roden M: Mechanism of amino acid-induced skeletal muscle
insulin resistance in humans. Diabetes. 51:599–605. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Pi-Sunyer FX: The epidemiology of central
fat distribution in relation to disease. Nutr Rev. 62((7 Pt2)):
S120–S126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Despres JP: Intra-abdominal obesity: An
untreated risk factor for type 2 diabetes and cardiovascular
disease. J Endocrinol Invest. 29 (3 Suppl):S77–S82. 2006.
|
|
305
|
Klein S, Allison DB, Heymsfield SB, Kelley
DE, Leibel RL, Nomas C and Kahn R; Association for Weight
Management and Obesity Prevention; NASSO, the Obesity Society;
American Society for Nutrition, : American Diabetes Association:
Waist circumference and cardiometabolic risk: A consensus statement
from shaping America's health: Association for weight management
and obesity pevention; NAASO, the obesity society; the American
society for nutrition; and the American diabetes association.
Diabetes Care. 30:1647–1652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Ashwell M, Gumn P and Gibson S:
Waist-to-height is a better screening tool than waist cincumference
and BMI for adult cardiometabolic risk factors: Systemic review and
meta-analysis. Obes Rev. 13:275–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Kouli GM, Panagiotakos DB, Kyrou I,
Georgousopoulou EN, Chryoshoou C, Tsigos C, Tousoulis D and
Pitsavos C: Visceral adiposity index and 10-year cardiovascular
disease incidence. The ATTICA study. Nutr Metab Cardiovasc Dis.
27:881–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Weiss R: Fat distribution and storage: How
much, where, and how? Eur J Endocrinol. 157 (Suppl 1):S39–S45.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Montague CT and O'Rahilly S: The perils of
portliness: Causes and consequences of viscelar adiposity.
Diabetes. 49:883–888. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
310
|
Yang X and Smith U: Adipose tissue
distribution and risk of metabolic disease: Does
thiazolidinedione-induced adipose tissue redistribution provide a
clue to the anwer? Diagetologia. 50:1127–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Peraldi P and Spiegelman B: TNF-α and
insulin resistance: Summary and future prospects. Mol Cell Biochem.
182:169–175. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Pickup JC: Inflammation and activated
innate immunity in the pathogenesis of type 2 diabetes. Diabetes
Care. 27:813–823. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Han CY: Roles of reactive oxygen species
on insulin resistance in adipose tissue. Diabetes Metab J.
40:272–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
314
|
Yan LJ: Redox imbalance stress in diabetes
mellitus: Role of the polyol pathway. Animal Model Exp Med. 1:7–13.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
315
|
Dutta BJ, Singh S, Seksaria S, Gupta GD
and Singh A: Inside the diabetic brain: Insulin resistance and
molecular mechanism associated with cognitive impairment and its
possible therapeutic strategies. Pharmacol Res. 182:1063582022.
View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Li H, Ren J, Li Y, Wu Q and Wei J:
Oxidative stress: The nexus of obesity and cognitive dysfunction in
diabetes. Front Endocrinol (Lausanne). 14:11340252023. View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Emanuela F, Grazia M, Marco DR, Paola LM,
Giorgio F and Marco B: Inflammation as a link between obesity and
metabolic syndrome. J Nutr Metab. 2012:4763802012. View Article : Google Scholar : PubMed/NCBI
|
|
318
|
Böni-Schnetzler M and Meier DT: Islet
inflammation in type 2 diabetes. Semin Immunopathol. 41:501–513.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
319
|
Larsen CN, Faulenbach A, Vaag Α, Vølund A,
Ehses JA, Seifert B, Mandrup-Poulsen T and Donath MY:
Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N
Engl J Med. 356:1517–1526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
320
|
Larsen CM, Faulenbach M, Vaag A, Ehses JA,
Donath MY and Mandrup-Poulsen T: Sustained effects of interleukin-1
receptor antagonist treatment in type 2 diabetes. Diabetes Care.
32:1663–1668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
321
|
Infante M, Padilla N, Alejandro R, Caprio
M, Della-Morte D, Fabbri A and Ricordi C: Diabetes-modifying
antirheumatic drugs: The roles of DMARDs as glucose-lowering
agents. Medicina (Kaunas). 58:5712022. View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Powers NE, Swartzwelter B, Marchetti C, de
Graaf DM, Lerchner A, Schlapschy M, Datar R, Binder U, Edwards CK
III, Skerra A and Dinarell CA: PASylation of IL-1 receptor
antagonist (IL-1Ra) retains IL-1 blockade and extends its duration
in mouse urate crystal-induced peritonitis. J Biol Chem.
295:868–882. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
323
|
Tegtmeyer K, Atassi G, Zhao J, Maloney NJ
and Lio PA: Off-Label studies on anakinra in dermatology: A review.
J Dermatolog Treat. 33:73–86. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
324
|
van Asseldonk EJ, Stienstra R, Koenen TB,
Joosten LA, Netea MG and Tack CJ: Treatment with Anakinra improves
disposition index but not insulin sensitivity in nondiabetic
subjects with the metabolic syndrome: A randomized, double-blind,
placebo-controlled study. J Clin Endocrinol Metab. 96:2119–2126.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
325
|
van Poppel PCM, van Asseldonk EJP, Holst
JJ, Vilsbøll T, Netea MG and Tack CJ: The interleukin-1 receptor
antagonist anakinra improves first-phase insulin secretion and
insulinogenic index in subjects with impaired glucose tolerance.
Diabetes Obes Metab. 16:1269–1273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
326
|
Cucak H, Hansen G, Vrang N, Skarsfeldt T,
Steiness E and Jelsing J: The IL-1β receptor antagonist SER140
postpones the onset of diabetes in female nonobese diabetic mice. J
Diabetes Res. 2016:74846012016. View Article : Google Scholar : PubMed/NCBI
|
|
327
|
Cavelti-Weder C, Babians-Brunner A, Keller
C, Stahel MA, Kurz-Levin M, Zayed H, Solinger AM, Mandrup-Poulsen
T, Dinarello CA and Donath MY: Effects of gevokizumab on glycemia
and inflammatory markers in type 2 diabetes. Diabetes Care.
35:1654–1662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
328
|
Rissanen A, Howard CP, Botha J and Thuren
T; Global Investigators, : Effects of anti-IL-1β antibody
(canakinumab) on insulin secretion rates in impaired glucose
tolerance or type 2 diabetes: Results of a randomized
placebo-controlled trial. Diabetes Obes Metab. 14:1088–1096. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
329
|
Hensen J, Howard CP, Walter V and Thuren
T: Impact of interleukin-1β antibody (canakinumab) on glycemic
indicators in patients with type 2 diabetes mellitus: Results of
secondary endpoints from a randomized, placebo-controlled trial.
Diabetes Metab. 39:524–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
330
|
Ridker PM, Howard CP, Walter V, Everett B,
Libby P, Hensen J and Thuren T; on the behalf of CANTOS Pilot
Investigative Group, : Effects of Interleukin-1β Inhibition with
Canakinumab on Hemoglobin A1c, Lipids, C-Reactive Protein,
Interleukin-6, and Fibrinogen: A Phase IIb Randomized,
Placebo-Controlled Trial. Circulation. 126:2739–2748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
331
|
Choudhury RP, Birks JS, Manii V, Biasiolli
L, Robson MD, L'Allier PL, Gingras MA, Alie N, McLaughlin MA,
Basson CT, et al: Artherial effects of canakinumab in patients with
atherosclerosis and type 2 diabetes or glucose intolerance. J Am
Coll Cardiol. 68:1769–1780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
332
|
Noe A, Howard C, Thuren T, Taylor A and
Skerjanec A: Pharmacokinetic and pharmacodynamics characteristics
of single-dose canakinumab in patients with type 2 diabetes
mellitus. Clin Ther. 36:1625–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
333
|
Sloan-Lancaster J, Abu-Raddad E, Polzer J,
Miller JW, Schere JC, De Gaetano A, Berg JK and Landschulz WH:
Double blind, randomized study evaluating the glycemic and
anti-inflammatory effects of subcutaneous LY2189102, a neutralizing
IL-1 antibody, in patients with type 2 diabetes. Diabetes Care.
36:2239–2246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
334
|
Everett BM, Donath MY, Pradhan AD, Thuren
T, Pais P, Nicolau JC, Glynn RJ, Libby P and Ridker PM:
Anti-inflimmatory therapy with canakinumad for the prevention and
management of diabetes. J Am Coll Cardiol. 71:2392–2401. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
335
|
Olson NC, Callas PW, Hanley AJG, Festa A,
Haffner SM, Wagenknecht LE and Tracy RP: Circulating levels of
TNF-α are associated with impaired glucose tolerance, increased
insulin resistance, and ethnicity: The insulin resistance
atherosclerosis study. J Clin Endocrinol Metab. 97:1032–1040. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
336
|
Wascher TC, Lindeman JHN, Sourij H,
Kooistra T, Pacini G and Roden M: Chronic TNF-α neutralization does
not improve insulin resistance or endothelial function in ‘healthy’
men with metabolic syndrome. Mol Med. 17:189–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
337
|
van den Oever IAM, Baniaamam M, Simsek S,
Raterman HG, van Denderen JC, van Eijk IC, Peters MJL, van der
Horst-Bruinsma IE, Smulders YM and Nurmohamed MT: The effect of
anti-TNF treatment on body composition and insulin resistance in
patients with rheumatoid arthritis. Rheumatol Int. 41:319–328.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
338
|
Kiortsis DN, Mavridis AK, Vasakos S, Nikas
SN and Drosos AA: Effects of infliximab treatment on insulin
resistance in patients with rheumatoid arthritis and ankylosing
spondylitis. Ann Rheum Dis. 64:765–766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
339
|
Gonzalez-Gay MA, De Matias JM,
Gonzalez-Juanatey C, Garcia-Porrua G, Sanchez-Andrade A, Martin J
and Llorca J: Anti-tumor necrosis factor-alpha blockade improves
insulin resistance in patients with rheumatoid arthritis. Clin Exp
Rheumatol. 24:83–86. 2006.PubMed/NCBI
|
|
340
|
Haida KS, Bertachini G, Tavoni T,
Guilhermetti M, Loures MR and Bazotte RB: Infliximab treatment
prevents hyperglycemia and the intensification of hepatic
gluconeogenesis in an animal model of high fat diet-induced liver
glucose overproduction. Braz Arch Biol Technol. 55:389–393. 2012.
View Article : Google Scholar
|
|
341
|
Méndez-García LA, Trejo-Millán F,
Martínez-Reyes CP, Majarrez-Reyna AN, Esquivel-Velázquez M,
Melendez-Mier G, Islas-Andrade S, Rojas-Bernbé A, Kzhyshkowska J
and Escobedo G: Infliximab ameriorates tumor necrosis
factor-alpha-induced insulin resistance by attenuating PTP1B
activation in 3T3L1 adipocytes in vitro. Scan J Immunol.
88:e127162018. View Article : Google Scholar : PubMed/NCBI
|
|
342
|
Abdelhamid YA, Elyamany MF, Al-Shorbagy MY
and Badary OA: Effects of TNF-α antagonist infliximad on
fructose-induced metabolic syndrome in rats. Hum Exp Toxicol.
40:801–811. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
343
|
Bernstein LE, Berry J, Kim S, Canavan B
and Grinspoon SK: Effects of etanercept in patients with the
metabolic syndrome. Arch Intern Med. 166:902–908. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
344
|
Lo J, Bernstein LE, Canavan B, Torriani M,
Jackson MB, Ahima RS and Grinspoon SK: Effects of TNF-alpha
neutralization on adipocytokines and skeletal muscle adiposity in
the metabolic syndrome. Am J Physiol Endocrinol Metabol.
293:E102–E109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
345
|
Bravo C, Cataldo LR, Galgani J, Parada J
and Santos JL: Leptin/Adiponectin ratios using either total or high
molecular weight adiponectin as biomarkers of systemic insulin
sensitivity in normoglycemic women. Diabetes Res.
2017:90310792017.PubMed/NCBI
|
|
346
|
Stanley TL, Zanni MV, Johnsen S, Rasheed
S, Makimura H, Lee H, Khor VK, Ahima RS and Grinspoon SK: TNF-α
antagonism with etanercept decreases glucose and increases the
proportion of high molecular weight adiponectin in obese subjects
with features of the metabolic syndrome. J Clin Endocrinol Metab.
96:E146–E150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
347
|
Paquot N, Castillo MJ, Lefèbvre PJ and
Scheen AJ: No increased insulin sensitivity after a single
intravenous administration of a recombinant human tumor necrosis
factor receptor: Fc fusion protein in obese insulin-resistant
patients. J Clin Endocrinol Metab. 85:1316–1319. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
348
|
Dominguez H, Storgaard H, Rask-Madsen C,
Hermann TS, Ihlemann N, Nielsen DB, Spohr C, Kober L, Vaag A and
Torp-Pedersen C: Metabolic and vascular effects of tumor necrosis
factor-α blockade with etanercept in obese patients with type 2
diabetes. J Vasc Res. 42:517–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
349
|
Ronti T, Lupattelli G and Mannarino E: The
endocrine function of adipose tissue: An update. Clin Endocrinol
(Oxf). 64:355–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
350
|
Hu D, Russell RD, Remash D, Greenaway T,
Rattigan S, Squibb KA, Jones G, Ross RM, Roberts CK, Premilovac D,
et al: Are the metabolic benefits of resistance in type 2 diabetes
linked to improvement in adipose tissue microvascular blood flow?
Am J Physiol Endocrinol Metab. 315:E1242–E1250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
351
|
Ruscitti P, Berardicurti O, Cipriani P and
Giacomelli R; TRACK Study Group, : Benefits of anakinra versus TNF
inhibitors in rheumatoid arthritis and type 2 diabetes: Long-term
findings from participants furtherly followed-up in the TRACK
study, a multicentre, open-label, randomized, controlled trial.
Clin Exp Rheumatol. 39:403–406. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
352
|
Ramos-Zavala MG, Gonzalez-Ortiz M,
Martinez-Abundis E, Robles-Cervantes JA, Gonzalez-Lopez R and
Santiago-Hernandez NJ: Effect of diacerein on insulin secretion and
metabolic control in drug-naïve patients with type 2 diabetes.
Diabetes Care. 34:1591–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
353
|
Cardoso CRL, Leite NC, Carlos FO, Loureiro
AA, Viegas BB and Salles GF: Efficacy and safety of diacerein in
patients with inadequately controlled type 2 diabetes: A randomized
controlled trial. Diabetes Care. 40:1356–1363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
354
|
Tres GS, Fuchs SC, Piovesan F,
Koehler-Santos P, Pereira FD, Camey S, Lisboa HK and Moreira LB:
Effect of diacerein on metabolic control and inflammatory markers
in patients with type 2 diabetes using antidiabetic agents: A
randomized controlled trial. J Diabetes Res. 2018:42465212018.
View Article : Google Scholar : PubMed/NCBI
|
|
355
|
Jangsiripornpakorn J, Srisuk S, Chailurkit
L, Nimitphong H, Saetung S and Ongphiphadhanakul B: The
glucose-lowering effect of low-dose diacerein and its
responsiveness metabolic markers in uncontrolled diabetes. BMC Res
Notes. 15:912022. View Article : Google Scholar : PubMed/NCBI
|
|
356
|
Piovesan F, Tres GS, Moreira LB, Andrades
ME, Lisboa HK and Fucks SC: Effects of diacerein on renal function
and inflammatory cytokines in participants with type 2 diabetes
mellitus and chronic kidney disease: A randomized controlled trial.
PLoS One. 12:e01865542017. View Article : Google Scholar : PubMed/NCBI
|
|
357
|
Di Prospero NA, Artis E, Andrade-Gordon P,
Johnson DL, Vaccaro N, Xi L and Rothenberg P: CCR2 antagonism in
patients with type 2 diabetes mellitus: A randomized,
placebo-controlled study. Diabetes Obes Metab. 16:1055–1064. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
358
|
Mulder P, van den Hoek AM and Kleemann R:
The CCR2 inhibitor propagermanium attenuates diet-induced insulin
resistance, adipose tissue inflammation and non-alcoholic
steatohepatitis. PLoS One. 12:e01697402017. View Article : Google Scholar : PubMed/NCBI
|
|
359
|
Huh JH, Kim HM, Lee ES, Kwon MH, Lee BR,
Ko HJ and Chung CH: Dual CCR2/5 antagonist attenuates
obesity-induced insulin resistance by regulating macrophage
recruitment and M1/M2 status. Obesity (Silver Spring). 26:378–386.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
360
|
Tuttle KR, Brosius FC III, Adler SG,
Kretzler M, Mehta RL, Tumlin JA, Tanaka Y, Haneda M, Liu J, Silk
ME, et al: JAK1/JAK2 inhibition by baricitinib in diabetic kidney
disease: Results from a phase 2 randomized controlled clinical
trial. Nephrol Dial Transplant. 33:1950–1959. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
361
|
Faghihimani E, Amnorroaya A, Rezvanian H,
Adibi P, Ismail-Beigi F and Amini M: Salsalate improves glycemic
control in patients with newly diagnosed type 2 diabetes. Acta
Diabetol. 50:537–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
362
|
Goldfine AB, Fonseca V, Jablonski KA, Chen
YD, Tipton L, Staten MA and Steven E; Targeting Inflammation Using
Salsalate in Type 2 Diabetes Study Team, : Salicylate (Salsalate)
in patients with type 2 diabetes: A randomized trial. Ann Intern
Med. 159:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
363
|
Li D, Zhong J, Zhang Q and Zhang J:
Effects of anti-inflammatory therapies on glycemic control in type
2 diabetes mellitus. Front Immunol. 14:11251162023. View Article : Google Scholar : PubMed/NCBI
|
|
364
|
Raimondo MG, Biggioggero M, Crotti C,
Becciolini A and Favalli EG: Profile of sarilumab and its potential
in the treatment of rheumatoid arthritis. Drug Design Dev Ther.
11:1593–1603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
365
|
Klinder A, Waletzko-Hellwig J, Sellin ML,
Seyfarth-Sehlke A, Wolfien M, Prehn F, Bader R and Jonitz-Heincke
A: Effects of the interleukin-6 receptor blocker sarilumab on
metabolic activity and differentiation capacity of primary human
osteoblasts. Pharmaceutics. 14:13902022. View Article : Google Scholar : PubMed/NCBI
|
|
366
|
Genovese MC, Burmester GR, Hagino O,
Thangavelu K, Iglesias-Rodriguez M, John GT, González-Gay MA,
Mandrup-Poulsen T and Fleischmann R: Interleukin-6 receptor
blockade or TNFα inhibition for reducing glycaemia in patients with
RA and diabetes: Post hoc analyses of three randomised, controlled
trials. Arthritis Res Ther. 22:2062020. View Article : Google Scholar : PubMed/NCBI
|
|
367
|
Drutskaya MS, Efimou GA, Kruglou AA and
Nedospasou SA: Can we design a better anti-cytokine therapy? Semin
Arthritis and Rhematism. 49:S39–S42. 2019.PubMed/NCBI
|
|
368
|
Nosenko MA, Atretkhany KSN, Mokhonov VV,
Vasilenko EA, Kruglov AA, Tillib SV, Drutskaya MS and Nedospasov
SA: Moduatation of bioavailability of proinflammatory cytokines
produced by myeloid cells. Semin Arthritis Rheum. 49:S39–S42. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
369
|
Velikova TV, Kabakchieva PP, Assyov YS and
Georgiev TA: Targeting inflammatory cytokines to improve type 2
diabetes control. Biomed Res Int. 2021:72974192021. View Article : Google Scholar : PubMed/NCBI
|
|
370
|
Achari A and Jain SK: Adiponectin, a
therapeutic target for obesity, diabetes, and endothelial
dysfunction. Int J Mol Sci. 18:13212017. View Article : Google Scholar : PubMed/NCBI
|
|
371
|
Yamauchi T, Kamon J, Waki H, Terauchi Y,
Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka T,
et al: The fat-derived hormone adiponectin reverses insulin
resistance associated with both lipoatrophy and obesity. Nat Med.
7:941–946. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
372
|
Combs TP, Wagner JA, Berger J, Doebber T,
Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, et al:
Induction of adipocyte complement-related protein of 30 kilodaltons
by PPRgamma agonists: A potential mechanism of insulin
sensitization. Endocrinology. 143:998–1007. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
373
|
Kolak M, Yki-Järvinen H, Kannisto K,
Tiikkainen M, Hamsten A, Eriksson P and Fisher RM: Effects of
chronic rosiglitazone therapy on gene expression in human adipose
tissue in vivo in patients with type 2 diabetes. J Clin Endocrinol
Metab. 92:720–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
374
|
Peraldi P, Xu M and Spiegelman BM:
Thiazolidinediones block tumor necrosis factor-alpha-incuded
inhibition of insulin signaling. J Clin Invest. 100:1863–1869.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
375
|
Wolf AM, Wolf D, Rumpold H, Enrich B and
Tilg H: Adiponectin induces the anti-inflammatory cytokines IL-10
and IL-1RA in human leukocytes. Biochem Biophys Res Commun.
323:630–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
376
|
Mosser DM and Zhang X: Interleukin-10: New
perspectives on an old cytokine. Immunol Rev. 226:205–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
377
|
Maclsaac RJ and Jerum G: Clinical
indications for thiazolidinediones. Aust Prescr. 27:70–74. 2004.
View Article : Google Scholar
|
|
378
|
Quinn CE, Hamilton PK, Lockhart CJ and
McVeigh GE: Thiazolidinediones: Effects on insulin resistance and
the cardiovascular system. Br J Pharmacol. 153:636–645. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
379
|
Graham DJ, Green L, Senior JR and Nourjah
P: Troglitazone-induced liver failure: A case study. Am J Med.
114:299–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
380
|
Tuccori M, Filion KB, Yin H, Yu OH, Platt
RW and Azoulay L: Pioglitazone use and risk of bladder cancer:
Population based cohort study. BMJ. 352:i15412016. View Article : Google Scholar : PubMed/NCBI
|
|
381
|
Aronoff S, Rosenblatt S, Braithwaite S,
Egan JW, Mathisen AL and Schneider RL: Pioglitazone hydrochloride
monotherapy improves glycemic control in the treatment of patients
with type 2 diabetes: A 6-month randomized placebo-controlled
dose-response study. The pioglitazone 001 study group. Diabetes
Care. 23:1605–1611. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
382
|
Mudaliar S, Chang AR and Henry RR:
Thiazolidinediones, peripheral edema, and type 2 diabetes:
Incidence, pathophysiology, and clinical implications. Endocr
Pract. 9:406–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
383
|
Arnold SV, Inzucchi SE, Echouffo-Tcheugui
JB, Tang F, Lam CSP, Sperling LS and Kosiborod M: Understanding
contemporary use of thiazolidinediones. Cir Heart Fail.
12:e0058552019. View Article : Google Scholar : PubMed/NCBI
|
|
384
|
Ferris FL III and Patz A: Macular edema. A
complication of diabetic retinopathy. Surv Ophthalmol. 28:452–461.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
385
|
Ryan EH Jr, Han DP, Ramsay RC, Cantrill
HL, Bennett SR, Dev S and Williams DF: Diabetic macular edema
associated with glitazone use. Retina. 26:562–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
386
|
Vestergaard P: Discrepacies in bone
mineral density and fracture risk in patients with type 1 and type
2 diabetes-a meta-analysis. Osteoporos Int. 18:427–444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
387
|
Fonseca V: Effect of thiazolidinediones on
body weight in patients with diabetes mellitus. Am J Med.
115:42–48. 2003. View Article : Google Scholar
|
|
388
|
Ko KD, Kim KK and Lee KR: Does weight gain
associated with thiazolidinedione use negatively affect
cardiometabolic health? J Obes Metab Syndr. 26:102–106. 2017.
View Article : Google Scholar : PubMed/NCBI
|